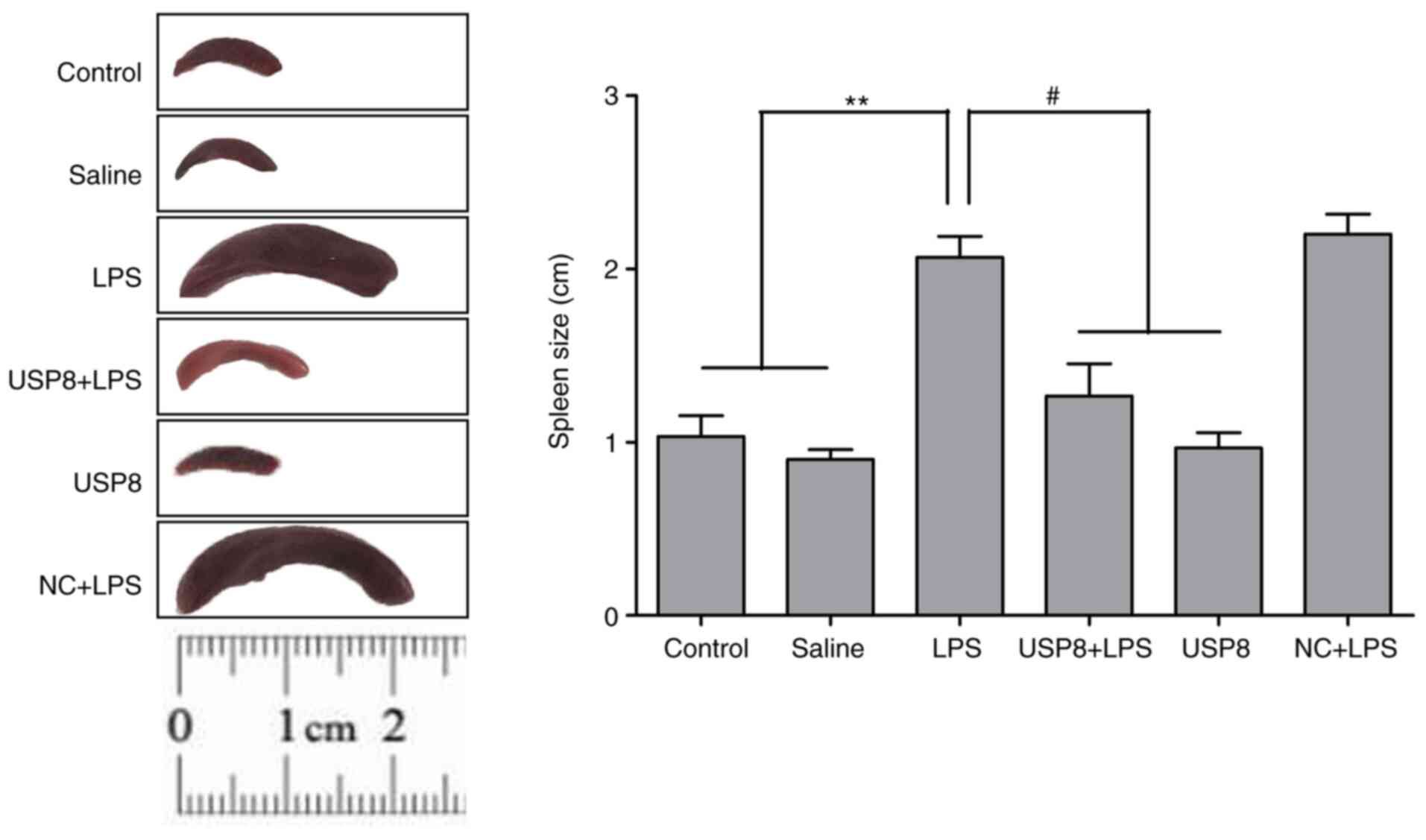
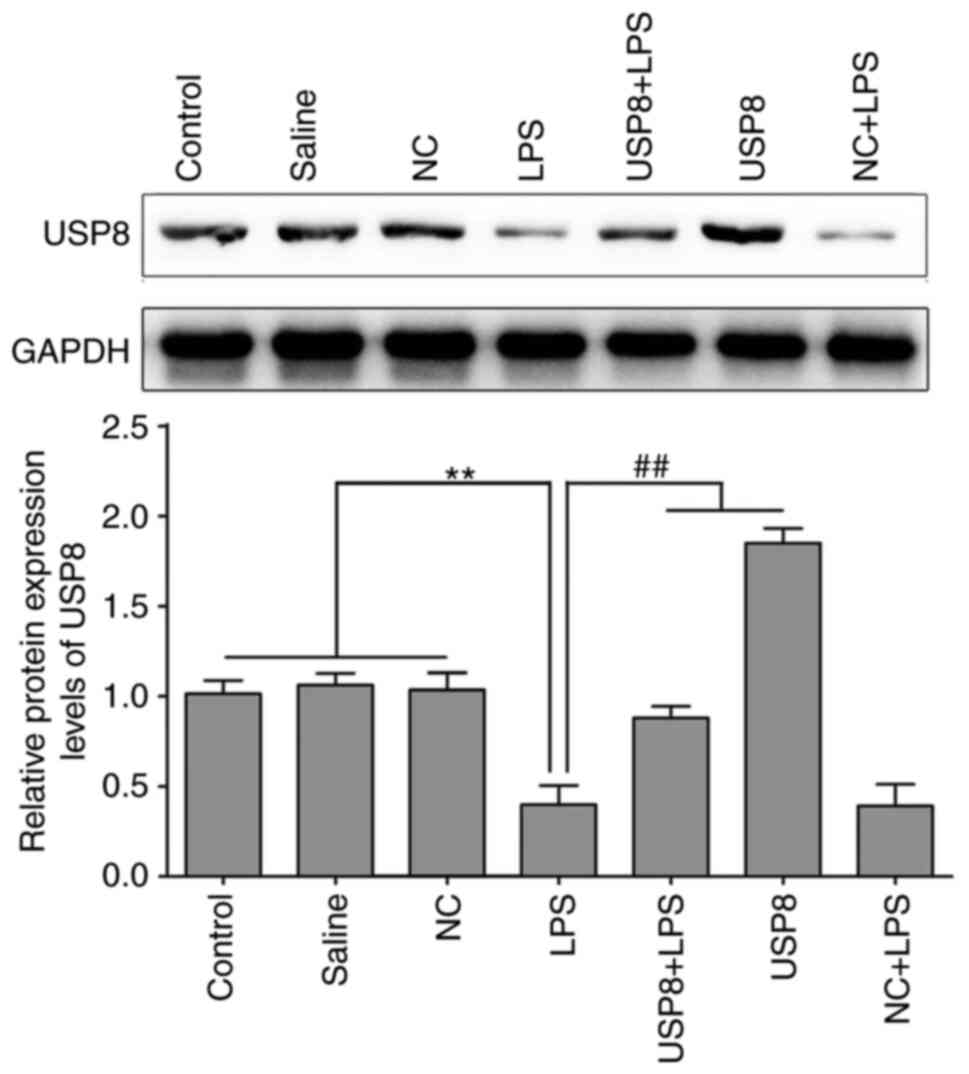
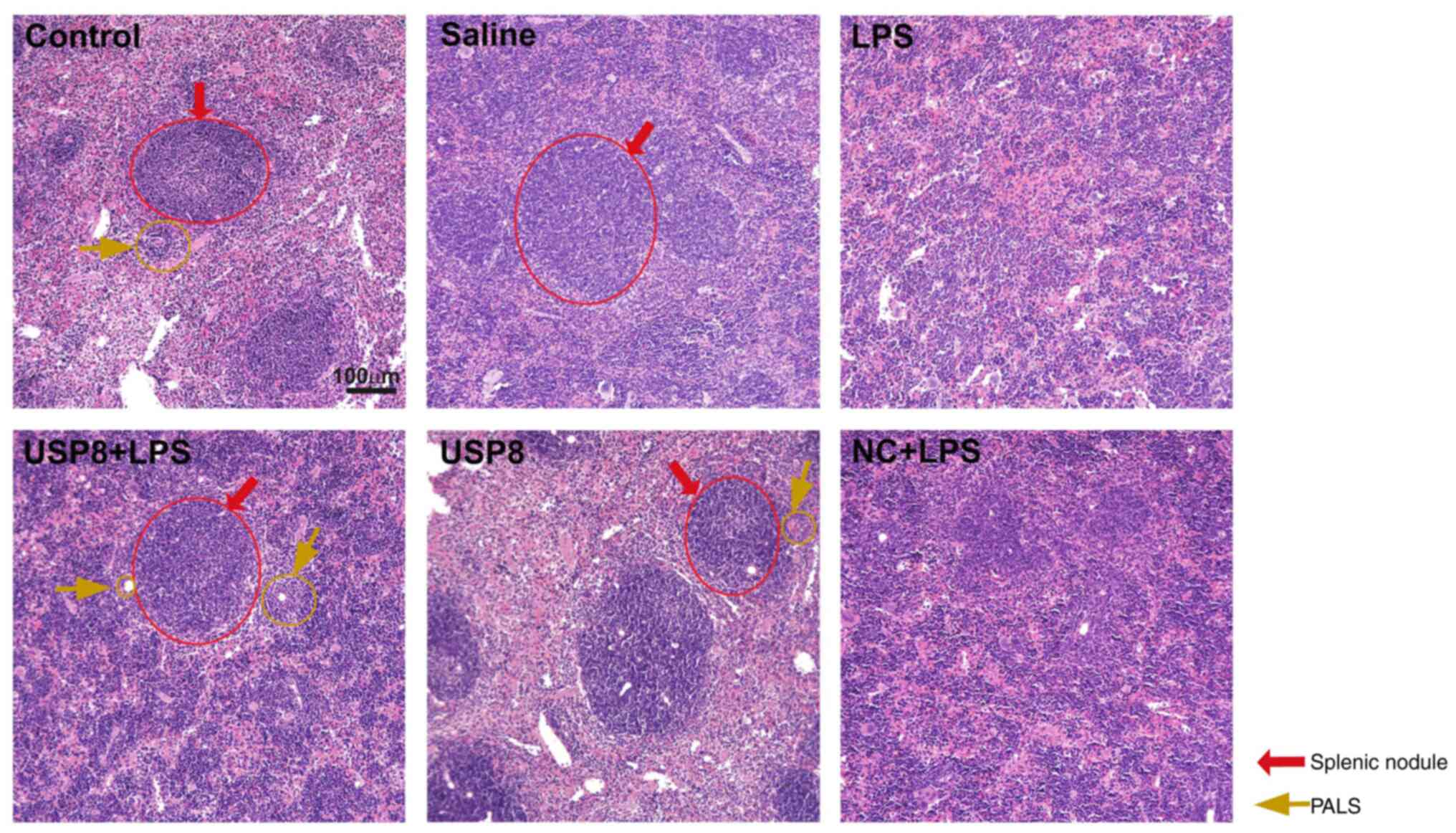
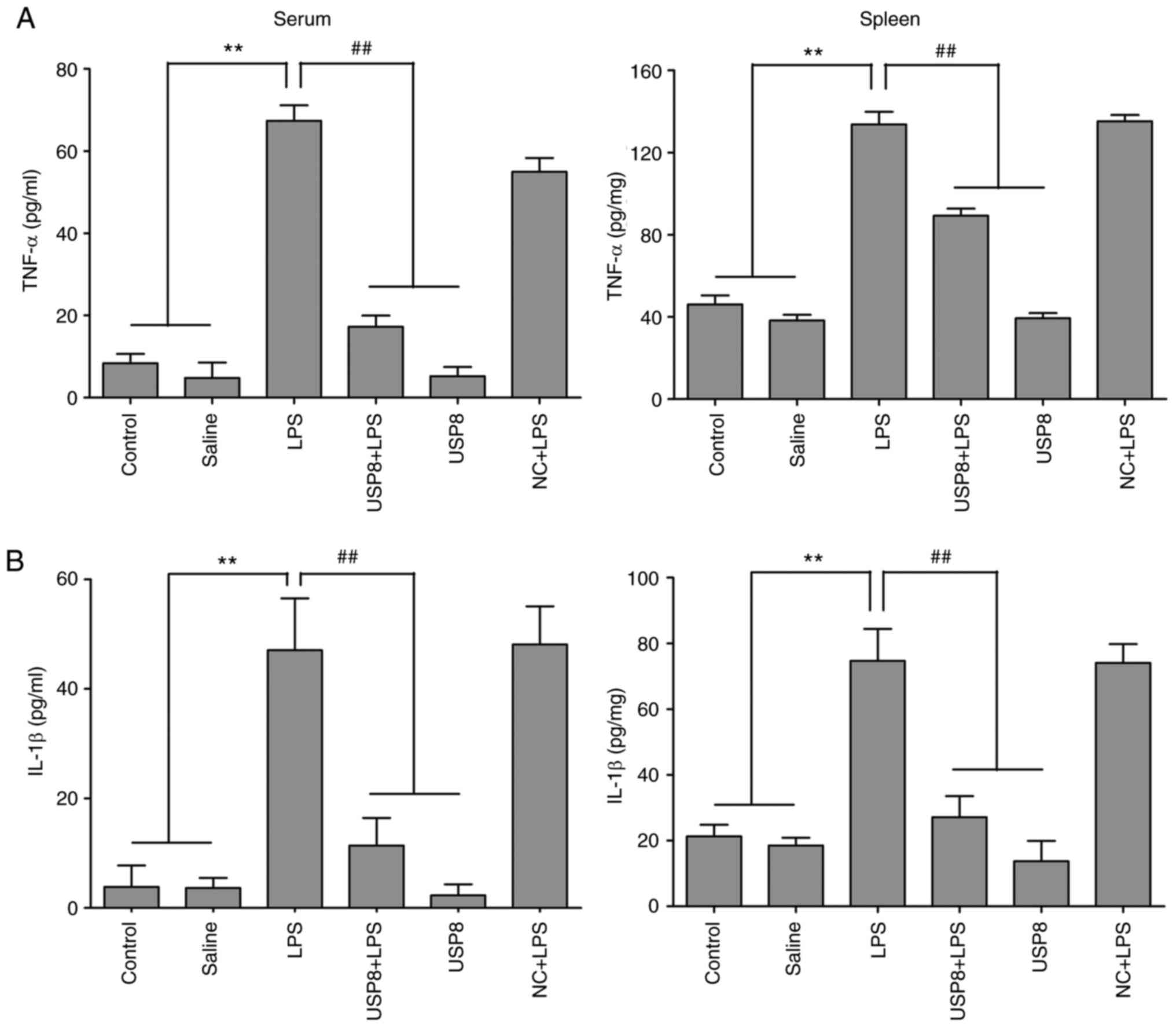
|
1
|
Jiang LN, Yao YM and Sheng ZY: The role of
regulatory T cells in the pathogenesis of sepsis and its clinical
implication. J Interferon Cytokine Res. 32:341–349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altamura M, Caradonna L, Amati L,
Pellegrino NM, Urgesi G and Miniello S: Splenectomy and sepsis: The
role of the spleen in the immune-mediated bacterial clearance.
Immunopharmacol Immunotoxicol. 23:153–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noh H, Jeon J and Seo H: Systemic
injection of LPS induces region-specific neuroinflammation and
mitochondrial dysfunction in normal mouse brain. Neurochem Int.
69:35–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Badshah H, Ali T and Kim MO: Osmotin
attenuates LPS-induced neuroinflammation and memory impairments via
the TLR4/NFκB signaling pathway. Sci Rep. 6:244932016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahimifard M, Maqbool F, Moeini-Nodeh S,
Niaz K, Abdollahi M, Braidy N, Nabavi SM and Nabavi SF: Targeting
the TLR4 signaling pathway by polyphenols: A novel therapeutic
strategy for neuroinflammation. Ageing Res Rev. 36:11–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao K, Zou WH, Yang Z, Rehman ZU, Ansari
AR, Yuan HR, Zhou Y, Cui L, Peng KM and Song H: The role of
visfatin on the regulation of inflammation and apoptosis in the
spleen of LPS-treated rats. Cell Tissue Res. 359:605–618. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nijman SM, Luna-Vargas MP, Velds A,
Brummelkamp TR, Dirac AM, Sixma TK and Bernards R: A genomic and
functional inventory of deubiquitinating enzymes. Cell.
123:773–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soares L, Seroogy C, Skrenta H,
Anandasabapathy N, Lovelace P, Chung CD, Engleman E and Fathman CG:
Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat
Immunol. 5:45–54. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naviglio S, Mattecucci C, Matoskova B,
Nagase T, Nomura N, Di Fiore PP and Draetta GF: UBPY: A
growth-regulated human ubiquitin isopeptidase. EMBO J.
17:3241–3250. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niendorf S, Oksche A, Kisser A, Löhler J,
Prinz M, Schorle H, Feller S, Lewitzky M, Horak I and Knobeloch KP:
Essential role of ubiquitin-specific protease 8 for receptor
tyrosine kinase stability and endocytic trafficking in vivo. Mol
Cell Biol. 27:5029–5039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu L, Bi W and Lu D, Zhang C, Shu X, Wang
H, Qi R, Shi Q and Lu D: Regulation of ubiquitin-specific
processing protease 8 suppresses neuroinflammation. Mol Cell
Neurosci. 64:74–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Bi W, Zhang J, Xiao S, Zhou R,
Tsang CK, Lu D and Zhu L: USP8 protects against
lipopolysaccharide-induced cognitive and motor deficits by
modulating microglia phenotypes through TLR4/MyD88/NF-κB signaling
pathway in mice. Brain Behav Immun. 88:582–596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. National Academies Press (US); Washington DC,
USA: pp. 21–48. 1996
|
|
14
|
Haley TJ and Mccormick WG: Pharmacological
effects produced by intracerebral injection of drugs in the
conscious mouse. Br J Pharmacol Chemother. 12:12–15. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tunstall ME and Sheikh A: Comparison of
1.5% enflurane with 1.25% isoflurane in oxygen for caesarean
section: Avoidance of awareness without nitrous oxide. Br J
Anaesth. 62:138–143. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao Z, Pan X, Zhang H, Sun J, Li J, Lu T,
Gao M, Liu S, Yu D and Ding Z: Isoflurane preconditioning
alleviated murine liver ischemia and reperfusion injury by
restoring AMPK/mTOR-Mediated Autophagy. Anesth Analg.
125:1355–1363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin S, Yin Q, Zhong Q, Lv FL, Zhou Y, Li
JQ, Wang JZ, Su BY and Yang QW: Heme activates TLR4-mediated
inflammatory injury via MyD88/TRIF signaling pathway in
intracerebral hemorrhage. J Neuroinflamm. 9:462012. View Article : Google Scholar
|
|
18
|
Hernández-Romero MC, Delgado-Cortés MJ,
Sarmiento Ml, de Pablos RM, Espinosa-Oliva AM, Argüelles S, Bández
MJ, Villarán RF, Mauriño R, Santiago M, et al: Peripheral
inflammation increases the deleterious effect of CNS inflammation
on the nigrostriatal dopaminergic system. Neurotoxicology.
33:347–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ozato K, Tsujimura H and Tamura T:
Toll-like receptor signaling and regulation of cytokine gene
expression in the immune system. Biotechniques. (Suppl):66–68. 7072
passim. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koistinaho M and Koistinaho J: Role of p38
and p44/42 mitogen-activated protein kinases in microglia. Glia.
40:175–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallastegui N and Groll M: The 26S
proteasome: Assembly and function of a destructive machine. Trends
Biochem Sci. 35:634–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu X, Yen L, Irwin L, Sweeney C and
Carraway KL III: Stabilization of the E3 ubiquitin ligase Nrdp1 by
the deubiquitinating enzyme USP8. Mol Cell Biol. 24:7748–7757.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Chen T, Zhang J, Yang M, Li N, Xu
X and Cao X: The E3 ubiquitin ligase Nrdp1 ‘preferentially’
promotes TLR-mediated production of type I interferon. Nat Immunol.
10:744–752. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bi W, Lan X, Zhang J, Xiao S, Cheng X,
Wang H, Lu D and Zhu L: USP8 ameliorates cognitive and motor
impairments via microglial inhibition in a mouse model of
sepsis-associated encephalopathy. Brain Res. 1719:40–48. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang WH, Yao YM, Shi ZG, Yu Y, Wu Y, Lu LR
and Sheng ZY: Effect of recombinant
bactericidal/permeability-increasing protein on endotoxin
translocation and lipopolysaccharide-binding protein/CD14
expression in rats after thermal injury. Crit Care Med.
29:1452–1459. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mallard C: Innate immune regulation by
toll-like receptors in the brain. ISRN Neurol. 2012:7019502012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nair AR, Masson GS, Ebenezer PJ, Del Piero
F and Francis J: Role of TLR4 in lipopolysaccharide-induced acute
kidney injury: Protection by blueberry. Free Radic Biol Med.
71:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Petrova TV, Akama KT and Van Eldik LJ:
Cyclopentenone prostaglandins suppress activation of microglia:
Down-regulation of inducible nitric-oxide 12,14-synthase by
15-deoxy-prostaglandin J2. Proc Natl Acad Sci USA. 96:4668–4673.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye SM and Johnson RW: Regulation of
interleukin-6 gene expression in brain of aged mice by nuclear
factor kappaB. J Neuroimmunol. 117:87–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nithianandarajah-Jones GN, Wilm B,
Goldring CE, Müller J and Cross MJ: ERK5: Structure, regulation and
function. Cell Signal. 24:2187–2196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meng AH, Ling YL, Zhang XP, Zhao XY and
Zhang JL: CCK-8 inhibits expression of TNF-alpha in the spleen of
endotoxic shock rats and signal transduction mechanism of p38 MAPK.
World J Gastroenterol. 8:139–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mormède C, Palin K, Kelley KW, Castanon N
and Dantzer R: Conditioned taste aversion with lipopolysaccharide
and peptidoglycan does not activate cytokine gene expression in the
spleen and hypothalamus of mice. Brain Behav Immun. 18:186–200.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bland T, Sahin GS, Zhu M, Dillon C, Impey
S, Appleyard SM and Wayman GA: USP8 deubiquitinates the leptin
receptor and is necessary for leptin-mediated synapse formation.
Endocrinology. 160:1982–1998. 2019. View Article : Google Scholar : PubMed/NCBI
|