Introduction
Asthma has long been a global health issue,
affecting approximately 262 million people in 2019 (1) and causing 455,000 mortalities
(2). The established asthma risk
factors (3) includes air
pollutants, allergens, microbial products and obesity. According to
the updated National Asthma Education Prevention Program guidelines
(4), the combination of inhaled
corticosteroids and long-acting β-agonist is the current mainstream
asthma treatment. Asthma is a heterogeneous disease with various
phenotypes or endotypes, including eosinophilic asthma and
non-eosinophilic asthma (5).
Eosinophilic airway inflammation is a hallmark of allergic asthma;
this type of inflammation may explain the symptoms of asthma,
including airway hyperresponsiveness (AHR) (5), airway remodeling (5) and mucus overproduction (6).
Oxidative stress is a cellular condition in which
the production of reactive oxygen species (ROS) exceeds the
antioxidant content of the cell (7). This process is known to serve a
major role in the pathogenesis and progression of asthma, and
although it is considered to be a significant part of the
inflammatory response, it can also stimulate inflammation in asthma
(8). The inflammatory cells,
notably eosinophils, are an important source of ROS following
antigenic challenge; excessive production of ROS can cause damage
to the lipids, proteins and DNA in cells (9). Increased ROS production is closely
related to a decrease in the forced expiratory volume in 1 sec. It
has also been reported that pulmonary function is negatively
related to oxidative stress markers and positively related to serum
antioxidant levels (10). The
levels of antioxidants, such as malondialdehyde (MDA), and
oxidative stress markers, such as reduced glutathione (GSH), have
also been reported to be linked to the severity of asthma (11).
The cellular antioxidant system, which maintains
redox homeostasis, consists of both enzymatic and non-enzymatic
antioxidants. The transcription factor nuclear factor erythroid
2-related factor (Nrf2) is crucial in ROS scavenging (12). As a result of oxidative stress,
Nrf2 is dissociated from kelch-like ECH-associated protein-1
(Keap1) and translocated to the nucleus where it can activate
>200 antioxidant genes. These gene products are crucial for the
antioxidant, anti-inflammatory and cytoprotective functions of the
cells (13). Superoxide dismutase
(SOD), heme oxygenase-1 (HO-1), quinone oxidoreductase-1 and
glutathione peroxidase (GPx) are the main antioxidant molecules.
Accumulating evidence has demonstrated the importance of the Nrf2
pathway in asthma, as eosinophilic inflammation; AHR and
inflammatory cytokine production have been reported in Nrf2
knockout mice (14–16).
Dexmedetomidine (DEX) is a selective α2-adrenergic
receptor agonist, which is commonly used as a sedative in clinical
practice (17). Several studies
have reported organ-protective effects and anti-inflammatory
effects of DEX. For example, it has been reported that DEX
attenuates neuroinflammation (18), protects the lung from injury
induced by limb ischemia/reperfusion (19), protects hepatic cells from
oxygen-glucose deprivation/reperfusion injury (20) and reduces the levels of
proinflammatory mediators (IL-6, TNF-α, IL-10 and IL-1) in an acute
lung injury model in vivo (21). Oxidative stress serves a major
role in inducing organ injury and inflammation, and the
antioxidative effects of DEX have also been reported by a number of
studies (22–24). Our previous study demonstrated
that administration of DEX could reduce airway eosinophilic
inflammation, AHR and mucus production in an ovalbumin
(OVA)-induced murine asthma model (25). In light of the potential role of
oxidative stress in the pathogenesis of asthma, it was hypothesized
that DEX could reduce the symptoms of allergic asthma by decreasing
oxidative stress. To evaluate this hypothesis, the present study
assessed the antioxidative effect of DEX on an allergic murine
asthma model.
Materials and methods
Animals
A total of 25 female Balb/c mice (age, 6–7 weeks;
weight, 15–20 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. The mice were kept in the animal
experimental center of the Plastic Surgery Hospital Chinese Academy
of Medical Sciences and Peking Union Medical College (Beijing,
China) under a controlled temperature (25°C) and humidity (45–55%),
with a 12-h light-dark cycle. The mice were fed with sterilized
food and water ad libitum and were acclimated for 1 week
prior to the experiment. The protocol of the animal experiments was
approved by the Institutional Animal Care and Use Committee of
Plastic Surgery Hospital, Chinese Academy of Medical Sciences and
Peking Union Medical College [approval no. 2022(201)].
Animal experiments
The mice were divided into five groups (n=5) as
follows: i) Control group; ii) OVA group; iii) DEX group; iv) OVA +
DEX group; and v) OVA + DEX + ML385 group. The protocol used for
the OVA-induced murine asthma model was described in our previous
work (25). In brief, the mice in
the control group received an intraperitoneal (i.p.) injection of
0.2 ml saline on days 0, 7 and 14 for sensitization, and were
administered 30 µl saline intranasally for the challenge on days
21–28. The mice in the DEX group were sensitized with i.p.
injection of 0.2 ml saline on days 0, 7 and 14, and received an
i.p. injection of 30 µg/kg DEX 1 h prior to intranasal
administration of 30 µl saline on days 21–28. The mice in the OVA,
OVA + DEX and OVA + DEX + ML385 groups received an i.p. injection
of 0.2 ml saline containing 100 µg OVA (cat. no. A5503;
Sigma-Aldrich; Merck KGaA) and 10 mg aluminum hydroxide (cat. no.
239186; Sigma-Aldrich; Merck KGaA) on days 0, 7 and 14 for
sensitization. From day 21 to 28, the mice in the OVA group were
administered 30 µl saline containing 200 µg OVA intranasally for
the challenge. The mice in the OVA + DEX group were injected i.p.
with 30 µg/kg DEX and were challenged with 30 µl saline containing
200 µg OVA intranasally 1 h after DEX injection; the mice in the
OVA + DEX+ML385 group were injected i.p. with 30 mg/kg ML385
working solution (cat. no. 846557-71-9; MedChemExpress) 1 h prior
to the i.p. injection of 30 µg/kg DEX and were challenged with 30
µl saline containing 200 µg OVA intranasally 1 h following DEX
injection. The preparation method for the ML385 working solution
was as follows: ML385 was diluted with DMSO (cat. no. K91281;
Beijing Kerhui Technology Co., Ltd.) and corn oil to a
concentration of 2 mg/ml; the final concentration of DMSO was 1%.
Following the assessment of AHR, bronchoalveolar lavage fluid
(BALF) was collected on day 29 for subsequent analysis.
Assessment of AHR
The respiratory resistance (Rrs) (cm
H2O.s/ml) was assessed for the mice in each group using
a flexiVent lung function system (SCIREQ) 24 h following the last
challenge. Prior to the tracheotomy and intubation, the mice were
i.p. anesthetized with 2% pentobarbital sodium (50 mg/kg).
Following intubation, the mice were fixed to a platform and allowed
to inhale aerosolized methacholine (Mch) at different
concentrations (0, 6, 12, 24 and 48 mg/ml) through the tube for 6
min. The results presented for the different concentrations of Mch
inhaled by each mouse comprised the mean absolute value of the data
collected during the 6 min period.
Analysis of BALF
Immediately following AHR assessment, the mice were
euthanized by i.p. injection of 2% pentobarbital sodium (100
mg/kg); the mice were considered to be at a terminally-anesthetized
state when they stopped breathing, the heart stopped beating and
they did not response to stimuli (26). Subsequently, a tube was inserted
into the trachea of each mouse for lung lavage. The lungs were
lavaged three times using 0.8 ml Dulbecco's phosphate-buffered
saline (DPBS) and ~90% of the lavage volume was collected.
Following centrifugation at 187 × g at 4°C for 10 min, the
supernatant of the BALF was collected and stored at −80°C for use
in ELISA and the cells were resuspended in 500 µl DPBS for cell
counting. The total cell count in the BALF samples was assessed
using a chamber slide and Wright-Giemsa-stained BALF smears were
made for eosinophil and lymphocyte counts. The assay was performed
using a Wright-Giemsa staining kit (cat. no. G1020; Beijing
Solarbio Science & Technology Co., Ltd.) according to the
manufacturer's protocol (staining at room temperature for 1.5 min).
The smears were assessed under ×200 magnification using a BX53
upright light microscope (Olympus Corporation). The eosinophil and
lymphocyte counts were assessed using ImageJ software (version
1.8.0; National Institutes of Health).
Measurement of cytokines in BALF
The levels of Th2 cytokines (including IL-4, IL-5,
and IL-13) were detected using commercial ELISA kits purchased from
Boster Biological Technology (IL-4: cat. no. EK0405; IL-5: cat. no.
EK0408; IL-13: cat. no. EK0425). Detections were performed
according to the manufacturer's instructions. The concentrations of
cytokines were determined by measuring absorbance at 450 nm using a
multimode plate reader (cat. no. HH34000000; PerkinElmer).
Hematoxylin and eosin (H&E) and
periodic acid-Schiff (PAS) staining
The right lobes of the lung were collected 24 h
after the last challenge, following euthanasia, and fixed using 4%
paraformaldehyde (cat. no. P1110; Beijing Solarbio Science &
Technology Co., Ltd.) for 48 h at room temperature. The following
procedures were all performed at room temperature. H&E and PAS
staining were performed on different lung sections. For H&E
staining, the lung tissues were embedded in paraffin and
subsequently cut into 5 µm thick sections. Deparaffinization of the
sections was performed using xylene and hydration was performed by
passing the sections through changes of ethanol with different
concentrations (100% ethanol for 2 min; 100% ethanol for 2 min; 95%
ethanol for 2 min). The sections were subsequently stained with
hematoxylin for 1 min at room temperature. Following rinsing in tap
water for 5 min, nuclear staining was finished following incubation
in an alkaline solution (Scott's Bluing Solution; cat. no. G1865;
Beijing Solarbio Science & Technology Co., Ltd.) for 20 sec.
Following differentiation of the sections for 3 sec, they were
stained using alcoholic-eosin for 1 min and subsequently dehydrated
using an ascending alcohol series.
PAS staining was performed using the Periodic
Acid-Schiff (PAS) stain kit (cat. no. G1280; Beijing Solarbio
Science & Technology Co., Ltd.) according to the manufacturer's
protocol. In brief, after being fixed using 4% paraformaldehyde
(cat. no. P1110; Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 48 h and then placed in 30% sucrose
(1X PBS) until lung tissues sank, the lung tissues were
subsequently embedded in OTC and frozen at −20°C. Then, 5 µm-thick
frozen sections were washed with tap water for 2 min and rinsed
with double-distilled H2O once. The sections were
incubated with the oxidizing agent contained in the PAS stain kit
for 6.5 min and rinsed with tap water for 10 min. The slides were
incubated with Schiff reagent for 15 min and subsequently washed
with tap water for 15 min. The nuclei were stained using
hematoxylin for 50 sec. Following washing with tap water for 2 min,
the slides were re-stained with an alkaline solution (Scott's
Bluing Solution; cat. no. G1865; Beijing Solarbio Science &
Technology Co., Ltd.) for 20 sec and differentiated for 3 sec. Five
fields of view were assessed by two experienced pathologists in a
blinded study design at ×200 magnification using a BX53 upright
light microscope (Olympus Corporation). The color imbalance was
corrected as described by Marty (27). In brief, auto exposure was used to
capture a tissue image and a blank image which were saved and
processed with Adobe Photoshop CS6 (Adobe, Inc.) as follows: i) The
blank image was inverted and the layer duplicated to the original
tissue image; ii) in the window with the tissue image, Color Dodge
in the Layers Palette was to achieve a uniform white background;
iii) the image was flattened and then saved as the final
version.
The scoring systems for H&E and PAS staining
were described in our previous work (25). In brief, the score for H&E
staining represented the infiltration of inflammatory cells around
the airways and was presented as follows: 0, normal; 1, a low
number of cells present around the airways; 2, one cell layer was
present around the airways; 3, 2–4 cell layers were present around
the airways; 4, 5–7 cell layers were present around the airways; 5,
8–10 cell layers were present around the airways; and 6, >10
cell layers were present around the airways. The percentage of
PAS-positive cells in each slide was assessed using ImageJ software
(version 1.8.0; National Institutes of Health) and scored as
follows: 0, <2%; 1, ≥2 to <20%; 2, ≥20 to <40%; 3, ≥40 to
<60%; 4, ≥60 to <80%; and 5, ≥80% PAS-positive cells.
Dihydroethidium (DHE) staining
DHE (cat. no. S0063) was purchased from Beyotime
Institute of Biotechnology and the staining process was performed
according to the manufacturer's protocols. Briefly, frozen lung
sections were prepared as those used for PAS staining and were
treated with DHE working reagent (5 µM) at 37°C in the dark for 30
min. The slides were sealed with a sealing reagent containing DAPI
(cat. no. P0131; Beyotime Institute of Biotechnology) at room
temperature for 1 min. The slides were assessed using a BX53
fluorescence microscope (Olympus Corporation) at 535 nm.
Unprocessed frozen sections of lung tissues were used as an
endogenous control to exclude autofluorescence and no
autofluorescence was observed in the unprocessed frozen sections at
488 nm (data not shown).
Measurement of MDA, SOD and GSH
levels
Specific commercial kits (purchased from Beyotime
Institute of Biotechnology) were used to determine the MDA content
(cat. no. S0131S), the activity levels of SOD (cat. no. S0103) and
the GSH content (cat. no. S0053) in the supernatant of the
homogenized lung tissues. Lung tissues (30 mg) were homogenized in
the sample preparation solution which was contained in the kits
using a Multi-sample tissue grinder (TissueLyser-24L; Shanghai
Jingxin). The assays were performed according to the manufacturer's
protocols. Total protein concentration was determined using the BCA
method (cat. no. P0012S; Beyotime Institute of Biotechnology)
according to the manufacturer's protocols.
Reverse transcription-quantitative PCR
(RT-qPCR)
A total of 50 mg lung tissue was homogenized in
TRIzol® (cat. no. 15596-026; Invitrogen; Thermo Fisher
Scientific, Inc.), which was used for total RNA extraction
according to the manufacturer's protocol. The total RNA was
resuspended in DNase/RNase-free water (cat. no. R1600; Beijing
Solarbio Science & Technology Co., Ltd.), and its concentration
was determined using a NanoDrop® 2000 ultraviolet
spectrophotometer (Thermo Fisher Scientific, Inc.). RNA samples
with a 260/280 absorbance ratio between 1.8 and 2.0 were used. A
total of 5 µg RNA was revers transcribed using the
TransScript® First-Strand cDNA Synthesis SuperMix (cat.
no. AT301-02; Beijing Transgen Biotech Co., Ltd.) in 20 µl reaction
volumes according to the manufacturer's protocol. The qPCR primer
sequences (Invitrogen; Thermo Fisher Scientific, Inc.) used were as
follows: Nrf2 forward (F), 5′-AGATGACCATGAGTCGCTTGC-3′ and reverse
(R), 5′-CCTGATGAGGGGCAGTGAAG-3′; Keap1 F,
5′-GCCCCGGGACTCTTATTGTG-3′ and R, 5′-TTAGGGGCCCCGCCAT-3′; HO-1 F,
5′-GCTAGCCTGGTGCAAGATACT-3′ and R, 5′-AAGCTGAGAGTGAGGACCCA-3′; GPx4
F, 5′-CCTCCCCAGTACTGCAACAG-3′ and R, 5′-GCACACGAAACCCCTGTACT-3′;
and β-actin F, 5′-CTCTTTTCCAGCCTTCCTTCTT-3′ and R,
5′-AGGTCTTTACGGATGTCAACGT-3′. The relative mRNA expression levels
of Nrf2, Keap1, HO-1 and GPx4 were normalized to those of β-actin.
The LightCycler® 480 SYBR Green I Master mix (cat. no.
04707516001) and the LightCycler® 96 Instrument for qPCR
were purchased from Roche Diagnostics GmbH and all the experimental
procedures were performed according to the manufacturer's protocol.
The thermocycling conditions for qPCR were as follows: Initial
denaturation at 95°C for 180 sec; a two-step amplification of 40
cycles of denaturation at 95°C for 10 sec and extension at 60°C for
30 sec. The data were quantified using the 2−ΔΔCq method
(28) and LightCycler®
96 software version 1.1 (Roche Diagnostics GmbH).
Western blotting
The lung tissues were washed with cold PBS and lysed
using RIPA lysis buffer (cat. no. C1053; Applygen Technologies,
Inc.) in the presence of Protease Phosphatase Inhibitor Cocktail
(cat. no. P1045; Beyotime Institute of Biotechnology). The
extraction of nuclear proteins was performed using a Nuclear and
Cytoplasmic Protein Extraction Kit (cat. no. P0028; Beyotime
Institute of Technology). The total and nuclear protein
concentrations were determined using the BCA Protein Assay Kit
(cat. no. P0012S; Beyotime Institute of Biotechnology). The
proteins were dissolved using SDS-PAGE Protein Sample Loading
Buffer (cat. no. P0286-15 ml; Beyotime Institute of Biotechnology)
and subsequently denatured. Protein samples (20 µg) were separated
using SDS-PAGE (4–20%). The protein bands were subsequently
transferred to nitrocellulose membranes and blocked using 5%
skimmed milk for 2 h at room temperature. The membranes were
incubated with primary antibodies overnight at 4°C. The membranes
were washed and incubated with the appropriate secondary antibodies
for 1 h at room temperature. Finally, the protein bands were
visualized using BeyoECL Plus detection reagent (cat. no. P0018S;
Beyotime Biotechnology Institute of Biotechnology). Band images
were obtained using Chemi-Doc Imaging System (cat. no. 12003153;
Bio-Rad Laboratories, Inc.). The semi-quantification of the bands
was performed using ImageJ software (version 1.8.0) according to a
publicly available protocol (29). The primary antibodies used in the
experiments were as follows: anti-Nrf2 (1:1,000; cat. no.
16396-1-AP; ProteinTech Group, Inc.), anti-Keap1 (1:1,000; cat. no.
10503-2-AP; ProteinTech Group, Inc.), anti-HO-1 (1:500; cat. no.
sc-390991; Santa Cruz Biotechnology, Inc.), anti-GPx4 (1:1,000;
cat. no. 67763-1-Ig; ProteinTech Group, Inc.), anti-β-actin
(1:1,000; cat. no. 66009-1-Ig; ProteinTech Group, Inc.),
anti-β-tubulin (1:2,000; cat. no. 10094-1-AP; ProteinTech Group,
Inc.) and anti-PCNA (1:2,000; cat. no. 10205-2-AP; ProteinTech
Group, Inc.). The secondary antibodies used for the experiments
were as follows: HRP-conjugated Affinipure goat anti-mouse IgG
(H+L). (1:2,000; cat. no. SA00001-1; ProteinTech Group, Inc.;),
HRP-conjugated Affinipure goat anti-rabbit IgG (H+L) (1:2,000; cat.
no. SA00001-2; ProteinTech Group, Inc.) and anti-mouse-IgGκ binding
protein-HRP (1:2,000; cat. no. sc-516102; Santa Cruz Biotechnology,
Inc.).
Statistical analysis
The data were analyzed using GraphPad Prism 9
(GraphPad Software, Inc.). Quantitative and semi-quantitative data
are presented as mean ± SD, and one-way ANOVA followed by
Bonferroni's multiple comparisons test was performed to assess the
differences between the groups. The histopathological scores are
presented as median + interquartile range, and the Kruskal-Wallis
test followed by Dunn's multiple comparisons test was performed to
assess the differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
DEX attenuates airway inflammation and
mucus overproduction
Allergic asthma is characterized by eosinophilic
airway inflammation and mucus overproduction (30). To assess airway inflammation and
mucus production in the lung tissues, BALF was collected for total
cell counting and Wright-Giemsa-stained BALF smears were used for
differential cell counts. Significant increases were demonstrated
in the total cell counts (P<0.0001), eosinophil counts
(P<0.0001) and lymphocyte counts (P<0.01) in the OVA group
compared with the control group (Figs. 1A-C and S1). In comparison with the OVA group,
DEX treatment significantly reduced the total cell and eosinophil
counts (both P<0.01) in the BALF; however no significant
difference was demonstrated for the lymphocyte count. Total cell,
eosinophil and lymphocyte counts in the DEX group were not
significantly different to that of the control group.
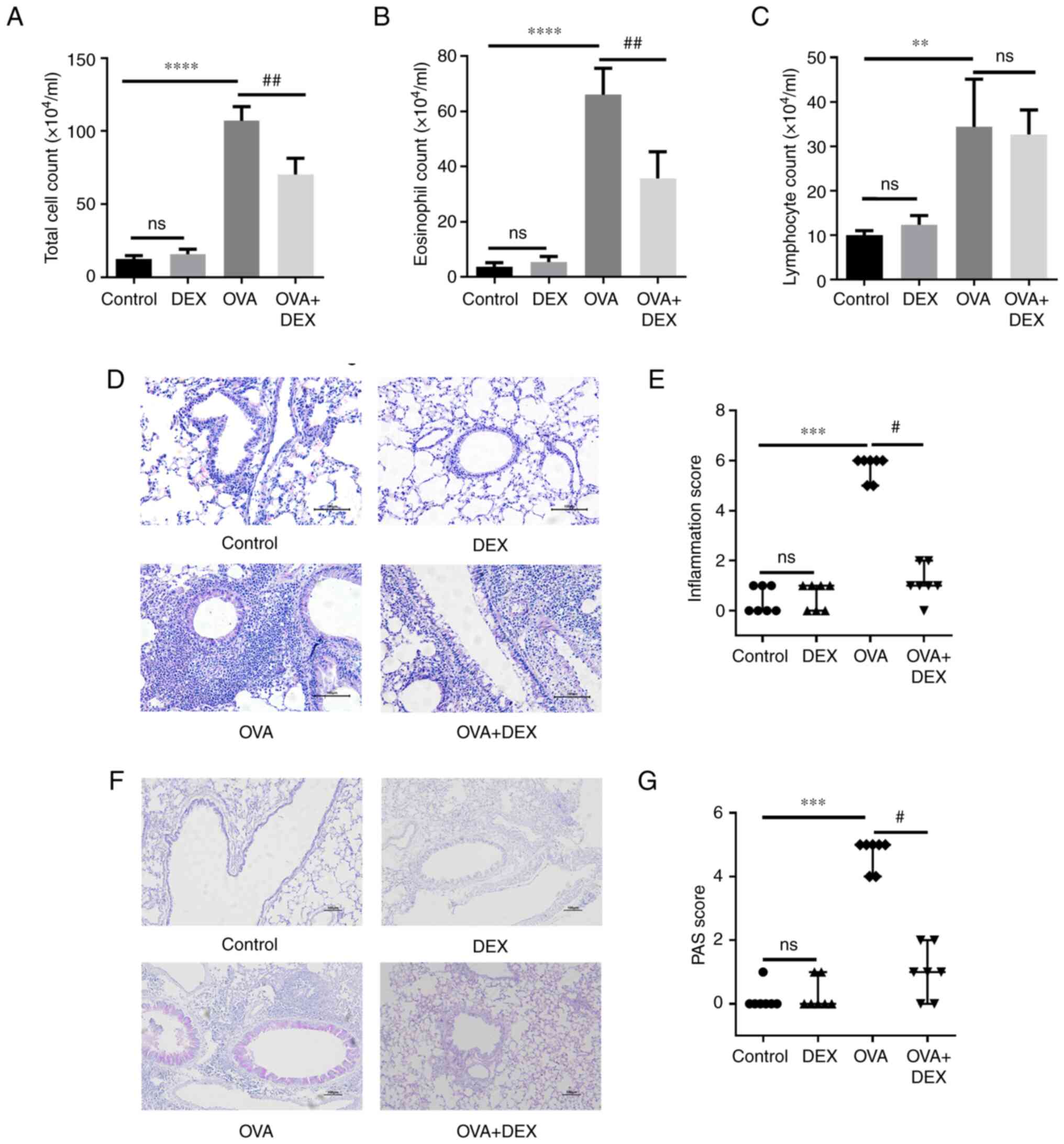 | Figure 1.DEX attenuates eosinophilic airway
inflammation and mucus overproduction. (A) Total cell, (B)
eosinophil and (C) lymphocyte counts in bronchoalveolar lavage
fluid samples. (D) H&E and (F) PAS staining were performed to
evaluate inflammatory cell infiltration and mucus production in
lung tissues, respectively. Magnification for H&E staining,
×200; magnification for PAS staining, ×100; scale bar, 100 µm. The
color imbalance was corrected as described by Marty (27). Histological scoring of (E)
inflammatory cell infiltration and (G) mucus production were based
on the layers of cells around the airways and the percentage of
PAS-positive cells, respectively. Quantitative data are presented
as the mean ± SD (n=3). Ordinal data are presented as median +
interquartile range (n=7). **P<0.01, ***P<0.001 and
****P<0.0001 vs. control; #P<0.05 and
##P<0.01 vs. OVA. DEX, dexmedetomidine; H&E,
hematoxylin and eosin; ns, not significant; OVA, ovalbumin; PAS,
periodic acid-Schiff. |
Lung sections were used for H&E and PAS staining
to evaluate the histopathological characteristics. The number of
the rings of the inflammatory cells infiltrating around the
airways, which were assessed as inflammation scores, was
significantly higher in the OVA group compared with the control
group (Fig. 1D and E;
P<0.001); DEX was able to significantly inhibit inflammatory
cell infiltration (P<0.05) compared with the OVA group. A
significant increase in the PAS-positive cells was demonstrated in
the OVA group compared with the control (Fig. 1F and G; P<0.001), and a
significant reduction in mucus overproduction was demonstrated in
the OVA + DEX group compared with the OVA group (P<0.05). DEX
treatment alone failed to induce histopathological changes in the
lung tissues.
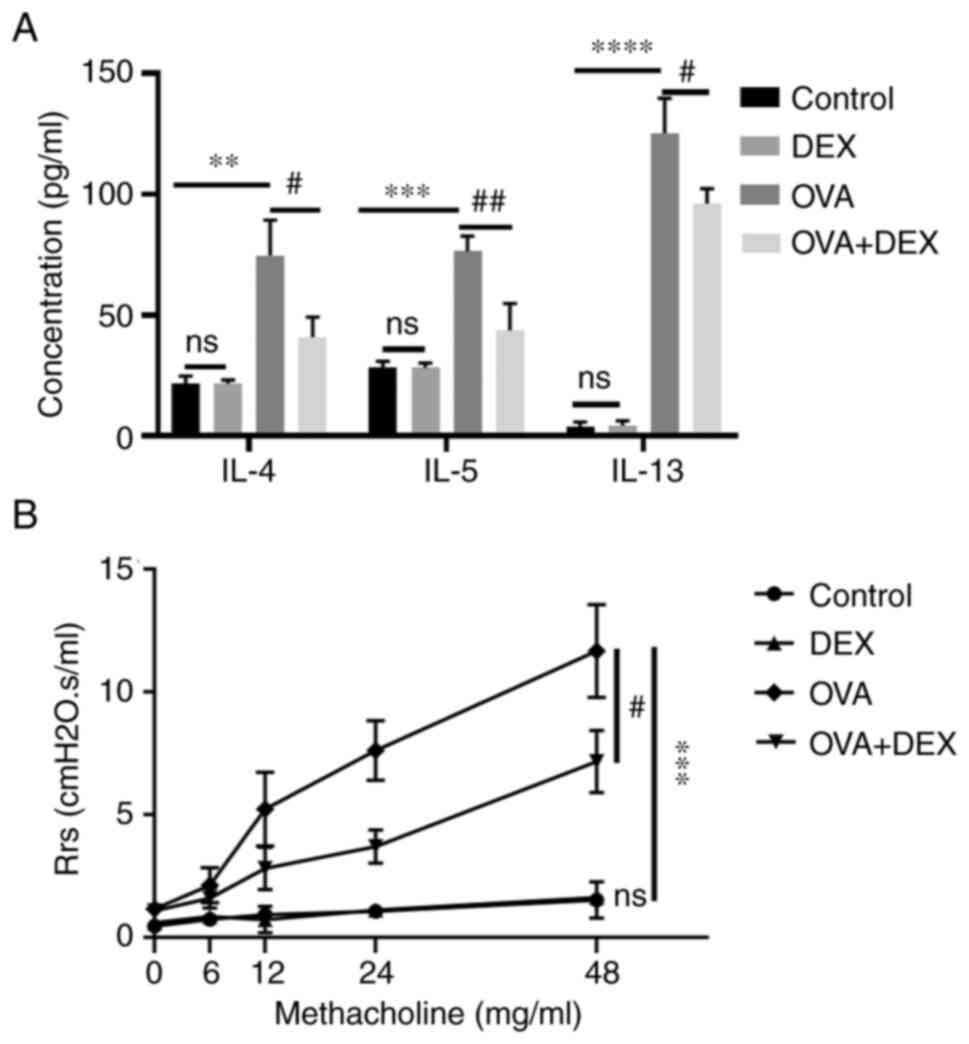
DEX reduces T helper (Th)2 cytokine
production and AHR
A Th2-dominant asthma model was established and the
content of the Th2 cytokines IL-4, IL-5 and IL-13 was assessed
using ELISA. The levels of these cytokines in BALF were
significantly elevated in the OVA group compared with those in the
control group (P<0.01 for IL-4, P<0.001 for IL-5, P<0.0001
for IL-13; Fig. 2A). The levels
of IL-4, IL-5 and IL-13 in the BALF were significantly decreased
following DEX administration compared with the OVA group (P<0.05
for IL-4, P<0.01 for IL-5, P<0.05 for IL-13; Fig. 2A). DEX administration alone did
not significantly affect the number of Th2 cytokines.
AHR is a crucial feature of asthma; both the airway
inflammation and Th2 cytokines (particularly IL-13) contribute to
its pathogenesis (31). Compared
with the control group, the respiratory resistance to inhaled Mch
(48 mg/ml; the Rrs for each concentration of Mch inhaled by each
animal was collected every minute for 6 min and the results were
presented as the mean absolute value of the data collected during
the 6 min period.) was significantly increased in the OVA group
(P<0.001; Fig. 2B). DEX
treatment significantly reduced the airway resistance compared with
the OVA group (P<0.05). DEX administration alone did not
significantly affect airway responsiveness compared with the
control (Fig. 2).
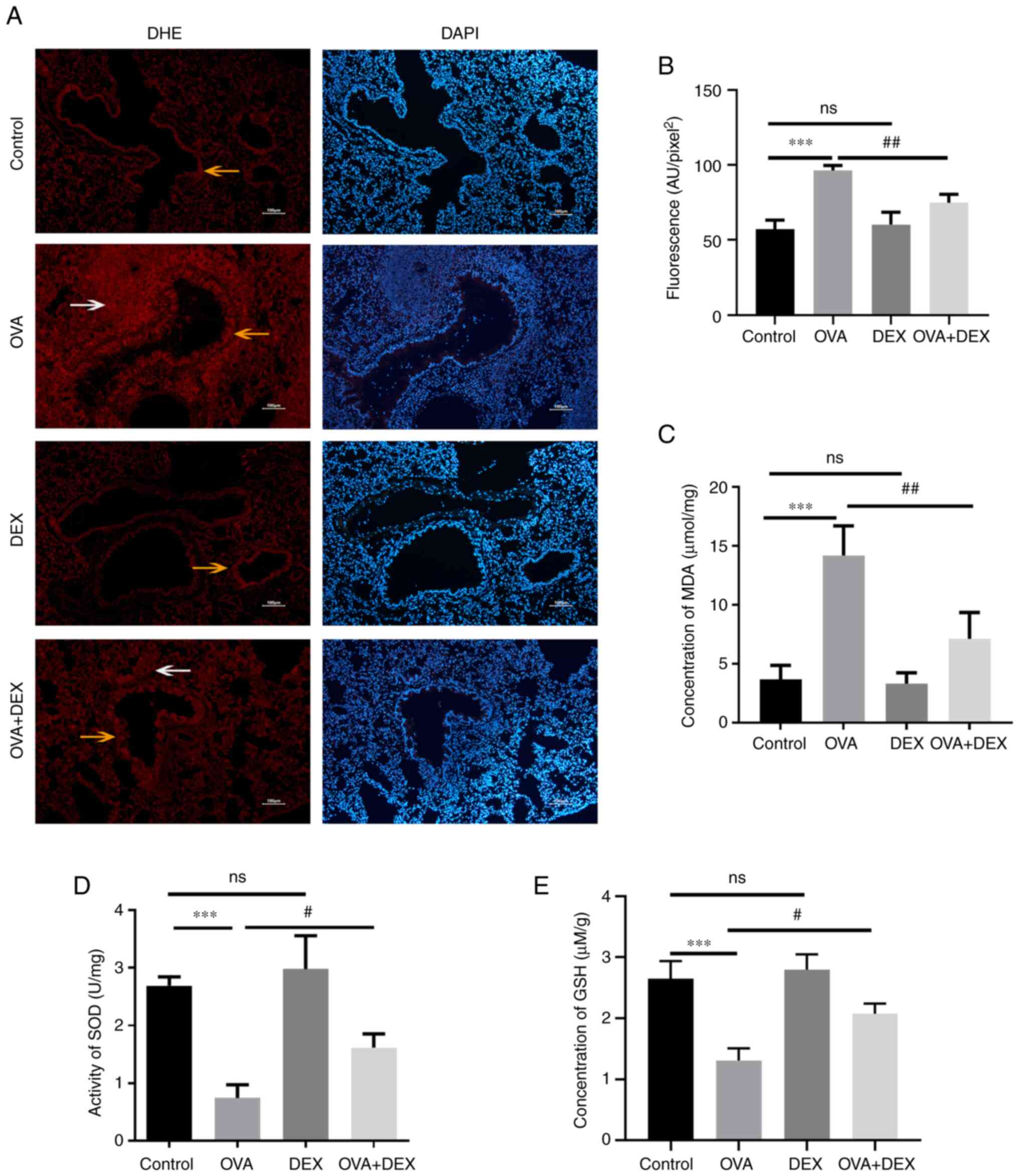
DEX reduces oxidative stress and
restores antioxidant capacity in the murine model of asthma
It has been reported that redox imbalance is
associated with the development of asthma (32). To evaluate the redox state in the
present study, the levels of oxidative stress markers (ROS and MDA)
and the antioxidant capacity of the cells (GSH level and SOD
activity) were assessed. Lung sections were stained with DHE to
evaluate ROS. The data indicated that ROS were mainly generated in
airway epithelial cells (yellow arrows) and inflammatory cells
(white arrows) around the airways (Fig. 3A). Subsequently, the average
fluorescence intensity was calculated (Fig. 3B). ROS levels were significantly
increased in the OVA group compared with the control (P<0.001),
and DEX treatment significantly decreased ROS levels in the lung
compared with the OVA group (P<0.01). The MDA levels in the lung
tissue were assessed to evaluate lipid peroxidation (Fig. 3C). In agreement with the ROS
results, these data demonstrated that the MDA levels were
significantly elevated in the OVA group compared with the control
(P<0.001), whereas DEX treatment significantly reduced MDA
compared with the OVA group (P<0.01). The activity of SOD and
level of GSH were reduced in asthmatic mice compared with the
control (both P<0.001), and DEX treatment restored their content
compared with the OVA group (both P<0.05; Fig. 3D and E). In comparison with the
control group, administration of DEX alone demonstrated no
significant effect on the redox state of the lung tissues compared
with the control group.
Nrf2 signaling pathway and its
downstream antioxidant genes are activated by DEX treatment
Nrf2 signaling and its downstream genes (HO-1 and
GPx4) serve an important role in maintaining redox balance in
asthma (33). The mRNA expression
levels of Nrf2 in the OVA + DEX group were significantly higher
compared with those demonstrated in the OVA group (P<0.01;
Fig. 4A), whereas no significant
differences were demonstrated between the OVA and the control
group. As the activation of Nrf2 is regulated by modification of
Keap1 (34), the expression level
of Keap1 was detected. The mRNA expression levels of Keap1 were
significantly decreased in the OVA group compared with the control
(P<0.01), and DEX treatment significantly reduced its levels
compared with the OVA group (P<0.01). The mRNA expression levels
of HO-1 and GPx4 were significantly reduced in the OVA group
compared with the control (P<0.05; Fig. 4B), and DEX administration led to a
significant increase in HO-1 (P<0.001) and GPx4 (P<0.01) mRNA
expression levels compared with the OVA group. Western blotting
demonstrated that although Nrf2 (nuclear and total) and antioxidant
(HO-1 and GPX4) protein expression levels were increased and Keap1
protein expression level was reduced in the OVA group, the data
were not statistically significant compared with those of the
control group (Fig. 4C and D),
which suggested that Nrf2 might be activated to a limited extent in
asthmatic mice. The presence of DEX further activated Nrf2 and
demonstrated significantly increased nuclear and total Nrf2 protein
expression levels and significantly decreased Keap1 protein
expression levels compared with the OVA group (P<0.05; Fig. 4C). In addition, the protein
expression levels of HO-1 and GPx4 were significantly elevated in
the OVA + DEX group compared with the OVA group (P<0.05;
Fig. 4D).
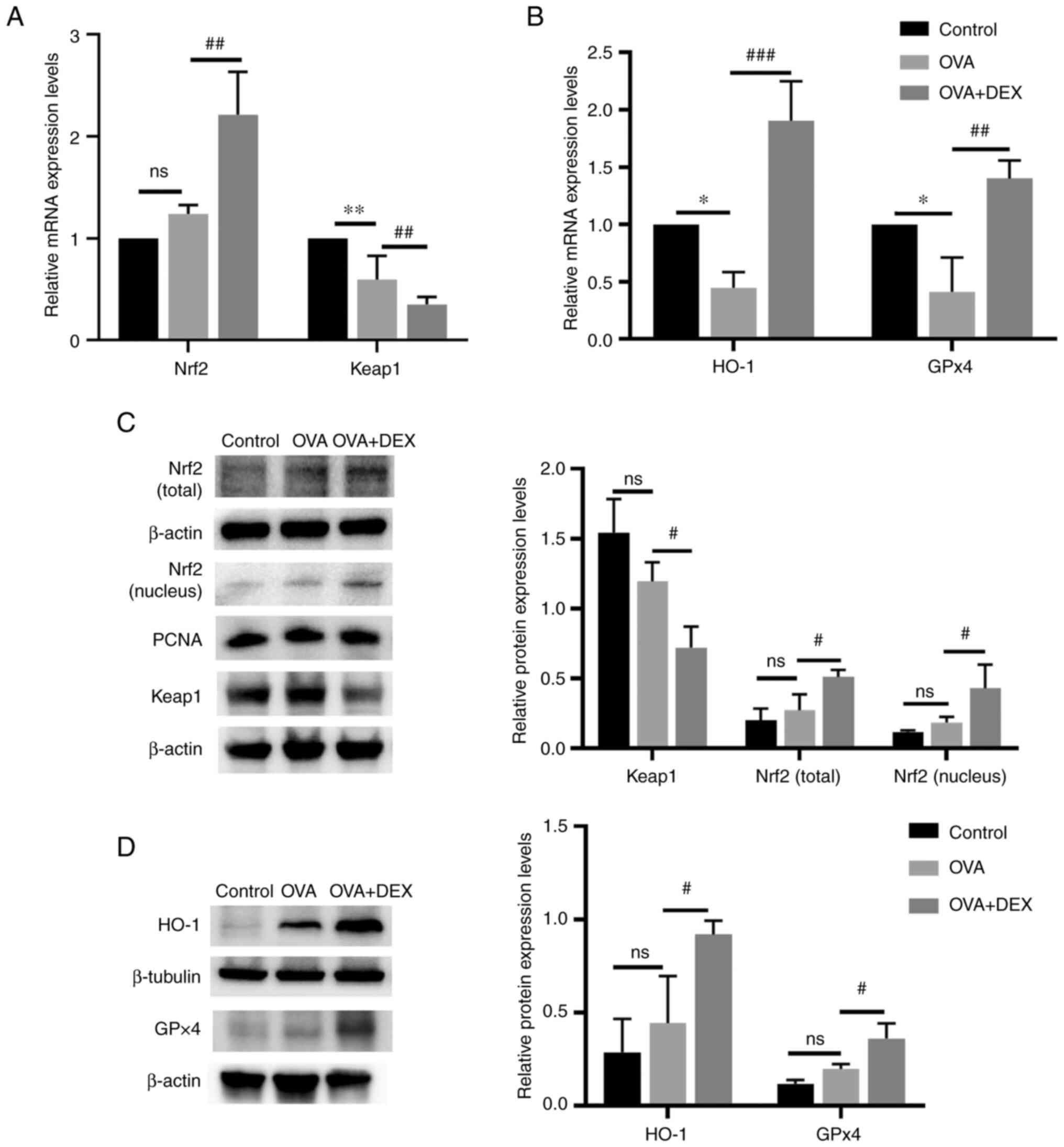 | Figure 4.Nrf2 signaling and its downstream
antioxidant genes are activated by DEX. mRNA expression levels of
(A) Nrf2 and Keap1, and (B) HO-1 and GPx4 in lung tissues were
assessed using reverse transcription-quantitative PCR. Protein
expression levels of (C) Nrf2 (cytoplasm and nucleus), Keap1, (D)
HO-1 and GPx4 were assessed by western blotting. Data are presented
as the mean ± SD (n=3). #P<0.05,
##P<0.01 and ###P<0.001 vs. OVA;
*P<0.05 and **P<0.01 vs. control. DEX, dexmedetomidine; GPx,
glutathione peroxidase; HO-1, heme oxygenase 1; Keap1, kelch-like
ECH-associated protein-1; Nrf2, nuclear factor erythroid 2-related
factor 2; OVA, ovalbumin. |
Therapeutic and antioxidative effects
of DEX in asthma are partially diminished by ML385
The presence of Nrf2 activation was further explored
in the current model by the administration of ML385 (an Nrf2
inhibitor) prior to DEX administration. As demonstrated using
western blotting (Fig. 5A), ML385
significantly reduced the total and nuclear Nrf2 protein expression
levels (P<0.05), significantly upregulated the protein
expression level of Keap1 (P<0.05) and significantly
downregulated the protein expression levels of HO-1 and GPx4
(P<0.01) compared with the OVA + DEX group. The MDA content was
significantly increased by ML385 treatment compared with the OVA +
DEX group (Fig. 5B). Moreover,
the increased GSH content and the increased activity levels of SOD
caused by DEX treatment were significantly suppressed following
administration of ML385 compared with the OVA + DEX group (SOD,
P<0.05; GSH, P<0.01; Fig. 5C
and D). As shown in Fig. 5E and
F, administration of OVA significantly increased inflammatory
cells infiltration around airways and mucus production (both
P<0.001, vs. the control group), and DEX treatment effectively
decreased airway inflammation and mucus overproduction (both
P<0.05; vs. the OVA group). Subsequently, ML385 treatment
reversed these effects of DEX (P<0.05; Fig. 5E and F). However, administration
of ML385 did not significantly increase the airway resistance,
which was reduced by DEX treatment (Fig. S2). The concentration of Th2
cytokines in the BALF was also assessed (Fig. 5G); the concentrations of IL-4
(P<0.05) and IL-5 (P<0.01) were significantly elevated
following the administration of ML385 compared with the OVA + DEX
group. However, the concentration of IL-13 in the OVA + DEX + ML385
group was not significantly different compared with those of the
OVA + DEX group.
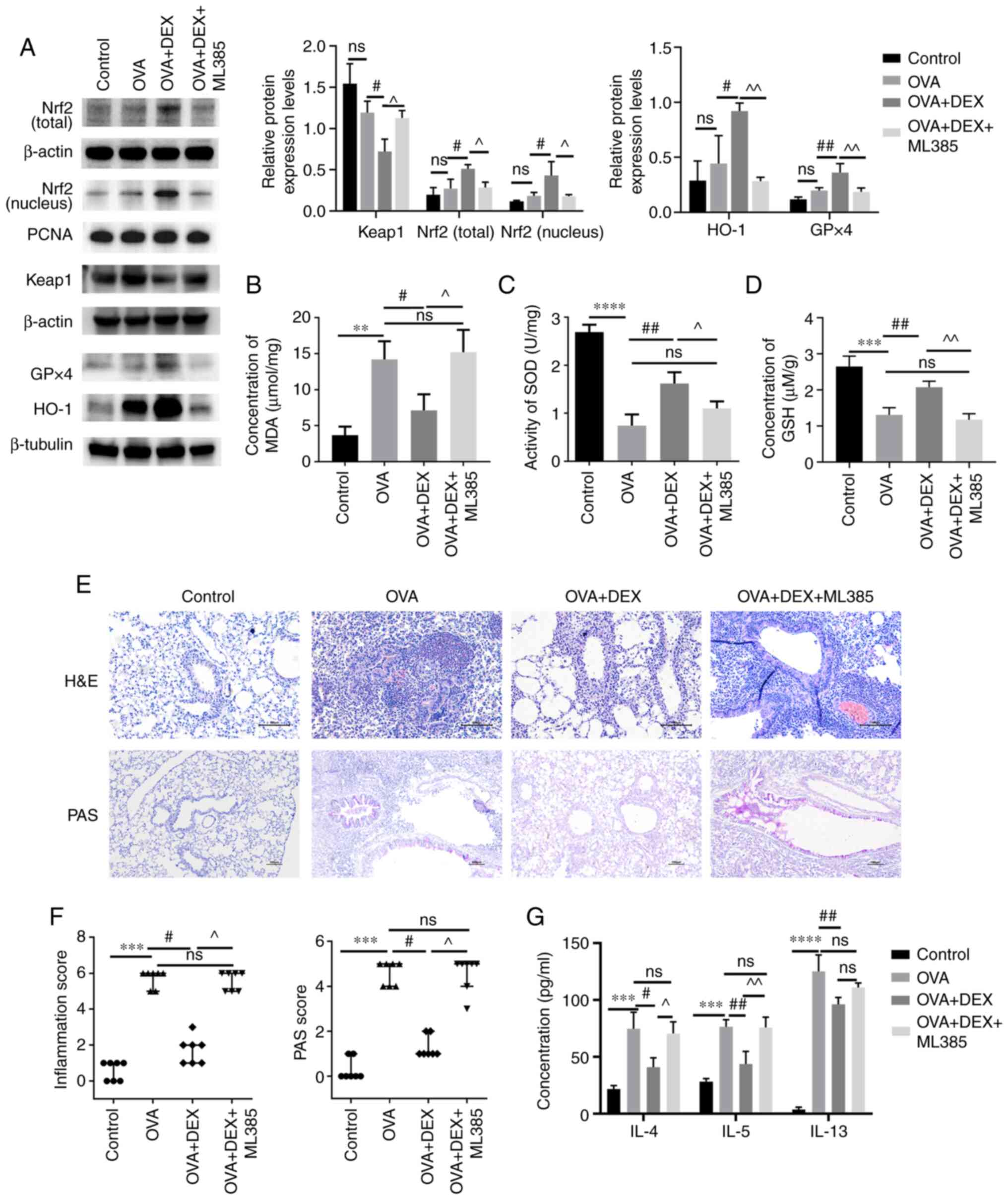 | Figure 5.Therapeutic and antioxidative effects
of DEX in asthma are partly diminished by ML385. (A) The protein
expression levels of Nrf2 (cytoplasmic and nuclear), Keap1, HO-1
and GPx4 were assessed using western blotting. (B) MDA content (C)
activity levels of SOD and (D) GSH content in lung tissues were
assessed using commercial kits. (E) H&E and PAS staining were
performed to evaluate inflammatory cell infiltration and mucus
production in lung tissues, respectively. Magnification for H&E
staining, ×200; magnification for PAS staining, ×100; scale bar,
100 µm. Color imbalance was corrected as described by Marty
(27). (F) Histological scoring
of inflammatory cell infiltration and mucus production were based
on the layers of the cells around the airways and the percentage of
PAS-positive cells, respectively. (G) The concentration of IL-4,
IL-5 and IL-13 in bronchoalveolar lavage fluid were assessed using
commercial ELISA kits. Quantitative data are presented as the mean
± SD (n=3). Ordinal data are presented as the median +
interquartile range (n=7). **P<0.01, ***P<0.001 and
****P<0.0001; #P<0.05 and ##P<0.01;
^P<0.05 and ^^P<0.01. DEX,
dexmedetomidine; GPx, glutathione peroxidase; GSH, reduced
glutathione; H&E, hematoxylin and eosin; HO-1, heme oxygenase
1; Keap1, kelch-like ECH-associated protein-1; MDA,
malondialdehyde; Nrf2, nuclear factor erythroid 2-related factor 2;
ns, not significant OVA, ovalbumin; PAS, periodic acid-Schiff; SOD,
superoxide dismutase. |
Discussion
In our previous study, it was demonstrated that 30
µg/kg DEX exerted the optimal therapeutic effect on a murine model
of asthma induced by OVA (25).
DEX has been reported to have exerted anti-inflammatory and
antioxidative effects on brain injury (22), neuroinflammation (24) and hepatic ischemia/reperfusion
injury (35) via activation of
the Nrf2 signaling pathway. DEX was also reported to lessen
oxidative stress-induced alveolar epithelial cell apoptosis in the
lung (36). Since oxidative
stress serves an important role in the pathogenesis of asthma
(10), the present study
evaluated the antioxidative effect of DEX on a murine model of
allergic asthma. The data demonstrated that DEX significantly
reduced the levels of oxidative stress in lung tissues and
inhibited the symptoms of asthma via activation of the Nrf2
signaling pathway and significantly promoted the expression of
antioxidant factors. To verify that the Nrf2 signaling pathway
served a vital role in the antioxidative effect of DEX, the Nrf2
inhibitor ML385 was used. These data demonstrated that the
therapeutic effect of DEX was partially reduced following treatment
with ML385. However, a limitation of the present study was that a
ML385 + OVA group was not included as a control. In general, the
present study indicated that DEX may suppress inflammation in the
lung through activation of the antioxidant signaling pathway.
Allergic asthma is characterized by eosinophilic
airway inflammation, AHR and excessive mucus production (30). Th2 cells and Th2 cytokines serve
key roles in allergic asthma development, and the OVA-induced
murine model of asthma used in the present study represented a
classical allergic asthma model in which Th2 cells were the
dominant cell type (37).
Cytokines production is one of the hallmarks of Th2 cell activation
(38). The secretion of cytokines
from activated Th2 cells contributed to the symptoms of asthma by
recruiting eosinophils (IL-5), inducing excessive mucus production
(IL-4) and leading to airway smooth muscle contraction (IL-13)
(30). The results of the present
study suggested that DEX treatment lowered the levels of
Th2-secreted cytokines prior to each challenge, which in turn
attenuated the infiltration of eosinophils in BALF samples and lung
tissues, and alleviated mucus overproduction. It was a limitation
of the present study that the levels of Th2 cells were not assessed
and this should be incorporated in future studies. In addition, no
significant differences were observed in the levels of the
inflammatory markers between the DEX and the control groups.
The critical role of oxidative stress in the
development of asthma has been reported by numerous studies.
Oxidative stress has been reported to regulate T cell
differentiation (39) and as
being closely related to inflammation (40). Eosinophils, which participate in
the allergic airway inflammation, are one of the main sources of
oxidative stress in asthma; eosinophils produce ROS by releasing
eosinophil peroxidase, which can induce bromotyrosine and
nitrotyrosine. Both of these two compounds are oxidation products
of eosinophils which have been reported to indicate a high risk of
asthma exacerbation (41). In
addition to excessive production of oxidants, asthmatic lungs
possess lower activity levels of antioxidant enzymes (such as SOD
and catalase) compared with those levels in normal lungs (32). Certain factors involved in
oxidative stress, such as ROS and H2O2, can
serve a major role in signal transduction (42). However, the overproduction of
oxidants that exceeds the capacity of the cellular antioxidant
system can contribute to the symptoms of asthma (43). It has been reported that ROS
induces the activation of EGFR, which leads to goblet cell
metaplasia (44). ROS have also
been reported to result in AHR by induction of airway remodeling
(airway stenosis) (45),
increased intracellular Ca2+ concentration (46) and vagal tone (47). Furthermore, ROS have been reported
to decrease β-adrenergic function in the lungs, which may result in
a poor response to traditional bronchodilators (15). Certain oxidative stress biomarkers
are reported to be highly related to asthma, including MDA, GSH
(11) and SOD (48). MDA is a common indicator of lipid
peroxidation and its levels are considerably higher in patients
with asthma compared with those in healthy subjects during both
acute attacks and symptom-free periods (49). The levels of MDA have also been
reported to be positively correlated with the infiltration of
eosinophils (8). GSH is the most
abundant antioxidant in the airway epithelial lining fluid and is
involved in various important functions relevant to asthma, such as
detoxification, scavenging of free radicals and modulation of
apoptosis and immune function (50). SOD levels are positively
correlated with certain lung functions (48), and its inactivation was reported
in asthmatic patients (51). In
the present study, the levels of these oxidative biomarkers were
evaluated, and the data indicated that DEX administration improved
the maintenance of the redox balance by significantly reducing the
levels of MDA and ROS, and significantly increasing the activity of
SOD and the levels of GSH.
Nrf2 is a transcription factor that regulates the
majority of antioxidant genes, including HO-1, GPx4 and NAD(P)H
quinone dehydrogenase 1 (52).
Under oxidative stress conditions, Nrf2 can be activated by
phosphorylation (53) or Keap1
modification (54). Subsequently,
it translocates to the nucleus by dissociating from Keap1 (55). Park et al (53) reported the involvement of protein
kinase C (PKC) in the antioxidant effect of DEX in a rat model of
ischemia. PKC phosphorylated Nrf2 and activated its detachment from
Keap1. ERK1/2 has also been reported to have mediated the
antioxidative effect of DEX by participating in Nrf2 activation and
translocation into the nucleus (56). In addition to the two
aforementioned kinases, the MAPK signaling pathway (57) and certain microRNAs (miRNAs), such
as miRNA (miR)-27a, miR-153, miR142-5p and miR-144 (58), have also been reported to have
mediated the regulation of Nrf2 expression and its activation.
However, their potential to participate in the regulation of Nrf2
by DEX remains to be elucidated. The modification of Keap1 is used
to regulate the activation of the Nrf2 pathway. Several mechanisms
have been reported that explain the regulation of the concentration
and activity levels of Keap1, such as transcriptional regulation
(STAT6 and hypoxia-inducible factor) (59), miRNAs (miR-7 and miR-24-3p)
(54), post-translational
regulation, such as ubiquitination (60) and S-nitrosylation (61), as well as degradation of the Keap1
protein (p62-dependent autophagy) (62). Liu et al (63) reported the impact of DEX on the
regulation of Keap1; however, the underlying mechanism of this
effect remains unknown. The possible mechanism of DEX activation of
the Nrf2/Keap1 signaling pathway in a murine model of asthma needs
further evaluation.
The anti-inflammatory effects of Nrf2 have been
reported in numerous disease models, including asthma (64). Rangasamy et al (15) reported that Nrf2 knockout mice
were more susceptible to asthma, and Sussan et al (16) reported the role of Nrf2 in the
cytoprotective effect on the airway epithelia of subjects with
asthma. To assess whether the antioxidant effects of DEX on asthma
were mediated by Nrf2, the mRNA and protein expression levels of
Nrf2 and its downstream genes were evaluated. Owing to the
cytoprotective effect of HO-1 and GPx4 on the airway epithelia
(33) and their crucial role as
in the cellular antioxidant system (65), the mRNA and protein expression
levels of HO-1 and GPx4 were assessed. The results of the present
study indicated that DEX treatment significantly increased the
expression levels of HO-1 and GPx4 compared with those of the
untreated OVA group. Administration of ML385, which significantly
inhibited the activation of Nrf2, partly abrogated the antioxidant
and anti-inflammatory effects of DEX. The results of the present
study indicated that the therapeutic effect of DEX on asthma partly
depended on the activation of the Nrf2 signaling pathway.
In conclusion, results from the present study
indicated that DEX attenuated airway inflammation, AHR and mucus
overproduction in the lung by the reduction of oxidative stress.
This effect was at least partially mediated by the Nrf2 signaling
pathway. The results suggested the potential protective effects of
DEX in asthma. However, this was just a preliminary study on the
antioxidant effect of DEX in a murine asthma model with Th2
dominance; further investigation of the mechanisms involved and the
evaluation of other endotypes of asthma are required.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Plastic Surgery Hospital,
Chinese Academy of Medical Sciences and Peking Union Medical
College (Beijing, China; grant no. YS202006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX designed the study and drafted the manuscript. SX
and DY critically revised it for important intellectual content. HG
and YZ performed the animal experiments and were major contributors
to writing the manuscript. SX, HG and YZ performed the experiments
and analyzed the data. YZ and DY made substantial contributions to
the study conception. SX and DY confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Experimental animals were handled under a protocol
approved by the Institutional Animal Care and Use Committee of
Plastic Surgery Hospital, Chinese Academy of Medical Sciences and
Peking Union Medical College [Beijing, China; approval no.
2022(201)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ribeiro A, Aguiar R and Morais-Almeida M:
Biological therapies, asthma and coronavirus disease 2019. Curr
Opin Allergy Clin Immunol. 21:597–601. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Byrne P, Fabbri LM, Pavord ID, Papi A,
Petruzzelli S and Lange P: Asthma progression and mortality: The
role of inhaled corticosteroids. Eur Respir J. 54:19004912019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller RL, Grayson MH and Strothman K:
Advances in asthma: New understandings of asthma's natural history,
risk factors, underlying mechanisms, and clinical management. J
Allergy Clin Immunol. 148:1430–1441. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chipps BE, Murphy KR and Oppenheimer J:
2020 NAEPP guidelines update and GINA 2021-asthma care differences,
overlap, and challenges. J Allergy Clin Immunol Pract. 10((1S)):
S19–S30. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakagome K and Nagata M: Involvement and
possible role of eosinophils in asthma exacerbation. Front Immunol.
9:22202018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Djukanovic R: Airway inflammation in
asthma and its consequences: Implications for treatment in children
and adults. J Allergy Clin Immunol. 109 (6 Suppl):S539–S548. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017:84167632017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Groot LES, Sabogal Piñeros YS, Bal SM,
van de Pol MA, Hamann J, Sterk PJ, Kulik W and Lutter R: Do
eosinophils contribute to oxidative stress in mild asthma? Clin Exp
Allergy. 49:929–931. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Checa J and Aran JM: Airway redox
homeostasis and inflammation gone awry: From molecular pathogenesis
to emerging therapeutics in respiratory pathology. Int J Mol Sci.
21:93172020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riedl MA and Nel AE: Importance of
oxidative stress in the pathogenesis and treatment of asthma. Curr
Opin Allergy Clin Immunol. 8:49–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fatani SH: Biomarkers of oxidative stress
in acute and chronic bronchial asthma. J Asthma. 51:578–584. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang P, Geng J, Gao J, Zhao H, Li J, Shi
Y, Yang B, Xiao C, Linghu Y, Sun X, et al: Macrophage achieves
self-protection against oxidative stress-induced ageing through the
Mst-Nrf2 axis. Nat Commun. 10:7552019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dang X, He B, Ning Q, Liu Y, Guo J, Niu G
and Chen M: Alantolactone suppresses inflammation, apoptosis and
oxidative stress in cigarette smoke-induced human bronchial
epithelial cells through activation of Nrf2/HO-1 and inhibition of
the NF-κB pathways. Respir Res. 21:952020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Xu Y, Yan M, Yu Y and Guo Y:
18β-Glycyrrhetinic acid suppresses allergic airway inflammation
through NF-κB and Nrf2/HO-1 signaling pathways in asthma mice. Sci
Rep. 12:31212022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rangasamy T, Guo J, Mitzner WA, Roman J,
Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN and
Biswal S: Disruption of Nrf2 enhances susceptibility to severe
airway inflammation and asthma in mice. J Exp Med. 202:47–59. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sussan TE, Gajghate S, Chatterjee S,
Mandke P, McCormick S, Sudini K, Kumar S, Breysse PN, Diette GB,
Sidhaye VK and Biswal S: Nrf2 reduces allergic asthma in mice
through enhanced airway epithelial cytoprotective function. Am J
Physiol Lung Cell Mol Physiol. 309:L27–L36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gertler R, Brown HC, Mitchell DH and
Silvius EN: Dexmedetomidine: A novel sedative-analgesic agent. Proc
(Bayl Univ Med Cent). 14:13–21. 2001.PubMed/NCBI
|
|
18
|
Bao Y, Zhu Y, He G, Ni H, Liu C, Ma L,
Zhang L and Shi D: Dexmedetomidine attenuates neuroinflammation In
LPS-stimulated BV2 microglia cells through upregulation of miR-340.
Drug Des Devel Ther. 13:3465–3475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue BB, Chen BH, Tang YN, Weng CW and Lin
LN: Dexmedetomidine protects against lung injury induced by limb
ischemia-reperfusion via the TLR4/MyD88/NF-κB pathway. Kaohsiung J
Med Sci. 35:672–678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Z, Chen Q, Wan L, Zheng D, Li Z and
Wu Z: Dexmedetomidine protects hepatic cells against oxygen-glucose
deprivation/reperfusion injury via lncRNA CCAT1. Cell Biol Int.
42:1250–1258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geng Y, Li R, He SX, Yang HH, Deng QT,
Shao XY, Wu YS, Xu WW and Ma Q: Dexmedetomidine attenuates acute
lung injury induced by heatstroke and improve outcome. Shock.
52:532–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng X, Ma W, Zhu J, Jiao W and Wang Y:
Dexmedetomidine alleviates early brain injury following traumatic
brain injury by inhibiting autophagy and neuroinflammation through
the ROS/Nrf2 signaling pathway. Mol Med Rep. 24:6612021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao Y, Kong GY, Pei WM, Zhou B, Zhang QQ
and Pan BB: Dexmedetomidine alleviates hepatic injury via the
inhibition of oxidative stress and activation of the Nrf2/HO-1
signaling pathway. Eur Cytokine Netw. 30:88–97. 2019.PubMed/NCBI
|
|
24
|
Li F, Wang X, Zhang Z, Zhang X and Gao P:
Dexmedetomidine attenuates neuroinflammatory-induced apoptosis
after traumatic brain injury via Nrf2 signaling pathway. Ann Clin
Transl Neurol. 6:1825–1835. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao S, Wang Q, Gao H, Zhao X, Zhi J and
Yang D: Dexmedetomidine alleviates airway hyperresponsiveness and
allergic airway inflammation through the TLR4/NF-κB signaling
pathway in mice. Mol Med Rep. 25:742022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Hoecke L, Job ER, Saelens X and Roose
K: Bronchoalveolar lavage of murine lungs to analyze inflammatory
cell infiltration. J Vis Exp. 4:553982017.PubMed/NCBI
|
|
27
|
Marty GD: Blank-field correction for
achieving a uniform white background in brightfield digital
photomicrographs. Biotechniques. 42:7167187202007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davarinejad H: Quantifications of western
blots with imageJ. http://www.yorku.ca/yisheng/Internal/Protocols/ImageJ.pdf
|
|
30
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Townley RG and Horiba M: Airway
hyperresponsiveness: A story of mice and men and cytokines. Clin
Rev Allergy Immunol. 24:85–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Comhair SA and Erzurum SC: Redox control
of asthma: Molecular mechanisms and therapeutic opportunities.
Antioxid Redox Signal. 12:93–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagasaki T, Schuyler AJ, Zhao J, Samovich
SN, Yamada K, Deng Y, Ginebaugh SP, Christenson SA, Woodruff PG,
Fahy JV, et al: 15LO1 dictates glutathione redox changes in
asthmatic airway epithelium to worsen type 2 inflammation. J Clin
Invest. 132:e1516852022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baird L and Yamamoto M: The molecular
mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol.
40:e00099–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu Y, Qiu G, Zhang H, Zhu L, Cheng G, Wang
Y, Li Y and Wu W: Dexmedetomidine alleviates hepatic
ischaemia-reperfusion injury via the PI3K/AKT/Nrf2-NLRP3 pathway. J
Cell Mol Med. 25:9983–9994. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cui J, Zhao H, Wang C, Sun JJ, Lu K and Ma
D: Dexmedetomidine attenuates oxidative stress induced lung
alveolar epithelial cell apoptosis in vitro. Oxid Med Cell Longev.
2015:3583962015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Casaro M, Souza VR, Oliveira FA and
Ferreira CM: OVA-induced allergic airway inflammation mouse model.
Methods Mol Biol. 1916:297–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bosnjak B, Stelzmueller B, Erb KJ and
Epstein MM: Treatment of allergic asthma: Modulation of Th2 cells
and their responses. Respir Res. 12:1142011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
King MR, Ismail AS, Davis LS and Karp DR:
Oxidative stress promotes polarization of human T cell
differentiation toward a T helper 2 phenotype. J Immunol.
176:2765–2772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mishra V, Banga J and Silveyra P:
Oxidative stress and cellular pathways of asthma and inflammation:
Therapeutic strategies and pharmacological targets. Pharmacol Ther.
181:169–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu W, Samoszuk MK, Comhair SA, Thomassen
MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC and Hazen SL:
Eosinophils generate brominating oxidants in allergen-induced
asthma. J Clin Invest. 105:1455–1463. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reczek CR and Chandel NS: ROS-dependent
signal transduction. Curr Opin Cell Biol. 33:8–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cho YS and Moon HB: The role of oxidative
stress in the pathogenesis of asthma. Allergy Asthma Immunol Res.
2:183–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Casalino-Matsuda SM, Monzón ME and Forteza
RM: Epidermal growth factor receptor activation by epidermal growth
factor mediates oxidant-induced goblet cell metaplasia in human
airway epithelium. Am J Respir Cell Mol Biol. 34:581–591. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wiegman CH, Michaeloudes C, Haji G, Narang
P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J,
et al: Oxidative stress-induced mitochondrial dysfunction drives
inflammation and airway smooth muscle remodeling in patients with
chronic obstructive pulmonary disease. J Allergy Clin Immunol.
136:769–780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Q, Zhou Y, Zhou L, Fu Z, Yang C, Zhao
L, Li S, Chen Y, Wu Y, Ling Z, et al: TRPC6-dependent
Ca2+ signaling mediates airway inflammation in response
to oxidative stress via ERK pathway. Cell Death Dis. 11:1702020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park CS, Kim TB, Lee KY, Moon KA, Bae YJ,
Jang MK, Cho YS and Moon HB: Increased oxidative stress in the
airway and development of allergic inflammation in a mouse model of
asthma. Ann Allergy Asthma Immunol. 103:238–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Comhair SA, Ricci KS, Arroliga M, Lara AR,
Dweik RA, Song W, Hazen SL, Bleecker ER, Busse WW, Chung KF, et al:
Correlation of systemic superoxide dismutase deficiency to airflow
obstruction in asthma. Am J Respir Crit Care Med. 172:306–313.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sharma A, Bansal S and Nagpal RK: Lipid
peroxidation in bronchial asthma. Indian J Pediatr. 70:715–717.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fitzpatrick AM, Jones DP and Brown LA:
Glutathione redox control of asthma: From molecular mechanisms to
therapeutic opportunities. Antioxid Redox Signal. 17:375–408. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Comhair SA, Bhathena PR, Dweik RA, Kavuru
M and Erzurum SC: Rapid loss of superoxide dismutase activity
during antigen-induced asthmatic response. Lancet. 355:6242000.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Q, Gao Y and Ci X: Role of Nrf2 and
its activators in respiratory diseases. Oxid Med Cell Longev.
2019:70905342019.PubMed/NCBI
|
|
53
|
Park YH, Park HP, Kim E, Lee H, Hwang JW,
Jeon YT and Lim YJ: The antioxidant effect of preischemic
dexmedetomidine in a rat model: Increased expression of Nrf2/HO-1
via the PKC pathway. Braz J Anesthesiol. S0104-0014(21)00331-6.
2021.(Epub ahead of print).
|
|
54
|
Kopacz A, Kloska D, Forman HJ, Jozkowicz A
and Grochot-Przeczek A: Beyond repression of Nrf2: An update on
Keap1. Free Radic Biol Med. 157:63–74. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kansanen E, Kuosmanen SM, Leinonen H and
Levonen AL: The Keap1-Nrf2 pathway: Mechanisms of activation and
dysregulation in cancer. Redox Biol. 1:45–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu W, Du Z and Wu L: Dexmedetomidine
attenuates hypoxia-induced cardiomyocyte injury by promoting
telomere/telomerase activity: Possible involvement of ERK1/2-Nrf2
signaling pathway. Cell Biol Int. 46:1036–1046. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun Z, Huang Z and Zhang DD:
Phosphorylation of Nrf2 at multiple sites by MAP kinases has a
limited contribution in modulating the Nrf2-dependent antioxidant
response. PLoS One. 4:e65882009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cheng D, Wu R, Guo Y and Kong AN:
Regulation of Keap1-Nrf2 signaling: The role of epigenetics. Curr
Opin Toxicol. 1:134–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee OH, Jain AK, Papusha V and Jaiswal AK:
An auto-regulatory loop between stress sensors INrf2 and Nrf2
controls their cellular abundance. J Biol Chem. 282:36412–36420.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cullinan SB, Gordan JD, Jin J, Harper JW
and Diehl JA: The Keap1-BTB protein is an adaptor that bridges Nrf2
to a Cul3-based E3 ligase: Oxidative stress sensing by a Cul3-Keap1
ligase. Mol Cell Biol. 24:8477–8486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kopacz A, Klóska D, Proniewski B, Cysewski
D, Personnic N, Piechota-Polańczyk A, Kaczara P, Zakrzewska A,
Forman HJ, Dulak J, et al: Keap1 controls protein S-nitrosation and
apoptosis-senescence switch in endothelial cells. Redox Biol.
28:1013042020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Taguchi K, Fujikawa N, Komatsu M, Ishii T,
Unno M, Akaike T, Motohashi H and Yamamoto M: Keap1 degradation by
autophagy for the maintenance of redox homeostasis. Proc Natl Acad
Sci USA. 109:13561–13566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu Y, Liu W, Wang XQ, Wan ZH, Liu YQ and
Zhang MJ: Dexmedetomidine relieves neuropathic pain in rats with
chronic constriction injury via the Keap1-Nrf2 pathway. Front Cell
Dev Biol. 9:7149962021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rockwell CE, Jin Y, Boss AP, Kaiser LM and
Awali S: The complicated role of nuclear factor erythroid-derived
2-like 2 in allergy and asthma. Drug Metab Dispos. 50:500–507.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ammar M, Bahloul N, Amri O, Omri R, Ghozzi
H, Kammoun S, Zeghal K and Ben Mahmoud L: Oxidative stress in
patients with asthma and its relation to uncontrolled asthma. J
Clin Lab Anal. 36:e243452022. View Article : Google Scholar : PubMed/NCBI
|