Ursolic acid (UA) is a natural product present in
plants, including apples, bilberries, cranberries, elder flower,
peppermint, lavender, oregano, thyme, hawthorn and prunes (5,6),
which has been reported to have chemotherapeutic effects against
numerous types of cancer, including CRC (7–11).
UA demonstrates pharmacological properties that affect cancer
development, including cytotoxic (12,13), antitumor (8,14)
and anti-metastatic activity (15,16). UA has been reported to regulate
signaling pathways, including STAT3 signaling pathway (17), TGF-β1/ZEB1 signaling pathway
(18) and AMPK signaling pathway
(19), to exert these antitumor
effects, including inhibition of proliferation (20), promotion of apoptosis (21,22), modulation of cell cycle arrest
(23,24), suppression of
epithelial-to-mesenchymal transition (EMT) (8,25,26) and induction of autophagy (22,27,28). However, the precise underlying
mechanisms of UA remain unknown.
Doxorubicin (DOX), an anthracycline chemotherapeutic
agent, is one of the most effective chemotherapy drugs used to
treat numerous types of cancer including breast, lung, gastric,
ovarian, thyroid, non-Hodgkin's and Hodgkin's lymphoma, multiple
myeloma, sarcoma and pediatric cancers (29–34). DOX exerts its cytotoxic activity
through DNA damage by inhibiting DNA topoisomerase II and
generating reactive oxygen species (32,35). DOX is the first-line anticancer
agent used to treat numerous types of cancer; however, the
widespread clinical use of DOX is limited by severe dose-dependent
toxicity, such as renal toxicity (36), cardiotoxicity (37), hepatotoxicity (38) and neurotoxicity (39). Therefore, minimizing DOX toxicity
should be considered in clinical applications.
The Hippo/yes-associated protein 1 (Yap) signaling
pathway is a highly conserved adjuster of histogenesis, cell fate
regulation and organ size (40),
which serves essential roles in tumorigenesis, including cell
proliferation, apoptosis, viability and migration (41). The core upstream units of the
Hippo pathway are mammalian Ste20-like kinase (Mst) 1/2 and large
tumor suppressor kinase 1/2, which form a complex that inhibits the
nuclear translocation of Yap by phosphorylating Yap in the
cytoplasm (42). Yap is a
transcriptional co-activator that serves a crucial role in
sustaining intestinal homeostasis (43). Hippo signaling pathway
inactivation and Yap hyperactivation have been reported to be
associated with common human malignancies, including lung cancer
(44), gastric cancer (45), breast cancer (46), liver cancer (47), gliomas (48) and pancreatic cancer (49). Previous studies have demonstrated
that the Hippo signaling pathway promotes stem cell properties and
intestinal tumorigenesis (50–52). Mst1/2 downregulation in the
intestinal epithelium has been shown to induce activation of Yap,
and may contribute to tumor proliferation and metastasis (53). Moreover, the overall and
disease-free survival rates of patients with CRC with Yap
upregulation have been shown to be markedly decreased (54,55). There is accumulating evidence that
shows that the PI3K/Akt/mTOR signaling pathway is a key survival
pathway that is unconventionally activated in numerous types of
malignant tumor, including CRC (56,57). Furthermore, aberrant Akt signaling
pathway activation has been reported to suppress the Hippo
signaling pathway, leading to increased tumorigenesis (58,59). However, the association between
the Akt and Hippo signaling pathways, and its implications for the
use of combined treatment with first-line chemotherapy (DOX) and a
natural anticancer product (UA) remain unclear in CRC. Therefore,
the present study aimed to evaluate the therapeutic effects of an
anticancer herbal component-based drug in combination with
chemotherapy in CRC cells.
The human colon cancer HCT116 cell line (cat. no.
10247) and CRC HT-29 cell line (cat. no. 30038) originating from
colorectal adenocarcinoma (60)
were purchased from the Korean Cell Line Bank (Korean Cell Line
Research Foundation) and were authenticated by Procell Life Science
& Technology Co., Ltd. using STR analysis. The two cell lines
were cultured in RPMI-1640 (cat. no. 23400-021; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (cat.
no. S001-07; Welgene Co., Ltd.), 1% penicillin-streptomycin (cat.
no. P4333-100ml; Sigma-Aldrich; Merck KGaA) in a humidified chamber
at 37°C under 5% CO2. UA (purity, >95%; cat. no.
10072) was purchased from Cayman Chemical Company. DOX
hydrochloride (purity, 98–100%; cat. no. D1515-10MG) and SC79 (AKT
activator) (purity, ≥97%; cat. no. 305834-79-1) were purchased from
Sigma-Aldrich; Merck KGaA. LY294002 hydrochloride (AKT inhibitor)
(purity, ≥98%; cat. no. 934389-88-5) was purchased from LKT
Laboratories, Inc. Following pre-treatment with AKT inhibitor
LY294002 (1 µM) for 2 h or AKT activator SC79 (5 µM) for 2 h, UA +
DOX combination treatment was applied for 48 h.
Briefly, a base layer containing 1% agar (micro
agar, cat. no. M1002.1; Duchefa Biochemie B.V.) and a top layer
containing 0.7% agar (Agarose SPI, cat. no. A1203.01; Duchefa
Biochemie B.V.) were inoculated with 1×105 cells per
well. The medium, supplemented with UA (15 µM) and/or DOX (1.5 µM),
was changed three times/week and 6-well plates were incubated for
14 days at 37°C under 5% CO2. Colonies were defined as
>30 cells, were counted manually and were observed using an IX71
fluorescence microscope (Olympus).
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Jeonbuk National
University (approval no. CBNU2017-0001; Jeonju, South Korea) under
National Institutes of Health guidelines (65). The present study was performed in
compliance with the Animal Research: Reporting of In Vivo
Experiments guidelines 2.0 (66).
The present study was performed according to the guidelines on the
welfare and use of animals in cancer research (67). Male SPF/BALB/c nu/nu
immunodeficient mice were purchased from Orient Bio Inc. Athymic
nude mice (age, 4 weeks; weight, 20±3 g; n=20) were acclimated to
the animal housing conditions for 1 week. Mice were housed in an
experimental animal facility under standard laboratory conditions
(20–25°C, 40–60% humidity, 12-h light/dark cycle) with free access
to food and water. HCT116 cells (1×107) in 100 µl
Matrigel (Corning Matrigel Basement Membrane Matrix; cat. no.
356234; Corning, Inc.) were injected into the flank area of the
mice. After inoculation, HCT116 tumor-bearing animals were assigned
to four groups (n=5/group) as follows: i) vehicle; ii) UA; iii)
DOX; and iv) UA + DOX and housed in individually ventilated cages
with litter and nesting material. The mice in the vehicle group
were administered 20 µl sterile DMSO twice weekly. The mice in the
UA group were treated with 10 mg/kg/day UA in 100 µl PBS. The mice
in the DOX group were administered 2 mg/kg DOX in 20 µl DMSO twice
weekly. The mice in the DOX + UA combination group were
administered 2 mg/kg DOX in 20 µl DMSO twice weekly and 10
mg/kg/day UA in 100 µl PBS. All agents were administered via
intraperitoneal injection. The average volume of the tumors was
calculated as follows: Tumor volume=width2 × length/2.
The body weight of mice was monitored every 2 days. Mouse welfare
was assessed daily and palpable tumors were assessed every other
day. All mice could reach food and water, and all had a fairly
normal gait. Mice moved freely in the cage with normal body and
tail position and in a forward motion; the abdomen of the mice was
soft with a mild resistance to pressure when touched and abdominal
distention was not observed in the mice following injection of the
drugs. All mice demonstrated a slightly curved back and did not
have a hunched position when sitting or during rest. One mouse had
bite wounds on the tail and back; however, blood stains were absent
in the cage and the mouse was separated to an individual cage on
day 13. Wounds were locally disinfected daily until healed and the
mouse was isolated until the end of the procedure. All mice had no
ocular or nasal discharge. When the skin of the neck of the mice
was pinched, one mouse in the control group demonstrated poor skin
turgor and the tumor diameter of this mouse reached 2 cm on day 22.
The poor turgor indicated that this mouse was dehydrated and all
mice were sacrificed on day 22. Mice were placed in a chamber with
4% isoflurane (cat. no. 1349003; Sigma-Aldrich; Merck KGaA) until
animals lost consciousness and CO2 (50% of the chamber
vol/min) was used to euthanize the mice. Death was confirmed by
loss of heartbeat, toe reflex, muscle tone, breathing and corneal
reflex, and rigor mortis; subsequently, tumor tissues were
harvested.
Serum concentrations of aspartate aminotransferase
(AST; cat. no. 10103; Asan Pharmaceuticals Co., Ltd.), alanine
aminotransferase (ALT; cat. no. 10102; Asan Pharmaceuticals Co.,
Ltd.), blood urea nitrogen (BUN; cat. no. K024-H1; Arbor Assays LLC
Co., Ltd.) and creatinine (cat. no. KB02-H1; Arbor Assays LLC Co.,
Ltd.) were determined using commercial kits.
At the end of the animal experiment, mouse tumor
tissue samples were embedded in 10% formaldehyde at 4°C for 24 h,
sliced into 4-µm sections and stained using hematoxylin and eosin
(H&E) following a standard protocol for histopathological
examination (68). For
immunohistochemistry staining, sections from mouse tumor tissue
were prepared according to the manufacturer's protocol using the
Rabbit HRP/DAB Detection IHC kit (cat. no. ab64261; Abcam). Samples
were deparaffinized with xylene, rehydrated with a descending
ethanol series l (100, 95, 90, 80 and 70% ethanol) at room
temperature for 5 min and subjected to antigen retrieval by boiling
samples in decreasing concentrations of EDTA buffer (pH 9.0) at
120°C for 2 min, followed by cooling to room temperature for 30
min. Slides were blocked using hydrogen peroxide blocking buffer
(supplied in the kit) for 15 min to quench endogenous peroxidase
and 10% goat serum (cat. no. 16210064; Thermo Fisher Scientific,
Inc.) at room temperature for 30 min each. Slides were washed with
PBS and incubated with rabbit anti-Ki-67 (1:100; cat. no.
PA5-16785; Invitrogen; Thermo Fisher Scientific, Inc.) primary
antibodies overnight at 4°C and biotinylated goat anti-rabbit IgG
and streptavidin peroxidase (cat. no. ab64261; Abcam) at room
temperature for 2 h. Slides were washed with PBS and
counterstaining was performed using 100 µl diaminobenzidine at room
temperature for 1 min and sufficient hematoxylin to cover the
slides at room temperature for 1 min. Histopathological assessment
and immunohistochemistry staining analysis were performed using a
light microscope.
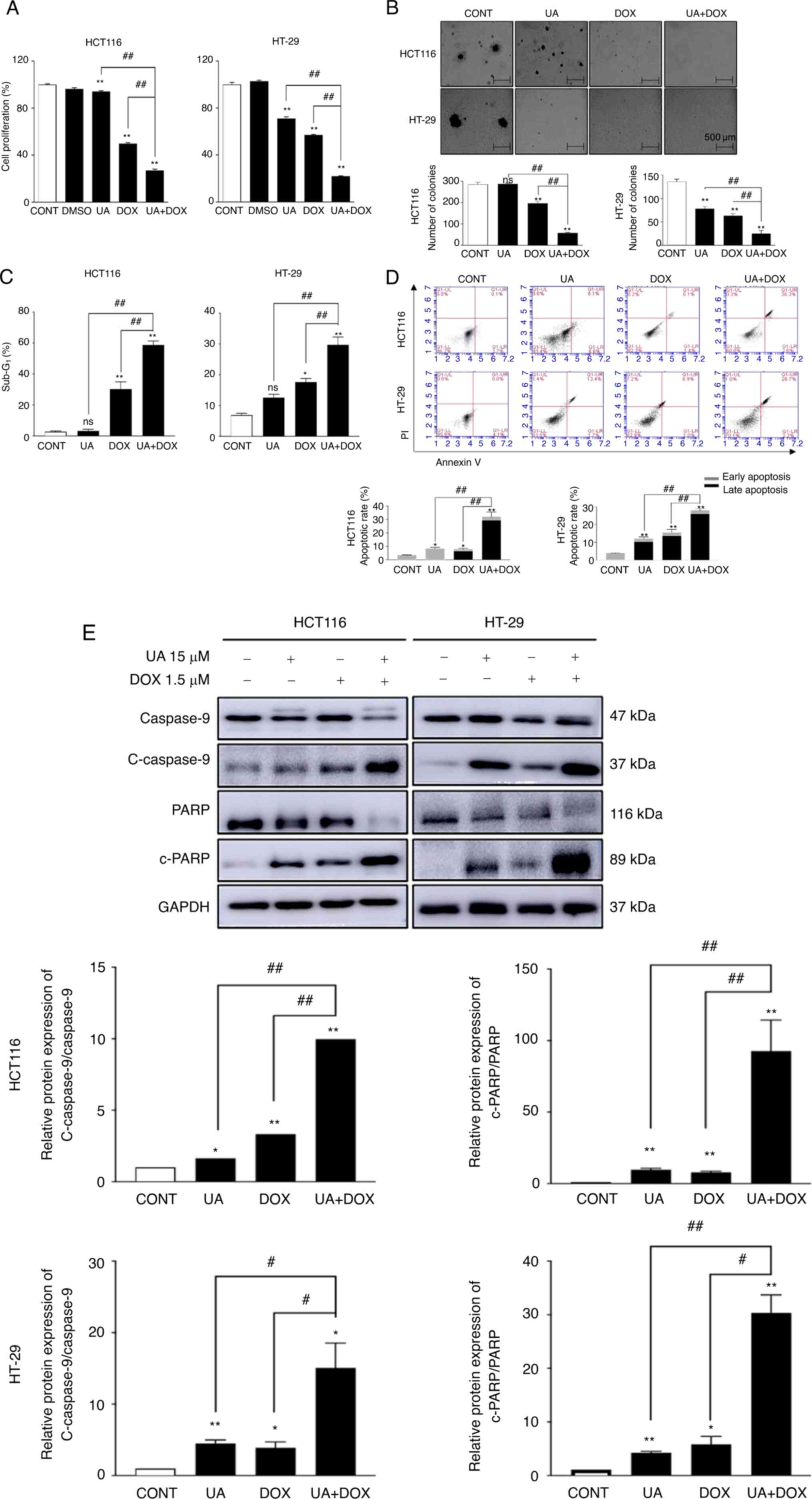
In the present study, the anti-proliferative effects
of UA and DOX were assessed by cell proliferation assay to evaluate
cytotoxicity. The UA and DOX concentrations used in the in
vitro experiments were based on the cell proliferation assay
results. Following 48 h of exposure, UA or DOX markedly inhibited
the cell proliferation of both HCT116 and HT29 cells in a
concentration-dependent manner compared with the control (Fig. S1). DOX (1.5 µM) treatment of
HCT116 cells caused significant cell proliferation suppression
compared with in the control group (Fig. 1A). The UA (15 µM) + DOX (1.5 µM)
combination treatment significantly inhibited cell proliferation
compared with in the UA and DOX groups. HT-29 cell proliferation
was significantly decreased (75–85% after 48 h) by co-treatment
compared with either agent alone. These data suggested that cell
proliferation was significantly suppressed by the combination of UA
+ DOX compared with either agent alone. Subsequently, a soft agar
colony formation assay was performed using CRC cells to assess the
effect of combination treatment on colony formation. UA + DOX
markedly decreased the size and significantly decreased the number
of colonies compared with each drug alone in both CRC cell lines
(Fig. 1B).
Flow cytometry was used to assess the apoptotic
status of cells following treatment with different drugs. It has
been reported that the sub-G1 phase is characterized by
apoptosis induction (69). The
proportion of cells in sub-G1 phase was significantly
increased following treatment with the combination of 15 µM UA +
1.5 µM DOX compared with the control and either agent alone
(Figs. 1C and S2A). PI/FITC assay results demonstrated
that the combination treatment significantly induced apoptosis
compared with the control and either agent alone (Fig. 1D). Furthermore, western blotting
demonstrated markedly decreased caspase-9, significantly decreased
PARP, and significantly increased cleaved caspase-9/caspase-9 and
cleaved-PARP/PARP protein expression levels following treatment
with the combination of 15 µM UA + 1.5 µM DOX compared with the
single treatments in HCT116 and HT-29 cells (Fig. 1E). These data were consistent with
the flow cytometry results. The present study indicated that
combination treatment triggered the apoptosis of CRC cells.
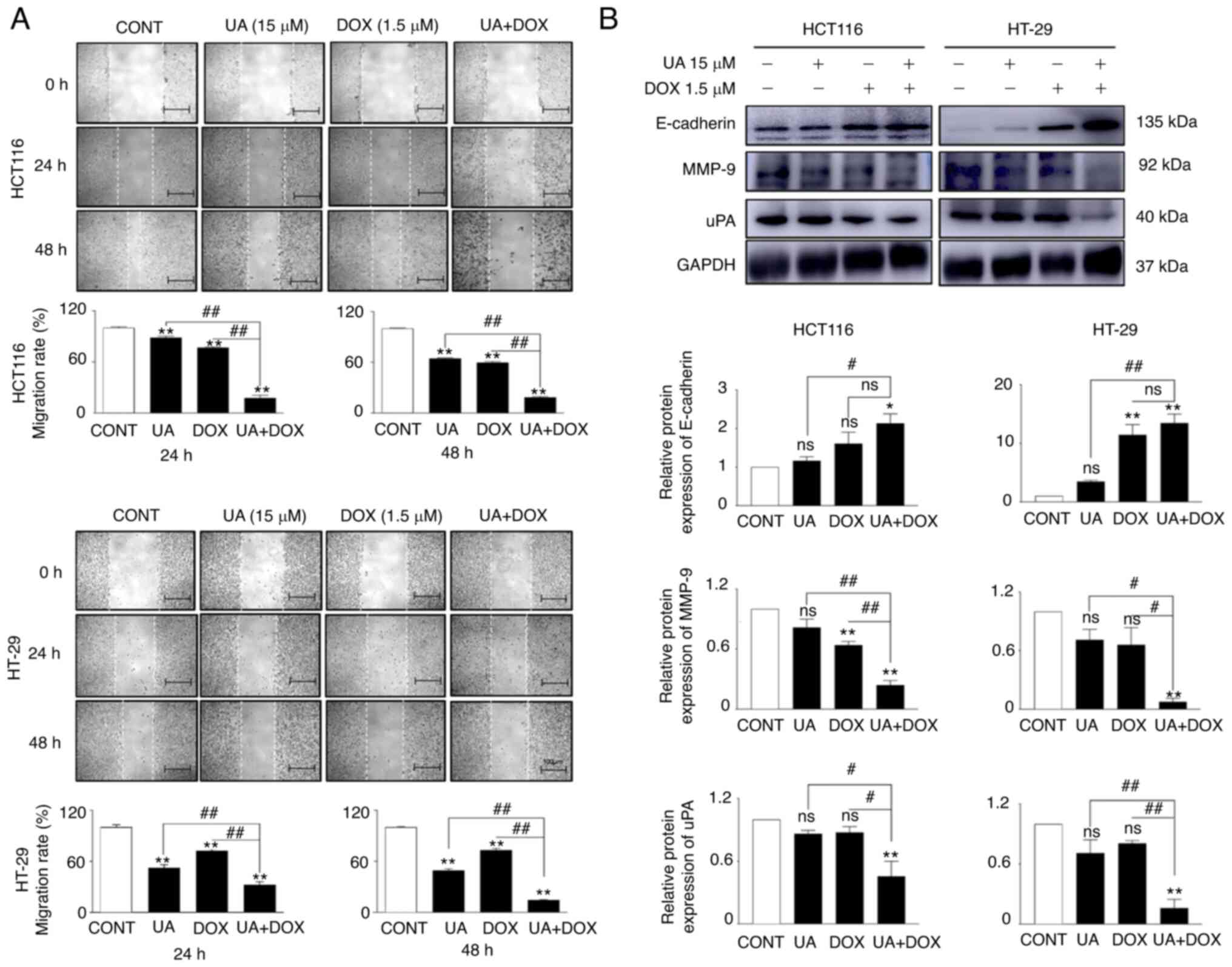
Distant cancer cell metastasis is a key step that
includes migration, invasion and EMT, which represents an advanced
malignancy stage (70). The
migratory ability of CRC cells, as demonstrated using wound healing
assay, was significantly decreased following treatment with the
combination of UA + DOX compared with UA or DOX alone (Fig. 2A). Investigation of EMT-associated
regulators was performed using western blotting. The protein
expression levels of E-cadherin, an epithelial marker, were
markedly increased, whereas the protein expression levels of
mesenchymal markers (MMP-9 and uPA) were significantly decreased in
the combination groups in both cell lines compared with the control
and either agent alone (Fig. 2B).
These data demonstrated that combination treatment with UA + DOX
may have inhibited cell migration via disruption of the EMT
process.
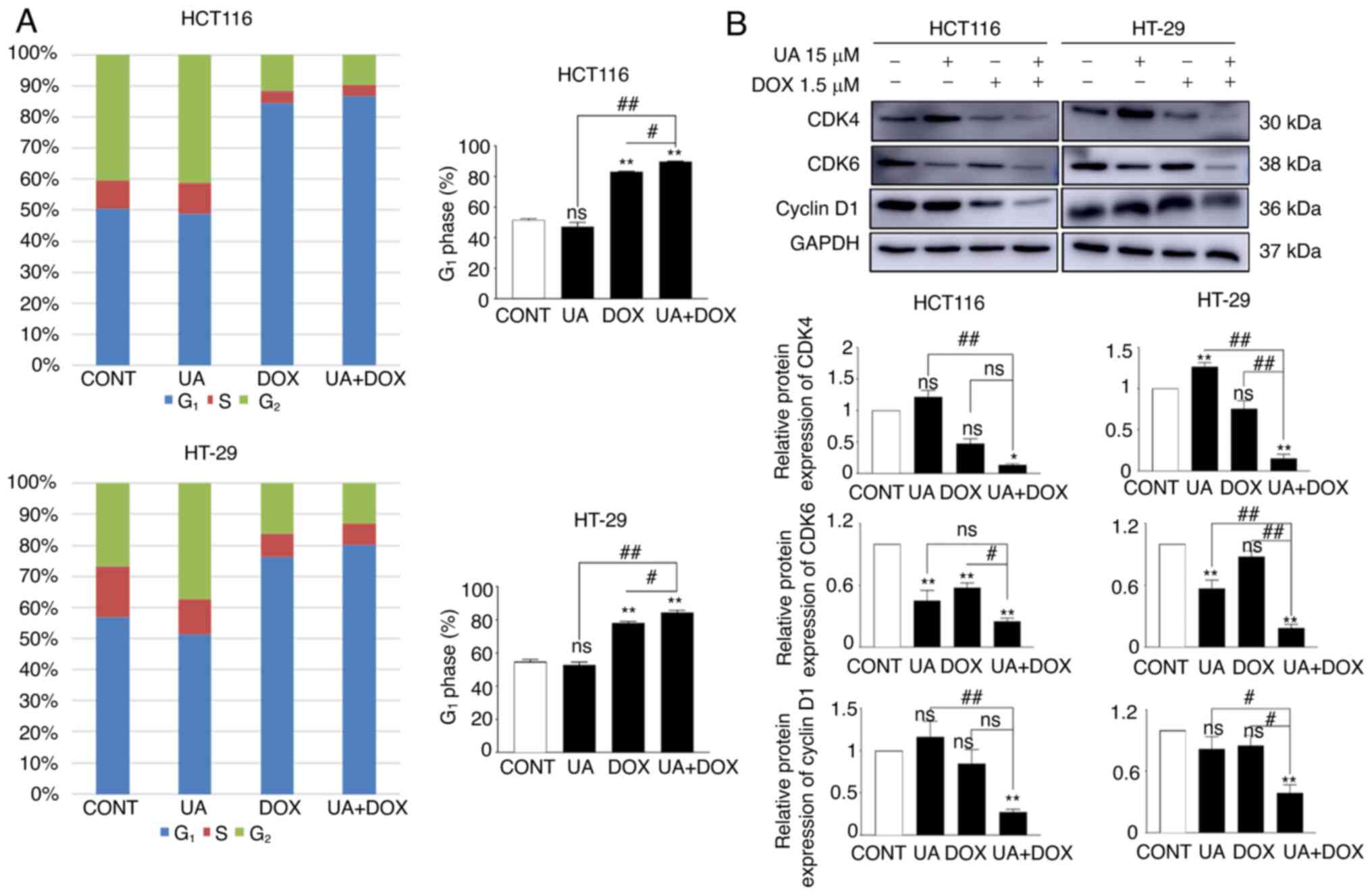
In the present study, flow cytometry was used to
assess cell cycle progression. The results suggested that UA did
not affect G1 phase in HCT116 and HT29 cell lines,
whereas cell accumulation in G1 phase was markedly
increased following DOX treatment compared with in the control
group. Furthermore, combination treatment with UA + DOX in HCT116
and HT-29 cells further induced G1 phase arrest,
followed by a marked decrease in the number of cells in
G2 phase compared with in the control group (Figs. 3A and S2B). Western blotting demonstrated that
cyclin-dependent kinase (CDK)4/6 and cyclin D1 protein expression
levels, which are essential for DNA synthesis (71), were significantly downregulated
following combination treatment compared with in the control group
(Fig. 3B). Collectively, these
data indicated that UA further enhanced DOX-mediated cell cycle
arrest in the G1 phase in HCT116 and HT-29 cell lines.
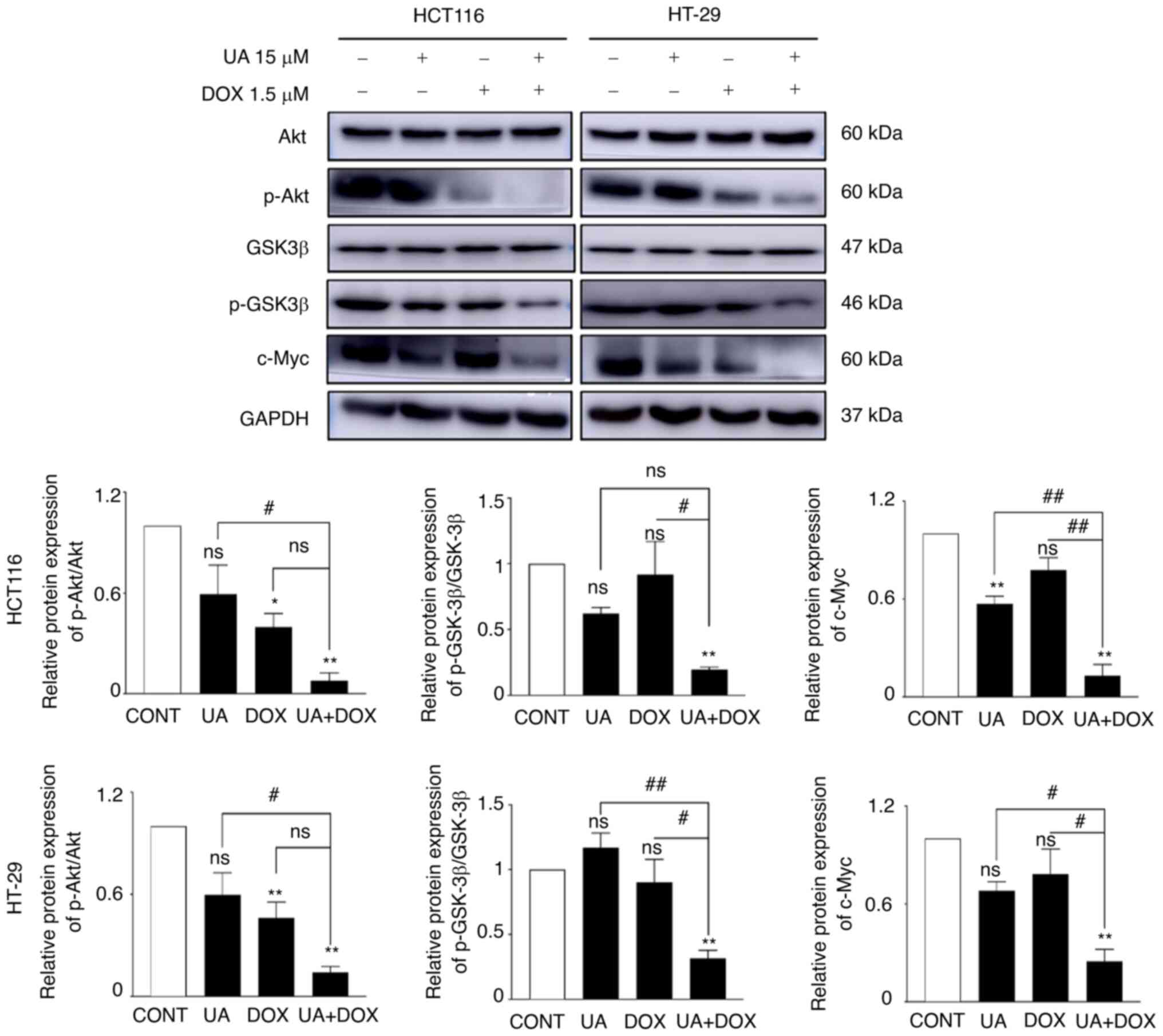
Growing evidence has indicated that overactive
PI3K/Akt signaling serves a crucial role in cell migration and
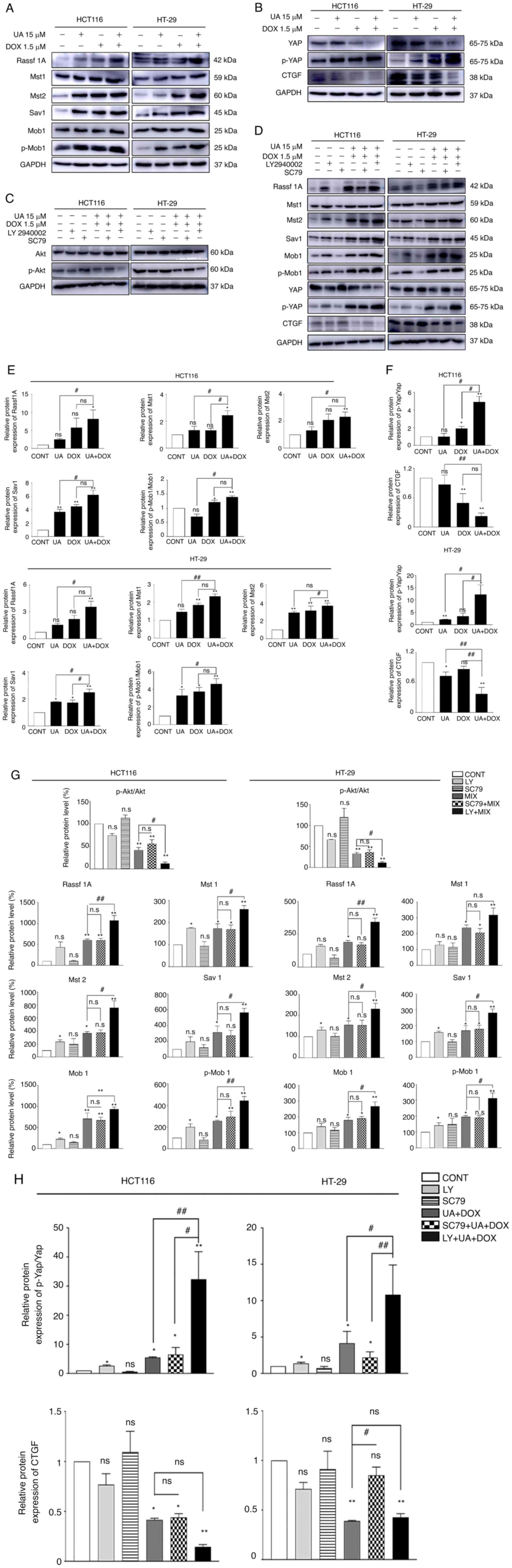
proliferation, as well as apoptosis inhibition (72–74). In the present study, the protein
expression levels of p-Akt, p-Gsk3β and c-Myc were assessed using
western blotting to evaluate the effects of UA and DOX treatment on
the Akt signaling pathway. The combination treatment of UA + DOX
significantly decreased the protein expression levels of p-Akt and
c-Myc, which is downstream of the Akt signaling pathway (75), compared with the control, whereas
the total level of Akt remained unchanged (Fig. 4). Consistently, p-Gsk3β (S9)
expression levels were markedly decreased by combination treatment
compared with UA or DOX alone (Fig.
4). Collectively, these results demonstrated that combination
treatment with UA + DOX exerted anticancer effects by inhibiting
the Akt signaling pathway.
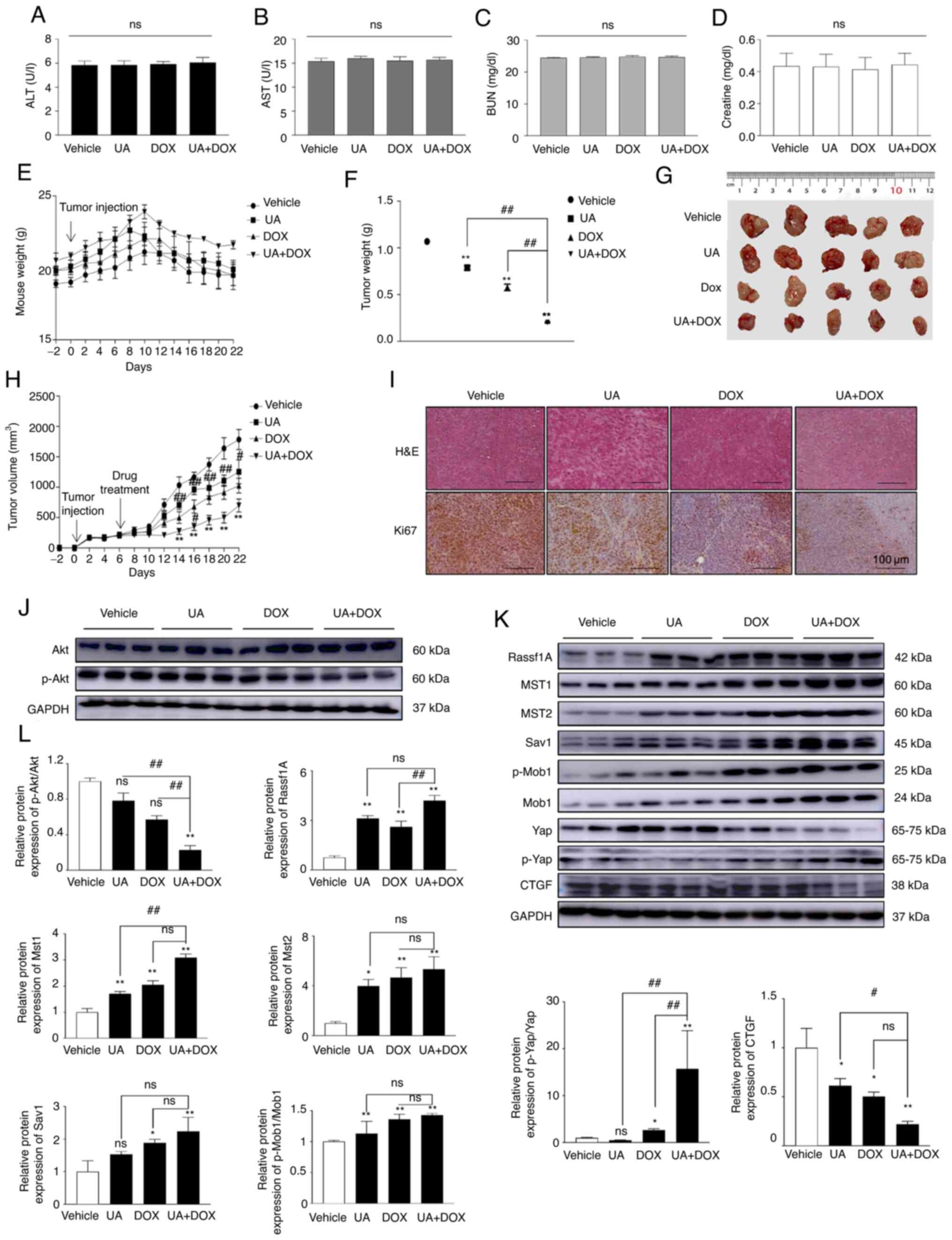
In the present study, levels of the biochemical
markers ALT, AST, BUN and creatinine were assessed in mouse serum
to evaluate the organ toxicity of UA and DOX. UA and DOX treatment
did not significantly affect the ALT, AST, BUN or creatinine level
between the four groups (Fig.
6A-D). This result indicated that the doses of UA and DOX did
not impair liver or kidney function. As presented in Fig. 6E, the weight of most of the
tumor-bearing mice remained unchanged throughout the study period,
with only three mice losing <5% of their weight in the vehicle
and UA treatment groups. UA and DOX treatment alone significantly
decreased tumor weight (Fig. 6F),
tumor size (Fig. 6G) and tumor
volume (Fig. 6H) compared with in
the vehicle group. UA and DOX treatment demonstrated notable
inhibition of tumor growth compared with the control group in the
xenograft model. Furthermore, a marked different histology was
demonstrated using H&E staining (Fig. 6I). Aggressive tumor cell
proliferation with a high nucleus/cytoplasm ratio was observed in
the control group, whereas the combination of UA + DOX treatment
induced lymphocyte infiltration around tumor cells and increased
the areas of apoptosis (Fig. 6I).
Moreover, immunohistochemistry staining demonstrated that
expression of the proliferation marker Ki-67 in the UA + DOX
treatment group was lower than that in the control group, which
suggested that the combination of UA + DOX suppressed tumor growth
and development in vivo. Tumor samples were harvested and
western blot analysis was performed to assess the molecular
mechanisms underlying the effects of combination treatment in
vivo. The combination treatment significantly decreased the
p-Akt/Akt ratio compared with UA or DOX alone while markedly
activating the Hippo signaling pathway compared with UA or DOX
alone (Fig. 6J-L). Collectively,
these in vivo results demonstrated that UA augmented the
antitumor effects of DOX via inactivation of Akt and activation of
the Hippo signaling pathway in the mouse xenograft model.
The precision of CRC diagnosis has improved in
recent years; however, CRC is the fourth most commonly diagnosed
and the third deadliest cancer worldwide (88). The primary biological
characteristics of metastatic CRC are tumor cell proliferation and
migration, and this type of cancer has limited therapeutic options
and is thus associated with a higher risk of mortality (89,90). With rapid development of molecular
genetic analysis of human metastatic CRC, treatments that target
specific signaling pathways, including TGF-β (transforming growth
factor-β)/SMAD (91),
Wnt/β-catenin (92), EGFR-related
pathway (93) and VEGF/VEFGR
pathway (94) have been reported
as a potential approach for CRC therapy (95). Studies on the anticancer efficacy
of UA (96,97) or DOX (98) have been performed; however, to the
best of the authors' knowledge, the effects of UA on the efficacy
of DOX in human CRC cells have not been reported. The present study
demonstrated that UA and DOX served a key role in the crosstalk
between the Hippo and Akt signaling pathways, resulting in the
inactivation of tumorigenesis in colon cancer cells.
In this study, HCT116 and HT-29 were used because
HCT116 is a highly aggressive cell line with little or no capacity
to differentiate, whereas HT-29 has an intermediate capacity to
differentiate into enterocytes and mucin-expressing lineages. The
HCT116 cell line was originally isolated from a colon primary tumor
(99,100) and the HT-29 cell line originated
from a colorectal adenocarcinoma (60). Ahmed et al (101) assessed the genetic mutation
status of the HCT116 and HT-29 cell lines. It was reported that
HT-29 exhibited deficient TP53 expression, whereas HCT116
had gained a KRAS mutation (101), which resulted in constitutive
stimulation of the KRAS signaling pathway. KRAS signaling pathway
activation has a high oncogenic potential and a very aggressive
nature (102). For these
reasons, the use of HCT116 and HT-29 cell lines enabled the
comparison of a broad spectrum of features, which were
characteristic of these types of cells. Furthermore, this allowed
the present study to evaluate the therapeutic effects of an
anticancer herbal component-based drug in combination with
chemotherapy in CRC.
In the present study, UA and DOX significantly
inhibited the proliferation, colony formation and migration of the
human CRC HCT116 and HT-29 cell lines. It has been reported that
DOX can arrest the cell cycle in G1 phase in human CRC
cells (103). Furthermore, cell
cycle-associated regulators, such as CDKs and cyclins,
dysregulation of which result in uncontrolled cellular
proliferation and malignancy, are regulated by DOX (104). The present study demonstrated
that UA enhanced DOX-mediated upregulation in the G1
population of CRC cells. Apoptosis, a form of programmed cell
death, is a promising target for anticancer therapy (105). Numerous treatments with natural
products, such as herbal compounds, have been evaluated for their
ability to induce cell apoptosis in CRC (106–109). Previous studies have assessed
whether cell apoptosis is induced by UA in cancer, such as CRC and
breast and esophageal cancer, via different mechanisms (27,110,111). The present study demonstrated
that combined UA + DOX treatment promoted CRC cell late apoptosis
and increased the sub-G1 apoptotic fraction as assessed
by FITC/PI staining and flow cytometry. UA + DOX promoted cleavage
of PARP and activated caspase-9, which also suggested that
combination treatment enhanced the effect on programmed cell death,
compared with each drug alone.
The Akt signaling pathway serves a key role in
tumorigenesis, and regulates cancer cell proliferation, migration
and apoptosis (72).
Overactivated PI3K/Akt signaling has been reported in numerous
types of cancer, including CRC (112–114). Akt signaling pathway activation
upregulates the expression of downstream genes c-Myc and cyclin D1
(115). Moreover, accumulating
studies have reported that the Akt signaling pathway serves an
important role in EMT and metastasis by inhibiting E-cadherin
expression and upregulating the mesenchymal marker vimentin
(116,117). Gsk3β is the downstream regulator
of Akt signaling in malignant cells. Akt signaling inhibition has
been reported to induce apoptosis via inhibition of Gsk3β (Ser9) by
phosphorylation (118).
Furthermore, there have been increasing reports that have suggested
that cyclin/CDKs, which act as the master regulator for cell cycle
progression (119), are
modulated by Gsk3β (120,121).
In the present study, DOX markedly inhibited the protein expression
levels of p-Akt and combination treatment further attenuated the
activity of the Akt signaling pathway. These results demonstrated
that the therapeutic effect primarily occurred via downregulation
of the Akt signaling pathway and decreased the p-Gsk3β (S9)
expression level following combined treatment in CRC cells.
Consistent with these results, in vivo results demonstrated
that UA and DOX treatment caused marked necrosis and morphological
changes, and significantly decreased tumor volume compared with UA
or DOX alone in the HCT116 ×enograft tumor model. Moreover, the
combination treatment at the dosage used in the present study did
not significantly affect liver or kidney function, as evaluated by
the detection of biochemical marker levels. Cardiotoxicity is the
primary limitation of DOX-based chemotherapy (122). In a recent study, early protein
markers of DOX toxicity were detected in mice treated with DOX at
cumulative doses of ≥12 mg/kg (123). In the present study, low-dose
DOX (2 mg/kg twice/week) treatment with a cumulative dose of <10
mg/kg was used and the heart was not assessed. However, serological
analysis demonstrated that the dosage of UA and DOX did not
significantly impair liver or kidney function. Therefore, these
data suggested that UA and DOX treatment inhibited tumor
progression without any toxicity to the liver or kidney at the
endpoint of the animal experiment. Furthermore, the p-Akt/Akt
protein expression levels were decreased in UA and DOX-treated
xenograft animal tissue, which demonstrated that UA enhanced the
efficacy of DOX by targeting the Akt signaling pathway, thus
indicating that UA + DOX may serve as a potential therapeutic
strategy for CRC.
The tumor-suppressive Hippo/Yap signaling pathway
has attracted attention following reports that inhibits
tumorigenesis (124–126). Activation of the Hippo/Yap
signaling pathway primarily involves tumor suppressors (the Mst1/2
kinase cascade) and the following downstream regulators: following
downstream regulators: Yap, transcriptional coactivator with PDZ
postsynaptic density protein (TAZ), Drosophila disk large
tumor suppressor and zonula occludens-1 protein-binding motif
(127). It has been reported
that tumor suppressor dysfunction and Yap activity overactivation
can induce carcinogenesis, cancer proliferation and metastasis
(128,129). Our previous study demonstrated
that UA significantly upregulated the protein expression levels of
tumor suppressors associated with the Hippo signaling pathway, such
as Rassf1A, Mst1, Mst2 and Sav1 (130). In agreement with the
aforementioned study, the present study demonstrated that DOX
treatment stimulated the Hippo signaling pathway and UA markedly
enhanced DOX-mediated activation of the Hippo signaling pathway
both in vivo and in vitro and the activity of Yap was
significantly inactivated by combination treatment in CRC cells.
Numerous studies have reported the crosstalk between the Akt and
Hippo/Yap signaling pathways in numerous types of cancer (131–136). For example, Akt has been
reported to phosphorylate Mst1 and inhibit its activity, thereby
preventing cell apoptosis (137). Upstream tumor suppressor
phosphatase and tensin homolog inactivation has been reported to
promote the PI3K/Akt signaling pathway in a Hippo pathway-dependent
manner in human gastric cancer (136). Akt hyperactivation may inhibit
the Hippo pathway and stabilize downstream transcriptional factor
Yap/TAZ, thereby maintaining liver homeostasis (131). Previous studies regarding the
Mst2 kinase reported that phosphorylation of Akt prevents Rassf1A
binding and decreases Mst2 kinase activity (138,139). Rassf1A is a Hippo signaling
pathway regulator that is highly silenced in human gastric
(130), esophageal (140), lung (141), breast (142) and liver cancer (143). Akt signaling inhibition using a
PI3K inhibitor can demethylate the promoter of Rassf1A and increase
the protein expression levels of Rassf1A, resulting in Hippo
signaling pathway activation (144,145). Therefore, inhibition of Akt is
important for regulating the Hippo pathway on multiple levels.
Notably, the present study indicated that the Akt signaling pathway
was associated with the Hippo pathway and crosstalk occurred in
CRC. There was no significant difference between UA + DOX
combination treatment and SC79 + UA + DOX combination treatment in
the expression levels of Akt/p-Akt and Hippo pathway proteins
(Rassf1A, Mst1 and Sav1). The PI3K inhibitor LY294002 was used to
assess the effects of the Akt signaling pathway and its association
with the Hippo signaling pathway and UA and DOX-mediated anticancer
effects. LY294002 further suppressed p-Akt expression, and induced
Mst1/2, Sav1 and p-Yap expression, which led to decreased CTGF
protein expression levels following UA and DOX combination
treatment. In agreement with the in vitro data, the in
vivo data demonstrated that the protein expression levels of
p-Akt were significantly decreased, whereas Hippo
pathway-associated protein expression levels (Rassf1A, Mst1, Mst2,
Sav1, Mob1 and p-Mob1) were markedly increased in UA and
DOX-treated xenograft mouse tumor samples.
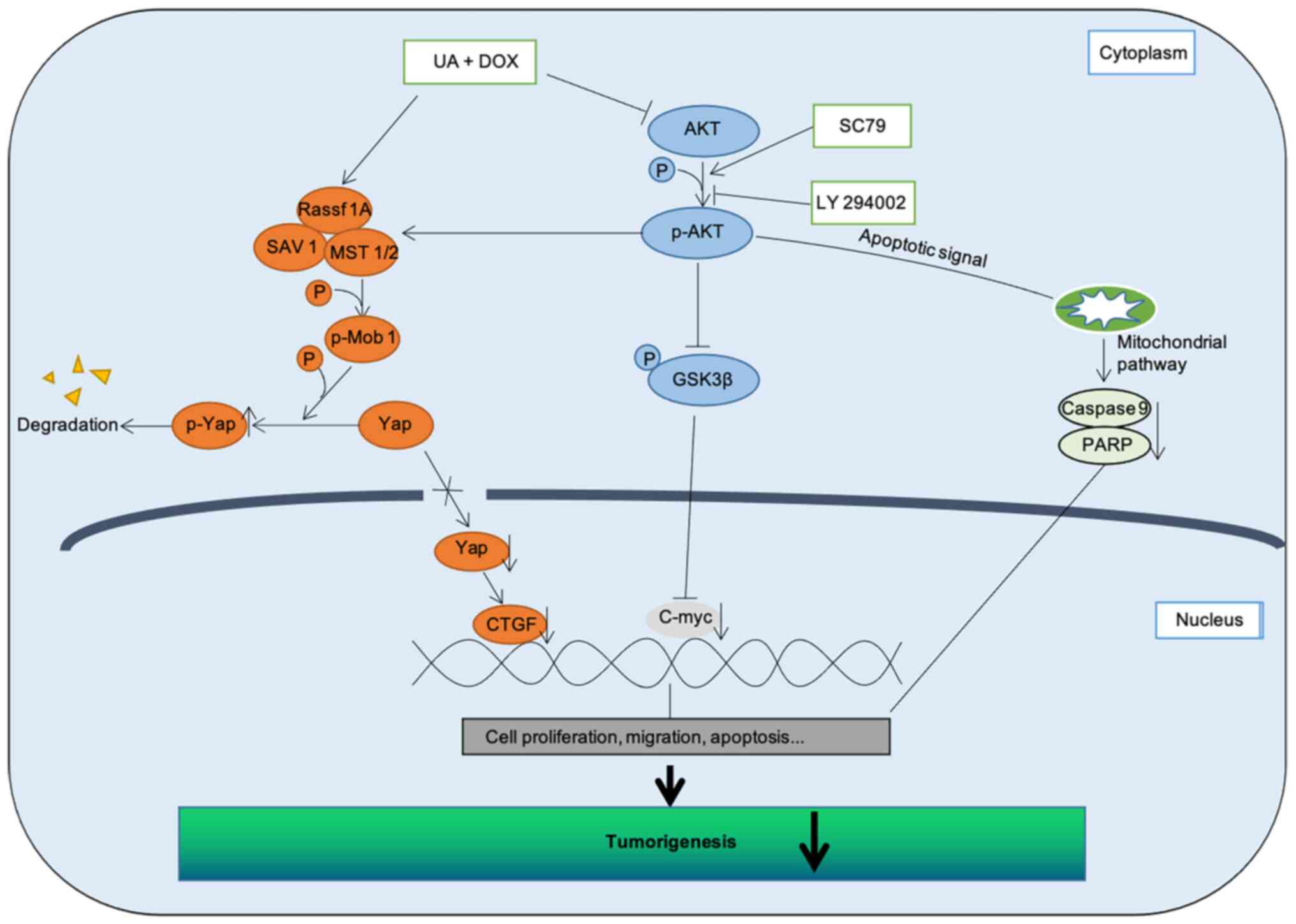
In conclusion, these data indicated that Hippo
signaling pathway stimulation may rely on Akt signaling pathway
inhibition, with UA and DOX combination treatment effectively
arresting tumor growth and metastasis, and triggering apoptosis in
CRC cells. To delineate the molecular mechanisms and assess whether
Akt directly or indirectly binds with tumor suppressors that serve
a key role in the Hippo signaling pathway and affect the activation
of downstream factors following UA and DOX treatment, a more
in-depth study is required. Collectively, the present study
demonstrated that a natural product-derived agent (UA) enhanced
chemotherapy (DOX) outcomes by targeting PI3K/Akt signaling and
activating the Hippo signaling pathway (Fig. 7), thus demonstrating the potential
of UA in combination with DOX as a novel, more efficient strategy
for CRC treatment.
Not applicable.
The present study was supported by The National University
Development Project in 2020.
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
DH performed assays and wrote the manuscript. RYM
performed analysis of the data. TVN and OHC performed H&E
staining. BHP designed in vivo study and JSL performed the
animal experiments. SMK conceived the experiments, and wrote and
revised the manuscript. All authors have read and approved the
final manuscript. DH and SMK confirm the authenticity of all the
raw data.
All animal care procedures and experiments were
performed in accordance with the Animal Research: Reporting of
In Vivo Experiments guidelines 2.0 and were approved by the
Institutional Animal Care and Use Committee of Jeonbuk National
University (approval no. CBNU2017-0001).
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shin DW, Chang D, Jung JH, Han K, Kim SY,
Choi KS, Lee WC and Park JH and Park JH: Disparities in the
participation rate of colorectal cancer screening by fecal occult
blood test among people with disabilities: A national database
study in South Korea. Cancer Res Treat. 52:60–73. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang SY, Cho MS and Kim NK: Difference
between right-sided and left-sided colorectal cancers: From
embryology to molecular subtype. Expert Rev Anticancer Ther.
18:351–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McQuade RM, Stojanovska V, Bornstein JC
and Nurgali K: Colorectal cancer chemotherapy: The evolution of
treatment and new approaches. Curr Med Chem. 24:1537–1557. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cargnin ST and Gnoatto SB: Ursolic acid
from apple pomace and traditional plants: A valuable triterpenoid
with functional properties. Food Chem. 220:477–489. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu C, Liao Y, Fang C, Tsunoda M, Zhang Y,
Song Y and Deng S: Simultaneous analysis of ursolic acid and
oleanolic acid in guava leaves using QuEChERS-based extraction
followed by high-performance liquid chromatography. J Anal Methods
Chem. 2017:29845622017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng JL, Wang SS, Shen KP, Huang XW, Li
M, Chen L, Peng X, An HM and Hu B: Ursolic acid potentiated
oxaliplatin to induce apoptosis in colorectal cancer RKO cells.
Pharmazie. 75:246–249. 2020.PubMed/NCBI
|
|
8
|
Wang X, Wang T, Yi F, Duan C, Wang Q, He
N, Zhu L, Li Q and Deng W: Ursolic acid inhibits tumor growth via
epithelial-to-mesenchymal transition in colorectal cancer cells.
Biol Pharm Bull. 42:685–691. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai Q, Lin J, Zhang L, Lin J, Wang L, Chen
D and Peng J: Comparative proteomics-network analysis of proteins
responsible for ursolic acid-induced cytotoxicity in colorectal
cancer cells. Tumour Biol. 39:10104283176950152017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Shu L, Zhang C, Li W, Wu R, Guo Y,
Yang Y and Kong AN: Histone methyltransferase Setd7 regulates Nrf2
signaling pathway by phenethyl isothiocyanate and ursolic acid in
human prostate cancer cells. Mol Nutr Food Res. 62:e17008402018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang K, Chen Y, Zhou J, Ma L, Shan Y,
Cheng X, Wang Y, Zhang Z, Ji X, Chen L, et al: Ursolic acid
promotes apoptosis and mediates transcriptional suppression of
CT45A2 gene expression in non-small-cell lung carcinoma harbouring
EGFR T790M mutations. Br J Pharmacol. 176:4609–4624. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mendes VIS, Bartholomeusz GA, Ayres M,
Gandhi V and Salvador JAR: Synthesis and cytotoxic activity of
novel A-ring cleaved ursolic acid derivatives in human non-small
cell lung cancer cells. Eur J Med Chem. 123:317–331. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan EWC, Soon CY, Tan JBL, Wong SK and
Hui YW: Ursolic acid: An overview on its cytotoxic activities
against breast and colorectal cancer cells. J Integr Med.
17:155–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim K, Shin EA, Jung JH, Park JE, Kim DS,
Shim BS and Kim SH: Ursolic acid induces apoptosis in colorectal
cancer cells partially via upregulation of MicroRNA-4500 and
inhibition of JAK2/STAT3 phosphorylation. Int J Mol Sci.
20:1142018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prasad S, Yadav VR, Sung B, Reuter S,
Kannappan R, Deorukhkar A, Diagaradjane P, Wei C,
Baladandayuthapani V, Krishnan S, et al: Ursolic acid inhibits
growth and metastasis of human colorectal cancer in an orthotopic
nude mouse model by targeting multiple cell signaling pathways:
Chemosensitization with capecitabine. Clin Cancer Res.
18:4942–4953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu P, Du R and Yu X: Ursolic acid
exhibits potent anticancer effects in human metastatic melanoma
cancer cells (SK-MEL-24) via apoptosis induction, inhibition of
cell migration and invasion, cell cycle arrest, and inhibition of
mitogen-activated protein kinase (MAPK)/ERK signaling pathway. Med
Sci Monit. 25:1283–1290. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu T, Ma H, Shi W, Duan J, Wang Y, Zhang
C, Li C, Lin J, Li S, Lv J and Lin L: Inhibition of STAT3 signaling
pathway by ursolic acid suppresses growth of hepatocellular
carcinoma. Int J Oncol. 51:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Cai QY, Liu J, Peng J, Chen YQ,
Sferra TJ and Lin JM: Ursolic acid suppresses the invasive
potential of colorectal cancer cells by regulating the
TGF-β1/ZEB1/miR-200c signaling pathway. Oncol Lett. 18:3274–3282.
2019.PubMed/NCBI
|
|
19
|
Cheng J, Liu Y, Liu Y, Liu D, Liu Y, Guo
Y, Wu Z, Li H and Wang H: Ursolic acid alleviates lipid
accumulation by activating the AMPK signaling pathway in vivo and
in vitro. J Food Sci. 85:3998–4008. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim GH, Kan SY, Kang H, Lee S, Ko HM, Kim
JH and Lim JH: Ursolic acid suppresses cholesterol biosynthesis and
exerts anti-cancer effects in hepatocellular carcinoma cells. Int J
Mol Sci. 20:47672019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin CW, Chin HK, Lee SL, Chiu CF, Chung
JG, Lin ZY, Wu CY, Liu YC, Hsiao YT, Feng CH, et al: Ursolic acid
induces apoptosis and autophagy in oral cancer cells. Environ
Toxicol. 34:983–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin JH, Chen SY, Lu CC, Lin JA and Yen GC:
Ursolic acid promotes apoptosis, autophagy, and chemosensitivity in
gemcitabine-resistant human pancreatic cancer cells. Phytother Res.
34:2053–2066. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin W and Ye H: Anticancer activity of
ursolic acid on human ovarian cancer cells via ROS and MMP mediated
apoptosis, cell cycle arrest and downregulation of PI3K/AKT
pathway. J BUON. 25:750–756. 2020.PubMed/NCBI
|
|
24
|
Li W, Zhang H, Nie M, Tian Y, Chen X, Chen
C, Chen H and Liu R: Ursolic acid derivative FZU-03,010 inhibits
STAT3 and induces cell cycle arrest and apoptosis in renal and
breast cancer cells. Acta Biochim Biophys Sin (Shanghai).
49:367–373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruan JS, Zhou H, Yang L, Wang L, Jiang ZS,
Sun H and Wang SM: Ursolic acid attenuates TGF-β1-induced
epithelial-mesenchymal transition in NSCLC by targeting integrin
αVβ5/MMPs signaling. Oncol Res. 27:593–600. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sohn EJ, Won G, Lee J, Yoon SW, Lee I, Kim
HJ and Kim SH: Blockage of epithelial to mesenchymal transition and
upregulation of let 7b are critically involved in ursolic acid
induced apoptosis in malignant mesothelioma cell. Int J Biol Sci.
12:1279–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee NR, Meng RY, Rah SY, Jin H, Ray N, Kim
SH, Park BH and Kim SM: Reactive oxygen species-mediated autophagy
by ursolic acid inhibits growth and metastasis of esophageal cancer
cells. Int J Mol Sci. 21:94092020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park HJ, Jo DS, Choi DS, Bae JE, Park NY,
Kim JB, Chang JH, Shin JJ and Cho DH: Ursolic acid inhibits
pigmentation by increasing melanosomal autophagy in B16F1 cells.
Biochem Biophys Res Commun. 531:209–214. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arcamone F, Cassinelli G, Fantini G, Grein
A, Orezzi P, Pol C and Spalla C: Adriamycin, 14-hydroxydaunomycin,
a new antitumor antibiotic from S. peucetius var. caesius.
Reprinted from biotechnology and bioengineering, Vol. XI, Issue 6,
Pages 1101–1110 (1969). Biotechnol Bioeng. 67:704–713. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cortés-Funes H and Coronado C: Role of
anthracyclines in the era of targeted therapy. Cardiovasc Toxicol.
7:56–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weiss RB: The anthracyclines: Will we ever
find a better doxorubicin? Semin Oncol. 19:670–686. 1992.PubMed/NCBI
|
|
32
|
Sarmento-Ribeiro AB, Scorilas A, Goncalves
AC, Efferth T and Trougakos IP: The emergence of drug resistance to
targeted cancer therapies: Clinical evidence. Drug Resist Updat.
47:1006462019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rui M, Xin Y, Li R, Ge Y, Feng C and Xu X:
Targeted biomimetic nanoparticles for synergistic combination
chemotherapy of paclitaxel and doxorubicin. Mol Pharm. 14:107–123.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fan YP, Liao JZ, Lu YQ, Tian DA, Ye F,
Zhao PX, Xiang GY, Tang WX and He XX: MiR-375 and doxorubicin
co-delivered by liposomes for combination therapy of hepatocellular
carcinoma. Mol Ther Nucleic Acids. 7:181–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo NF, Cao YJ, Chen X, Zhang Y, Fan YP,
Liu J and Chen XL: Lixisenatide protects doxorubicin-induced renal
fibrosis by activating wNF-κB/TNF-α and TGF-β/Smad pathways. Eur
Rev Med Pharmacol Sci. 23:4017–4026. 2019.PubMed/NCBI
|
|
37
|
Saleh D, Abdelbaset M, Hassan A, Sharaf O,
Mahmoud S and Hegazy R: Omega-3 fatty acids ameliorate
doxorubicin-induced cardiorenal toxicity: In-vivo regulation of
oxidative stress, apoptosis and renal Nox4, and in-vitro
preservation of the cytotoxic efficacy. PLoS One. 15:e02421752020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Prasanna PL, Renu K and Valsala
Gopalakrishnan A: New molecular and biochemical insights of
doxorubicin-induced hepatotoxicity. Life Sci. 250:1175992020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou X, Xu P, Dang R, Guo Y, Li G, Qiao Y,
Xie R, Liu Y and Jiang P: The involvement of autophagic flux in the
development and recovery of doxorubicin-induced neurotoxicity. Free
Radic Biol Med. 129:440–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim CL, Choi SH and Mo JS: Role of the
Hippo pathway in fibrosis and cancer. Cells. 8:4682019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size-control mechanism in
Drosophila and mammals. Cell. 130:1120–1233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Avruch J, Zhou D and Bardeesy N: YAP
oncogene overexpression supercharges colon cancer proliferation.
Cell Cycle. 11:1090–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu XF, Han Q, Rong XZ, Yang M, Han YC, Yu
JH and Lin XY: ANKHD1 promotes proliferation and invasion of
non-small-cell lung cancer cells via regulating YAP oncoprotein
expression and inactivating the Hippo pathway. Int J Oncol.
56:1175–1185. 2020.PubMed/NCBI
|
|
45
|
Niu K, Liu Y, Zhou Z, Wu X, Wang H and Yan
J: Antitumor effects of paeoniflorin on Hippo signaling pathway in
gastric cancer cells. J Oncol. 2021:47249382021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hou L, Chen L and Fang L: Scutellarin
inhibits proliferation, invasion, and tumorigenicity in human
breast cancer cells by regulating HIPPO-YAP signaling pathway. Med
Sci Monit. 23:5130–5138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Driskill JH and Pan D: The Hippo pathway
in liver homeostasis and pathophysiology. Annu Rev Pathol.
16:299–322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Masliantsev K, Karayan-Tapon L and Guichet
PO: Hippo signaling pathway in gliomas. Cells. 10:1842021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ansari D, Ohlsson H, Althini C, Bauden M,
Zhou Q, Hu D and Andersson R: The Hippo signaling pathway in
pancreatic cancer. Anticancer Res. 39:3317–3321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Llado V, Nakanishi Y, Duran A,
Reina-Campos M, Shelton PM, Linares JF, Yajima T, Campos A,
Aza-Blanc P, Leitges M, et al: Repression of intestinal stem cell
function and tumorigenesis through direct phosphorylation of
β-catenin and Yap by PKCζ. Cell Rep. 10:740–754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen L, Qin F, Deng X, Avruch J and Zhou
D: Hippo pathway in intestinal homeostasis and tumorigenesis.
Protein Cell. 3:305–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gu Y, Zhang L and Yu FX: Functions and
regulations of the Hippo signaling pathway in intestinal
homeostasis, regeneration and tumorigenesis. Yi Chuan. 39:588–596.
2017.PubMed/NCBI
|
|
53
|
Zhou D, Zhang Y, Wu H, Barry E, Yin Y,
Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD and
Avruch J: Mst1 and Mst2 protein kinases restrain intestinal stem
cell proliferation and colonic tumorigenesis by inhibition of
Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA.
108:E1312–E1320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xiao Y, Liu Q, Peng N, Li Y, Qiu D, Yang
T, Kang R, Usmani A, Amadasu E, Borlongan CV and Yu G: Lovastatin
inhibits RhoA to suppress canonical Wnt/β-catenin signaling and
alternative Wnt-YAP/TAZ signaling in colon cancer. Cell Transplant.
31:96368972210757492022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Touil Y, Igoudjil W, Corvaisier M, Dessein
AF, Vandomme J, Monté D, Stechly L, Skrypek N, Langlois C, Grard G,
et al: Colon cancer cells escape 5FU chemotherapy-induced cell
death by entering stemness and quiescence associated with the
c-Yes/YAP axis. Clin Cancer Res. 20:837–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shamekhi S, Abdolalizadeh J, Ostadrahimi
A, Mohammadi SA, Barzegari A, Lotfi H, Bonabi E and Zarghami N:
Apoptotic effect of saccharomyces cerevisiae on human colon cancer
SW480 cells by regulation of Akt/NF-ĸB signaling pathway.
Probiotics Antimicrob Proteins. 12:311–319. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Goel S, Huang J and Klampfer L: K-Ras,
intestinal homeostasis and colon cancer. Curr Clin Pharmacol.
10:73–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tumaneng K, Schlegelmilch K, Russell RC,
Yimlamai D, Basnet H, Mahadevan N, Fitamant J, Bardeesy N, Camargo
FD and Guan KL: YAP mediates crosstalk between the Hippo and
PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol.
14:1322–1329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu FX, Zhao B, Panupinthu N, Jewell JL,
Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al:
Regulation of the Hippo-YAP pathway by G-protein-coupled receptor
signaling. Cell. 150:780–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kawai K, Viars C, Arden K, Tarin D,
Urquidi V and Goodison S: Comprehensive karyotyping of the HT-29
colon adenocarcinoma cell line. Genes Chromosomes Cancer. 34:1–8.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Grada A, Otero-Vinas M, Prieto-Castrillo
F, Obagi Z and Falanga V: Research techniques made simple: Analysis
of collective cell migration using the wound healing assay. J
Invest Dermatol. 137:e11–e16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vang Mouritzen M and Jenssen H: Optimized
scratch assay for in vitro testing of cell migration with an
automated optical camera. J Vis Exp. 576912018.PubMed/NCBI
|
|
63
|
Martinotti S and Ranzato E: Scratch wound
healing assay. Methods Mol Biol. 2109:225–229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Meng RY, Jin H, Nguyen TV, Chai OH, Park
BH and Kim SM: Ursolic acid accelerates paclitaxel-induced cell
death in esophageal cancer cells by suppressing Akt/FOXM1 signaling
cascade. Int J Mol Sci. 22:114862021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Stephenson W: Deficiencies in the national
institute of health's guidelines for the care and protection of
laboratory animals. J Med Philos. 18:375–88. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. Osteoarthritis
Cartilage. 20:256–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Spangenberg EM and Keeling LJ: Assessing
the welfare of laboratory mice in their home environment using
animal-based measures-a benchmarking tool. Lab Anim. 50:30–38.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Clayden EC: Practical section cutting and
staining. 5th edition. Edinburgh: (15 Teviot Place, Edinburgh 1).
Churchill Livingstone; 7. pp. pp2701971
|
|
69
|
Kim DH, Kang DY, Sp N, Jo ES, Rugamba A,
Jang KJ and Yang YM: Methylsulfonylmethane induces cell cycle
arrest and apoptosis, and suppresses the stemness potential of
HT-29 cells. Anticancer Res. 40:5191–5200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ombrato L and Malanchi I: The EMT
universe: Space between cancer cell dissemination and metastasis
initiation. Crit Rev Oncog. 19:349–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang C, Li Z, Lu Y, Du R, Katiyar S, Yang
J, Fu M, Leader JE, Quong A, Novikoff PM and Pestell RG: Cyclin D1
repression of nuclear respiratory factor 1 integrates nuclear DNA
synthesis and mitochondrial function. Proc Natl Acad Sci USA.
103:11567–11572. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bishnupuri KS, Alvarado DM, Khouri AN,
Shabsovich M, Chen B, Dieckgraefe BK and Ciorba MA: IDO1 and
kynurenine pathway metabolites activate PI3K-Akt signaling in the
neoplastic colon epithelium to promote cancer cell proliferation
and inhibit apoptosis. Cancer Res. 79:1138–1150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ji J, Wang Z, Sun W, Li Z, Cai H, Zhao E
and Cui H: Effects of cynaroside on cell proliferation, apoptosis,
migration and invasion though the MET/AKT/mTOR axis in gastric
cancer. Int J Mol Sci. 22:121252021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang P, Yuan X, Yu T, Huang H, Yang C,
Zhang L, Yang S, Luo X and Luo J: Lycorine inhibits cell
proliferation, migration and invasion, and primarily exerts in
vitro cytostatic effects in human colorectal cancer via
activating the ROS/p38 and AKT signaling pathways. Oncol Rep.
45:192021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang L, Liu Y, Wang M, Qian Y, Dong X, Gu
H, Wang H, Guo S and Hisamitsu T: Quercetin-induced apoptosis of
HT-29 colon cancer cells via inhibition of the Akt-CSN6-Myc
signaling axis. Mol Med Rep. 14:4559–4566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Guo C, Zhang X and Pfeifer GP: The tumor
suppressor RASSF1A prevents dephosphorylation of the mammalian
STE20-like kinases MST1 and MST2. J Biol Chem. 286:6253–6261. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kim M, Kim M, Lee MS, Kim CH and Lim DS:
The MST1/2-SAV1 complex of the Hippo pathway promotes ciliogenesis.
Nat Commun. 5:53702014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shome D, von Woedtke T, Riedel K and Masur
K: The HIPPO transducer YAP and its targets CTGF and Cyr61 drive a
paracrine signalling in cold atmospheric plasma-mediated wound
healing. Oxid Med Cell Longev. 2020:49102802020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang Y, Kuramitsu Y, Baron B, Kitagawa T,
Tokuda K, Akada J, Maehara SI, Maehara Y and Nakamura K: PI3K
inhibitor LY294002, as opposed to wortmannin, enhances AKT
phosphorylation in gemcitabine-resistant pancreatic cancer cells.
Int J Oncol. 50:606–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lin J, Chen Y, Wei L, Hong Z, Sferra TJ
and Peng J: Ursolic acid inhibits colorectal cancer angiogenesis
through suppression of multiple signaling pathways. Int J Oncol.
43:1666–1674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang Y, Huang L, Shi H, Chen H, Tao J,
Shen R and Wang T: Ursolic acid enhances the therapeutic effects of
oxaliplatin in colorectal cancer by inhibition of drug resistance.
Cancer Sci. 109:94–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zeng Q, Che Y, Zhang Y, Chen M, Guo Q and
Zhang W: Thymol isolated from thymus vulgaris L. inhibits
colorectal cancer cell growth and metastasis by suppressing the
Wnt/β-catenin pathway. Drug Des Devel Ther. 14:2535–2547. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yao M, Ma X, Zhang X, Shi L, Liu T, Liang
X, Zhao H, Li X, Li L, Gao H, et al: Lectin-mediated pH-sensitive
doxorubicin prodrug for pre-targeted chemotherapy of colorectal
cancer with enhanced efficacy and reduced side effects.
Theranostics. 9:747–760. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
O'Bryan RM, Baker LH, Gottlieb JE, Rivkin
SE, Balcerzak SP, Grumet GN, Salmon SE, Moon TE and Hoogstraten B:
Dose response evaluation of adriamycin in human neoplasia. Cancer.
39:1940–1948. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gabizon A, Shmeeda H and Barenholz Y:
Pharmacokinetics of pegylated liposomal doxorubicin: Review of
animal and human studies. Clin Pharmacokinet. 42:419–436. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Marina NM, Cochrane D, Harney E, Zomorodi
K, Blaney S, Winick N, Bernstein M and Link MP: Dose escalation and
pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in
children with solid tumors: A pediatric oncology group study. Clin
Cancer Res. 8:413–418. 2002.PubMed/NCBI
|
|
87
|
Nair AB and Jacob S: A simple practice
guide for dose conversion between animals and human. J Basic Clin
Pharm. 7:27–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xie YH, Chen YX and Fang JY: Comprehensive
review of targeted therapy for colorectal cancer. Signal Transduct
Target Ther. 5:222020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li J, Ma X, Chakravarti D, Shalapour S and
DePinho RA: Genetic and biological hallmarks of colorectal cancer.
Genes Dev. 35:787–820. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tiwari A, Saraf S, Verma A, Panda PK and
Jain SK: Novel targeting approaches and signaling pathways of
colorectal cancer: An insight. World J Gastroenterol. 24:4428–4435.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen Z, Oh D, Dubey AK, Yao M, Yang B,
Groves JT and Sheetz M: EGFR family and Src family kinase
interactions: Mechanics matters? Curr Opin Cell Biol. 51:97–102.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lopez A, Harada K, Vasilakopoulou M,
Shanbhag N and Ajani JA: Targeting angiogenesis in colorectal
carcinoma. Drugs. 79:63–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ahmad R, Singh JK, Wunnava A, Al-Obeed O,
Abdulla M and Srivastava SK: Emerging trends in colorectal cancer:
Dysregulated signaling pathways (Review). Int J Mol Med. 47:142021.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kassi E, Sourlingas TG, Spiliotaki M,
Papoutsi Z, Pratsinis H, Aligiannis N and Moutsatsou P: Ursolic
acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast
cancer cells. Cancer Invest. 27:723–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yim EK, Lee KH, Namkoong SE, Um SJ and
Park JS: Proteomic analysis of ursolic acid-induced apoptosis in
cervical carcinoma cells. Cancer Lett. 235:209–220. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Argenziano M, Gigliotti CL, Clemente N,
Boggio E, Ferrara B, Trotta F, Pizzimenti S, Barrera G, Boldorini
R, Bessone F, et al: Improvement in the anti-tumor efficacy of
doxorubicin nanosponges in in vitro and in mice bearing breast
tumor models. Cancers (Basel). 12:1622020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Brattain MG, Brattain DE, Fine WD, Khaled
FM, Marks ME, Kimball PM, Arcolano LA and Danbury BH: Initiation
and characterization of cultures of human colonic carcinoma with
different biological characteristics utilizing feeder layers of
confluent fibroblasts. Oncodev Biol Med. 2:355–366. 1981.PubMed/NCBI
|
|
100
|
Brattain MG, Fine WD, Khaled FM, Thompson
J and Brattain DE: Heterogeneity of malignant cells from a human
colonic carcinoma. Cancer Res. 41:1751–1756. 1981.PubMed/NCBI
|
|
101
|
Ahmed D, Eide PW, Eilertsen IA, Danielsen
SA, Eknaes M, Hektoen M, Lind GE and Lothe RA: Epigenetic and
genetic features of 24 colon cancer cell lines. Oncogenesis.
2:e712013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bazan V, Migliavacca M, Zanna I, Tubiolo
C, Grassi N, Latteri MA, La Farina M, Albanese I, Dardanoni G,
Salerno S, et al: Specific codon 13 K-ras mutations are predictive
of clinical outcome in colorectal cancer patients, whereas codon 12
K-ras mutations are associated with mucinous histotype. Ann Oncol.
13:1438–1446. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lupertz R, Watjen W, Kahl R and Chovolou
Y: Dose- and time-dependent effects of doxorubicin on cytotoxicity,
cell cycle and apoptotic cell death in human colon cancer cells.
Toxicology. 271:115–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Nie W, Zan X, Yu T, Ran M, Hong Z, He Y,
Yang T, Ju Y and Gao X: Synergetic therapy of glioma mediated by a
dual delivery system loading α-mangostin and doxorubicin through
cell cycle arrest and apoptotic pathways. Cell Death Dis.
11:9282020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tilija Pun N, Jang WJ and Jeong CH: Role
of autophagy in regulation of cancer cell death/apoptosis during
anti-cancer therapy: Focus on autophagy flux blockade. Arch Pharm
Res. 43:475–488. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lin YJ, Liang WM, Chen CJ, Tsang H, Chiou
JS, Liu X, Cheng CF, Lin TH, Liao CC, Huang SM, et al: Network
analysis and mechanisms of action of Chinese herb-related natural
compounds in lung cancer cells. Phytomedicine. 58:1528932019.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Doğan Şiğva ZÖ, Balci Okcanoğlu T, Biray
Avci Ç, Yilmaz Süslüer S, Kayabaşi Ç, Turna B, Dodurga Y, Nazli O
and Gündüz C: Investigation of the synergistic effects of
paclitaxel and herbal substances and endemic plant extracts on cell
cycle and apoptosis signal pathways in prostate cancer cell lines.
Gene. 687:261–271. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Aiello P, Sharghi M, Mansourkhani SM,
Ardekan AP, Jouybari L, Daraei N, Peiro K, Mohamadian S, Rezaei M,
Heidari M, et al: Medicinal plants in the prevention and treatment
of colon cancer. Oxid Med Cell Longev. 2019:20756142019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Phan T, Nguyen VH, A'Lincourt Salazar M,
Wong P, Diamond DJ, Yim JH and Melstrom LG: Inhibition of autophagy
amplifies baicalein-induced apoptosis in human colorectal cancer.
Mol Ther Oncolytics. 19:1–7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Mandal S, Gamit N, Varier L, Dharmarajan A
and Warrier S: Inhibition of breast cancer stem-like cells by a
triterpenoid, ursolic acid, via activation of Wnt antagonist, sFRP4
and suppression of miRNA-499a-5p. Life Sci. 265:1188542021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zheng JL, Wang SS, Shen KP, Chen L, Peng
X, Chen JF, An HM and Hu B: Ursolic acid induces apoptosis and
anoikis in colorectal carcinoma RKO cells. BMC Complement Med Ther.
21:522021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yang S, Zhang X, Qu H, Qu B, Yin X and
Zhao H: Cabozantinib induces PUMA-dependent apoptosis in colon
cancer cells via AKT/GSK-3β/NF-κB signaling pathway. Cancer Gene
Ther. 27:368–377. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Qin J, Fu M, Wang J, Huang F, Liu H,
Huangfu M, Yu D, Liu H, Li X, Guan X and Chen X: PTEN/AKT/mTOR
signaling mediates anticancer effects of epigallocatechin-3-gallate
in ovarian cancer. Oncol Rep. 43:1885–1896. 2020.PubMed/NCBI
|
|
114
|
Zhu ML, Zhang PM, Jiang M, Yu SW and Wang
L: Myricetin induces apoptosis and autophagy by inhibiting
PI3K/Akt/mTOR signalling in human colon cancer cells. BMC
Complement Med Ther. 20:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Li W, Li C, Ma L and Jin F: Resveratrol
inhibits viability and induces apoptosis in the small-cell lung
cancer H446 cell line via the PI3K/Akt/c-Myc pathway. Oncol Rep.
44:1821–1830. 2020.PubMed/NCBI
|
|
116
|
Tian J, Zhang H, Mu L, Wang M, Li X, Zhang
X, Xie E, Ma M, Wu D and Du Y: The miR-218/GAB2 axis regulates
proliferation, invasion and EMT via the PI3K/AKT/GSK-3β pathway in
prostate cancer. Exp Cell Res. 394:1121282020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Qi X, Sun L, Wan J, Xu R, He S and Zhu X:
Tensin4 promotes invasion and migration of gastric cancer cells via
regulating AKT/GSK-3β/snail signaling pathway. Pathol Res Pract.
216:1530012020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chang YX, Lin YF, Chen CL, Huang MS, Hsiao
M and Liang PH: Chaperonin-containing TCP-1 promotes cancer
chemoresistance and metastasis through the AKT-GSK3β-β-catenin and
XIAP-survivin pathways. Cancers (Basel). 12:38652020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ding L, Cao J, Lin W, Chen H, Xiong X, Ao
H, Yu M, Lin J and Cui Q: The roles of cyclin-dependent kinases in
cell-cycle progression and therapeutic strategies in human breast
cancer. Int J Mol Sci. 21:19602020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Xu S, Zhang H, Liu T, Yang W, Lv W, He D,
Guo P and Li L: 6-Gingerol induces cell-cycle G1-phase arrest
through AKT-GSK 3β-cyclin D1 pathway in renal-cell carcinoma.
Cancer Chemother Pharmacol. 85:379–390. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhou C, Du J, Zhao L, Liu W, Zhao T, Liang
H, Fang P, Zhang K and Zeng H: GLI1 reduces drug sensitivity by
regulating cell cycle through PI3K/AKT/GSK3/CDK pathway in acute
myeloid leukemia. Cell Death Dis. 12:2312021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu
YL, Liu LF and Yeh ET: Identification of the molecular basis of
doxorubicin-induced cardiotoxicity. Nat Med. 18:1639–1642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Desai VG, Lee T, Moland CL, Vijay V, Han
T, Lewis SM, Herman EH and Fuscoe JC: Candidate early predictive
plasma protein markers of doxorubicin-induced chronic
cardiotoxicity in B6C3F1 mice. Toxicol Appl Pharmacol.
363:164–173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kuenzi BM and Ideker T: Author correction:
A census of pathway maps in cancer systems biology. Nat Rev Cancer.
21:2122021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kuenzi BM and Ideker T: A census of
pathway maps in cancer systems biology. Nat Rev Cancer. 20:233–246.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chang YC, Wu JW, Wang CW and Jang AC:
Hippo signaling-mediated mechanotransduction in cell movement and
cancer metastasis. Front Mol Biosci. 6:1572020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Kennedy MB: Origin of PDZ (DHR, GLGF)
domains. Trends Biochem Sci. 20:3501995. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zheng Y and Pan D: The Hippo signaling
pathway in development and disease. Dev Cell. 50:264–282. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Furth N and Aylon Y: The LATS1 and LATS2
tumor suppressors: Beyond the Hippo pathway. Cell Death Differ.
24:1488–1501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kim SH, Jin H, Meng RY, Kim DY, Liu YC,
Chai OH, Park BH and Kim SM: Activating Hippo pathway via Rassf1 by
ursolic acid suppresses the tumorigenesis of gastric cancer. Int J
Mol Sci. 20:47092019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Jeong SH, Kim HB, Kim MC, Lee JM, Lee JH,
Kim JH, Kim JW, Park WY, Kim SY, Kim JB, et al: Hippo-mediated
suppression of IRS2/AKT signaling prevents hepatic steatosis and
liver cancer. J Clin Invest. 128:1010–1025. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhang S, Chen Q, Liu Q, Li Y, Sun X, Hong
L, Ji S, Liu C, Geng J, Zhang W, et al: Hippo signaling suppresses
cell ploidy and tumorigenesis through Skp2. Cancer Cell.
31:669–684.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ahmed AA, Abedalthagafi M, Anwar AE and
Bui MM: Akt and Hippo pathways in Ewing's sarcoma tumors and their
prognostic significance. J Cancer. 6:1005–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Berthold R, Isfort I, Erkut C, Heinst L,
Grunewald I, Wardelmann E, Kindler T, Åman P, Grünewald TGP,
Cidre-Aranaz F, et al: Fusion protein-driven IGF-IR/PI3K/AKT
signals deregulate Hippo pathway promoting oncogenic cooperation of
YAP1 and FUS-DDIT3 in myxoid liposarcoma. Oncogenesis. 11:202022.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ma W, Han C, Zhang J, Song K, Chen W, Kwon
H and Wu T: The histone methyltransferase G9a promotes
cholangiocarcinogenesis through regulation of the Hippo pathway
kinase LATS2 and YAP signaling pathway. Hepatology. 72:1283–1297.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Xu W, Yang Z, Xie C, Zhu Y, Shu X, Zhang
Z, Li N, Chai N, Zhang S, Wu K, et al: PTEN lipid phosphatase
inactivation links the hippo and PI3K/Akt pathways to induce
gastric tumorigenesis. J Exp Clin Cancer Res. 37:1982018.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Jang SW, Yang SJ, Srinivasan S and Ye K:
Akt phosphorylates MstI and prevents its proteolytic activation,
blocking FOXO3 phosphorylation and nuclear translocation. J Biol
Chem. 282:30836–30844. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Romano D, Matallanas D, Weitsman G,
Preisinger C, Ng T and Kolch W: Proapoptotic kinase MST2
coordinates signaling crosstalk between RASSF1A, Raf-1, and Akt.
Cancer Res. 70:1195–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Kim D, Shu S, Coppola MD, Kaneko S, Yuan
ZQ and Cheng JQ: Regulation of proapoptotic mammalian ste20-like
kinase MST2 by the IGF1-Akt pathway. PLoS One. 5:e96162010.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kim SM, Ye S, Rah SY, Park BH, Wang H, Kim
JR, Kim SH, Jang KY and Lee KB: RhBMP-2 activates Hippo signaling
through RASSF1 in esophageal cancer cells. Sci Rep. 6:268212016.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Pankova D, Jiang Y, Chatzifrangkeskou M,
Vendrell I, Buzzelli J, Ryan A, Brown C and O'Neill E: RASSF1A
controls tissue stiffness and cancer stem-like cells in lung
adenocarcinoma. EMBO J. 38:e1005322019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Gupta V, Agarwal P and Deshpande P: Impact
of RASSF1A gene methylation on clinico-pathological features of
tumor and non-tumor tissue of breast cancer. Ann Diagn Pathol.
52:1517222021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Lee NH, Kim SJ and Hyun J: MicroRNAs
regulating Hippo-YAP signaling in liver cancer. Biomedicines.
9:3472021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Agarwal S, Amin KS, Jagadeesh S, Baishay
G, Rao PG, Barua NC, Bhattacharya S and Banerjee PP: Mahanine
restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in
prostate cancer cells. Mol Cancer. 12:992013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Blanchard TG, Lapidus R, Banerjee V,
Bafford AC, Czinn SJ, Ahmed H and Banerjee A: Upregulation of
RASSF1A in colon cancer by suppression of angiogenesis signaling
and Akt activation. Cell Physiol Biochem. 48:1259–1273. 2018.
View Article : Google Scholar : PubMed/NCBI
|