Introduction
Spinal cord injury (SCI) is known as a fatal
neurological disorder characterized by motor, sensory, or autonomic
function deficits and has led to a heavy economic burden on
patients and society (1,2). As reported in 2016, there was a
total of 282,000 individuals suffering from SCI in the United
States and the rate of annual occurrence was found to be 54 cases
per million individuals (~17,000 new cases per year) (3). Although great progress has been made
in the development of strategies for neuroprotection and
neuroregeneration with the development of drugs (1), surgery (4), stem cell transplantation (5) and tissue engineering technology
(6), these strategies have not
achieved satisfactory therapeutic efficacy clinically (7). This suggests that the understanding
of the mechanisms of SCI progression remain to be elucidated.
The main pathogenesis of SCI is the inflammation
dominated by activated microglia and astrocytes in the injured site
after the primary mechanical injury (8). It has been reported that the levels
of inflammatory factors, including TNF-α, IL-1β and IL-6 are
elevated following SCI (9).
Furthermore, the excessive production of pro-inflammatory cytokines
can promote the generation of reactive oxygen species (ROS),
leading to oxidative damage (10). On the other hand, the loss of
blood supply to the injured site directly leads to an increase in
ROS generation (11). This
microenvironment composed of various inflammatory mediators leads
directly to the apoptosis of functional cells, such as neurons
(12). Therefore, a comprehensive
understanding of the inflammatory mechanisms following SCI is
necessary in order to providing a more theoretical basis for the
treatment of SCI in the future.
S100A1, belonging to the S100 protein family, has
been reported to regulate the release, uptake and transport of
calcium by regulating calcium ATPase, leading to the activity of
cellular signals (13). An
imbalance in S100A1 has been revealed to induce the further
impairment of myocardial viability and function on hypoxia-induced
cell dysfunction in cardiovascular disease (13–15). Furthermore, S100A1 can bind to
Toll-like receptor 4 (TRL4), which in turn activates the nuclear
factor κB (NF-κB) and mitogen-activated protein kinases (MAPK)
pathways, well-known drivers of inflammation, in cardiovascular and
respiratory diseases (15,16),
suggesting that S100A1 is a crucial inflammatory mediator,
particularly under the conditions of ischemia and hypoxia. At the
same time, S100A1 plays a critical role in the nervous system. A
previous study demonstrated that S100A1 aggravated
neuroinflammation and disease histopathology in a mouse model of
Alzheimer's disease (17). In
addition, the ablation of S100A1 in PC12 cells has been found to
maintain cell viability and increase resistance to amyloid-β
peptide-induced cell apoptosis (18). Extracellular signal-regulated
kinase (ERK), downstream of NF-κB, has been reported to be a
critical target of the apoptosis of nerve cells. Blocking the
activity of ERK can attenuate neuroinflammation and neuroapoptosis
(19). However, whether S100A1
can affect the function of neuronal cells through ERK activity
remains to be fully determined.
In the present study, S100A1 expression was detected
in a rat SCI model in vivo and a PC12 cell model in
vitro of lipopolysaccharide (LPS)-induced inflammation. Next,
it was determined whether ERK signaling activity was a downstream
target of S100A1 by observing whether ERK signaling activity
changed with S100A1 expression. Finally, whether S100A1 could
affect the inflammation, oxidative stress and apoptosis of PC12
induced by LPS through ERK signaling pathway was explored. The
present study revealed the expression and mechanism of S100A1 in
SCI, providing a new reference for the study of SCI.
Materials and methods
Establishment of rat model of SCI
A total of 10 male Sprague-Dawley rats (age, 11–12
weeks; weight, 200–230 g) were obtained from the Experimental
Animal Center of Harbin Medical University and were divided into
the sham-operated (Sham; n=5) and SCI group (n=5). All rats were
raised in the isolated ventilation cage at 22–26°C, 50% relative
humidity with a 12/12-h light/dark cycle and had free access to
sterile water and granular food. The rats in the sham group were
only subjected to dorsal laminectomy of the T10 vertebral body,
while the rats in the SCI group were subjected to spinal cord
contusion surgery at the level of T10 as previously described
(20). All rats were observed for
health and behavior status once a day for seven consecutive days
after surgery and were administered with penicillin (1 ml; 160,000
U/ml) and meloxicam (4 mg/kg) in the first three days to prevent
infection and relieve pain. Uncontrollable inflammatory response at
the wound site was set as the humane endpoints in the study
(21). The study was reviewed and
approved by the Ethics Committee of The First Affiliated Hospital
of Harbin Medical University (Harbin, China; approval no. 2021080)
and all experiments were carried out in accordance with the
guidelines of Animal Care and Use Committee of the Harbin Medical
University.
Euthanasia of experimental animals and
sample handling
The animal experiment lasted for 7 days. On the 7th
day after the surgery, all 10 rats had survived without
infection/death and were anesthetized by an intraperitoneal
injection of 1% sodium pentobarbital (40 mg/kg, MilliporeSigma).
Death was confirmed by observing lack of heartbeat, breathing and
pupils and nerve reflexes of the rats for 5 min (22). Then, 1 ml blood was collected
through the orbital venous plexus for ELISA. Subsequently, the rats
were euthanized via excessive anesthesia (3% sodium pentobarbital;
160 mg/kg), as previously described (23). After the whole body was perfused
with cool saline, the site of injury was removed together with 5 mm
of upper and lower spinal cord tissue. The samples used for western
blot analysis and reverse transcription-quantitative PCR (RT-qPCR)
were stored in liquid nitrogen, while the samples used for
histochemistry were stored in 4% paraformaldehyde.
PC12 cell culture and treatment
LPS can induce inflammation in PC12 cells via TLR4
activity and it is widely used for SCI model in vitro
(24–26). In the present study, the PC12
cells induced by LPS was used for the SCI model in vitro.
PC12 cells, harvested from the pheochromocytoma of the rat adrenal
medulla, no adrenaline secretion and comprised of eosinophilic and
neuroblastic cells (27), were
obtained from the Cell Resource Center of the Chinese Academy of
Sciences (Shanghai Institute of Biological Sciences) and cultured
in RPMI-1640 medium (HyClone; Cytiva) containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) in 5% CO2
and a 37°C humidified atmosphere. The culture medium was changed
every 2–3 days and the cells were sub-cultured when the cell
density reached 90%. LPS, extracted from Escherichia coli
055:B5, at 1, 5 and 10 µg/ml (cat. no. L8880; Beijing Solarbio
Science & Technology Co., Ltd.), configured one night in
advance and stored overnight, was used to establish a PC12 cell
model of inflammation for subsequent research.
RT-qPCR
The mRNA expression levels of S100A1, IL-1β, IL-6,
IL-10 and TNF-α were evaluated using RT-qPCR. Total RNA from the
spinal cord tissue and PC12 cells was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. A total of 1 µg
RNA was used for cDNA reverse transcription with the reverse
transcription kit (Takara Bio, Inc.) following the manufacturer's
instructions. Lastly, the TB Green® Premix Ex Taq II kit
(Takara Bio, Inc.) was used for qPCR with the following
thermocycling conditions: Pre-heating at 95°C for 10 min, followed
by 45 cycles at 95°C for 15 sec, at 60°C for 30 sec and at 72°C for
30 sec as previously described (22). The primer sequences used in the
present study are listed in Table
I. GAPDH was used as the internal reference. The relative
expression levels of target genes were calculated using the
2−ΔΔCq formula (28).
 | Table I.Primers sequences used in reverse
transcription-quantitative PCR. |
Table I.
Primers sequences used in reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| S100A1 |
ggtcggcagtaaagacaggt |
atttcagcagcacacggttg |
| IL-1β |
gcacagttccccaactggta |
ggagactgcccattctcgac |
| IL-6 |
acaagtccggagaggagact |
acagtgcatcatcgctgttc |
| TNF-α |
atgggctccctctcatcagt |
gcttggtggtttgctacgac |
| IL-10 |
ttccctgggagagaagctga |
gacacctttgtcttggagctta |
| CAT |
gctccgcaatcctacaccat |
ggacatcgggtttctgaggg |
| MnSOD |
caccgaggagaagtaccacg |
tgggttctccaccaccctta |
| GAPDH |
gcatcttcttgtgcagtgcc |
ggtaaccaggcgtccgatac |
Western blot analysis
Briefly, total protein from the spinal cord tissue
and PC12 cells was isolated using RIPA buffer (Beyotime Institute
of Biotechnology) containing 1 mM PMSF (Dalian Meilun Biology
Technology Co., Ltd.) on the ice for 30 min. After the protein
concentration was detected using a BCA kit (Beyotime Institute of
Biotechnology), 60–80 µg protein was loaded on 12.5 or 15% SDS-PAGE
for separation, followed by transfer onto a PVDF membrane (Merck
KGaA). The membrane was first blocked with 5% skimmed milk in 0.05%
PBS-Tween 20 for 1 h at room temperature. The membranes were then
incubated with primary antibodies for S100A1 (1:500; cat. no.
16027-1-AP; ProteinTech Group, Inc.), nuclear factor erythroid
2-related factor 2 (Nrf2; 1:1,000; cat. no. 80593-1-RR; ProteinTech
Group, Inc.), ERK1/2 (1:1,000; cat. no. 4695), p-ERK1/2 (1:2,000;
cat. no. 4370), cleaved caspase-3 (1:1,000; cat. no. 9664), Bax
(1:1,000; cat. no. 2772), Bcl2 (1:1,000; cat. no. 3498) (all from
Cell Signaling Technology, Inc.) and β-actin (1:1,000; cat. no.
20536-1-AP; ProteinTech Group, Inc.) overnight at 4°C followed by
incubation with HRP-conjugated secondary antibody (1:10,000; cat.
no. 7074, Cell Signaling Technology, Inc.) at room temperature for
1 h. Lastly, the bands were visualized using ECL reagent (Beyotime
Institute of Biotechnology) as per the manufacturer's protocol with
the ChemiDoc MP System (Bio-Rad Laboratories, Inc.). The
semi-quantitative analysis was performed with Image-Pro Plus
(version 6.0; Media Cybernetics, Inc.).
Cell transfection
S100A1 overexpression (ov-S100A1) plasmid (plasmid
primer: Forward: 5′-GAAGATTCTAGAGCTAGCGAATTCATGGGCTCTGAGCTGGAG-3′;
Reverse: 5′-CGCAGATCCTTGCGGCCGCGGATCCTCAACTGTTCTCCCAGAA-3′)
encoding the full-length open reading frame of rat S100A1 and the
S100A1 short-interfering (si) RNA plasmid targeting sequence
(5′-UGGAGACCCUCAUCAAUGUdTdT-3′) for silencing S1001A (si-S100A1),
as well as their corresponding blank plasmid vectors were prepared
by Shanghai Genechem Co., Ltd. The overexpression and silencing of
S100A1 in PC12 cells were carried out using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. At
48 h post-transfection, the PC12 cells with gene intervention were
exposed to LPS and were used in other experiments. In addition, the
cells transfected with ov-S100A1 plasmid were treated with the ERK
inhibitor, MK-8353 (100 nM, MedChemExpress), 12 h prior to exposure
to LPS, as previously described (29). The cell groups were designed as
follows: The control group (without any treatment), LPS group
(cells stimulated with 5 µg/ml LPS), LPS + si-S100A1, LPS +
ov-S100A1 and ov-S100A1 with MK-8353 treatment (LPS + ov-S100A1 +
MK) groups.
Hematoxylin and eosin (HE)
staining
The HE staining was performed to compare the
histomorphological differences between the SCI and Sham groups.
Briefly, the spinal cord tissues were fixed with 4%
paraformaldehyde at 4°C for 24 h and prepared as paraffin-embedded
tissues after dehydration using xylene and gradient alcohol
solution. Next, 5-µm paraffin sections were prepared, heated in the
60°C oven for 30 min and washed with xylene I and II, for 5 min
each time at room temperature. After being treating with 100, 100,
95, 95 and 80% gradient alcohol at room temperature for 5 min each
time, the sections were stained using the HE stain kit (Beijing
Solarbio Science & Technology Co., Ltd.) according to the
manufacturer's instructions. Firstly, the sections were treated
with hematoxylin for 1 min at room temperature, followed by being
differentiated in 1% hydrochloric acid for 30 sec at room
temperature. Lastly, the sections were stained with eosin for 2 min
at room temperature. Finally, the images were harvested by light
microscope (Leica Microsystems GmbH).
Tissue immunofluorescence
Immunofluorescence assay was performed to explore
the changes in S100A1 expression in SCI in vivo. The spinal
cord tissues were fixed with 4% paraformaldehyde at 4°C for 24 h,
then dehydrated by sucrose solution gradient and embedded in
optimal cutting temperature compound to yield 5-µm-thick frozen
sections. Following incubation with 0.3% H2O2
at room temperature for 30 min, 0.5% Triton X-100 was used for
permeabilizing the sections. Normal goat serum (10%; Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.) was then used to
incubate the sections at room temperature for 30 min. After the
primary antibody of S100A1 (1:50; cat. no. 16027-1-AP; ProteinTech
Group, Inc.) was used to incubate the sections overnight at 4°C,
the fluorescent secondary antibody (1:500; cat. no. ab150113,
Abcam) was used to incubate the sections for 1 h at room
temperature. Lastly, the cell nuclei were stained with DAPI
(Beijing Solarbio Science & Technology Co., Ltd.) for 5 min at
room temperature and the sections were visualized using a
fluorescence microscope (Olympus Corporation). Image-Pro Plus
(version 6.0; Media Cybernetics, Inc.) was used for statistical
analysis.
Cell immunofluorescence
In order to detect the changes in the expression of
S100A1 in PC12 cells stimulated with various concentrations of LPS,
a density of 2×105 cells/well was seeded in six-well
plates and cultured overnight followed by stimulation with 1, 5 and
10 µg/ml LPS for 12 h at 37°C. The cells were then fixed with 4%
paraformaldehyde for 30 min at room temperature and permeabilized
with 0.5% Triton X-100 for 30 min at room temperature.
Subsequently, the cells were blocked with 5% BSA for 30 min
(Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature and incubated with S100A1 primary antibody (1:100; cat.
no. 16027-1-AP; ProteinTech Group, Inc.) overnight at 4°C. The
fluorescent secondary antibody (1:500; cat. no. ab150113, Abcam)
and DAPI were used to treat the cells for 1 h and 5 min at room
temperature, respectively. Images were obtained using a
fluorescence microscope (Olympus Corporation). Image-Pro Plus
(version 6.0; Media Cybernetics, Inc.) was used for analysis.
ELISA
ELISA was performed to examine the effects of S100A1
on inflammation levels in the PC12 cell culture supernatant. The
cell supernatant among different groups was collected and the
expression levels of IL-1β (cat. no. KE20005; ProteinTech Group,
Inc.), IL-6 (cat. no. KET9004-96T; EliKine; Abbkine Scientific Co.,
Ltd.), TNF-α (cat. no. KE20001; ProteinTech Group, Inc.) and IL-10
(cat. no. KE20003; ProteinTech Group, Inc.) were detected using the
respective kits according to the manufacturer's instructions.
ROS level evaluation
The levels ROS among different groups were evaluated
using the 2′-7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Briefly, the cells from the different
groups were collected following trypsin digestion and incubated
with serum-free medium (Gibco; Thermo Fisher Scientific, Inc.)
containing DCFH-DA (10 µM) probes for 30 min in the dark at room
temperature. The cells were then resuspended by PBS following
centrifugation (201 × g) at room temperature for 5 min. The
DCFH-DA-positive cells were detected using a flow cytometer (BD
FACSCalibur; BD Biosciences).
Activity detection of catalase (CAT)
and manganese superoxide dismutase (MnSOD)
The activities of CAT and MnSOD among different
groups were evaluated using CAT and MnSOD Assay kits (Nanjing
Jiancheng Bioengineering Institute) based on the cell lysate.
Briefly, the cells from the different groups were harvested
followed by resuspension with 0.3 ml PBS. Next, after the
resuspended cells being broken by ultrasound (frequency, once every
2 sec) on ice for 1 min (Scientz-IID), the protein was harvested
with centrifugation (13,887 × g for 25 min at 4°C). The total
protein concentration was detected using a BCA kit (Beyotime
Institute of Biotechnology). The contents of CAT and MnSOD in the
samples were examined according to the corresponding manufacturer's
instructions.
Apoptosis assay
The cellular apoptosis levels of the control,
si-S100A1, ov-S100A1 and ov-S100A1 + MK groups were evaluated using
an apoptosis kit (cat. no. CA1020 Beijing Solarbio Science &
Technology Co., Ltd.) according to the manufacturer's instructions.
First, the cells from the different groups were collected following
trypsin digestion and incubated with solution containing propidium
iodide and Annexin V for 10 min in the dark at room temperature.
The sample was then resuspended in PBS following centrifugation
(201 × g) at room temperature for 5 min and detected using a flow
cytometer (BD FACSCalibur; BD Biosciences).
Statistical analysis
All experiments in the present study were conducted
at least three times. The data were analyzed using GraphPad Prism
6.0 software (GraphPad Software, Inc.) and are presented as the
mean ± standard deviation. An unpaired t-test was used for
comparisons between two groups. For comparisons between multiple
groups, when n<6, Kruskal Wallis test of multiple independent
samples were performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
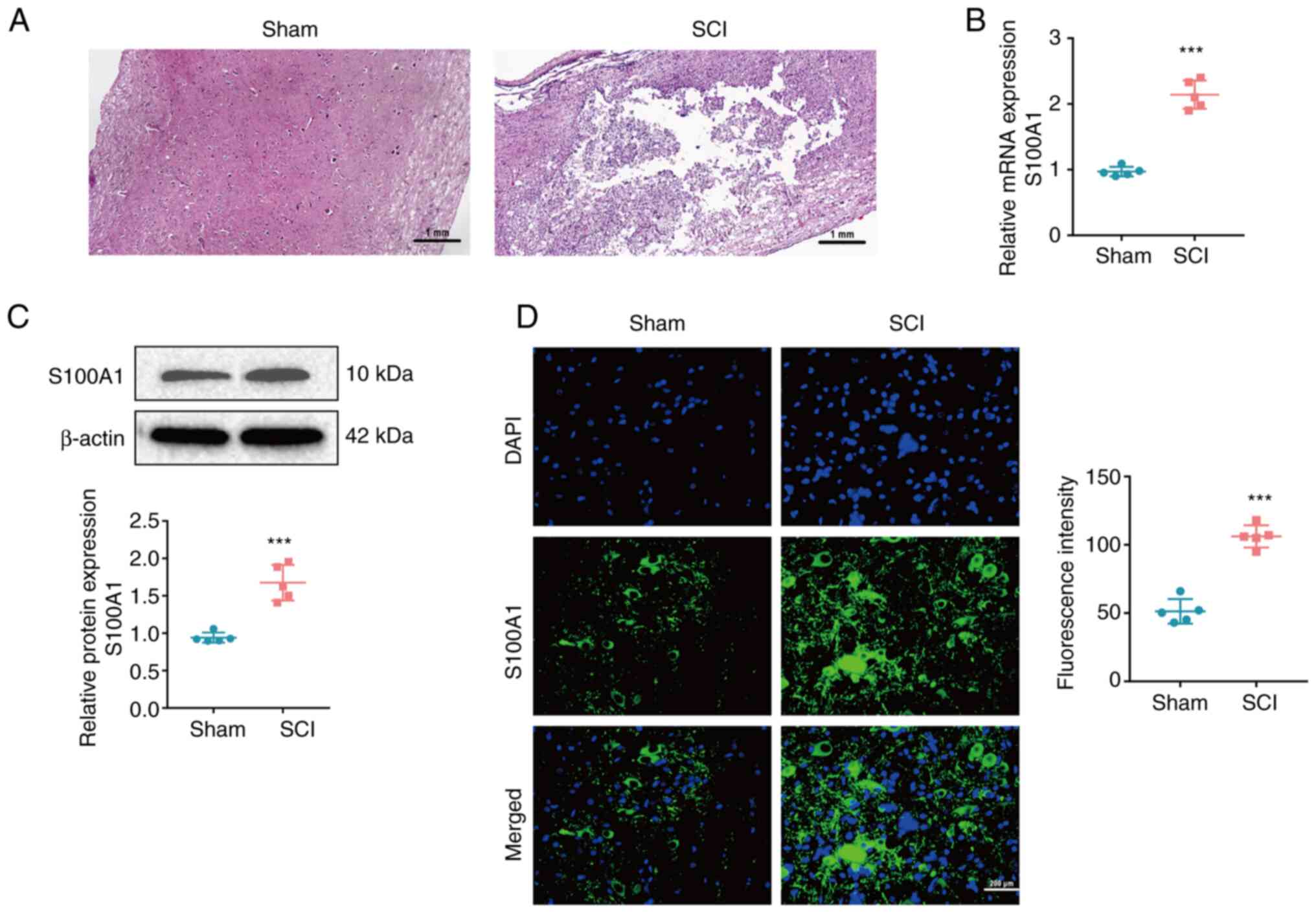
S100A1 expression is elevated in
tissue from rats with SCI
With the aim of exploring the expression of S100A1
in SCI, a rat model of SCI was first established. Subsequently, the
morphological examination revealed that there were evident tissue
defects, hemorrhaging, necrosis and inflammatory cell infiltration,
as well as nerve cell atrophy and vacuolization in the SCI group
compared with the Sham group (Fig.
1A); this suggested that the model of SCI was successfully
established. The mRNA and protein expression levels of S100A1 were
found to be significantly upregulated in the SCI group compared
with the Sham group (Fig. 1B and
C). Furthermore, immunofluorescence staining confirmed that
S100A1 was highly expressed in the tissue of rats with SCI,
compared with that of the rats in the Sham group (Fig. 1D). Finally, the aforementioned
results confirmed that S100A1 was significantly upregulated in the
tissue of rats with SCI.
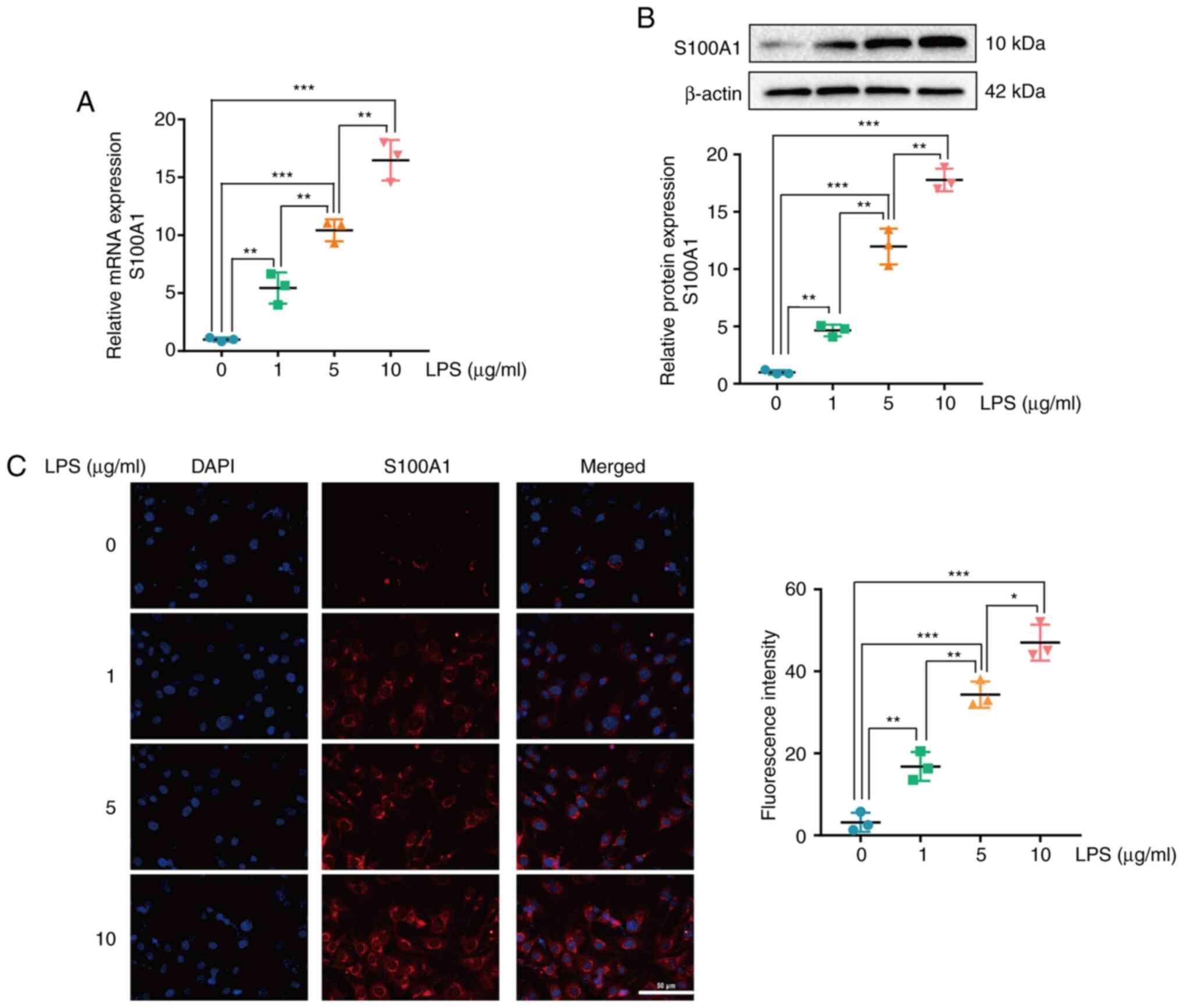
S100A1 expression is upregulated in
the PC12 cell model of LPS-induced inflammation
PC12 cells are widely used in neural
dysfunction-related research, including SCI (30). In the present study, S100A1
expression was detected in the PC12 cell model of LPS-induced
inflammation. After the PC12 cells were stimulated with various
concentrations of LPS (0, 1, 5 and 10 µg/ml) for 12 h, the mRNA and
protein expression levels of S100A1 exhibited a significant
increase in a concentration-dependent manner (Fig. 2A and B). The results of
immunofluorescence staining also revealed that the expression of
S100A1 was elevated with the increasing LPS concentration (Fig. 2C). On the whole, these results
suggested that S100A1 expression was upregulated in the PC12 cell
model of LPS-induced inflammation. Subsequently, 5 µg/ml LPS was
used for PC12 cell model establishment to explore the biological
function of S00A1 in LPS induced SCI model in vitro as the
reported studies (30–32).
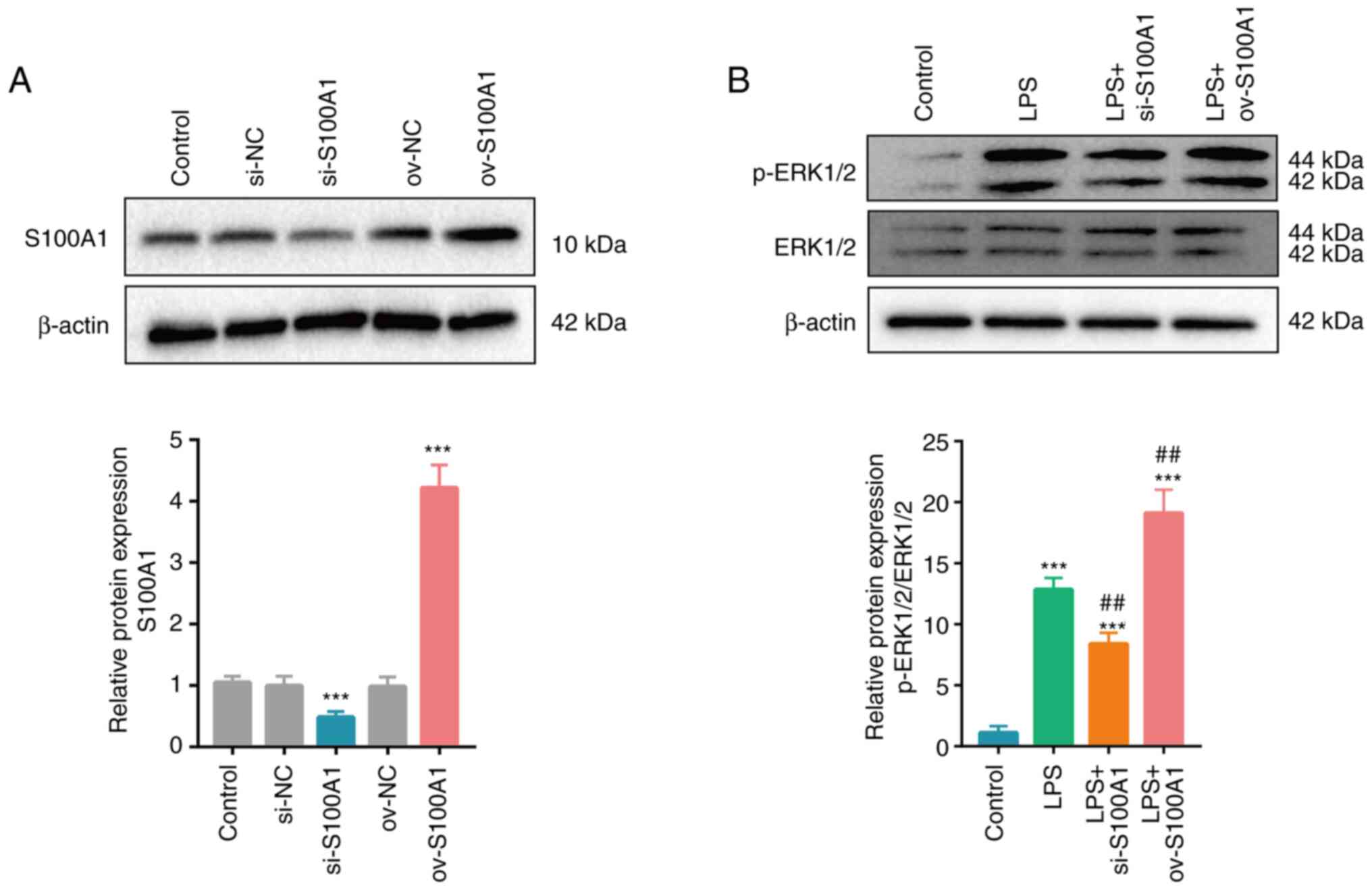
S100A1 expression mediates the
activity of the ERK signaling pathway in PC12 cells stimulated with
LPS
S100A1 expression was upregulated in the rats with
SCI and in cells with stimulated with LPS in vitro. However,
the potential function of S100A1 in PC12 cells stimulated with LPS
remained unclear. Plasmids containing siRNA targeting S100A1 and
the full length of S100A1 were used to silence and overexpress
S100A1, respectively and the results were verified using western
blot analysis. S100A1 expression was significantly decreased in the
si-S100A1 group and significantly increased in the ov-S100A1 group,
compared with the control group (Fig.
3A). The ERK signaling pathway has been verified to be
associated with the inflammation process and blocking its activity
can effectively attenuate inflammatory injury induced by
oxygen-glucose deprivation and the reperfusion of PC12 cells
(33–35). Similarly, in the present study,
the silencing or overexpression of S100A1 was accompanied by the
partially decreased or increased expression of p-ERK1/2,
respectively, indicating that changes in S100A1 expression could
mediate the activity of the ERK signaling pathway in PC12 cells
(Fig. 3B).
S100A1 regulates the inflammation of
PC12 cells by mediating the ERK signaling pathway
As SCI is accompanied by the inflammatory cascade,
effective strategies for suppressing inflammatory injury may
contribute to functional recovery following SCI (8). Whether S100A1 could regulate the
inflammation level of PC12 cells in vitro was explored.
First, the mRNA and protein secretion levels of pro-inflammatory
cytokines (IL-1β, IL-6 and TNF-α), as well as anti-inflammatory
cytokines (IL-10) were detected. As was expected, following
stimulation with LPS, the expression levels of IL-1β, IL-6 and
TNF-α in the LPS group were significantly higher in comparison with
the control group, while the expression of IL-10 exhibited the
opposite trend (Fig. 4A and B).
However, the silencing of S100A1 partially attenuated the levels of
inflammatory mediators in the cells and improved their
anti-inflammatory ability (Fig. 4A
and B). The overexpression of S100A1 aggravated the levels of
inflammation and these effects were partially abolished by the
ERK1/2 molecular inhibitor, MK-8353 (Fig. 4A and B). These results suggested
that S100A1 could mediate the levels of inflammation of PC12 cells
by targeting the ERK signaling pathway.
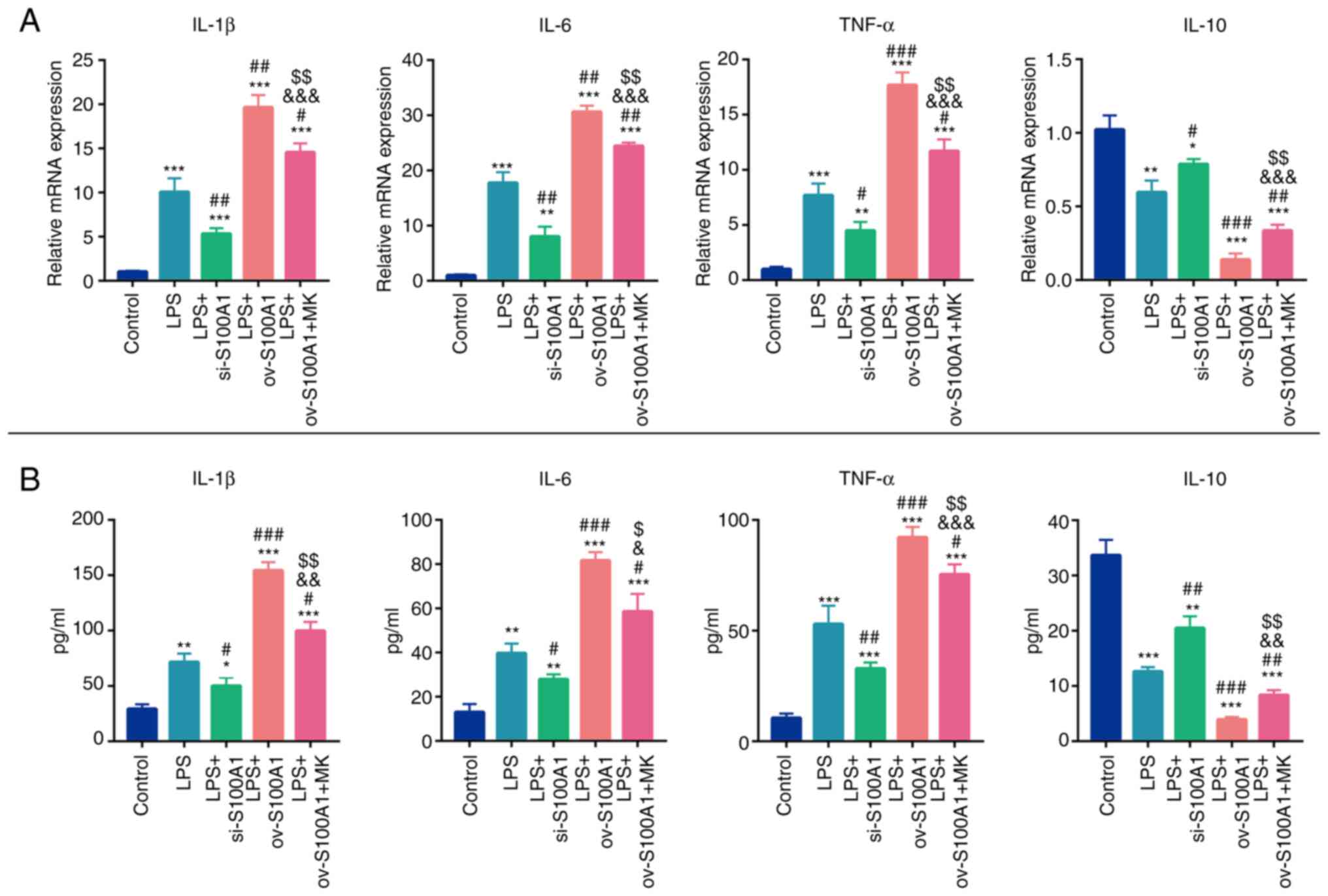 | Figure 4.S100A1 regulates the level of
inflammation in PC12 cells through the ERK signaling pathway. (A)
The mRNA expression levels of inflammatory cytokines (IL-1β, IL-6
and TNF-α) and anti-inflammatory cytokines (IL-10) were detected
using reverse transcription-quantitative PCR in the Control, LPS,
LPS with S100A1 silencing/overexpression, LPS with S100A1
overexpression and EKR inhibitor groups. (B) The protein levels of
inflammatory cytokines (IL-1β, IL-6 and TNF-α) and
anti-inflammatory cytokines (IL-10) were detected using ELISA in
the Control, LPS, LPS with S100A1 silencing/overexpression, LPS
with S100A1 overexpression and EKR inhibitor groups. n=3.
*P<0.05, **P<0.01, ***P<0.001 vs. Control group.
#P<0.05, ##P<0.01,
###P<0.001 vs. LPS group. &P<0.05,
&&P<0.01 vs. LPS + si-S100A1 group.
$P<0.05, $$P<0.01 vs. LPS + ov-S100A1
group. LPS, lipopolysaccharide; ov, overexpression; si, short
interfering. |
S100A1 regulates the oxidative-stress
damage of PC12 cells by mediating the ERK signaling pathway
Oxidative stress-induced damage is highly associated
with inflammation and tissue ischemia and necrosis caused by SCI
are the common causes of oxidative stress-induced injury (36). In the present study, as expected,
the levels of ROS were found to be significantly elevated in the
LPS group (Fig. 5A and B). At the
same time, the silencing of S100A1 partially decreased the level of
ROS and the overexpression of S100A1 promoted the generation of
ROS. Similarly, the ERK1/2 inhibitor, MK-8353, partially prevented
the promoting effects of the overexpression of S100A1 on ROS
generation (Fig. 5A and B).
Furthermore, the effects of S100A1 on the antioxidant capacity of
cells were evaluated. The enzymatic activities, CAT and MnSOD,
decreased significantly in the LPS group. The silencing expression
of S100A1 promoted the gene expression and enzyme activity of CAT
and MnSOD, while the overexpression of S100A1 further decreased the
levels of CAT and MnSOD. In addition, MK-8353 partially
counteracted the inhibitory effects of S100A1 overexpression on CAT
and MnSOD levels (Fig. 5C and D).
Nrf2, is a transcription factor of anti-oxidative stress element
and its increased expression can transfer it to the nucleus and
participate in the transcription of CAT and SOD, leading to the
enhancement of cell antioxidant capacity (37). Herein, the protein expression of
Nrf2 was detected among the groups and found that Nrf2 expression
decreased significantly in the LPS group. Silencing and
overexpression of S100A1 accompanied by up- and down-regulated Nrf2
expression. MK-8353 partially counteracted the inhibitory effects
of S100A1 overexpression on Nrf2 level (Fig. 5E) Thus, these results suggested
that S100A1 regulated the oxidative stress levels in PC12 cells by
targeting the ERK signaling pathway.
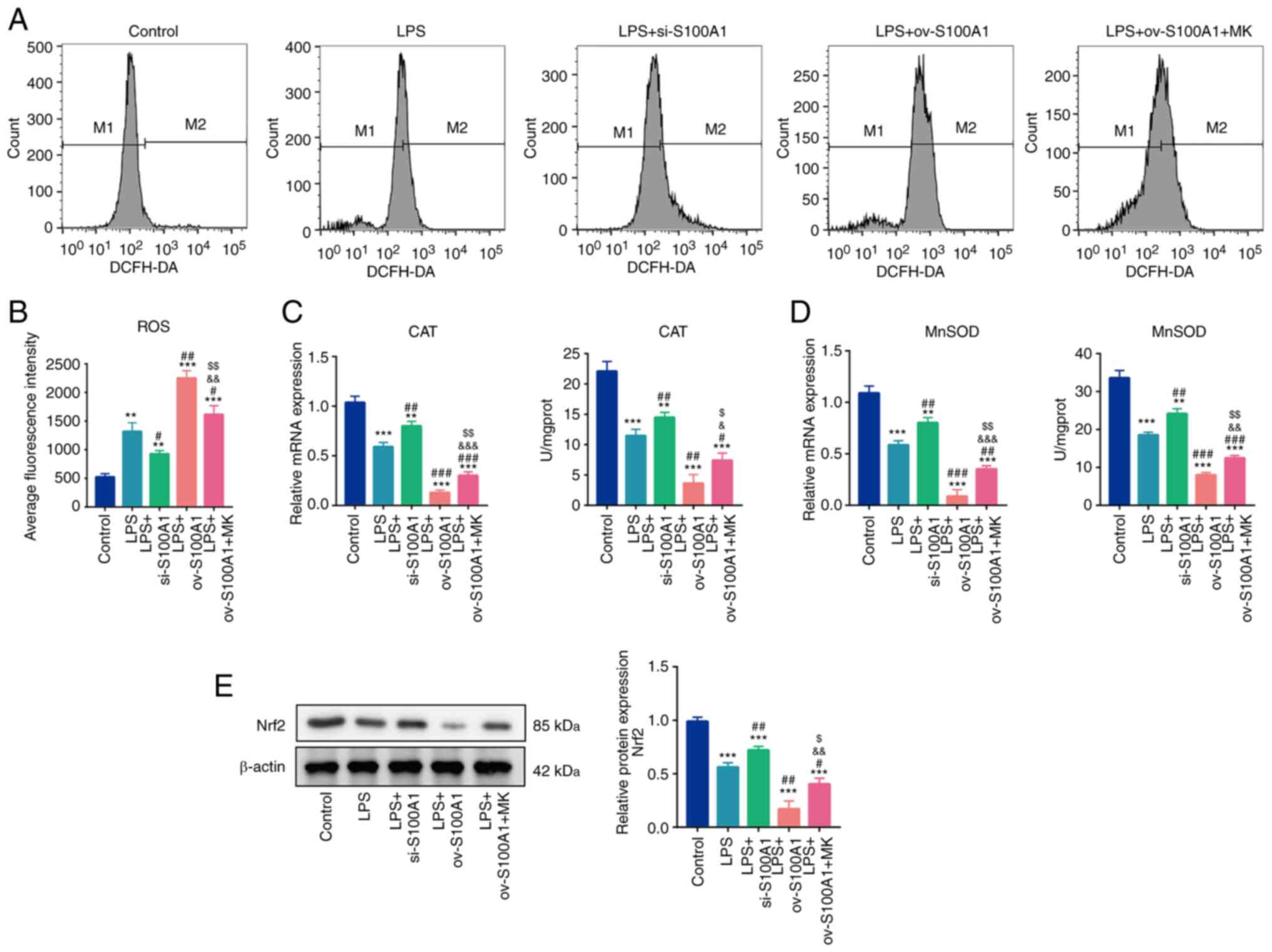 | Figure 5.S100A1 regulates the oxidative stress
levels of PC12 cells through the ERK signaling pathway. (A and B)
ROS levels in the Control, LPS, LPS + S100A1
silencing/overexpression, LPS with S100A1 overexpression and EKR
inhibitor groups. (C) mRNA and enzyme activity of CAT in the
different groups. (D) mRNA and enzyme activity of MnSOD in the
different groups. (E) The protein expression of Nrf2 in differnet
groups, n=3. **P<0.01, ***P<0.001 vs. Control group.
#P<0.05, ##P<0.01,
###P<0.001 vs. LPS group. &P<0.05,
&&P<0.01,
&&&P<0.001 vs. LPS + si-S100A1.
$P<0.05, $$P<0.01 vs. LPS + ov-S100A1
group. LPS, lipopolysaccharide; ROS, reactive oxygen species; CAT,
catalase; MnSOD, manganese superoxide dismutase; ov,
overexpression; si, short interfering; Nrf2, nuclear factor
erythroid 2-related factor 2. |
S100A1 regulates the apoptosis of PC12
cells by mediating the ERK signaling pathway
Retaining a sufficient number of viable cells is the
crucial factor limiting the progression of SCI (38). Thus, the present study
investigated the effects of S100A1 on the level of apoptosis. It
was found that the level of apoptosis was elevated by LPS
stimulation. The silencing and overexpression of S100A1
significantly decreased and increased apoptosis level,
respectively. In addition, MK-8353 partly abolished the promoting
effects of S100A1 overexpression on apoptosis (Fig. 6A and B). Subsequently, the effects
of S100A1 on apoptosis related proteins were also detected. The
ratio of Bax/Bcl2 was increased in the LPS group, whereas it was
partially decreased in the LPS + si-S100A1 group and further
increased in the LPS + ov-S100A1 group (Fig. 6C). In addition, the expression of
cleaved caspase-3 also exhibited a similar trend of Bax/Bcl2
(Fig. 6D). As was expected,
MK-8353 partially abolished the promoting effect of S100A1
overexpression on the expression of Bax/Bcl2 and cleaved caspase-3
(Fig. 6C and D). These results
suggested that S100A1 regulated the apoptosis of PC12 cells by
targeting the ERK signaling pathway.
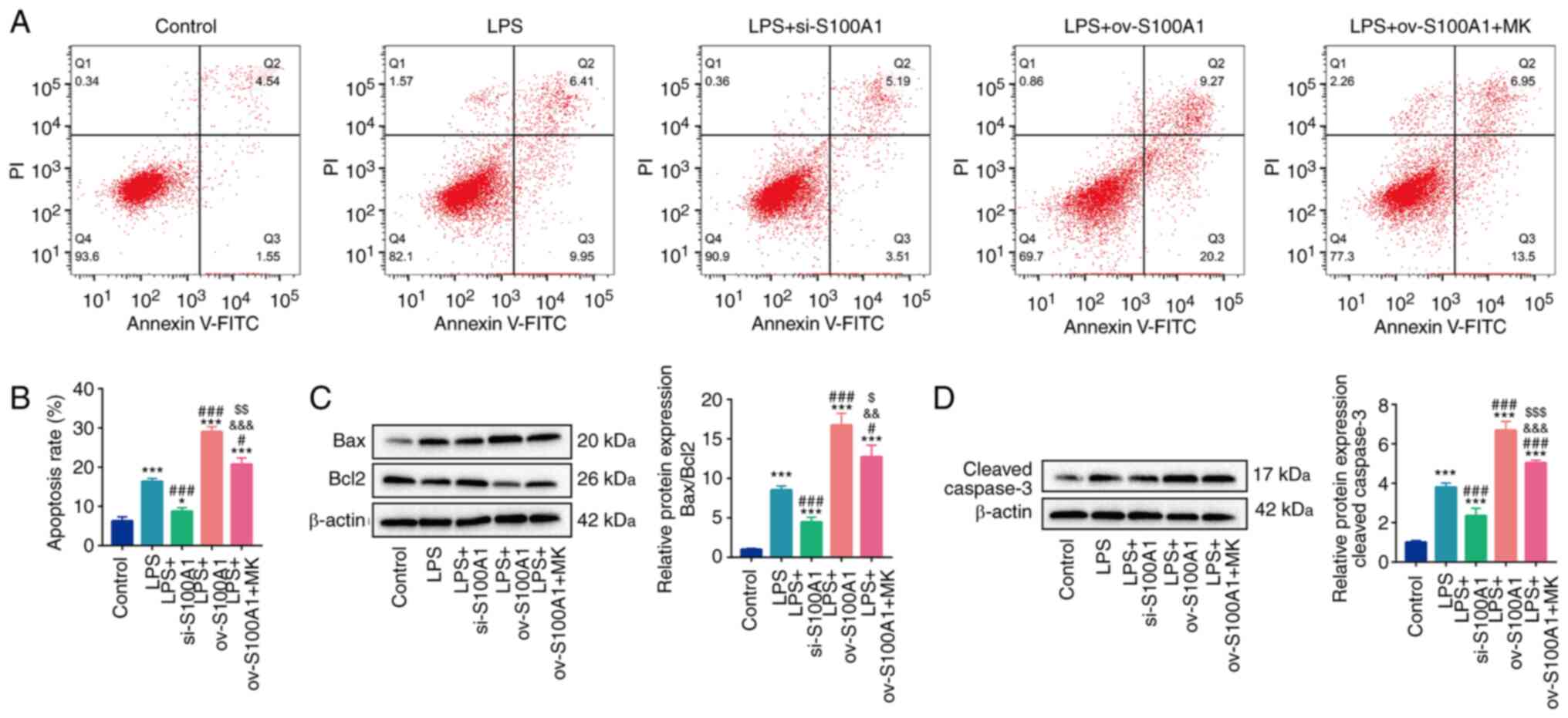 | Figure 6.S100A1 regulates the apoptosis of
PC12 cells through the ERK signaling pathway. (A and B) The
apoptosis levels in the Control, LPS, LPS with S100A1
silencing/overexpression, LPS with S100A1 overexpression and EKR
inhibitor groups. (C and D) The protein levels of Bax, Bcl2 and
cleaved caspase-3 in the different groups. n=3. *P<0.05,
***P<0.001 vs. Control group. #P<0.05,
###P<0.001 vs. LPS group.
&&P<0.01,
&&&P<0.001 vs. LPS + si-S100A1.
$P<0.05, $$P<0.01,
$$$P<0.001 vs. LPS + ov-S100A1 group. LPS,
lipopolysaccharide; ov, overexpression; si, short interfering. |
Discussion
With the development of modern medical technology,
SCI remains a significant source of mortality and is associated
with increasing costs for society, as a result of long-term
disability. Although there some preclinical studies on stem cells
(39), gene therapy (40) or tissue engineering technology
(41) for neuroprotection and
regeneration have been performed, few investigations have reached
clinic practice (35). Thus, a
more in-depth understanding of the molecular basis of SCI is still
required. In the present study, S100A1 was found to be
significantly highly expressed in the damaged spinal cord tissue.
In addition, S100A1 was also found to be highly expressed in the
PC12 cell model of LPS induced-inflammation. Furthermore, silencing
the expression of S100A1 partially inhibited inflammation,
oxidative stress-induced damage and apoptosis via the ERK signaling
pathway in PC12 cells stimulated with LPS.
The main pathological structures of SCI include
primary injury followed by the secondary injury. The primary injury
involves mechanical compression and damage caused by fractured
and/or displaced bone fragments. Current clinical approaches
towards primary injury mainly include early surgical decompression
and stabilization (42). The
spinal cord tissue squeezed by external forces is followed by
ischemia, hypoxia and the excessive release of inflammatory
cytokines and prostaglandins, resulting in oxidative stress-induced
injury and more severe tissue apoptosis and necrosis (43,44). Nevertheless, at present, there are
no available effective treatments for the inflammatory process of
SCI (42), even though a number
of research teams have verified that anti-inflammatory treatment
can exert an effective neuroprotective effect in animal
experimental models (45–47). However, the majority of research
results are still far from real clinical transformation. Thus, the
investigation of the inflammatory pathological process of SCI is
still necessary so as to develop practical and effective
treatments.
S100A1 is a member of the S100 protein family and
serves as an alarmin in injured tissue, such as the heart or/and
cardiomyocytes (15). S100A1 is
mainly involved in the regulation of sarcoplasmic reticulum
Ca2+ activity, as well as mitochondrial function and
participates in cellular activities (48). In a previous study, S100A1 was
found to be released from the ischemic myocardium to the
circulation of patients and mice with acute ST-segment (15). At the same time, S100A1 plays an
early immunomodulatory role in cardiac fibroblasts of injured
hearts (15). In addition, the
inhibition of S100A1 expression has been shown to mitigate
oxidative stress and the apoptosis of cardiomyocytes (49,50). However, the expression of S100A1
in the injured spinal cord and its regulatory effects on
inflammation, oxidative stress and apoptosis remain to be
elucidated.
In the present study, S100A1 was verified to be
highly expressed in vivo and in PC12 cells stimulated with
LPS in vitro, accompanied by increased levels of
inflammation, oxidative stress-induced damage and apoptosis. The
silencing of S100A1 partially reduced the LPS-induced inflammation,
oxidative stress and apoptosis, whereas the overexpression of
S100A1 aggravated inflammation, oxidative stress and the apoptosis
of PC12 in vitro.
ERK1/2 signaling, as a crucial component of the MAPK
pathway, is an evolutionary conserved signaling cascade,
responsible for transmitting signals from the cell surface to
inside the cells (33).
Furthermore, it has been reported that ERK1/2 signaling can mediate
the development process of the neuronal system and can participate
in certain neurological activities by regulating nerve growth,
elastic properties, neurological and cognitive processing in nerve
injuries (51). In addition, the
inhibition of ERK1/2 can block the cellular inflammatory and
apoptosis response of PC12 (33).
However, whether S100A1 can regulate the ERK signaling pathway in
PC12 cells remains unclear.
In the present study, it was found that the
stimulation of PC12 cells with LPS activated the ERK signaling
pathway. The silencing of S100A1 partially reduced the activity of
ERK and the overexpression of S100A1 promoted the activation of the
ERK signaling pathway, suggesting that S100A1 can regulate the
activity of ERK signaling as upstream signal. In addition, the ERK
inhibitor, MK-8353 partly abolished the promoting effects of the
overexpression of S100A1 on inflammation, oxidative stress and the
apoptosis of PC12 cells. These results verified that S100A1
regulated the inflammation, oxidative stress and apoptosis of PC12
cells via the ERK signaling pathway.
It is worth noting that although MK-8353 application
significantly reduced the levels of inflammation, oxidative stress
and apoptosis compared with LPS + ov-S100A1 group, MK-8353
application did not decrease these phenotypes levels of cells like
LPS + si-S100A1 group, suggesting the increase of S100A1 leaded to
the increase of inflammation, oxidative stress and apoptosis of
PC12 cells partly through ERK signaling pathway. As expected for
the ERK signaling pathway in the present study, PI3K,
Wnt/β-catenin, NF-κB signaling and others are all reported to be
involved in SCI development (52–54). MK-8353 application blocked the
activity of ERK but did not block other possible mechanisms that
were potential regulated by SA100A1. This may be the reason why the
application of MK-8353 can only partially reduce the inflammation,
oxidative stress and apoptosis levels of cells. The typical
downstream mechanisms of S100A1 will continue to be explored in
subsequent studies.
There were still some limitations in the present
study. First, LPS was used to induce PC12 cells to simulate SCI
in vitro. LPS causes inflammatory reaction by activating
TLR4 molecules in cells (55).
Although TLR4 activation is also a mechanism of SCI (56), the LPS induced cell model does not
fully represent the mechanism of SCI in vivo. In addition,
the results of the present study have not been supplemented in
vivo, which is another limitation of the study.
In conclusion, in the present study, S100A1
expression was upregulated in rats with SCI and in LPS-stimulated
PC12 cells. The overexpression of S100A1 was accompanied by an
increase in inflammation, oxidative stress injury and apoptosis.
The silencing of S100A1 attenuated inflammation, oxidative stress
and apoptosis by blocking the ERK signaling pathway. Thus, the
present study revealed the expression and mechanisms of S100A1 in
SCI. These findings may provide more references for the future
theoretical research of SCI.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YB and ZB were substantially responsible for the
experiment conception and design. YB, NG, ZX and YC contributed to
data acquisition and statistical analysis. YB, WZ and QC were
responsible for the interpretation of the experimental data,
drafting the manuscript and revising it critically for important
intellectual content. YB and ZB confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study obtained the approval of the
ethics committee of The First Affiliated Hospital of Harbin Medical
University (grant no. 2021080).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahuja CS, Wilson JR, Nori S, Kotter MRN,
Druschel C, Curt A and Fehlings MG: Traumatic spinal cord injury.
Nat Rev Dis Primers. 3:170182017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alizadeh A, Dyck SM and Karimi-Abdolrezaee
S: Traumatic spinal cord injury: An overview of pathophysiology,
models and acute injury mechanisms. Front Neurol. 10:2822019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spinal Cord Injury (SCI) 2016 facts and
figures at a glance. J Spinal Cord Med. 39:493–494. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahuja CS, Nori S, Tetreault L, Wilson J,
Kwon B, Harrop J, Choi D and Fehlings MG: Traumatic spinal cord
injury-repair and regeneration. Neurosurgery. 80 (Suppl 1):S9–S22.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sabapathy V, Tharion G and Kumar S: Cell
therapy augments functional recovery subsequent to spinal cord
injury under experimental conditions. Stem Cells Int.
2015:1321722015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raspa A, Pugliese R, Maleki M and Gelain
F: Recent therapeutic approaches for spinal cord injury. Biotechnol
Bioeng. 113:253–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou
X, Zhou H, Ning G, Kong X and Feng S: Microenvironment imbalance of
spinal cord injury. Cell Transplant. 27:853–866. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutson TH and Di Giovanni S: The
translational landscape in spinal cord injury: Focus on
neuroplasticity and regeneration. Nat Rev Neurol. 15:732–745. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bareyre FM and Schwab ME: Inflammation,
degeneration and regeneration in the injured spinal cord: Insights
from DNA microarrays. Trends Neurosci. 26:555–563. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Filippin LI, Vercelino R, Marroni NP and
Xavier RM: Redox signalling and the inflammatory response in
rheumatoid arthritis. Clin Exp Immunol. 152:415–422. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hazzaa SM, Abdou AG, Ibraheim EO, Salem
EA, Hassan MHA and Abdel-Razek HAD: Effect of L-carnitine and
atorvastatin on a rat model of ischemia-reperfusion injury of
spinal cord. J Immunoassay Immunochemistry. 42:596–619. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Donnelly DJ and Popovich PG: Inflammation
and its role in neuroprotection, axonal regeneration and functional
recovery after spinal cord injury. Exp Neurol. 209:378–388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu J, Lu Y, Li Y, Xiao L, Xing Y, Li Y and
Wu L: Role of S100A1 in hypoxia-induced inflammatory response in
cardiomyocytes via TLR4/ROS/NF-κB pathway. J Pharm Pharmacol.
67:1240–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bashir M, Frigiola A, Iskander I, Said HM,
Aboulgar H, Frulio R, Bruschettini P, Michetti F, Florio P,
Pinzauti S, et al: Urinary S100A1B and S100BB to predict hypoxic
ischemic encephalopathy at term. Front Biosci (Elite Ed).
1:560–567. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rohde D, Schön C, Boerries M, Didrihsone
I, Ritterhoff J, Kubatzky KF, Völkers M, Herzog N, Mähler M,
Tsoporis JN, et al: S100A1 is released from ischemic cardiomyocytes
and signals myocardial damage via Toll-like receptor 4. EMBO Mol
Med. 6:778–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haw TJ, Starkey MR, Pavlidis S, Fricker M,
Arthurs AL, Nair PM, Liu G, Hanish I, Kim RY, Foster PS, et al:
Toll-like receptor 2 and 4 have opposing roles in the pathogenesis
of cigarette smoke-induced chronic obstructive pulmonary disease.
Am J Physiol Lung Cell Mol Physiol. 314:L298–L317. 2018.PubMed/NCBI
|
|
17
|
Afanador L, Roltsch EA, Holcomb L,
Campbell KS, Keeling DA, Zhang Y and Zimmer DB: The Ca2+
sensor S100A1 modulates neuroinflammation, histopathology and Akt
activity in the PSAPP Alzheimer's disease mouse model. Cell
Calcium. 56:68–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zimmer DB, Chaplin J, Baldwin A and Rast
M: S100-mediated signal transduction in the nervous system and
neurological diseases. Cell Mol Biol (Noisy-le-Grand). 51:201–214.
2005.PubMed/NCBI
|
|
19
|
Park JH, Seo YH, Jang JH, Jeong CH, Lee S
and Park B: Asiatic acid attenuates methamphetamine-induced
neuroinflammation and neurotoxicity through blocking of
NF-kB/STAT3/ERK and mitochondria-mediated apoptosis pathway. J
Neuroinflammation. 14:2402017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jing Y, Yu Y, Bai F, Wang L, Yang D, Zhang
C, Qin C, Yang M, Zhang D, Zhu Y, et al: Effect of fecal microbiota
transplantation on neurological restoration in a spinal cord injury
mouse model: Involvement of brain-gut axis. Microbiome. 9:592021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Howard B, Nevalainen T and Perretta G: The
COST manual of laboratory animal care and use:Refinement.
Reduction, and Research. Crc Press; 2010
|
|
22
|
Sun F, Zhang H, Huang T, Shi J, Wei T and
Wang Y: S100A9 blockade improves the functional recovery after
spinal cord injury via mediating neutrophil infiltration. Ex Ther
Med. 23:2912022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Liu P and Wei F: Long non-coding RNA
MBI-52 inhibits the development of liver fibrosis by regulating the
microRNA-466g/SMAD4 signaling pathway. Mol Med Rep. 25:332022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang R, Liu Y and Jing L: MiRNA-99a
alleviates inflammation and oxidative stress in
lipopolysaccharide-stimulated PC-12 cells and rats post spinal cord
injury. Bioengineered. 13:4248–4259. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo K, Chang Y, Jin Y, Yuan H and Che P:
circ-Ncam2 (mmu_circ_0006413) Participates in LPS-Induced Microglia
Activation and Neuronal Apoptosis via the TLR4/NF-κB Pathway. J Mol
Neurosci. 72:1738–1748. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He X, Zhang J, Guo Y, Yang X, Huang Y and
Hao D: Exosomal miR-9-5p derived from BMSCs alleviates apoptosis,
inflammation and endoplasmic reticulum stress in spinal cord injury
by regulating the HDAC5/FGF2 axis. Mol Immunol. 145:97–108. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiatrak B, Kubis-Kubiak A, Piwowar A and
Barg E: PC12 cell line: Cell types, coating of culture vessels,
differentiation and other culture conditions. Cells. 9:9582020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li R, Ng TSC, Wang SJ, Prytyskach M,
Rodell CB, Mikula H, Kohler RH, Garlin MA, Lauffenburger DA,
Parangi S, et al: Therapeutically reprogrammed nutrient signalling
enhances nanoparticulate albumin bound drug uptake and efficacy in
KRAS-mutant cancer. Nat Nanotechnol. 16:830–839. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou J, Li Z, Zhao Q, Wu T, Zhao Q and Cao
Y: Knockdown of SNHG1 alleviates autophagy and apoptosis by
regulating miR-362-3p/Jak2/stat3 pathway in LPS-injured PC12 cells.
Neurochemical Res. 46:945–956. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Y, Li S, Chen H, Feng L, Yuan W and
Han T: Butorphanol reduces the neuronal inflammatory response and
apoptosis via inhibition of p38/JNK/ATF2/p53 signaling. Exp Ther
Med. 23:2292022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma Z, Lu Y, Yang F, Li S, He X, Gao Y,
Zhang G, Ren E, Wang Y and Kang X: Rosmarinic acid exerts a
neuroprotective effect on spinal cord injury by suppressing
oxidative stress and inflammation via modulating the Nrf2/HO-1 and
TLR4/NF-κB pathways. Toxicol Appl Pharmacol. 397:1150142020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samatar AA and Poulikakos PI: Targeting
RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug
Dis. 13:928–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Shen R, Shu Z, Zhang Q and Chen
Z: S100A12 promotes inflammation and apoptosis in
ischemia/reperfusion injury via ERK signaling in vitro study using
PC12 cells. Pathol International. 70:403–412. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoo SR, Kim Y, Lee MY, Kim OS, Seo CS,
Shin HK and Jeong SJ: Gyeji-tang water extract exerts
anti-inflammatory activity through inhibition of ERK and NF-κB
pathways in lipopolysaccharide-stimulated RAW 264.7 cells. BMC
Complement Altern Med. 16:3902016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Biswas SK: Does the interdependence
between oxidative stress and inflammation explain the antioxidant
paradox? Oxid Med Cell Longev. 2016:56989312016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ganner A, Pfeiffer ZC, Wingendorf L, Kreis
S, Klein M, Walz G and Neumann-Haefelin E: The acetyltransferase
p300 regulates Nrf2 stability and localization. Biochem Biophys Res
Commun. 524:895–902. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi Z, Yuan S, Shi L, Li J, Ning G, Kong X
and Feng S: Programmed cell death in spinal cord injury
pathogenesis and therapy. Cell Prolif. 54:e129922021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zipser CM, Cragg JJ, Guest JD, Fehlings
MG, Jutzeler CR, Anderson AJ and Curt A: Cell-based and
stem-cell-based treatments for spinal cord injury: Evidence from
clinical trials. Lancet Neurol. 21:659–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cunningham CJ, Viskontas M, Janowicz K,
Sani Y, Håkansson ME, Heidari A, Huang W and Bo X: The potential of
gene therapies for spinal cord injury repair: A systematic review
and meta-analysis of pre-clinical studies. Neural Regen Res.
18:299–305. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chaudhari LR, Kawale AA, Desai SS, Kashte
SB and Joshi MG: Pathophysiology of spinal cord injury and tissue
engineering approach for its neuronal regeneration: Current status
and future prospects. Adv Exp Med Bio. Aug 30–2022.(Epub ahead of
print) doi: 10.1007/5584_2022_731. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karsy M and Hawryluk G: Modern medical
management of spinal cord injury. Curr Neurol Neurosci Rep.
19:652019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anjum A, Yazid MD, Fauzi Daud M, Idris J,
Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK and Lokanathan
Y: Spinal cord injury: Pathophysiology, multimolecular
interactions, and underlying recovery mechanisms. Int J Mol Sci.
21:75332020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li X, Zhan J, Hou Y, Hou Y, Chen S, Luo D,
Luan J, Wang L and Lin D: Coenzyme Q10 regulation of apoptosis and
oxidative stress in H2O2 induced BMSC death
by modulating the Nrf-2/NQO-1 signaling pathway and its application
in a model of spinal cord injury. Oxid Med Cell Longev.
2019:64930812019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen S, Ye J, Chen X, Shi J, Wu W, Lin W,
Lin W, Li Y, Fu H and Li S: Valproic acid attenuates traumatic
spinal cord injury-induced inflammation via STAT1 and NF-κB pathway
dependent of HDAC3. J Neuroinflammation. 15:1502018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shen K, Sun G, Chan L, He L, Li X, Yang S,
Wang B, Zhang H, Huang J, Chang M, et al: Anti-inflammatory
nanotherapeutics by targeting matrix metalloproteinases for
immunotherapy of spinal cord injury. Small. 17:e21021022021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rodríguez-Cal Y Mayor A,
Castañeda-Hernández G, Favari L, Martinez-Cruz A, Guízar-Sahagún G
and Cruz-Antonio L: Pharmacokinetics and anti-inflammatory effect
of naproxen in rats with acute and subacute spinal cord injury.
Naunyn Schmiedebergs Arch Pharmacol. 393:395–404. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun B and Kekenes-Huskey PM: Molecular
basis of S100A1 activation and target regulation within
physiological cytosolic Ca(2+) levels. Front Mol Biosci. 7:772020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alanazi AM, Fadda L, Alhusaini A, Ahmad R,
Hasan IH and Mahmoud AM: Liposomal resveratrol and/or carvedilol
attenuate doxorubicin-induced cardiotoxicity by modulating
inflammation, oxidative stress and S100A1 in Rats. Antioxidants
(Basel). 9:1592020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zeng Z, Huang N, Zhang Y, Wang Y, Su Y,
Zhang H and An Y: CTCF inhibits endoplasmic reticulum stress and
apoptosis in cardiomyocytes by upregulating RYR2 via inhibiting
S100A1. Life Sci. 242:1171582020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sahu R, Upadhayay S and Mehan S:
Inhibition of extracellular regulated kinase (ERK)-1/2 signaling
pathway in the prevention of ALS: Target inhibitors and influences
on neurological dysfunctions. Eur J Cell Biol. 100:1511792021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ge X, Tang P, Rong Y, Jiang D, Lu X, Ji C,
Wang J, Huang C, Duan A, Liu Y, et al: Exosomal miR-155 from
M1-polarized macrophages promotes EndoMT and impairs mitochondrial
function via activating NF-κB signaling pathway in vascular
endothelial cells after traumatic spinal cord injury. Redox Biol.
41:1019322021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cheng RD, Ren W, Sun P, Tian L, Zhang L,
Zhang J, Li JB and Ye XM: Spinal cord injury causes insulin
resistance associated with PI3K signaling pathway in hypothalamus.
Neurochem Int. 140:1048392020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xiang Z, Zhang S, Yao X, Xu L, Hu J, Yin
C, Chen J and Xu H: Resveratrol promotes axonal regeneration after
spinal cord injury through activating Wnt/β-catenin signaling
pathway. Aging. 13:23603–23619. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ciesielska A, Matyjek M and Kwiatkowska K:
TLR4 and CD14 trafficking and its influence on LPS-induced
pro-inflammatory signaling. Cell Mol Life Sci. 78:1233–1261. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen J, Wang Z, Zheng Z, Chen Y, Khor S,
Shi K, He Z, Wang Q, Zhao Y, Zhang H, et al: Neuron and
microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt
signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent
neuroinflammation to improve functional recovery after spinal cord
injury. Cell Death Dis. 8:e30902017. View Article : Google Scholar : PubMed/NCBI
|