Introduction
Three types of hedgehog (Hh) ligands, Sonic hedgehog
(Shh), Indian hedgehog (Ihh) and Desert hedgehog (Dhh) have been
found in mammals exhibiting different expression patterns and
biological functions (1). Shh is
extensively expressed in a number of tissues in both the embryo and
adult. However, Ihh is specifically expressed in hematopoietic
cells, bones, cartilage and the eyes, whereas Dhh is restricted to
the gonads, external genitalia, eyes and peripheral nerves
(2–5). Hh ligands that bind to a
transmembrane protein, patched (Ptch), transduce an intracellular
signal that is widely involved in the differentiation,
proliferation and survival of numerous cells at a series of
developmental stages from embryogenesis to adulthood (1). It has been reported that Hh signaling
spatially and temporally paves the way for the development and
differentiation of hematopoietic stem and progenitor cells (HSPCs)
and blood cells in embryonic and adult hematopoietic system
developing tissues. Defining the role of Hh signaling in
hematopoiesis, and the potential impact in embryonic hematopoiesis
and the development of multi-lineage blood cells in adults may
provide a novel theoretical basis for improving regeneration
following injury or hematopoietic stem cell (HSC)
transplantation.
Hh signaling pathway in mammals
In mammals, canonical Hh signaling initiates from
the binding of Hh ligands to a 12-transmembrane domain receptor
protein, Ptch. This binding leads to alleviation of the
Ptch-induced suppressive effects on smoothened (Smo). Smo is
subsequently uninhibited, and activates zinc finger transcription
factor glioma-associated oncogene (Gli) proteins, which are
dissociated from a suppressive complex-containing scaffold protein,
suppressor of fused (SuFu), and translocate into the nucleus to
promote or depress target gene transcription (5,6),
including that of Gli1, CCND, Ptch, BCL2 and Hhip. Three Gli
proteins (Gli1, Gli2 and Gli3) are expressed in vertebrates with
overlapping and partially redundant domains. Only Gli1 usually acts
as a transcriptional activator, while Gli2 and Gli3 act as either
activators or repressors, which is determined by
post-transcriptional and post-translational modifications (7–9).
Lack of SuFu leads to a decrease in the stability of Gli2/3 protein
(10). Kinesin-like protein KIF7
(Kif7) negatively regulates Smo; however, it enhances Hh signaling
in certain cases (Fig. 1)
(11).
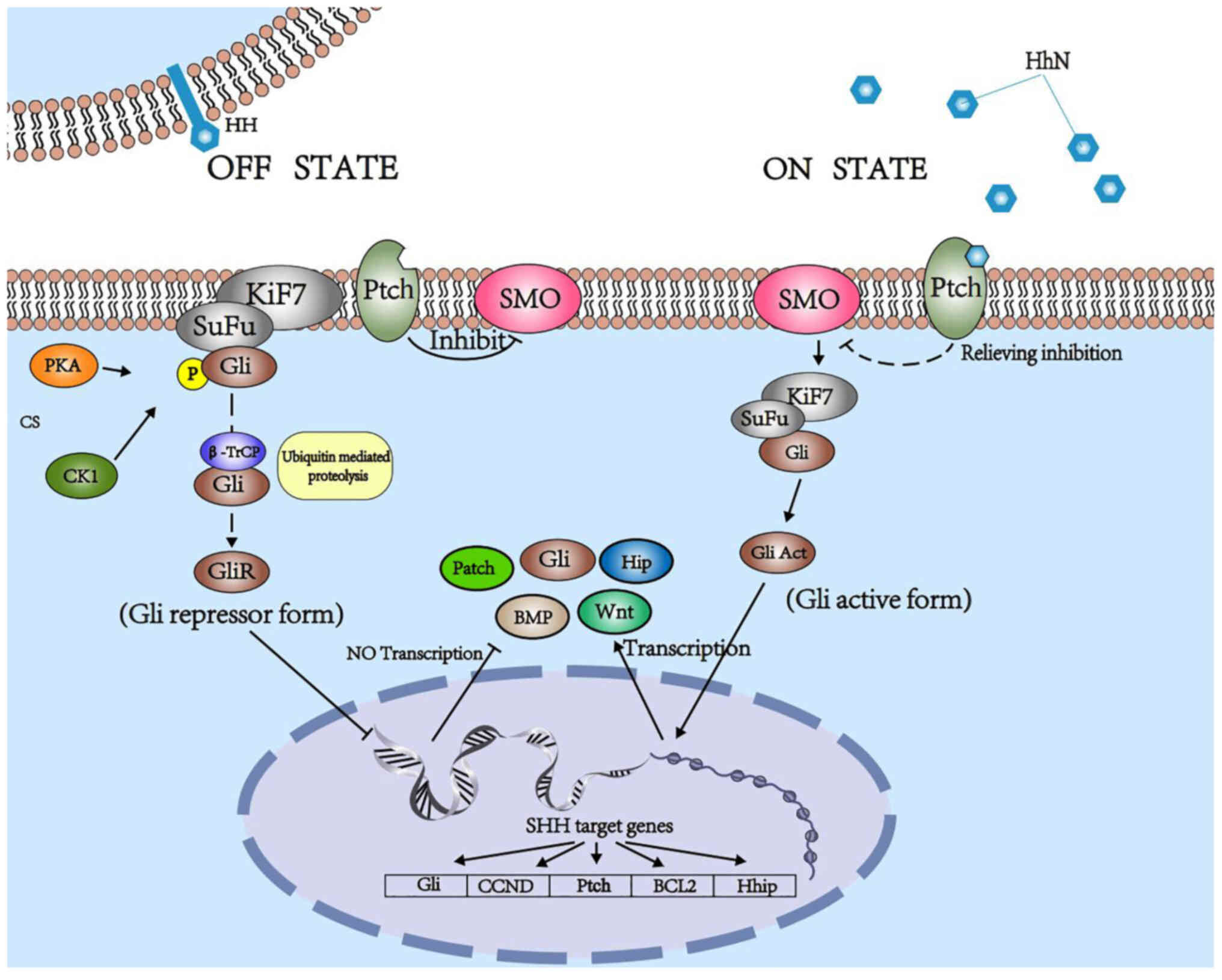 | Figure 1.Mechanism of Hh signal transduction
in mammals. The canonical pathway starts from the binding of Hh to
receptor patched. Hh, hedgehog; Ptch, patched; Smo, smoothened;
SuFu, suppressor of fused; PKA, protein kinase A; CK1, casein
kinase I; β-TrCP, β-transducin repeat-containing protein; Hhip,
Hh-interacting protein; Kif7, kinesin-like protein KIF7; Gli, zinc
finger transcription factor glioma-associated oncogene; CCND,
cyclin D. |
Hh signaling promotes embryonic
hematopoiesis
Hh signaling is predominantly involved in the later
stages of embryonic hematopoiesis, which includes two spatially and
temporally distinguishable hematogenic waves, namely pro-definitive
and definitive hematopoiesis, to individually give rise to bipotent
hepatic progenitor cells (HPCs) or HSCs (12). Mutants in the Hh pathway (the shh
mutant and the slow muscle-omitted, smu, mutant) or treatment with
Hh signaling inhibitor, cyclopamine, in zebrafish embryos severely
reduced the number of runt-related transcription factor
(runx1)+ definitive blood cells, while the number of
βE1+ primitive erythrocytes were unaffected. This
requirement for Hh signaling coincides with the time of three
consecutive steps in dorsal aorta formation and intersomitic vessel
sprouting, indicating that Hh signaling is required for HSC
formation (13). Using mouse
embryonic explants, Dyer et al (14) demonstrated that Ihh secreted by
visceral endoderm and mature yolk sacs alone are sufficient in
inducing endothelial and hematopoietic differentiation, following
increased Ptch, Smo and Gli1, as well as Bmp4 within anterior
epiblasts (15). HSC numbers in
the aorta-gonad-mesonephros region (AGM) increased, leading to
increased colony-forming units spleen day 11 (CFU-S11)
in the AGM with ventral tissues explant culture and in vivo
transplantation compared with AGM explants, suggesting an important
role of ventral tissues in the AGM HSC activity. Considering the
increased level of Gli1 expression in AGM
(c-Kit−CD34−) mesenchymal cells, positively
regulating HSC activity by both Ihh and Shh proteins, the Hh
signaling pathway is identified as a HSC inducing signal for
definitive hematopoietic development in embryonic day 10 AGM
(16).
Through a combined analysis of differentiating mouse
embryonic stem cells (ESCs), mouse embryo cultures and zebrafish
embryos, Kim et al (17)
constructed a model of later embryonic hematopoietic development
from mesoderm stage to hematopoietic cell differentiation. This
hierarchy of differentiation consists of Flk1+ mesoderm
patterning to the endothelium with arterial identity
(VE-cadherin+CD41−CD45−) through
the activation of Notch signaling, and promoting blood formation
(CD41/45+) from hemogenic endothelial cells upon
upregulation of the protein, stem cell leukemia.
Expression of transcriptional factors that promote
hemogenic endothelium differentiation, such as BRACHYURY, GATA2 and
RUNX1, was increased in human ESCs following treatment with a Smo
agonist, purmorphamine, for 6 days (18,19).
However, treatment with a Smo antagonist, SANT-1, reduced the
expression of RUNX1 and BRACHURY in hESCs, and increased the
expression of markers of endocardiogenic endothelium
differentiation, suggesting a crucial role of Shh for
differentiation of hESCs toward a hemogenic lineage (19,20).
Hh signaling regulates HSPC proliferation
and differentiation
The role of Hh signaling in HSPCs remains
controversial. Bhardwaj et al (21) showed that Hh signaling components,
including Shh, Ptch and Smo, were expressed in primitive and mature
CD19+, CD33+ and CD3+ cells, as
well as stromal cells isolated from adult bone marrow (BM) and
endothelial cells from human umbilical veins, while Gli1, Gli2 and
Gli3 were absent in both myeloid and lymphoid lineages. In NOD-SCID
mice transplanted with Shh-treated human
CD34+CD38−Lin− cells, the
proliferation of CD34+CD38−Lin−
cells was enhanced and multi-lineage blood cells, including myeloid
CD15+CD33+, lymphoid
CD19+CD20+, CD34+ and rare
CD34+CD38− cells increased. In addition, the
total number of HPCs increased when Hh signaling was blocked by an
anti-Hh antibody, suggesting a positive regulatory role in HSPCs.
This effect was also observed in vitro. The promoter action
of Shh on HSPCs and hematopoietic reconstitution is dependent on
downstream Bmp4 signaling, as noggin, a specific inhibitor of Bmp4,
is capable of inhibiting Shh-induced proliferation in a similar
manner to anti-Hh antibody. Together with mesenchymal stem cells,
Shh protein promotes the proliferation of HSCs, which is associated
with increased expression of angiogenic factor receptor Tie-2 in
HSCs cells, and angiogenic factors VEGF and Ang1 in MSCs (22). Expansion of human HSCs in
vitro and in vivo stimulated by Ihh in the stromal
supportive culture system was observed in an independent study
(23).
Using a Ptch-1+/− mouse model, which
exhibited increased Hh signaling activity, Trowbridge et al
(24) found that Hh signaling
induced cycling and expansion of primitive BM HPCs during
homeostasis and stress at the expense of HSC exhaustion. Compared
with wild-type (WT) mice, Ptch-1+/− mice had
significantly higher numbers of
Lin−Sca-1+c-Kit+ (LSK) cells and
CFUs, and exhibited accelerated recovery of peripheral blood
leukocytes after 5-fluorouracil (5-FU) treatment.
Ptch-1+/−Lin− BM cells had a greater
short-term regeneration capability but reduced long-term
reconstitution efficiency. Downregulation of cell cycle-related
genes (Tfdp2, Skp1a, CyclinA2, Rad51, CyclinH, CDC16, CDC2a, Mre11a
and RPA3) in the Ptch-1+/− group was associated with the
absence of long-term hematopoiesis. The regenerative capacity of
Ptch-1+/− HSCs could be restored by cyclopamine through
the upregulation of cycling Lin−Sca-1+ cells.
Homozygous deletion of Gli1 (Gli1null) increased the
number of long-term HSCs, which survived for a prolonged period
following engraftment. However, in vitro colony-forming
assays showed that the number of granulocyte CFUs derived from
Gli1null BM was almost 2-fold lower compared with those
derived from Gli1WT mice. Furthermore, the number of
neutrophils and platelets in Gli1null mice recovered at
a reduced rate following 5-FU treatment, indicating that loss of
Gli1 affected myeloid progenitor function and impaired their
subsequent ability to recover (25).
An independent study demonstrated that Smo deletion
or overexpression exerted no significant effect on HSC
self-renewal, differentiation and reconstitution ability. In
Smo-deficient LSKs, <10% (70/739) of HSC gene signatures changed
(upregulated or downregulated) and the expression of genes closely
associated with long-term HSC activity was not altered (26). The number of T and B cells,
erythroid and myeloid cells, and megakaryocytes and LSK cells in
SmoNull mice were not changed, similar to the results
obtained using SmoWT mice. Furthermore, deletion of Smo
did not affect BM reconstitution after transplantation, and no
significant difference was observed in hematopoietic colony
formation potential, long-term survival ability of competitive
transplantation and recovery ability of BM under 5-FU-induced
stress (27).
Hh signaling participates in lineage
differentiation of blood cells
T-cell differentiation
Hh signaling components are expressed in the human
and mouse thymus, and participate in T-cell development at a series
of stages (28–32). Hh signaling may affect the
differentiation, maturation and distribution of γδ or αβ T cells in
the thymus. After treatment with recombinant Shh in a fetal thymus
organ culture system, CD3+γδ T-cell receptor
(TCR+) and mature CD44+ cells were
significantly increased. By contrast, treatment with a
Hh-neutralizing antibody reduced the number of γδ T cells and
promoted αβ T-cell maturation (33,34).
Targeted deletion has demonstrated that Shh, Ihh and
Ptch expressed in the mouse thymus are necessary for αβ T cell
differentiation from double-negative (DN;
CD4−CD8−) cells to double-positive (DP;
CD4+CD8+) cells (31). Results of a previous study
demonstrated that the proportion of
CD44+CD25− (DN1) was increased, and the
proportion of CD44+CD25+ (DN2) and DP cells
was decreased. In addition, single-positive (SP; CD4+ or
CD8+) T-cell production were observed in
Shh−/− and Ihh−/−
embryonic thymi as well as Ptc−/− thymi, suggesting that
the Hh signaling pathway positively regulates the transition from
DN1 to DN2 and the transition from CD44−CD25−
(DN4) to DP without affecting mature SP cells (31,32,35).
Results of a previous study using mutants of Smo, Shh and Ihh
demonstrated that Gli2 and Gli3 are required for transitions from
DN1 to DN2 mediated by Smo-dependent Shh signaling (36–38).
In addition, the Hh pathway also showed negative regulation of
pre-TCR induced differentiation from
CD44−CD25+ (DN3) to DP, in a Shh- and
Ihh-mediated and Gli2-dependent manner upon analysis of
Gli2−/−, Shh−/−, Ihh−/−,
Gli2ΔN2-transgenic and Gli2ΔC2-transgenic thymi (30,36,38).
Gli1 was shown to promote the differentiation of DN thymocytes
prior to pre-TCR signal transduction and exerted an inhibitory
function following pre-TCR signaling. Introduction of a class
I-restricted transgenic TCR into the adult Gli1-deficient and
embryonic Gli2-deficient thymus identified that both Gli1 and Gli2
influence its selection to the CD8 lineage (39). A significant reduction in the
proportion of SP CD4+ (SP4) cells and the ratio of
CD4/CD8 SP thymocytes was detected in the Gli2 transgenic mouse
thymus (40). Furthermore, rShh
significantly increased the proportion of CD3high DP
cells and decreased the proportion of CD3high SP4 and
the ratio of CD4/CD8 SP cells in thymus explants (41). Analysis of Gli3−/− fetal
mice thymocytes showed that the expression of Shh protein was
significantly increased and that the development of DP to SP was
impaired. Neutralization of Hh protein in the Gli3−/−
thymus enhanced SP4 differentiation, suggesting that Gli3 expressed
in mouse thymic epithelial cells stimulates the differentiation of
thymocytes from DP to SP, through inhibition of Shh (42). In particular, the Hh pathway
activation regulator, Kif7, is required for healthy T-cell
development, by which differentiation of DP and mature
CD8+ T cells is enhanced and T-cell activation in
vitro is promoted (31).
B lymphopoiesis
Shh has an effect on the proliferation of
B220+CD43+ pro-B cells in a dose-dependent
manner (43). Smo and Gli3 are
also important regulators in the development of B lymphocytes.
Depletion of Smo from osteoblastic cells, significantly reduced BM
pro-B and pre-B cells, and recovery of BM B progenitors was delayed
in the chemical ablation of the BM (44). Gli3 deficiency increased Shh
transcription and the expression of Hh-target genes in the fetal
liver, leading to the inhibition of early B-cell maturation. The
proportion of CD19+, B220+,
CD19+B220+, CD19+B220−
and CD19+BP1+ in Shh−/− and
Shh+/− E14.5 fetal hepatocytes was significantly higher
than that in Shh+/+ (WT) mice. The proportion of
CD19+ cells decreased significantly following treatment
with rShh, suggesting that Gli3 promotes B-cell development through
repression of Shh (45).
Myelopoiesis and erythropoiesis
Three Hh ligands have been reported to modulate
myelopoiesis and erythropoiesis through different actions under
normal or stress conditions at multiple stages of differentiation,
despite sharing a common signaling pathway.
Dhh is expressed in stromal cells in the BM and by
non-hematopoietic cells of the spleen stroma (46,47).
The population of Sca-1−c-kit+ progenitors
was increased in Dhh−/− BM compared with that in the WT.
Following expression of CD34 and FcγRII/III, the earlier common
myeloid progenitor (CMP) was demonstrated to increase in
Dhh−/− mouse BM. Furthermore, there was a statistically
significant increase in megakaryocyte-erythrocyte progenitor cell
proportion and a concomitant reduction in the granulocyte-monocyte
progenitor proportion. These data therefore suggest that Dhh acts
on the CMP population and negatively regulates early erythroid
differentiation, but is required for normal granulocyte production
(48). Shh was reported to play a
critical role in the granulopoietic response to bacterial
infection. Expression of Shh mRNA and protein in BM cells,
consistent with increased Gli1 expression in HSPCs and
BrdU+ cells in the LKS cell subpopulation, was markedly
increased in mice with bacteremia induced by injection of E.
coli. Gli1 deletion did not affect the baseline activity of
BrdU incorporation into LKS cells; however, attenuated bacteremia
induced activation of BrdU incorporation into LKS cells, following
an inhibition of the increase in granulocytes in the bloodstream
after systemic E. coli infection. These findings
demonstrated that Shh/Gli1 pathway positively regulated
granulopoietic response to serious bacterial infection through the
activation of HSPCs (49).
Numerous components of the Hh signaling pathway are
present in the fetal liver, with high expression of Ihh and Gli1 in
the stroma, and high expression of Ptc1 in HSPCs. Cridland et
al (50) showed that nearly
one-half of the Ihh knockout (Ihh−/−) embryos did not
survive between E13.5 and E14.5. Circulating red blood cells in
Ihh−/− embryos were significantly reduced, consistent
with a significant reduction in α-globin gene expression in E13.5
fetal livers, which were small and pale upon observation. Embryonic
red cells in Ihh−/− embryos were less terminally
differentiated with an abnormal structure. The expression of Gli1,
but not Ptch-1, was significantly downregulated, suggesting that
Ihh acts upon Gli1 transcription factors during definitive
erythropoiesis. However, an independent study showed that Dhh was a
negative regulator of normal and stress-induced erythropoiesis. In
Dhh-deficient mice, erythrocyte progenitor cell counts in the BM
were increased. Erythroblasts were reliant on Dhh both in
vitro and ex vivo, and were negatively regulated by Dhh.
Erythrocyte differentiation was accelerated in both the spleen and
BM in Dhh-deficient mice under irradiation (48).
Hh signaling is involved in hematopoietic
injury
Expression of Shh, Ptch-1, Smo and Gli1 was
significantly reduced in mice with pesticide-induced aplasia
(51). Expression of Shh, Ihh and
Dhh of Hh ligands was markly reduced in BM stromal cells.
Supplementation of the recombinant mouse Shh (rmShh) in
vitro promoted CFUs of granulocytes, erythrocytes, monocytes
and megakaryocytes, and CFUs of granulocyte-macrophage progenitors,
and augmented fibroblastic colony formation, suggesting that rmShh
can minimize the suppression of different pesticide mixtures on
hematopoiesis and rescue stromal and hematopoietic precursors from
pesticide-induced cytotoxicity in vitro (52). Downregulation of Hh signaling may
be involved in hematopoietic injury and failure. Results of our
previous study demonstrated that expression of Shh in the BM of
patients with aplastic anemia was significantly lower than that in
patients with iron deficiency anemia. The expression level of Shh
was positively associated with white blood cell count, lymphocyte
count and CD4+/CD8+ ratio (unpublished
data).
Conclusion
While progress has been made in elucidating the role
of Hh signaling on hematopoiesis (Fig.
2), there are still a number of questions that need to be
addressed. The disagreement between results may be attributed to a
set of methodological differences, such as different hematopoietic
microenvironments (AGM, fetal liver or BM), developmental stages
(primitive, early or late definitive hematopoiesis), species
(zebrafish or mouse) and physiological state (stress damage or
normal hematopoiesis). Whether the Hh signaling pathway exhibits
potential as a target for the diagnosis and treatment of blood
disorders requires further investigation.
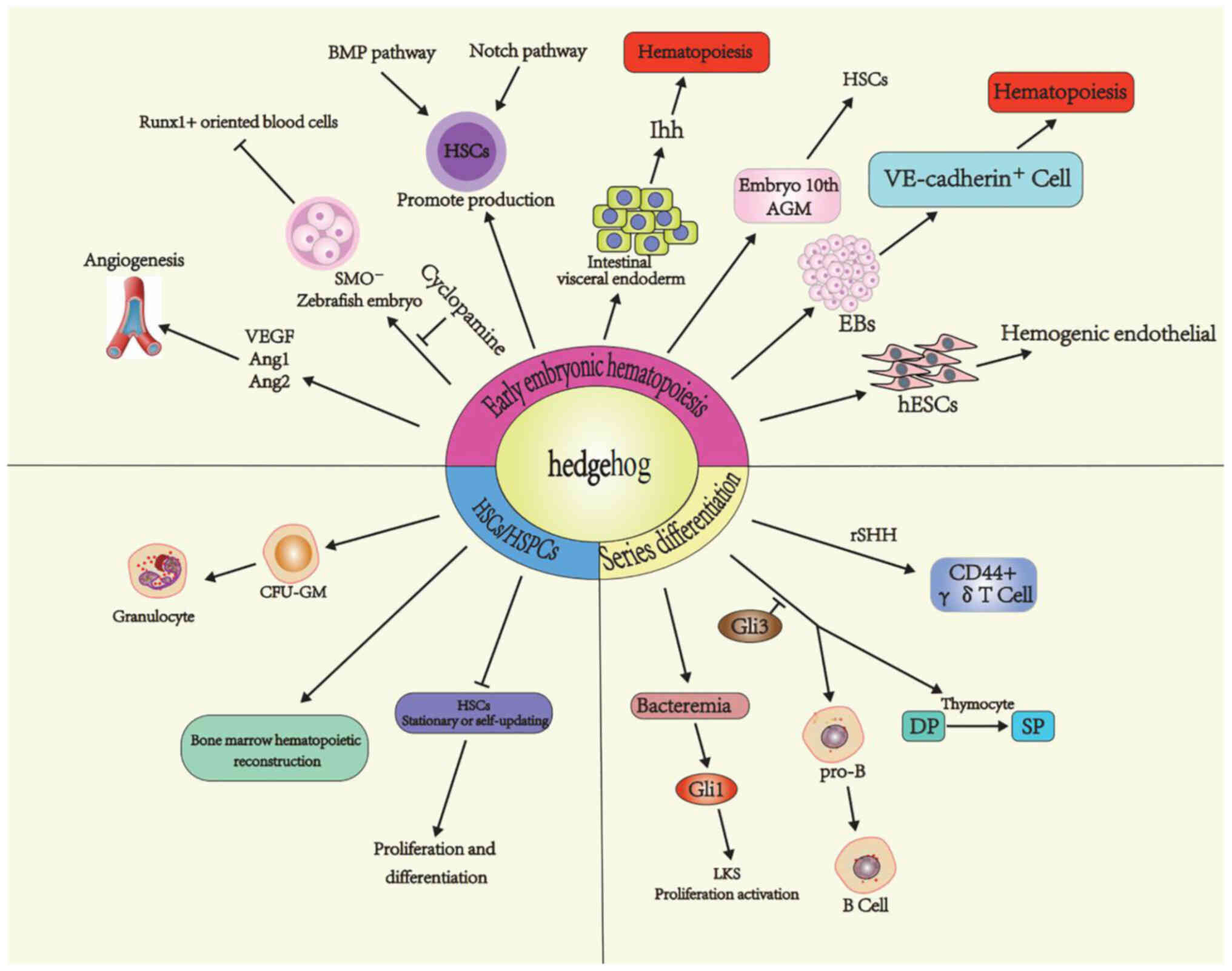 | Figure 2.Regulation of hematopoiesis by Hh
signaling. The Hh signal regulates hematopoiesis in three stages:
Early embryonic hematopoiesis, HSC/HSPC proliferation and
differentiation, and lineage blood cell differentiation. Hh,
hedgehog; HSC, hematopoietic stem cell; HSPC, hematopoietic stem
and progenitor cell; VEGF, vascular endothelial growth factor;
ANG1, angiopoietin1; ANG2, angiopoietin2; Ihh, Indian hedgehog;
hESC, human embryonic stem cell; CFU-GM, colony forming units of
granulocyte-macrophages; DP, double-positive; SP,
single-positive. |
Acknowledgements
Not applicable.
Funding
Research grants were obtained from the following non-commercial
foundations: The Innovation Training Program for College Students
(grant no. 202010573023) and the China Postdoctoral Science
Foundation (grant no. 2017M612848).
Availability of data and materials
Not applicable.
Authors' contributions
ZC was responsible for study conception and design.
JC and YS drafted and revised the manuscript. All authors have read
and approved the final manuscript. Supervision of the study was
provided by ZC. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Petrova R and Joyner AL: Roles for
hedgehog signaling in adult organ homeostasis and repair.
Development. 141:3445–3457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pachernegg S, Georges E and Ayers K: The
desert hedgehog signalling pathway in human gonadal development and
differences of sex development. Sex Dev. 16:98–111. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohba S: Hedgehog signaling in skeletal
development: Roles of Indian hedgehog and the mode of its action.
Int J Mol Sci. 21:66652020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pathi S, Pagan-Westphal S, Baker DP,
Garber EA, Rayhorn P, Bumcrot D, Tabin CJ, Blake Pepinsky R and
Williams KP: Comparative biological responses to human sonic,
Indian, and desert hedgehog. Mech Dev. 106:107–117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sasai N, Toriyama M and Kondo T: Hedgehog
signal and genetic disorders. Front Genet. 10:11032019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carballo GB, Honorato JR, de Lopes GPF and
Spohr TCLSE: A highlight on sonic hedgehog pathway. Cell Commun
Signal. 16:112018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim J, Kato M and Beachy PA: Gli2
trafficking links hedgehog-dependent activation of smoothened in
the primary cilium to transcriptional activation in the nucleus.
Proc Natl Acad Sci USA. 106:21666–21671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varjosalo M and Taipale J: Hedgehog:
Functions and mechanisms. Genes Dev. 22:2454–2472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matissek SJ and Elsawa SF: GLI3: A
mediator of genetic diseases, development and cancer. Cell Commun
Signal. 18:542020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen MH, Wilson CW, Li YJ, Law KK, Lu CS,
Gacayan R, Zhang X, Hui CC and Chuang PT: Cilium-independent
regulation of Gli protein function by Sufu in hedgehog signaling is
evolutionarily conserved. Genes Dev. 23:1910–1928. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu SH, Zhang X, Yu C, Li ZJ, Wunder JS,
Hui CC and Alman BA: Kif7 promotes hedgehog signaling in growth
plate chondrocytes by restricting the inhibitory function of Sufu.
Development. 138:3791–3801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tesanovic S, Krenn PW and Aberger F:
Hedgehog/GLI signaling in hematopoietic development and acute
myeloid leukemia-from bench to bedside. Front Cell Dev Biol.
10:9447602022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gering M and Patient R: Hedgehog signaling
is required for adult blood stem cell formation in zebrafish
embryos. Dev Cell. 8:389–400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dyer MA, Farrington SM, Mohn D, Munday JR
and Baron MH: Indian hedgehog activates hematopoiesis and
vasculogenesis and can respecify prospective neurectodermal cell
fate in the mouse embryo. Development. 128:1717–1730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farrington SM, Belaoussoff M and Baron MH:
Winged-helix, hedgehog and Bmp genes are differentially expressed
in distinct cell layers of the murine yolk sac. Mech Dev.
62:197–211. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peeters M, Ottersbach K, Bollerot K,
Orelio C, de Bruijn M, Wijgerde M and Dzierzak E: Ventral embryonic
tissues and hedgehog proteins induce early AGM hematopoietic stem
cell development. Development. 136:2613–2621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim PG, Albacker CE, Lu YF, Jang IH, Lim
Y, Heffner GC, Arora N, Bowman TV, Lin MI, Lensch MW, et al:
Signaling axis involving hedgehog, notch, and Scl promotes the
embryonic endothelial-to-hematopoietic transition. Proc Natl Acad
Sci USA. 110:E141–E150. 2013.PubMed/NCBI
|
|
18
|
Sinha S and Chen JK: Purmorphamine
activates the hedgehog pathway by targeting smoothened. Nat Chem
Biol. 2:29–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pethe P, Noel VS and Kale V: Deterministic
role of sonic hedgehog signalling pathway in specification of
hemogenic versus endocardiogenic endothelium from differentiated
human embryonic stem cells. Cells Dev. 166:2036852021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rohatgi R, Milenkovic L, Corcoran RB and
Scott MP: Hedgehog signal transduction by smoothened: Pharmacologic
evidence for a 2-step activation process. Proc Natl Acad Sci USA.
106:3196–3201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhardwaj G, Murdoch B, Wu D, Baker DP,
Williams KP, Chadwick K, Ling LE, Karanu FN and Bhatia M: Sonic
hedgehog induces the proliferation of primitive human hematopoietic
cells via BMP regulation. Nat Immunol. 2:172–180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo J, Wang SY, Zhu XF, Li ST, Lin FL, Li
XM and Huang CL: Effect of Shh and BM-MSC synergism on the
proliferation of hematopoietic stem cells. Zhongguo Shi Yan Xue Ye
Xue Za Zhi. 26:1523–1530. 2018.(In Chinese). PubMed/NCBI
|
|
23
|
Kobune M, Ito Y, Kawano Y, Sasaki K,
Uchida H, Nakamura K, Dehari H, Chiba H, Takimoto R, Matsunaga T,
et al: Indian hedgehog gene transfer augments hematopoietic support
of human stromal cells including NOD/SCID-beta2m-/- repopulating
cells. Blood. 104:1002–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trowbridge JJ, Scott MP and Bhatia M:
Hedgehog modulates cell cycle regulators in stem cells to control
hematopoietic regeneration. Proc Natl Acad Sci USA.
103:14134–14139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Merchant A, Joseph G, Wang Q, Brennan S
and Matsui W: Gli1 regulates the proliferation and differentiation
of HSCs and myeloid progenitors. Blood. 115:2391–2396. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao J, Graves S, Koch U, Liu S, Jankovic
V, Buonamici S, El Andaloussi A, Nimer SD, Kee BL, Taichman R, et
al: Hedgehog signaling is dispensable for adult hematopoietic stem
cell function. Cell Stem Cell. 4:548–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hofmann I, Stover EH, Cullen DE, Mao J,
Morgan KJ, Lee BH, Kharas MG, Miller PG, Cornejo MG, Okabe R, et
al: Hedgehog signaling is dispensable for adult murine
hematopoietic stem cell function and hematopoiesis. Cell Stem Cell.
4:559–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sacedón R, Varas A, Hernández-López C,
Gutiérrez-deFrías C, Crompton T, Zapata AG and Vicente A:
Expression of hedgehog proteins in the human thymus. J Histochem
Cytochem. 51:1557–1566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gutiérrez-Frías C, Sacedón R,
Hernández-López C, Cejalvo T, Crompton T, Zapata AG, Varas A and
Vicente A: Sonic hedgehog regulates early human thymocyte
differentiation by counteracting the IL-7-induced development of
CD34+ precursor cells. J Immunol. 173:5046–5053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Outram SV, Varas A, Pepicelli CV and
Crompton T: Hedgehog signaling regulates differentiation from
double-negative to double-positive thymocyte. Immunity. 13:187–197.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Outram SV, Hager-Theodorides AL, Shah DK,
Rowbotham NJ, Drakopoulou E, Ross SE, Lanske B, Dessens JT and
Crompton T: Indian hedgehog (Ihh) both promotes and restricts
thymocyte differentiation. Blood. 113:2217–2228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shah DK, Hager-Theodorides AL, Outram SV,
Ross SE, Varas A and Crompton T: Reduced thymocyte development in
sonic hedgehog knockout embryos. J Immunol. 172:2296–2306. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mengrelis K, Lau CI, Rowell J, Solanki A,
Norris S, Ross S, Ono M, Outram S and Crompton T: Sonic hedgehog is
a determinant of γδ T-cell differentiation in the thymus. Front
Immunol. 10:16292019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Furmanski AL, Saldana JI, Rowbotham NJ,
Ross SE and Crompton T: Role of hedgehog signalling at the
transition from double-positive to single-positive thymocyte. Eur J
Immunol. 42:489–499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Uhmann A, Dittmann K, Nitzki F, Dressel R,
Koleva M, Frommhold A, Zibat A, Binder C, Adham I, Nitsche M, et
al: The hedgehog receptor patched controls lymphoid lineage
commitment. Blood. 110:1814–1823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hager-Theodorides AL, Dessens JT, Outram
SV and Crompton T: The transcription factor Gli3 regulates
differentiation of fetal CD4-CD8-double-negative thymocytes. Blood.
106:1296–1304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
El Andaloussi A, Graves S, Meng F, Mandal
M, Mashayekhi M and Aifantis I: Hedgehog signaling controls
thymocyte progenitor homeostasis and differentiation in the thymus.
Nat Immunol. 7:418–426. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rowbotham NJ, Hager-Theodorides AL,
Furmanski AL, Ross SE, Outram SV, Dessens JT and Crompton T: Sonic
hedgehog negatively regulates pre-TCR-induced differentiation by a
Gli2-dependent mechanism. Blood. 113:5144–5156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Drakopoulou E, Outram SV, Rowbotham NJ,
Ross SE, Furmanski AL, Saldana JI, Hager-Theodorides AL and
Crompton T: Non-redundant role for the transcription factor Gli1 at
multiple stages of thymocyte development. Cell Cycle. 9:4144–4152.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rowbotham NJ, Hager-Theodorides AL,
Cebecauer M, Shah DK, Drakopoulou E, Dyson J, Outram SV and
Crompton T: Activation of the hedgehog signaling pathway in
T-lineage cells inhibits TCR repertoire selection in the thymus and
peripheral T-cell activation. Blood. 109:3757–3766. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saldaña JI, Solanki A, Lau CI, Sahni H,
Ross S, Furmanski AL, Ono M, Holländer G and Crompton T: Sonic
hedgehog regulates thymic epithelial cell differentiation. J
Autoimmun. 68:86–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Solanki A, Yanez DC, Ross S, Lau CI,
Papaioannou E, Li J, Saldaña JI and Crompton T: Gli3 in fetal
thymic epithelial cells promotes thymocyte positive selection and
differentiation by repression of Shh. Development.
145:dev1469102018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cooper CL, Hardy RR, Reth M and Desiderio
S: Non-cell-autonomous hedgehog signaling promotes murine B
lymphopoiesis from hematopoietic progenitors. Blood. 119:5438–5448.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu W, Dordai D, Huso D and Desiderio S:
Smoothened signaling in the mouse osteoblastoid lineage is required
for efficient B lymphopoiesis. Blood. 131:323–327. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Solanki A, Lau CI, Saldaña JI, Ross S and
Crompton T: The transcription factor Gli3 promotes B cell
development in fetal liver through repression of Shh. J Exp Med.
214:2041–2058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Perry JM, Harandi OF, Porayette P, Hegde
S, Kannan AK and Paulson RF: Maintenance of the BMP4-dependent
stress erythropoiesis pathway in the murine spleen requires
hedgehog signaling. Blood. 113:911–918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hegde GV, Peterson KJ, Emanuel K, Mittal
AK, Joshi AD, Dickinson JD, Kollessery GJ, Bociek RG, Bierman P,
Vose JM, et al: Hedgehog-induced survival of B-cell chronic
lymphocytic leukemia cells in a stromal cell microenvironment: A
potential new therapeutic target. Mol Cancer Res. 6:1928–1936.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lau CI, Outram SV, Saldaña JI, Furmanski
AL, Dessens JT and Crompton T: Regulation of murine normal and
stress-induced erythropoiesis by desert hedgehog. Blood.
119:4741–4751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shi X, Wei S, Simms KJ, Cumpston DN, Ewing
TJ and Zhang P: Sonic hedgehog signaling regulates hematopoietic
stem/progenitor cell activation during the granulopoietic response
to systemic bacterial infection. Front Immunol. 9:3492018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cridland SO, Keys JR, Papathanasiou P and
Perkins AC: Indian hedgehog supports definitive erythropoiesis.
Blood Cells Mol Dis. 43:149–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chaklader M, Das P, Pereira JA, Chaudhuri
S and Law S: Altered canonical hedgehog-gli signalling axis in
pesticide-induced bone marrow aplasia mouse model. Arh Hig Rada
Toksikol. 63:271–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chaklader M and Law S: Alteration of
hedgehog signaling by chronic exposure to different pesticide
formulations and unveiling the regenerative potential of
recombinant sonic hedgehog in mouse model of bone marrow aplasia.
Mol Cell Biochem y. 401:115–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
















