Introduction
Oral cancer is one of the leading causes of
mortality worldwide, with a reported 5-year survival rate of ~50%
after treatment (1). Of the total
oral malignancies ~90% are squamous cell carcinomas and the
etiological basis of oral cancer is tobacco intake, smoking,
smokeless tobacco (snuff or chewing tobacco), alcohol and areca nut
intake, excessive sunlight exposure, passive smoking and human
papillomavirus (HPV) (2). The
management of oral cancer is a multidisciplinary endeavor, as each
patient presents with a unique set of challenges, the management of
which influences both overall survival and quality of life
(2). The treatment measures for
oral cancer are expensive and affordability is therefore low. Thus,
it is necessary to develop more effective therapies to treat this
difficult disease.
MicroRNAs (miRNAs) have emerged as a stimulating
area of basic and translational biomedical study, owing to their
influence on gene expression, robust presence in bodily tissues and
fluids and their potential usefulness as disease biomarkers
(3). A number of cancer studies
have found that miRNAs are invasive biomarkers and have therapeutic
potential for a variety of cancers. For example, miR106a was
reported to promote the growth of breast cancer and suppress the
sensitivity of transplanted tumors to cisplatin (4). miR-126 was found to regulate
angiogenesis in breast cancer by targeting VEGF-A-mRNA, which was
proposed as a novel therapeutic approach in breast cancer treatment
(5). Therefore, it is worthwhile
to investigate the effects of miRNAs for the development of novel
biomarkers and anticancer drugs.
miRNAs are found to regulate different signaling
pathways. As one of the important signaling pathways controlling a
variety of cellular activities, the phosphoinositide-3 kinase
(PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway
serves an important role in various aspects of cancer initiation
and progression, including proliferation, apoptosis, metastasis,
angiogenesis and drug resistance interacting with different miRNAs
(6). The Notch signaling pathway
serves an essential role in differentiation and development and is
found to cross-regulate with miRNAs in cancer
initiation/progression via regulating the expression of multiple
oncogenes and tumor suppressor genes (7). Wnt signaling pathway is well-known to
be involved in numerous fundamental processes essential for
embryonic development and normal adult homeostasis (8). Dysfunctional Wnt signaling has been
related to the evolution of and maintenance of leukemic stem cells
as well as a number of other different cancers (9). In chronic lymphocytic leukemia, it
was found that miRNAs and signaling cascades of Wnt pathway had
strong interaction (10).
Therefore, it is worthwhile to explore the interaction of miRNAs
and Wnt signaling and its effects on oral cancer.
As one member of miRNAs, miR-216a-3p has been
explored in various diseases. Wang et al (11) found that miR-216a-3p serves an
important role in Parkinson's disease. Chang and Kan (12) found that mesenchymal stem cell
originated exosomal circular RNA circFBXW7 could obviously reduce
cell proliferation, migration and inflammation via interacting
miR-216a-3p in rheumatoid arthritis. Regarding tumors, a previous
study found that miR-216a-3p markedly inhibited the proliferation
and invasion of cervical cancer via suppressing ACTL6A-mediated YAP
signaling (13). Moreover, Wang
et al (14) found that
miR-216a-3p significantly suppressed the proliferation of
colorectal cancer cell proliferation via regulating genes including
COX-2 and ALOX5. Thus, it is attractive to explore the effects of
miR-216a-3p on oral cancer.
In the present study, two oral cancer lines, HSC-6
and CAL-27, were used to investigate the effects of rapamycin on
oral cancer growth. It was found that expression level of
miR-216a-3p was higher in oral cancer patients and positively
correlated with tumor stages and inhibition of miR-216a-3p potently
suppressed cell viability and induced apoptosis of oral cancer
cells. In principle, it was found that effects of miR-216a-3p on
oral cancer were through Wnt3a. It was also found that the
expression level of β-catenin was higher in oral cancer patients
and positively correlated to tumor stages and effects of
miR-216a-3p on oral cancer were through β-catenin. The findings of
the present study may provide important insight for treating oral
cancers.
Materials and methods
Ethical statement
All patients agreed to participate in the study and
provided written informed consent. The present study was approved
by the ethical board of The Fourth Hospital of Hebei Medical
University (Shijiazhuang, China; approval no. 2022KY392).
Patients' basic information and
peripheral blood mononuclear cells (PBMC) isolation
PBMCs were isolated from whole blood that was
obtained from the blood biobank of Fourth Affiliated Hospital,
Hebei Medical University. In total, there were 30 patients with
oral cancer and 30 healthy controls included in the study. Blood
(~5 ml) was obtained from patients before they received any
intervention. The clinicopathological characteristics of the
patients with oral cancer are listed in Table SI.
Cell culture
The two oral cancer cell lines, HSC-6 and CAL-27,
were purchased from Tianjin IB Biology (https://www.innovationbiotechnology.com.cn/;
Tianjin, China). The two cell lines were maintained in Dulbecco's
modified Eagle's medium (DMEM; cat. no. D6429-500ML;
MilliporeSigma) supplemented with 10% fetal bovine serum (FBS; cat.
no. MFCD00132239; MilliporeSigma), 1% L-glutamine (cat. no.
25030081; Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin solution (cat. no. V900929-100ML;
MilliporeSigma) at 37°C in a 5% CO2 incubator. The cells
were sub-cultured when they were 80% confluent. The HSC-6 cell line
and CAL-27 cell line were regularly tested for Mycoplasma by
using the MycoAlert Plus kit (Lonza Group, Ltd.) to make sure they
were mycoplasma negative.
Western blot analysis
RIPA lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) was used to lyse the cells using
SDS/PAGE sample buffer [50 mM Tris-HCl (pH 6.8), 2% SDS, 0.1%
bromophenol blue, 10% glycerol and 1 mM dithiothreitol]. Five
microliters of the lysates were removed for protein concentration
measurement (Bio-Rad Protein Assay Dye Reagent Concentrate; Bio-Rad
Laboratories, Inc.). Samples were boiled at 95°C for denature for
10 min. Subsequently, total protein (30 µg per lane) was separated
by 10% SDS-PAGE. Post transferring, PVDF membranes (Beyotime
Institute of Biotechnology) were blocked by 5% non-fat milk TBST
buffer containing 0.5 ml/l TWEEN 20 for 45 min at room temperature,
followed by incubation with primary antibodies (1:1,000 dilution),
including anti-pro-caspase-3 (cat. no. ab32499; Abcam),
anti-Caspase-3 (cat. no. ab2302; Abcam), anti-Wnt3a antibody
[EPR21889] (cat. no. ab219412; Abcam), anti-β catenin antibody
(IGX4794R-3; cat. no. ab223075; Abcam) and anti-β actin antibody
(cat. no. mAbcam 8226; Abcam) at 4°C overnight. After incubation
with horseradish-peroxidase-coupled secondary antibodies (1:5,000
dilution; HRP-labeled goat anti-rabbit IgG H+L; cat. no. A0208;
Beyotime Institute of Biotechnology) at room temperature for 1 h,
the immunoblots were visualized using BeyoECL Plus (cat. no.
P0018S; Beyotime Institute of Biotechnology). Densitometry was
measured using ImageJ software (version: ImageJ bundled with 64-bit
Java 8; National Institutes of Health).
Flow cytometric analysis of Annexin
V/propidium iodide (PI) staining
HSC-6 or CAL-27 cells were seeded in 6 well plates
(Costar; Corning, Inc.; 150,000 cells/well). When the confluence
reached 60–70%, cells were treated with miR-216a-30 inhibitor 1 for
24 h in the cell culture incubator (37°C, 5% CO2). After
the treatment, cells were harvested with trypsin/EDTA and stained
for 15 min at room temperature using FITC Annexin V apoptosis
Detection kit I (cat. no. 556547; BD Pharmingen). Results were
analyzed by a FACSCanto II (BD Biosciences). As illustrated in the
Results section below, PI+ Quadrants Q1 and Q2
respectively represent necrosis and late-stage apoptosis/secondary
necrosis; Quadrant Q4 represents viability
(AnnV−/PI−) and Quadrant Q3
(AnnV+/PI−) represents early-stage
apoptosis.
RNA isolation and reverse
transcription-quantitative (RT-q)PCR
TRIzol (Beyotime Institute of Biotechnology) was
used for total RNA isolation from both cell lines (seeded on
96-well plates, seeding density of 6,000 cells per well) following
the manufacturer's protocol. The BeyoRT First Strand cDNA Synthesis
kit (cat. no. D7166; Beyotime Institute of Biotechnology) was used
for cDNA synthesis from total RNA. RT-qPCR was performed using
BeyoFast SYBR Green qPCR Mix (2X; cat. no. D7260-25 ml; Beyotime
Institute of Biotechnology) on a 7500 Fast Real-time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was
performed at 50°C for 2 min and 95°C for 2 min, followed by 40
cycles at 95°C for 15 sec, 60°C for 1 min and extension at 72°C for
1 min, and a final extension step at 72°C for 10 min. GAPDH was
used as an internal control. The relative gene expression levels
were calculated using the 2−ΔΔCq method (15). Primers used in the study are listed
in Table I. This experiment was
repeated three times.
 | Table I.Primers of reverse
transcription-quantitative PCR used in the present study. |
Table I.
Primers of reverse
transcription-quantitative PCR used in the present study.
| Gene | Primer | Sequence | Product length
(nt) |
|---|
| Caspase 3 | Sense |
TGAGGCGGTTGTAGAAGAGTTTCG | 153 |
|
| Anti-sense |
TTATTAACGAAAACCAGAGCGCC |
|
| Wnt3A | Sense |
ATAGGCTCCTTCCTGTGGGT | 188 |
|
| Anti-sense |
GAACCTTACAGGGGGTTGGG |
|
| β-catenin | Sense |
CTGAGGAGCAGCTTCAGTCC | 161 |
|
| Anti-sense |
CCATCAAATCAGCTTGAGTAGCC |
|
| miR-216 | Sense |
CTCAGCTGGCAACTGTG |
|
|
| Anti-sense |
GAACATGTCTGCGTATCTC |
|
| GAPDH | Sense |
AATGGGCAGCCGTTAGGAAA | 168 |
|
| Anti-sense |
GCGCCCAATACGACCAAATC |
|
Small interfering (si)RNA-based
knockdown assay
Gene knockdown was performed using the siRNA
approach. The sequence of siRNAs against mTOR was designed using
siRNA-Target-Finder (GeneScript), followed by being synthesized and
purchased from Synbio Technologies. The sequence of the empty
vector siRNA-negative control (NC) was as
5′-UUCUCCGAACGUGUCACGU-3′, that of siRNA-WNT3a was
5′-GCTTCTGCAGGAACTACGTGGAGAT-3′ and the sequence of siRNA-β-catenin
was 5′-CAGTTATGGTCCATCAGCTTTCTAA-3′. The sequences of Wnt3a and
β-catenin primers, respectively, were as follows: Forward,
5′-AAGACATGCTGGTGGTCGCAA-3′ and reverse,
5′-AACCGCCACAACAACGAGGCT-3′; and forward,
5′-AAGTAGCTGATATTGATGGAC-3′ and reverse,
5′-AAGCTCATCATACTGGCTAGT-3′.
The siRNAs (non-targeting Control siRNA and target
siRNA) were transiently transfected into the HSC-6 and CAL-27 cell
lines and 3D spheroids using FuGENE HD Transfection Reagent (cat.
no. E2311; Promega Corporation) according to the manufacturer's
instructions in the cell culture incubator (37°C, 5%
CO2). The time of transfection of siRNA was 24 h before
the subsequent experiments. The knockdown efficiency was evaluated
using RT-qPCR and western blot assay following protocols described
in this study.
Immunohistochemistry (IHC)
The β-catenin protein expression levels in
paraffin-embedded hepatocellular carcinoma tissues were examined by
IHC as described previously (16).
Briefly, IHC was performed on 4-µm sections. Following
deparaffinization and rehydration, the endogenous peroxidase
activity was blocked using 3% H2O2 (reagent
A; UltraSensitive SP IHC kit; Maxim Biotech Inc.). Next, antigen
retrieval was performed and normal serum (reagent B; UltraSensitive
SP IHC kit; Maxim Biotech Inc.) was applied to the sections to
block non-specific binding. Sections were then incubated at 4°C
overnight with the primary antibodies, including an anti-β-catenin
antibody (E247)-ChIP grade (1:300 dilution; cat. no. ab32572;
Abcam). Subsequently, the sections were incubated with the
secondary antibody (reagent C; UltraSensitive SP IHC kit; Maxim
Biotech Inc.) for 15 min, followed by incubation with
streptavidin-peroxidase (reagent D, UltraSensitive SP IHC kit;
Maxim Biotech Inc.) and 3,3-diaminobenzidine (DAB) was used to
stain the sections for 30 sec at room temperature. Finally,
sections were counterstained with hematoxylin for 5 min at room
temperature and mounted. Sections of oral cancer tissue showing
strong staining with the respective proteins during antibody
optimization served as the positive controls.
Measurement of cytotoxicity using Cell
Counting Kit-8 (CCK8) assay
The Cell Counting Kit-8 (cat no. C0037; Beyotime
Institute of Biotechnology) was used to measure cytotoxicity of
both cell lines, according to the manufacturer's instructions.
Briefly, the HSC-6 cell line and CAL-27 cell line were seeded into
96-well plates at a cell density of 5×104/ml) overnight.
After 24 h, the cell culture medium was replaced by indicated
concentrations of chemicals and the treatment was continued for 48
h. CCK8 solution (0.5 mg/ml; 100 µl) was added into each well and
incubated for 3 h at 37°C, followed by detection of optical density
(OD) values at 450 nm using an Infinite M200 PRO Multimode
Microplate Reader (Tecan Group, Ltd.). The percentage of live cells
was calculated relative to the control.
Target genes of miRNA analysis using
TargetScan
Target genes of miRNA analysis were evaluated using
an online bioinformatics tool, i.e. TargetScan (https://www.targetscan.org/vert_80/).
Statistical analysis
All data were presented as mean ± standard error of
the mean. One-way ANOVA and Tukey's post-hoc test were used for
statistical analysis of continuous variables and categorical
variables were analyzed by Fisher's exact tests. Correlation
analysis (Pearson) and statistical analysis were performed with
GraphPad Prism 5.0 software (GraphPad Software, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression level of miR-216a-3p is
higher in oral cancer patients and positively associated with tumor
stage
To evaluate the effects of miR-216a-3p on oral
cancer, the expression level of miR-216a-3p in oral cancer patients
and healthy controls was measured using RT-qPCR. It was found that
the expression level of miR-216a-3p in oral cancer patients was
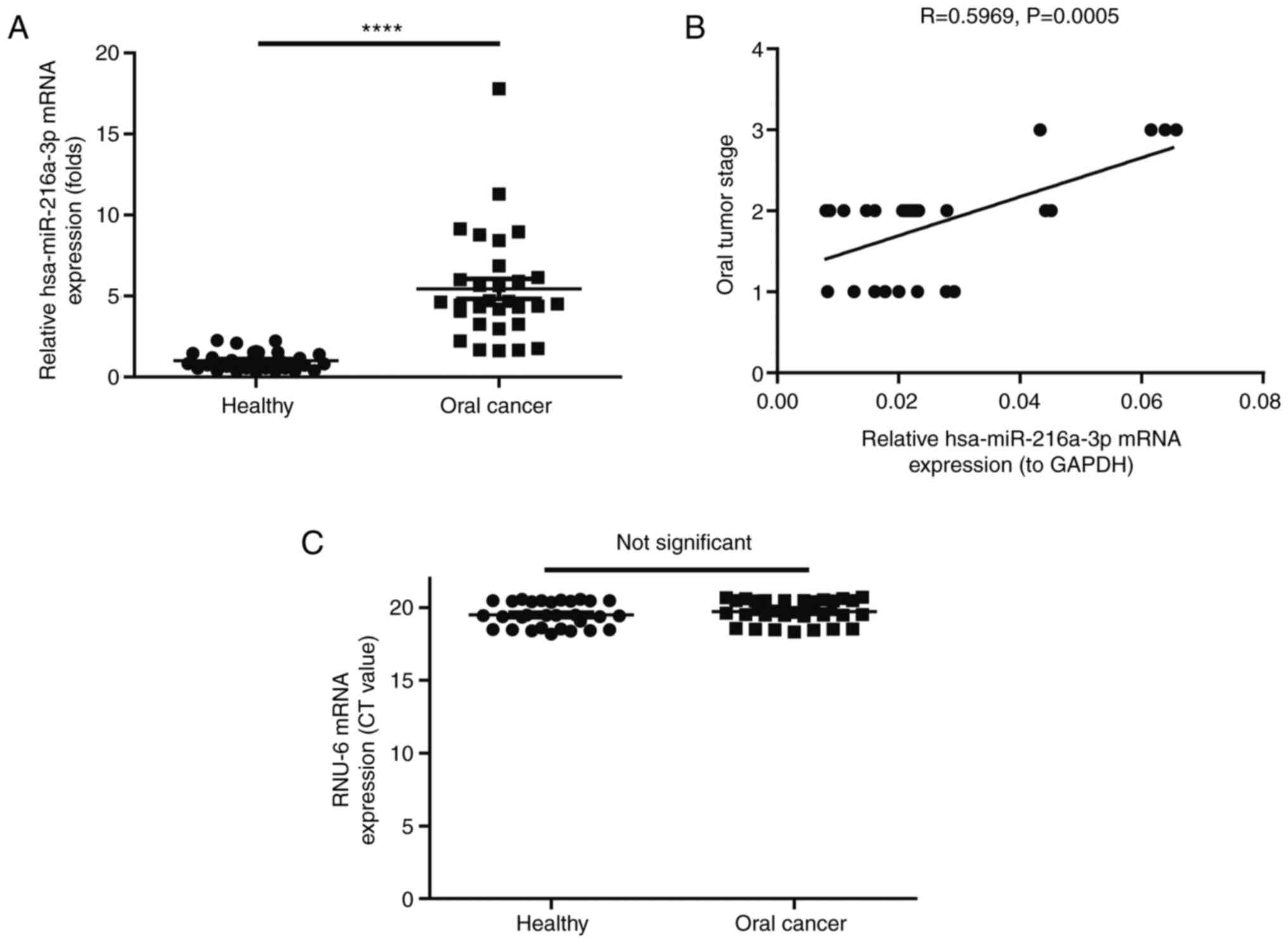
much higher than in healthy controls (P<0.0001; Fig. 1A). Of note, the expression level of
miR-216a-3p was positively correlated with the oral cancer stage
(correlation coefficient R=0.5969, P=0.0005; Fig. 1B). No significant difference was
found between healthy and oral cancer patients regarding expression
level of RNU-6 (Fig. 1C).
Furthermore, the association between the expression level of
miR-216a-3p and clinicopathological factors, including sex, age and
smoking status, was examined using Fisher's exact tests (Table II). It was found that the
expression level of miR-216a-3p was significantly associated with
sex (P=0.0494) and oral cancer stage (P=0.0028) and two
complications, including difficulty swallowing (P=0.0494) and
speech problems (P=0.0608). However, the expression level of
miR-216a-3p had no significant correlation with the smoking status
and free bleeding in the mouth.
 | Table II.Association of the expression level
of miR-216a-3p and clinicopathological factors including sex, age
and smoking status using Fisher's exact test. |
Table II.
Association of the expression level
of miR-216a-3p and clinicopathological factors including sex, age
and smoking status using Fisher's exact test.
| Variable | n=30 | Expression level of
miR-216a-3p (fold) | P-value |
|---|
| Sex |
|
| 0.0494 |
|
Male | 20 | 0.022323192 |
|
|
Female | 10 | 0.027622647 |
|
| Oral cancer
stage |
|
| 0.0028 |
| I | 10 | 0.021805101 |
|
| II | 17 | 0.020385862 |
|
|
III | 3 | 0.058574635 |
|
| Age, years |
|
| 0.1429 |
|
<50 | 2 | 0.01580415 |
|
|
50-60 | 15 | 0.027476579 |
|
|
>60 | 13 | 0.025532913 |
|
| Smoking status |
|
| 0.5100 |
|
Yes | 13 | 0.021847226 |
|
| No | 17 | 0.031098617 |
|
| Complications |
|
|
|
|
Difficulty swallowing |
|
| 0.0002 |
|
(Dysphagia) |
|
|
|
|
Yes | 8 | 0.042074396 |
|
| No | 22 | 0.019958622 |
|
| Speech
problems |
|
| 0.0608 |
|
Yes | 10 | 0.028519873 |
|
| No | 20 | 0.024314014 |
|
| Free bleeding in
the mouth |
|
| 0.3981 |
|
Yes | 15 | 0.030593512 |
|
| No | 15 | 0.021118813 |
|
Inhibition of miR-216a-3p potently
suppresses cell viability and induces apoptosis of oral cancer
cells
To further investigate the effects of miR-216a-3p on
oral cancer, inhibitors of miR-216a-3p were used. It was found that
two miR-216a-3p inhibitors (cat. no. miR2150818102526-1-5;
Guangzhou RiboBio Co., Ltd.) could significantly inhibit
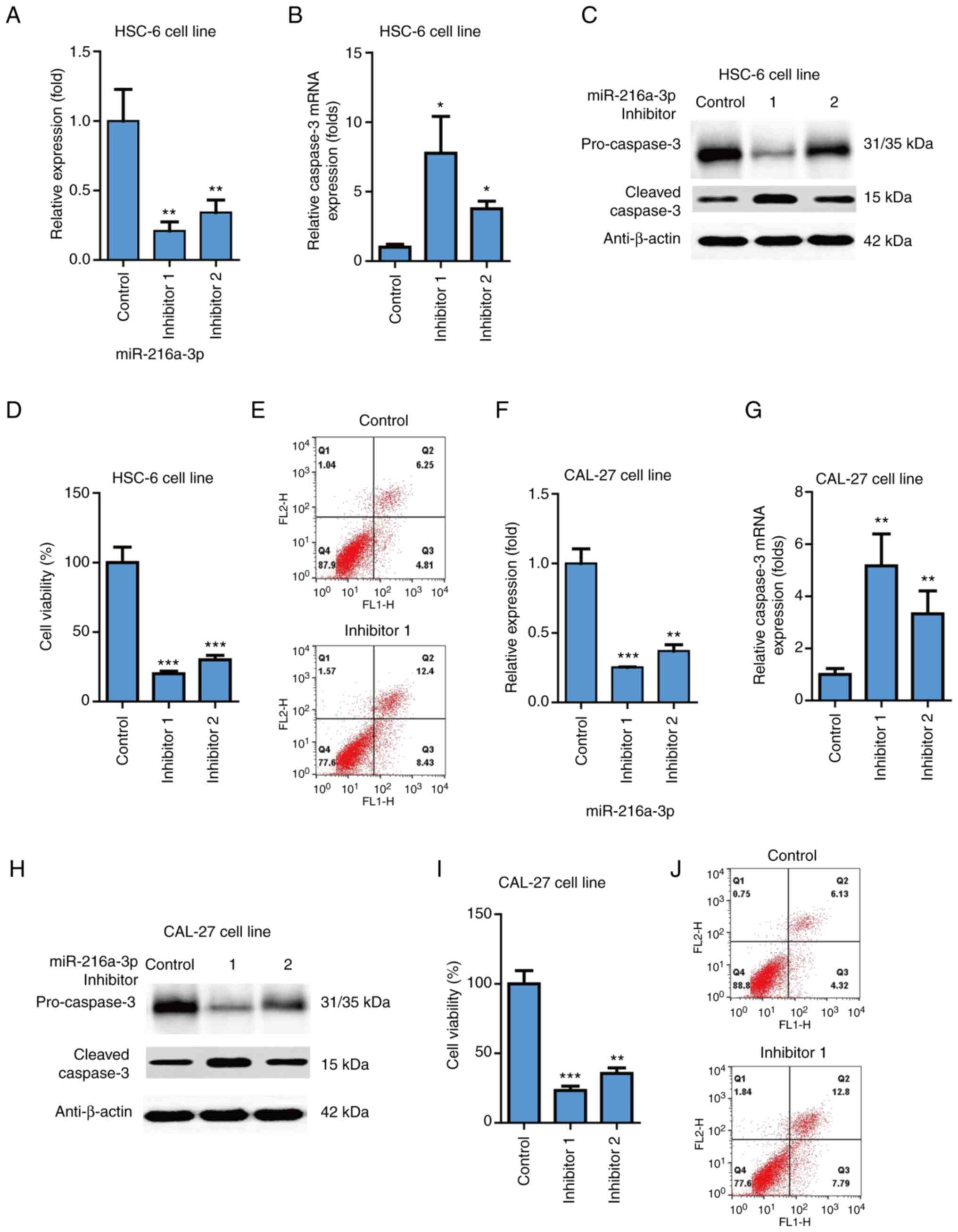
miR-216a-3p level on HSC-6 cells (P<0.01; Fig. 2A). Moreover, it was found that two
miR-216a-3p inhibitors could significantly increase expression
levels of apoptosis marker caspase3 in the HSC-6 cells (P<0.05;
Fig. 2B). It was also found that
the two miR-216a-3p inhibitors could significantly increase the
protein level of apoptosis marker cleaved caspase3 in HSC-6 cells
and decrease the protein level of pro-caspase 3 in HSC-6 cells
(Fig. 2C). The two miR-216a-3p
inhibitors could significantly decrease cell viability of HSC-6
cells (P<0.001; Fig. 2D).
Apoptosis was also evaluated using flow cytometry, which indicated
that miR-216a-3p inhibitor increased apoptosis in HSC-6 cells
(Fig. 2E). Similarly, it was found
that the two miR-216a-3p inhibitors significantly inhibited
miR-216a-3p level on CAL-27 cells (P<0.01; Fig. 2F). It was found that the two
miR-216a-3p inhibitors could significantly increase expression
level of apoptosis marker caspase3 in CAL-27 cells (P<0.05;
Fig. 2G); the two miR-216a-3p
inhibitors could significantly increase the protein level of
apoptosis marker cleaved caspase3 and decrease the protein level of
pro-caspase 3 in CAL-27 cells (Fig.
2H); the two miR-216a-3p inhibitors could significantly
decrease the cell viability of CAL-27 cells (P<0.001; Fig. 2I). Apoptosis was also evaluated
using flow cytometry and indicated that miR-216a-3p inhibitor
increased apoptosis in CAL-27 cells (Fig. 2J). Thus, inhibition of miR-216a-3p
potently suppressed cell viability and induced apoptosis of oral
cancer cells.
Effects of miR-216a-3p on oral cancer
through Wnt3a
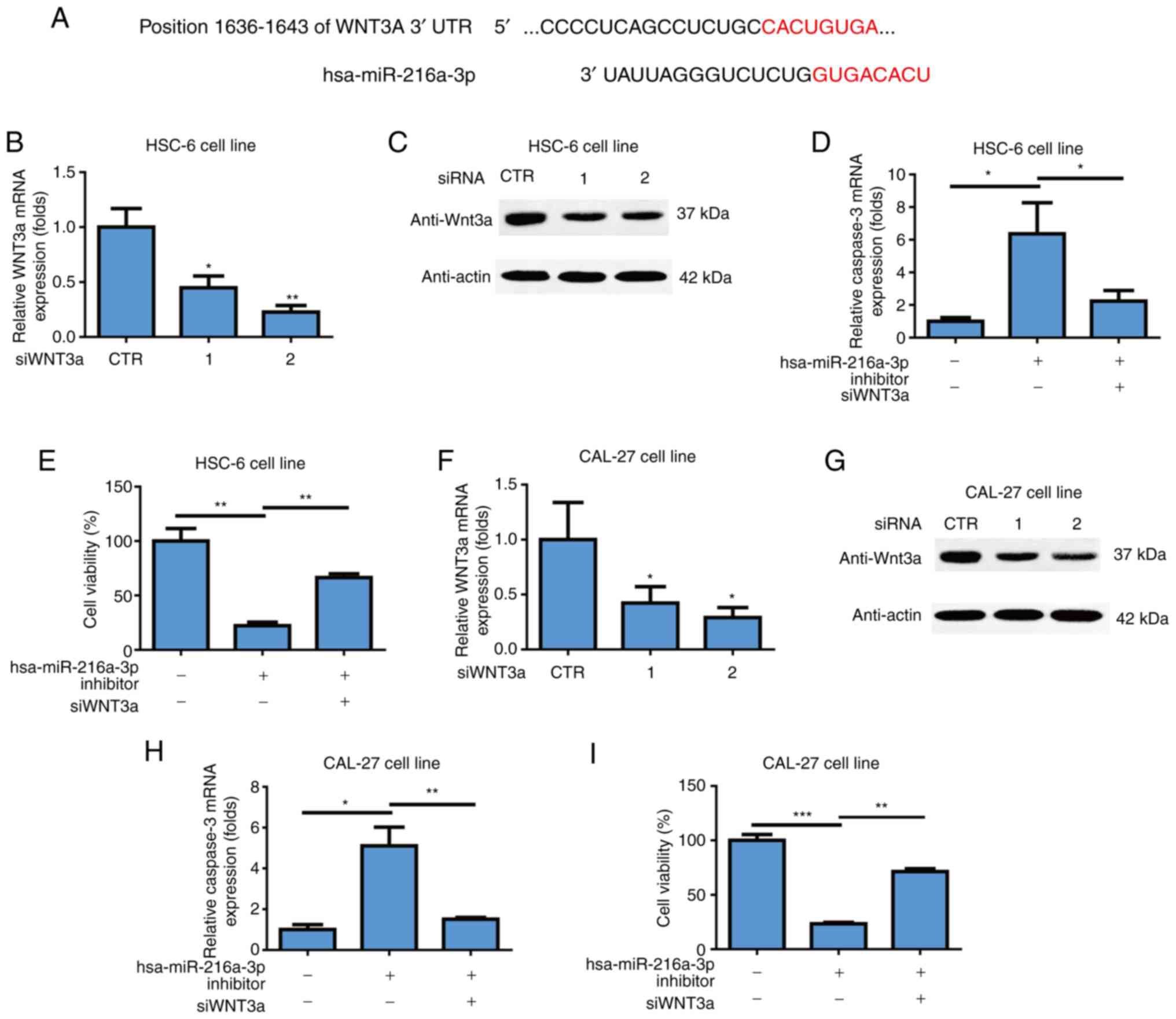
To evaluate the mechanism of action of miR-216a-3p
on oral cancer, an in silico method of TargetScan
(https://www.targetscan.org/vert_80/)
was used to analyze the target gene of miR-216a-3p, which showed
that Wnt3a was a target gene of miR-216a-3p (Fig. 3A). To investigate effects of Wnt3a
on oral cancer, two siRNAs against Wnt3a were used to knockdown the
gene and it was found that siRNAs could successfully inhibit mRNA
level of Wnt3a in HSC-6 cell line (P<0.05; P<0.01; Fig. 3B). siRNAs could successfully
inhibit the protein level of Wnt3a in HSC-6 cell line (Fig. 3C). Wnt3a knockdown attenuated
induction of miR-216a-3p inhibitors on apoptosis of oral cancer
cells in the HSC-6 cell line (P<0.05; P<0.01; Fig. 3D). It was also found that Wnt3a
knockdown attenuated the inhibitory effects of miR-216a-3p
inhibitors on cell viability of oral cancer cells in HSC-6 cells
(P<0.01; Fig. 3E). The two
Wnt3a siRNAs successfully inhibited the mRNA level of Wnt3a in the
CAL-27 cell line (P<0.05; Fig.
3F). siRNAs could successfully inhibit the protein level of
Wnt3a in CAL-27 cells (Fig. 3G).
Wnt3a knockdown attenuated induction of miR-216a-3p inhibitors on
apoptosis of oral cancer cells in CAL-27 cells (P<0.05;
P<0.01; Fig. 3H). Further,
Wnt3a knockdown attenuated the inhibitory effects of miR-216a-3p
inhibitors on cell viability of oral cancer cells in CAL-27 cells
(P<0.01; Fig. 3I). While miR126
does not affect mRNA expression of WNT3a in both HSC-6 (Fig. S1A) and CAL-27 cells (Fig. S1B), knockdown of WNT3a
significantly inhibits cell viability of HSC-6 cells (Fig. S1C) and CAL-27 cells (Fig. S1D), thus, the effects of
miR-216a-3p on oral cancer was through Wnt3a.
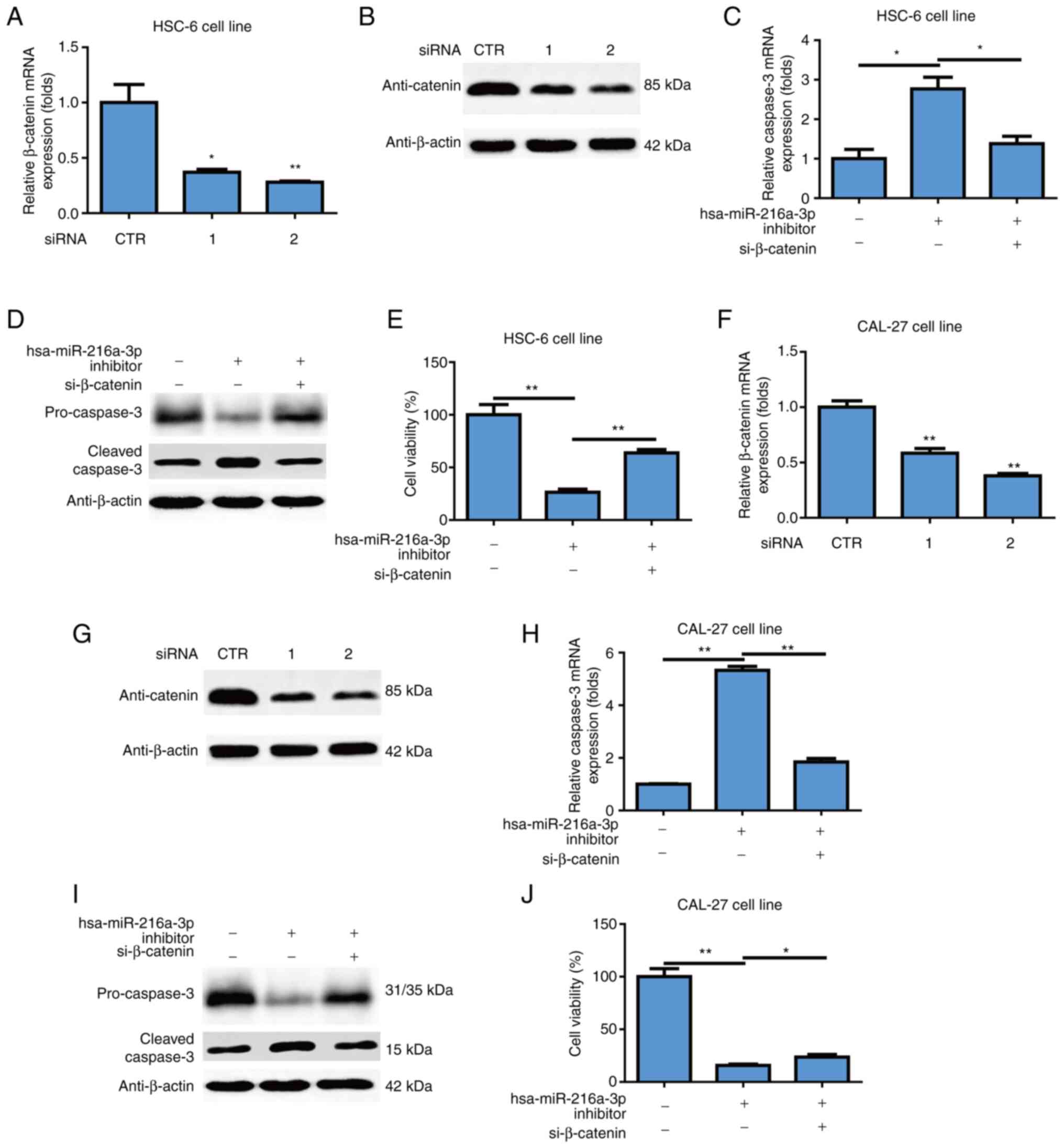
Expression level of β-catenin is
higher in oral cancer patients and positively correlated with tumor
stage
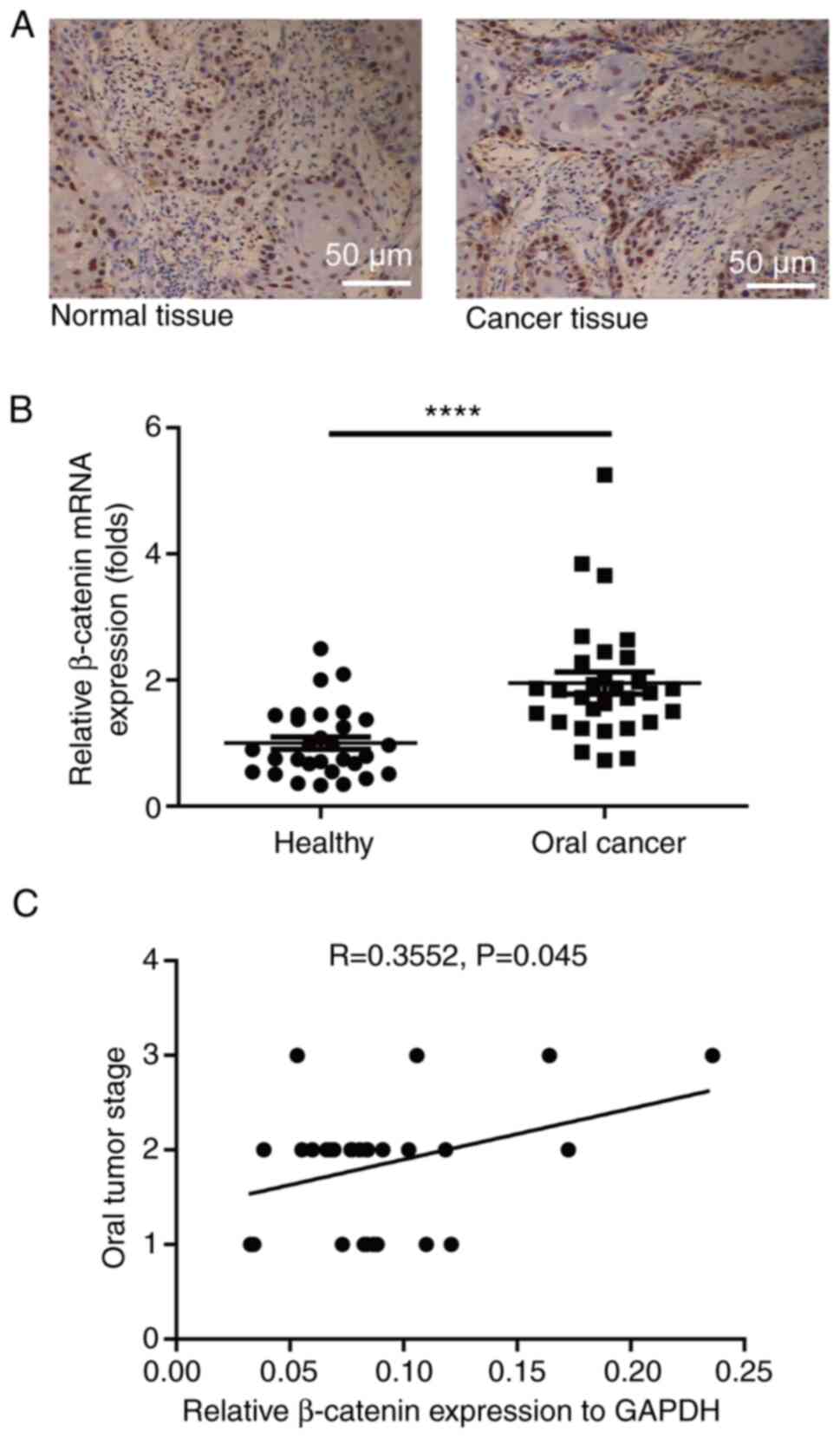
The present study measured the expression level of
miR-216a-3p in oral cancer patients and healthy controls using
RT-qPCR. The protein level of β-catenin in oral cancer patients was
much higher than in healthy controls, as measured using IHC method
(Fig. 4A). The mRNA expression
level of β-catenin in oral cancer patients was much higher than in
healthy controls (P<0.0001; Fig.
4B). Of note, it was found that the expression level of
β-catenin positively correlated with oral cancer stage (correlation
coefficient R=0.3552, P=0.045; Fig.
4C).
Effects of miR-216a-3p on oral cancer
through β-catenin
To further investigate the effects of β-catenin on
oral cancer, siRNAs against β-catenin were used and it was found
that siRNAs could successfully inhibit mRNA level of β-catenin in
the HSC-6 cell line (P<0.05; P<0.01; Fig. 5A). siRNAs could successfully
inhibit the protein level of β-catenin in HSC-6 cells (Fig. 5B). β-catenin knockdown attenuated
induction of miR-216a-3p inhibitors on mRNA level of caspase3 in
HSC-6 cells (*P<0.05; Fig. 5C).
β-catenin knockdown attenuated induction of miR-216a-3p inhibitors
on the protein level of cleaved caspase3 in HSC-6 cells and
decrease the protein level of pro-caspase 3 in HSC-6 cells
(Fig. 5D). Moreover, β-catenin
knockdown attenuated the inhibitory effects of miR-216a-3p
inhibitors on cell viability of oral cancer cells in the HSC-6 cell
line (P<0.01; Fig. 5E). The two
β-catenin siRNAs successfully inhibited the mRNA level of β-catenin
in the CAL-27 cell line (P<0.01; Fig. 5F). Two β-catenin siRNAs
successfully inhibited the protein level of β-catenin in CAL-27
cells (Fig. 5G). β-catenin
knockdown attenuated induction of miR-216a-3p inhibitors on mRNA
level of caspase3 in CAL-27 cells (P<0.01; Fig. 5H). β-catenin knockdown attenuated
induction of miR-216a-3p inhibitors on protein level of cleaved
caspase3 and decrease the protein level of pro-caspase 3 in CAL-27
cells (Fig. 5I). Furthermore,
β-catenin knockdown attenuated the inhibitory effects of
miR-216a-3p inhibitors on cell viability of oral cancer cells in
the CAL-27 cell line (P<0.05; P<0.01; Fig. 5J), while miR-126 does not affect
mRNA expression of β-catenin in HSC-6 cells (Fig. S1E) and CAL-27 cells (Fig. S1F).
Discussion
Oral cancer is a severe public health concern and is
widespread in a number of countries, especially developing
countries (17). More efforts are
needed to uncover the underlying pathogenesis and develop novel
drugs for treatment. miRNAs are considered important targets for
developing anti-cancer drugs. The Wnt signaling pathway serves an
important role in activities of cancer stem cells (18). In the present study, two oral
cancer lines, iHSC-6 and CAL-27, were used. Effects of miR-216a-3p
on oral cancer and the corresponding mechanism were
investigated.
As small single-stranded non-coding RNA molecules,
miRNAs have been confirmed to exert various functions such as cell
growth, apoptosis, development, differentiation and inflammation,
via RNA silencing and post-transcriptional regulation of gene
expression (19). An increasing
number of studies have confirmed that miRNAs are essential to
regulate the activities of a variety of tumors. Terkelsen et
al (20) found that miR-146a
and miR-494 are richly expressed in the tumor interstitial fluid of
breast cancer patients and several miRNAs are associated with tumor
grade. In another study, authors found that 8 of the 10 miRNAs
(miR-139-5p, miR-10b-5p, miR-486-5p, miR-455-3p, miR-107,
miR-146b-5p, miR-324-5p and miR-20a-5p) are highly correlated with
prognosis of breast cancer (21).
Grzelczyk et al (22) found
that the levels of serum expression of miR-31, miR-141, miR-149a,
miR-182, LET-7a, miR-4853p, miR-122 and miR-33 are upregulated,
which might be used to diagnose laryngeal squamous cell carcinoma
with high sensitivity and specificity. For oral cancer, Li et
al (23) found that miR-34a-5p
binds to its direct downstream target AXL to suppress oral squamous
cell carcinoma cell proliferation and metastasis. Cai et al
(24) found that exosome-enclosed
miR-29a-3p promotes tumor growth in nude mice with xenograft of
oral squamous cell carcinoma. Similarly, the present study found
that the expression level of miR-216a-3p was much higher in oral
cancer patients than healthy controls and positively correlated
with tumor grades. Thus, it appears that miR-216a-3p is a potential
biomarker for oral cancer. Notably, it was also found that
inhibition of miR-216a-3p potently inhibited cell viability and
induced apoptosis in oral cancer cell lines. The results showed the
important role of miR-216a-3p in oral cancer.
The Wnt signaling pathway is one of important
pathways controlling various biological activities (17). In cancer studies, accumulating
evidence has indicated that Wnt signaling serves an important role
in regulating cancer activities. In colorectal cancer (CRC) with
mutations of activated KRAS and SLC25A22, which indicates increased
DNA methylation, activation of Wnt signaling to β-catenin increased
expression of LGR5, proliferation, stem cell features and
resistance to 5-fluorouracil (25). In hepatocellular carcinoma, it was
found that activation of autophagy can induce MCT1 expression by
activating Wnt/β-catenin signaling to promote metastasis and
glycolysis (26). Regarding breast
carcinomas, it was found that increased relative mRNA expression
levels of Wnt3 were found in 54% cases (27). Deng et al (28) found that activation of Wnt
signaling can facilitate pancreatic cancer progression. The present
study found that Wnt was the target gene of miR-216a-3p. More
importantly, it was found that the effects of miR-216a-3p on oral
cancer were via Wnt3a. Li et al (29) found that serum β-catenin levels in
the CRP and CRC patients are significantly higher compared with
those in the healthy control group. Xu et al (30) found that β-catenin expression is
higher in hepatocellular carcinoma patients compared with that in
healthy controls and increased β-catenin expression is closely
correlated with tumor differentiation, tumor size, serum
α-fetoprotein level and transarterial chemoembolization treatment
frequency. The present study found that the expression level of
β-catenin was much higher in oral cancer patients compared with in
healthy controls and positively correlated with tumor stages. The
effect of miR-216a-3p on oral cancer was via β-catenin. Therefore,
the Wnt-β-catenin signaling pathway probably serves an important
role in oral cancer.
In conclusion, the present study demonstrated that
the expression level of miR-216a-3p was higher in oral cancer
patients compared with healthy controls and positively correlated
with tumor stage. Inhibition of miR-216a-3p could potently inhibit
the growth of oral cancer cells and this effect was through the
Wnt-β-catenin signaling pathway. β-Catenin expression level was
higher in oral cancer patients compared with healthy controls and
positively correlated with tumor stage. Therefore, miR-216a-3p and
Wnt-β-catenin signaling pathway might be appealing candidates for
the development of effective therapies for oral cancers. It is
considered that the findings of the present study will provide
useful insights for developing novel therapies of oral cancer.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Medical Science Research
Project of Hebei Provincial Healthcare Commission (grant no.
20221256).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, SL, YD, YQ, FL, WZ and WW performed the
experiments. WZ and WW designed the research. YW and SL wrote the
manuscript and YD and YQ supervised the project. YW and YQ confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures performed involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Approval was obtained from the ethical board of The
Fourth Hospital of Hebei Medical University (approval no.
2022KY392). Informed consent was obtained from all volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Montero PH and Patel SG: Cancer of the
oral cavity. Surg Oncol Clin N Am. 24:491–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neville BW and Day TA: Oral cancer and
precancerous lesions. CA Cancer J Clin. 52:195–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu M, Wang G, Tian W, Deng Y and Xu Y:
MiRNA-based therapeutics for lung cancer. Curr Pharm Des.
23:5989–5996. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
You F, Li J, Zhang P, Zhang H and Cao X:
miR106a promotes the growth of transplanted breast cancer and
decreases the sensitivity of transplanted tumors to cisplatin.
Cancer Manag Res. 12:233–246. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alhasan L: MiR-126 modulates angiogenesis
in breast cancer by targeting VEGF-A-mRNA. Asian Pac J Cancer Prev.
20:193–197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akbarzadeh M, Mihanfar A, Akbarzadeh S,
Yousefi B and Majidinia M: Crosstalk between miRNA and
PI3K/AKT/mTOR signaling pathway in cancer. Life Sci.
285:1199842021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majidinia M, Darband SG, Kaviani M, Nabavi
SM, Jahanban-Esfahlan R and Yousefi B: Cross-regulation between
Notch signaling pathway and miRNA machinery in cancer. DNA Repair
(Amst). 66–67. 30–41. 2018.PubMed/NCBI
|
|
8
|
Gajos-Michniewicz A and Czyz M: WNT
signaling in melanoma. Int J Mol Sci. 21:48522020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polakis P: WNT signaling in cancer. Cold
Spring Harb Perspect Biol. 4:a0080522012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhattacharya M, Sharma AR, Sharma G, Patra
BC, Lee SS and Chakraborty C: Interaction between miRNAs and
signaling cascades of Wnt pathway in chronic lymphocytic leukemia.
J Cell Biochem. 121:4654–4666. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Zhang M, Wei T, Zhou J, Zhang Y
and Guo D: Long non-coding RNA SNHG1 mediates neuronal damage in
Parkinson's disease model cells by regulating
miR-216a-3p/Bcl-2-associated X protein. Ann Transl Med. 9:8512021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang L and Kan L: Mesenchymal stem
cell-originated exosomal circular RNA circFBXW7 attenuates cell
proliferation, migration and inflammation of fibroblast-like
synoviocytes by targeting miR-216a-3p/HDAC4 in rheumatoid
arthritis. J Inflamm Res. 14:6157–6171. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao J, Li L and Yang T: MiR-216a-3p
suppresses the proliferation and invasion of cervical cancer
through downregulation of ACTL6A-mediated YAP signaling. J Cell
Physiol. 235:9718–9728. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang D, Li Y, Zhang C, Li X and Yu J:
MiR-216a-3p inhibits colorectal cancer cell proliferation through
direct targeting COX-2 and ALOX5. J Cell Biochem. 119:1755–1766.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin Y, Bijvelds M, Dang W, Xu L, van der
Eijk AA, Knipping K, Tuysuz N, Dekkers JF, Wang Y, de Jonge J, et
al: Modeling rotavirus infection and antiviral therapy using
primary intestinal organoids. Antiviral Res. 123:120–131. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Divisato G, Piscitelli S, Elia M, Cascone
E and Parisi S: MicroRNAs and stem-like properties: The complex
regulation underlying stemness maintenance and cancer development.
Biomolecules. 11:10742021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Terkelsen T, Russo F, Gromov P, Haakensen
VD, Brunak S, Gromova I, Krogh A and Papaleo E: Secreted breast
tumor interstitial fluid microRNAs and their target genes are
associated with triple-negative breast cancer, tumor grade, and
immune infiltration. Breast Cancer Res. 22:732020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong HC, Chuang CH, Huang WC, Weng SL,
Chen CH, Chang KH, Liao KW and Huang HD: A panel of eight microRNAs
is a good predictive parameter for triple-negative breast cancer
relapse. Theranostics. 10:8771–8789. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grzelczyk WL, Szemraj J, Kwiatkowska S and
Józefowicz-Korczyńska M: Serum expression of selected miRNAs in
patients with laryngeal squamous cell carcinoma (LSCC). Diagn
Pathol. 14:492019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li YY, Tao YW, Gao S, Li P, Zheng JM,
Zhang SE, Liang J and Zhang Y: Cancer-associated fibroblasts
contribute to oral cancer cells proliferation and metastasis via
exosome-mediated paracrine miR-34a-5p. EBioMedicine. 36:209–220.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai J, Qiao B, Gao N, Lin N and He W: Oral
squamous cell carcinoma-derived exosomes promote M2 subtype
macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am
J Physiol Cell Physiol. 316:C731–C740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong CC, Xu J, Bian X, Wu JL, Kang W, Qian
Y, Li W, Chen H, Gou H, Liu D, et al: In colorectal cancer cells
with mutant KRAS, SLC25A22-mediated glutaminolysis reduces DNA
demethylation to increase WNT signaling, stemness, and drug
resistance. Gastroenterology. 159:2163–2180.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong
L, Zong Z, Hua X, Su D, Li H and Liu J: Autophagy promotes
metastasis and glycolysis by upregulating MCT1 expression and
Wnt/β-catenin signaling pathway activation in hepatocellular
carcinoma cells. J Exp Clin Cancer Res. 37:92018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Michelli M, Zougros A, Chatziandreou I,
Michalopoulos NV, Lazaris AC and Saetta AA: Concurrent Wnt pathway
component expression in breast and colorectal cancer. Pathol Res
Pract. 216:1530052020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deng J, Zhang J, Ye Y, Liu K, Zeng L,
Huang J, Pan L, Li M, Bai R, Zhuang L, et al:
N6-methyladenosine-mediated upregulation of WTAPP1
promotes WTAP translation and Wnt signaling to facilitate
pancreatic cancer progression. Cancer Res. 81:5268–5283. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Huang M, Liu Q, Wang D, Wu R, Zhang
X, Chen W and Duan L: Serum expression of β-catenin is a potential
detection marker in patients with colorectal cancer. Dis Markers.
2019:50705242019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu X, Gao D, Yuan X, Liu LI, Zhang X,
Liang X, Chen S, Ai M, Chen BO, Shi D, et al: β-Catenin expression
correlates with prognosis in hepatocellular carcinoma patients
treated with transcatheter arterial chemoembolization. Anticancer
Res. 39:1129–1134. 2019. View Article : Google Scholar : PubMed/NCBI
|