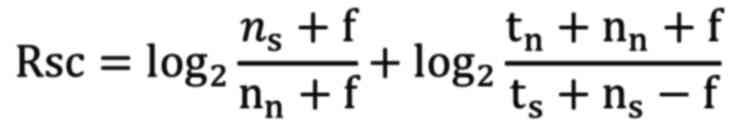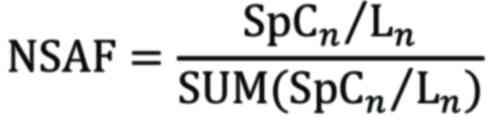Introduction
Nuclear cataracts usually develop slowly over time
and represent the most common form of age-related cataracts
globally (1,2). They are considered the primary form
of senile cataracts, leading to visual disturbances characterized
by increasing forward light scattering, higher-order aberrations,
and backward light scattering, even in the early stages (3). Heister (4) reported cataracts arising in
glassblowers due to infrared light in 1739. In addition, residents
of regions with warmer climates tend to experience presbyopia at an
earlier age (5).
A number of studies have investigated the
relationship between environmental temperature and the development
of nuclear cataracts, suggesting that prolonged exposure to hot
environments may increase the risk of developing this condition
(5–7). Moreover, as environmental temperature
increases, UV exposure generally increases, in turn damaging eye
tissue and increasing the risk of nuclear cataracts (8). Furthermore, exposure to hot
environments exposes ocular tissues to thermal stress, which can
lead to oxidative stress. Epidemiological studies have shown a
markedly higher prevalence of grade 1 or higher nuclear cataracts,
as per the World Health Organization cataract grading system, in
tropical and subtropical regions compared with temperate and
subarctic areas, irrespective of ethnicity (7,9). The
relationship between environmental temperature and the development
of nuclear cataracts is therefore complex, involving a myriad of
factors.
In our previous study, using a comprehensive
computational approach, it was observed that the temperature of the
eye lens may rise to 37.5°C or beyond with an increase in
environmental temperatures. By setting the lens temperature
threshold at 37.5°C, we found a positive correlation between
cumulative heat exposure, defined as the cumulative temperature
difference exceeding 37°C, and the incidence of nuclear cataracts
over a decade. Additionally, there was a connection between the
temporal pattern of mean lens temperature increase and the
incidence of nuclear cataracts (10). However, the precise relationship
between temperature and the development of nuclear cataracts
remains to be elucidated.
The present study applied a proteomics approach to
investigate the relationship between temperature and nuclear
cataracts in the present study. Shotgun liquid chromatography/mass
spectrometry-based global proteomic analysis has emerged as a
valuable technique for various applications (11–13).
Previously, we applied this technique to assess alterations in
protein expression within the cornea and lens of
streptozotocin-induced diabetic rats. The findings revealed that a
reduction in superoxide dismutase levels contributes to the
progression of diabetic cataracts. Additionally, upregulation of
lumican was observed, which resulted in delayed corneal wound
healing in corneas affected by diabetic keratopathy (14,15).
This shotgun proteomic approach was applied to investigate the
cataract-inducing factors in lens incubated at physiologically
normal and warmer ocular temperatures in the present study.
Materials and methods
Materials
Urea was obtained from Cytiva. Thiourea and Triton
X-100 were procured from Nacalai Tesque, Inc. All other reagents
and solvents used were of analytical or HPLC grade.
Animals
Wistar rats (male; age, 6 weeks; weight, 214±3 g;
n=3) were supplied by Sankyo Labo Service Corporation, Inc. All
experiments were conducted in accordance with the regulations
approved by the Ethics Committee of Kindai University Faculty of
Pharmacy (approval no. KAPS-2021-004, April 1, 2021). The rats were
kept in a room at 25°C and 55±10% humidity with a 12-h light/dark
cycle (3 rats per cage) with unlimited access to food and water.
The present study adhered to the ARRIVE guidelines (16) and the Guiding Principles sanctioned
by The Japanese Pharmacological Society (17). Euthanasia was performed by
administering pentobarbital (200 mg/kg, i.p.) in line with the AVMA
2020 guidelines (18).
Ex-vivo culture of rat lenses
All experiments and animal handling were conducted
in accordance with the Association for Research in Vision and
Ophthalmology Statement for the Use of Animals in Ophthalmic and
Vision Research (19). Male Wistar
rats purchased at 6 weeks of age were housed for 1 week, and those
aged 7 weeks were used for the experiments (weight, 231±7 g; n=3).
The three rats were housed in groups in suspended wire-bottomed
cages with unlimited access to food and water, maintained under a
12-h light/dark cycle (temperature, 25°C; humidity, 55±10%).
Euthanasia was performed by administering pentobarbital (200 mg/kg,
i.p.) in line with the AVMA 2020 guidelines (18). Lenses were then excised from the
rats and were cultured as previously described (20), with some modifications. In brief,
the six lenses extracted from the three rats were divided into two
groups (3 lenses/group) and cultured in serum-free M199 medium
(Thermo Fisher Scientific, Inc.), supplemented with 0.1% (w/v)
bovine serum albumin, 100 IU/ml penicillin, 100 µg/ml streptomycin,
and 250 ng/ml fungizone (Thermo Fisher Scientific, Inc.) at either
35.0°C or 37.5°C in a humidified atmosphere of 5% CO2
for 48 h.
Tryptic digestion of proteins
extracted from rat lenses
In the present study, three lenses were combined
into a single sample and were homogenized. The proteins were
extracted using RIPA Lysis Buffer (Santa Cruz Biotechnology, Inc.)
and the supernatant was collected following centrifugation at
13,000 × g at 4°C for 10 min. Protein concentrations were measured
using the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Inc.).
Gel-free trypsin digestion was performed following a previously
established protocol (21). In
brief, 10 µg of protein extract from each sample was reduced at
37°C for 30 min using tris (2-carboxyethyl) phosphine (20 mM) in
ammonium bicarbonate buffer (50 mM) and dithiothreitol (45 mM). The
proteins were then alkylated with iodoacetamide (100 mM) in
ammonium bicarbonate buffer (50 mM) at 37°C for 30 min. After
alkylation, the samples were digested at 37°C for 24 h with
MS-grade trypsin gold (Promega Corporation) at a trypsin/protein
ratio of 1:100 (w:w). Finally, the digested peptides were purified
using PepClean C-18 Spin Columns (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions.
Liquid chromatography with tandem mass
spectrometry (LC-MS/MS) identification of proteins
The semiquantitative analysis of protein expression
by using LC-MS/MS analysis were performed following method of
Kawamura et al (22).
Peptide samples (2 µg) were injected using a peptide L-trap column
(Chemicals Evaluation and Research Institute) with an HTC PAL
autosampler (CTC Analytics AG). The peptides were then separated on
a Paradigm MS4 system (AMR Inc.) fitted with a reverse-phase C18
column (Sunniest, 3 µm diameter gel particles, 120 Å pore size,
0.23×150 mm; Chromanik Technologies Inc.). The mobile phase
comprised 0.1% formic acid in water (solution A) and acetonitrile
(solution B), with a gradient increasing from 5–40% solution B over
120 min at a constant flow rate of 1 µl/min. The gradient-eluted
peptides were directed into the mass spectrometer via a
nanoelectrospray ionization (NSI) interface, with the separation
column outlet directly connected to the NSI needle. Peptide
analysis was performed using an LTQ Orbitrap XL mass spectrometer
(Thermo Fisher Scientific, Inc.) without sheath or auxiliary gas.
The mass spectrometry scan sequence involved a full-scan MS for
precursor ions over a 300–2,000 m/z range at a resolution of
60,000, followed by MS/MS in normal/centroid mode. Positive ion
mass spectra were acquired in a data-dependent manner, with MS/MS
fragmentation of the most intense peaks in each MS scan using
Collision-Induced Dissociation, and a dynamic exclusion window of
30 sec. All MS/MS data were analysed against the SwissProt Rattus
database selected from SwissProt_2022_01 (https://www.uniprot.org/uniprotkb?query=*&facets=reviewed%3Atrue),
which contains 566,996 peptides and 22,502 proteins using Mascot
version 2.4.01 (Matrix Science). The search parameters included
trypsin digestion with up to two missed cleavages, a mass tolerance
of ±30 ppm, an MS/MS tolerance of ±0.8 Da, cysteine
carbamidomethylation as a fixed modification and methionine
oxidation as a variable modification.
Semiquantitative analysis of
identified proteins
The data were presented as the cumulative values
from three measurements and the fold change in expression was
determined as the log2-transformed ratio of protein
abundance (Rsc) and assessed through spectral counting (23). The value of Rsc was determined
utilizing the subsequent equation:
Where, nn and ns represent
spectral counts for proteins in rat lenses cultured at 35.0°C and
37.5°C, respectively. The tn and ts denote
the total numbers of spectra for all proteins in the respective
samples (35.0°C and 37.5°C), respectively. The ƒ is a correction
factor set to 1.25.
For comparative analysis, relative protein abundance
was computed by the normalized spectral abundance factor (NSAF)
(24). The value of NSAF was
determined using the subsequent equation:
Where, SpCn refers to the spectral count of proteins
in rat lenses incubated at 35.0°C and 37.5°C, while Ln denotes the
length of these proteins in the lenses at the same temperatures.
Proteins with differential expression were identified when the
ratio Rsc was >1 or <-1, indicating fold changes >2 or
<0.5, respectively. Table SI
shows the raw data of semiquantitative analysis in the present
study.
Bioinformatics
The present study explored the role of proteins that
exhibit notable changes in expression in nuclear cataracts. Their
sequences were annotated using Gene Ontology (GO) terms related to
molecular function, cellular component and biological process, as
well as the Kyoto Encyclopedia of Genes and Genomes (KEGG)
signalling pathways, using the Database for Annotation,
Visualization, and Integrated Discovery (https://david.ncifcrf.gov/tools.jsp) (25–27).
The P-values from the GO analysis were also calculated using this
database tool.
Results
Changes in protein expression in
cultured rat lenses
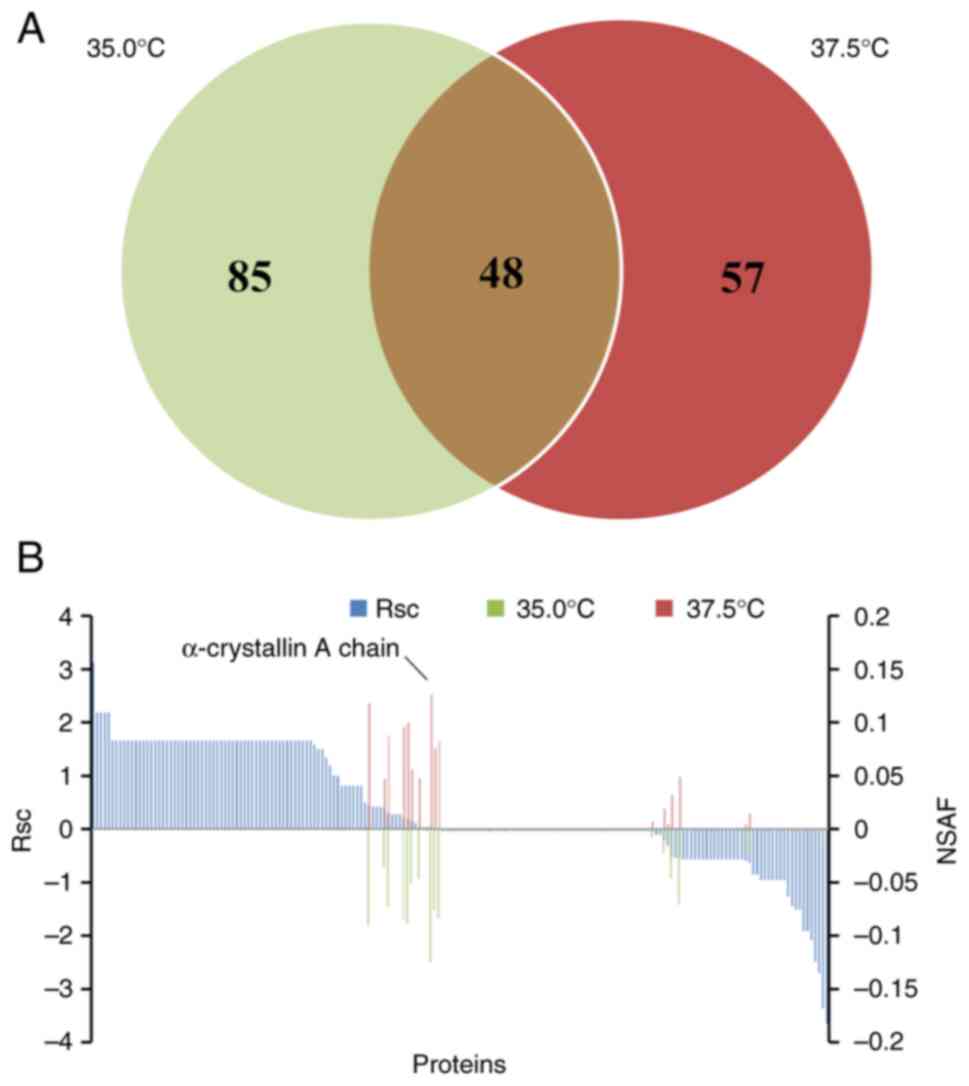
A total of 133 and 105 proteins were detected in the
rat lenses cultured at 35.0°C and 37.5°C, respectively. Of these,
190 proteins were identified, including 48 (25.3%) present in both
lenses cultured at 35.0°C and 37.5°C, 85 (44.7%) unique to those
cultured at 35.0°C, and 57 (30.0%) unique to those cultured at
37.5°C (Fig. 1A). The proteins
expressed in rat lenses were next assessed using a label-free
semi-quantitative approach based on spectral counting. Fig. 1B shows the Rsc values for proteins
identified in lenses cultured at 35.0°C and 37.5°C. A positive Rsc
value indicates increased expression in lenses cultured at 37.5°C,
while a negative Rsc value signifies reduced expression.
Additionally, the NSAF value was determined for each identified
protein at both temperatures. Proteins with Rsc values >1 or
<-1 were considered as candidates for differential regulation
depending on the culture temperature. The levels of housekeeping
proteins, such as α-crystallin A chain, remained unchanged across
different culture temperatures (Fig.
1B).
GO analysis was conducted on the candidate proteins
regulated in rat lenses cultured at 37.5°C. This involved searching
for GO terms associated with molecular function (Table I), cellular component (Table II) and biological process
(Table III), and KEGG pathways
(Table IV), with resultant
detected counts of 10, 17, 19 and 2, respectively. Among these, the
most abundant terms in each category were ‘protein binding,’
‘cytoplasm,’ ‘intermediate filament organization,’ and ‘estrogen
signaling pathway,’ respectively. In addition, a cutoff value for
spectral counting was set to 1, to exclude proteins with low
expression levels, and listed proteins with expression changes
following culture at 37.5°C showed Rsc >1 or <-1 in the
label-free semiquantitative method based on spectral counting
(Table V). A total of 22 proteins
showed Rsc >1 or <-1 and, at 37.5°C, the expression levels of
11 proteins was upregulated, while that of the other 11 was
downregulated. The present study focused on the downregulated
proteins at 37.5°C since they were more likely to be affected than
the overexpressed ones. In conjunction with GO analysis results,
the factor associated with both ‘protein binding’ and ‘intermediate
filament organization’ in the cytoplasm was filensin and vimentin.
No factors related to ‘estrogen signaling pathway’ were
identified.
 | Table I.GO analysis of identified proteins in
molecular function category. |
Table I.
GO analysis of identified proteins in
molecular function category.
| Molecular function
category | Relative abundance
(%) | P-value |
|---|
| Protein
binding | 23.0 | 0.001 |
| ATP binding | 18.9 | 0.006 |
| Structural
constituent of cytoskeleton | 8.11 |
0.065×10−5 |
| Ubiquitin protein
ligase binding | 8.11 | 0.010 |
| GTPase
activity | 6.76 | 0.037 |
| Structural
constituent of epidermis | 5.41 |
0.022×10−2 |
| Structural molecule
activity | 5.41 | 0.020 |
| Double-stranded RNA
binding | 4.05 | 0.043 |
| NAD binding | 4.05 | 0.029 |
| Keratin filament
binding | 2.70 | 0.024 |
 | Table II.GO analysis of identified proteins in
cellular component category. |
Table II.
GO analysis of identified proteins in
cellular component category.
| Cellular component
category | Relative abundance
(%) | P-value |
|---|
| Cytoplasm | 60.8 |
0.013×10−6 |
| Cytosol | 40.5 |
0.032×10−3 |
| Synapse | 12.2 | 0.008 |
| Intermediate
filament | 10.8 |
0.077×10−7 |
| Keratin
filament | 9.46 |
0.012×10−4 |
| Axon | 9.46 | 0.010 |
| Cytoskeleton | 8.11 | 0.015 |
| Cell periphery | 6.76 |
0.055×10−2 |
| Cornified
envelope | 6.76 |
0.042×10−3 |
| Cell body | 5.41 | 0.012 |
| Cell
projection | 5.41 | 0.027 |
| Lamellipodium | 5.41 | 0.031 |
| Chloride channel
complex | 4.05 | 0.014 |
| Filopodium | 4.05 | 0.034 |
| Intermediate
filament cytoskeleton | 4.05 | 0.019 |
| Phagocytic
vesicle | 4.05 | 0.036 |
| Mitotic spindle
microtubule | 2.70 | 0.050 |
 | Table III.GO analysis of identified proteins in
biological process category. |
Table III.
GO analysis of identified proteins in
biological process category.
| Biological process
category | Relative abundance
(%) | P-value |
|---|
| Intermediate
filament organization | 14.9 |
0.031×10−12 |
| Process positive
regulation of gene expression | 9.46 | 0.028 |
| Cell division | 8.11 | 0.002 |
| Epithelial cell
differentiation | 6.76 |
0.089×10−2 |
| Keratinization | 6.76 |
0.015×10−2 |
| Axonogenesis | 5.41 | 0.009 |
| Cell cycle | 5.41 | 0.023 |
| Mitotic cell
cycle | 5.41 | 0.014 |
| Neuron projection
development | 5.41 | 0.033 |
| Regulation of
protein localization | 5.41 | 0.008 |
| Collagen fibril
organization | 4.05 | 0.019 |
| Excitatory
postsynaptic potential | 4.05 | 0.047 |
| Glucose metabolic
process | 4.05 | 0.037 |
| Intermediate
filament-based process | 4.05 |
0.049×10−2 |
| Protein
deubiquitination | 4.05 | 0.039 |
| Response to
electrical stimulus | 4.05 | 0.022 |
| Sensory perception
of pain | 4.05 | 0.038 |
| Lens fiber cell
development | 2.70 | 0.033 |
| Regulation of
protein complex stability | 2.70 | 0.048 |
 | Table IV.Kyoto Encyclopedia of Genes and
Genomes pathway analysis of identified proteins. |
Table IV.
Kyoto Encyclopedia of Genes and
Genomes pathway analysis of identified proteins.
| Pathway
category | Relative abundance
(%) | P-values |
|---|
| Estrogen signaling
pathway | 8.11 |
0.048×10−2 |
| Staphylococcus
aureus infection | 6.76 | 0.001 |
 | Table V.Differentially expressed proteins in
the rat lenses incubated with 35.0°C and 37.5°C. |
Table V.
Differentially expressed proteins in
the rat lenses incubated with 35.0°C and 37.5°C.
|
|
|
|
| Spectral
counting |
|---|
| ID | Accession
number | Description | Number of amino
acids |
|
|---|
| 37.5°C | 35.0°C | Fold change,
Rsc |
|---|
| K1C27_RAT | Q6IFW8 | Keratin, type I
cytoskeletal 27 | 449 | 5 | 0 | 3.134039 |
| LPAR3_RAT | Q8K5E0 | Lysophosphatidic
acid receptor 3 | 354 | 2 | 0 | 2.188507 |
| PER1_RAT | Q8CHI5 | Period circadian
protein homolog 1 | 1,293 | 2 | 0 | 2.188507 |
| VPS4A_RAT | Q793F9 | Vacuolar protein
sorting-associated protein 4A | 437 | 2 | 0 | 2.188507 |
| PLCD4_RAT | Q62711 |
1-phosphatidylinositol 4,5-bisphosphate
phosphodiesterase delta-4 | 772 | 2 | 0 | 2.188507 |
| K1C14_RAT | Q6IFV1 | Keratin, type I
cytoskeletal 14 | 485 | 6 | 3 | 1.582128 |
| CLCN2_RAT | P35525 | Chloride channel
protein 2 | 907 | 4 | 2 | 1.502479 |
| K2C1B_RAT | Q6IG01 | Keratin, type II
cytoskeletal 1b | 519 | 4 | 2 | 1.502479 |
| ACNT2_RAT | Q5FVR5 | Acyl-coenzyme A
amino acid N-acyltransferase 2 | 418 | 2 | 1 | 1.340108 |
| UCHL1_RAT | Q00981 | Ubiquitin
carboxyl-terminal hydrolase isozyme L1 | 223 | 3 | 2 | 1.196919 |
| K2C1_RAT | Q6IMF3 | Keratin, type II
cytoskeletal 1 | 625 | 15 | 13 | 1.003428 |
| K1C17_RAT | Q6IFU8 | Keratin, type I
cytoskeletal 17 | 433 | 0 | 4 | −1.263412 |
| BFSP1_RAT | Q02435 | Filensin
(Fragment) | 617 | 3 | 19 | −1.449344 |
| K2C5_RAT | Q6P6Q2 | Keratin, type II
cytoskeletal 5 | 576 | 2 | 15 | −1.517975 |
| TBB3_RAT | Q4QRB4 | Tubulin β-3
chain | 450 | 0 | 5 | −1.515353 |
| K1C15_RAT | Q6IFV3 | Keratin, type I
cytoskeletal 15 | 447 | 0 | 7 | −1.916696 |
| K2C7_RAT | Q6IG12 | Keratin, type II
cytoskeletal 7 | 457 | 0 | 7 | −1.916696 |
| TBB5_RAT | P69897 | Tubulin β-5
chain | 444 | 0 | 8 | −2.082158 |
| VIME_RAT | P31000 | Vimentin | 466 | 0 | 11 | −2.488624 |
| ACTC_RAT | P68035 | Actin, α cardiac
muscle 1 | 377 | 0 | 13 | −2.707610 |
| FABP5_RAT | P55053 | Fatty acid-binding
protein 5 | 135 | 1 | 39 | −3.367463 |
| ACTG_RAT | P63259 | Actin, cytoplasmic
2 | 375 | 0 | 26 | −3.648159 |
Discussion
We previously reported that computer simulation
analyses using a supercomputer and a biothermal transport equation
have shown a strong relationship between the cumulative thermal
dose estimation in the crystalline lens and the prevalence of
nuclear cataracts (10). However,
the precise relationship between temperature and nuclear cataracts
remains unclear. The present study investigated the
cataract-inducing factors associated with physiologically normal
and warmer ocular temperatures using a shotgun proteomic approach
and found a decrease in filensin and vimentin and an increase in
chloride channel protein 2 under the latter temperature
(37.5°C).
Temperature is crucial in the formation of nuclear
cataracts. In this regard, we previously reported that simulated
lens temperatures ranged from 35.0–37.5°C, which corresponds to
ambient eye temperatures of 19–35°C in a typical setting Based on
these results, the experimental temperatures used in the present
study were 35.0°C and 37.5°C, mirroring lens temperatures in
vivo.
Although the quantitative values obtained via
spectral counting may not be accurate, they reflect expression
discrepancies and have been used in previous studies investigating
novel diagnostic biomarkers and biological mechanisms (12,14,15,28–31).
Thus, the present proteome analysis was semi-quantitative and, for
quantitative analysis, more detailed investigations such as western
blotting are essential. However, the present study aimed to
elucidate how temperature changes, as determined by computer
simulation analyses using a supercomputer and a biothermal
transport equation, are related to cataract formation by conducting
a comprehensive proteome analysis. Therefore, this method was
chosen for its ability to provide insights into the connection
between temperature changes and cataract development. First, the
changes in proteins expression from rat lenses cultured at 35.0°C
and 37.5°C were identified and their functions scrutinized through
analysis against four GO terms. GO analysis revealed that the most
prevalent factors identified in the cellular component, biological
Process and molecular function categories were ‘cytoplasm,’
‘intermediate filament organization’ and ‘protein binding’
respectively. In addition, the effect on the expression system is
generally more pronounced when a protein is underexpressed than
when it is overexpressed. Taken together, the present study
identified factors that satisfied two criteria: A significant
number of proteins expressed in response to temperature changes and
reduced protein expression levels to half or less at 37.5°C. As a
result, the factors associated with filensin and vimentin were
identified in the ‘cytoplasm’ category and were related to both
‘intermediate filament organization’ and ‘protein binding.’
Therefore, variations in the expression of filensin and vimentin
were the focus of the present study.
Decreased filensin expression was observed at
37.5°C. Filensin is an important protein for maintaining water
balance in the lens and its decrease affects lens function.
Moreover, filensin interacts with aquaporin 0 and is involved in
water transport and retention (32). Reduced filensin expression may
weaken this interaction, resulting in reduced water transport and
lens regulation. Thus, reduced expression of filensin and its
weakened interaction with aquaporin 0 under temperature conditions
>35°C may disrupt the water balance within the lens and cause
over-entry of water. This may be the key mechanism associated with
lens dysfunction and pathology. On the other hand, it is known that
phakinin often works in concert with filensin (33). Therefore, the changes in expression
of phakinin protein was also measured. The Rsc value of phakinin
was −0.85 and phakinin also decreased at 37.5°C.
To prevent the excessive entry of water into the
lens, the expression of voltage-dependent chloride channel protein
2 may be increased. Voltage-dependent chloride channel protein 2
plays an important role in regulating intracellular and
extracellular salt concentration, as well as intracellular water
content (34). Therefore, its
increased expression is expected to regulate the amount and balance
of water in lens cells and prevent excessive water entry. Through
this mechanism, water balance within the lens may be maintained and
damage to the lens and disease progression may be inhibited. In the
present study, voltage-dependent chloride channel protein 2
expression was enhanced; therefore, decreased filensin expression
may be related to water transport abnormalities, potentially
compensated for by upregulating the expression of voltage-dependent
chloride channel protein 2, which is responsible for expelling
excess water from the lenses.
In addition, the expression of vimentin in
37.5°C-incubated lenses was lower than that in those incubated at
35.0°C. Vimentin is an intracellular, intermediate-diameter
filament that plays an important role in maintaining cell
morphology and motility. In particular, it plays an important role
in intraocular tissues such as lens fibroblasts and dendritic cells
(35,36). It is possible that decreased
vimentin expression results in alterations in the intracellular
skeleton within the lens. This may alter lens cell morphology and
function and increase the risk of developing nuclear cataracts
(35,36). Thus, reduced vimentin expression is
associated with the development of nuclear cataracts and this
mechanism may be primarily due to changes in the intracellular
skeleton within the lens.
It is important to investigate whether overexpressed
proteins and other decreased proteins at 37.5°C are related to lens
dysfunction. Further studies are required to investigate the
relationship between the onset of nuclear cataracts and changes in
vimentin and filensin levels. Additionally, the present proteome
analysis is semi-quantitative and more detailed investigations such
as western blotting are essential in a further study. Therefore,
the authors are planning to determine the localization and
expression of vimentin and filensin at 37.5°C using western
blotting and immunostaining.
In conclusion, shotgun proteomic analysis revealed
that increased ocular temperatures decrease the expression of
filensin and vimentin in rat lenses. The present study may serve as
a valuable indicator for elucidating the relationship between
temperature and onset of nuclear cataracts. Nonetheless, additional
research, such as performing western blotting and immunostaining,
is necessary to elucidate the molecular mechanisms that underpin
the relationships among these factors.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The proteomics data generated in the present study
may be found in the jPOSTrepo under accession number PXD056067 or
at the following URL: https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD056067.
The other data generated in the present study may be requested from
the corresponding author.
Author's contributions
The experiments were conducted by HO, SM, and NN.
Data analysis and interpretation were performed by HO, SM, TY, YM,
YN and NY. TY, YM, YN and NY further investigated the experimental
methodologies. AT, HS, and NN made substantial contributions to the
conceptualization and design of the present study. AT, HS, and NN
devised the experimental framework and provided the final approval
for the publication of the manuscript. NN, HO and TY confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the regulations approved by the Ethics Committee of the Kindai
University Faculty of Pharmacy (approval no. KAPS-2021-004; April
1, 2021). The present study adhered to the ARRIVE guidelines and
the Guiding Principles endorsed by The Japanese Pharmacological
Society. Animal sacrifice was performed following the AVMA 2020
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GO
|
Gene Ontology
|
|
LC-MS/MS
|
liquid chromatography with tandem mass
spectrometry
|
|
NSAF
|
normalized spectral abundance
factor
|
|
NSI
|
nanoelectrospray ionization
|
|
Rsc
|
log2-transformed ratio of
protein abundances
|
References
|
1
|
Vashist P, Talwar B, Gogoi M, Maraini G,
Camparini M, Ravindran RD, Murthy GV, Fitzpatrick KE, John N,
Chakravarthy U, et al: Prevalence of cataract in an older
population in India: The India study of age-related eye disease.
Ophthalmology. 118:272–278. e1–2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klein BE, Klein R and Lee KE: Incidence of
age-related cataract over a 10-year interval: The Beaver Dam Eye
Study. Ophthalmology. 109:2052–2057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee J, Kim MJ and Tchah H: Higher-order
aberrations induced by nuclear cataract. J Cataract Refract Surg.
34:2104–2109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heister L: Institutiones Chirurgicae. I.
Janssonius-Waesbergius; Amsterdam: pp. 5981739
|
|
5
|
Miranda MN: The geographic factor in the
onset of presbyopia. Trans Am Ophthalmol Soc. 77:603–621.
1979.PubMed/NCBI
|
|
6
|
Sasaki H, Shui YB, Kojima M, Chew SJ, Ono
M, Katoh N, Cheng HM, Takahashi N and Sasaki K: Characteristics of
cataracts in the Chinese Singaporean. J Epidemiol. 11:16–23. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki K, Sasaki H, Jonasson F, Kojima M
and Cheng HM: Racial differences of lens transparency properties
with aging and prevalence of age-related cataract applying a WHO
classification system. Ophthalmic Res. 36:332–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasaki H, Jonasson F, Shui YB, Kojima M,
Ono M, Katoh N, Cheng HM, Takahashi N and Sasaki K: High prevalence
of nuclear cataract in the population of tropical and subtropical
areas. Dev Ophthalmol. 35:60–69. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyashita H, Hatsusaka N, Shibuya E, Mita
N, Yamazaki M, Shibata T, Ishida H, Ukai Y, Kubo E and Sasaki H:
Association between ultraviolet radiation exposure dose and
cataract in Han people living in China and Taiwan: A
cross-sectional study. PLoS One. 14:e02153382019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kodera S, Hirata A, Miura F, Rashed EA,
Hatsusaka N, Yamamoto N, Kubo E and Sasaki H: Model-based approach
for analyzing prevalence of nuclear cataracts in elderly residents.
Comput Biol Med. 126:1040092020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joseph R, Srivastava OP and Pfister RR:
Differential epithelial and stromal protein profiles in keratoconus
and normal human corneas. Exp Eye Res. 92:282–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto T, Kudo M, Peng WX and Naito Z:
Analysis of protein expression regulated by lumican in PANC-1 cells
using shotgun proteomics. Oncol Rep. 30:1609–1621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meade ML, Shiyanov P and Schlager JJ:
Enhanced detection method for corneal protein identification using
shotgun proteomics. Proteome Sci. 7:232009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagai N, Yamamoto T, Mitamura K and Taga
A: Proteomic profile of the lens in a streptozotocin-induced
diabetic rat model using shotgun proteomics. Biomed Rep. 7:445–450.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto T, Otake H, Hiramatsu N, Yamamoto
N, Taga A and Nagai N: A proteomic approach for understanding the
mechanisms of delayed corneal wound healing in diabetic keratopathy
using diabetic model rat. Int J Mol Sci. 19:36352018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Biol. 18:e30004102020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
The Japanese Pharmacological Society, .
The Japanese Pharmacological Society Guidlones for Animal
Experiments. Available from:. https://pharmacol.or.jp/cms/wp-content/uploads/2020/03/animal.pdfSeptember
4–2024
|
|
18
|
Leary S, Underwood W, Anthony R, Cartner
S, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R,
Miller D, et al: AVMA Guidelines for the Euthanasia of Animals.
American Veterinary Medical Association; Schaumburg, IL: 2020
|
|
19
|
The Association for Research in Vision and
Ophthalmology, . ARVO Statement for the Use of Animals in
Ophthalmic and Vision Research. Available from:. https://www.arvo.org/globalassets/arvo/advocacy/advocacy-resources/other-toolkits/updated-arvo-statement-_revised_dec_2021.pdfSeptember
4–2024
|
|
20
|
Hales AM, Chamberlain CG and McAvoy JW:
Cataract induction in lenses cultured with transforming growth
factor-beta. Invest Ophthalmol Vis Sci. 36:1709–1713.
1995.PubMed/NCBI
|
|
21
|
Bluemlein K and Ralser M: Monitoring
protein expression in whole-cell extracts by targeted label-and
standard-free LC-MS/MS. Nat Protoc. 6:859–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawamura T, Nomura M, Tojo H, Fujii K,
Hamasaki H, Mikami S, Bando Y, Kato H and Nishimura T: Proteomic
analysis of laser-microdissected paraffin-embedded tissues: (1)
Stage-related protein candidates upon non-metastatic lung
adenocarcinoma. J Proteomics. 73:1089–1099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Old WM, Meyer-Arendt K, Aveline-Wolf L,
Pierce KG, Mendoza A, Sevinsky JR, Resing KA and Ahn NG: Comparison
of label-free methods for quantifying human proteins by shotgun
proteomics. Mol Cell Proteomics. 4:1487–1502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zybailov B, Coleman MK, Florens L and
Washburn MP: Correlation of relative adundance ratios derived from
peptide ion chromatograms and spectrum counting for quantitative
proteomic analysis using stable isotope labeling. Anal Chem.
77:6218–6224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:R602003.
View Article : Google Scholar
|
|
26
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang da W, Sherman BT, Zheng X, Yang J,
Inamichi T, Stephens R and Lempicki RA: Extracting biological
meaning from large gene lists with DAVID. Curr Protoc
Bioinformatics Chapter. 13:Unit13.11. 2009.PubMed/NCBI
|
|
28
|
Takaya A, Peng WX, Ishino K, Kudo M,
Yamamoto T, Wada R, Takeshita T and Naito Z: Cystatin B as a
potential diagnostic biomarker in ovarian clear cell carcinoma. Int
J Oncol. 46:1573–1581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanzaki A, Kudo M, Ansai S, Peng WX,
Ishino K, Yamamoto T, Wada R, Fujii T, Teduka K, Kawahara K, et al:
Insulin-like growth factor 2 mRNA-binding protein-3 as a marker for
distinguishing between cutaneous squamous cell carcinoma and
keratoacanthoma. Int J Oncol. 48:1007–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto T, Kudo M, Peng WX, Takata H,
Takakura H, Teduka K, Fujii T, Mitamura K, Taga A, Uchida E and
Naito Z: Identification of aldolase A as a potential diagnostic
biomarker for colorectal cancer based on proteomic analysis using
formalin-fixed paraffin-embedded tissue. Tumor Biol.
37:13595–13606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takata H, Kudo M, Yamamoto T, Ueda J,
Ishino K, Peng WX, Wada R, Taniai N, Yoshida H, Uchida E and Naito
Z: Increased expression of PDIA3 and its association with cancer
cell proliferation and poor prognosis in hepatocellular carcinoma.
Oncol Lett. 12:4896–4904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakazawa Y, Oka M, Furuki K, Mitsuishi A,
Nakashima E and Takehana M: The effect of the interaction between
aquaporin 0 (AQP0) and the filensin tail region on AQP0 water
permeability. Mol Vis. 17:3191–3199. 2011.PubMed/NCBI
|
|
33
|
Tashiro M, Nakamura A, Kuratani Y, Takada
M, Iwamoto S, Oka M and Ando S: Effects of truncations in the N-
and C-terminal domains of filensin on filament formation with
phakinin in cell-free conditions and cultured cells. FEBS Open Bio.
13:1990–2004. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thiemann A, Gründer S, Pusch M and Jentsch
TJ: A chloride channel widely expressed in epithelial and
non-epithelial cells. Nature. 356:57–60. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Müller M, Bhattacharya SS, Moore T,
Prescott Q, Weding T, Hermann H and Magin TM: Dominant cataract
formation in association with a vimentin assembly disrupting
mutation. Hum Mol Genet. 18:1052–1057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsuyama M, Tanaka H, Inoko A, Goto H,
Yonemura S, Kobori K, Hayashi Y, Kondo E, Itohara S, Izawa I and
Inagaki M: Defect of mitotic vimentin phosphorylation causes
microophthalmia and cataract via aneuploidy and senescence in lens
epithelial cells. J Biol Chem. 288:35626–35635. 2013. View Article : Google Scholar : PubMed/NCBI
|