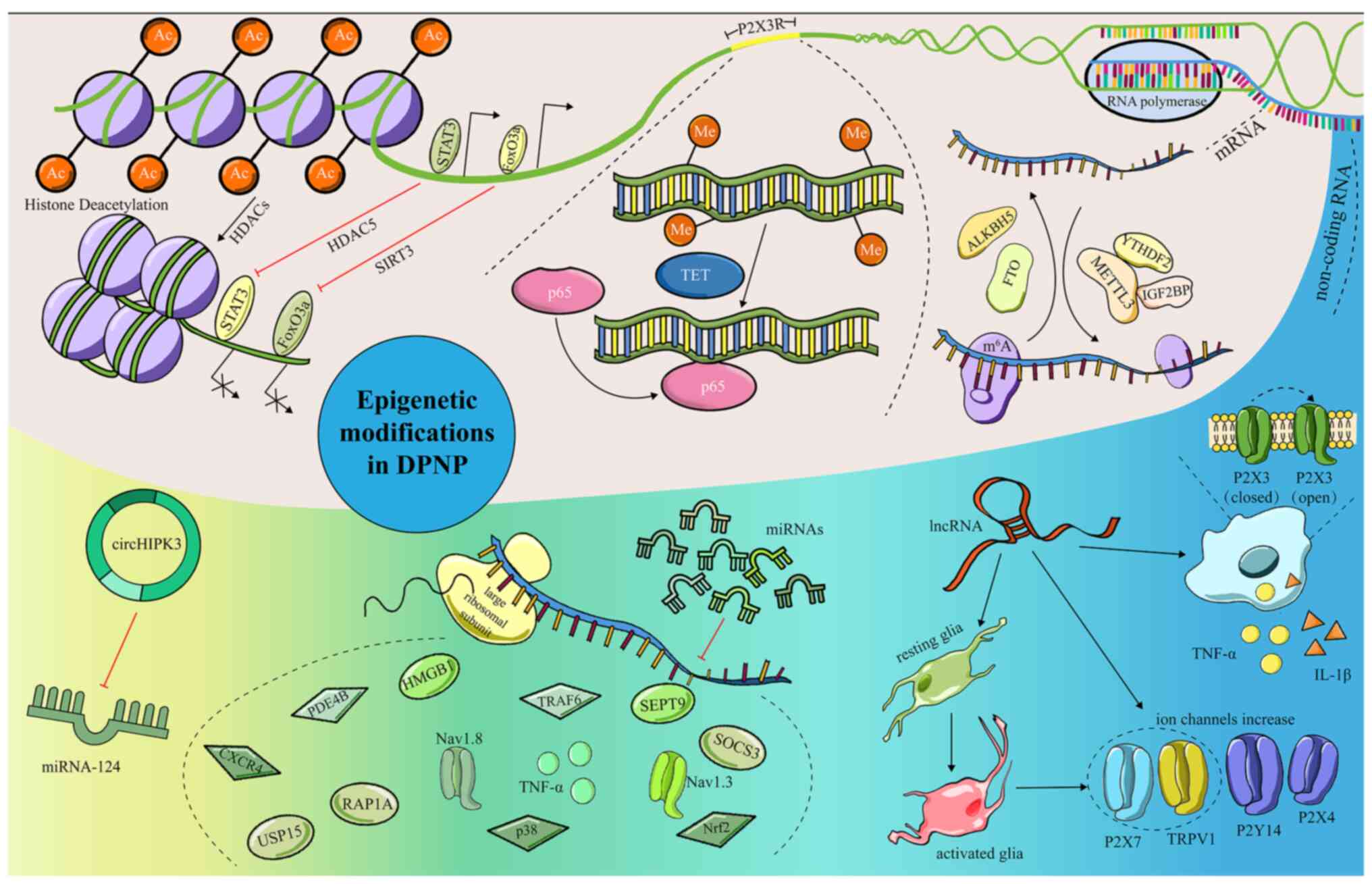
|
1
|
Behl T, Gupta A, Sehgal A, Sharma S, Singh
S, Sharma N, Diaconu CC, Rahdar A, Hafeez A, Bhatia S, et al: A
spotlight on underlying the mechanism of AMPK in diabetes
complications. Inflamm Res. 70:939–957. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abbott CA, Malik RA, van Ross ERE,
Kulkarni J and Boulton AJM: Prevalence and characteristics of
painful diabetic neuropathy in a large community-based diabetic
population in the U.K. Diabetes Care. 34:2220–2224. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feldman EL, Nave KA, Jensen TS and Bennett
DLH: New horizons in diabetic neuropathy: Mechanisms,
bioenergetics, and pain. Neuron. 93:1296–1313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sloan G, Selvarajah D and Tesfaye S:
Pathogenesis, diagnosis and clinical management of diabetic
sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 17:400–420.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frank T, Nawroth P and Kuner R:
Structure-function relationships in peripheral nerve contributions
to diabetic peripheral neuropathy. Pain. 160 (Suppl 1):S29–S36.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tesfaye S, Sloan G, Petrie J, White D,
Bradburn M, Julious S, Rajbhandari S, Sharma S, Rayman G, Gouni R,
et al: Comparison of amitriptyline supplemented with pregabalin,
pregabalin supplemented with amitriptyline, and duloxetine
supplemented with pregabalin for the treatment of diabetic
peripheral neuropathic pain (OPTION-DM): A multicentre,
double-blind, randomised crossover trial. Lancet. 400:680–690.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berger SL, Kouzarides T, Shiekhattar R and
Shilatifard A: An operational definition of epigenetics. Genes Dev.
23:781–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Shen X, Xu Y, Xu S, Xia F, Zhu B,
Liu Y, Wang W, Wu H and Wang F: The etiological changes of
acetylation in peripheral nerve injury-induced neuropathic
hypersensitivity. Mol Pain. 14:17448069187984082018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khangura RK, Bali A, Jaggi AS and Singh N:
Histone acetylation and histone deacetylation in neuropathic pain:
An unresolved puzzle? Eur J Pharmacol. 795:36–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lutz BM, Bekker A and Tao YX: Noncoding
RNAs: New players in chronic pain. Anesthesiology. 121:409–417.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang L, Lutz BM, Bekker A and Tao YX:
Epigenetic regulation of chronic pain. Epigenomics. 7:235–245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo D, Li X, Tang S, Song F, Li W, Xie G,
Liang J and Zhou J: Epigenetic modifications in neuropathic pain.
Mol Pain. 17:174480692110567672021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Brien PD, Sakowski SA and Feldman EL:
Mouse models of diabetic neuropathy. ILAR J. 54:259–272. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Devendra D, Liu E and Eisenbarth GS: Type
1 diabetes: Recent developments. BMJ. 328:750–754. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eleazu CO, Eleazu KC, Chukwuma S and
Essien UN: Review of the mechanism of cell death resulting from
streptozotocin challenge in experimental animals, its practical use
and potential risk to humans. J Diabetes Metab Disord. 12:602013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agarwal N, Helmstädter J, Rojas DR, Bali
KK, Gangadharan V and Kuner R: Evoked hypoalgesia is accompanied by
tonic pain and immune cell infiltration in the dorsal root ganglia
at late stages of diabetic neuropathy in mice. Mol Pain.
14:17448069188179752018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Obrosova IG, Ilnytska O, Lyzogubov VV,
Pavlov IA, Mashtalir N, Nadler JL and Drel VR: High-fat diet
induced neuropathy of pre-diabetes and obesity: Effects of
‘healthy’ diet and aldose reductase inhibition. Diabetes.
56:2598–2608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moore LD, Le T and Fan G: DNA methylation
and its basic function. Neuropsychopharmacology. 38:23–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun L, Gu X, Pan Z, Guo X, Liu J, Atianjoh
FE, Wu S, Mo K, Xu B, Liang L, et al: Contribution of DNMT1 to
neuropathic pain genesis partially through epigenetically
repressing Kcna2 in primary afferent neurons. J Neurosci.
39:6595–6607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Zhao X, Wang J, Jin Y, Gong M, Ye
Y and Li P: METTL3 suppresses neuropathic pain via modulating
N6-methyladenosine-dependent primary miR-150 processing. Cell Death
Discov. 8:802022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jang JH, Song EM, Do YH, Ahn S, Oh JY,
Hwang TY, Ryu Y, Jeon S, Song MY and Park HJ: Acupuncture
alleviates chronic pain and comorbid conditions in a mouse model of
neuropathic pain: The involvement of DNA methylation in the
prefrontal cortex. Pain. 162:514–530. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Tao W, Hou YY, Wang W, Kenny PJ
and Pan ZZ: MeCP2 repression of G9a in regulation of pain and
morphine reward. J Neurosci. 34:9076–9087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weng YL, An R, Cassin J, Joseph J, Mi R,
Wang C, Zhong C, Jin SG, Pfeifer GP, Bellacosa A, et al: An
intrinsic epigenetic barrier for functional axon regeneration.
Neuron. 94:337–346.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen W, Lan T, Sun Q, Zhang Y, Shen D, Hu
T, Liu J, Chong Y, Wang P, Li Q, et al: Whole genomic DNA
methylation profiling of CpG sites in promoter regions of dorsal
root ganglion in diabetic neuropathic pain mice. J Mol Neurosci.
71:2558–2565. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang HH, Hu J, Zhou YL, Qin X, Song ZY,
Yang PP, Hu S, Jiang X and Xu GY: Promoted interaction of nuclear
factor-κB with demethylated purinergic P2X3 receptor gene
contributes to neuropathic pain in rats with diabetes. Diabetes.
64:4272–4284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen W, Wang X, Sun Q, Zhang Y, Liu J, Hu
T, Wu W, Wei C, Liu M, Ding Y, et al: The upregulation of NLRP3
inflammasome in dorsal root ganglion by ten-eleven translocation
methylcytosine dioxygenase 2 (TET2) contributed to diabetic
neuropathic pain in mice. J Neuroinflammation. 19:3022022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Penas C and Navarro X: Epigenetic
modifications associated to neuroinflammation and neuropathic pain
after neural trauma. Front Cell Neurosci. 12:1582018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bannister AJ and Kouzarides T: Regulation
of chromatin by histone modifications. Cell Res. 21:381–395. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Black JC, Van Rechem C and Whetstine JR:
Histone lysine methylation dynamics: Establishment, regulation, and
biological impact. Mol Cell. 48:491–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Jiao B, Yu S, Zhang C, Zhang K,
Liu B and Zhang X: Histone deacetylase as emerging pharmacological
therapeutic target for neuropathic pain: From epigenetic to
selective drugs. CNS Neurosci Ther. 30:e147452024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morales S, Monzo M and Navarro A:
Epigenetic regulation mechanisms of microRNA expression. Biomol
Concepts. 8:203–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qi R, Cao J, Sun Y, Li Y, Huang Z, Jiang
D, Jiang XH, Snutch TP, Zhang Y and Tao J: Histone
methylation-mediated microRNA-32-5p down-regulation in sensory
neurons regulates pain behaviors via targeting Cav3.2 channels.
Proc Natl Acad Sci USA. 119:e21172091192022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan T, Yu Y, Chen YL, Gu P, Wong S, Xia
ZY, Liu JA and Cheung CW: Histone deacetylase 5-induced deficiency
of signal transducer and activator of transcription-3 acetylation
contributes to spinal astrocytes degeneration in painful diabetic
neuropathy. Glia. 71:1099–1119. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou C, Zhang Y, Jiao X, Wang G, Wang R
and Wu Y: SIRT3 alleviates neuropathic pain by deacetylating FoxO3a
in the spinal dorsal horn of diabetic model rats. Reg Anesth Pain
Med. 46:49–56. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding H, Wang F, Shi X, Ma H, Du Y, Hou L
and Xing N: LncRNA MALAT1 induces the dysfunction of β cells via
reducing the histone acetylation of the PDX-1 promoter in type 1
diabetes. Exp Mol Pathol. 114:1044322020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thakur V, Gonzalez MA, Parada M, Martinez
RD and Chattopadhyay M: Role of histone deacetylase inhibitor in
diabetic painful neuropathy. Mol Neurobiol. 61:2283–2296. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Elsherbiny NM, Ahmed E, Kader GA,
Abdel-Mottaleb Y, ElSayed MH, Youssef AM and Zaitone SA: Inhibitory
effect of valproate sodium on pain behavior in diabetic mice
involves suppression of spinal histone deacetylase 1 and
inflammatory mediators. Int Immunopharmacol. 70:16–27. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Michelson D, Chin WW, Dworkin RH, Freeman
R, Herrmann DN, Mazitschek R, Pop-Busui R, Shaibani A, Vornov J,
Jones M, et al: A randomized, double-blind, placebo-controlled
study of histone deacetylase type 6 inhibition for the treatment of
painful diabetic peripheral neuropa. Pain Rep. 8:e11142023.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roundtree IA, Evans ME, Pan T and He C:
Dynamic RNA modifications in gene expression regulation. Cell.
169:1187–1200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bokar JA, Shambaugh ME, Polayes D, Matera
AG and Rottman FM: Purification and cDNA cloning of the
AdoMet-binding subunit of the human mRNA
(N6-adenosine)-methyltransferase. RNA. 3:1233–1247. 1997.PubMed/NCBI
|
|
41
|
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han
D, Fu Y, Parisien M, Dai Q, Jia G, et al:
N6-methyladenosine-dependent regulation of messenger RNA stability.
Nature. 505:117–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Smith PR and Campbell ZT: RNA-binding
proteins in pain. Wiley Interdiscip Rev RNA. 15:e18432024.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu T, Wang J, Wu Y, Wu JY, Lu WC, Liu M,
Zhang SB, Xie D, Xin WJ and Xie JD: Ac4C enhances the translation
efficiency of vegfa mRNA and mediates central sensitization in
spinal dorsal horn in neuropathic pain. Adv Sci (Weinh).
10:e23031132023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Q, Weng W, Gu X, Xiang J, Yang Y,
Zhu MX, Gu W, He Z and Li Y: hnRNPA1 SUMOylation promotes cold
hypersensitivity in chronic inflammatory pain by stabilizing TRPA1
mRNA. Cell Rep. 42:1134012023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang Z, Zhang Y, Wang S, Qi R, Tao Y, Sun
Y, Jiang D, Jiang X and Tao J: FOXD3-mediated transactivation of
ALKBH5 promotes neuropathic pain via m6A-dependent
stabilization of 5-HT3A mRNA in sensory neurons. Proc Natl Acad Sci
USA. 121:e23128611212024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu W, Yang X, Zhong W, Chen G, Guo X, Ye
Q, Xu Y, Qi Z, Ye Y, Zhang J, et al: METTL14-mediated m6A
epitranscriptomic modification contributes to chemotherapy-induced
neuropathic pain by stabilizing GluN2A expression via IGF2BP2. J
Clin Invest. 134:e1748472024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang K, Li P, Jia Y, Liu M and Jiang J:
Non-coding RNA and n6-methyladenosine modification play crucial
roles in neuropathic pain. Front Mol Neurosci. 15:10020182022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang XL, Wei X, Yuan JJ, Mao YY, Wang ZY,
Xing N, Gu HW, Lin CH, Wang WT, Zhang W and Xing F: Downregulation
of fat mass and obesity-related protein in the anterior cingulate
cortex participates in anxiety- and depression-like behaviors
induced by neuropathic pain. Front Cell Neurosci. 16:8842962022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zeng F, Cao J, Hong Z, Lu Y, Qin Z and Tao
T: Epigenetic combined with transcriptomic analysis of the m6A
methylome after spared nerve injury-induced neuropathic pain in
mice. Neural Regen Res. 18:2545–2552. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang W, Qiao SC, Wu XB, Sun B, Yang JG, Li
X, Zhang X, Qian SJ, Gu YX and Lai HC: Circ_0008542 in osteoblast
exosomes promotes osteoclast-induced bone resorption through m6A
methylation. Cell Death Dis. 12:6282021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hulse RP, Beazley-Long N, Ved N, Bestall
SM, Riaz H, Singhal P, Ballmer Hofer K, Harper SJ, Bates DO and
Donaldson LF: Vascular endothelial growth factor-A165b prevents
diabetic neuropathic pain and sensory neuronal degeneration. Clin
Sci (Lond). 129:741–756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Quattrini C, Jeziorska M, Boulton AJM and
Malik RA: Reduced vascular endothelial growth factor expression and
intra-epidermal nerve fiber loss in human diabetic neuropathy.
Diabetes Care. 31:140–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hulse RP, Beazley-Long N, Hua J, Kennedy
H, Prager J, Bevan H, Qiu Y, Fernandes ES, Gammons MV,
Ballmer-Hofer K, et al: Regulation of alternative VEGF-A mRNA
splicing is a therapeutic target for analgesia. Neurobiol Dis.
71:245–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bestall SM, Hulse RP, Blackley Z, Swift M,
Ved N, Paton K, Beazley-Long N, Bates DO and Donaldson LF: Sensory
neuronal sensitisation occurs through HMGB-1-RAGE and TRPV1 in
high-glucose conditions. J Cell Sci. 131:jcs2159392018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Diederichs S: Non-coding RNA and disease.
RNA Biol. 9:701–702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Good DJ: Non-coding RNAs in human health
and diseases. Genes (Basel). 14:14292023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li C, Ni YQ, Xu H, Xiang QY, Zhao Y, Zhan
JK, He JY, Li S and Liu YS: Roles and mechanisms of exosomal
non-coding RNAs in human health and diseases. Signal Transduct
Target Ther. 6:3832021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nemeth K, Bayraktar R, Ferracin M and
Calin GA: Non-coding RNAs in disease: From mechanisms to
therapeutics. Nat Rev Genet. 25:211–232. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Roganović J and Petrović N: Clinical
perspectives of non-coding RNA in oral inflammatory diseases and
neuropathic pain: A narrative review. Int J Mol Sci. 23:82782022.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jiang BC, Cao DL, Zhang X, Zhang ZJ, He
LN, Li CH, Zhang WW, Wu XB, Berta T, Ji RR and Gao YJ: CXCL13
drives spinal astrocyte activation and neuropathic pain via CXCR5.
J Clin Invest. 126:745–761. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang B, Ma L, Guo X, Du S, Feng X, Liang
Y, Govindarajalu G, Wu S, Liu T, Li H, et al: A sensory
neuron-specific long non-coding RNA reduces neuropathic pain by
rescuing KCNN1 expression. Brain. 146:3866–3884. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wu M, Feng Y and Shi X: Advances with long
non-coding RNAs in diabetic peripheral neuropathy. Diabetes Metab
Syndr Obes. 13:1429–1434. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Du H, Liu Z, Tan X, Ma Y and Gong Q:
Identification of the genome-wide expression patterns of long
non-coding RNAs and mRNAs in mice with streptozotocin-induced
diabetic neuropathic pain. Neuroscience. 402:90–103. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang S, Xu H, Zou L, Xie J, Wu H, Wu B, Yi
Z, Lv Q, Zhang X, Ying M, et al: LncRNA uc.48+ is involved in
diabetic neuropathic pain mediated by the P2X3 receptor in the
dorsal root ganglia. Purinergic Signal. 12:139–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Peng H, Zou L, Xie J, Wu H, Wu B, Zhu G,
Lv Q, Zhang X, Liu S, Li G, et al: lncRNA NONRATT021972 siRNA
decreases diabetic neuropathic pain mediated by the P2X3
receptor in dorsal root ganglia. Mol Neurobiol. 54:511–523. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wu B, Zhou C, Xiao Z, Tang G, Guo H, Hu Z,
Hu Q, Peng H, Pi L, Zhang Z, et al: LncRNA-UC.25 + shRNA alleviates
P2Y14 receptor-mediated diabetic neuropathic pain via
STAT1. Mol Neurobiol. 59:5504–5515. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sun M, Zhang M, Yin H, Tu H, Wen Y, Wei X,
Shen W, Huang R, Xiong W, Li G and Gao Y: Long non-coding RNA
MSTRG.81401 short hairpin RNA relieves diabetic neuropathic pain
and behaviors of depression by inhibiting P2X4 receptor expression
in type 2 diabetic rats. Purinergic Signal. 19:123–133. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhan T, Tang S, Du J, Liu J, Yu B, Yang Y,
Xie Y, Qiu Y, Li G and Gao Y: Implication of lncRNA MSTRG.81401 in
hippocampal pyroptosis induced by P2X7 receptor in type 2 diabetic
rats with neuropathic pain combined with depression. Int J Mol Sci.
25:11862024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu S, Zou L, Xie J, Xie W, Wen S, Xie Q,
Gao Y, Li G, Zhang C, Xu C, et al: LncRNA NONRATT021972 siRNA
regulates neuropathic pain behaviors in type 2 diabetic rats
through the P2X7 receptor in dorsal root ganglia. Mol Brain.
9:442016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang A, Shi X, Yu R, Qiao B, Yang R and Xu
C: The P2X7 receptor is involved in diabetic neuropathic
pain hypersensitivity mediated by TRPV1 in the rat dorsal root
ganglion. Front Mol Neurosci. 14:6636492021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu C, Li C, Deng Z, Du E and Xu C: Long
non-coding RNA BC168687 is involved in TRPV1-mediated diabetic
neuropathic pain in rats. Neuroscience. 374:214–222. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wen CH, Berkman T, Li X, Du S,
Govindarajalu G, Zhang H, Bekker A, Davidson S and Tao YX: Effect
of intrathecal NIS-lncRNA antisense oligonucleotides on neuropathic
pain caused by nerve trauma, chemotherapy, or diabetes mellitus. Br
J Anaesth. 130:202–216. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yu W, Zhao GQ, Cao RJ, Zhu ZH and Li K:
LncRNA NONRATT021972 was associated with neuropathic pain scoring
in patients with type 2 diabetes. Behav Neurol. 2017:29412972017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang C, Gao R, Zhou R, Chen H, Liu C, Zhu
T and Chen C: The emerging power and promise of non-coding RNAs in
chronic pain. Front Mol Neurosci. 15:10379292022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kaur P, Kotru S, Singh S and Munshi A:
Role of miRNAs in diabetic neuropathy: Mechanisms and possible
interventions. Mol Neurobiol. 59:1836–1849. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Meydan C, Üçeyler N and Soreq H:
Non-coding RNA regulators of diabetic polyneuropathy. Neurosci
Lett. 731:1350582020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bali KK, Gandla J, Rangel DR, Castaldi L,
Mouritzen P, Agarwal N, Schmelz M, Heppenstall P and Kuner R: A
genome-wide screen reveals microRNAs in peripheral sensory neurons
driving painful diabetic neuropathy. Pain. 162:1334–1351. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
He J, Wang HB, Huang JJ, Zhang L, Li DL,
He WY, Xiong QM and Qin ZS: Diabetic neuropathic pain induced by
streptozotocin alters the expression profile of non-coding RNAs in
the spinal cord of mice as determined by sequencing analysis. Exp
Ther Med. 22:7752021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ma F, Wang C, Yoder WE, Westlund KN,
Carlson CR, Miller CS and Danaher RJ: Efficacy of herpes simplex
virus vector encoding the human preproenkephalin gene for treatment
of facial pain in mice. J Oral Facial Pain Headache. 30:42–50.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu B, Guo Y, Chen Q, Xiong Q and Min S:
MicroRNA-193a downregulates HMGB1 to alleviate diabetic neuropathic
pain in a mouse model. Neuroimmunomodulation. 26:250–257. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wu D, Zhong S, Du H, Han S, Wei X and Gong
Q: MiR-184-5p represses neuropathic pain by regulating CCL1/CCR8
signaling interplay in the spinal cord in diabetic mice. Neurol
Res. 46:54–64. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang X, Xia L, Xie A, Liao O, Ju F and
Zhou Y: Low concentration of bupivacaine ameliorates painful
diabetic neuropathy by mediating miR-23a/PDE4B axis in microglia.
Eur J Pharmacol. 891:1737192021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Aghdam AM, Shahabi P, Karimi-Sales E,
Ghiasi R, Sadigh-Eteghad S, Mahmoudi J and Alipour MR: Swimming
exercise induced reversed expression of miR-96 and its target gene
NaV1.3 in diabetic peripheral neuropathy in rats. Chin J Physiol.
61:124–129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yan J, Yu H, Shen J, Han C, Li C, Shen X
and Li B: Early over-expressing of microRNA-145 effectively
precludes the development of neuropathic mechanical hyperalgesia
via suppressing Nav1.8 in diabetic rats. Pain Physician.
23:E673–E686. 2020.PubMed/NCBI
|
|
87
|
Wu Y, Gu Y and Shi B: miR-590-3p
Alleviates diabetic peripheral neuropathic pain by targeting RAP1A
and suppressing infiltration by the T cells. Acta Biochim Pol.
67:587–593. 2020.PubMed/NCBI
|
|
88
|
Zhang T, Wang L and Chen L: Alleviative
effect of microRNA-497 on diabetic neuropathic pain in rats in
relation to decreased USP15. Cell Biol Toxicol. 39:1–16. 2023.
View Article : Google Scholar
|
|
89
|
Guo Y, Zeng J, Zhuang Y, Jiang C and Xie
W: MiR-503-5p alleviates peripheral neuropathy-induced neuropathic
pain in T2DM mice by regulating SEPT9 to inhibit astrocyte
activation. Sci Rep. 14:143612024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kasimu A, Apizi X, Talifujiang D, Ma X,
Fang L and Zhou X: miR-125a-5p in astrocytes attenuates peripheral
neuropathy in type 2 diabetic mice through targeting TRAF6.
Endocrinol Diabetes Nutr (Engl Ed). 69:43–51. 2022.PubMed/NCBI
|
|
91
|
Ashjari D, Karamali N, Rajabinejad M,
Hassani SS, Afshar Hezarkhani L, Afshari D, Gorgin Karaji A, Salari
F and Rezaiemanesh A: The axis of long non-coding RNA
MALAT1/miR-1-3p/CXCR4 is dysregulated in patients with diabetic
neuropathy. Heliyon. 8:e091782022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li YB, Wu Q, Liu J, Fan YZ, Yu KF and Cai
Y: miR-199a-3p is involved in the pathogenesis and progression of
diabetic neuropathy through downregulation of SerpinE2. Mol Med
Rep. 16:2417–2424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wu X, Wang X, Yin Y, Zhu L, Zhang F and
Yang J: Investigation of the role of miR-221 in diabetic peripheral
neuropathy and related molecular mechanisms. Adv Clin Exp Med.
30:623–632. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chang LL, Wang HC, Tseng KY, Su MP, Wang
JY, Chuang YT, Wang YH and Cheng KI: Upregulation of miR-133a-3p in
the sciatic nerve contributes to neuropathic pain development. Mol
Neurobiol. 57:3931–3942. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen J, Li C, Liu W, Yan B, Hu X and Yang
F: miRNA-155 silencing reduces sciatic nerve injury in diabetic
peripheral neuropathy. J Mol Endocrinol. 63:227–238. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chattopadhyay M, Zhou Z, Hao S, Mata M and
Fink DJ: Reduction of voltage gated sodium channel protein in DRG
by vector mediated miRNA reduces pain in rats with painful diabetic
neuropathy. Mol Pain. 8:172012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Haque S and Harries LW: Circular RNAs
(circRNAs) in health and disease. Genes (Basel). 8:3532017.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang HH, Zhang Y, Wang X, Yang P, Zhang
BY, Hu S, Xu GY and Hu J: Circular RNA profile in diabetic
peripheral neuropathy: Analysis of coexpression networks of
circular RNAs and mRNAs. Epigenomics. 12:843–857. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang SB, Lin SY, Liu M, Liu CC, Ding HH,
Sun Y, Ma C, Guo RX, Lv YY, Wu SL, et al: CircAnks1a in the spinal
cord regulates hypersensitivity in a rodent model of neuropathic
pain. Nat Commun. 10:41192019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang L, Luo T, Bao Z, Li Y and Bu W:
Intrathecal circHIPK3 shRNA alleviates neuropathic pain in diabetic
rats. Biochem Biophys Res Commun. 505:644–650. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang Y, Ma H, Bai Y, Hou X, Yang Y, Wang
G and Li Y: Chronic neuropathic pain and comorbid depression
syndrome: From neural circuit mechanisms to treatment. ACS Chem
Neurosci. 15:2432–2444. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Song Q, Wei A, Xu H, Gu Y, Jiang Y, Dong
N, Zheng C, Wang Q, Gao M, Sun S, et al: An ACC-VTA-ACC
positive-feedback loop mediates the persistence of neuropathic pain
and emotional consequences. Nat Neurosci. 27:272–285. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Huang J, Gadotti VM, Chen L, Souza IA,
Huang S, Wang D, Ramakrishnan C, Deisseroth K, Zhang Z and Zamponi
GW: A neuronal circuit for activating descending modulation of
neuropathic pain. Nat Neurosci. 22:1659–1668. 2019. View Article : Google Scholar : PubMed/NCBI
|