|
1
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pavlova NN, Zhu J and Thompson CB: The
hall marks of cancer metabolism: Still emerging. Cell Metab.
34:355–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li F and Simon MC: Cancer Cells don't live
alone: Metabolic communication within tumor microenvironments. Dev
Cell. 54:183–195. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koizume S and Miyagi Y: Lipid droplets: A
key cellular organelle associated with cancer cell survival under
normoxia and hypoxia. Int. J Mol Sci. 17:14302016. View Article : Google Scholar
|
|
5
|
Broadfield LA, Pane AA, Talebi A, Swinnen
JV and Fendt SM: Lipid metabolism in cancer: New perspectives and
emerging mechanisms. Dev Cell. 56:1363–1393. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riscal R, Skuli N and Simon MC: Even
cancer cells watch their cholesterol! Mol Cell. 76:220–231. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiang W, Lv H, Xing F, Sun X, Ma Y, Wu L,
Lv G, Zong Q, Wang L, Wu Z, et al: Inhibition of ACLY overcomes
cancer immunotherapy resistance via polyunsaturated fatty acids
peroxidation and cGAS-STING activation. Sci Adv. 9:eadi24652023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vanauberg D, Schulz C and Lefebvre T:
Involvement of the pro-oncogenic enzyme fatty avid synthase in the
hallmarks of cancer: A promising target in anti-cancer therapies.
Oncogenesis. 12:162023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freitas FP, Alborzinia H, dos Santos AF,
Nepachalovich P, Pedrera L, Zilka O, Inague A, Klein C, Aroua N,
Kaushal K, et al: 7-dehydrocholesterol is endogenous suppressor of
ferroptosis. Nature. 626:401–410. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Ran Q, Duan Q, Jin J, Wang Y, Yu L,
Wang C, Zhu Z, Chen X, Weng L, et al: 7-Dehydrocholesterol dictates
ferroptosis sensitivity. Nature. 626:411–418. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumor. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Macklin PS, Yamamoto A, Browning L, Hofer
M, Adam J and Pugh CW: Recent advances in the biology of tumor
hypoxia with relevance to diagnostic practice and tissue-based
research. J Pathol. 250:593–611. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haga N, Saito S, Tsukumo Y, Sakurai J,
Furuno A, Tsuruo T and Tomida A: Mitochondria regulate the unfolded
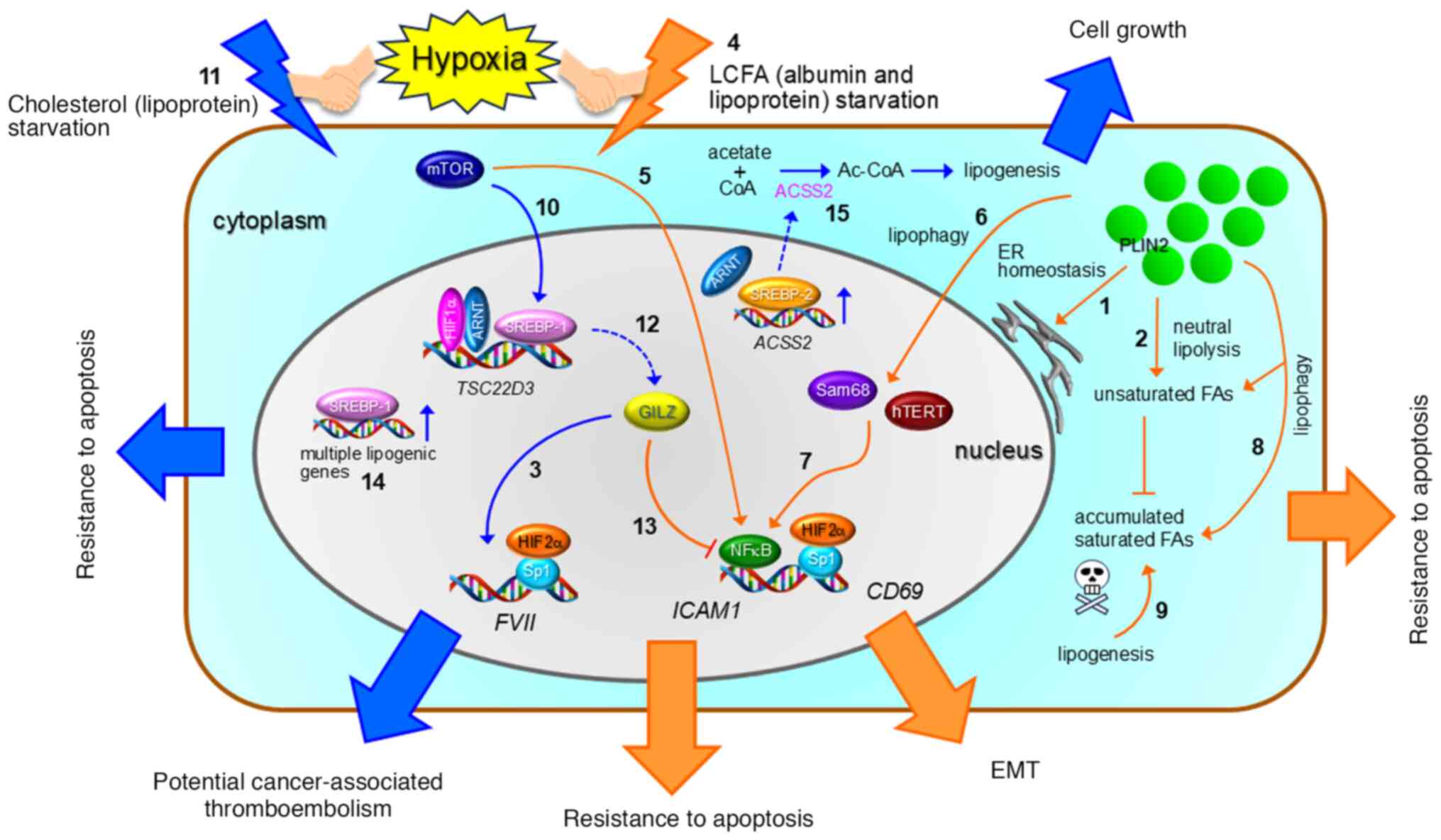
protein response leading to cancer cell survival under glucose
deprivation conditions. Cancer Sci. 101:1125–1132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwon SJ and Lee YJ: Effect of low
glutamine/glucose on hypoxia-induced elevation of hypoxia-inducible
factor-1a in human pancreatic cancer MiaPaCa-2 and human prostatic
cancer DU-145 cells. Clin. Cancer Res. 11:4694–4700. 2005.
|
|
15
|
Kikuchi D, Tanimoto K and Nakayama K: CREB
is activated by ER stress and modulates the unfolded protein
response by regulating the expression of IRE1α and PERK. Biochem.
Biophys. Res Commun. 469:243–250. 2016.PubMed/NCBI
|
|
16
|
Wang HF, Wang ZQ, Ding Y, Piao MH, Feng
CS, Chi GF, Luo YN and Ge PF: Endoplasmic reticulum stress
regulates oxygen-glucose deprivation-induced parthanatos in human
SH-SY5Y cells via improvement of intracellular ROS. CNS Neurosci
Ther. 24:29–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Natsuizaka M, Ozasa M, Darmanin S,
Miyamoto M, Kondo S, Kamada S, Shindoh M, Higashino F, Suhara W,
Koide H, et al: Synergistic up-regulation of Hexokinase-2, glucose
transporters and angiogenic factors in pancreatic cancer cells by
glucose deprivation and hypoxia. Exp Cell Res. 313:3337–3348. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumor growth and progression.
Nat Rev Cancer. 12:9–22. 2012. View Article : Google Scholar
|
|
19
|
Wouters BG and Koritzinsky M: Hypoxia
signalling through mTOR and the unfolded protein response in
cancer. Nat Rev Cancer. 8:851–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee P, Chandel N and Simon MC: Cellular
adaptation to hypoxia through hypoxia inducible factors and beyond.
Nat Rev Mol Cell Biol. 21:268–283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Kobayashi M, Darmanin S, Qiao Y,
Gully C, Zhao R, Kondo S, Wang H, Wang H, Yeung SC, et al:
Hypoxia-mediated up-regulation of Pim-1 contributes to solid tumor
formation. Am J Pathol. 175:400–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu W, Glunde K, Bhujwalla ZM, Raman V,
Sharma A and Phang JM: Proline oxidase promotes tumor cell survival
in hypoxic tumor microenvironments. Cancer Res. 72:3677–3686. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Ilipoulos D, Zhang Q, Tang Q,
Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, et
al: XBP1 promotes triple-negative breast cancer by controlling the
HIF1a pathway. Nature. 508:103–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang C, Li Y, Li Z, Xu Y, Li N, Ge Y,
Dong J, Chang A, Zhao T, Wang X, et al: LIMS1 promotes pancreatic
cancer cell survival under oxygen-glucose deprivation conditions by
enhancing HIF1A protein translation. Clin Cancer Res. 25:4091–4103.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saggese P, Pandey A, Alcaraz M, Fung E,
Hall A, Yanagawa J, Rodriguez EF, Grogan TR, Giurato G, Nassa G, et
al: Glucose deprivation promotes pseudohypoxia and
de-differentiation in lung adenocarcinoma. Cancer Res. 84:305–327.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao T, Jiang T, Li X, Chang S, Sun Q,
Kong F, Kong X, Wei F, He J, Hao J, et al: Nuclear GRP78 promotes
metabolic reprogramming and therapeutic resistance in pancreatic
ductal adenocarcinoma. Clin Cancer Res. 29:5183–5195. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishimoto A, Kugiyama N, Hosoyama T, Enoki
T, Li TS and Hamano K: HIF-1α activation under glucose deprivation
plays a central role in the acquisition of anti-apoptosis in human
colon cancer cells. Int J Oncol. 44:2077–2084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takai M, Takauchi M, Kuribayashi M and
Tsujiuchi T: LPA receptor-mediated signaling regulates cell
motility and survival to anticancer drug of pancreatic cancer cells
under glucose-deprived and hypoxic conditions. Biochem Biophys Res
Commun. 661:21–27. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davern M, Fitzgerald MC, Buckley CE,
Heeran AB, Donlon NE, McGrath J, O'Connel F, Deshpande MR, Hayes C,
MacDonald J, et al: PD-1 and TIGIT blockade differentially affect
tumour cell survival under hypoxia and glucose deprived conditions
in oesophageal adenocarcinoma; implications for overcoming
resistance to PD-1 blockade in hypoxic tumors. Transl Oncol.
19:1013812022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Buzzai M, Bauer DE, Jones RG, DeBerardinis
RJ, Hatzivassiliou G, Elstrom RL and Tompson CB: The glucose
dependence of Akt-transformed cells can be reversed by
pharmacologic activation of fatty acid β-oxidation. Oncogene.
24:4165–4173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leithner K, Triebl A, Trotzmuller M,
Hinteregger B, Leko P, Wieser BI, Grasmann G, Bertsch AL, Züllig T,
Stacher E, et al: The glycerol backbone of phospholipids derives
from noncarbohydrate precursors in staved lung cancer cells. Proc
Natl Acad Sci USA. 115:6225–6230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai M, He J, Xiong J, Tay LWR, Wang Z, Rog
C, Wang J, Xie Y, Wang G, Banno Y, et al: Phospholipase
D1-regulated autophagy supplies free fatty acids to counter
nutrient stress in cancer cells. Cell Death Dis. 7:e24482016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khan AUH, Salehi H, Alexia C, Valdivielso
JM, Bozic M, Lopez-Mejia IC, Fajas L, Gerbal-Chaloin S,
DaujatChavanieu M, Gitenay D, et al: Glucose starvation or pyruvate
dehydrogenase activation induce a broad, ERK5-mediated, metabolic
remodeling leading to fatty acid oxidation. Cells. 11:13922022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoang-Minh LB, Siebzehnrubl FA, Yang C,
Suzuki-Hatano S, Dajac K, Loche T, Andrews N, Massari MS, Patel J,
Amin K, et al: Infiltrative and drug-resistant slow-cycling cells
support metabolic heterogeneity in glioblastoma. EMBO J.
37:e987722018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Haas MA, Yeo SK, Paul R, Yang F,
Vallabhapurapu S, Qi X, Plas DR and Guan JL: Autophagy mediated
lipid catabolism facilitates glioma progression to overcome
bioenergetic crisis. Br J Cancer. 124:1711–1723. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu R, Lee JH, Li J, Yu R, Tan L, Xia Y,
Zheng Y, Bian XL, Lorenzi PL, Chen Q, et al: Choline kinase alpha 2
acts as a protein kinase to promote lipolysis of lipid droplets.
Mol Cell. 81:2722–2735. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chauhan SS, Casillas AL, Vizzerra AD, Liou
H, Clements AN, Flores CE, Prevost CT, Kashatus DF, Snider AJ,
Snider JM, et al: PIM1 drives lipid droplet accumulation to promote
proliferation and survival in prostate cancer. Oncogene.
43:406–419. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cao B, Deng H, Cui H, Zhao R, Li H, Wei B
and Chen L: Knockdown of PGM1 enhances anticancer effects of
orlistat in gastric cancer under glucose deprivation. Cancer Cell
Int. 21:4812021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Monteiro-Cardoso VF, Silva AM, Oliveira
MM, Peixoto F and Videira RA: Membrane lipid profile alterations
are associated with the metabolic adaptation of the Caco-2 cells to
a glycemic nutritional condition. J Bioenerg Biomembr. 46:45–57.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Spencer AG, Woods JW, Arakawa T, Singer II
and Smith WI: Subcellular localization of prostaglandin
endoperoxide H synthase-1 and −2 by immunoelectron microscopy. J
Biol Chem. 273:9886–9893. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roberts HR, Smartt HJM, Greenhough A,
Moore AE, Williams AC and Paraskeva C: Colon tumor cells increase
PGE2 by regulating COX-2 and 15-PGDH to promote survival during the
microenvironmental stress of glucose deprivation. Carcinogenesis.
32:1741–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hwang SH, Yang Y, Jung JH and Kim Y: Oleic
acid from cancer-associated fibroblast promotes cancer cell
stemness by stearoyl-CoA desaturase under glucose-deficient
condition. Cancer Cell Int. 22:4042022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kamphorst JJ, Nofal M, Commisso C, Hackett
SR, Lu W, Grabocka E, Heiden MGV, Miller G, Drebin JA, Bar-Sagi D,
et al: Human pancreatic cancer tumors are nutrient poor and tumor
cells actively scavenge extracellular protein. Cancer Res.
75:544–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gameiro PA, Yang J, Metelo AM, Perez-Carro
R, Baker R, Wang Z, Arreola A, Rathmell WK, Olumi A, López-Larrubia
P, et al: In vivo HIF-mediated reductive carboxylation is regulated
by citrate levels and sensitizes VHL-deficient cells to glutamine
deprivation. Cell Metab. 17:372–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kamphorst JJ, Cross JR, Fan J, de
Stanchina E, Mathew R, White EP, Thompson CB and Rabinowitz JD:
Hypoxic and Ras-transformed cells support growth by scavenging
unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci
USA. 110:8882–8887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miess H, Dankworth B, Gouw AM, Rosenfeldt
M, Schmitz W, Jiang M, Saunders B, Howell M, Downward J, Felsher
DW, et al: The glutathione redox system is essential to prevent
ferroptosis caused by impaired lipid metabolism in clear cell renal
cell carcinoma. Oncogene. 37:5435–5450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Byun JK, Choi YK, Kim JH, Jeong JY, Jeon
HJ, Kim MK, Hwang I, Lee SY, Lee YM, Lee IK, et al: A positive
feedback loop between Sestrin 2 and mTORC2 is required for the
survival of glutamine-depleted lung cancer cells. Cell Rep.
20:586–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Babbar M, Huang Y, An J, Landas SK and
Sheikh MS: CHTM1, a novel metabolic marker deregulated in human
malignancies. Oncogene. 37:2052–2066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Juh JW, Yan JB, Lin ZH, Lin SC and Peng
IC: SREBP1-induced glutamine synthetase triggers a feedforward loop
to upregulate SREBP1 through Sp1 O-GlcNAcylation and augments lipid
droplet formation in cancer cells. Int J Mol Sci. 22:98142021.
View Article : Google Scholar
|
|
50
|
Zhu R, Yang Y, Shao F, Wang J, Gao Y, He J
and Lu Z: Choline kinase alpha2 promotes lipid droplet lipolysis in
non-small-cell lung carcinoma. Front Oncol. 22:8484832022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guo X, Wang A, Wang W, Wang Y, Chen H, Liu
X, Xia T, Zhang A, Chen D, Qi H, et al: HRD1 inhibits fatty acid
oxidation and tumorigenesis by ubiquitinating CPT2 in triple
negative breast cancer. Mol Oncol. 15:642–656. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
De Santis MC, Gozzelino L, Margaria JP,
Costamagna A, Ratto E, Gulluni F, Gregorio ED, Mina E, Lorito N,
Bacci M, et al: Lysosomal lipid switch sensitises to nutrient
deprivation and mTOR targeting in pancreatic cancer. Gut.
72:360–371. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kong Y, Wu M, Wan X, Sun M, Zhang Y, Wu Z,
Li C, Liang X, Gao L, Ma C, et al: Lipophagy-mediated cholesterol
synthesis inhibition is required for the survival of hepatocellular
carcinoma under glutamine deprivation. Redox Biol. 63:1027322023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nedara K, Reinhardt C, Lebraud E, Arena G,
Gracia C, Buard V, Pioche-Durieu C, Castelli F, Colsch B, Bénit P,
et al: Relevance of the TRIAP1/p53 axis in colon cancer cell
proliferation and adaptation to glutamine depletion. Front Oncol.
12:9581552022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lorenz NI, Sittig ACM, Urban H, Luger AL,
Engel AL, Munch C, Steinbach JP and Ronellenfitsch MW: Activating
transcription factor 4 mediates adaptation of human glioblastoma
cells to hypoxia and temozolomide. Sci Rep. 11:141612021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pan M, Reid MA, Lowman XH, Kulkarni RP,
Tran TQ, Liu X, Yang Y, Hernandez-Davies JE, Rosales KK, Li H, et
al: Regional glutamine deficiency in tumors promotes
dedifferentiation through inhibition of histone demethylation. Nat
Cell Biol. 18:1090–1101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jain M, Nilsson R, Sharma S, Madhusudhan
N, Kiytami T, Souza AL, Kafri R, Kirschner MW, Clish CB and Mootha
VK: Metabolite profiling identifies a key role for glycine in rapid
cancer cell proliferation. Science. 336:1040–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gao X, Lee K, Reid MA, Sanderson SM, Qiu
C, Li S, Liu J and Locasale JW: Serine availability influences
mitochondrial dynamics and function through lipid metabolism. Cell
Rep. 22:3507–3520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Maddocks OD, Berkers CR, Mason SM, Zheng
L, Blyth K, Gottlieb E and Vousden KH: Serine starvation induces
stress and p53-dependent metabolic remodelling in cancer cells.
Nature. 493:542–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Muthusamy T, Cordes T, Handzlik MK, You L,
Lim EW, Gengatharan J, Pinto AFM, Badur MG, Kolar MJ, Wallace M, et
al: Serine restriction alters sphingolipid diversity to constrain
tumour growth. Nature. 586:790–795. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Truman JP, Ruiz CF, Montal E,
Garcia-Barros M, Mileva I, Snider AJ, Hannun YA, Obeid LM and Mao
C: 1-Deoxysphinganine initiates adaptive responses to serine and
glycine starvation in cancer cells via proteolysis of sphingosine
kinase. J Lipid Res. 63:1001542022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yun HJ, Li M, Guo D, Jeon SM, Park SH, Lim
JS, Lee SB, Liu R, Du L, Kim SH, et al: AMPK-HIF-1α signaling
enhances glucose-derived de novo serine biosynthesis to promote
glioblastoma growth. J Exp Clin Cancer Res. 42:3402023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bonifacio VDB, Pereira SA, Serpa J and
Vicente JB: Cysteine metabolic circuitries: Druggable targets in
cancer. Br J Cancer. 124:862–879. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cunningham A, Oudejans LL, Geugien M,
Pereira-Martins DA, Wierenga ATJ, Erdem A, Sternadt D, Huls G and
Schuringa JJ: The nonessential amino acid cysteine is required to
prevent ferroptosis in acute myeloid leukemia. Blood Adv. 8:56–69.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Upadhyayula PS, Higgins DM, Mela A, Banu
M, Dovas A, Zandkarimi F, Patel P, Mahajan A, Humala N, Nguyen TTT,
et al: Dietary restriction of cysteine and methionine sensitizes
gliomas to ferroptosis and induces alterations in energetic
metabolism. Nature Commun. 14:11872023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kerimoglu B, Lamb C, McPherson RD, Ergen
E, Stone EM and Ooi A: Cysteinase-rapamycin combination induces
ferroptosis in both in vitro and in vivo models of hereditary
leiomyomatosis and renal cell carcinoma. Mol Caner Ther.
21:419–426. 2022. View Article : Google Scholar
|
|
67
|
Liu N, Lin X and Huang C: Activation of
the reverse transsulfuration pathway through NRF2/CBS confers
erastin-induced ferroptosis resistance. Br J Cancer. 122:279–92.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Armenta DA, Laqtom NN, Alchemy G, Dong W,
Morrow D, Poltorack CD, Nathanson DA, Abu-Remaileh M and Dixon S:
Ferroptosis inhibition by lysosome-dependent catabolism of
extracellular protein. Cell Chem Biol. 29:1588–1600. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen PH, Wu J, Ding CKC, Lin CC, Pan S,
Bossa N, Xu Y, Yang WH, Mathey-Prevot B and Chi JT: Kinome screen
of ferroptosis reveals a novel role of ATM in regulating iron
metabolism. Cell Death Differ. 27:1008–1022. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Y, Wang X, Huang Z, Zhou Y, Xia J, Hu
W, Wang X, Du J, Tong X and Wang Y: CISD3 inhibition drives
cysteine-deprivation induced ferroptosis. Cell Death Dis.
12:8392021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu D, Liang C, Huang B, Zhuang X, Cui W,
Yang L, Yang Y, Zhang Y, Fu X, Zhang X, et al: Tryptophan
metabolism acts as a new anti-ferroptotic pathway to mediate tumor
growth. Adv Sci. 10:22040062023. View Article : Google Scholar
|
|
72
|
Hong SE, Kim MR, Jang SK, Seong MK, Kim
HA, Noh WC, Jin HO and Park IC: Hypoxia suppresses cysteine
deprivation-induced cell death via ATF4 regulation in MDA-MB-231
breast cancer cells. Anticancer Res. 40:1387–1394. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Qiu F, Chen YR, Liu X, Chu CY, Shen LJ, Xu
J, Gaur S, Forman HJ, Zhang H, Zheng S, et al: Arginine starvation
impairs mitochondrial respiratory function in ASS1-deficient breast
cancer cells. Sci Signal. 7:ra312014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu Q, Dai J, Zhang Z, Yu H, Zhang J, Zhu
X, Qin Y, Zhang L and Zhang P: ASS1-mediated reductive
carboxylation of cytosolic glutamine confers ferroptosis resistance
in cancer cells. Cancer Res. 83:1646–1665. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Brashears CB, Barlin M, Ehrhardt WR,
Rathore R, Schultze M, Tzeng SC, Tine BAV and Held JM: Systems
level profiling of arginine starvation reveals MYC and ERK adaptive
metabolic reprogramming. Cell Death Dis. 11:6622020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Long Y, Tsai WB, Wangpaichitr M, Tsukamoto
T, Savaraj N, Feun LG and Kuo MT: Arginine deiminase resistance in
melanoma cells is associated with metabolic reprogramming, glucose
dependence, and glutamine addiction. Mol Cancer Ther. 12:2581–2590.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Burrows N, Cane G, Robson M, Gaude E,
Howat WJ, Szlosarek PW, Pedley RB, Frezza C, Ashcroft M and Maxwell
PH: Hypoxia-induced nitric oxide production and tumour perfusion is
inhibited by pegylated arginine deiminase (ADI-PEG20). Sci Rep.
6:229502016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Guenin S, Morvan D, Thivat E, Stepien G
and Demidem A: Combined methionine deprivation and
chloroethylnitrosourea have time-dependent therapeutic synergy on
melanoma tumors that NMR spectroscopy-based metabolomics explains
by methionine and phospholipid metabolism reprogramming. Nutr
Cancer. 61:518–529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Harada S, Taketomi Y, Aiba T, Kawaguchi M,
Hirabayashi T, Uranbileg B, Kurano M, Yatomi Y and Murakami M: The
lisophospholipase PNPLA7 controls hepatic choline and methionine
metabolism. Biomolecules. 13:4172023. View Article : Google Scholar
|
|
80
|
Yokogami K, Kikuchi T, Watanabe T,
Nakatake Y, Yamashita S, Mizuguchi A and Takeshima H: Methionine
regulates self-renewal pluripotency, and cell death of GIC through
cholesterol-rRNA axis. BMC Cancer. 22:13512022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Young RM, Ackerman D, Quinn ZL, Mancuso A,
Gruber M, Liu L, Giannoukos DN, Bobrovnikova-Marjon E, Diehl JA,
Keith B, et al: Dysregulated mTORC1 renders cells critically
dependent on desaturated lipids for survival under tumor-like
stress. Genes Dev. 27:1115–1131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li Z, Ji BW, Dixit PD, Tchourine K, Lien
EC, Hosios AM, Abbott KL, Rutter JC, Westermark AM, Gorodetsky EF,
et al: Cancer cells depend on environmental lipids for
proliferation when electron acceptors are limited. Nat Metab.
4:711–723. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Qiu B, Ackermann D, Sanchez DJ, Li B,
Ochocki JD, Grazioli A, Bobrovnikova-Marjon E, Diehl A, Keith B and
Simon C: HIF2α-dependent lipid storage promotes endoplasmic
reticulum homeostasis in clear-cell renal cell carcinoma. Cancer
Discov. 5:652–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Du W, Zhang L, Brett-Morris A, Aguila B,
Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, et
al: HIF drives lipid deposition and cancer in ccRCC via repression
of fatty acid metabolism. Nat Commun. 8:17692017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen C, Zhao W, Lu X, Ma Y, Zhang P, Wang
Z, Cui Z and Xia Q: AUP1 regulates lipid metabolism and induces
lipid accumulation to accelerate the progression of renal clear
cell carcinoma. Cancer Sci. 113:2600–2615. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Brezis M and Rosen S: Hypoxia of the renal
medulla-Its implications for disease. N Eng J Med. 332:647–655.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Little RA, Jamin Y, Boult JKR, Naish JH,
Watson Y, Cheung S, Holliday KF, Lu H, McHugh DJ, Irlam J, et al:
Mapping hypoxia in renal carcinoma with oxygen-enhanced MRI.
Radiology. 288:739–747. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ackerman D, Tumanov S, Qiu B,
Michalopoulou E, Spata M, Azzam A, Xie H, Simon MC and Kamphorst
JJ: Triglycerides promote lipid homeostasis during hypoxic stress
by balancing fatty acid saturation. Cell Rep. 24:2596–2605. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ji JX, Wang YK, Cochrane DR and Huntsman
DG: Clear cell carcinomas of the ovary and kidney: Clarity through
genomics. J Pathol. 244:550–564. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lee S, Garner EIO, Welch WR, Berkowitz RS
and Mok SC: Over-expression of hypoxia-inducible factor 1 alpha in
ovarian clear cell carcinoma. Gynecol Oncol. 106:311–317. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Anglesio MS, George J, Kulbe H,
Friedlander M, Rischin D, Lemech C, Power J, Coward J, Cowin PA,
House CM, et al: IL6-STAT3-HIF signaling and therapeutic response
to the angiogenesis inhibitor sunitinib in ovarian clear cell
carcinoma. Clin Cancer Res. 17:2538–2548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Spowart JE, Townsend KN, Huwait H, Eshragh
S, West NR, Ries JN, Kalloger S, Anglesio M, Gorski SM, Watson PH,
et al: The autophagy protein LC3A correlates with hypoxia and is a
prognostic marker of patient survival in clear cell ovarian cancer.
J Pathol. 228:437–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Koizume S, Ito S, Miyagi E, Hirahara F,
Nakamura Y, Sakuma Y, Osaka H, Takano Y, Ruf W and Miyagi Y:
HIF2α-Sp1 interaction mediates a deacetylation-dependent FVII-gene
activation under hypoxic condition in ovarian cancer cells. Nucleic
Acid Res. 40:5389–5401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Koizume S and Miyagi Y: Tissue factor in
cancer-associated thromboembolism: Possible mechanisms and clinical
applications. Br J Cancer. 127:2099–2107. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Koizume S, Ito S, Nakamura Y, Yoshihara M,
Furuya M, Yamada R, Miyagi E, Hirahara F, Takano Y and Miyagi Y:
Lipid starvation and hypoxia synergistically activate ICAM1 and
multiple genes in an Sp1-dependent manner to promote the growth of
ovarian cancer. Mol Cancer. 14:772015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Koizume S, Kanayama T, Kimura Y, Hirano H,
Takahashi T, Ota Y, Miyazaki K, Yoshihara M, Nakamura Y, Yokose T,
et al: Cancer cell-derived CD69 induced under lipid and oxygen
starvation promotes ovarian cancer progression through fibronectin.
Cancer Sci. 114:2485–2498. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Trigatti BL and Gerber GE: A direct role
for serum albumin in the cellular uptake of long-chain fatty acids.
Biochem. J. 308:155–159. 1995.PubMed/NCBI
|
|
98
|
Koizume S, Takahashi T, Nakamura Y,
Yoshihara M, Ota Y, Sato S, Tadokoro H, Yokose T, Kato H, Miyagi E,
et al: Lipophagy-ICAM-1 pathway associated with fatty acid and
oxygen deficiencies is involved in poor prognoses of ovarian clear
cell carcinoma. Br J Cancer. 127:462–473. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Koizume S, Takahashi T, Yoshihara M,
Nakamura Y, Ruf W, Takenaka K, Miyagi E and Miyagi Y: Cholesterol
starvation and hypoxia activate the FVII gene via the SREBP1-GILZ
pathway in ovarian cancer cells to produce procoagulant
microvesicles. Thromb Haemost. 119:1058–1071. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lewis CA, Peck B, Bensaad K, Griffiths B,
Mitter R, Chakravarty P, East P, Dankworth B, Alibhai D, Harris AL
and Schulze A: SREBP maintains lipid biosynthesis and viability of
cancer cells under lipid- and oxygen-deprived conditions and
defines a gene signature associated with poor survival in
glioblastoma multiforme. Oncogene. 34:5128–5140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Schug ZT, Peck B, Jones DT, Zhang Q,
Grosskurth S, Alam IS, Goodwin LM, Smethurst E, Mason S, Blyth K,
et al: Acetyl-CoA synthetase 2 promotes acetate utilization and
maintains cancer cell growth under metabolic stress. Cancer Cell.
27:57–71. 2015. View Article : Google Scholar : PubMed/NCBI
|