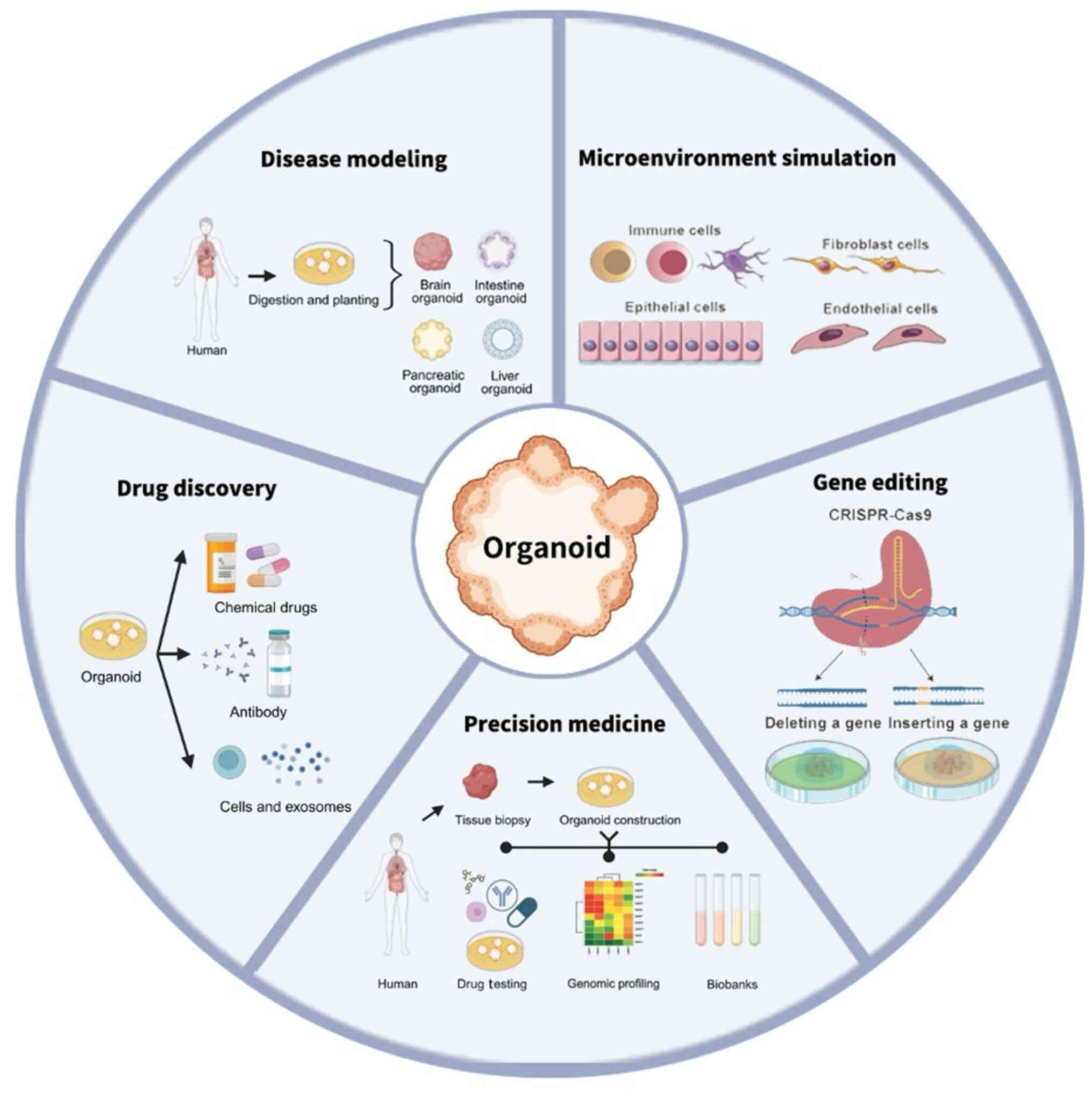
Cancer, a leading cause of mortality, notably
decreases life expectancy. In 2022, there were ~20.0 million new
cases and ~9.7 million cancer-associated mortalities worldwide
(1). Despite advances in cancer
diagnosis and treatment, mechanisms underlying tumorigenesis,
progression and drug resistance have not been fully elucidated. Due
to tumor heterogeneity, different individuals with the same type of
cancer may exhibit different responses to the same therapy; thus
models need to be established that can recapitulate the tumors to
study the mechanisms of tumorigenesis, progression and drug
resistance.
Preclinical models include two-dimensional (2D) cell
lines, patient-derived xenografts (PDXs) and organoids. Despite
simple operation and culture, 2D cell lines cannot definitively
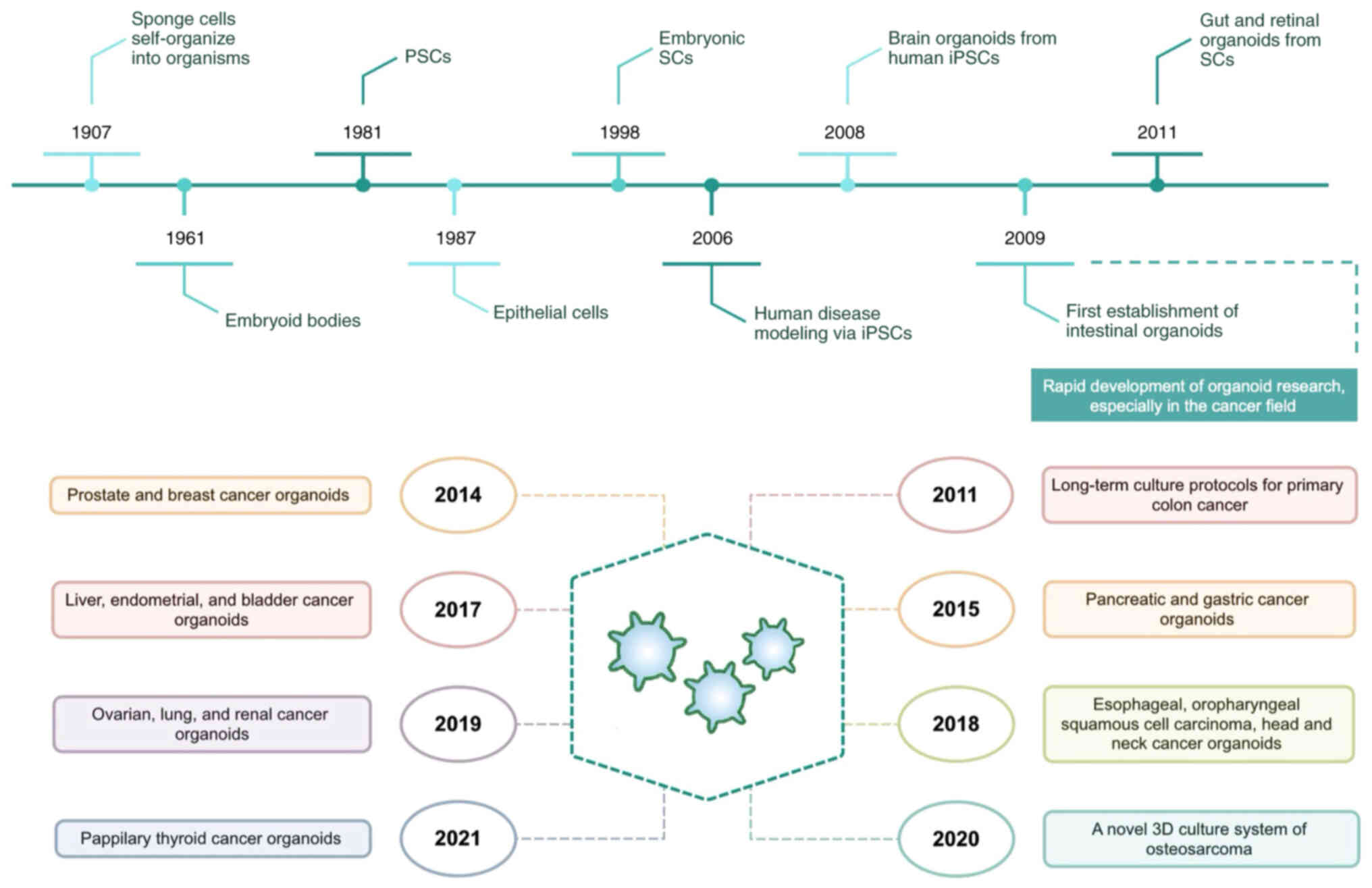
predict the drug response of patients due to accumulation of gene
mutations during passaging (2).
Additionally, 2D cell lines are unreliable compared with in
vivo models because of variations in cell phenotypical
behaviors (3). PDXs, which are
created by engraftment of patient tumor tissue into immunocompetent
mice, recapitulate the tumor heterogeneity while preserving the
biological and molecular features of original tumors (4,5), but
they are time-consuming, expensive and may undergo mouse-specific
tumor evolution rendering them unable to reflect the pathogenic
process of patients (6,7). Therefore, application of PDXs is
limited by the complex operation, duration, high cost and low
success rate. Organoids, a novel type of three-dimensional (3D)
miniature structure derived from adult or embryonic stem cells
(SCs), not only retains in vivo tumor characteristics and
heterogeneity, but also can predict the sensitivity of multiple
drugs simultaneously, with the advantages of high success rate of
generation, short time-frame and low cost (8) (Table
I). Currently, organoids from multiple types of cancer have
been established, including colorectal cancer (CRC), breast,
pancreatic and lung cancer (9–12).
These tumor organoids not only preserve the features of original
tumors at genomic, molecular and epigenetic levels, but also
contribute to predicting patient responses to therapies, thus
offering potential for unveiling the biology of tumorigenesis,
promoting drug discovery and personalized treatment in cancer.
The present review aimed to summarize evolution of
tumor organoids and their combination with advanced technologies,
such as organ-on-a-chip, 3D-bioprinting, tissue-engineered cell
scaffolds and clustered regularly interspaced short palindromic
repeats (CRISPR)/CRISPR-associated protein 9 (CRISPR-Cas9), as well
as the application of tumor organoids in basic and clinical
research.
Tumor organoids reflect diverse key characteristics
of tumor progression, but they lack characteristics such as
vasculature, stomal components and tissue-resident immune cells
(42). Moreover, multiple
biophysical and biochemical factors from the tumor microenvironment
(TME) are difficult to replicate accurately using conventional 3D
organoid culture. Therefore, technologies, including
organ-on-a-chip, 3D bio-printing, tissue-engineered cell scaffolds
and CRISPR-Cas9 (Table II), are
combined with tumor organoids as more precise models to study the
mechanisms of tumorigenesis, progression and drug resistance
(Fig. 2).
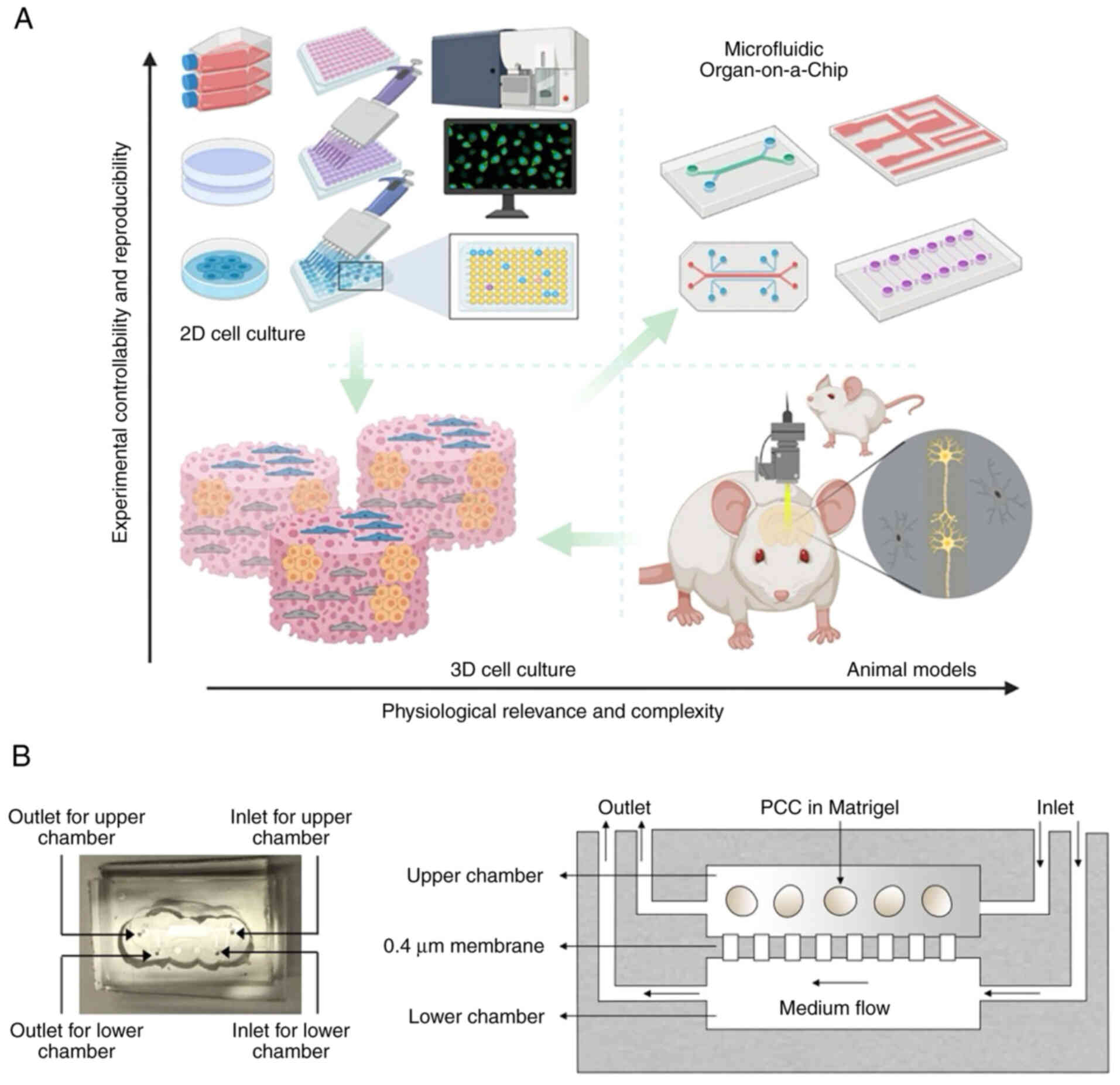
As a microfabricated device, the organ-on-chip is
designated to integrate the culture of extracellular matrix (ECM),
living cells and microstructures imitating organs or tissue
(43,44). Integrating living human cells into
a synthetically produced microenvironment models physiological
homeostasis and the process of complex diseases (Fig. 3A) (43). Demers et al (45) developed a versatile microfluidic
platform that mimics in vivo spatial and temporal chemical
environments during neural tube development. Using similar
techniques, Wang et al (46) developed a brain organoid-on-a-chip
system from human iPSCs, promoting 3D culture, in situ
neural differentiation and self-organization of brain organoids
under continuous perfusion of neural differentiation medium in a
controlled manner.
The organ-on-a-chip has the advantages of specific
stroma, requiring a small amount of tissue for analysis,
high-resolution optical measurement, real-time tracking of organoid
morphogenesis and inexpensive manufacture. Although tumor
microsystems are used to explore the cancer-specific hallmarks, the
TME complexity cannot be recapitulated due to simultaneous or
successive occurrence of cancer-specific hallmarks. Shirure et
al (47) designed a
tumor-on-a-chip microfluidic platform and that this could
simultaneously model the hallmark characteristics of tumor
progression in both cell lines and patient-derived organoids
(PDOs), such as proliferation, migration, angiogenesis and
intravasation.
At present, the culture environment of organoids
lacks vascularization, leading to decreased organoid lifespan and
changeability in tissue-specific functionality and architecture
(48). In a previous study, 3D
vascularized liver organoids comprising induced hepatic cells and
decellularized liver ECM were developed based on the microfluidic
system, exhibiting improved liver functionality, biosynthetic and
metabolic activity, as well as drug response; this study also
confirmed the feasibility of vascularized liver organ-on-a-chip
systems as a high-throughput drug screening platform (49). To study tumor angiogenesis, the
human primary clear cell renal cell carcinoma (ccRCC) cells are
combined with endothelial cells in a vascularized, flow-directed,
3D culture system. Under continuous flow, cRCC clusters preserve
the key angiogenic signaling axis between ccRCC and endothelial
cells by promoting endothelial cell sprouting. This system
signifies a vascularized tumor model with adjustable perfusate,
input cells and matrices (50).
The PDOs established in a multicellular microfluidic chip may
prolong cellular function and longevity and construct an intricate
organotypic TME (Fig. 3B).
Targeting stroma in a tumor-chip model notably increases response
to chemotherapy in cancer cells, further verifying the application
of the tumor-chip device in drug testing (51). Additionally, multiorgan models of
coculture with SC-derived stomach, intestinal and liver organoids
have also been established, promoting the discovery of interorgan
crosstalk characteristics (52).
The aforementioned findings indicate how the organoids combined
with organ-on-a-chip technique replicate cell maturity.
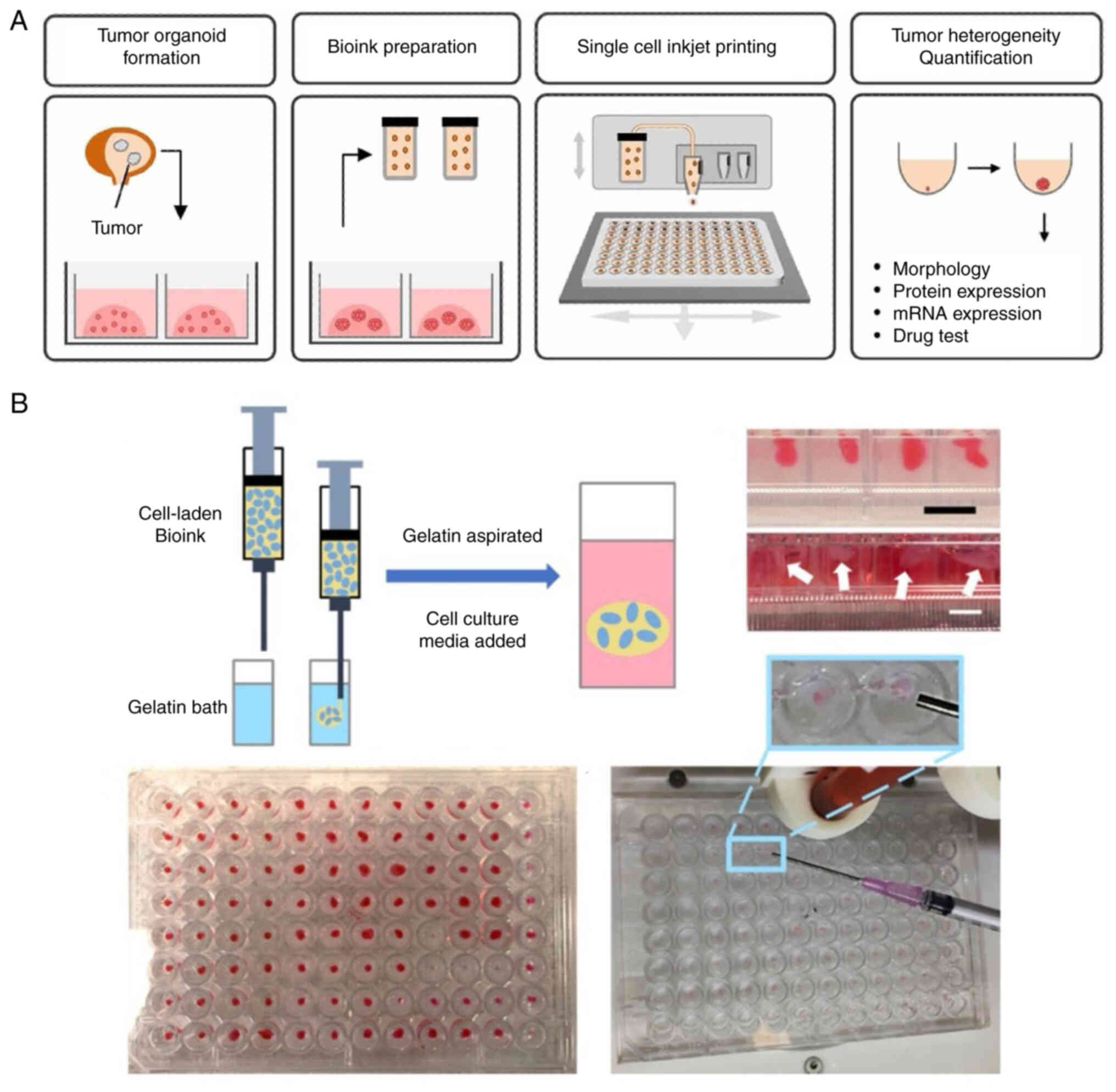
3D bio-printing accurately controls the spatial
arrangement of cells, biomaterials and soluble factors, forming
intricate multicellular structures (53,54).
By offering tumor-specific ECM, accurate geometric architecture and
biophysical properties, bio-printing can replicate the TME, thus
promoting the establishment of complicated and controllable 3D
tissue models. In most studies, however, monodispersed tumor cells
used as bio-printing building blocks do not effectively replicate
the tumor progression due to the rare presence of volumetric tumor
cells in isolation (55,56). The combination of 3D bio-printing
and tumor organoids allows for the introduction of miniaturized
tumor aggregations into a heterogeneous 3D niche containing stromal
cells and hydrogels, which are more cell-specific for simulation of
TME features and high-throughput drug screening (Fig. 4A) (57,58).
Matrigel is key for the culture of most organoids,
but it may elevate the risk of animal-derived microbial infection
and batch-to-batch variability in organoids, leading to
unreproducible experimental results (64,65).
Tissue-engineered cell scaffolds support cell proliferation and
attachment and simulate ECM function in vivo (66). For cell- and tissue-derived
matrices, synthetic scaffolds, such as polyethylene glycol
(PEG)-based hydrogel scaffolds, allow control over the growth
conditions.
For conventional organoid cultures, generic matrices
are usually applied, but they are difficult to adjust to replicate
the unique TME. Ng et al (67) used gelatin-based hydrogels to
demonstrate CRC organoid sensitivity to multiple drugs in
vivo, and found that these hydrogels may be a promising
platform for biochemically and mechanically defined matrices used
in multiple types of tumor organoid. In a previous study, a fully
synthetic hydrogel scaffold was constructed based on the 8-arm PEG
and pancreatic cancer organoids were generated successfully
(68). Through regulation of
hydrogel properties, the proliferation of pancreatic cancer
organoids is controlled, and the phenotypic traits of the TME in
vivo are effectively replicated when stromal cells are
incorporated into the hydrogels (68). These findings suggest that
synthetic scaffolds replicate a pathologically remodeled TME for
studying normal and pancreatic cancer cells in vitro.
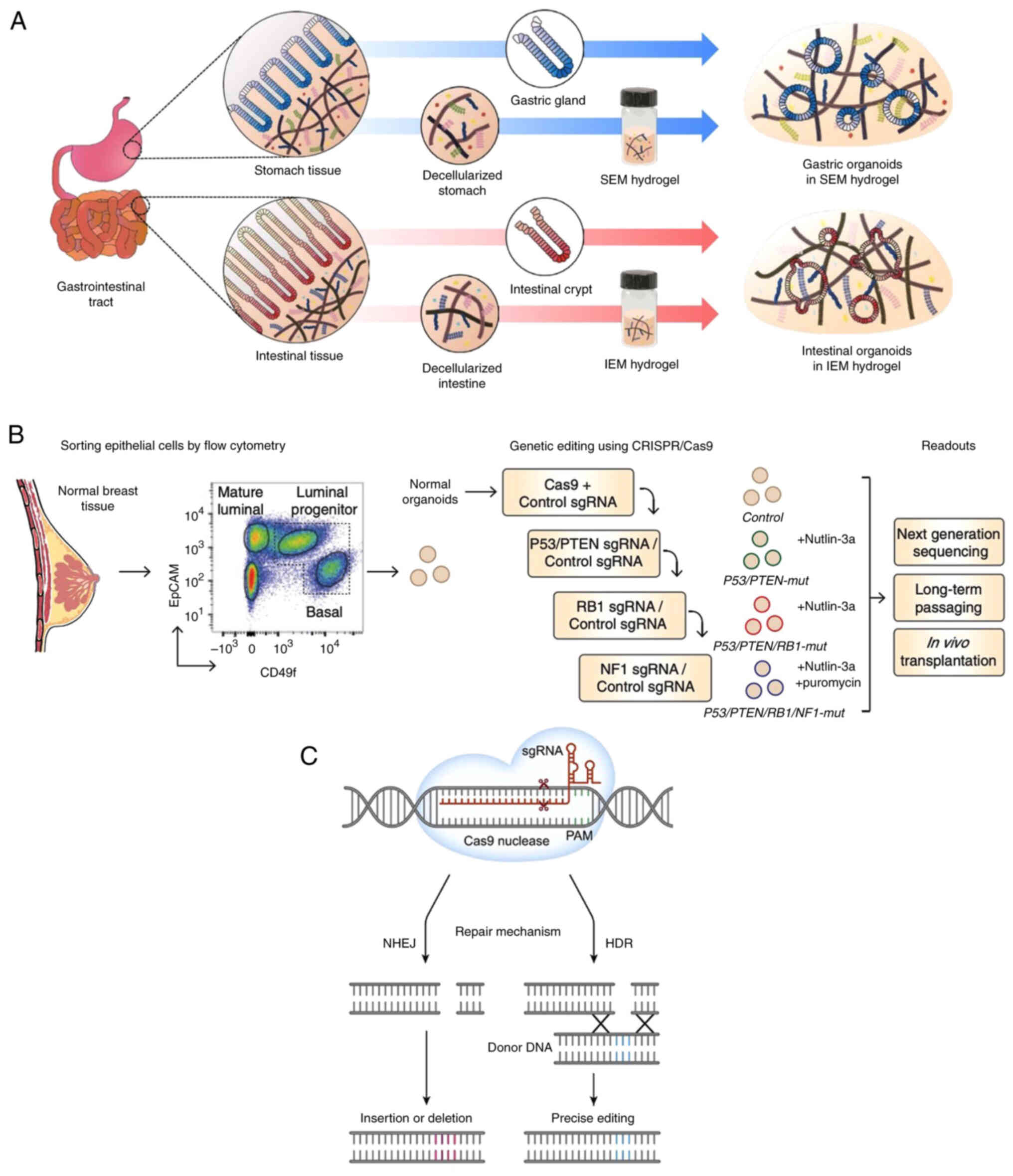
Another study showed that ECM hydrogels generated organoids
appropriate for gastrointestinal disease modeling, tissue
regeneration and drug development (Fig. 5A), which may serve as effective
alternatives to Matrigel (69).
Accordingly, tissue-engineered cell scaffolds are promising
next-generation materials for organoid technology to understand
organ-based developmental biology and predict drug response in
tumor organoids (67–69).
CRISPR-Cas9 enables more efficient gene knockout and
knock-in than other types of genome editor through introduction of
DNA double-strand breaks at specific genomic loci (70). In addition to identification of
novel targets for cancer therapy, CRISPR-Cas9 is also used to
produce genetically inhibited animal models for drug development
(71,72). CRISPR-Cas9 combined with 3D
organoid systems facilitates development of precise cancer models
for studying diverse mechanisms of tumor progression, metastasis,
interactions and drug resistance. Organoid systems not only mimic
the human disease and tailor therapeutic strategies, but also serve
as an experimental platform for mechanistically studying the gene
function in humans (73).
Tumorigenesis and progression primarily depend on
the accumulation of genetic alterations. Understanding the
mutational process is key to analyzing the mechanism of
tumorigenesis. Multiple studies have demonstrated the feasibility
of introducing pathological mutations into normal organoids using
genetic modification to simulate tumorigenesis (74,76,82,83)
(Fig. 6). As reported by Matano
et al (74), isogenic
organoids with mutations show tumorigenicity in mice when gene
mutations from driver pathways are introduced into organoids
derived from normal human intestinal epithelium (84). By establishing AT-rich binding
domain protein 1A-deficient human gastric cancer organoids using
CRISPR/Cas9 technology, modes of oncogenic transformation are
revealed, including essential transcriptional forkhead box protein
M1/baculoviral IAP repeat-containing 5-stimulated proliferation and
non-essential Wnt-inhibited mucinous differentiation (82). As a key cause of mortality in
patients with cancer, the migration and invasion of tumor cells
also serve important roles in tumor progression. Through coculture
with mammary tumor organoids, a tissue-engineered model with
physiologically realistic microvessels was created, which allows
quantitative and real-time evaluation of tumor-vessel interactions
under the conditions that retain various in vivo
characteristics to identify targetable mechanisms of vascular
recruitment and intravasation (84). In the patient-derived breast cancer
organoids, CD homophilic interactions and subsequent
CD44-p21-activated kinase 2 interactions mediate tumor cluster
migration and aggregation (85).
Moreover, invasion could also be triggered in breast cancer
subtypes by basal epithelial gene expression (86).
Tumor organoids not only contribute to understanding
the mechanism of tumorigenesis, but also predict the response to
therapies, including chemotherapy, radiotherapy and targeted
therapy (91–93). Vlachogiannis et al (92) established organoids from metastatic
gastrointestinal cancer to predict the response to targeted drugs
or chemotherapy, showing sensitivity of 100 and specificity of 93%.
Gastric cancer organoids faithfully reflect responses to commonly
used chemotherapy drugs, such as irinotecan, oxaliplatin,
docetaxel, epirubicin and 5-fluerouracil (91). Tiriac et al (94) generated a pancreatic cancer
organoid library and found that PDO profiling based on the
next-generation sequencing of DNA and RNA and pharmacotyping may
predict responses to chemotherapy in pancreatic cancer. Ji et
al (95) identified potential
drug combination therapies based on pharmaco-proteogenomic
profiling of liver cancer organoids, offering guidance for clinical
patient selection and drug combination therapies. Ganesh et
al (93) revealed the
heterogeneity of rectal cancer organoids in chemoradiation and
ex vivo responses to clinically relevant chemoradiation
associated with clinical responses. Moreover, KRAS-wild-type
rectal cancer organoids are sensitive to cetuximab, while
KRAS-mutant organoids are resistant, which is consistent
with the results of a clinical trial that KRAS mutations are
associated with resistance to EGFR-targeted therapy (93). Importantly, in a prospective,
interventional clinical trial of the last-line systemic therapy
based on PDOs, improved clinical outcomes were observed in patients
with CRC compared with those receiving the best supportive care
alone (96). In addition, the
association between tumor organoids and clinical response has been
identified in other types of cancer. By establishing PDOs from
different stages of bladder cancer, Minoli et al (97) demonstrated that the PDOs exhibit
heterogenous drug responses to standard-of-care treatment and drug
screening showed sensitivity to targeted therapy. In a real-world
study, lung cancer organoids were used to validate the response to
osimertinib, chemotherapy and dual-targeted therapy and a high
concordance was identified between lung cancer organoids and
clinical response (12). PDO
pharmaco-phenotyping not only reflects previous treatment responses
of patients with advanced breast cancer but also serves as a
potential platform to guide personalized treatment (9). Breast cancer organoids may also be
used to predict patient-specific response to drug treatment
(98).
Coculture of tumor organoids with immune components
may generate tumor-reactive T cells, which may promote the
prediction and evaluation of tumor responses at an individual level
by blocking PD-1/PD-L1 (98,99).
Cattaneo et al (100)
described the generation and function of tumor-reactive T cells
based on the coculture of tumor organoids with peripheral blood
mononuclear cells (PBMCs), demonstrating the feasibility of
establishing ex vivo models of T cell immunotherapy at an
individual level. Meng et al (101) developed a platform for the
expansion of tumor-targeted T cells from peripheral blood and
revealed that the coculture of tumor organoids with PBMCs generates
tumor-reactive T cells, thereby promoting personalized
immunotherapy. Moreover, tumor organoids cocultured with PBMCs are
also used to enrich tumor-reactive T cells from peripheral blood of
patients with mismatch repair-deficient CRC and non-small-cell lung
cancer (SCLC); these T cells are useful for assessing the killing
efficiency of matched tumor organoids (102). In certain organoids established
from immunotherapy-responsive tumors, activation of T cells and
tumor killing activity have been identified using PD-1/PD-L1
blockade (98). Meanwhile,
Votanopoulos et al (103)
developed an immune-enhanced tumor organoid model with a high
clinical association between these organoids and response to
checkpoint inhibitors. Collectively, tumor organoids can predict
the response to immunotherapy in cancer.
In a longitudinal, observational co-clinical study,
second mitochondria-derived activator of caspase mimetic LCL161 was
shown to serve as a treatment target in the organoids derived from
recurrent, KRAS-mutated liver metastases from rectal cancer
(104). Compared with THZ1,
YPN-005, a potent inhibitor of CDK7, shows potent antitumor effects
in SCLC organoids, suggesting its treatment value in SCLC (105). Based on the interaction of breast
cancer organoids and tumor-specific cytotoxic T cells, epigenetic
inhibitors GSK-LSD1, CUDC-101 and BML-210 identified via
high-throughput screening show antitumor activities (106). Furthermore, BML-210 promotes the
efficacy of PD-1-based immune checkpoint blockage.
The tumor-suppressing function and efficient
delivery of drugs have key roles in cancer treatment. In
multicellular hepatocellular carcinoma organoids (MCHOs) with
activated Yes-associated protein (YAP) and transcriptional
coactivator with PDZ-binding motif (TAZ) signaling, there is
stromal activation and damaged penetration of verteporfin;
inhibiting YAP/TAZ transcriptional activity in hepatocellular
carcinoma (HCC) cells may facilitate the penetration of drugs into
MCHOs. These findings suggest that the treatment targeting
activated tumor stroma may promote drug delivery into HCC cells
with increased YAP/TAZ activity (107).
To improve understanding of angiogenic signaling
pathways and investigate effective treatment strategies, tumor
vasculature must be preserved in organoid cultures. For organoid
vascularization, implantation of organoids into highly vascularized
tissue is a frequently used method (108,109). Another method is coculture with
cells including vascular smooth muscle and epithelial cells (ECs)
based on gene editing or microfluidic platforms (110). By integrating mesodermal
progenitor cells into organoids, Wörsdörfer et al (111) found that the vascularized
organoids formed following coculture with tumor cells or neural
spheroids and the vessels in tumor organoids are associated with
the host vessels after transplantation. Breast cancer organoids
with ECs and immune cells show an obvious angiogenic response when
cultured with the vascular network (112). Similarly, in the collagen- and
hyaluronic acid-enriched ECM with human fibroblasts and MCF-7
cells, vascularized breast cancer organoids have been established
successfully (113).
Additionally, in the coculture system of organoids and ECs,
vascularization is triggered by vascular endothelial growth factors
and hypoxia gradients based on compartmentalized microfluidic chips
(109,114). The aforementioned findings
underscore the importance of coculture models in organoid
vascularization.
Coculture of tumor organoids with immune components,
such as fibroblasts, stroma, ECs and immune cells, models the
tumor-immune interactions, which provides insights into cancer
immunotherapy (115). Using the
tumor organoid culture for expansion and characterization of
tumor-reactive T cells, Dijkstra et al (102) developed a multifunctional
platform to study tumor-immune interactions and concluded that
CD8+ T cells in PBMCs of the same patient are activated
in half of the CRC organoids, with similar results in non-SCLC
organoids. Meanwhile, a platform for expanding tumor-targeted T
cells has been reported in patients with pancreatic cancer
(116). By coculturing PBMCs and
autologous tumor organoids, this platform enables recognition and
expansion of tumor-targeted cytotoxic T cells (99). Based on the coculture of tumor
organoids with PBMCs, the establishment and functional assessment
of tumor-reactive T cells has also been described (98).
By generating organoids from surgically resected
types of cancer based on the air-liquid interface, Neal et
al (98) demonstrated that
these organoid cultures retain various endogenous immune cell types
and non-immune matrix components; immune checkpoint blockade with
anti-PD-1 and/or anti-PD-L1 kills the tumor cells through induction
of the expansion and activation of tumor antigen-specific T cells
in organoid cultures. Moreover, a previous study suggested the
potential of the organoid culture system in predicting adoptive
immunotherapy responses following incorporation of patient-specific
mature lymph node antigen-presenting cells into organoids (103).
There are limitations to the application of tumor
organoids that need to be addressed. First, there are no
standardized evaluation criteria and culture protocols. Currently,
the culture conditions of tumor organoids are diverse, leading to
large differences in results between laboratories and teams. These
differences may arise from inconsistent tissue dissociation,
undefined formulation of culture medium and different matrices. To
promote the standardization and reproducibility of tumor organoid
cultures, culture conditions and laboratory operations should be
unified as much as possible. Organoid culture is also affected by
tumor cell composition, cell activity and tumor heterogeneity. For
certain types of cancer, such as prostate cancer (26), the low success rate hinders
repeatability and reproducibility, thereby affecting
high-throughput screening. Hence, development of standard culture
and evaluation protocols and application of well-defined materials
are required for improving the success rate of organoid generation.
Second, due to potential inclusion of normal cells, TME
reconstruction is challenging. Tumor organoid models lack certain
in vivo components, such as endothelial and immune cells and
fibroblasts. Although it is challenging to establish the organoids
comprising immune and vascular cells, this limitation may be
resolved in the future with the development of organoid technology.
Third, the currently established tumor organoids are primarily from
ECs. In the future, studies should establish organoids from
non-ECs, which may further optimize the treatment of tumors such as
CRC and lung cancer (117,118).
Additionally, during the long-term culture and passage of tumor
organoids epigenetic drift may occur (43). To avoid normal cells being
contaminated and make organoids more mature, investigating the
mechanisms underlying epigenetic drift is needed. Shi et al
assessed the tumor purity in long-term cultures and found that none
were contaminated with normal or non-human cells (119). In addition to recapitulating the
biology that drives histologic appearance of original tumors, their
organoid models had not drifted at the molecular level. More
importantly, tumor organoids should be improved to model the
interactions between cells, tissue and organs. Although TME can be
replicated through coculture with stromal cells and ECM, the role
of peripheral immune systems is not evaluated (77). Combination of tumor organoids with
advanced technologies allows modeling of a more complex and
realistic state, which may overcome the aforementioned challenges
and create more appropriate model systems for cancer treatment.
Patient-derived tumor organoids are more advanced at
physiological and clinical levels compared with conventional cancer
cell lines and PDXs. Despite challenges, tumor organoids show
potential in the treatment of cancer. Tumor organoids may be
combined with advanced technologies, such as organ-on-a-chip,
3D-bioprinting, tissue-engineered cell scaffolds and CRISPR-Cas9,
which may not only overcome defects of conventional culture
methods, but also expand the application range, offering insights
into the treatment strategies in cancer. Combined application of
tumor organoids and advanced technologies allows accurate
simulation of tumor heterogeneity, vascularization and tumor-immune
interactions, facilitating comprehensive high-throughput drug
screening to predict drug responses and optimize treatment options
to promote personalized treatment in cancer.
Not applicable.
Funding: No funding was received.
Not applicable.
YW, FZ, JH and SW conceived and designed the study
and wrote the manuscript. FD acquired data and revised the
manuscript critially. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu C, Qin T, Huang Y, Li Y, Chen G and
Sun C: Drug screening model meets cancer organoid technology.
Transl Oncol. 13:1008402020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Magré L, Verstegen MMA, Buschow S, van der
Laan LJW, Peppelenbosch M and Desai J: Emerging organoid-immune
co-culture models for cancer research: From oncoimmunology to
personalized immunotherapies. J Immunother Cancer. 11:e0062902023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drapkin BJ, George J, Christensen CL,
Mino-Kenudson M, Dries R, Sundaresan T, Phat S, Myers DT, Zhong J,
Igo P, et al: Genomic and functional fidelity of small cell lung
cancer patient-derived xenografts. Cancer Discov. 8:600–615. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Izumchenko E, Paz K, Ciznadija D, Sloma I,
Katz A, Vasquez-Dunddel D, Ben-Zvi I, Stebbing J, McGuire W, Harris
W, et al: Patient-derived xenografts effectively capture responses
to oncology therapy in a heterogeneous cohort of patients with
solid tumors. Ann Oncol. 28:2595–2605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saito R, Kobayashi T, Kashima S, Matsumoto
K and Ogawa O: Faithful preclinical mouse models for better
translation to bedside in the field of immuno-oncology. Int J Clin
Oncol. 25:831–841. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ben-David U, Ha G, Tseng YY, Greenwald NF,
Oh C, Shih J, McFarland JM, Wong B, Boehm JS, Beroukhim R and Golub
TR: Patient-derived xenografts undergo mouse-specific tumor
evolution. Nat Genet. 49:1567–1575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang XY, Wu S, Wang D, Chu C, Hong Y, Tao
M, Hu H, Xu M, Guo X and Liu Y: Human organoids in basic research
and clinical applications. Signal Transduct Target Ther. 7:1682022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen P, Zhang X, Ding R, Yang L, Lyu X,
Zeng J, Lei JH, Wang L, Bi J, Shao N, et al: Patient-derived
organoids can guide personalized-therapies for patients with
advanced breast cancer. Adv Sci (Weinh). 8:e21011762021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu KS, Adileh M, Martin ML, Makarov V,
Chen J, Wu C, Bodo S, Klingler S, Sauvé CG, Szeglin BC, et al:
Colorectal cancer develops inherent radiosensitivity that can be
predicted using patient-derived organoids. Cancer Res.
82:2298–2312. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seppälä TT, Zimmerman JW, Suri R, Zlomke
H, Ivey GD, Szabolcs A, Shubert CR, Cameron JL, Burns WR, Lafaro
KJ, et al: Precision medicine in pancreatic cancer: Patient-derived
organoid pharmacotyping is a predictive biomarker of clinical
treatment response. Clin Cancer Res. 28:3296–3307. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang HM, Zhang CY, Peng KC, Chen ZX, Su
JW, Li YF, Li WF, Gao QY, Zhang SL, Chen YQ, et al: Using
patient-derived organoids to predict locally advanced or metastatic
lung cancer tumor response: A real-world study. Cell Rep Med.
4:1009112023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drost J and Clevers H: Organoids in cancer
research. Nat Rev Cancer. 18:407–418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilson HV: A new method by which sponges
may be artificially reared. Science. 25:912–915. 1907. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lindberg K, Brown ME, Chaves HV, Kenyon KR
and Rheinwald JG: In vitro propagation of human ocular surface
epithelial cells for transplantation. Invest Ophthalmol Vis Sci.
34:2672–2679. 1993.PubMed/NCBI
|
|
16
|
Pellegrini G, Traverso CE, Franzi AT,
Zingirian M, Cancedda R and De Luca M: Long-term restoration of
damaged corneal surfaces with autologous cultivated corneal
epithelium. Lancet. 349:990–993. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JY, Nam Y, Rim YA and Ju JH: Review of
the current trends in clinical trials involving induced pluripotent
stem cells. Stem Cell Rev Rep. 18:142–154. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng H, Liu C, Cai X, Lu Y, Xu Y and Yu
X: iPSCs derived from malignant tumor cells: Potential application
for cancer research. Curr Stem Cell Res Ther. 11:444–450. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orqueda AJ, Giménez CA and Pereyra-Bonnet
F: iPSCs: A minireview from bench to bed, including organoids and
the crispr system. Stem Cells Int. 2016:59347822016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dimos JT, Rodolfa KT, Niakan KK,
Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R,
Goland R, et al: Induced pluripotent stem cells generated from
patients with ALS can be differentiated into motor neurons.
Science. 321:1218–1221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato T, Vries RG, Snippert HJ, van de
Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters
PJ and Clevers H: Single Lgr5 stem cells build crypt-villus
structures in vitro without a mesenchymal niche. Nature.
459:262–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuratnik A and Giardina C: Intestinal
organoids as tissue surrogates for toxicological and
pharmacological studies. Biochem Pharmacol. 85:1721–1726. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spence JR, Mayhew CN, Rankin SA, Kuhar MF,
Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn
AM, et al: Directed differentiation of human pluripotent stem cells
into intestinal tissue in vitro. Nature. 470:105–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eiraku M, Takata N, Ishibashi H, Kawada M,
Sakakura E, Okuda S, Sekiguchi K, Adachi T and Sasai Y:
Self-organizing optic-cup morphogenesis in three-dimensional
culture. Nature. 472:51–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sato T, Stange DE, Ferrante M, Vries RG,
Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J,
Siersema PD and Clevers H: Long-term expansion of epithelial
organoids from human colon, adenoma, adenocarcinoma, and Barrett's
epithelium. Gastroenterology. 141:1762–1772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao D, Vela I, Sboner A, Iaquinta PJ,
Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora
VK, et al: Organoid cultures derived from patients with advanced
prostate cancer. Cell. 159:176–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu M, Bardia A, Aceto N, Bersani F, Madden
MW, Donaldson MC, Desai R, Zhu H, Comaills V, Zheng Z, et al:
Cancer therapy. Ex vivo culture of circulating breast tumor cells
for individualized testing of drug susceptibility. Science.
345:216–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boj SF, Hwang CI, Baker LA, Chio II, Engle
DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, et al:
Organoid models of human and mouse ductal pancreatic cancer. Cell.
160:324–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartfeld S, Bayram T, van de Wetering M,
Huch M, Begthel H, Kujala P, Vries R, Peters PJ and Clevers H: In
vitro expansion of human gastric epithelial stem cells and their
responses to bacterial infection. Gastroenterology. 148:126–136.e6.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hubert CG, Rivera M, Spangler LC, Wu Q,
Mack SC, Prager BC, Couce M, McLendon RE, Sloan AE and Rich JN: A
three-dimensional organoid culture system derived from human
glioblastomas recapitulates the hypoxic gradients and cancer stem
cell heterogeneity of tumors found in vivo. Cancer Res.
76:2465–2477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Broutier L, Mastrogiovanni G, Verstegen
MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R,
Sidorova O, Gaspersz MP, et al: Human primary liver cancer-derived
organoid cultures for disease modeling and drug screening. Nat Med.
23:1424–1435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Girda E, Huang EC, Leiserowitz GS and
Smith LH: The use of endometrial cancer patient-derived organoid
culture for drug sensitivity testing is feasible. Int J Gynecol
Cancer. 27:1701–1707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pauli C, Hopkins BD, Prandi D, Shaw R,
Fedrizzi T, Sboner A, Sailer V, Augello M, Puca L, Rosati R, et al:
Personalized in vitro and in vivo cancer models to guide precision
medicine. Cancer Discov. 7:462–477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kijima T, Nakagawa H, Shimonosono M,
Chandramouleeswaran PM, Hara T, Sahu V, Kasagi Y, Kikuchi O, Tanaka
K, Giroux V, et al: Three-Dimensional organoids reveal therapy
resistance of esophageal and oropharyngeal squamous cell carcinoma
cells. Cell Mol Gastroenterol Hepatol. 7:73–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka N, Osman AA, Takahashi Y, Lindemann
A, Patel AA, Zhao M, Takahashi H and Myers JN: Head and neck cancer
organoids established by modification of the CTOS method can be
used to predict in vivo drug sensitivity. Oral Oncol. 87:49–57.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kopper O, de Witte CJ, Lõhmussaar K,
Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost
N, Begthel H, et al: An organoid platform for ovarian cancer
captures intra- and interpatient heterogeneity. Nat Med.
25:838–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ,
Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, et al: Patient-derived
lung cancer organoids as in vitro cancer models for therapeutic
screening. Nat Commun. 10:39912019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grassi L, Alfonsi R, Francescangeli F,
Signore M, De Angelis ML, Addario A, Costantini M, Flex E, Ciolfi
A, Pizzi S, et al: Organoids as a new model for improving
regenerative medicine and cancer personalized therapy in renal
diseases. Cell Death Dis. 10:2012019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen D, Tan Y, Li Z, Li W, Yu L, Chen W,
Liu Y, Liu L, Guo L, Huang W and Zhao Y: Organoid cultures derived
from patients with papillary thyroid cancer. J Clin Endocrinol
Metab. 106:1410–1426. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wörsdörfer P, Takashi I, Asahina I, Sumita
Y and Ergün S: Do not keep it simple: Recent advances in the
generation of complex organoids. J Neural Transm (Vienna).
127:1569–1577. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Demyan L, Habowski AN, Plenker D, King DA,
Standring OJ, Tsang C, St Surin L, Rishi A, Crawford JM, Boyd J, et
al: Pancreatic cancer patient-derived organoids can predict
response to neoadjuvant chemotherapy. Ann Surg. 276:450–462. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beato F, Reverón D, Dezsi KB, Ortiz A,
Johnson JO, Chen DT, Ali K, Yoder SJ, Jeong D, Malafa M, et al:
Establishing a living biobank of patient-derived organoids of
intraductal papillary mucinous neoplasms of the pancreas. Lab
Invest. 101:204–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma C, Peng Y, Li H and Chen W:
Organ-on-a-Chip: A new paradigm for drug development. Trends
Pharmacol Sci. 42:119–133. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kutluk H, Bastounis EE and Constantinou I:
Integration of extracellular matrices into organ-on-chip systems.
Adv Healthc Mater. 12:e22032562023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Demers CJ, Soundararajan P, Chennampally
P, Cox GA, Briscoe J, Collins SD and Smith RL: Development-on-chip:
In vitro neural tube patterning with a microfluidic device.
Development. 143:1884–1892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Wang L, Zhu Y and Qin J: Human
brain organoid-on-a-chip to model prenatal nicotine exposure. Lab
Chip. 18:851–860. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shirure VS, Bi Y, Curtis MB, Lezia A,
Goedegebuure MM, Goedegebuure SP, Aft R, Fields RC and George SC:
Tumor-on-a-chip platform to investigate progression and drug
sensitivity in cell lines and patient-derived organoids. Lab Chip.
18:3687–3702. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Garreta E, Kamm RD, de Sousa Lopes SM,
Lancaster MA, Weiss R, Trepat X, Hyun I and Montserrat N:
Rethinking organoid technology through bioengineering. Nat Mater.
20:145–155. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jin Y, Kim J, Lee JS, Min S, Kim S, Ahn
DH, Kim YG and Cho SW: Vascularized liver organoids generated using
induced hepatic tissue and dynamic liver-specific microenvironment
as a drug testing platform. Adv Funct Mater. 28:18019542018.
View Article : Google Scholar
|
|
50
|
Miller CP, Tsuchida C, Zheng Y, Himmelfarb
J and Akilesh S: A 3D human renal cell carcinoma-on-a-chip for the
study of tumor angiogenesis. Neoplasia. 20:610–620. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Haque MR, Wessel CR, Leary DD, Wang C,
Bhushan A and Bishehsari F: Patient-derived pancreatic
cancer-on-a-chip recapitulates the tumor microenvironment.
Microsyst Nanoeng. 8:362022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Picollet-D'hahan N, Zuchowska A, Lemeunier
I and Le Gac S: Multiorgan-on-a-Chip: A systemic approach to model
and decipher inter-organ communication. Trends Biotechnol.
39:788–810. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mandrycky C, Wang Z, Kim K and Kim DH: 3D
bioprinting for engineering complex tissues. Biotechnol Adv.
34:422–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Datta P, Barui A, Wu Y, Ozbolat V, Moncal
KK and Ozbolat IT: Essential steps in bioprinting: From pre- to
post-bioprinting. Biotechnol Adv. 36:1481–1504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang X, Luo Y, Ma Y, Wang P and Yao R:
Converging bioprinting and organoids to better recapitulate the
tumor microenvironment. Trends Biotechnol. 42:648–663. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ding S, Feng L, Wu J, Zhu F, Tan Z and Yao
R: Bioprinting of stem cells: Interplay of bioprinting process,
bioinks, and stem cell properties. ACS Biomater Sci Eng.
4:3108–3124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gaspar VM, Lavrador P, Borges J, Oliveira
MB and Mano JF: Advanced bottom-up engineering of living
architectures. Adv Mater. 32:e19039752020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yoon WH, Lee HR, Kim S, Kim E, Ku JH, Shin
K and Jung S: Use of inkjet-printed single cells to quantify
intratumoral heterogeneity. Biofabrication. 12:0350302020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mollica PA, Booth-Creech EN, Reid JA,
Zamponi M, Sullivan SM, Palmer XL, Sachs PC and Bruno RD: 3D
bioprinted mammary organoids and tumoroids in human mammary derived
ECM hydrogels. Acta Biomater. 95:201–213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Reid JA, Palmer XL, Mollica PA, Northam N,
Sachs PC and Bruno RD: A 3D bioprinter platform for mechanistic
analysis of tumoroids and chimeric mammary organoids. Sci Rep.
9:74662019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Maloney E, Clark C, Sivakumar H, Yoo K,
Aleman J, Rajan SAP, Forsythe S, Mazzocchi A, Laxton AW, Tatter SB,
et al: Immersion bioprinting of tumor organoids in multi-well
plates for increasing chemotherapy screening throughput.
Micromachines (Basel). 11:2082020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mao S, He J, Zhao Y, Liu T, Xie F, Yang H,
Mao Y, Pang Y and Sun W: Bioprinting of patient-derived in vitro
intrahepatic cholangiocarcinoma tumor model: Establishment,
evaluation and anti-cancer drug testing. Biofabrication.
12:0450142020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bernal PN, Bouwmeester M, Madrid-Wolff J,
Falandt M, Florczak S, Rodriguez NG, Li Y, Größbacher G, Samsom RA,
van Wolferen M, et al: Volumetric bioprinting of organoids and
optically tuned hydrogels to build liver-like metabolic
biofactories. Adv Mater. 34:e21100542022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kleinman HK and Martin GR: Matrigel:
Basement membrane matrix with biological activity. Semin Cancer
Biol. 15:378–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Talbot NC and Caperna TJ: Proteome array
identification of bioactive soluble proteins/peptides in Matrigel:
Relevance to stem cell responses. Cytotechnology. 67:873–883. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Aisenbrey EA and Murphy WL: Synthetic
alternatives to Matrigel. Nat Rev Mater. 5:539–551. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ng S, Tan WJ, Pek MMX, Tan MH and Kurisawa
M: Mechanically and chemically defined hydrogel matrices for
patient-derived colorectal tumor organoid culture. Biomaterials.
219:1194002019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Below CR, Kelly J, Brown A, Humphries JD,
Hutton C, Xu J, Lee BY, Cintas C, Zhang X, Hernandez-Gordillo V, et
al: A microenvironment-inspired synthetic three-dimensional model
for pancreatic ductal adenocarcinoma organoids. Nat Mater.
21:110–119. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim S, Min S, Choi YS, Jo SH, Jung JH, Han
K, Kim J, An S, Ji YW, Kim YG and Cho SW: Tissue extracellular
matrix hydrogels as alternatives to Matrigel for culturing
gastrointestinal organoids. Nat Commun. 13:16922022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Komor AC, Badran AH and Liu DR:
CRISPR-Based technologies for the manipulation of eukaryotic
genomes. Cell. 169:5592017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Aguirre AJ, Meyers RM, Weir BA, Vazquez F,
Zhang CZ, Ben-David U, Cook A, Ha G, Harrington WF, Doshi MB, et
al: Genomic copy number dictates a gene-independent cell response
to CRISPR/Cas9 targeting. Cancer Discov. 6:914–929. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sharma G, Sharma AR, Bhattacharya M, Lee
SS and Chakraborty C: CRISPR-Cas9: A preclinical and clinical
perspective for the treatment of human diseases. Mol Ther.
29:571–586. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Artegiani B, van Voorthuijsen L, Lindeboom
RGH, Seinstra D, Heo I, Tapia P, López-Iglesias C, Postrach D,
Dayton T, Oka R, et al: Probing the tumor suppressor function of
BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell.
24:927–943.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Matano M, Date S, Shimokawa M, Takano A,
Fujii M, Ohta Y, Watanabe T, Kanai T and Sato T: Modeling
colorectal cancer using CRISPR-Cas9-mediated engineering of human
intestinal organoids. Nat Med. 21:256–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Erlangga Z, Wolff K, Poth T, Peltzer A,
Nahnsen S, Spielberg S, Timrott K, Woller N, Kühnel F, Manns MP, et
al: Potent antitumor activity of liposomal irinotecan in an
organoid- and CRISPR-Cas9-based murine model of gallbladder cancer.
Cancers (Basel). 11:19042019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dekkers JF, Whittle JR, Vaillant F, Chen
HR, Dawson C, Liu K, Geurts MH, Herold MJ, Clevers H, Lindeman GJ
and Visvader JE: Modeling breast cancer using CRISPR-Cas9-mediated
engineering of human breast organoids. J Natl Cancer Inst.
112:540–544. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhan T, Rindtorff N, Betge J, Ebert MP and
Boutros M: CRISPR/Cas9 for cancer research and therapy. Semin
Cancer Biol. 55:106–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Vaishnavi A, Juan J, Jacob M, Stehn C,
Gardner EE, Scherzer MT, Schuman S, Van Veen JE, Murphy B, Hackett
CS, et al: Transposon mutagenesis reveals RBMS3 silencing as a
promoter of malignant progression of BRAFV600E-driven lung
tumorigenesis. Cancer Res. 82:4261–4273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Takeda H, Kataoka S, Nakayama M, Ali MAE,
Oshima H, Yamamoto D, Park JW, Takegami Y, An T, Jenkins NA, et al:
CRISPR-Cas9-mediated gene knockout in intestinal tumor organoids
provides functional validation for colorectal cancer driver genes.
Proc Natl Acad Sci USA. 116:15635–15644. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Artegiani B, Hendriks D, Beumer J, Kok R,
Zheng X, Joore I, de Sousa Lopes SC, van Zon J, Tans S and Clevers
H: Fast and efficient generation of knock-in human organoids using
homology-independent CRISPR-Cas9 precision genome editing. Nat Cell
Biol. 22:321–331. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hendriks D, Artegiani B, Hu H, de Sousa
Lopes SC and Clevers H: Establishment of human fetal hepatocyte
organoids and CRISPR-Cas9-based gene knockin and knockout in
organoid cultures from human liver. Nat Protoc. 16:182–217. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lo YH, Kolahi KS, Du Y, Chang CY,
Krokhotin A, Nair A, Sobba WD, Karlsson K, Jones SJ, Longacre TA,
et al: A CRISPR/Cas9-engineered ARID1A-deficient human gastric
cancer organoid model reveals essential and nonessential modes of
oncogenic transformation. Cancer Discov. 11:1562–1581. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yao Q, Cheng S, Pan Q, Yu J, Cao G, Li L
and Cao H: Organoids: Development and applications in disease
models, drug discovery, precision medicine, and regenerative
medicine. MedComm (2020). 5:e7352024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Silvestri VL, Henriet E, Linville RM, Wong
AD, Searson PC and Ewald AJ: A tissue-engineered 3D microvessel
model reveals the dynamics of mosaic vessel formation in breast
cancer. Cancer Res. 80:4288–4301. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu X, Taftaf R, Kawaguchi M, Chang YF,
Chen W, Entenberg D, Zhang Y, Gerratana L, Huang S, Patel DB, et
al: Homophilic CD44 interactions mediate tumor cell aggregation and
polyclonal metastasis in patient-derived breast cancer models.
Cancer Discov. 9:96–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cheung KJ, Gabrielson E, Werb Z and Ewald
AJ: Collective invasion in breast cancer requires a conserved basal
epithelial program. Cell. 155:1639–1651. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Boos SL, Loevenich LP, Vosberg S,
Engleitner T, Öllinger R, Kumbrink J, Rokavec M, Michl M, Greif PA,
Jung A, et al: Disease modeling on tumor organoids implicates AURKA
as a therapeutic target in liver metastatic colorectal cancer. Cell
Mol Gastroenterol Hepatol. 13:517–540. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sánchez-Botet A, Quandt E, Masip N,
Escribá R, Novellasdemunt L, Gasa L, Li VSW, Raya Á, Clotet J and
Ribeiro MPC: Atypical cyclin P regulates cancer cell stemness
through activation of the WNT pathway. Cell Oncol (Dordr).
44:1273–1286. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Harada K, Sakamoto N, Ukai S, Yamamoto Y,
Pham QT, Taniyama D, Honma R, Maruyama R, Takashima T, Ota H, et
al: Establishment of oxaliplatin-resistant gastric cancer
organoids: Importance of myoferlin in the acquisition of
oxaliplatin resistance. Gastric Cancer. 24:1264–1277. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mosquera MJ, Kim S, Bareja R, Fang Z, Cai
S, Pan H, Asad M, Martin ML, Sigouros M, Rowdo FM, et al:
Extracellular matrix in synthetic hydrogel-based prostate cancer
organoids regulate therapeutic Response to EZH2 and DRD2
Inhibitors. Adv Mater. 34:e21000962022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L,
Xia F, Fu G, Deng Y, Pan M, et al: Patient-Derived organoids
predict chemoradiation responses of locally advanced rectal cancer.
Cell Stem Cell. 26:17–26.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Vlachogiannis G, Hedayat S, Vatsiou A,
Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford
I, Burke R, et al: Patient-derived organoids model treatment
response of metastatic gastrointestinal cancers. Science.
359:920–926. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ganesh K, Wu C, O'Rourke KP, Szeglin BC,
Zheng Y, Sauvé CG, Adileh M, Wasserman I, Marco MR, Kim AS, et al:
A rectal cancer organoid platform to study individual responses to
chemoradiation. Nat Med. 25:1607–1614. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tiriac H, Belleau P, Engle DD, Plenker D,
Deschênes A, Somerville TDD, Froeling FEM, Burkhart RA, Denroche
RE, Jang GH, et al: Organoid profiling identifies common responders
to chemotherapy in pancreatic cancer. Cancer Discov. 8:1112–1129.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ji S, Feng L, Fu Z, Wu G, Wu Y, Lin Y, Lu
D, Song Y, Cui P, Yang Z, et al: Pharmaco-proteogenomic
characterization of liver cancer organoids for precision oncology.
Sci Transl Med. 15:eadg33582023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jensen LH, Rogatto SR, Lindebjerg J,
Havelund B, Abildgaard C, do Canto LM, Vagn-Hansen C, Dam C,
Rafaelsen S and Hansen TF: Precision medicine applied to metastatic
colorectal cancer using tumor-derived organoids and in-vitro
sensitivity testing: A phase 2, single-center, open-label, and
non-comparative study. J Exp Clin Cancer Res. 42:1152023.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Minoli M, Cantore T, Hanhart D, Kiener M,
Fedrizzi T, La Manna F, Karkampouna S, Chouvardas P, Genitsch V,
Rodriguez-Calero A, et al: Bladder cancer organoids as a functional
system to model different disease stages and therapy response. Nat
Commun. 14:22142023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Neal JT, Li X, Zhu J, Giangarra V,
Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, et
al: Organoid Modeling of the Tumor Immune Microenvironment. Cell.
175:1972–1988.e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bozkus CC and Bhardwaj N: Tumor
organoid-originated biomarkers predict immune response to PD-1
blockade. Cancer Cell. 39:1187–1189. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Cattaneo CM, Dijkstra KK, Fanchi LF,
Kelderman S, Kaing S, van Rooij N, van den Brink S, Schumacher TN
and Voest EE: Tumor organoid-T-cell coculture systems. Nat Protoc.
15:15–39. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Meng Q, Xie S, Gray GK, Dezfulian MH, Li
W, Huang L, Akshinthala D, Ferrer E, Conahan C, Perea Del Pino S,
et al: Empirical identification and validation of tumor-targeting T
cell receptors from circulation using autologous pancreatic tumor
organoids. J Immunother Cancer. 9:e0032132021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dijkstra KK, Cattaneo CM, Weeber F,
Chalabi M, van de Haar J, Fanchi LF, Slagter M, van der Velden DL,
Kaing S, Kelderman S, et al: Generation of tumor-reactive T cells
by co-culture of peripheral blood lymphocytes and tumor organoids.
Cell. 174:1586–1598.e12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Votanopoulos KI, Forsythe S, Sivakumar H,
Mazzocchi A, Aleman J, Miller L, Levine E, Triozzi P and Skardal A:
Model of patient-specific immune-enhanced organoids for
immunotherapy screening: Feasibility study. Ann Surg Oncol.
27:1956–1967. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kryeziu K, Moosavi SH, Bergsland CH, Guren
MG, Eide PW, Totland MZ, Lassen K, Abildgaard A, Nesbakken A, Sveen
A and Lothe RA: Increased sensitivity to SMAC mimetic LCL161
identified by longitudinal ex vivo pharmacogenomics of recurrent,
KRAS mutated rectal cancer liver metastases. J Transl Med.
19:3842021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Choi YJ, Lee H, Kim DS, Kim DH, Kang MH,
Cho YH, Choi CM, Yoo J, Lee KO, Choi EK, et al: Discovery of a
novel CDK7 inhibitor YPN-005 in small cell lung cancer. Eur J
Pharmacol. 907:1742982021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhou Z, Van der Jeught K, Fang Y, Yu T, Li
Y, Ao Z, Liu S, Zhang L, Yang Y, Eyvani H, et al: An organoid-based
screen for epigenetic inhibitors that stimulate antigen
presentation and potentiate T-cell-mediated cytotoxicity. Nat
Biomed Eng. 5:1320–1335. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Cho K, Ro SW, Lee HW, Moon H, Han S, Kim
HR, Ahn SH, Park JY and Kim DY: YAP/TAZ suppress drug penetration
into hepatocellular carcinoma through stromal activation.
Hepatology. 74:2605–2621. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Grebenyuk S and Ranga A: Engineering
organoid vascularization. Front Bioeng Biotechnol. 7:392019.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Strobel HA, Moss SM and Hoying JB:
Vascularized tissue organoids. Bioengineering (Basel). 10:1242023.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bhat SM, Badiger VA, Vasishta S,
Chakraborty J, Prasad S, Ghosh S and Joshi MB: 3D tumor
angiogenesis models: Recent advances and challenges. J Cancer Res
Clin Oncol. 147:3477–3494. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wörsdörfer P, Dalda N, Kern A, Krüger S,
Wagner N, Kwok CK, Henke E and Ergün S: Generation of complex human
organoid models including vascular networks by incorporation of
mesodermal progenitor cells. Sci Rep. 9:156632019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Shirure VS, Bi Y, Curtis MB, Lezia A,
Goedegebuure MM, Goedegebuure SP, Aft R, Fields RC and George SC:
Tumor-on-a-chip platform to investigate progression and drug
sensitivity in cell lines and patient-derived organoids. Lab Chip.
18:3687–3702. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mazio C, Casale C, Imparato G, Urciuolo F
and Netti PA: Recapitulating spatiotemporal tumor heterogeneity in
vitro through engineered breast cancer microtissues. Acta Biomater.
73:236–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Nashimoto Y, Hayashi T, Kunita I, Nakamasu
A, Torisawa YS, Nakayama M, Takigawa-Imamura H, Kotera H, Nishiyama
K, Miura T and Yokokawa R: Integrating perfusable vascular networks
with a three-dimensional tissue in a microfluidic device. Integr
Biol (Camb). 9:506–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Grönholm M, Feodoroff M, Antignani G,
Martins B, Hamdan F and Cerullo V: Patient-Derived organoids for
precision cancer immunotherapy. Cancer Res. 81:3149–3155. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Meng Q, Liu Z, Rangelova E, Poiret T,
Ambati A, Rane L, Xie S, Verbeke C, Dodoo E, Del Chiaro M, et al:
Expansion of tumor-reactive T cells from patients with pancreatic
cancer. J Immunother. 39:81–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Almeqdadi M, Mana MD, Roper J and Yilmaz
ÖH: Gut organoids: Mini-tissues in culture to study intestinal
physiology and disease. Am J Physiol Cell Physiol. 317:C405–C419.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Vazquez-Armendariz AI and Tata PR: Recent
advances in lung organoid development and applications in disease
modeling. J Clin Invest. 133:e1705002023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shi R, Radulovich N, Ng C, Liu N, Notsuda
H, Cabanero M, Martins-Filho SN, Raghavan V, Li Q, Mer AS, et al:
Organoid cultures as preclinical models of non-small cell lung
cancer. Clin Cancer Res. 26:1162–1174. 2020. View Article : Google Scholar : PubMed/NCBI
|