Introduction
Melanin, synthesized within the melanosomes of
melanocytes, serves a key role in determining skin pigmentation
(1). In the epidermis, melanin is
transferred to adjacent keratinocytes, where it serves as a
protective barrier against ultraviolet radiation damage (2). However, abnormal melanin production
can lead to pigmentary disorders, such as melasma,
hyperpigmentation, freckles and vitiligo (3). While agents such as arbutin, kojic
acid and hydroquinone are employed to treat these disorders, their
efficacy is typically limited owing to poor skin penetration and
absorption (4). Moreover, these
agents are associated with side effects, including dryness,
inflammation and a potentially increased risk of cancer (5,6).
Some agents, such as hydroquinone, have been banned or restricted
in certain countries owing to their potential health risks, further
highlighting the limitations of current treatments (7). Therefore, there is a need for the
development of efficient and safe transdermal agents and research
has increasingly focused on using natural substances with fewer
side effects for treating skin disease (5–7).
Plant-derived extracellular vesicles (EVs) sourced
from edible plants, such as vegetables and fruits, are rich in
bioactive molecules, including nucleic acid, protein, lipid and
metabolites (8). These vesicles,
ranging in size from 50 to 1,000 nm, have potential as therapeutic
agents for various diseases because of their biocompatibility,
safety and high functional efficiency (9,10).
For example, EVs isolated from grapes, grapefruit and carrots have
demonstrated anti-inflammatory properties in the treatment of
severe inflammatory bowel disease (11–13).
Additionally, ginseng root-derived EVs exhibit protective and
anti-aging effects on cells damaged by ultraviolet B radiation,
effectively reducing reactive oxygen species (ROS), decreasing cell
mortality and mitigating oxidative stress responses (14). Notably, these plant-derived EVs are
non-pathogenic and cost-effective to produce compared with
mammalian counterparts (15).
Moreover, they contain bioactive molecules, such as phytochemicals,
which are beneficial to human health, making them promising
candidates for treating or alleviating numerous diseases (16–18).
Cannabis sativa, commonly referred to as
hemp, is one of the oldest medicinal plants and is also used in
textiles (19). Cannabis
extracts can carry out various biological activities, including
antioxidant, anticancer and anti-inflammatory effects; however,
previous studies have focused on seeds or leaves, limiting the
research scope (20–23). Therefore, the present study aimed
to explore the potential of C. sativa stem EV-like
nanoparticles (CSS-NPs) as therapeutic agents or pharmaceuticals
for treating skin disorders. Additionally, the present study aimed
to investigate the cosmetic properties of CSS-NPs, particularly
their whitening and antioxidant activity in melanoma cells, and to
elucidate the underlying mechanism.
Materials and methods
Ethics approval
Experiments were conducted in compliance with the
Narcotics Control Act of the Republic of Korea. The plant species
used is not classified as protected or endangered.
Isolation of CSS-NPs
Stems of 2-month-old CS variety Cheungsam were
collected in August 2022 from an open field at Jay Hemp Korea.
Initially, stems were washed three times with sterile distilled
water. After cutting into 2-cm pieces, 200 g CSS was mixed with 1 l
PBS and ground in a mixer grinder at a 30-sec for a total of three
cycles. The isolation of CSS-NPs involved centrifugation at 1,800,
3,500 and 10,000 × g for 20 min each at 4°C. Supernatants were
filtered using a 0.45-µm membrane filter (GVS S.p.A.). Filtered
supernatants were first concentrated to a 15-ml using a tangential
flow filtration (TFF) system equipped with a 500-kDa ultrafilter
membrane (Pall Life Sciences). For purification, sucrose cushioned
ultracentrifugation was performed using Optiprep solution (60%
iodixanol; Stemcell Technologies). Specifically, 15 ml concentrated
supernatant was transferred to a 17 ml ultracentrifuge tube
(Beckman Coulter, Inc.) with 1 ml Optiprep using a Pasteur pipette
(MilliporeSigma). The tubes were ultracentrifuged at 120,000 × g
for 60 min at 4°C (Optima XE-100; Beckman Coulter Inc.). The highly
purified CSS-NPs were collected and dialyzed with 5 ml cold PBS.
Finally, CSS-NPs were filtered using a 0.45-µm syringe filter
(Advantec) and stored at −80°C until further use. The protein
concentration of CSS-NPs was determined using a BCA protein assay
kit (Thermo Fisher Scientific, Inc.).
Physicochemical assessment of
CSS-NPs
Size distribution and ζ potential of CSS-NPs were
confirmed using qNano-Exoid (Izon Science Limited) according to the
manufacturer's instructions using the NP200 nanopore (size; 85–500
nm). To visualize the morphology of CSS-NPs, scanning electron
microscopy (SEM) was used. Samples were first mixed at 20°C for 60
min with 4% paraformaldehyde solution for fixation. CSS-NPs were
washed twice with PBS and dehydrated in 70% ethanol before the
evaporation of ethanol. Following dehydration, the samples were
coated with gold-palladium sputtering and assessed using a S-4700
scanning electron microscope (Hitachi, Ltd.). To evaluate the
stability of CSS-NPs against external stimuli, CSS-NPs were exposed
to different pH concentrations (pH 2.0 or 12.1) for 30 min at 37°C.
Additionally, CSS-NPs were incubated with 3 DNase (Thermo Fisher
Scientific, Inc.), 6 RNase and 100 µg/ml proteinase K (both
BioLabs) at 37°C for 30 min, after which changes in size and ζ
potential were measured.
Biochemical composition of
CSS-NPs
Biochemical composition of CSS-NPs was evaluated
using quadrupole time-of-flight mass spectrometry (Q-TOF-MS) and
ultra-high-performance liquid chromatography (UPLC; both Waters
Corporation). Briefly, 1 mg CSS-NPs was dried using a
freezing/vacuum-drying system (VD-800F; TAITEC CORPORATION) and
resuspended with 100 µl 80% methanol. UPLC was conducted by
injecting 10 µl reconstituted sample onto an Acquity UPLC BEH C18
column (Waters Corporation). Q-TOF-MS and tandem MS spectra were
acquired as previously described (24). For gas chromatography (GC)-MS
analysis, 1 mg CSS-NPs were mixed with 70 µl hydroxymethyl amine
solution, incubated at 37°C for 90 min and then methylated with 70
µl N,O-bis(trimethylsilyl)trifluoroacetamide at 90°C for 30 min.
After cooling, 1.5 saturated NaCl solution and 1.0 ml n-hexane were
added. Next, the mixture was centrifugated at 180 × g at 20°C for 2
min, and the upper organic phase was transferred to a GC vial.
Analysis was performed on a Shimadzu GC-2010 Plus GC-MS-TQ 8030
(Shimadzu) device with a DB-5 MS column (30 m × 0.25 mm; film
thickness; 0.25 µm). GC-MS spectra were acquired as previously
described (25).
Cell lines and immune cell
culture
Mouse skin-derived B16F10 melanoma (Korean Cell Line
Bank; Korean Cell Line Research Foundation) and the HaCaT human
keratinocyte cell line (Cytion) were cultured in DMEM supplemented
with 10% FBS and 1% penicillin-streptomycin (all Gibco; Thermo
Fisher Scientific, Inc.). Cells were maintained at 37°C in a 5%
CO2 atmosphere and sub-cultured every 3 days by seeding
1×106 cells/T-75 flask. All experiments were conducted
using cells at passages 5–8. Mycoplasma contamination testing was
performed regularly to ensure cell line integrity.
Bone marrow-derived dendritic cells (BMDCs) were
generated from C57BL/6 mice (Orient Bio Inc.). Female mice (n=4;
age 7 weeks, weight, ~18 g) were acclimated for 1 week at the Korea
Research Institute of Bioscience and Biotechnology animal facility
under specific pathogen-free conditions (22±2°C; 12/12 h light/dark
cycle; 55±5% humidity), with ad libitum access to food and
water. Mice were anesthetized with 2–3 % isoflurane (Hana Pharm
Co., Ltd.) and euthanized by cervical dislocation. Bone marrow
cells were isolated from femurs and tibias and cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS, 2.5 ng/ml IL-4 and 20 ng/ml
granulocyte-macrophage colony-stimulating factor (both JW
CreaGene). Cells were incubated at 37°C with 5% CO2 for
8 days to induce BMDC differentiation.
Cell viability
B16F10 and HaCaT cells and BMDCs (1×104
cells/well) were placed in a 96-well plate, incubated for 24 h,
then treated with CSS-NPs (5–100 µg/ml) for 24 h at 37°C in a 5%
CO2 incubator. Subsequently, the effect of CSS-NPs on
cell viability was assessed using an EZ-Cytox assay kit (Dogenbio
Co., Ltd.) according to the manufacturer's instructions. The
EZ-Cytox solution (1X) was added to each well, and the plates were
incubated at 37°C for 30 min. Absorbance was measured at 450 nm
using a SpectraMax iD5 microplate reader (Molecular Devices, LLC).
To evaluate the cytotoxicity of CSS-NPs, B16F10 and HaCaT cells and
BMDCs were seeded in a 12-well plate (1×106 cells/well)
and treated with CSS-NPs at concentrations of 50 and 100 µg/ml,
respectively, for 24 h at 37°C. Cells were stained using an
apoptosis and necrosis detection kit (Thermo Fisher Scientific,
Inc.), which included Annexin V and propidium iodide, according to
the manufacturer's instructions. Live and dead cells were counted
using a Life Launch Attune Nxt Flow Cytometer (Thermo Fisher
Scientific, Inc.) and FlowJo software (Version 10; Tree Star,
Inc.).
Analysis of melanin content
B16F10 cells were seeded at a density of
1×105 cells/well in a six-well plate for 24 h. Then, the
cells were incubated with 5, 25, 50 and 100 µg/ml CSS-NPs at 37°C
for 3 days in the presence of α-melanocyte stimulating hormone
(α-MSH; 100 nM). Cells were collected via trypsin solution (Gibco;
Thermo Fisher Scientific, Inc.) and centrifuged at 8,000 × g at
20°C for 3 min. The supernatant was removed, and cell pellets were
washed twice with PBS. Cell pellets were lysed with 100 µl mixed
lysis solution (1 M NaOH and 10% DMSO) and heated to 100°C for 1 h.
The solubilized melanin was transferred to a 96-well plate and the
absorbance at 405 nm was measured using a SpectraMax iD5 microplate
reader. The amount of solubilized melanin was normalized to the
total protein content of the lysate using Pierce™ BCA Protein Assay
kit.
Tyrosinase (TYR) activity
B16F10 cells (1.5×105/well in a six-well
plate) were treated with 25 µg/ml CSS-NPs for 2 days at 37°C in the
presence of α-MSH (100 nM), harvested and washed twice in PBS. TYR
activity was measured using the TYR assay kit (cat. no. MAK550-1KT;
MilliporeSigma) according to the manufacturer's instructions.
Western blotting
B16F10 cells (0.5×105/per well in a
six-well plate) were incubated with 5 or 25 µg/ml CSS-NPs for 2
days at 37°C in the presence of α-MSH (100 nM). Cells were lysed in
M-PER Mammalian Protein Extraction Reagent and Halt Protease &
Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The protein was
quantified using the BCA kit, and protein samples were denatured
for 10 min at 70°C. Subsequently, 25 µg/lane protein was separated
using 4–12% SDS-PAGE, transferred onto PVDF membranes (Thermo
Fisher Scientific, Inc.) and blocked with EveryBlot Blocking Buffer
(cat. no. #12010020; Bio-Rad Laboratories, Inc.) at 25°C for 1 h.
The membrane was then incubated with primary antibodies at 4°C for
24 h (Table I). The membrane was
incubated with anti-mouse (1:10,000; cat. no. 7076S) or rabbit IgG
(1:10,000; Cat. no. 7074S; both Cell signaling technology, Inc.)
HRP-linked secondary antibodies at 25°C for 1 h. Protein bands were
detected using enhanced SuperSignal West Pico PLUS
chemiluminescence substrates (Thermo Fisher Scientific, Inc.) and
visualized using Amersham ImageQuant™ 800 (Cytiva, Amersham,
England). Densitometry was quantified using the ImageQuant TL
analysis software (Cytiva, version 10.0.261).
 | Table I.Antibodies used for western
blotting. |
Table I.
Antibodies used for western
blotting.
| Name | Supplier | Cat. no. | Dilution |
|---|
|
Microphthalmia-associated transcription
factor | Santa Cruz
Biotechnology, Inc. | sc-515925 | 1:1,000 |
| Tyrosinase | Santa Cruz
Biotechnology, Inc. | sc-20035 | 1:1,000 |
| TRP-1 | Santa Cruz
Biotechnology, Inc. | sc-58438 | 1:1,000 |
| TRP-2 | Santa Cruz
Biotechnology, Inc. | sc-74439 | 1:1,000 |
| p-Akt | Cell Signaling
Technology, Inc. | 4060S | 1:1,000 |
| p-GSK3β | Cell Signaling
Technology, Inc. | 5558S | 1:1,000 |
| p-ERK | Cell Signaling
Technology, Inc. | 4370S | 1:1,000 |
| ERK | Cell Signaling
Technology, Inc. | 4695S | 1:1,000 |
| p-p38 | Cell Signaling
Technology, Inc. | 4511S | 1:1,000 |
| p38 | Cell Signaling
Technology, Inc. | 8690S | 1:1,000 |
| p-JNK | Cell Signaling
Technology, Inc. | 4668S | 1:1,000 |
| JNK | Cell Signaling
Technology, Inc. | 9252S | 1:1,000 |
| β-actin | Santa Cruz
Biotechnology, Inc. | sc-8432 | 1:2,000 |
mRNA analysis
B16F10 cells (1×105/well in a 12-well
plate) were incubated with CSS-NPs (5 and 25 µg/ml) and α-MSH (100
nM) for 24 h at 37°C. Cells were washed twice in PBS, and total RNA
was isolated using a RNeasy Mini kit (Cat no. 74106; Qiagen GmbH).
For reverse transcription-quantitative (RT-q)PCR, 10 µl RNA was
reverse-transcribed into cDNA using the iScript Adv cDNA kit for
RT-qPCR (Cat. no. #1725038; Bio-Rad Laboratories, Inc.), according
to the manufacturer's instructions. Next, target cDNA levels were
quantified by RT-qPCR using the CFX96 Touch RT-PCR detection system
and SYBR green (Bio-Rad Laboratories, Inc.). The PCR steps for all
genes were as follows: For a hot start, 40 cycles of 95°C for 5
min, 95°C for 20 sec, 60°C for 20 sec and 72°C for 30 s, melting
curve at 95°C for 1 min, 55°C for 1 min, and 30°C for 1 min. The
quantification of gene expression was performed using the 2^-ΔΔCq
method (26). Specific forward and
reverse primers are presented in Table II.
 | Table II.Primers used for reverse
transcription-quantitative PCR. |
Table II.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence,
5′→3′ |
|---|
|
Microphthalmia-associated transcription
factor |
CCCGTCTCTGGAAACTTGATCG (forward) |
|
|
CTGTACTCTGAGCAGCAGGTG (reverse) |
| Tyrosinase |
GTCGTCACCCTGAAAATCCTAACT (forward) |
|
|
CATCGCATAAAACCTGATGGC (reverse) |
| TRP-1 |
GCTGCAGGAGCCTTCTTTCT (forward) |
|
|
AAGACGCTGCACTGCTGGTC (reverse) |
| TRP-2 |
TTGCCCTACTGGAACTTTGC (forward) |
|
|
TGGGTCATCTTGTCTTGCTG (reverse) |
| β-actin |
TATTGGCAACGAGCGGTTCC (forward) |
|
|
GGCATAGAGGTCTTTACGGATGTC (reverse) |
Inhibitor assay
B16F10 cells were seeded (0.5×105/per
well in a six-well plate) and pretreated with PD98059 (ERK
inhibitor, 20 µM) or LY294002 (PI3K inhibitor, 10 µM) at 37°C for 1
h before treatment with 25 µg/ml CSS-NPs and α-MSH (100 nM) at
37°C. After 2 days, cells were harvested for subsequent
experiments.
2,2-diphenyl-1-picrylhydrazyl (DPPH)
assay
DPPH radical scavenging activity of CSS-NPs was
determined as previously described (27). The samples, diluted to specific
concentrations (0.1, 0.2, 0.5 and 1 mg/ml) with distilled water,
were mixed with 0.2 mM DPPH solution (MilliporeSigma) at a 1:1
ratio (100 µl each). The mixture was stirred and left to stand in
the dark for 30 min at 20°C, after which the absorbance was
measured at 517 nm using a SpectraMax iD5 microplate reader.
2,2′-Azino-bis
(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS)
assay
ABTS radical scavenging activity of CSS-NPs was
determined as previously described (28). To prepare the ABTS solution, 88 µl
140 mM potassium persulfate (Samchun Pure Chemical Co., Ltd.) and
two ABTS diammonium salt tablets (MilliporeSigma) were added to 5
ml distilled water, mixed thoroughly, and allowed to react in the
dark for 12–16 h at 20°C. ABTS solution was diluted with 95%
ethanol at a ratio of 1:88 to achieve an absorbance of 0.7±0.02 at
734 nm. A total of 50 µl sample, diluted to specific concentrations
(0.1, 0.2, 0.5 and 1.0 mg/ml) with distilled water, was mixed with
1 ml ABTS solution. Finally, the mixture was stirred and allowed to
react in the dark for 5 min at 20°C, and the absorbance was
measured at 734 nm.
Measurement of ROS
B16F10 cells (1/105/well) were seeded in
12-well plates and incubated with CSS-NPs and vitamin C (Vit C;
both 50 µg/ml) in the presence of α-MSH (100 nM) for 24 h at 37°C.
Following cell harvesting, intracellular ROS levels were measured
using a CellROX kit (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol.
Antioxidant activity
Total antioxidant capacity (TAC) and catalase (CAT)
enzyme activity in sonicated (40 kHz at 25°C for 5 min) lysate of
B16F10 cells treated with CSS-NPs and Vit C (both 50 µg/ml) for 24
h at 37°C in the presence of α-MSH (100 nM) were measured using
EZ-Total Antioxidant Capacity (Dogenbio Co., Ltd.) and Catalase
Colorimetric Assay (Thermo Fisher Scientific, Inc.). kits,
respectively, according to the manufacturers' protocols.
Statistical analysis
All data are expressed as the mean ± standard
deviation of three independent experiments. Significance of groups
was evaluated using one-way ANOVA followed by Tukey's multiple
comparison test. Data were analyzed using GraphPad Prism 9
(Dotmatics), P<0.05 was considered to indicate a statistically
significant difference.
Results
Profiling and stability of
CSS-NPs
CSS-NPs were isolated from CSS using TFF and sucrose
cushioned ultracentrifugation, resulting in highly purified
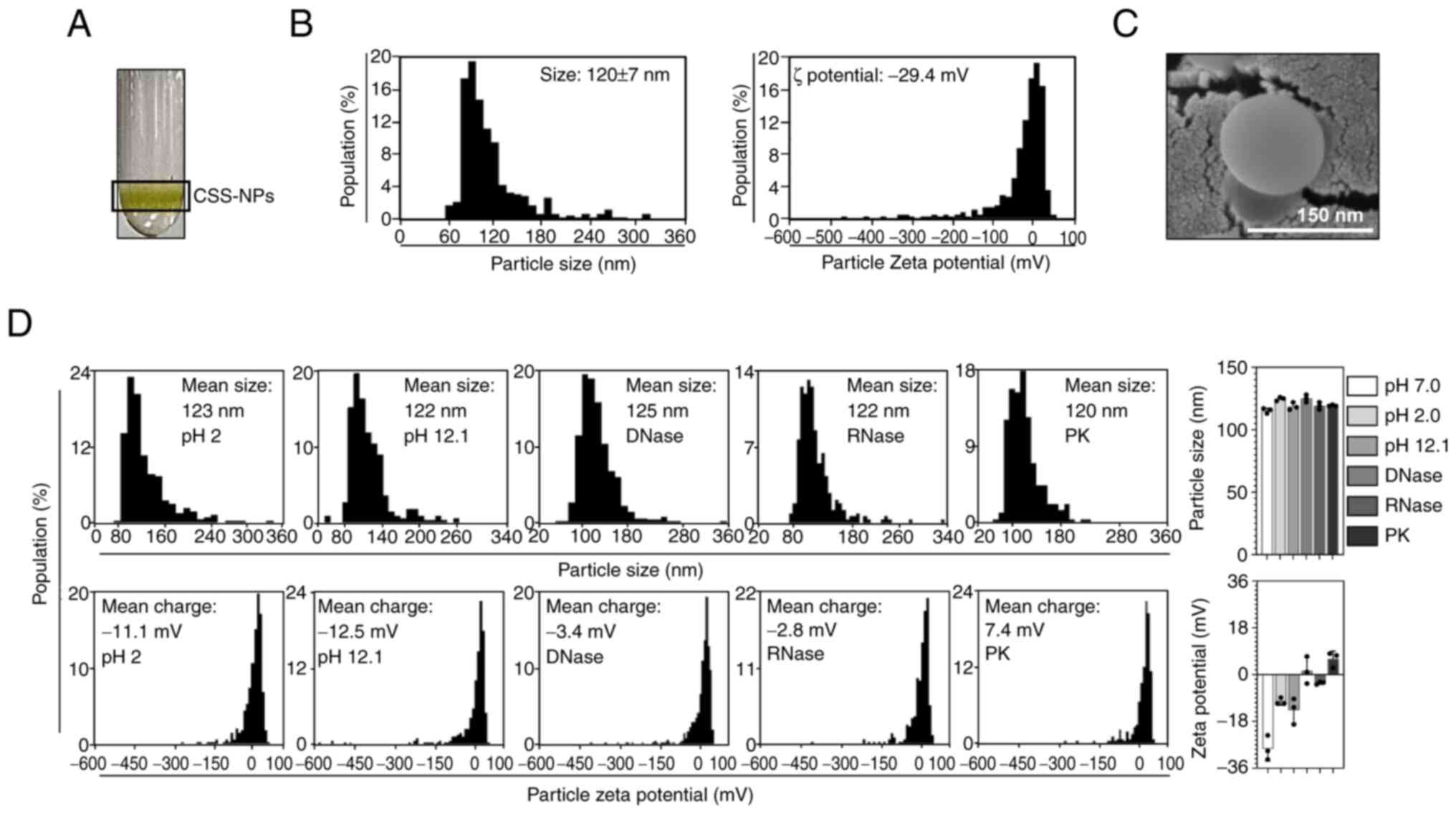
nanoparticles (Fig. 1A). The
purified CSS-NPs were characterized by size, ζ potential and
morphological features using qNano-Exoid (Fig. 1B) and SEM (Fig. 1C). CSS-NPs, when resuspended in PBS
at a pH of 7.0, had a mean diameter of 120±7 nm. The ζ potential
value was −29.4 mV (Fig. 1B),
which falls within the stable range (−30-30 mV) for nanoparticles
(29). SEM imaging confirmed that
CSS-NPs possessed typical nanovesicle structure characterized by
spherical morphology (Fig. 1C).
Notably, CSS-NPs maintained consistent particle size and ζ
potential values within the stable range across acidic (pH 2.0) and
alkaline (pH 12.1) conditions, compared with those in the neutral
condition (pH 7.0), and following exposure to DNase, RNase and
proteinase K (Fig 1D). This
demonstrated that CSS-NPs exhibit stability under external stress
conditions.
Biochemical composition analysis of
CSS-NPs
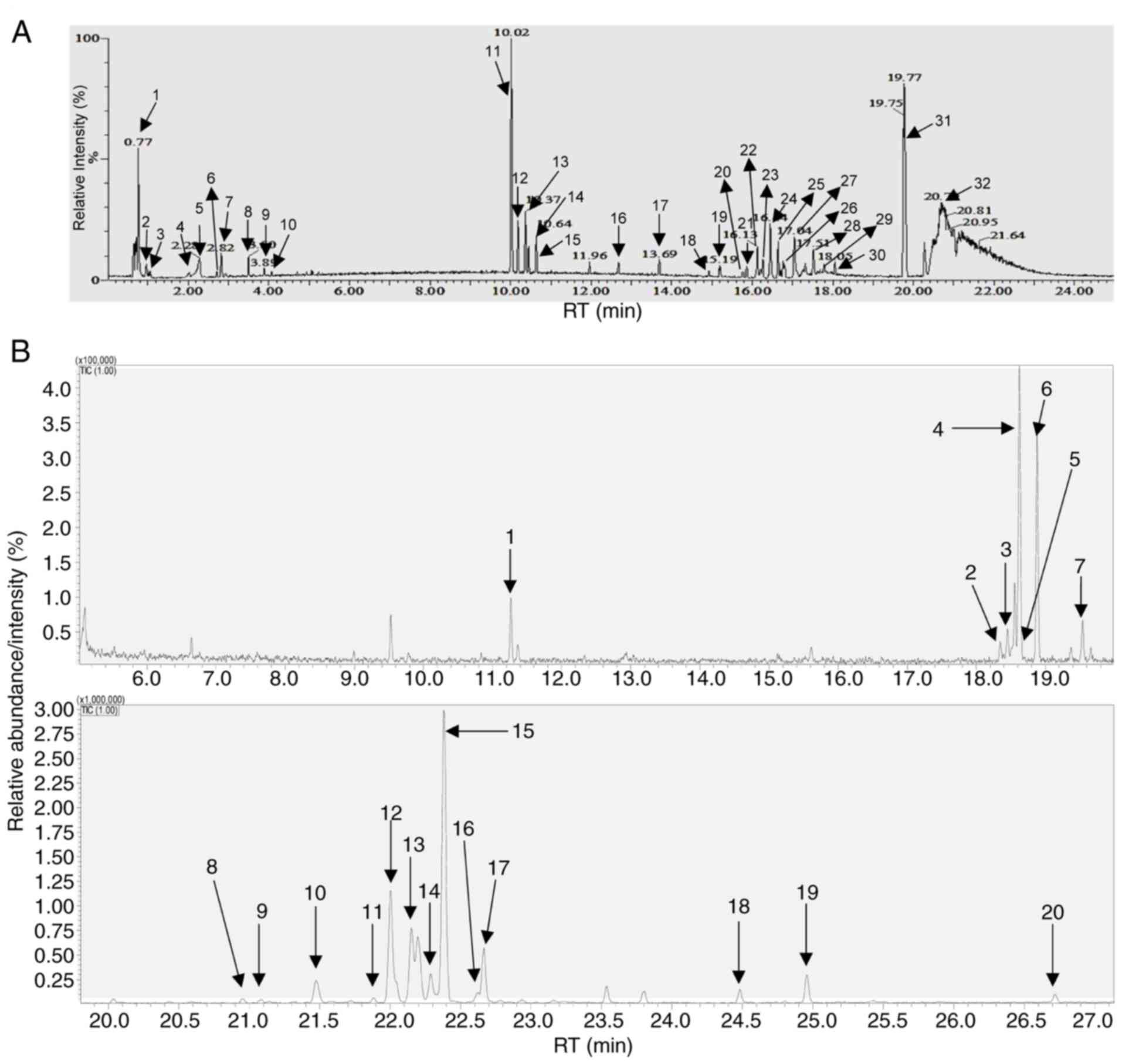
Next, the biochemical composition of CSS-NPs was
analyzed using UPLC/Q-TOF-MS (Fig.
2A and Table III) and GC-MS
(Fig. 2B and Table IV). A total of 48 metabolites were
detected within the CSS-NPs. UPLC/Q-TOF-MS analysis identified four
nucleosides, three amino acids, two flavonoids and 20 lipids and
chlorophyll derivatives (Fig. 2A;
Table II). The nucleosides were
guanine, adenosine, guanosine and 1-methyladenosine; the amino
acids were N-(1-deoxy-1-fructosyl) valine, L-phenylalanine and
L-tryptophan and the flavonoids were pterosin E and pterosin K.
Furthermore, CSS-NPs contained pheophorbide A, a chlorophyll
derivative. CSS-NPs contained various lipid compositions including
dehydrophytosphingosine, 3-ketosphingosine, phytosphingosine,
stearoylethanolamide, lysophosphatidylcholine 16:0 (lysoPC 16:0),
2-O-protocatechuoylalphitolic acid, PC[18:1(9Z)/2:0], methyl
4,8-decadienoate, lysoPC(18:0), lysoPC[16:1(9Z)],
PC[16:1(9Z)/17:1(9Z)], lysoPC[18:1(9Z)], PC[17:1(9Z)/17:1(9Z)],
lysoPC[22:6(4Z,7Z,10Z,13Z,16Z,19Z)], PC(18:0/2:0), 13-docosenamide,
octadecanamide, oleoylethanolamide, stigmastane-3,6-dione and
PC[18:3(9Z,12Z,15Z)/16:0]. In GC-MS analysis, an additional 18
metabolites were identified, including glycerol, ribose, threose,
xylose, lyxose, arabinose, rhamnose, fructofuranose, psicofuranose,
tagatose, pinitol, fructose, talose, glucose, galactose, palmitic
acid, myo-inositol and stearic acid (Fig. 2B and Table IV).
 | Table III.Ultra-high-performance liquid
chromatography quadrupole time-of-flight mass spectrometry analysis
of Cannabis sativa stem-derived nanoparticles. |
Table III.
Ultra-high-performance liquid
chromatography quadrupole time-of-flight mass spectrometry analysis
of Cannabis sativa stem-derived nanoparticles.
| No. | Retention time
(min) | Identification | Mass-to-charge
ratio | Fragment ions
(m/z) |
|---|
| 1 | 0.77 | Guanine | 152.05 | 136 |
| 2 | 0.96 |
|
| 136,119 |
| 3 | 1.05 |
N-(1-Deoxy-1-fructosyl)valine | 280.14 | 262, 244, 118, 112,
91, 87, 84 |
| 4 | 2.03 | Adenosine | 268.10 | 136, 119 |
| 5 | 2.28 | Guanosine | 284.10 | 152, 135, 110 |
| 6 | 2.68 |
1-Methyladenosine | 282.12 | 136, 119 |
| 7 | 2.82 |
L-phenylalanine | 166.08 | 120, 103, 91,
77 |
| 8 | 3.50 | L-tryptophan | 205.0 | 188, 146, 118, 115,
91 |
| 9 | 3.89 | Pterosin E | 233.05 | 215, 203, 177, 169,
147, 79 |
| 10 | 4.08 | Pterosin K | 267.13 | 237, 217 |
| 11 | 10.02 |
Dehydrophytosphingosine | 316.28 | 298, 280, 209,
125 |
| 12 | 10.18 |
3-Ketosphingosine | 298.27 | 280, 109, 95, 81,
69 |
| 13 | 10.37 |
|
| 280, 193, 95, 83,
81, 69 |
| 14 | 10.45 |
Phytosphingosine | 318.30 | 300, 69 |
| 15 | 10.64 |
Stearoylethanolamide | 328.29 | 251, 83 |
| 16 | 12.68 | LysoPC(16:0) | 496.33 | 184, 104 |
| 17 | 13.69 |
2-O-Protocatechuoylalphitolic acid | 609.34 | 177 |
| 18 | 14.91 |
PC(18:1(9Z)/2:0) | 564.42 | 184 |
| 19 | 15.19 | Methyl
4,8-decadienoate | 183.08 | 165, 153, 109, 107,
95, 81 |
| 20 | 15.77 | Pheophorbide A | 593.28 | 577, 533, 519, 475,
447, 433 |
| 21 | 15.85 | LysoPC(18:0) | 524.51 | 313, 184 |
| 22 | 16.13 |
LysoPC(16:1(9Z)) | 494.56 | 337, 313, 184 |
| 23 | 16.26 |
PC16:1(9Z)/17:1(9Z)) | 744.64 | 337, 184 |
| 24 | 16.44 |
LysoPC(18:1(9Z)) | 522.60 | 337, 184 |
| 25 | 16.65 |
PC(17:1(9Z)/17:1(9Z)) | 758.56 | 337, 184 |
| 26 | 16.78 | LysoPC(22:6(4Z, 7Z,
10Z, 13Z, 16Z, 19Z)) | 568.43 | 184 |
| 27 | 17.04 | PC(18:0/2:0) | 566.55 | 184 |
| 28 | 17.51 |
13-Docosenamide | 338.34 | 184, 121, 109, 95,
81 |
| 29 | 17.80 | Octadecanamide | 284.29 | 243, 211, 201,
95 |
| 30 | 18.05 |
Oleoylethanolamide | 326.34 | 237, 209, 109,
95 |
| 31 | 19.77 |
Stigmastane-3,6-dione | 429.37 | 177, 139, 121, 107,
95, 81 |
| 32 | 20.70 | PC(18:3(9Z, 12Z,
15Z)/16:0) | 756.55 | 337, 313, 184 |
 | Table IV.Gas chromatography mass spectrometry
analysis of Cannabis sativa stem-derived nanoparticles. |
Table IV.
Gas chromatography mass spectrometry
analysis of Cannabis sativa stem-derived nanoparticles.
| No. | Compound | Retention time,
min) | Retention
index | Area |
|---|
| 1 | Glycerol | 11.265 | 1269 | 173000 |
| 2 | Ribose | 18.349 | 1640 | 54664 |
| 3 | Threose | 18.45 | 1646 | 108640 |
| 4 | Xylose | 18.554 | 1652 | 239424 |
| 5 | Lyxose | 18.624 | 1656 | 934454 |
| 6 | Arabinose | 18.879 | 1671 | 687705 |
| 7 | Rhamnose | 19.539 | 1709 | 110111 |
| 8 | Fructofuranose | 20.954 | 1795 | 97657 |
| 9 | Psicofuranose | 21.082 | 1803 | 73027 |
| 10 | Tagatose | 21.475 | 1829 | 580484 |
| 11 | Pinitol | 21.883 | 1855 | 87359 |
| 12 | Fructose | 22.003 | 1863 | 2721573 |
| 13 |
| 22.151 | 1872 | 1688900 |
| 14 | Talose | 22.287 | 1881 | 677544 |
| 15 | Glucose | 22.382 | 1887 | 5921962 |
| 16 |
| 22.621 | 1903 | 198221 |
| 17 | Galactose | 22.665 | 1906 | 1046570 |
| 18 | Palmitic acid | 24.482 | 2043 | 241181 |
| 19 | myo-inositol | 24.958 | 2082 | 533891 |
| 20 | Stearic acid | 26.717 | 2239 | 165370 |
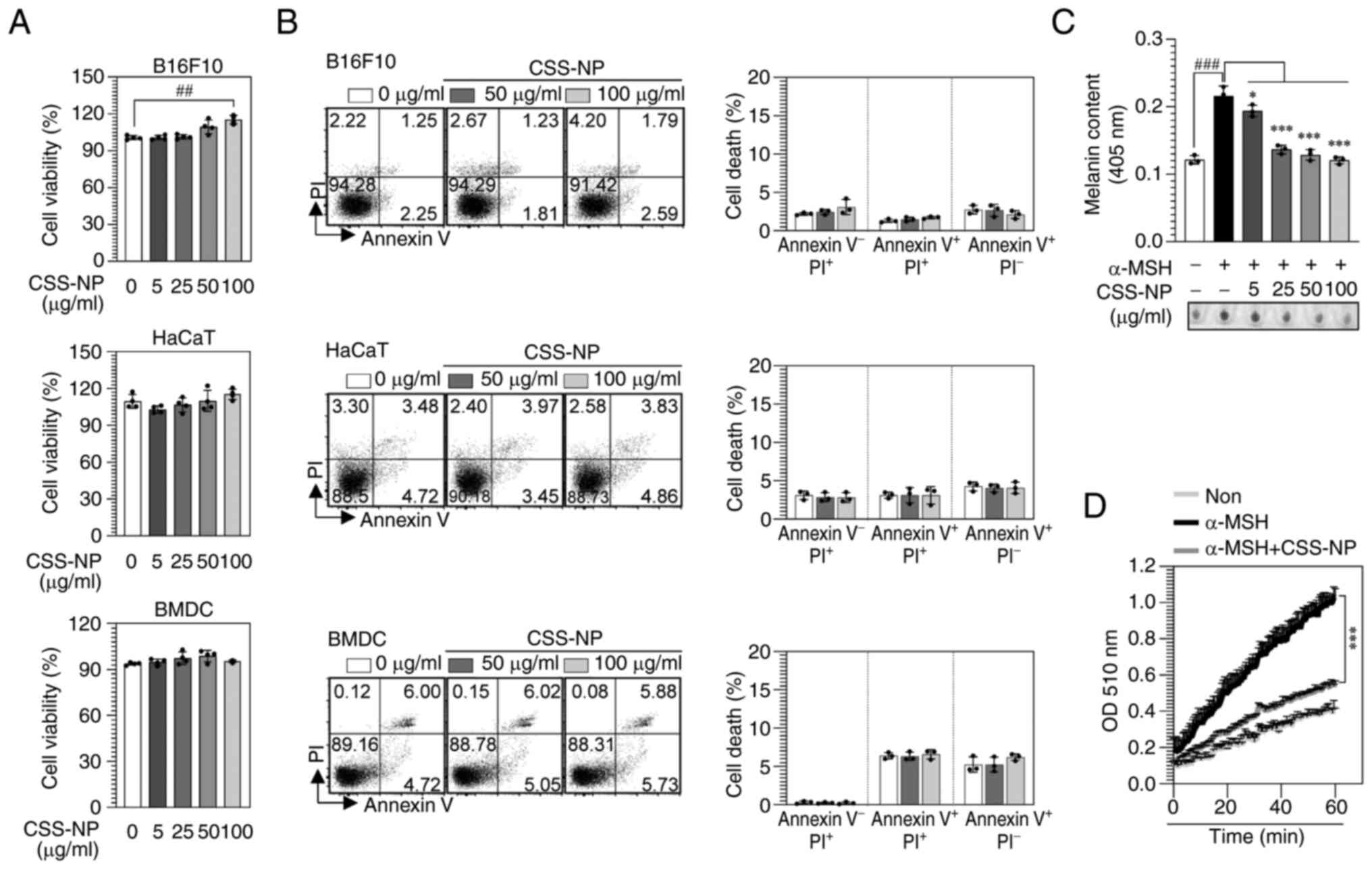
Cytotoxic effects of CSS-NPs in
different cell types
Cytotoxic effects of CSS-NPs were observed in B16F10
and HaCaT cells and BMDCs. CSS-NPs treatment did not affect
cellular cytotoxicity, and B16F10 cell viability remained unchanged
at concentrations of 5–50 µg/ml but revealed a significant increase
at a concentration of 100 µg/ml. By contrast, HaCaT cells and BMDCs
exhibited no significant changes in viability up to 100 µg/ml
(Fig. 3A). Annexin V and PI
staining confirmed that no death occurred in any of the cells
treated with CSS-NPs at 50 or 100 µg/ml (Fig. 3B). These findings demonstrate that
CSS-NPs did not exhibit cytotoxicity and that the selected
concentrations were appropriate for assessing the whitening and
antioxidant activity of CSS-NPs in melanoma cells.
CSS-NPs suppress melanin synthesis in
α-MSH-stimulated B16F10 cells
CSS-NPs inhibited melanin formation in a
dose-dependent manner (Fig. 3C).
Melanin synthesis is catalyzed by three enzymes: TYR and
tyrosinase-related protein (TRP)-1 and TRP-2, with TYR being an
enzyme that initiates melanin synthesis (30,31).
Therefore, TYR activity was measured in B16F10 cells treated with
CSS-NPs. Compared with the control (α-MSH-treated B16F10 cells),
low-dose CSS-NPs (25 µg/ml) resulted in decrease TYR activity
(Fig. 3D), suggesting that CSS-NPs
regulate melanin synthesis by modulating TYR activity.
CSS-NPs downregulate the melanogenic
signaling pathway and gene expression in α-MSH-stimulated B16F10
cells
Microphthalmia-associated transcription factor
(MITF) is a key transcription factor that controls the activity
levels of TYR, TRP-1 and TRP-2 in melanogenesis (32). Thus, to explore how CSS-NPs affect
the regulatory mechanisms of melanin synthesis induced by α-MSH in
B16F10 cells, western blot analysis was performed out. Protein
expression levels of MITF, TYR, TRP-1 and TRP-2 decreased in cells
treated with 5 and 25 µg/ml CSS-NPs compared with those treated
with α-MSH alone (Fig. 4A).
CSS-NPs significantly inhibited the expression of melanin
synthesis-associated genes including MITF, TYR, TRP-1 and TRP-2
(Fig. 4B). These results suggested
that the anti-melanogenic effects of CSS-NPs in B16F10 cells were
induced by downregulation of melanogenic genes and protein
including MITF, TYR, TRP-1 and TRP-2.
 | Figure 4.Melanin synthesis-associated mRNA and
protein levels induced by CSS-NPs in α-MSH-treated B16F10 cells.
B16F10 cells were treated with CSS-NPs (5 and 25 µg/ml) in the
presence of α-MSH (100 nM). (A) Protein expression of MITF, TYR,
TRP-1 and TRP-2 determined by western blotting. β-actin was used as
a protein loading control. (B) mRNA levels of MITF, TYR, TRP-1 and
TRP-2 determined by reverse transcription-quantitative PCR.
*P<0.05, **P<0.01, ***P<0.001 vs. α-MSH-treated cells and
#P<0.05, ##P<0.01,
###P<0.001 vs. non-treated cells. CSS-NP, Cannabis
sativa stem-derived nanoparticle; MITF,
microphthalmia-associated transcription factor; TYR, tyrosinase;
TRP, tyrosinase-related protein; α-melanocyte stimulating hormone,
α-MSH; Con, control. |
CSS-NPs activate ERK and Akt signaling
in α-MSH-stimulated B16F10 cells
MAPK signaling regulates cellular processes by
transducing external signals into intracellular responses (33). Therefore, whether MAPK signaling
regulated the anti-melanogenic activity induced by CSS-NPs was
evaluated. CSS-NPs dose-dependently increased the phosphorylation
of ERK, but not JNK and p38 (Fig.
5A). To elucidate the functional role of CSS-NPs in activating
ERK signaling in α-MSH-stimulated B16F10 cells, melanin content and
biosynthesis proteins in the presence of PD98059 (an ERK inhibitor)
were assessed. Melanin content (Fig
5B) and biosynthesis protein levels (MITF, TYR, TRP-1 and
TRP-2; Fig. 5C), which were
decreased by CSS-NPs in α-MSH-treated B16F10 cells, increased when
ERK signaling (PD98059-treated condition) was inhibited.
Subsequently, the PI3K/Akt signaling pathway was investigated
because this signaling pathway regulates melanin synthesis by
promoting the degradation of MITF (34). CSS-NPs promoted an increase of Akt
phosphorylation and consequent decrease of GSK3β phosphorylation in
α-MSH-treated B16F10 cells (Fig.
6A). Following inhibition of Akt signaling (LY294002-treated
condition), CSS-NPs restored the melanin content (Fig. 6B). Additionally, expression of
melanin biosynthesis proteins increased following α-MSH treatment
(Fig. 6C). These results indicate
that CSS-NPs suppressed melanin synthesis by activating the ERK and
Akt signaling pathway.
 | Figure 5.Changes in MAPK signaling pathway
induced by CSS-NPs in α-MSH-treated B16F10 cells. (A) Western blot
analysis of protein expression of MAPKs (ERK, p38, and JNK) in
B16F10 cells treated with CSS-NPs at 5 and 25 µg/ml for 2 days in
the presence of 100 nM α-MSH. (B) Melanin content and (C) western
blot analysis of expression of MITF, TYR, TRP-1 and TRP-2 in
α-MSH-treated B16F10 cells co-treated with PD98059 (ERK inhibitor;
10 µM) and CSS-NPs (25 µg/ml) for 3 days. *P<0.05, **P<0.01,
***P<0.001 vs. α-MSH-treated or α-MSH + CSS-NPs;
###P<0.001 vs. non-treated cells. CSS-NP, Cannabis
sativa stem-derived nanoparticle; α-MSH, α-melanocyte
stimulating hormone; MITF, microphthalmia-associated transcription
factor; TYR, tyrosinase; TRP, tyrosinase-related protein; Con,
control; p-, phosphorylated. |
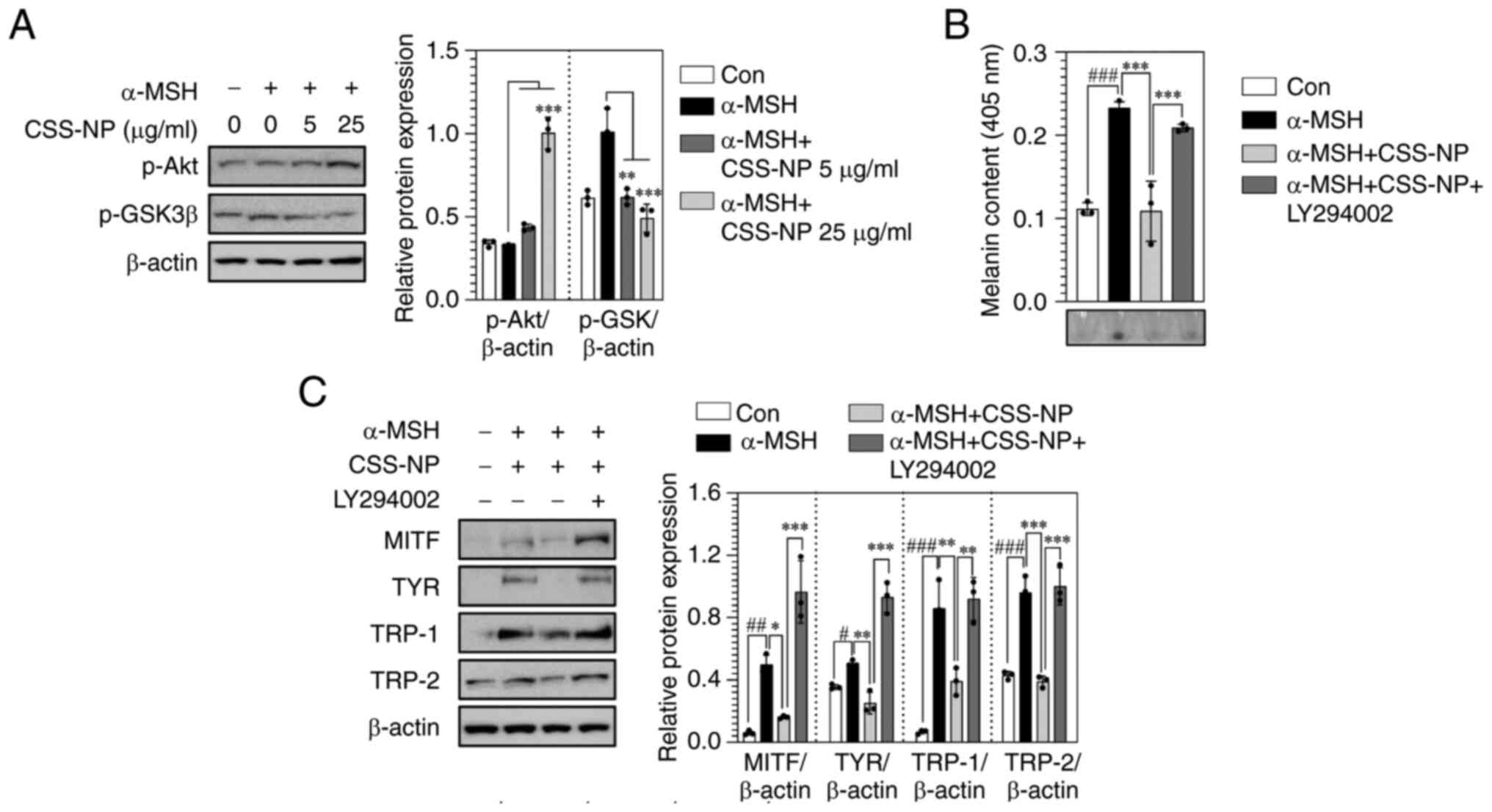 | Figure 6.Change in the Akt signaling pathway
induced by CSS-NPs in α-MSH-treated B16F10 cells. (A) Protein
expression of p-Akt and p-GSK3β determined by western blotting in
B16F10 cells treated with CSS-NPs at 5 and 25 µg/ml for 2 days in
the presence of 100 nM α-MSH. (B) Melanin content and (C) protein
expression of MITF, TYR, TRP-1 and TRP-2 determined using western
blotting in α-MSH-treated B16F10 cells co-treated with LY294002
(Akt inhibitor; 20 µM) and CSS-NPs (25 µg/ml) for 3 days. β-actin
was used as a protein loading control. *P<0.05, **P<0.01,
***P<0.001 vs. α-MSH-treated or α-MSH + CSS-NPs;
#P<0.05, ##P<0.01,
###P<0.001 vs. non-treated cells. CSS-NP, Cannabis
sativa stem-derived nanoparticle; GSK3β, glycogen synthase
kinase 3β; α-MSH, α-melanocyte stimulating hormone; MITF,
microphthalmia-associated transcription factor; TYR, tyrosinase;
TRP, tyrosinase-related protein; Con, control; p-,
phosphorylated. |
CSS-NPs restore antioxidant activity
in α-MSH-stimulated B16F10 cells
Synthesis of melanin leads to an increase in ROS
within skin cells, which is associated with skin aging,
pigmentation alteration and the onset of inflammatory reactions
(35–37). To determine whether CSS-NPs exert
antioxidant effects by controlling ROS generated during melanin
synthesis, the indirect antioxidant potential of CSS-NPs was
assessed using DPPH and ABTS radical scavenging assays, with Vit C
serving as a positive control. CSS-NPs exhibited lower direct
radical scavenging activity than Vit C; however, the activity of
CSS-NPs increased in a concentration-dependent manner; these
results were not significant (Fig.
7A). Whether CSS-NPs could provide protection in
α-MSH-stimulated B16F10 cells at non-toxic concentrations was
evaluated. CSS-NPs restored cell viability in a dose-dependent
manner (Fig. 7B). The antioxidant
functionality of CSS-NPs was explored by comparing their effects on
cellular toxicity induced during melanin synthesis with those of
Vit C. CSS-NPs effectively reduced α-MSH-induced cytotoxicity,
demonstrating effects comparable with treatment with Vit C
(Fig. 7C). Finally, to investigate
the underlying mechanism, intracellular ROS levels (Fig. 7D) and antioxidant enzyme activity
(TAC and CAT; Fig. 7E) were
measured. In α-MSH-stimulated B16F10 cells treated with CSS-NPs,
intracellular ROS levels were significantly decreased, while TAC
and CAT activities were increased compared with α-MSH-stimulated
B16F10 cells. These effects were similar to those observed with Vit
C treatment. These results suggest that CSS-NPs preserved
melanocyte survival by suppressing free radicals generated during
excessive melanin formation and alleviating impaired antioxidant
activity.
 | Figure 7.Antioxidant activity induced by
CSS-NPs in α-MSH-treated B16F10 cells. (A) Radical scavenging
activity assessed by DPPH and ABTS. (B) Viability of B16F10 cells
co-treated with 5, 25, 50 and 100 µg/ml CSS-NPs for 2 days in the
presence of 100 nM α-MSH, measured using an EZ-Cytox assay kit. (C)
Cytotoxic effects in α-MSH-treated B16F10 cells co-stimulated with
CSS-NPs or Vit C (both 50 µg/ml) were evaluated using Annexin V and
propidium iodide staining. (D) Reactive oxygen species levels in
α-MSH-treated B16F10 cells co-stimulated with CSS-NPs or Vit C
analyzed using a CellROX kit. (E) TAC and CAT activity in
α-MSH-treated B16F10 cells co-stimulated with CSS-NPs or Vit C (50
µg/ml) detected using TAC and CAT detection kits. *P<0.05,
**P<0.01, ***P<0.001 vs. α-MSH-treated;
##P<0.01, ###P<0.001 vs. non-treated
cells. CSS-NP, Cannabis sativa stem-derived nanoparticle;
α-MSH, α-melanocyte stimulating hormone, Vit C, vitamin C; Con,
control; TAC, total antioxidant capacity; CAT, catalase; DDPH,
2,2-diphenyl-1-picrylhydrazyl; ABTS,
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
diammonium. |
Discussion
The present study suggested that CSS-NPs are a
reliable natural material with potential as a cosmetic ingredient.
CSS-NPs contained 48 primary bioactive metabolites and exhibited
significant resistance to pH fluctuations and enzymatic reactions,
such as DNase, RNase and proteinase K. CSS-NPs decreased the
expression of key factors involved in melanin synthesis by
activating the ERK and Akt signaling pathways under excessive
melanin production in B16F10 cells. Additionally, the present study
demonstrated that CSS-NPs alleviated melanocyte toxicity induced
during excessive melanin synthesis, which may be attributed to the
suppression of ROS and the upregulation of antioxidant enzymes.
CSS-NPs exerted an anti-melanogenic effect by
downregulating TYR activity, as well as TRP-1, TRP-2 and MITF gene
and protein expressions. TYR, TRP-1 and TRP-2 are key enzymes in
melanin biosynthesis. MITF, the key activator of these genes or
proteins, is a central transcription factor that regulates melanin
synthesis and governs the proliferation, differentiation and
survival of melanocytes (38,39).
Materials capable of controlling these melanin synthesis factors
can offer considerable advantages in developing effective
skin-whitening products, such as enhancing skin brightening
efficacy, reducing hyperpigmentation, and providing a safer
alternative to conventional depigmenting agents like
hydroquinone.
The anti-melanogenic effect induced by CSS-NPs may
be associated with the activation of the ERK and Akt signaling
pathways. The ERK and Akt signaling pathways are associated with
the transcriptional activation and stabilization of MITF (40,41).
Accordingly, research aims to identify candidate substances that
regulate the ERK and Akt signaling pathways; however, this faces
challenges owing to the potential for side effects (42–45).
Examples of such candidate substances include gomisin N and
berberine, which is a component of various plant species (42,43).
These compounds inhibit melanin production through ERK and Akt
pathway activation; however, clinical research is still in its
early phases, and cytotoxicity has been observed at high
concentrations (44,45). The present study revealed that
CSS-NPs significantly inhibited melanin production without
exhibiting toxicity. Additionally, mechanistic experiments using
ERK- and Akt-specific pharmacological inhibitors revealed that the
decrease in key elements associated with melanin synthesis induced
by CSS-NPs could be reversed by activating the ERK and Akt
pathways. Thus, CSS-NPs, with non-cytotoxic properties, may serve
as novel natural cosmetic ingredients with the potential to treat
skin pigmentation disorder and serve as an alternative to
chemical-based cosmetics.
CSS-NPs are a multifunctional natural material,
possessing not only anti-melanogenic effects but also antioxidant
properties. Melanin is involved in ROS production, which causes
melanocytes undergoing melanin synthesis to maintain higher ROS
levels than other cell types (35). Elevated levels of ROS damage the
DNA, proteins and lipids within skin cells, leading to impaired
cellular function. This oxidative stress contributes to skin aging,
pigmentary alteration and inflammatory responses (35,46,47).
CSS-NPs could scavenge free radicals, and in conditions of
excessive melanin synthesis, CSS-NPs restored antioxidant capacity
within the cellular antioxidant defense system. These findings
suggested that CSS-NPs can be employed as a cosmetic ingredient
with multifunctional effects, capable of not only alleviating skin
pigmentation disorder caused by excessive melanin synthesis but
also protecting skin cells.
Plants can produce various phytochemicals beneficial
to human health, which can be encapsulated within plant-derived
exosomes to exert bioactive functions (48–50).
GC-MS and LC-MS data provided insights into the chemical
composition and potential bioactive functions of CSS-NPs. The
metabolic product analysis of CSS-NPs identified 10 phospholipids,
which may be involved in the nanostructure formation of CSS-NPs.
CSS-NPs incorporate three compounds known for their
anti-melanogenic activity (adenosine, phytosphingosine and
oleoylethanolamide) and four compounds recognized for their
antioxidant effects (2-O-protocatechuoylalphitolic acid,
pheophorbide A, myo-inositol and stigmastane-3,6-dione).
Consequently, the decreased melanin intracellular melanin synthesis
by CSS-NPs and the enhancement of antioxidant effects may be due to
metabolites in CSS-NPs that induce anti-melanogenic and antioxidant
properties.
Despite the present study demonstrating the
functional potential of CSS-NPs as a cosmetic ingredient at the
cellular level, additional studies are needed to establish their
practical value and reliability. To enhance the credibility and
applicability of CSS-NPs, it is essential to obtain safety data
through clinical studies addressing potential side effects and
toxicity in humans. Once safety is confirmed, evaluation of the
clinical value of CSS-NPs for various skin conditions (such as,
wrinkles, hyperpigmentation, dry skin, telangiectasia and skin
damage) is key for enhancing their practical value. Toxicological
and clinical data may determine the value and function of CSS-NPs
as a cosmetic ingredient.
The present study confirmed that CSS-NPs, which
contain metabolites with anti-melanogenic and antioxidant
properties, inhibited excessive melanin synthesis by inducing ERK
and Akt activation. Moreover, CSS-NPs induced antioxidant effects
in melanocytes by decreasing ROS levels and restoring antioxidant
activity during the melanin synthesis process. These findings
indicated that CSS-NPs may be used as a multifunctional ingredient
in the treatment of hyperpigmentation disorder as they suppress
excessive melanin production in skin cells and offer protective
effects against cellular toxicity.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Korea Research Institute
of Bioscience and Biotechnology Research Initiative Programs (grant
nos. KGM5242423 and KGM5382414).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HJL performed experiments, analyzed data and wrote
the manuscript. YHK performed experiments and analyzed data. SJL
and JCJ analyzed data. SHP designed and performed experiments. JMY
performed experiments and facilitated data interpretation. JCJ, WSK
and YBR designed experiments. WSK conceived and supervised the
study, performed experiments and wrote and reviewed the manuscript.
YBR and WSK confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of the Korea Research Institute of
Bioscience and Biotechnology (approval no. KRIBB-AEC-24094).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EV
|
extracellular vesicle
|
|
ROS
|
reactive oxygen species
|
|
CSS-NP
|
Cannabis sativa stem-derived
EV-like nanoparticle
|
|
TFF
|
tangential flow filtration
|
|
SEM
|
scanning electron microscopy
|
|
Q-TOF-MS
|
quadrupole time-of-flight mass
spectrometry
|
|
UPLC
|
ultra-high-performance liquid
chromatography
|
|
GC
|
gas chromatography
|
|
α-MSH
|
α-melanocyte stimulating hormone
|
|
TYR
|
tyrosinase
|
|
DPPH
|
2,2-diphenyl-1-picrylhydrazyl
|
|
ABTS
|
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
diammonium
|
|
TAC
|
total antioxidant capacity
|
|
CAT
|
catalase
|
|
MITF
|
microphthalmia-associated
transcription factor
|
|
TRP
|
tyrosinase-related protein
|
References
|
1
|
D'Mello SA, Finlay GJ, Baguley BC and
Askarian-Amiri ME: Signaling pathways in melanogenesis. Int J Mol
Sci. 17:11442016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Solano F: Photoprotection and skin
pigmentation: Melanin-Related molecules and some other new agents
obtained from natural sources. Molecules. 25:15372020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee A, Kim JY, Heo J, Cho DH, Kim HS, An
IS, An S and Bae S: The inhibition of melanogenesis via the PKA and
ERK signaling pathways by chlamydomonas reinhardtii extract in
B16F10 melanoma cells and artificial human skin equivalents. J
Microbiol Biotechnol. 28:2121–2132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarkar R, Arora P and Garg KV:
Cosmeceuticals for hyperpigmentation: What is available? J Cutan
Aesthet Surg. 6:4–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pillaiyar T, Manickam M and Namasivayam V:
Skin whitening agents: Medicinal chemistry perspective of
tyrosinase inhibitors. J Enzyme Inhib Med Chem. 32:403–425. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Solano F, Briganti S, Picardo M and Ghanem
G: Hypopigmenting agents: An updated review on biological, chemical
and clinical aspects. Pigment Cell Res. 19:550–571. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kooyers T and Westerhof W: Toxicology and
health risks of hydroquinone in skin lightening formulations. J
European academy of Dermatology and Venereology. 20:777–780. 2006.
View Article : Google Scholar
|
|
8
|
Sarasati A, Syahruddin MH, Nuryanti A, Ana
ID, Barlian A, Wijaya CH, Ratnadewi D, Wungu TDK and Takemori H:
Plant-derived exosome-like nanoparticles for biomedical
applications and regenerative therapy. Biomedicines. 11:10532023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sall IM and Flaviu TA: Plant and
mammalian-derived extracellular vesicles: A new therapeutic
approach for the future. Front Bioeng Biotechnol. 11:12156502023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lian MQ, Chng WH, Liang J, Yeo HQ, Lee CK,
Belaid M, Tollemeto M, Wacker MG, Czarny B and Pastorin G:
Plant-derived extracellular vesicles: Recent advancements and
current challenges on their use for biomedical applications. J
Extracell Vesicles. 11:e122832022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ju S, Mu J, Dokland T, Zhuang X, Wang Q,
Jiang H, Xiang X, Deng ZB, Wang B, Zhang L, et al: Grape
exosome-like nanoparticles induce intestinal stem cells and protect
mice from DSS-induced colitis. Mol Ther. 21:1345–1357. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B, Zhuang X, Deng ZB, Jiang H, Mu J,
Wang Q, Xiang X, Guo H, Zhang L, Dryden G, et al: Targeted drug
delivery to intestinal macrophages by bioactive nanovesicles
released from grapefruit. Mol Ther. 22:522–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li DF, Tang Q, Yang MF, Xu HM, Zhu MZ,
Zhang Y, Tian CM, Nie YQ, Wang JY, Liang YJ, et al: Plant-derived
exosomal nanoparticles: Potential therapeutic for inflammatory
bowel disease. Nanoscale Adv. 5:3575–3588. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi W, Cho JH, Park SH, Kim DS, Lee HP,
Kim D, Kim HS, Kim JH and Cho JY: Ginseng root-derived exosome-like
nanoparticles protect skin from UV irradiation and oxidative stress
by suppressing activator protein-1 signaling and limiting the
generation of reactive oxygen species. J Ginseng Res. 48:211–219.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim M, Jang H, Kim W, Kim D and Park JH:
Therapeutic applications of plant-derived extracellular vesicles as
antioxidants for oxidative stress-related diseases. Antioxidants
(Basel). 12:12862023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morgan C and Nigam Y: Naturally derived
factors and their role in the promotion of angiogenesis for the
healing of chronic wounds. Angiogenesis. 16:493–502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roleira FM, Tavares-da-Silva EJ, Varela
CL, Costa SC, Silva T, Garrido J and Borges F: Plant derived and
dietary phenolic antioxidants: Anticancer properties. Food Chem.
183:235–258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui X, Lin Q and Liang Y: Plant-derived
antioxidants protect the nervous system from aging by inhibiting
oxidative stress. Front Aging Neurosci. 12:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hussain T, Jeena G, Pitakbut T, Vasilev N
and Kayser O: Cannabis sativa research trends, challenges, and
new-age perspectives. iScience. 24:1033912021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stasilowicz-Krzemien A, Sip S, Szulc P and
Cielecka-Piontek J: Determining antioxidant activity of cannabis
leaves extracts from different varieties-unveiling Nature's
Treasure Trove. Antioxidants (Basel). 12:13902023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koltai H and Shalev N: Anti-Cancer
activity of Cannabis Sativa Phytocannabinoids: Molecular mechanisms
and potential in the fight against ovarian cancer and stem cells.
Cancers (Basel). 14:42992022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaiachuk M, Suryavanshi SV, Pryimak N,
Kovalchuk I and Kovalchuk O: The anti-inflammatory effects of
cannabis sativa extracts on LPS-induced cytokines release in human
macrophages. Molecules. 28:49912023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zagorska-Dziok M, Bujak T, Ziemlewska A
and Niziol-Lukaszewska Z: Positive effect of Cannabis sativa L.
Herb extracts on skin cells and assessment of Cannabinoid-Based
Hydrogels properties. Molecules. 26:8022021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim WS, Ha JH, Jeong SH, Lee JI, Lee BW,
Jeong YJ, Kim CY, Park JY, Ryu YB, Kwon HJ and Lee I: Immunological
effects of aster yomena callus-derived extracellular vesicles as
potential therapeutic agents against allergic Asthma. Cells.
11:28052022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HJ, Shin KW, Lee SJ, Park JY, Lee IC,
Kwon HJ, Jeong HJ, Yuk JM, Ryu YB and Kim WS: Immunomodulation by
extracellular vesicle-like nanoparticles from marine macroalgae
Sargassum fusiforme: Enhancing type 1 T helper and cytotoxic T
lymphocyte-mediated immune responses. J Funct Foods.
112:1059812024. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blois MS: Antioxidant determinations by
the use of a stable free radical. Nature. 181:1199–1200. 1958.
View Article : Google Scholar
|
|
28
|
Re R, Pellegrini N, Proteggente A, Pannala
A, Yang M and Rice-Evans C: Antioxidant activity applying an
improved ABTS radical cation decolorization assay. Free Radic Biol
Med. 26:1231–1237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ou X, Wang H, Tie H, Liao J, Luo Y, Huang
W, Yu R, Song L and Zhu J: Novel plant-derived exosome-like
nanovesicles from Catharanthus roseus: Preparation,
characterization, and immunostimulatory effect via
TNF-alpha/NF-kappaB/PU.1 axis. J Nanobiotechnology. 21:1602023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niu C and Aisa HA: Upregulation of
melanogenesis and tyrosinase activity: Potential agents for
Vitiligo. Molecules. 22:13032017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
El-Nashar HAS, El-Din MIG, Hritcu L and
Eldahshan OA: Insights on the inhibitory power of flavonoids on
tyrosinase activity: A survey from 2016 to 2021. Molecules.
26:75462021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim JW, Ha JH, Jeong YJ and Park SN:
Anti-melanogenesis effect of dehydroglyasperin C through the
downregulation of MITF via the reduction of intracellular cAMP and
acceleration of ERK activation in B16F1 melanoma cells. Pharmacol
Rep. 70:930–935. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jang JY, Lee JH, Kang BW, Chung KT, Choi
YH and Choi BT: Dichloromethane fraction of Cimicifuga
heracleifolia decreases the level of melanin synthesis by
activating the ERK or AKT signaling pathway in B16F10 cells. Exp
Dermatol. 18:232–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Emanuelli M, Sartini D, Molinelli E,
Campagna R, Pozzi V, Salvolini E, Simonetti O, Campanati A and
Offidani A: The double-edged sword of oxidative stress in skin
damage and melanoma: From physiopathology to therapeutical
approaches. Antioxidants (Basel). 11:6122022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakai K and Tsuruta D: What are reactive
oxygen species, free radicals, and oxidative stress in skin
diseases? Int J Mol Sci. 22:107992021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ansary TM, Hossain MR, Kamiya K, Komine M
and Ohtsuki M: Inflammatory molecules associated with ultraviolet
radiation-mediated skin aging. Int J Mol Sci. 22:39742021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawakami A and Fisher DE: The master role
of microphthalmia-associated transcription factor in melanocyte and
melanoma biology. Lab Invest. 97:649–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gelmi MC, Houtzagers LE, Strub T, Krossa I
and Jager MJ: MITF in normal melanocytes, cutaneous and uveal
melanoma: A delicate balance. Int J Mol Sci. 23:60012022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ko GA and Cho SK: Phytol suppresses
melanogenesis through proteasomal degradation of MITF via the
ROS-ERK signaling pathway. Chem Biol Interact. 286:132–140. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cheng ZJ, Dai GF, Hsu JL, Lin JJ, Wu WT,
Su CC and Wu YJ: Antimelanogenesis effect of methyl gallate through
the regulation of PI3K/Akt and MEK/ERK in B16F10 melanoma cells.
Evid. Based Complement Alternat Med. 2022:50926552022.PubMed/NCBI
|
|
42
|
Chae JK, Subedi L, Jeong M, Park YU, Kim
CY, Kim H and Kim SY: Gomisin N inhibits melanogenesis through
regulating the PI3K/Akt and MAPK/ERK signaling pathways in
Melanocytes. Int J Mol Sci. 18:4712017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song YC, Lee Y, Kim HM, Hyun MY, Lim YY,
Song KY and Kim BJ: Berberine regulates melanin synthesis by
activating PI3K/AKT, ERK and GSK3β in B16F10 melanoma cells. Int J
Mol Med. 35:1011–1016. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smejkal K, Slapetova T, Krmencik P, Babula
P, Dall'Acqua S, Innocenti G, Vančo J, Casarin E, Carrara M,
Kalvarová K, et al: Evaluation of cytotoxic activity of Schisandra
Chinensis Lignans. Planta Med. 76:1672–1677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Singh N and Sharma B: Toxicological
effects of Berberine and Sanguinarine. Front Mol Biosci. 5:212018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu HM, Cheng MY, Xun MH, Zhao ZW, Zhang
Y, Tang W, Cheng J, Ni J and Wang W: Possible mechanisms of
oxidative stress-induced skin cellular senescence, inflammation,
and cancer and the therapeutic potential of plant polyphenols. Int
J Mol Sci. 24:37552023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wei M, He X, Liu N and Deng H: Role of
reactive oxygen species in ultraviolet-induced photodamage of the
skin. Cell Div. 19:12024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rodriguez-Casado A: The health potential
of fruits and vegetables phytochemicals: Notable examples. Crit Rev
Food Sci Nutr. 56:1097–1107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Barbieri R, Coppo E, Marchese A, Daglia M,
Sobarzo-Sánchez E, Nabavi SF and Nabavi SM: Phytochemicals for
human disease: An update on plant-derived compounds antibacterial
activity. Microbiol Res. 196:44–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gonzalez-Vallinas M, Gonzalez-Castejon M,
Rodriguez-Casado A and de Molina AR: Dietary phytochemicals in
cancer prevention and therapy: A complementary approach with
promising perspectives. Nutr Rev. 71:585–599. 2013. View Article : Google Scholar : PubMed/NCBI
|