Introduction
Globally, cancers are considered as one of the
highest leading causes of mortality. The pathogenesis of cancer is
initiated when normal cells acquire the ability to uncontrollably
grow and eventually invade and damage the normal tissues of the
body (1). Cancer development is
comprised of multiple stages, that is, from precancerous changes to
malignant tumors. Furthermore, some cancer cells may spread from
their original location to other places in the body via the
bloodstream or lymphatic system, referred to as metastasis
(2). Embryonic cancer stem cells
(CSCs) are known as a small subpopulation of whole cancer cells
which exhibit high tumorigenic potential (3,4). In
the past, CSCs were simply considered as tumor-initiating cells and
also known to be the primary cause for tumorigenesis (5,6).
CSCs are known to have additional features including a self-renewal
capacity, differentiation into all types of cancers, invasion and
metastasis into other tissues and drug resistance (7). These characteristics induce cancer
aggressiveness and play a pivotal role in increasing cancer therapy
difficulty. Additionally, CSCs have the fundamental properties of
all types of cancers including high tumorigenicity, high metastasis
potential, immune surveillance evasion, drug resistance and cancer
relapse (3,5,6,8,9).
Thus, several therapeutic attempts targeting these CSCs have been
employed to improve the therapeutic effects and long-term clinical
outcome, including efforts to overcome the unique carcinogenic
properties of CSCs.
The transcription pluripotency factors, also known
as stem cell markers, such as NANOG, SOX2 and OCT4, contribute in
maintaining the phenotype of pluripotent embryonic stem (ES) cells
in embryonic stem cells (ESCs) and enterochromaffin cells (10–12).
Some studies suggest that the abnormal ES cell self-renewal through
the hyper-expression of these transcription pluripotency factors
promotes carcinogenesis (13–15).
Particularly, NANOG overexpression enhances poor prognosis in
gastric adenocarcinoma indicating tumor progression (16). Reactive oxygen species (ROS) are
known to be a subset of free radicals, which promote tumorigenesis,
resulting in oxidative damages of DNA, protein or lipid in normal
cells. In the prolonged stress state, cells activate DNA repair
pathway, also known as a DNA damage response (DDR), for the repair
of damaged DNA and maintaining genome fidelity (17–19).
The DDR mechanism is initiated with the induction of
phosphorylation in ATM, ATR and DNA-PKcs kinase (20,21).
Moreover, Chk1/2 and BRCA1 are phosphorylated, thus activating
p53-dependent pathway in the repair response to DNA damage, which
leads to the induction of cell cycle arrests, DDR activation and
apoptotic mechanism (22).
Cell cycle checkpoints are precisely controlled by
both activators and inhibitors to evade normal cell carcinogenesis.
Generally, the failed checkpoints for oncogenesis or tumorigenesis
allow progression of uncontrolled cell cycle, induce failure of
apoptosis and downregulate tumor suppressor factors.
Cyclin-dependent kinases, such as CDK4, are considered to be
significant cell cycle regulators through positively regulating
multiple checkpoints (23,24). However, CDK inhibitors (CKIs) such
as p21 (p21Cip1/Waf1) and p53 are known as tumor
suppressor factors that inhibit the abnormal cell cycle and
carcinogenesis (25–27). Therefore, carcinogenesis in normal
cells is related to abnormal operation of CDKs or CKIs, which
indicates loss of the ability to induce DNA repair or apoptosis.
These cell cycle checkpoints are being studied as target sites for
anticancer treatment. Matrix metalloproteinases (MMPs), such as
MMP-2, −3 or −9, are knowns as the family of metzincin proteases,
which play a role in the degradation of various proteins in the
extracellular matrix (ECM) (28,29).
In tumor cells, it plays an essential role in a wide range of
tumorigenesis, tumor growth, tumor invasion and metastasis
(30–32). Additionally, MMPs are emerging as a
key player in the entrance or exit of circulating cancer cells in
the blood vessels (33). Hence,
tumor-associated MMPs are good targets for developing MMP
inhibitors for anticancer therapy.
Chemotherapy using various types of drugs has been
used to effectively treat several types of cancer. Cancer cells are
known to grow faster than normal cells, which indicates that it is
easier for drugs to attack these fast-growing cells. However,
similar to other cancer treatments, it often causes various side
effects, including fatigue, nausea, vomiting, various pains or
memory loss (34,35). Additionally, although chemotherapy
is initiated depending on the cancer type, location, drugs and
health condition of the patient, chronic drug exposure has been
linked to serious side effects, including neutropenia, arrhythmia,
neuropathy and cardiovascular breakdown. Thus, cancer treatment
using natural ingredients could be a more optimal alternative as it
has been known to alleviate side effects compared with
chemotherapeutic drugs (36–40).
Recently, anticancer chemotherapy using natural ingredients has
been widely employed to extend the therapeutic domain in
chemotherapeutic agents, decrease side effects or drug resistance
(41–44).
Natural gallic acid (3,4,5-trihydroxybenzoic acid;
GA) is a secondary metabolite, which is widely distributed in
natural plants, vegetables, fruits and green tea (45–47).
GA is considered to have numerous pharmacological properties,
including antibacterial, anti-inflammatory, antioxidant, antiviral
and antitumor activities (48–55).
Furthermore, it has anticancer properties, including apoptosis
induction and suppression of angiogenesis and metastasis. Some
studies have reported that GA selectively activates the induction
of cancer cell apoptosis in HeLa, HCT-15, SH-SY5Y and NSCLC cells
(56–60). In addition, it suppresses cell
proliferation and metastasis through the increase or decrease of
fatty acid synthase in estrogen receptor (ER) alpha level in
TSGH-8301 bladder cancer cells (60). A study suggests that GA decreases
the progression of T24 bladder cancer cell via inducing
mitochondrial dysfunction and suppressing the PI3K/Akt and NF-κB
signaling pathway (61).
The present study revealed that natural GA showed
multiple anticancer functions in two NTERA-2 and NCCIT human
embryonic carcinoma cells. The anticancer properties of GA in terms
of proliferation, apoptosis, cell cycle distribution, migration and
invasion were assessed. It was demonstrated that GA effectively
suppressed the cancer characteristics of CSC cells. Additionally,
it was revealed that the reduction of MMPs by GA was associated
with EGFR/JAK2/STAT5 signaling inhibition.
Materials and methods
Cell Culture
Embryonic carcinoma cells (NCCIT and NTERA-2 cells
with biomarkers of NANOG, SOX2 and OCT4) were obtained from the
Korea Cell Line Bank. NTERA-2 cells were maintained using
Dulbecco's Modified Eagle's Medium (DMEM; cat. no. L0103; Biowest)
and NCCIT cells were cultured using RPMI-1640 media (cat. no.
L0498; Biowest) containing 10% fetal bovine serum (FBS; cat. no.
A5670801; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (cat. no. 15140122; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in 5% CO2; 5×105
cells/60 mm dish were cultured up to 70–80% confluence for 48 h and
the adherent cells were harvested with trypsin-EDTA solution (cat.
no. T4049; MilliporeSigma). The cells were either treated or left
untreated with GA and were then further cultured for 24 h.
Reagents and antibodies
GA was bought from MilliporeSigma and dissolved in
distilled water (stock concentration of 100 mM). MitoSOX and
CM-H2DCFDA were obtained from Invitrogen (Thermo Fisher
Scientific, Inc.). The primary antibodies were purchased from Santa
Cruz Biotechnology, Inc.: p21 (cat. no. sc-756), p53 (cat. no.
sc-126), cyclin E (cat. no. sc-481), MMP9 (cat. no. sc-13520),
STAT5b (cat. no. sc-1656), CDK4 (cat. no. sc-260) and β-actin (cat.
no. sc-47778). Cell Signaling Technology, Inc. supplied: p27 Kip1
(cat. no. 3686), p-EGFR (cat. no. 3777), EGFR (cat. no. 4267),
phosphorylated (p-)JAK2 (cat. no. 3776), JAK2 (cat. no. 3230),
p-STAT5 (cat. no. 9351), pCHK1 (cat. no. 2348), p-CHK2 (cat. no.
2197), p-ATM (cat. no. 5883), p-Histone (cat. no. 9718) and p-BRCA1
(cat. no. 9009). MilliporeSigma supplied MMP3 (cat. no. AB2963),
OCT4 (cat. no. MABD76), SOX2 (cat. no. MAB4423) and NANOG (cat. no.
MABD24) Abcam supplied Cyclin D1 (cat. no. ab6152) and EnoGene MMP2
(cat. no. E90317). Secondary antibodies were purchased from Cell
Signaling Technology (cat. nos. 7074; anti-rabbit and 7076;
anti-mouse). All antibodies were incubated at 4°C for 12–16 h.
Morphological analysis
NTERA-2 and NCCIT cells were cultured at a density
of 1.5×105 cells per well and treated with or without GA
for 24 h, followed by washing with phosphate-buffered saline (PBS)
twice and again incubated with DAPI staining solution for 1 h. A
fluorescence microscope was used to capture the images (Olympus
IX71/DP72; Olympus Corporation).
Cell viability assay
MTT (cat. no. M6494; Thermo Fisher Scientific, Inc.)
is used to detect reductive metabolism in cells for viability,
proliferation and cytotoxicity assays. Cells (3×103
cells per well) were cultured in a 96-well plate for 24 h.
Thereafter, they were incubated using dimethyl sulfoxide (DMSO) as
vehicle control or using different concentrations of GA (20–400 µM)
at 37°C for 24 h. The next day, treatment with 5 mg/ml of MTT
reagent was performed and incubation for 4 h at 37°C. After washing
with 1X PBS, the formazan product was then dissolved in DMSO. At a
560-nm wavelength, the formazan product absorbance was measured
with an Ultra Multifunctional Microplate Reader (Tecan Group,
Ltd.).
Apoptosis analysis
An Apoptosis assay kit (cat. no. 556570; BD
Biosciences) was used to measure apoptosis in NCCIT and NTERA-2
cells. First, the GA-treated or GA-untreated cells were washed with
PBS and resuspended in a binding buffer at a concentration of
1×106 cells. Thereafter, cells were stained with Annexin
V-FITC and propidium iodide for 10 min in a dark box at room
temperature. Finally, the percentage of apoptotic cells was
measured using flow cytometry using a FACSCalibur flow cytometer
(BD Biosciences) and dead cells are initially filtered through a
‘singlets’ gate that excludes aggregates, using the height vs. area
signals of the same parameter (such as side scatter or forward
scatter). Then, cells were gated for detecting apoptotic cells on
Annexin V-FITC (FL1) vs. propidium iodide (PI) (FL3). FlowJo v10
software (FlowJo LLC) was used to perform the analysis. The
apoptotic rates were calculated with apoptotic cells (Annexin
V+/PI-), late apoptotic cells (Annexin V+/PI+), and necrotic cells
(Annexin V-/PI+).
Cell cycle analysis
The DNA content from cells with or without GA was
determined using a BD Cycletest Plus DNA Reagent Kit (BD
Biosciences) according to the manufacturer's protocol. The samples
(5×105 cells) were analyzed using a FACSCalibur flow
cytometer (BD Biosciences).
Western blot analysis
All protein samples were obtained using a
radioimmunoprecipitation assay lysis buffer (cat. no. 20–188;
MilliporeSigma) on ice for 20 min and determined by the Bradford
assay. The proteins isolated (10 µg/well) were resolved on sodium
dodecyl sulfate (SDS)-polyacrylamide gels (6–15%) and transferred
to nitrocellulose western blotting membrane (cat. no. 10600002;
Cytiva). Then 2% bovine serum albumin (BSA; cat. no. 5217; R&D
systems) was used for blocking at 4°C overnight. Target proteins
were identified using immunoblotting assay at 4°C overnight. The
primary antibodies (1:1,000 dilution) and secondary antibodies
(1:5,000-10,000) used are in the Reagents and antibodies section.
The iBright™ CL1500 Imaging System (cat. no. A44114; Invitrogen;
Thermo Fisher Scientific, Inc.) was used to capture images and
analyze data from immunoblotting assay.
Reverse transcription-quantitative
(RT-q) PCR
At between 70–80% confluence, cells were gently
washed twice with PBS. Using TRIzol® (cat. no. 15596018;
Thermo Fisher Scientific, Inc.), total RNAs were isolated and
quantified by Nanodrop (ND-1000; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Subsequently, a
thermal cycler was used to prepare cDNA from the total RNA using a
first-strand cDNA synthesis kit (Bioneer Corporation) for RT-qPCR
according to the manufacturer's instructions. The qPCR was
performed using a LightCycler 480II (Roche Diagnostics). It was
used for qPCR as follows: 2 µl diluted cDNA was mixed with 10 µl TB
Green Advantage Premix (cat. no. 639676; Takara Bio, Inc.) and 1 µl
(100 pM) each of forward and reverse primers according to the
manufacturer's instructions. The cycling conditions were: 95°C for
5 min for the initial denaturation, followed by 40 cycles of 95°C
for 40 sec, 58°C for 40 sec, 72°C for 40 sec and, finally,
extension for 5 min at 72°C. All reactions were conducted three
times and normalized to GAPDH and quantifications were conducted
using obtained Cp values. The sequences of all primers with GAPDH
are in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| CDK4 | Sense |
5′-CCCGAAGTTCTTCTGCAGTC-3′ |
|
| Antisense |
5′-CTGGTCGGCTTCAGAGTTTC-3′ |
| Cyclin D1 | Sense |
5′-TGTTTGCAAGCAGGACTTTG-3′ |
|
| Antisense |
5′-TCATCCTGGCAATGTGAGAA-3′ |
| Cyclin E | Sense |
5′-ATCCTCCAAAGTTGCACCAG-3′ |
|
| Antisense |
5′-AGGGGACTTAAACGCCACTT-3′ |
| p21 | Sense |
5′-ATGAAATTCACCCCCTTTCC-3′ |
|
| Antisense |
5′-AGGTGAGGGGACTCCAAAGT-3′ |
| p27 | Sense |
5′-CCGGCTAACTCTGAGGACAC-3′ |
|
| Antisense |
5′-TTGCAGGTCGCTTCCTTATT-3′ |
| SOX2 | Sense |
5′-CTGCAGTACAACTCCATGAC-3′ |
|
| Antisense |
5′-GAGTGGGAGGAAGAGGTAAC-3′ |
| Nanog | Sense |
5′-ACCAGTCCCAAAGGCAAACA-3′ |
|
| Antisense |
5′-TCTGCTGGAGGCTGAGGTAT-3′ |
| Oct4 | Sense |
5′-CAAAGCAGAAACCCTCGTGC-3′ |
|
| Antisense |
5′-AACCACACTCGGACCACATC-3′ |
| MMP2 | Sense |
5′-TGATGGCATCGCTCAGATCC-3′ |
|
| Antisense |
5′-GGCCTCGTATACCGCATCAA-3′ |
| MMP3 | Sense |
5′-CACAGACCTGACTCGGTTCC-3′ |
|
| Antisense |
5′-AGGTTCTGGAGGGACAGGTT-3′ |
| MMP9 | Sense |
5′-GGACAAGCTCTTCGGCTTCT-3′ |
|
| Antisense |
5′-TCGCTGGTACAGGTCGAGTA-3′ |
| GAPDH | Sense |
5′-CCCACTCCTCCACCTTTGAC-3′ |
|
| Antisense |
5′-TCCTCTTGTGCTCTTGCTGG-3′ |
Tumorsphere formation assay
Cells were cultured in DMEM/F-12-containing growth
supplements, epidermal growth factor, basic fibroblast growth
factor and B27 in low attachment six-well plates together with or
without GA for 14 days. Cell status was checked on days 0, 7 or 14
using an optical microscope (BX-51; Olympus Corporation) at 100×
and 200× magnification, and cells harvested for immunoblotting
assay.
Mitochondrial and cellular ROS
Analysis
1×106 cells were stained with 5 µM of
MitoSOX to evaluate mROS or 5 µM of CM-H2DCFDA to
evaluate cellular ROS for 20 min at 37°C. Then, the stained cells
were used for ROS analysis using a FACSCalibur flow cytometer (BD
Biosciences). FlowJo v10 software (FlowJo LLC) was used for the
analysis.
Comet assay
Cellular DNA damage was measured using the comet
assay kit (cat. no. 238544; Abcam) following the manufacturer's
protocol. This assay is a single-cell gel electrophoresis method
for a simple evaluation of cellular DNA damage. First, a base layer
of comet agarose was created on a slide and then a layer of cells
and agarose was added, followed by lysis. Next, under neutral
conditions, electrophoresis was performed and cells were stained
using a DNA dye. Finally, a fluorescence microscope was used to
observe cell morphology (Olympus IX71/DP72; Olympus
Corporation).
Invasion assay
Matrigel was used to pre-coat plates overnight at
4°C. For the invasion assay, 5×104 cells with or without
GA were inserted into each invasion chamber (BD Biocoat; BD
Biosciences) and further incubated at 37°C for 24 h. Then, cells
were stained with crystal violet at room temperate for 30 min and
the invaded cells were observed using an optical microscope (BX-51;
Olympus Corporation) at 100× and 200× magnification and
counted.
Wound healing assay
NTERA-2 and NCCIT cells (1×105 per well)
were seeded and incubated in six-well plates. After a confluent
monolayer was attained, the cell layers were scratched using a
1,000 µl pipette tip and immediately incubated with GA (0 or 200
µM) for 24 h. Images were captured at different time intervals to
assess the wound edges through an optical microscope (BX-51;
Olympus Corporation) at 100× and 200× magnification and closure of
the wound was quantified by ImageJ (v1.51; National Institutes of
Health). All cells were cultured with culture medium without
FBS.
Chromatin immunoprecipitation (ChIP) assay. A
ChIP assay kit was purchased from MilliporeSigma (cat. no. 17–295)
and the ChIP assay was performed according to the manufacturer's
protocol. Cells (1×106) were fixed in 1% formaldehyde
and quenched with 1.25 M glycine, washed with ice-cold PBS,
suspended in a nuclear preparation and shearing buffer, and then
sonicated (on/off 20 sec during 20 min on ice). The sheared DNA was
centrifuged at 13,000 × g for 15 min at 4°C, followed by
protein/DNA immunoprecipitation from the cleared supernatant as
follows. The clarified supernatant was diluted with buffer (1:1
ratio), and 5 µl aliquots of the diluted samples were used as
internal controls. Next, the diluted supernatant was incubated with
STAT5b anti-bodies in pre-coated wells for 90 min at room
temperature. The controls were incubated with normal goat-IgG and
anti-RNA polymerase II. Unbound DNA was removed and bound DNA
collected using the cross-link reversal method with DNA release
buffer containing proteinase K. The released DNA and internal
control DNA were purified using the GenElute Binding Column G (cat.
no. 17-295; MilliporeSigma). DNAs from each cell were quantified
using a PCR instrument with the following primers for MMP2: Sense;
5′-TGATGGCATCGCTCAGATCC-3′ and Antisense;
5′-GGCCTCGTATACCGCATCAA-3′.
Statistical analyses
All experiments were performed in triplicate. The
values of three independent experiments conducted in triplicates
(n=3) were represented as mean ± standard error of the mean. The
controls were set to 100. One way analysis of variance (ANOVA) or
Student's t-test were used for the statistical analyses. One-way
ANOVA was also performed using Tukey's post hoc test. The analyses
were performed using the SAS 9.3 software (SAS Institute, Inc.).
The relative density of proteins by the effects of GA was compared
with the GA non-treated control. P<0.05 was considered to
indicate a statistically significant difference.
Results
GA inhibits the propagation of
embryonic CSCs and induces apoptosis
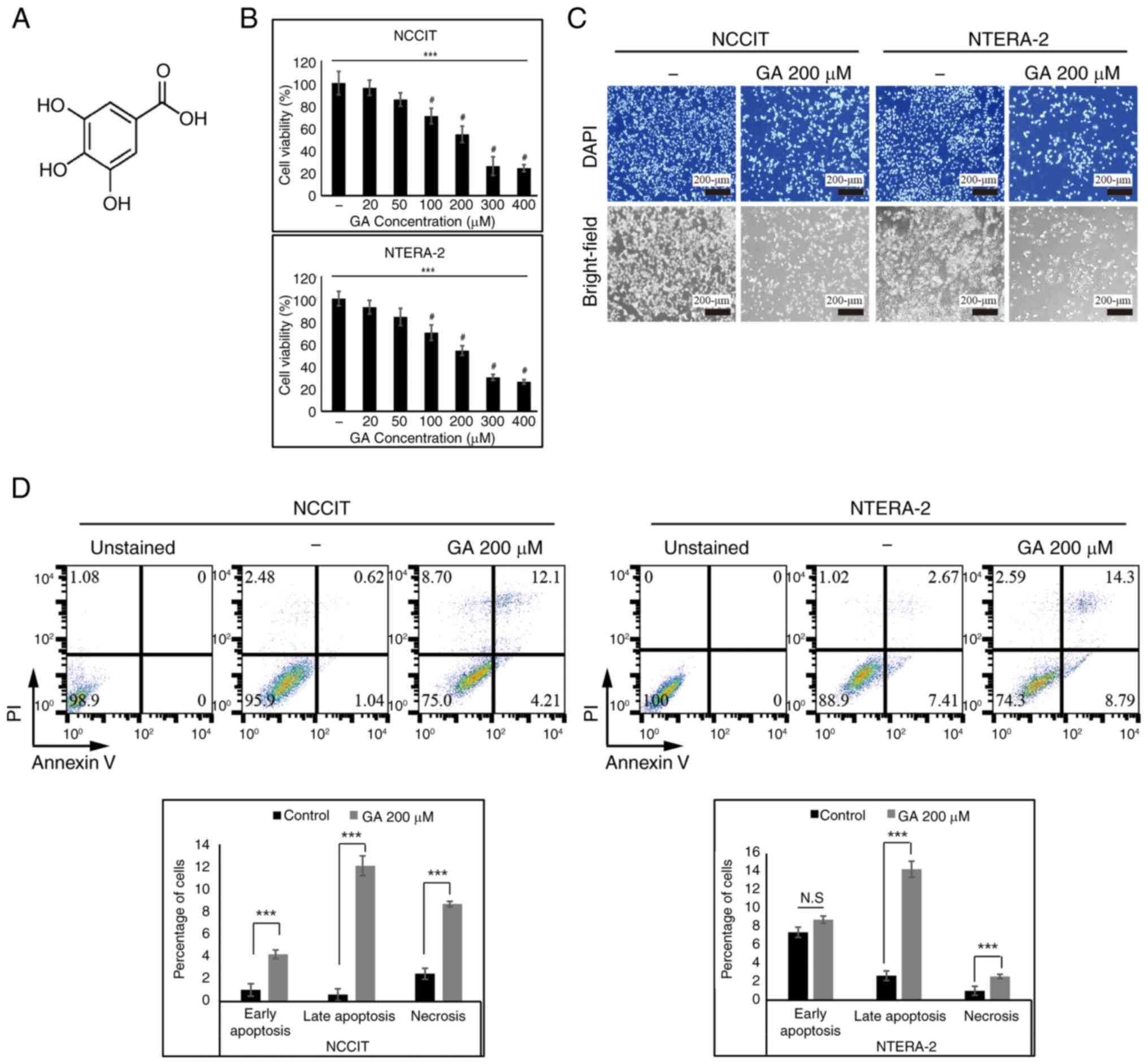
The structure of GA, also known as 3,4,5-trihydroxy
benzoic acid, is shown by Fig. 1A.
First, the present study performed the MTT assay for confirming the
inhibition ability of cell proliferation by GA in embryonic CSCs.
It confirmed a dose-dependent inhibition of cell viability through
GA treatment in CSCs (Fig. 1B).
The present study used 200 µM GA as the IC50 dose and
100- or 200 µM GA concentrations for dose-based studies. Then, the
status of embryonic CSCs following 200 µM GA treatment was
estimated using DAPI staining. This confirmed the characteristic
features of apoptosis, such as the nuclear shrinkage and
fragmentation as well as the chromatin condensation in CSCs treated
with GA. A decrease in cell number following GA treatment was
clearly observed compared with non-treated control (Fig. 1C). The inhibition of CSCs growth
via GA treatment was verified with bright-field microscopic
analysis. These results may provide evidence that GA can induce
apoptosis. FACS-based Annexin V/PI staining assay was applied to
assess the effects of GA on the apoptosis and necrosis of NTERA-2
and NCCIT cells. The assay may show that GA induced the increase in
early-stage apoptosis (Annexin V+/PI-cells), late-stage apoptosis
(Annexin V+/PI+ cells) and necrosis (Annexin V-/PI+ cells).
Treatment with 200-µM GA into CSCs demonstrated the inhibitory
effect of GA, suggesting that GA may induce apoptosis. In addition,
increased cell death by 200-µM GA was observed and results revealed
the induction of cell apoptosis by GA in both NTERA-2 and NCCIT
cells (Fig. 1D).
GA induces cell cycle arrest in
embryonic CSCs
The present study used 100- or 200-µM GA for further
studies. Previous results suggested that GA may induce cell cycle
arrest. The present study verified that cell cycle arrest in
G0/G1 phase, which was increased through the
administration of 200 µM GA, was found in CSCs through using a flow
cytometry (Fig. 2A). To confirm
the inhibitory induction of cell cycle by GA treatment, western
blotting assay was performed to identify the lower-expression of
cell cycle markers (CDK4 or cyclin D1 and E) and hyper-expression
of tumor suppressor proteins (p21, p27 or p53) was confirmed
(Fig. 2B). To confirm the RNA
levels, qPCR was performed to assess for the expression of markers
(p21, p27, CDK4 or cyclin D and E) and these results clearly
demonstrated the inhibition of cell cycle arrest by GA (Fig. 2C). These results suggest anticancer
activity of GA against CSCs.
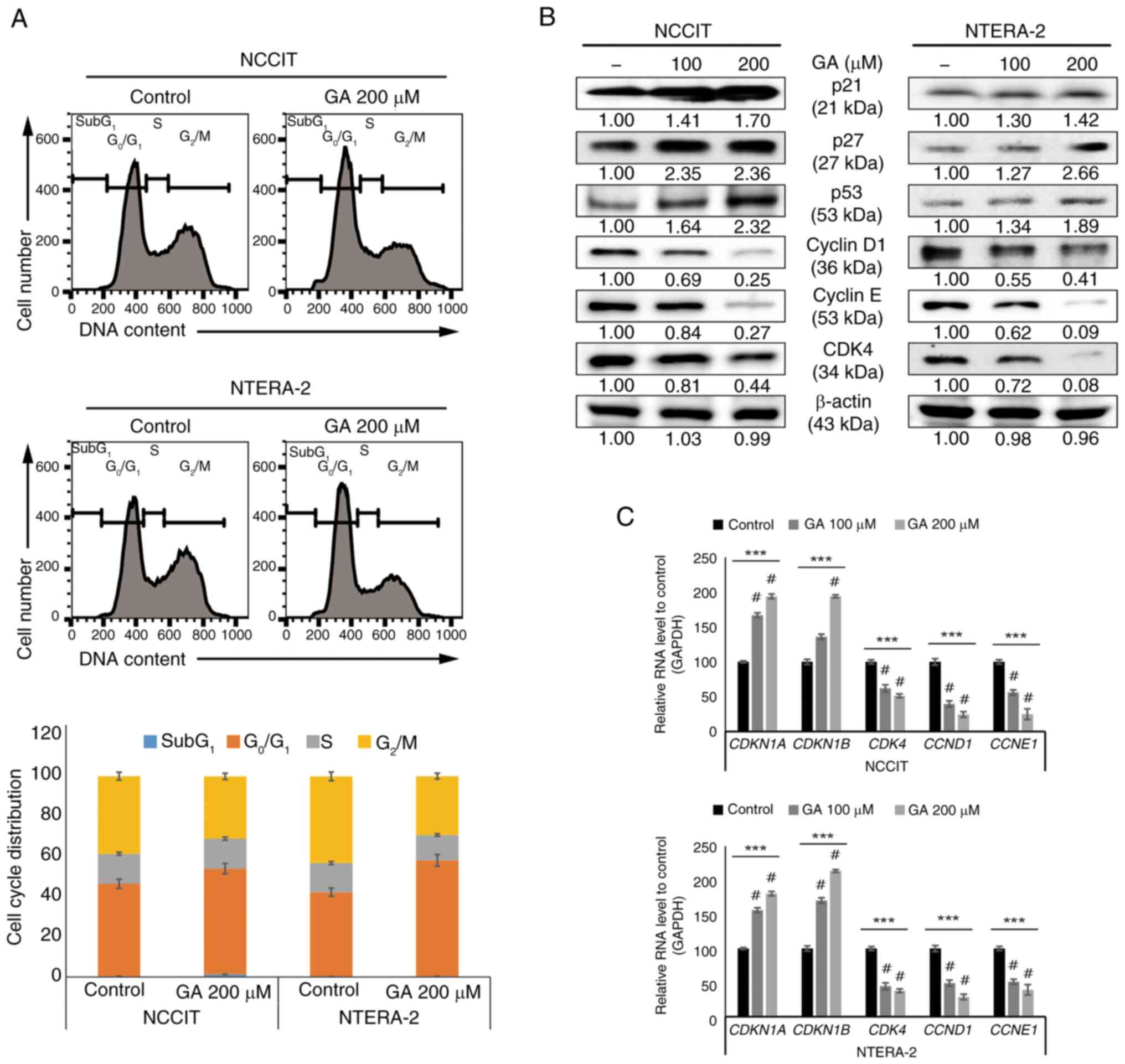 | Figure 2.GA enhances the arrest of
G0/G1 cell cycle in CSCs. (A) The flow
cytometry results demonstrated the cell cycle distribution with 0
or 200 µM GA, respectively, for 24 h. Graphics display a
G0/G1 arrest by GA in embryonic CSCs. (B)
Immunoblotting analysis showed the expression patterns of p21, p27,
p53, CDK4 or cyclin D1 and E with 0, 100, or 200 µM GA,
respectively, for 24 h. All proteins were normalized to β-actin
levels. (C) Reverse transcription-quantitative PCR represented the
genetic expression of cell cycle factors, including CDKN1A,
CDKN1B, CDK4, CCND1 or CCNE1 mRNA. ***P<0.001 (ANOVA
test) and #P<0.001 vs. control. GA, gallic acid;
CSCs, cancer stem cells. |
GA suppresses cancer stemness through
the inhibition of tumorsphere formation in embryonic CSCs
For an improved replication of the conditions in
vivo, tumorsphere formation assay was performed for 3D culture.
It was determined whether GA could downregulate the expression of
CSC markers. In the analysis of the CSC markers, NANOG, OCT4 or
SOX2, in NTERA-2 and NCCIT cells upon GA treatment, a decrease in
the expression level of CSC markers was clearly observed (Fig. 3A). Using RT-qPCR, CSC marker
repression by GA was also confirmed at the level of mRNA
transcripts (Fig. 3B). These
results indicated inhibition of proliferation of CSCs by GA. A
tumorsphere formation assay was further performed using NTERA-2 and
NCCIT cells to evaluate the GA ability to inhibit the two CSCs.
NTERA-2 and NCCIT cells were cultured in the tumorsphere medium
using low attachment plates with GA for 14-days. Images were
captured using a microscope, which indicated a significant
reduction in tumorspheres following the treatment of 200 µM GA
compared with untreated controls (Fig.
3C). To confirm CSCs suppression by GA on the protein level
using these samples, western blotting was used to identify the
expression levels of specific CSC markers after sphere formation
culture for 14 days. A significant downregulation of stem cell
markers, NANOG, OCT4 or SOX2, by GA was observed (Fig. 3D). These results demonstrated the
repression of target CSCs by GA.
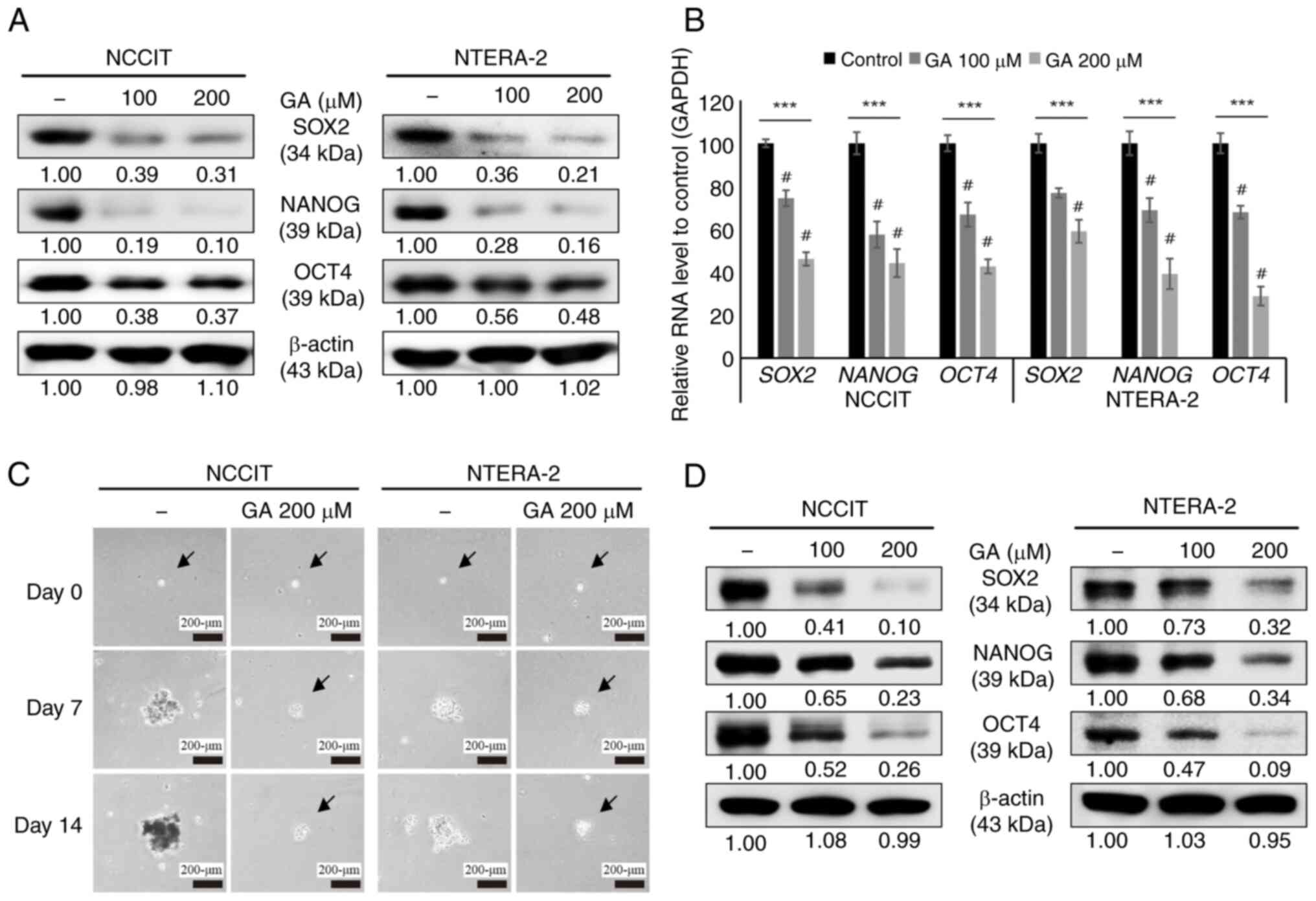 | Figure 3.GA inhibits cancer stemness in CSCs.
(A) The expression patterns of CSC markers, NANOG, SOX2 or OCT4,
were incubated with 0 or 200 µM GA, respectively, for 24 h. (B)
Reverse transcription-quantitative PCR of CSC marker mRNAs was
performed and normalized to GAPDH mRNA. ***P<0.001 (ANOVA test)
and #P<0.001 vs. control. (C) The tumorsphere
formation assay of CSCs were performed with 0 or 200 µM GA,
respectively, for 14 days (scale bar, 200 µm). (D) After 14 days,
the CSC marker expression levels of NANOG, SOX2 or OCT4 were
measured via western blotting. GA, gallic acid; CSCs, cancer stem
cells. |
GA activates ROS mediated-DNA damage
in embryonic CSCs
The present study identified GA activity that
induces cell cycle arrest and apoptosis in CSCs. Thus, it further
confirmed whether ROS was also induced by GA, as this is crucial in
anticancer study or application. MitoSOX and CM-H2DCFDA
reagents are widely used to identify mitochondrial and cellular
ROS. MitoSOX and CM-H2DCFDA reagents were used for
detecting the mitochondrial and cellular ROS by GA. It was found
that the treatment of 200-µM GA can show the considerable induction
of cellular ROS levels in both NTERA-2 and NCCIT cells (Fig. 4A). As the mitochondria are known as
the major source of cellular ROS in cells, an increase in cellular
ROS levels by GA may imply ROS induction in mitochondria. The
induction of higher mitochondrial ROS as in the case of cellular
ROS by 200-µM GA in CSCs was also detected by flow cytometry
analysis (Fig. 4B). Based on the
hypothesis that the induction of cell death, cell cycle arrest or
apoptosis was a result of DNA damage, presence of DNA damage was
therefore confirmed. Excessive ROS product is known to cause DNA
damage and induce the DDR pathway. To confirm this, the induction
of DDR protein phosphorylation was investigated and confirmed
upregulation in DDR proteins, p-BRCA1, ATM, CHK1, CHK2 or histone
when treated with increased GA concentrations, focusing on NTERA-2
and NCCIT cells (Fig. 4C).
Furthermore, the activity of GA to promote DDR mechanism was
evaluated using a comet assay for detecting DNA fragmentation. A
significant increase in comet-positive cells was also noted,
including the length of comet in GA treated-cells compared with
untreated-control cells (Fig. 4D).
These results may validate the capability of GA to cause DNA damage
in embryonic CSCs.
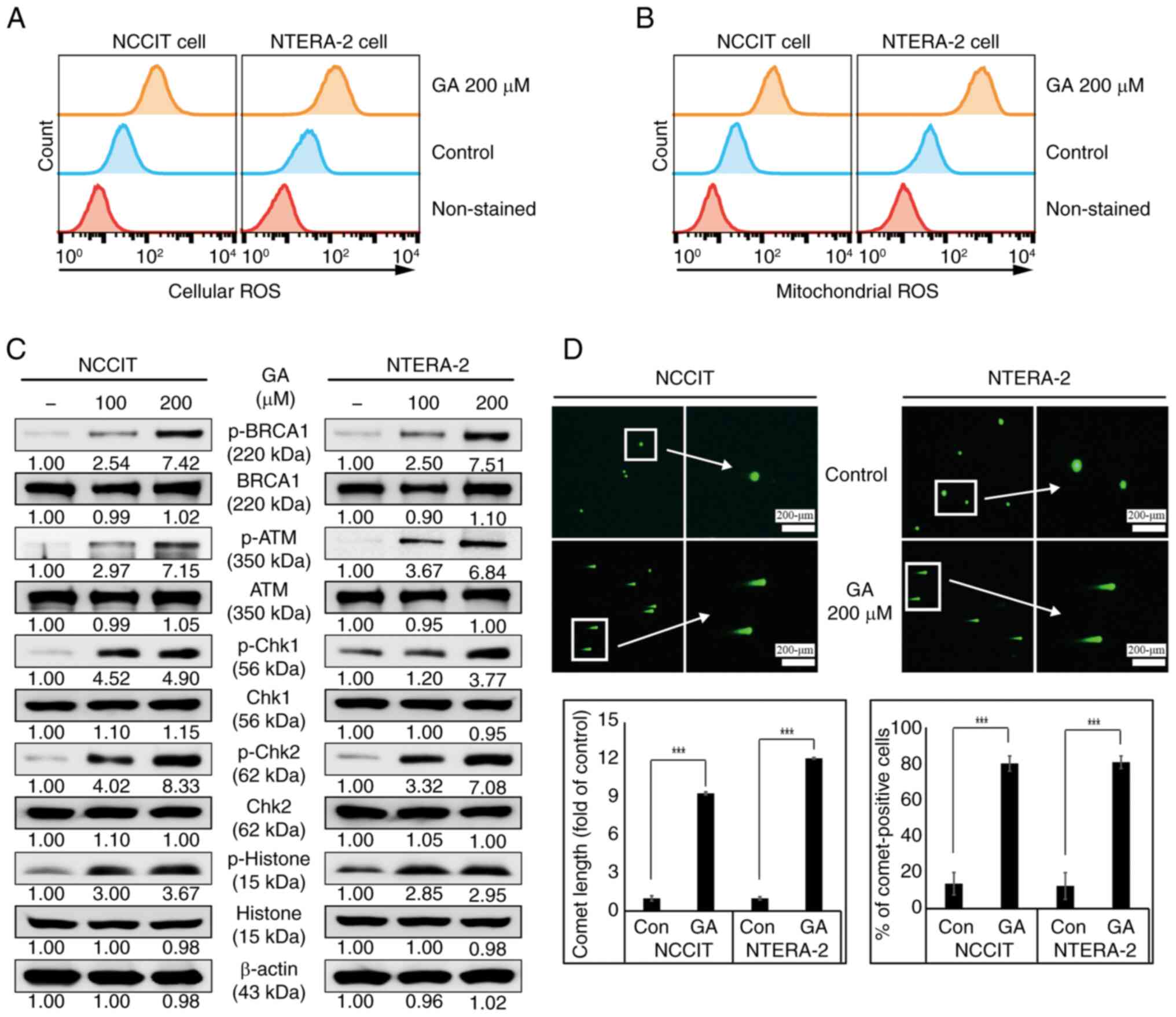 | Figure 4.GA enhances ROS generation and DNA
damage induction in CSCs. (A) The flow cytometry analysis for
detecting cellular ROS with 0 or 200 µM GA, respectively, for 24 h.
The image showed the embryonic CSCs with induction of cellular ROS.
(B) The flow cytometry analysis for detecting mitochondrial ROS
with 0 or 200 µM GA, respectively, for 24 h. The graphical image
revealed embryonic CSCs with the induction of mitochondrial ROS.
(C) The western blotting analysis results showed the induction of
the DDR factors, p-BRCA1, p-ATM, p-Chk1, pChk2 and p-Histone with
0, 100 or 200 µM GA, respectively, for 24 h. (D) The comet assay
results indicated the fragmented DNA migration from the nucleoid
body and the percentage of comet-positive CSCs with 0 or 200 µM GA,
respectively, for 24 h. ***P<0.001 (ANOVA test) vs. control. GA,
gallic acid; ROS, reactive oxygen species; CSCs, cancer stem cells;
p-, phosphorylated. |
GA inhibits migration and invasion of
embryonic CSCs
GA prevented CSCs from normally expanding and
growing through DNA damage and inhibition of sphere culture growth.
Hence, it was hypothesized that GA can inhibit tumor metastasis and
invasion. The ability of GA to suppress CSCs migration and invasion
was analyzed to confirm metastasis. First, the present study
analyzed for molecular markers to confirm the capacity for invasion
and the decrease of MMP proteins, MMP-2, −3 or −9, by GA was
identified, suggesting the reduction of CSC progression and
metastasis (Fig. 5A). These
results were then validated via the analysis of gene expression
patterns at the level of mRNA transcripts and GA induced the
downregulated mRNA levels of MMPs in two CSCs (Fig. 5B). An invasion assay was also
performed to confirm whether GA can induce the inhibition of CSCs
metastasis and the results indicated a significant suppression of
invaded cells in GA treated-CSCs (Fig.
5C). Moreover, to determine whether CSCs can migrate after
exposure to GA, migration-inhibiting effects by GA were confirmed
through a wound-healing assay (Fig.
5D). Overall, these results indicated that GA effectively
repressed cell migration and invasion through MMPs expression
inhibition in embryonic CSCs.
 | Figure 5.GA weakens migration and invasion in
embryonic CSCs. (A) Immunoblotting analysis reveals the expression
patterns of MMP-2, −3 or −9 protein with 0, 100 or 200 µM GA,
respectively, for 24 h. (B) The analysis of RT-qPCR indicates the
genetic expression of cell cycle factors including MMP-2, −3
or −9 mRNA. (C) The results of a Matrigel invasion assay indicate
the suppression of embryonic CSCs invasion with 0 or 200 µM GA,
respectively, for 24 h (scale bar, 100 µm). (D) The wound healing
assay shows the inhibitory migration of embryonic CSC with 0 or 200
µM GA, respectively, for 24 h (scale bar, 200 µm). ***P<0.001,
**P<0.01 and *P<0.05 (ANOVA test); #P<0.001 vs.
control. GA, gallic acid; CSCs, cancer stem cells; MMP, matrix
metalloproteinase. |
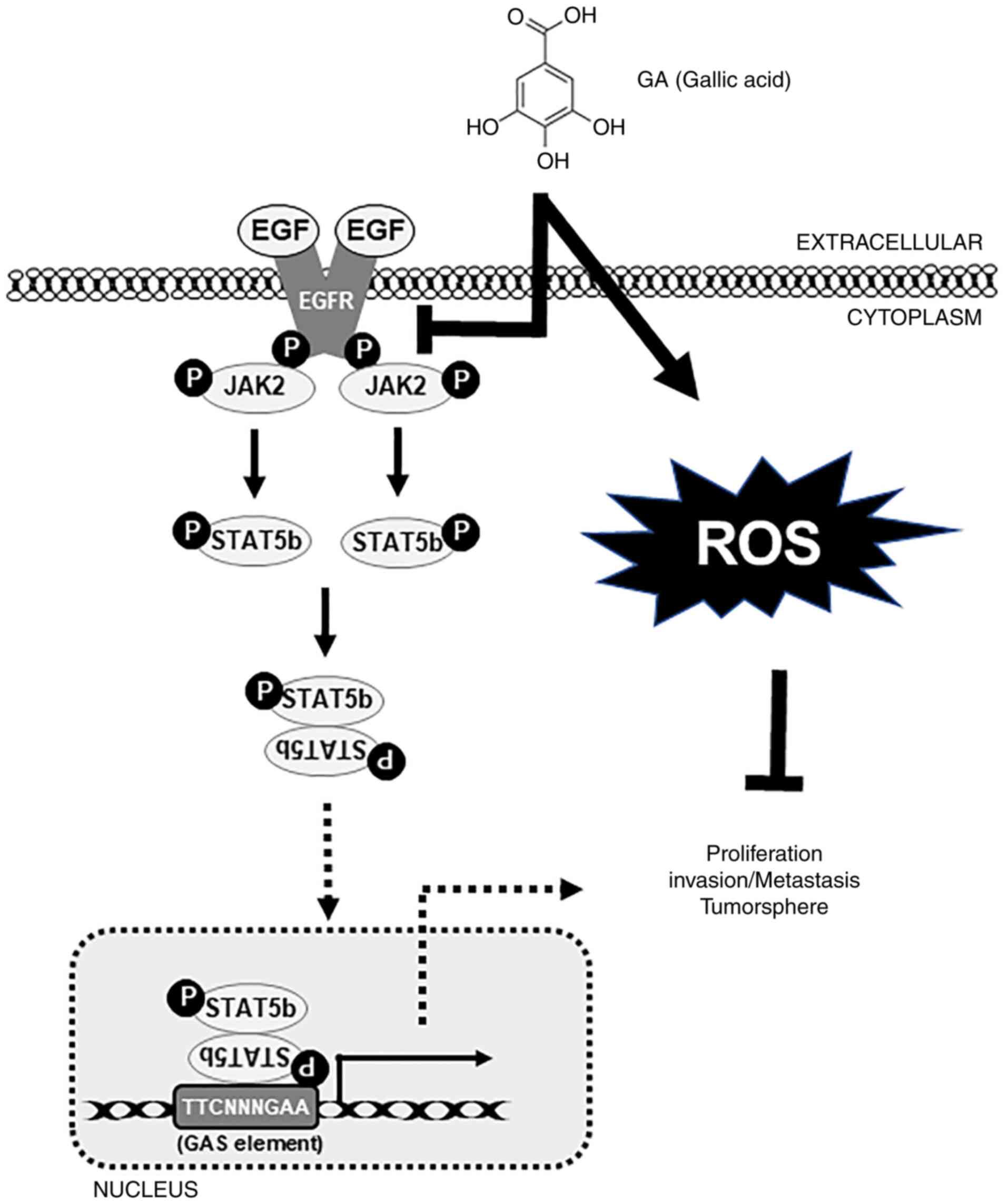
GA inhibits the EGFR-STAT5 pathway and
DNA-protein binding activity by STAT5
After confirmation that GA can inhibit migration and
invasion, the mechanism of molecular signaling by GA was evaluated.
The EGFR/JAK2/STAT5b pathway is known to be associated with tumor
invasion and migration. To validate these results, the EGFR-STAT
pathway was assessed through GA treatment and the suppression of
EGFR phosphorylation blocked cellular signaling toward the
downstream targets of EGFR (Fig.
6A). Additionally, to assess the relationship between STAT5 and
MMP-2, GA and/or a specific STAT5 siRNA was used to confirm the
STAT5-mediated MMP-2 expression (Fig.
6B). These results showed a significant inhibition of p-STAT5
and MMP-2 expression in GA-treated samples and its combination with
STAT5 siRNA samples (Fig. 6B). To
demonstrate the p-STAT5 binding onto the MMP-2 promoter region by
GA inhibition, primers specific to the MMP-2 genome were designed
and its binding was confirmed using ChIP assay. GA-treated samples
indicated the considerable suppression of STAT5/MMP-2 binding
(Fig. 6C). This result clearly
suggested the role of the EGFR/STAT5/MMP-2 signaling cascade in the
anticancer ability of GA against embryonic CSCs. Altogether, GA can
inhibit cancer hallmarks against NCCIT and NTERA-2 human embryonic
carcinoma cells through EGFR-mediated JAK2/STAT5 signaling
mechanisms (Fig. 7).
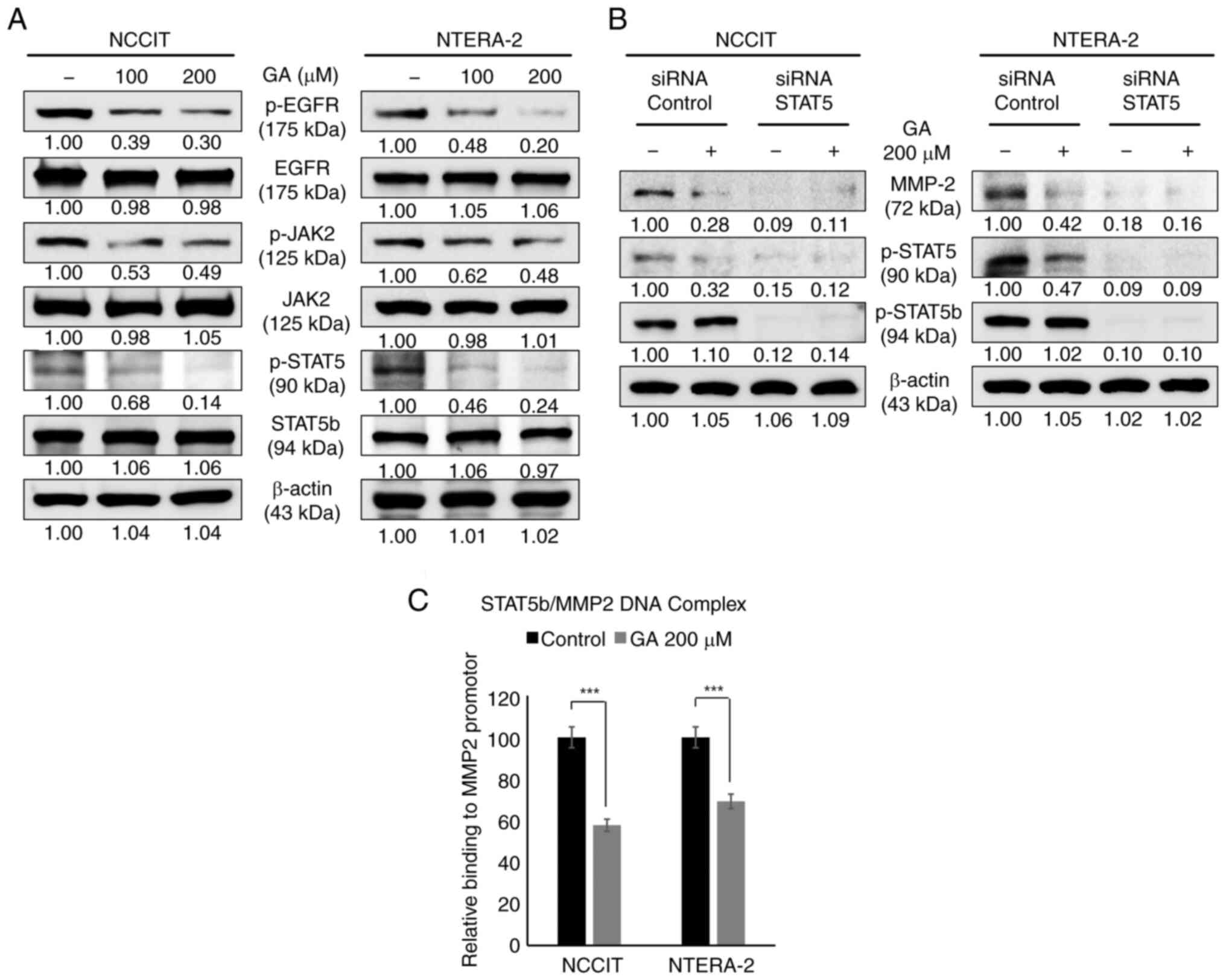 | Figure 6.GA inhibits migration and invasion
through the decrease of the EGFR-STAT5 pathway in embryonic CSCs.
(A) The western blotting assay in embryonic CSCs with 0, 100 or 200
µM GA, respectively, for 24 h for p-EGFR, JAK2 or STAT5. (B)
Immunoblotting analysis showed that treatment with 30 pM STAT5
siRNA or 200 µM GA for 24 h demonstrated the decrease of MMP-2,
STAT5 or p-STAT5b levels. (C) The ChIP assay showed that GA
inhibited the formation of the STAT5b/MMP2 complexes in embryonic
CSCs with 200 µM GA, respectively, for 24 h. ***P<0.001
(Student's t-test). GA, gallic acid; CSCs, cancer stem cells; p-,
phosphorylated; MMP, matrix metalloproteinase. |
Discussion
CSCs are considered to be a subpopulation of tumor
cells, commonly observed in a majority of liquid and solid cancers
(3–6). CSCs feature specific characteristics,
including self-renewal, tumorigenesis, multilineage differentiation
potential and tumorigenicity (7,62).
Success in cancer treatment using drugs is mainly dependent not
only on how it affects targeted cancer cells but also on its
ability to prevent cancer recurrence of CSCs. Although several
types of chemotherapeutic drugs successfully inhibit tumor
progression, in a number of cases these fail to target CSCs which
ultimately results in cancer recurrence (63,64).
CSCs possess a formidable ability to evade or resist the killing
effect of chemotherapeutic drugs through genetic instability,
hypoxic stability and expression of drug efflux transports and
antiapoptotic proteins (65–68).
It has been shown that various types of natural compounds have
anticancer effects, indicating their potential as a drug (11,36,69).
It has also been shown that a chemotherapeutic drug and a natural
compound exhibit optimal efficacy when used in combination compared
with using the drug alone (70).
Therefore, chemotherapy using natural compounds may be an
attractive method as it reduces side effects in both cancer cells
and CSCs.
GA is a natural phenolic compound, well-recognized
to have anticancer ability against diverse types of cancer cells
in vitro and in vivo (48,56–58,71).
Some studies have shown that GA can suppress cell proliferation by
inducing cell cycle arrest and apoptosis in lung and ovarian cancer
cells (72,73). The present study revealed that GA
induced late apoptosis in both NTERA-2 and NCCIT cells compared
with others (that is, early apoptosis and necrosis). Furthermore,
GA induced the arrest of G0/G1 cell cycle
through the downregulation of CDK4 or cyclin D1 and E and
upregulation of p21, p27 or p53, suggesting its anticancer activity
against CSCs.
CSC markers play a role in transcription factors,
essential in maintaining the pluripotent ESC phenotype in CSCs
(10). Furthermore, they are
important factors in forming CSC tumorspheres. Thus, CSC markers
are deemed good targets for drug development. In previous studies,
a natural compound exhibited inhibition of sphere formation in CSCs
(11) and suppressed the
expression of Wnt/β-catenin-mediated CSC markers (74). The present study found that GA
inhibited cancer stemness through the suppression of tumorsphere
formation in CSCs. GA effectively downregulated CSC markers in mRNA
and protein levels and inhibited CSC marker-mediated tumorsphere
formation. Generally, prolonged ROS generation is known to induce
to DNA fragmentation, genomic instability and loss of
heterozygosity. ROS play a pivotal role in tumorigenesis by
inducing DNA damage that results in DDR mechanism. A number of
natural compounds can activate the induction of DDR mechanism in
cancer cells, finally leading to cell cycle arrest or apoptosis,
suggesting that they can be possible candidates for further
studies. Prior studies have demonstrated that GA prompts the
induction of DNA damage and the inhibition of DNA repair-associated
protein expression, resulting in DDR mechanism in some cancer cells
(75–77). The present study observed that GA
elevated cellular ROS and mitochondrial ROS in CSCs, suggesting an
anticancer activity of GA. Furthermore, a high ROS generation by GA
upregulated the DDR mechanism proteins, such as BRCA1, ATM or CHK1
kinase which are central sensors or regulator in DDR in cells. The
results indicated that the increase in the expression of these
kinases by GA enhanced the arrest of G0/G1
cell cycle and apoptosis in CSCs. MMPs perform a key role in the
decrease of various proteins in the ECM, resulting in
tumorigenesis, tumor growth and tumor invasion and metastasis
(30,31). Although the PI3K/AKT/mTOR pathway
is related to tumor cell invasion and migration, the JAK/STAT
pathway regulates the expression of MMPs, playing a crucial role in
cancer progression via invasion or angiogenesis (33,78).
Its regulation could be considered as one of the potential targets
for halting tumor invasion or migration and thus cancer
progression. Our previous study shows that a natural compound
markedly inhibits cell invasion in compound-treated cancer cell
(39). Thus, the present study
demonstrated the decrease of these transmembrane signals that
finally control MMPs in CSCs through GA. Eventually, GA decreased
the EGFR/STAT5 pathway that enhanced MMP expression, leading to a
significant inhibition of the STAT5/MMP2 binding in the nucleus.
The present study confirmed that GA has anti-cell invasion and
anti-migration properties by inhibiting the JAK/STAT pathway.
However, it is necessary to additionally conform that GA suppresses
invasion and migration of CSCs by inhibiting PI3K/AKT/mTOR
pathway.
The present study revealed that GA targets embryonic
CSCs through the inhibition of cell proliferation. In addition, it
was also evident that GA enhanced the induction of cellular and
mitochondrial ROS, leading to DDR against to CSCs. In addition, it
revealed that GA resulted in late apoptosis via the arrest of
G0/G1 cell cycle. Finally, the significant
suppression of MMPs, which are key factors for tumor invasion, was
demonstrated by GA. Altogether, GA could be an attractive agent for
preventing cancer recurrence by targeting CSCs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MSIT) to KJ. (grant no. RS-2024-00450676). It was also supported
by the Basic Science Research Program to Research Institute for
Basic Sciences (RIBS) of Jeju National University through the
National Research Foundation of Korea (NRF) funded by the Ministry
of Education to SB. (grant no. 2019R1A6A1A10072987) and the Korea
Basic Science Institute (National research Facilities and Equipment
Center) grant funded by the Ministry of Education. (grant no.
2023R1A6C101A045). The present study was also supported by the
faculty research fund of Sejong University in 2024.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
KJ designed the experiments and wrote the
manuscript. DK and SB performed all the experiments and analyzed
the data. DK, SB and KJ confirm the authenticity of all the raw
data and helped to revise the manuscript. All authros read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heron M and Anderson RN: Changes in the
leading cause of death: Recent patterns in heart disease and cancer
mortality. NCHS Data Brief. 254:1–8. 2016.PubMed/NCBI
|
|
2
|
Steeg PS: Targeting metastasis. Nat Rev
Cancer. 16:201–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maiuthed A, Chantarawong W and
Chanvorachote P: Lung cancer stem cells and cancer stem
cell-targeting natural compounds. Anticancer Res. 38:3797–3809.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koren E and Fuchs Y: The bad seed: Cancer
stem cells in tumor development and resistance. Drug Resist Updat.
28:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adorno-Cruz V, Kibria G, Liu X, Doherty M,
Junk DJ, Guan D, Hubert C, Venere M, Mulkearns-Hubert E, Sinyuk M,
et al: Cancer stem cells: Targeting the roots of cancer, seeds of
metastasis, and sources of therapy resistance. Cancer Res.
75:924–929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nassar D and Blanpain C: Cancer stem
cells: Basic concepts and therapeutic implications. Annu Rev
Pathol. 11:47–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andrews PW, Damjanov I, Berends J, Kumpf
S, Zappavigna V, Mavilio F and Sampath K: Inhibition of
proliferation and induction of differentiation of pluripotent human
embryonal carcinoma cells by osteogenic protein-1 (or bone
morphogenetic protein-7). Lab Invest. 71:243–251. 1994.PubMed/NCBI
|
|
9
|
Donovan PJ and Gearhart J: The end of the
beginning for pluripotent stem cells. Nature. 414:92–97. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B,
Ng HH and Robson P: Transcriptional regulation of nanog by Oct4 and
Sox2. J Biol Chem. 280:24731–24737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sp N, Kang DY, Kim DH, Park JH, Lee HG,
Kim HJ, Darvin P, Park YM and Yang YM: Nobiletin inhibits
CD36-dependent tumor angiogenesis, migration, invasion, and sphere
formation through the CD36/Stat3/NF-kB signaling axis. Nutrients.
10:7722018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pitrone M, Pizzolanti G, Tomasello L,
Coppola A, Morini L, Pantuso G, Ficarella R, Guarnotta V, Perrini
S, Giorgino F and Giordano C: NANOG plays a hierarchical role in
the transcription network regulating the pluripotency and
plasticity of adipose tissue-derived stem cells. Int J Mol Sci.
18:11072017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang YS, Eades G, Yao Y, Li QL and Zhou
Q: Estrogen receptor alpha signaling regulates breast
tumor-initiating cells by down-regulating miR-140 which targets the
transcription factor SOX2. J Biol Chem. 287:41514–41522. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eini R, Stoop H, Gillis AJ, Biermann K,
Dorssers LC and Looijenga LH: Role of SOX2 in the etiology of
embryonal carcinoma, based on analysis of the NCCIT and NT2 cell
lines. PLoS One. 9:e835852014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeter CR, Yang T, Wang JC, Chao HP and
Tang DG: Concise review: NANOG in cancer stem cells and tumor
development: An update and outstanding questions. Stem Cells.
33:2381–2390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin T, Ding YQ and Li JM: Overexpression
of Nanog protein is associated with poor prognosis in gastric
adenocarcinoma. Med Oncol. 29:878–885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Zhao XY, Tang M, Li L, Lei Y,
Cheng P, Guo W, Zheng Y, Wang W, Luo N, et al: The role of ROS and
subsequent DNA-damage response in PUMA-induced apoptosis of ovarian
cancer cells. Oncotarget. 8:23492–23506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Srinivas US, Tan BWQ, Vellayappan BA and
Jeyasekharan AD: ROS and the DNA damage response in cancer. Redox
Biol. 25:1010842019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye Z, Shi Y, Lees-Miller SP and Tainer JA:
Function and molecular mechanism of the DNA damage response in
immunity and cancer immunotherapy. Front Immunol. 12:7978802021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maréchal A and Zou L: DNA damage sensing
by the ATM and ATR kinases. Cold Spring Harb Perspect Biol.
5:a0127162013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou BB and Elledge SJ: The DNA damage
response: Putting checkpoints in perspective. Nature. 408:433–439.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banin S, Moyal L, Shieh S, Taya Y,
Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y
and Ziv Y: Enhanced phosphorylation of p53 by ATM in response to
DNA damage. Science. 281:1674–1677. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Visconti R, Della Monica R and Grieco D:
Cell cycle checkpoint in cancer: A therapeutically targetable
double-edged sword. J Exp Clin Cancer Res. 35:1532016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malumbres M: Cyclin-dependent kinases.
Genome Biol. 15:1222014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng C, Zhang P, Harper JW, Elledge SJ and
Leder P: Mice lacking P21Cip1/WAF1 undergo normal development, but
are defective in G1 checkpoint CONTROL. Cell. 82:675–684. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong B, van den Heuvel AP, Prabhu VV,
Zhang S and El-Deiry WS: Targeting tumor suppressor p53 for cancer
therapy: Strategies, challenges and opportunities. Curr Drug
Targets. 15:80–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stöcker W, Grams F, Baumann U, Reinemer P,
Gomis-Rüth FX, McKay DB and Bode W: The metzincins-topological and
sequential relations between the astacins, adamalysins,
serralysins, and matrixins (collagenases) define a superfamily of
zinc-peptidases. Protein Sci. 4:823–840. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lohi J, Wilson CL, Roby JD and Parks WC:
Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed
in testis and keratinocytes and in response to injury. J Biol Chem.
276:10134–10144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu JS, Sheng SR, Liang XH and Tang YL: The
role of tumor microenvironment in collective tumor cell invasion.
Future Oncol. 13:991–1002. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Russo S, Cinausero M, Gerratana L, Bozza
C, Iacono D, Driol P, Deroma L, Sottile R, Fasola G and Puglisi F:
Factors affecting patient's perception of anticancer treatments
side-effects: An observational study. Expert Opin Drug Saf.
13:139–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wamukaya JW and Philis PB: Outcome of
supportive management in the prevention of chemotherapy induced
nausea and vomiting in a resource limited set up-nurse experience.
Asia-Pac J Clin Oncol. 10:194–196. 2014.
|
|
36
|
Sp N, Kang DY, Joung YH, Park JH, Kim WS,
Lee HK, Song KD, Park YM and Yang YM: Nobiletin inhibits
angiogenesis by regulating Src/FAK/STAT3-mediated signaling through
PXN in ER+ breast cancer cells. Int J Mol Sci. 18:9352017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sp N, Kang DY, Lee JM, Bae SW and Jang KJ:
Potential antitumor effects of 6-gingerol in p53-dependent
mitochondrial apoptosis and inhibition of tumor sphere formation in
breast cancer cells. Int J Mol Sci. 22:46602021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin SR, Fu YS, Tsai MJ, Cheng H and Weng
CF: Natural compounds from herbs that can potentially execute as
autophagy inducers for cancer therapy. Int J Mol Sci. 18:14122017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rugamba A, Kang DY, Sp N, Jo ES, Lee JM,
Bae SW and Jang KJ: Silibinin regulates tumor progression and
tumorsphere formation by suppressing PD-L1 expression in non-small
cell lung cancer (NSCLC) cells. Cells. 10:16322021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sp N, Kang DY, Jo ES, Lee JM, Bae SW and
Jang KJ: Pivotal role of iron homeostasis in the induction of
mitochondrial apoptosis by 6-gingerol through pten regulated PD-L1
expression in embryonic cancer cells. Front Oncol. 11:7817202021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ouyang L, Luo Y, Tian M, Zhang SY, Lu R,
Wang JH, Kasimu R and Li X: Plant natural products: From
traditional compounds to new emerging drugs in cancer therapy. Cell
Prolif. 47:506–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang M, Lu JJ and Ding J: Natural
products in cancer therapy: Past, present and future. Nat Prod
Bioprospect. 11:5–13. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ali Abdalla YO, Subramaniam B, Nyamathulla
S, Shamsuddin N, Arshad NM, Mun KS, Awang K and Nagoor NH: Natural
products for cancer therapy: A review of their mechanism of actions
and toxicity in the past decade. J Trop Med. 2022:57943502022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Talib WH, Alsalahat I, Daoud S, Abutayeh
RF and Mahmod AI: Plant-derived natural products in cancer
research: Extraction, mechanism of action, and drug formulation.
Molecules. 25:53192020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shahrzad S, Aoyagi K, Winter A, Koyama A
and Bitsch I: Pharmacokinetics of gallic acid and its relative
bioavailability from tea in healthy humans. J Nutr. 131:1207–1210.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nabavi SF, Habtemariam S, Di Lorenzo A,
Sureda A, Khanjani S, Nabavi SM and Daglia M: Post-stroke
depression modulation and in vivo antioxidant activity of gallic
acid and its synthetic derivatives in a murine model system.
Nutrients. 8:2482016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Abdelwahed A, Bouhlel I, Skandrani I,
Valenti K, Kadri M, Guiraud P, Steiman R, Mariotte AM, Ghedira K,
Laporte F, et al: Study of antimutagenic and antioxidant activities
of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia
Lentiscus. Confirmation by microarray expression profiling. Chem
Biol Interact. 165:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Velderrain-Rodríguez GR, Torres-Moreno H,
Villegas-Ochoa MA, Ayala-Zavala JF, Robles-Zepeda RE, Wall-Medrano
A and González-Aguilar GA: Gallic acid content and an antioxidant
mechanism are responsible for the antiproliferative activity of
‘Ataulfo’ mango peel on LS180 cells. Molecules. 23:6952018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim SW, Han YW, Lee ST, Jeong HJ, Kim SH,
Kim IH, Lee SO, Kim DG, Kim SH, Kim SZ and Park WH: A superoxide
anion generator, pyrogallol, inhibits the growth of HeLa cells via
cell cycle arrest and apoptosis. Mol Carcinog. 47:114–125. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sorrentino E, Succi M, Tipaldi L, Pannella
G, Maiuro L, Sturchio M, Coppola R and Tremonte P: Antimicrobial
activity of gallic acid against food-related pseudomonas strains
and its use as biocontrol tool to improve the shelf life of fresh
black truffles. Int J Food Microbiol. 266:183–189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Couto AG, Kassuya CAL, Calixto JB and
Petrovick PR: Anti-inflammatory, antiallodynic effects and
quantitative analysis of gallic acid in spray dried powders from
Phyllanthus Niruri leaves, stems, roots and whole plant. Rev Bras
Farmacogn. 23:124–131. 2013. View Article : Google Scholar
|
|
52
|
Lee JH, Oh M, Seok JH, Kim S, Lee DB, Bae
G, Bae HI, Bae SY, Hong YM, Kwon SO, et al: Antiviral effects of
black raspberry (Rubus coreanus) seed and its gallic acid against
influenza virus infection. Viruses. 8:1572016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rasooly R, Choi HY, Do P, Morroni G,
Brescini L, Cirioni O, Giacometti A and Apostolidis E:
whISOBAXTM inhibits bacterial pathogenesis and enhances
the effect of antibiotics. Antibiotics (Basel). 9:2642020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schimites PI, Segat HJ, Teixeira LG,
Martins LR, Mangini LT, Baccin PS, Rosa HZ, Milanesi LH, Burger ME
and Soares AV: Gallic acid prevents ketamine-induced oxidative
damages in brain regions and liver of rats. Neurosci Lett.
714:1345602020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang TX, Ma LJ, Wu PF, Li W, Li T, Gu R,
Dan X, Li Z, Fan X and Xiao Z: Gallic acid has anticancer activity
and enhances the anticancer effects of cisplatin in non-small cell
lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol
Rep. 41:1779–1788. 2019.PubMed/NCBI
|
|
56
|
You BR, Moon HJ, Han YH and Park WH:
Gallic acid inhibits the growth of HeLa cervical cancer cells via
apoptosis and/or necrosis. Food Chem Toxicol. 48:1334–1340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Subramanian AP, Jaganathan SK, Mandal M,
Supriyanto E and Muhamad II: Gallic acid induced apoptotic events
in HCT-15 colon cancer cells. World J Gastroenterol. 22:3952–3961.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tang HM and Cheung PCK: Gallic acid
triggers iron-dependent cell death with apoptotic, ferroptotic, and
necroptotic features. Toxins (Basel). 11:4922019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Phan AN, Hua TN, Kim MK, Vo VT, Choi JW,
Kim HW, Rho JK, Kim KW and Jeong Y: Gallic acid inhibition of
Src-Stat3 signaling overcomes acquired resistance to EGF receptor
tyrosine kinase inhibitors in advanced non-small cell lung cancer.
Oncotarget. 7:54702–54713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liao CC, Chen SC, Huang HP and Wang CJ:
Gallic acid inhibits bladder cancer cell proliferation and
migration via regulating fatty acid synthase (FAS). J Food Drug
Anal. 26:620–627. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zeng M, Su Y, Li K, Jin D, Li Q, Li Y and
Zhou B: Gallic acid inhibits bladder cancer T24 cell progression
through mitochondrial dysfunction and PI3K/Akt/NF-ĸB signaling
suppression. Front Pharmacol. 11:12222020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li Z, Bao S, Wu Q, Wang H, Eyler C,
Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al:
Hypoxia-inducible factors regulate tumorigenic capacity of glioma
stem cells. Cancer Cell. 15:501–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dawood S, Austin L and Cristofanilli M:
Cancer stem cells: Implications for cancer therapy. Oncology
(Williston Park). 28:1101–1107. 11102014.PubMed/NCBI
|
|
64
|
Brehmer B, Kauffmann C, Blank C,
Heidenreich A and Bex A: Resection of metastasis and local
recurrences of renal cell carcinoma after presurgical targeted
therapy: Probability of complete local control and outcome. World J
Urol. 34:1061–1066. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ewald B, Sampath D and Plunkett W:
Nucleoside analogs: Molecular mechanisms signaling cell death.
Oncogene. 27:6522–6537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wilson TR, Johnston PG and Longley DB:
Anti-apoptotic mechanisms of drug resistance in cancer. Curr Cancer
Drug Targets. 9:307–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rochat B: Importance of influx and efflux
systems and xenobiotic metabolizing enzymes in intratumoral
disposition of anticancer agents. Curr Cancer Drug Targets.
9:652–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kang DY, Darvin P, Yoo YB, Joung YH, Sp N,
Byun HJ and Yang YM: Methylsulfonylmethane inhibits HER2 expression
through STAT5b in breast cancer cells. Int J Oncol. 48:836–842.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sp N, Darvin P, Yoo YB, Joung YH, Kang DY,
Kim DN, Hwang TS, Kim SY, Kim WS, Lee HK, et al: The combination of
methylsulfonylmethane and tamoxifen inhibits the Jak2/STAT5b
pathway and synergistically inhibits tumor growth and metastasis in
ER-positive breast cancer xenografts. BMC Cancer. 15:4742015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kang DY, Sp N, Jo ES, Rugamba A, Hong DY,
Lee HG, Yoo JS, Liu Q, Jang KJ and Yang YM: The inhibitory
mechanisms of tumor PD-L1 expression by natural bioactive gallic
acid in non-small-cell lung cancer (NSCLC) cells. Cancers (Basel).
12:7272020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ko EB, Jang YG, Kim CW, Go RE, Lee HK and
Choi KC: Gallic acid hindered lung cancer progression by inducing
cell cycle arrest and apoptosis in A549 lung cancer cells via
PI3K/Akt pathway. Biomol Ther (Seoul). 30:151–161. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
He Z, Liu X, Wu F, Wu S, Rankin GO,
Martinez I, Rojanasakul Y and Chen YC: Gallic acid induces S and G2
phase arrest and apoptosis in human ovarian cancer cells in vitro.
Appl Sci (Basel). 11:38072021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sp N, Kang DY, Jo ES, Lee JM and Jang KJ:
Iron metabolism as a potential mechanism for inducing
TRAIL-mediated extrinsic apoptosis using methylsulfonylmethane in
embryonic cancer stem cells. Cells. 10:28472021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Weng SW, Hsu SC, Liu HC, Ji BC, Lien JC,
Yu FS, Liu KC, Lai KC, Lin JP and Chung JG: Gallic acid induces DNA
damage and inhibits DNA repair-associated protein expression in
human oral cancer SCC-4 cells. Anticancer Res. 35:2077–2084.
2015.PubMed/NCBI
|
|
76
|
Liu KC, Ho HC, Huang AC, Ji BC, Lin HY,
Chueh FS, Yang JS, Lu CC, Chiang JH, Meng M, et al: Gallic acid
provokes DNA damage and suppresses DNA repair gene expression in
human prostate cancer PC-3 cells. Environ Toxicol. 28:579–587.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Setayesh T, Nersesyan A, Mišík M,
Noorizadeh R, Haslinger E, Javaheri T, Lang E, Grusch M, Huber W,
Haslberger A and Knasmüller S: Gallic acid, a common dietary
phenolic protects against high fat diet induced dna damage. Eur J
Nutr. 58:2315–2326. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lockhart AC, Braun RD, Yu D, Ross JR,
Dewhirst MW, Humphrey JS, Thompson S, Williams KM, Klitzman B, Yuan
F, et al: Reduction of wound angiogenesis in patients treated with
BMS-275291, a broad spectrum matrix metalloproteinase inhibitor.
Clin Cancer Res. 9:586–593. 2003.PubMed/NCBI
|