Introduction
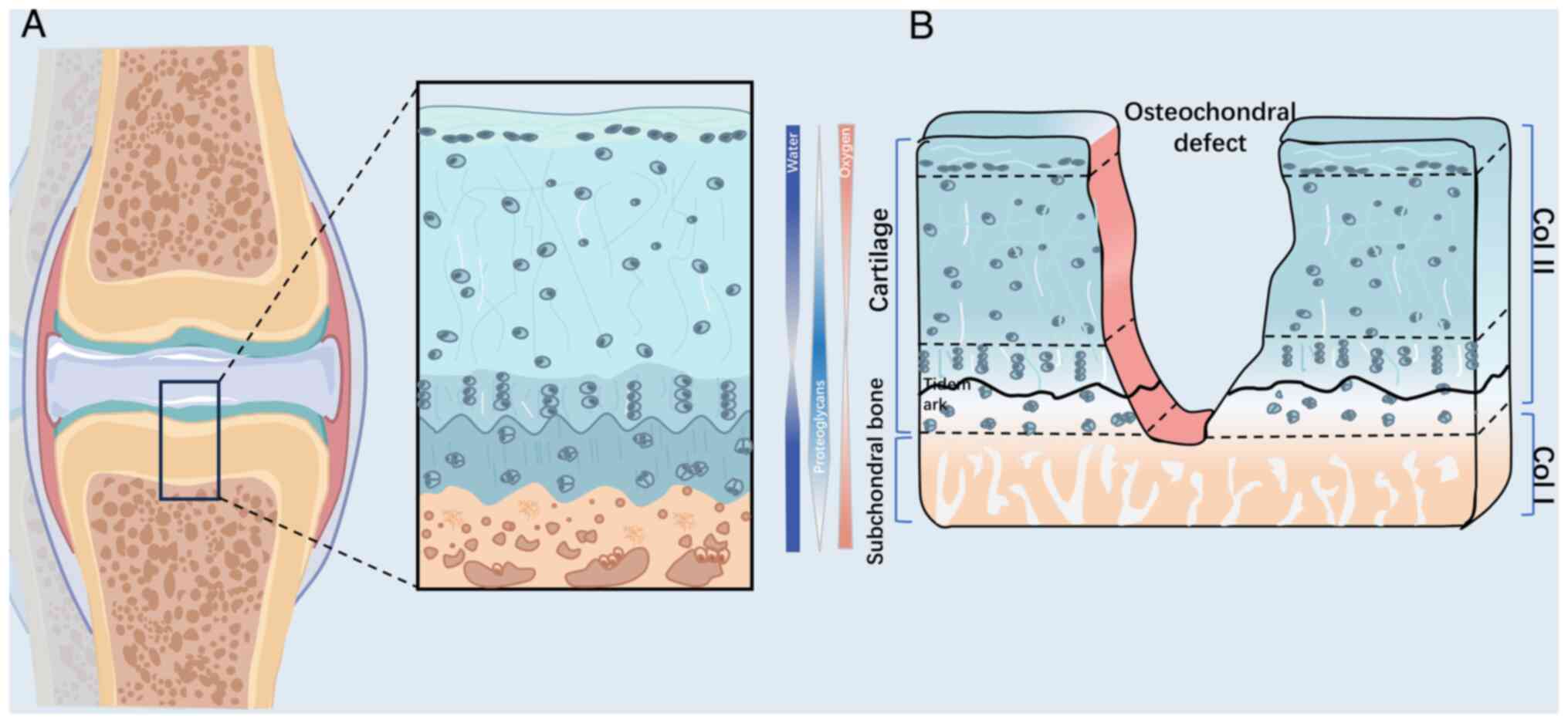
Articular cartilage is a durable tissue capable of
load transmission and articulation of joints. It is primarily
composed of hyaline cartilage, which provides low friction and
shock absorption in synovial joints (1). Hyaline cartilage benefits from the
presence of collagen (Col)-II-based dense extracellular matrix
(ECM), which provides resistance against complex loading patterns
including compression, shear and friction (2). Together, the upper articular
cartilage, subchondral bone plate (SBP) and underlying trabecular
bone create an intact structural and functional osteochondral unit
(Fig. 1) (3).
There are a number of ways to evaluate osteochondral
regeneration, including the International Cartilage Repair Society,
Wakitani and O'Driscoll scoring systems (4,5), all
of which place greater emphasis on cartilage regeneration. However,
the evaluation of subchondral bone repair has been less thoroughly
investigated (4,5). Subchondral bone consists of two
anatomical entities, the SBP and trabecular bone. The SBP is a thin
cortical bone plate with a permeable porous structure located
beneath the calcified cartilage (3). The cancellous bone structure within
the trabecular bone is located beneath the SBP. The porous
structure in subchondral bone provides nutritional support to the
osteochondral unit through numerous nerves and vessels. Currently,
osteoarthritis-related subchondral bone damage is a highly
prevalent pathological condition. The supply of blood and nutrients
is limited after injury and osteoarthritis has a major adverse
impact on the preservation of osteochondral unit function. Thus,
subchondral bone regeneration requires further recognition and
investigation.
Osteochondral defects are a considerable symptomatic
and functional burden to patients and lead to decreased quality of
life. In particular, younger patients lack long-term treatment
solutions, may require numerous surgeries and may experience
unwanted effects throughout their lives because of the inevitable
progression of osteoarthritis (6).
Various clinical strategies and techniques have been developed to
improve repair efficacy. These can be classified into bone
marrow-stimulating techniques (drilling, abrasion and
microfracture), direct chondral replacement (mosaicplasty and
osteochondral allograft transplantation), cell culture-based
treatment [autologous chondrocyte implantation (ACI),
matrix-induced autologous chondrocyte implantation (MACI)] and
total joint arthroplasty (7,8)
(Fig. 2B). Total joint
arthroplasty is considered to be the most useful strategy for both
cartilage and bone repair (9).
Although the aforementioned treatments are common clinical
procedures, obvious drawbacks and limitations remain for long-term
joint preservation (7,8).
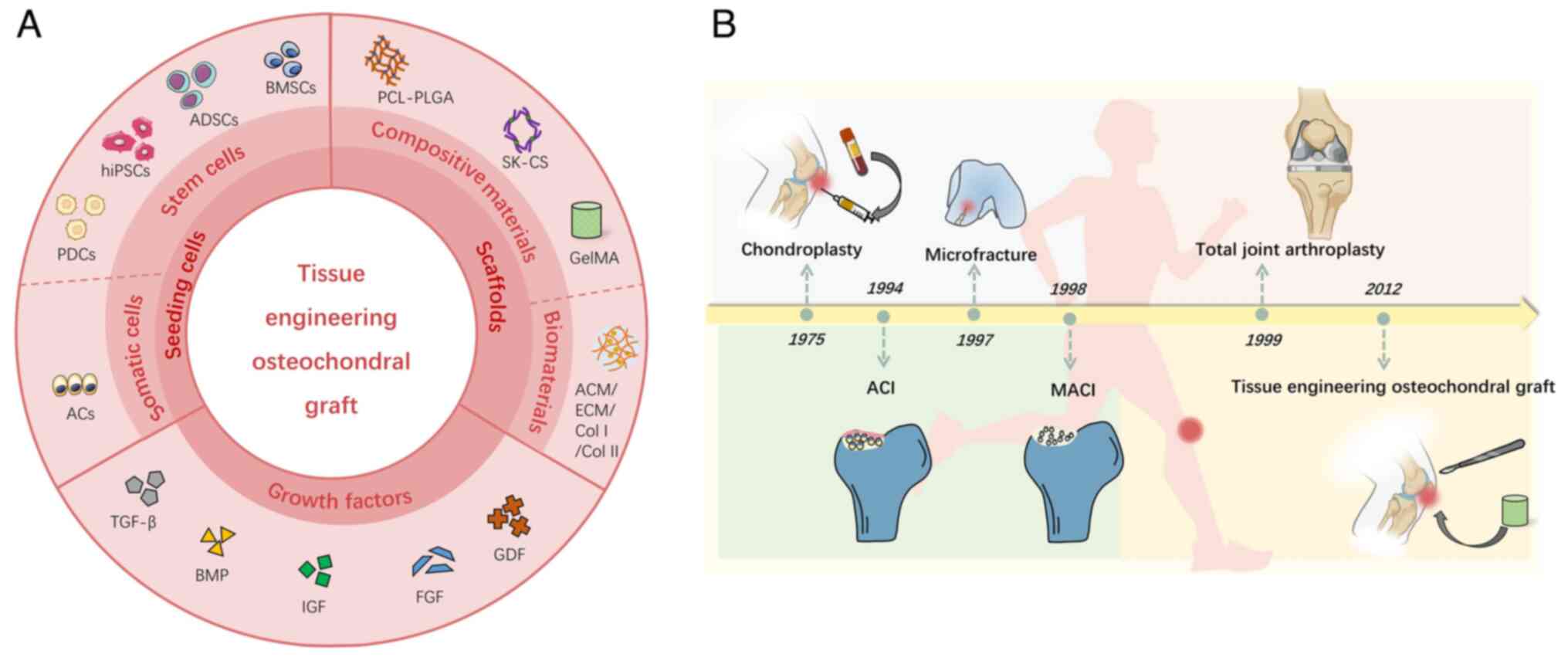 | Figure 2.Overview of composition of tissue
engineering osteochondral graft and clinical treatments for
osteochondral lesion. (A) Graphical illustration of tissue
engineering graft, including seeding cells, scaffolds and growth
factors. Moreover, the cells are divided into two types, stem cells
and somatic cells. Scaffolds are also divided into composite
scaffolds and biomaterial scaffolds. (B) Summary of the development
history of clinically utilized methods for the repair or/and
regeneration of osteochondral lesions. PDCs, periosteum-derived
cells; hiPSCs, human-induced pluripotent stem cells; ADSCs,
adipose-derived stem cells; BMSCs, bone marrow stem cells; ACs,
articular cells; PCL-PLGA, polycaprolactone-poly lactic-co-glycolic
acid; SK-CS, silk-chitosan; GelMA, gelatin methacryloyl; ACM,
acellular cartilage matrix; ECM, extracellular matrix; GDF, growth
differentiation factor; FGF, fibroblast growth factor; IGF,
insulin-like growth factor; BMP, bone morphogenetic protein; TGF,
transforming growth factor. |
Microfracture treatment involves drilling tiny holes
that permit the cartilage and subchondral bone to take blood and
bone marrow components from the underlying tissues (7). This induces cartilage and bone
regeneration/remodeling following the introduction of stem cells
and biomolecules at the defect site (9). However, the procedure may lead to the
formation of fibrocartilage, which has inferior biofunctional and
mechanical properties compared with hyaline cartilage (10). Similarly, use of ACI and MACI
commonly results in the production of fibrocartilage rather than
hyaline cartilage, which hinders the joint from recovering normal
function (3). For 20 years,
articular cartilage has been successfully regenerated using ACI,
with positive surgical results. Nevertheless, drawbacks remain,
including a shortage of chondrocyte sources, long chondrocyte
harvesting time, difficulty of chondrocyte solution fixation,
periosteal hypertrophy and ablation (10), as well as limited effectiveness in
aged patients. Also, osteochondral lesions require simultaneous
healing of the subchondral bone, which ACI cannot repair (8,11).
Allografts have numerous disadvantages, including limited tissue
supply, immune rejection, insufficient host-graft integration, low
cell viability due to graft storage and potential for disease
transmission (12). Osteochondral
autografts may be able to overcome the shortcomings of allografts;
however, insufficient integration and a deficient tissue source,
additional surgery and donor site morbidity limit extensive
clinical application (10). For
cell culture-based treatment, technical disadvantages such as poor
preparation of cell sources, donor site morbidity, inadequate time
for cell expansion, poor retention and de-differentiation of
cultured cells, decline of intrinsic activity and functionality of
senescent cells, as well as inconsistent quality control for
large-scale cell production may block progression of mesenchymal
stem cell or chondrocyte transplantation (12). Consequently, more sophisticated
treatments that take different architecture and regeneration
potential into account, namely structurally and functionally
biomimetic tissue-engineered strategies, have emerged as promising
options for the simultaneous regeneration of subchondral bone and
cartilage lesions (Table I).
 | Table I.Clinical studies for
cartilage/subchondral repair. |
Table I.
Clinical studies for
cartilage/subchondral repair.
| First author/s,
year | Clinical
strategy | Basic process | Advantages | Limitations | Phase | Corresponding
accession number | (Refs.) |
|---|
| Kwon et al,
2019; Maia et al, 2018 | Microfracture | Creating small
holes in the subchondral bone and stimulating bone marrow | Minimally
invasive | Cause the formation
of fibrocartilage | III | NCT03696394 | (7,8) |
| Yang et al,
2017 | ACI | Implant the
patient's autologous chondrocytes, harvested from healthy patients,
into chondral lesions | Enhance the
probability of hyaline-like cartilage compared with
microfracture | Long chondrocyte
harvesting time, periosteal hypertrophy and ablation | III | NCT01947374 | (10) |
| Maia et al,
2018; Campos et al, 2018 | MACI | Autologous
chondrocytes placed onto the surface of a purified film and then
the same implantation | More sufficient
source of autologous chondrocytes than ACI | Inevitable
fibrocartilage formation and poor maintenance for long-term
evaluation | III | NCT00719576 | (8,11) |
| Kim et al,
2020 | Osteochondral
allograft transplantation | Osteochondral
tissue from a donor is transplanted into osteochondral defect | Suitable for large
defects, avoids donor site morbidity | Immune rejection,
poor host-graft integration and risk of disease transmission | III | NCT04236492 | (12) |
| Zhao et al,
2019 | Tissue engineering
graft | Combines cells,
biomaterials and growth factors to promote the regeneration of both
cartilage and bone | Offer the prospect
of the both functional and structural regeneration of osteochondral
defect | Lack of actual
clinical application, challenge in scaffold integration | II | NCT06163573 | (13) |
Significant progress has been made in the field of
tissue engineering over the last two decades with numerous studies
demonstrating the construction of de novo cartilage and bone
both in vitro and in vivo (13). The three important components
involved in the tissue engineering of osteochondral grafts are
biomaterials, cells and growth factors (Fig. 2A). Currently, very few studies
focus specifically on the reconstruction of the SBP during
osteochondral regeneration. However, a growing body of research
suggests that creating the right microenvironment using tissue
engineering approaches and further stimulating SBP regeneration are
essential for regenerating the entire osteochondral unit (14–16).
Incorporating the three components aforementioned into the process
should provide the conditions required to establish the ideal
microenvironment and heal the osteochondral lesion.
Achieving satisfactory osteochondral regeneration in
small animal models does not guarantee success in large animal
models. As both the joint size and the burden increase,
osteochondral regeneration must meet the mechanical demands of the
new tissues. SBP regeneration, an important indicator of recovery
from osteochondral stiffness, may predict the performance of
tissue-engineered osteochondral grafts during the translational
process. However, very few studies have examined SBP regeneration
and almost all have only evaluated the methodology
descriptively.
The present study reviewed in vivo
osteochondral repair with tissue-engineered osteochondral grafts.
This includes i) summarizing the design of tissue-engineered
osteochondral grafts to demonstrate their role in promoting
osteochondral regeneration in terms of cells, scaffolds and growth
factors, with a focus on the regeneration of SBPs. ii) Discussing
both normal histology and SBP reconstruction. iii) Summarizing
current limitations and challenges associated with SBP regeneration
during osteochondral defect repair to guide future translational
research and accelerate the ‘bench to bedside’ process.
Evaluating SBP regeneration
Defining satisfactory SBP repair
Histological assessment is commonly employed to
evaluate osteochondral tissue regeneration. The regenerated tissue,
including the SBP, is compared with corresponding host tissue to
further assess repair quality. In addition, microcomputed
tomography (µ-CT) is an acknowledged ‘gold standard’ for SBP
evaluation, with its global view of bone architecture providing a
detailed assessment. However, criteria for satisfactory SBP
regeneration have not been established. Typical SBPs share common
features, such as a suitable height, a flat and smooth surface,
good interface integration and dense texture (17); SBP regeneration is not considered
successful unless all four features are present. Other literature
has summarized the histological scores of SBP repair by scoring
abnormal activity following the repair (18). The standard for scoring abnormal
activity is that a SBP with a low score has good mobility and good
mechanical properties. However, a low score for repaired SBP is not
representative (18), because few
studies have used this scoring system and it cannot quantify
performance appropriately.
Typical indicators of
insufficient/unsatisfactory SBP repair
In contrast to the aforementioned four criteria for
adequate SBP regeneration, the four typical indicators of a failed
repair are abnormal height, uneven surface, poor integration and
loose lacunae structure (Fig. 3).
When three or more indicators are present, defects filled with
fibrous tissue have been reported, along with either tissue
resembling collapsed cartilage or scarcely any cartilage
regeneration. When one or two indicators were found in the repaired
SBP, regenerated hyaline cartilage with sufficient integration with
surrounding host cartilage was observed (Fig. 3). while a plate with one or two
indicators of failure was defined as a moderate repair. Among these
four indicators of failed repair, abnormal height was the most
frequent. Moreover, when abnormal height was apparent, it was often
accompanied by two or three other indicators, which suggests that
abnormal height has a clear influence on SBP repair and that SBP
may be required to reach an appropriate height during regeneration.
These four histological findings provide a path to understanding
the underlying causes of inadequate osteochondral regeneration from
the perspective of the SBP.
Cell selection for tissue-engineered
osteochondral grafts
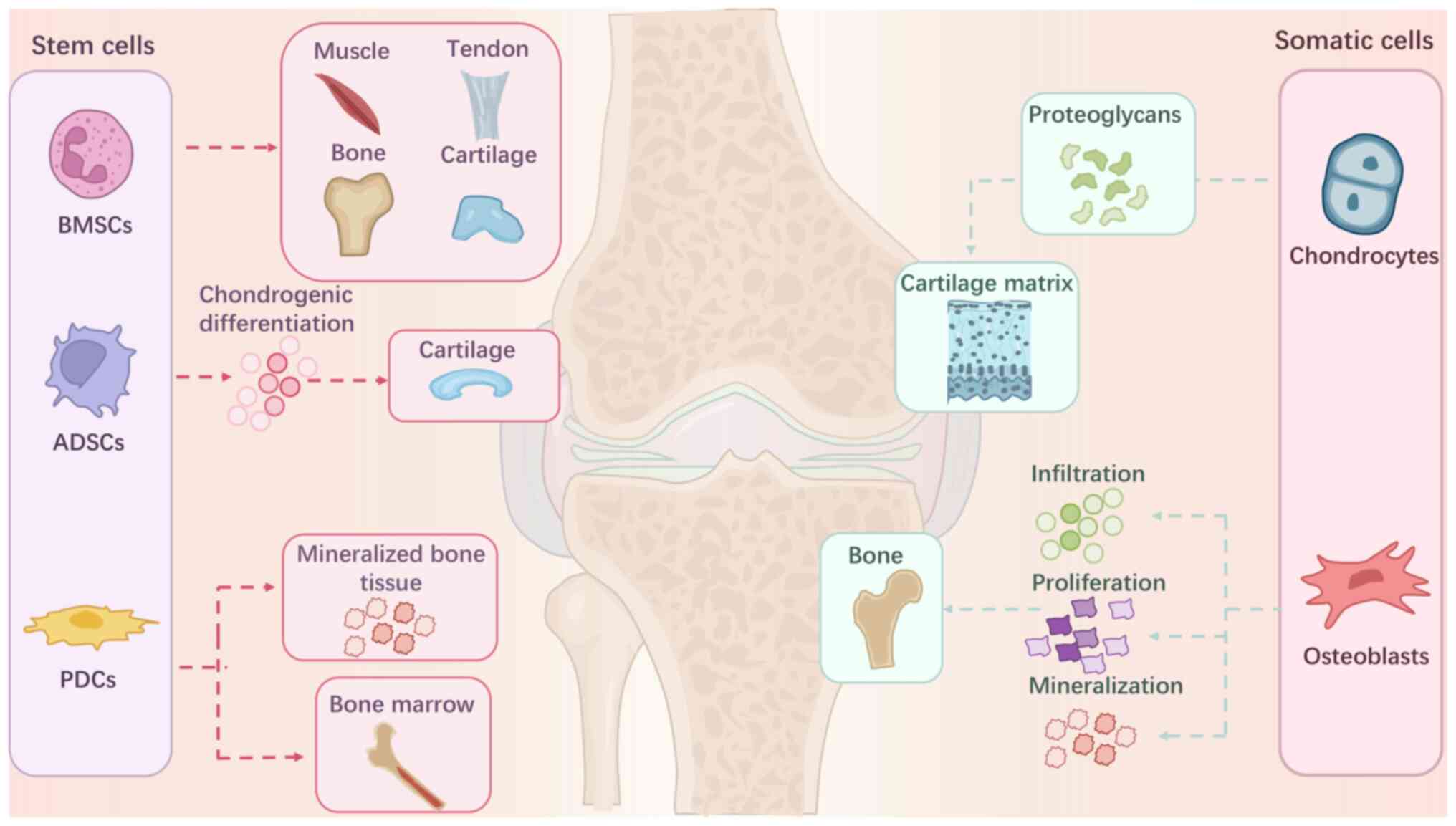
Cells with specific differentiation and
proliferation capacities are used in osteochondral tissue
engineering. Bone marrow-derived mesenchymal stem cells (BMSCs) are
the most common seed cells used in both monolayer and multilayer
grafts. BMSCs can differentiate into various cell types, including
osteocytes and chondrocytes, which then form osteochondral units
(19). Adipose-derived stem cells
(ADSCs) are another important source of stem cells for
osteochondral transplants. Along with chondrogenic potential, ADSCs
have demonstrated extraordinary potential for invasion, migration
and proliferation (19) and
provide a suitable microenvironment for osteochondral regeneration.
In addition to stem cells, somatic cells are also used.
Chondrocytes are the primary option as they promote chondral
portion regeneration in osteochondral grafts (8,11,14)
(Fig. 4). Recently, genetically
modified cells were introduced in osteochondral tissue engineering
(13,20). These cells upregulate gene
expression, thus promoting cell proliferation or
differentiation.
Cells for cartilage repair
One of the most significant functions of seeding
cells is to promote the regeneration of cartilage. Research has
indicated that BMSCs have a distinct advantage over ADSCs in
cartilage repair because of their greater capacity for cartilage
differentiation (21,22). Compared with chondrocytes, BMSCs
can produce ECM with higher mechanical strength (21,22).
However, the aforementioned advantages do not consider the effect
of the graft itself. When widely applied to osteochondral grafts,
BMSCs occasionally result in the formation of fibrocartilage
instead of hyaline cartilage (23). This unsatisfactory repair should
not be attributed to the cell type since the same limited cell
types are used in bilayer and trilayer grafts. The bilayer graft,
which is usually composed of chondrogenic and osteogenic layers,
resembles a normal osteochondral unit, with the top layer
encouraging cartilage repair. BMSCs can be inserted into the
cartilage layer to restore the ECM. Alternatively, chondrocytes can
be implanted in the chondrogenic layer to improve chondrogenesis
because of their outstanding ability to synthesize cartilage matrix
(22). Similarly, BMSCs and
chondrocytes are utilized in trilayer or multilayer grafts for
cartilage regeneration. Under these circumstances, the neocartilage
is always reconstructed with firm and compact cell lineage. BMSCs
and chondrocytes have significant potential to promote cartilage
regeneration in both bilayer and multilayer grafts.
Cells for trabecular bone repair
Although bone regeneration appears easier than
cartilage regeneration over an extended period, bone tissue
reconstruction must be relatively rapid and provide early support
for superficial chondral tissue regeneration (24,25).
For this purpose, cells were incorporated into tissue-engineered
grafts to adjust the speed of bone reconstruction. BMSCs have been
widely used in this process because of their self-renewal and
differentiation capacities (1).
Other studies have reported the use of periosteum-derived
progenitor cells, which can induce the formation of subchondral
bone. These cells can also stimulate differentiation of mineralized
bone tissue and bone marrow (Fig.
4) (19,26). Furthermore, osteoblasts in the
underlying layer of bone can promote the growth, differentiation
and infiltration of new bone (27)
and are mainly responsible for the synthesis, secretion and
mineralization of bone matrix (19). When cultured on osteochondral
tissue grafts, the regenerated bone tissue stimulated by
osteoblasts is firm with good mechanical properties.
Cells for SBP repair
No existing literatures discuss the function of a
certain cell to promote the regeneration of SBP exclusively, as SBP
is often regarded as part of subchondral bone. Previous studies
viewed that cells which can regenerate bone certainly can repair
SBP (28–30). However, the SBP can be
satisfactorily rebuilt when specific cell types are incorporated
into a well-integrated graft that contains growth factors. It has
been found in previous studies that BMSCs embedded in bi-layer or
tri-layer grafts can obtain flat and compact SBP regeneration
(28–30), which no is doubt related to the
polypotent and powerful differentiation potential of BMSCs
(22). Others have shown that
ADSCs can achieve good SBP regeneration after implantation in
bi-layer grafts (28–30). Besides these pluripotent stem
cells, chondrocytes may have the similar function to repair SBP
(24). A flat and neat SBP
structure well integrated with surrounding tissues can be detected.
This is also closely related to the chondrocyte's function of
secretion, synthesis and induction of calcified cartilage matrix
(22). Previous research revealed
that the regeneration of the SBP could not be independently
stimulated by any one cell type, including stem cells or somatic
cells. In general, SBP, cortical bone above trabecular bone, can
usually achieve ideal repair results right after subchondral
trabecular bone is well repaired (Table II).
 | Table II.Cell types for subchondral bone plate
repair. |
Table II.
Cell types for subchondral bone plate
repair.
| First author/s,
year | Cell types | Mechanism | Application | (Refs.) |
|---|
| Mendes et
al, 2020 | BMSCs | Multipotent
differentiation into osteoblasts, secretion of growth factors,
angiogenesis promotion | Bilayered
PLGA/PLGA-HAp composite scaffold | (19) |
| Nie et al,
2019 | ADSCs | Chondrogenic
differentiation and provision of settlement for SBP |
Cartilage-dECM-decorated nanofibrils | (24) |
| Lu et al,
2018 | iPSCs | Capable of
differentiating into osteoblasts, improving the tissue repair
micro-environment, promoting angiogenesis | - | (23) |
| Kim et al,
2020 | Chondrocytes | Secretion of
extracellular matrix components (such as chondroitin sulfate and
type II collagen) and support of the foundation of SBP | Sole graft of
tissue-engineered hyaline cartilage | (29) |
| Xu et al,
2019 | Osteoblasts | Secretion of bone
matrix proteins (such as type I collagen) and mineralization to
form new bone, which indirectly improves the formation of SBP | Bi-layered
composite chitosan/chitosan-tricalcium phosphate (CS/CS-s-TCP)
scaffold | (27) |
Subsequent research revealed that the
biocompatibility of the three-layer/multi-layer graft with the
typical osteochondral unit structure gave it unique benefits in the
repair of osteochondral lesions. Three-layer graft, distinguished
from the two-layer graft, adds a transition layer between the
chondrogenesis layer and the osteogenesis layer. It was expected
that the SBP would undergo good regeneration when the cells were
grown into the transition layer (30). Some stem cells with osteogenic
potential, such as BMSCs and ADSCs, are commonly applied into the
translational layer. For instance, these investigations all share
the construction of the calcified cartilage layer (CCZ) and the
successful SBP regeneration that results from the addition of BMSCs
to the CCZ (31–33). On the other hand, the SBP typically
heals poorly if these cells are denied access to the intermediate
transition layer (30). These
results can prove that osteogenesis is one of the necessary factors
for the SBP. The trabecular bone structure can provide a stable
biomechanical environment and mechanical support for the
regeneration of the SBP. Also, translational layer of grafts
provides enough space to accommodate neo SBP.
Scaffold design for osteochondral
repair
Monolayer scaffold design
As a pivotal part of tissue engineering strategy,
seeding cells can promote regeneration of the osteochondral unit,
but cannot be effective without supportive scaffolds. The critical
concept of the supportive scaffold design is to induce cell growth,
proliferation and differentiation at the defect sites (34). As research rapidly progressed,
monolayer scaffolds were the first to be designed and studied.
Owing to their inherent limitations, monolayer grafts cannot
regenerate the entire osteochondral structure; instead, their
primary goal is to stimulate cartilage regeneration (35–37).
The most recent tissue engineering approaches for the development
of monolayer scaffolds include acellular cartilage matrix (ACM) and
hydrogels, which are shown in Fig.
5 (38–41). Of these, ACM is the most frequently
used material (38–41) because cartilage matrix clearly
promotes cartilage regeneration and has demonstrated the ability to
mimic various distinctive requirements of an ECM-like
microenvironment. Hydrogels can be made from natural or synthetic
polymers, depending on their unique characteristics and specific
functions (36). They both offer
conditions suitable for cell proliferation and differentiation.
Hydrogels based on natural polymers including silk fibroin protein,
sodium alginate, porous chitosan and hyaluronic acid have been
extensively documented (41–45).
Widely reported synthetic polymers include polycaprolactone (PCL),
poly lactic-co-glycolic acid (PLGA) and polyurethane (43). These hydrogels are often a good
choice for scaffold design due to good biocompatibility and
biodegradability (46). In
addition to these single-component hydrogel scaffolds, other
scaffolds using combinations of two or more biomaterials, such as
sodium alginate-gelatin, collagen-fibroin, hyaluronic
acid-chitosan, have been used (23,29,47–49).
These composite scaffolds are not only more beneficial to cartilage
reconstruction but also markedly enhance the biomechanical
properties of scaffolds (47).
However, only a portion of cartilage tissue may be restored by
monolayer grafts and subchondral bone is frequently disregarded
(35,50). Although monolayer grafts do not
appear to improve the repair of osteochondral lesions, their
creation provides a foundation for additional study and the
development of bi- and trilayer grafts.
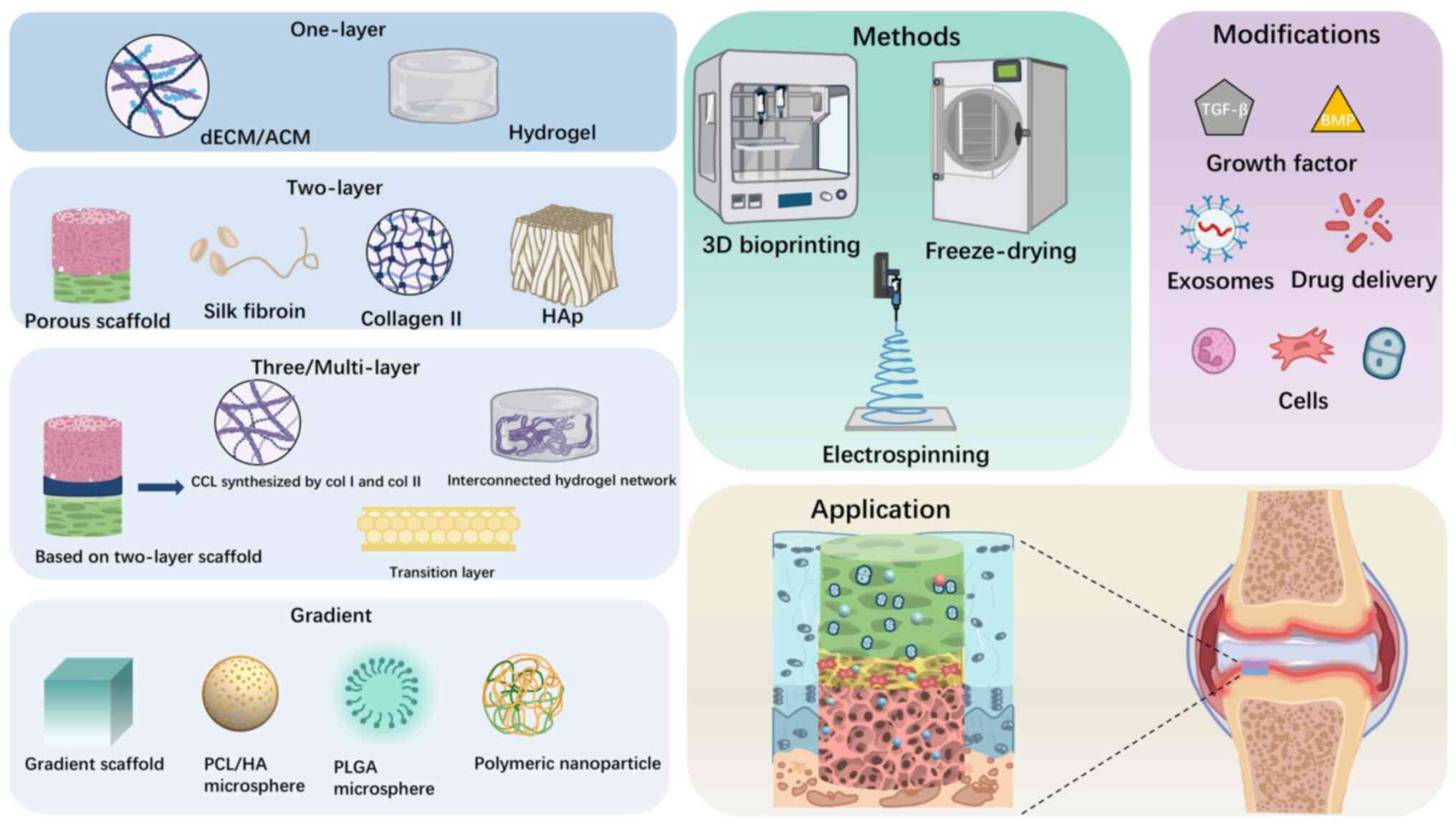 | Figure 5.Preparation methods, materials types,
structures and modifications of scaffolds for osteochondral repair.
The illustration of preparation methods including 3D-printing,
freeze-drying and electrospinning, scaffold designs including
single, double, triple/multilayer and gradient and the main
materials used in them respectively, as well as the functional
modifications including growth factors, cells and exosomes. ECM,
extracellular matrix; ACM, acellular cartilage matrix; HAp,
hydroxyapatite; CCL, calcified cartilage layer; PCL/HA,
polycaprolactone/hyaluronic acid; PLGA, poly lactic-co-glycolic
acid. |
Bilayer scaffold design
Typically, monolayer grafts only partly repair the
defect and cannot achieve overall regeneration of the osteochondral
unit. Due to the distinct mechanical strengths and biological
environments of the cartilage and bone layers, the design of
bilayer scaffolds is much more complicated. The cartilage layer of
bilayer scaffolds shares a common structure with monolayer
scaffolds, that is, polymer and ACM hydrogels (18,47,51).
Due to the robust mechanical properties, excellent biocompatibility
and slow biodegradability, cartilage regeneration was evident
(38) and the arrangement of newly
formed chondrocytes was similar to natural cartilage (52,53).
The design of the osteogenesis layer of the bilayer scaffold is
also important. To provide a suitable microenvironment and ensure
bone regeneration, hydrogels and some inorganic materials became
the main source for the osteogenesis layer (54–56).
Commonly used materials, including bioceramics and bioglass
(54–56), have been improved over the past few
decades, with excellent osteoconductive and inductive properties,
stiff mechanical strength and extraordinary biodegradability,
allowing these materials to demonstrate superiority for subchondral
bone repair (54). The
osteogenesis layer of scaffolds constructed with hydroxyapatite
(HAp) (57) show good
bioplasticity (58) and a pattern
similar to trabecular bone structure can be shaped using
three-dimensional (3D) printing and other technologies. Bilayer
scaffolds tend to achieve better repair results for both the
cartilage layer and the subchondral bone layer compared with
monolayer scaffolds.
Among bilayer graft studies, only a few reported
that the SBP regenerated with an uneven surface or anomalous tissue
formation between the cartilage and subchondral bone layer
(58–60). At present, however, no phase of
bilayer scaffolds has been developed that can precisely repair the
SBP. Nonetheless, it has been demonstrated that offering a somewhat
stable 3D microenvironment allows the bilayer scaffold to
accomplish SBP regeneration (58).
Proper porosity is one of the key conditions for creating a
microenvironment and is also important for scaffold design
(61,62). Relevant studies have reported that
bilayer scaffolds with specific porosities can enhance SBP
regeneration (55,63–65).
Using different porosities, the microenvironment can support the
growth and multiplication of encapsulated seeding cells as well as
osteogenic and chondrogenic differentiation and can also improve
structural stability and enhance mineralization in the subchondral
bone region (66,67). For example, a bilayer scaffold with
an upper porosity of 200 µm and a lower porosity of 400 µm
(66,67) provided clear evidence of improving
SBP regeneration (68). Although
it is not entirely convincing to rely solely on designing different
porosity to achieve SBP regeneration (55), these designs provide insight into
how best to repair the SBP. If trilayer or multilayer scaffolds
achieve unsatisfactory reconstruction, porosity may be a potential
target to improve the SBP regeneration.
Trilayer/multilayer scaffold
design
Trilayer grafts are designed based on bilayer
grafts. The biggest advantage of the trilayer design is that it
addresses the structural flaws in bilayer grafts by incorporating
more structure into the graft. A transitional layer between the
cartilage and bone layers often plays a pivotal role, with its
functions including isolation of components and provision of a
physicochemical barrier, cross-linking network and mechanical
support (69). These grafts have
two types of components; one that is similar to the composition of
cartilage and another similar to the composition of bone (31,34).
For the cartilage-like phase, Col-II-based scaffolds have been the
most used. A dense isolated layer produced by Col-II/PLGA, with a
small enough pore size and porosity, prevents excessive downward
cartilage growth (34) and at the
same time inhibits bone hyperplasia and hypertrophy (70). Others synthesized the calcified
cartilage layer with Col-II/HAp and Col-I/HAp as a transitional
layer (71) that served as a vital
physical barrier, separating nutrients and cells within their
respective spaces, avoiding crosstalk and interference between the
cartilage and subchondral bone layers and ensuring a distinction
between the cartilage and bone environments (72). For the bone-like phase, bioceramic
was the most common material. PCL-β-tricalcium phosphate is a
prepared transitional layer that has been reported to match the
local Young's modulus in the middle and also provides steady
support with excellent mechanical properties (31). Additionally, this transitional
layer may offer sufficient space to regenerate the SBP. Trilayer
scaffolds reported in the literature thus distinguish themselves
from bilayer scaffolds. Together with the creation of stable
cartilage and trabecular bone structures, trilayer
tissue-engineered osteochondral grafts yield good repair of the
SBP.
Compared with trilayer grafts, multilayer grafts
could provide a more sophisticated structure to promote SBP
regeneration (70,73). Typically, multilayer grafts are
multifunctional, with each layer capable of performing a distinct
function. Of those reported, the most common type includes a top
layer promoting cartilage regeneration, a second calcified
cartilage layer, a third transitional layer and a bottom layer that
mainly mimics the porous structure of trabecular bone (33). This complex architecture creates a
biomimetic environment for the entire osteochondral tissue
(74). The calcified cartilage
layer not only balances the differential physical load between
upper cartilage and lower subchondral bone (34), but also helps stabilize the overall
mechanical properties of the scaffold. In addition to the isolation
and barrier function aforementioned (34), the intermediate transitional layer
can release growth factors to the upper and lower layers of the
scaffold (75). Notably, in both
small and large animals, SBP regeneration results were good with a
flat and even surface achieved (30,33,76).
In general, the closer the structure of the artificial scaffold is
to normal osteochondral structure, the more improved the repair
efficacy. Multilayer grafts may thus result in improved
osteochondral unit regeneration compared with bilayer grafts.
Given the complex structure of multilayer scaffolds,
fabrication techniques have limited their progress. 3D printing has
become a standard technique for fabricating biomimetic scaffolds.
To date, a variety of 3D printing methods such as fused deposition
modeling (FDM), inkjet printing, light-assisted bioprinting
(digital light processing), stereolithography and laser-based
printing have been used to engineer different tissue repair
scaffolds (67,68) (Fig.
5). To mimic the structural and mechanical characteristics of
subchondral bone and improve the hydrophilicity of the bone
scaffold, some previous researchers fabricated the core-sheath
structure bone layer using FDM (30). Other researchers have produced the
chondral and osteochondral phases with 3D bioplotting; these have
been designed with a cross-linking network and high porosity
(71,73). These structures had surprising
biocompatibility and could be adjusted according to the lesion
position. Moreover, with 3D printing technologies, the fabrication
of patient-specific scaffolds that perfectly match the size and
shape of the defect would come true. And targeting porosity,
layers, integration would be designed by 3D-printing technology,
all these provide the possibility for future customized strategies.
Consequently, multilayer scaffolds are unquestionably of higher
quality because of 3D printing. Moreover, additional techniques for
multiphase scaffolds are anticipated in the future.
Non-layer/gradient scaffold
design
Gradient scaffolds have gained increasing popularity
over the past decades. Gradient scaffolds involved two types:
Compositional gradient scaffolds and structural gradient scaffolds
(77). First, as with other kinds
of stratified scaffolds, the materials applied in gradient
component scaffold included collagen or extracellular matrix
(77–79) and inorganic polymers such as PCL,
PLGA and gelatin methacryloyl (GelMA) (80,81):
With their suitable biodegradability and mechanical properties,
scaffolds physicochemical stability could be sustained continuously
(77). In contrast to stratified
scaffolds, the primary characteristic of gradient scaffolds was the
smooth transition between layers. Gradient scaffolds can prevent
sudden component changes in distinct zones since the components in
the osteochondral micro-environment vary gradually as depths
increased (81). A gradient
component scaffold was designed with PLGA/TCP and the upper
cartilage layer was constructed of a microtubule-like structure.
These microtubules were interconnected and parallel arranged in
perpendicular plane (diameter, 84.2±20.7 µm). The bone layer had a
highly interconnected porous structure with large pores (450.5±47.2
µm) (76). The whole osteochondral
structure was repaired integrally and both cartilage layer and
trabecular bone layer showed perfect reconstruction. Other studies
have also proposed a biodegradable hydroxyapatite-collagen
(HAp/Coll) gradient distribution scaffold with collagen matrix
synthesized in four different weight ratios as follows: 0:100,
10:90, 30:70 and 50:50 to simulate the normal amount of cartilage
and bone components (82). As well
as cartilage and trabecular bone regeneration, regeneration of SBP
could be also detected (82). The
special benefits of the compositional gradient scaffold, structural
stability, a biocompatible interior environment and successive
compositional alteration, were the main reason of all these
satisfactory results (77,82).
Microsphere scaffolds, another common construction
prototype as gradient scaffolds, organize a three-dimension and
porous structure for cells proliferation and tissue formation. In
addition to the inherent advantages such as biocompatible structure
and smooth translation between layers, the microsphere scaffolds
show unique features to repair osteochondral defect. First is the
architectural and mechanical variations throughout their 3D
structure. Chitosan/mesoporous silica nanoparticles was designed as
microsphere scaffold by Yuan et al (83). The nanoparticles possess excellent
bio-remodeling activity, different patterns can be arranged in the
chondrogenesis zone and osteogenesis zone. With their 3D structure
and porous formation, the differentiation of chondrocytes can be
obviously promoted (83) (Fig. 5). By adjusting the arrangement of
nanoparticles, the mechanical properties of the scaffolds can mimic
the transition from soft cartilage tissue to the calcified
cartilage and ultimately subchondral bone (84). Along with the feasibility of
nanoparticles, osteochondral defects could gain regeneration. The
second characteristic of microsphere scaffolds is their ability to
create interfacial cohesiveness between several layers. PCL/HA was
used to construct a composite microsphere scaffold by Gu et
al (85). Due to their tiny
spherical 3D structure, these microspheres have the potential to
improve scaffold transitions while also strengthening the interface
integration (77). Regeneration of
cartilage and bone tissue was observed following implantation of
these microsphere scaffolds (84,85).
With their unlimited and promising prospect, the design of gradient
grafts will be markedly improved. After long-term observation and
evaluation, the satisfactory result of osteochondral repair would
be achieved with the gradient grafts application.
Growth factors for osteochondral repair
Incorporation of therapeutic growth factors into
tissue-engineered grafts allows modulation of the local
microenvironment (making it chondro- or osteo-inducive), which
improves differentiation and increases matrix production (86,87).
The transforming growth factor-β (TGF-β) superfamily includes
TGF-β, bone morphogenetic protein (BMP) and growth differentiation
factors. Of these, TGF-β3, TGF-β1 and BMP-2 are the three most
widely used (86–88). TGF-β has a stimulatory effect at
the early stage of chondrogenesis, promoting cartilage matrix
synthesis, cell proliferation and upregulation of
chondrogenic-specific genes (86,87).
Moreover, it inhibits the terminal differentiation of hypertrophic
chondrocytes (5). BMP-2 can
stimulate bone regeneration by promoting the deposition of Col-I,
inducing osteocyte differentiation and initiating angiogenesis in
trabecular bone (89). In addition
to the TGF superfamily, other frequently used growth factors
include insulin-like growth factor-1, fibroblast growth factor and
platelet-derived growth factor (PDGF) (19,26,36,50,90).
Their functions include stimulating cell proliferation, triggering
chondrogenesis gene expression and regulating apoptosis (36,50).
Additionally, PDGF is important for vascularization, since it
induces angiogenesis, regulates cell migration and supports vessel
maturation and stabilization.
These growth factors can encourage the regeneration
of cartilage or trabecular bone, but their specific efficacy in
promoting SBP regeneration has not yet been reported. There are
circumstances in which the SBP may be repaired. The plate is
composed of cortical bone tissue (91). Growth factors, which can stimulate
the repair of trabecular bone, can similarly regenerate the SBP.
BMP encapsulated into the osteogenic layer contributes to the
regeneration of the SBP (19) not
only because of its excellent osteogenic differentiation
properties, but also because of its ability to maintain homeostasis
in the joint (19,33,36).
Furthermore, SBP repair can be indirectly accomplished if the bone
and cartilage layers are repaired together. Multilayer scaffolds
encapsulating TGF-β1 and BMP-2 induce more uniform osteochondral
tissue regeneration than scaffolds without growth factors (19,33,75).
This indirectly stimulates the regeneration of the SBP. Use of
growth factors may prove to be a useful strategy for SBP
regeneration, despite the lack of evidence of a direct role for
growth factors in SBP repair (Table
III).
 | Table III.Summary of growth factors about their
function and application for osteochondral repair. |
Table III.
Summary of growth factors about their
function and application for osteochondral repair.
|
|
|
| Influence on tissue
regeneration |
|
|---|
|
|
|
|
|
|
|---|
| First author/s,
year | Growth factor | Function | Cartilage | Trabecular
bone | Subchondral bone
plate | (Refs.) |
|---|
| Qasim et al,
2019; Chen et al, 2020 | TGF-β3 | Provocation of
glycosaminoglycan deposition; Assistance in chondrogenesis;
Induction of chondrocyte proliferation | Promotion | No influence | No influence | (86,87) |
| Spencer et
al, 2018 | TGF-β1 | Provocation of
glycosaminoglycan deposition; Assistance in chondrogenesis;
Induction of chondrocyte proliferation; Stimulation of mineralized
bone tissue synthesis | Promotion | Promotion | Indirect
promotion | (5) |
| Sun et al,
2020 | BMP-2 | Promotion of the
deposition of type I collagen; Induction of osteocyte
differentiation; Induction of subchondral bone tissue
integration | No functioning | Promotion | Indirect
promotion | (89) |
| Zhai et al,
2018; Xue et al, 2018 | FGF | Stimulation of
chondrocytes proliferation | Promotion | Unclear | Unclear | (34,36) |
| Zhai et al,
2018; Xue et al, 2018 | GDF | Regulation of
apoptosis Promotion of cartilage differentiation | Promotion | Unclear | Unclear | (34,36) |
Translating animal models for SBP
repair
Animal studies are an important stage between in
vitro studies and clinical application. The choice of an
appropriate animal model is fundamental to making appropriate
conclusions (92). Animal studies
usually include two groups according to size, small animal models
and large animal models (Table
IV). Small animal models, including rabbit, rat, dog and mouse
models, are widely used in preclinical studies. These smaller
models are low-cost, easy to handle and house and studies are easy
to implement (5). Rabbits are the
most used small animal model. Short- and long-term evaluations can
be performed easily due to their light weight, robust exercise
capacity and low load on the defect location (5). Compared with small animal models,
large animal models, including pig, sheep, goat and horse models,
have the advantage of similarity to humans in joint size, cartilage
thickness and lesions (5). In
addition, their bone tissue macro- and microstructure, composition,
biochemical properties and mineral density are closer to humans
(4,7,93).
Of the large animal models, pigs are used most. As they are heavier
than sheep and goats, the injury site bears a larger load and
requires a longer evaluation period (17); however, satisfactory repair of
osteochondral lesions is rarely achieved in large animals.
Therefore, achievement of satisfactory osteochondral regeneration
in large animal models could indicate potential strategies for
further clinical work.
 | Table IV.Overview and characterization of
experimental studies with a focus on alterations of the subchondral
bone plate and osteochondral unit. |
Table IV.
Overview and characterization of
experimental studies with a focus on alterations of the subchondral
bone plate and osteochondral unit.
|
|
|
|
| Defect
information |
|
|
|
|
|---|
|
| Model |
|
|
|
|
|
|---|
| First author/s,
year |
| Location | Geometry (diameter
× depth) | Subchondral bone
plate alterations | Osteochondral unit
alterations | Untreated defect
alterations | (Refs.) |
|---|
| Animal | Animal age | Animal type |
|---|
| Liu et al,
2017 | Rabbit | 4–5 months | Small animal | On the patellar
groove | 4×3 | Sufficient
thickness regenerated and seamless interface in the sub-chondral
bone plate region | Satisfying
regeneration of osteochondral tissue | The repair of
cartilage defect failed but trabecular bone tissue slightly grew in
the defect location | (47) |
| Nie et al,
2019 | Rabbit | 16 weeks | Small animal | On the patellar
groove | 3×2 | Subchondral bone
plate layers were revealed with compact and flat surface | The osteochondral
defects were completely healed. The traumatic dents have
vanished | Huge fibrous tissue
filled in the defect location | (24) |
| Zhang et al,
2019 | Rabbit | 12 weeks | Small animal | On the medial
femoral condyle | 4×3 | The surface
appeared relatively flat and thickness increased | Entire structure
was regenerated with orderly and compact components | The newly repaired
tissue was thinner than treatment group but cartilage and
subchondral bone were reshaped initially | (97) |
| Critchley et
al, 2020 | Sheep | 1.5–2 years | Large animal | In the condyle of
femur | 6×6 | Deep clefts and
obvious blank interface space were detected | Nearly blank fill
in the defect location | Entire defect
location was filled with tangled fibrous tissue or worse no tissue
filled in with deep clefts | (18) |
| Zhai et al,
2018 | Goat | 10 months | Large animal | In the condyle of
femur | 6×9 | A clearly visible
transition in the interface could be detected | Whole structure
tended to be collapsed | Deep clefts and
huge interface in the defect location, big hollow can be detected
in the depths | (34) |
| Xiao et al,
2019 | Miniature pig | 6 months | Large animal | In the femoral
trochlea | 7×3 | The thickness was
under expectation and nearly no subchondral bone plate existed | The defect location
was filled with amount of fibrous tissue | A large amount of
fibrous tissue filled in the site | (98) |
| Korthagen et
al, 2019 | Shetland pony | 7.3±3.2 years | Large animal | In the trochlea of
femur | 5.9×7.5 | A clearly visible
transition in the interface could be detected | Merely slight
repair of osteochondral tissue | Only fibrous tissue
occupied into the site and the thickness was thinner than
surrounding tissue | (99) |
It is necessary to establish standard criteria for
animal models used in translational studies. Generally, the
criteria for osteochondral defects include defect location, defect
size (depth and diameter) and the age of the animal. At present,
the creation of an osteochondral defect is based on the protocol
used in small animal models, with the defects mostly located in the
femoral trochlea, patellar trochlea and condyle of the femur
(92,94). The induced defect is usually 2–4 mm
in diameter and 3–6 mm deep; this depth can generally lead to
formation of a full-scale osteochondral defect. Selected animals
must have mature skeletons (92,94).
Although several studies have reported osteochondral tissue
regeneration using small animal models, use of these models has
been restricted due to the major disparities between human and
animal joints (92). By contrast,
large animal model-based research is likely to be more useful in
the future. Defects in large animal models are always induced in
the condyle or trochlea of the femur (17). The defect diameter is usually 6–10
mm and the depth is also 6–10 mm, which generally reaches the depth
of a full-scale osteochondral defect (92). Due to the similarities to human
disease development and pathophysiological changes, large animal
models are ideal for studying osteochondral defects. Future
research should establish uniform standards for selecting animal
models. First, large animals make more suitable models. Second,
large animals are ideal for the choice of defect location; most
human knee joint injuries occur on the femoral condyle, which is a
suitable option for the defect site in the model (92) and the defect size must reach that
of a full-scale osteochondral defect. Furthermore, adult animals
should be used, which is consistent with the current trend of human
disease; osteoarthritis is more common in elderly adults than in
adolescents (8,11).
Challenges and perspectives
Previous studies have illustrated various failures
in osteochondral transplants (30,33).
Such unsatisfactory consequences comprise two main classifications:
Functional failure and pathological abnormalities. The most
perplexing findings for pathological abnormalities are the
collapsed structure of the osteochondral unit (18). Cartilage hypertrophy, fibro tissue
hyperplasia, concavity formation and inconsistency among phases
(47) are common in osteochondral
regeneration studies. Even structural collapsing directly indicates
the functional failure of osteochondral transplants. Other
pathological changes, such as cartilage hypertrophy, fibro tissue
hyperplasia, cause difficulty for long maintenance.
In the complicated progression of osteochondral
repair and design of transplants variation, it is difficult to
explain a single failure in performance comprehensively. However,
inherent deficiency for chondral regeneration and temporal disorder
among chondral/osteo-regeneration have been attributed to the
aforementioned pathological abnormalities and functional failures.
Compositional and structural insufficient for neo formed cartilage
are strongly associated with cartilage hypertrophy, fibro tissue
hyperplasia and concavity formation. On the other hand, a relative
earlier completion of bone tissue repair is equally important for
reducing pathological abnormalities. Immunological rejection
related insufficient interfacial integration is another potential
reason for transplants failure (58).
Neo-formed SBP, with proper location and acceptable
architecture, is used as a crucial standard to determine successful
osteochondral regeneration. The present review summarized four
common SBP pathological performances from previous studies with
failed osteochondral regeneration including abnormal height, uneven
surface, poor integration and loose internal structure. Abnormal
height was the most common presentation of poor repair and markedly
affected SBP reconstruction. With the help of our pathological
classification, transplants failure interpretation may be possible
for future studies.
To achieve satisfactory SBP during osteochondral
regeneration, multi-layer TE grafts with a transition layer or
calcified layer was introduced into current studies. However, graft
layer quantity is limited to a certain extent. With the increase of
the number of layers, the manufacturing cost gradually increases,
as well as the difficulty of the fabrication techniques. However,
the anticipated improvement in the ultimate repair has not been
achieved (30,33), which is contrary to the concept of
high efficiency and low consumption. The structure of gradient
scaffolds is different from that of traditional layered scaffolds.
The gradient grafts can obtain satisfactory osteochondral repair by
virtue of its unique advantages. However, the shortcomings of the
gradient scaffold cannot be ignored. Gradually changed components
in the graft could not acquire the distinct regions at the defect
site. In addition, the degradation rate of gradient scaffolds is
faster than the stratified scaffolds (95) and such rapid degradation rate is
not conducive to the load-bearing capacity of neo tissues at the
defect site.
Tissue-engineered osteochondral grafts have promise
for repairing osteochondral defects. Nonetheless, barriers remain
throughout the translational process and must be overcome. First,
the restrictive microenvironment in vitro may be a crucial
factor in cultivating a successful graft, including appropriate
culture medium, suitable temperature and sophisticated culture
techniques (13). Second, because
improper biomaterial selection may result in the premature collapse
of the scaffold or, conversely, late degeneration, adaptive
biomaterials may potentially be a barrier to further deployment
(3). Multilayer graft with proper
biomaterial to regenerate SBP is a promising way to obtain
reasonable chronological order for chondral/osteo-regeneration.
Third, the translational animal model selected also affects the
repair results depending on such factors as species, age, defect
location and defect geometry (96). Lack of a unified standard for
translational animal types will lead to a biased interpretation of
regeneration results. Security concerns and expense also limit the
repair efficiency of tissue-engineered grafts. Although these
grafts show promise for healing osteochondral lesions, several
obstacles to successful clinical application remain; these
obstacles may guide breakthroughs in the creation of new tissue
engineering strategies (97–99).
There are also several limitations in current
reviews. As the limited quantity of studies focus on SBP
regeneration, the present study merely focused on the local
regeneration of SBP, instead of establishing connection between SBP
pathological changes and the regeneration of other parts. Second,
the evaluation of SBP was based on histological and radiological
performance. The summary of poor SBP repair was subjective and
additional research is required to address this issue. Third, no
conclusion for the TE graft with ideal SBP regeneration was drawn
in the present review. Even multilayer scaffold with cocktail
growth factors to promote SBP regeneration was suggested. Further
study to provide proper biomaterials allowing fine control for
chondral and osteo-portion regeneration as well as biocompatibility
are required.
Conclusion
SBP is a new promising path to understand the
osteochondral regeneration and to interpret osteochondral
transplants destiny. In the present review, the histology of the
SBP was discussed and four common histological manifestations of
poor repair were established, including abnormal height, uneven
surface, poor integration and loose internal structure. The impact
of different tissue engineering graft designs on osteochondral unit
repair was also discussed. Incorporating mesenchymal stem cells
into trilayer/multilayer scaffolds, supplemented by appropriate
growth factors, can produce satisfactory osteochondral unit repair.
Moreover, the SBP has also been repaired. Finally, future studies
should focus on large animal models given their physical
similarities to humans; such models will inspire future clinical
research on tissue-engineered grafts. This review has shed light on
potential standards for the construction of future animal models of
osteochondral defects.
Acknowledgements
Not applicable.
Funding
The present study was funded by Natural Science Foundation of
Jilin Province (grant no. YDZJ202201ZYTS281)
Availability of data and materials
Not applicable.
Authors' contributions
MC and XZ made substantial contributions to the
conception and design of the work. XZ, WJ, QD and YS drafted the
manuscript. MC, XZ, WJ, QD and YS revised the manuscript critically
for important intellectual content. Data authentication is not
applicable. All authors read and approved the final manuscript. All
co-authors agree to be accountable for all aspects of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Francis SL, Di Bella C, Wallace GG and
Choong PFM: Cartilage tissue engineering using stem cells and
bioprinting technology-barriers to clinical translation. Front
Surg. 5:702018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel JM, Saleh KS, Burdick JA and Mauck
RL: Bioactive factors for cartilage repair and regeneration:
Improving delivery, retention, and activity. Acta Biomater.
93:222–238. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou L, Gjvm VO, Malda J, Stoddart MJ, Lai
Y, Richards RG, Ho KKW and Qin L: Innovative tissue-engineered
strategies for osteochondral defect repair and regeneration:
Current progress and challenges. Adv Healthc Mater. 9:e20010082020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacob G, Shimomura K and Nakamura N:
Osteochondral injury, management and tissue engineering approaches.
Front Cell Dev Biol. 8:5808682020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spencer V, Illescas E, Maltes L, Kim H,
Sathe V and Nukavarapu S: Osteochondral tissue engineering:
Translational research and turning research into products. Adv Exp
Med Biol. 1058:373–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu H, Feng M, Mao G, Li Q, Zhang Z, Bian W
and Qiu Y: Implementation of photosensitive, injectable,
interpenetrating, and kartogenin-modified GELMA/PEDGA biomimetic
scaffolds to restore cartilage integrity in a full-thickness
osteochondral defect model. ACS Biomater Sci Eng. 8:4474–4485.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwon H, Brown WE, Lee CA, Wang D, Paschos
N, Hu JC and Athanasiou KA: Surgical and tissue engineering
strategies for articular cartilage and meniscus repair. Nat Rev
Rheumatol. 15:550–570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maia FR, Carvalho MR, Oliveira JM and Reis
RL: Tissue engineering strategies for osteochondral repair. Adv Exp
Med Biol. 1059:353–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Z, Li J, Bai X, Wang Y, Wang Q, Lv N,
Gao H, Guo Z, Zhu H, Guo Q and Li Z: Microfracture augmentation
with direct in situ radial shockwave stimulation with appropriate
energy has comparable repair Performance with tissue engineering in
the porcine osteochondral defect model. Am J Sports Med.
50:3660–3670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Zhang YS, Yue K and Khademhosseini
A: Cell-laden hydrogels for osteochondral and cartilage tissue
engineering. Acta Biomater. 57:1–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Campos Y, Almirall A, Fuentes G, Bloem HL,
Kaijzel EL and Cruz LJ: Tissue engineering: An alternative to
repair cartilage. Tissue Eng Part B Rev. 25:357–373. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim YG, Choi J and Kim K: Mesenchymal stem
cell-derived exosomes for effective cartilage tissue repair and
treatment of osteoarthritis. Biotechnol J. 15:e20000822020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Z, Fan C, Chen F, Sun Y, Xia Y, Ji A
and Wang DA: Progress in articular cartilage tissue engineering: A
review on therapeutic cells and macromolecular scaffolds. Macromol
Biosci. 20:e19002782020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Meng H, Guo Q, Sun B, Zhang K, Yu
W, Liu S, Wang Y, Jing X, Zhang Z, et al: Tissue-derived scaffolds
and cells for articular cartilage tissue engineering:
Characteristics, applications and progress. Cell Tissue Res.
372:13–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Y, Chen X, Wang S, Jing Y and Su J:
Subchondral bone microenvironment in osteoarthritis and pain. Bone
Res. 9:202021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu W, Chen Y, Dou C and Dong S:
Microenvironment in subchondral bone: Predominant regulator for the
treatment of osteoarthritis. Ann Rheum Dis. 80:413–422. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orth P and Madry H: Advancement of the
subchondral bone plate in translational models of osteochondral
repair: Implications for tissue engineering approaches. Tissue Eng
Part B Rev. 21:504–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Critchley S, Sheehy EJ, Cunniffe G,
Diaz-Payno P, Carroll SF, Jeon O, Alsberg E, Brama PAJ and Kelly
DJ: 3D printing of fibre-reinforced cartilaginous templates for the
regeneration of osteochondral defects. Acta Biomater. 113:130–143.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mendes LF, Bosmans K, Van Hoven I, Viseu
SR, Marechal M and Luyten FP: Developmental engineering of living
implants for deep osteochondral joint surface defects. Bone.
139:1155202020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song H and Park KH: Regulation and
function of SOX9 during cartilage development and regeneration.
Semin Cancer Biol. 67:12–23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Yu J, Ren K, Zuo J, Ding J and
Chen X: Thermosensitive hydrogels as scaffolds for cartilage tissue
engineering. Biomacromolecules. 20:1478–1492. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lesage C, Lafont M, Guihard P, Weiss P,
Guicheux J and Delplace V: Material-Assisted strategies for
osteochondral defect repair. Adv Sci (Weinh). 9:e22000502022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu J, Shen X, Sun X, Yin H, Yang S, Lu C,
Wang Y, Liu Y, Huang Y, Yang Z, et al: Increased recruitment of
endogenous stem cells and chondrogenic differentiation by a
composite scaffold containing bone marrow homing peptide for
cartilage regeneration. Theranostics. 8:5039–5058. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nie X, Yang J, Chuah YJ, Zhu W, Peck Y, He
P and Wang DA: Full-Scale osteochondral regeneration by sole graft
of tissue-engineered hyaline cartilage without co-engraftment of
subchondral bone substitute. Adv Healthc Mater. 9:e19013042020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu F, Li M, Yuan Z, Rao F, Fang X, Jiang
B, Wen Y and Zhang P: Mechanism research on a bioactive
resveratrol- PLA-gelatin porous nano-scaffold in promoting the
repair of cartilage defect. Int J Nanomedicine. 13:7845–7858. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mendes LF, Katagiri H, Tam WL, Chai YC,
Geris L, Roberts SJ and Luyten FP: Advancing osteochondral tissue
engineering: Bone morphogenetic protein, transforming growth
factor, and fibroblast growth factor signaling drive ordered
differentiation of periosteal cells resulting in stable cartilage
and bone formation in vivo. Stem Cell Res Ther. 9:422018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu D, Cheng G, Dai J and Li Z: Bi-layered
composite scaffold for repair of the osteochondral defects. Adv
Wound Care (New Rochelle). 10:401–414. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang X, Duan P, Gao J, Guo R, Qu Z, Li X,
He Y, Yao H and Ding J: Bilayered PLGA/PLGA-HAp composite scaffold
for osteochondral tissue engineering and tissue regeneration. ACS
Biomater Sci Eng. 4:3506–3521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HS, Mandakhbayar N, Kim HW, Leong KW
and Yoo HS: Protein-reactive nanofibrils decorated with
cartilage-derived decellularized extracellular matrix for
osteochondral defects. Biomaterials. 269:1202142021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang T, Zhang H, Zhang L, Jia S, Liu J,
Xiong Z and Sun W: Biomimetic design and fabrication of
multilayered osteochondral scaffolds by low-temperature deposition
manufacturing and thermal-induced phase-separation techniques.
Biofabrication. 9:0250212017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Ding X, Dong Y, Sun X, Wang L, Ma
X, Zhu M, Xu B and Yang Q: Role of the calcified cartilage layer of
an integrated trilayered silk fibroin scaffold used to regenerate
osteochondral defects in rabbit knees. ACS Biomater Sci Eng.
6:1208–1216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Y, Fan H, Gong X, Yang L and Wang F:
Scaffold with natural calcified cartilage zone for osteochondral
defect repair in minipigs. Am J Sports Med. 49:1883–1891. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen T, Bai J, Tian J, Huang P, Zheng H
and Wang J: A single integrated osteochondral in situ composite
scaffold with a multi-layered functional structure. Colloids Surf B
Biointerfaces. 167:354–363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhai C, Fei H, Hu J, Wang Z, Xu S, Zuo Q,
Li Z, Wang Z, Liang W and Fan W: Repair of articular osteochondral
defects using an integrated and biomimetic trilayered scaffold.
Tissue Eng Part A. 24:1680–1692. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin H, Wang Y, Sun X, Cui G, Sun Z, Chen
P, Xu Y, Yuan X, Meng H, Xu W, et al: Functional tissue-engineered
microtissue derived from cartilage extracellular matrix for
articular cartilage regeneration. Acta Biomater. 77:127–141. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xue J, He A, Zhu Y, Liu Y, Li D, Yin Z,
Zhang W, Liu W, Cao Y and Zhou G: Repair of articular cartilage
defects with acellular cartilage sheets in a swine model. Biomed
Mater. 13:0250162018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Feng G, Xu G and Qi Y:
Microporous acellular extracellular matrix combined with
adipose-derived stem cell sheets as a promising tissue patch
promoting articular cartilage regeneration and interface
integration. Cytotherapy. 21:856–869. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu S, Chen P, Chen Y, Li M, Chen C and Lu
H: 3D-Printed extracellular matrix/polyethylene glycol diacrylate
hydrogel incorporating the anti-inflammatory phytomolecule honokiol
for regeneration of osteochondral defects. Am J Sports Med.
48:2808–2818. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Z, Li Z, Li Z, Wu B, Liu Y and Wu W:
Cartilaginous extracellular matrix derived from decellularized
chondrocyte sheets for the reconstruction of osteochondral defects
in rabbits. Acta Biomater. 81:129–145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bahrami N, Bordbar S, Hasanzadeh E,
Goodarzi A, Ai A and Mohamadnia A: The effect of decellularized
cartilage matrix scaffolds combined with endometrial stem
cell-derived osteocytes on osteochondral tissue engineering in
rats. In Vitro Cell Dev Biol Anim. 58:480–490. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang W, Zhang Y, Zhang A, Ling C, Sheng
R, Li X, Yao Q and Chen J: Enzymatically crosslinked
silk-nanosilicate reinforced hydrogel with dual-lineage bioactivity
for osteochondral tissue engineering. Mater Sci Eng C Mater Biol
Appl. 127:1122152021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feng X, Xu P, Shen T, Zhang Y, Ye J and
Gao C: Influence of pore architectures of silk fibroin/collagen
composite scaffolds on the regeneration of osteochondral defects in
vivo. J Mater Chem B. 8:391–405. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salonius E, Muhonen V, Lehto K, Järvinen
E, Pyhältö T, Hannula M, Aula AS, Uppstu P, Haaparanta AM, Rosling
A, et al: Gas-foamed poly(lactide-co-glycolide) and
poly(lactide-co-glycolide) with bioactive glass fibres demonstrate
insufficient bone repair in lapine osteochondral defects. J Tissue
Eng Regen Med. 13:406–415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Petrovova E, Tomco M, Holovska K, Danko J,
Kresakova L, Vdoviakova K, Simaiova V, Kolvek F, Hornakova P, Toth
T, et al: PHB/CHIT scaffold as a promising biopolymer in the
treatment of osteochondral defects-an experimental animal study.
Polymers (Basel). 13:12322021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou F, Zhang X, Cai D, Li J, Mu Q, Zhang
W, Zhu S, Jiang Y, Shen W, Zhang S and Ouyang HW: Silk
fibroin-chondroitin sulfate scaffold with immuno-inhibition
property for articular cartilage repair. Acta Biomater. 63:64–75.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kabirkoohian A, Bakhshi H, Irani S and
Sharifi F: Chemical immobilization of carboxymethyl chitosan on
polycaprolactone nanofibers as osteochondral scaffolds. Appl
Biochem Biotechnol. 195:3888–3899. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang
Y, Bao C, Xie Z, Lin Q and Zhu L: Integration of stem cell-derived
exosomes with in situ hydrogel glue as a promising tissue patch for
articular cartilage regeneration. Nanoscale. 9:4430–4438. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zuo Q, Cui W, Liu F, Wang Q, Chen Z and
Fan W: Utilizing tissue-engineered cartilage or BMNC-PLGA
composites to fill empty spaces during autologous osteochondral
mosaicplasty in porcine knees. J Tissue Eng Regen Med. 10:916–926.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang X, Song X, Li T, Chen J, Cheng G,
Yang L and Chen C: Aptamer-Functionalized bioscaffold enhances
cartilage repair by improving stem cell recruitment in
osteochondral defects of rabbit knees. Am J Sports Med.
47:2316–2326. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He A, Liu L, Luo X, Liu Y, Liu Y, Liu F,
Wang X, Zhang Z, Zhang W, Liu W, et al: Repair of osteochondral
defects with in vitro engineered cartilage based on autologous bone
marrow stromal cells in a swine model. Sci Rep. 7:404892017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Perez-Silos V, Moncada-Saucedo NK,
Pena-Martinez V, Lara-Arias J, Marino-Martínez IA, Camacho A,
Romero-Díaz VJ, Banda ML, García-Ruiz A, Soto-Dominguez A, et al: A
cellularized biphasic implant based on a bioactive silk fibroin
promotes integration and tissue organization during osteochondral
defect repair in a porcine model. Int J Mol Sci. 20:51452019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang KH, Wan R, Chiu LH, Tsai YH, Fang CL,
Bowley JF, Chen KC, Shih HN and Lai W: Effects of collagen matrix
and bioreactor cultivation on cartilage regeneration of a
full-thickness critical-size knee joint cartilage defects with
subchondral bone damage in a rabbit model. PLoS One.
13:e01967792018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yan J, Liu C, Tu C, Zhang R, Tang X, Li H,
Wang H, Ma Y, Zhang Y, Wu H and Sheng G:
Hydrogel-hydroxyapatite-monomeric collagen type-I scaffold with
low-frequency electromagnetic field treatment enhances
osteochondral repair in rabbits. Stem Cell Res Ther. 12:5722021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xing J, Peng X, Li A, Chen M, Ding Y, Xu
X, Yu P, Xie J and Li J: Gellan gum/alginate-based Ca-enriched
acellular bilayer hydrogel with robust interface bonding for
effective osteochondral repair. Carbohydr Polym. 270:1183822021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shen T, Dai Y, Li X, Xu S, Gou Z and Gao
C: Regeneration of the osteochondral defect by a wollastonite and
macroporous fibrin biphasic scaffold. ACS Biomater Sci Eng.
4:1942–1953. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin D, Cai B, Wang L, Cai L, Wang Z, Xie
J, Lv QX, Yuan Y, Liu C and Shen SG: A viscoelastic PEGylated
poly(glycerol sebacate)-based bilayer scaffold for cartilage
regeneration in full-thickness osteochondral defect. Biomaterials.
253:1200952020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kumai T, Yui N, Yatabe K, Sasaki C, Fujii
R, Takenaga M, Fujiya H, Niki H and Yudoh K: A novel,
self-assembled artificial cartilage-hydroxyapatite conjugate for
combined articular cartilage and subchondral bone repair:
Histopathological analysis of cartilage tissue engineering in rat
knee joints. Int J Nanomedicine. 14:1283–1298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ruan SQ, Yan L, Deng J, Huang WL and Jiang
DM: Preparation of a biphase composite scaffold and its application
in tissue engineering for femoral osteochondral defects in rabbits.
Int Orthop. 41:1899–1908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu Y, Yang Z, Denslin V, Ren X, Lee CS,
Yap FL and Lee EH: Repair of osteochondral defects with
predifferentiated mesenchymal stem cells of distinct phenotypic
character derived from a nanotopographic platform. Am J Sports Med.
48:1735–1747. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin TH, Wang HC, Cheng WH, Hsu HC and Yeh
ML: Osteochondral tissue regeneration using a tyramine-modified
bilayered PLGA scaffold combined with articular chondrocytes in a
porcine model. Int J Mol Sci. 20:3262019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Browe DC, Diaz-Payno PJ, Freeman FE,
Schipani R, Burdis R, Ahern DP, Nulty JM, Guler S, Randall LD,
Buckley CT, et al: Bilayered extracellular matrix derived scaffolds
with anisotropic pore architecture guide tissue organization during
osteochondral defect repair. Acta Biomater. 143:266–281. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Seong YJ, Kang IG, Song EH, Kim HE and
Jeong SH: Calcium phosphate-collagen scaffold with aligned pore
channels for enhanced osteochondral regeneration. Adv Healthc
Mater. 6:242017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ding X, Gao J, Yu X, Shi J, Chen J, Yu L,
Chen S and Ding J: 3D-Printed porous scaffolds of hydrogels
modified with TGF-β1 binding peptides to promote in vivo cartilage
regeneration and animal gait restoration. ACS Appl Mater
Interfaces. 14:15982–15995. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gao J, Ding X, Yu X, Chen X, Zhang X, Cui
S, Shi J, Chen J, Yu L, Chen S and Ding J: Cell-Free bilayered
porous scaffolds for osteochondral regeneration fabricated by
continuous 3D-printing using nascent physical hydrogel as ink. Adv
Healthc Mater. 10:e20014042021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wei X, Liu B, Liu G, Yang F, Cao F, Dou X,
Yu W, Wang B, Zheng G, Cheng L, et al: Mesenchymal stem cell-loaded
porous tantalum integrated with biomimetic 3D collagen-based
scaffold to repair large osteochondral defects in goats. Stem Cell
Res Ther. 10:722019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang Y, Ling C, Chen J, Liu H, Mo Q, Zhang
W and Yao Q: 3D-printed composite scaffold with gradient structure
and programmed biomolecule delivery to guide stem cell behavior for
osteochondral regeneration. Biomater Adv. 140:2130672022.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fang J, Liao J, Zhong C, Lu X and Ren F:
High-Strength, biomimetic functional chitosan-based hydrogels for
full-thickness osteochondral defect repair. ACS Biomater Sci Eng.
8:4449–4461. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Steele JAM, Moore AC, St-Pierre JP,
McCullen SD, Gormley AJ, Horgan CC, Black CR, Meinert C, Klein T,
Saifzadeh S, et al: In vitro and in vivo investigation of a zonal
microstructured scaffold for osteochondral defect repair.
Biomaterials. 286:1215482022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li M, Song P, Wang W, Xu Y, Li J, Wu L,
Gui X, Zeng Z, Zhou Z, Liu M, et al: Preparation and
characterization of biomimetic gradient multi-layer cell-laden
scaffolds for osteochondral integrated repair. J Mater Chem B.
10:4172–4188. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nie X, Chuah YJ, He P and Wang DA:
Engineering a multiphasic, integrated graft with a biologically
developed cartilage-bone interface for osteochondral defect repair.
J Mater Chem B. 7:6515–6525. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Orth P, Cucchiarini M, Kaul G, Ong MF,
Gräber S, Kohn DM and Madry H: Temporal and spatial migration
pattern of the subchondral bone plate in a rabbit osteochondral
defect model. Osteoarthritis Cartilage. 20:1161–1169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Findlay DM and Kuliwaba JS: Bone-cartilage
crosstalk: A conversation for understanding osteoarthritis. Bone
Res. 4:160282016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nordberg RC, Huebner P, Schuchard KG,
Mellor LF, Shirwaiker RA, Loboa EG and Spang JT: The evaluation of
a multiphasic 3D-bioplotted scaffold seeded with adipose derived
stem cells to repair osteochondral defects in a porcine model. J
Biomed Mater Res B Appl Biomater. 109:2246–2258. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yucekul A, Ozdil D, Kutlu NH, Erdemli E,
Aydin HM and Doral MN: Tri-layered composite plug for the repair of
osteochondral defects: In vivo study in sheep. J Tissue Eng.
8:20417314176975002017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Qiao Z, Lian M, Han Y, Sun B, Zhang X,
Jiang W, Li H, Hao Y and Dai K: Bioinspired stratified
electrowritten fiber-reinforced hydrogel constructs with
layer-specific induction capacity for functional osteochondral
regeneration. Biomaterials. 266:1203852021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jia S, Wang J, Zhang T, Pan W, Li Z, He X,
Yang C, Wu Q, Sun W, Xiong Z and Hao D: Multilayered scaffold with
a compact interfacial layer enhances osteochondral defect repair.
ACS Appl Mater Interfaces. 10:20296–20305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Du Y, Liu H, Yang Q, Wang S, Wang J, Ma J,
Noh I, Mikos AG and Zhang S: Selective laser sintering scaffold
with hierarchical architecture and gradient composition for
osteochondral repair in rabbits. Biomaterials. 137:37–48. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jiang LB, Su DH, Liu P, Ma YQ, Shao ZZ and
Dong J: Shape-memory collagen scaffold for enhanced cartilage
regeneration: Native collagen versus denatured collagen.
Osteoarthritis Cartilage. 26:1389–1399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Parisi C, Salvatore L, Veschini L, Serra
MP, Hobbs C, Madaghiele M, Sannino A and Di Silvio L: Biomimetic
gradient scaffold of collagen-hydroxyapatite for osteochondral
regeneration. J Tissue Eng. 11:20417314198960682020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Asensio G, Benito-Garzon L,
Ramirez-Jimenez RA, Guadilla Y, Gonzalez-Rubio J, Abradelo C, Parra
J, Martín-López MR, Aguilar MR, Vázquez-Lasa B and Rojo L:
Biomimetic gradient scaffolds containing hyaluronic acid and Sr/Zn
folates for osteochondral tissue engineering. Polymers (Basel).
14:122021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Idaszek J, Costantini M, Karlsen TA,
Jaroszewicz J, Colosi C, Testa S, Fornetti E, Bernardini S, Seta M,
Kasarełło K, et al: 3D bioprinting of hydrogel constructs with cell
and material gradients for the regeneration of full-thickness
chondral defect using a microfluidic printing head. Biofabrication.
11:0441012019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Oshima T, Nakase J, Toratani T, Numata H,
Takata Y, Nakayama K and Tsuchiya H: A scaffold-free allogeneic
construct from adipose-derived stem cells regenerates an
osteochondral defect in a rabbit model. Arthroscopy. 35:583–593.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yuan Z, Lyu Z, Zhang W, Zhang J and Wang
Y: Porous bioactive prosthesis with Chitosan/Mesoporous silica
nanoparticles microspheres sequentially and sustainedly releasing
platelet-derived growth factor-BB and kartogenin: A new treatment
strategy for osteoarticular lesions. Front Bioeng Biotechnol.
10:8391202022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gupta V, Lyne DV, Laflin AD, Zabel TA,
Barragan M, Bunch JT, Pacicca DM and Detamore MS: Microsphere-based
osteochondral scaffolds carrying opposing gradients of
decellularized cartilage and demineralized bone matrix. ACS
Biomater Sci Eng. 3:1955–1963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gu X, Zha Y, Li Y, Chen J, Liu S, Du Y,
Zhang S and Wang J: Integrated polycaprolactone microsphere-based
scaffolds with biomimetic hierarchy and tunable vascularization for
osteochondral repair. Acta Biomater. 141:190–197. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Qasim M, Chae DS and Lee NY:
Bioengineering strategies for bone and cartilage tissue
regeneration using growth factors and stem cells. J Biomed Mater
Res A. 108:394–411. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen L, Liu J, Guan M, Zhou T, Duan X and
Xiang Z: growth factor and its polymer scaffold-based delivery
system for cartilage tissue engineering. Int J Nanomedicine.
15:6097–6111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kazemi M and Williams JL: Properties of
cartilage-subchondral bone junctions: A narrative review with
specific focus on the growth plate. Cartilage. 13:16S–33S. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sun J, Lyu J, Xing F, Chen R, Duan X and
Xiang Z: A biphasic, demineralized, and Decellularized allograft
bone-hydrogel scaffold with a cell-based BMP-7 delivery system for
osteochondral defect regeneration. J Biomed Mater Res A.
108:1909–1921. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bothe F, Deubel AK, Hesse E, Lotz B, Groll
J, Werner C, Richter W and Hagmann S: Treatment of focal cartilage
defects in minipigs with zonal chondrocyte/mesenchymal progenitor
cell constructs. Int J Mol Sci. 20:6532019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chen L, Wei L, Su X, Qin L, Xu Z, Huang X,
Chen H and Hu N: Preparation and characterization of biomimetic
functional scaffold with gradient structure for osteochondral
defect repair. Bioengineering (Basel). 10:2132023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hurtig MB, Buschmann MD, Fortier LA,
Hoemann CD, Hunziker EB, Jurvelin JS, Mainil-Varlet P, McIlwraith
CW, Sah RL and Whiteside RA: Preclinical studies for cartilage
repair. Cartilage. 2:137–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Confalonieri D, Schwab A, Walles H and
Ehlicke F: Advanced therapy medicinal products: A guide for bone
marrow-derived MSC application in bone and cartilage tissue
engineering. Tissue Eng Part B Rev. 24:155–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Dargoush SA, Hanaee-Ahvaz H, Irani S,
Soleimani M, Khatami SM and Sohi AN: A composite bilayer scaffold
functionalized for osteochondral tissue regeneration in rat animal
model. J Tissue Eng Regen Med. 16:559–574. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Aisenbrey EA, Tomaschke A, Kleinjan E,
Muralidharan A, Pascual-Garrido C, McLeod RR, Ferguson VL and
Bryant SJ: A stereolithography-based 3D printed hybrid scaffold for
in situ cartilage defect repair. Macromol Biosci.
18:10.1002/mabi.201700267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zlotnick HM, Locke RC, Hemdev S, Stoeckl
BD, Gupta S, Peredo AP, Steinberg DR, Carey JL, Lee D, Dodge GR and
Mauck RL: Gravity-based patterning of osteogenic factors to
preserve bone structure after osteochondral injury in a large
animal model. Biofabrication. 14:10.1088/1758–5090/ac79cd. 2022.
View Article : Google Scholar
|
|
97
|
Zhang J, Zhang D, Wu C, Liu A, Zhang C,
Jiao J and Shang M: Icariin-conditioned serum engineered with
hyaluronic acid promote repair of articular cartilage defects in
rabbit knees. BMC Complement Altern Med. 19:1552019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xiao SP, Tang LS, Chen JY, Li ZT, Cheng
GH, Chen QQ, Liu SH and Liu WG: Effect of cross-linked hyaluronate
scaffold on cartilage repair: An in vivo study. Orthop Surg.
11:679–689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Korthagen NM, Brommer H, Hermsen G, Plomp
SGM, Melsom G, Coeleveld K, Mastbergen SC, Weinans H, van Buul W
and van Weeren PR: A short-term evaluation of a thermoplastic
polyurethane implant for osteochondral defect repair in an equine
model. Vet J. 251:1053402019. View Article : Google Scholar : PubMed/NCBI
|