Introduction
Ulcerative colitis (UC) is a nonspecific
inflammatory bowel disease that primarily affects the mucosal and
submucosal layers of the colon and rectum. It is clinically
characterized by recurrent episodes of abdominal pain, diarrhea and
bloody stools. The global incidence of UC is generally increasing
(1), particularly in newly
industrialized countries, and it contributes to a substantial
economic burden (2). Furthermore,
patients with UC have a 2.4-fold increased risk of developing
colorectal cancer compared with the general population (3). These findings underscore the critical
importance of research in inflammatory bowel disease. Owing to its
chronic and recurrent nature with idiopathic etiologies (4), UC presents significant challenges in
achieving a cure and necessitates prolonged, often lifelong,
pharmacological management. The primary therapeutic agents employed
in the treatment of UC include sulfasalazine, glucocorticoids,
immunomodulators and biologics (5). While these medications offer some
clinical efficacy, they are frequently associated with adverse
effects and the potential for drug dependence. Consequently, the
discovery and development of novel and more effective
pharmacological treatments for UC are of paramount importance.
The mechanical barrier, as a critical component of
intestinal barrier function, primarily consists of intestinal
epithelial cells, intercellular junctions and the mucus layer
(6). Intestinal epithelial cells
are closely organized and play a pivotal role in nutrient
absorption, mucus secretion and antimicrobial production.
Intercellular junctions, predominantly tight junctions, ensure the
cohesion and integrity of the epithelial cell layer. The mucus
layer overlays the surface of the intestinal epithelial cells,
providing a protective shield against direct damage from harmful
substances.
Cell death serves a crucial role in maintaining
homeostasis within organisms. Recently, necroptosis has emerged as
a novel mode of cell death, exhibiting morphological similarities
to necrosis while possessing distinct programmed characteristics at
the molecular level. Although both apoptosis and necroptosis are
forms of programmed cell death (7), necroptosis is distinguished as a
programmed, inflammatory mode of cell demise. It is characterized
by organelle swelling, cell membrane rupture and the subsequent
release of cellular contents, which provoke a substantial
inflammatory response (8).
Necroptosis serves a critical role in the pathogenesis, progression
and prognosis of neurodegenerative (9), intestinal (10) and viral infectious diseases
(11). In instances where cells
are unable to activate the key apoptosis initiator caspase-8, tumor
necrosis factor-α (TNF-α) engages with TNF receptor 1, resulting in
the activation of receptor-interacting protein kinase 1 (RIPK1).
Upon activation, RIPK1 undergoes autophosphorylation at several
residues. Within the necrosome, receptor-interacting protein kinase
3 (RIPK3) undergoes phosphorylation, which subsequently facilitates
the recruitment and phosphorylation of mixed lineage kinase
domain-like protein (MLKL). Following phosphorylation, MLKL
oligomerizes and translocates from the cytoplasm to the cell
membrane, where it compromises membrane integrity and triggers cell
necroptosis (12).
Necroptosis serves a multifaceted role in UC,
primarily through the disruption of cellular membrane integrity.
This disruption compromises the mechanical barrier function of the
intestinal epithelium, facilitating the infiltration of harmful
substances into the intestinal tissues, which subsequently triggers
further inflammatory responses and tissue damage (13). Consequently, inhibiting necroptosis
may alleviate the severity of colitis and protect the intestinal
tract (14), thereby offering a
therapeutic approach for UC.
Traditional Chinese Medicine (TCM) has demonstrated
significant advancements in the treatment of UC, attributed to its
multi-component and multi-target properties, which result in
effective therapeutic outcomes, as well as low recurrence rates and
minimal adverse reactions. Traditional formulations, individual
herbal components and active constituents function by modulating
inflammatory cytokines, safeguarding the intestinal epithelium and
tight junctions and maintaining the integrity of the intestinal
barrier (15). In addition, TCM
influences the intestinal microecology to modulate immune responses
and the composition of the intestinal flora, among other
mechanisms, thereby achieving therapeutic efficacy in the
management of UC (16).
Consequently, there has been increasing scholarly interest in
natural compounds derived from TCM. Pristimerin, a naturally
occurring pentacyclic triterpenoid compound sourced from the plant
Tripterygium wilfordii Hook.f., has been documented in the
literature to exhibit therapeutic effects against UC (17). Nonetheless, research focusing on
the treatment of UC through the inhibition of necroptosis by
pristimerin remains unexplored. The present study investigated the
effect of the natural active ingredient pristimerin on UC and
further validated, through both in vivo and in vitro
analyses, that the potential mechanism by which pristimerin
ameliorates intestinal barrier damage in the treatment of UC
involves the inhibition of necroptosis in intestinal epithelial
cells.
Materials and methods
Reagents
Dextran sulfate sodium (DSS; molecular weight range:
36,000-50,000; cat. no. CD4421) was obtained from Beijing Coolaber
Science & Technology Co., Ltd. Pristimerin (purity: 99.22%, CAS
No.: 1258-84-0), sourced from Med Chem Express, was prepared in
dimethyl sulfoxide. Necrostain-1 (Nec-1; cat. no. S8037) was
purchased from Selleck Chemicals. Human TNF-α (cat. no. C008),
SM-164 Hydrochloride (cat. no. HY-15989A) and z-VAD-fmk (cat. no.
T6013) were acquired from Novoprotein, Med Chem Express and
TargetMol Chemicals, respectively. Human-derived antibodies
included anti-RIPK1 (cat. no. 3493S) and anti-phosphorylated
(p-)RIPK1 (cat. no. 65746S) from Cell Signaling Technology, Inc.;
anti-RIPK3/p-RIPK3 (cat. no. ab209384), anti-MLKL (cat. no.
ab184718) and anti-phospho-MLKL (cat. no. ab187091) from Abcam.
Mouse-derived antibodies included: anti-p-RIPK1 (cat. no. BX60008)
from Hangzhou Bailing (Biolynx) Biotechnology Co., Ltd.; anti-RIPK1
(cat. no. 3493S) from Cell Signaling Technology, Inc.; anti-p-RIPK3
(cat. no. ab195117) from Abcam; RIPK3 polyclonal antibody (cat. no.
17563-1-A) and anti-MLKL (cat. no. 66675-1-Ig) from Proteintech
Group, Inc.; anti-phospho-MLKL (cat. no. bsm-54104R) from Bioss;
occludin (cat. no. GB111401) and Zonula Occludens-1 (ZO-1; cat. no.
GB111402) from Wuhan Servicebio Technology Co., Ltd.; and
anti-GAPDH (cat. no. ab181602) from Abcam. Secondary antibodies
included HRP Goat anti-rabbit IgG (cat. no. abs20040) from Absin
Bioscience Inc. and HRP-conjugated Goat anti-Mouse IgG (H+L) (cat.
no. AS003) from ABclonal Biotech Co., Ltd.
Cell culture
The HT-29 cell line, a widely used model for
investigating necroptosis, is derived from human colorectal cancer
(18–20). This cell line was obtained from
Procell Life Science & Technology Co., Ltd. (cat. no. CL-0118)
and authenticated by short tandem repeat DNA profiling.
Cell culture was typically performed in McCoy's 5A
medium (cat. no. CM-0118; Procell Life Science & Technology
Co., Ltd.) supplemented with 10% fetal bovine serum (cat. no.
164210-50; Procell Life Science & Technology Co., Ltd.) and 1%
Penicillin-Streptomycin Solution (cat. no. 15140-122; Gibco; Thermo
Fisher Scientific, Inc.). The cells were cultured and grown at a
temperature of 37°C and in air with 5% CO2.
Anti-necroptosis effect of
pristimerin
HT-29 cells were seeded in 96-well plates at a
density of 1×104 cells/well and cultured for 12 h.
Pristimerin was diluted in culture medium supplemented with 10 nM
SM-164 and 20 µM Z-VAD-FMK to achieve final concentrations ranging
from 10–0.075 µM. After 12 h of cell culture, the culture medium
was removed and replaced with the prepared pristimerin solutions.
After a 30-min pre-treatment with pristimerin, cells were
stimulated with 20 ng/ml recombinant human (h-) TNF-α. This point
marked the initiation of TNF-α-SMAC-z-VAD-FMK, necroptosis inducer
(TSZ) treatment, which consisted of h-TNF-α in the presence of
SM-164 and Z-VAD-FMK. Cell activity was detected after 12 h using
CellTiter-Lumi II Luminescent Cell Viability Assay Kit (cat. no.
C0056S; Beyotime Institute of Biotechnology).
Double staining of live/dead
cells
HT-29 cells were treated with TSZ and pristimerin as
aforementioned. Following a 12-h incubation period, 100 µl of a
1:1,000 dilution of calcein-AM/Propidium Iodide (calcein-AM/PI)
working solution was added to each well. The plates were then
incubated for 30 min at 37°C in the dark. Cellular viability within
each group was subsequently assessed under a fluorescence
microscope (TS-2; Nikon Corporation). Red fluorescence indicated
dead cells, while green fluorescence denoted live cells.
Western blotting
HT29-cells were seeded at a density of
1×106 cells/well in 6-well plates and incubated for 12
h. Subsequently, cells were treated with TSZ and different
concentrations of pristimerin (10, 5 and 2.5 µM) for durations
ranging from 0–6 h. Following treatment, cells were lysed with RIPA
lysis buffer (Jiangsu Kangwei Biology Co., Ltd.) for 30 min and
centrifuged at 12,000 × g for 10 min at 4°C. Protein (20 µg/lane)
was extracted from the supernatant and protein concentration was
determined using a BCA protein assay kit (Jiangsu Kangwei Biology
Co., Ltd.). After denaturation by boiling, proteins were resolved
by 10% SDS-PAGE and transferred to a PVDF membrane
(MilliporeSigma). The membrane was blocked with 5% bovine serum
albumin (BSA; Wuhan Servicebio Technology Co., Ltd.) for 2 h at
room temperature, washed with phosphate-buffered saline and 0.1%
Tween-20 (PBST) and then incubated with the primary antibodies at a
1:1,000 dilution overnight at 4°C. The primary antibodies used in
this study included the following: Anti-RIPK1 (cat. no. 3493S;
1:1,000) and anti-phosphorylated (p-)RIPK1 (cat. no. 65746S;
1:1,000) from Cell Signaling Technology, Inc., anti-RIPK3/p-RIPK3
(cat. no. ab209384; 1:1,000), anti-MLKL (cat. no. ab184718;
1:1,000), anti-p-MLKL (cat. no. ab187091; 1:1,000) and anti-GAPDH
(cat. no. ab181602; 1:50,000) from Abcam. After incubation, the
membrane was washed three times with PBST, followed by incubation
with horseradish peroxidase (HRP-labeled)-conjugated secondary
antibodies for 1 h at room temperature. Secondary antibodies
included HRP Goat anti-rabbit IgG (cat. no. abs20040; 1:10,000)
from Absin Bioscience Inc. and HRP-conjugated Goat anti-Mouse IgG
(H+L) (cat. no. AS003; 1:10,000) from ABclonal Biotech Co., Ltd.
After three additional washes with PBST, the membrane was treated
with ECL chemiluminescent substrate (Tannen) and imaged using a
Bio-Rad imaging system (Bio-Rad Laboratories, Inc.). Band
intensities were quantified using ImageJ software (version 1.52;
National Institutes of Health).
Animals
A total of 36 C57BL/6J male mice (aged 6–7 weeks;
weighing 22–23 g) were obtained from Liaoning Changsheng
Biotechnology Co., Ltd. The animals were maintained in a controlled
environment with a temperature of 23–25°C, 50–60% relative humidity
and a 12-h light/dark cycle. Standard rodent chow and tap water
were provided ad libitum. All animal procedures were
approved by the Animal Care and Use Committee of Henan University
of Chinese Medicine (approval no. DWLLGZR202200023; Henan,
China).
Mice model of DSS-induced UC
Following a 1-week acclimation period with standard
chow, mice were randomly assigned to a control group (Con) and a
dextran sulfate sodium (DSS)-induced colitis model group (DSS). The
control group received standard chow and water ad libitum.
The DSS group received 2.5% DSS in drinking water for 7 days to
induce UC. Subsequently, the DSS group was further randomized into
five treatment groups: DSS-only, high-dose pristimerin (P-H; 3
mg/kg), low-dose pristimerin (P-L; 1 mg/kg), mesalazine (100 mg/kg)
and Nec-1 (5 mg/kg), with six mice per group. The doses of
pristimerin were selected based on previous studies and preliminary
experiments (21,22). The DSS-only group was maintained on
a standard chow diet and water. Treatment groups received their
corresponding medications once daily for 5 consecutive days via the
following routes: P-H and P-L and mesalazine were administered
orally by gavage, while Nec-1 was administered intraperitoneally.
On day 12, all animals were sacrificed. The process was performed
by certified personnel using an overdose of sodium pentobarbital
(150 mg/kg, intraperitoneally). Following the loss of
consciousness, cervical dislocation was performed as a secondary
method to ensure the cessation of all brain activity. All
euthanized animals were confirmed deceased through assessment of
the following cessation parameters: Absence of detectable cardiac
activity and the loss of corneal reflexes. The predefined humane
endpoints used were the inability to access food and water and no
animals reached the humane endpoints before the end of the
experiment. All animal experiments were conducted at the Animal
Experimental Center of Henan University of Chinese Medicine. Blood
and colonic tissue samples were collected. Colonic length was
measured, and colonic tissue was processed for histological
analysis using paraffin-embedded sections. Tissues were fixed in 4%
paraformaldehyde fixative (cat. no. G1101; Wuhan Servicebio
Technology Co., Ltd.) for 24 h at room temperature and then
transferred to a dehydration box for dehydration using a graded
alcohol series. After dehydration, the tissues were immersed in wax
and embedded using an embedding machine. The wax blocks were cooled
at −20°C, trimmed and sectioned to a thickness of 4 µm.
Disease activity index (DAI)
The DAI is a validated scoring system used to assess
disease activity in experimental models of UC. It is derived from
an understanding of UC pathophysiology gleaned from previous
clinical and experimental studies, encompassing a comprehensive
evaluation of disease activity (23,24).
The DAI is calculated by summing scores from three key indicators:
Weight loss, stool consistency and rectal bleeding. Body mass loss
of 1–5% was scored as 1, 5–10% as 2, 10–20% as 3 and >20% as 4.
Stools were scored according to the nature of the blood in the
stools, with occult blood negative scoring 0, weakly positive
occult blood scoring 1, moderately positive occult blood scoring 2,
strongly positive occult blood scoring 3 and hemorrhage scoring 4.
Normal feces was scored as 0, soft scoring 1, soft and thick
scoring 2 and soft adherence to the perineal region scoring 3, with
diarrhea scoring 4. Daily monitoring of mice included meticulous
observation and measurement of body weight, hair condition, mental
status and stool characteristics.
Hematoxylin and eosin (H&E)
staining
Mouse colon, heart, lung, liver, spleen and kidney
tissues were fixed, dehydrated, embedded in sections and
deparaffinized in water. Sections were then stained with
hematoxylin for 3–5 min at room temperature, rinsed and blued.
Following this, the sections were dehydrated in 95% alcohol for 1
min and counterstained with eosin for 15 sec at room temperature.
Finally, the sections were dehydrated, mounted and coverslipped for
subsequent imaging and analysis.
Periodic acid-schiff staining
Paraffin sections were dewaxed and rehydrated. Next,
these sections were immersed in Periodic acid-Schiff (PAS) staining
solution B for 10–15 min at room temperature, followed by thorough
washing. Subsequently, sections were immersed in immersion staining
solution A for 25–30 min at room temperature, with meticulous
attention paid to avoiding exposure to light. Following thorough
cleaning, sections were immersed in staining solution C for 30 sec
at room temperature to neutralize any residual ammonia and restore
the blue color. After rinsing, sections were dehydrated and
mounted. An upright light microscope (NIKON ECLIPSE E100; Nikon
Coporation) was used for microscopic examination and images were
captured for subsequent analysis. The overall morphology was
observed, followed by detailed examination at ×18 magnification. To
ensure representative sampling, ≥3 fields of view were analyzed for
each sample.
Immunohistochemistry
Paraffin sections of colon tissue were
deparaffinized in xylene and subsequently rehydrated through graded
alcohols (100, 95 and 70%). Endogenous peroxidase activity was
blocked by incubation in 3% H2O2 for 10 min,
followed by two 5-min washes in phosphate-buffered saline (PBS).
The sections were blocked with 3% BSA for 30 min at room
temperature. After overnight incubation with the primary antibodies
targeting Muc2 (cat. no. GB11344; 1:500; Wuhan Servicebio
Technology Co., Ltd.), Occludin (cat. no. GB111401; 1:500; Wuhan
Servicebio Technology Co., Ltd.) and ZO-1 (cat. no. GB1114021;
1:2,000; Wuhan Servicebio Technology Co., Ltd.) at 4°C, sections
were washed three times in PBS for 5 min each. Following incubation
with the appropriate horseradish peroxidase (HRP)-conjugated Goat
Anti-Rabbit IgG (H+L) secondary antibodies (cat. no. GB23303;
1:200; Wuhan Servicebio Technology Co., Ltd.) for 50 min at room
temperature, protected from light, sections were washed three times
in PBS for 5 min each. Color development was achieved using freshly
prepared diaminobenzidine (DAB) solution, monitored microscopically
and terminated with distilled water rinses. Finally, sections were
counterstained with hematoxylin for 3 min at room temperature and
dehydrated for mounting. The samples were imaged at ×25
magnification using a light microscope (E100; Nikon
Corporation).
TUNEL fluorescence assay
Paraffin sections of mouse colon tissue were
deparaffinized to water, followed by a 20 min incubation at 37°C
with proteinase K working solution for antigen retrieval.
Subsequent washes were performed thrice with PBS for 5 min each at
room temperature. Membrane-permeabilization solution was then added
to cover the tissue and incubated for 20 min at room temperature,
followed by another PBS wash. The sections were subsequently
incubated in the TUNEL reaction solution for 1 h at 37°C. The
amount of TDT enzyme in the TUNEL kit (cat. no. G1502; Wuhan
Servicebio Technology Co., Ltd.) was used according to the number
of slides and tissue size, mixed with dUTP and buffer at a 1:5:50
ratio. After thorough washing with PBS, the sections were stained
with DAPI for 10 min at room temperature, protected from light.
Following a final PBS wash, the sections were mounted with an
anti-fluorescence quenching mounting medium. Images were
subsequently acquired using a fluorescence microscope (×25
magnification; NIKON ECLIPSE C1; Nikon Coporation).
Immunofluorescence assay
Paraffin-embedded intestinal tissue sections were
sectioned into thin slices and subjected to deparaffinization prior
to immunofluorescence staining of epithelial monolayers. Following
antigen retrieval, sections were blocked with 5% BSA for 30 min at
room temperature. Subsequently, primary antibodies against p-RIPK3
(cat. no. ab195117; 1:200; Abcam) and p-MLKL (cat. no. bsm-54104R;
1:300; BIOSS). were applied and incubated overnight at 4°C. After
four washes with PBS, sections were incubated with secondary
antibodies for 1 h in the dark at room temperature. Nuclear
staining was performed using DAPI (cat. no. G1012, Xavier, China)
for 10 min at room temperature. Following subsequent washes with
PBS, sections were mounted with an antifluorescent mounting medium.
Secondary antibodies employed were the Cy3 conjugated Goat
Anti-Rabbit IgG (H+L) (cat. no. GB21303; 1:300; Wuhan Servicebio
Technology Co., Ltd.) antibodies. Samples were imaged using a
fluorescent microscope at ×25 magnification (NIKON ECLPISE C1;
Nikon Corporation).
Statistical analysis
Statistical analysis was performed using one-way
ANOVA, followed by Tukey's post hoc test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference. Data were analyzed using SPSS (version 25; IBM
Corp.).
Results
Pristimerin exhibits anti-necroptosis
activity in HT-29 cells
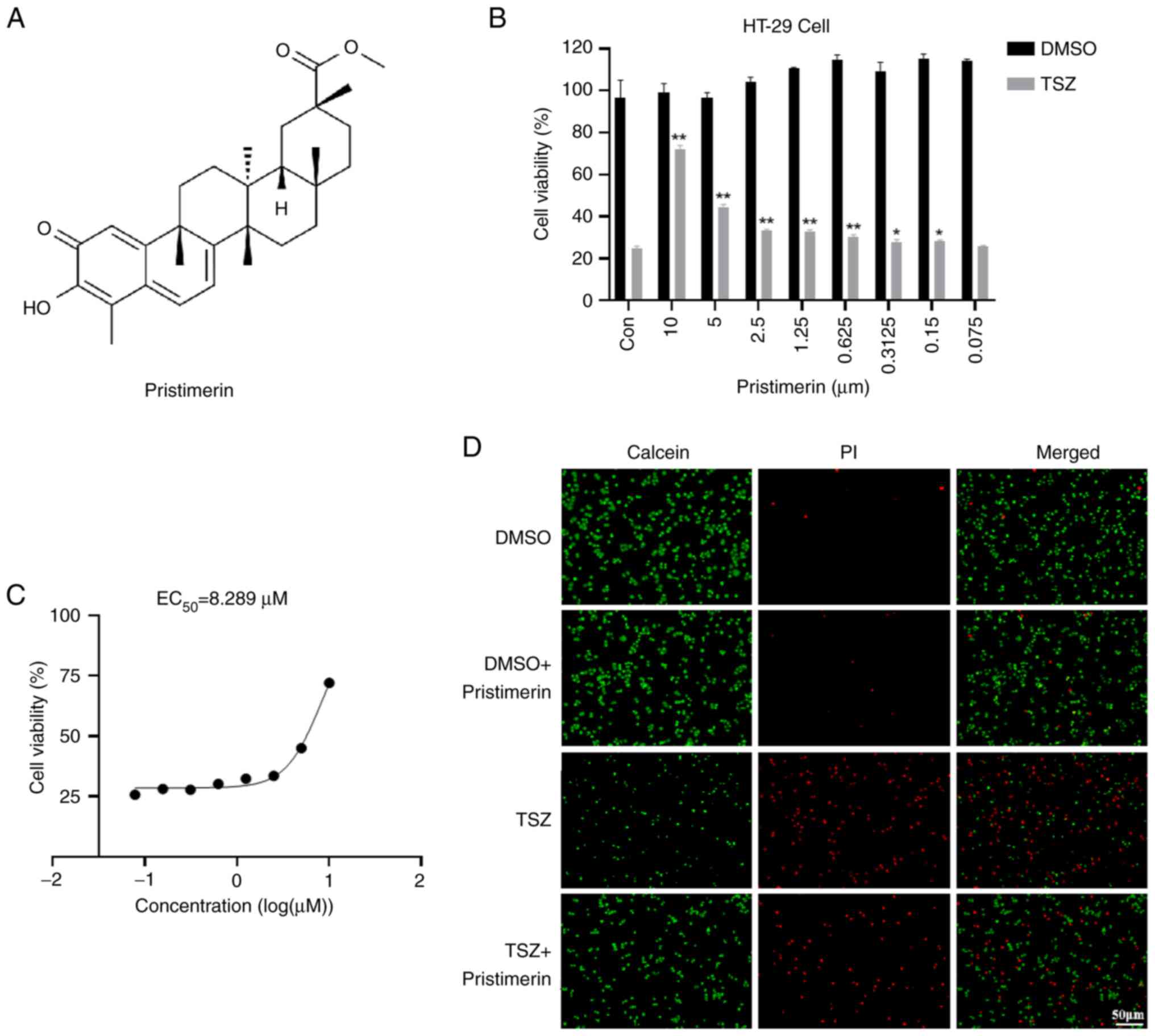
The chemical structure of Pristimerin is shown in
Fig. 1A. An in vitro model
of TSZ-induced necroptosis was established utilizing HT-29 cells to
assess the anti-necroptotic effects of pristimerin. The cells were
co-incubated with varying concentrations of pristimerin for a 12-h
period. Cell viability was assessed using the CellTiter-Lumi™ II
Luminescent Cell Viability Assay Kit. The findings indicated that
pristimerin markedly increased cell viability in a dose-dependent
manner (Fig. 1B). The half-maximal
effective concentration (EC50) was determined to be
8.289 µM based on cell viability data (Fig. 1C). Following pristimerin treatment,
there was a notable enhancement in cell death, accompanied by a
discernible increase in the number of viable cells, as evidenced by
staining with the Calcein/PI Cell Viability/Cytotoxicity Assay Kit
(Fig. 1D). In this assay, Calcein
AM selectively stained viable cells with green fluorescence, while
Propidium Iodide marked dead cells with red fluorescence.
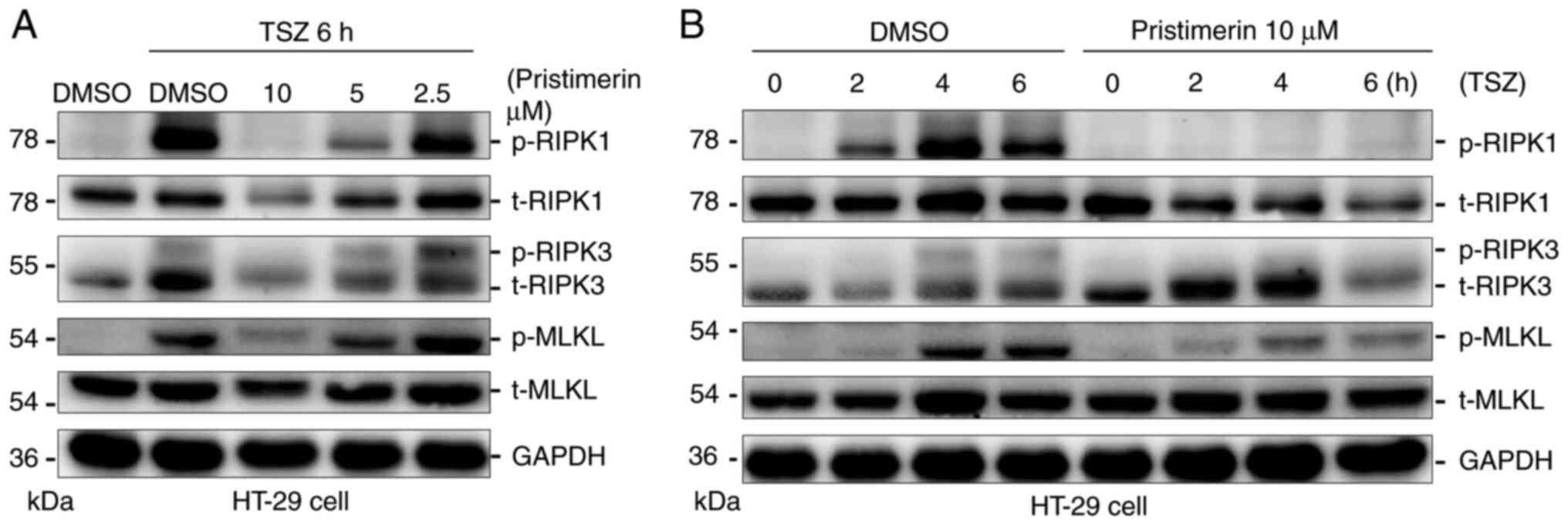
Pristimerin inhibits the expression of
necroptosis-related proteins
RIPK1 and RIPK3 modulate cell death through
processes of polyubiquitination and deubiquitination, thereby
playing a crucial role in the mechanism of necroptosis (25). The phosphorylation of MLKL induces
its oligomerization, which subsequently leads to the disruption of
membrane integrity and the translocation of necroptosomes to
cellular or organelle membranes (26). The present study investigated the
effect of pristimerin on the phosphorylation status of RIPK1, RIPK3
and MLKL. The experimental findings demonstrated that pristimerin
inhibited the phosphorylation of RIPK1, RIPK3 and MLKL in a
dose-dependent manner (Fig. 2A).
Furthermore, a time-dependent decline in the phosphorylation levels
of these proteins upon pristimerin treatment was observed (Fig. 2B).
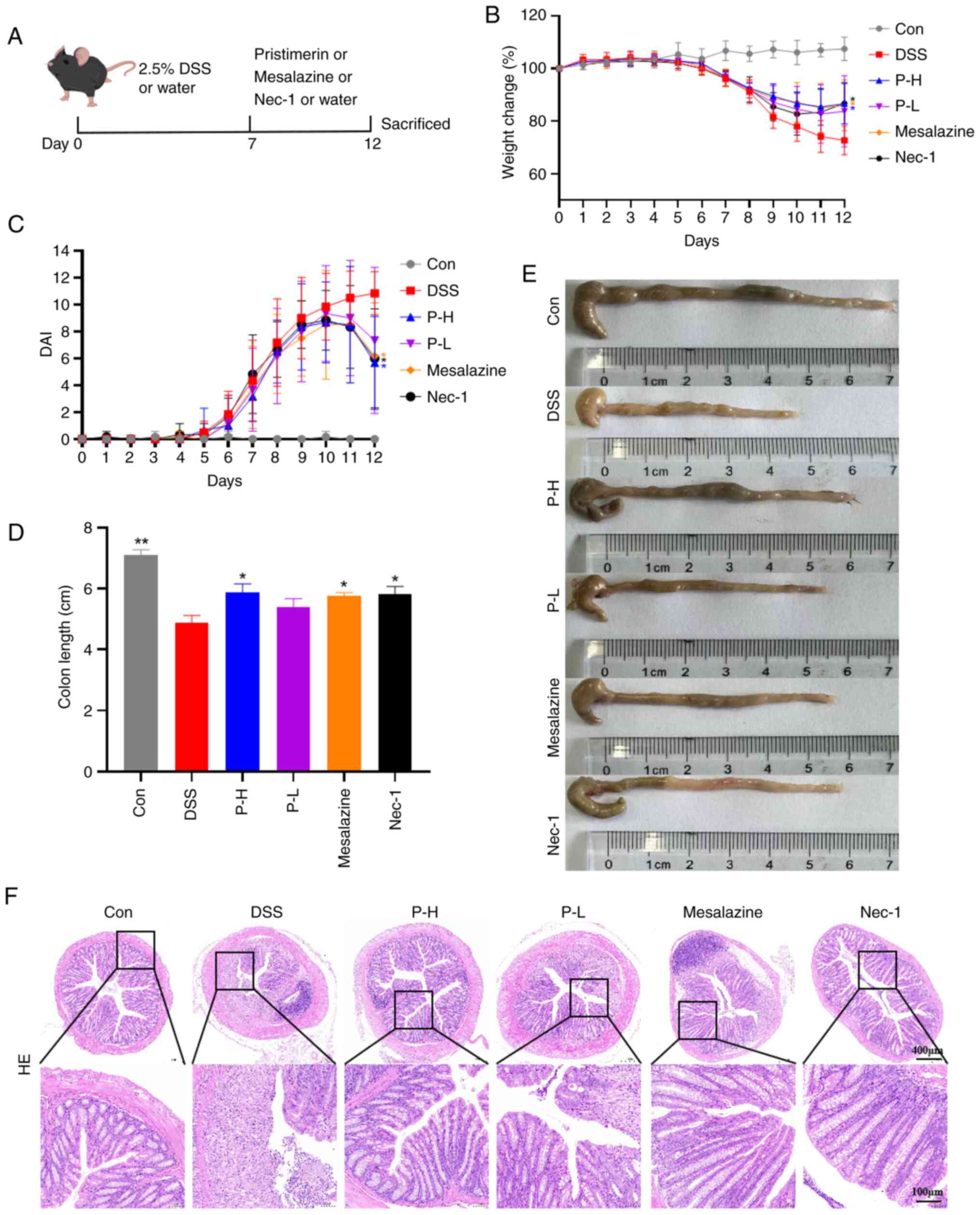
Pristimerin ameliorates symptoms in
DSS-induced UC mice
The DSS-induced UC model was established to evaluate
the therapeutic efficacy of pristimerin. Following 7 days of 2.5%
DSS administration, all mice exhibited weight loss, hematochezia
and decreased stool output, confirming the successful induction of
colitis. Subsequently, these mice were randomly assigned into five
groups, ensuring no significant baseline differences in body weight
or DAI among them. The groups comprised: i) DSS-treated group
(DSS), ii) high-dose pristimerin group (P-H; 3 mg/kg), iii)
low-dose pristimerin group (P-L, 1 mg/kg), iv) positive control
group treated with the standard-of-care UC therapeutic agent
mesalazine (100 mg/kg) and v) positive control group treated with
the necroptosis inhibitor Nec-1 (5 mg/kg).
Experimental findings demonstrated that pristimerin
exerted a significant effect on DSS-induced UC-related symptoms in
mice. A schematic diagram of the DSS-induced UC mouse model and
dosing regimen is presented in Fig.
3A. Compared with the model group, the pristimerin high-dose
(P-H) group exhibited a slower rate of weight decline on day nine
and achieved weight recovery by day 12 (Fig. 3B). This was accompanied by a
reduction in DAI scores (Fig. 3C),
amelioration of clinical symptoms including blood in stool and
stool consistency and an increase in colon length (Fig. 3D and E). The therapeutic efficacy
of the P-H group was comparable to that of the positive control
groups (Nec-1 and mesalazine) and superior to the P-L group. To
evaluate the extent of intestinal injury, histopathological
analysis was conducted using H&E staining. The findings
indicated that mice in the model group exhibited more severe damage
to the colonic mucosal layer, characterized by diffuse infiltration
of inflammatory cells within the submucosal layer, along with
disruption of crypt architecture and a reduction in crypt numbers.
The P-H and P-L groups both demonstrated amelioration of colonic
crypt damage and quantity, with the P-H group exhibiting effects
compared with the positive control group. This suggested that
pristimerin has the potential to repair DSS-induced colonic tissue
damage in UC mice (Fig. 3F).
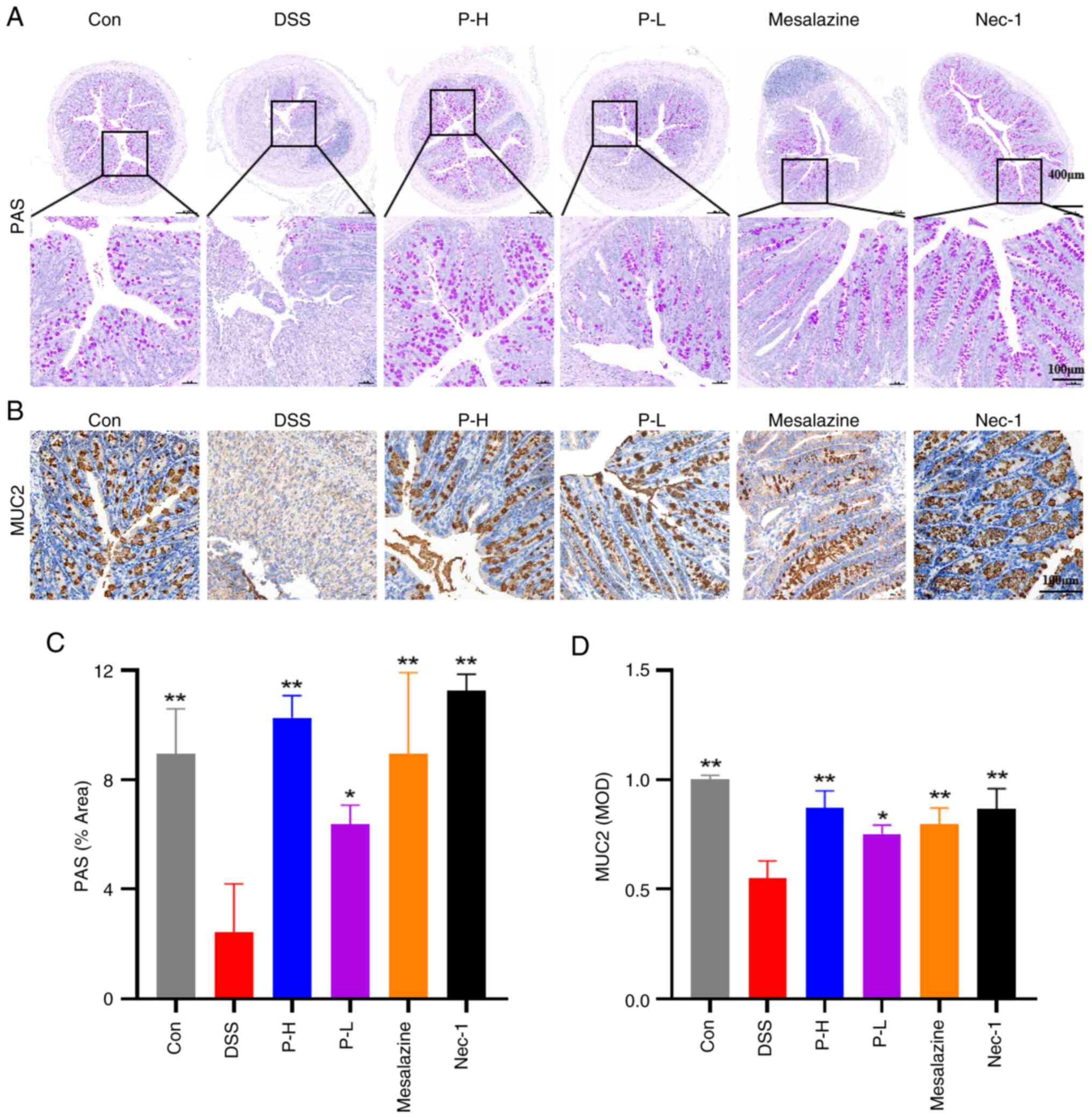
Pristimerin protects the mucus barrier
of colonic tissue in DSS-induced UC mice
Goblet cells are integral to the pathogenesis of UC
(27). These cells are responsible
for the secretion of mucin proteins, particularly mucoprotein 2
(MUC2) (28), which form a
substantial component of the intestinal mucus layer, thereby
serving as a critical physical barrier, protecting the intestinal
epithelium against pathogens and harmful substances. Murine models
of DSS-induced UC exhibit a notable impairment of intestinal
barrier function, predominantly characterized by the loss of goblet
cells and a reduction in glycoprotein synthesis, particularly
mucins (29). PAS staining was
employed to assess the distribution of polysaccharides and
inflammatory damage within colonic tissue. The present study
indicated a significant reduction in goblet cell numbers
attributable to inflammatory injury, leading to a decrease in
PAS-positive areas in the DSS model. Conversely, treatment with
pristimerin demonstrated a notable restoration of goblet cell
numbers, suggesting that pristimerin may ameliorate DSS-induced
colonic tissue damage in UC mice, as illustrated in Fig. 4A. Immunohistochemical analysis
revealed a reduction in MUC2 expression within the model group. In
contrast, pristimerin administration markedly enhanced MUC2
expression in a dose-dependent manner, with the P-H group
exhibiting effects comparable to those of the positive control
drug, Nec-1 (Fig. 4B).
Pristimerin enhances the expression
and distribution of tight junction proteins in DSS-induced
intestinal epithelial cells of UC mice
Tight junctions between intestinal epithelial cells
play a crucial role in preserving the integrity of the intestinal
epithelium, with Occludin and ZO-1 being the primary proteins
comprising these junctions (30).
Immunohistochemical analyses revealed a reduction in Occludin and
ZO-1 protein expression in the colonic tissues of mice subjected to
the DSS treatment, compared with the control group. This reduction
suggested a disruption of the intestinal mucosal barrier in these
mice. Notably, the administration of pristimerin, mesalazine and
Nec-1 treatments resulted in a significant upregulation of Occludin
and ZO-1 proteins (Fig. 5). These
findings indicated that pristimerin could promote the repair of the
intestinal mucosal barrier and maintain the integrity of the
intestinal barrier by upregulating the expression of intestinal
tight junction proteins in UC mice.
Pristimerin inhibits decrease of
intestinal epithelial cells in UC mice
TUNEL staining was employed to assess apoptosis in
intestinal epithelial cells. The model group exhibited a
significant increase in the number of TUNEL-positive cells within
the intestinal epithelium compared with the control group. However,
a reduction in TUNEL-positive cells was observed in both
pristimerin-treated groups, with the effect in the P-H group being
comparable with that of the positive control groups, mesalazine and
Nec-1 (Fig. 6A and D). These
findings suggested that pristimerin ameliorated apoptosis in
intestinal epithelial cells. Furthermore, increased phosphorylation
of RIPK3 and MLKL is recognized as a critical pathological
mechanism in necroptosis (31)
with a prevalence exceeding 400 per 100 000 in North America.
Individuals with UC have a lower life expectancy and are at
increased risk for colectomy and colorectal cancer.nOBSERVATIONS:
UC impairs quality of life secondary to inflammation of the colon
causing chronic diarrhea and rectal bleeding. Extraintestinal
manifestations, such as primary sclerosing cholangitis, occur in
approximately 27% of patients with UC. People with UC require
monitoring of symptoms and biomarkers of inflammation (eg, fecal
calprotectin. To evaluate the effect of pristimerin on necroptosis,
the distribution of phosphorylated RIPK3 (pRIPK3) and
phosphorylated MLKL (pMLKL) in colonic tissues was analyzed
(Fig. 6B and C). These findings
indicated that the expression levels of phosphorylated RIPK3 and
MLKL were elevated in the colonic tissues of DSS-treated mice.
However, administration of pristimerin resulted in a dose-dependent
inhibition of these phosphorylation levels. Notably, the effect of
P-H was comparable with that observed in the Nec-1 and mesalazine
treatment groups (Fig. 6E and F),
indicating that pristimerin inhibited necroptosis in the colonic
tissue of mice with UC, thereby enhancing intestinal barrier
function and exerting therapeutic effects on the condition.
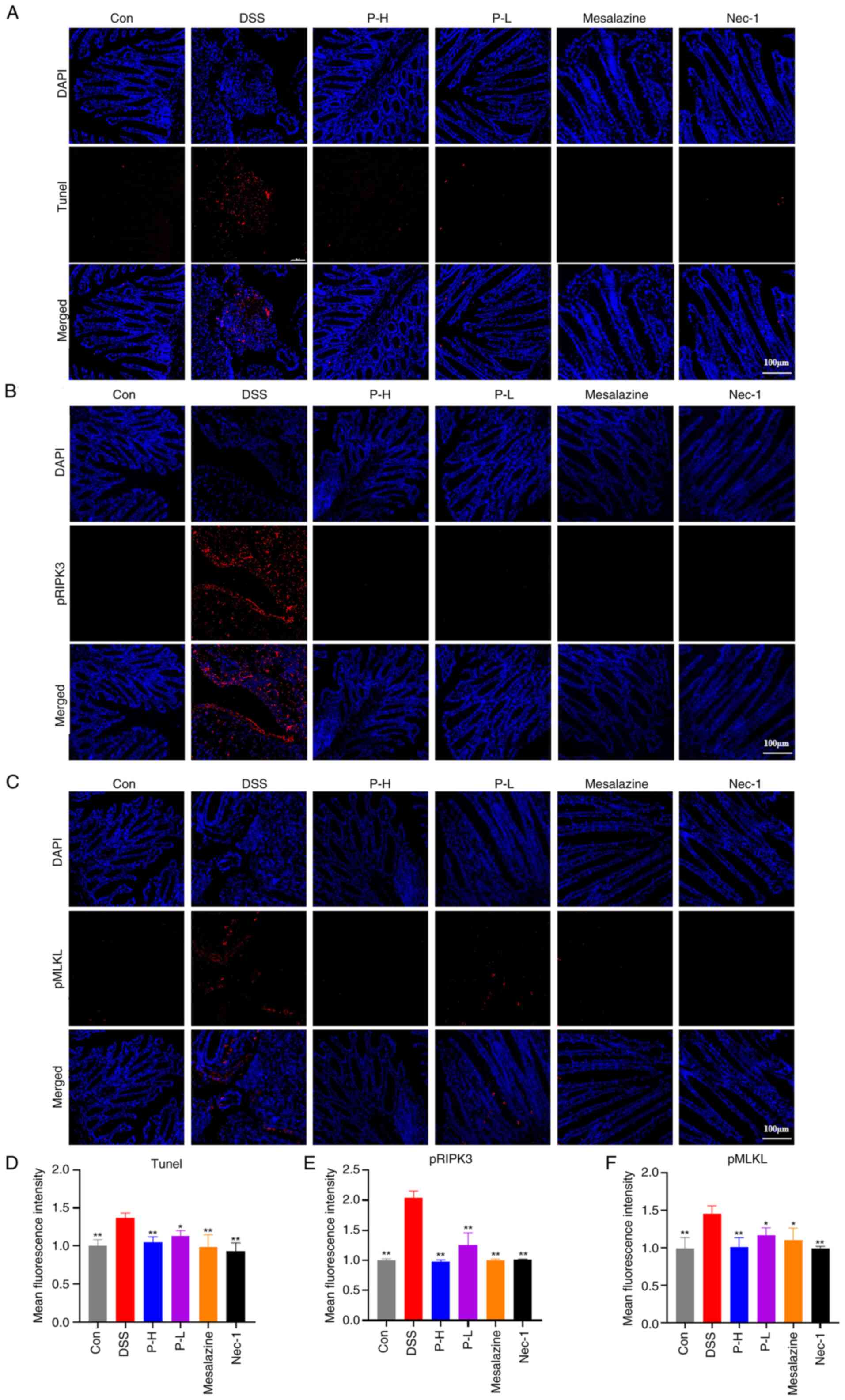 | Figure 6.Pristimerin inhibits
necroptosis-related protein expression in UC mice. (A) Fluorescence
TUNEL detection of apoptosis in intestinal epithelial cells. (B)
Immunofluorescence detection of the effects of pRIPK3 and pMLKL.
(C) Distribution and expression in intestinal epithelial cells. (D)
Quantitative results after normalization of TUNEL, (E) pRIPK3 and
(F) pMLKL. **P<0.01, *P<0.05 vs. the DSS group. UC,
ulcerative colitis; p, phosphorylated; RIPK3, receptor-interacting
protein kinase 3; MLKL, mixed lineage kinase domain-like protein;
DSS, dextran sulfate sodium; UC, ulcerative colitis; ZO-1, Zonula
Occludens-1; Con, control; P-H, high-dose pristimerin group; P-L,
low-dose pristimerin group; Nec-1, necrostain-1. |
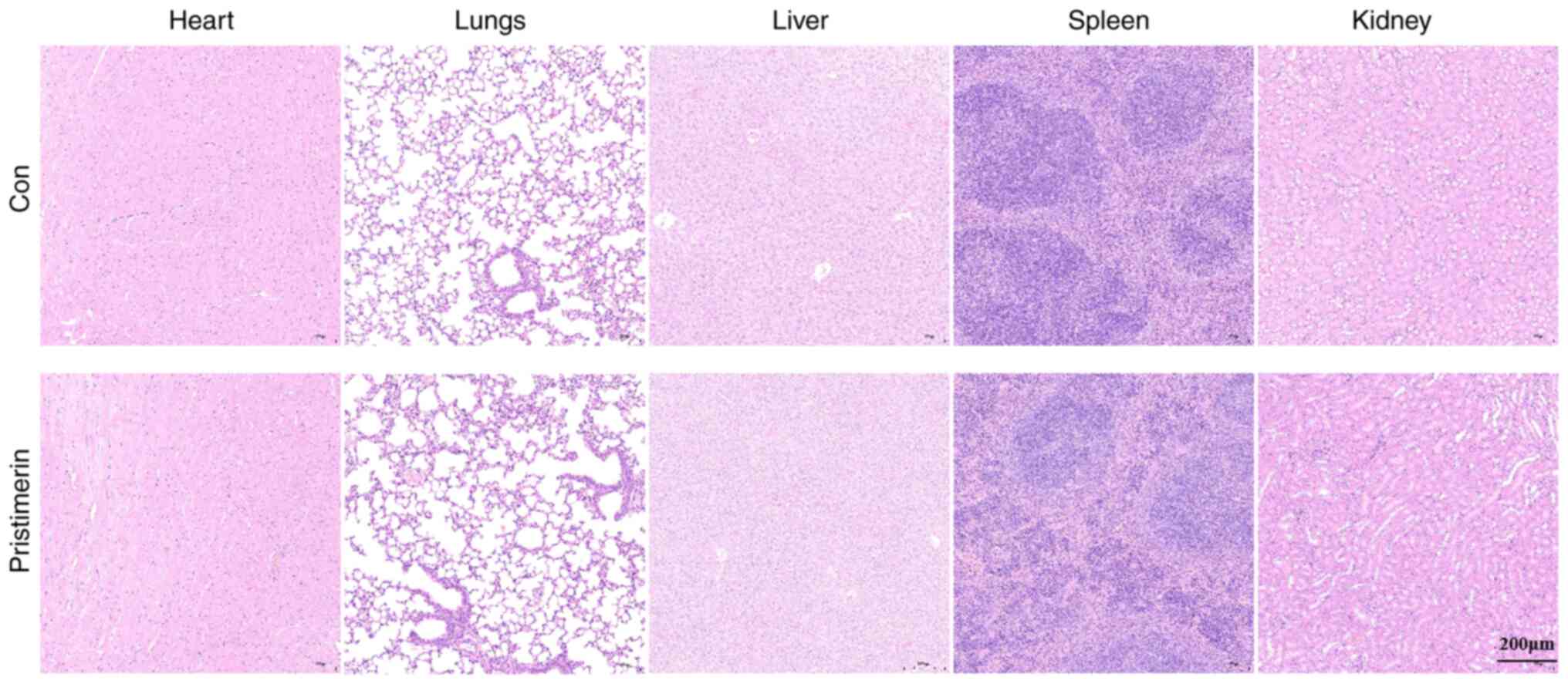
Pristimerin yields no toxic effects in
UC mice
The present study evaluated the pathology of vital
organs, including the heart, liver, spleen, lungs and kidneys, in
UC mice administered with a high dose of pristimerin.
Histopathological analysis, using H&E staining, revealed no
significant differences between the control group and the
pristimerin-treated group at the high dose. These findings indicate
that pristimerin did not exert toxic effects in mice at the
administered dosage (Fig. 7).
Discussion
Despite the availability of certain treatments for
UC, their clinical efficacy remains suboptimal. Research indicates
that the one-year clinical remission rate with current therapies is
~40%. Moreover, prolonged use of these medications often leads to
diminished effectiveness and necessitates alterations in treatment
strategies (32). Consequently, to
enhance therapeutic outcomes, improve patient quality of life and
effectively prevent and manage complications (33), the investigation of novel
pharmacological agents is imperative. TCM has exhibited significant
therapeutic efficacy in the management of chronic diseases,
attributed to its distinctive advantages. Specifically, monomeric
components with well-defined chemical structures and specific
activities, extracted from TCM, have demonstrated notable
therapeutic effects. Research has identified that herbal monomers
such as berberine (34), baicalin
(35), triptolide (36) and astragalus polysaccharide
(37) mitigate colonic
inflammation through various mechanisms. Consequently, further
investigation into herbal monomers may offer modern medicine novel
insights and therapeutic strategies for the management of UC.
Necroptosis, a regulated form of necrotic cell
death, markedly contributes to various pathological conditions,
particularly inflammatory responses (38). In UC, necroptosis serves a crucial
role by triggering the release of pro-inflammatory mediators. This
activation initiates the RIPK1/RIPK3/MLKL signaling cascade,
leading to cellular necroptosis and subsequent exacerbation of the
disease (39). Therefore,
inhibiting the RIPK1/RIPK3/MLKL signaling pathway presents a
promising therapeutic strategy for mitigating UC severity (40). The discovery of necroptosis
inhibitors not only advances our understanding of the necroptosis
signaling pathway but also provides a valuable avenue for
pharmaceutical research in diseases associated with necroptosis
(41). These inhibitors,
particularly those targeting RIPK1, have demonstrated therapeutic
potential in models of dermatosis, colitis, arthritis and
neurodegenerative diseases (42)
by suppressing RIPK1 activity and preventing necroptotic signaling.
Inhibitors targeting RIPK3 have been shown to suppress
TNF-α-induced necroptosis in human intestinal epithelial cells,
thereby enhancing their proliferative capacity (43). Furthermore, the inhibition of MLKL
has been observed to mitigate colonic inflammation and reduce
colitis-associated tumorigenesis (44). Consequently, the identification and
development of novel necroptosis inhibitors hold potential for
therapeutic intervention in UC.
In the present study, a murine model of UC was
established by administering a 2.5% DSS solution ad libitum,
a model that more accurately reflects the pathogenesis and clinical
manifestations observed in human UC patients. Following treatment
with pristimerin, the mice exhibited amelioration in weight loss,
reduced DAI scores and a gradual resolution of symptoms,
demonstrating the therapeutic efficacy of pristimerin in UC. UC is
characterized by dysregulation of epithelial barrier integrity and
mucosal damage, accompanied by a marked reduction in mucins and
tight junction proteins, as well as increased intestinal
permeability (45). In the current
study, pristimerin augmented the number of goblet cells and
elevated the expression levels of MUC2, Occludin and ZO-1. These
findings suggested that pristimerin may mitigate DSS-induced damage
to the intestinal mucosal barrier by enhancing the tight junctions
between epithelial cells and ameliorating mucosal injury. In HT-29
cells, pristimerin was found to inhibit the phosphorylation of
RIPK1, RIPK3 and MLKL in a time- and dose-dependent manner.
Furthermore, pristimerin demonstrated a dose-dependent inhibition
of p-RIPK3 and p-MLKL expression levels in colonic tissues.
Therefore, pristimerin may exert a therapeutic effect on UC by
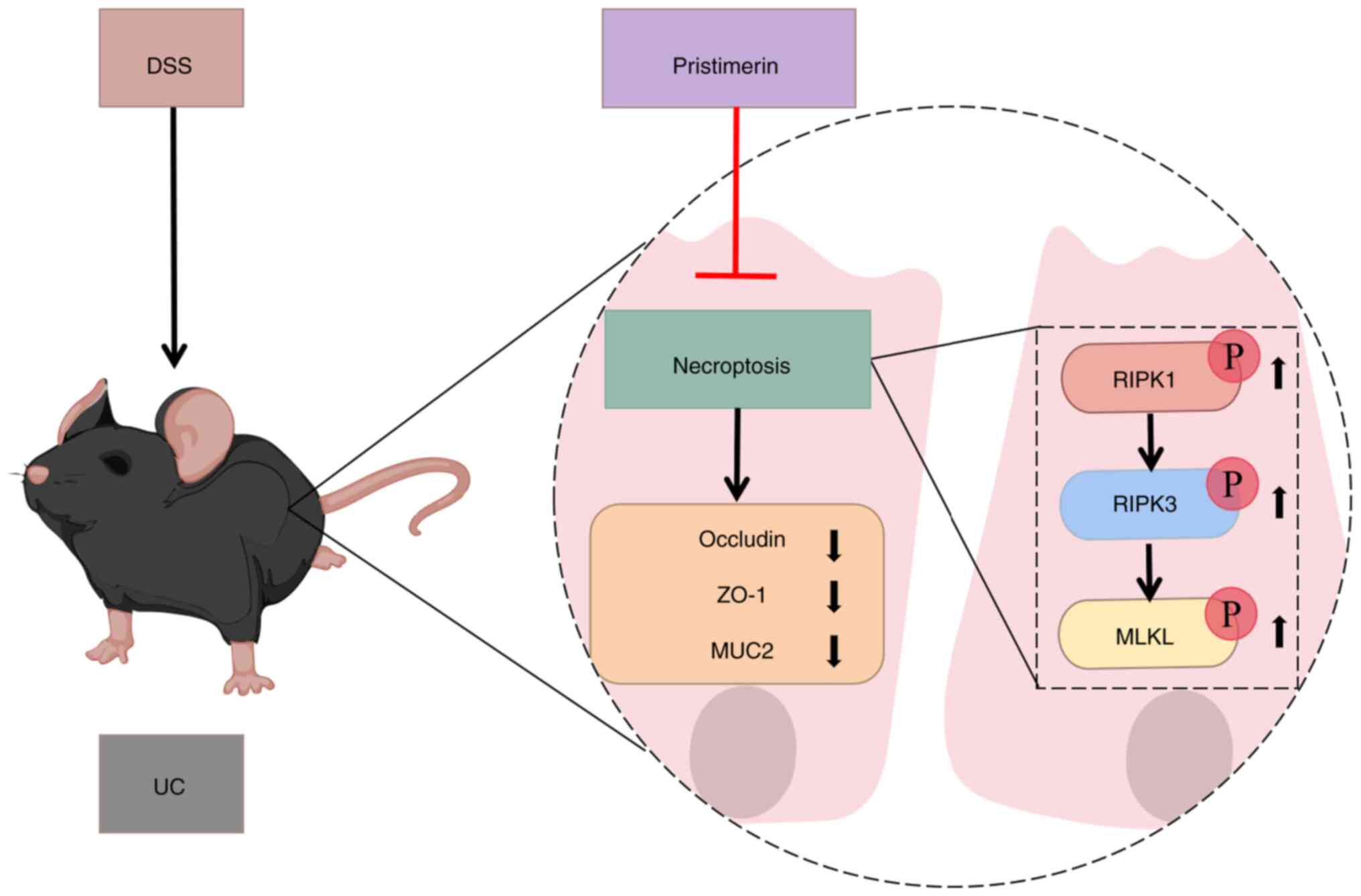
inhibiting the necroptotic signaling pathway. The proposed
mechanism is depicted in Fig.
8.
Although pristimerin showed protective effects in UC
mouse models, limitations remain. DSS is a synthetic sulfated
polysaccharide closely linked to the severity, duration and dosage
of colitis. DSS can directly affect colonic epithelial cells,
compromising mucosal integrity and animal models of DSS-induced
colitis can simulate the symptoms of human UC. Nevertheless, the
etiology of human UC is complex, involving immune dysregulation,
genetic predispositions and environmental influences, which cannot
be entirely replicated in DSS models. The dosage of pristimerin
administered in this study was determined to be non-toxic; however,
due to the limited range of doses investigated, future research
should include more comprehensive dose-response studies. Given the
substantial gaps in the existing research on pristimerin, further
investigations into its metabolism, distribution and
pharmacokinetics in vivo are essential. Such studies will
facilitate the optimization of clinical dosing regimens and the
reduction of potential toxicity. Although pristimerin showed
anti-necroptosis effects in in vitro and in vivo
experiments, necroptosis was assessed only by phosphorylation of
RIPK1, RIPK3 and MLKL. The specific targets of pristimerin and its
effects on upstream and downstream key regulators remain unclear.
In future experiments, additional assays are necessary to more
comprehensively evaluate the occurrence and inhibitory effects of
necroptosis.
In summary, the present study demonstrated that
pristimerin can inhibit the phosphorylation of RIPK1, RIPK3 and
MLKL, thereby alleviating the symptoms in UC mice and enhancing
intestinal barrier function. These findings suggested that
pristimerin holds promise as a therapeutic agent for UC. However,
the mechanisms underlying cell death are complex and may involve
multiple synergistic pathways. Further research is necessary to
comprehensively elucidate the precise mechanisms by which
pristimerin inhibits cell death in the colonic tissues of UC
mice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Henan Provincial Science
and Technology Research and Development Joint Fund (grant no.
222301420021), the National Natural Science Foundation of China
(grant no. 82274496), the start-up funds from Henan University of
Chinese Medicine (grant no. 03104150-2024-1-85) and the Shanghai
Sailing Program (grant no. 21YF1444700).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SL and YW conceived the study. EX and ZW determined
the design of this study. SL, KL and YS performed experiments and
analyzed data. SL and YW wrote and revised the manuscript. EX and
ZW contributed to critical revision of this article and supervised
the study. SL and YW confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Animal Care
and Use Committee of Henan University of Traditional Chinese
Medicine (approval no. DWLLGZR202200023; Henan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UC
|
ulcerative colitis
|
|
RIPK1
|
receptor-interacting protein kinase
1
|
|
RIPK3
|
receptor-interacting protein kinase
3
|
|
MLKL
|
mixed lineage kinase domain-like
protein
|
|
TCM
|
Traditional Chinese Medicine
|
|
DSS
|
dextran sulfate sodium
|
|
Nec-1
|
necrostain-1
|
|
Con
|
control
|
|
P-L
|
low-dose pristimerin group
|
|
DAI
|
Disease Activity Index
|
|
EC50
|
half-maximal effective
concentration
|
|
H&E
|
hematoxylin and eosin
|
|
PAS
|
Periodic acid-Schiff
|
|
MUC2
|
mucoprotein 2
|
References
|
1
|
Du L and Ha C: Epidemiology and
pathogenesis of ulcerative colitis. Gastroenterol Clin North Am.
49:643–654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ng SC, Shi HY, Hamidi N, Underwood FE,
Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, et
al: Worldwide incidence and prevalence of inflammatory bowel
disease in the 21st century: A systematic review of
population-based studies. Lancet. 390:2769–2778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopez A, Pouillon L, Beaugerie L, Danese S
and Peyrin-Biroulet L: Colorectal cancer prevention in patients
with ulcerative colitis. Best Prac Res Clin Gastroenterol.
32-33:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang Y, Li Y, Lee C, Yu Z, Chen C and
Liang C: Ulcerative colitis: Molecular insights and intervention
therapy. Mol Biomed. 5:422024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asgharzadeh F, Yaghoubi A, Nazari SE,
Hashemzadeh A, Hasanian SM, Avan A, Javandoost A, Ferns GA,
Soleimanpour S and Khazaei M: The beneficial effect of combination
therapy with sulfasalazine and valsartan in the treatment of
ulcerative colitis. EXCLI J. 20:236–247. 2021.PubMed/NCBI
|
|
6
|
Chelakkot C, Ghim J and Ryu SH: Mechanisms
regulating intestinal barrier integrity and its pathological
implications. Exp Mol Med. 50:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khy MK, Gupta K, Franco SR and Liu B:
Necroptosis in the pathophysiology of disease. Am J Pathol.
190:272–285. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Negroni A, Colantoni E, Cucchiara S and
Stronati L: Necroptosis in intestinal inflammation and cancer: New
concepts and therapeutic perspectives. Biomolecules. 10:14312020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gaba A, Xu F, Lu Y, Park H-S, Liu G and
Zhou Y: The NS1 protein of influenza a virus participates in
necroptosis by interacting with MLKL and increasing its
oligomerization and membrane translocation. J Virol. 93:e01835–18.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeb S, Ye H, Liu Y, Du HP, Guo Y, Zhu YM,
Ni Y, Zhang HL and Xu Y: Necroptotic kinases are involved in the
reduction of depression-induced astrocytes and fluoxetine's
inhibitory effects on necroptotic kinases. Front Pharmacol.
13:10609542023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Wang H, Wang Z and Geng Y: Pine
pollen polysaccharides' and sulfated polysaccharides' effects on UC
mice through modulation of cell tight junctions and RIPK3-dependent
necroptosis pathways. Molecules. 27:76822022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SH, Kwon JY, Moon J, Choi J, Jhun J,
Jung K, Cho KH, Darlami O, Lee HH, Jung ES, et al: Inhibition of
RIPK3 pathway attenuates intestinal inflammation and cell death of
inflammatory bowel disease and suppresses necroptosis in peripheral
mononuclear cells of ulcerative colitis patients. Immune Network.
20:e162020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Li BG, Su YH, Zhao RX, Song P, Li
H, Cui XH, Gao HM, Zhai RX, Fu XJ and Ren X: Potential activity of
traditional Chinese medicine against ulcerative colitis: A review.
J Ethnopharmacol. 289:1150842022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang M, Fu R, Xu D, Chen Y, Yue S, Zhang S
and Tang Y: Traditional Chinese medicine: A promising strategy to
regulate the imbalance of bacterial flora, impaired intestinal
barrier and immune function attributed to ulcerative colitis
through intestinal microecology. J Ethnopharmacol. 318:1168792024.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Q, Bi Y, Zhong J, Ren Z, Liu Y, Jia
J, Yu M, Tan Y, Zhang Q and Yu X: Pristimerin suppresses colorectal
cancer through inhibiting inflammatory responses and Wnt/β-catenin
signaling. Toxicol Appl Pharmacol. 386:1148132020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai Z, Zhang A, Choksi S, Li W, Li T,
Zhang XM and Liu ZG: Activation of cell-surface proteases promotes
necroptosis, inflammation and cell migration. Cell Res. 26:886–900.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu K, Liang W, Ma Z, Xu D, Cao S, Lu X,
Liu N, Shan B, Qian L and Yuan J: Necroptosis promotes
cell-autonomous activation of proinflammatory cytokine gene
expression. Cell Death Dis. 9:5002018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen X, Chen H, Wen T, Liu L, Yang Y, Xie
F and Wang L: A natural chalcone cardamonin inhibits necroptosis
and ameliorates dextran sulfate sodium (DSS)-induced colitis by
targeting RIPK1/3 kinases. Eur J Pharmacol. 954:1758402023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang LN, Wang Y, Lu Y, Yin ZF, Zhang YH,
Aslanidi GV, Srivastava A, Ling CQ and Ling C: Pristimerin enhances
recombinant adeno-associated virus vector-mediated transgene
expression in human cell lines in vitro and murine hepatocytes in
vivo. J Integr Med. 12:20–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang J, Yuan S, Wang X, Lei Y, Zhang X,
Huang M and Ouyang H: Attenuation of pristimerin on TNF-α-induced
endothelial inflammation. Int Immunopharmacol. 82:1063262020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang D, Ge F, Ji J, Li YJ, Zhang FR, Wang
SY, Zhang SJ, Zhang DM and Chen M: β-sitosterol alleviates dextran
sulfate sodium-induced experimental colitis via inhibition of
NLRP3/Caspase-1/GSDMD-mediated pyroptosis. Front Pharmacol.
14:12184772023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan YX, Shao MJ, Qi Q, Xu YS, Yang XQ, Zhu
FH, He SJ, He PL, Feng CL, Wu YW, et al: Artemisinin analogue SM934
ameliorates DSS-induced mouse ulcerative colitis via suppressing
neutrophils and macrophages. Acta Pharmacol Sin. 39:1633–1644.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Newton K: RIPK1 and RIPK3: Critical
regulators of inflammation and cell death. Trends Cell Biol.
25:347–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo J, Nam YW, Kim S, Oh DB and Song J:
Necroptosis molecular mechanisms: Recent findings regarding novel
necroptosis regulators. Exp Mol Med. 53:1007–1017. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh V, Johnson K, Yin J, Lee S, Lin R,
Yu H, In J, Foulke-Abel J, Zachos NC, Donowitz M and Rong Y:
Chronic inflammation in ulcerative colitis causes long-term changes
in goblet cell function. Cell Mol Gastroenterol Hepatol.
13:219–232. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao D, Dai W, Dong M, Dai C and Wu S: MUC2
and related bacterial factors: Therapeutic targets for ulcerative
colitis. EBioMedicine. 74:1037512021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van der Sluis M, De Koning BA, De Bruijn
AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J,
Van Seuningen I, Renes IB and Einerhand AW: Muc2-deficient mice
spontaneously develop colitis, indicating that MUC2 is critical for
colonic protection. Gastroenterology. 131:117–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li ZY, Lin LH, Liang HJ, Li YQ, Zhao FQ,
Sun TY, Liu ZY, Zhu JY, Gu F, Xu JN, et al: Lycium barbarum
polysaccharide alleviates DSS-induced chronic ulcerative colitis by
restoring intestinal barrier function and modulating gut
microbiota. Ann Med. 55:22902132023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu T, Dai Y, Xue L, Sheng Y, Xu L and Xue
Y: Expression of receptor interacting protein 3 and mixed lineage
kinase domain-like protein-key proteins in necroptosis is
upregulated in ulcerative colitis. Ann Palliat Med. 8:483–489.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feuerstein JD, Moss AC and Farraye FA:
Ulcerative colitis. Mayo Clin Proc. 94:1357–1373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gros B and Kaplan GG: Ulcerative colitis
in adults: A review. JAMA. 330:951–965. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong Y, Fan H, Zhang Z, Jiang F, Li M,
Zhou H, Guo W, Zhang Z, Kang Z, Gui Y, et al: Berberine ameliorates
DSS-induced intestinal mucosal barrier dysfunction through
microbiota-dependence and Wnt/β-catenin pathway. Int J Biol Sci.
18:1381–1397. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu YJ, Xu B, Huang SW, Luo X, Deng XL, Luo
S, Liu C, Wang Q, Chen JY and Zhou L: Baicalin prevents LPS-induced
activation of TLR4/NF-κB p65 pathway and inflammation in mice via
inhibiting the expression of CD14. Acta Pharmacol Sin. 42:88–96.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rao Q, Ma G, Li M, Wu H, Zhang Y, Zhang C,
Ma Z and Huang L: Targeted delivery of triptolide by dendritic
cell-derived exosomes for colitis and rheumatoid arthritis therapy
in murine models. Br J Pharmacol. 180:330–346. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huo Z, Li J, Li X, Xiao H, Lin Y, Ma Y, Li
J, Yang H and Zhang C: Functional fractions of Astragalus
polysaccharides as a potential prebiotic to alleviate ulcerative
colitis. Int J Biol Macromol. 271:1325802024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Degterev A, Ofengeim D and Yuan J:
Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad
Sci USA. 116:9714–9722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Günther C, Martini E, Wittkopf N, Amann K,
Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF
and Becker C: Caspase-8 regulates TNF-α-induced epithelial
necroptosis and terminal ileitis. Nature. 477:335–339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang J, Lei H, Hu X and Dong W:
Hesperetin ameliorates DSS-induced colitis by maintaining the
epithelial barrier via blocking RIPK3/MLKL necroptosis signaling.
Eur J Pharmacol. 873:1729922020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou Y and Cai Z, Zhai Y, Yu J, He Q, He
Y, Jitkaew S and Cai Z: Necroptosis inhibitors: Mechanisms of
action and therapeutic potential. Apoptosis. 29:22–44. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Newton K: Multitasking kinase RIPK1
regulates cell death and inflammation. Cold Spring Harb Perspect
Biol. 12:a0363682020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Duan C, Xu X, Lu X, Wang L and Lu Z: RIP3
knockdown inhibits necroptosis of human intestinal epithelial cells
via TLR4/MyD88/NF-κB signaling and ameliorates murine colitis. BMC
Gastroenterol. 22:1372022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao Q, Yu X, Li M, Liu Y, Han Y, Zhang X,
Li XM, Wu X, Qin J, Fang J and Zhang H: MLKL attenuates colon
inflammation and colitis-tumorigenesis via suppression of
inflammatory responses. Cancer Lett. 459:100–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Martini E, Krug SM, Siegmund B, Neurath MF
and Becker C: Mend your fences: The epithelial barrier and its
relationship with mucosal immunity in inflammatory bowel disease.
Cell Mol Gastroenterol Hepatol. 4:33–46. 2017. View Article : Google Scholar : PubMed/NCBI
|