Introduction
Uterine leiomyosarcoma (Ut-LMS) is a rare and
aggressive malignant tumor that originates from the smooth muscle
cells of the uterus (1). Ut-LMS
constitutes approximately 1–2% of all uterine malignancies and is
characterized by poor prognosis, high recurrence rates, and a
tendency to metastasize (2,3).
Pathologically, Ut-LMS is characterized by atypical spindle cells
with considerable nuclear pleomorphisms, high mitotic activity, and
areas of necrosis. These characteristics distinguish it from benign
leiomyomas (fibroids) that lack malignant potential (4,5).
Ut-LMS diagnosis typically requires histopathological examinations,
which are often supplemented by immunohistochemical staining, to
confirm the smooth muscle origin of the tumor cells (6). Clinically, patients with Ut-LMS may
present with nonspecific symptoms, such as abnormal uterine
bleeding, pelvic pain, and a palpable mass (1). Owing to its aggressive nature, Ut-LMS
often presents at an advanced stage, and early diagnosis is
challenging. Imaging techniques, including ultrasound, magnetic
resonance imaging, and computed tomography, aid in the evaluation
of tumor size, extent, and metastatic spread; however, definitive
diagnosis relies on tissue biopsy (7).
Depending on the extent of the disease, Ut-LMS
treatment primarily involves surgical resection, often in the form
of total hysterectomy with or without bilateral
salpingo-oophorectomy (1).
Conservative treatment for patients who wish to preserve fertility
may be an option; however, it requires close and intensive
follow-up (8). Although
laparoscopic approaches provide minimally invasive options, the
abdominal incision approach remains commonly used owing to its
lower cost and ease of application (9). Despite surgical intervention, the
high recurrence rate of Ut-LMS necessitates additional therapeutic
strategies. Owing to the limited data on this rare tumor,
preoperative diagnosis is challenging, and uncertainty regarding
optimal postoperative management render treatment decisions complex
(8). Adjuvant therapies, including
radiation and chemotherapy, have been explored; however, their
efficacy remains limited, and the optimal treatment regimen remains
a subject of ongoing research (10). Therefore, factors, such as safety,
fertility preservation, long-term prognosis, and cost should be
carefully considered when determining the best surgical approach
(8).
Proteasome inhibitors are a class of compounds that
target the ubiquitin-proteasome system, which is a critical pathway
for degrading misfolded, damaged, or unnecessary proteins within
cells (11). By inhibiting
proteasome activity, these inhibitors disrupt cellular protein
homeostasis and trigger stress responses, which results in
programmed cell death (apoptosis) in some cases (12). MG132 is a potent peptide
aldehyde-based inhibitor that primarily blocks the
chymotrypsin-like activity of proteasomes, thereby resulting in
accumulation of ubiquitinated proteins (13,14).
Hence, MG132 is widely used in experimental settings to study
proteasome function, apoptosis induction, and stress response
pathways (15,16). Furthermore, MG132 is utilized to
investigate signaling mechanisms, such as nuclear factor kappa B
(NF-κB) pathway inhibition, which is crucial in inflammation and
immune responses (17,18).
Clinically, proteasome inhibitors, including MG132,
have shown notable therapeutic potential, particularly in cancer
treatment (19,20). Moreover, MG132 serves as a
foundational compound for developing Food and Drug Administration
(FDA)-approved drugs, such as bortezomib and carfilzomib, which are
used to treat multiple myeloma and mantle cell lymphoma (21). However, in patients with metastatic
sarcomas, including leiomyosarcoma, single-agent bortezomib showed
minimal efficacy and led to early study termination after the first
stage of patient accrual (22). In
addition to oncology, MG132 has been used in preclinical studies to
explore its role in neurodegenerative diseases, such as Alzheimer's
and Parkinson's disease, where protein aggregation is a hallmark
(23,24). Furthermore, its anti-inflammatory
properties and ability to modulate oxidative stress suggest its
potential application in the treatment of inflammatory,
cardiovascular, and metabolic disorders (25–27).
Accordingly, continued research on MG132 and its related inhibitors
may uncover new therapeutic avenues and deepen our understanding of
proteasome biology.
However, the effect of MG132 on the growth of Ut-LMS
remains poorly understood. Therefore, this study aimed to
investigate the effects of MG132 on Ut-LMS cells by focusing on the
relevant molecular mechanisms and cellular responses. By
elucidating the underlying processes, this study will provide
valuable insights that will contribute to the development of more
effective therapeutic strategies for Ut-LMS. This will ultimately
enhance patient outcomes and offer renewed hope to individuals
affected by this challenging malignancy.
Materials and methods
Cell lines and reagents
Human Ut-LMS cancer cell lines (SK-LMS-1, SK-UT-1,
and SK-UT-1B) were obtained from the American Type Culture
Collection (ATCC, Manassas, VA, USA). These cells were cultured in
Minimal Essential Medium (LM007-07; Gyeongsan, South Korea,
Welgene) supplemented with 10% fetal bovine serum (SH30919.03;
Hyclone, Logan, UT, USA) and 1% streptomycin-penicillin (15140122;
Gibco, Waltham, MA, USA) and were maintained in a humidified
incubator at 37°C and 5% CO2. MG132 was obtained from
Selleckchem (S2619; Houston, TX, USA), and dimethyl sulfoxide
(DMSO; LPS Solution) was used as the control. N-acetyl-L-cysteine
(NAC; A7250) and catalase (CAT; C1345) were purchased from
Sigma-Aldrich (St. Louis, MI, USA).
The 2,5-diphenyl-2H-tetrazolium
bromide (MTT) assay
Cells were seeded in 96-well plates at a density of
5,000 cells/well and allowed to adhere overnight. After treatment
with various concentrations of MG132 for 24 h, 20 µl of MTT
solution (5 mg/ml, M2128; Sigma-Aldrich) was added to each well and
incubated for 2 h at 37°C. The resulting formazan crystals were
dissolved by adding 150 µl of DMSO to each well, and the absorbance
was measured at 570 nm using an INNO microplate spectrophotometer
(LTek, Seongnam, South Korea). Cell viability was calculated as a
percentage of the untreated control group.
Lactate dehydrogenase (LDH) release
assay
The cells were cultured in 96-well plates and
treated with MG132 for 24 h. After treatment, LDH release was
measured using a Dyne LDH Plus Cytotoxicity Assay Kit (GBL-P500;
Dyne Bio, Seongnam, South Korea) following the manufacturer's
instructions. LDH PLUS Reaction Mixture (100 µl) was added to each
well, and the plate was gently mixed. Thereafter, the reaction was
allowed to proceed in the dark for 30 min at room temperature. The
absorbance of the samples was measured at 490 nm using a microplate
reader. Absorbance values were normalized to those of the control
group, thus allowing for comparison between treated and untreated
samples.
Protein preparation and western
blotting analysis
Ut-LMS cells were treated with varying
concentrations of MG132 (0–2 µM) for 24 h. Following treatment, the
cells were lysed using radioimmunoprecipitation assay buffer
(R2002; Biosesang, Yongin, South Korea) supplemented with a
protease inhibitor cocktail (04693132001; Roche, Basel,
Switzerland). Subsequently, equal amounts of protein were denatured
by heating and were resolved using 12–15% sodium dodecyl-sulfate
polyacrylamide gel electrophoresis. The separated proteins were
transferred onto polyvinylidene fluoride membranes (IPVH00010;
Merck Millipore, Burlington, MA, USA) and blocked with 5% skim milk
(262100; BD Difco, Franklin Lakes, NJ, USA) for 1 h at room
temperature. The membranes were then incubated overnight at 4°C
with gentle shaking using primary antibodies. The following primary
antibodies were used: caspase-3 (9665S), cleaved caspase-3 (9664S),
caspase-9 (9502S), poly-adenosine diphosphate ribose polymerase
(PARP; 9542S), p21 (2947S), p27 (3686T), p53 (2527S), light chain 3
(LC3) beta (2775S), β-actin (4967S; Cell Signaling Technology,
Danvers, MA, USA), and ubiquitin (BML-PW0930; Enzo Biochem Inc.,
Farmingdale, NY, USA). After washing with Tris-buffered saline with
0.1% Tween® 20 detergent, membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (7074S or
7076S; Cell Signaling Technology) for 1 h at room temperature.
Protein signals were detected using the Western Pico ECL Kit
(PICO-250; LPS Solution) and visualized using an Azure c280
chemiluminescent imaging system. The intensity of the protein bands
was quantified using ImageJ version 1.53a software to analyze
relative protein expression levels.
Apoptotic profile analysis
The apoptotic profile of Ut-LMS cells was assessed
using the Muse Annexin V & Dead Cell Kit (MCH100105; Luminex,
Austin, TX, USA) according to the manufacturer's instructions.
Ut-LMS cells were seeded in six-well plates at a density of 5×10
cells/well and treated with various concentrations of MG132 for 24
h. After treatment, the cells were detached using trypsin,
harvested by centrifugation, and resuspended in 100 µl of fresh
medium. Subsequently, 100 µl of Muse Annexin V & Dead Cell
reagent was added, and the mixture was incubated for 20 min at room
temperature in the dark. The stained cells were then analyzed using
a Guava Muse Cell Analyzer to evaluate apoptotic and dead cell
populations.
Flow cytometric evaluation of cell
cycle distribution
Cell cycle distribution was analyzed using the Muse
Cell Cycle Kit (MCH100106; Luminex) following the manufacturer's
protocol. Cells were harvested, centrifuged to form a pellet, and
fixed in 70% ethanol at −20°C for 3 h. After fixation, the cells
were stained with the Muse Cell Cycle Reagent and incubated in the
dark for 30 min. Thereafter, the stained samples were analyzed
using a Guava Muse Cell Analyzer to evaluate the distribution of
cells across different cell cycle phases.
Assessment of autophagy using flow
cytometry
Autophagy induction by MG132 was assessed using the
Muse Autophagy LC3-Antibody Based Kit (MCH200109; Luminex) and Muse
Cell Analyzer following the manufacturer's instructions. Both
treated and untreated cells were cultured, detached, and incubated
with anti-LC3 Alexa Fluor 555 and 1X Autophagy Reagent for 30 min
in the dark. After incubation, the cells were resuspended in 1X
assay buffer and analyzed using a Muse Cell Analyzer. The level of
autophagy was then quantified by calculating the ratio of
fluorescence intensity in the treated samples relative to the
controls.
ROS quantification using flow
cytometry
ROS production was quantified using flow cytometry
with the Muse Oxidative Stress Kit (cat. no. MCH100111; Luminex)
according to manufacturer's instructions. Cells were seeded at
5×104 cells/well in six-well plates and cultured at 37°C
for 24 h. The cells were then treated with 0, 0.25, 0.5, or 1 µM
MG132, diluted in fresh medium, and incubated for 24 h. After
treatment, the cells were detached, resuspended in 1X Assay Buffer,
and incubated with the Muse Oxidative Stress Reagent working
solution for 30 min at 37°C. Samples were then analyzed using a
Guava Muse Cell Analyzer (cat. no. 0500-3115; Luminex), and the
data were processed using Muse analysis software (version 1.5;
Luminex).
Statistical analyses
Statistical analyses were performed using GraphPad
Prism software (version 8.0). Data are expressed as the mean ±
standard deviation. One-way analysis of variance was used to
compare multiple groups, followed by Tukey's post hoc test or
Dunnett's multiple comparison test, as appropriate. Statistical
significance was set at *P<0.05 and **P<0.01.
Results
Assessment of MG132 cytotoxicity in
Ut-LMS cell lines
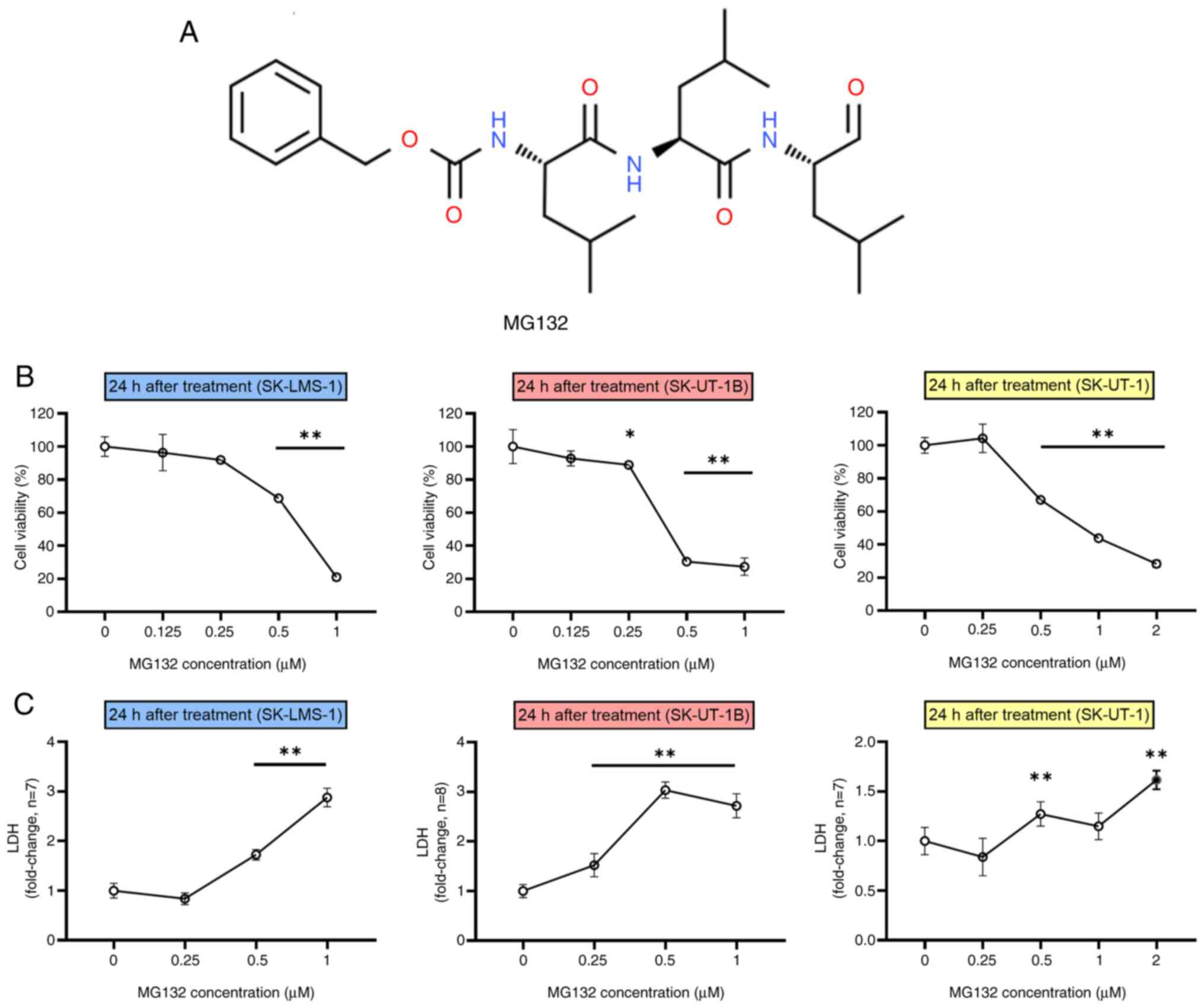
To evaluate the cytotoxic effects of MG132 (Fig. 1A), Ut-LMS cell lines (SK-LMS-1,
SK-UT-1B, and SK-UT-1) were treated with varying concentrations
(0–1 or 2 µM) of MG132 for 24 h. Cytotoxicity was then evaluated
using the MTT and LDH release assays. As shown in Fig. 1B, all three Ut-LMS cell lines
exhibited a dose-dependent decrease in viability as MG132
concentrations increased. This indicated that MG132 effectively
reduced cell viability in a concentration-dependent manner.
Additionally, LDH release assays were conducted on Ut-LMS cells
treated with MG132 for 24 h, which confirmed enhanced membrane
damage. As the concentration of MG132 increased, all three cell
lines showed a considerable increase in LDH release (Fig. 1C). Moreover, a relative increase in
ubiquitinated proteins was observed in Ut-LMS cells treated with
0.5 and 1 µM MG132 for 24 h compared with those in control cells
(Fig. S1). This indicated that
Ut-LMS cell lines experienced reduced cell viability and membrane
damage. These findings demonstrate that MG132 induces cytotoxicity
in Ut-LMS cells, rendering it a promising therapeutic agent for
uterine smooth muscle tumors.
Assessment of apoptosis induction by
MG132 in Ut-LMS cell lines
To investigate the pro-apoptotic effects of MG132 in
Ut-LMS cells, apoptotic markers were examined using Annexin V and
7-AAD staining via flow cytometry after treating SK-LMS-1,
SK-UT-1B, and SK-UT-1 cell lines with MG132 (0, 0.25, 0.5, or 1 µM)
for 24 h. Apoptosis markedly increased in a dose-dependent manner
upon MG132 treatment in all three cell lines (Fig. 2A). To further confirm apoptosis
induction, western blot analysis was performed to evaluate the
expression of apoptosis-related proteins including PARP, caspase-3,
and caspase-9. The concentrations of MG132 (0.5 and 1 µM) were
carefully selected based on the cell viability data. Following
MG132 treatment, increased cleaved PARP and cleaved caspase-3
levels were observed in all three cell lines, whereas no changes in
cleaved caspase-9 levels were detected in any of the cell lines
(Fig. 2B). These results confirmed
that MG132 induced apoptosis via activation of apoptosis-related
markers in all three Ut-LMS cell lines.
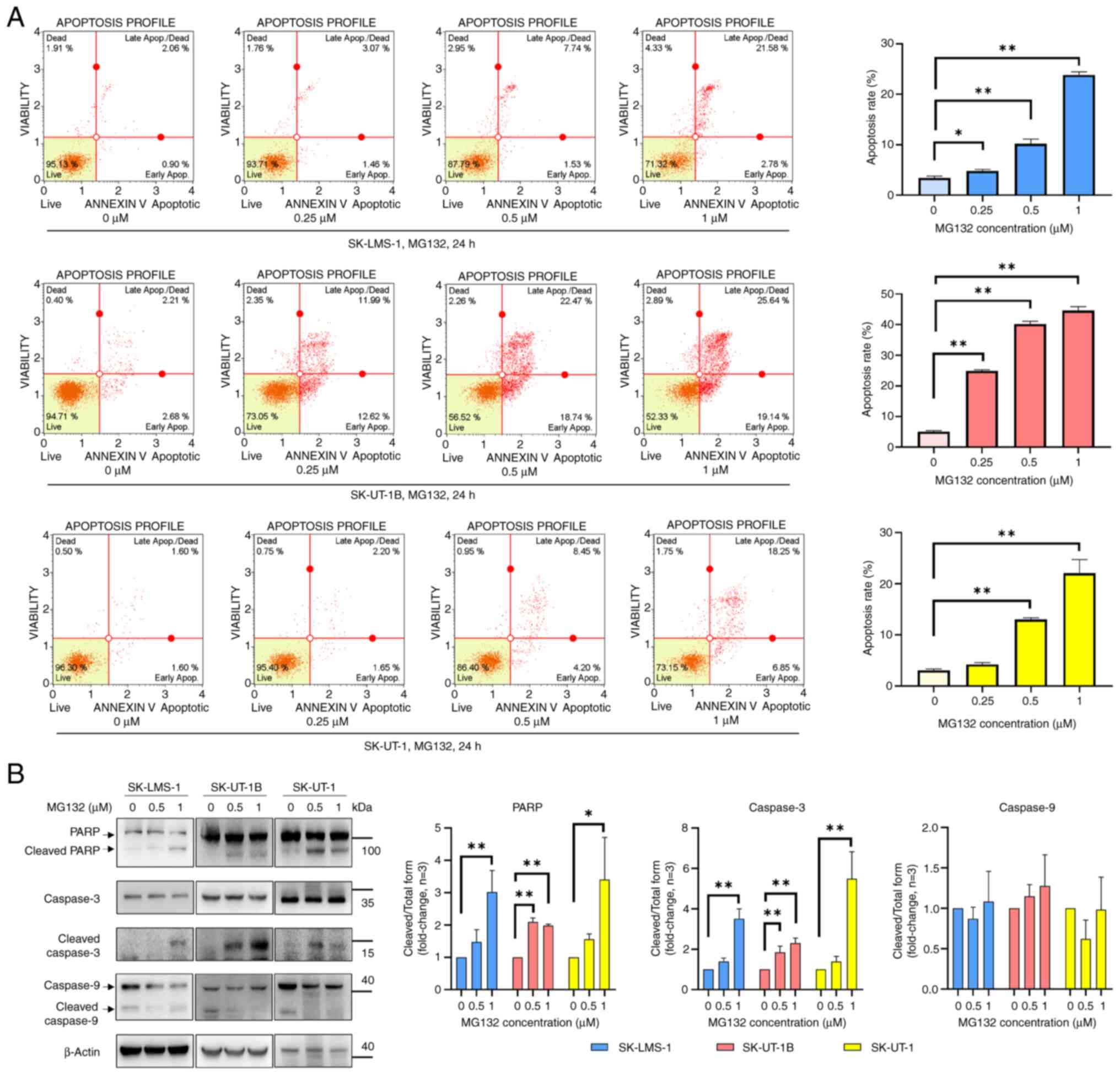 | Figure 2.MG132 induces apoptosis and cell
death in Ut-LMS cells. (A) Annexin V and 7-AAD analysis of
apoptosis and cell death in MG132-treated SK-LMS-1 (upper panels),
SK-UT-1B (middle panels) and SK-UT-1 (bottom panels) cells. Cells
were treated with MG132 (0.25, 0.5 and 1 µM) for 24 h, followed by
flow cytometric analysis to determine the percentage of cells in
the live, early apoptosis, late apoptosis and dead cell phases.
Quantitative data are presented in the right panel. (B) Western
blot analysis of apoptosis-related proteins, including PARP,
cleaved caspase-3 and caspase-9, in MG132-treated Ut-LMS cell
lines. Cells were treated with MG132 (0.5 and 1 µM) for 24 h, and
protein expression levels were assessed using specific antibodies.
Band intensities are shown in the right panel, with β-actin serving
as a loading control. Representative results from three independent
experiments are shown. Data are expressed as means ± SD. *P<0.05
and **P<0.01. Ut-LMS, uterine leiomyosarcoma; SD, standard
deviation; PARP, poly-adenosine diphosphate ribose polymerase. |
Effects of MG132 on cell cycle
progression and regulatory proteins in Ut-LMS cells
To investigate the impact of MG132 on cell cycle
progression in Ut-LMS cells, cell cycle distribution was analyzed
using propidium iodide staining, followed by flow cytometry after
treating the cells with varying concentrations of MG132 for 24 h.
As shown in Fig. 3A, MG132
treatment induced a G2/M phase arrest at concentrations of 0.5 and
1 µM in SK-LMS-1 and SK-UT-1 cells, whereas no significant changes
in cell cycle distribution were observed in SK-UT-1B cells. Western
blot analysis was performed to evaluate the expression of cell
cycle regulatory proteins, including p21, p27, and p53, in Ut-LMS
cells (Fig. 3B). The p21
expression was markedly upregulated by MG132 in all three cell
lines. In contrast, p27 levels pronouncedly decreased in all three
cell lines following MG132 treatment. However, a differential
response was observed in p53 expression; its expression decreased
in SK-LMS-1 cells, increased in SK-UT-1B cells, and showed no
significant changes in SK-UT-1 cells. These findings suggest that
MG132 differentially regulates cell cycle progression and
expression of cell cycle-related proteins in Ut-LMS cell lines,
thereby highlighting the distinct cellular responses to
treatment.
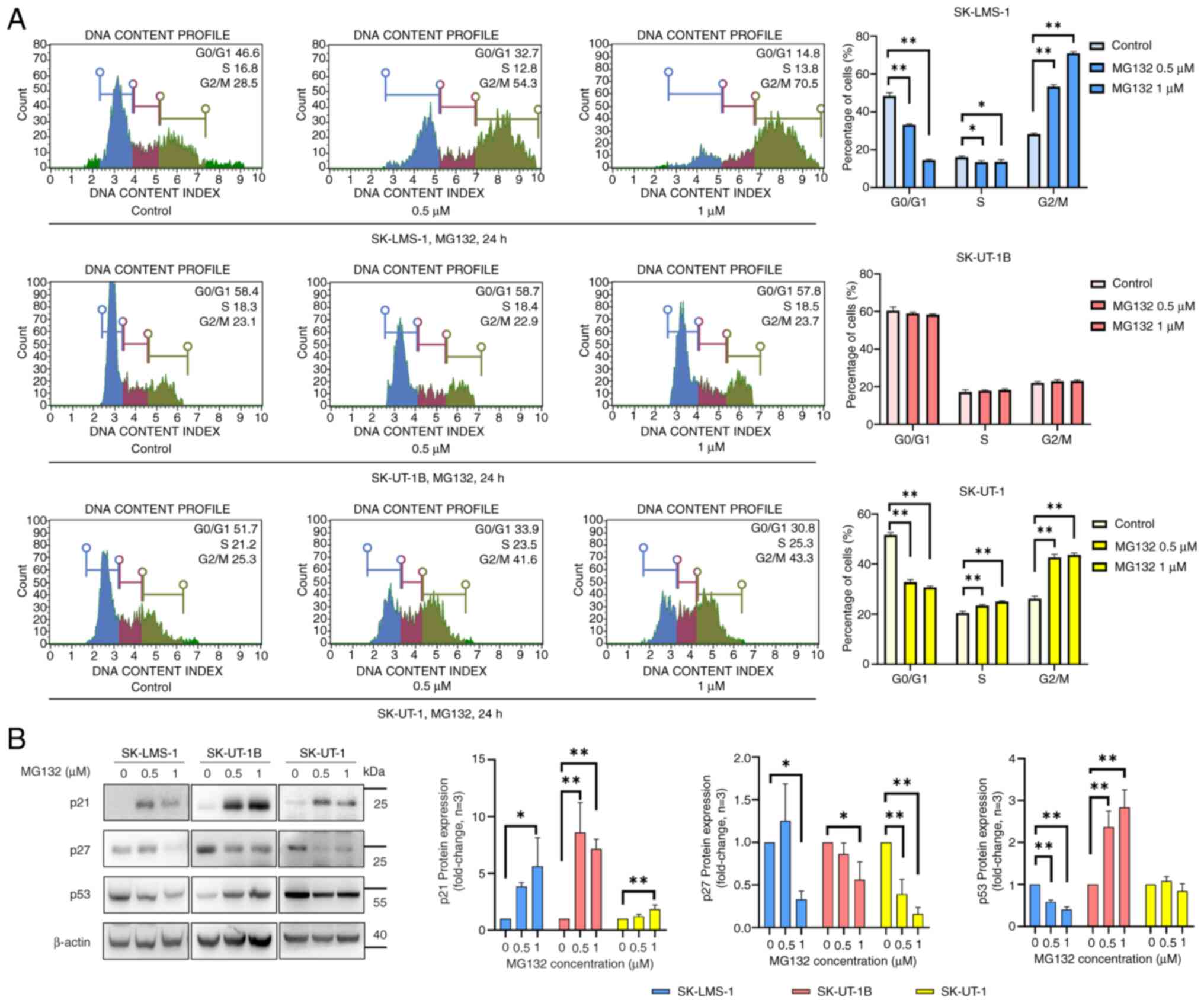 | Figure 3.MG132 regulates cell cycle arrest and
modulates the expression of cell cycle regulatory proteins in
Ut-LMS cells. (A) Cell cycle distribution in SK-LMS-1, SK-UT-1B and
SK-UT-1 cells treated with MG132 (0.5 and 1 µM) for 24 h, analyzed
using PI staining and flow cytometry. The subG1 peak was
removed through gating to focus solely on the pure cell cycle
changes. Quantitative data representing the percentage of cells in
each phase of the cell cycle (G0/G1, S and
G2/M) are shown on the right panel. (B) Western blot
analysis of cell cycle regulatory proteins, including p21, p27 and
p53, in MG132-treated Ut-LMS cell lines. Cells were treated with
MG132 (0.5 and 1 µM) for 24 h, and protein expression levels were
analyzed using specific antibodies. Band intensities are shown in
the right panel, with β-actin serving as a loading control. Data
are presented as means ± SD. *P<0.05 and **P<0.01. Ut-LMS,
uterine leiomyosarcoma; SD, standard deviation; PI, propidium
iodide. |
Effects of MG132 on autophagy in
Ut-LMS cell lines
Autophagy is an essential cellular mechanism that
maintains homeostasis under normal and stressful conditions
(28). To assess the induction of
autophagy, LC3 protein levels, a marker of autophagosome formation,
were evaluated using flow cytometry and western blotting. Following
MG132 treatment at the indicated concentrations (0.5 and 1 µM) for
24 h, flow cytometry analysis revealed an increase in autophagy
induction in SK-LMS-1, SK-UT-1B, and SK-UT-1 cells (Fig. 4A). Consistent with the flow
cytometry results, western blot analysis showed elevated LC3 II
levels in all three cell lines, thereby indicating the conversion
of LC3 I to LC3 II, which is a hallmark of autophagy induction
(Fig. 4B). These findings suggest
that MG132 promotes autophagy in Ut-LMS cells, thus potentially
contributing to its cellular effects.
Effects of MG132 on intracellular ROS
levels in Ut-LMS cells
Excessive ROS accumulation in cells leads to
oxidative stress, which damages nucleic acids, lipids, proteins,
membranes, and mitochondria (29).
To assess whether MG132 increases ROS levels in Ut-LMS cells, cells
were treated with varying concentrations of MG132 (0.5 and 1 µM)
for 24 h, followed by flow cytometry analysis. In SK-LMS-1 cells,
no significant changes in ROS levels were observed after MG132
treatment (Fig. 5A, upper panels).
Based on the results shown in Fig.
2A and these findings, apoptosis induced by MG132 in SK-LMS-1
cells appeared to occur independently of ROS production. However,
ROS levels were increased in SK-UT-1B and SK-UT-1 cells treated
with MG132 at the indicated concentrations for 24 h (Fig. 5A, middle and bottom panels).
Therefore, to investigate whether ROS production contributes to
MG132-induced apoptosis in SK-UT-1B and SK-UT-1 cells, cells were
treated with MG132 (1 µM) with or without the ROS scavengers NAC
and CAT (30,31). In SK-UT-1B cells, NAC and CAT had
no significant effect on MG132-induced apoptosis (Fig. S2A). However, NAC effectively
reduced MG132-induced apoptosis (Fig.
5B), whereas CAT had no significant effect (Fig. S2B) in SK-UT-1 cells. These results
suggest that MG132 induced ROS-mediated apoptosis in SK-UT-1 cells,
whereas MG132-induced apoptosis in SK-LMS-1 and SK-UT-1B cells
occurs in an ROS-independent manner.
Discussion
The findings of this study (Fig. 6) provide valuable insights into the
molecular mechanisms underlying the effects of MG132 on Ut-LMS cell
lines. The study emphasizes the therapeutic potential of MG132 and
the distinct responses observed in different cell lines. Ut-LMS is
a rare and aggressive malignancy of the uterine smooth muscle
cells, characterized by poor prognosis and considerable challenges
in treatment and management. Thus, the study findings contribute to
the growing understanding of this disease and offer new
perspectives on potential therapeutic strategies for
difficult-to-treat malignancies.
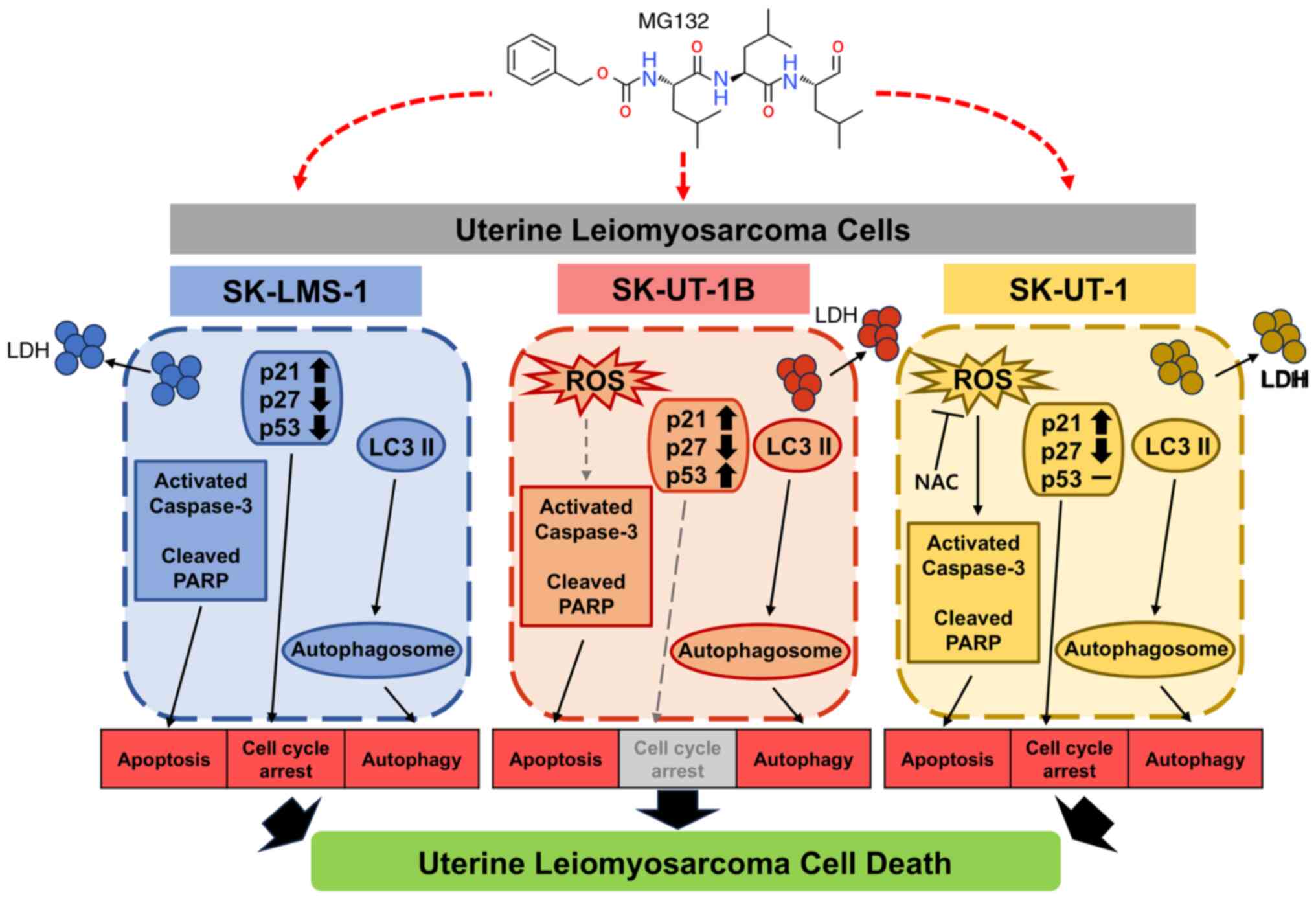 | Figure 6.MG132 exerts its antitumor effects in
Ut-LMS cells by modulating multiple cellular pathways, including
apoptosis, cell cycle regulation, apoptosis and ROS-mediated
effects, in a cell-line-specific manner. LDH, lactate
dehydrogenase; ROS, reactive oxygen species; NAC,
N-acetyl-L-cysteine; Ut-LMS, uterine leiomyosarcoma; PARP,
poly-adenosine diphosphate ribose polymerase; LC3, light chain 3;
NAC, N-acetyl-L-cysteine. |
This study demonstrated that MG132 induces various
forms of cell death in Ut-LMS cell lines. MG132 induced
cytotoxicity in SK-LMS-1, SK-UT-1B, and SK-UT-1 cells, with a
dose-dependent decrease in cell viability and increased LDH release
observed in all three cell lines. Furthermore, MG132 treatment
affected cell cycle progression, resulting in G2/M arrest in
SK-LMS-1 and SK-UT-1 cells. However, no significant changes were
observed in SK-UT-1B cells. In addition, MG132 induced apoptosis,
with ROS-independent apoptosis observed in SK-LMS-1 and SK-UT-1B
cells, and ROS-dependent apoptosis in SK-UT-1 cells. Additionally,
MG132 differentially regulated the expression of cell cycle
proteins (p21, p27, and p53) in each cell line and induced
autophagy, as evidenced by increased LC3 II levels. This suggests
that MG132 simultaneously activates multiple pathways, thereby
disrupting key survival mechanisms.
This study also revealed crucial differences in
protein expression and cellular behavior across these cell lines.
MG132 treatment decreased p53 expression in SK-LMS-1 cells, whereas
it increased p53 expression in SK-UT-1B cells. Furthermore, ROS
analysis demonstrated variability; SK-LMS-1 cells exhibited higher
basal ROS levels, whereas MG132 treatment elevated ROS levels in
SK-UT-1B and SK-UT-1 cells solely. These findings underscore the
molecular heterogeneity of Ut-LMS cell lines and align with
previous reports of variability in cell doubling times and tumor
formation rates among these models (32).
The differences between MG132-treated SK-LMS-1,
SK-UT-1, and SK-UT-1 cell lines in this study could be attributed
to several key factors. A detailed comparison of the cell line
characteristics based on ATCC product sheets supports these
observations. SK-UT-1B and SK-UT-1, which are derived from the
uterus of a 75-year-old female, differ in pathology. SK-UT-1B is
associated with Grade III endometrial leiomyosarcoma (LMS), whereas
SK-UT-1 originates from a mesodermal mixed tumor. In contrast,
SK-LMS-1 cells, which are derived from the vulva of a 43-year-old
female patient with LMS, exhibited fibroblast morphology indicative
of its stromal tissue origin. However, SK-UT-1B and SK-UT-1 display
epithelial morphology consistent with uterine and endometrial
tissues. Therefore, these differences in tissue origin are likely
to contribute to variations in molecular pathways that subsequently
influence drug responses. For example, the expression levels of key
proteins, such as p21 and p27, and ROS levels, play key roles in
the response of each cell line to MG132-induced proteasome
inhibition and stress. Additionally, according to the ATCC product
sheet, SK-UT-1B is a subclone derived from SK-UT-1. Although
SK-UT-1, similar to SK-LMS-1, harbors two independent p53 point
mutations, some reports suggest that SK-UT-1B retains wild-type p53
(33,34). Owing to these conflicting findings,
the p53 status of SK-UT-1B remains unclear, and further sequencing
or validation may be necessary for confirmation. These factors
collectively contributed to the variability in MG132-induced
signaling observed in this study. Therefore, future studies should
investigate these differences in detail to gain a comprehensive
understanding of the diverse mechanisms underlying Ut-LMS
progression and therapeutic responses.
In addition to MG132, research on proteasome
inhibitors has markedly expanded, thereby contributing to
advancements in cancer treatment by targeting the
ubiquitin-proteasome system (19,20).
Proteasome inhibitors, such as bortezomib, carfilzomib, ixazomib,
and marizomib, have demonstrated efficacy in inducing apoptosis and
disrupting critical cellular processes in various malignancies
(20,35). Bortezomib is the first FDA-approved
proteasome inhibitor and is notably effective in treating multiple
myeloma (MM) by inhibiting the NF-κB pathway and stabilizing
pro-apoptotic factors, thereby improving survival rates (36). Despite the associated
cardiovascular risks, carfilzomib, which is a second-generation
inhibitor, provides sustained proteasome inhibition and has shown
enhanced efficacy in combination therapies for relapsed/refractory
MM (37,38). Ixazomib is the first oral
proteasome inhibitor that is convenient for outpatient treatment
and is effective in extending progression-free survival in patients
with MM (39). Furthermore,
marizomib has emerged as a promising candidate because of its
ability to cross the blood-brain barrier, thus showing potential
for treating glioblastoma by inducing oxidative stress and
apoptosis in cancer cells (40).
Research on the mechanisms of action of these proteasome inhibitors
is ongoing to optimize treatment protocols and expand their use to
other cancer types, including Ut-LMS. These efforts highlight the
critical role of proteasome inhibition in contemporary cancer
therapy, with ongoing studies focusing on improving efficacy,
reducing toxicity, and enhancing patient outcomes.
This study had some limitations. Although the cell
death mechanisms and therapeutic potential of MG132 were
investigated, the full extent of its effects across different
Ut-LMS cell lines is not yet clear. In addition, the long-term
toxicity of MG132 and its potential synergistic effects in
combination with other treatments have not yet been explored.
Therefore, further research is needed to address these aspects and
enhance current understanding of the mechanism by which MG132 can
be effectively and safely incorporated into treatment strategies
for Ut-LMS.
In conclusion, this study demonstrates the
anticancer potential of MG132 in Ut-LMS cell lines, thereby
revealing diverse and cell-specific responses. The variability in
the effects of MG132 on these cell lines underscores the molecular
heterogeneity of this aggressive malignancy and emphasizes the
importance of personalized therapeutic approaches. Therefore, the
study findings provide a strong foundation for further research to
elucidate the precise mechanisms underlying MG132-induced cell
death and to explore its synergistic potential in combination
therapies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Basic Science Research Program
through the National Research Foundation of Korea, funded by the
Ministry of Education (grant no. NRF-2022R1l1A1A01053069) and the
Korean Government (Ministry of Science and Information and
Communication Technology) (grant no. RS-2022-00166501).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL and SS designed the experiments and revised the
manuscript accordingly. HL and HJ conducted the experiments and
wrote the manuscript. HJ and SS performed the data analysis. All
authors have read and approved the final manuscript. HL and HJ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Ut-LMS
|
uterine leiomyosarcoma
|
|
LDH
|
lactate dehydrogenase
|
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-acetylcysteine
|
|
CAT
|
catalase
|
|
PARP
|
poly-adenosine diphosphate ribose
polymerase
|
|
NF-κB
|
nuclear factor κB
|
|
FDA
|
Food and Drug Administration
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
2,5-diphenyl-2H-tetrazolium
bromide
|
|
LC3
|
light chain 3
|
|
LMS
|
leiomyosarcoma
|
|
MM
|
multiple myeloma
|
References
|
1
|
Byar KL and Fredericks T: Uterine
leiomyosarcoma. J Adv Pract Oncol. 13:70–76. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahuja A, Agarwal P, Sardana R and Bhaskar
S: Extensively metastasizing leiomyosarcoma: A diagnostic
challenge. J Midlife Health. 8:148–150. 2017.PubMed/NCBI
|
|
3
|
Chapel DB, Sharma A, Lastra RR, Maccio L,
Bragantini E, Zannoni GF, George S, Quade BJ, Parra-Herran C and
Nucci MR: A novel morphology-based risk stratification model for
stage I uterine leiomyosarcoma: An analysis of 203 cases. Mod
Pathol. 35:794–807. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chapel DB, Nucci MR, Quade BJ and
Parra-Herran C: Epithelioid leiomyosarcoma of the uterus: Modern
outcome-based appraisal of diagnostic criteria in a large
institutional series. Am J Surg Pathol. 46:464–475. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menon G, Mangla A and Yadav U:
Leiomyosarcoma. StatPearls [Internet]. StatPearls Publishing;
Treasure Island, FL: 2024
|
|
6
|
Soares Queirós C, Filipe P and Soares de
Almeida L: Cutaneous leiomyosarcoma: A 20-year retrospective study
and review of the literature. An Bras Dermatol. 96:278–283. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bathan AJ, Constantinidou A, Pollack SM
and Jones RL: Diagnosis, prognosis, and management of
leiomyosarcoma: Recognition of anatomic variants. Curr Opin Oncol.
25:384–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giannini A, Golia D'Augè T, Bogani G,
Laganà AS, Chiantera V, Vizza E, Muzii L and Di Donato V: Uterine
sarcomas: A critical review of the literature. Eur J Obstet Gynecol
Reprod Biol. 287:166–170. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giannini A, Cuccu I, D'Auge TG, De Angelis
E, Laganà AS, Chiantera V, Caserta D, Vitale SG, Muzii L, D'Oria O,
et al: The great debate: Surgical outcomes of laparoscopic versus
laparotomic myomectomy. A meta-analysis to critically evaluate
current evidence and look over the horizon. Eur J Obstet Gynecol
Reprod Biol. 297:50–58. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kyriazoglou A, Liontos M,
Ntanasis-Stathopoulos I and Gavriatopoulou M: The systemic
treatment of uterine leiomyosarcomas: A systematic review. No news
is good news? Medicine (Baltimore). 100:e253092021.PubMed/NCBI
|
|
11
|
Bhat SA, Vasi Z, Adhikari R, Gudur A, Ali
A, Jiang L, Ferguson R, Liang D and Kuchay S: Ubiquitin proteasome
system in immune regulation and therapeutics. Curr Opin Pharmacol.
67:1023102022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nunes AT and Annunziata CM: Proteasome
inhibitors: Structure and function. Semin Oncol. 44:377–380. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji MM, Lee JM, Mon H, Xu J, Tatsuke T and
Kusakabe T: Proteasome inhibitor MG132 impairs autophagic flux
through compromising formation of autophagosomes in Bombyx cells.
Biochem Biophys Res Commun. 479:690–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Momose I and Kawada M: The therapeutic
potential of microbial proteasome inhibitors. Int Immunopharmacol.
37:23–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo N and Peng Z: MG132, a proteasome
inhibitor, induces apoptosis in tumor cells. Asia Pac J Clin Oncol.
9:6–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bulangalire N, Claeyssen C, Agbulut O and
Cieniewski-Bernard C: Impact of MG132 induced-proteotoxic stress on
αB-crystallin and desmin phosphorylation and O-GlcNAcylation and
their partition towards cytoskeleton. Biochimie. 226:121–135. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
La Frazia S, Amici C and Santoro MG:
Antiviral activity of proteasome inhibitors in herpes simplex
virus-1 infection: Role of nuclear factor-kappaB. Antivir Ther.
11:995–1004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ortiz-Lazareno PC, Hernandez-Flores G,
Dominguez-Rodriguez JR, Lerma-Diaz JM, Jave-Suarez LF,
Aguilar-Lemarroy A, Gomez-Contreras PC, Scott-Algara D and
Bravo-Cuellar A: MG132 proteasome inhibitor modulates
proinflammatory cytokines production and expression of their
receptors in U937 cells: Involvement of nuclear factor-kappaB and
activator protein-1. Immunology. 124:534–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaplan GS, Torcun CC, Grune T, Ozer NK and
Karademir B: Proteasome inhibitors in cancer therapy: Treatment
regimen and peripheral neuropathy as a side effect. Free Radic Biol
Med. 103:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manasanch EE and Orlowski RZ: Proteasome
inhibitors in cancer therapy. Nat Rev Clin Oncol. 14:417–433. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldberg AL: Development of proteasome
inhibitors as research tools and cancer drugs. J Cell Biol.
199:583–588. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maki RG, Kraft AS, Scheu K, Yamada J,
Wadler S, Antonescu CR, Wright JJ and Schwartz GK: A multicenter
phase II study of bortezomib in recurrent or metastatic sarcomas.
Cancer. 103:1431–1438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steinhilb ML, Turner RS and Gaut JR: The
protease inhibitor, MG132, blocks maturation of the amyloid
precursor protein Swedish mutant preventing cleavage by
beta-secretase. J Biol Chem. 276:4476–4484. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun F, Anantharam V, Zhang D,
Latchoumycandane C, Kanthasamy A and Kanthasamy AG: Proteasome
inhibitor MG-132 induces dopaminergic degeneration in cell culture
and animal models. Neurotoxicology. 27:807–815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma Y, Chen B, Liu D, Yang Y, Xiong Z, Zeng
J and Dong Y: MG132 treatment attenuates cardiac remodeling and
dysfunction following aortic banding in rats via the NF-κB/TGFβ1
pathway. Biochem Pharmacol. 81:1228–1236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng W, Qi W, Mu J, Wei Y, Yang LL, Zhang
Q, Wu Q, Tang JY and Feng B: MG132 protects against renal
dysfunction by regulating Akt-mediated inflammation in diabetic
nephropathy. Sci Rep. 9:20492019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shu Z, Li X, Zhang W, Huyan Z, Cheng D,
Xie S, Cheng H, Wang J and Du B: MG-132 activates sodium
palmitate-induced autophagy in human vascular smooth muscle cells
and inhibits senescence via the PI3K/AKT/mTOR axis. Lipids Health
Dis. 23:2822024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gómez-Virgilio L, Silva-Lucero MD,
Flores-Morelos DS, Gallardo-Nieto J, Lopez-Toledo G,
Abarca-Fernandez AM, Zacapala-Gómez AE, Luna-Muñoz J, Montiel-Sosa
F, Soto-Rojas LO, et al: Autophagy: A key regulator of homeostasis
and disease: An overview of molecular mechanisms and modulators.
Cells. 11:22622022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo C, Sun L, Chen X and Zhang D:
Oxidative stress, mitochondrial damage and neurodegenerative
diseases. Neural Regen Res. 8:2003–2014. 2013.PubMed/NCBI
|
|
30
|
Zhitkovich A: N-acetylcysteine:
Antioxidant, aldehyde scavenger, and more. Chem Res Toxicol.
32:1318–1319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anwar S, Alrumaihi F, Sarwar T, Babiker
AY, Khan AA, Prabhu SV and Rahmani AH: Exploring therapeutic
potential of catalase: Strategies in disease prevention and
management. Biomolecules. 14:6972024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mills J, Matos T, Charytonowicz E, Hricik
T, Castillo-Martin M, Remotti F, Lee FY and Matushansky I:
Characterization and comparison of the properties of sarcoma cell
lines in vitro and in vivo. Hum Cell. 22:85–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smardová J, Pavlová S, Svitáková M,
Grochová D and Ravcuková B: Analysis of p53 status in human cell
lines using a functional assay in yeast: Detection of new non-sense
p53 mutation in codon 124. Oncol Rep. 14:901–907. 2005.PubMed/NCBI
|
|
34
|
Coley HM, Shotton CF, Kokkinos MI and
Thomas H: The effects of the CDK inhibitor seliciclib alone or in
combination with cisplatin in human uterine sarcoma cell lines.
Gynecol Oncol. 105:462–469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Potts BC, Albitar MX, Anderson KC,
Baritaki S, Berkers C, Bonavida B, Chandra J, Chauhan D, Cusack JC
Jr, Fenical W, et al: Marizomib, a proteasome inhibitor for all
seasons: Preclinical profile and a framework for clinical trials.
Curr Cancer Drug Targets. 11:254–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alwahsh M, Farhat J, Talhouni S, Hamadneh
L and Hergenröder R: Bortezomib advanced mechanisms of action in
multiple myeloma, solid and liquid tumors along with its novel
therapeutic applications. EXCLI J. 22:146–168. 2023.PubMed/NCBI
|
|
37
|
Latif A, Kapoor V, Lateef N, Ahsan MJ,
Usman RM, Malik SU, Ahmad N, Rosko N, Rudoni J, William P, et al:
Incidence and management of carfilzomib-induced cardiovascular
toxicity; A systematic review and meta-analysis. Cardiovasc Hematol
Disord Drug Targets. 21:30–45. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhai Y, Ye X, Hu F, Xu J, Guo X, Cao Y,
Lin Z, Zhou X, Guo Z and He J: Cardiovascular toxicity of
carfilzomib: The real-world evidence based on the adverse event
reporting system database of the FDA, the United States. Front
Cardiovasc Med. 8:7354662021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Richardson PG, Zweegman S, O'Donnell EK,
Laubach JP, Raje N, Voorhees P, Ferrari RH, Skacel T, Kumar SK and
Lonial S: Ixazomib for the treatment of multiple myeloma. Expert
Opin Pharmacother. 19:1949–1968. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Di K, Lloyd GK, Abraham V, MacLaren A,
Burrows FJ, Desjardins A, Trikha M and Bota DA: Marizomib activity
as a single agent in malignant gliomas: Ability to cross the
blood-brain barrier. Neuro Oncol. 18:840–848. 2016. View Article : Google Scholar : PubMed/NCBI
|