Introduction
The tumor microenvironment (TME) encompasses a
complex network of tumor cells, surrounding tissue cells,
extracellular matrix (ECM) and various immune cells and cytokines.
The ECM, a 3D structure, plays a pivotal role in maintaining tissue
and organ homeostasis (1). The
non-cellular component of the TME is the extracellular matrix
(ECM), which is a complex 3D structure, a universal scaffold for
maintaining tissue and organ homeostasis and a complex network
composed of fibrils or non-fibrillar collagen, elastin,
proteoglycans, glycoproteins, laminin, fibronectin (FN) and other
matrix proteins (2). FN, a key
component of the ECM, with its extra domain B (FN-EDB), regulates
tumor cell interactions with the ECM, influencing tumor cell
adhesion, migration and proliferation (3). The role of FN-EDB in the TME includes
promoting tumor cell adhesion, aiding in tumor angiogenesis and
modulating the activity of tumor-associated immune cells.
FN-EDB provides an anchor effect for the survival
and spread of tumor cells by promoting the adhesion of tumor cells
and ECM (4); second, FN-EDB
participates in the formation of tumor neovascularization, provides
nutrition and oxygen for tumor and promotes its growth (5); in addition, FN-EDB can also regulate
the activity of tumor-related immune cells and affect the
anti-tumor immune response (6).
FN-EDB has emerged as an important target for in targeted
immunotherapy studies of tumors (7). Overexpression of FN-EDB is closely
related to the malignancy, aggressiveness of tumors and the poor
prognosis of patients (8). By
inhibiting the function of FN-EDB, it is possible to effectively
block the interaction between tumor cells and ECM and inhibit tumor
growth and metastasis. Immunotherapy strategies against FN-EDB,
such as antibody drug conjugates (ADC) and chimeric antigen
receptor T cell therapy (CAR-T), have shown promising application
in clinical trials (9). However,
the study of the FN-EDB remains controversial. On one hand, the
expression and mechanism of FN-EDB in different types of tumors may
differ, which poses a challenge for the targeted therapy of FN-EDB.
On the other hand, the role of FN-EDB in tumor development is not
fully understood, especially when FN-EDB interacts with multiple
integrins and signaling pathways, such as FAK/PI3K/AKT and MAPK/ERK
to promote tumor cell survival and angiogenesis, making it
difficult to design potent inhibitors that specifically target
FN-EDB without affecting normal cell function (10). Thus, this may present potential
difficulties in inhibiting the function of the tumor cells.
Therefore, the present review aimed to summarize the
role of FN-EDB in tumor progression, including its molecular
mechanisms, biological functions, and clinical implications, with a
focus on elucidating potential mechanisms such as tumor
angiogenesis and vascular cell signaling. It also summarized the
latest targeted therapy and diagnostic strategies in the field to
provide necessary guidance for researchers, clinicians and drug
developers to facilitate informed decision making. The present
study could provide important information for future research
directions and treatment strategies that could guide the
development of new diagnostic and treatment modalities in cancer
treatment in the future.
Molecular structural properties and
biological characteristics of FN and FN-EDB
Structural features of the FN-EDB
molecules
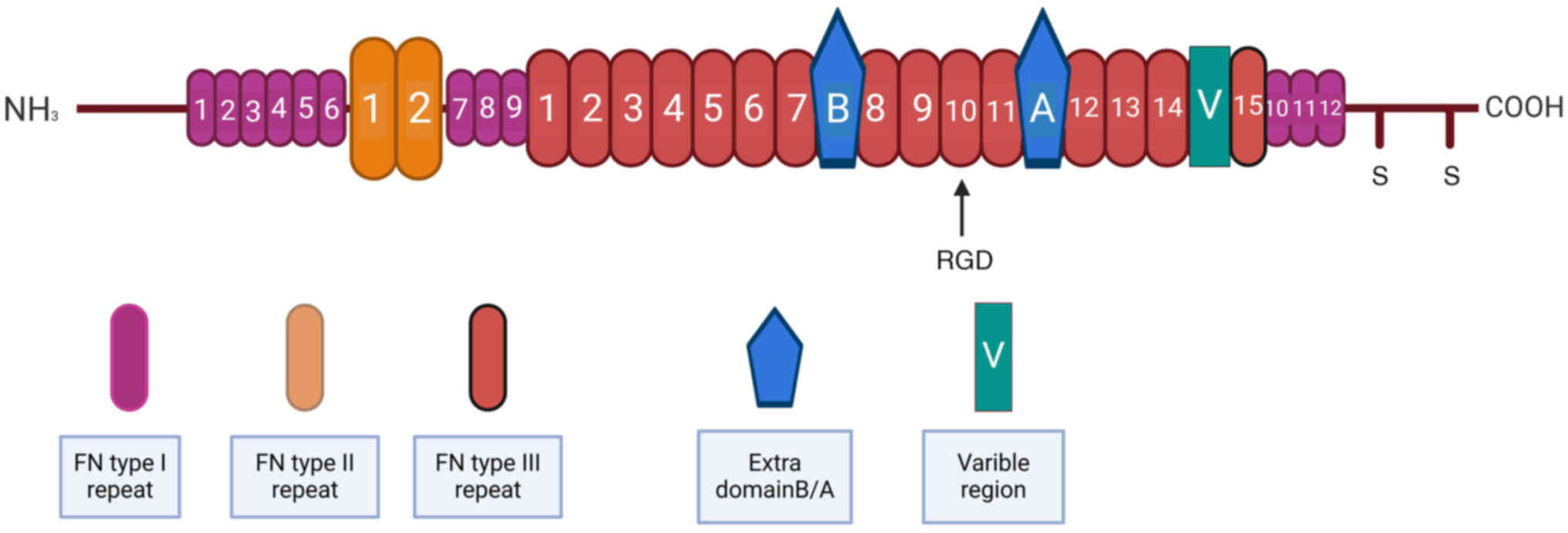
FN is a large glycoprotein found in the ECM. It
plays a key role in a variety of biological processes, including
cell adhesion, migration, proliferation, differentiation and tissue
repair and regeneration of (11).
The versatility of FN is attributed in part to its complex
molecular structure and multiple isoforms generated by alternative
splicing. FN is composed of two similar subunits linked by
disulfide bonds, each containing multiple domains such as the
N-terminal domain, multiple types of repeats (such as type I, II,
III and V repeats) and linker domain and C-terminal domain (EIIIA,
EIIA, EIIIB and EDB) (12). The
type I and II repeats are small and composed of 45 and 60 amino
acids, respectively, which contain key cysteine residues that form
disulfide bonds within the domain (13). These repeats are involved in the
formation of stable domains in FN molecules and are essential for
maintaining the overall structure of FN and function. Unlike type I
and II repeats, type III repeats are large, each consisting of 90
amino acids and containing no cysteine (14). These repeats are organized into two
antiparallel β folds, forming a sandwich conformation with a
hydrophobic core. This structure of the FNIII repeats allows it to
extend when subjected to mechanical strain, thus playing an
important role in cell adhesion and migration (15). In addition to the standard type III
repeat, two additional variant domains exist; EDB and EDA (16). EDB is an alternative splice variant
of FN and its structure and function are particularly important in
tumor biology. The EDB domain consists of about 90 amino acids,
located between the sea urchin-derived domain (EIIIA) and the rod
helical domain of the FN molecule (17). Compared with the full-length FN,
the FN molecules containing EDB show an enhanced cell adhesion and
proliferation activity, especially in tumor cells (Fig. 1).
Biological properties of FN-EDB
FN-EDB is an ECM protein upregulated in the TME,
playing a multifaceted role in cancer progression. It interacts
with integrin receptors αvβ3 and αvβ5, activating downstream
signaling pathways including focal adhesion kinase (FAK), PI3K, AKT
and MAPK/ERK, which enhance tumor cell adhesion and migration
(18). FN-EDB influences cell
cycle progression and gene expression, promoting cell proliferation
and participating in differentiation processes such as skeletal
muscle cells (19). It contributes
to ECM remodeling, maintaining its structure and function through
interaction with cells and matrix proteins (20). FN-EDB also promotes tumor
angiogenesis by upregulating factors like VEGF and matrix
metalloproteinases, providing necessary nutrients and oxygen for
tumor growth (10). It may
modulate the tumor immune microenvironment, potentially affecting
immune checkpoint molecules like PD-L1 and cytotoxic T lymphocyte
antigen 4, thereby facilitating immune evasion (21). FN-EDB can influence the expression
of Cyclin D1 and apoptosis inhibitors like Bcl-2, contributing to
tumor cell proliferation and apoptosis suppression (22). Additionally, it plays a role in the
inflammatory response within the TME, interacting with cytokines
such as TNF-α and IL-6 to promote tumor development (23). Given its involvement in critical
biological processes of cancer cells, FN-EDB serves not only as a
key factor in tumor biology but also as a potential biomarker for
diagnosis and prognostic assessment, making it an attractive target
for therapeutic intervention (24).
FN-EDB is highly expressed in the tumor
tissues
RNA-Seq data from The Cancer Genome Atlas (TCGA) and
Genotype-Tissue Expression projects reveal that FN and its splicing
isoforms, particularly FN-EDB, are significantly overexpressed
across various types of cancer (25). Bioinformatics analyses correlate
FN-EDB expression patterns in tumor tissues with cancer cell
biological behaviors, with FN-EDB levels notably higher in cancer
cell lines than in normal solid tumor cells (26). Further comparative analysis shows
that FN-EDB expression is markedly different from normal tissue in
15 out of 17 types of cancer, especially in head and neck squamous
cell carcinoma and malignant glioma (MG) (8,12).
Experimental validations at the tissue and cellular levels confirm
FN-EDB overexpression in malignant glioma, suggesting its utility
as a diagnostic and prognostic biomarker (27). In oral squamous cell carcinoma,
high FN-EDB expression is associated with increased invasiveness
(28). In breast cancer, EDB-FN
overexpression correlates with poor overall survival and is
upregulated in invasive cell populations with acquired chemotherapy
resistance following long-term TGF-β treatment (29). These findings underscore the
variable FN-EDB expression across types of cancer and its intimate
link with tumor development and progression, warranting further
investigation into its expression patterns and functions in
different types of cancer.
FN-EDB and the TME
The TME includes all non-cancerous host cells in
tumors, including fibroblasts, endothelial cells, adipocytes,
adaptive and innate immune cells including T cells, macrophages and
its noncellular components, including ECM and soluble products such
as chemokines, cytokines, growth factors and extracellular vesicle
(30). Dynamic remodeling of the
ECM is crucial for influencing the TME. Fibronectin components in
ECM play a role in tumor angiogenesis, cell invasion and metastasis
development. In general, the role of FN-EDB in the TME is
multi-dimensional, which not only directly promotes the malignant
behavior of tumor cells, but also indirectly supports the tumor
progression and metastasis of by affecting the tumor angiogenesis,
immune escape and stem cell properties (31).
FN-EDB participates in the regulated
and regulated signaling pathways in the TME
In the TME, FN-EDB as a key ECM protein, through a
regulation of multiple signaling pathways, activated the series of
signaling pathways closely related to tumor cell behavior (Fig. 2), including integrin signaling
pathways, vascular formation signaling pathways, signaling
pathways, immune regulatory signaling pathways, tumor stem cell
signaling pathway, inflammatory signaling pathways and
epithelial-stromal transformation (EMT) signaling pathway promote
the occurrence and development of tumor and provides the
theoretical basis for regulating targeted immunotherapy of tumor
under various pathological conditions.
 | Figure 2.ECM signaling and integrin
activation. There is complex interplay between the ECM and cell
surface integrins, leading to intracellular signaling cascades that
regulate various cellular processes. The central motif is the
activation of integrins by specific ECM components, such as
fibronectin, through the recognition of the RGD sequence by
integrin receptors. Integrin activation; inactive integrins
are depicted as receptors that, upon binding to ECM ligands,
undergo a conformational change to become active integrins. This
activation is crucial for the initiation of downstream signaling.
The RGD motif is highlighted as a key recognition site for integrin
binding, which is essential for mediating cell adhesion and
signaling. Signaling pathways; PKC and FAK are early
signaling molecules that become activated upon integrin engagement
with the ECM. SH2 domain-containing proteins such as Grb2 and Crk,
along with Cas, are involved in the propagation of signals from the
cell membrane to the cytoplasm. SOS, a guanine nucleotide exchange
factor, activates the small GTPase RAS, which in turn activates the
Raf-MEK1/2-ERK1/2 (MAPK/ERK) pathway, leading to cell proliferation
and survival. PI3K generates PIP3, which recruits AKT/PKB and PAK1
to the membrane, promoting cell survival and cytoskeletal
rearrangements. Rac, a small GTPase, activates PAK1 and NF-kB,
contributing to cell migration and inflammation. Cyclin-D1and JNK
are also implicated in cell cycle progression and stress response,
respectively. Cell Functions; Activation of these signaling
pathways ultimately leads to a range of cellular responses,
including cell proliferation, cell survival, cell migration,
angiogenesis and chemotherapy resistance in tumor cells. The figure
also includes a schematic representation of focal adhesion
complexes, with key proteins such as paxillin, DOCK and Pix/GIT,
which are involved in the formation and regulation of these
structures. Figure created with BioRender.com, the software was
developed by Biorender, Inc. ECM, extracellular matrix; RGD,
arginine-glycine-aspartic acid; PKC, Protein Kinase C; FAK, focal
adhesion kinase; SH2, Src Homology 2; SOS, Son of Sevenless. |
Integrin signaling pathway
Molecular characteristics of integrin
Integrins are heterodimeric cell surface adhesion
molecules composed of 18α and 8β subunits, forming 24 distinct
heterodimers with functional and tissue-specific properties. The
high-affinity interaction of integrins with the central cell
binding domain (CCBD) of fibronectin requires the Arg-Gly-Asp (RGD)
sequence within the 10th III-type repeat and a second site within
the adjacent 9th III-type repeat, which synergistically promote
cell adhesion and signaling (18,32).
Three β1 integrins (α4, α5, α8) and three αv integrins (β1, β3, β6)
serve as receptors for fibronectin. A total of genes bind to the
RGD sequence in fibronectin III-10 and one gene, α4β1, recognizes
the Leu-Asp-Val-Pro-like sequence in fibronectin III-CS1 (33).
Despite the non-traditional RGD binding site of
FN-EDB, it interacts with integrins through its unique amino acid
sequence, displaying ligand recognition specificity (34). Conformational changes of integrins
are also crucial for their binding with FN-EDB, as signaling events
induced by cellular activation can lead to integrin conformational
changes, increasing their affinity for FN-EDB. Integrins bind to
various ECM ligands with different affinities, inducing integrin
clustering and significant conformational changes in their external
domains, thereby activating intracellular signaling pathways
(35).
Binding characteristics of different
subtypes of integrins to FN-EDB
The interaction between integrins αvβ3 and αvβ5 with
FN-EDB is a finely regulated process involving multiple layers of
interaction. First, structural complementarity forms the basis of
this interaction, with FN-EDB's specific three-dimensional
structure complementing the binding sites of integrins, promoting
tight molecular interactions (36). Secondly, the αv subunit of
integrins contains specific domains responsible for recognizing
FN-EDB, while the β3 or β5 subunits contribute to the overall
structural stability of the integrin. FN-EDB can also interact with
other integrin subtypes, albeit with differing binding
characteristics (37). α5β1
integrin binds to the RGD sequence in fibronectin and participates
in cell adhesion, while α8β1 integrin primarily interacts with
other domains of fibronectin but can also interact with FN-EDB
(38). αvβ1 integrin has the
ability to bind to various ECM proteins, including FN-EDB (39) and αvβ6 and αvβ8 integrin subtypes,
especially their interactions with epithelial and tumor cells,
suggest their potential binding with FN-EDB under specific cell
types and pathological conditions (40). Additionally, α4β1 (VLA-4) integrin,
primarily interacting with VCAM-1 and the CS-1 region of
fibronectin, may also interact with FN-EDB. α9β1 integrin, although
less studied in its direct interaction with FN-EDB, can also bind
to some domains of fibronectin (41).
The diversity of integrin subtypes and their
interactions with FN-EDB highlight the complexity and importance of
the ECM in cell function and pathology. The dynamic nature of the
ECM, including its composition and physical state, such as rigidity
or disassembly, can also regulate integrin activity and
subsequently influence their interactions with FN-EDB (42). This intricate network of
interactions between FN-EDB and integrins underscores the
multifaceted role of the ECM in cellular behavior and disease
progression.
Transmembrane signaling mode of
integrins
Integrins are a bidirectional signaling molecule
(Fig. 3) and integrins are
transmembrane receptor that play a key role in cell-ECM
interactions. On one hand, they transduce information from the
extracellular environment to modulate cellular responses, a process
often referred to as ‘inside-out’ signaling. On the other hand, the
active state of integrins can also be modulated by intracellular
signaling molecules that, when integrins are bound to their
ligands, can trigger an intracellular signaling pathway, a process
known as ‘outside-in’ signaling (33). Integrins mediate inside-in and
inside-out signaling, required for various cellular processes such
as cell adhesion, migration, survival, proliferation and gene
expression (36).
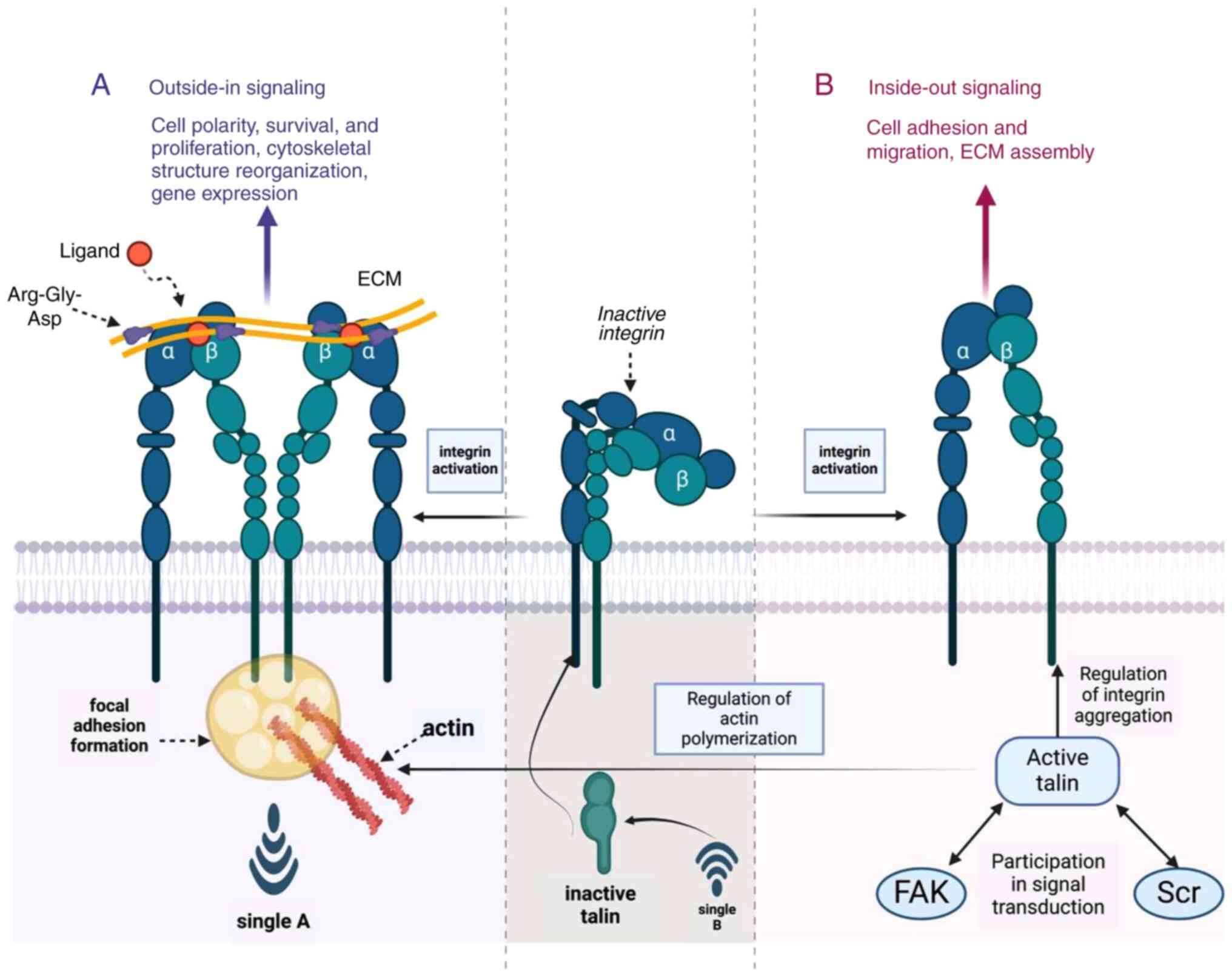 | Figure 3.Signaling mechanisms of integrins in
cell-matrix interactions. The schematic represents integrin's role
in bidirectional signaling, influencing cell adhesion, migration,
survival and proliferation. (A) Outside-in signaling is initiated
by ECM ligands, such as fibronectin, binding to inactive integrins,
triggering conformational changes and activation. Key players
include talin, which facilitates integrin activation and FAK,
crucial for signal transduction and cytoskeletal reorganization.
(B) Inside-out signaling modulates integrin affinity for ECM,
regulated by intracellular signaling and cytoskeletal dynamics.
This bidirectional communication is essential for maintaining cell
polarity and ECM assembly, underpinning cellular homeostasis.
Figure created with BioRender.com (Biorender, Inc. ECM, extracellular
matrix; FAK, focal adhesion kinase; Scr, Src homology 2
domain-containing protein. |
In outside-in signaling inactive integrins are
described as their basal state, waiting for the activation of ECM
ligands, such as fibronectin, which contains the RGD sequence. Upon
ligand binding, integrins undergo a conformational change to become
active integrins, initiating a cascade of intracellular signaling
events. FAK and Src homology 2 (SH2) domain-containing proteins
(Src) are key components of the signaling complex, which assemble
following integrin activation, leading to the recruitment of
various signaling molecules and the subsequent activation of
downstream signaling pathways, including the FAK/PI3K/AKT and
MAPK/ERK pathways (35); for
example, the monomeric form of talin protein, which then regulates
actin polymerization and affects cell morphology and motility.
The inside-out signaling pathway involves the
regulation of intracellular signaling molecules on the activation
of integrins, thereby regulating the affinity of integrins for
their ligands (43). Talin is a
cytoskeletal protein considered a key regulator of integrin
activation and is critical for integrin activation and the
formation of focal adhesions that are essential for the
transmission of mechanical and biochemical signals between the ECM
and the cell interior (44). By
interacting with integrin and its enhanced ability to bind to ECM,
it participates in the transmission of signals, ultimately
realizing the directed movement of cells and the remodeling of
cellular structure (45). In
addition, talin activation in turn affects the actin cytoskeleton
assembly and the formation of focal adhesions. Actin polymerization
is thought to be a critical process downstream of integrin
activation, which contributes to cell polarity, survival, migration
and the assembly of the ECM (46).
FN-EDB promotes cell adhesion to the ECM by binding
to specific integrin isoforms such as α v β 3 and α v β 5. This
adhesion is fundamental to cell stability and is critical for
cellular sensing and response to external signals (47). The interaction of FN-EDB with
integrins activates a series of downstream signaling events that
triggers the activation of integrin signaling pathways, including
cellular signaling cascade in FAK, PI3K, AKT and MAPK/ERK (48).
The reorganization of the cytoskeleton underlies
cell migration and morphological changes; FN-EDB positively
regulates this process through a signaling pathway activated by
integrins. This is extremely critical for the physiological and
pathological processes such as maintaining cell adhesion,
cytoskeletal structure reorganization, gene expression, tumor cell
invasion and metastasis (49).
FN-EDB modulation of classical
integrin signaling pathways
FN-EDB plays a pivotal role in regulating classical
integrin signaling pathways within the TME. This process is
initiated by the binding of integrins to ECM components,
particularly FN-EDB, leading to the activation of FAK at Tyr397,
resulting in its autophosphorylation [phosphorylated (p-)FAK-Y397]
(50). This event marks the
initiation of intracellular signaling events crucial for tumor
progression.
Abnormal activation of FAK signaling can be achieved
through various mechanisms, including integrin-mediated
autophosphorylation and interactions with kinase families such as
Src and Grb2. FAK promotes activation of Ras, which in turn
activates RAF, MEK and ultimately ERK1/2, initiating the ERK/MAPK
signaling pathway (48,51). This phosphorylation ultimately
leads to the translocation of ERK1/2 to the nucleus, where it
regulates the expression of genes involved in cell proliferation,
differentiation and survival. The activation state of ERK1/2 is
closely associated with the invasiveness and metastatic potential
of tumor cells, with its high expression often correlating with
poor prognosis in various tumors (52).
Parallel to the ERK/MAPK pathway, FAK also
participates in the PI3K-Akt pathway, a key regulator of cell
survival, proliferation and metabolism. FAK activation of PI3K
leads to the phosphorylation and activation of Akt, which then
phosphorylates multiple downstream targets, influencing cellular
metabolism and survival signals. The sustained activation of Akt is
also associated with tumor invasiveness and chemoresistance, making
it a significant target for cancer therapy (51–53).
In clinical research, the activation status of FAK,
ERK1/2 and Akt signaling pathways has become an important biomarker
for assessing tumor progression and prognosis. Targeted therapeutic
strategies against these signaling pathways, such as the use of FAK
inhibitors, MEK inhibitors and PI3K/Akt inhibitors, have shown
potential therapeutic value in clinical trials for various types of
cancer (54).
FN-EDB modulation of non-canonical
β1-integrin signaling pathways
FN-EDB not only regulates classical integrin
signaling pathways but also modulates atypical β1-integrin
signaling pathways, which are critical for tumor cell plasticity
and metastatic potential. Unlike the classical FAK-ERK/MAPK or
PI3K-Akt signaling axes, the atypical β1-integrin signaling
mechanism involves integrin-mediated endocytosis, leading to the
internalization of integrin-ECM complexes into endosomes (55). This process allows for the
continued signaling within the cell through the formation of
endosomal vesicles.
In endosomes, activated integrins co-localize with
phosphorylated FAK (p-FAK-Y397), indicating the sustained
transmission of integrin signals within the cell (56). The presence of integrin ligands
like fibronectin and integrin-activating proteins such as talin in
endosomes further confirms the connection between functional
receptor-ligand coupling and active FAK pools associated with
endosomes (57).
In endosomal lumen, integrin signaling continues
through the activation of FAK, which can occur independently of its
typical activation at the plasma membrane. This atypical activation
of FAK in endosomes is associated with the regulation of
cytoskeletal dynamics and cell migration, processes essential for
tumor invasion (58). Key
regulators such as early endosome antigen 1 and Ras-related protein
Rab21 play crucial roles in the endocytosis and signaling of
integrins, potentially promoting the assembly of specific signal
complexes on endosomes (59).
Inhibition of endocytosis, for example through
dynamin inhibitors, significantly reduces the activation of FAK and
ERK1/2, highlighting the central role of endocytosis in
integrin-ECM signaling (60).
Additionally, endocytosis is crucial for maintaining cell
resistance to anoikis, revealing its protective role in cell
survival signaling (61).
Integrin signaling on endosomes may involve
different molecular mechanisms and regulatory proteins than those
at focal adhesions on the cell membrane, such as direct interaction
with integrins and the assembly of unique signaling complexes.
Furthermore, FN-EDB-mediated integrin activation can activate Rho
GTPases signaling pathways, leading to the activation of RhoA and
its downstream effector ROCK, which are crucial for regulating cell
contraction, migration and invasion (62).
Endosomal integrin signaling also intersects with
the JNK signaling pathway, potentially affecting cell stress
responses and apoptosis (63).
Non-classical integrin signaling through FN-EDB can activate NF-κB,
which is associated with inflammation and cell survival regulation,
further highlighting the multifaceted role of FN-EDB in tumor
biology (64).
In clinical research, atypical β1-integrin signaling
mechanisms are related to the development and progression of
various types of tumor. For example, in breast cancer, atypical
β1-integrin signaling enhances tumor invasiveness and metastatic
potential by promoting EMT and angiogenesis (65). In non-small cell lung cancer,
atypical integrin signaling is associated with tumor cell
proliferation, drug resistance and metastasis. Integrin-mediated
signaling can enhance tumor cell invasiveness and promote immune
evasion within the TME (66). In
glioblastoma (67), atypical
β1-integrin signaling is linked to the maintenance of tumor stem
cell properties, which may contribute to tumor recurrence and
treatment resistance.
In summary, the regulation of both classical and
atypical integrin signaling pathways by FN-EDB underlines its
multifaceted role in tumor progression. Understanding the complex
interactions between FN-EDB, integrins and their downstream
signaling molecules is key for deciphering the intricate regulatory
networks that control tumor angiogenesis, invasion and metastasis.
Future research aimed at dissecting these pathways may reveal new
therapeutic targets for cancer treatment, providing strategies to
disrupt the complex signaling networks regulated by FN-EDB in the
TME.
Angiogenic signaling pathways
FN-EDB is a key regulator of tumor
angiogenesis
FN-EDB is a unique structural module of fibronectin
involved in the pathogenesis of various malignant tumors,
especially due to its ability to regulate the TME. The core of this
action is the interaction of FN-EDB with integrin receptors, such
as αVβ3 found on the surface of angiogenic endothelial cells, known
for their key functions in cell adhesion and signal transduction.
Immunohistochemical and histological analyses provide morphological
evidence of FN-EDB's involvement in tumor angiogenesis, showing the
co-localization of FN-EDB and integrin αvβ3 in tumor tissues and
increased vascular density (68).
After contact with integrins, FN-EDB initiates a series of
intracellular signaling events that ultimately promote
angiogenesis.
Mechanisms of the signaling pathways
for angiogenesis
Integrins in the activation of signaling pathways
that mediate angiogenesis
Binding of FN-EDB to integrins triggers a
phosphorylation event involving FAK, a non-receptor tyrosine kinase
that acts as a node of extracellular signaling and activates a
plethora of downstream signaling molecules. Notably, p-FAK is
involved and phosphorylated with other substrates, including
members of MAPK pathway, such as ERK1/2 and the serine/threonine
kinase Akt (51–53). These kinases contribute to the
transduction of proangiogenic signals, thereby promoting the
proliferation, migration and survival of endothelial cells. FN-EDB
also affects tumor vascularization and maturation by regulating the
DLL4/Notch and Wnt/β-catenin signaling pathways (69). The DLL4/Notch pathway is crucial
for the regulation of vessel size and patterning during
angiogenesis. It influences the balance between Tip and Stalk cells
in the angiogenic sprouts. FN-EDB may promote the polarization of
vascular endothelial cells through DLL4/Notch signaling, a key step
in tip formation and trailing cells of vascular endothelial cells
during angiogenesis (Fig. 4).
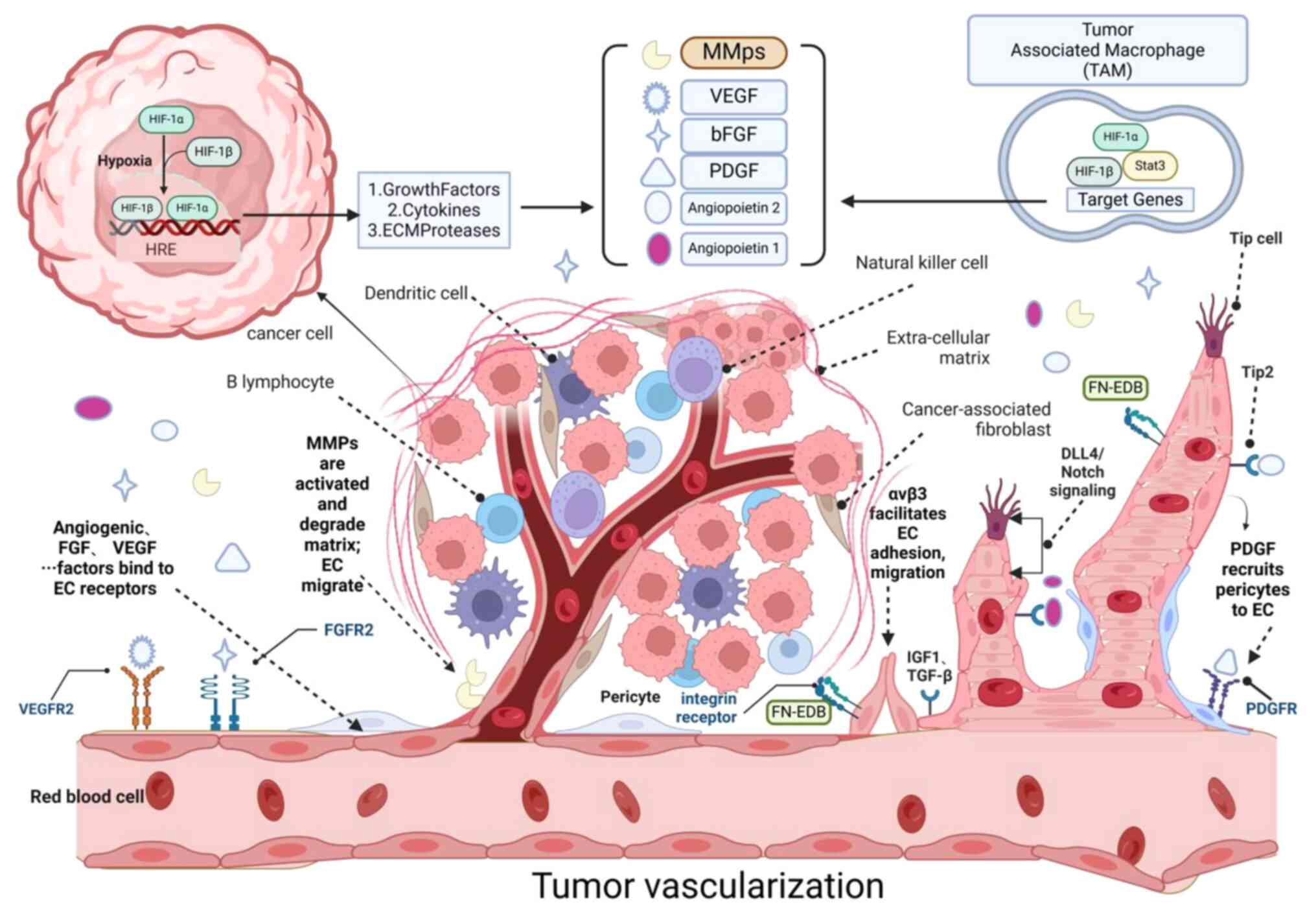 | Figure 4.Molecular mechanisms of tumor.
Hypoxia and HIF-1α; Hypoxia, a condition of low oxygen,
activates HIF-1α, a master regulator of the cellular response to
oxygen deprivation. HIF-1α, in collaboration with HIF-1β, forms the
HIF-1 complex that binds to hypoxia response elements in the
promoter regions of target genes, leading to their transcription.
Angiogenic factors; VEGF and its receptor VEGFR2 are pivotal
in angiogenesis. VEGF signaling promotes endothelial cell
proliferation, migration and survival. Angiopoietin 1 and 2
regulate the maturation and stabilization of newly formed blood
vessels, interacting with Tie-2 receptors on endothelial cells.
ECM and proteases; MMPs facilitate tumor vascularization by
degrading the basement membrane, allowing endothelial cells to
migrate and form new vessels. ECM proteases, such as FN-EDB, are
involved in the proteolytic processing of ECM components, promoting
endothelial cell migration and tube formation. Cytokines and
growth factors; Insulin-like Growth Factor 1 and TGF-β
contribute to the angiogenic process by modulating endothelial cell
function and ECM remodeling. Endothelial cells release PDGF to
recruit pericytes to endothelial cells: This process contributes to
the maturation and stabilization of new blood vessels.
tumor-associated macrophages TAMs; TAMs secrete angiogenic
factors, such as growth factors, cytokines and ECM proteases and
contribute to the degradation of the ECM, thereby promoting tumor
vascularization. Notch signaling; The DLL4/Notch pathway is
crucial for the regulation of vessel size and patterning during
angiogenesis. It influences the balance between Tip and Stalk cells
in angiogenic sprouting. Cell types and functions; EPCs are
mobilized and incorporated into the growing vasculature,
contributing to vessel formation. Tip cells guide the direction of
sprouting vessels, while Stalk cells proliferate to elongate the
vessel. Regulation of tumor vascularization; The interplay
between hypoxia, growth factors, ECM remodeling and cellular
interactions is essential for the regulation of tumor
vascularization, which supports tumor growth, invasion and
resistance to therapy. Figure created with BioRender.com, the
software was developed by Biorender, Inc. HIF-1α, hypoxia-inducible
factor-1 alpha; ECM, extracellular matrix; FN, fibronectin EDB,
extra domain B; PDGF, platelet-derived growth factor; TAMs,
tumor-associated macrophages EPCs, endothelial progenitor
cells. |
Transcriptional regulation in
angiogenesis
The activation of ERK1/2 and Akt not only regulates
immediate cellular responses but also extends to the regulation of
gene expression. Specifically, these kinases promote the nuclear
translocation of transcription factors such as NF-κB and
hypoxia-inducible factor-1alpha (HIF-1α). In the hypoxic
environment within tumor masses, the HIF-1α dimeric protein complex
remains stable and activates the expression of numerous genes
involved in angiogenic processes (70). HIF-1-induced proteins include VEGF
and basic fibroblast growth factor (bFGF), which promotes vascular
permeability, while the latter promotes endothelial cell growth
(71). Other secreted factors such
as platelet-derived growth factor (PDGF), angiopoietin-1 (ANG-1)
and angiopoietin-2 (ANG-2) promote chemotaxis, while hepatocellular
signaling controls migration and cell-cell adhesion, thereby
guiding the formation and stabilization of newly formed blood
vessels (72). Other HIF-1-induced
gene products include MMP, where MMP resolves the ECM, promoting
endothelial cell migration and releasing associated growth factors.
Once activated, these factors bind to the promoter regions of genes
encoding angiogenic factors, including VEGF, to amplify the
angiogenic response (73).
Endothelial cell behavior and
angiogenesis
Endothelial cells and pericytes are fundamental
cellular components of new blood vessels and their interactions
regulate angiogenesis (74).
FN-EDB has been shown to disrupt the interaction between pericytes
and endothelial cells, leading to pericyte detachment and vascular
system instability, resulting in further angiogenesis and
remodeling of the tumor vasculature (75). FN-EDB-induced signaling pathways
enhance endothelial cell proliferation, migration and invasion,
which are crucial for vascular sprout formation (76). Pericytes provide support by
covering the basal surface of endothelial cells and regulate
vascular contraction and relaxation under normal physiological
conditions. Newly formed vessels often lack pericytes, but
endothelial cells recruit these pericytes to provide additional
structural support, enhancing tumor vascularization (77).
FN-EDB promotes formation of tubular structures by
promoting the proliferation and organization of endothelial cells.
It achieves this by activating FAK and subsequent downstream
signaling molecules, including ERK/MAPK and PI3K-Akt pathways that
enhance cell adhesion and cytoskeletal rearrangements, required for
tube morphogenesis (78).
In the TME, in addition to endothelial cells, many
other cell types contribute to angiogenesis. FN-EDB interacts with
tumor-associated macrophages (TAMs) and other immune cells,
promoting ECM remodeling and creating favorable physical and
chemical conditions for angiogenesis (79). Factors secreted into the TME
activate TAMs, producing VEGF and MMP, further promoting
angiogenesis. Neutrophils, a significant component of immune cell
infiltration, promote tumor angiogenesis through various
mechanisms, including the release of MMP into the TME, triggering
the release of VEGF and other angiogenic factors (80). Other immune cell types (such as B
and T cells) secrete VEGF-A, bFGF, MMP9, interferon γ (IFNγ) and
interleukin (IL)-17, indirectly affecting angiogenesis (81). Adipocytes release a variety of
cytokines, chemokines and hormones (collectively referred to as
adipokines), many of which are pro-angiogenic factors (82).
A positive feedback loop in
angiogenesis
FN-EDB expression and activity can be further
enhanced by tumor-secreted cytokines, forming a positive feedback
loop that drives tumor angiogenesis. This positive feedback
mechanism amplifies the impact of FN-EDB on tumor angiogenesis
(83). VEGF and other growth
factors respond to the upregulation of FN-EDB signaling, creating a
feedforward mechanism that strengthens the angiogenic process. This
autocrine and paracrine signaling enhances the recruitment of
endothelial cells, pericytes and smooth muscle cells, promoting the
stability and maturation of newly formed blood vessels (84).
Targeting FN-EDB in tumor diagnosis
In the field of tumor diagnosis, FN-EDB is a key
protein in the TME and its expression in tumor tissues is closely
related to the occurrence and development of multiple types of
cancer (85). Tissue-expression
type FN-EDB is mainly found in tumor stroma and its high expression
is correlated with tumor malignancy and poor prognosis (86). Therefore, examining the expression
level of FN-EDB in tumor tissue can be used as a biomarker for to
assess tumor aggressiveness and predict patient prognosis (87) (Table
I).
 | Table I.Molecular diagnostic applications
targeting FN-EDB. |
Table I.
Molecular diagnostic applications
targeting FN-EDB.
| First author/s,
year | Targeting EDB
molecule | Molecule type | Conjugate | Full name | Detection
method | Tumor
application | (Refs.) |
|---|
| Rossin et
al, 2007 | L19 | Monoclonal
antibody |
76Br |
76Br-L19-SIP | PET | Dermoid tumor | (111) |
| Tijink et
al, 2009 |
|
|
124I |
124I-L19SIP | PET | Brain metastases
from solid cancer | (180) |
| Petrini et
al, 2022 |
|
|
131I |
131I-L19-SIP | PET | TETs | (183) |
| Berndorff et
al, |
|
|
99mTc |
99mTc-AP39 | PET |
Teratocarcinoma | (112) |
| 2006 |
|
|
|
99mTc-L19-His |
|
|
|
|
|
|
|
|
99mTc-L19-Hi20 |
|
|
|
| Ye et al,
2017 | ZD2 | Aptamer
peptide |
99mTc |
99mTc-HYNIC-ZD2 | SPECT | Breast cancer | (98) |
| Qiao et al,
2020 |
|
| Gd | ZD2-N3-Gd
(HP-DO3A) | MRMI | Cancer of
pancreas | (88) |
| Feng et al,
2023 |
|
| GVs | ZD2-GVs | Unique molecular
identifier | Carcinoma of
urinary bladder | (89) |
| Lu et al,
2022 |
|
| N3-Gd | ZD2-N3-Gd
(HP-DO3A) | MRI | Prostatic
carcinoma | (95) |
| Han et al,
2019 |
|
| Gd | ZD2-Gd
(HP-DO3A) | MRI | Prostate
cancer | (94) |
| Sergeeva et
al, 2023 |
|
|
68Ga-NOTA |
ZD2-(68Ga-NOTA) | PET | Hepatocellular
carcinoma | (93) |
| Zhang et al,
2024 |
|
| Cys-TVRTSAD |
ZD2-Gd-DOTA-Cy7 | MRI/fluorescence
molecular imaging EDB | Ductal | (99) |
| Yang et al,
2024 |
|
| Gd-DOTA |
CREKA-Cy7-(Gd-DOTA) | MR/near-infrared
fluorescence | GC | (100) |
| Han et al,
2019 |
|
|
64Cu |
ZD2-64Cu-DOTA | PET | Prostatic
carcinoma | (96) |
| Park et al,
2012 | APTEDB | Nano
antibodies | SPION | APT
(EDB)-SPIONs | MRI | Mammary cancer | (108) |
| Sun et al,
2014 |
|
| SPION |
APTEDB-TCL-SPION | MRI | Mammary cancer | (106) |
| Jailkhani et
al, 2019 |
|
| NJB2 |
64Cu-NJB2 | SPECT | Ductal
adenocarcinoma of pancreas | (109) |
| Ranjbar et
al, 2020 |
|
|
99mTc |
99mTc-APTEDB | Radio-isotope
thin-layer chromatography scanner | Brain cancer and
colorectal cancer | (102) |
| Li et al,
2023 | EDBp | Aptamer
peptide |
18F/177Lu |
18F-NOTA-PEG4-EDBp
177Lu- DOTA-P EG4-EDBp | PET | TC | (179) |
Targeting of the FN-EDB aptamer
polypeptide Thr-Val-Arg-Thr-Ser-Ala-Asp (ZD2)
ZD2, a small peptide aptamer, selectively targets
the TME-specific fibronectin extra domain B (FN-EDB), a protein
overexpressed in various types of cancer. Its tumor-targeting
capability positions ZD2 as a promising agent for diagnostic
imaging and therapeutic applications (88,89).
Recent advances include the development of a Tc-99m-labeled aptamer
(EDB) as an effective tumor imaging agent, demonstrating high
specificity for EDB-positive cell lines in vitro and
favorable pharmacokinetics in vivo, with rapid blood
clearance and renal excretion. The potential of this agent to
reflect tumor metastasis status offers a valuable tool for early
cancer diagnosis and therapeutic response monitoring (90,91).
FN-EDB, a splice variant of fibronectin, has emerged
as a promising target for the design of clinically translatable
magnetic resonance imaging (MRI) and positron emission tomography
(PET) contrast agents. A study investigated the MRI dose-response
effect of ZD2-N3-Gd (HP-DO3A) (MT218), a targeted contrast agent,
in a rat prostate cancer xenograft model and its detection
potential in mouse lung and pancreatic cancer models (92). MT218 exhibited high relaxivities
(r1 and r2), favorable physicochemical properties, pharmacokinetics
and safety, leading to effective tumor enhancement at reduced
contrast agent doses. In the realm of PET imaging, a
ZD2-(68Ga-NOTA) conjugate was developed for
hepatocellular carcinoma (HCC) imaging, demonstrating high affinity
for FN-EDB in a marmoset model, rapid tumor accumulation and a
stable state within 5 min post-injection (93). Additionally, a 64Cu-DOTA
conjugated to the ZD2 peptide was created for sensitive detection
and risk stratification of prostate cancer (94). These findings validate the
feasibility of using ZD2 peptide-based radiopharmaceuticals for PET
imaging, offering new perspectives for clinical management of
patients with HCC and prostate cancer.
ZD2-Gd (HP-DO3A), a molecular MRI contrast agent
targeting FN-EDB, has demonstrated significant contrast enhancement
in primary and metastatic triple-negative breast cancer tumors,
outperforming the nonspecific clinical agent Gd (HP-DO3A) (95–97).
The high expression of this agent in the tumor ECM positions it as
a promising tool for precise cancer diagnosis. Furthermore,
Cy5-EDBp, (18F)-EDBp and (177Lu)-EDBp have
emerged as potential candidates for surgical navigation,
radionuclide imaging and targeted radionuclide therapy,
respectively (98).
The development of novel targeted contrast agents,
such as the EDB-FN-targeted imaging probe (ZD2-Gd-DOTA-Cy7), has
shown good performance in dual-modality MRI and near-infrared
fluorescence imaging for pancreatic cancer, as well as in the
precise assessment of chemotherapy efficacy (99). This probe requires only half the
dose of conventional clinical gadolinium contrast agents to achieve
accurate cancer imaging and evaluation of therapeutic responses. By
conjugating ZD2 with commonly used clinical MRI gadolinium contrast
agents (Gd-DOTA) and the near-infrared fluorescence dye Cy7,
researchers have created an EDB-FN-targeted imaging probe. This
probe, combined with dual-modality imaging techniques, has been
used to assess the efficacy of chemotherapy regimens, offering a
new approach for personalized treatment of pancreatic cancer
patients (100).
In summary, ZD2 peptide and its derived targeted
probes have shown considerable potential and application prospects
in the field of tumor diagnosis and therapy targeting FN-EDB. With
continuing technological advances and clinical trials, ZD2 peptide
and its associated targeted probes are expected to play an
increasingly vital role in future cancer therapy.
Targeting peptide, APTEDB
APTEDB Peptide, a molecule designed based on
structural features of FN-EDB, ensures high affinity and selective
binding to the TME by recognition of key amino acid residues
(101). Ranjbar et al
(102) achieved specific binding
and high tumor uptake in colorectal cancer models by Tc-APTEDB
probe, demonstrating its potential as a diagnostic imaging probe.
The high binding specificity of the APTEDB peptide, which
selectively targets the fibronectin extra domain B (FN-EDB)
overexpressed in the tumor microenvironment, enables the
development of novel tumor imaging modalities. For example, APTEDB
has been conjugated to imaging agents, such as fluorescent dyes,
quantum dots, and radionuclides, to create targeted probes that
enhance the detection and visualization of tumors with high
contrast and resolution (103).
These targeted imaging modalities have shown promise in preclinical
models, providing a non-invasive approach to cancer detection and
monitoring. In the field of optical imaging, the coupling of APTEDB
with fluorescent labels or quantum dots enables the visualization
of tumor regions with high contrast and resolution (104). In PET and MRI, the coupling of
APTEDB to a radionuclide or contrast agent, respectively, enhances
the detection of tumors in preclinical models (105). Using FN-EDB-specific peptide [APT
(EDB)] Sun et al (106)
conjugated thermal cross crosslinked superparamagnetic iron oxide
nanoparticles [APT (EDB)-TCL-SPIONs] in a breast cancer
stem-cell-like cell xenograft mouse model. The results of these
studies confirm the broad application of APTEDB peptides in
tumor-targeted therapy and imaging (107).
Other diagnostic methods for targeting
the FN-EDB
Recent advances in nanotechnology have facilitated
the development of FN-EDB-targeting nanoparticles for enhanced
diagnostic and therapeutic capabilities in oncology (108). Surface modification of these
nanoparticles has been instrumental in augmenting their
accumulation and internalization within the TME. Jailkhani et
al (109) identified a
high-affinity nanobody, alpaca-derived libraries of nanobodies
(NJB2), which selectively bind to FN-EDB expressed in the ECM of
tumors and metastases, offering a non-invasive diagnostic tool for
cancer detection and a targeted approach to fibrotic diseases.
Using immunoPET/CT, 64Cu-NJB2 nanobodies demonstrate the
ability to detect primary and metastatic tumors across various
cancer models, highlighting their potential as robust tools for
oncological imaging (110). In
parallel, Rossin et al (111) successfully radiolabeled an
anti-ED-B fibronectin human antibody derivative, L19-SIP, using an
enzymatic radiobromination method. In vivo studies in animal
models revealed rapid and specific tumor targeting by (76) Br-L19-SIP, with subsequent
validation of its potential in tumor imaging through small animal
PET. Berndorff et al (112) developed 99 mtc-tagged L19
derivatives to target ED-B fibronectin for the angiogenesis imaging
in tumors. Compounds 99 mTc-AP39,99 mTc-L19-His and 99
mTc-L19-Hi20, were synthesized and evaluated in vivo.
99mTc-AP39 shows high tumor uptake, rapid blood clearance and
excellent imaging properties, providing a promising tool for
visualizing tumor-expressing ED-B.
These studies underline the value of targeted
molecular imaging with radiolabeled antibodies in enhancing the
detection and monitoring of cancer, paving the way for more precise
and personalized therapeutic strategies.
Application of targeted FN-EDB in tumor
immunotherapy
Targeting FN-EDB, a tumor-specific marker, has
emerged as a promising strategy in cancer immunotherapy. This
approach exploits the unique expression pattern of FN-EDB in the
TME to enhance the selective delivery of therapeutic agents,
thereby improving treatment efficacy and minimizing damage to
healthy tissues.
The rationale behind FN-EDB-targeted immunotherapy
is to direct cancer treatments specifically to tumor cells, sparing
healthy cells that do not express the target. This strategy
encompasses a range of therapeutic modalities (Table II), including small molecule
inhibitors, monoclonal antibodies (mAbs), antibody-drug conjugates
(ADCs) and gene therapy, all designed to exploit molecular
characteristics or cell surface proteins associated with cancer
cells (113).
 | Table II.Tumor immunotherapy strategies
targeting FN-EDB. |
Table II.
Tumor immunotherapy strategies
targeting FN-EDB.
| First author/s,
year | Treatment | Antibody | Conjugate | Name | Combination | Tumor therapy | Research
progress | Study
identifier | (Refs.) |
|---|
| Danielli et
al, 2015 | Antibody
therapy | L19 | IL2 | L19-IL-2 +
L19-TNF-α | L19-TNF | GMs | Clinical Phase
I/II | NCT02076633 | (137) |
| Saif et al,
2022 |
| L19 | IL2 | L19IL2 +
L19TNF | L19-TNF | Cutaneous
melanoma | Clinical Phase
IIIB/IIIC | NCT03567889 | (141) |
| Spitaleri et
al, 2013 |
| L19 |
| L19+TNF | TNF | Colorectal
cancer | Clinical Phase
I/II | NCT01253837 | (139) |
| Yu et al,
2022 |
| Anticotinine
antibody | VEGF | HyPEP (EDB-VEGF) +
PD-1 | PD-1 | Metastatic melanoma
pancreatic cancer | Animal
experiment | - | (147) |
| Johannsen et
al, 2010 |
| L19 | IL2 | L19-IL2 |
| Renal cell
carcinoma | Clinical phase
I/II | NCT01058538 | (118) |
| Kim et al,
2019 |
| Abcot | APTEDB | HC
(cot-APTEDB-Stereotactic ablative body radiotherapy/Abcot) | Stereotactic
ablative body radiotherapy/Abcot | MGs | Animal
experiment | - | (149) |
| Van Limbergen et
al, 2021 |
| L19 | IL2 | L19-IL2 +
stereotactic body radiation therapy | Stereotactic body
radiation therapy | NSCLC | Clinical Phase
I/II | NCT02086721 | (123) |
| Schliemann et
al, 2009 |
| L19 | IL2 | L19-IL2 +
rituximab | Rituximab | B cell NHL | Clinical phase
II | NCT02957019 | (121) |
| Weide et al,
2019 |
| L19 | IL2 | L19-IL2 +
dacarbazine | Dacarbazine | Stage IV
melanoma | Clinical phase
II | NCT02076646 | (122) |
| Ongaro et
al, 2022 |
| L19-L19 | IL2 |
L19-L19-IL2+PD-1 | PD-1 | solid tumor | Animal
experiment | - | (129) |
| Orecchia et
al, 2019 |
| L19 | IL2 | L19-IL2 +
46F2SIP | Anti-SDC1 46F2SIP
(small immuno protein) antibody | EOC | Animal
experiment | - | (120) |
| Papadia et
al, 2013 |
| L19 | TNF | L19-TNF +
melphalan | Melphalan | extremity
melanoma | Clinical Phase
II | NCT01213732. | (138) |
| Lieverse et
al, 2020 |
| L19 | IL2 | L19-IL2 + SABR | SABR | NSCLC | phase IV | NCT03705403 | (124) |
| Trachsel et
al, 2007 |
| L19 | IL-10 | L19-IL-10 | IL-10 | RA | Animal
experiment | - | (132) |
| Kaspar et
al, 2007 |
| L19 | IL-15/GM-CSF | L19-IL-15
L19-GM-CSF | IL-15/GM-CSF | Teratocarcinoma and
colon carcinoma | Animal
experiment | - | (133) |
| Niu et al,
2023 |
| L12 | IFN-γ + PD-1 | IL-12-IFN-γ +
PD-1 | PD-1 | Solid tumor | Clinical phase
I/II | NCT04370587 | (136) |
| Spaeth et
al, 2006 | Radiation
therapy | L19-SIP |
131I,124I |
131I-L19-SIP | RIT | Colorectal
tumour | Animal
experiment | - | (140) |
| Tijink et
al, 2009 |
| L19-SIP |
131I | 131
I-L19-SIP | RIT squamous cell
carcinoma | Head and neck | Animal
experiment | - | (180) |
| Tang et al,
2023 | Cell Therapy | CAR-T | APT0/CGS2 | APT0 CAR-T CGS2
CAR-T |
| Solid tumor
cells | Cell
experiment | - | (163) |
| Zhang et al,
2022 |
| CAR-T | rTCR | rTCR-CAR-T |
| Solid tumor
cells | Xenograft
models | - | (167) |
| Xiong et al,
2024 |
| CAR-T | CD70 | CD70 CAR-T |
| Renal cell
carcinoma | Xenograft model and
in vivo tests | - | (170) |
| Zhu et al,
2022 |
| CAR-T | CD70 | CD70 CAR-T+
oncolytic herpes simplex virus-1 | Oncolytic herpes
simplex virus-1 | GBM | Animal
experiment | - | (171) |
| Li et al,
2023 |
| CAR-T | PD-L1 | CAR-T +PD-L1 |
| Hematological
malignancies | Cell
experiment | - | (173) |
| Li et al,
2023 |
| CAR-T | Rituximab | CAR-T +
rituximab |
| Relapsed/refractory
B cell acute lymphoblastic leukemia | Retrospective
study | - | (174) |
| Noh et al,
2021 |
Nanoparticle-drug |
| cyNP |
APTEDB-cyNP@CpG | CpG adjuvant | Colon tumor | Animal
experiment | - | (104) |
| Saw et al,
2021 |
|
|
| APT
EDB-DSPE-DTX | DSPE-DTX | MGs | Gene expression
profiles and survival analysis | - | (154) |
| Saw et al,
2018 |
|
| NPs | APT-EDB
NPs-experiments) |
| GBM | In vitro and
in vivo | - | (152) |
Antibodies or antibody-like molecules that target
FN-EDB can inhibit tumor cell adhesion and migration by binding
specifically to FN-EDB, thereby suppressing tumor progression
(7). Additionally, small molecule
compounds can disrupt tumor cell interactions with the ECM,
inhibiting tumor growth and metastasis by targeting FN-EDB's
functional roles. These molecules can also interfere with signaling
pathways within the TME, such as FAK/PI3K/AKT and MAPK/ERK, which
are crucial for tumor cell proliferation, survival, angiogenesis
and immune cell infiltration (114).
mAbs can induce antibody-dependent cellular
cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) upon
binding to FN-EDB, leading to the direct killing of tumor cells
(115). Gene therapies, using
clustered regularly interspaced short palindromic repeats
(CRISPR)/CRISPR-associated protein 9 and similar editing
technologies, can knock out or downregulate FN-EDB expression, thus
inhibiting the malignant behavior of tumor cells (116). Furthermore, gene-edited T cells
with enhanced specificity for FN-EDB-positive tumor cells can
improve immune clearance through approaches such as T cell receptor
(TCR) or chimeric antigen receptor (CAR) modification.
Conjuome-mediated targeted therapy of
FN-EDB tumor-specific carriers
At present, antibodies (L19) are often targeted with
cytokines in FN-EDB for tumor treatment and diagnosis. These other
antitumor drugs are composed of mAbs and cytokines (such as IL-12,
IL-15 and IL-21), TNF-α, IFN-γ and tissue factor to deliver
cytokines directly to the TME to activate the immune response
(117).
The L19-interleukin-2 fusion
protein
L19-IL2, a novel fusion protein consisting of the
single-chain variable fragment of the humanized monoclonal antibody
L19 targeting FN-EDB and IL-2 represents a promising advancement in
cancer therapeutics (118). This
targeted approach enhances the delivery of IL-2 directly to the
TME, thereby mitigating the systemic side effects associated with
conventional IL-2 therapy.
The L19 antibody, which selectively binds to FN-EDB
overexpressed in various malignancies, has been effectively fused
with IL-2 to create darleukin. This fusion protein not only
exhibits high tumor specificity but also activates cytotoxic T
lymphocytes and natural killer (NK) cells, bolstering the immune
response against cancer cells (119). Moreover, L19-IL2 may reshape the
immunosuppressive TME, facilitating the activation and
proliferation of tumor-specific T cells and the maturation and
antigen presentation of dendritic cells.
Preclinical studies have demonstrated the efficacy
of L19-IL2 in suppressing the growth of FN-EDB-positive tumors and
extending survival in animal models, including nude and SCID mice
(120). The transition of L19-IL2
into clinical trials marks a significant step, with ongoing
research aimed at assessing its safety, tolerability and efficacy
in human subjects. Initial clinical investigations are focused on
determining the maximum tolerated dose, recommended phase II dose
and characterizing the pharmacokinetic and pharmacodynamic profiles
of L19-IL2 (121).
The potential for L19-IL2 to be combined with other
therapeutic modalities, such as immune checkpoint inhibitors,
chemotherapy and radiotherapy, is currently being explored in
clinical trials. For example, in a randomized phase II clinical
trial, one group of patients received dacarbazine (DTIC; 1,000
mg/m2 on day 1 of a 21-day cycle) as a single agent,
while in the other two groups, L19IL2 was increased (22.5 million
international units of IL 2 equivalents) depending on two different
dosing regimens. Analysis of efficacy results showed that patients
receiving L19IL2 combined with DTIC had statistically significant
results for overall response rate and median progression-free
survival compared with DTIC monotherapy (122). The synergy between L19-IL2 and
stereotactic ablation body radiotherapy (SABR) is of particular
interest, as evidenced by a phase II clinical trial investigating
their combined use in patients with oligometastatic non-small cell
lung cancer (NSCLC) (123). This
multicenter, randomized controlled trial, involving 126 patients,
is designed to evaluate the 1.5-year progression-free survival as
the primary endpoint, highlighting the potential of integrating
L19-IL2 with SABR to enhance local tumor control and systemic
immune responses (124).
The L19-interleukin-12 fusion
protein
IL-12 was covalently linked with a monoclonal
antibody to produce an IL-12 antibody drug conjugate (ADC) to
deliver IL-12 to tumor cells enriched at the target antigen level.
This IL-12 delivery targeting a specific antigen minimizes IL-12
exposure in normal tissues, allowing it to show lower toxicity and
an improved therapeutic index (125). The development of ADC therapy
underwent several stages. The first generation ADC has limited
therapeutic efficacy due to problems such as linker instability and
insufficient payload toxicity. The second generation ADC improved
efficacy by improving the stability of the linker and using more
payloads. The third generation of ADC further optimized the
selectivity of antibodies, the design of linker and the toxicity
and bystander effects of payload, so that ADC therapy has achieved
significant therapeutic effects in some hematological tumors and
solid tumors (126,127).
AS1409 is formed by covalently linking the humanized
anti-FN-EDB antibody BC-1 with IL-12, aiming to deliver IL-12 to
the tumor-associated vasculature (128). Ongaro et al (129) developed a tandem-dimer form of a
full-human fusion protein, L19-L19-IL12, created by fusing human
IL-12 as a tandem dimer with the L19 antibody using protein
engineering techniques. This variant, equipped with an optimized
linker, demonstrated enhanced tumor targeting, prolonged retention
at the tumor site and rapid clearance from blood and normal organs,
suggesting its potential as a promising alternative to L19-IL12 and
warranting further clinical investigation.
Clinical trials are currently underway to evaluate
ADCs targeting FN-EDB across different types of tumor for safety,
pharmacokinetics and antitumor effects. PYX-201, an ADC comprising
a monoclonal antibody against FN-EDB, a cleavable linker and an
auristatin payload, is being assessed in Phase I clinical trials
for various solid tumors, including squamous cell carcinoma of the
head and neck, non-small cell lung cancer, ovarian cancer, soft
tissue sarcoma and pancreatic ductal adenocarcinoma (130). The ADC's internalization and
endosomal-lysosomal pathway release the payload, achieving precise
cytotoxicity against tumor cells.
EDB-ADCs have demonstrated antitumor activity and
enhanced immune cell infiltration in multiple cancer models, with
improved efficacy when combined with immune checkpoint blockade and
have shown good safety profiles in non-human primates (131). These findings underscore the
potential of FN-EDB-targeting ADCs as a valuable tool in the
oncology therapeutic arsenal, providing a rationale for their
further development and clinical application.
L19 with other immune cytokines
L19 can also be coupled to other cytokines,
including IL-10 (132), IL-15
(133) and granulocyte-macrophage
colony-stimulating factor (granulocyte-macrophage colony
stimulating factor; GM-CSF) (133), IFN-γ (134–136), and TNF-α (137–140), Most of these cytokines are
produced by immune cells and act on the tumor.
Trachsel et al (132) demonstrated that L19-IL-10 fusion
protein, when delivered to rheumatoid arthritis models, exhibits
superior efficacy in alleviating arthritis symptoms and inhibiting
disease progression compared with non-targeted IL-10. This targeted
approach suggests a potential therapeutic benefit in chronic
inflammatory conditions. Kaspar et al (133) investigated the antitumor
properties of L19-IL-15 and L19-GM-CSF, revealing potent antitumor
activities in both subcutaneous and metastatic cancer models. These
immunocytokines, by selectively binding to tumor vasculature,
effectively inhibited tumor growth and metastasis, underscoring the
potential of L19-based targeted therapy in oncology (133). IFNγ, a critical immunomodulatory
cytokine, has been explored in the context of L19 fusion proteins
to enhance tumor immunogenicity and promote immune cell
infiltration. Ruan et al (134) developed an L19-IFNγ variant
fusion protein with reduced affinity for its cognate receptor,
thereby minimizing off-target organ capture and enhancing
tumor-specific localization. Di Nitto et al (135) designed an L19-IFNγ KRG fusion
protein that demonstrated improved tumor homing and pharmacokinetic
properties in preclinical models. Niu et al (136) show that the combination of
L19-IFNγ KRG with immune checkpoint inhibitors, such as anti-PD-1
antibodies, induce tumor growth delay and increase intra-tumoral
concentrations of T cells and NK cells, highlighting the
synergistic potential of this combination therapy.
TNF-α is known for its potent antitumor effects.
L19-TNF is an immune cytokine fusion protein targeting tumor
vasculature resulting from the fusion of the human monoclonal
antibody L19 to TNF. L19 antibody can specifically bind FN-EDB on
the surface of tumor vascular endothelial cells and accurately
deliver TNF to tumor tissue, which has also been studied in the
form of L19-mTNF fusion protein (140). Spitaleri et al (139) reported on the efficacy and safety
of L19-TNF monotherapy in patients with advanced solid tumors in a
phase I/II clinical trial (139).
A total of 34 patients were enrolled into six dose groups, with
L19-TNF doses increasing from 1.3–13 µg/kg, every 3 weeks,
administered on days 1, 3 and 5. The aforementioned study showed
that in terms of safety, the patients mainly experienced mild
chills, nausea and vomiting but no hematology or unexpected serious
toxicity. In terms of efficacy, no objective tumor response was
observed, but out of 31 evaluable patients, 19 had transient stable
disease-states (137). Therefore,
its potential for combination chemotherapy to play a greater role
in the treatment of advanced solid tumors can be further explored.
In an additional exploratory clinical trial, the investigators
evaluated the safety and clinical activity of L19-TNF in
combination with melphalam-isolated limb perfusion (ILP) containing
melphalam in patients with locally advanced limb melanoma (138). A total of 17 patients were
included, of which 7 received 325 µg L19-TNF and 10 received 650 µg
L19-TNF. The results showed that the non-hematologic toxicity of
L19-TNF ILP was low, but four patients developed severe
myelosuppression. Although the TNF equivalent dose of L19-TNF was
only 3.13 and 6.25% of the approved TNF (Beromun®) dose
of 4 mg, the clinical efficacy was marked. In the 650 µg dose
group, 89% of patients had objective responses, five achieved
complete response (CR) and four patients had CR status until 12
months; no CR was observed in the 325 µg group. This suggests that
L19-TNF ILP has promising clinical activity and potential
application in the treatment of locally advanced limb melanoma
(138). Danielli et al
(137) fused two immune
cytokines, IL-2 and TNF-α, to a combination of monoclonal L19
antibody that binds to FN-EDB and this selectively targeting L19
antibody could allow the drug to accumulate in the injected lesion,
potentially producing a more durable immunoreactive. Melanoma is an
aggressive skin malignancy (141), especially in the local advanced
stage (stage IIIB/C). Traditional surgical resection may not
achieve ideal results, and patients face a high risk of recurrence
and metastasis. New treatments are needed. The Neo-DREAM trial is a
phase III study to evaluate the efficacy of neoadjuvant
intratumoral injection of Darleukin/Fibromun (L19IL2 + L19TNF) in
patients with clinical stage IIIB/C melanoma. The study randomizes
patients into two groups: Darleukin/Fibromun, followed by surgery
and adjuvant therapy and surgery and adjuvant therapy alone
(142). Darleukin/Fibromun
precisely targets FN-EDB in the tumor extracellular matrix (ECM) by
using immune cytokines IL-2 and TNF-α with the monoclonal antibody
L19 to stimulate the local immune response. According to the June
2022 issue of Ann Surg Oncol, the study observed significant
patient objective response rates (ORR) and excellent complete
response rates (CR) and partial response rates (PR) (143). Notably, the incidence of distant
effect (abscopal effect) in non-injected lesions was ≤53.8% (7/13),
indicating that Darleukin/Fibromun can not only effectively control
tumors at the injection site, but also produce immune killing in
distant uninjected lesions. In terms of safety, the side effects
recorded in the study were relatively few and controllable and
Darleukin/Fibromun showed improved safety compared with traditional
treatments.
These studies collectively illustrate the
versatility and potential of L19-based immunocytokines in
modulating the TME and enhancing antitumor immune responses. The
ongoing clinical development of L19-IL2 and other L19-based fusion
proteins signifies a promising direction in cancer immunotherapy,
with the potential to improve patient outcomes through targeted
delivery of therapeutic agents to the tumor site. The synergistic
effects observed with combination therapies further emphasize the
importance of exploring multimodal treatment strategies to maximize
clinical benefits.
Tumor-specific peptide-drug conjugate
(PDC)
Tumor-specific PDCs) represent a burgeoning class of
therapeutics in cancer immunotherapy, harnessing the targeting
capabilities of tumor-specific peptides to deliver cytotoxic agents
directly to cancer cells (144).
This strategy aims to enhance therapeutic efficacy while minimizing
damage to healthy tissues, offering a promising approach to improve
cancer treatment.
The design of PDCs necessitates careful selection of
a linker that ensures drug stability and is cleavable within the
TME to release the active drug. Equally critical is the choice of
toxin, which must possess potent antitumor activity and retain its
pharmacological properties post-conjugation. Researchers have
developed a bispecific peptide, HyPEP (EDB-VEGF), capable of
binding to cancer cells overexpressing EDB and VEGF (145). In animal models, this conjugate
has demonstrated the ability to inhibit tumor growth, with enhanced
effects when combined with anti-PD-1 antibodies, suggesting its
potential in immunotherapy (146). The PL1 peptide, identified for
its ability to bind to FN-EDB and Tenascin C subtypes in the tumor
ECM, triggers cellular uptake primarily through electrostatic
interactions.
This finding provides a novel perspective for
developing targeted cancer therapeutics. By conjugating carboplatin
(CBT) with a cyclic cell-penetrating peptide, a new drug delivery
system has been developed. This conjugate selectively binds to
cancer cells overexpressing integrins and EDB-FN and releases CBT
in the acidic tumor environment, exhibiting high selectivity and
low toxicity for cancer cells (147). In the treatment of prostate
cancer, PDCs targeting EDB-FN have shown therapeutic effects in
animal models by providing selective cytotoxicity (148). To overcome the short circulation
half-life of PDCs, a strategy involving the formation of hybrid
complexes with anti-hapten antibodies has been developed,
significantly extending the PDC's circulation half-life, enhancing
tumor accumulation and penetration, thereby inhibiting tumor growth
(149). The expression of VEGF,
closely associated with the development of various tumors, has been
targeted by cetuximab (Erbitux), a monoclonal antibody that
inhibits tumor angiogenesis by blocking the binding of VEGF to its
receptors, thus exerting antitumor effects (150).
Current research on PDCs in cancer therapy has made
significant progress. As clinical trials continue to assess the
safety and efficacy of PDCs, their potential to revolutionize
cancer treatment strategies continues to grow.
Nanoparticle-drug conjugates
In the medical research domain, innovative
nanosystems have been developed to enhance drug targeting and
bioavailability while minimizing systemic side effects (104,151,152). Gold nanoparticles (NPs) with
distinct surface modifications have demonstrated the ability to
selectively bind various structural domains of fibronectin (FN),
with interaction strengths influenced by the NPs' physicochemical
properties. Particularly, cationic NPs (NP-NH3+) interact with
acidic domain-rich regions of FN, potentially impairing its
function (153).
For instance, a system encapsulating bevacizumab
within immunoglobulin-1 functionalized PLGA nanoparticles has been
investigated for the treatment of atherosclerosis, showing improved
drug targeting and bioavailability with reduced systemic adverse
effects (151). Noh et al
(104) developed APTEDB-cyNP @
CpG nanomedicine, which coupled APTEDB to PEG and DSPE to build
cyanine nanoparticles coated with CpG adjuvant for photothermal
therapy and immunotherapy of cancer. In addition,
APTEDB-DSPE-docetaxel (DTX) nanoparticles formed by combining
APTEDB peptide and DTX show excellent targeting and anti-tumor
effects in glioma treatment (154). These nanodrugs have exhibited
significant therapeutic effects in tumor models, inducing
immunogenic cell death and enhancing T-cell antitumor activity.
To enhance the therapeutic efficiency for malignant
glioblastoma multiforme (152),
aptamer-like peptide-modified liposomal nanoplatforms for systemic
small interfering RNA (siRNA) delivery have been developed. These
nanoplatforms specifically target tumor cells and suppress tumor
growth by silencing the expression of key genes, such as
cyclophilin A. Furthermore, FN-EDB expression in atherosclerotic
plaques has been explored, leading to the design of FN-EDB-specific
nanoparticles for enhanced plaque detection and localized drug
delivery, demonstrating favorable targeting and drug delivery
outcomes in animal models (155).
These studies collectively highlight the potential
of nanotechnology in improving drug delivery efficiency and tumor
therapy, offering new directions for future clinical
applications.
Small molecule inhibitors
Small molecule inhibitors targeting FN-EDB disrupt
FN-EDB interactions with integrin receptors to impede tumor cell
adhesion, migration and invasion. These inhibitors mitigate tumor
growth and metastasis by attenuating interactions between cancer
cells and the ECM, modulating signaling pathways within the TME
that influence angiogenesis and immune cell infiltration (156).
BRAF/MEK inhibitors exemplify cell growth
suppressants that induce immunogenic cell death, activating
antitumor T cell responses, particularly in melanomas harboring
BRAF (V600E) mutations (157).
VEGF-VEGFR inhibitors, such as bevacizumab, disrupt the VEGF
pathway, curtailing recruitment of immunosuppressive cells and
enhancing T cell tumor infiltration, demonstrating significant
efficacy in combination with atezolizumab for non-small cell lung
cancer (158).
CSF1R inhibitors, by modulating immune cell
activity in the TME, reduce the number of TAMs, bolstering the
antitumor immune response (159).
Metabolic pathway inhibitors, including glutaminase and arginase
inhibitors, are under investigation to regulate the metabolism of
both tumor and immune cells, further potentiating immune responses
(160).
The advent of small molecule immune checkpoint
inhibitors offers a novel avenue for directly targeting the
PD-1/PD-L1 axis, promising similar therapeutic effects to antibody
drugs with improved pharmacokinetics and oral bioavailability.
Emerging small molecule immunotherapeutic candidates, such as
ABBV-CLS-484, target both tumor and immune cells, enhancing immune
cell activity and sensitizing tumors to immune attack, showing
significant antitumor effects in animal models (161).
These advances highlight the key role of small
molecule inhibitors in activating and augmenting the immune system
against cancer, offering new therapeutic prospects for precision
oncology.
Chimeric antigen receptor T-cell
(CAR-T) therapy
CAR-T cell therapy has revolutionized cancer
treatment by genetically engineering the T cells of patients to
recognize and attack specific tumor cells. This approach has
demonstrated marked efficacy in hematological malignancies, leading
to the approval of several CAR-T products. The evolution of CAR-T
therapy, from the first generation with a single-chain variable
fragment to the fourth generation incorporating multiple signaling
pathways, has been driven by the pursuit of enhanced efficacy and
safety.
Despite the success in blood cancers, CAR-T cell
therapy faces challenges in solid tumors due to the suppressive TME
and the lack of specific tumor-associated antigens (162). To address this, researchers have
turned to FN-EDB, a cancer-fetal antigen highly expressed in the
tumor vasculature and cancer cells, making it a potential target
for CAR-T cell therapy in solid tumors (163).
Investigators have developed recombinant T cells
expressing T cell receptors (rTCRs) fused with CARs (rTCR-CAR T
cells) that target FN-EDB (164).
These rTCR-CAR T cells have shown the ability to bypass TME
suppression by combining the single-chain variable fragment with
CD3ε. Preclinical studies have shown that rTCR-CAR T cells exert
antitumor effects on various solid tumors, including thyroid cancer
and breast cancer, by inhibiting the effects of FN-EDB in cell
adhesion, migration and angiogenesis (165,166). These rTCR-CAR T cells exhibited
cytotoxicity against EDB-positive tumor cells in vitro and
effectively inhibited tumor growth and reduced tumor vascular
density in vivo without significant toxicity.
Further research has constructed a BBz CAR
targeting EDB-FN, using lentiviral transduction to express CAR
molecules on T cells (167).
In vitro and in vivo experiments confirm the ability
of CAR T cells to activate and lyse EDB-positive cells, inhibiting
tumor growth and vascular density in U-87 MG xenograft models.
The innovative rTCR-CAR T cell therapy is currently
being evaluated in multiple clinical trials for its safety and
efficacy in cancer treatment, particularly in targeting FN-EDB
within the TME (168,169). Combination therapy strategies
under exploration include the use of CD70 CAR-T cells with PARP
inhibitors, aiming to enhance efficacy by optimizing CAR-T cell
structures and constructing bispecific CAR-T cells (170).
Additionally, the combination of CD70 CAR-T cells
with oncolytic viruses (OVs) exploits the selective replication and
destructive capacity of OVs within tumor cells, promoting an
inflammatory TME to improve treatment outcomes for solid tumors
(171). Epigenetic modulators,
such as decitabine and givinostat, have shown potential in
enhancing the efficacy of CD70 CAR-T cells by increasing CD70
expression levels (172).
The combination of CAR-T cells with immune
checkpoint inhibitors, such as anti-PD-1 and anti-PD-L1 antibodies,
restores the function of tumor-infiltrating lymphocytes, enhancing
their ability to attack cancer cells and improving the persistence
and efficacy of CAR-T cells within the TME (173). Furthermore, the co-expression of
cytokines such as IL-12, IL-15 and IL-18 with CAR-T cells has
demonstrated advantages in boosting their activity and durability
(174).
Moreover, the combination of CAR-T cells with small
molecule drugs, such as the BTK inhibitor ibrutinib, has potential
to improve patient responses in clinical trials (175). The scope of combination therapies
for CAR-T cells extends beyond the aforementioned strategies to
include radiotherapy, chemotherapy, hematopoietic stem cell
transplantation and targeted therapies, all aiming to enhance
treatment efficacy.
In summary, CAR-T cell therapy targeting FN-EDB
holds significant potential in the treatment of solid tumors, with
ongoing clinical trials exploring its effectiveness. The future of
cancer treatment may benefit from more effective and personalized
options offered by CAR-T cell therapies. As research progresses,
optimizing CAR-T cell design, enhancing treatment efficacy,
minimizing side effects and exploring combination applications with
other therapeutic modalities will remain a focal point in this
field.
Radiotherapy for targeting the FN-EDB
FN-EDB, a tumor-associated antigen, is highly
expressed in the TME and serves as an ideal target for
radiotherapy, particularly in radioimmunotherapy (RIT). RIT
combines the precision of mAbs with the cytotoxic effects of
radioactive isotopes, offering a targeted approach to cancer
treatment (176).
The L19 mAb, which selectively binds to FN-EDB, has
been instrumental in RIT. Variants such as L19-SIP, L19 (ScFv)2 and
AP39, have demonstrated significant success in imaging and treating
various types of cancer (177).
These antibodies have been conjugated with radioisotopes like
131I, 124I, 125I, 90Y
and 111In, showing promising results in preclinical
models by significantly inhibiting tumor growth (178).
Radiotherapy strategies targeting FN-EDB have
potential in several types of tumor. The EDBp probes developed by
Li et al (179), including
Cy 5-PEG 4-EDBp, (18F)-NOTA-PEG 4-EDBp and
(177Lu)-DOTA-PEG 4-EDBp, respectively, for surgical
navigation, radionuclide imaging and treatment. These probes showed
high tumor uptake and good therapeutic effects in thyroid cancer
models, providing new tools for the diagnosis and treatment of
various types of tumors. In a study by Spaeth et al
(140), 131I-labeled
L19 (SIP) was evaluated in a C6 glioma rat model, revealing a
significant extension in median survival time compared with the
control group, underscoring the potential of 131I-SIP
(L19)-RIT in glioma treatment. Tijink et al (180) prepared high-purity
124I-L19-SIP for immuno-PET imaging of tumor
angiogenesis, supporting its use as a preclinical tool to predict
the biodistribution of 131I-L19-SIP in RIT.
Moosmayer et al (181) compared the therapeutic potential
of directly radioiodinated L19-SIP with a pretargeting approach
using 111In-labeled HSG peptide and AP39×m679 bispecific
mAb. The pretargeting strategy showed superior tumor accumulation
and dose delivery, suggesting that using 90Y-labeled
half-antigen peptides could enhance therapeutic outcomes.
A phase I study by Del Conte et al (182) evaluated the safety and tumor
targeting of 131I-L19SIP in 51 patients with soft tissue
sarcoma (SC) and hematological malignancies. The treatment
exhibited acceptable toxicity with thrombocytopenia as the main
side effect. Partial responses and disease stabilization were
observed in Hodgkin's lymphoma patients, confirming the potential
of EDBF as a target for angiogenesis-based antibody therapy.
Research on FN-EDB expression in thymic epithelial
tumors (TET) and its application in R-RIT has been conducted
through prospective clinical trials, demonstrating the feasibility
of using ED-B FN-specific human recombinant antibody radretumab for
the treatment of recurrent TET patients (183).
In summary, RIT targeting FN-EDB has shown
considerable potential in various types of cancer, with ongoing
clinical trials aimed at optimizing treatment strategies. Future
research should focus on refining RIT approaches, enhancing
treatment efficacy, reducing side effects and exploring combination
therapies with other modalities such as chemotherapy, immunotherapy
and targeted agents to improve patient outcomes.
Discussion
Summary and outlook
The present study focused on the involvement of
FN-EDB and FN-EDB and its diagnostic role as a biomarker in tumors
and FN-EDB inhibition as a strategy for immunotherapy. FN-EDB has
emerged as a pivotal player in tumorigenesis, with its elevated
expression levels in malignant tissues correlating with aggressive
tumor behavior, including invasion, lymph node metastasis and poor
prognosis. This association positions FN-EDB as a potential
biomarker for cancer diagnosis and a therapeutic target. The
utility of FN-EDB as a biomarker has been harnessed in the
development of various imaging techniques, such as MRI contrast
agents using FN-EDB-specific peptides like ZD2, enhancing the
specificity and sensitivity of tumor detection. Significant
advances have been made in targeting FN-EDB for cancer
immunotherapy, with approaches including ADCs, CAR-T cell therapy
and immune checkpoint blockade. Clinical trials for several
therapeutic and diagnostic modalities targeting FN-EDB are
underway, with some, like the phase III trial of daromun for
neoadjuvant treatment, showing promising results (184,185).
Despite these developments, challenges remain in
translating FN-EDB-targeted therapies into clinical practice. The
precise mechanisms by which FN-EDB contributes to the TME are not
fully understood and its complex interactions with cancer cell
signaling pathways require further investigation. The differential
expression of FN-EDB across tumor types hints at diverse roles in
oncogenesis, necessitating a deeper exploration of the underlying
biological mechanisms. While preclinical studies have shown promise
for FN-EDB-targeted conjugate therapies, the transition from animal
models to clinical settings demands rigorous clinical trials to
validate safety and efficacy in humans. Future research should
focus on elucidating the role of FN-EDB in tumor biology,
dissecting its signaling pathways in oncogenesis and understanding
the basis for its variable expression across different tumors.
A thorough understanding of FN-EDB's function will
pave the way for the development of more effective and safer
immunotherapies targeting FN-EDB. Concurrently, the pursuit of
safer and more effective conjugate drugs, along with the
development of non-invasive and precise diagnostic strategies for
tumor grading, will be crucial for enhancing cancer treatment and
diagnosis outcomes. Although challenges persist, ongoing research
into FN-EDB holds the promise of yielding more potent
immunotherapeutic agents and clinical strategies, offering renewed
hope for patients with cancer.
Conclusion
FN-EDB, a key player in tumor aggression,
angiogenesis and immune evasion, presents a promising therapeutic
target. Strategies targeting FN-EDB, including peptide-drug
conjugates, antibody-drug conjugates, nanoparticles and
immunotherapies, have demonstrated significant antitumor effects in
various cancer models. Future research should focus on optimizing
targeting ligands, exploring combination therapies, accelerating
clinical translation and understanding resistance mechanisms to
enhance the precision and efficacy of cancer treatments.
Despite the growing interest in tumor-associated
FN-EDB as a potential therapeutic target, there is a lack of
articles available for a comprehensive review of the field. Thus,
the present study provided a broad body of knowledge carefully
compiled for the current research, highlighting the role of FN-EDB
in tumor progression and its implications for clinical oncology. By
elucidating the underlying mechanisms, including tumor
angiogenesis, internal and external cell signaling and summarizing
the latest targeted therapy and diagnostic strategies in the field,
it provided necessary guidance for researchers, clinicians and
pharmaceutical developers and to promote informed decision-making.
In conclusion, the present study could provide important
information for future research directions and treatment strategies
that could guide the development of new diagnostic and treatment
modalities in cancer treatment in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YZ wrote the first draft and reviewed and edited
the article. JL helped to conceive the article, guided the writing
direction and ideas, designed the research content, and drafted the
article. YP and TC performed the literature review, were
responsible for collecting and organizing the relevant literature,
and performed a preliminary analysis of the literature. In addition
they participated in the initial collation and analysis of the
data, providing valuable insights into the conception and design of
this study. YZ, TC, YP and JL contributed to revising the
manuscript, provided important intellectual content. All authors
agree to be accountable for all aspects of the work, ensuring that
questions related to the accuracy or integrity of the work are
appropriately investigated and resolved and have read and approved
the final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
124I/131I
|
Iodine-124//131 radioisotope
|
|
131I-L19-SIP
|
131I-labeled L19 small
immunoprotein
|
|
18F
|
Fluorine-18 radioisotope
|
|
64Cu
|
copper-64 radioisotope
|
|
68Ga
|
gallium-68 radioisotope
|
|
76Br
|
bromine-76 radioisotope
|
|
99mTc
|
technetium-99m radioisotope
|
|
ADC
|
Antibody-Drug Conjugate
|
|
ADCC
|
Antibody-Dependent Cellular
Cytotoxicity
|
|
ANG-1
|
Angiopoietin-1
|
|
ANG-2
|
Angiopoietin-2
|
|
APTEDB
|
aptamer targeting EDB; fibronectin
extra domain B specific peptide
|
|
CAR-T
|
chimeric antigen receptor T
|
|
CD70
|
Cluster of Differentiation 70
|
|
CRISPR/Cas9
|
Clustered Regularly interspaced short
palindromic repeats/CRISPR-associated protein 9
|
|
Cy7
|
near-infrared dye Cy7
|
|
DOTA
|
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid
|
|
ECM
|
extracellular matrix
|
|
FAK
|
focal adhesion kinase
|
|
FN
|
fibronectin
|
|
FN-EDB
|
fibronectin extra domain B
|
|
GM-CSF
|
granulocyte-macrophage colony
stimulating factor
|
|
HCC
|
hepatocellular carcinoma
|
|
HIF-1α/β
|
hypoxia-inducible factor-1 α/β
|
|
HP-DO3A
|
hydroxypropyl-dodecylamine chelating
agent for Gd
|
|
MG
|
malignant glioma
|
|
MRI
|
magnetic resonance imaging
|
|
NJB2
|
alpaca-derived libraries of
nanobodies
|
|
NK
|
natural killer
|
|
NOTA
|
1,4,7-triazacyclononane-1,4,7-triacetic acid
|
|
NPs
|
nanoparticles
|
|
NSCLC
|
non-small cell lung cancer
|
|
OVs
|
oncolytic viruses
|
|
PD-1
|
programmed death-1
|
|
PD-L1
|
programmed death-ligand 1
|
|
PDCs
|
tumor-specific peptide-drug
conjugates
|
|
PDGF
|
platelet-derived growth factor
|
|
PET
|
positron emission tomography
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
RA
|
rheumatoid arthritis
|
|
RGD
|
Arg-Gly-Asp
|
|
rhFEB
|
recombinant fusion protein
|
|
RIT
|
radioimmunotherapy
|
|
rTCR
|
recombinant T cell receptor
|
|
TAMs
|
tumor-associated macrophages
|
|
TC
|
thyroid cancer
|
|
TME
|
tumor microenvironment
|
|
TNF-α
|
tumor necrosis factor-alpha
|
References
|
1
|
Tiwari A, Trivedi R and Lin SY: Tumor
microenvironment: Barrier or opportunity towards effective cancer
therapy. J Biomed Sci. 29:832022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kolesnikoff N, Chen CH and Samuel MS:
Interrelationships between the extracellular matrix and the immune
microenvironment that govern epithelial tumour progression. Clin
Sci (Lond). 136:361–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Efthymiou G, Saint A, Ruff M, Rekad Z,
Ciais D and Van Obberghen-Schilling E: Sha up the tumor
microenvironment with cellular fibronectin. Front Oncol.
10:6412020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SE, Yun S and Doh J: Effects of
extracellular adhesion molecules on immune cell mediated solid
tumor cell killing. Front Immunol. 13:10041712022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parmar D and Apte M: Angiopoietin
inhibitors: A review on targeting tumor angiogenesis. Eur J
Pharmacol. 899:174021. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng Z, Lv X and Huang S: Recent progress
on the role of fibronectin in tumor stromal immunity and
immunotherapy. Curr Top Med Chem. 22:2494–2505. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neri D and Sondel PM: Immunocytokines for
cancer treatment: Past, present and future. Curr Opin Immunol.
40:96–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Li R, Li M and Wang C: Fibronectin
and colorectal cancer: Signaling pathways and clinical
implications. J Recept Signal Transduct Res. 41:313–320. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wagner J, Wickman E, Shaw TI, Anido AA,
Langfitt D, Zhang J, Porter SN, Pruett-Miller SM, Tillman H,
Krenciute G and Gottschalk S: Antitumor effects of CAR T cells
redirected to the EDB Splice variant of fibronectin. Cancer Immunol
Res. 9:279–290. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou F, Sun J, Ye L, Jiang T, Li W, Su C,
Ren S, Wu F, Zhou C and Gao G: Fibronectin promotes tumor
angiogenesis and progression of Non-small-cell lung cancer by
elevating WISP3 expression via FAK/MAPK/HIF-1α axis and activating
wnt signaling pathway. Exp Hematol Oncol. 12:612023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumra H and Reinhardt DP:
Fibronectin-targeted drug delivery in cancer. Adv Drug Deliv Rev.
97:101–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang K, Seo BR, Fischbach C and Gourdon D:
Fibronectin mechanobiology regulates tumorigenesis. Cell Mol
Bioeng. 9:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kraft S, Klemis V, Sens C, Lenhard T,
Jacobi C, Samstag Y, Wabnitz G, Kirschfink M, Wallich R, Hänsch GM
and Nakchbandi IA: Identification and characterization of a unique
role for EDB fibronectin in phagocytosis. J Mol Med (Berl).
94:567–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dalton CJ and Lemmon CA: Fibronectin:
Molecular structure, fibrillar structure and mechanochemical
signaling. Cells. 10:24432021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh P, Carraher C and Schwarzbauer JE:
Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev
Biol. 26:397–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ventura E, Sassi F, Parodi A, Balza E,
Borsi L, Castellani P, Carnemolla B and Zardi L: Alternative
splicing of the angiogenesis associated Extra-domain B of
fibronectin regulates the accessibility of the B-C loop of the type
III repeat 8. PLoS One. 5:e91452010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barrow-McGee R, Kishi N, Joffre C, Ménard
L, Hervieu A, Bakhouche BA, Noval AJ, Mai A, Guzmán C,
Robbez-Masson L, et al: Beta 1-integrin-c-Met cooperation reveals
an inside-in survival signalling on autophagy-related
endomembranes. Nat Commun. 7:123922016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dinesh NEH, Campeau PM and Reinhardt DP:
Fibronectin isoforms in skeletal development and associated
disorders. Am J Physiol Cell Physiol. 323:C536–C549. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guerrero-Barberà G, Burday N and Costell
M: Shaping oncogenic microenvironments: Contribution of
fibronectin. Front Cell Dev Biol. 12:13630042024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rick JW, Chandra A, Dalle Ore C, Nguyen
AT, Yagnik G and Aghi MK: Fibronectin in malignancy:
Cancer-specific alterations, protumoral effects and therapeutic
implications. Semin Oncol. 46:284–290. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JH, Ryu JM and Han HJ: Involvement of
caveolin-1 in fibronectin-induced mouse embryonic stem cell
proliferation: Role of FAK, RhoA, PI3K/Akt and ERK 1/2 pathways. J
Cell Physiol. 226:267–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fei D, Meng X, Yu W, Yang S, Song N, Cao
Y, Jin S, Dong L, Pan S and Zhao M: Fibronectin (FN) cooperated
with TLR2/TLR4 receptor to promote innate immune responses of
macrophages via binding to integrin β1. Virulence. 9:1588–1600.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Menrad A and Menssen HD: ED-B fibronectin
as a target for antibody-based cancer treatments. Expert Opin Ther
Targets. 9:491–500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hall RC, Vaidya AM, Schiemann WP, Pan Q
and Lu ZR: RNA-Seq analysis of extradomain a and extradomain B
fibronectin as extracellular matrix markers for cancer. Cells.
12:6852023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Faisal SM, Comba A, Varela ML, Argento AE,
Brumley E, Abel C II, Castro MG and Lowenstein PR: The complex
interactions between the cellular and non-cellular components of
the brain tumor microenvironmental landscape and their therapeutic
implications. Front Oncol. 12:10050692022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen CW, Yang CH, Lin YH, Hou YC, Cheng
TJ, Chang ST, Huang YH, Chung ST, Chio CC, Shan YS, et al: The
fibronectin expression determines the distinct progressions of
malignant gliomas via transforming growth Factor-beta pathway. Int
J Mol Sci. 22:3782. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hall RC, Ayat NR, Qiao PL, Vaidya AM, Ma
D, Aminoshariae A, Stojanov I and Lu ZR: Preclinical assessment of
the effectiveness of magnetic resonance molecular imaging of
Extradomain-B fibronectin for detection and characterization of
oral cancer. Mol Imaging Biol. 22:1532–1542. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vaidya A, Wang H, Qian V, Gilmore H and Lu
ZR: Overexpression of Extradomain-B fibronectin is associated with
invasion of breast cancer cells. Cells. 9:18262020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nail HM, Chiu CC, Leung CH, Ahmed MMM and
Wang HD: Exosomal miRNA-mediated intercellular communications and
immunomodulatory effects in tumor microenvironments. J Biomed Sci.
30:692023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu
X, Zhang Z, Yang S and Xiao M: Extracellular matrix remodeling in
tumor progression and immune escape: From mechanisms to treatments.
Molecular Cancer. 22:482023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burrows L, Clark K, Mould AP and Humphries
MJ: Fine mapping of inhibitory anti-α5 monoclonal antibody epitopes
that differentially affect integrin-ligand binding. Biochem J.
344:527. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Farndale RW and Jarvis GE: Integrins in
GtoPdb v.2023.1. IUPHAR/BPS guide to pharmacology CITE. Apr
26–2023.doi:10.2218/gtopdb/f760/2023.1. View Article : Google Scholar
|
|
34
|
Takagi J: Structural basis for ligand
recognition by RGD (Arg-Gly-Asp)-dependent integrins. Biochem Soc
Trans. 32:403–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kolasangiani R, Bidone TC and Schwartz MA:
Integrin conformational dynamics and mechanotransduction. Cells.
11:35842022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moro L, Venturino M, Bozzo C, Silengo L,
Altruda F, Beguinot L, Tarone G and Defilippi P: Integrins induce
activation of EGF receptor: Role in MAP kinase induction and
adhesion-dependent cell survival. EMBO J. 17:6622–6632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su Y, Xia W, Li J, Walz T, Humphries MJ,
Vestweber D, Cabañas C, Lu C and Springer TA: Relating conformation
to function in integrin α 5 β 1. Proc Natl Acad Sci USA.
113:E3872–E3881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Widmaier M, Rognoni E, Radovanac K,
Azimifar SB and Fässler R: Integrin-linked kinase at a glance. J
Cell Sci. 125:1839–1843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuura S, Thompson CR, Kah NS,
Karagianni A, Torres CW, Mazzeo CS, Leiva O, Malara A, Balduini A
and Ravid K: Integrin-mediated adhesion to extracellular matrix
protein fibronectin drives megakaryocytosis in
JAK2V617F+ primary myelofibrosis. Blood. 134 (Suppl
1):S42052019. View Article : Google Scholar
|
|
40
|
Andreucci E, Bugatti K, Peppicelli S,
Ruzzolini J, Lulli M, Calorini L, Battistini L, Zanardi F, Sartori
A and Bianchini F: Nintedanib-αVβ6 integrin ligand conjugates
reduce TGFβ-Induced EMT in human Non-small cell lung cancer. Int J
Mol Sci. 24:14752023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Haarmann A, Nowak E, Deiß A, van der Pol
S, Monoranu CM, Kooij G, Müller N, van der Valk P, Stoll G, de
Vries HE, et al: Soluble VCAM-1 impairs human brain endothelial
barrier integrity via integrin α-4-transduced outside-in
signalling. Acta Neuropathologica. 129:639–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Su Y, Iacob RE, Li J, Engen JR and
Springer TA: Dynamics of integrin α5β1, fibronectin and their
complex reveal sites of interaction and conformational change. J
Biol Chem. 298:1023232022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Durrant TN, van den Bosch MT and Hers I:
Integrin αIIbβ3 outside-in signaling. Blood. 130:1607–1619. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pang X, He X, Qiu Z, Zhang H, Xie R, Liu
Z, Gu Y, Zhao N, Xiang Q and Cui Y: Targeting integrin pathways:
Mechanisms and advances in therapy. Signal. 8:12023.
|
|
45
|
Shams H and Mofrad MRK: Molecular
mechanisms underlying the inside-out signaling through focal
adhesions. Biophysical J. 106:574a2014. View Article : Google Scholar
|
|
46
|
Huang C, Rajfur Z, Yousefi N, Chen Z,
Jacobson K and Ginsberg MH: Talin phosphorylation by Cdk5 regulates
Smurf1-mediated talin head ubiquitylation and cell migration. Nat
Cell Biol. 11:624–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han SB, Lee G, Kim D, Kim JK, Kim IS, Kim
HW and Kim DH: Selective suppression of Integrin-ligand binding by
single molecular tension probes mediates directional cell
migration. Adv Sci (Weinh). 11:e23064972024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim HJ, You SJ, Yang DH, Chun HJ and Kim
MS: Preparation of novel RGD-conjugated thermosensitive mPEG-PCL
composite hydrogels and in vitro investigation of their impacts on
Adhesion-dependent cellular behavior. J Industrial Engineering
Chemistry. 84:226–235. 2020. View Article : Google Scholar
|
|
49
|
Legate KR, Wickström SA and Fässler R:
Genetic and cell biological analysis of integrin outside-in
signaling. Genes Dev. 23:397–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Betriu N, Andreeva A, Alonso A and Semino
CE: Increased stiffness downregulates focal adhesion kinase
expression in pancreatic cancer cells cultured in 3D
Self-assembling peptide scaffolds. Biomedicines. 10:1835. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Martinez OE and Sudhamsu J: Abstract A025:
Investigating the molecular mechanisms of regulation of the RAS
guanine nucleotide exchange factor, SOS1 by Grb2 and 14-3-3. Mol
Cancer Res. 21 (5_Suppl):A0252023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lagarrigue F and Gingras AR: Src-mediated
phosphorylation of RIAM promotes integrin activation. Structure.
29:305–307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kang BW and Chau I: Molecular target:
Pan-AKT in gastric cancer. ESMO Open. 5:e0007282020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu W, Xue X, Chen Y, Zheng N and Wang J:
Targeting prolyl isomerase Pin1 as a promising strategy to overcome
resistance to cancer therapies. Pharmacol Res. 184:1064562022.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Alanko J and Ivaska J: Endosomes: Emerging
platforms for Integrin-mediated FAK signalling. Trends Cell Biol.
26:391–398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wan J: The regulatory role of the mitotic
checkpoint component Mad1 in interphase and tumor progression.
University of Wisconsin-Madison; 2019
|
|
57
|
Liu J, Lu F, Ithychanda SS, Apostol M, Das
M, Deshpande G, Plow EF and Qin J: A mechanism of platelet integrin
αIIbβ3 outside-in signaling through a novel integrin αIIb
subunit-filamin-actin linkage. Blood. 141:2629–2641.
2023.PubMed/NCBI
|
|
58
|
Shams H and Mofrad MRK: α-Actinin induces
a kink in the transmembrane domain of β3-Integrin and impairs
activation via talin. Biophys J. 113:948–956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fraser J, Simpson J, Fontana R,
Kishi-Itakura C, Ktistakis NT and Gammoh N: Targeting of early
endosomes by autophagy facilitates EGFR recycling and signalling.
EMBO Rep. 20:e477342019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Miao M, Collins J, Moreira Bahnson ES,
Chubinskaya S and Loeser RF: Reactive oxygen species regulate
fibronectin fragments-induced map kinase signaling and
metalloproteinase 13 release in human chondrocytes through nadph
oxidase 2 and endocytosis of chondrocyte integrins. Osteoarthritis.
28 (Suppl):S45–S46. 2020. View Article : Google Scholar
|
|
61
|
Teran OY, Zanotelli MR, Joy Lin MC,
Cerione RA and Wilson KF: Dock7 regulates AKT and mTOR/S6K activity
required for the transformed phenotypes and survival of cancer
cells. bioRxiv. Jan 3–2023.doi: 10.1101/2023.01.03.522657.
PubMed/NCBI
|
|
62
|
Gagné D, Benoit YD, Groulx JF, Vachon PH
and Beaulieu JF: ILK supports RhoA/ROCK-mediated contractility of
human intestinal epithelial crypt cells by inducing the
fibrillogenesis of endogenous soluble fibronectin during the
spreading process. BMC Mol Cell Biol. 21:142020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nadel G, Maik-Rachline G and Seger R: JNK
Cascade-induced Apoptosis-A unique role in GqPCR signaling. Int J
Mol Sci. 24:13527. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Karunakaran D, Nguyen MA, Geoffrion M,
Vreeken D, Lister Z, Cheng HS, Otte N, Essebier P, Wyatt H, Kandiah
JW, et al: RIPK1 expression associates with inflammation in early
atherosclerosis in humans and can be therapeutically silenced to
reduce NF-κB activation and atherogenesis in mice. Circulation.
143:163–177. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang H, Meng F, Wu S, Kreike B, Sethi S,
Chen W, Miller FR and Wu G: Engagement of I-branching {beta}-1,
6-N-acetylglucosaminyltransferase 2 in breast cancer metastasis and
TGF-{beta} signaling. Cancer Res. 71:4846–4856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang S, Liu Y, Li MY, Ng CSH, Yang SL,
Wang S, Zou C, Dong Y, Du J, Long X, et al: FOXP3 promotes tumor
growth and metastasis by activating Wnt/β-catenin signaling pathway
and EMT in non-small cell lung cancer. Mol Cancer. 16:1242017.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Weller M, Silginer M, Goodman SL,
Hasenbach K, Thies S, Schraml P, Tabatabai G, Moch H, Tritschler I
and Roth P: Effect of the integrin inhibitor cilengitide on
TGF-beta signaling. J Clin Oncol. 30 (15_suppl):S20552012.
View Article : Google Scholar
|
|
68
|
Valdembri D and Serini G: Angiogenesis:
The importance of RHOJ-mediated trafficking of active integrins.
Current Biology. 30:R652–R654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nilsson M and Heymach JV: Vascular
endothelial growth factor (VEGF) pathway. J Thorac Oncol.
1:768–770. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu Z and Dong Z: A cross talk between HIF
and NF-κB in AKI. Am J Physiol Renal Physiol. 321:F255–F256. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhao Y, Wang J, Qin W, Hu Q, Li J, Qin R,
Ma N, Zheng F, Tian W, Jiang J, et al: Dehydroepiandrosterone
promotes ovarian angiogenesis and improves ovarian function in a
rat model of premature ovarian insufficiency by up-regulating
HIF-1α/VEGF signalling. Reprod Biomed Online. 49:1039142024.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chatterjee S and Naik UP:
Pericyte-endothelial cell interaction. Cell Adh Migr. 6:157–159.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Surazynski A, Donald SP, Cooper SK,
Whiteside MA, Salnikow K, Liu Y and Phang JM: Extracellular matrix
and HIF-1 signaling: The role of prolidase. Int J Cancer.
122:1435–1440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Caporali A, Martello A, Miscianinov V,
Maselli D, Vono R and Spinetti G: Contribution of pericyte
paracrine regulation of the endothelium to angiogenesis. Pharmacol
Ther. 171:56–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Harrell CR, Simovic Markovic B, Fellabaum
C, Arsenijevic A, Djonov V and Volarevic V: Molecular mechanisms
underlying therapeutic potential of pericytes. J Biomed Sci.
25:122028.
|
|
76
|
Simonavicius N, Ashenden M, van Weverwijk
A, Lax S, Huso DL, Buckley CD, Huijbers IJ, Yarwood H and Isacke
CM: Pericytes promote selective vessel regression to regulate
vascular patterning. Blood. 120:1516–1527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Smart N: Understanding the recruitment
process. Arteriosclerosis Thrombosis Vascular Biol. 40:2564–2565.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Senger DR and Davis GE: Angiogenesis. Cold
Spring Harb Perspect Biol. 3:a0050902011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Huang Y, Snuderl M and Jain RK:
Polarization of Tumor-associated macrophages: A novel strategy for
vascular normalization and antitumor immunity. Cancer Cell. 19:1–2.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Poto R, Cristinziano L, Modestino L, de
Paulis A, Marone G, Loffredo S, Galdiero MR and Varricchi G:
Neutrophil extracellular traps, angiogenesis and cancer.
Biomedicines. 10:431. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Van Hinsbergh VW and Koolwijk P:
Endothelial sprouting and angiogenesis: Matrix metalloproteinases
in the lead. Cardiovasc Res. 78:203–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhou X, Zhang J, Lv W, Zhao C, Xia Y, Wu Y
and Zhang Q: The pleiotropic roles of adipocyte secretome in
remodeling breast cancer. J Exp Clin Cancer Res. 41:2032022.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tang KH, Ma S, Lee TK, Chan YP, Kwan PS,
Tong CM, Ng IO, Man K, To KF, Lai PB, et al: CD133(+) liver
tumor-initiating cells promote tumor angiogenesis, growth, and
self-renewal through neurotensin/interleukin-8/CXCL1 signaling.
Hepatology. 55:807–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Engelmann D, Mayoli-Nüssle D, Mayrhofer C,
Fürst K, Alla V, Stoll A, Spitschak A, Abshagen K, Vollmar B, Ran S
and Pützer BM: E2F1 promotes angiogenesis through the
VEGF-C/VEGFR-3 axis in a feedback loop for cooperative induction of
PDGF-B. J Mol Cell Biol. 5:391–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Azizi M, Dianat-Moghadam H, Salehi R,
Farshbaf M, Iyengar D, Sau S, Iyer AK, Valizadeh H, Mehrmohammadi M
and Hamblin MR: Interactions between tumor biology and targeted
nanoplatforms for imaging applications. Adv Funct Mater.
30:19104022020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Vaidya A, Ayat N, Buford M, Wang H,
Shankardass A, Zhao Y, Gilmore H, Wang Z and Lu ZR: Noninvasive
assessment and therapeutic monitoring of drug-resistant colorectal
cancer by MR molecular imaging of extradomain-B fibronectin.
Theranostics. 10:111272020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lewandowski S, Diao L, Quigley A,
Crochiere M and Pinkas J: EDB+ FN is an attractive therapeutic
target in oncology: Insights from protein expression analysis of
solid tumors. Cancer Res. 84 (6_Suppl):S29082004. View Article : Google Scholar
|
|
88
|
Qiao P, Ayat NR, Vaidya A, Gao S, Sun W,
Chou S, Han Z, Gilmore H, Winter JM and Lu ZR: Magnetic resonance
molecular imaging of extradomain B fibronectin improves imaging of
pancreatic cancer tumor xenografts. Front Oncol. 10:5867272020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Feng Y, Hao Y, Wang Y, Song W, Zhang S, Ni
D, Yan F and Sun L: Ultrasound molecular imaging of bladder cancer
via extradomain B Fibronectin-targeted biosynthetic GVs. Int J
Nanomedicine. 18:4871–4884. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang Y, Zheng X, Huang Y, Li S, Li X and
Zhu L: EDB-FN-targeted probes for near infrared fluorescent imaging
and positron emission tomography imaging of breast cancer in mice.
Sci Rep. 14:220562024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mohammadgholi M, Sadeghzadeh N, Erfani M,
Abediankenari S, Abedi SM, Emrarian I, Jafari N and Behzadi R:
Human fibronectin Extra-domain B (EDB)-Specific aptide (APTEDB)
radiolabelling with technetium-99m as a potent targeted
Tumour-imaging agent. Anticancer Agents Med Chem. 18:277–285. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Qiao PL, Gargesha M, Liu Y, Laney VEA,
Hall RC, Vaidya AM, Gilmore H, Gawelek K, Scott BB, Roy D, et al:
Magnetic resonance molecular imaging of extradomain B fibronectin
enables detection of pancreatic ductal adenocarcinoma metastasis.
Magn Reson Imaging. 86:37–45. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sergeeva O, Zhang Y, Gao S, Chan ER,
Sergeev M, Iyer R, Sexton S, Avril N, Lu ZR and Lee Z: PET imaging
of hepatocellular carcinoma using ZD2-(Ga-NOTA). J Hepatocell
Carcinoma. 10:291–301. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Han Z, Sergeeva O, Roelle S, Cheng H, Gao
S, Li Y, Lee Z and Lu ZR: Preparation and evaluation of ZD2 peptide
Cu-DOTA conjugate as a positron emission tomography probe for
detection and characterization of prostate cancer. ACS Omega.
4:1185–1190. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lu ZR, Laney V and Li Y: Targeted contrast
agents for magnetic resonance molecular imaging of cancer. Acc Chem
Res. 55:2833–2847. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Han Z, Sergeeva O, Roelle S, Cheng H, Gao
S, Li Y, Lee Z and Lu ZR: Preparation and evaluation of ZD2 Peptide
64Cu-DOTA conjugate as a positron emission tomography
probe for detection and characterization of prostate cancer. ACS
Omega. 4:1185–1190. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Han Z, Li Y, Roelle S, Zhou Z, Liu Y,
Sabatelle R, DeSanto A, Yu X, Zhu H, Magi-Galluzzi C and Lu ZR:
Targeted contrast agent specific to an oncoprotein in tumor
microenvironment with the potential for detection and risk
stratification of prostate cancer with MRI. Bioconjug Chem.
28:1031–1040. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ye XX, Zhao YY, Wang Q, Xiao W, Zhao J,
Peng YJ, Cao DH, Lin WJ, Si-Tu MY, Li MZ, et al: EDB
Fibronectin-Specific SPECT Probe 99mTc-HYNIC-ZD2 for breast cancer
detection. ACS Omega. 2:2459–2468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang W, Liang X, Zhang X, Tong W, Shi G,
Guo H, Jin Z, Tian J, Du Y and Xue H: Magnetic-optical
dual-modality imaging monitoring chemotherapy efficacy of
pancreatic ductal adenocarcinoma with a low-dose
fibronectin-targeting Gd-based contrast agent. Eur J Nucl Med Mol
Imaging. 51:1841–1855. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yang N, Huang Y, Wang X, Wang D, Yao D and
Ren GL: Fibronectin-targeting Dual-Modal MR/NIRF imaging contrast
agents for diagnosis of gastric cancer and peritoneal metastasis.
Bioconjug Chem. 35:843–854. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang MD, Lv GT, An HW, Zhang NY and Wang
H: In Situ Self-assembly of bispecific peptide for cancer
immunotherapy. angewandte chemie international edition. Angew Chem
Int Ed Engl. 61:e2021136492022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ranjbar L, Maleki F, Sadeghzadeh N,
Abediankenari S, Mardanshahi A and Masteri Farahani A: In vitro/in
vivo assessment of the targeting ability of [99mTc] Tc-labeled an
aptide specific to the extra domain B of fibronectin (APTEDB) for
colorectal cancer. Ann Nucl Med. 34:460–466. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ghaffari H, Atashzar MR and Abdollahi H:
Molecular imaging in tracking cancer stem cells: A review. Med J
Islam Repub Iran. 34:902020.PubMed/NCBI
|
|
104
|
Noh I, Son Y, Jung W, Kim M, Kim D, Shin
H, Kim YC and Jon S: Targeting the tumor microenvironment with
amphiphilic near-infrared cyanine nanoparticles for potentiated
photothermal immunotherapy. Biomaterials. 275:1209262021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Henze J: Immunotherapy of solid tumors:
Multimodal imaging strategies for chimeric antigen receptor T cell
tracking in the tumor microenvironment. Dissertation Göttingen:
Georg-August Universität; 2021
|
|
106
|
Sun Y, Kim HS, Park J, Li M, Tian L, Choi
Y, Choi BI, Jon S and Moon WK: MRI of breast tumor initiating cells
using the extra domain-B of fibronectin targeting nanoparticles.
Theranostics. 4:845–857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sun Y, Kim HS, Kang S, Piao YJ, Jon S and
Moon WK: Magnetic resonance Imaging-guided drug delivery to breast
cancer Stem-like cells. Adv Healthc Mater. 7:e18002662018.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Park J, Kim S, Saw PE, Lee IH, Yu MK, Kim
M, Lee K, Kim YC, Jeong YY and Jon S: Fibronectin extra domain
B-specific aptide conjugated nanoparticles for targeted cancer
imaging. J Control Release. 163:111–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jailkhani N, Ingram JR, Rashidian M,
Rickelt S, Tian C, Mak H, Jiang Z, Ploegh HL and Hynes RO:
Noninvasive imaging of tumor progression, metastasis and fibrosis
using a nanobody targeting the extracellular matrix. Proc Natl Acad
Sci USA. 116:187452019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Reeves KM: Applying PET imaging to cancer
immunotherapy to improve clinical outcomes. The University of
Alabama; Birmingham: 2022
|
|
111
|
Rossin R, Berndorff D, Friebe M,
Dinkelborg LM and Welch MJ: Small-animal PET of tumor angiogenesis
using a (76)Br-labeled human recombinant antibody fragment to the
ED-B domain of fibronectin. J Nucl Med. 48:1172–1179. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Berndorff D, Borkowski S, Moosmayer D,
Viti F, Müller-Tiemann B, Sieger S, Friebe M, Hilger CS, Zardi L,
Neri D and Dinkelborg LM: Imaging of tumor angiogenesis using
99mTc-labeled human recombinant anti-ED-B fibronectin antibody
fragments. J Nucl Med. 47:1707–1716. 2006.PubMed/NCBI
|
|
113
|
Zhao Q, Zong H, Zhu P, Su C, Tang W, Chen
Z and Jin S: Crosstalk between colorectal CSCs and immune cells in
tumorigenesis and strategies for targeting colorectal CSCs. Exp
Hematol Oncol. 13:62024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Morgos DT, Stefani C, Miricescu D, Greabu
M, Stanciu S, Nica S, Stanescu-Spinu II, Balan DG,
Balcangiu-Stroescu AE, Coculescu EC, et al: Targeting PI3K/AKT/mTOR
and MAPK signaling pathways in gastric cancer. Int J Mol Sci.
25:18482024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yu J, Li S, Chen D, Liu D, Guo H, Yang C,
Zhang W, Zhang L, Zhao G, Tu X, et al: SIRPα-Fc fusion protein
IMM01 exhibits dual anti-tumor activities by targeting CD47/SIRPα
signal pathway via blocking the ‘don't eat me’ signal and
activating the ‘eat me’ signal. J Hematol Oncol. 15:1672022.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li R, Wang Q, She K, Lu F and Yang Y:
CRISPR/Cas systems usher in a new era of disease treatment and
diagnosis. Mol Biomed. 3:312022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Dabas P and Danda A: Revolutionizing
cancer treatment: A comprehensive review of CAR-T cell therapy. Med
Oncol. 40:2752023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Johannsen M, Spitaleri G, Curigliano G,
Roigas J, Weikert S, Kempkensteffen C, Roemer A, Kloeters C,
Rogalla P, Pecher G, et al: The tumour-targeting human L19-IL2
immunocytokine: Preclinical safety studies, phase I clinical trial
in patients with solid tumours and expansion into patients with
advanced renal cell carcinoma. Eur J Cancer. 46:2926–2935. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Schliemann C, Börschel N, Schwöppe C,
Liersch R, Kessler T, Dreyling M, Klapper W, Menssen HD, Neri D,
Berdel WE and Mesters RM: Targeting Interleukin-2 to the
neovasculature potentiates Rituximab's activity against mantle cell
lymphoma in mice. Blood. 120:3716. 2012. View Article : Google Scholar
|
|
120
|
Orecchia P, Balza E, Pietra G, Conte R,
Bizzarri N, Ferrero S, Mingari MC and Carnemolla B: L19-IL2
immunocytokine in combination with the anti-syndecan-1 46F2SIP
antibody format: A new targeted treatment approach in an ovarian
carcinoma model. Cancers (Basel). 11:12322019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Schliemann C, Palumbo A, Zuberbühler K,
Villa A, Kaspar M, Trachsel E, Klapper W, Menssen HD and Neri D:
Complete eradication of human B-cell lymphoma xenografts using
rituximab in combination with the immunocytokine L19-IL2. Blood.
113:2275–2283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Weide B, Eigentler T, Catania C, Ascierto
PA, Cascinu S, Becker JC, Hauschild A, Romanini A, Danielli R,
Dummer R, et al: A phase II study of the L19IL2 immunocytokine in
combination with dacarbazine in advanced metastatic melanoma
patients. Cancer Immunol Immunother. 68:1547–1559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Van Limbergen EJ, Hoeben A, Lieverse RIY,
Houben R, Overhof C, Postma A, Zindler J, Verhelst F, Dubois LJ, De
Ruysscher D, et al: Toxicity of L19-Interleukin 2 Combined with
Stereotactic Body Radiation Therapy: A Phase 1 Study. Int J Radiat
Oncol Biol Phys. 109:1421–1430. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lieverse RIY, Van Limbergen EJ, Oberije
CJG, Troost EGC, Hadrup SR, Dingemans AC, Hendriks LEL, Eckert F,
Hiley C, Dooms C, et al: Stereotactic ablative body radiotherapy
(SABR) combined with immunotherapy (L19-IL2) versus standard of
care in stage IV NSCLC patients, ImmunoSABR: A multicentre,
randomised controlled open-label phase II trial. BMC Cancer.
20:5572020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang S, Mills D, Kim JY, Knudsen N,
Nelson J, Buechler Y and Skidmore L: 41P Evaluation of ARX517: A
next-generation anti-PSMA antibody drug conjugate for prostate
cancer treatment, in preclinical enzalutamide-resistant and
enzalutamide-sensitive pharmacology models and in toxicology
models. Ann Oncol. 34 (Suppl 2):S1992023. View Article : Google Scholar
|
|
126
|
Nakada T, Sugihara K, Jikoh T, Abe Y and
Agatsuma T: The latest research and development into the
antibody-drug conjugate, (fam-)Trastuzumab Deruxtecan (DS-8201a),
for HER2 cancer therapy. Chem Pharm Bull (Tokyo). 67:173–185. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ceci C, Lacal PM and Graziani G:
Antibody-drug conjugates: Resurgent anticancer agents with
multi-targeted therapeutic potential. Pharmacol Ther.
236:1081062022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Rudman SM, Jameson MB, McKeage MJ, Savage
P, Jodrell DI, Harries M, Acton G, Erlandsson F and Spicer JF: A
phase 1 study of AS1409, a novel Antibody-cytokine fusion protein,
in patients with malignant melanoma or renal cell carcinoma. Clin
Cancer Res. 17:1998–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ongaro T, Gouyou B, Stringhini M,
Corbellari R, Neri D and Villa A: A novel format for recombinant
antibody-interleukin-2 fusion proteins exhibits superior
tumor-targeting properties in vivo. Oncotarget. 11:3698–3711. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wilks S, Carneiro BA, Coté GM, Henry J,
Sen S, Spira AL, Tsai F YC, Wang JS, Crochiere M, He S, et al: 762
A first-in-human phase 1 clinical study evaluating safety,
tolerability, pharmacokinetics, pharmacodynamics and efficacy of
the EDB+FN targeting ADC PYX-201 in participants with advanced
solid tumors. J Immunother Cancer. 11 (Suppl 1):A1–A1731. 2023.
|
|
131
|
Hooper AT, Marquette K, Chang CB, Golas J,
Jain S, Lam MH, Guffroy M, Leal M, Falahatpisheh H, Mathur D, et
al: Anti-extra Domain B splice variant of fibronectin antibody-drug
conjugate eliminates tumors with enhanced efficacy when combined
with checkpoint blockade. Mol Cancer Ther. 21:1462–1472. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Trachsel E, Bootz F, Silacci M, Kaspar M,
Kosmehl H and Neri D: Antibody-mediated delivery of IL-10 inhibits
the progression of established collagen-induced arthritis.
Arthritis Res Ther. 9:R92007. View
Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kaspar M, Trachsel E and Neri D: The
antibody-mediated targeted delivery of interleukin-15 and GM-CSF to
the tumor neovasculature inhibits tumor growth and metastasis.
Cancer Res. 67:4940–4948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ruan JI: Blocking leptin-STAT3
axis-induced fatty acid oxidation: A novel approach to activate
CD8+ T effector cells in breast cancer. Thoracic Cancer.
11:3422–3424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Di Nitto C, Gilardoni E, Mock J, Nadal L,
Weiss T, Weller M, Seehusen F, Libbra C, Puca E, Neri D and De Luca
R: An engineered IFNγ-antibody fusion protein with improved
tumor-homing properties. Pharmaceutics. 15:3772023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Niu J, Kaufman HL, Kichenadasse G, Haydon
AM, Barve MA, Ganju V, Iannotti Buchbinder E, Spira AI, Pang W, Fu
W, et al: Updated results from an ongoing phase 1/2a study of
T3011, an oncolytic HSV expressing IL-12 and PD-1 antibody,
administered via IT injection as monotherapy or combined with
pembrolizumab in advanced solid tumors. J Clin Oncol. 41
(16_suppl):95352023. View Article : Google Scholar
|
|
137
|
Danielli R, Patuzzo R, Di Giacomo AM,
Gallino G, Maurichi A, Di Florio A, Cutaia O, Lazzeri A, Fazio C,
Miracco C, et al: Intralesional administration of L19-IL2/L19-TNF
in stage III or stage IVM1a melanoma patients: Results of a phase
II study. Cancer Immunol Immunother. 64:999–1009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Papadia F, Basso V, Patuzzo R, Maurichi A,
Di Florio A, Zardi L, Ventura E, González-Iglesias R, Lovato V,
Giovannoni L, et al: Isolated limb perfusion with the
tumor-targeting human monoclonal antibody-cytokine fusion protein
L19-TNF plus melphalan and mild hyperthermia in patients with
locally advanced extremity melanoma. J Surg Oncol. 107:173–179.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Spitaleri G, Berardi R, Pierantoni C, De
Pas T, Noberasco C, Libbra C, González-Iglesias R, Giovannoni L,
Tasciotti A, Neri D, et al: Phase I/II study of the
tumour-targeting human monoclonal antibody-cytokine fusion protein
L19-TNF in patients with advanced solid tumours. J Cancer Res Clin
Oncol. 139:447–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Spaeth N, Wyss MT, Pahnke J, Biollaz G,
Trachsel E, Drandarov K, Treyer V, Weber B, Neri D and Buck A:
Radioimmunotherapy targeting the extra domain B of fibronectin in
C6 rat gliomas: A preliminary study about the therapeutic efficacy
of iodine-131-labeled SIP (L19). Nucl Med Biol. 33:661–666. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Saif A, Rossi AJ, Sarnaik A, Hernandez JM
and Zager JS: Efficacy of neoadjuvant intratumoral
Darleukin/Fibromun (L19IL2 + L19TNF) in patients with clinical
stage IIIB/C melanoma (Neo-DREAM). Ann Surg Oncol. 29:3377–3378.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Hauschild A, Hassel JC, Ziemer M,
Rutkowski P, Meier FE, Flatz L, Gaudy-Marqueste C, Santinami M,
Russano F, von Wasielewski I, et al: Phase 3 study (PIVOTAL) of
neoadjuvant intralesional daromun vs. immediate surgery in fully
resectable melanoma with regional skin and/or nodal metastases. J
Clin Oncol. 42 (17_suppl):LBA95012024. View Article : Google Scholar
|
|
143
|
Borga G, Sucre S, Sucre O, Vivas L,
Salazar H and Sucre CE: Use of tumor mutational burden as a
predictive marker of response to immuno-oncology agents: Initial
experience at an academic center in Venezuela. J Clin Oncol. 41
(16_suppl):e146132023. View Article : Google Scholar
|
|
144
|
Park SE: Design and Sythesis of conjugages
of amphiphilic cell-penetrating peptides containing anticancer drug
and ligand for extra cellular matrix biomarker to provide efficient
tumor-targeting. Irvine, CA: Chapman University; 2021
|
|
145
|
Yu B, Hwang D, Jeon H, Kim H, Lee Y, Keum
H, Kim J, Lee DY, Kim Y, Chung J and Jon S: A hybrid platform based
on a bispecific peptide-antibody complex for targeted cancer
therapy. Angew Chem Int Ed Engl. 58:2005–2010. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Park SE, El-Sayed NS, Shamloo K, Lohan S,
Kumar S, Sajid MI and Tiwari RK: Targeted delivery of cabazitaxel
using cyclic cell-penetrating peptide and biomarkers of
extracellular matrix for prostate and breast cancer therapy.
Bioconjug Chem. 32:1898–1914. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yu B, Yoo D, Kim KH, Kim TW, Park S, Kim
Y, Son Y, Kim J, Noh I, Whang CH, et al: Effective combination
immunotherapy through vessel normalization using a cancer-targeting
antiangiogenic peptide-antibody hybrid. Advanced Therapeutics:.
5:21001512022. View Article : Google Scholar
|
|
148
|
Park SE, Shamloo K, Kristedja TA, Darwish
S, Bisoffi M, Parang K and Tiwari RK: EDB-FN targeted peptide-drug
conjugates for use against prostate cancer. Int J Mol Sci. 20:3291.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Kim H, Hwang D, Choi M, Lee S, Kang S, Lee
Y, Kim S, Chung J and Jon S: Antibody-assisted delivery of a
peptide-drug conjugate for targeted cancer therapy. Mol Pharm.
16:165–172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kim YJ, Bae J, Shin TH, Kang SH, Jeong M,
Han Y, Park JH, Kim SK and Kim YS: Immunoglobulin Fc-fused,
neuropilin-1-specific peptide shows efficient tumor tissue
penetration and inhibits tumor growth via anti-angiogenesis. J
Control Release. 216:56–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hong Y, Gao D, Zhao B, Ma J, Yang Z and
Guo H: Thermal immuno-nanomedicine: A new strategy for cancer
treatment. Clin Transl Med. 23:e12562023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Saw PE, Zhang A, Nie Y, Zhang L, Xu Y and
Xu X: Tumor-associated fibronectin targeted liposomal nanoplatform
for cyclophilin A siRNA delivery and targeted malignant
glioblastoma therapy. Front Pharmacol. 9:11942018. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Javid H, Oryani MA, Rezagholinejad N,
Hashemzadeh A and Karimi-Shahri M: Unlocking the potential of
RGD-conjugated gold nanoparticles: A new frontier in targeted
cancer therapy, imaging, and metastasis inhibition. J Mater Chem B.
12:10786–10817. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Saw PE, Xu X, Kang BR, Lee J, Lee YS, Kim
C, Kim H, Kang SH, Na YJ, Moon HJ, et al: Extra-domain B of
fibronectin as an alternative target for drug delivery and a cancer
diagnostic and prognostic biomarker for malignant glioma.
Theranostics. 11:941–957. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Yu M, Leitao R, Xu X, Saw PE, Ortega CA,
Si K, Ahn S, Liu J, Lotfi A, Lee I-H, et al: Fibronectin
Extradomain B (FN-EDB) expression is specific to the
atherosclerotic lesion types III, IV, and V, and the FN-EDB
targeting nanomedicine enhances atherosclerotic plaque detection
and local delivery of model drug cargo. Arteriosclerosis Thrombosis
Vascular Biol. 37 (Suppl_1):A4672017. View Article : Google Scholar
|
|
156
|
Zhou Y, Qian M, Li J, Ruan L, Wang Y, Cai
C, Gu S and Zhao X: The role of tumor-associated macrophages in
lung cancer: From mechanism to small molecule therapy. Biomed
Pharmacother. 170:116014. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Lee JH, Carlino MS and Rizos H: Dosing of
BRAF and MEK inhibitors in melanoma: No point in taking a break.
Cancer Cell. 38:779–781. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Kapoor AR and Mittal V: Immunoregulatory
role of club cell secretory proteins in non-small cell lung cancer.
Cancer Res. 83 (7_Suppl):44242023. View Article : Google Scholar
|
|
159
|
Ji P, Gong Y, Jin M, Hu X, Di G and Shao
Z: 23P in vivo multi-dimensional CRISPR screens identify LGALS2 as
an immunotherapy target in triple-negative breast cancer. Ann
Oncol. 33:S1332022. View Article : Google Scholar
|
|
160
|
Canè S, Barouni RM, Fabbi M, Cuozzo J,
Fracasso G, Adamo A, Ugel S, Trovato R, De Sanctis F, Giacca M, et
al: Neutralization of NET-associated human ARG1 enhances cancer
immunotherapy. Sci Transl Med. 15:eabq62212023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Baumgartner CK, Ebrahimi-Nik H,
Iracheta-Vellve A, Hamel KM, Olander KE, Davis TGR, McGuire KA,
Halvorsen GT, Avila OI, Patel CH, et al: The PTPN2/PTPN1 inhibitor
ABBV-CLS-484 unleashes potent anti-tumour immunity. Nature.
622:850–862. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Jogalekar MP, Rajendran RL, Khan F, Dmello
C, Gangadaran P and Ahn BC: CAR T-cell-based gene therapy for
cancers: New perspectives, challenges, and clinical developments.
Front Immunol. 13:9259852022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Tang J, Liu N, Zhu Y, Li Y and Zhao X:
CAR-T therapy targets extra domain b of fibronectin positive solid
tumor cells. Immunol Invest. 52:985–996. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Zhang Z, Liu C, Wang M, Sun R, Yang Z, Hua
Z, Wu Y, Wu M, Wang H, Qiu W, et al: Treating solid tumors with
TCR-based chimeric antigen receptor targeting extra domain
B-containing fibronectin. J Immunother Cancer. 11:e0071992023.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Gentile D, Orlandi P, Banchi M and Bocci
G: Preclinical and clinical combination therapies in the treatment
of anaplastic thyroid cancer. Med Oncol. 37:192020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhang S, Hao R, Wang H, Yi QY, Yantao Y,
Zhong Y and Sun M: Peri cruiser CAR-T: An innovative platform to
reduce on-target off-tumor toxicity of CAR-T therapy. J Clin Oncol.
41 (16_suppl):25392023. View Article : Google Scholar
|
|
167
|
Zhang Z, Liu C, Yang Z and Yin H:
CAR-T-cell therapy for solid tumors positive for fibronectin extra
Domain B. Cells. 11:28632022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Martínez Bedoya D, Gustave R, Corlazzoli
F, Dutoit V and Migliorini D: 53P A multispecific non-integrating
RNA CAR T platform to overcome the clinical challenge of
glioblastoma heterogeneity. Ann Oncol. 32:S13952021. View Article : Google Scholar
|
|
169
|
Parikh RH and Lonial S: Chimeric antigen
receptor T-cell therapy in multiple myeloma: A comprehensive review
of current data and implications for clinical practice. CA Cancer J
Clin. 73:275–285. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Xiong Q, Wang H, Shen Q, Wang Y, Yuan X,
Lin G and Jiang P: The development of chimeric antigen receptor
T-cells against CD70 for renal cell carcinoma treatment. J Transl
Med. 22:3682024. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Zhu G, Zhang J, Zhang Q, Jin G, Su X, Liu
S and Liu F: Enhancement of CD70-specific CAR T treatment by IFN-γ
released from oHSV-1-infected glioblastoma. Cancer Immunol
Immunother. 71:2433–2448. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Wu G, Guo S, Luo Q, Wang X, Deng W, Ouyang
G, Pu JJ, Lei W and Qian W: Preclinical evaluation of CD70-specific
CAR T cells targeting acute myeloid leukemia. Front Immunol.
14:10937502023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Li X, Zhu T, Wang R, Chen J, Tang L, Huo
W, Huang X and Cao Q: Genetically programmable vesicles for
enhancing CAR-T therapy against solid tumors. Adv Mater.
35:e22111382023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Li Y, Liu Y, Cui Q, Liu L, Li Z, Cui W, Li
M, Zhu X, Kang L, Yu L, et al: Rituximab improves clinical outcomes
of CAR-T therapy for r/r B-ALL via sensitizing leukemia cells to
CAR-T-mediated cytotoxicity and reducing CAR-T exhaustion. Blood.
142 (Suppl 1):68032023. View Article : Google Scholar
|
|
175
|
Simmons ME, McIntosh J, Zhang T, Li Y, Yan
F, Yao Y, Nie L, Lee HH, Wang W, Jiang VC, et al: The reversible
BTK inhibitor nemtabrutinib demonstrates favorable antitumor
efficacy and enhances the function of CAR T cells in mantle cell
lymphoma. Blood. 142 (Suppl 1):57892023. View Article : Google Scholar
|
|
176
|
Gholamrezanezhad A, Shooli H, Jokar N,
Nemati R and Assadi M: Radioimmunotherapy (RIT) in brain tumors.
Nucl Med Mol Imaging. 53:374–381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Leung K: I-Human recombinant anti-ED-B
fibronectin antibody small immunoprotein. Molecular Imaging and
Contrast Agent Database (MICAD) Bethesda (MD): National Center for
Biotechnology Information (US); April 17–2007, PubMed/NCBI
|
|
178
|
Borsi L, Balza E, Bestagno M, Castellani
P, Carnemolla B, Biro A, Leprini A, Sepulveda J, Burrone O, Neri D
and Zardi L: Selective targeting of tumoral vasculature: Comparison
of different formats of an antibody (L19) to the ED-B domain of
fibronectin. Int J Cancer. 102:75–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Li R, He H, Li X, Zheng X, Li Z, Zhang H,
Ye J, Zhang W, Yu C, Feng G and Fan W: EDB-FN targeted probes for
the surgical navigation, radionuclide imaging and therapy of
thyroid cancer. Eur J Nucl Med Mol Imaging. 50:2100–2113. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Tijink BM, Perk LR, Budde M, Stigter-van
Walsum M, Visser GW, Kloet RW, Dinkelborg LM, Leemans CR, Neri D
and van Dongen GA: (124)I-L19-SIP for immuno-PET imaging of tumour
vasculature and guidance of (131)I-L19-SIP radioimmunotherapy. Eur
J Nucl Med Mol Imaging. 36:1235–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Moosmayer D, Berndorff D, Chang CH,
Sharkey RM, Rother A, Borkowski S, Rossi EA, McBride WJ, Cardillo
TM, Goldenberg DM and Dinkelborg LM: Bispecific antibody
pretargeting of tumor neovasculature for improved systemic
radiotherapy of solid tumors. Clin Cancer Res. 12:5587–5595. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Del Conte G, Erba PA, Fasolo A, Chiesa C,
Grana C, Menssen H, Neri D, Mariani G, Bombardieri E and Gianni L:
Radioimmunotherapy (RIT) with 131l-L19SIP in solid
cancers (SC) and lymphoproliferative diseases: Final results of the
first human trial. J Clin Oncol. 28 (15_suppl):25232010. View Article : Google Scholar
|
|
183
|
Petrini I, Sollini M, Bartoli F, Barachini
S, Montali M, Pardini E, Burzi IS and Erba PA: ED-B-containing
isoform of fibronectin in tumor microenvironment of thymomas: A
target for a theragnostic approach. Cancers (Basel). 14:25922022.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Miura JT and Zager JS: Neo-DREAM study
investigating Daromun for the treatment of clinical stage IIIB/C
melanoma. Future Oncol. 15:3665–3674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Vaidya AM, Wang H, Qian V and Lu ZR:
Extradomain-B Fibronectin is a molecular marker of invasive breast
cancer cells. bioRxiv. Aug 22–2019.doi: 10.1101/743500.
|