Perimenopause is the stage through which women pass
as they move from adulthood to old age. During this period, due to
the decline in ovarian function and the decrease in estrogen
levels, women develop a group of syndromes called perimenopausal
syndromes, which are characterized by disorders of the vegetative
nervous system accompanied by neuropsychological symptoms (1). Perimenopausal syndrome is commonly
accompanied by significant mood disorder problems (2,3).
Depression and anxiety are considered a harmful mood disorder
characterized by persistent feelings of worry, despair, tension and
distress, accompanied by physical symptoms such as tachycardia,
nervousness and an inability to relax (4–6).
Medication and psychotherapy are the conventional treatments for
perimenopausal depression, with the former being considered the
standard treatment and the latter being insufficient on its own in
most cases (7). Tricyclic
antidepressants, monoamine oxidase inhibitors and selective
serotonin reuptake inhibitors are main conventional antidepressant
medications used to treat perimenopausal depression (8–10).
New pharmacotherapies, including serotonin and norepinephrine
reuptake inhibitors, glutamatergic agents and selective estrogen
receptor modulators, have also been developed in recent years
(11,12).
The monoamine hypothesis, currently important in the
field of depression, was proposed after the discovery of the
antidepressant effects of monoamine oxidase inhibitors (MAOIs)
(13). According to it, depression
is caused by low levels of serotonin, norepinephrine and dopamine
in the central nervous system. By increasing these neurotransmitter
concentrations, MAOIs and tricyclic antidepressants alleviate
depression symptoms (14).
Furthermore, patients with depression have lower plasma tryptophan
levels and less serotonin in the cerebrospinal fluid. As a result,
serotonin was also included in this hypothesis, leading to the
development of selective serotonin reuptake inhibitors (15). It seems most individuals are in
agreement that boosting monoamine levels would alleviate depressive
symptoms. However, we still do not understand more about this
hypothesis or know how to find new drugs that target the central
monoamine system.
In recent years, an increasing number of studies
have focused on the role of neuroinflammation in the perimenopausal
depression (16–19). There are several processes
associated with neurodegeneration that may contribute to
neuroprogression, including apoptosis, decreased neurogenesis,
reduced neuronal plasticity and an increase in autoimmune responses
(20). Elevated oxidative stress
markers and a neuroinflammatory signature are consistently observed
in the blood of individuals with depression (21). The present study mainly introduced
the roles of neuroinflammation and oxidative stress in
perimenopausal depression and then analyzed potential
anti-inflammatory and anti-oxidative stress mechanisms for
estrogen, acupuncture and Chinese medicines.
Recently, the peripheral immune system has been
found to play an important role in neuroinflammation. Activated
endothelial cells cause disruption of the blood-brain barrier,
allowing peripheral immune cells to infiltrate the CNS and
transendothelial migration of immune cells tends to exacerbate
neuroinflammation (22).
Inflammatory differentiation of monocytes in the central nervous
system by dendritic cells and macrophages drives neuroinflammation
and transforms neuroglia into a deleterious phenotype, even leading
directly to nerve damage (23).
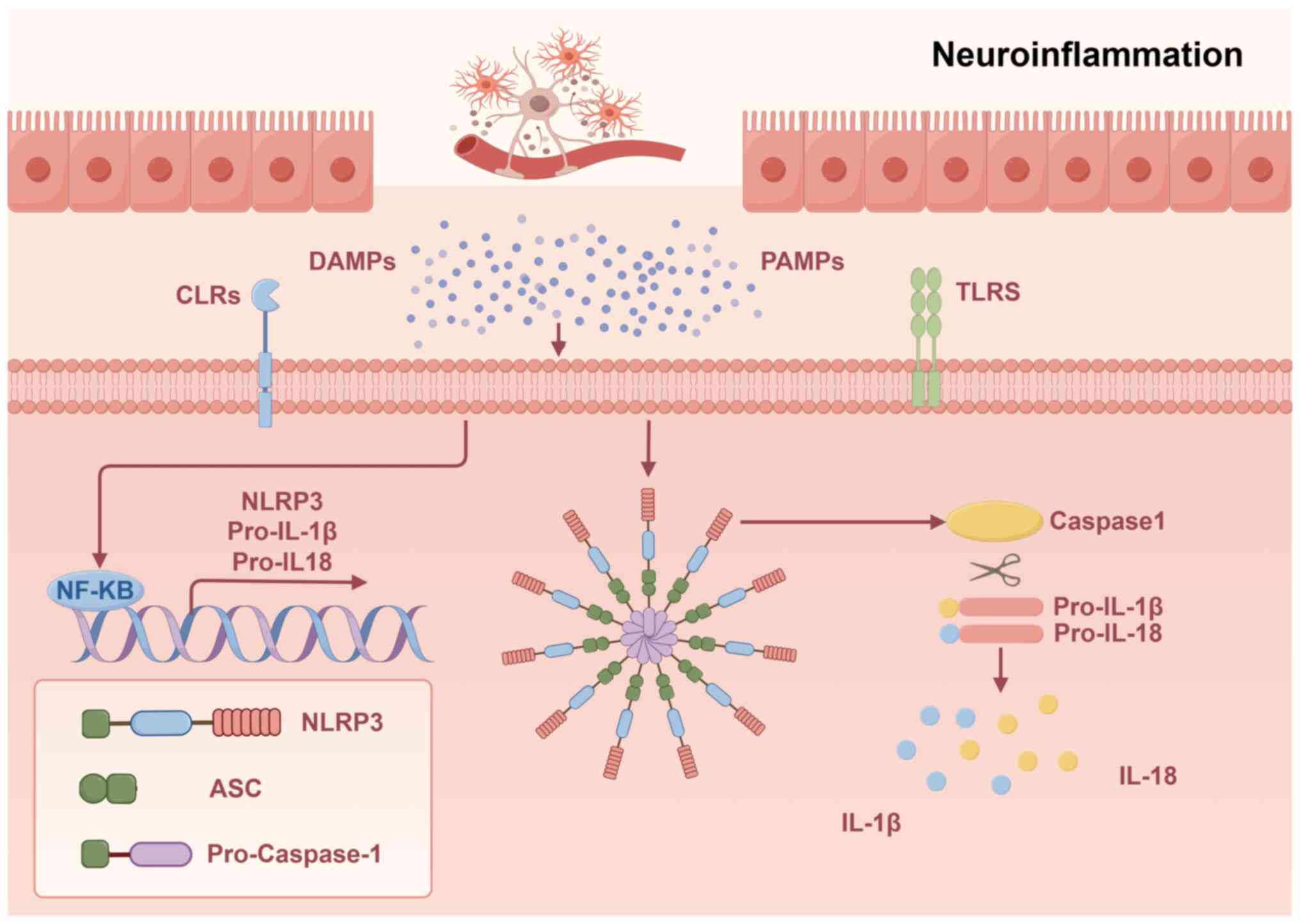
Over-activated microglia release TNF-α and IL-1β, while activating
peripheral T cells into the CNS, creating a positive feedback loop.
A significant increase in proinflammatory cytokines secreted by CD4
T cells is associated with perimenopause, including IL-8 and TNF-α
(24). Among perimenopausal women,
estradiol levels in peripheral serum correlate inversely with serum
IL-8, TNF-α and microglial and astrocytic reactivity (25). Moreover, estrogen deficiency can
activate immune cells, resulting in proinflammatory cytokine
milieus, such as IL-1, IL-6 and TNF-α (26). The interleukins area very important
family of cytokines. IL-1 is one of the most common and potent
inflammatory factors of the family and IL-1β is the major secreted
form of IL-1, which plays an important role in the inflammatory
process (Fig. 1).
As an important part of the limbic system, the
hippocampus plays an important role in spatial and contextual
memory. There is relatively high expression of IL-1, its receptor
(IL-1R) and the natural IL-1R antagonist (IL-1RA) in the
hippocampus, indicating their potential to modulate hippocampal
memory functions (27). As a
result, excessive or dysregulated IL-1 signaling may be associated
with deficits in hippocampus-dependent memory processes (28). Glutamate, a neurotransmitter
converted into gamma-aminobutyric acid (GABA) by glutamic acid
decarboxylase (GAD), can be affected by pro-inflammatory cytokines
(29). The influence of
pro-inflammatory cytokines occurs through complex interactions with
N-methyl-D-aspartate (NMDA) and
amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors (30). It has been shown that chronic
elevations in IL-1 induced by intraventricular infusions of
lipopolysaccharide (LPS) reduce both NMDAR-dependent and
NMDAR-independent forms of long-term potentiation in the
hippocampal region (31).
Hippocampal-dependent memory is mediated by long-term potentiation,
which is triggered by high-frequency stimulation and theta bursts.
In an autoimmune encephalomyelitis mouse model, activated microglia
secreted IL-1β that suppressed GABAergic inhibitory transmission,
which may lead to neuroplastic damage (32). IL-6 plays an important role in
regulating the age-related loss of critical GABAergic interneurons
that express parvalbumin by increasing the superoxide production of
neuronal NADPH-oxidase and cognitive performance in aging mice is
preserved by this effect (33). A
key role for cytokines is their ability to mediate interactions
between immune cells and the neuroendocrine system, especially
through the hypothalamus-pituitary-adrenal (HPA) axis and the
effects it has on immunosuppressive mechanisms (34,35).
The increase in dialysate noradrenaline induced by IL-1β
corresponds partly to the increase in plasma adrenocorticotropic
hormone (ACTH) and corticosterone, supporting an association
between noradrenaline, IL-1β and HPA axis activation (26). It has also been demonstrated that
IL-1 can stimulate the hypothalamic corticotropin-releasing factor,
thereby increasing ACTH and glucocorticoids secretion and, finally,
can cause glucocorticoid resistance and disrupt HPA axis function
(36).
The aforementioned inflammatory cytokines may not be
the only cytokines related to perimenopausal depression. TGF-β may
play an important role as well. In multicellular organisms,
TGF-signaling promotes embryonic development, tissue homeostasis
and damage repair through coordinated effects on cell
proliferation, phenotypic plasticity, migration and metabolic
adaptation (37–40). Defective TGF-β signaling
specifically affects epithelial cells, tissue fibroblasts and
immune cells, disrupting immune tolerance, promoting inflammation
and making disease treatment more difficult. When adult mice are
deficient in TGF-β, they are more susceptible to excitotoxic damage
and develop an increased number of degenerating neurons.
Synaptophysin and laminin are also reduced (41).
Oxidative stress is an important pathological factor
in depression and aging and is a common feature of various chronic
diseases (42). Depressive
disorders are associated with oxidative stress, an imbalance
between oxidative and antioxidant reactions in the body. Excess
reactive oxygen species (ROS) play a significant role in this
process (43,44). Perimenopause in women is the
process of aging, which is accompanied by a weakening of
antioxidant activity, allowing the accumulation of ROS, which can
trigger processes such as inflammation, neurodegeneration, tissue
impairment and cell death (45).
Another study has shown that individuals with lower intake of
antioxidants have a higher risk of depression (46). Other researchers have suggested
that an imbalance between 5-hydroxytryptaminergic and noradrenergic
neurotransmitters plays a key role in the pathogenesis of
depression (47). Some researchers
have found that chronically activated microglia in the central
nervous system release large amounts of inflammatory cytokines
(48). Activated microglia produce
a variety of pro-inflammatory cytokines in response to inflammation
and stress that contribute to depressive behaviors by evoking an
imbalance of 5-hydroxytryptaminergic and noradrenergic
neurotransmission via the HPA axis or by decreasing
5-hydroxytryptamine levels through increased
indoleamine-2,3-deoxygenase activity (49).
In spite of the fact that oxygen is essential for
neurons, some of its products can be neurotoxic in the brain
(50). An excessive level of ROS
disrupts neural cytoarchitecture and affects the function of
multiple biomolecules, including lipids, proteins and nucleic
acids. This results in some undergoing modifications and becoming
anti-inflammatory, while others forming and becoming
pro-inflammatory (20). ROS also
damage nuclear and mitochondrial DNA by modifying bases, reducing
purines, destroying ribose sugar and altering DNA-protein cross
connections, as well as damaging DNA repair systems (51). As a result of all of these changes,
genetic regulation can be altered and programmed cell death can
occur. When DNA damage is not repaired, neurons may silence
expression of the affected genomic region, which is important for
cell survival (52). During a
specific transformation of one of the DNA bases,
8-hydroxydeoxyguanosine is produced, which is widely used as a
marker of DNA damage in clinical studies and elevated in those who
are depressed (53,54).
A significant increase in oxidative stress is
observed in postmenopausal women compared with premenopausal women
by detecting derivatives of reactive oxygen metabolites and the
biological antioxidant potential (55). In addition, higher levels of
malonaldehyde, 4-hydroxynenal and oxidized lipoproteins and
decreased levels of glutathione peroxidase are shown in
postmenopausal women compared with fertile women, indicating that
oxidative stress levels rise while antioxidant enzymes are
synthesized less during postmenopause (56). Similarly, women who undergo total
hysterectomy with bilateral salpingo-oophorectomy show increased
oxidative stress and reduced mRNA expression of both superoxide
dismutase and glutathione peroxidases. By contrast, estrogen
replacement therapy can prevent and counteract these modifications
by regulating antioxidant gene expression (57). According to these results, estrogen
regulates antioxidant enzyme synthesis in the body, reducing
oxidative stress levels in women.
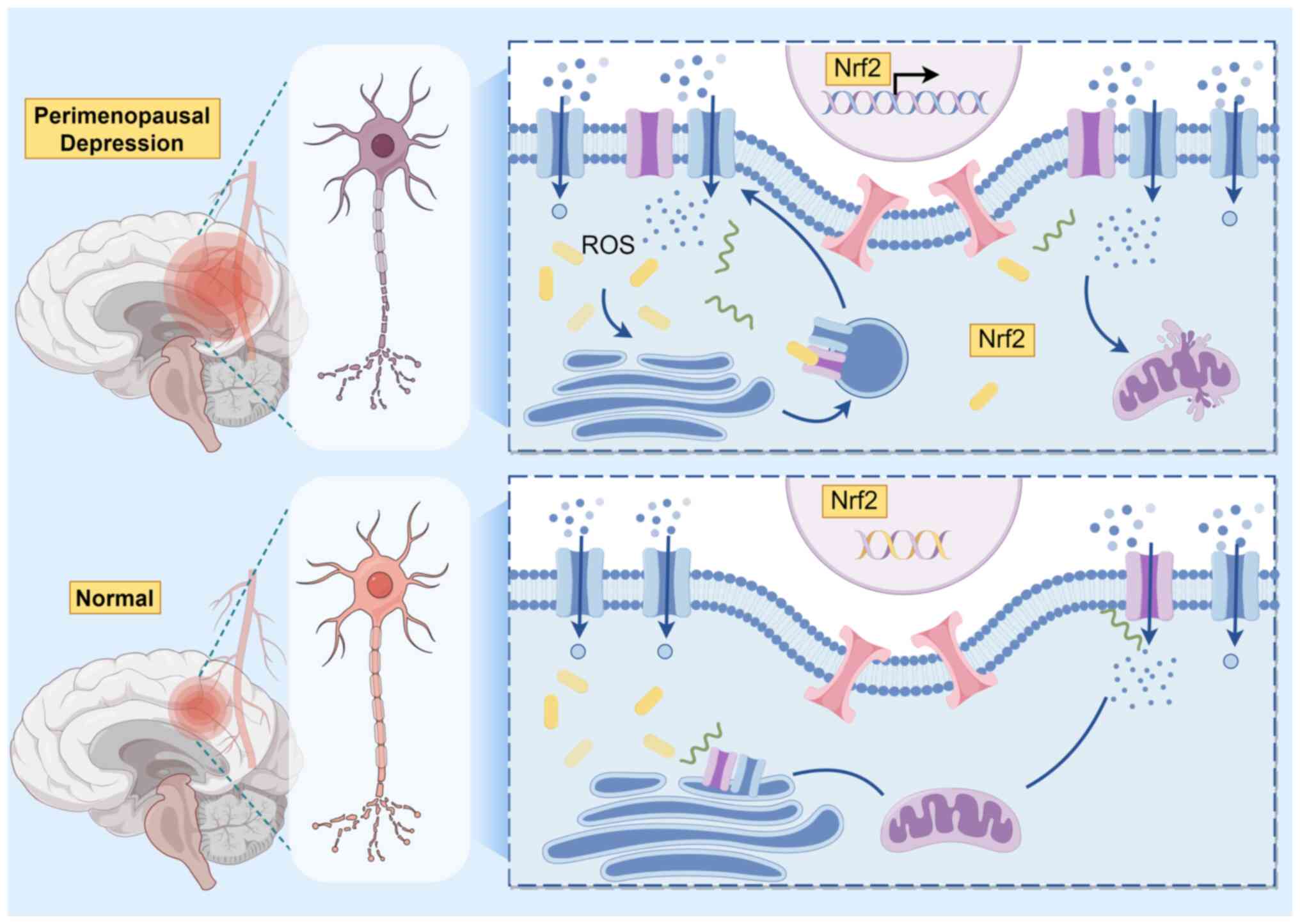
Nuclear factor erythroid 2-related factor 2 (Nrf2),
the main cellular defense pathway that is activated in a wide range
of cell types because of oxidative stress, induces the production
of antioxidants and enzymes to fight apoptosis (58). The Kelch-like ECH-associated
protein 1 binds to Nrf2 in the cytoplasm, degrading it through
ubiquitination in unstressed conditions (20). When oxidative stress occurs, Nrf2
moves to nucleus where initiates genes transcription coding for
proteins with cytoprotective effects (Fig. 2) (59). It also regulates key regulatory
molecules, including protective proteins, such as brain-derived
neurotrophic factor and IL-10 (60). It has been suggested that Nrf2
might be a potential target in the treatment of neurodegenerative
diseases and depression because of its wide range of antioxidant
effects (61–63). Using microarrays, researchers
discovered that Nrf2 plays an important role in regulating immune
and inflammation genes, along with growth factors, signaling
proteins and neuron-specific genes (64). Inflammation caused by a deletion of
Nrf2 can produce a depressive-like phenotype, while induction of
Nrf2 may provide a new approach to developing antidepressants
(65). Recent findings indicate
that a menopause depression mouse model induced by activation of
the Nrf2/HO-1 pathway shows significant amelioration of
depression-like behaviors (66).
A growing body of research suggests that estrogen
has a protective effect on the central nervous system and that
estrogen hormone therapy may play a role in the treatment of
perimenopausal depression (9).
Decreased estrogen levels can lead to the activation of immune
cells resulting in the production of a pro-inflammatory cytokine
milieu and these peripheral cytokines can negatively affect the
central nervous system by entering the brain directly or indirectly
through various pathways (67). A
study by Maggioli et al (68) found that elevated levels of the
anti-inflammatory protein Annexin A1 in the central nervous system
of model mice treated with estradiol modulate the blood-brain
barrier, reduce parietal endothelial cell permeability and inhibit
the migration of lymphocytes to the brain.
Estrogen is derived from the adrenal glands in
addition to the ovaries. When a woman enters perimenopause, the
production of estrogen by the ovaries decreases and estrogen is
mainly regulated by the adrenal glands, where the role of the
hypothalamus-pituitary-adrenal (HPA axis) is particularly important
(69). Decreased estrogen levels
exacerbate hyperactivity of the HPA axis, which is commonly seen in
patients with depression (70).
The inflammatory cytokines IL-1, IL-6 and TNF-α have been shown to
activate the HPA axis (71).
Decreased estrogen levels lead to decreased levels of serotonin and
dopamine which further stimulate the HPA axis while also making the
inflammatory response in vivo more persistent (72).
A therapeutic effect of estrogen on perimenopausal
depression or clinical symptoms of perimenopausal depression has
been demonstrated in several previous clinical trials (73,74).
In addition, a clinical trial has shown that the combination of
estrogen and antidepressants is more effective in alleviating
perimenopausal depression than estrogen or antidepressants alone
(75,76).
Previous research has shown that depression in women
is associated with dramatic fluctuations in ovarian hormones in
both clinical and preclinical studies (89–91).
However, the controversy over hormone replacement therapy has never
ceased in recent years because of the risk of cancer and other
cardiovascular diseases (92–94).
In China, individuals have been using Chinese medicines to
alleviate illness for thousands of years. Modern medical research
has shown that traditional compound Chinese medicines can
effectively treat perimenopausal depression (95,96).
Erxian decoction (EXD) is a well-known and empirical
Chinese herbal formula of six herbs developed by Bernard Chang in
the early 1950s. It is used for the treatment of perimenopausal
syndrome in women, including perimenopausal depression (98). Zhang et al (99) investigated the antidepressant
effects of EXD on perimenopausal mice. In behavioral tests, EXD
improved spatial memory in mice with depressive symptoms. EXD
reduced serum levels of FSH, luteinizing hormone and IL-6. This
meant that EXD reduced oxidative stress as well as inflammation
levels in mice. In addition, this study found that EXD markedly
upregulated the expression of brain-derived neurotrophic factor and
Bcl-2 in the hippocampus, as well as the expression of estrogen
receptor in the uterus and adrenal glands, antagonizing the
symptoms of estrogen deficiency in mice. In another study by Zhang
et al (100) it was shown
that EXD antagonized the damage of corticosterone on PC12 cells and
improved depression-like behavior in mice. In this study, the EXD
was neuroprotective against corticosterone-injured PC12 cells in
vitro. EXD can effectively increase the levels of serotonin,
dopamine and norepinephrine in the hypothalamus, reduce the
inflammatory response to protect the neural tissues and effectively
improve the depression-like behavior in mice. Furthermore, EXD
markedly increased cell viability, inhibited corticosterone-induced
apoptosis and modulated the expression of apoptosis-associated
proteins Bcl-2, Bax, cystatinase-3 and cystatinase-8. Although some
current studies have shown that EXD has good therapeutic effects on
perimenopausal depression (101,102), its specific mechanism of action
and active ingredients still need further research.
In addition, there are numerous researchers working
on the drugs or chemically active ingredients in traditional
Chinese medicine formulas that play a major role in treating or
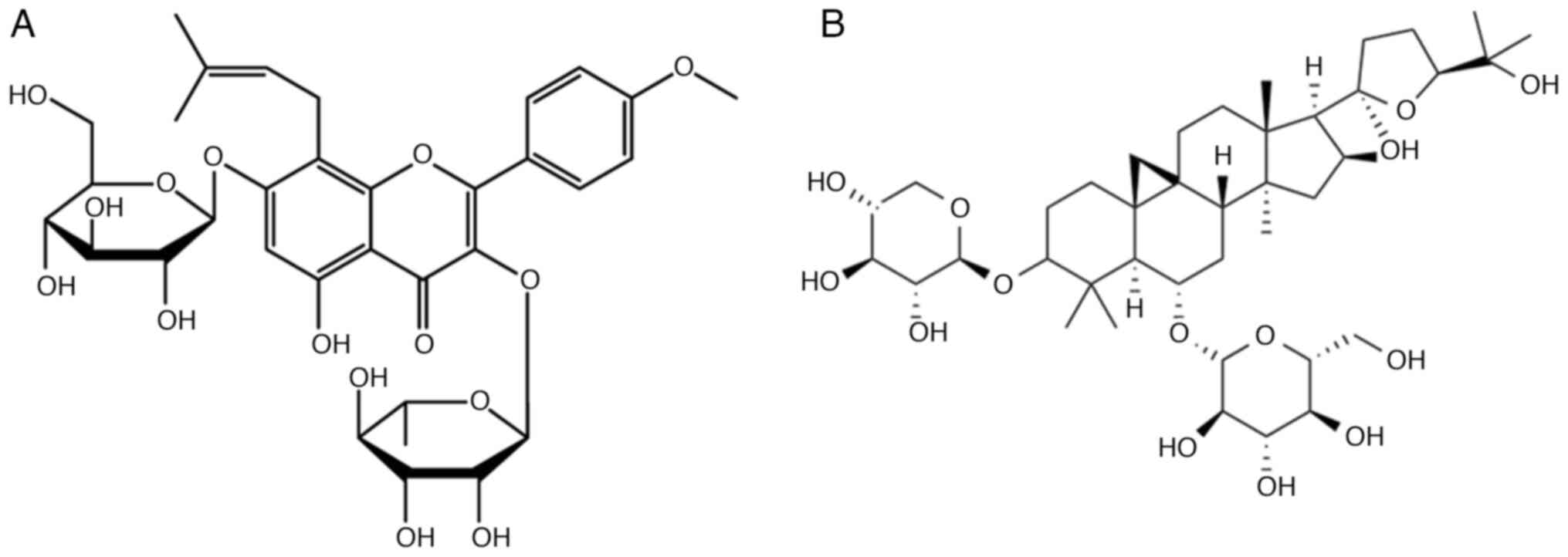
ameliorating perimenopausal depression. Icariin
(C33H40O15; Mw=676.67;
Fig. 3A) has been shown to be the
main bioactive component of the traditional Chinese medicine
Epimedium and its antidepressant mechanism of action has been
increasingly evaluated and confirmed (103,104). Icariin is a type of prenylated
flavonoid, showing extensive bioactivities such as antioxidant
(105). Cao et al
(106) explored the effects of
epimedium glycosides on the expression of PI3K/AKT pathway-related
proteins in a rat model of perimenopausal depression. It was found
that epimedium glycosides markedly ameliorated apparent symptoms,
increased organ indices of the uterus, spleen and thymus and
ameliorated pathological changes in the ovaries in model rats. The
results of the study showed that administration of epimedium
glycosides could re-regulate the disordered sex hormones, regulate
the secretion of neurotransmitters in the brain, enhance the immune
function and ameliorate the perimenopausal syndrome in
perimenopausal depressed rats.
Astragalus contains a variety of active ingredients,
such as astragaloside, astragaloside polysaccharides and
flavonoids. Among them, astragaloside IV
(C41H68O14; MW=784.97;
Fig. 3B) a tetracyclic
triterpenoid saponin in the form of lanolin ester alcohol, is the
most biologically active component (107). Studies have shown that
astragaloside can exert antidepressant effects through the
regulation of neurotransmitters and improvement of nerve cell
damage (108,109). Yao et al (110) noted that Astragalus can be used
for the recovery and treatment of perimenopausal depression from
the perspective of M2 microglia polarization to alleviate
neuroinflammation and thus promote the recovery of perimenopausal
depression. The report investigated the effects of Astragalus on
mice with simulated perimenopausal depression by modulating
microglia polarization and examined markers of microglia
polarization and their regulatory signals.
Curculiginis is a common Chinese herb that is known
to strengthen bones and muscles. Previous studies have shown that
curculiginis has a wide range of pharmacological effects and can be
used for neuroprotective, anti-inflammatory, antioxidant and
estrogenic effects (111,112).
Chaihu, a Chinese herb, is present in some of the
herbal formulas for depression, such as Chaihu Jia Longgu Muli
decoction (113–115), Chaihu-Shugan-San (116,117) and Chaihu Anxin Capsule (118). Chaihu saponin A, an active
component of Chaihu, inhibits IL-6 and TNF-α in the intestinal
tract of septic rats (119). Chen
et al (120) demonstrated
the antidepressant-like effect of Chaihu saponin A on
perimenopausal rats by behavioral tests, serum
corticotropin-releasing hormone, ACTH and corticosterone levels, as
well as hypothalamic adrenocorticotropic hormone and hippocampal
glucocorticoid receptors. Studies on the nervous system of rats
have shown that Chai Hu Saponin A produces antidepressant effects
in perimenopausal rats, possibly resulting from the restoration of
neuroendocrine, neuroinflammatory and neurotrophic systems in their
hippocampus (121,122).
Depressive patients during perimenopause have been
demonstrated to possess high levels of ROS and inflammatory
biomarkers and activation of stress kinases, promoting further
oxidative stress and neuroinflammation and cell death, which may
all contribute to depression. Therefore, the exploration of these
pathways can provide potential therapeutic strategies for
perimenopausal depression. It would be possible to develop
potential therapeutic strategies aimed at treating perimenopausal
depression by identifying ROS and inflammatory levels in patients.
However, measuring ROS remains highly challenging, particularly
in vivo, due to their short half-life. Given the cell
type-specific and context-dependent nature of ROS functions and
harmful effects, further research is needed in brain regions
affected by perimenopausal depression to delineate specific
mechanisms. As well, an understanding of the interactions between
redox status and the immune-inflammatory system could help identify
the correct targets for neuroprogression in perimenopausal
depression.
Chinese medicines have been passed down in China for
thousands of years and with the rise of modern medicine, the
active/effective components in Chinese medicines as well as their
mechanisms of action still need to be further investigated. The
components in Chinese medicines are very complex and accurate
extraction and purification of their active ingredients is a great
challenge. The modernization of Chinese traditional medicine still
has a long way to go and, although it is difficult, it has a bright
future.
The present study was supported by Heilongjiang Postdoctoral
Fund (grant no. LBH-Z22283) and the Scientific Research Fund of
Heilongjiang University of Chinese Medicine (grant no.
2024XJJ-QNCX020).
Not applicable.
YY, TYu, KL, YLi, HC and XW contributed to writing
and editing of this review. YLu, TYa and WL revised the article.
Data authentication is not applicable. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Maki PM, Kornstein SG, Joffe H, Bromberger
JT, Freeman EW, Athappilly G, Bobo WV, Rubin LH, Koleva HK, Cohen
LS and Soares CN: Guidelines for the evaluation and treatment of
perimenopausal depression: summary and recommendations. J Womens
Health (Larchmt). 28:117–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toffol E, Heikinheimo O and Partonen T:
Hormone therapy and mood in perimenopausal and postmenopausal
women: A narrative review. Menopause. 22:564–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams K: Perimenopausal depression:
Review of recent findings and implications for future research.
Curr Opin Obstet Gynecol. 35:150–153. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang D, Dong H, Shen Y, Ou J and Zhao J:
The clinical application of Chinese herbal medication to
depression: A narrative review. Front Public Health.
11:11206832023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dobrek L and Głowacka K: Depression and
its phytopharmacotherapy-a narrative review. Int J Mol Sci.
24:47722023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braillon A, Fried EI, Cristea IA, Cosgrove
L and Naudet F: Treatments for major depression. Lancet.
401:21102023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao FY, Fu QQ, Spencer SJ, Kennedy GA,
Conduit R, Zhang WJ and Zheng Z: Acupuncture: A promising approach
for comorbid depression and insomnia in perimenopause. Nat Sci
Sleep. 13:1823–1863. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bromberger JT and Epperson CN: Depression
during and after the perimenopause: Impact of hormones, genetics,
and environmental determinants of disease. Obstet Gynecol Clin
North Am. 45:663–678. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herson M and Kulkarni J: Hormonal agents
for the treatment of depression associated with the menopause.
Drugs Aging. 39:607–618. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiang X, Palasuberniam P and Pare R:
Exploring the feasibility of estrogen replacement therapy as a
treatment for perimenopausal depression: A comprehensive literature
review. Medicina (Kaunas). 60:10762024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gilmor ML, Owens MJ and Nemeroff CB:
Inhibition of norepinephrine uptake in patients with major
depression treated with paroxetine. Am J Psychiatry. 159:1702–1710.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garay RP, Charpeaud T, Logan S, Hannaert
P, Garay RG, Llorca PM and Shorey S: Pharmacotherapeutic approaches
to treating depression during the perimenopause. Expert Opin
Pharmacother. 20:1837–1845. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Pan F, Tang Y and Huang JH:
Editorial: Early life stress-induced epigenetic changes involved in
mental disorders. Front Genet. 12:6848442021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kasper S and Hamon M: Beyond the
monoaminergic hypothesis: Agomelatine, a new antidepressant with an
innovative mechanism of action. World J Biol Psychiatry.
10:117–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moncrieff J, Cooper RE, Stockmann T,
Amendola S, Hengartner MP and Horowitz MA: The serotonin theory of
depression: A systematic umbrella review of the evidence. Mol
Psychiatry. 28:3243–3256. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Y, Gu S, Li Y, Qian X, Wang F and
Huang JH: Neuroendocrine pathogenesis of perimenopausal depression.
Front Psychiatry. 14:11625012023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Xu Y, Sheng H, Ni X and Lu J:
Exercise amelioration of depression-like behavior in OVX mice is
associated with suppression of NLRP3 inflammasome activation in
hippocampus. Behav Brain Res. 307:18–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HJ, Shim HS and Shim I: The
differential role of cytokines on stress responses in a menopause
rat model. Front Psychiatry. 11:5775612020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo Y, Chen X, Gong P, Li Z, Wu Y, Zhang
J, Wang J, Yao W, Yang W and Chen F: Advances in the mechanisms of
polysaccharides in alleviating depression and its complications.
Phytomedicine. 109:1545662023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bakunina N, Pariante CM and Zunszain PA:
Immune mechanisms linked to depression via oxidative stress and
neuroprogression. Immunology. 144:365–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lopresti AL, Maker GL, Hood SD and
Drummond PD: A review of peripheral biomarkers in major depression:
The potential of inflammatory and oxidative stress biomarkers. Prog
Neuropsychopharmacol Biol Psychiatry. 48:102–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Gao Y, Zhao Y, Huang TY, Zheng Q
and Wang X: Peripheral and central neuroimmune mechanisms in
Alzheimer's disease pathogenesis. Mol Neurodegener. 20:222025.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garofalo S, Cocozza G, Bernardini G,
Savage J, Raspa M, Aronica E, Tremblay ME, Ransohoff RM, Santoni A
and Limatola C: Blocking immune cell infiltration of the central
nervous system to tame Neuroinflammation in Amyotrophic lateral
sclerosis. Brain Behav Immun. 105:1–14. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garofalo S, Cocozza G, Porzia A,
Inghilleri M, Raspa M, Scavizzi F, Aronica E, Bernardini G, Peng L,
Ransohoff RM, et al: Natural killer cells modulate motor
neuron-immune cell cross talk in models of Amyotrophic Lateral
Sclerosis. Nat Commun. 11:17732020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malutan AM, Dan M, Nicolae C and Carmen M:
Proinflammatory and anti-inflammatory cytokine changes related to
menopause. Prz Menopauzalny. 13:162–168. 2014.PubMed/NCBI
|
|
26
|
Liang G, Kow ASF, Yusof R, Tham CL, Ho YC
and Lee MT: Menopause-associated depression: impact of oxidative
stress and neuroinflammation on the central nervous system-a
review. Biomedicines. 12:1842024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Avital A, Goshen I, Kamsler A, Segal M,
Iverfeldt K, Richter-Levin G and Yirmiya R: Impaired interleukin-1
signaling is associated with deficits in hippocampal memory
processes and neural plasticity. Hippocampus. 13:826–834. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patterson SL: Immune dysregulation and
cognitive vulnerability in the aging brain: Interactions of
microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology.
96((Pt A)): 11–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee MT, Peng WH, Kan HW, Wu CC, Wang DW
and Ho YC: Neurobiology of depression: Chronic stress alters the
glutamatergic system in the brain-focusing on AMPA receptor.
Biomedicines. 10:10052022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Viviani B and Boraso M: Cytokines and
neuronal channels: A molecular basis for age-related decline of
neuronal function? Exp Gerontol. 46:199–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Min SS, Quan HY, Ma J, Han JS, Jeon BH and
Seol GH: Chronic brain inflammation impairs two forms of long-term
potentiation in the rat hippocampal CA1 area. Neurosci Lett.
456:20–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nisticò R, Mango D, Mandolesi G, Piccinin
S, Berretta N, Pignatelli M, Feligioni M, Musella A, Gentile A,
Mori F, et al: Inflammation subverts hippocampal synaptic
plasticity in experimental multiple sclerosis. PLoS One.
8:e546662013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dugan LL, Ali SS, Shekhtman G, Roberts AJ,
Lucero J, Quick KL and Behrens MM: IL-6 mediated degeneration of
forebrain GABAergic interneurons and cognitive impairment in aged
mice through activation of neuronal NADPH oxidase. PLoS One.
4:e55182009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu C, Chu D, Kalantar-Zadeh K, George J,
Young HA and Liu G: Cytokines: From clinical significance to
quantification. Adv Sci (Weinh). 8:e20044332021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang YT, Wang XL, Wang ZZ, Lei L, Hu D and
Zhang Y: Antidepressant effects of the traditional Chinese herbal
formula Xiao-Yao-San and its bioactive ingredients. Phytomedicine.
109:1545582023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Webster JC, Oakley RH, Jewell CM and
Cidlowski JA: Proinflammatory cytokines regulate human
glucocorticoid receptor gene expression and lead to the
accumulation of the dominant negative beta isoform: A mechanism for
the generation of glucocorticoid resistance. Proc Natl Acad Sci
USA. 98:6865–6870. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng Z, Fan T, Xiao C, Tian H, Zheng Y, Li
C and He J: TGF-β signaling in health, disease, and therapeutics.
Signal Transduct Target Ther. 9:612024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo W, Liu H, Yan Y, Wu D, Yao H, Lin K
and Li X: Targeting the TGF-β signaling pathway: An updated patent
review (2021-present). Expert Opin Ther Pat. 34:99–126. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Connolly EC, Freimuth J and Akhurst RJ:
Complexities of TGF-β targeted cancer therapy. Int J Biol Sci.
8:964–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morikawa M, Derynck R and Miyazono K:
TGF-β and the TGF-β Family: Context-dependent roles in cell and
tissue physiology. Cold Spring Harb Perspect Biol. 8:a0218732016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brionne TC, Tesseur I, Masliah E and
Wyss-Coray T: Loss of TGF-beta 1 leads to increased neuronal cell
death and microgliosis in mouse brain. Neuron. 40:1133–1145. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jomova K, Raptova R, Alomar SY, Alwasel
SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species,
toxicity, oxidative stress, and antioxidants: chronic diseases and
aging. Arch Toxicol. 97:2499–2574. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bhatt S, Nagappa AN and Patil CR: Role of
oxidative stress in depression. Drug Discov Today. 25:1270–1276.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Correia AS, Cardoso A and Vale N:
Oxidative stress in depression: The link with the stress response,
neuroinflammation, serotonin, neurogenesis and synaptic plasticity.
Antioxidants (Basel). 12:4702023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bajpai A, Verma AK, Srivastava M and
Srivastava R: Oxidative stress and major depression. J Clin Diagn
Res. 8:CC04–7. 2014.PubMed/NCBI
|
|
46
|
Ferriani LO, Silva DA, Molina MDCB, Mill
JG, Brunoni AR, da Fonseca MJM, Moreno AB, Benseñor IM, de Aguiar
OB, Barreto SM and Viana MC: Associations of depression and intake
of antioxidants and vitamin B complex: Results of the Brazilian
longitudinal study of adult health (ELSA-Brasil). J Affect Disord.
297:259–268. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Massart R, Mongeau R and Lanfumey L:
Beyond the monoaminergic hypothesis: Neuroplasticity and epigenetic
changes in a transgenic mouse model of depression. Philos Trans R
Soc Lond B Biol Sci. 367:2485–2494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Venneti S, Lopresti BJ and Wiley CA:
Molecular imaging of microglia/macrophages in the brain. Glia.
61:10–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang H, Wang J, Liu T, Leng Y and Yang W:
Stem cell-derived exosomal MicroRNAs: Potential therapies in
diabetic kidney disease. Biomed Pharmacother. 164:1149612023.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gandhi S and Abramov AY: Mechanism of
oxidative stress in neurodegeneration. Oxid Med Cell Longev.
2012:4280102012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kohen R and Nyska A: Oxidation of
biological systems: Oxidative stress phenomena, antioxidants, redox
reactions, and methods for their quantification. Toxicol Pathol.
30:620–650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bishop NA, Lu T and Yankner BA: Neural
mechanisms of ageing and cognitive decline. Nature. 464:529–535.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Forlenza MJ and Miller GE: Increased serum
levels of 8-hydroxy-2′-deoxyguanosine in clinical depression.
Psychosom Med. 68:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Irie M, Miyata M and Kasai H: Depression
and possible cancer risk due to oxidative DNA damage. J Psychiatr
Res. 39:553–560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ishikawa A, Matsushita H, Shimizu S,
Morita N, Hanai R, Sugiyama S, Watanabe K and Wakatsuki A: Impact
of menopause and the menstrual cycle on oxidative stress in
Japanese women. J Clin Med. 12:8292023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Signorelli SS, Neri S, Sciacchitano S,
Pino LD, Costa MP, Marchese G, Celotta G, Cassibba N, Pennisi G and
Caschetto S: Behaviour of some indicators of oxidative stress in
postmenopausal and fertile women. Maturitas. 53:77–82. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bellanti F, Matteo M, Rollo T, De Rosario
F, Greco P, Vendemiale G and Serviddio G: Sex hormones modulate
circulating antioxidant enzymes: Impact of estrogen therapy. Redox
Biol. 1:340–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Petri S, Körner S and Kiaei M: Nrf2/ARE
signaling pathway: Key mediator in oxidative stress and potential
therapeutic target in ALS. Neurol Res Int. 2012:8780302012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Baird L and Dinkova-Kostova AT: The
cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol.
85:241–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hybertson BM, Gao B, Bose SK and McCord
JM: Oxidative stress in health and disease: The therapeutic
potential of Nrf2 activation. Mol Aspects Med. 32:234–246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yu C and Xiao JH: The Keap1-Nrf2 System: A
mediator between oxidative stress and aging. Oxid Med Cell Longev.
2021:66354602021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee JM, Shih AY, Murphy TH and Johnson JA:
NF-E2-related factor-2 mediates neuroprotection against
mitochondrial complex I inhibitors and increased concentrations of
intracellular calcium in primary cortical neurons. J Biol Chem.
278:37948–37956. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Martín-de-Saavedra MD, Budni J, Cunha MP,
Gómez-Rangel V, Lorrio S, Del Barrio L, Lastres-Becker I, Parada E,
Tordera RM, Rodrigues AL, et al: Nrf2 participates in depressive
disorders through an anti-inflammatory mechanism.
Psychoneuroendocrinology. 38:2010–2022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Samy DM, Mostafa DK, Saleh SR, Hassaan PS,
Zeitoun TM, Ammar GAG and Elsokkary NH: Carnosic acid mitigates
depression-like behavior in ovariectomized mice via activation of
Nrf2/HO-1 pathway. Mol Neurobiol. 60:610–628. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Khan D and Ansar Ahmed S: The immune
system is a natural target for estrogen action: Opposing effects of
estrogen in two prototypical autoimmune diseases. Front Immunol.
6:6352015.PubMed/NCBI
|
|
68
|
Maggioli E, McArthur S, Mauro C, Kieswich
J, Kusters DHM, Reutelingsperger CPM, Yaqoob M and Solito E:
Estrogen protects the blood-brain barrier from inflammation-induced
disruption and increased lymphocyte trafficking. Brain Behav Immun.
51:212–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Iob E, Kirschbaum C and Steptoe A:
Persistent depressive symptoms, HPA-axis hyperactivity, and
inflammation: The role of cognitive-affective and somatic symptoms.
Mol Psychiatry. 25:1130–1140. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Keller J, Gomez R, Williams G, Lembke A,
Lazzeroni L, Murphy GM Jr and Schatzberg AF: HPA axis in major
depression: Cortisol, clinical symptomatology and genetic variation
predict cognition. Mol Psychiatry. 22:527–536. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen X, Gianferante D, Hanlin L, Fiksdal
A, Breines JG, Thoma MV and Rohleder N: HPA-axis and inflammatory
reactivity to acute stress is related with basal HPA-axis activity.
Psychoneuroendocrinology. 78:168–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Stamper CE, Hassell JE Jr, Kapitz AJ,
Renner KJ, Orchinik M and Lowry CA: Activation of 5-HT(1A)
receptors in the rat dorsomedial hypothalamus inhibits
stress-induced activation of the hypothalamic-pituitary-adrenal
axis. Stress. 20:223–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Schmidt PJ, Wei SM, Martinez PE, Dor RRB,
Guerrieri GM, Palladino PP, Harsh VL, Li HJ, Wakim P, Nieman LK and
Rubinow DR: The short-term effects of estradiol, raloxifene, and a
phytoestrogen in women with perimenopausal depression. Menopause.
28:369–383. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pulst SM: Prenatal diagnosis of the
neurofibromatoses. Clin Perinatol. 17:829–844. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Westlund Tam L and Parry BL: Does estrogen
enhance the antidepressant effects of fluoxetine? J Affect Disord.
77:87–92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Morgan ML, Cook IA, Rapkin AJ and Leuchter
AF: Estrogen augmentation of antidepressants in perimenopausal
depression: A pilot study. J Clin Psychiatry. 66:774–780. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang M, Liu W, Ge J and Liu S: The
immunomodulatory mechanisms for acupuncture practice. Front
Immunol. 14:11477182023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wen J, Chen X, Yang Y, Liu J, Li E, Liu J,
Zhou Z, Wu W and He K: Acupuncture medical therapy and its
underlying mechanisms: A systematic review. Am J Chin Med. 49:1–23.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Amorim D, Amado J, Brito I, Fiuza SM,
Amorim N, Costeira C and Machado J: Acupuncture and
electroacupuncture for anxiety disorders: A systematic review of
the clinical research. Complement Ther Clin Pract. 31:31–37. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cheuk DK, Yeung WF, Chung KF and Wong V:
Acupuncture for insomnia. Cochrane Database Syst Rev.
2012:CD0054722012.PubMed/NCBI
|
|
81
|
Yeung WF, Chung KF, Leung YK, Zhang SP and
Law AC: Traditional needle acupuncture treatment for insomnia: A
systematic review of randomized controlled trials. Sleep Med.
10:694–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang XW, Hou WB, Pu FL, Wang XF, Wang YR,
Yang M, Cheng K, Wang Y, Robinson N and Liu JP: Acupuncture for
cancer-related conditions: An overview of systematic reviews.
Phytomedicine. 106:1544302022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Baumelou A, Liu B, Wang XY and Nie GN:
Perspectives in clinical research of acupuncture on menopausal
symptoms. Chin J Integr Med. 17:893–897. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chon TY and Lee MC: Acupuncture. Mayo Clin
Proc. 88:1141–1146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Smith CA, Armour M, Lee MS, Wang LQ and
Hay PJ: Acupuncture for depression. Cochrane Database Syst Rev.
3:CD0040462018.PubMed/NCBI
|
|
86
|
Xu MM, Guo P, Ma QY, Zhou X, Wei YL, Wang
L, Chen Y and Guo Y: Can acupuncture enhance therapeutic
effectiveness of antidepressants and reduce adverse drug reactions
in patients with depression? A systematic review and meta-analysis.
J Integr Med. 20:305–320. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jing Q, Ren L, Deng X, Zhang N, Fu M, Wang
G, Jiang XR, Lin SR and Ming CR: Electroacupuncture promotes neural
proliferation in hippocampus of perimenopausal depression rats via
Wnt/β-catenin signaling pathway. J Acupunct Meridian Stud.
13:94–103. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhou JH, Zhang DL, Ning BL, Xue XJ, Zhao
L, Wu Q, Yan LD, Liu M and Fu WB: The role of acupuncture in
hormonal shock-induced cognitive-related symptoms in perimenopausal
depression: A randomized clinical controlled trial. Front
Psychiatry. 12:7725232022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lokuge S, Frey BN, Foster JA, Soares CN
and Steiner M: Depression in women: Windows of vulnerability and
new insights into the link between estrogen and serotonin. J Clin
Psychiatry. 72:e1563–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pengo MF, Won CH and Bourjeily G: Sleep in
women across the life Span. Chest. 154:196–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tao C, Zhang GW, Huang JJ, Li Z, Tao HW
and Zhang LI: The medial preoptic area mediates depressive-like
behaviors induced by ovarian hormone withdrawal through distinct
GABAergic projections. Nat Neurosci. 26:1529–1540. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Johansson T, Fowler P, Ek WE, Skalkidou A,
Karlsson T and Johansson Å: Oral contraceptives, hormone
replacement therapy, and stroke risk. Stroke. 53:3107–3115. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Rozenberg S, Di Pietrantonio V, Vandromme
J and Gilles C: Menopausal hormone therapy and breast cancer risk.
Best Pract Res Clin Endocrinol Metab. 35:1015772021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tandon VR, Sharma S, Mahajan A, Mahajan A
and Tandon A: Menopause and sleep disorders. J Midlife Health.
13:26–33. 2022.PubMed/NCBI
|
|
95
|
Di YM, Yang L, Shergis JL, Zhang AL, Li Y,
Guo X, Xue CC and Lu C: Clinical evidence of Chinese medicine
therapies for depression in women during perimenopause and
menopause. Complement Ther Med. 47:1020712019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Xiao M, Xie K, Yuan L, Wang J, Liu X and
Chen Z: Effects of huolisu oral solution on depression-like
behavior in rats: Neurotransmitter and HPA Axis. Front Pharmacol.
13:8932832022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cao XJ, Huang XC and Wang X: Effectiveness
of Chinese herbal medicine granules and traditional Chinese
medicine-based psychotherapy for perimenopausal depression in
Chinese women: A randomized controlled trial. Menopause.
26:1193–1203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhong D, Cheng H, Pan Z, Ou X, Liu P, Kong
X, Liu D, Chen J and Li J: Efficacy of scalp acupuncture combined
with conventional therapy in the intervention of post-stroke
depression: A systematic review and meta-analysis. Complement Ther
Med. 77:1029752023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang L, Li J, Chen Q, Di L and Li N:
Erxian decoction, a famous Chinese medicine formula, ameliorate
depression-like behavior in perimenopausal mice. Endocr Metab
Immune Disord Drug Targets. 21:2203–2212. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang L, Yang Y, Di L, Li JL and Li N:
Erxian decoction, a famous Chinese medicine formula, antagonizes
corticosterone-induced injury in PC12 cells, and improves
depression-like behaviours in mice. Pharm Biol. 58:498–509. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zeng NX, Li H, Su MY, Chen X, Yang XY and
Shen M: Therapeutic potential of Erxian decoction and its special
chemical markers in depression: A review of clinical and
preclinical studies. Front Pharmacol. 15:13770792024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang Y, Lou XT, Shi YH, Tong Q and Zheng
GQ: Erxian decoction, a Chinese herbal formula, for menopausal
syndrome: An updated systematic review. J Ethnopharmacol. 234:8–20.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Luo Z, Dong J and Wu J: Impact of Icariin
and its derivatives on inflammatory diseases and relevant signaling
pathways. Int Immunopharmacol. 108:1088612022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang S, Ma J, Zeng Y, Zhou G, Wang Y, Zhou
W, Sun X and Wu M: Icariin, an Up-and-coming bioactive compound
against neurological diseases: Network pharmacology-based study and
literature review. Drug Des Devel Ther. 15:3619–3641. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Seyedi Z, Amiri MS, Mohammadzadeh V,
Hashemzadeh A, Haddad-Mashadrizeh A, Mashreghi M, Qayoomian M,
Hashemzadeh MR, Simal-Gandara J and Taghavizadeh Yazdi ME: Icariin:
A Promising Natural Product in Biomedicine and Tissue Engineering.
J Funct Biomater. 14:442023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Cao LH, Qiao JY, Huang HY, Fang XY, Zhang
R, Miao MS and Li XM: PI3K-AKT signaling activation and icariin:
The potential effects on the perimenopausal depression-like rat
model. Molecules. 24:37002019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang Y, Zhang Z, Cheng Z, Xie W, Qin H and
Sheng J: Astragaloside in cancer chemoprevention and therapy. Chin
Med J (Engl). 136:1144–1154. 2023.PubMed/NCBI
|
|
108
|
Rao Y, Li J, Qiao R, Luo J and Liu Y:
Synergistic effects of tetramethylpyrazine and astragaloside IV on
spinal cord injury via alteration of astrocyte A1/A2 polarization
through the Sirt1-NF-κB pathway. Int Immunopharmacol.
131:1116862024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang YL, Chio CC, Kuo SC, Yeh CH, Ma JT,
Liu WP, Lin MT, Lin KC and Chang CP: Exercise rehabilitation and/or
astragaloside attenuate amyloid-beta pathology by reversing
BDNF/TrkB signaling deficits and mitochondrial dysfunction. Mol
Neurobiol. 59:3091–3109. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yao G, Bai Z, Niu J, Zhang R, Lu Y, Gao T
and Wang H: Astragalin attenuates depression-like behaviors and
memory deficits and promotes M2 microglia polarization by
regulating IL-4R/JAK1/STAT6 signaling pathway in a murine model of
perimenopausal depression. Psychopharmacology (Berl).
239:2421–2443. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chauhan NS, Rao ChV and Dixit VK: Effect
of curculigo orchioides rhizomes on sexual behaviour of male rats.
Fitoterapia. 78:530–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Shi K, Chen L, Chen L, Tan A, Xie G, Long
Q, Ning F, Lan Z and Wang P: Epimedii folium and curculiginis
rhizoma ameliorate lipopolysaccharides-induced cognitive impairment
by regulating the TREM2 signaling pathway. J Ethnopharmacol.
284:1147662022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Jia X, Chen J, Huang R, Wang D and Wang X:
Effect-enhancing and toxicity-reducing effects of Chaihu Jia Longgu
Muli decoction in the treatment of multimorbidity with depression:
A systematic review and meta-analysis. Pharm Biol. 61:1094–1106.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wan R, Song R, Fan Y, Li L, Zhang J, Zhang
B, Li X and Wang S: Efficacy and safety of chaihu jia longgu muli
decoction in the treatment of poststroke depression: A systematic
review and meta-analysis. Evid Based Complement Alternat Med.
2021:76045372021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhao Y, Xu D, Wang J, Zhou D, Liu A, Sun
Y, Yuan Y, Li J and Guo W: The pharmacological mechanism of
chaihu-jia-longgu-muli-tang for treating depression: integrated
meta-analysis and network pharmacology analysis. Front Pharmacol.
14:12576172023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fan Q, Liu Y, Sheng L, Lv S, Yang L, Zhang
Z, Guo J, Fan Y and Hu D: Chaihu-Shugan-San inhibits
neuroinflammation in the treatment of post-stroke depression
through the JAK/STAT3-GSK3β/PTEN/Akt pathway. Biomed Pharmacother.
160:1143852023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Han SK, Kim JK, Park HS, Shin YJ and Kim
DH: Chaihu-Shugan-San (Shihosogansan) alleviates restraint
stress-generated anxiety and depression in mice by regulating
NF-κB-mediated BDNF expression through the modulation of gut
microbiota. Chin Med. 16:772021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhao D, Yang K, Guo H, Zeng J, Wang S, Xu
H, Ge A, Zeng L, Chen S and Ge J: Mechanisms of ferroptosis in
Alzheimer's disease and therapeutic effects of natural plant
products: A review. Biomed Pharmacother. 164:1143122023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ,
Liao M and Chen JX: Saikosaponin a and its epimer saikosaponin d
exhibit anti-inflammatory activity by suppressing activation of
NF-κB signaling pathway. Int Immunopharmacol. 14:121–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chen XQ, Chen SJ, Liang WN, Wang M, Li CF,
Wang SS, Dong SQ, Yi LT and Li CD: Saikosaponin A attenuates
perimenopausal depression-like symptoms by chronic unpredictable
mild stress. Neurosci Lett. 662:283–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang AR, Mi LF, Zhang ZL, Hu MZ, Zhao ZY,
Liu B, Li YB and Zheng S: Saikosaponin A improved depression-like
behavior and inhibited hippocampal neuronal apoptosis after
cerebral ischemia through p-CREB/BDNF pathway. Behav Brain Res.
403:1131382021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Bi Y, Li M, Wang Y, Yao J, Wang Y, Wang S,
Zhuang L, Liu S, Li Z, Hao Z, et al: Saikosaponins from Bupleurum
scorzonerifolium Willd. alleviates microglial pyroptosis in
depression by binding and inhibiting P2X7 expression.
Phytomedicine. 136:1562402025. View Article : Google Scholar : PubMed/NCBI
|