Introduction
Breast cancer (BC) is one of the most common
malignancies worldwide, with 2.26 million new cases in 2022,
representing 23.8% of all cases of cancer in women (1). BC is the leading cause of
cancer-associated mortality in women, having caused 666,103
mortalities in 2022 worldwide (1).
In Mexico, 31,043 new cases and 8,195 mortalities from BC were
reported in 2022 (1), making it
one of the most prevalent and fatal types of cancer in Mexico. The
estimated national incidence of BC age-standardized rate (ASR) is
40 cases per 100,000 person-years (1). Likewise, the first population-based
cancer registry in Southeastern Mexico revealed an ASR incidence of
49.3 cases per 100,000 person-years (2). Despite the high incidence of BC,
emerging therapeutic targets, diagnostic and prognostic biomarkers
have managed a 5-year overall survival rate of 90% in the USA
(3,4). However, long-term chemotherapy and
targeted therapy can induce resistance and cancer progression
(5). Nevertheless, for individuals
without social security in emerging countries, such as Mexico, 55%
BC cases are typically diagnosed already at locally advanced
stages, with a 5-year survival of 69.9% (6). By contrast, patients with social
security, who often have greater access to comprehensive medical
care and timely treatments, have a 5-year overall survival rate of
90.4% (7); however, upon analyzing
data according to stages of the disease, a progressive decrease in
survival can be observed. Stage I has a survival rate of 98.8%,
stage II has a survival rate of 97.5%, stage III has a survival
rate of 89.4% and stage IV has a survival rate of 22.7% (7).
BC is highly heterogeneous and is classified based
on expression of the estrogen receptor (ER), progesterone receptor
(PR) and human epidermal growth factor receptor 2 (HER2) (8). Molecular classification of BC based
on global expression analysis has contributed to determining
prognosis and appropriate treatment options (9–13).
BC tumors can be divided into luminal A, luminal B, HER2, basal,
claudin-low and normal-like (14).
Basal and claudin-low subtypes are clinically known as
triple-negative BC (TNBC), an aggressive subtype of BC
characterized by the lack of ER, PR and HER2 expression (15) Clinically, TNBC presents additional
challenges compared with other subtypes of BC, including high
aggressiveness and a high recurrence rate due to the lack of
specific therapeutic options and molecular targets for targeted
therapy, exerting a considerable negative impact on the survival of
patients with TNBC (14).
Consequently, efforts have been focused on
characterizing TNBC to identify predictive biomarkers and
therapeutic targets. Sub-classification of TNBC has been proposed,
namely basal-like 1, basal-like 2, mesenchymal, mesenchymal
stem-like, immunomodulatory and luminal androgen receptor (16). Molecular classification, biomarkers
and therapeutic approaches have been designed based on the profile
of protein-coding RNAs. However, these transcripts only represent
2% of the total transcriptome in a human cell (17). The remaining 98% of transcripts
consist of non-coding RNAs (ncRNAs) that may exert relevant
clinical and biological functions. A diverse range of non-coding
RNAs have been identified in tumor cells, among which long
non-coding RNAs (lncRNAs) represent a tissue-specific group of
transcripts exhibiting diverse functions in tumor progression and
metastasis (18). LncRNAs are
transcripts consisting of >200 nucleotides that lack protein
coding potential (19). These
transcripts can interact with DNA, mRNA, microRNAs (miRNA) and
protein molecules to control gene and protein expression on various
levels, such as epigenetic, transcriptional, post-transcriptional,
translational and post-translational levels (20). LncRNAs can be located in the
nucleus or cytoplasm of a cell (21). In the cytoplasm, lncRNAs can
modulate mRNA stability, regulate translation, mediate protein
modifications, act as competing endogenous RNAs, function as a
sponge of miRNA by binding miRNAs and sequestering them and can
also be precursors of miRNAs (22,23).
By contrast, nuclear lncRNAs can control chromatin states,
transcriptional and posttranscriptional gene expression, RNA
stability and RNA processing and splicing (24). LncRNAs exert a plethora of
functions in a cell, implying roles in a variety of normal and
pathological cellular processes, including tumorigenesis, invasion
and metastasis (18). Expression
of lncRNAs is commonly dysregulated during tumorigenesis, which
suggests oncogenic or tumor-suppressive functions and
chemo-resistance (25,26). Several studies have previously
focused on describing the role of lncRNAs in the development and
progression of cancer, such as breast, colon, melanoma, pancreas
and liver cancers (27), which
have been attributed to oncogenic or tumor-suppressor functions
(28–30), such as metastasis-associated lung
adenocarcinoma transcript 1, homeobox transcript antisense RNA,
urothelial cancer associated 1, growth arrest-specific transcript
5, nuclear paraspeckle assembly transcript and lincRNAp21.
The present study aimed to identify the molecular
profile of lncRNAs in a series of BC tumors from Mexican patients,
according to the expression of routine biomarkers ER, PR, HER2.
TNBC (ER-, PR- and HER2-) was then compared with luminal B HER2+
(ER+, PR+ and HER2+) BC. Previous studies have reported an
increased prevalence of TNBC tumors in Mexico compared with USA
populations (31,32). In these studies, it was reported
that the prevalence of TNBC in Mexico was 23.1%, compared with the
10–13% in USA, making it essential to find suitable prognostic
biomarkers and therapeutic options. The aim of the present study
was to find a lncRNA signature associated with TNBC prognosis,
since TNBC is known to be the most aggressive form of BC (15). Differentially expressed lncRNAs
were validated using a cohort from The Cancer Genome Atlas (TCGA),
through a cohort obtained from the Gene Expression Omnibus database
(accession no. GSE134359), and an independent cohort of Mexican
patients with BC. These samples were different from those used in
the microarray analyses of the present study; although they were
selected based on the same characteristics, they were independent
samples collected from UMAE Oncology Hospital. It is hoped that
this approach can allow for the identification of lncRNA profiles
with a potential impact on the overall survival of patients with
TNBC.
Materials and methods
Sample collection
Approval for the present study was obtained from the
Research and Ethics committee of the Mexican Institute of Social
Security under approval nos. R-2013-785-045 and R-2020-785-154. A
total of 192 BC samples with histopathological reports were
collected from pathology records at the Unidad Médica de Alta
Especialidad Oncology Hospital, XXI National Medical Center of the
Mexican Institute of Social Security (Mexico City, Mexico). These
samples were collected retrospectively from cases registered
between January 2009 and December 2013, following project
authorization in July 2013. Clinical data, including age, sex,
grade and stage, were compiled from the medical records at the UMAE
Oncology Hospital (Table SI).
Histological samples processing
BC tissues were processed at the Pathology
department, UMAE Oncology Hospital (Mexico City, Mexico) as
follows: Tumor tissue biopsies were previously fixed in 10% neutral
formaldehyde buffer for 24 h at room temperature. Subsequently, the
tissue was processed using a tissue processor. The tissues were
dehydrated for 1 h at room temperature in each of the following
solvents: Ethanol 70%, two changes of 96% ethanol, two changes of
absolute ethanol, one change of a 50% mixture of absolute ethanol
and absolute xylene, two changes of xylene for clearing and three
changes of paraffin at 58°C, concluding with the embedding process.
Afterwards, tissue sections were cut to 5-m thickness using a
microtome and deparaffinized at 58°C for 20 min and immersed in
xylene. The tissues were then rehydrated by immersion in alcohol
baths of 100, 96 and 70% alcohol, followed by water.
H&E staining
Following the deparaffinization and rehydration of
the tissue sections, they were stained with hematoxylin at room
temperature for 6 min, rinsed with water and differentiated with
acid alcohol. Afterward, they were rinsed in tap water, blued in
mildly alkaline water and rinsed with tap water. Subsequently,
eosin staining was performed at room temperature for 1 min. The
slides were dehydrated in baths of 70 and 96% alcohol, absolute
alcohol and xylene. The slides were then mounted using synthetic
resin. Light microscopic evaluation of the samples was performed by
a pathologist (author AM) for diagnosis.
Tumor microarray construction
For subsequent microarray (n=30) and reverse
transcription-quantitative PCR (RT-qPCR) (n=21) analysis, only
samples that met the following criteria were used: Sufficient tumor
tissue, samples belonging to the TNBC or luminal B HER2 subtypes
and samples with a high RNA quality. A high RNA quality was classed
as an absorbance value at 260/280 of 1.9–2.2, as well as an RNA
Integrity Number (RIN) >6. Histological evaluation was performed
by a pathologist (author AM) who confirmed diagnosis and selected
representative fragments for the construction of tissue microarrays
(TMAs). TMAs were built using Chemicon ATA-100 equipment (Leica
Biosystems) and representative cylinders of 1 mm in diameter.
Histological sections that were 4-mm thick were cut and mounted on
electrocharged slides (cat. no. AMS90-Color; Cancer Diagnostics,
Inc.) for H&E staining and marker detection by
immunohistochemistry.
Immunohistochemistry
Immunohistochemistry was performed on Ventana
BenchMark XT using the UltraView Universal DAB Kit (cat. no.
760-500; Roche Tissue Diagnostics) following the provider's
protocol. Key steps included a deparaffinization process using
EZprep at 76°C in 4-min intervals over a total of 16 min, followed
by antigen exposure in cell conditioning solution 1 (cat. no.
950-124) at 95°C for 30 min. Endogenous peroxidase activity was
subsequently blocked with UV INHIBITOR for 4 min at 37°C. The
antibodies used were intended for in vitro diagnostic use,
were ready to use and it was not necessary to perform any dilution.
The samples were then incubated with Ventana primary antibodies
specific to the target antigens: CONFIRM ER (SP1) (cat. no.
790-4324), CONFIRM PR (1E2) (cat. no. 790-2223) and PATHWAY
anti-HER2 (4B5) (cat. no 790-4493), each incubated at 37°C for 20
min. Next, the samples were incubated with Amplification Kit (cat.
no. 760-080; Roche Diagnostics) for 20 min at room temperature and
treated with UV HRP UNIV MULT for 8 min at room temperature,
followed by detection using UV DAB and UV DAB
H2O2 for another 8 min. Additionally, they
were incubated with UV COPPER for 4 min. Finally, the samples were
counterstained with hematoxylin incubated at room temperature for 6
min, dehydrated and mounted in synthetic resin.
Cases were classified based on the expression of ER,
PR and HER2 into the following subtypes: Luminal A (ER+, PR+ and
HER2-); luminal B HER2+ (ER+, PR+ and HER2+); non-luminal HER2+
(Er-, PR- and HER2+); and triple-negative (ER-, PR- and HER2-;
Fig. S1).
Image analysis
Immunohistochemistry images were scanned using
Aperio equipment (Leica Microsystems, Inc.) and quantified using
the ImageJ software (version 1.47; National Institutes of Health)
following parameters described by Tuominen et al (33). The ImageJ plugin ‘ImmunoRatio’
(version 1.0c; 14.2.2011) and ‘ImmunoMembrane’ (version 1.0i;
8.7.2011) were used to analyze nuclear and membrane location,
respectively, of the analyzed molecules. Receptor status was
considered positive when ≥1% cells expressed the corresponding
proteins, according to the American Society of Clinical
Oncology/College of American Pathologists guideline recommendations
(34,35). Chromogenic in situ
hybridization (Ventana Her2 dual ISH DNA; cat. no. 760-6072) was
performed on Her2+ samples with an equivocal result of >2. This
suggested that the HER2 expression results obtained through
immunohistochemistry were inconsistent as the presence of complete
or incomplete membrane staining with weak to moderate intensity or
complete and strong membrane staining was observed in <10% of
the cells. Therefore, the evaluation of HER2 amplification using
ISH evaluation was required. The HER2 detection process involves a
cocktail of two DNA probes (cat. no. 800-4422; Ventana), with one
targeting the HER2 gene, labeled with dinitrophenol, and another
targeting the centromere of chromosome 17, labeled with digoxigenin
(DIG). Probe hybridization were visualized using the Ventana
UltraView SISH DNSP kit (cat. no. 800-098) and the Ventana
UltraView Red ISH DIG detection kit (cat. no. 800-505).
Expression analysis
A transcriptome analysis of both luminal B HER2+
(ER+, PR+ and HER2+; n=15) and triple-negative (n=15) BC samples
was performed to analyze critical molecular differences between
aggressive tumors and improve understanding of the tumor biology of
TNBC. RNA was extracted using TRIzol® Reagent (cat. no.
15596026; Invitrogen; Thermo Fisher Scientific, Inc.). The RT kit
used was the GeneChip® Human Transcriptome Array 2.0
(cat. no. 902310; Applied Biosystems; Thermo Fisher Scientific,
Inc.). A total of 30/197 samples were chosen for subsequent
expression analysis as they contained sufficient tumor tissue, the
appropriate molecular subtype [luminal B HER2+ (ER+, PR+ and HER2+)
and TNBC (ER-, PR- and HER2-)] and high RNA quality (absorbance
value at 260/280 of 1.9–2.2 and RIN >6). This analysis was
conducted using the Human Transcriptome Array 2.0 platform (cat.
no. 902162) developed by Affymetrix (Thermo Fisher Scientific,
Inc.), with the capacity to identify a total of 44,699 transcripts
associated with coding genes, as well as 22,829 transcripts
corresponding to non-coding genes.
Transcriptome Analysis Console (version 4.0.1;
Applied Biosystems; Thermo Fisher Scientific, Inc.) was
subsequently used to identify differentially expressed (DE) genes
(DEGs) between TNBC and luminal samples. Quality control and
normalization were conducted using Robust Multichip Average
(36). Transcripts were considered
as DEGs if their fold change (FC) was found to be >2 or <-2
with P-values and a false discovery rate (FDR) <0.05. Transcript
annotation was verified with the ‘BioMart-Ensembl’ tool (version
GRCh38p.14; http://www.ensembl.org/biomart/martview/4cb2962c2a96efbcf3aa813eb2716e42).
Raw data was deposited into gene expression omnibus (GEO) database
with submission number GSE270721.
To visualize expression levels of DEGs in Mexican
patients with BC, clustering analysis using ‘clustVis’ (https://biit.cs.ut.ee/clustvis/) (37), a variant of the R heatmap package
(version 0.7.7). Heatmaps were generated for both coding and
non-coding transcripts, comparing samples between luminal B, HER2+
and TNBC groups. Sample grouping was conducted using an
unsupervised approach based on Euclidean correlation distances
(38).
In silico validation and survival
analysis using public databases
To validate DEGs found between TNBC and luminal B
HER2+ BC from Mexican patients, data from a BC cohort from TCGA was
used. This was carried out through the Genomic Data Commons Data
Portal, using the term ‘breast cancer’ for the data search.
Expression levels of mRNA transcripts in both TNBC and luminal B
HER2+ BC were analyzed using the UCSC Xena browser platform,
(version 2.0; http://xena.ucsc.edu/). Additionally,
expression of lncRNA from the same tumor samples was evaluated
using ‘The Atlas of Noncoding RNAs in Cancer’ (version: 2.2.1;
http://www.tanric.org/), an open platform for
exploring lncRNAs associated with TCGA data. LncRNAs not validated
in TCGA cohort were considered exclusive to the Mexican patients of
the present study. In addition, the 14 lncRNAs exclusive to Mexican
patients were validated in a second cohort of Mexican patients from
the GEO database (accession no. GSE134359) (39). To compare the present data with the
GSE134359 study, transcripts were considered DE if they showed a FC
>2 or FC <-2, with P-values and FDR values <0.05.
Overall survival of patients with
BC
The impact of lncRNA expression levels (low or high)
on the overall survival of breast cancer patients was evaluated.
Furthermore, the impact of lncRNA expression on overall survival of
patients was categorized according to molecular subtype (luminal A.
luminal B and basal subtypes), through the use of tanric (40). The overall survival analysis was
conducted by grouping patients according to lncRNA expression into
low or high categories. For this purpose, the samples were divided
using the median as a cutoff point. Subsequently, cox regression
and log-rank P-values were calculated to assess the differences
between the groups.
Key pathway analysis
DEGs were used to infer biological processes
enriched in TNBC tumors. Ingenuity pathway analysis (IPA) software
(version 122103623; Qiagen GmbH) was used to identify enriched
pathways and to infer upstream regulators. Ensemble gene IDs, FC
and FDR values were used as input parameters for IPA analysis.
Results with z-scores ≥2 and a P<0.05 were considered
statistically significant.
In silico exploration of the long
intergenic non-coding RNA (LINC)01087 interactome
To explore the interaction of LINC01087 with other
molecules, the online RNA interactome database from RNA inter
(version 3.0; http://www.rnainter.org/) was used, which integrates
RNA interaction data, including RNA-RNA, RNA-protein, RNA-DNA and
RNA-histone modification. This method relies on confidence scores,
which are derived from three different levels of supporting
evidence: Interactions backed by strong experimental evidence,
those supported by weak experimental evidence and predicted
evidence. Each interacting molecule is assigned a confidence score,
meaning that molecules with higher confidence scores are more
likely to interact with LINC01087. RNA inter integrates information
from literature and databases 10.1093/nar/gkab997, such as LncTarD
(https://lnctard.bio-database.com/),
NPInter (http://bigdata.ibp.ac.cn/npinter5), NoncoRNA
(http://www.ncdtcdb.cn:8080/NoncoRNA/), miRDB
(https://mirdb.org/), oRNAment (https://rnabiology.ircm.qc.ca/oRNAment) and tRFtarget
(http://trftarget.net/). An overrepresentation
analysis of the top molecules interacting with LINC01087 was also
conducted with g:profiler software (version e112_eg59_p19_25aa4782;
ELIXIR infrastructure). GProfiler performs gene set enrichment
analysis interrogating DEGs using Gene Ontology, KEGG, Reactome and
WikiPathways pathways; miRNA targets from miRTarBase and regulatory
motif matches from TRANSFAC.
Cell culture
To validate the clinical findings using an
experimental model, the present study examined whether some
lncRNAs, previously identified in tumor biopsies, were
differentially expressed and similarly regulated in a controlled
environment, such as cell lines. Luminal (MCF7, ZR75 and T47D) and
basal (MDA-MB-231, MDA-MB-468 and BT20) cell lines were acquired
from American Type Culture Collection (ATCC). MCF7, T47D, ZR75 and
BT20 cell lines were cultured in RPM1 medium (cat. no. 10-040-CV;
Corning Inc) and MDA-MB-231 and MDA-MB-468 cell lines were cultured
in DMEM medium (cat. no. 10-013-CV; Corning Inc). Cell cultures
were supplemented with 5% FBS from ATCC (cat. no. 30-2020) and
maintained at 37°C with 5% CO2.
RNA extraction
Total RNA was obtained and purified from tissue
sections embedded in paraffin and fixed with formalin for 24 h at
room temperature with the RNeasy FFPE Kit (cat. no. 73504; Qiagen
GmbH), following the manufacturer's instructions. Cellularity of
tumors was analyzed unblinded by a pathologist and only samples
with ≥70% tumor cells were selected for subsequent experiments. RNA
was quantified using spectrophotometry on the EPOCH system (Norgen
BioTek Corp) and PicoGreen (cat. no. DNAQF; Sigma-Aldrich; Merck
KGaA). Samples with high RNA quantity and purity were processed
using the Sensation Plus kit (Affymetrix; Thermo Fisher Scientific,
Inc.). After RNA extraction, samples underwent DNase digestion
using the RQ1 RNase-Free DNase kit (cat. no. M6101; Promega
Corporation). A single unit of DNase was used to degrade 1 µg of
DNA in 10 min at 37°C.
RT-qPCR
RNA was reverse transcribed into cDNA using the
High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.), following the manufacturer's
instructions. The following thermocycling conditions were used:
25°C for 10 min, 50°C for 120 min and 85°C for 5 min. Finally, qPCR
was performed using the SYBR-select Master Mix kit (cat. no.
4472908; Applied Biosystems; Thermo Fisher Scientific, Inc.) and
the LightCycler 480 system [Roche Diagnostics (Shanghai) Co.,
Ltd.]. The expression of several lncRNAs that showed differential
expression were subsequently analysed. The primers used for RT-qPCR
were as follows: LINC01087 forward, 5′-CGGTCTTTGTCATCGAGGCA-3′ and
reverse, 5′-GGCAAAAGGATGGCTTGGAC-3′; Lnc-peroxidasin (Lnc-PXDN)-3:1
forward, 5′-CAGCGTCTCGTTCACCTCTT-3′ and reverse,
5′-GCTCCCTGAAGCACTGACAT-3′; SOX9-AS1 forward,
5′-AGCTGTCCCATGAGTGAAGC-3′ and reverse, 5′-CACTGGATGTCAAGGCTGGT-3′;
Lnc-SYDE forward, 5′-AGAGAGGCTAGGTCGTCAGA-3′ and reverse,
5′-ACATGAGGCTGCTTAGTGACA-3′ and GAPDH forward,
5′-AGCCACATCGCTCAGACAC-3′ and reverse,
5′-GCCCAATACGACCAAATCC-3′.
The PCR amplification conditions for lncRNAs were:
50°C for 2 min, 95°C for 2 min, followed by 40 cycles of 95°C for
15 sec, 60°C for 15 sec and 60°C for 1 min. For GAPDH, the same
initial conditions were used, followed by 40 cycles of 95°C for 15
sec, 58°C for 15 sec and 60°C for 1 min.
The primers designed aligned 100% with the
corresponding target sequences. RT-PCR was performed and a single
product was verified using melting curves. Product size was
verified by gel electrophoresis. The expression level of each gene
was normalized to GAPDH and calculated using the 2−ΔΔCq
method (41).
Statistical analysis
Statistical analyses were performed in GraphPad
Prism version 10.3.1 (Dotmatics). Before conducting the ANOVA, the
normality of the data within each group was assessed using the
Shapiro-Wilk test. To calculate statistical significance between
two groups, an unpaired two-tailed student's t-test with Welch
correction was used in case both groups had unequal variances. For
analysis involving two groups or more, a one-way ANOVA parametric
or non-parametric test was performed. and the mean rank of each
column was compared with the mean rank of every other column. The
normality of the data within each group was assessed using the
Shapiro-Wilk test. If data were normally distributed, a parametric
one-way ANOVA was used and the Brown-Forsythe test applied. If the
normality assumption was violated, a non-parametric alternative
ANOVA was used, followed by the Kruskal-Wallis test. Data were
presented as mean ± SEM.
Results
Clinical and pathological
characteristics
In the present study, of the initial 192 samples
selected, 98.4% (n=189) of all analyzed cases were found in women.
Age range varied from 25 to 88 years, with a mean of 57±12.6 years.
Notably, 34.4% (n=66) patients studied were aged <50 years. In
total, 97.9% (n=188) of the cases were pathologically diagnosed as
invasive ductal carcinoma, whereas 73% (n=140) were classified as
high-grade histological cases, based on standardized histological
criteria (Scarff-Bloom Richardson scheme) (42,43)
that evaluates cellular characteristics such as the degree of
cellular differentiation, mitotic index and irregularities in the
size and shape of the nuclei of tumor cells. Analysis of expression
biomarkers revealed that 58% (n=111) exhibited luminal A
characteristics, 21% (n=40) were identified as luminal B, 7% (n=13)
were non-luminal HER2+ and 14% (n=26) were classified as TNBC.
TNBC exhibits different expression
profiles to luminal B HER2+ tumors
To improve understanding of the biological
characteristics of TNBC, 15 samples were selected and their
transcriptomic profiles (mRNA and lncRNA) were compared with those
in luminal B HER2+ tumors (n=15), which have one of the most
favorable prognosis among aggressive breast tumors (44,45).
Most of the selected samples corresponded to stages ranging from
IIB to IIIC, with an average age of 58.4±12.6 years for the luminal
B HER2+ type and 52.8±15.4 years for TNBC samples (Table SI).
The transcriptional portrait of TNBCs compared with
luminal B HER2+ tissues revealed a total of 746 DEGs. DE RNAs were
defined as transcripts with a fold change >2 or <-2 harboring
P-values and an FDR <0.05. Among these DE transcripts, 555 were
protein-coding (mRNAs), whilst 191 were non-coding RNAs (ncRNAs).
The DE ncRNAs were then classified into 102 long non-coding RNAs
(lncRNAs), 16 miRNAs, 36 small nucleolar RNAs (snoRNAs), 29
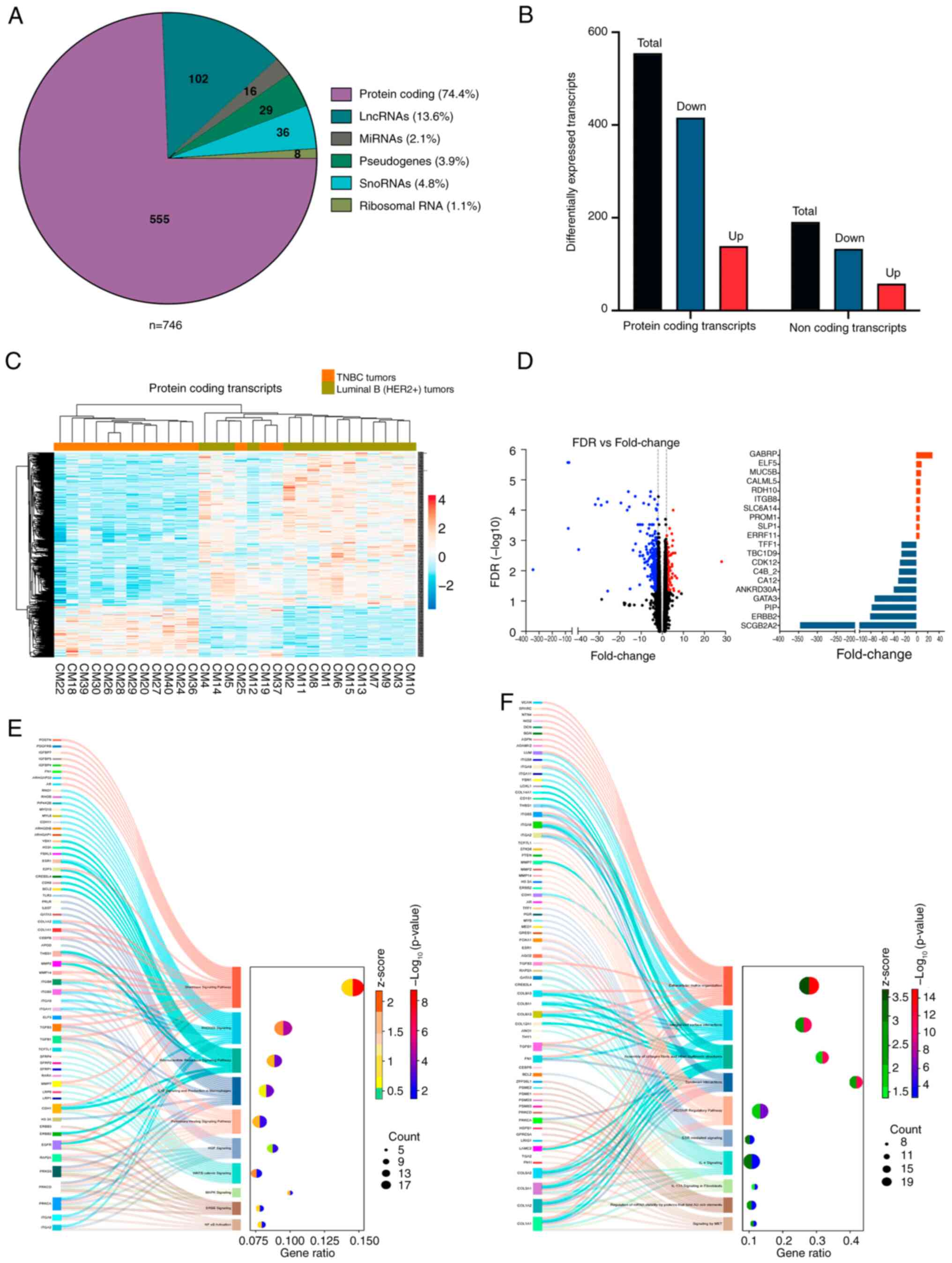
pseudogenes and 8 rRNAs (Fig. 1A).
Amongst the DE mRNAs, 139 show increased expression, whereas 416
presented decreased expression in the TNBC samples, compared with
those in luminal B HER2+ samples. Analysis of the DE lncRNAs
revealed 27 upregulated DEGs and 75 downregulated DEGs (Fig. 1B).
Unsupervised hierarchical clustering analysis
revealed that the samples segregated into two distinct groups,
luminal B HER2+ and TNBC. Heatmap illustrating expression patterns
of protein coding DE transcripts are depicted in Fig. 1C. The expression profile of lncRNAs
provided a clearer distinction in transcriptional profiles between
the two groups compared with the coding RNA profile.
The mRNAs with the highest change were GABRP
(FC=28.19), ELF5 (FC=9.1), MUC5B (FC=14.34), CALML5 (FC=8.93),
PROM1 (FC=5.52), SCGB2A2 (FC=−345.93), ERBB2 (FC=−81.24), PIP
(FC=−78.39), GATA3 (FC=71.2), ANKRD30A (FC=−39.66), CDK12
(FC=−28.95) and TBC1D9 (FC=−26.19; Fig. 1D). A total of 57 DE transcripts are
involved in processes that contribute to invasion and metastasis of
BC, by promoting cell migration and remodeling of the extracellular
matrix (data not shown).
A pathway enrichment analysis was also conducted on
the DE transcripts to compare TNBC with luminal B HER2+ tumors
using the IPA software. Analysis revealed activation of several
signaling pathways, such as ‘Wnt/β-catenin’, ‘Rho GDP Dissociation
Inhibitor (RHOGDI)’, ‘MAPK’ and ‘NF-κB’ (Fig. 1E). These pathways have been
previously described to be the signaling pathways that can promote
proliferation, migration and invasion of triple negative breast
cancer cell lines, which are characteristics of TNBC tumors
(46,47). Additionally, pathways and processes
were identified that were considerably inhibited in TNBC, such as
‘extracellular matrix organization’, ‘integrin cell surface
interactions’, ‘estrogen receptor signaling’, ‘Interleukin-4 (IL-4)
signaling’, ‘mesenchymal-epithelial transition’ and ‘homeobox
antisense intergenic RNA regulatory pathways’. These processes and
pathways revealed the nature of TNBC tumors. Unlike luminal tumors,
which exhibit strong adhesion properties that influence their
movement, TNBC tumors possess weaker adhesion traits. This
difference affects how triple-negative cancer cells migrate,
interact with tumor microenvironment and metastasize (Fig. 1F).
To confirm the gene expression results retrieved
from the microarrays, expression changes of DE mRNAs were next
analyzed on the UCSC Xena platform. This software integrates
accurate and consistent data sources and clinical information,
including TCGA, information that has been validated by various
independent research teams (48).
A cohort of 758 TCGA BC samples was used, where only samples with a
basal or luminal molecular profile were considered. Expression of
DEGs was evaluated in basal (n=142), luminal B (n=194) and luminal
A (n=422) tumors. The data of the present study were successfully
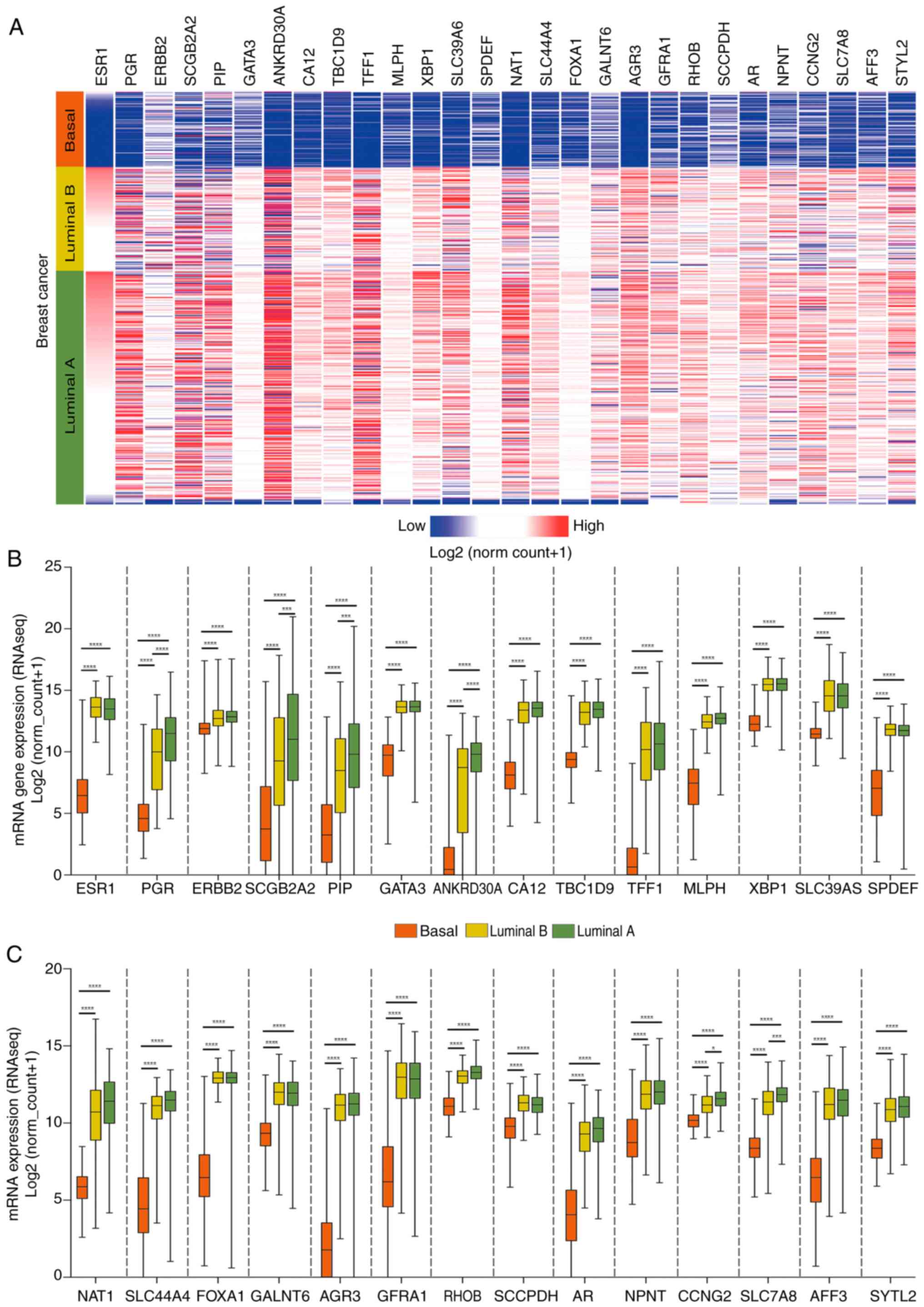
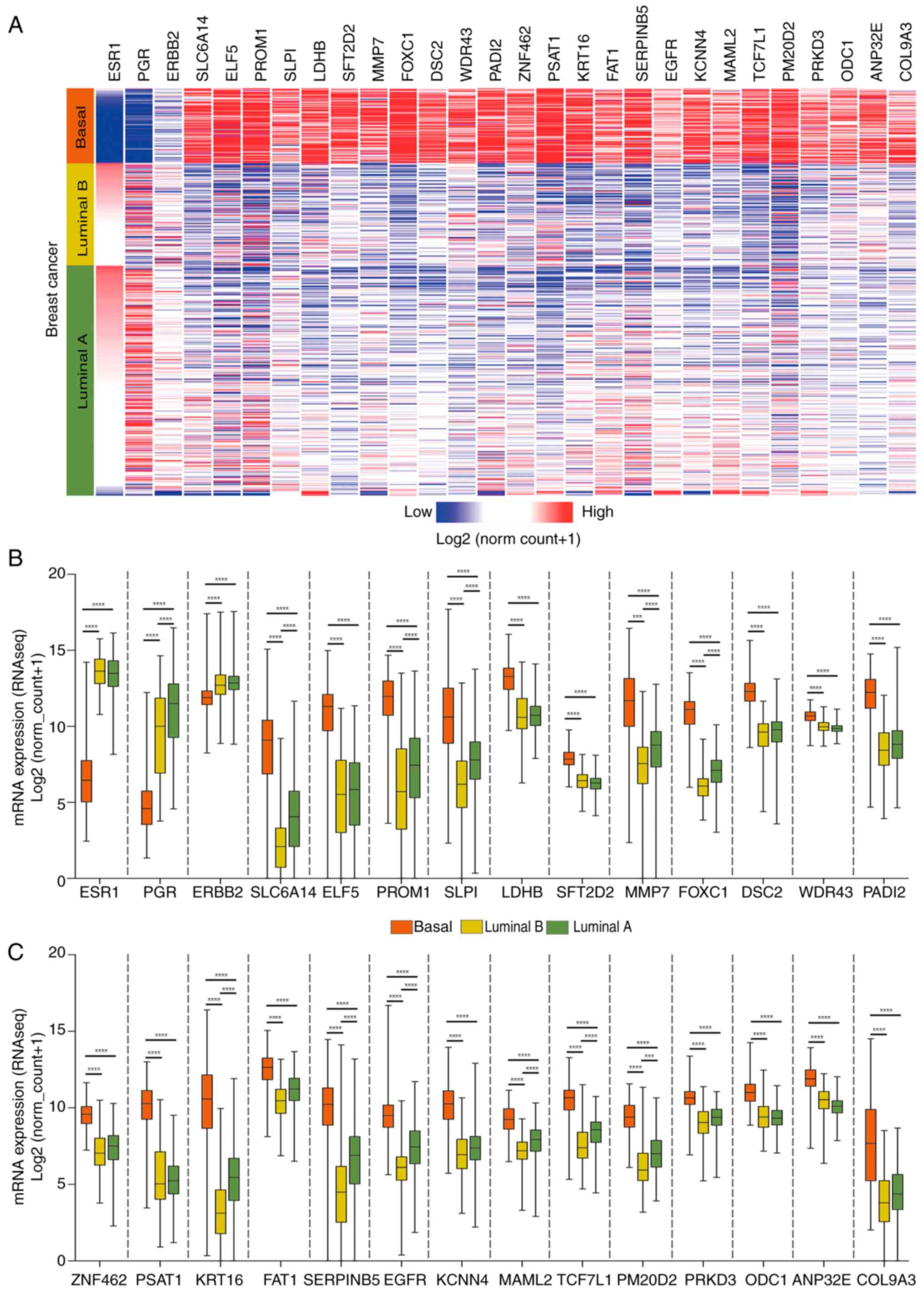
validated in luminal B samples and luminal A samples. Figs. 2 and 3 display the top 25 transcripts with the
highest rate of change, regulated both negatively (Fig. 2A-C) and positively (Fig. 3A-C), in patients with basal BC
compared with those with the luminal subtype. As expected, reduced
expression of ESR1, PGR and ERBB2 was identified in basal tumors.
The genes showing the highest fold change between basal and luminal
samples were anterior gradient protein 3, ankyrin repeat
domain-containing protein 30a (ANKRD30A) and arylamine
n-acetyltransferase 1 (downregulated in basal tumors), whereas
phosphoserine aminotransferase 1, prominin-1 (PROM1) and forkhead
box c1 were upregulated in basal tumors.
Expression profiles of lncRNAs in TNBC
and luminal subtypes of BC
Heatmap illustrating expression patterns of
non-coding DE transcripts are depicted in Fig. 4A. LncRNAs exhibiting the most
significant changes between TNBC and luminal B HER2+ were as
follows: AL157387.1 (FC=−39.39), AC093001.1 (FC=−28.74), LINC01087
(FC=−13.22), AC044784.1 (FC=−12.83), SOX9-AS1 (FC=12.9), mucin
5B-AS1 (FC=8.45), AC092168.2 (FC=5.46) and small nucleolar RNA host
gene 16 (FC=4.97). Although some of these molecules have been
described in certain types of tumors, they have not been fully
characterized and their functions therefore remain undefined
(Fig. 4B). For example, it has
been reported that AC093001.1 may serve an important role in clear
cell renal cell carcinoma (49)
and the lncRNA Sox9-AS1 may be important in hepatocellular
carcinoma and breast cancer (50).
LINC01087 has been reported to increase the aggressiveness of
breast cancer and to predict patient outcome, however the mechanism
of action of this LINC01087 is poorly understood (51), Mucin 5B-AS1 has been involved in
promoting cell migration and invasion processes in lung cancer
cells through interaction with MUC5B (52).
 | Figure 4.Analysis and validation of lncRNAs in
patients with triple-negative breast cancer versus luminal B
(HER2+) using Mexican and TCGA datasets. (A) Heatmap showing DE
lncRNAs in TNBC tissue samples vs. luminal B HER2+ samples. Range
of gene expression spans from red (increased) to blue (decreased).
(B) Volcano plots and top DE lncRNAs. (C) Overexpressed LncRNAs in
basal tumors vs. luminal B (HER2+). Each point on the graph
represents a patient, either with Basal (orange points) or luminal
B (HER2+) tumors (green points). (D) Inhibited LncRNAs in basal
tumors compared with luminal B (HER2+). (E) LncRNA signature found
only in the cohort of Mexican patients. Changes in expression
levels in TNBC samples are shown in blue (decreased) and red
(increased), whereas green rectangles highlight lncRNAs validated
in a second cohort of Mexican patients (accession no. GSE134359).
Additionally, the font size represents the magnitude of the change
in expression. Larger font sizes indicate more pronounced changes,
while smaller font sizes correspond to more subtle changes. (F)
Overall survival of LINC00174 in TNBC tumors using TCGA data.
****P<0.0001, ***P<0.001, **P<0.01 and *P<0.05. LINC,
long intergenic non-coding RNA; LncRNA, long non-coding RNA; TNBC,
with triple-negative breast cancer; HR, hazard ratio; DE,
differentially expressed; FDR, false discovery rate; HER2, human
epidermal growth factor receptor 2. |
Next, the expression of DEGs in patients with
luminal BC and TNBC tumors from TCGA were assessed. Amongst the 102
lncRNAs, only 48 well-annotated lncRNAs had information available.
Of these lncRNAs, 40/48 (83.3%) were successfully validated with
statistical significance. Representative validated lncRNAs in
Fig. 4C and D were those
exhibiting the most pronounced change. The integration of in
silico and experimental analysis enabled the identification of
potential lncRNAs associated with basal BC.
These DE lncRNAs were validated using TCGA samples
originating from non-Mexican patients. The majority of these
differences were made to emphasize the discrepancies found in the
analysis of the present study of Mexican patients compared with
TCGA data. Additionally, a set of 14 lncRNAs were identified which
exhibited DE patterns in the cohort of Mexican patients that were
not identified in the European TCGA cohorts (Fig. 4E). Additionally, a second cohort of
Mexican patients (accession no. GSE134359) was analyzed in the same
manner as the DEGs in basal and luminal B (HER2+) samples (cut-off
criteria: FC=>2 or <-2/P-values and FDR <0.05). Fig. 4E revealed that AC011676.1,
Lnc-lactalbumin α-1, Lnc-zinc finger protein 200-1 and LINC00174
were successfully validated, meaning the same expression pattern
(increased or decreased according to the established criteria) was
observed in this second cohort. Although the remaining 10 lncRNAs
showed a similar trend, they failed to meet established cut-off
criteria.
To assess the influence of these lncRNAs in the
prognosis of patients with BC, TCGA data was used to determine
whether any of the lncRNAs validated in the second cohort were
associated with overall survival. The findings revealed that the
increased expression of LINC00174 was associated with worse overall
survival in patients harboring basal breast tumors. However, this
association was not statistically significant (Fig. 4F). High and low LINC00174
expression was determined by dividing the expression data into
percentiles.
Additionally, four lncRNAs were selected and their
expression was analyzed in luminal and TNBC cell lines. To validate
the clinical findings using an experimental model, the expression
of the lncRNAs was examined to determine whether they are expressed
and upregulated or downregulated in a controlled environment, such
as in cell lines, in a similar manner to how they behave in
previously identified tumor biopsies from Mexican patients in the
present study. Fig. 5A, D, G and J
revealed the expression levels of DE lncRNAs between luminal B
HER2+ and TNBC subtypes derived from Mexican patients with BC.
These data originated from the expression analysis using Human
Transcriptome Array 2.0 microarrays. Subsequently, Fig. 5B, E, H and K illustrate the
expression levels of these lncRNAs in a panel of BC cell lines
belonging to luminal (MCF7, ZR75 and T47D) and triple-negative
(MDA-MB-231 and MDA-MB-468, BT20) subtypes. Specifically, SOXA9_AS1
exhibited increased expression levels in TNBC cell lines compared
with those in the luminal cell lines, whilst Lnc-PXDN-3:1, Lnc-SYDE
and LINC01087 exhibited decreased expression levels in TNBC cell
lines. These findings are consistent with those found by the
microarray analysis. Expression of downregulated lncRNA SYDE was
found to be reduced in TNBC cell lines, but the results were not
statistically significant (Fig.
5H).
 | Figure 5.Analysis of lncRNA expression in cell
lines across several breast cancer subtypes. Relative expression of
the (A) lncRNA SOX9_AS, based on microarray data obtained from
Mexican patients with BC, (B) between luminal and TNBC cell lines
evaluated by RT-qPCR and (C) in luminal vs. basal, Claudin-low and
HER2 breast cancer cell lines evaluated by RT-qPCR. (D) Relative
expression of the lncRNA PXDN-3:1, based on microarray data
obtained from Mexican patients with BC, (E) between luminal and
TNBC cell lines evaluated by RT-qPCR and (F) in luminal vs. basal,
Claudin-low and HER2 breast cancer cell lines evaluated by RT-qPCR.
(G) Relative expression of the lncRNA SYDE, based on microarray
data obtained from Mexican patients with BC, (H) between luminal
and TNBC cell lines evaluated by RT-qPCR and (I) in luminal vs.
basal, Claudin-low and HER2 breast cancer cell lines evaluated by
RT-qPCR. (J) Relative expression of the LINC01087, based on
microarray data obtained from Mexican patients with BC, (K) between
luminal and TNBC cell lines evaluated by RT-qPCR and (L) in luminal
vs. basal, Claudin-low and HER2 breast cancer cell lines evaluated
by RT-qPCR.. Each data point on the graph represents a biological
replicate, which is composed of the mean of two technical
replicates. Statistical analysis was conducted using two groups
with an unpaired student's t-test followed by the Welch correction
in case of unequal variances or a one-way ANOVA followed by the
Brown-Forsythe test for non-parametric data. ****P<0.0001,
***P<0.001, **P<0.01 and *P<0.05. lncRNA or lnc, long
non-coding RNA; LINC, long intergenic non-coding RNA; PXDN,
peroxidasin; TNBC, triple-negative breast cancer; RT-qPCR, reverse
transcription-quantitative PCR. |
Since TNBC can harbor both basal and claudin-low
molecular subtypes (53), cell
lines were next grouped according to their molecular subtypes,
namely Basal (MDA-MB-468 and BT20), claudin-low (MDA-MB-231),
luminal (MCF7, T47D and ZR75) and HER2 (SKBR3 and MDA-MB-453)
(Fig. 5C, F, I and L). Expression
of lncRNA SOX9-AS was revealed to be increased in basal and
claudin-low cell lines compared with that in luminal and HER2 cell
lines (Fig. 5C). LINC01087 and
SYDE exhibited different expression patterns in basal and
claudin-low cell lines, with elevated levels observed particularly
in basal cell lines. In the case of lncPXDN, significant
differences were identified when comparing luminal cell lines with
basal and claudin-low cell lines. Furthermore, HER2 cell lines also
showed significant differences compared with claudin-low and basal
cell lines (Fig. 5F).
Specifically, LINC01087 exhibited decreased expression in basal,
but it was absent in claudin-low cells (Fig. 5L), suggesting that it may only be
expressed in the basal molecular subtype of TNBC. Therefore,
expression of LINC01087 could be used to differentiate between
basal and claudin-low subtypes. Furthermore, SYDE was expressed at
increased levels in luminal cell lines compared with the other
cells. The expression of SYDE was significantly decreased in
claudin-low and HER2 cells compared with luminal cels. Basal cell
lines had a slightly decreased expression of SYDE compared with
luminal cells, although this was not statistically significant
(Fig. 5I).
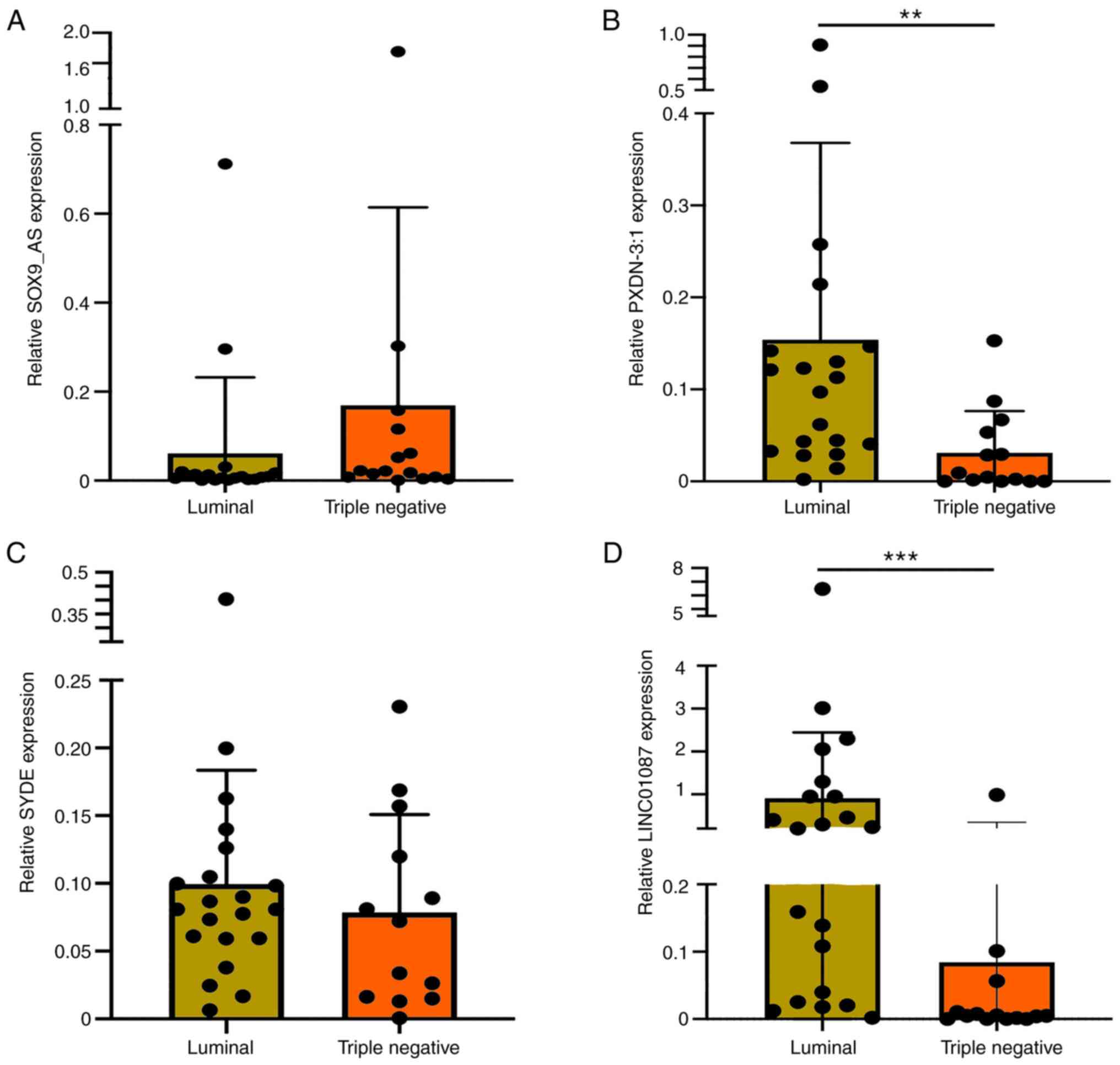
DE lncRNAs were also evaluated in an independent
validation cohort of 21 samples obtained from formalin-fixed
paraffin-embedded tumors from Mexican patients. Samples were
divided into TNBC (n=8) and luminal B HER2+ tumors (n=13). Results
revealed a significant decrease in the expression of lncRNAs
PXDN-3:1 and LINC01087 in TNBC samples compared with those in
luminal B HER2+ tumors. Furthermore, a decrease in the expression
of lncRNA SYDE was observed in TNBC tumors, whilst that of
lncRNA-SOX9-AS1 was also increased, though no statistical
significance was reached (Fig.
6).
In summary, the validation graphs depicting the
expression of lncRNAs across the different cell lines are
consistent with findings from the Mexican patient samples and TCGA
data. These findings suggest that the differential expression
patterns of identified lncRNAs can serve as a key biomarker for the
molecular classification of BC, while possibly serving a
fundamental functional role in TNBC progression.
LncRNAs with clinical significance in
patients with BC
Clinical implication of lncRNAs in the survival of
patients with BC holds importance (54). A previous study has demonstrated
that certain lncRNAs may be associated with diagnosis, prediction
or treatment response in patients with BC (54). Therefore, overall survival of DE
lncRNAs was next assessed using TCGA BC data.
The relationship between 102 DE lncRNAs and overall
survival were evaluated using TCGA BC data. Validation of lncRNAs
in both cell lines and a second set of tissue samples different to
the samples used for microarray, revealed that the decreased
expression of genes, such as LINC01087, LINC02568, ACO22196.1 and
eosinophil granule ontogeny transcript (EGOT), are associated with
poor overall survival in samples of BC, regardless of molecular
subtype (Fig. 7). Furthermore,
overall survival analysis revealed a significant impact of
LINC01087 on the survival of patients with the luminal B subtype of
BC (Fig. 8C), where the decreased
expression of this lncRNA was found to be associated with worse
prognosis. No significant associations were found between overall
survival of patients with BC and the expression of lncRNAs
SOX9-AS1, lncRNA-PXDN-3:1 and SYDE (data not shown). When the
samples were classified by molecular subtype, lncRNA-PXDN-3:1
revealed a significant association in ER+ samples, where worse
survival was associated with the high expression of lncRNA-PXDN-3:1
(Fig. 8A).
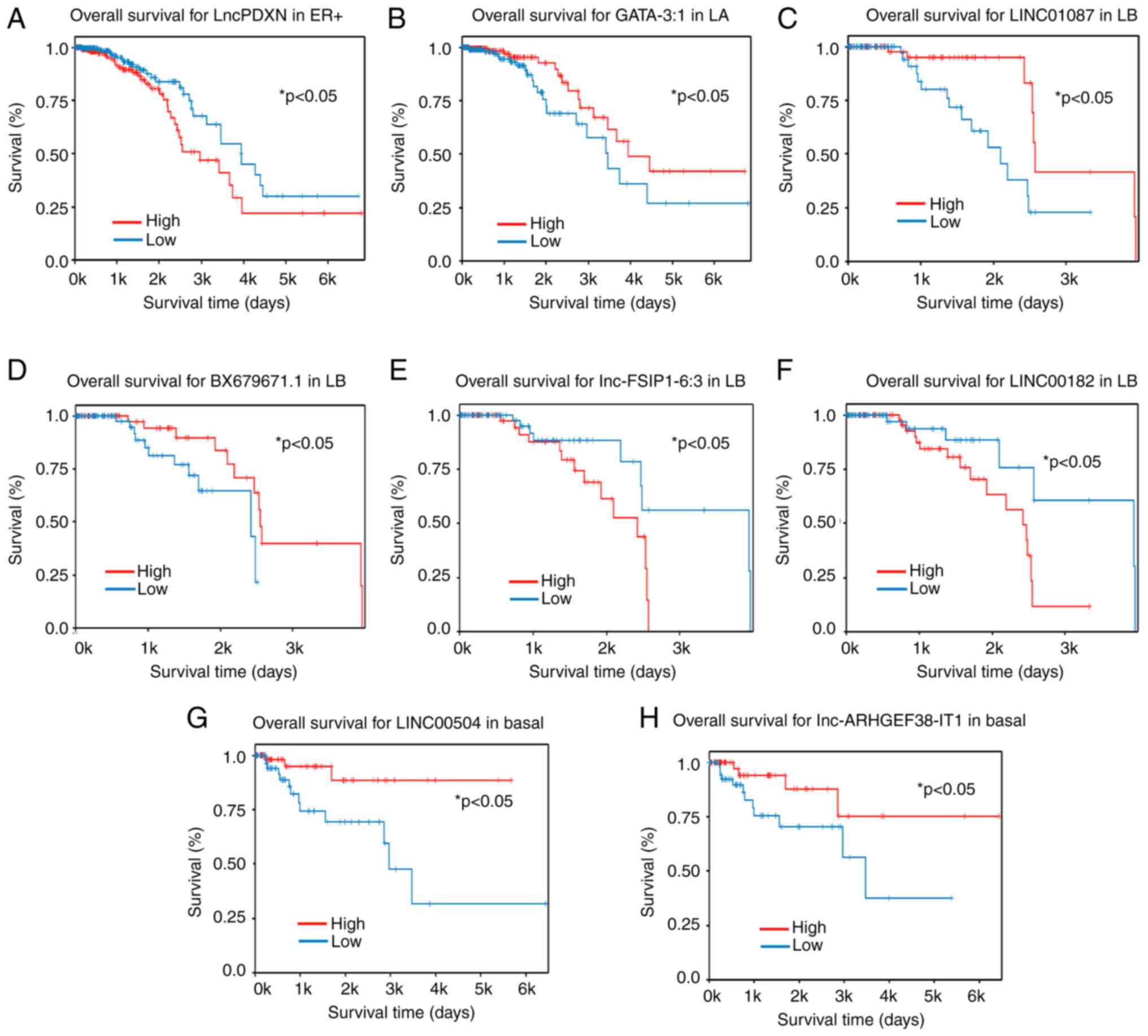 | Figure 8.LncRNAs involved in the survival of
patients with breast cancer with specific molecular subtypes.
Kaplan-Meier plots displaying lncRNAs involved in the overall
survival of patients with (A) ER+, specifically the PXDN-3.1 (B)
Luminal A, specifically the Lnc-GATA-3-1 (C) Luminal B, the
LINC01087 (D) Luminal B, the Lnc- BX679671.1 (E) Luminal B, the
Lnc-FSIP1.6:3 (F) Luminal B, the LINC00182 (G) basal, the LINC00504
and (H) basal, Lnc-ARHGEF38-IT1. Blue lines indicate low expression
of lncRNA, whilst red lines indicate high expression. Blue lines
indicate low expression of lncRNA, whilst red lines indicate high
expression. LA, luminal A, LB, luminal B; LncRNA, long non-coding
RNA; LINC, long intergenic non-coding RNA; PXDN, peroxidasin;
GATA-3-1, GATA binding protein 3-1; FSIP1, fibrous sheath
interacting protein 1; ARHGEF38-IT1, rho guanine nucleotide
exchange factor 38 intronic transcript 1. |
In addition, the overall survival of patients with
distinct molecular subtypes of BC according to the low or high
expression of other lncRNAs was assessed. In luminal A samples, low
expression of lncRNA-GATA-3-1 was found to associate with worse
overall survival (Fig. 8B). In
luminal B samples, low expression of lncRNA BX679671.1 was
associated with worse overall survival (Fig. 8D), whilst high expression of
lncRNAs fibrous sheath interacting protein 1-6:3 and LINC00182 was
associated with reduced survival (Fig.
8E and F). In basal samples, low expression of lncRNAs
LINC00504 and ARHGEF38-IT1 were associated with worse survival.
Notably, these lncRNAs exhibited decreased expression in TNBC
samples compared with luminal B HER2 samples, consistent with the
aggressive characteristics of TNBC (Fig. 8G and H).
LINC01087 interacts with histones and
transcription factors to possibly regulate ESR-mediated
signaling
In silico analysis was next conducted to
characterize SOX9-AS1, PXDN-3.1 and LINC01087 using the RNA
interactome repository (55), to
investigate potential interactions of this lncRNA with other
molecules (Fig. 9A-C). Analysis
revealed that the majority of interactions with LINC01087 strongly
associated with chromatin marks, such as H3K27me3, H3K4me3, H327ac
and H3K4me1/2 (Fig. 9C).
Additionally, interactions of LINC01087 with several transcription
factors were identified, including CCCTC-binding factor, estrogen
receptor 1, Forkhead box (FOX) A1, SOX2, STAT3 and Krüppel-like
factor. Additionally, analysis of LINC01087 revealed interactions
with other lncRNAs, such as non-coding RNA activated by DNA damage
and AP000526.1 (Fig. 9C). Analysis
of co-regulated gene patterns to explore any possible functional
relationships is shown according to gene color and size (Fig. 9D).
 | Figure 9.Prediction of SOX-AS1, PXDN-3.1 and
LINC01087 interactions with associated biological processes and
signaling pathways. Circus plot indicating interactions of (A)
SOX-AS1, (B) PXDN-3.1 and (C) LINC01087 with molecules, such as
histone marks (light green dots), transcription factors (yellow
dots), lncRNAs (dark blue dots), G protein-coupled proteins (red
dots), chromatin remodeling factors (light blue dots), RNA-binding
proteins (dark green dots). (D) Gene co-regulation analysis, with
the font size reflecting the degree of correlation between
molecules and the color reflects the degree of expression, with red
as the highest degree of expression. (E) Processes and signaling
pathways enriched by the presence of LINC1087. LINC, long
intergenic non-coding RNA. |
Since LINC01087 has been associated with
aggressiveness in several types of cancers, including breast cancer
(56–59), the possible function of this lncRNA
was explored by investigating the involvement of biological
processes and signaling pathways associated with LINC01087.
Functional enrichment analysis successfully identified relevant
signaling pathways where LINC01087 may participate. Results
revealed engagement of this transcript in various biological
functions, such as ‘signaling by nuclear receptors’, ‘ESR-mediated
signal’, ‘transcription factor binding’, ‘estrogen-dependent gene
expression’, ‘chromatin binding’ and ‘protein dimerization
activity’, suggesting that LINC01087 is involved in nuclear
receptor signaling. These analyses suggest that molecules
interacting with LINC01087 participate in ER-mediated signaling,
which suggests that this lncRNA may regulate the expression of
ER-related genes and have a role in luminal tumors (Fig. 9E).
Discussion
BC is a notably heterogeneous disease (60) and the TNBC subtype is particularly
distinguished for its aggressive nature (44). Since the different molecular
subtypes of breast cancer have distinct clinical behaviors and
specific therapies, research has been focused on the identification
of biomarkers to stratify patients both therapeutically and
prognostically (15). Results of
the data generated in the present study are comparable with other
studies conducted worldwide, due to the expression profiles of
these tumors and the signaling pathways and processes dysregulated
in these tumors (61,62). However, the observed frequency of
TNBC was lower when compared with that reported in previous studies
in the Mexican population (31,32).
This discrepancy may be attributed to the cut-off points in the
present study used to positively diagnose TNBC. The official cutoff
value was used to assess the expression of estrogen and
progesterone receptors in BC using IHC, following American Society
of Clinical Oncology/College of American Pathologists guidelines
(35). A result was considered
positive if ≥1% of tumor cell nuclei showed staining. The
transcriptomic analysis, using cutoff values based on fold change
and statistical analysis, confirmed the expression findings. No
hormonal receptors were observed in TNBC, whereas they were
expressed in luminal tumors.
In TNBC, lncRNAs have emerged as promising markers
for diagnosis, prognosis, treatment resistance and monitoring the
spread of cancer. These lncRNAs interact with a variety of
molecules, such as DNA, RNA and proteins, to activate the
metastatic transcriptional network (18). Although luminal subtypes are
generally characterized by a more favorable prognosis in comparison
with TNBC, it is important to highlight that the luminal B subgroup
is also associated with a poor prognosis (45,54,63).
Given that transcriptomic profiles and molecular classification
panels for BC rely heavily on mRNA, the present study focused on
the investigation of lncRNAs.
The present study aimed to elucidate differences in
the gene expression levels of lncRNAs and mRNAs among tissue
samples between patients with TNBC and luminal B HER2+ BC. To
ensure robustness, reliability and accurate interpretation of the
microarray data, gene expression changes were validated in patient
samples using TCGA database. Results confirmed the consistency of
both coding and non-coding RNA data. In Fig. 4C and D, the representative lncRNAs,
that is, those with the most pronounced and significant changes
between luminal B and TNBC samples, were shown. Thus, the graphs
only show 19 lncRNAs, chosen for their higher statistical
robustness and clearer representation of the observed trends.
Regarding AC011676, no significant difference was
found between Luminal B and TNBC Samples. Since only 26 lncRNAs
were upregulated, information was available for only 8/26 lncRNAs
in the TCGA cohort. As a result, AC011676 was one of the 8 lncRNAS
that failed validation.
Expression of lncRNAs in TNBC has been previously
described in other studies, which have identified specific lncRNAs
differentially expressed across the different molecular subtypes
(54,63). However, some transcripts are
difficult to identify due to their low expression levels and
tissue-specific patterns. In particular, some transcripts may not
be abundant enough in the sample to be reliably detected,
especially in heterogeneous tissues such as breast tumors.
Additionally, variability in expression patterns across different
tissue types makes it difficult to distinguish signals from
non-cancerous tissues within the sample. Therefore, the present
study provided specific information from Mexican patients and
contributed support for future clinical considerations. A total of
14 lncRNAs were identified that were not DE in TCGA cohorts, which
mainly included data from European patients. Among these, 4 lncRNAs
were validated in a different Mexican cohort, including TNBC and
luminal B HER2+ tumors. However, since several lncRNAs remain
unidentified and their functions remain poorly defined, the
presented study integrated coding and non-coding data to
investigate potential signaling pathways associated with the TNBC
tumor phenotype.
Several signaling pathways were found to be
activated in TNBC that are known for regulating various processes
during tumor progression and metastasis (18)Specifically, the present study
identified that the NF-κB, Wnt/β-catenin, MAPK and RHOGDI signaling
pathways are activated in TNBC. NF-κB and Wnt/β-catenin are
established signaling axes that serve key roles in cell
proliferation, maintenance of stemness and development of drug
resistance in TNBC (64). Their
activation considerably affects the survival of patients with TNBC.
MAPK pathways are involved in signal transduction cascades that
promote tumor progression, invasion and metastasis of TNBC
(65). Constitutive activation of
MAPKs has been observed in aggressive TNBC, which is associated
with worse overall survival (66).
By contrast, RHOGDIs regulates the Rho family of GTPases, which
control several signal transduction pathways, such as the actin
cytoskeleton remodeling pathway and the cell cycle regulatory
pathway. RHOGDI can both inhibit or activate Rho GTPases,
potentially serving a dual role in cancer (67). Notably, RhoGDI-2 is overexpressed
in TNBC and its inhibition can induce apoptosis whilst sensitizing
BC cells to cisplatin treatment (46).
The present study revealed several processes that
are inhibited, such as extracellular matrix organization, integrins
and syndecan interactions, ESR and IL-4 signaling. The altered
pathways reflected changes in adhesion properties of TNBCs. TNBC
cells have fewer adhesive properties, which facilitates cell
migration and invasion (18). In
this regard, several clinical trials are currently underway to
evaluate the efficacy of pharmacological agents that can regulate
components of the extracellular matrix in TNBC (68), For example, Lucitanib, which
inhibits angiogenesis and reduces matrix metalloproteases and
collagen, or Reparixin, an interleukin-8 receptor C-X-C chemokine
receptor type 1 and 2 inhibitor (69,70).
LncRNAs have emerged as key components in BC biology, serving a key
role in the regulation of gene expression and disruption of key
biological pathways in the development and progression of BC
(71,72). For example, the AKT/PI3K/mTOR
pathway is mediated by lncRNAs such as HOX transcript antisense
intergenic RNA (HOTAIR), metastasis associated lung adenocarcinoma
transcript 1 and lnc-urothelial carcinoembryonic antigen 1.
Additionally, HOTAIR and lncRNA maternally expressed gene 3
(lncRNA-MEG3) regulate NF-κB signaling (72) and lncRNA growth arrest-specific
transcript 5 influences apoptosis through the
miR-378a-5p/suppressor of fused homolog pathway in TNBC (73). In addition, the diversity and
specificity of lncRNAs in different molecular subtypes of BC
suggest potential applications in patient stratification and
personalized therapies (25,74)
It is noteworthy that the transcription profile of
lncRNAs is more consistent compared with that of mRNAs. This is
reflected by the reduced variability of data among molecular
subgroups, in addition to a marked difference in the diversity of
molecular subtype expression. Congruence in validation results
using RT-qPCR and expression microarray data serves to underscore
the steadfastness and robustness of the findings. Reduction in the
expression of PXDN-3:1 and LINC01087 in TNBC cell lines supports
their potential role in characterizing this subtype. LncRNA
PXDN-3:1 was also highly expressed in the luminal and HER2
molecular subtypes compared with that in the basal and claudin-low
cell lines, where its expression was scarce. Similarly, increased
expression of lncRNA-SOX9-AS1 in this subtype suggests its
potential contribution to the molecular characteristics of
TNBC.
Although SYDE results were not initially significant
in TNBC cell lines, subgroup analysis highlighted its potential
role in specific subtypes. This detailed approach could provide an
improved reflection of biological variability in TNBC cell lines.
Certain lncRNAs may exhibit such sensitivity in their expression
levels to enable classification of subtypes, particularly within
the TNBC subtype. This sensitivity can lead to more precise
stratification in terms of classification and survival outcomes.
Claudin-low samples have been observed to display a more aggressive
behavior compared with basal samples, where this variability has
been reported to be associated with different mutations that can
impact drug response (75). In the
present study, LncRNA SYDE exhibited reduced expression in
claudin-low and HER2 cell lines compared with that in basal cell
lines. This suggests the possibility that SYDE may be involved in
proliferation processes, given that basal lines tend to display a
higher proliferation rate compared with claudin-low lines (76). However, the functional role of
lncRNA SYDE remains unknown. Therefore, additional studies are
required to deepen the understanding of this lncRNA. In addition, a
significant decrease in the expression of PXDN-3:1 and LINC1087
transcripts was observed in an independent set of FFPE TNBC tissues
compared with luminal tissues. These results support the validity
of these lncRNAs as potential biomarkers for characterizing tumor
subtypes.
The decreasing trend in SYDE expression observed in
TNBC tumors underscores the importance of using a larger sample
size to detect more pronounced differences. Similarly, the
increasing trend in lncRNA-SOX9-AS1 expression in TNBC tumors,
whilst not reaching statistical significance in the present study,
merits further exploration. Previous studies have also revealed
that SOX9-AS1 is highly expressed in TNBC tumors compared with
other BRCA subtypes (77,78). Among the various TNBC molecular
subtypes, the overexpression of SOX9-AS1 is associated with a
favorable prognosis (77).
Furthermore, other studies suggest that SOX9-AS1 may be involved in
migration, invasion and lipid metabolic reprogramming in TNBC cells
(77,79). Previously, SOX9-AS1 was identified
to regulate cellular senescence resistance in TNBC cells through
the Wnt pathway (78). SOX9-AS1
knockdown promotes senescence and immune cell infiltration,
suggesting its involvement in modulating the immune
microenvironment (78). However,
databases report different isoforms of SOX9-AS1 that are
susceptible to more robust biological analysis to determine if they
have any clinical implications.
Several studies have revealed the potential of the
association of LINC01087 with carcinogenesis and suggest that it
may possess significant diagnostic value (56–58).
This lncRNA has been previously investigated in esophageal,
gastric, ovarian and breast cancers (59,80).
It has been reported that LINC01087, located in the cytoplasm,
functions as an endogenous competitor (ceRNA) by binding to
specific miRNAs to prevent interaction with their mRNA targets. In
the case of gastric cancer (GC), it has been observed that
LINC01087 modulates tumorigenesis through the miR-135a-5p/caspase
activity and apoptosis inhibitor 1 (CAAP1) signaling pathway
(56). LINC01087 is highly
expressed in GC and acts as a molecular sponge for miR-135a-5p,
allowing CAAP1 expression to increase the migration and invasion
capacity of tumor cells (56). The
analysis of LINC01087 was deepened owing to the growing evidence of
its relevance in the context of BC (51,58,59,80).
In TNBC, in silico experiments previously revealed that
LINC01087 can form a ceRNA network with LINC01087/miR-135b-5p by
co-expressing with sulfite oxidase, E6-AP carboxyl terminus domain
E3 ubiquitin protein ligase 2 and solute carrier family 39 member 6
(79).
However, interaction analysis conducted in the
present study suggested that besides interacting with miRNAs,
LINC01087 may interact with nuclear molecules, such as
transcription factors ER2, PR, Runt-related transcription factor 1
and GATA. This finding may provide novel insights into the function
of this lncRNA in the nucleus and its role in BC. The data from our
study support findings, indicating a decrease in LINC01087 in TNBC,
in addition to upregulation in luminal subtypes (51). Therefore, LINC01087 may have a
considerable role in the classification of luminal and TNBC
samples. Furthermore, data of the present study indicated that
increased LINC01087 expression is associated with a favorable
prognosis in BC. Consequently, low expression levels of LINC01087
were associated with reduced survival, as seen in TNBC cases
(51). No information was found
regarding the lncRNA lnc-Syde, which could present a novel research
opportunity.
In the present study, low expression of LINC01087,
ACO22196.1 and EGOT is associated with a reduction in 5-year
survival. Furthermore, it was demonstrated that the decreased
expression of FOXP1-IT1, LINC02568 and Z9330.2 predicts worse
20-year survival, highlighting their impact on long-term prognosis.
These data suggest that these lncRNAs may have a role in
controlling tumor progression. Survival analysis specific to
molecular subtypes provides additional information on the
relationship between lncRNA expression and the nature of each
molecular subtype, reinforcing the potential clinical relevance of
these findings.
Association between low expression levels of several
lncRNAs and worse overall survival, along with specific
associations in molecular subtypes, emphasizes the significance of
considering these lncRNAs as potential biomarkers for patient
stratification and development of more personalized therapeutic
approaches in BC. In this sense, it is relevant to classify and
identify biological subtypes of clinical importance using different
approaches, such as mutational profiles of mitochondrial DNA, in
addition to both radiological and histopathological image analysis
(81,82). Nevertheless, further studies are
warranted. Despite the use of additional tissue samples to validate
results, the sample size remains limited, calling for the expansion
of analysis to larger sample sizes to confirm these findings and
achieve more robust statistical significance, thereby
substantiating and further exploring these prognostic
associations.
The present study identified a set of lncRNAs that
are DE in Mexican patients with TNBC and luminal BC. However, some
limitations hindered the ability to obtain a unique profile of
lncRNAs specifically associated with TNBC. Analysis focused on only
two of the five molecular subtypes, meaning that confirmation on
whether the DE lncRNAs reported in the present study are
predominantly associated with TNBC remains unconfirmed. Another
limitation is the small sample size that was analyzed in the
microarray, which can be less representative and may not allow
generalization to a broader population. A strength of the present
study is the representative selection of tumor tissues in the
presence or absence of immunohistochemical biomarkers for
transcriptomic analysis. Another strength is that the
transcriptomic analysis was performed in a group of tumors from
Mexican patients, providing more specific information about
transcriptomic signatures of clinical relevance in Mexico.
Additionally, the present analysis identified transcriptomic data
that, regardless of the population, were consistent with those of
European tumors according to TCGA data. Likewise, the lncRNA
signature enriched in the Mexican population was identified, which
was validated in another independent Mexican cohort (GSE134359).
Additionally, previous studies have compared transcriptomic
profiles of TNBC and HER2-low or lncRNA expression profiles in
luminal B associated with neoadjuvant chemoresistance (83,84).
However, to the best of our knowledge, studies that analyzed the
DEG profile between TNBC and luminal B HER2+ could not be
identified. Therefore, the present study provided information to
identify the signature of lncRNAs associated with prognosis.
Further genomic and epigenomic studies must be
conducted on Latin American populations to achieve a more
representative landscape, since exposure to environmental factors
may vary between populations, in addition to genetic
differences.
The present study identified transcripts associated
with tumor aggressiveness, including lncRNAs linked to poorer
prognosis in patients with TNBC. Furthermore, it identified
molecules involved in signaling pathways that could drive the more
aggressive phenotype of TNBC compared with the luminal B HER2+
subtype, contributing to the discovery of potentially useful
biomarkers for patient prognosis. Since TNBC is the most aggressive
subtype with limited therapeutic options, generating insights into
its biology is key for improving clinical management (85).
There are studies comparing TNBC and luminal B HER2+
or triple-positive (TPBC) subtypes, analyzing clinicopathological
differences, such as tumor stage, metastatic sites and
lymphovascular invasion (LVI) (61,62).
In a study by Mandor et al (61), these comparisons aimed to evaluate
possible associations between these clinical characteristics and
patient recurrence or survival. The results showed no significant
differences between the two groups in terms of disease progression.
However, significant changes in progression, such as LVI, were
observed within each group separately (61).
A study previously conducted in Korea highlighted
differences in the clinical courses of TNBC and TPBC. The authors
found significant variations in histologic grade, nuclear grade,
lymphatic invasion and long-term survival after treatment in
patients with these BC subtypes (62). However, to the best of our
knowledge, studies specifically focusing on transcriptomic
evaluations that compared only these two molecular subtypes could
not be identified, underscoring the relevance of the work in the
present study.
To conclude, BC subtypes are highly heterogeneous,
such that comparing the transcriptomes of each subtype may
facilitate understanding into how molecular differences can
influence their clinical behavior. The present study focused on
comparing TNBC and luminal B HER2+ subtypes because whilst luminal
B HER2+ is an aggressive subtype, it shows improved outcomes
compared with TNBC (44,86) due to the availability of targeted
therapies. By contrast, the TNBC subtype has a worse prognosis
because of its aggressiveness and the lack of specific
treatments.
Collectively, these discoveries underscore the
intricate involvement of mRNAs and lncRNAs in the landscape of BC
biology as a whole and in the context of each one of the molecular
subtypes. The findings reveal that specific mRNAs may have a key
influence on BC progression, impacting key signaling pathways.
Furthermore, emphasis is placed on the significance of select
lncRNAs as biomarkers for patient stratification and their
potential use as prognostic indicators for individuals with BC.
Indeed, quantitative assessment of lncRNAs is considered to hold
clinical relevance for the diagnosis and prognosis of patients with
TNBC. Whilst promising associations have been uncovered, a deeper
exploration into these prognostic relationships is necessary.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Instituto Mexicano del Seguro
Social through the Health Research Coordination (grant nos.
FIS/IMSS/PROT/PRIO/13/027 ‘Temas prioritarios IMSS 2013’) and
funded by ‘Protocolos de investigación multidisciplinarios de
Cohorte de Largo Aliento sobre Temas Prioritarios en el IMSS 2023’
(grant no. R-2020-785-154).
Availability of data and materials
The data generated in the present study may be
found in the GEO under accession number GSE270721. (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE270721).
The data generated in the present study may be requested from the
corresponding author.
Authors' contributions
RCO conducted RT-qPCR experiments, bioinformatic
analysis, analyzed results and wrote the manuscript. CVV obtained
RNA from samples and conducted IHC experiments. KVS conducted
RT-qPCR experiments, bioinformatics analysis and wrote the
manuscript. AMM confirmed the histopathological diagnosis and
selected representative areas of tumor for molecular analysis.
MERT, JT, NRS and HM analyzed the results. PPS conceived the
present study, conducted experiments, analyzed and revised results
and wrote the manuscript. RCO and PPS confirm the authenticity of
all the raw data. All authors have approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee and by the National Committee for Scientific
Research from Mexican Institute of Social Security (approval nos.
R-2013-785-045 and R-2020-785-154).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leal YA, Torres J, Gamboa R,
Mantilla-Morales A, Piña-Sanchez P, Arrieta O, Bonifaz L, Meneses
A, Duque C and Piñeros M: Cancer incidence in Merida, Mexico
2015–2018: First report from the population-based cancer registry.
Arch Med Res. 53:859–866. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000-14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nicolini A and Ferrari P: Targeted
therapies and drug resistance in advanced breast cancer,
alternative strategies and the way beyond. Cancers (Basel).
16:4662024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Unger-Saldaña K, Bandala-Jacques A,
Huerta-Gutierrez R, Zamora-Muñoz S, Hernández-Ávila JE,
Cabrera-Galeana P, Mohar A and Lajous M: Breast cancer survival in
Mexico between 2007 and 2016 in women without social security: A
retrospective cohort study. Lancet Reg Health Am.
23:1005412023.PubMed/NCBI
|
|
7
|
Grajales-Alvarez R, Gutiérrez-Mata A,
Pichardo-Piña C, Gutiérrez-De la Barrera M and Dip-Borunda K:
Survival outcomes of patients with breast cancer in a Mexican
population. JCO Glob Oncol. 10:e23002332024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calhoun BC and Collins LC: Predictive
markers in breast cancer: An update on ER and HER2 testing and
reporting. Semin Diagn Pathol. 32:362–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vieira AF and Schmitt F: An update on
breast cancer multigene prognostic tests-emergent clinical
biomarkers. Front Med (Lausanne). 5:2482018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sørlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karsli-Ceppioglu S, Dagdemir A, Judes G,
Lebert A, Penault-Llorca F, Bignon YJ and Bernard-Gallon D: The
epigenetic landscape of promoter genome-wide analysis in breast
cancer. Sci Rep. 7:65972017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manjunath M and Choudhary B:
Triple-negative breast cancer: A run-through of features,
classification and current therapies (Review). Oncol Lett.
22:5122021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohammed AA: The clinical behavior of
different molecular subtypes of breast cancer. Cancer Treat Res
Commun. 29:1004692021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of triple-negative breast cancer molecular subtypes:
Implications for neoadjuvant chemotherapy selection. PLoS One.
11:e01573682016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmad M, Weiswald LB, Poulain L, Denoyelle
C and Meryet-Figuiere M: Involvement of lncRNAs in cancer cells
migration, invasion and metastasis: Cytoskeleton and ECM crosstalk.
J Exp Clin Cancer Res. 42:1732023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sahu A, Singhal U and Chinnaiyan AM: Long
noncoding RNAs in cancer: From function to translation. Trends
Cancer. 1:93–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen LL: Linking long noncoding RNA
localization and function. Trends Biochem Sci. 41:761–772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mattick JS, Amaral PP, Carninci P,
Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME,
Fitzgerald KA, et al: Long non-coding RNAs: Definitions, functions,
challenges and recommendations. Nat Rev Mol Cell Biol. 24:430–447.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh D, Assaraf YG and Gacche RN: Long
non-coding RNA mediated drug resistance in breast cancer. Drug
Resist Updat. 63:1008512022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taghvimi S, Abbaszadeh S, Banan FB, Fard
ES, Jamali Z, Najafabadi MA, Savardashtaki A and Movahedpour A:
LncRNAs roles in chemoresistance of cancer cells. Curr Mol Med.
22:691–702. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tano K and Akimitsu N: Long non-coding
RNAs in cancer progression. Front Genet. 3:2192012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guzel E, Okyay TM, Yalcinkaya B,
Karacaoglu S, Gocmen M and Akcakuyu MH: Tumor suppressor and
oncogenic role of long non-coding RNAs in cancer. North Clin
Istanb. 22:81–86. 2019.
|
|
29
|
Kaushik AC, Mehmood A, Wang X, Wei DQ and
Dai X: Globally ncRNAs expression profiling of TNBC and screening
of functional lncRNA. Front Bioeng Biotechnol. 8:5231272021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia M, Zu X, Chen Z, Wen G and Zhong J:
Noncoding RNAs in triple negative breast cancer: Mechanisms for
chemoresistance. Cancer Lett. 523:100–110. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lara-Medina F, Pérez-Sánchez V,
Saavedra-Pérez D, Blake-Cerda M, Arce C, Motola-Kuba D,
Villarreal-Garza C, González-Angulo AM, Bargalló E, Aguilar JL, et
al: Triple-negative breast cancer in Hispanic patients: High
prevalence, poor prognosis, and association with menopausal status,
body mass index, and parity. Cancer. 117:3658–3669. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Macari A, Soberanis-Pina P, Varela-Santoyo
E, Valle-Sanchez MA, Leal-Hidalgo JL, Torres-Guillen VM,
Motola-Kuba D, Ruiz-Morales JM and Dorantes-Heredia R: Prevalence
and molecular profile of breast carcinoma using
immunohistochemistry markers in Mexican women. World J Oncol.
12:119–123. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tuominen VJ, Ruotoistenmäki S, Viitanen A,
Jumppanen M and Isola J: ImmunoRatio: A publicly available web
application for quantitative image analysis of estrogen receptor
(ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res.
12:R562010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hammond MEH, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer (Unabridged Version). Arch Pathol Lab Med.
134:e48–e72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allison KH, Hammond MEH, Dowsett M,
McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR,
Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone
receptor testing in breast cancer: ASCO/CAP guideline update. J
Clin Oncol. 38:1346–1366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McCall MN, Bolstad BM and Irizarry RA:
Frozen robust multiarray analysis (fRMA). Biostatistics.
11:242–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Metsalu T and Vilo J: ClustVis: A web tool
for visualizing clustering of multivariate data using principal
component analysis and heatmap. Nucleic Acids Res. 43:W566–W570.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tiessen A, Cubedo-Ruiz EA and Winkler R:
Improved representation of biological information by using
correlation as distance function for heatmap cluster analysis. Am J
Plant Sci. 8:502–516. 2017. View Article : Google Scholar
|
|
39
|
Cedro-Tanda A, Ríos-Romero M,
Romero-Córdoba S, Cisneros-Villanueva M, Rebollar-Vega RG,
Alfaro-Ruiz LA, Jiménez-Morales S, Domínguez-Reyes C,
Villegas-Carlos F, Tenorio-Torres A, et al: A lncRNA landscape in
breast cancer reveals a potential role for AC009283.1 in
proliferation and apoptosis in HER2-enriched subtype. Sci Rep.
10:131462020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Han L, Roebuck P, Diao L, Liu L,
Yuan Y, Weinstein JN and Liang H: TANRIC: An interactive open
platform to explore the function of lncRNAs in cancer. Cancer Res.
75:3728–3737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meyer JS, Alvarez C, Milikowski C, Olson
N, Russo I, Russo J, Glass A, Zehnbauer BA, Lister K and Parwaresch
R; Cooperative Breast Cancer Tissue Resource, : Breast carcinoma
malignancy grading by Bloom-Richardson system vs proliferation
index: Reproducibility of grade and advantages of proliferation
index. Mod Pathol. 18:1067–1078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bloom HJG and Richardson WW: Histological
grading and prognosis in breast cancer; a study of 1,409 cases of
which 359 have been followed for 15 years. Br J Cancer. 11:359–377.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Afifi N and Barrero CA: Understanding
breast cancer aggressiveness and its implications in diagnosis and
treatment. J Clin Med. 12:13752023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Holowatyj AN, Ruterbusch JJ, Ratnam M,
Gorski DH and Cote ML: HER2 status and disparities in luminal
breast cancers. Cancer Med. 5:2109–2116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lino MA, Palacios-Rodríguez Y,
Rodríguez-Cuevas S, Bautista-Piña V, Marchat LA, Ruíz-García E,
Astudillo-de la Vega H, González-Santiago AE, Flores-Pérez A,
Díaz-Chávez J, et al: Comparative proteomic profiling of
triple-negative breast cancer reveals that up-regulation of
RhoGDI-2 is associated to the inhibition of caspase 3 and caspase
9. J Proteomics. 111:198–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu X, Zhang Q, Xing W and Wang W: Role of
microRNA/lncRNA intertwined with the Wnt/β-Catenin axis in
regulating the pathogenesis of triple-negative breast cancer. Front
Pharmacol. 13:8149712022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Goldman MJ, Craft B, Hastie M, Repečka K,
McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, et al:
Visualizing and interpreting cancer genomics data via the Xena
platform. Nat Biotechnol. 38:675–678. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang G, Liu P, Li J, Jin K, Zheng X and
Xie L: Novel prognosis and therapeutic response model of
immune-related lncRNA pairs in clear cell renal cell carcinoma.
Vaccines (Basel). 10:11612022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang W, Wu Y, Hou B, Wang Y, Deng D, Fu Z
and Xu Z: A SOX9-AS1/miR-5590-3p/SOX9 positive feedback loop drives
tumor growth and metastasis in hepatocellular carcinoma through the
Wnt/β-catenin pathway. Mol Oncol. 13:2194–2210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
De Palma FDE, Del Monaco V, Pol JG, Kremer
M, D'Argenio V, Stoll G, Montanaro D, Uszczyńska-Ratajczak B, Klein
CC, Vlasova A, et al: The abundance of the long intergenic
non-coding RNA 01087 differentiates between luminal and
triple-negative breast cancers and predicts patient outcome.
Pharmacol Res. 161:1052492020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yuan S, Liu Q, Hu Z, Zhou Z, Wang G, Li C,
Xie W, Meng G, Xiang Y, Wu N, et al: Long non-coding RNA MUC5B-AS1
promotes metastasis through mutually regulating MUC5B expression in
lung adenocarcinoma. Cell Death Dis. 9:4502018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xie Y, Han J, Xie K and Gou Q: LncRNAs as
biomarkers for predicting radioresistance and survival in cancer: A
meta-analysis. Sci Rep. 12:184942022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kang J, Tang Q, He J, Li L, Yang N, Yu S,
Wang M, Zhang Y, Lin J, Cui T, et al: RNAInter v4.0: RNA
interactome repository with redefined confidence scoring system and
improved accessibility. Nucleic Acids Res. 50:D326–D332. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang KX, Ding C, Liu QH and Zhu DM:
Knockdown of LINC01087 inhibits gastric cancer malignant behavior
by regulating the miR-135a-5p/CAAP1 axis. Funct Integr Genomics.
23:2482023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yin Y, Huang J, Shi H, Huang Y, Huang Z,
Song M and Yin L: LINC01087 promotes the proliferation, migration,
and invasion of thyroid cancer cells by upregulating PPM1E. J
Oncol. 2022:1–12. 2022. View Article : Google Scholar
|
|
58
|
She JK, Fu DN, Zhen D, Gong GH and Zhang
B: LINC01087 is highly expressed in breast cancer and regulates the
malignant behavior of cancer cells through miR-335-5p/Rock1. Onco
Targets Ther. 13:9771–9783. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
De Palma FDE, Carbonnier V, Salvatore F,
Kroemer G, Pol JG and Maiuri MC: Systematic investigation of the
diagnostic and prognostic impact of LINC01087 in human cancers.
Cancers (Basel). 14:59802022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Polyak K: Heterogeneity in breast cancer.
J Clin Invest. 121:3786–3788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mandor M, Atef MM, El-Sayed FM and
Abdel-Mohsen SE: Comparison of survival rate of triple negative
versus luminal B HER2 neu-positive breast cancer patients in
oncology medicine center in Suez Canal University Hospital. Suez
Canal University Medical J. 26:20–31. 2023. View Article : Google Scholar
|
|
62
|
Baeg S, Park I, Kim J, Park C, Cho H, Yang
K, Kim J, Shin Y, Park K and Gwak G: Comparative study for clinical
outcomes of triple-positive and triple-negative breast cancer:
Long-term results in 161 patients followed in a single center. J
Breast Dis. 8:78–84. 2020. View Article : Google Scholar
|
|
63
|
Bjørklund SS, Aure MR, Häkkinen J,
Vallon-Christersson J, Kumar S, Evensen KB, Fleischer T, Tost J;
OSBREAC; Sahlberg KK, ; et al: Subtype and cell type specific
expression of lncRNAs provide insight into breast cancer. Commun
Biol. 5:8342022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Merikhian P, Eisavand MR and Farahmand L:
Triple-negative breast cancer: Understanding wnt signaling in drug
resistance. Cancer Cell Int. 21:4192021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Menck K, Heinrichs S, Wlochowitz D, Sitte
M, Noeding H, Janshoff A, Treiber H, Ruhwedel T, Schatlo B, von der
Brelie C, et al: WNT11/ROR2 signaling is associated with tumor
invasion and poor survival in breast cancer. J Exp Clin Cancer Res.
40:3952021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang W, Wang X, Zhang C, Xue L and Yang
L: Expression and clinical significance of MAPK and EGFR in
triple-negative breast cancer. Oncol Lett. 19:1842–184.
2020.PubMed/NCBI
|
|
67
|
Crosas-Molist E, Samain R, Kohlhammer L,
Orgaz JL, George SL, Maiques O, Barcelo J and Sanz-Moreno V: Rho
GTPase signaling in cancer progression and dissemination. Physiol
Rev. 102:455–510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kvokačková B, Remšík J, Jolly MK and
Souček K: Phenotypic heterogeneity of triple-negative breast cancer
mediated by epithelial-mesenchymal plasticity. Cancers (Basel).
13:21882021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hui R, Pearson A, Cortes J, Campbell C,
Poirot C, Azim HA Jr, Fumagalli D, Lambertini M, Daly F, Arahmani
A, et al: Lucitanib for the treatment of HR+/HER2-metastatic breast
cancer: Results from the multicohort phase II FINESSE study. Clin
Cancer Res. 26:354–363. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Goldstein LJ, Perez RP, Yardley D, Han LK,
Reuben JM, Gao H, McCanna S, Butler B, Ruffini PA, Liu Y, et al: A
window-of-opportunity trial of the CXCR1/2 inhibitor reparixin in
operable HER-2-negative breast cancer. Breast Cancer Research.
22:42020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xu Y, Ren W, Li Q, Duan C, Lin X, Bi Z,
You K, Hu Q, Xie N, Yu Y, et al: LncRNA Uc003×sl.1-mediated
activation of the NFκB/IL8 axis promotes progression of
triple-negative breast cancer. Cancer Res. 82:556–570. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Thapa R, Afzal O, Gupta G, Bhat AA,
Almalki WH, Alzarea SI, Kazmi I, Altamimi ASA, Subramaniyan V,
Thangavelu L, et al: Unveiling the connection: Long-chain
non-coding RNAs and critical signaling pathways in breast cancer.
Pathol Res Pract. 249:1547362023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zheng S, Li M, Miao K and Xu H: lncRNA
GAS5-promoted apoptosis in triple-negative breast cancer by
targeting miR-378a-5p/SUFU signaling. J Cell Biochem.
121:2225–2235. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang Y, Bu N, Luan X, Song QQ, Ma BF, Hao
W, Yan JJ, Wang L, Zheng XL and Maimaitiyiming Y: Harnessing the
potential of long non-coding RNAs in breast cancer: From etiology
to treatment resistance and clinical applications. Front Oncol.
14:13375792024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pommier RM, Sanlaville A, Tonon L,
Kielbassa J, Thomas E, Ferrari A, Sertier AS, Hollande F, Martinez
P, Tissier A, et al: Comprehensive characterization of claudin-low
breast tumors reflects the impact of the cell-of-origin on cancer
evolution. Nat Commun. 11:34312020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pan C, Xu A, Ma X, Yao Y, Zhao Y, Wang C
and Chen C: Research progress of Claudin-low breast cancer. Front
Oncol. 13:1226112023. View Article : Google Scholar
|
|
77
|
Cisneros-Villanueva M, Fonseca-Montaño MA,
Ríos-Romero M, López-Camarillo C, Jiménez-Morales S, Langley E,
Rosette-Rueda AS, Cedro-Tanda A, Hernández-Sotelo D and
Hidalgo-Miranda A: LncRNA SOX9-AS1 triggers a transcriptional
program involved in lipid metabolic reprogramming, cell migration
and invasion in triple-negative breast cancer. Sci Rep.
14:14832024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ye X, Cen Y, Li Q, Zhang Y, Li Q and Li J:
Immunosuppressive
<scp>SOX9</scp>-<scp>AS1</scp> resists
triple-negative breast cancer senescence via regulating wnt
signalling pathway. J Cell Mol Med. 28:e702082024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Naorem LD, Prakash VS, Muthaiyan M and
Venkatesan A: Comprehensive analysis of dysregulated lncRNAs and
their competing endogenous RNA network in triple-negative breast
cancer. Int J Biol Macromol. 145:429–436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tripathi R, Aier I, Chakraborty P and
Varadwaj PK: Unravelling the role of long non-coding RNA-LINC01087
in breast cancer. Noncoding RNA Res. 5:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li Y, Sundquist K, Zhang N, Wang X,
Sundquist J and Memon AA: Mitochondrial related genome-wide
Mendelian randomization identifies putatively causal genes for
multiple cancer types. EBioMedicine. 88:1044322023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang Y, Yao Z, Li L, Mao R, Huang W, Hu
Z, Hu Y, Wang Y, Guo R, Tang X, et al: Deep learning radiopathomics
based on preoperative US images and biopsy whole slide images can
distinguish between luminal and non-luminal tumors in early-stage
breast cancers. EBioMedicine. 94:1047062023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Atallah NM, Haque M, Quinn C, Toss MS,
Makhlouf S, Ibrahim A, Green AR, Alsaleem M, Rutland CS, Allegrucci
C, et al: Characterisation of luminal and triple-negative breast
cancer with HER2 low protein expression. Eur J Cancer.
195:1133712023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
González-Woge M, Contreras-Espinosa L,
García-Gordillo JA, Aguilar-Villanueva S, Bargallo-Rocha E,
Cabrera-Galeana P, Vasquez-Mata T, Cervantes-López X, Vargas-Lías
DS, Montiel-Manríquez R, et al: The expression profiles of lncRNAs
are associated with neoadjuvant chemotherapy resistance in locally
advanced, luminal B-type breast cancer. Int J Mol Sci. 25:80772024.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cocco S, Piezzo M, Calabrese A, Cianniello
D, Caputo R, Lauro VD, Fusco G, Gioia GD, Licenziato M and De
Laurentiis M: Biomarkers in triple-negative breast cancer:
State-of-the-art and future perspectives. Int J Mol Sci.
21:45792020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Price TJ, Hardingham JE, Lee CK, Townsend
AR, Wrin JW, Wilson K, Weickhardt A, Simes RJ, Murone C and Tebbutt
NC: Prognostic impact and the relevance of PTEN copy number
alterations in patients with advanced colorectal cancer (CRC)
receiving bevacizumab. Cancer Med. 2:277–285. 2013. View Article : Google Scholar : PubMed/NCBI
|