Introduction
Liver cancer, the predominant histological type of
liver malignancy, accounts for the majority of global liver cancer
diagnoses and mortalities (1). In
total, >50% of the reported liver cancer cases worldwide occur
in China (2). Despite advances in
medical technology, including surgery, ablation therapy, liver
transplantation, radiation therapy, interventional therapy,
immunotherapy and systemic treatment options (3,4), the
prognosis remains suboptimal due to the low rates of operability,
transplantation and frequent recurrence (5). Furthermore, drug resistance in liver
cancer continues to pose a considerable challenge. Numerous
clinical studies are focused on overcoming this resistance
(6–10). Hence, there is a need to identify
novel diagnostic and therapeutic targets, as well as biomarkers to
enhance treatment efficacy and overcome drug resistance in liver
cancer.
Genomic instability is a key factor in cancer,
contributing to the accumulation of mutations in tumor suppressor
genes and oncogenes (11). Several
of these genetic mutations serve as key features of cancer and
prognostic biomarkers, such as isocitrate dehydrogenase mutations
in glioma and chondrosarcoma (12), titin (TTN) mutations in melanoma,
EGFR mutations in non-small cell lung cancer (NSCLC) and TP53
mutations in breast, ovarian, small cell lung cancer and NSCLC
(13,14). Genetic mutations can lead to DNA
repair deficiencies, altered protein conformations, dysregulated
tumor growth and proliferation, as well as resistance to
chemotherapeutic agents (15,16).
In liver cancer, previous studies linked tumor initiation and
progression to genomic mutations that impact tumor cell
proliferation, invasion, metastasis and drug resistance (17,18).
The progression of liver cancer is influenced by a variety of
factors, including radiation exposure, genetic mutations and
epigenetic or transcriptional variations. Sequencing of human liver
tumors has revealed potential driver gene mutations and key
carcinogenic pathways in liver cancer (19).
The TTN gene, the largest in the human genome with
363 exons, encodes a considerable amount of myosin in striated
muscle. Due to its large molecular size, complexity and plasticity,
TTN is particularly susceptible to dysregulation and mutations
within this gene can lead to myosin dysfunction and associated
diseases (20,21). TTN mutations have been associated
with various skeletal and cardiomyopathies, with truncated TTN
variants primarily associated with dilated cardiomyopathy (22). TTN mutations also carry out a role
in cancer development and resistance (23). TTN mutations have been frequently
observed across diverse tumor types, including ocular surface
squamous neoplasia, lung squamous cell carcinoma, ovarian serous
cystadenocarcinoma and thyroid cancer (24,25).
The impact of TTN mutations varies between different types of
cancer; for instance, patients with wild-type TTN exhibit markedly
longer overall survival compared with patients with TTN mutations
in immunotherapy for endometrioid endometrial carcinoma and
melanoma, positioning TTN mutations as an independent marker of
poor prognosis (26). By contrast,
TTN mutations in other solid tumors, such as lung squamous cell
carcinoma and gastric cancer, are associated with improved
chemotherapy responses and longer overall survival (27–29).
Despite these findings, the landscape and implications of TTN
mutations in liver cancer remain largely unexplored. Therefore, the
present study aimed to investigate the relationship between TTN
mutations and liver cancer, with a particular focus on mechanisms
of drug resistance.
Materials and methods
Acquisition of somatic cell mutation
spectrum and mutation analysis
RNA-sequencing (seq), microRNA (miR/miRNA)-seq,
somatic mutation and clinical data from 274 patients with liver
cancer in The Cancer Genome Atlas (TCGA; accession no. TCGA-LIHC;
portal.gdc.cancer.gov/) cohort were obtained. Somatic mutation data
were analyzed using the ‘maftools’ R package (version 2.18.0;
bioconductor.org/packages/release/bioc/html/maftools.html) to
calculate the frequency of variant classifications in liver cancer
tissues and to determine the distribution of different variant gene
types. Somatic variant profiles of liver cancer tissues were
detected using the VarScan algorithm (30), and the top 10 most frequently
mutated genes in liver cancer were visualized. A forest plot was
generated to display the hazard ratio (HR) and P-value for each
mutation, categorizing patients into wild-type and mutated
groups.
Acquisition and analysis of gene
expression profiles and identification of differentially expressed
genes (DEGs)
RNA-seq profiles from TCGA liver cancer cohort were
downloaded, and the matrix was normalized using the limma package
(version 3.6.4;
bioconductor.org/packages/release/bioc/html/limma.html). Probe
names were converted to gene names prior to analysis. Based on the
negative binomial distribution model, edgeR is able to model gene
expression count data more accurately, thereby improving the
accuracy of differential expression analysis (31). Differential gene expression
analysis was carried out using edgeR (version 3.44.0;
bioconductor.org/packages/release/bioc/html/edgeR.html) between
liver cancer tissues with TTN mutations and wild-type controls.
DEGs were defined as genes with a log2 fold change ≥1
and P<0.05.
Functional enrichment analysis
Functional annotation and visualization of the DEGs
was carried out using the DAVID Bioinformatics Resources
(david.ncifcrf.gov/), enabling the identification of enriched Kyoto
Encyclopedia of Genes and Genomes (KEGG; kegg.jp/) pathways and
Biological Process (BP) categories. It not only provides rapid
access to a variety of heterogeneous annotation data in a
centralized location, but also enriches the bio-informative level
of individual genes. By using Benjamini-Hochberg multiplex testing,
adjust P-values to control False Discovery Rate (FDR), terms with
P<0.05 were considered to indicate a statistically significant
difference and a bubble plot was used to visualize the top five
terms.
Drug sensitivity calculation
In vivo drug responses to 198 drugs in
patients with liver cancer were predicted using the OncoPredict R
package (version 1.2; URL:
cran.r-project.org/web//packages/oncoPredict/index.html) (32). This package calculates drug scores
by fitting the gene expression matrix of each liver cancer sample
into the gene expression matrix of cancer cell lines from the Broad
Institute's Cancer Cell Line Encyclopedia (CCLE;
portals.broadinstitute.org/cclelegacy/home). The corresponding
half-maximal inhibitory concentration (IC50) for each
drug was determined. A higher drug score indicates greater drug
resistance (32). Drug scores
between patients with liver cancer exhibiting wild-type and mutant
genes were compared using unpaired t-test, with P<0.05
considered to indicated a statistically significant difference.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 5×106 liver
cancer cells (SNU182, HuH7 and JHH7 from Procell Life Science &
Technology Co., Ltd.; HepG2, Hep3B and Li7 cells were purchased
from EIAab Group.) using the Total RNA Extraction Kit (Tiangen
Biotech Co., Ltd.). According to the manufacturer's protocol, the
Rever Tra Ace qPCR RT Master Mix (cat. no. RR037A; Takara Bio Inc.)
was used to reverse transcribe total RNA into complementary DNA
(cDNA). RT-qPCR was carried out using a real-time PCR instrument
(cat. no. 12011319; CFX Opus 96 Real-Time PCR System), and relative
expression was calculated using the 2−ΔΔCq method
(33). GAPDH was used as a loading
control. The primers used were as follows: GAPDH, forward primer
5′-GTCTCCTCTGACTTCAACAGCG-3′, and reverse primer
5′-ACCACCCTGTTGCTGTAGCCAA-3′, TTN forward primer
5′-CCCCATCGCCCATAAGACAC-3′, and reverse primer
5′-CCACGTAGCCCTCTTGCTTC-3′. The thermocycling conditions were as
follows: Initial denaturation at 95.0°C for 30 sec, followed by 40
cycles of 95.0°C for 5 sec, 62.0°C for 30 sec and 67.5°C for 5
sec.
Cell culture and knockdown or
overexpression of TTN
Liver cancer cell lines SNU182, HuH7 and JHH7 were
obtained from Procell (Procell Life Science & Technology Co.,
Ltd.), while HepG2, Hep3B and Li7 cells were purchased from EIAab
Group. According to mutation data from the CCLE database, JHH7,
HepG2 and Li7 exhibit TTN mutations, whereas HuH7, Hep3B and SNU182
have wild-type forms of TTN. The authenticity of all cell lines
used in the present study has been confirmed by STR analysis. All
cell lines were cultured in Dulbecco's Modified Eagle Medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (Gibco, Thermo Fisher Scientific, Inc.) at 37°C and 5%
CO2.
TTN overexpression was induced via transfection with
pcDNA3.1(+) plasmids (Shanghai GeneChem Co., Ltd.) using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). Empty vector
was used as a negative control. Transfection was performed at 37°C,
5% CO2 incubator for 4–6 h and switch to fresh complete
medium. After 48 h, the expression level of TTN protein was
detected by Western blot to verify the overexpression effect.
Stable TTN knockdown was achieved through
transduction with TTN-targeting lentivirus (iGene Biotechnology
Co., Ltd.). Using a second-generation lentiviral generation system,
lentiviral vector plasmids (10 µg), packaging plasmids, and
envelope plasmids were co-transfected into HEK293T cells (Procell
Life Science & Technology Co., Ltd.) at 50–70% confluence
(4:3:1), incubated at 37°C for 6–8 h, and then replaced with fresh
complete medium. Viral supernatants were collected 48 h after
transfection and viral particles were collected by
ultracentrifugation (4°C, 50,000 g, 2 h). JHH7 and HepG2 cells
(50–60% confluence, lentiviral particles (MOI: 20) were added to
the cell culture medium along with polybrene (5 µg/ml) and after 24
h of incubation at 37°C, 5% CO2 incubator, they were
replaced with fresh complete medium. 48 h after transfection,
puromycin (1 µg/ml; cat. no. A1113803; Thermo Fisher Scientific,
Inc.) was added for 1 weeks of screening, followed by a maintenance
culture using puromycin at a concentration of 0.5 µg/ml. Western
blot was used to measure the expression level of TTN protein to
verify the knockdown effect. All shRNAs (Shanghai Genechem Co.,
Ltd.) used are as follows for the sense (SS) and antisense strands
(AS): sh-NC SS sequence 5′-TTCTCCGAACGTGTCACGT-3′, and AS sequence
5′-ACGTGACACGTTCGGAGAA-3′; sh1-TTN SS sequence GGTTGTTTATGTTATGTTA,
and AS sequence TAACATAACATAAACAACC; sh2-TTN SS sequence
GCTTGTTACTAATATAATA, and AS sequence TATTATATTAGTAACAAGC;
sh3-TTN-SS sequence CACAGAAGTTAGAGTTCAA, and AS sequence
TTGAACTCTAACTTCTGTG.
Western blotting
Total protein was extracted from liver cancer cells
using phenylmethanesulfonyl fluoride (1:100) in high-strength RIPA
lysis buffer (Sangon Biotech Co., Ltd.). The protein concentration
was determined using a BCA protein assay. SDS-PAGE electrophoresis
was carried out using 12% gels (20 µg/lane), first at 80 V for 30
min and then at 120 V for 90 min. Proteins were transferred to a
0.45 µm polyvinylidene fluoride membrane for 90 min at 300 mA. The
membrane was blocked with 5% skim milk powder for 1 h at room
temperature, followed by overnight incubation at 4°C with primary
antibodies against GAPDH (1:5,000; cat. no. AB-2937024; Abmart
Pharmaceutical Technology Co., Ltd.) and TTN (1:2,000; cat. no.
H00007273-M09; Thermo Fisher Scientific, Inc.). After washing with
TBS with 0.1% Tween-20, the membrane was incubated with
HRP-conjugated goat anti-rabbit IgG (H+L) (1:10,000; cat. no.
SA00001-2; Proteintech Group, Inc.) or HRP-conjugated Goat
Anti-Mouse IgG (H+L) (1:10,000; cat. no. SA00001-1; Proteintech
Group, Inc.) for 2 h at room temperature. The membrane was then
treated with enhanced chemiluminescence (ECL; Omni-ECL™,
Shanghai Epizyme Biotech Co., Ltd.) solution for ~1 min, followed
by imaging using Tanon 5200 automatic chemiluminescence image
analysis system. All experiments were carried out in triplicate as
previously described (34).
Optical density analysis was performed using ImageJ version 1.53t
software (National Institutes of Health).
Protein stability assay
Liver cancer cells were cultured to 50–70%
confluence, the old medium was removed and a fresh medium
containing cycloheximide (CHX; cat. no. S7418; Selleck Chemicals)
with a final concentration of 1.0 µM was added at 37°C and 5%
CO2, cells were collected at 0, 1, 2, 3 and 4 h and
protein was extracted, and the expression of TTN was detected by
western blotting.
Detection of cell proliferation and
viability
Cell viability was assessed using the Cell Counting
Kit-8 (CCK-8; Shanghai Yeasen Biotechnology Co., Ltd.). Cells were
seeded at a density of 4×103 cells/well in a 96-well
plate and incubated at 37°C with 5% CO2. Once cells
adhered, Nilotinib (cat. no. HY-10159; 0.00, 0.62, 1.25, 2.50,
5.00, 10.00 and 20.00 µM), GSK1904529A (cat. no. HY-10524; 0.000,
0.031, 0.062, 0.125, 0.250, 0.500 and 1.000 µM), 5-Fluorouracil
(5-FU; cat. no. HY-90006; 0.00, 0.62, 1.25, 2.50, 5.00, 10.00 and
20.00 µM) and Sapitinib (cat no. HY-13050; 0.0, 0.5, 1.0,2.0, 4.0,
8.0 and 16.0 nM) were added to the wells for 48 h. All drugs were
purchased from MedChemExpress. After treatment, the culture medium
containing the drug was discarded, and 100 µl of DMEM containing
1/10 of the CCK-8 reagent (Shanghai Yeasen Biotechnology Co., Ltd.)
was added to each well and incubated for 2 h. Absorbance was
measured at 450 nm to assess cell viability.
Tumor cell sphere forming assay
Liver cancer cells were suspended in 200 µl of DMEM
(Gibco; Thermo Fisher Scientific, Inc.) at a density of 1,000 cells
per well, cultured in 96-well clear ultra-low attachment U-plates
(Invitrogen; Thermo Fisher Scientific, Inc.) was incubated at 37°C
for 2 days. Culture medium was refreshed with a complete medium
containing 5-FU (Huh7: 4.5 µM; Hep3B: 6.5 µM; JHH7: 2.0 µM; HepG2:
2.5 µM) or with hMAO-B-IN-9 (1.58 µM) (cat. no. HY-163879,
MedChemExpress). The culture medium was replaced every 3 days, and
the cells were continuously cultured in a cell incubator at 37°C
and 5% CO2 for 13 days. The size of the spheroids
derived from liver cancer cells were captured using an inverted
microscope.
5-Ethynyl-2-deoxyuridine assay
To assess cell proliferation, the
BeyoClick™ EdU-555 kit (cat. no. C0075S, Beyotime
Institute of Biotechnology) was used to evaluate DNA synthesis in
liver cancer cells. The experiment was carried out according to the
manufacturer's instructions. All experiments were carried out in
triplicate, as described previously (34).
Levels of malondialdehyde (MDA) and
glutathione (GSH) peroxidase (GPX) in liver cancer cells
Liver cancer cells were seeded in culture dishes and
collected when the cell density reached 80%. Cells were lysed using
Western and IP cell lysis buffer (cat. no. P0013; Beyotime
Institute of Biotechnology), and then the supernatant was collected
by centrifugation at 12,000 g at 4°C for 10 min for subsequent
assays. MDA content in the cells was measured using the Lipid
Peroxidation (MDA) Assay Kit (cat. no. HY-K0319; MedChemExpress)
according to the manufacturer's instructions. Absorbance was
measured at 532 nm using a microplate reader, and the resulting
values were compared with a standard curve to determine the
concentration of MDA. GSH and GPX4) activity were also tested using
Reduced Glutathione (GSH) Colorimetric Assay (cat. no. E-BC-K030-M)
and Glutathione Peroxidase 4 (GPX4) Activity Assay Kit
(E-BC-K883-M; both Elabscience Biotechnology Co., Ltd.) following
the manufacturer's instructions.
Colony formation assay
Control Huh7 and Hep3B cells, and TTN-overexpressing
Huh7 and Hep3B cells were seeded in 6-well plates at 500 cells per
well and cultured for 2 weeks with replacement of the culture
medium containing 5-FU (Huh7: 4.5 µM; Hep3B: 6.5 µM) every 3 days.
After 2 weeks of incubation, cells were gently washed with PBS
three times (3 min each), fixed with 4% paraformaldehyde for 30 min
at room temperature, and washed three times with PBS for 3 min
each. Cells were then stained with 1% crystal violet for 30 min at
room temperature. After washing with PBS, the dishes were dried,
and colonies with diameter >100 µm were counted. Clone count and
size measurements were performed using ImageJ version 1.53t
software (National Institutes of Health).
In vivo experiments
All animal experiments were conducted following the
‘3R’ principles (replacement, reduction and refinement) and were
approved by the Animal Care Committee of Guizhou Medical University
under approval no. 2400641. A total of 10 female and 10 male (age,
4–6 weeks of 16–18 g BALB/c nude mice were purchased from Beijing
Huafukang Biotechnology Co., Ltd., and after receiving the mice,
they were acclimatized for 2 weeks in a specific pathogen-free
environment at 25°C and humidity of 30%. The mice were exposed to a
12-h light/dark cycle, and were given free access to mouse feed
sterilized and water sterilized by autoclaving. For tumor growth
experiments, BALB/c mice were randomly assigned to shNC and shTTN
groups, 10 mice per group). HepG2/shNC or HepG2/shTTN cells
(6×105 cells/100 µl) were resuspended in PBS containing
1% Matrigel and subcutaneously injected into the right abdomen of
BALB/c mice. After 1 week, when tumors reached ~10 mm3,
the mice were randomly divided into two groups: One treated with
Di-methyl sulfoxide (DMSO) control, and the other with 5-FU (25
mg/kg; 100 µl; intraperitoneal injections, once daily for 3 weeks).
Tumor size was measured every 3 days, and tumor volume was
calculated using the formula: Volume=1/2 (length ×
width2). The mice were monitored for signs of poor
health, including weight loss, postural abnormality, changes in
activity levels, breathlessness, and signs of distress. Humane
endpoints included maximum tumor volume >2,000 mm3,
ulceration, necrosis or infection on the surface of the tumor,
weight loss >20%, abnormal posture, tumor affecting motor
function, dyspnea, inability to eat and drink normally, the
experiment was terminated, all mice were anesthetized with sodium
pentobarbital at 5.0 mg/100 g body weight and sacrificed by
cervical dislocation, and death was confirmed without breathing and
heartbeat for more than 5 min. Tumors were harvested for weight
measurement and histological analysis.
Immunohistochemical (IHC)
staining
The tumor tissue was fixed in 4% paraformaldehyde at
room temperature for 24 h, dehydrated in graded ethanol and
embedded in paraffin, embedded in paraffin, and sectioned into 4 µm
slices. After paraffin was removed with xylene at room temperature,
and the tissue sections were gradually hydrated using ethanol.
Antigen retrieval was performed using tris-EDTA antigen retrieval
solution (pH 9.0; tris-EDTA antigen retrieval solution; Beyotime
Institute of Biotechnology) at 95–100°C for 15 min for antigen
retrieval. To reduce non-specific binding, tissues were blocked
with a solution containing 3% hydrogen peroxide for 10 min at room
temperature, and 5% bovine serum albumin (Beijing Solarbio Science
& Technology Co., Ltd.) for 1 h at room temperature. Tissues
were incubated overnight at 4°C with primary antibodies: Anti-Ki67
(1:400; cat. no. 2807-1-AP) and anti-PCNA (1:400; cat. no.
60097-1-IG) (both from Proteintech Group, Inc.). After washing with
PBS, tissues were incubated with the secondary antibody (goat
anti-rabbit/mouse HRP-labeled polymer; cat. no. PR30009 Proteintech
Group, Inc.) for 2 h at 37°C. The tissues were then stained with
3,3′-Diaminobenzidine tetrahydrochloride (Shanghai Yeasen
Biotechnology Co., Ltd.) and counterstained with hematoxylin for 3
min at room temperature to visualize the cell nuclei. The
antigen-antibody complex binding was detected using a light
microscope.
Statistical analysis
Statistical analysis was carried out using Prism
software (version 6.0; Dotmatics). Data are presented as the mean ±
standard deviation. All experiments were conducted in triplicate.
Statistical significance was determined using an unpaired Student's
t-test or one-way ANOVA with Tukey's post hoc test. The
Benjamini-Hochberg method was used to control the false discovery
rates. P<0.05 was considered to indicate a statistically
significant difference.
Results
Landscape of somatic variant and
identification of molecular mechanisms affected by TTN mutations in
liver cancer
Genome-wide mutation profiling in liver cancer was
conducted by analyzing somatic mutation data, revealing that
missense mutations were the most common variant types, followed by
nonsense and frameshift deletion mutations, with single nucleotide
polymorphisms (SNPs) accounting for the majority of variant types.
The C>T transition emerged as the most prevalent single
nucleotide variant (SNV; Fig. 1A).
The number of mutant bases per patient was quantified, with a
median value of 1. TTN was identified as the gene with the highest
mutation frequency in liver cancer. The top 10 most frequently
mutated genes-TP53, TTN, CTNNB1, MUC16, ALB, PCLO, MUC4, RYR2,
ABCA13 and APOB-demonstrated high mutation frequencies and distinct
somatic mutation patterns (Fig.
1B). Analysis of tissue mutation characteristics in TCGA
database revealed a positive correlation between TTN mutations and
poor prognosis in liver cancer (HR=136.69; 95% CI=1.36–13,766.33;
Fig. 1C). To elucidate the
molecular mechanisms underlying TTN mutations in liver cancer,
differential expression analysis was conducted on TTN-mutant and
TTN-wild-type liver cancer tissues from TCGA database. The analysis
revealed 140 downregulated genes and 30 upregulated genes (Fig. 1D and E). in TTN-mutant and
-wild-type liver cancer (Fig. 1E).
BP term enrichment analysis of the upregulated DEGs identified
significant enrichment in terms such as ‘Complement activation’,
‘positive regulation of cell activation’, ‘stress response to metal
ions’, ‘glutathione metabolism’ and ‘cellular response to metal
ions’ (Fig. 1F). KEGG pathway
analysis further indicated enrichment in pathways including
‘mineral absorption’, ‘neuroactive ligand-receptor interaction’,
‘ferroptosis’, ‘fat digestion and absorption’ and ‘pancreatic
secretion’ (Fig. 1G). Notably, the
BP terms impacted by TTN-mutant DEGs were associated with stress
responses to metal ions and GSH metabolism. Additionally, KEGG
pathway enrichment highlighted the association of these
differential genes with ferroptosis. Given that numerous studies
have established a connection between GSH metabolism and
ferroptosis (35,36), these observations suggest that TTN
mutations may influence liver cancer progression through the
modulation of ferroptosis-related metabolic processes.
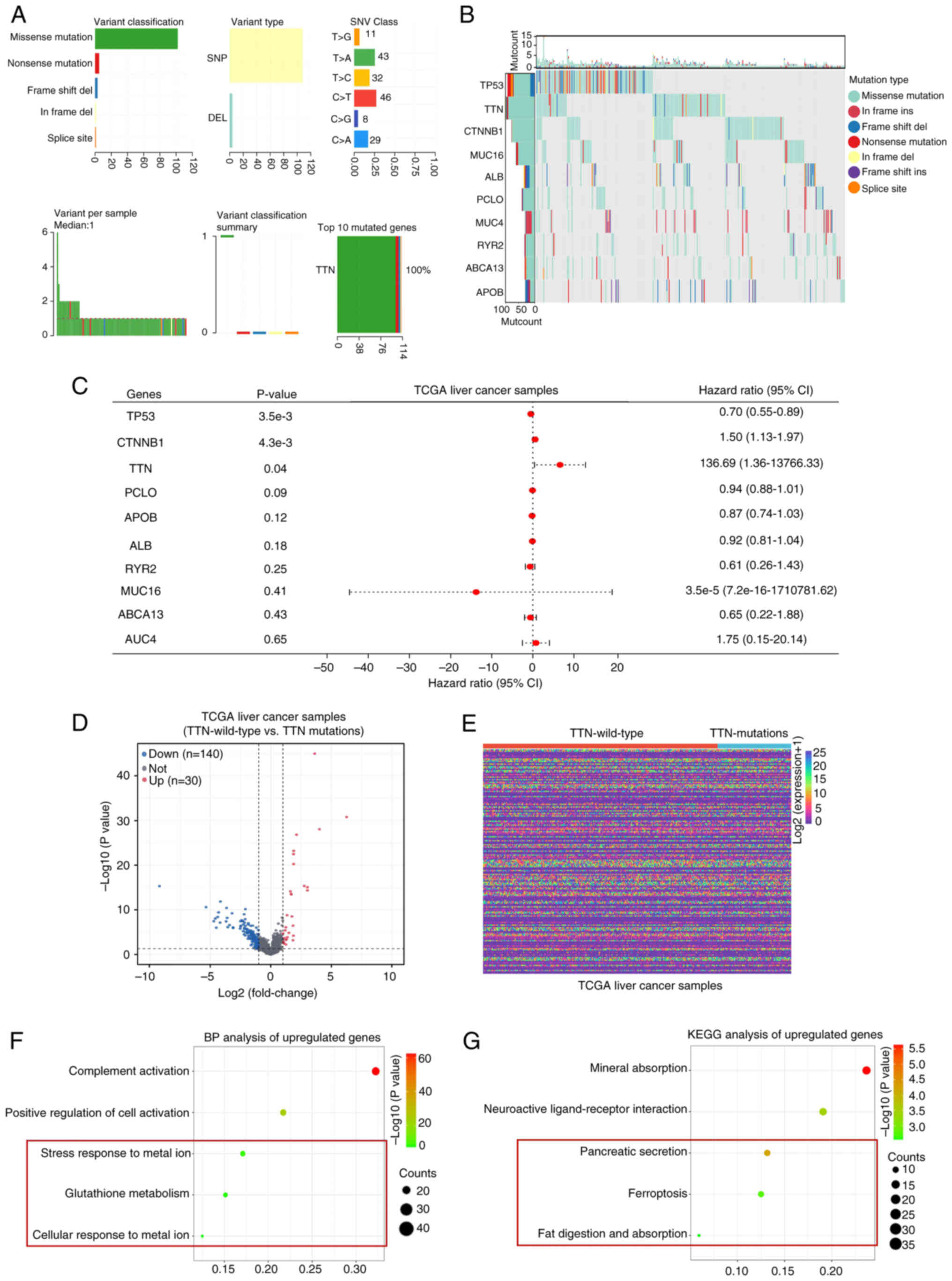 | Figure 1.Comprehensive profiling of somatic
mutation data and DEGs between samples of liver cancer with either
mutated or wild-type TTN, with enrichment analysis. (A) Variant
classification in liver cancer, highlighting missense mutations as
the most frequent, with SNPs comprising the majority of variant
types. C > T is the most common type of SNV. The number of
mutated bases per patient is displayed, with a median of 1. The top
mutated gene, TTN, is indicated. (B) Waterfall plot illustrating
the distribution of variant classifications across all patients
with liver cancer. (C) Overall survival analysis of patients with
liver cancer carried out using the Cox proportional hazards model.
(D) Volcano plot depicting gene expression changes between TCGA
liver cancer datasets harboring either TTN mutations or wild-type
TTN. (E) Heatmap displaying the DEGs between TCGA liver cancer
datasets harboring either TTN mutations or wild-type TTN. (F) BP
analysis and (G) KEGG pathway enrichment analysis of the 30
upregulated genes common to both conditions. DEL, Deletion; SNP,
single nucleotide polymorphism; SNV, single nucleotide variation;
TTN, titin; TCGA, The Cancer Genome Atlas; CI, confidence interval;
BP, biological processes; KEGG; Kyoto Encyclopedia of Genes and
Genomes; DEGs, differentially expressed genes; Mut, mutation. |
TTN mutations increase the sensitivity
of liver cancer cells to GSK1904529A and nilotinib
To assess the impact of TTN mutations on drug
sensitivity in liver cancer, the OncoPredict algorithm was used. A
total of 198 drugs were analyzed, revealing significant changes in
the drug scores of four drugs in liver cancer tissues with TTN
mutations (Fig. 2A and B).
Specifically, the drug scores for GSK1904529A and nilotinib were
decreased in TTN-mutant liver cancer tissues, while 5-FU and
sapitinib scores were increased (Fig.
2C and D). This suggests that TTN mutations may increase
sensitivity to GSK1904529A and nilotinib while conferring
resistance to 5-FU and sapitinib. To validate these observations,
liver cancer cell lines with and without TTN mutations were
analyzed using the CCLE database. Three TTN-mutant cell lines
(Huh7, Hep3B and SNU182) and three wild-type cell lines (JHH7,
HepG2 and LI7) were selected for further experiments. CCK-8 assays
were carried out to determine IC50 values. For
GSK1904529A, the IC50 values in 48 h were as follows:
JHH7 (0.4156 µM), HepG2 (0.3501 µM), LI7 (0.2572 µM), Huh7 (0.0720
µM), Hep3B (0.0836 µM) and SNU182 (0.1107 µM). For nilotinib,
IC50 values were: JHH7 (10.470 µM), HepG2 (6.200 µM),
LI7 (5.049 µM), Huh7 (3.342 µM), Hep3B (3.675 µM) and SNU182 (2.010
µM). The IC50 values of 5-FU were as follows: JHH7
(2.251 µM), HepG2 (2.831 µM), LI7 (2.347 µM), Huh7 (4.618 µM),
Hep3B (6.624 µM) and SNU182 (4.950 µM). Finally, for sapitinib,
IC50 values were: JHH7 (2.140 nM), HepG2 (1.946 nM), LI7
(1.737 nM), Huh7 (7.749 nM), Hep3B (5.203 nM) and SNU182 (4.375 nM;
Fig. 2E). Statistical analysis
using the unpaired student's t-test indicated that the
IC50 values for 5-FU and sapitinib were increased in
TTN-mutant liver cancer cells, while the IC50s for
GSK1904529A and nilotinib were decreased when compared with their
wild-type counterparts (Fig. 2F).
These results suggest that TTN mutations may enhance sensitivity to
GSK1904529A and nilotinib while contributing to resistance against
5-FU and sapitinib.
 | Figure 2.TTN mutations increase liver cancer
cell sensitivity to GSK1904529A and nilotinib. (A) Heatmap showing
the differential drug sensitivity in liver cancer cells with TTN
mutation vs. TTN wild-type. (B) OncoPredict algorithm analysis of
drug response scores for 198 drugs in liver cancer tissues with TTN
mutations compared with TTN wild-type. (C) Drug response scores for
GSK1904529A and nilotinib in liver cancer tissues with mutated and
wild-type TTN. (D) Drug response scores for fluorouracil and
sapitinib in liver cancer tissues with either mutated or wild-type
TTN. Huh7, Hep3B and SNU182 harbor TTN mutations, while JHH7, HepG2
and Li7 are TTN wild-type. (E) CCK-8 assays used to assess the
IC50 of GSK1904529A, nilotinib, fluorouracil and
sapitinib in JHH7, HepG2, Li7, Huh7, Hep3B and SNU182 liver cancer
cell lines over 48 h. (F) Comparison of IC50 values for
GSK1904529A, nilotinib, fluorouracil and sapitinib between liver
cancer cells with mutated or wild-type TTN. *P<0.05, n=3. The
control group was used for comparison. Data are presented as mean ±
SD. TTN, titin; TTN-WT, wild-type titin; TTN-MUT, mutated titin;
CCK-8, Cell Counting Kit-8. |
TTN mutations enhance the stability of
the TTN protein
To validate the effect of TTN mutations on gene
expression, the transcription levels of TTN were analyzed in liver
cancer tissues from TCGA cohort. No significant difference was
observed in the transcriptional levels of TTN between mutant and
wild-type liver cancer tissues (Fig.
3A). Similarly, RT-qPCR experiments revealed no marked
difference in TTN mRNA expression between TTN-mutant and wild-type
liver cancer cell lines (Fig. 3B).
However, despite similar mRNA levels, TTN protein expression was
notably higher in TTN-mutant liver cancer cells (JHH7, HepG2 and
Li7) (Fig. 3C). To explore the
underlying mechanisms, protein synthesis was inhibited using
cycloheximide (CHX; 1 µM), revealing that TTN protein stability was
increased in TTN-mutant cells, with slower degradation when
compared with wild-type cells (Fig.
3D). These results indicate that single nucleotide missense
mutations in TTN enhance the stability of the encoded protein.
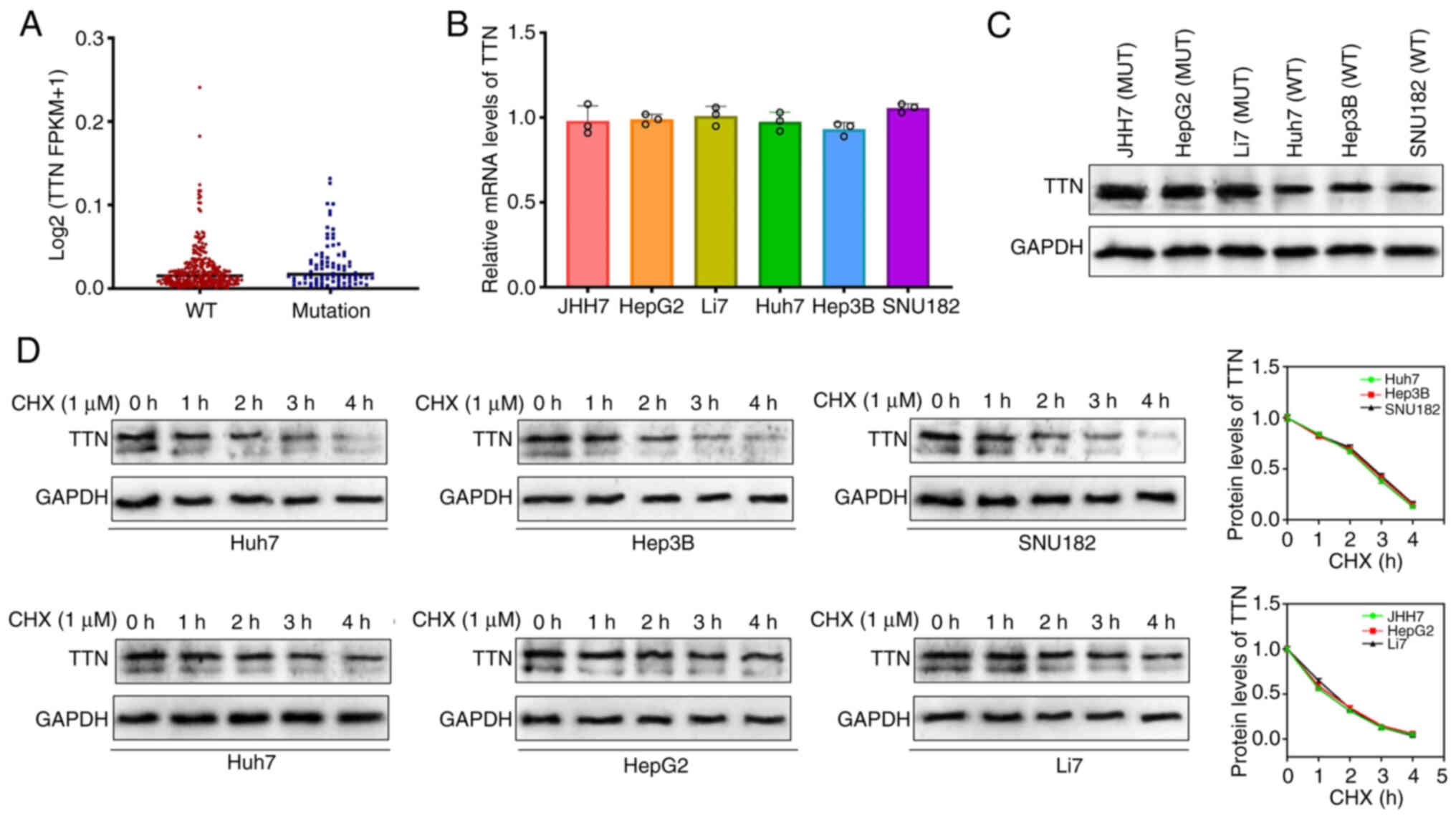 | Figure 3.TTN mutation enhances the stability
of the TTN protein. (A) TTN mRNA expression levels in liver cancer
tissues with mutated TTN (blue dots) and wild-type TTN (red dots)
from TCGA databases. A total of 86 patients with liver cancer with
TTN-MUT and 274 with TTN-WT were included. (B) TTN mRNA expression
levels in liver cancer cell lines: JHH7, HepG2, Li7, Huh7, Hep3B
and SNU182. (C) TTN protein expression levels in JHH7, HepG2, Li7,
Huh7, Hep3B and SNU182 cell lines (D) TTN protein degradation rate
in JHH7, HepG2, Li7, Huh7, Hep3B and SNU182 cells (uncropped images
available in the Supplementary file). Data are presented as mean ±
SD. TTN, titin; TTN-WT, wild-type titin; TTN-MUT, mutated titin;
TCGA, The Cancer Genome Atlas. |
Increased TTN protein in liver cancer
cells with TTN wild-type reduce the sensitivity to 5-FU
5-FU, an uracil analog, is an effective anti-cancer
drug. Previous studies have demonstrated its potent antitumor
activity, both as a monotherapy and in combination with other
chemotherapeutic agents, across a variety of different types of
cancer, including lung cancer, hepatocellular carcinoma, colorectal
cancer and gastric cancer (37–40).
The present study further investigated the role of TTN protein in
mediating 5-FU resistance in liver cancer cells. To enhance
expression of TTN, plasmids were introduced into Huh7 and Hep3B
cells (Fig. 4A). Studies have
highlighted the role of metal ions in various physiological
processes, including cellular homeostasis, metabolic regulation,
biosynthesis, signal transduction and energy conversion (41,42),
As studies examining the relationship between metal ions and cancer
treatment progress, several metal ions have been found to induce
apoptosis and enhance sensitivity to chemotherapeutic drugs
(43,44). BP and KEGG pathway analyses
revealed associations between TTN mutations and both metal ion
metabolism and GSH metabolism (Fig.
1F), with ferroptosis pathway enrichment also observed
(Fig. 1F). Building on these
findings, the effects of TTN overexpression on the levels of
ferrous and other metal ions in liver cancer cells were explored.
The results revealed that TTN overexpression or 5-FU treatment had
minimal impact on the levels of zinc, calcium and copper ions in
the cells (Fig. 4B). Notably, TTN
overexpression significantly inhibited the increase in ferrous ion
levels in 5-FU-treated Huh7 and Hep3B cells compared with
5-FU-treated negative control (Fig.
4C). Further analysis of ferroptosis-related markers revealed
that TTN overexpression reduced MDA content in 5-FU-treated cells
and increased levels of GSH and GPX4 compared with the 5-FU-treated
negative control group, effectively reversing 5-FU-induced
ferroptosis (Fig. 4D).
Additionally, while TTN overexpression did not significantly impact
colony formation, tumor spheroid formation or DNA synthesis rates,
it notably mitigated the inhibitory effects of 5-FU on these
processes (Fig. 4E-G). These
results suggest that increased TTN expression levels may inhibit
ferroptosis in tumor cells, thereby enhancing liver cancer
resistance to 5-FU.
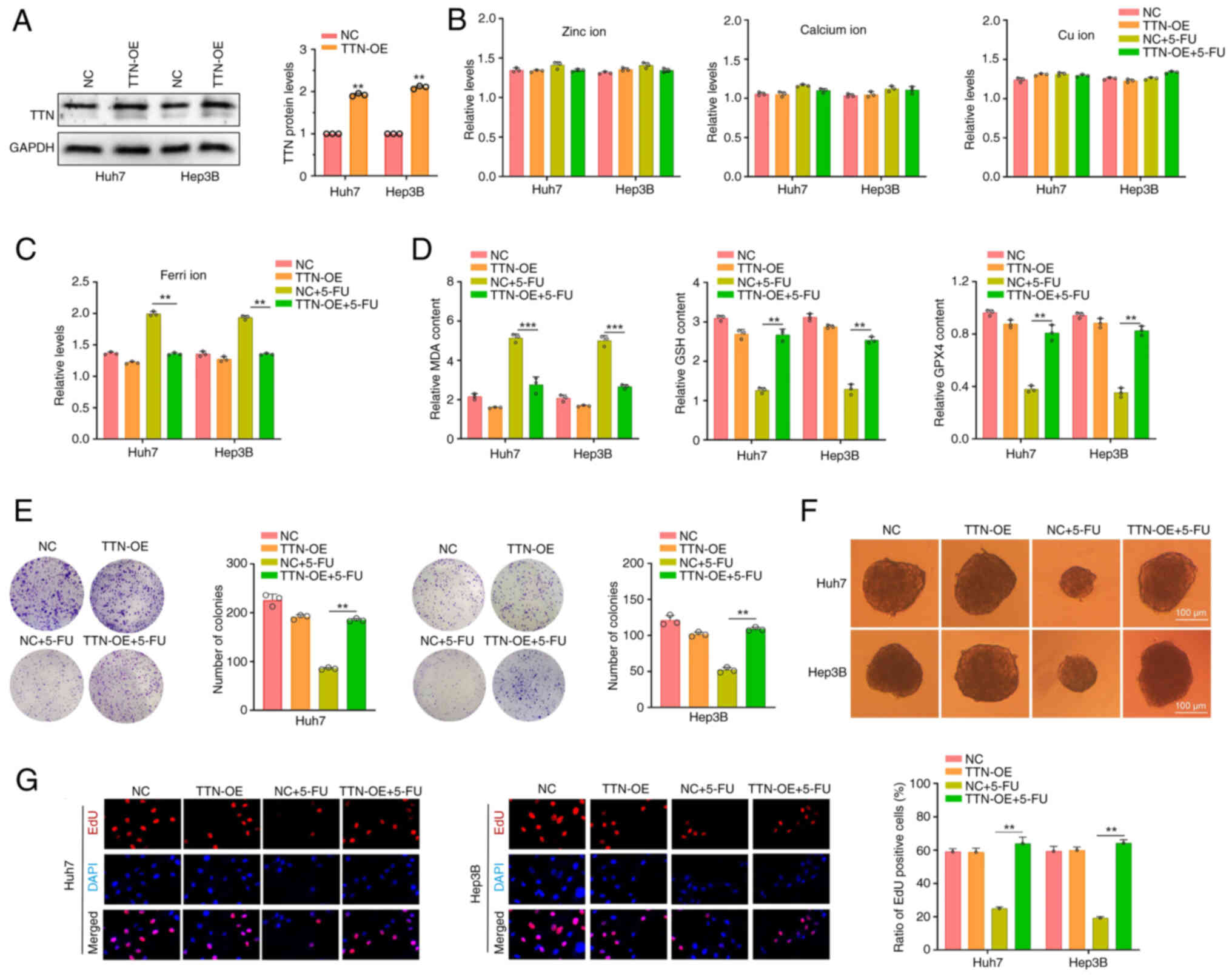 | Figure 4.Overexpression of TTN protein in
liver cancer cells with wild-type TTN reduces sensitivity to 5-FU.
(A) TTN plasmid transfection into Huh7 and Hep3B cells to establish
TTN overexpression models. NC group: Cells transfected with an
empty vector pCDNA3.1 without the target gene. (B)
Spectrophotometric analysis of zinc, calcium, cu, and (C) ferri ion
levels in Huh7 and Hep3B cells following TTN overexpression and
5-FU treatment. (D) MDA, GSH and GPX4 content in Huh7 and Hep3B
cells after TTN overexpression and 5-FU treatment. (E) Colony
formation assay in Huh7 and Hep3B cells following TTN
overexpression and 5-FU treatment. (F) Tumor sphere formation assay
in Huh7 and Hep3B cells after TTN overexpression and 5-FU
treatment. (G) EdU staining of Huh7 and Hep3B cells following TTN
overexpression and 5-FU treatment. **P<0.01, ***P<0.001, n=3.
Data are presented as mean ± SD. TTN, titin; 5-FU, 5-fluorouracil;
NC, negative control; TTN-OE, titin overexpression; Cu, copper;
Ferri, ferrous ions; MDA, malondialdehyde; GSH, glutathione; GPX4,
glutathione peroxidase 4. |
TTN knockdown enhances sensitivity to
5-FU in liver cancer cells with TTN mutations
TTN protein function in liver cancer cells harboring
TTN mutations was further investigated. Three specific shRNAs were
employed to knockdown TTN expression in JHH7 and HepG2 cells
(Fig. 5A and B). TTN knockdown did
not significantly affect the cellular levels of zinc, calcium,
copper or ferrous ions. However, following 5-FU treatment, ferrous
ion levels in the sh2 and sh3 groups increased significantly
compared with shNC group (Fig. 5C and
D). Additionally, MDA levels were significantly higher in the
sh2 and sh3 groups following 5-FU treatment compared with shNC,
while GPX4 and GSH levels were significantly decreased (Fig. 5E).
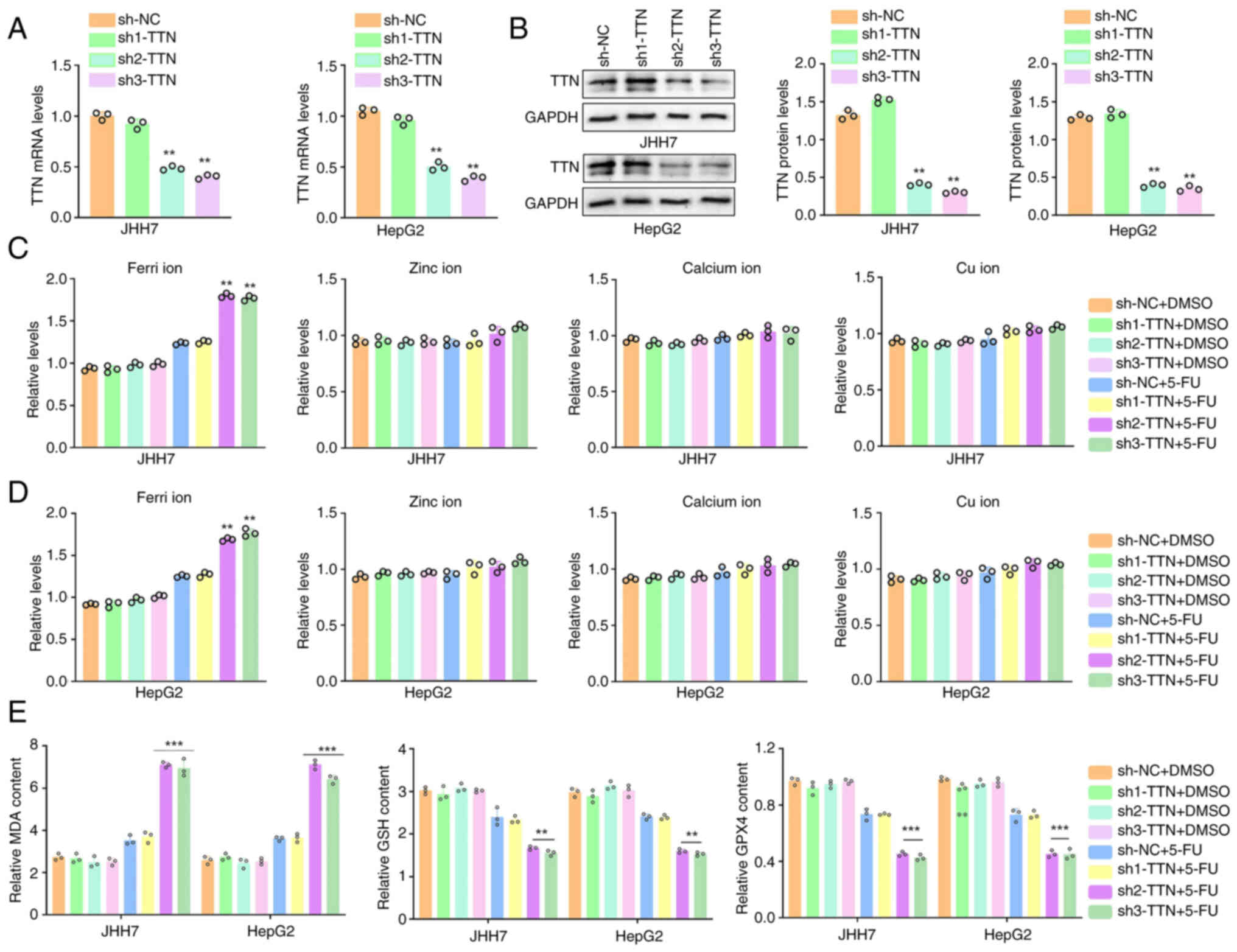 | Figure 5.TTN knockdown enhances sensitivity to
5-FU in liver cancer cells with mutated TTN. TTN shRNAs were
transfected into JHH7 and HepG2 cells to generate TTN knockdown
models (uncropped images available in the Supplementary file).
Efficiency of shRNAs was assessed by (A) RT-qPCR and (B) western
blotting. Spectrophotometric analysis of zinc ion, calcium ion, cu
ion and ferri ion levels in (C) JHH7 and (D) HepG2 cells following
TTN knockdown and 5-FU treatment. (E) Measurement of MDA, GSH and
GPX4 levels in JHH7 and HepG2 cells after TTN knockdown and 5-FU
treatment. **P<0.01, ***P<0.001, n=3. Data are presented as
mean ± SD. TTN, titin; FU, 5-fluorouracil; NC, negative control;
malondialdehyde, MDA; GSH, glutathione; GPX4, glutathione
peroxidase 4. |
Knockdown of TTN enhances the
suppressive effects of 5-FU on cell proliferation
TTN knockdown in JHH7 and HepG2 cells also enhanced
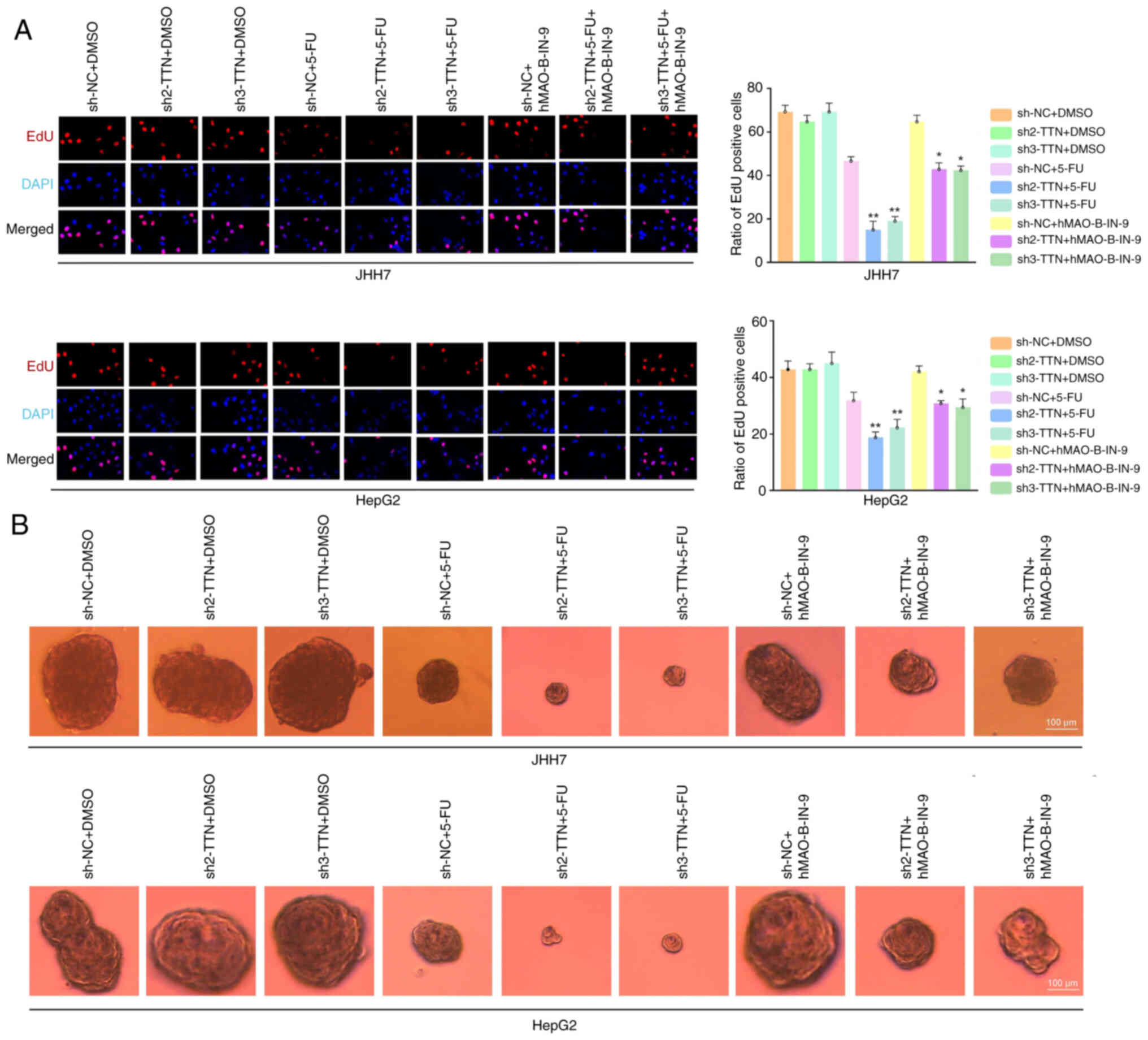
the inhibitory effect of 5-FU on DNA synthesis rates (Fig. 6A), and tumor spheroid proliferation
(Fig. B). Treatment with the
ferrous ion chelator hMAO-B-IN-9 slightly reversed these inhibitory
effects (Fig. 6A and B).
Knockdown of TTN enhances the
therapeutic effect of 5-FU in vivo
In vivo, the synergistic effect of TTN
deletion with 5-FU was explored by transplanting HepG2/shNC or
HepG2/shTTN cells into the subcutaneous tissue of immunocompromised
BABL/c mice. Tumor-bearing mice were treated with DMSO or 5-FU (25
mg/kg; 100 µl) via intraperitoneal injection once daily for 21 days
(Fig. 7A). TTN knockdown did not
significantly impact tumor growth in HepG2/shNC cells (Fig. 7B-D). However, stable TTN knockdown
increased the sensitivity of tumors to 5-FU, significantly
inhibiting tumor growth in mice transplanted with HepG2/shTTN cells
(Fig. 7B-D). IHC staining of tumor
tissue revealed that, similar to the in vitro results, the
levels of Ki67 and PCNA were significantly lower in TTN-knockdown
tumors compared with the control following 5-FU treatment (Fig. 7E-G). These results underscore the
potential role of TTN mutations in modulating 5-FU sensitivity in
liver cancer.
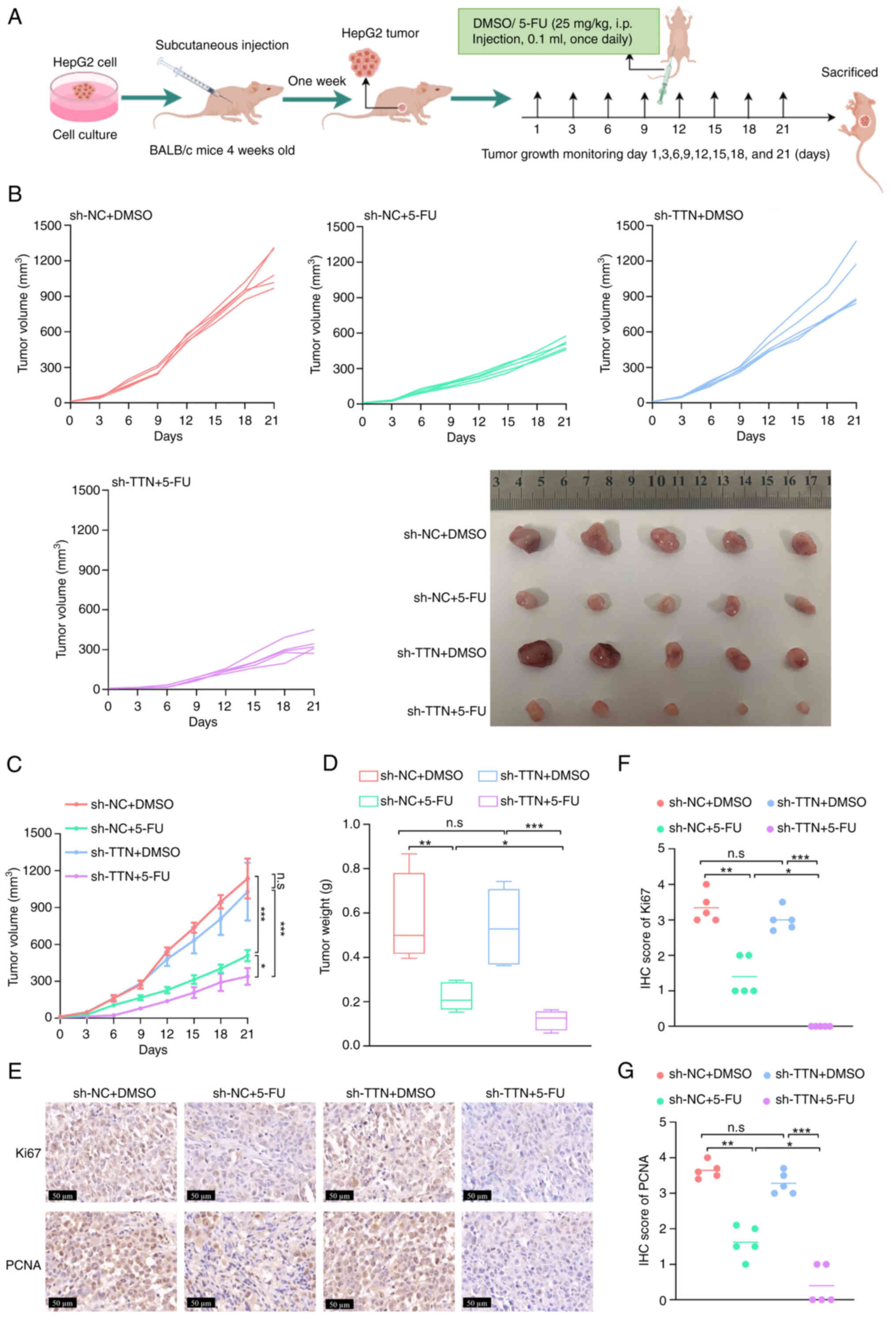 | Figure 7.Knockdown of TTN enhances the
therapeutic effect of 5-FU in vivo. (A) Tumor growth of
HepG2/shNC and HepG2/shTTN cells in subcutaneous xenografts of
immunocompromised BABL/c mice. Tumor-bearing mice were treated with
intraperitoneal injections of DMSO (control) or 5-FU (25 mg/kg; 100
µl) once daily for 21 days. (B) Tumor growth curves (n=5). Tumors
were harvested after 21 days of DMSO or 5-FU treatment (n=5). (C)
Tumor volume and (D) weight (n=5). (E) Representative
immunohistochemistry images showing Ki67 and PCNA expression in
tumor tissues (magnification, 400×; scale bars, 50 µm; n=5).
Quantification of (F) Ki67 and (G) PCNA expression levels in tumor
tissues using ImageJ software. All results are shown as mean ± SD.
*P<0.05, **P<0.01, ***P<0.001, n=3. Data are presented as
mean ± SD. TTN, titin; 5-FU, 5-fluorouracil; NC, negative control;
i.p., intraperitoneal; IHC, immunohistochemistry; n.s. not
significant. |
Discussion
Liver cancer continues to be a major global health
issue, characterized by rising morbidity and mortality; there were
~865,000 new cases and 757,948 deaths worldwide in 2022 (45). The onset and progression of liver
cancer exhibits considerable molecular heterogeneity, with complex
underlying mechanisms involving various genetic abnormalities, such
as SNPs, genomic instability, somatic mutations and dysregulated
signaling pathways (46,47). Genomic instability is a hallmark of
liver cancer, and an accumulation of gene mutations is associated
with tumorigenesis. These mutations contribute to the acquisition
of cancer cell traits, including uncontrolled proliferation, immune
evasion, invasion, metastasis and resistance to therapies (18,48).
The advent of high-throughput sequencing technologies has enhanced
understanding of the pathological processes underlying liver cancer
and facilitated the identification of key genes involved in its
carcinogenesis (18,49). However, the precise biological
effects and molecular mechanisms associated with liver cancer gene
mutations remain poorly understood.
The present study demonstrates that TTN mutations
are prevalent in liver cancer, as evidenced by the analysis of
somatic mutation data from liver cancer tissues in TCGA cohort.
These mutations associate with prognosis and resistance to
treatment in liver cancer. Differential gene expression analysis
and enrichment analysis revealed that TTN mutations significantly
influence metal ion metabolism, GSH metabolism and ferroptosis.
High frequencies of TTN mutations have also been identified in
several other types of cancer, including non-small cell lung
cancer, ovarian cancer, breast cancer, small cell lung cancer and
colon adenocarcinoma (24,50). Findings of the present study
revealed an association between TTN mutations and dysregulated iron
ion metabolism and GSH metabolism, along with enrichment in the
ferroptosis pathway.
Ferroptosis, an iron-dependent form of cell death
distinct from autophagy, apoptosis and necrosis (51), has a pivotal role in inhibiting
tumorigenesis and offers new therapeutic avenues for the treatment
of cancer (52). TTN mutations may
indirectly affect the occurrence of ferroptosis by affecting
various mechanisms such as cytoskeletal stability, calcium
homeostasis, mitochondrial function, iron metabolism and gene
expression (53–56). The present study revealed that the
increase in protein stability after TTN mutation in liver cancer
mainly affected the expression of GPX4 and GSH, key regulators of
ferroptosis, and reduced intracellular iron ion concentration,
thereby inhibiting ferroptosis. However, the relationship between
TTN mutation and ferroptosis has not been fully elucidated, and
future work explore the association between TTN mutations and
ferroptosis.
Despite advances in liver cancer treatment, drug
resistance remains a major clinical challenge. Several chemotherapy
agents have been found to induce ferroptosis, and resistance to
treatment is associated with the dysregulation of this process
(57). Studies indicate that
cancer cells become resistant through GPX4-mediated regulation of
lipid peroxidation, further implicating ferroptosis in treatment
failure (58–60). Building on these findings, the
present study suggests that TTN mutations may enhance liver cancer
resistance by inhibiting the ferroptosis pathway. Moreover,
OncoPredict analysis and CCK-8 assays revealed that TTN mutations
increased sensitivity to GSK1904529A and nilotinib, while
conferring resistance to 5-FU and saptinib in liver cancer cells.
However, there are limitations to the association between drug
susceptibility scores and clinical outcomes. At present, the
evaluation method of the drug susceptibility score is not fully
standardized, and different detection techniques and scoring
systems may produce different results. In addition, factors such as
tumor heterogeneity, individual differences in patients, and other
factors can also affect the correlation between drug sensitivity
scores and clinical outcomes.
Predictions made by OncoPredict are based on network
databases and do not fully capture the molecular changes in
individual patients, highlighting the need for additional in
vitro cell line testing and patient-derived xenograft models.
Since its discovery, 5-FU has remained a cornerstone in the
treatment of solid tumors, including liver cancer (61). Upon entering the cell, 5-FU is
metabolized into several active metabolites, such as
fluorodeoxyuridine triphosphate and fluorodeoxyuridine
monophosphate, which inhibit thymidylate synthetase and exert
cytotoxic effects (62). Results
of the present study indicate that TTN mutations stabilize TTN
protein, suppress ferroptosis by reducing intracellular iron ion
levels and significantly diminish the sensitivity of liver cancer
cells to 5-FU both in vitro and in vivo. The present
study provides preliminary evidence suggesting that TTN mutations
may serve as a prognostic biomarker for liver cancer and contribute
to treatment resistance. The specific effects of TTN gene mutations
vary depending on factors such as the type of mutation, the
location of the mutation and the genetic background of the
individual. For example, missense mutations cause one amino acid to
be replaced with another, altering the function of a protein. A
nonsense mutation produces a stop codon that results in an early
termination of protein synthesis. Deletion mutations and
insertional mutations may introduce alterations in the reading
frame, resulting in severe alterations or deletions of protein
sequences, resulting in loss of function (63). At present, there is still
insufficient research on the effects of different types of TTN
mutations on liver cancer, warranting further exploration of the
biological or therapeutic implications of the different types of
TTN mutations in liver cancer in future studies.
The present study has limitations, particularly the
absence of clinical validation and the reliance on animal and
cellular models for experimental evidence. Future studies should
focus on clinical trials and xenotransplantation models derived
from patient samples to validate the in vitro and in
vivo findings of the present study and strengthen the clinical
relevance of these observations.
In conclusion, TTN mutations represent a
high-frequency genetic alteration in liver cancer, enhancing TTN
protein stability. Depletion of TTN leads to reduced intracellular
iron ion levels, inhibiting ferroptosis and significantly
decreasing liver cancer sensitivity to 5-FU both in vitro
and in vivo. These findings highlight the potential role of
TTN mutations in diminishing the effectiveness of 5-FU in liver
cancer treatment. The present study is limited by the lack of
clinical validation and is based solely on animal and cellular
models. Further research is needed to confirm the therapeutic
potential of targeting TTN mutations in overcoming liver cancer
drug resistance.
Acknowledgements
Not applicable.
Funding
This research was funded by the National Natural Science
Foundation of China (grant no. 82103681), the Guizhou Medical
University National Natural Science Foundation Cultivation Project
(grant nos. 20NSP020 and 19NSP034), the Guizhou Provincial Science
and Technology Projects [grant no. ZK (2024)159], the Guizhou
Provincial Science and Technology Projects [grant no. ZK (2024)169]
and the Continuous Support Fund for Excellent Scientific Research
Platforms of Colleges and Universities in Guizhou Province [grant
no. QJJ (2022)020].
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SL and TC contributed to conceptualization; SL
stored and backed-up all data in the article; ZZh, YS and ZZe
analyzed data. DL and WC performed bioinformatics analysis; ZZh and
YS contributed to the methodology; SL contributed to the resources;
SL and TC contributed to the software; SL and TC contributed to
validation; ZZh contributed to writing the original draft; SL
contributed to writing, reviewing and editing. ZZh and SL confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Ethics Committee of Guizhou Medical University (approval no.
2400641). Tissue sample data used in the present study were sourced
from public databases, such as TCGA and did not involve human
ethics.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer. 1873:1883142020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh SP, Arora V, Madke T and Sarin SK:
Hepatocellular carcinoma-southeast asia updates. Cancer J.
29:259–265. 2013. View Article : Google Scholar
|
|
3
|
Mazzaferro V, Citterio D, Bhoori S,
Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M,
Romagnoli R, Antonelli B, et al: Liver transplantation in
hepatocellular carcinoma after tumour downstaging (XXL): A
randomised, controlled, phase 2b/3 trial. Lancet Oncol. 21:947–956.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong TC, Lee VH, Law AL, Pang HH, Lam KO,
Lau V, Cui TY, Fong AS, Lee SW, Wong EC, et al: Prospective study
of stereotactic body radiation therapy for hepatocellular carcinoma
on waitlist for liver transplant. Hepatology. 74:2580–2594. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B,
Wu F, Wang Q, Wang S, Rong D, Reiter FP, et al: The mechanisms of
sorafenib resistance in hepatocellular carcinoma: Theoretical basis
and therapeutic aspects. Signal Transduct Target Ther. 5:872020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su X, Li Y, Ren Y, Cao M, Yang G, Luo J,
Hu Z, Deng H, Deng M, Liu B and Yao Z: A new strategy for
overcoming drug resistance in liver cancer: Epigenetic regulation.
Biomed Pharmacother. 176:1169022024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Wang Y, Gao P and Ding J: Immune
checkpoint inhibitor resistance in hepatocellular carcinoma. Cancer
Lett. 555:2160382023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ostroverkhova D, Przytycka TM and
Panchenko AR: Cancer driver mutations: Predictions and reality.
Trends Mol Med. 29:554–566. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pirozzi CJ and Yan H: The implications of
IDH mutations for cancer development and therapy. Nat Rev Clin
Oncol. 18:645–661. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shahbandi A, Nguyen HD and Jackson JG:
TP53 mutations and outcomes in breast cancer: Reading beyond the
headlines. Trends Cancer. 6:98–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prior IA, Hood FE and Hartley JL: The
frequency of ras mutations in cancer. Cancer Res. 80:2969–2974.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Timar J and Kashofer K: Molecular
epidemiology and diagnostics of KRAS mutations in human cancer.
Cancer Metastasis Rev. 39:1029–1038. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herzog SK and Fuqua SAW: ESR1 mutations
and therapeutic resistance in metastatic breast cancer: Progress
and remaining challenges. Br J Cancer. 126:174–186. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calderaro J, Couchy G, Imbeaud S, Amaddeo
G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage
P, et al: Histological subtypes of hepatocellular carcinoma are
related to gene mutations and molecular tumour classification. J
Hepatol. 67:727–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang P, Song Q, Ren J, Zhang W, Wang Y,
Zhou L, Wang D, Chen K, Jiang L, Zhang B, et al: Simultaneous
analysis of mutations and methylations in circulating cell-free DNA
for hepatocellular carcinoma detection. Sci Transl Med.
14:eabp87042022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calderaro J, Ziol M, Paradis V and
Zucman-Rossi J: Molecular and histological correlations in liver
cancer. J Hepatol. 71:616–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loescher CM, Hobbach AJ and Linke WA:
Titin (TTN): From molecule to modifications, mechanics, and medical
significance. Cardiovasc Res. 118:2903–2918. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ceyhan-Birsoy O, Agrawal PB, Hidalgo C,
Schmitz-Abe K, DeChene ET, Swanson LC, Soemedi R, Vasli N,
Iannaccone ST, Shieh PB, et al: Recessive truncating titin gene,
TTN, mutations presenting as centronuclear myopathy. Neurology.
81:1205–1214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kellermayer D, Smith JE III and Granzier
H: Titin mutations and muscle disease. Pflugers Arch. 471:673–682.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Djulbegovic MB, Uversky VN, Karp CL and
Harbour JW: Functional impact of titin (TTN) mutations in ocular
surface squamous neoplasia. Int J Biol Macromol. 195:93–101. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gomes FC, Figueiredo ERL, Araújo EN,
Andrade EM, Carneiro CDL, Almeida GM, Dias HAAL, Teixeira LIB,
Almeida MT, Farias MF, et al: Social, genetics and
histopathological factors related to titin (TTN) gene mutation and
survival in women with ovarian serous cystadenocarcinoma:
Bioinformatics analysis. Genes (Basel). 14:10922023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han X, Chen J, Wang J, Xu J and Liu Y: TTN
mutations predict a poor prognosis in patients with thyroid cancer.
Biosci Rep. 42:BSR202211682022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kodali N, Alomary S, Bhattaru A, Eldaboush
A, Schwartz RA and Lipner SR: Gender and melanoma subtype-based
prognostic implications of MUC16 and TTN co-occurrent mutations in
melanoma: A retrospective multi-study analysis. Cancer Med.
13:e701992024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue D, Lin H, Lin L, Wei Q, Yang S and
Chen X: TTN/TP53 mutation might act as the predictor for
chemotherapy response in lung adenocarcinoma and lung squamous
carcinoma patients. Transl Cancer Res. 10:1284–1294. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Z, Zhao X, Wang R, Tang X, Zhao Y,
Zhong G, Peng X and Zhang C: Heterogeneous pattern of gene
expression driven by TTN mutation is involved in the construction
of a prognosis model of lung squamous cell carcinoma. Front Oncol.
13:9165682023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen R, Yao Z and Jiang L: Construction
and validation of a TTN mutation associated immune prognostic model
for evaluating immune microenvironment and outcomes of gastric
cancer: An observational study. Medicine (Baltimore).
103:e389792024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mayakonda A, Lin DC, Assenov Y, Plass C
and Koeffler HP: Maftools: Efficient and comprehensive analysis of
somatic variants in cancer. Genome Res. 28:1747–1756. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maeser D, Gruener RF and Huang RS:
oncoPredict: An R package for predicting in vivo or cancer patient
drug response and biomarkers from cell line screening data. Brief
Bioinform. 22:bbab2602021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruiz-Villalba A, Ruijter JM and van den
Hoff MJB: Use and misuse of Cq in qPCR data analysis and
reporting. Life (Basel). 11:4962021.PubMed/NCBI
|
|
34
|
Lei S, Cao W, Zeng Z, Zhang Z, Jin B, Tian
Q, Wu Y, Zhang T, Li D, Hu C, et al: JUND/linc00976 promotes
cholangiocarcinoma progression and metastasis, inhibits ferroptosis
by regulating the miR-3202/GPX4 axis. Cell Death Dis. 13:9672022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen X, Li J, Kang R, Klionsky DJ and Tang
D: Ferroptosis: Machinery and regulation. Autophagy. 17:2054–2081.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang D, Feng Y, Zandkarimi F, Wang H,
Zhang Z, Kim J, Cai Y, Gu W, Stockwell BR and Jiang X: Ferroptosis
surveillance independent of GPX4 and differentially regulated by
sex hormones. Cell. 186:2748–2764.e22. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Propper DJ, Gao F, Saunders MP, Sarker D,
Hartley JA, Spanswick VJ, Lowe HL, Hackett LD, Ng TT, Barber PR, et
al: PANTHER: AZD8931, inhibitor of EGFR, ERBB2 and ERBB3
signalling, combined with FOLFIRI: A phase I/II study to determine
the importance of schedule and activity in colorectal cancer. Br J
Cancer. 128:245–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pereira M and Vale N: Repurposing alone
and in combination of the antiviral saquinavir with 5-fluorouracil
in prostate and lung cancer cells. Int J Mol Sci. 23:122402022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo J, Yu Z, Sun D, Zou Y, Liu Y and Huang
L: Two nanoformulations induce reactive oxygen species and
immunogenetic cell death for synergistic chemo-immunotherapy
eradicating colorectal cancer and hepatocellular carcinoma. Mol
Cancer. 20:102021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuan J, Khan SU, Yan J, Lu J, Yang C and
Tong Q: Baicalin enhances the efficacy of 5-fluorouracil in gastric
cancer by promoting ROS-mediated ferroptosis. Biomed Pharmacother.
164:1149862023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin J, Bjørk PK, Kolte MV, Poulsen E,
Dedic E, Drace T, Andersen SU, Nadzieja M, Liu H, Castillo-Michel
H, et al: Zinc mediates control of nitrogen fixation via
transcription factor filamentation. Nature. 631:164–169. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jomova K, Makova M, Alomar SY, Alwasel SH,
Nepovimova E, Kuca K, Rhodes CJ and Valko M: Essential metals in
health and disease. Chem Biol Interact. 367:1101732022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luan M, Feng Z, Zhu W, Xing Y, Ma X, Zhu
J, Wang Y and Jia Y: Mechanism of metal ion-induced cell death in
gastrointestinal cancer. Biomed Pharmacother. 174:1165742024.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun X, Zhang Y, Li J, Park KS, Han K, Zhou
X, Xu Y, Nam J, Xu J, Shi X, et al: Amplifying STING activation by
cyclic dinucleotide-manganese particles for local and systemic
cancer metalloimmunotherapy. Nat Nanotechnol. 16:1260–1270. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun Y, Wu P, Zhang Z, Wang Z, Zhou K, Song
M, Ji Y, Zang F, Lou L, Rao K, et al: Integrated multi-omics
profiling to dissect the spatiotemporal evolution of metastatic
hepatocellular carcinoma. Cancer Cell. 42:135–156.e17. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Garcia-Lezana T, Lopez-Canovas JL and
Villanueva A: Signaling pathways in hepatocellular carcinoma. Adv
Cancer Res. 149:63–101. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cheng X, Yin H, Fu J, Chen C, An J, Guan
J, Duan R, Li H and Shen H: Aggregate analysis based on TCGA: TTN
missense mutation correlates with favorable prognosis in lung
squamous cell carcinoma. J Cancer Res Clin Oncol. 145:1027–1035.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang C, Liu X, Jin S, Chen Y and Guo R:
Ferroptosis in cancer therapy: A novel approach to reversing drug
resistance. Mol Cancer. 21:472022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee SH, Oh J, Lee ST, Won D, Kim S, Choi
HK, Kim SJ, Han H, Yoon M, Choi JR, et al: Generation of a human
induced pluripotent stem cell line YCMi004-A from a patient with
dilated cardiomyopathy carrying a protein-truncating mutation of
the titin gene and its differentiation towards cardiomyocytes. Stem
Cell Res. 59:1026292022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Linke WA and Hamdani N: Gigantic business:
Titin properties and function through thick and thin. Circ Res.
114:1052–1068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hinson JT, Chopra A, Nafissi N, Polacheck
WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, et
al: HEART DISEASE. Titin mutations in iPS cells define sarcomere
insufficiency as a cause of dilated cardiomyopathy. Science.
349:982–986. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Richardson DR and Ponka P: The molecular
mechanisms of the metabolism and transport of iron in normal and
neoplastic cells. Biochim Biophys Acta. 1331:1–40. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zou Y, Palte MJ, Deik AA, Li H, Eaton JK,
Wang W, Tseng YY, Deasy R, Kost-Alimova M, Dančík V, et al: A
GPX4-dependent cancer cell state underlies the clear-cell
morphology and confers sensitivity to ferroptosis. Nat Commun.
10:16172019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Conrad M and Pratt DA: The chemical basis
of ferroptosis. Nat Chem Biol. 15:1137–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li SH, Mei J, Cheng Y, Li Q, Wang QX, Fang
CK, Lei QC, Huang HK, Cao MR, Luo R, et al: Postoperative adjuvant
hepatic arterial infusion chemotherapy With FOLFOX in
hepatocellular carcinoma with microvascular invasion: A
multicenter, phase III, randomized study. J Clin Oncol.
41:1898–1908. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Herman DS, Lam L, Taylor MR, Wang L,
Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B,
Sparks E, et al: Truncations of titin causing dilated
cardiomyopathy. N Engl J Med. 366:619–628. 2012. View Article : Google Scholar : PubMed/NCBI
|