Introduction
The endoplasmic reticulum (ER) is one of the
organelles of eukaryotic cells, which is named after its lumen-like
structure (1). The ER is mainly
responsible for protein synthesis, lipid synthesis, Ca2+
storage and signal transduction (2,3).
Intracellular and extracellular factors that interfere with any of
the homeostatic functions of the ER can induce ER stress (4,5). The
unfolded protein response (UPR) is induced to overcome ER stress,
and to reduce protein synthesis through transcription and
translation. In addition, the UPR increases ER protein assembly
ability, and reduces the aggregation of misfolded or unfolded
proteins, thus resulting in mitigation of stress, and recovery of
protein balance and ER homeostasis (6,7).
Long-term ER stress induces chronic UPR activation, which alters
homeostasis of the body, even though low-level UPR stimulation
protects cells (8–10). Notably, ER stress may propagate
between different cells (11,12).
Conditioned culture medium from ER-stressed tumor cells can cause
ER stress in bone marrow cells, which may then release unknown
molecules that promote tumor cell growth (13). Furthermore, in obesity, ER stress
propagates between liver cells and disrupts systemic metabolism
(14).
ER stress is directly related to the pathogenesis of
diseases of the central nervous system (CNS) (15). A previous study discovered that ER
stress markers, such as phosphorylated (p)-eIF-2α, activating
transcription factor (ATF4) and CHOP, are still present in the
cerebral cortex days after traumatic brain injury (16). Another study using a rat model of
neuropathic pain suggested that the spinal dorsal horn has
increased levels of ER stress markers, including glucose regulated
protein 78 (GRP78), p-PERK, p-eukaryotic translation initiation
factor 2α and ATF6, accompanied by NF-κB phosphorylation (17). In addition, in CNS diseases,
whether ER stress signaling transmission is the basic root cause of
neuronal death is not yet known.
Vitamin C (Vc) is a water-soluble antioxidant. It
neutralizes free radicals such as reactive oxygen species to
protect cells from oxidative damage and assists in collagen
synthesis, enhancing immunity. It can also be used as a standard
substance to help quantify the total antioxidant capacity of
samples. Its reducibility (providing electrons) makes it a key tool
for studying antioxidant mechanisms.
It has previously been confirmed that thapsigargin
(TG) can successfully induce ER stress in vitro (18). The present study aimed to
investigate whether ER stress signals can propagate between
different nerve cell types.
Materials and methods
Cell culture
Rat adrenal pheochromocytoma cell line 12 (PC12; The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences) and CTX TNA2 (ASCs; iCell Bioscience Inc.) were grown in
complete DMEM containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 U/ml
streptomycin at 37°C with 5% CO2 in a cell culture
incubator. The cells were passaged at 90% confluence. The medium
was discarded to initiate cell passage, and 0.25% trypsin was
introduced for cell detachment. The cells were then collected by
pipetting and subjected to centrifugation at 800 × g at room
temperature for 5 min. After removing the supernatant, the cells
were resuspended in fresh culture medium. The cells were evenly
distributed into T25 cell culture flask and passaged every 3 days
(19).
Observation of cell morphological
changes
Cells were seeded at a density of
5×104/ml in 6-well plates. After treatment, five fields
of view were selected for observation and photography under
high-power magnification of an optical microscope.
ASC ER stress supernatant
preparation
ASCs were treated with 0.5 µmol/l TG
(MilliporeSigma) at 37°C for 12 h, and the medium was removed. The
cells were washed three times with serum-free Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) and
then complete medium was added to continue culturing for 12 h.
After 12 h of culture, the cells were collected. The culture medium
was centrifuged at 2,000 × g at 4°C for 20 min to discard cell
debris. The supernatant was collected as ASC conditioned medium
(ACM) and used for subsequent experiments.
ACM filtrate (ACM-F) and ACM residue
(ACM-R) preparation
After collecting the ACM, it was added to an
ultrafiltration centrifuge tube containing a 100 K polyethersulfone
membrane. The mixture was then centrifuged at 5,000 × g for 40 min
at 4°C. The ACM was divided into two parts: The lower layer was
considered the ACM-F and an equal quantity of culture medium was
added into the upper layer, labelled the ACM-R.
Vesicle collection and
preparation
ACM was isolated and centrifuged at 2,000 × g for 5
min at 4°C to acquire the supernatant, which was centrifuged at
12,000 × g and 4°C for 15 min to discard cell debris and impurities
prior to discarding the pellet. The supernatant was then
ultracentrifuged at 100,000 × g, 4°C for 2 h. After rinsing with
PBS, a second ultracentrifugation step was performed under the same
conditions. The obtained precipitate is the vesicles. Resuspend in
200 µl of PBS buffer and store at −80°C for future use.
Experimental grouping
ASC ER stress validation experiment
ASCs were divided into four groups: i) Untreated
(UT): ASCs were incubated in complete medium at 37°C for 24 h; ii)
DMEM + complete medium group: ASCs were incubated in DMEM at 37°C
for 12 h, followed by incubation in complete medium for at 37°C 12
h; iii) TG + complete medium group: ASCs were incubated in 0.5
µmol/l TG at 37°C for 12 h, followed by incubation in complete
medium at 37°C for 12 h; and iv) TG group: ASCs were incubated in
0.5 µmol/l TG at 37°C for 24 h.
ER stress propagation experiment
PC12 cells were divided into four groups: i) UT
group: PC12 cells were incubated in complete medium at 37°C for 24
h; ii) UT-ACM group: PC12 cells were incubated in UT-ACM at 37°C
for 24 h; iii) TG-ACM group: PC12 cells were incubated in ACM
treated with 0.5 µmol/l TG at 37°C for 24 h; and iv) TG group: PC12
cells were incubated in 0.5 µmol/l TG at 37°C for 24 h.
Molecular weight assessment of ER
stress propagation mediator experiment
PC12 cells were divided into the following eight
groups: i) UT group: PC12 cells were incubated in complete medium
at 37°C for 24 h; ii) UT-ACM group: PC12 cells were incubated in
UT-ACM at 37°C for 24 h; iii) UT-ACM-F group: PC12 cells were
incubated in UT ACM-F at 37°C for 24 h; iv) UT-ACM-R group: PC12
cells were incubated in UT-ACM-R at 37°C for 24 h; v) TG-ACM group:
PC12 cells were incubated in ACM treated with 0.5 µmol/l TG at 37°C
for 24 h; vi) TG-ACM-F group: PC12 cells were incubated in ACM-F
treated with 0.5 µmol/l TG at 37°C for 24 h; vii) TG-ACM-R group:
PC12 cells were incubated with ACM-R treated with 0.5 µmol/l TG at
37°C for 24 h; viii) TG group: PC12 cells were incubated with 0.5
µmol/l TG at 37°C for 24 h.
Assessment of the vesicular nature of
the ER stress propagation mediator experiment
PC12 cells were divided into the following groups:
i) UT group: PC12 cells were incubated with complete medium at 37°C
for 24 h; ii) UT-ACM group: PC12 cells were incubated with UT-ACM
at 37°C for 24 h; iii) UT-ACM-(no) Vesicles group: PC12 cells were
incubated with UT de-vesicularized ACM at 37°C for 24 h; iv)
UT-ACM-Vesicles group: PC12 cells were incubated with UT
vesicularized ACM at 37°C for 24 h; v) TG-ACM group: PC12 cells
were incubated with 0.5 µmol/l TG-treated ACM at 37°C for 24 h; vi)
TG-ACM-(no) Vesicles group: PC12 cells were incubated with 0.5
µmol/l TG-treated de-vesicularized ACM at 37°C for 24 h; vii)
TG-ACM-Vesicles group: PC12 cells were incubated with 0.5 µmol/l
TG-treated vesicularized ACM at 37°C for 24 h; viii) TG group: PC12
cells were incubated with 0.5 µmol/l TG at 37°C for 24 h.
Assessment of the oxidative activity
of the ER stress propagation mediator experiment
PC12 cells were divided into the following five
groups: i) UT group: PC12 cells were incubated with complete medium
at 37°C for 24 h; ii) UT-ACM group: PC12 cells were incubated with
UT-ACM at 37°C for 24 h; iii) TG-ACM group: PC12 cells were
incubated with 0.5 µmol/l TG-treated ACM at 37°C for 24 h; iv)
TG-ACM + Vitamin C (Vc) group: PC12 cells were incubated with 0.5
µmol/l TG-treated ACM and supplemented with 100 µmol/l Vc
(MilliporeSigma) at 37°C for 24 h; v) TG group: PC12 cells were
incubated with 0.5 µmol/l TG at 37°C for 24 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from PC12 cells and ASCs
using the RNA-easy extraction kit (Vazyme Biotech Co., Ltd.)
following the manufacturer's instructions. Nucleic acid protein
detector (Eppendorf SE) was used to determine the quantity and
integrity of total RNA. The A260/A280 ratio obtained for RNA was
1.8–2.0, with a concentration of ~300 mg/ml. cDNA was generated
using the Quantscript RT Kit (Vazyme Biotech Co., Ltd.) according
to the manufacturer's instructions. The cDNA then underwent qPCR
using the SuperReal PreMix Plus SYBR Green kit (Vazyme Biotech Co.,
Ltd.). The gene amplification primer sequences are indicated in
Table I. Thermocycling conditions
were as follows: Initial denaturation at 95°C for 10 mins; 95°C for
denaturation for 15 s, 60°C for annealing for 15 s, and 72°C for
extension for 30 s. This process is repeated for a total of 40
cycles. Relative expression level of the target gene by using the
2−ΔΔCq method (20).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequence,
5′-3′ | Size, bp |
|---|
| β-actin |
F-GCTGTGCTATGTTGCCCTAGACTTC | 122 |
|
|
R-GGAACCGCTCATTGCCGATAGTG |
|
| GRP78 |
F-CGGAGGAGGAGGACAAGAAGGAG | 104 |
|
|
R-ATACGACGGTGTGATGCGGTTG |
|
| CHOP |
F-TGGCATCACCTCCTGTCTGTCTC | 95 |
|
|
R-CCCTCTCCTTTGGTCTACCCTCAG |
|
| ATF4 |
F-CTGCTTGCTCTGTGGTAGATGTCTC | 107 |
|
|
R-CTCTGCTGCCTCTAATACGCCATG |
|
Western blot analysis
PC12 cells and ASCs were rinsed twice with PBS. The
cells were lysed with ice-cold lysis buffer (100 µl RIPA + 1 µl
protease inhibitor; Beyotime Institute of Biotechnology). Total
protein concentration was calculated using the Nucleic acid protein
detector (Eppendorf SE). The protein samples (100 µg total
protein/lane) were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gels
for 2 h before transferring them to a PVDF membrane
(MilliporeSigma). After blocking the membrane with 5% skimmed milk
for 2 h at room temperature, it was incubated with primary
antibodies at 4°C overnight (Table
II). The membrane was then rinsed three times with 0.1%
Tris-buffered saline-Tween 20 (Biosharp Life Sciences) and was
incubated with a HRP-labeled secondary antibody (Suzhou Ruiying
Biotechnology Co., Ltd.; cat, RS0002, 1:5,000 dilution) at room
temperature for 1 h. The protein bands were identified using an ECL
substrate (Vazyme Biotech Co., Ltd.) and the optical intensity of
protein bands was determined by Lane1D Ver4.22 (19,20).
 | Table II.Antibodies used for
immunofluorescence and WB analyses. |
Table II.
Antibodies used for
immunofluorescence and WB analyses.
| Antibody | Catalog number and
research resource identifier (manufacturer) | Dilution |
|---|
| β-actin | Cat. no. 4970,
rabbit mAb, AB_2223172 (Cell Signaling Technology, Inc.) | WB 1:1,000 |
| GRP78 | Cat. no. ab108615,
rabbit pAb, AB_10890641 (Abcam) | WB 1:1,000 |
| ATF4 | Cat. no. BM5179,
rabbit mAb (Wuhan Boster Biological Technology, Ltd.) | WB 1:1,000 |
| CHOP | Cat. no. 2895,
mouse mAb, AB_2089254 (Cell Signaling Technology, Inc.) | WB 1:1,000 |
| PDI | Cat. no. ab154820,
rabbit pAb (Abcam) | Immunofluorescence
1:200 |
Protein disulfide isomerase (PDI)
immunofluorescence
PC12 cells were seeded in a 6-well plate at a
density of 5×104 cells/ml. Following treatment, the
culture medium was removed and cells were washed twice with PBS.
The cells were fixed with 4% paraformaldehyde solution at room
temperature for 30 min. Subsequently, the cells were rinsed three
times in PBS (5 min each) and permeabilized with 0.1% Triton X-100
at room temperature for 30 min. The cells were washed a further
three times with PBS (5 min each) and incubated with PDI primary
antibody (Abcam; cat. no. ab154820, 1:200) at 4°C overnight. Wash
the cells with PBS 3 times, each time for 5 min. A Cy™3-labeled
secondary antibody (The Jackson Laboratory; cat. no. 111-165-003,
1:200)) was introduced and incubated at room temperature for 1 h.
DAPI counterstaining was then carried out, followed by rinsing
three times with PBS (20 min each). Finally, the cells were viewed
under a fluorescence microscope (Olympus Corporation), images were
captured and observations were made.
Apoptosis probe staining
PC12 cells were seeded in a 6-well plate at a
density of 5×104 cells/ml. After intervention, the
culture medium was removed and cells were washed three times with
PBS. Subsequently, 1 ml of a solution comprising Annexin V-FITC (5
µl) and propidium iodide (PI; 5 µl) was added to each well (Vazyme
Biotech Co., Ltd.), and the cells were incubated in the dark at
37°C for 15 min. The cells were then examined under a fluorescence
microscope.
Determination of TG in the supernatant
using a full-wavelength spectrophotometer
TG has a distinctive absorption peak at 230 nm
(13). ACM samples were added to a
cuvette to be tested sequentially. Using a full-wavelength
spectrophotometer, the absorbance was measured at 230 nm. As a
positive control, TG added to DMEM was measured.
Measurement of the molecular weight of
ACM-F and ACM-R by Coomassie brilliant blue staining
After separating ACM-F and ACM-R, concentrations
were determined by nucleic acid-protein detector with 10 µl for
each lane, and analyzed by 8% SDS-PAGE. Electrophoresis was
conducted first at 70 V for 30 min and then increased to 110 V for
1 h until the electrophoresis process was completed. Subsequently,
the gel was stained with 2.5% Coomassie Brilliant Blue dye at room
temperature for 2 h on a shaker. The gel was incubated overnight at
room temperature in a decolorizing solution (50 ethanol, 100 acetic
acid and 850 ml distilled water) (21,22).
ACM-vesicle nanoparticle size
analysis
First, the ACM was isolated and centrifuged at 2,000
× g for 5 min at 4°C to acquire the supernatant, which was
centrifuged at 12,000 × g and 4°C for 15 min to discard cell debris
and impurities prior to discarding the pellet. The supernatant was
then ultracentrifuged at 100,000 × g, 4°C for 2 h. After rinsing
with PBS, a second ultracentrifugation step was carried out under
the same conditions. The supernatant was removed and 200 µl PBS was
introduced to resuspend the pellet. A total of 20 µl of the sample
was added to an equal volume of RIPA lysis buffer and lysed on ice
for 1 h. Concentration was determined using a nucleic acid protein
analyzer. The ACM-vesicles were appropriately diluted and injected
into the NTA nanoparticle size analyzer sample chamber, analyzed
and their particle size was determined through ZetaView PMX 110
(Particle Metrix Merge).
MTT assay to detect changes in cell
viability
Cell viability was assessed using the MTT assay
(Shanghai Macklin Biochemical Co., Ltd.). PC12 cells were seeded in
a 96-well plate at a density of 5×104 cells/ml. After
treatment, the medium was replaced with MTT (MTT:DMEM=1:9), and the
cells were incubated at 37°C for 4 h. The MTT mix was then
discarded, and 150 µl DMSO solution was added to each well. The
96-well plate was wrapped in tin foil and agitated at room
temperature for 10 min. Cell viability was assessed by measuring
the optical density (D) value of each well at 490 nm with a
microplate reader. Cell survival rate (%) was calculated as: (D
value of the experimental group-D value of the blank group)/(D
value of the control group-D value of the blank group) ×100.
Determination of Vc content in the
supernatant using a full-wavelength spectrophotometer
Vc was weighed out to 0.01 g, diluted in distilled
water and transferred to a 100-ml volumetric flask. The volume was
then adjusted to the 100 ml mark, stirred thoroughly and set aside.
A pipette was used to transfer 50, 40, 30, 20, 10 ml of the Vc
dilution to separate 100-ml volumetric flasks. To adjust the pH to
6, 1% hydrochloric acid and 0.1 mol/l NaOH were used. The solutions
were then diluted to the 100 ml mark using distilled water, shaken
well. Using a full-wavelength spectrophotometer, the absorbance was
measured at 243 nm to draw a standard curve. Finally, the pH of the
sample was adjusted, and absorbance was measured.
Statistical analysis
Data analysis was performed using GraphPad Prism
software (Version 8; Dotmatics). One-way analysis of variance was
applied to compare differences between three or more groups,
followed by a Tukey's post hoc test. If the assumption of variance
uniformity was not met, the Brown-Forsythe test was applied to
compare differences between >2 groups, followed by the
Games-Howell test for post hoc testing. P<0.05 was considered to
indicate a statistically significant difference. All quantitative
data are presented as the mean ± standard deviation. All
experiments were independently repeated three times.
Results
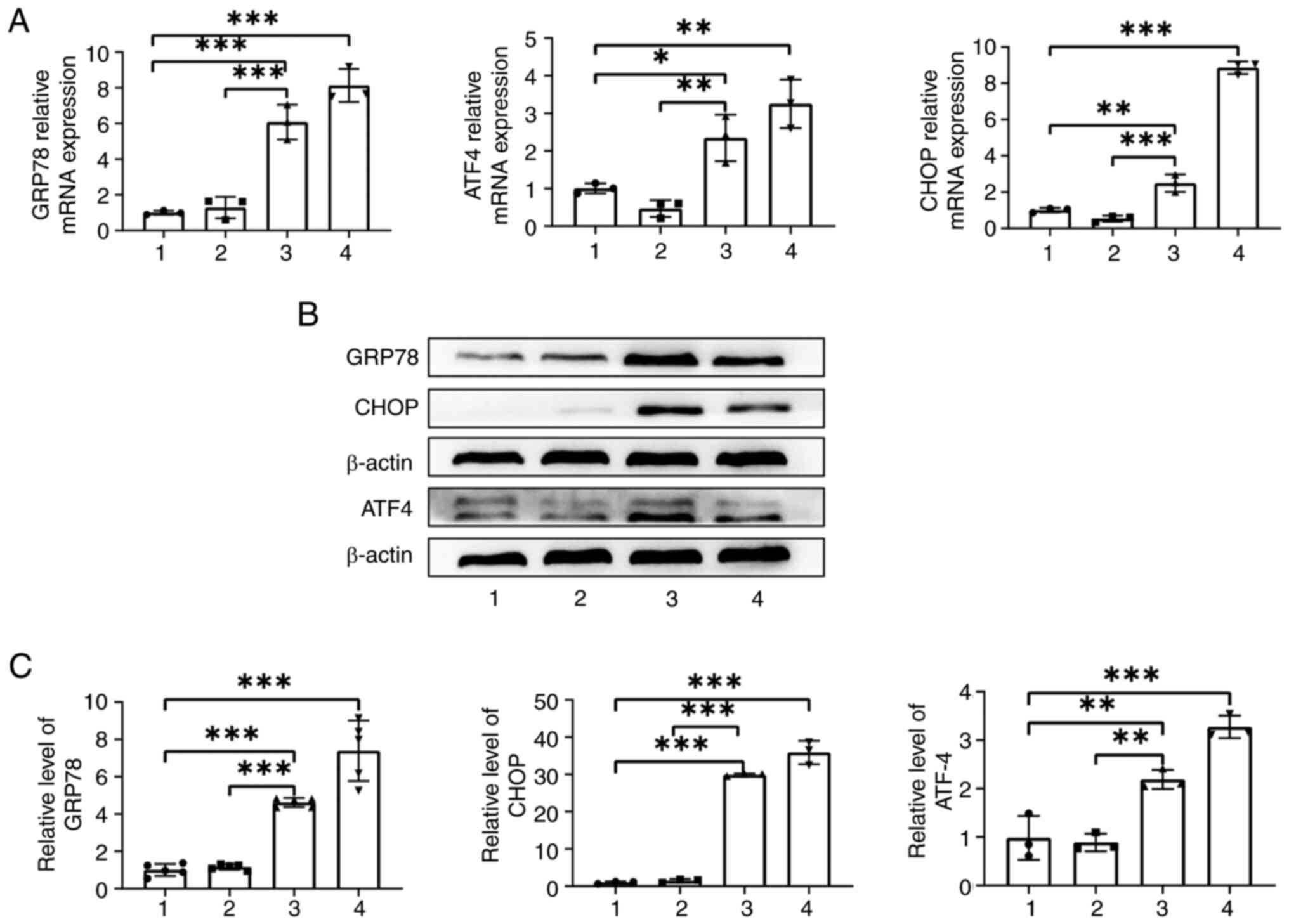
TG induces ER stress in ASCs
As shown in Fig.
1A, the RT-qPCR findings indicated that the mRNA expression
levels of GRP78, ATF4 and CHOP were significantly increased in the
TG + complete medium group (all P<0.05) when compared with those
in the UT group and DMEM + complete medium group. Western blot
analysis indicated that the protein levels of GRP78, ATF4 and CHOP
in the TG + complete medium group were also significantly increased
(all P<0.01) when compared with the UT group and DMEM + complete
medium group (Fig. 1B and C),
which indicated ER stress in ASCs.
To sum up, TG can induce ER stress in ASCs.
ER stress propagates between ASCs and
PC12 cells
Observation under a light microscope revealed that,
when compared with the UT group and UT-ACM group, the number of
cells in the TG-ACM group and TG group was markedly decreased, the
morphology was abnormal and the cells were damaged (Fig. 2A). In addition, the RT-qPCR
findings indicated that the mRNA expression levels of GRP78, ATF4
and CHOP in the TG-ACM group were significantly upregulated (all
P<0.001) when compared with those in the UT group and UT-ACM
group (Fig. 2B). Western blot
analysis revealed that the protein expression levels of GRP78, ATF4
and CHOP were also significantly upregulated in the TG-ACM group
(all P<0.01) compared with those in the UT group and UT-ACM
group (Fig. 2C and D). The
findings of PDI immunofluorescence staining confirmed that the PDI
fluorescence intensity of cells in the TG-ACM and TG groups was
markedly decreased, and appeared as granular spots, when compared
with the UT group and UT-ACM group (Fig. 2E), indicating that the ER was
severely damaged. Finally, PC12 cells were stained with apoptosis
probes. Analysis revealed that the TG-ACM and TG groups exhibited
more Annexin V and PI co-stained cells when compared with the UT
and UT-ACM groups (Fig. 2F). In
conclusion, ER stress propagates between ASCs and PC12 cells.
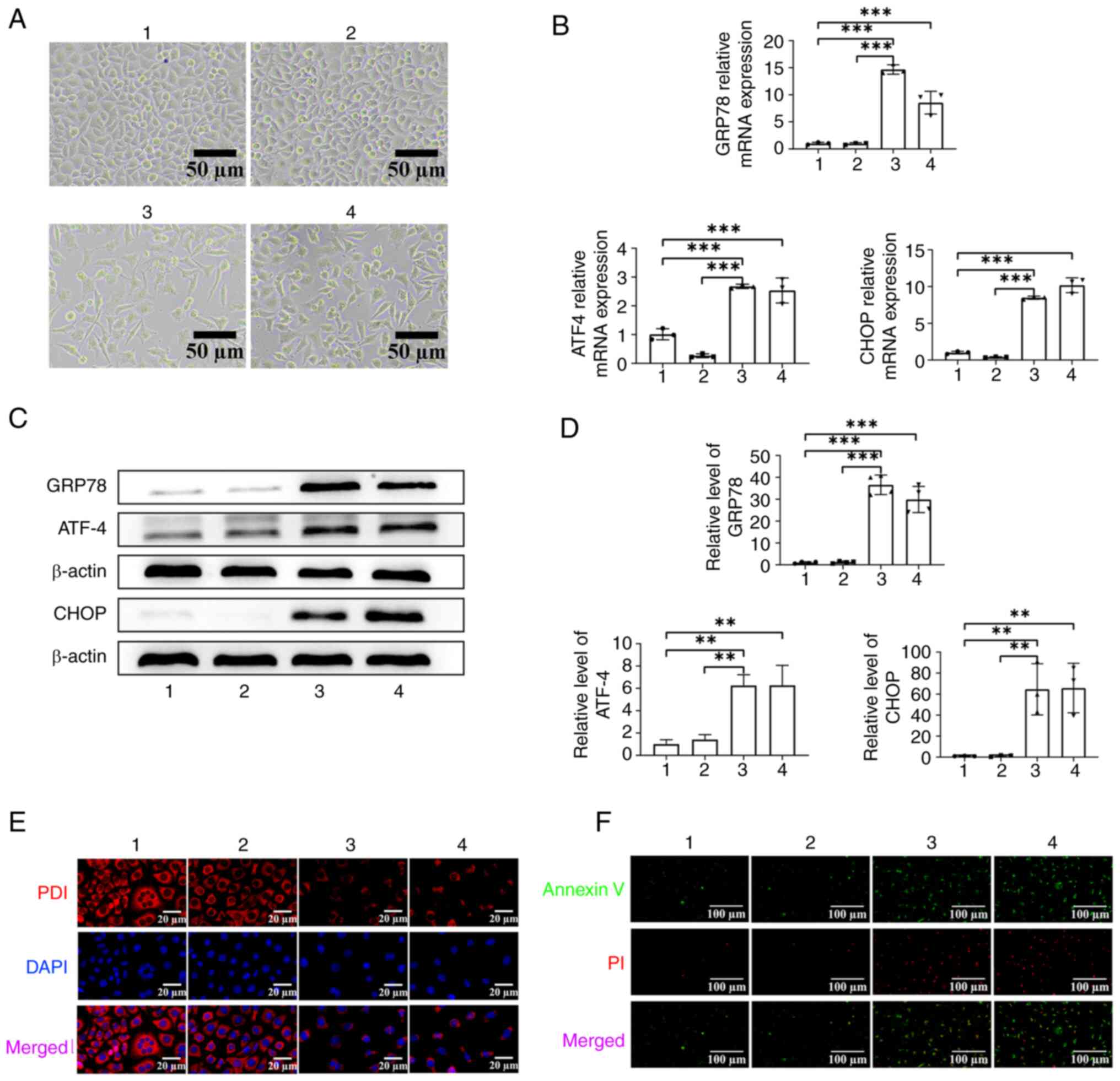 | Figure 2.Verification of propagation of ER
stress from ASCs to neurons. (A) Optical microscopy was performed
to observe the morphological changes of PC12 cells in each group,
scale bar, 50 µm. (B) Reverse transcription-quantitative PCR
analysis of ER stress-related mRNA expression in PC12 cells. (C)
Representative western blots of the ER stress-associated proteins
in PC12 cells in each group. (D) Semi-quantitative analysis of ER
stress-related proteins levels in PC12 cells in each group (one-way
ANOVA followed by Tukey's post hoc analysis; **P<0.01;
***P<0.001; n=3). (E) Immunofluorescence experiment of PDI
fluorescence intensity in PC12 cells. (F) Apoptosis probe analysis
of PC12 cell in each group, scale bar, 100 µm. 1: UT group; 2:
UT-ACM group; 3: TG-ACM group; 4: TG group. ER, endoplasmic
reticulum; ASCs, astrocytes; UT, untreated; ACM, astrocyte
conditioned medium; PDI, protein disulfide isomerase; TG,
thapsigargin; GRP78, glucose regulated protein 78; ATF4, activating
transcription factor 4. |
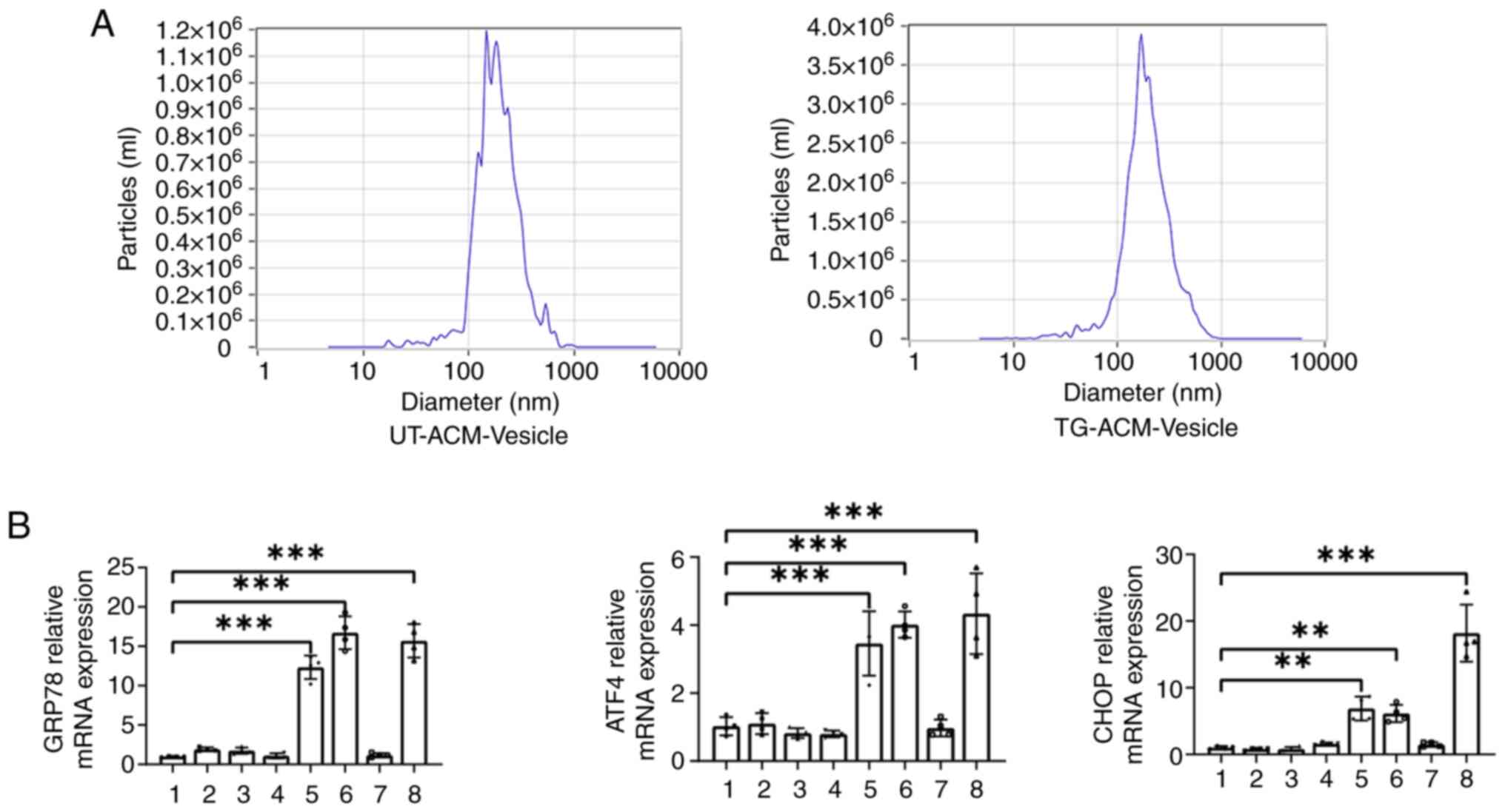
Molecular weight analysis of mediators
of ER stress propagation
As indicated in Fig.
3A, the results from the full-wavelength spectrophotometer
revealed that, when compared with the TG + DMEM group, the
absorption peaks in the supernatant of the UT-ACM group and TG-ACM
group almost completely disappeared, indicating that there was
almost no TG in the TG-ACM residue. Furthermore, ultrafiltration
was used to separate the components of the ACM-R and ACM-F
(Fig. 3B). Coomassie brilliant
blue staining experiments indicated that the molecular weight of
the filtrate was <100 kDa and the molecular weight of the filter
residue was >100 kDa (Fig. 3C).
The RT-qPCR results indicated that the mRNA expression levels of
GRP78, ATF4 and CHOP were significantly increased in the TG-ACM
group and TG-ACM-R group (all P<0.001) when compared with the UT
group (Fig. 3D).
In conclusion, the factors mediating the propagation
of endoplasmic reticulum stress are those with molecular weights
greater than 100 kDa.
Study of the vesicular/non-vesicular
properties of mediators of ER stress propagation
As shown in Fig 4A,
the nanoparticle size analyzer detected the particle size of the
supernatant vesicles, and the results revealed that the particle
sizes were all ~180 nm. The effects of supernatant
vesicle/non-vesicle components on ER stress in PC12 cells were
further examined. The RT-qPCR results indicated that the mRNA
expression levels of GRP78 ATF4 and CHOP were significantly
upregulated in the TG-ACM group and TG-ACM-(no) vesicles group,
(all P<0.01) when compared with the UT group (Fig. 4B). In conclusion, the factors
mediating the propagation of endoplasmic reticulum stress are
non-vesicular components.
Study of oxidative activity as a
mediator of ER stress propagation
As shown in Fig 5A,
observation with a light microscope revealed that compared with in
the TG-ACM group and TG group, the number of cells in the TG-ACM +
Vc group was increased and the morphology was restored. The
findings of the MTT experiment revealed that the cell viability of
the TG-ACM group was significantly decreased (P<0.001) when
compared with the UT group, whereas the cell viability of the
TG-ACM + Vc group was increased by ~10% compared with the TG-ACM
group (P<0.001) (Fig. 5B). In
addition, western blot analysis indicated that the protein
expression levels of GRP78 and ATF4 were significantly increased in
the TG-ACM group (all P<0.001) when compared with the UT group.
By contrast, the protein expression levels of GRP78 and ATF4 were
significantly decreased in the TG-ACM +Vc group (P<0.01 or
P<0.05) when compared with the TG-ACM group (Fig. 5C and D). Furthermore, the results
of PDI immunofluorescence staining indicated that the PDI
fluorescence intensity of cells in the TG-ACM + Vc group was
increased, and the punctate and granular staining areas were
reduced when compared with the TG-ACM group and TG group (Fig. 5E). Spectrophotometer results
indicated that the Vc concentration in the TG-ACM + Vc group
significantly decreased (P<0.001) when compared with the UT-ACM
+ Vc group (Fig. 5F and G). In
conclusion, the factor mediating the propagation of endoplasmic
reticulum stress is oxidative substances.
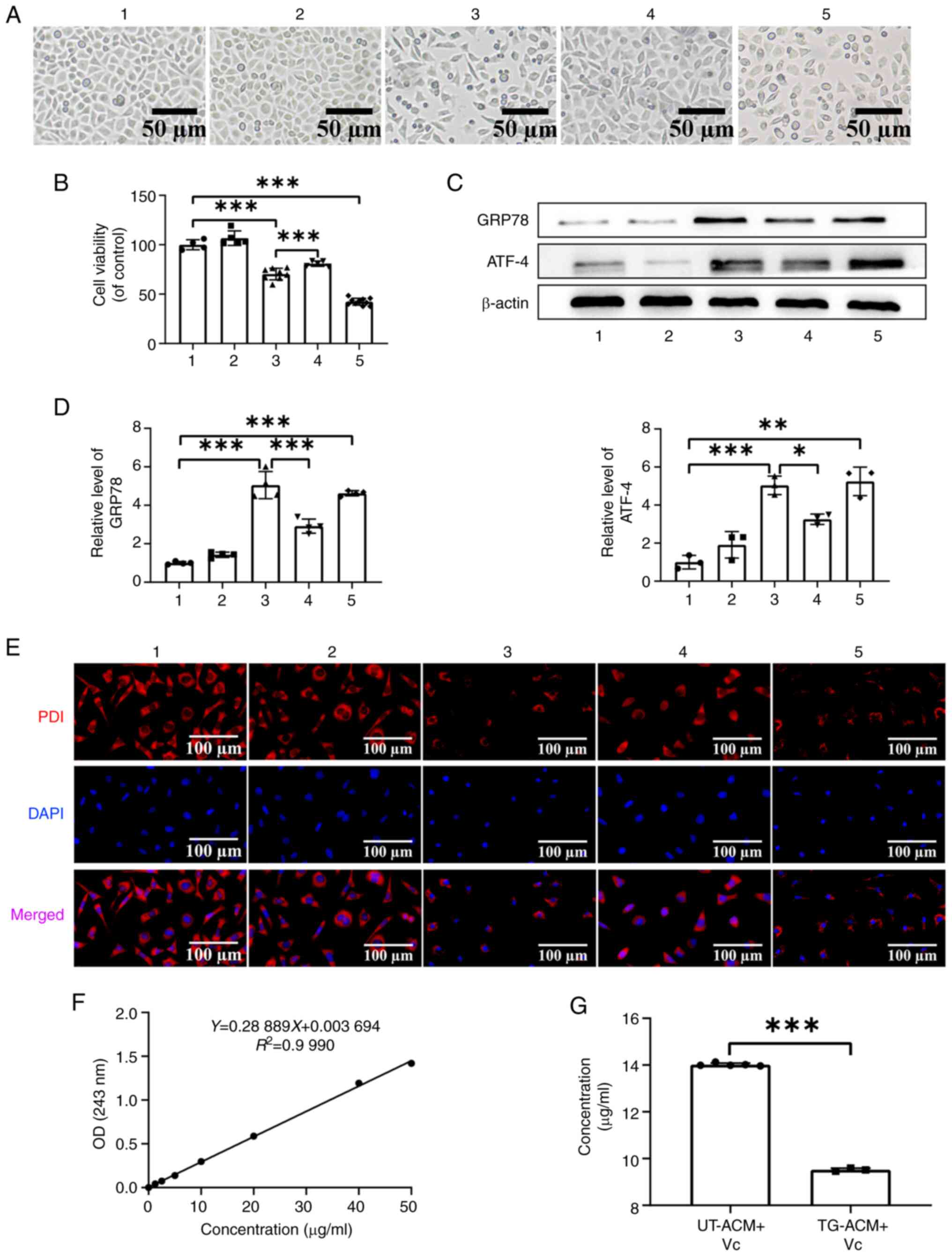 | Figure 5.Effect of supplementing Vc in the ACM
on ER stress propagation. (A) Morphological changes of PC12 cells
in each group observed under a light microscope; scale bar, 50 µm.
(B) MTT assay was performed to detect changes in PC12 cell
viability in each group (one-way ANOVA followed by Tukey's post hoc
analysis; n=5). (C) Representative western blots of ER
stress-related proteins in PC12 cells in each group. (D)
Semi-quantitative analysis of ER stress-related protein levels in
PC12 cells in each group (one-way ANOVA followed by Tukey's
multiple comparison; n=3). (E) Immunofluorescence experiment to
detect PDI fluorescence intensity of PC12 cells; scale bar, 100 µm.
(F) Full-wavelength spectrophotometry was performed to draw the Vc
standard curve. (G) Vc residue in the supernatant of each group was
measured using a spectrophotometer. *P<0.05, **P<0.01 and
***P<0.001. 1: UT group; 2: UT-ACM group; 3: TG-ACM group; 4:
TG-ACM + Vc group; 5: TG group. ER, endoplasmic reticulum; PDI,
protein disulfide isomerase; OD, optical density; UT, untreated;
ACM, astrocyte conditioned medium; TG, thapsigargin; GRP78, glucose
regulated protein 78; ATF4, activating transcription factor 4. |
Discussion
Cellular damage secondary to ER stress has been
identified as a central factor in the pathophysiology of various
diseases (23,24). Research has revealed that ER stress
may propagate between different cells and aggravate disease
progression. For example, ER stress can be propagated from cancer
cells to myeloid cells to promote tumor progression, or from
cardiomyocytes to macrophages to promote inflammatory infiltration
(11,13,25).
However, the cause and mechanism of ER stress propagation between
CNS cells remains unclear. Therefore, ER stress propagation between
CNS cells was the focus of the present study.
ASCs, which are glial cells found in the CNS, have
the largest volume and quantity among all regulating glial cells.
ASCs are one of the first cells to detect stress and transmit this
information to other cells (26,27).
Under normal and pathological conditions, ASCs, the resident cells
of the CNS, secrete membrane-bound nanoparticles known as vesicles
(28). A study evaluating prion
diseases revealed that abnormally folded prion aggregates could
bind to neuronal exocysts, and infect both neuronal and
non-neuronal cells without direct cell-to-cell contact (29). It may therefore be hypothesized
that the factors secreted from ER-stressed cells are extracellular
vesicles containing heterogeneous substances, such as RNA, proteins
or lipids, that induce ER stress in normal cells. However, the
findings of the present study demonstrated that vesicles secreted
by ER-stressed ASCs did not upregulate UPR markers in recipient
ASCs. Furthermore a previous study reported that components with
molecular weight >100 kDa mediate the development of ER stress
(27). In the present study, the
supernatant was separated to obtain ACM-F and ACM-R to treat PC12
cells. It was found that ACM-R with a molecular weight greater than
100 kDa could induce ER stress in the cells, which was consistent
with the results reported in previous studies (25,30).
Studies have shown that cells under sustained stress release
oxidative components into the supernatant, which further inhibits
their own growth (31,32). The present study revealed that
adding Vc to the supernatant partially blocked the role of the
supernatant in propagating ER stress. The spectrophotometry results
demonstrated that Vc was consumed in the supernatant of the TG-ACM
+ Vc group. Therefore, it may be hypothesized that Vc interacts
with oxidative substances in the supernatant to block the
propagation of ER stress.
ER stress has been demonstrated to carry out a key
role in maintaining important biological processes within the
brain. Mild stress activates the UPR to exert protective effects on
the organism. In the case of sustained stress, continuous
activation of UPR occurs, promoting neurotoxicity and accelerating
progression of the disease (33,34).
The findings of the present study indicated that ER
stress can cause damage to neurons through certain factors and be
transmitted between cell models. To determine whether this
ultimately exacerbates the progression of neurological diseases
requires further validation is required in animal models in
subsequent studies. Additionally, studies have tested the
transmissibility of ER stress in vivo. They demonstrated
that injecting transmissible ER stress factors intraperitoneally
into normal mice can cause widespread ER stress in the liver
(13,35,36).
Therefore, identifying the factors mediating ER
stress transmission are key for the treatment of diseases. The
present study demonstrated that ER stress can propagate from ASCs
to PC12 cells. The mediators that facilitate the propagation of ER
stress may be non-vesicular, oxidative-linked molecules with
molecular weight >100 kDa. However, whether this phenomenon
exists in animals is unknown. At present, we have successfully
produced an ER stress model in rats. Future studies will observe
the interaction between ASCs and neurons in brain tissue for
further verification in in vivo experiments. As for the
factors mediating ER stress, mass spectrometry will be used for
further analysis and corresponding inhibitors will be used for
verification.
The findings of the present study revealed the
influence of extracellular signaling pathways on cells of the CNS.
Given that persistent ER stress may contribute to the pathological
processes of diseases, understanding the molecular mechanisms
underlying the extracellular functions of UPR could provide new
therapeutic opportunities for treating neurodegenerative
diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81571221), the Science and
Technology Cooperation Foundation of Health Biomed (grant no.
20200605).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YTL conceived and designed the study, conducted the
experiments, collected and analyzed the data, produced the figures
and wrote the manuscript. MAH, XRL, DDN, YZ, YQ and BC analyzed the
data and revised the manuscript. JBH conceived and designed the
present study. All authors read and approved the final version of
the manuscript. YTL and MAH confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun S, Zhao G, Jia M, Jiang Q, Li S, Wang
H, Jiang Q, Li S, Wang H, Li W, et al: Stay in touch with the
endoplasmic reticulum. Sci China Life Sci. 67:230–257. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Celik C, Lee SYT, Yap WS and Thibault G:
Endoplasmic reticulum stress and lipids in health and diseases.
Prog Lipid Res. 89:1011982023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang SX, Wang JJ, Starr CR, Lee EJ, Park
KS, Zhylkibayev A, Medina A, Lin JH and Gorbatyuk M: The
endoplasmic reticulum: Homeostasis and crosstalk in retinal health
and disease. Prog Retin Eye Res. 98:1012312024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghemrawi R and Khair M: Endoplasmic
reticulum stress and unfolded protein pesponse in neurodegenerative
diseases. Int J Mol Sci. 21:61272020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hetz C, Zhang K and Kaufman RJ:
Mechanisms, regulation and functions of the unfolded protein
response. Nat Rev Mol Cell Biol. 21:421–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ajoolabady A, Kaplowitz N, Lebeaupin C,
Kroemer G, Kaufman RJ, Malhi H and Ren J: Endoplasmic reticulum
stress in liver diseases. Hepatology. 77:619–639. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li T, Zhao H, Guo G, Xia S and Wang L:
VMP1 affects endoplasmic reticulum stress sensitivity via
differential modulation of the three unfolded protein response
arms. Cell Rep. 42:1122092023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Yuan M, Guo YS, Shen XY, Gao ZK and
Bi X: Mechanism of endoplasmic reticulum stress in cerebral
ischemia. Front Cell Neurosci. 15:7043342021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marciniak SJ, Chambers JE and Ron D:
Pharmacological targeting of endoplasmic reticulum stress in
disease. Nat Rev Drug Discov. 21:115–140. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu X, Duan T, Xiong Y, He Z, Wei W and Cao
Z: Intercellular transmission of chronic ER stress: A new mechanism
of metabolic diseases. Acta Biochim Biophys Sin (Shanghai).
53:1109–1111. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Li M, Feng S, Xu Q, Zhang X, Xiong X
and Gu L: Ferroptosis and endoplasmic reticulum stress in ischemic
stroke. Neural Regen Res. 19:611–618. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mahadevan NR, Rodvold J, Sepulveda H,
Rossi S, Drew AF and Zanetti M: Transmission of endoplasmic
reticulum stress and pro-inflammation from tumor cells to myeloid
cells. Proc Natl Acad Sci USA. 108:6561–6566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tirosh A, Tuncman G, Calay ES, Rathaus M,
Ron I, Tirosh A, Yalcin A, Lee YG, Livne R, Ron S, et al:
Intercellular transmission of hepatic ER stress in obesity disrupts
systemic metabolism. Cell Metab. 33:17162021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sims SG, Cisney RN, Lipscomb MM and Meares
GP: The role of endoplasmic reticulum stress in astrocytes. Glia.
70:5–19. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun G, Zhao Z, Lang J, Sun B and Zhao Q:
Nrf2 loss of function exacerbates endoplasmic reticulum
stress-induced apoptosis in TBI mice. Neurosci Lett.
770:1364002022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiao B, Zhang W, Zhang C, Zhang K, Cao X,
Yu S and Zhang X: Protein tyrosine phosphatase 1B contributes to
neuropathic pain by aggravating NF-ĸB and glial cells
activation-mediated neuroinflammation via promoting endoplasmic
reticulum stress. CNS Neurosci Ther. 30:e146092024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Je S, Lee Y and Yamaoka Y: Effect of
Common ER stress-inducing drugs on the growth and lipid phenotypes
of chlamydomonas and arabidopsis. Plant Cell Physiol. 64:392–404.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ni W, Zhou J, Ling Y, Lu X, Niu D, Zeng Y,
Qiu Y, Si Y, Wang J, Zhang W, et al: Neural stem cell secretome
exerts a protective effect on damaged neuron mitochondria in
Parkinson's disease model. Brain Res. 1790:1479782022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou J, Ni W, Ling Y, Lv X, Niu D, Zeng Y,
Qiu Y, Si Y, Wang Z and Hu J: Human neural stem cell secretome
inhibits lipopolysaccharide-induced neuroinflammation through
modulating microglia polarization by activating peroxisome
proliferator-activated receptor gamma. Stem Cells Dev. 31:369–382.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao J, Zhang L, Wei Y, Chen T, Ji X, Ye K,
Yu J, Tang B, Sun X and Hu J: Human hair keratins promote the
regeneration of peripheral nerves in a rat sciatic nerve crush
model. J Mater Sci Mater Med. 30:822019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Gao J, Chen T, Chen X, Ji X, Ye
K, Yu J, Tang B, Wei Y, Xu H and Hu J: Microvesicles derived from
human embryonic neural stem cells inhibit the apoptosis of HL-1
cardiomyocytes by promoting autophagy and regulating AKT and mTOR
via transporting HSP-70. Stem Cells Int. 2019:64526842019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guillen-Samander A and De Camilli P:
Endoplasmic reticulum membrane contact sites, lipid transport, and
neurodegeneration. Cold Spring Harb Perspect Biol. 15:a0412572023.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Liu Y, Zhang X, Ye Y, Xiong X,
Zhang S, Gu L, Jian Z and Wang H: Endoplasmic reticulum stress and
the unfolded protein response in cerebral ischemia/reperfusion
injury. Front Cell Neurosci. 16:8644262022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei C, Yang X, Liu N, Geng J, Tai Y, Sun
Z, Mei G, Zhou P, Peng Y, Wang C, et al: Tumor microenvironment
regulation by the endoplasmic reticulum stress transmission
mediator golgi protein 73 in mice. Hepatology. 70:851–870. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frakes AE, Metcalf MG, Tronnes SU, Bar-Ziv
R, Durieux J, Gildea HK, Kandahari N, Monshietehadi S and Dillin A:
Four glial cells regulate ER stress resistance and longevity via
neuropeptide signaling in C. elegans. Science. 367:436–440. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith HL, Freeman OJ, Butcher AJ,
Holmqvist S, Humoud I, Schatzl T, Hughes DT, Verity NC, Swinden DP,
Hayes J, et al: Astrocyte unfolded protein response induces a
specific reactivity state that causes non-cell-autonomous neuronal
degeneration. Neuron. 105:855–866. e52020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta A and Pulliam L: Exosomes as
mediators of neuroinflammation. J Neuroinflammation. 11:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nunziante M, Ackermann K, Dietrich K, Wolf
H, Gadtke L, Gilch S, Vorberg I, Groschup M and Schätzl HM:
Proteasomal dysfunction and endoplasmic reticulum stress enhance
trafficking of prion protein aggregates through the secretory
pathway and increase accumulation of pathologic prion protein. J
Biol Chem. 286:33942–33953. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sprenkle NT, Lahiri A, Simpkins JW and
Meares GP: Endoplasmic reticulum stress is transmissible in vitro
between cells of the central nervous system. J Neurochem.
148:516–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang Z, Zhang G, Huang L, Yuan Y, Wu C
and Li Y: Transmissible endoplasmic reticulum stress: A novel
perspective on tumor immunity. Front Cell Dev Biol. 8:8462020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rodvold JJ, Chiu KT, Hiramatsu N,
Nussbacher JK, Galimberti V, Mahadevan NR, Willert K, Lin JH and
Zanetti M: Intercellular transmission of the unfolded protein
response promotes survival and drug resistance in cancer cells. Sci
Signal. 10:eaah71772017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Y, Lu D, Wang M, Liu G, Feng Y, Ren
Y, Sun X, Chen Z and Wang Z: Endoplasmic reticulum stress and the
unfolded protein response: Emerging regulators in progression of
traumatic brain injury. Cell Death Dis. 15:1562024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakka VP, Prakash-Babu P and Vemuganti R:
Crosstalk between endoplasmic reticulum stress, oxidative stress,
and autophagy: Potential therapeutic targets for acute CNS
injuries. Mol Neurobiol. 53:532–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang YM and Seki E: Global spread of a
local fire: Transmission of endoplasmic reticulum stress via
connexin 43. Cell Metab. 33:229–230. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tirosh A, Tuncman G, Calay ES, Rathaus M,
Ron I, Tirosh A, Yalcin A, Lee YG, Livne R, Ron S, et al:
Intercellular transmission of hepatic ER stress in obesity disrupts
systemic metabolism. Cell Metab. 33:319–333. e62021. View Article : Google Scholar : PubMed/NCBI
|