Global statistics indicate that there were ~20
million newly diagnosed cases of cancer and ~10 million cases of
cancer-associated mortality in 2022 (1). Projections based on demographics
indicate that the yearly incidence of new cancer cases might
increase to 35 million by 2050, representing a 77% increase
compared with 2022 (1). Current
cancer treatments, including surgery, radiation and chemotherapy,
have shown promising results; however, recurrence and metastasis
due to chemotherapy resistance remain the leading causes of death
in patients (2–4). Over the last few years, global
interest in traditional Chinese medicine (TCM) as cancer treatment
has rapidly increased (5–7). TCM is favored by an increasing number
of patients due to its holistic approach and possibility for
individualized treatment, as well as fewer side effects and its
applicability to long-term treatment. TCM has become an
indispensable part of comprehensive tumor therapy, and has been
shown to aid the relief of symptoms and improve the quality of life
of patients (8). The therapeutic
effects of the active ingredients of TCM on malignant growth,
including liver, lung and breast cancer growth, have been reported,
providing new potential for cancer therapy (9–11).
Furthermore, active ingredients of natural Chinese herbal medicines
have extensive use in the treatment of cancer due to their high
efficacy, lack of resistance, low toxicity and minimal side effects
(12,13). It is anticipated that they may work
around the disadvantages of currently available anticancer
medications, and serve as adjuvants to chemotherapy by enhancing
their effects and reducing toxicity. Consequently, they are gaining
increasing attention in cancer prevention and treatment.
The present review focuses on the anticancer effects
and mechanisms of the small molecule SAA from the traditional
Chinese medicine Salvia miltiorrhiza Bunge (Salvia
miltiorrhiza), also known as Danshen.
As a widely used traditional Chinese herb, Danshen
contains various active ingredients that can be categorized into
different groups according to their chemical and structural
characteristics (14). These
components are separated into two primary categories: Lipid-soluble
tanshinones and water-soluble phenolic acids (15). Salvianolic acid and rosmarinic acid
B make up the water-soluble phenolic acids, whereas the
lipid-soluble tanshinones include tanshinone I and II,
dihydrotanshinone I and cryptotanshinone (16). The salvianolic acids in Danshen
include many compounds, namely salvianolic acids A-H, J-N, T/U and
Y (17). Among these, SAA and
salvianolic acid B are particularly regarded as having the highest
antioxidant activity (18).
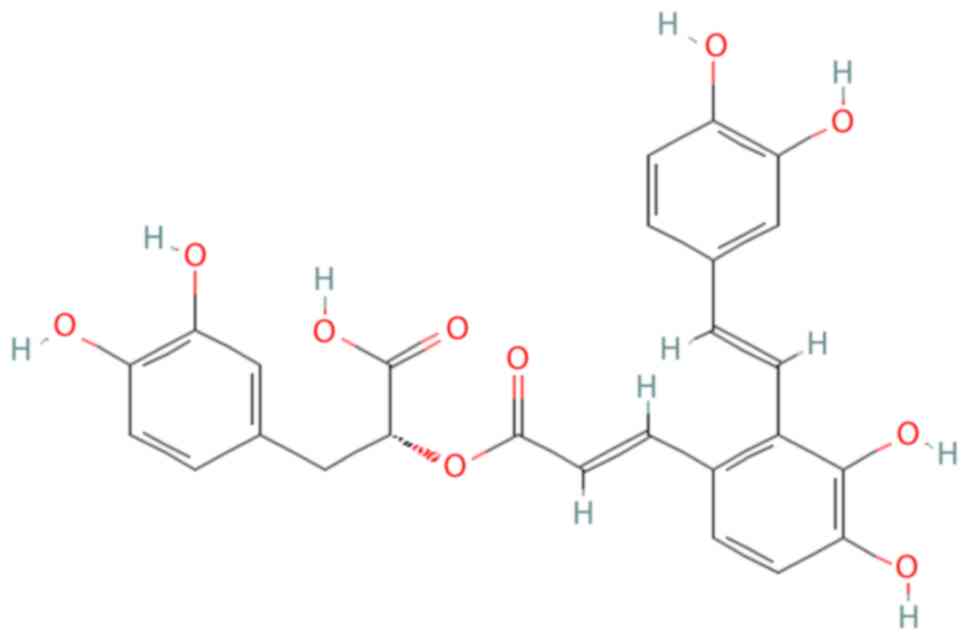
Chemically, SAA is identified as
(R)-3-(3,4-dihydroxyphenyl)-2-(((E)-3-(2-
((E)-3,4-dihydroxystyryl)-3,4-dihydroxyphenyl)acryloyl)oxy)propanoic
acid, with a molecular formula of
C26H22O10 (19,20).
Structurally, SAA is a trimer consisting of danshensu and caffeic
acid as its fundamental units. The molecular structure of SAA is
depicted in Fig. 1 (21).
The main water-soluble active ingredient in Danshen,
SAA, influences cell migration, invasion, apoptosis and
proliferation, all of which notably impact the occurrence and
development of tumors (29,30).
Unlike most chemotherapeutic drugs currently used in clinical
settings, which typically target a single pathway and are
susceptible to drug resistance (31), small-molecule components derived
from natural products often exhibit antitumor effects by modulating
multiple signaling pathways (32,33).
For example, natural compounds can influence pathways that are
critical for regulating tumor cell proliferation and growth, such
as PI3K/Akt (34,35), Wnt/β-catenin (36–38)
and NF-κB (39) pathways, thus
providing a multifaceted approach to cancer treatment (40–42).
In several tumor cells, including MCF-7 breast cancer cells, SCC-9
oral squamous cell carcinoma cells, and KG-1 and Kasumi-1 acute
myeloid leukemia cells, SAA exerts its antitumor effects by
inhibiting cell invasion, migration and proliferation, and promotes
apoptosis by targeting different signaling pathways, including the
PI3K/Akt, Raf/extracellular signal-regulated kinase (ERK) kinase
(MEK) and mammalian target of rapamycin (mTOR) pathways (43–45).
Through its electrophilic α,β-unsaturated ester moiety, SAA
functions as a covalent ligand to alter biological proteins
(46). The protein targets of SAA
have been identified using chemoproteomics and phosphoproteomics
(47). According to
chemoproteomics analysis, SAA covalently alters a minimum of 46
proteins, including the mTOR complex 1 (mTORC1) subunit Raptor to
inhibit mTORC1 (47). According to
the phosphoproteomics profile, SAA primarily interferes with the
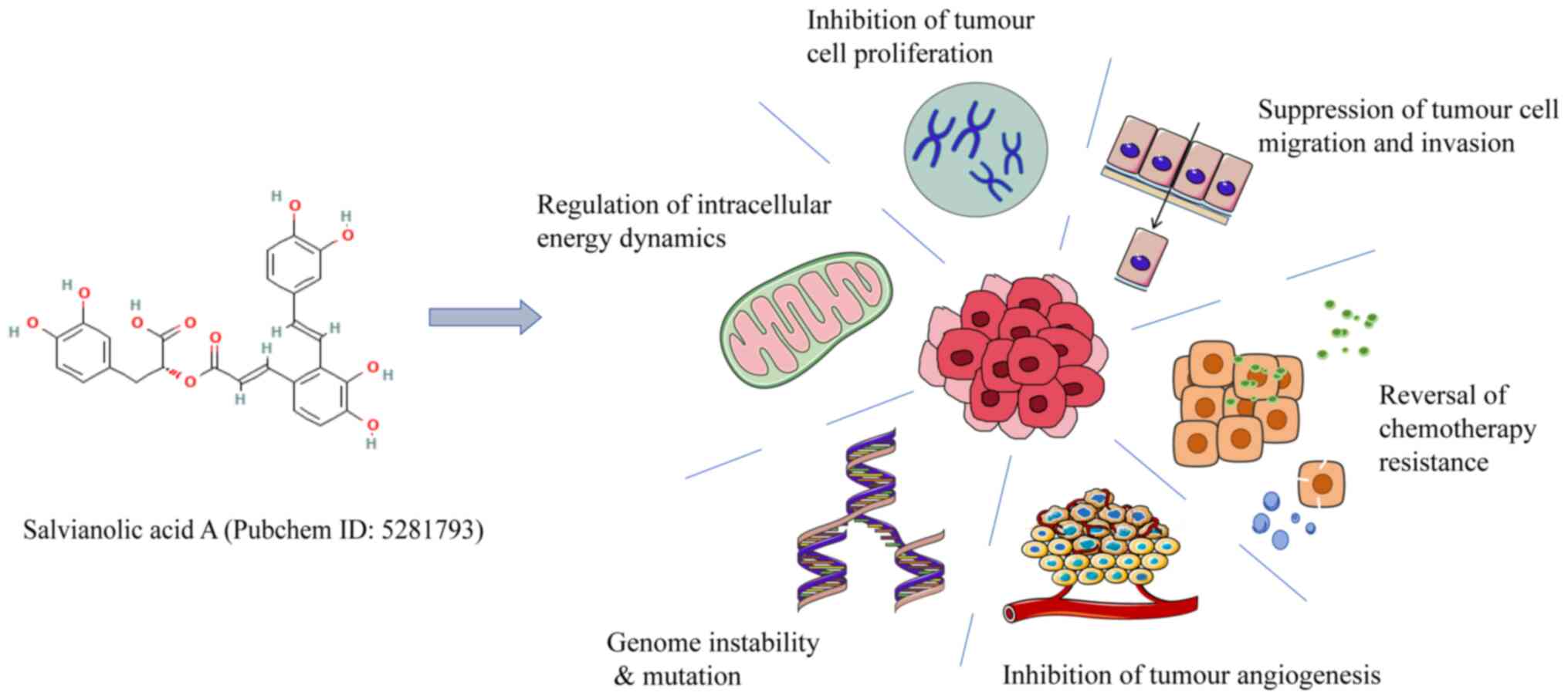
PI3K/Akt/mTOR signaling pathway (47). SAA exhibits multifaceted antitumour
properties. These include the regulation of intracellular energy
dynamics [e.g., induction of endogenous apoptosis (48) and suppression of mitochondrial
membrane potential (49)],
inhibition of tumour cell proliferation (50), suppression of tumour cell migratory
and invasive (51), enhancement of
chemotherapy sensitivity (49,52)
or reversal of chemotherapy resistance (53), inhibition of tumour angiogenesis
(54) and modulation of genomic
instability and mutations (55)
(Fig. 2).
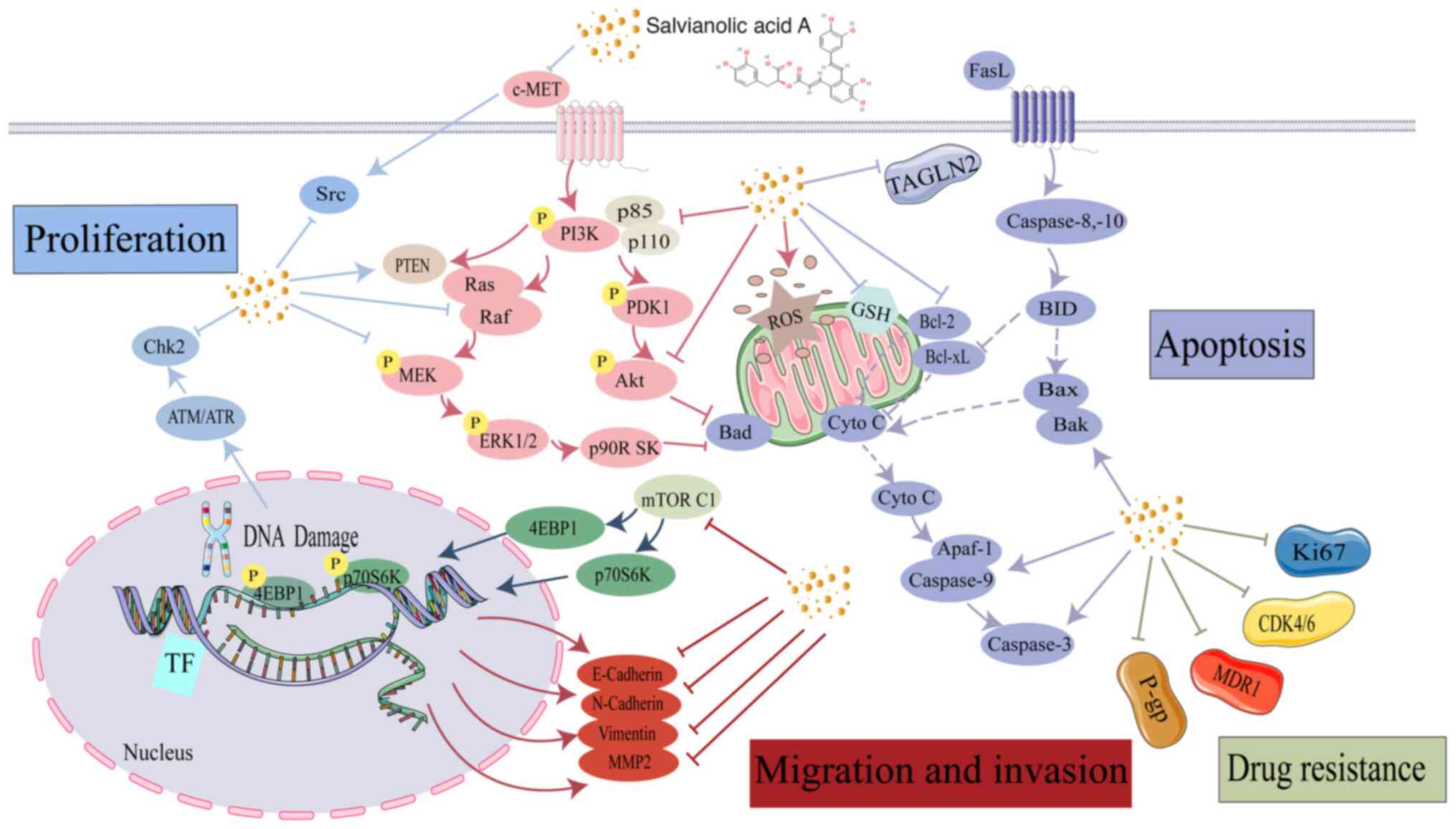
Research has indicated that the anticancer effect of
SAA is multifaceted and involves several molecular mechanisms. A
key aspect is activation of the caspase family, which is crucial
for the execution of apoptosis (48). In addition, the levels of proteins
that inhibit apoptosis have been shown to be decreased by SAA, such
as those of anti-apoptotic Bcl-2, while pro-apoptotic proteins such
as Bak and Bax are simultaneously activated (49). Furthermore, SAA may inhibit the
c-mesenchymal-to-epithelial transition factor (c-MET) and reduce
the activity of matrix metalloproteinases (MMPs), such as MMP-2 and
MMP-9, which are implicated in tumor invasion and metastasis
(50,51). In a previous study, SAA has been
shown to decrease the expression of drug-resistant proteins,
specifically P-glycoprotein and multidrug resistance-1 (MDR1)
(43). Finally, SAA has been found
to regulate critical signaling pathways, including PI3K/Akt/MAPK
and c-Raf/MEK/ERK pathways, which are essential for cell survival
and proliferation.
The process through which a cell stops growing and
dividing, and enters a phase that eventually leads to regulated
cell death, is called apoptosis or programmed cell death (56). The impairment of a series of
processes in cell apoptosis, as well as defects in death receptor
signaling, impacts the growth of tumor cells (57,58).
Notably, promoting cancer cell apoptosis is a strategy for treating
tumors, whereas inhibiting apoptosis or other cell death mechanisms
can directly impact tumor cell sensitivity to chemotherapy
(56,59).
Two mechanisms underlie apoptosis: The intrinsic
mechanism, which depends on mitochondrial components, and the
extrinsic mechanism, which is regulated by death receptors
(56,60,61).
A key event in intrinsic apoptosis is the release of cytochrome
c (Cyto C) from the mitochondria, which is regulated by the
Bcl-2 family (62). This family
consists of anti-apoptotic proteins, such as Bcl-2 and Bcl-xl, as
well as pro-apoptotic proteins, such as Bax, Bad, Bim and Bid. Bax
forms oligomers on the mitochondrial outer membrane, promoting Cyto
C release (61); this activates
the caspase family, which is split into initiators such as caspases
8, 9 and 10, and effectors such as caspases 3, 6 and 7 (63). Caspase 9 and apoptotic peptidase
activating factor 1 form apoptotic bodies, activating caspase 3 to
induce apoptosis (64). By
contrast, Bcl-2 inhibits apoptosis by sequestering Bax and
preventing Cyto C release (65–68).
After treating esophageal cancer cells with SAA, caspase 9, a
protease that causes DNA fragmentation, receives an upstream
apoptosis signal and activates downstream caspase 3, leading to
apoptosis (69). After treating
the esophageal cancer cell line KYSE-150 with SAA, while Bcl-2
expression levels were much lower, those of Bax, as well as
cleaved-caspase 9 and 3, were substantially greater (48). By upregulating Bak and
downregulating Bcl-xl, SAA therapy causes KG-1, THP-1 and Kasumi-1
acute myeloid leukemia cells to undergo apoptosis through the
intrinsic apoptotic pathway. Subsequently, poly ADP-ribose
polymerase is cleaved, which activates caspase 3 (Fig. 3) (45). In doxorubicin hydrochloride
(DOX)-resistant breast cancer cells (MCF-7/MDR), SAA causes
intrinsic apoptosis by caspase activation, Bax upregulation, Bcl-2
downregulation and a decrease in mitochondrial membrane potential
(49). Furthermore, SAA treatment
enhances Bax and decreases Bcl-2 expression in KYSE-150 esophageal
cancer cells, thereby inducing apoptosis (48). The aforementioned mechanisms likely
involve the inhibition of c-MET gene transcription and protein
expression in HepG-2 liver cancer cells, which in turn reduces the
degree of Akt phosphorylation in the downstream PI3K/Akt signaling
cascade (70) (Table I). These studies have suggested
that SAA mediates the intrinsic apoptotic pathway in various tumor
cells; however, whether SAA induces apoptosis via an extrinsic
pathway has not yet been reported, to the best of our
knowledge.
Tumor cells exhibit the hallmark characteristic of
unlimited proliferation. Capitalizing on this advantage, they not
only evade growth-inhibitory signals but also resist cell death,
often through the loss of tumor suppressor gene function (71). Phosphatase and tensin homolog
(PTEN) is a widely studied gene that suppresses tumor growth and
encodes a phosphatase that dephosphorylates phosphatidylinositol
(3,4,5)-trisphosphate, leading to the
inactivation of downstream effectors, particularly Akt (72). Essential for regulating various
biological functions, the PI3K/Akt signaling pathway is
particularly important for cell cycle progression, cell
proliferation and apoptosis prevention. This signaling cascade is
frequently found to be disrupted in cancer (73–75).
By reducing the expression of PI3K and Akt, cell cycle arrest and
effective inhibition of tumor cell growth can be accomplished
(76). Treatment of A549 lung
cancer cells with different concentrations of SAA (10, 20 and 30
µg/ml) for different durations (6, 12 and 24 h) has been shown to
significantly increase PTEN protein expression levels and
consistently reduce AKTS473 phosphorylation (Fig. 3). These findings indicate how
suppressing tumor cell proliferation and encouraging apoptosis have
concentration- and time-dependent effects (77).
Endothelin-1, a small vasoactive peptide that is
upregulated in the plasma and tissue specimens of various patients
with solid tumors, can activate endothelin A receptor (ETAR) and
endothelin B receptor through autocrine and paracrine interaction,
thereby regulating the proliferation of tumor cells (78). SAA, an ETAR antagonist,
significantly inhibits spontaneous tumor cell proliferation and
exhibits concentration-dependent effects (79). Furthermore, SAA and oxymatrine
together have been reported to exert a synergistic impact on
immortalized H8 cervical epithelial cells in a study of
precancerous lesions in cervical cancer (80). This combination effectively halts
the cell cycle in the G2/M phase in H8 cells, thereby
suppressing proliferation (80).
In vitro experiments have revealed that SAA suppresses
diffuse large B-cell lymphoma (DLBCL) proliferation though
regulating the MAPK signaling pathway; specifically, SAA
upregulates c-Jun N-terminal kinase, and downregulates
phosphorylated (p-)ERK and p-p38 MAPK (81). In vivo studies employing
xenograft mouse models have supported these findings, further
confirming that SAA can suppress the tumor growth of DLBCL OCI-Ly01
and SUDHL-8 cells (81). In
addition, the intermediate metabolite of SAA, S-3-1, inhibits the
spread of various types of cancer, including small cell lung
cancer, lung adenocarcinoma, oral squamous cell carcinoma, colon
cancer, gastric cancer and pancreatic cancer (82). The cell cycle is arrested at the
G2/M phase by SAA, which can also dose-dependently
reduce the proliferation of the human melanoma A2058 and A375 cell
lines. Notably, SAA causes checkpoint kinase (Chk)-2
phosphorylation to be selectively induced without impacting Chk-1;
this results in the degradation of the genes cell division cycle
(CDC)25A and CDC2, which are controlled by Chk-2, but has no effect
on the Chk1-CDC25C axis (29).
One major factor in cancer-associated mortality is
tumor metastasis, which is a complex and multidimensional process
(83). This progression is marked
by genetically unstable cancer cells that adapt to and thrive
within tissue microenvironments distant from the primary tumor site
(84). When cells with the ability
to metastasize invade new tissues, they undergo a selection process
that favors traits for survival and proliferation (85). Concurrently, recruitment of a
distinctive tumor stroma occurs, which serves a vital role in
facilitating the invasion and establishment of metastatic cells in
distant organs (86). A mechanism
known as epithelial-mesenchymal transition (EMT) causes epithelial
cancer cells to change from their standard epithelial phenotype to
a mesenchymal phenotype. Cancer cells that undergo EMT exhibit a
greater ability to migrate and invade, making them more likely to
metastasize (87). EMT is a
pivotal step towards invasiveness, orchestrated by many cell
adhesion molecules and intermediate filaments, including
E-cadherin, N-cadherin and vimentin (88–90).
MMPs are responsible for degrading the components of
the basement membrane and the extracellular matrix (91). Through interactions with a series
of cell adhesion molecules, they participate in altering the
adhesive properties between tumor cells and their environment,
facilitating the movement of tumor cells through the extracellular
matrix (92). Therefore, MMPs such
as MMP-2 and MMP-9 are required for cancer cell metastasis
(93–95). Targeting these crucial mediators of
metastasis can aid in inhibiting cancer cell migration and
invasion. SAA has been reported to reduce MMP-2 expression in SCC-9
oral squamous cell carcinoma cells through the Raf/MEK/ERK pathway
to regulate cell migration (44).
Similarly, SAA treatment can inhibit MMP-2 protein expression and
activity in HONE-1 and NPC-39 nasopharyngeal carcinoma cells in a
concentration-dependent manner, preventing migration and invasion
(96). SAA also inhibits MCF-7
cell migration and invasion by preventing EMT, leading to higher
E-cadherin expression, and reduced vimentin and N-cadherin levels
(30). Previous studies have
indicated that SAA inhibits cancer metastasis by regulating MMP-2
levels and important EMT markers, such as E-cadherin, vimentin and
N-cadherin; however, further research is required to ascertain
whether SAA can also impede tumor migration and invasion through
the modulation of additional EMT markers, such as zinc finger e-box
binding homeobox 1, snail family transcriptional repressor (Snail)
1, Snail 2, twist family bHLH transcription factor 1 and
fibronectin.
Resistance to chemotherapeutic drugs poses a notable
challenge for cancer therapy (97); therefore, it is essential to
overcome tumor resistance and enhance tumor cell sensitivity to
these drugs (98). c-MET is a
receptor tyrosine kinase that becomes activated upon binding to its
ligand, hepatocyte growth factor (HGF) (48). The HGF/c-MET axis is frequently
abnormally activated in gastric (99), lung (100) and pancreatic (101) cancer. This abnormal activation is
often associated with c-MET gene mutations, overexpression of the
c-MET protein and gene amplification (102). The activation of several
downstream signaling pathways, including PI3K/Akt, Ras/MAPK,
JAK/STAT, SRC and Wnt/β-catenin, can drive tumor progression and
enhance the resistance of tumor cells to therapeutic agents
(103–105). Thus, c-MET could potentially
serve as a crucial target for overcoming tumor cell resistance.
Drugs or small molecules that target c-MET can help overcome
chemotherapy resistance in tumor cells (106).
SAA is capable of partially reversing cisplatin
resistance in A549/DDP lung cancer cells by blocking the
c-MET/Akt/mTOR signaling axis (50). SAA has been shown to downregulate
MDR1 in A549 cells, while simultaneously upregulating the
expression of microRNAs (miRNAs) associated with this gene. This
dual action suggests that SAA may influence MDR1 expression by
regulating tumor cell miRNAs. Consequently, this regulatory
mechanism could potentially lead to the reversal of multidrug
resistance in A549 cells (107).
Furthermore, SAA may reverse drug resistance in lung cancer cells
through regulation of the PI3K/Akt signaling pathway (108). In MCF-7 breast cancer cells, SAA
decreases transgelin 2 (TAGLN2) and Bcl-2 levels, and increases
levels of PTEN, Bax, cleaved-PAPR, cleaved-caspase 3 and
cleaved-caspase 9. SAA enhances MCF-7 breast cancer cell
sensitivity to paclitaxel by inhibiting the PI3K/Akt signaling
pathway and activating the mitochondrial apoptosis pathway
(43,49). Furthermore, SAA can suppress the
migration and invasion of paclitaxel-resistant MCF-7 breast cancer
cells by downregulating TAGLN2 expression, thereby reversing
paclitaxel resistance in these cells (30). Furthermore, by blocking the
TAGLN2/PI3K/Akt signaling pathway, SAA can inhibit the malignant
growth of U87 glioma cells and increase their susceptibility to
temozolomide; therefore, SAA could potentially serve as an
adjunctive medication in clinical chemotherapy for glioma cells
(109). In adriamycin-resistant
MCF-7 breast cancer cells, protein arginine methyltransferase 1
(PRMT1) potentially contributes to the upregulation of MDR1, which
is activated by the pregnane X receptor (PXR). In addition, a PRMT
inhibitor may suppress MDR1 by interfering with the PRMT1 and PXR
interaction. Notably, SAA can act as a PRMT1 inhibitor to increase
the anticancer effects of DOX by increasing the sensitivity of
drug-resistant breast cancer cells to it (52). A previous study indicated that,
compared with the use of DOX alone, SAA alone or the SAA/DOX
combination, E-[c(RGDfK)2]/folic acid (FA) co-modification of
nanostructured lipid carriers (NLC) in conjunction with SAA and DOX
exhibit the most effective antitumor activity. These findings
suggested that SAA and DOX may have a synergistic effect in breast
cancer treatment (53).
Furthermore, the renal toxicity levels of
E-[c(RGDfK)2]/FA-NLC-SAA/DOX were revealed to be low, with no
abnormalities observed in the ex vivo kidney pathology
examination at the end of the study. In addition, SAA is capable of
antagonizing the nephrotoxic effects of DOX, highlighting its
significant potential in enhancing the tolerance of cancer patients
to chemotherapy (110).
Angiogenesis is the process through which
endothelial cells migrate and proliferate within preexisting small
blood vessels, or venules, to produce new blood vessels, mostly
capillaries (111). For tumors,
this mechanism is essential, as it provides the oxygen and
nutrients needed for development and spread (112). Furthermore, the blood circulation
makes it easier for tumor cells to spread to different areas of the
body (113). As a result, most
cancers promote tumor angiogenesis, the process through which new
capillaries grow in the surrounding tissue. Notably, not all
cancers depend on the development of new blood vessels, as some can
endure and proliferate by altering their metabolism (114,115). Therefore, inhibiting capillary
formation during tumor treatment can indirectly block the
development and metastasis of tumor cells (116). When it comes to inhibiting
tumor-secreted glucose-regulated protein 78 (GRP78) in DLD1 and
HCT-116 colon cancer cells, SAA has been shown to be the most
successful among Diosmin, Quertruicin, Vitexin, Baicain, SAA,
Isovitexin, Narigin and Platycodin D; SAA can encourage the
breakdown of GRP78 through the lysosomal pathway and prevent its
release into the tumor microenvironment (54). Furthermore, SAA has been shown to
specifically interact with GRP78 and inhibit tumor angiogenesis
(54). DOX, as one of the
anthracycline antibiotics, is extensively used in cancer therapy
(117). In a mouse lung cancer
model, it has been demonstrated that SAA is able to normalize the
tumor vasculature by targeting PKM2. This focused strategy affects
alterations to the shape and function of the tumor vasculature,
enabling the effective delivery of DOX and thereby enhancing its
therapeutic efficacy. Concurrently, SAA is capable of reducing the
cardiotoxicity caused by DOX (118).
The expression levels of two well-known hypoxia
indicators, hypoxia-inducible factor 1α and carbonic anhydrase IX
(121,122), have been shown to be
significantly reduced in mouse Lewis lung carcinoma cells LLC and
mouse melanoma high metastatic cells B16F10 following SAA
treatment. These findings suggested that SAA may have a marked
impact in lowering tumor hypoxia (118). Furthermore, the lactate levels in
LLC and B16F10 tumors from an SAA-treated group have been shown to
be significantly reduced compared with those in a vehicle control
group (118). These findings
collectively suggested that SAA could be essential in reducing
tumor growth by improving the hypoxic microenvironment (118). Through a caveolae-dependent
mechanism, SAA has been reported to increase the transcellular
permeability of the tumor blood-brain barrier while decreasing its
paracellular permeability; this can enhance the ability of DOX to
treat HCMEC/D3 immortalized human cerebral microvascular
endothelial cells and make it easier for the drug to enter brain
tumor tissues. Thus, SAA and DOX together might provide a new
method of treating glioma cells (123). In conclusion, SAA has shown
considerable promise as a drug monomer. Preclinical and
experimental studies have demonstrated its potential to enhance the
efficacy of antitumor drugs, providing new possibilities for cancer
treatment. The unique mechanism of action of SAA makes it a strong
candidate as an effective adjunctive antitumor agent, offering new
perspectives for future therapeutic strategies.
Chinese medicine has garnered notable attention from
experts and scholars, both domestically and internationally, due to
its distinctive characteristics and advantages in treating tumors.
Notably, it operates through multiple targets, effects and
pathways, contributing to its precise efficacy. Chinese medicine is
also widely available and well known for having few adverse effects
and low toxicity, which makes it a good substitute in oncology.
Research on drug delivery carriers has advanced,
particularly in the domain of specialized delivery systems. These
systems have attracted considerable attention due to their enhanced
in vivo stability, improved bioavailability, increased drug
efficacy, enhanced tissue targeting, reduced off-target adverse
reactions and ability to co-deliver drugs. For example, research
has shown that specific ligands, such as E-[c(RGDfK)2]/FA, can
effectively deliver drugs to target tissues, thereby enhancing the
therapeutic effects of the drugs (110).
In the field of TCM, as research into active
ingredients develops, it has been shown that terpenoids, alkaloids,
flavonoids, quinones, polysaccharides and other active ingredients
of TCM not only possess notable pharmacological activity but also
show potential as drug delivery nanocarriers (124). SAA, as a type of salvianolic
acid, is also seen as a promising candidate in drug delivery
systems; however, the extraction methods for SAA have certain
shortcomings (125). By
developing new extraction technologies and optimizing extraction
conditions, its extraction efficiency and safety can be improved.
In practice, the use of nanoparticles for targeted medication
delivery can minimize side effects, improve therapeutic benefits
and decrease toxicity, in addition to increasing the effectiveness
of SAA.
Notably, E-[c(RGDfK)2]/FA-NLC-SAA/DOX exhibits low
renal toxicity, as no abnormalities have been found in ex
vivo kidney pathology examinations. SAA is also capable of
antagonizing the nephrotoxic effects of DOX, further demonstrating
its low toxicity and lack of side effects (107). The pharmacological mechanisms of
SAA must be thoroughly studied to support widespread therapeutic
application. Currently, most studies on the use of SAA for tumor
treatment are in the preliminary stages, and many of the underlying
mechanisms of action have yet to be fully elucidated. A
comprehensive awareness of the antitumor mechanisms of SAA is
crucial for advancing TCM and improving tumor treatment
strategies.
Clinical trials of SAA have shown promise in the
fields of diabetic microangiopathy (CTR20210544) and acute
myocardial infarction (CTR20213269) (126). The clinical trial for diabetic
microangiopathy has progressed to phase III. The results of the
phase II trial (CTR20210544) have indicated that SAA tablets are
effective in alleviating the symptoms of diabetic peripheral
neuropathy, such as limb numbness and tingling, with good safety.
However, the current trials are subject to certain limitations,
such as strict inclusion and exclusion criteria (e.g., excluding
patients with severe cardiovascular and cerebrovascular diseases,
or abnormal liver and kidney function), which may restrict its
application in a broader population. In the field of acute
myocardial infarction, the phase II clinical trial (CTR20213269) of
Salvianolic acid A sodium salt monohydrate for injection is
ongoing, aiming to assess its efficacy and safety in myocardial
protection after percutaneous coronary intervention. The trial,
which follows a randomized, double-blind, placebo-controlled and
multicenter design, has shown that SAA sodium has potential
advantages in suppressing inflammatory responses and reducing
myocardial injury. However, the trial also had strict participant
selection criteria, such as excluding patients with severe liver
and kidney dysfunction, a history of malignant tumors or allergies
to other drugs, which may affect its clinical promotion. Future
research should focus on expanding the sample size, extending the
trial duration and further exploring the mechanism of action of SAA
to better advance its clinical application.
Not applicable.
The present study was funded by the Sichuan Science and
Technology Program (grant nos. 2022YFS0623, 2024YFFK0346 and
2024NSFSC0561), the Sichuan Science and Technology Program Joint
Innovation Grant (grant no. 2022YFS0623-B3), the Southwest Medical
University Grant (grant no. 2021ZKZD005), the Sichuan Science and
Technology Grant (grant no. 2020YFH0121), and the Southwest Medical
University College Student Innovation and Entrepreneurship Training
Program (grant no. 2024314).
Not applicable.
CXL and WLY conceptualized the review and wrote the
original draft. CXL, QX, STJ, DL, CT and WLY reviewed and edited
the review, and were involved in visualization. WLY was responsible
for supervision, project administration and funding acquisition.
Data authentication is not applicable. All authors have read and
approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Organization GWH, . Global Breast Cancer
Initiative Implementation Framework: Assessing, strengthening and
scaling-up of services for the early detection and management of
breast cancer. CC BY-NC-SA 3.0 IGO. 2023.
|
|
3
|
Xin J, Song M, Liu X, Zou H, Wang J, Xiao
L, Jia Y, Zhang G, Jiang W, Lei M, et al: A new strategy of using
low-dose caffeic acid carbon nanodots for high resistance to poorly
differentiated human papillary thyroid cancer. J Nanobiotechnology.
22:5712024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diefenhardt M, Martin D, Hofheinz RD,
Ghadimi M, Fokas E, Rödel C and Fleischmann M: Persistent lymph
node metastases after neoadjuvant chemoradiotherapy for rectal
cancer. JAMA Netw Open. 7:e24329272024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo J, Chen X, Wu M, Wang D, Zhao Y, Li Q,
Tang G, Che F, Xia Z, Liang Z, et al: Traditional Chinese medicine
FYTF-919 (Zhongfeng Xingnao oral prescription) for the treatment of
acute intracerebral haemorrhage: A multicentre, randomised,
placebo-controlled, double-blind, clinical trial. Lancet.
404:2187–2196. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang W, Wang J, Kuang M, Xiao Z, Fan B,
Sun G and Tan Z: Exploring global research status and trends in
anti-obesity effects of traditional Chinese medicine through
intestinal microbiota: A bibliometric study. Front Cell Infect
Microbiol. 13:12714732023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan Y, Liu J, Miao J, Zhang X, Yan Y, Bai
L, Chang J, Wang Y, Wang L, Bian Y and Zhou H: Anti-inflammatory
activity of the Tongmai Yangxin pill in the treatment of coronary
heart disease is associated with estrogen receptor and NF-κB
signaling pathway. J Ethnopharmacol. 276:1141062021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Fang C, Luo J, Gong C, Wang L and
Zhu S: Traditional Chinese medicine for cancer treatment. Am J Chin
Med. 52:583–604. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Wang S, Wang N, Zheng Y, Yang B,
Wang X, Zhang J, Pan B and Wang Z: Aiduqing formula inhibits breast
cancer metastasis by suppressing TAM/CXCL1-induced Treg
differentiation and infiltration. Cell Commun Signal. 19:892021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan X, Yao C, Fang C, Han M, Gong C, Hu D,
Shen W, Wang L, Li S and Zhu S: Rocaglamide promotes the
infiltration and antitumor immunity of NK cells by activating
cGAS-STING signaling in non-small cell lung cancer. Int J Biol Sci.
18:585–598. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Man S, Liu W, Bi J, Bai J, Wu Q, Hu B, Hu
J and Ma L: Smart mesoporous silica nanoparticles loading curcumin
inhibit liver cancer. J Agric Food Chem. 72:25743–25754. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Islam MS, Wang C, Zheng J, Paudyal N, Zhu
Y and Sun H: The potential role of tubeimosides in cancer
prevention and treatment. Eur J Med Chem. 162:109–121. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J, Wang J, Wan Y, Wang S and Jiang C:
Probiotic-fermented traditional Chinese herbal medicine, a
promising approach to maintaining the intestinal microecology. J
Ethnopharmacol. 337:1188152024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia Y, Yao D, Bi H, Duan J, Liang W, Jing
Z and Liu M: Salvia miltiorrhiza Bunge (Danshen) based
nano-delivery systems for anticancer therapeutics. Phytomedicine.
128:1555212024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang J, Zhang J, Sun C, Yang R, Sheng M,
Hu J, Kai G and Han B: Adjuvant role of Salvia miltiorrhiza
bunge in cancer chemotherapy: A review of its bioactive components,
health-promotion effect and mechanisms. J Ethnopharmacol.
318:1170222024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shan XX, Hong BZ, Liu J, Wang GK, Chen WD,
Yu NJ, Peng DY, Wang L and Zhang CY: Review of chemical
composition, pharmacological effects, and clinical application of
Salviae Miltiorrhizae Radix et Rhizoma and prediction of its
Q-markers. Zhongguo Zhong Yao Za Zhi. 46:5496–5511. 2021.(In
Chinese). PubMed/NCBI
|
|
18
|
Zhang J, Jin Q, Deng Y, Hou J, Wu W and
Guo D: New depsides from the roots of Salvia miltiorrhiza
and their radical-scavenging capacity and protective effects
against H2O2-induced H9c2 cells. Fitoterapia. 121:46–52. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Center for Biotechnology
Information, . ‘PubChem Compound Summary for CID 5281793.
Salvianolic acid A’ PubChem; https://pubchem.ncbi.nlm.nih.gov/compound/Salvianolic-acid-A2–January.
2024
|
|
20
|
Zhao H, Han B, Li X, Sun C, Zhai Y, Li M,
Jiang M, Zhang W, Liang Y and Kai G: Salvia miltiorrhiza in
breast cancer treatment: A review of its phytochemistry,
derivatives, nanoparticles, and potential mechanisms. Front
Pharmacol. 13:8720852022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim S, Chen J, Cheng T, Gindulyte A, He J,
He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, et al: PubChem 2023
update. Nucleic Acids Res. 51:D1373–D1380. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diao HY, Zhu W, Liu J, Yin S, Wang JH and
Li CL: Salvianolic acid a improves rat kidney injury by regulating
MAPKs and TGF-β1/Smads signaling pathways. Molecules. 28:36302023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang HF, Wang YL, Gao C, Gu YT, Huang J,
Wang JH, Wang JH and Zhang Z: Salvianolic acid A attenuates kidney
injury and inflammation by inhibiting NF-κB and p38 MAPK signaling
pathways in 5/6 nephrectomized rats. Acta Pharmacol Sin.
39:1855–1864. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Z, Li D, Wang T, Li Y, Qin P, Zhu H,
Zhang M, Li W, Yu L, Duan H, et al: Salvianolic acid A inhibits
pseudorabies virus infection by directly inactivating the virus
particle. Phytomedicine. 134:1560152024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meirelles LEF, Souza MVF, Carobeli LR,
Morelli F, Mari NL, Damke E, Shinobu Mesquita CS, Teixeira JJV,
Consolaro MEL and Silva VRSD: Combination of conventional drugs
with biocompounds derived from cinnamic acid: A promising option
for breast cancer therapy. Biomedicines. 11:2752023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang H, Wang S, Liu Y, Zheng C, Chen L,
Zheng K, Xu Z, Dai Y, Jin H, Cheng Z, et al: Targeting EFNA1
suppresses tumor progression via the cMYC-modulated cell cycle and
autophagy in esophageal squamous cell carcinoma. Discov Oncol.
14:642023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang LL, Li DY, Zhang YB, Zhu MY, Chen D
and Xu TD: Salvianolic acid A inhibits angiotensin II-induced
proliferation of human umbilical vein endothelial cells by
attenuating the production of ROS. Acta Pharmacol Sin. 33:41–48.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong W, Sun B, Gao W, Qin Y, Zhang H,
Huai L, Tang Y, Liang Y, He L, Zhang X, et al: Salvianolic acid A
targeting the transgelin-actin complex to enhance vasoconstriction.
EBioMedicine. 37:246–258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pu XY, Mei Y, Zheng Q and Ko CY:
Inhibition of melanoma cell growth by salvianolic acid A through
CHK2-CDC25A pathway modulation. Front Biosci (Landmark Ed).
29:2132024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng X, Chen S, Yang Q, Cai J, Zhang W,
You H, Xing J and Dong Y: Salvianolic acid A reverses the
paclitaxel resistance and inhibits the migration and invasion
abilities of human breast cancer cells by inactivating transgelin
2. Cancer Biol Ther. 16:1407–1414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin X, Guo J, Li H, He H, Cai F, Chen X,
Chen M, Chen T and Ma L: Selenium electrophilic center responsive
to biological electron donors for efficient chemotherapy. Adv Sci
(Weinh). e24120622025. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao M, Jiang X, Fang J, Lin Y, Li Y, Pei
R, Ye P, Lu Y and Jiang L: The kava chalcone flavokawain B exerts
inhibitory activity and synergizes with BCL-2 inhibition in
malignant B-cell lymphoma. Phytomedicine. 120:1550742023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hseu YC, Huang YC, Thiyagarajan V, Mathew
DC, Lin KY, Chen SC, Liu JY, Hsu LS, Li ML and Yang HL: Anticancer
activities of chalcone flavokawain B from Alpinia pricei Hayata in
human lung adenocarcinoma (A549) cells via induction of reactive
oxygen species-mediated apoptotic and autophagic cell death. J Cell
Physiol. 234:17514–17526. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang W, Dong J, Xu J, Qian Y, Chen D, Fan
Z, Yang H, Xiang J, Xue X, Luo X, et al: Columbianadin suppresses
glioblastoma progression by inhibiting the PI3K-Akt signaling
pathway. Biochem Pharmacol. 223:1161122024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang S, Wang P, Sun X, Zhang M, Zhang S,
Cao Y, Wang Y, Liu L and Gao X: Mechanistic study of leukopenia
treatment by Qijiao shengbai Capsule via the Bcl2/Bax/CASAPSE3
pathway. Front Pharmacol. 15:14515532024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ye C, Yao Z, Wang Y and Zhang C:
Asiaticoside promoted ferroptosis and suppressed immune escape in
gastric cancer cells by downregulating the Wnt/β-catenin pathway.
Int Immunopharmacol. 134:1121752024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shin N, Lee HJ, Sim DY, Ahn CH, Park SY,
Koh W, Koh J, Koh BS, Koh B and Koh SH: Anti-warburg mechanism of
ginsenoside F2 in human cervical cancer cells via activation of
miR193a-5p and inhibition of β-Catenin/c-Myc/hexokinase 2 signaling
axis. Int J Mol Sci. 25:94182024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nilkhet S, Vongthip W, Lertpatipanpong P,
Prasansuklab A, Tencomnao T, Chuchawankul S and Baek SJ: Ergosterol
inhibits the proliferation of breast cancer cells by suppressing
AKT/GSK-3beta/beta-catenin pathway. Sci Rep. 14:196642024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pan C, Xu Y, Jiang Z, Fan C, Chi Z, Zhang
Y, Miao M, Ren Y, Wu Z, Xu L, et al: Naringenin relieves
paclitaxel-induced pain by suppressing calcitonin gene-related
peptide signalling and enhances the anti-tumour action of
paclitaxel. Br J Pharmacol. 18:3136–3159. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin WS, Leland JV, Ho CT and Pan MH:
Occurrence, bioavailability, anti-inflammatory, and anticancer
effects of pterostilbene. J Agric Food Chem. 68:12788–12799. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Xu C, Weng W and Goel A: Combined
treatment with Aronia berry extract and oligomeric
proanthocyanidins exhibit a synergistic anticancer efficacy through
LMNB1-AKT signaling pathways in colorectal cancer. Mol Carcinog.
63:2145–2157. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Szoka L, Stocki M and Isidorov V:
Dammarane-Type 3,4-seco-triterpenoid from silver birch (Betula
pendula Roth) buds induces melanoma cell death by promotion of
apoptosis and autophagy. Molecules. 29:40912024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cai J, Chen S, Zhang W, Zheng X, Hu S,
Pang C, Lu J, Xing J and Dong Y: Salvianolic acid A reverses
paclitaxel resistance in human breast cancer MCF-7 cells via
targeting the expression of transgelin 2 and attenuating PI3 K/Akt
pathway. Phytomedicine. 21:1725–1732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fang CY, Wu CZ, Chen PN, Chang YC, Chuang
CY, Lai CT, Yang SF and Tsai LL: Antimetastatic potentials of
salvianolic acid A on oral squamous cell carcinoma by targeting
MMP-2 and the c-Raf/MEK/ERK pathway. Environ Toxicol. 33:545–554.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pei R, Si T, Lu Y, Zhou JX and Jiang L:
Salvianolic acid A, a novel PI3K/Akt inhibitor, induces cell
apoptosis and suppresses tumor growth in acute myeloid leukemia.
Leuk Lymphoma. 59:1959–1967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Péczka N, Orgován Z, Ábrányi-Balogh P and
Keserű GM: Electrophilic warheads in covalent drug discovery: An
overview. Expert Opin Drug Discov. 17:413–422. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng M, Zhang Y, Xu Y, Han Y, Wu Y and
Kang J: Chemoproteomics and phosphoproteomics profiling reveals
salvianolic acid a as a covalent inhibitor of mTORC1. J Proteome
Res. 22:2450–2459. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yin X, Feng Y and Kang W: Effect of
salvianolic acid A on the proliferation and apoptosis in esophageal
cancer cells and the underlying mechanisms. Zhong Nan Da Xue Xue
Bao Yi Xue Ban. 45:1269–1275. 2020.(In English, Chinese).
PubMed/NCBI
|
|
49
|
Wang X, Wang C, Zhang L, Li Y, Wang S,
Wang J, Yuan C, Niu J, Wang C and Lu G: Salvianolic acid A shows
selective cytotoxicity against multidrug-resistant MCF-7 breast
cancer cells. Anticancer Drugs. 26:210–223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang XL, Yan L, Zhu L, Jiao DM, Chen J and
Chen QY: Salvianolic acid A reverses cisplatin resistance in lung
cancer A549 cells by targeting c-met and attenuating Akt/mTOR
pathway. J Pharmacol Sci. 135:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang T, Xu J, Li D, Chen J, Shen X, Xu F,
Teng F, Deng Y, Ma H, Zhang L, et al: Salvianolic acid A, a matrix
metalloproteinase-9 inhibitor of Salvia miltiorrhiza,
attenuates aortic aneurysm formation in apolipoprotein E-deficient
mice. Phytomedicine. 21:1137–1145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li T, Kong AN, Ma Z, Liu H, Liu P, Xiao Y,
Jiang X and Wang L: Protein arginine methyltransferase 1 may be
involved in pregnane × receptor-activated overexpression of
multidrug resistance 1 gene during acquired multidrug resistant.
Oncotarget. 7:20236–20248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kebebe D, Wu Y, Zhang B, Yang J, Liu Y, Li
X, Ma Z, Lu P, Liu Z and Li J: Dimeric c(RGD) peptide conjugated
nanostructured lipid carriers for efficient delivery of Gambogic
acid to breast cancer. Int J Nanomedicine. 14:6179–6195. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang Y, Zhang L, La X, Li Z, Li H and Guo
S: Salvianolic acid A inhibits tumor-associated angiogenesis by
blocking GRP78 secretion. Naunyn Schmiedebergs Arch Pharmacol.
392:467–480. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang SH, Su J and Zhen YS: Salvianolic
acid A inhibits nucleoside transport and potentiates the antitumor
activity of chemotherapeutic drugs. Yao Xue Xue Bao. 39:496–499.
2004.(In Chinese). PubMed/NCBI
|
|
56
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Singh SP, Pathuri G, Asch AS, Rao CV and
Madka V: Stat3 inhibitors TTI-101 and SH5-07 suppress bladder
cancer cell survival in 3D tumor models. Cells. 13:14632024.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shaban NZ, Hegazy WA, Abu-Serie MM, Talaat
IM, Awad OM and Habashy NH: Seedless black Vitis vinifera
polyphenols suppress hepatocellular carcinoma in vitro and in vivo
by targeting apoptosis, cancer stem cells, and proliferation.
Biomed Pharmacother. 175:1166382024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Thinnes FP: Neuroendocrine differentiation
of LNCaP cells suggests: VDAC in the cell membrane is involved in
the extrinsic apoptotic pathway. Mol Genet Metab. 97:241–243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Conti Nibali S, De Siervi S, Luchinat E,
Magrì A, Messina A, Brocca L, Mantovani S, Oliviero B, Mondelli MU,
De Pinto V, et al: VDAC1-interacting molecules promote cell death
in cancer organoids through mitochondrial-dependent metabolic
interference. iScience. 27:1098532024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kulyar MF, Mo Q, Yao W, Li Y, Nawaz S,
Loon KS, Ahmed AE, Alsaegh AA, Al Syaad KM, Akhtar M, et al:
Modulation of apoptosis and Inflammasome activation in
chondrocytes: Co-regulatory role of Chlorogenic acid. Cell Commun
Signal. 22:22024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Soengas MS, Alarcón RM, Yoshida H, Giaccia
AJ, Hakem R, Mak TW and Lowe SW: Apaf-1 and caspase-9 in
p53-dependent apoptosis and tumor inhibition. Science. 284:156–159.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pettigrew CA and Cotter TG: Deregulation
of cell death (apoptosis): Implications for tumor development.
Discov Med. 8:61–63. 2009.PubMed/NCBI
|
|
66
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Peng H, Yuan X, Shi R, Wei X, Ren S, Yan
C, Ding Y, Lin Y, Fan D, Yang M, et al: PHII-7 inhibits cell growth
and induces apoptosis in leukemia cell line K562 as well as its
MDR-counterpart K562/A02 through producing reactive oxygen species.
Eur J Pharmacol. 718:459–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu J, Liu Y, Li H, Wei C, Mao A, Liu W
and Pan G: Polyphyllin D induces apoptosis and protective autophagy
in breast cancer cells through JNK1-Bcl-2 pathway. J
Ethnopharmacol. 282:1145912022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang S, Yadav AK, Han JY, Ahn KS and Jang
BC: Anti-Growth, Anti-angiogenic, and pro-apoptotic effects by
CX-4945, an inhibitor of casein kinase 2, on HuCCT-1 human
cholangiocarcinoma cells via control of caspase-9/3, DR-4,
STAT-3/STAT-5, Mcl-1, eIF-2α, and HIF-1α. Int J Mol Sci.
23:63532022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tang Z, Ding J and Xiao X: Salvianolic
acid a induces apoptosis and inhibits the C-Met expression in
hepatocellular carcinoma HepG2 cell line. Chin J Mod Appl Pharm.
31:537–541. 2014.
|
|
71
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Seront E, Pinto A, Bouzin C, Bertrand L,
Machiels JP and Feron O: PTEN deficiency is associated with reduced
sensitivity to mTOR inhibitor in human bladder cancer through the
unhampered feedback loop driving PI3K/Akt activation. Br J Cancer.
109:1586–1592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sun B, Zhao Y, Yang S, Li X, Li N, Wang Y,
Han Q, Liu X, Tu Q, Zheng J and Zhang X: Celecoxib as a potential
treatment for hepatocellular carcinoma in populations exposed to
high PFAS levels. J Hazard Mater. 489:1376132025. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li J, Bian X, Zhang C, Chen Y, Huang S,
Zhao S and Li Y: Identifying prognostic biomarkers and immune
interactions in ovarian cancer associated with perfluorooctanoic
acid exposure: Insights from comparative toxicogenomics and
molecular docking studies. Ecotoxicol Environ Saf. 291:1178312025.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hu M, Tao P, Wang Y, Zhu C, Ma Y, Liu X
and Cai H: Knockdown of CCNB2 inhibits the tumorigenesis of gastric
cancer by regulation of the PI3K/Akt pathway. Sci Rep. 15:57032025.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bi L, Chen J, Yuan X, Jiang Z and Chen W:
Salvianolic acid A positively regulates PTEN protein level and
inhibits growth of A549 lung cancer cells. Biomed Rep. 1:213–217.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wülfing P, Kersting C, Tio J, Fischer RJ,
Wülfing C, Poremba C, Diallo R, Böcker W and Kiesel L:
Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression
is correlated with vascular endothelial growth factor expression
and angiogenesis in breast cancer. Clin Cancer Res. 10:2393–2400.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang Q, Wang S, Yu Y, Sun S, Zhang Y,
Zhang Y, Yang W, Li S and Qiao Y: Salvianolic acid A, as a novel
ETA receptor antagonist, shows inhibitory effects on tumor in
vitro. Int J Mol Sci. 17:12442016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Leng X, Kan H, Wu Q, Li C, Zheng Y and
Peng G: Inhibitory effect of Salvia miltiorrhiza extract and
its active components on cervical intraepithelial neoplastic cells.
Molecules. 27:15822022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li S, Fang J, Si T, Lu Y and Jiang L:
Salvianolic acid A inhibits the growth of diffuse large B-cell
lymphoma through MAPK pathways. Exp Hematol. 94:60–68.e2. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li HY, Li Y, Yan CH, Li LN and Chen XG:
Inhibition of tumor growth by S-3-1, a synthetic intermediate of
salvianolic acid A. J Asian Nat Prod Res. 4:271–280. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xuan Z, Zhang Y, Li D, Wang K, Huang P and
Shi J: PLXNB1/SEMA4D signals mediate interactions between malignant
epithelial and immune cells to promote colorectal cancer liver
metastasis. J Cell Mol Med. 28:e701422024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xie S, Han S, Gong J, Feng Z, Sun Y, Yao H
and Shi P: Bee venom prompts the inhibition of gefitinib on
proliferation, migration, and invasion of non-small cell lung
cancer cells via EGFR-mediated autophagy. Toxicon. 251:1081492024.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li X, Sun Y, Guo J, Cheng Y, Lu W, Yang W,
Wang L and Cheng Z: Sodium bicarbonate potentiates the antitumor
effects of Olaparib in ovarian cancer via cGMP/PKG-mediated ROS
scavenging and M1 macrophage transformation. Biomed Pharmacother.
180:1175092024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu L, Meng T, Zheng X, Liu Y, Hao R, Yan
Y, Chen S, You H, Xing J and Dong Y: Transgelin 2 promotes
paclitaxel resistance, migration, and invasion of breast cancer by
directly interacting with PTEN and activating PI3K/Akt/GSK-3β
pathway. Mol Cancer Ther. 18:2457–2468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fares J, Fares MY, Khachfe HH, Salhab HA
and Fares Y: Molecular principles of metastasis: A hallmark of
cancer revisited. Signal Transduct Target Ther. 5:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mrozik KM, Blaschuk OW, Cheong CM,
Zannettino ACW and Vandyke K: N-cadherin in cancer metastasis, its
emerging role in haematological malignancies and potential as a
therapeutic target in cancer. BMC Cancer. 18:9392018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Saldanha R, Ho Thanh MT, Krishnan N,
Hehnly H and Patteson A: Vimentin supports cell polarization by
enhancing centrosome function and microtubule acetylation. J R Soc
Interface. 21:202306412024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xypolita ME, Goolam M, Bikoff EK,
Robertson EJ and Mould AW: The zinc-finger transcription factor
Blimp1/Prdm1 is required for uterine remodelling and repair in the
mouse. Nat Commun. 16:12202025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Curran S and Murray GI: Matrix
metalloproteinases: Molecular aspects of their roles in tumour
invasion and metastasis. Eur J Cancer. 36:1621–1630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tong Z, Zhang Y, Guo P, Wang W, Chen Q,
Jin J, Liu S, Yu C, Mo P, Zhang L and Huang J: Steroid receptor
coactivator 1 promotes human hepatocellular carcinoma invasiveness
through enhancing MMP-9. J Cell Mol Med. 28:e181712024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li K, Li D, Hafez B, Bekhit MMS, Jardan
YAB, Alanazi FK, Taha EI, Auda SH, Ramzan F and Jamil M:
Identifying and validating MMP family members (MMP2, MMP9, MMP12,
and MMP16) as therapeutic targets and biomarkers in kidney renal
clear cell carcinoma (KIRC). Oncol Res. 32:737–752. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Guo J, Song Z, Muming A, Zhang H and Awut
E: Cysteine protease inhibitor S promotes lymph node metastasis of
esophageal cancer cells via VEGF-MAPK/ERK-MMP9/2 pathway. Naunyn
Schmiedebergs Arch Pharmacol. 397:6051–6059. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chuang CY, Ho YC, Lin CW, Yang WE, Yu YL,
Tsai MC, Yang SF and Su SC: Salvianolic acid A suppresses MMP-2
expression and restrains cancer cell invasion through ERK signaling
in human nasopharyngeal carcinoma. J Ethnopharmacol.
252:1126012020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Stasiak P, Sopel J, Lipowicz JM,
Rawłuszko-Wieczorek AA, Korbecki J and Januchowski R: The role of
elacridar, a P-gp inhibitor, in the Re-sensitization of
PAC-resistant ovarian cancer cell lines to cytotoxic drugs in 2D
and 3D cell culture models. Int J Mol Sci. 26:11242025. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nikolaou M, Pavlopoulou A, Georgakilas AG
and Kyrodimos E: The challenge of drug resistance in cancer
treatment: A current overview. Clin Exp Metastasis. 35:309–318.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Jin Q, Ren Q, Chang X, Yu H, Jin X, Lu X,
He N and Wang G: Neuropilin-1 predicts poor prognosis and promotes
tumor metastasis through epithelial-mesenchymal transition in
gastric cancer. J Cancer. 12:3648–3659. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yao S, Liu X, Feng Y, Li Y, Xiao X, Han Y
and Xia S: Unveiling the role of HGF/c-Met signaling in Non-small
cell lung cancer tumor microenvironment. Int J Mol Sci.
25:91012024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xu J, Liu S, Yang X, Cao S and Zhou Y:
Paracrine HGF promotes EMT and mediates the effects of PSC on
chemoresistance by activating c-Met/PI3K/Akt signaling in
pancreatic cancer in vitro. Life Sci. 263:1185232020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shao Z, Pan H, Tu S, Zhang J, Yan S and
Shao A: HGF/c-Met Axis: The advanced development in digestive
system cancer. Front Cell Dev Biol. 8:8012020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bahrami A, Shahidsales S, Khazaei M,
Ghayour-Mobarhan M, Maftouh M, Hassanian SM and Avan A: C-Met as a
potential target for the treatment of gastrointestinal cancer:
Current status and future perspectives. J Cell Physiol.
232:2657–2673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Pilotto S, Carbognin L, Karachaliou N, Ma
PC, Rosell R, Tortora G and Bria E: Tracking MET de-addiction in
lung cancer: A road towards the oncogenic target. Cancer Treat Rev.
60:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wu JC, Wang CT, Hung HC, Wu WJ, Wu DC,
Chang MC, Sung PJ, Chou YW, Wen ZH and Tai MH: Heteronemin is a
Novel c-Met/STAT3 inhibitor against advanced prostate cancer cells.
Prostate. 76:1469–1483. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan
C, Wu Y, Li X, Li X, Li G, et al: Function of the c-Met receptor
tyrosine kinase in carcinogenesis and associated therapeutic
opportunities. Mol Cancer. 17:452018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen FY, Bi L, Qian L, Gao J, Jiang YC and
Chen WP: Identification of multidrug resistance gene MDR1
associated microRNA of salvianolic acid A reversal in lung cancer.
Zhongguo Zhong Yao Za Zhi. 41:3279–3284. 2016.(In Chinese).
PubMed/NCBI
|
|
108
|
Li H, Chen J, Xu C, Pang L and Cheng X:
Antitumor effect of salvianolic acid A and on its reversal of
multidrug resisitance in A549/MTX tumor. Chin J Clin Pharmacol
Ther. 22:12442017.
|
|
109
|
Ye T, Chen R, Zhou Y, Zhang J, Zhang Z,
Wei H, Xu Y, Wang Y and Zhang Y: Salvianolic acid A (Sal A)
suppresses malignant progression of glioma and enhances
temozolomide (TMZ) sensitivity via repressing transgelin-2 (TAGLN2)
mediated phosphatidylinositol-3-kinase (PI3K)/protein kinase B
(Akt) pathway. Bioengineered. 13:11646–11655. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang B, Zhang Y, Dang W, Xing B, Yu C,
Guo P, Pi J, Deng X, Qi D and Liu Z: The anti-tumor and
renoprotection study of E-[c(RGDfK)(2)]/folic acid co-modified
nanostructured lipid carrier loaded with doxorubicin
hydrochloride/salvianolic acid A. J Nanobiotechnology. 20:4252022.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xue L, Ouyang W, Qi P, Zhu Y, Qi X, Zhang
X, Zhang X, Wang L and Cui L: Key mechanisms of angiogenesis in the
infarct core: Association of macrophage infiltration with
venogenesis. Mol Brain. 18:122025. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yu Yan and Yuan E: Regulatory effect of
N6-methyladenosine on tumor angiogenesis. Front Immunol.
15:14537742024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sayed ZS, Khattap MG, Madkour MA, Yasen
NS, Elbary HA, Elsayed RA, Abdelkawy DA, Wadan AS, Omar I and
Nafady MH: Circulating tumor cells clusters and their role in
Breast cancer metastasis; a review of literature. Discov Oncol.
15:942024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Jin R, Neufeld L and McGaha TL: Linking
macrophage metabolism to function in the tumor microenvironment.
Nat Cancer. 6:239–252. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lim JX, Yong YK, Dewi FRP, Chan SY and Lim
V: Nanoscale strategies: Doxorubicin resistance challenges and
enhancing cancer therapy with advanced nanotechnological
approaches. Drug Deliv Transl Res. February 15–2025.(Epub ahead of
print). View Article : Google Scholar
|
|
118
|
Qian C, Zhou Y, Zhang T, Dong G, Song M,
Tang Y, Wei Z, Yu S, Shen Q, Chen W, et al: Targeting PKM2
signaling cascade with salvianic acid A normalizes tumor blood
vessels to facilitate chemotherapeutic drug delivery. Acta Pharm
Sin B. 14:2077–2096. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kaur T, Weadick B, Mace TA, Desai K, Odom
H and Govindarajan R: Nucleoside transporters and immunosuppressive
adenosine signaling in the tumor microenvironment: Potential
therapeutic opportunities. Pharmacol Ther. 240:1083002022.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Tang C, Jiang ST, Li CX, Jia XF and Yang
WL: The Effect of salvianolic acid a on Tumor-associated macrophage
polarization and its mechanisms in the tumor microenvironment of
Triple-negative breast cancer. Molecules. 29:14692024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Nan Y, Wu X, Luo Q, Chang W, Zhao P, Zhang
L and Liu Z: OTUB2 silencing promotes ovarian cancer via
mitochondrial metabolic reprogramming and can be synthetically
targeted by CA9 inhibition. Proc Natl Acad Sci USA.
121:e23153481212024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Liu X, Zhao J, Liu F, Xie Z, Lei X, Wang
Z, Yang Z, Zhou Y and Tang G: A Smart CA IX-targeting and
pH-responsive nano-mixed micelles for delivery of FB15 with
superior anti-breast cancer efficacy. Int J Nanomedicine.
19:10247–10262. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang C, Pan Y, Cai R, Guo S, Zhang X, Xue
Y, Wang J, Huang J, Wang J, Gu Y and Zhang Z: Salvianolic acid A
increases the accumulation of doxorubicin in brain tumors through
Caveolae endocytosis. Neuropharmacology. 167:1079802020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Qiu C, Zhang JZ, Wu B, Xu CC, Pang HH, Tu
QC, Lu YQ, Guo QY, Xia F and Wang JG: Advanced application of
nanotechnology in active constituents of Traditional Chinese
Medicines. J Nanobiotechnology. 21:4562023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lu L, Zhang H, Qian Y and Yuan Y:
Isolation of salvianolic acid A, a minor phenolic carboxylic acid
of Salvia miltiorrhiza. Nat Prod Commun. 5:805–808.
2010.PubMed/NCBI
|
|
126
|
Yang MY, Liu Y, Yu YW, Gong BF, Ruan J and
Fan HY: Application of targeted liposomes-based salvianolic acid A
for the treatment of ischemic stroke. Neurotherapeutics.
21:e003422024. View Article : Google Scholar : PubMed/NCBI
|