Introduction
After osteosarcoma, chondrosarcoma is the second
most common type of bone cancer (accounting for 20–30% of bone
cancer cases) and presents with a range of morphological and
clinical characteristics, from low-grade, biologically benign
tumors to aggressive high-grade variants (1,2).
Chondrosarcoma is the predominant malignancy of cartilage,
attributed to somatic mutations in the isocitrate dehydrogenase
(IDH) 1 and 2 genes (3). Surgery
is typically the first line treatment for chondrosarcoma. However,
surgical intervention is ineffective if the tumor has metastasized
to other parts of the body or is located in an unresectable
position such as the skull or pelvis (4). Between 50 and 70% of advanced-stage
chondrosarcoma cases involve metastasis to other organs, such as
the lung (5), markedly affecting
patient prognosis with median overall survival of 12.7 months in
the prospective database of the French Sarcoma Group (6,7).
Furthermore, numerous types of chemotherapy have limited efficacy
against chondrosarcoma (8). These
challenges highlight the need for alternative therapeutic
strategies to address chondrosarcoma metastasis.
The leading cause of mortality in patients with
cancer is metastases (9); the
involvement of MMPs is key to the metastatic process (10). MMP-7 is distributed in endothelial
and vascular smooth muscle cells (11). Additionally, it may be released by
epithelial cells or retained within the cytoplasm of breast cancer
cells (12). Furthermore, a direct
association between MMP-7 expression and the onset of cancer has
been demonstrated (13).
Specifically, chondrosarcomas exhibit high expression levels of
MMP-7, an enzyme that controls colony formation and cell invasion
in response to fluid shear stress (14). MicroRNA-520f-3p regulates the
activity of MMP-7, which is key for the progression and metastasis
of chondrosarcoma (15).
Therefore, MMP-7 may be a viable option for treating chondrosarcoma
metastases.
A previous study estimated that 60% of anticancer
drugs were wholly or partially derived from natural sources
(16). Researchers are screening
natural medications for active anticancer constituents and studying
the processes underlying their antitumor action, which is a growing
trend in the development of antitumor therapy (17–19).
Natural ingredients serve as a unique therapeutic alternative for
chondrosarcoma research (20).
Antrodia cinnamomea, a rare medicinal mushroom native to
Taiwan exhibits anti-inflammatory, hepatoprotective, anticancer,
immunomodulatory and antioxidative properties (21–23).
Antcin K, a triterpenoid derived from A. cinnamomea, has
been demonstrated to have anti-angiogenesis and anti-inflammatory
function in both in vitro and in vivo studies
(24–26). Antcin K has considerable
antiproliferative effects on liver cancer cells and induces cell
death by promoting the generation of reactive oxygen species and
ATP depletion, leading to endoplasmic reticulum stress and changes
in mitochondrial membrane permeability (27), and also inhibits hepatoma cancer
cell integrin-mediated adhesion, migration and invasion (28). Antcin K exerts anticancer
activities by regulating levels of MMP-2 and MMP-9 (28). The present study aimed to
investigate the effects of antcin K on chondrosarcoma progression
and metastasis. These findings may offer novel insight and
approaches for chondrosarcoma treatment in future.
Materials and methods
Materials
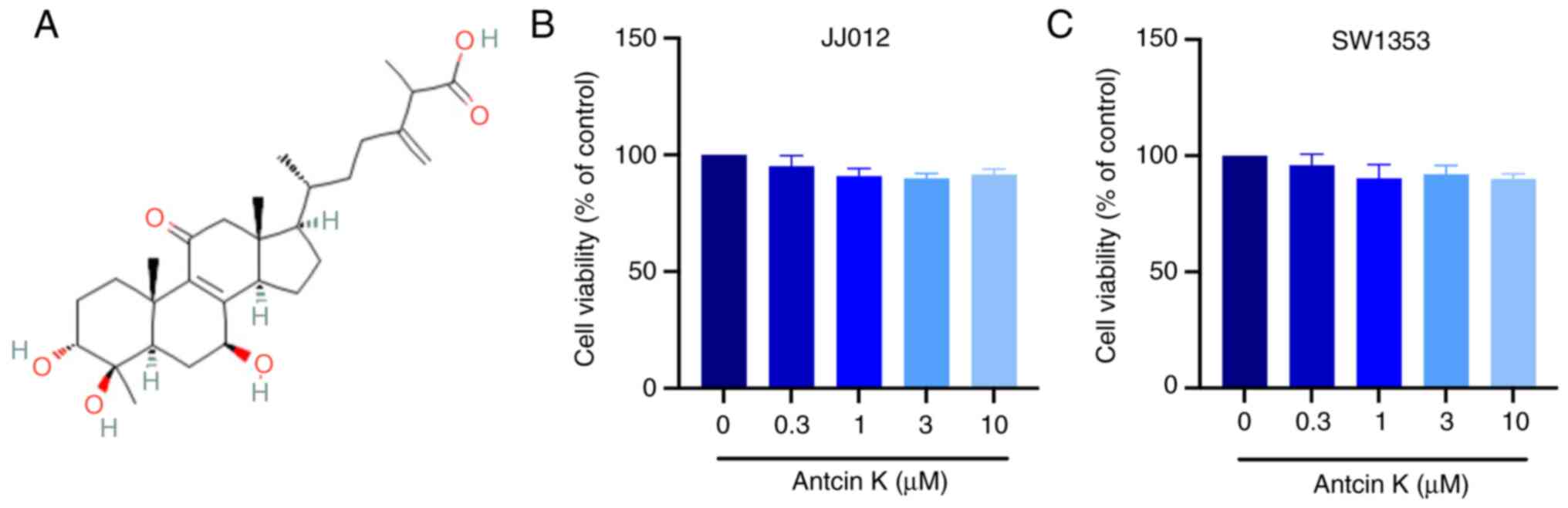
Antcin K (Fig. 1A)
was synthesized as previously described (27). The phosphorylated (p)-p85 (1:2,000;
cat. no. 4228S), p-Akt (1:2,000; cat. no. 4060S), p-mTOR (1:2,000;
cat. no. 5536S), mTOR (1:2,000; cat. no. 2983S) and p-p65 (1:2,000;
cat. no. 3033) antibodies were obtained from Cell Signaling
Technology, Inc. Antibodies for MMP-7 (1:500; cat. no. sc-515703),
Akt (1:500; cat. no. sc-5298) and p85 (1:500; cat. no. sc-1637)
were purchased from Santa Cruz Biotechnology, Inc. p65 (1:2,000;
cat. no. GTX102090) and β-actin (1:5,000; cat. no. GT5512)
antibodies were purchased from GeneTex International Corporation.
MTT was obtained from Sigma-Aldrich (Merck KGaA) and
Lipofectamine® 2000 was supplied by Invitrogen (Thermo
Fisher Scientific, Inc.).
Cell culture
Dr Sean P. Scully (Miller School of Medicine,
University of Miami; Miami; USA) gifted the chondrosarcoma JJ012
cell line. The chondrosarcoma SW1353 cell line was supplied by
American Type Culture Collection. The JJ012 and SW1353 cell culture
conditions were as previously described (29,30).
MTT assay
Chondrosarcoma cells (5×103 cells/well)
were seeded in 96-well culture plates and incubated at 37°C with or
without antcin K (0.3, 1.0, 3.0 or 10 µM) for 24 h.
Dimethylsulfoxide was used to dissolve the purple formazan crystals
formed by MTT solution, which was added at a concentration of 0.5
mg/ml for 2 h. Absorbance was measured at 570 nm using a BioTek
microplate reader (Agilent Technologies, Inc.), as previously
described (26,31).
Cell migration assay
For the migration assay, 48-well Micro Chemotaxis
Chambers (Neuro Probe Inc.) were used (32,33).
A total of 50 µl serum-free DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to seed ~2.5×103 cells into
the upper chamber. In the lower chamber, 30 µl DMEM (Invitrogen;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Corning, Inc.)
with or without antcin K (0.3, 1.0, 3 or 10 µM) was added.
Following a 24 h incubation, cells were fixed at room temperature
for 15 min using 3.7% formaldehyde and stained for 15 min at room
temperature using 0.1% crystal violet. PBS was used to wash the GVS
8 µm membrane (Data Support Company). The migrated cell was
visualised by an Olympus CKX53 microscope. ImageJ (version 1.53;
National Institutes of Health) was used to analyze the number of
migrated cells in one field of view.
Cell invasion assay
Invasion experiments were conducted using an 8-µm
pore-size Corning Costar Transwell chamber (Corning, Inc.). A total
of ~1×104 cells were inserted into the upper chamber,
which was pre-covered with a thin layer of matrix gel for 30 min at
37°C. In the lower chamber, 330 µl 10% FBS-containing medium with
or without antcin K (0.3, 1, 3 or 10 µM) were added for 24 h at
37°C. All invasive cells adhering to the lower surface were fixed
with 3.7% formaldehyde for 30 min, stained with 0.1% crystal violet
for 20 min at room temperature, and then washed with PBS at room
temperature. ImageJ (version 1.53; National Institutes of Health)
was used to evaluated the number of cell invasions.
RNA sequencing (RNA-seq)
Total RNA from the JJ012 cells treated for 30 min at
37°C with or without antcin K (10 µM) was isolated by
TRIzol® (cat. no. 12183555, Invitrogen; Thermo Fisher
Scientific, Inc.) for RNA-seq. Transcriptome sequencing experiments
included RNA extraction and quantity control, library construction,
purification, quality control and quantitation, sequencing cluster
generation and high through-put sequencing, were performed as
previously described (34).
Differentially expressed genes (DEGs) from RNA-seq analysis were
uploaded to Ingenuity Pathway Analysis (IPA;
digitalinsights.qiagen.com/) and Kyoto Encyclopedia Genes and
Genomes (KEGG; genome.jp/kegg/pathway.html) database to investigate
potential pathways and biological function analyses.
Western blotting
Total protein from JJ012 and SW1353 cells was
extracted using RIPA lysis buffer (cat. no. P0013; Beyotime
Institute of Biotechnology). A total of 25 µg protein calculated
using the BCA kit (Invitrogen; Thermo Fisher Scientific, Inc.) was
loaded into each lane. Proteins were separated using 8% or 10% of
resolving gels. Proteins were transferred to Immobilon®
PVDF membranes following separation via SDS-PAGE. The membrane was
blocked for 1 h at room temperature with a 5% non-fat milk
solution. The membranes were incubated with the primary antibodies
(MMP-7 (1:500), p-p85 (1:2,000), p85 (1:500), p-Akt (1:2,000), Akt
(1:500), p-mTOR (1:2,000), mTOR (1:2,000), p-p65 (1:2,000), p65
(1:2,000) and β-actin (1:5,000)) overnight at 4°C, followed by a
horseradish peroxidase-conjugated secondary antibodies (goat
anti-rabbit IgG, cat. no. sc-2357; 1:3,000; goat anti-mouse IgG,
cat. no. sc-516102; 1:3,000; Santa Cruz Biotechnology, Inc.) for 1
h at room temperature. An ECL kit (MilliporeSigma) was used to
detect immunoreactive bands by the chemiluminescent imaging system
(Invitrogen iBright CL1500 Imaging Systems). Following
normalization to β-actin, the optical density of the blot was
quantified using ImageJ v1.53 software (National Institutes of
Health).
Reverse transcription-quantitative
(RT-q)PCR
JJ012 and SW1353 cells (~1×104) were
seeded onto 6-well dishes and incubated with antcin K (0, 0.3, 1, 3
or 10 µM) for 24 h at 37°C. A total of 1 µg RNA was extracted using
TRIzol® (cat. no. 12183555, Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
RNA was transformed into cDNA using an oligo-DT primer. The KAPA
SYBR® FAST qPCR kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to mix 100 ng cDNA sample with primers
specific, with GAPDH used as the internal control. PCR primer
(5′-3′) sequences were as follows: MMP-7 forward,
GAGTGAGCTACAGTGGGAAC and reverse, CTATGACGCGGGAGTTTAACAT;
programmed cell death ligand 1 (PD-L1) forward,
TGCCGACTACAAGCGAATTACTG and reverse, CTGCTTGTCCAGATGACTTCGG; IL-6
forward, AGACAGCCACTCACCTCTTCAG and reverse,
TTCTGCCAGTGCCTCTTTGCTG; IL-1β forward, ATGATGGCTTATTACAGTGGCAA and
reverse, GTCGGAGATTCGTAGCTGGA; TNF-α forward,
CCTCTCTCTAATCAGCCCTCTG and reverse, GAGGACCTGGGAGTAGATGAG and GAPDH
forward, ACCACAGTCCATGCCATCAC and reverse, TCCACCACCCTGTTGCTGTA.
Thermocycling conditions were as follows: Initial denaturation at
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 1 min. A StepOnePlus sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to perform the
qPCR tests in triplicate. MMP-7 mRNA expression was determined
using the ∆Cq comparative methods (35–37).
Establishment of stable cell
lines
MMP-7-overexpressing chondrosarcoma cell lines were
established by transfecting JJ012 and SW1353 cells with 1 µg
pcDNA3.1(+)/MMP7 vector (MDBio Inc.) with Lipofectamine®
2000 at 37°C. After 24 h, transfectants were selected by 200 µg/ml
of G418 (Geneticin) (Life Technologies).
Luciferase activity assay
Chondrosarcoma cells were transfected with 1 µg of
NF-κB luciferase plasmid (Stratagene; Agilent Technologies, Inc.)
using Lipofectamine 2000® for 24 h at 37°C and treated
with antcin K (10 µM) for an additional 24 h at 37°C. The
Dual-Luciferase® Reporter Assay System (Promega) was
used to detect the luciferase activity following the company
protocol. Firefly luciferase activity was normalized to
Renilla luciferase activity.
Statistical analysis
Data were analyzed using GraphPad Prism 10
(Dotmatics). Statistical significance was assessed using an
unpaired Student's t-test for comparisons between two groups.
Comparisons involving a control group and multiple drug
concentrations were conducted using one-way ANOVA followed by
Dunnett's test. Comparisons involving >2 groups were analyzed
using one-way ANOVA followed by Tukey's post hoc test. Results are
expressed as the mean ± standard deviation of at least 3
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
Antcin K inhibits chondrosarcoma cell
migration and invasion
JJ012 (derived from a grade 2 chondrosarcoma tumor;
IDH1 mutation) and SW1353 (derived from a grade 2 chondrosarcoma
tumor; IDH2 mutation) cell lines (38) were used to evaluate the effect of
antcin K. Antcin K markedly influences the induction of apoptosis
in human hepatoma cells (27). MTT
assay was used to assess the cytotoxic effects of antcin K on JJ012
and SW1353 cell lines. Antcin K had no effect on the viability of
chondrosarcoma cells, including at the maximum dosage of 10 µM
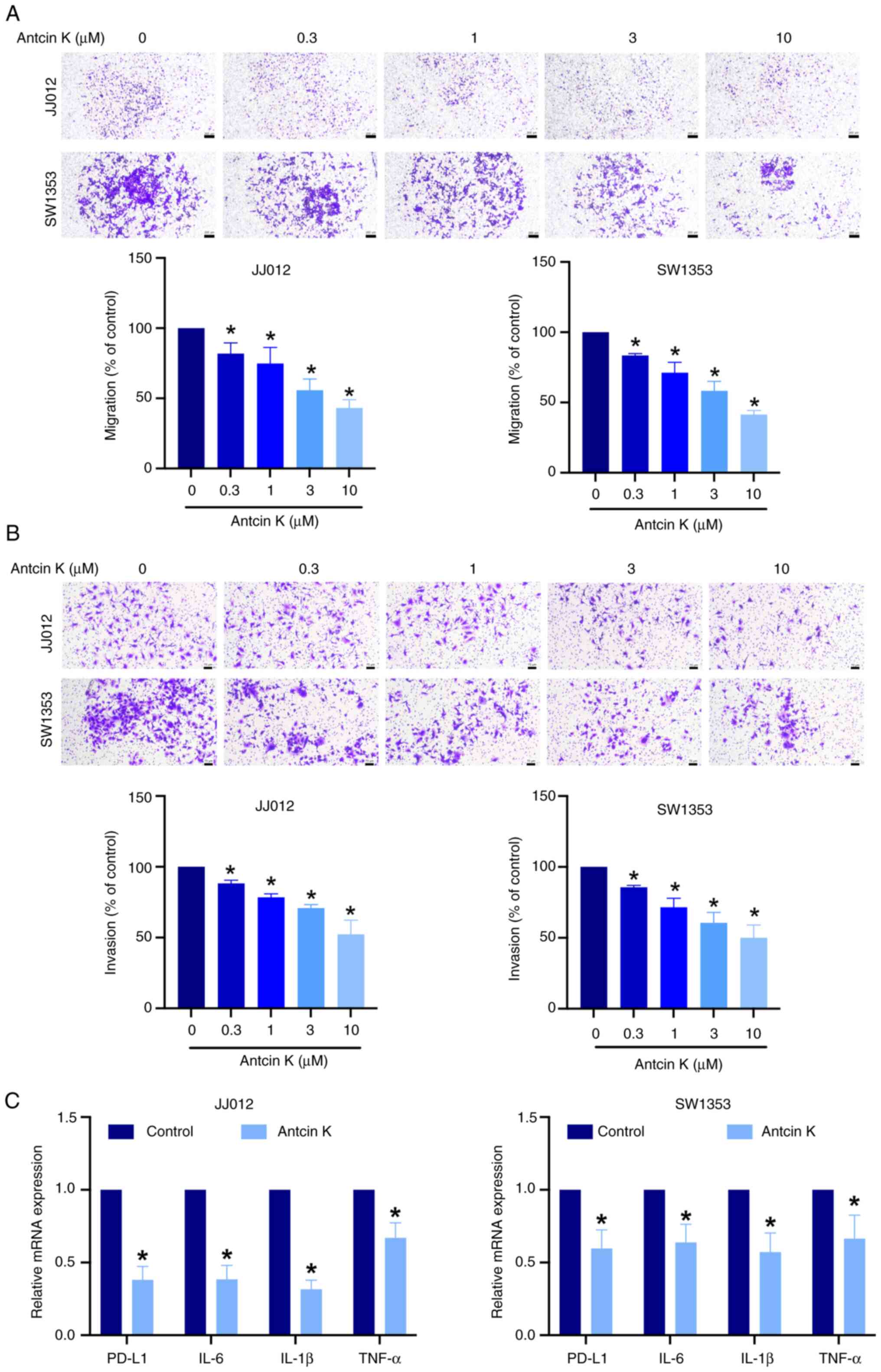
(Fig. 1B and C). Migration assay
was performed using the same concentration range to assess the
effects of antcin K on chondrosarcoma motility. Antcin K suppressed
migration in both chondrosarcoma cell lines (Fig. 2A). The invasion of chondrosarcoma
cells was also inhibited by antcin K (Fig. 2B). Antcin K exhibits
anti-inflammatory and immunomodulatory properties (39). Antcin K exerted a notable
inhibitory effect on the mRNA expression of genes associated with
immunomodulation (PD-L1) and inflammation (IL-6, IL-1β and TNF-α;
Fig. 2C). Collectively, these data
suggested that antcin K significantly reduces chondrosarcoma cell
motility.
Antcin K suppresses the motility of
chondrosarcoma cells by inhibiting the production of MMP-7
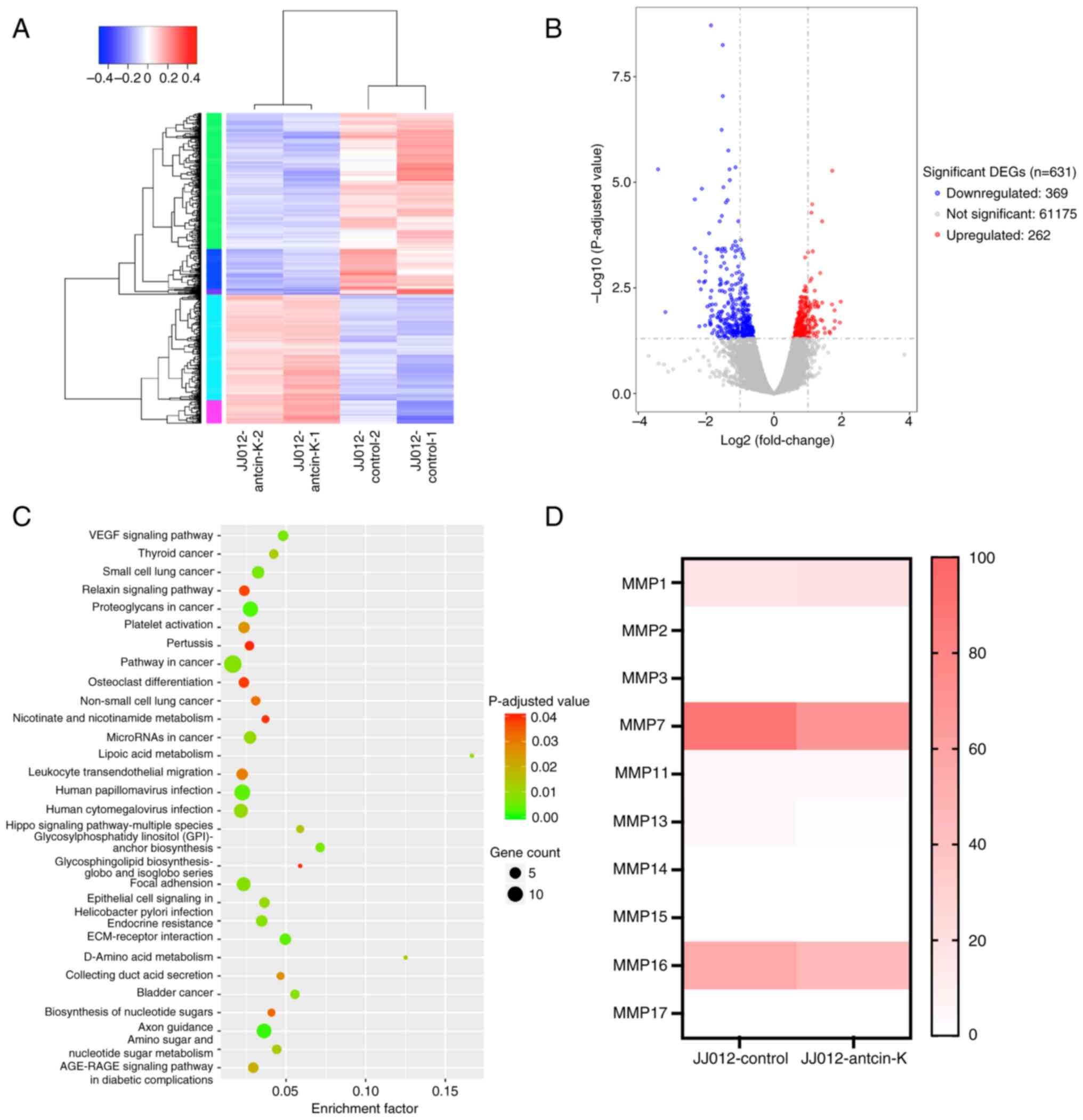
To investigate the molecules responsible for the
anti-motility effects of antcin K, RNA-seq analysis was performed
in JJ012 cells treated with or without antcin K. Following
treatment with antcin K, 369 genes were upregulated and 262 genes
were downregulated (Fig. 3A and
B). Kyoto Encyclopedia of Genes and Genomes analysis indicated
that ‘ECM-receptor interaction’ was enriched (Fig. 3C), which was associated with
metastasis clear cell renal cell carcinoma (40). Among the MMPs, MMP-7 expression was
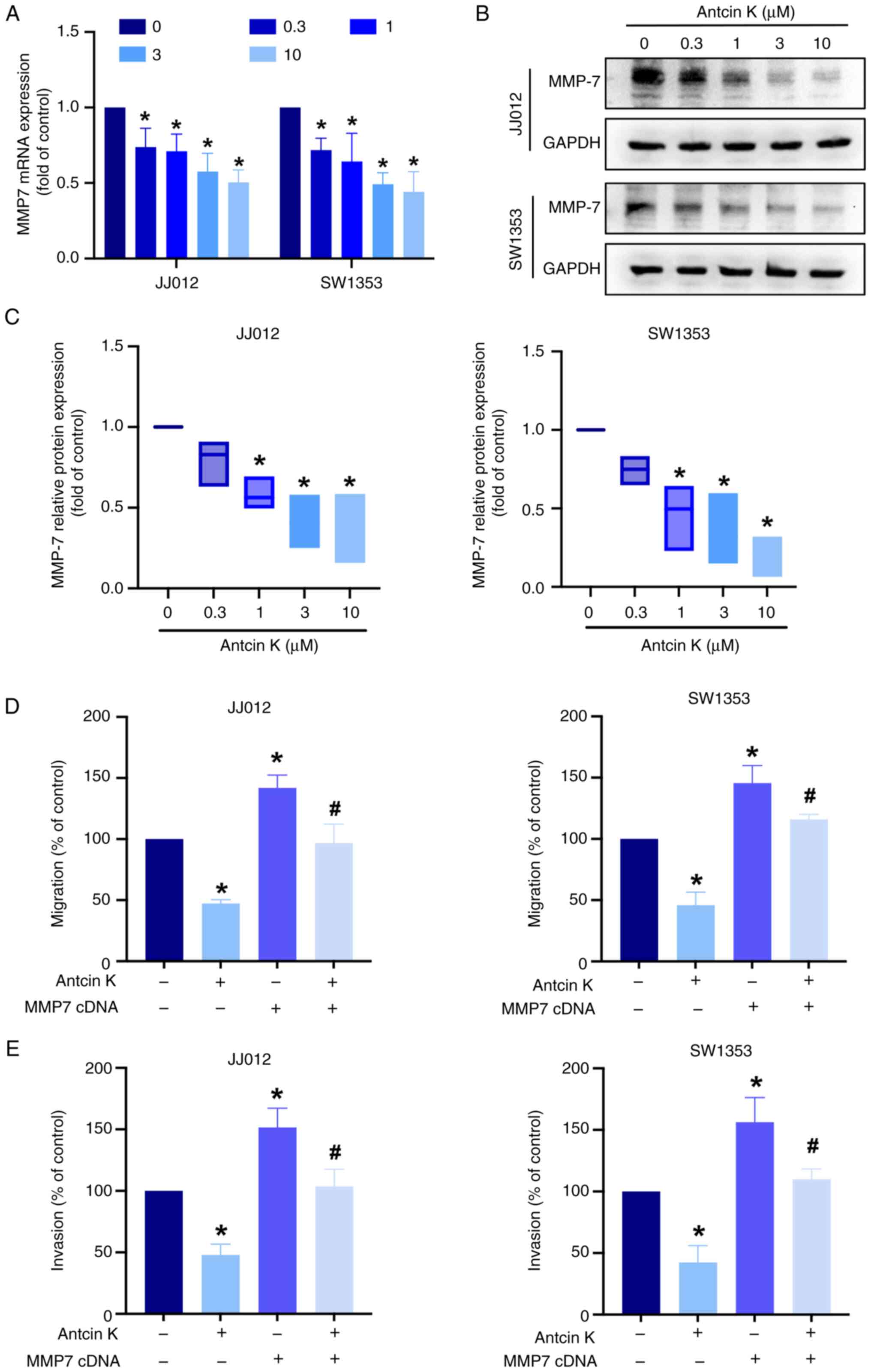
the most decreased following antcin K treatment (Fig. 3D). Antcin K inhibited MMP-7 mRNA
and protein production in a concentration-dependent manner
(Fig. 4A-C). Moreover, the
overexpression of MMP-7 antagonized the inhibitory effects of
antcin K on cell migration and invasion (Fig. 4D and E), indicating that antcin K
blocked chondrosarcoma motility by inhibiting MMP-7 production.
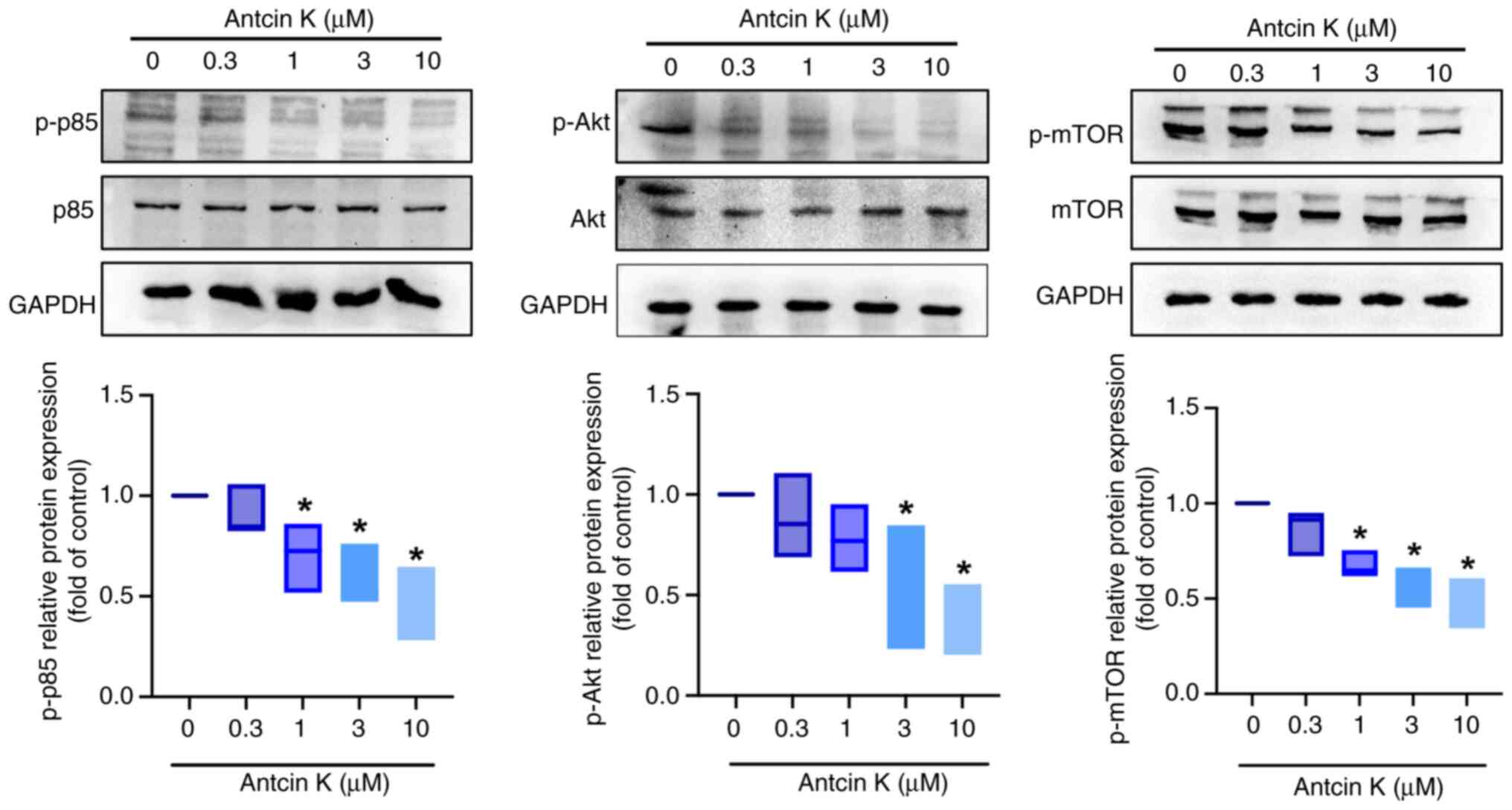
Antcin K downregulates PI3K, Akt, mTOR
and NF-κB signaling pathways in chondrosarcoma
Ingenuity Pathway Analysis (IPA) was used to
identify the enriched canonical pathways involved in chondrosarcoma
regulation to assess the molecular mechanism underlying the
regulatory effects of antcin K on chondrosarcoma cell motility.
‘Pulmonary fibrosis idiopathic signaling pathway’ demonstrated the
strongest downregulation (Fig.
5A), and was associated with the PI3K, Akt, mTOR and NF-κB
pathways (Fig. 5B). Antcin K
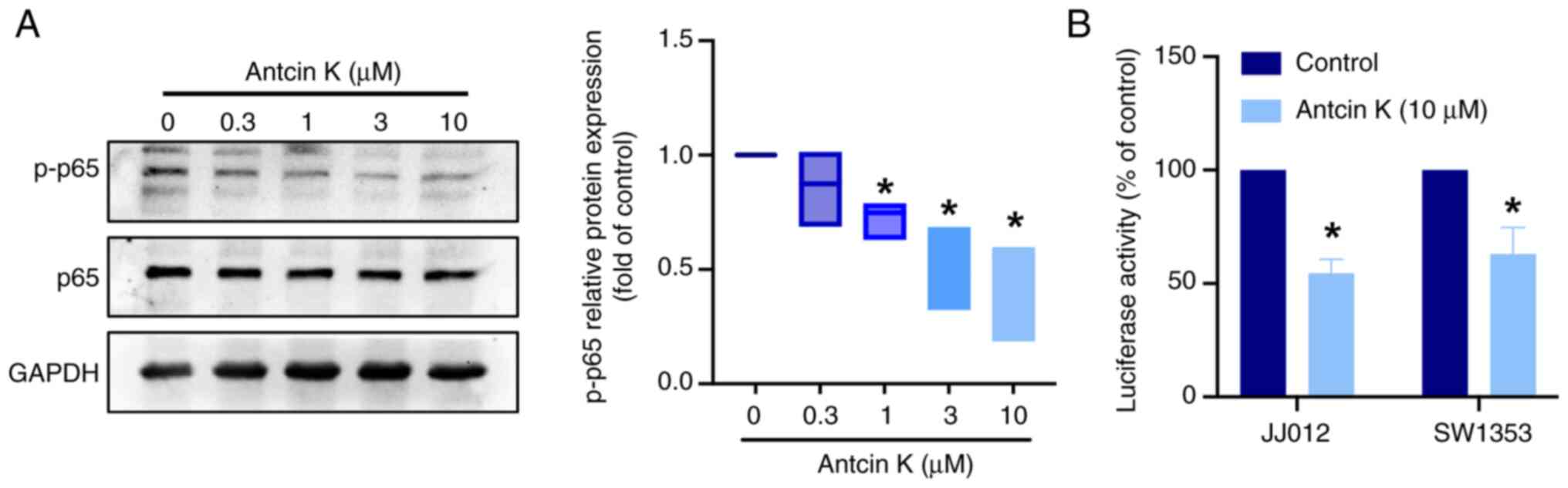
decreased the levels of p-p85, p-Akt and p-mTOR (Fig. 6). Furthermore, the phosphorylation
of p65 decreased following antcin K stimulation (Fig. 7A). NF-κB luciferase reporter assay
was performed to evaluate the effects of antcin K on NF-κB
activity. Antcin K decreased NF-κB luciferase activity (Fig. 7B). These data support the
hypothesis that antcin K abolishes the PI3K, Akt, mTOR and NF-κB
pathways in chondrosarcoma.
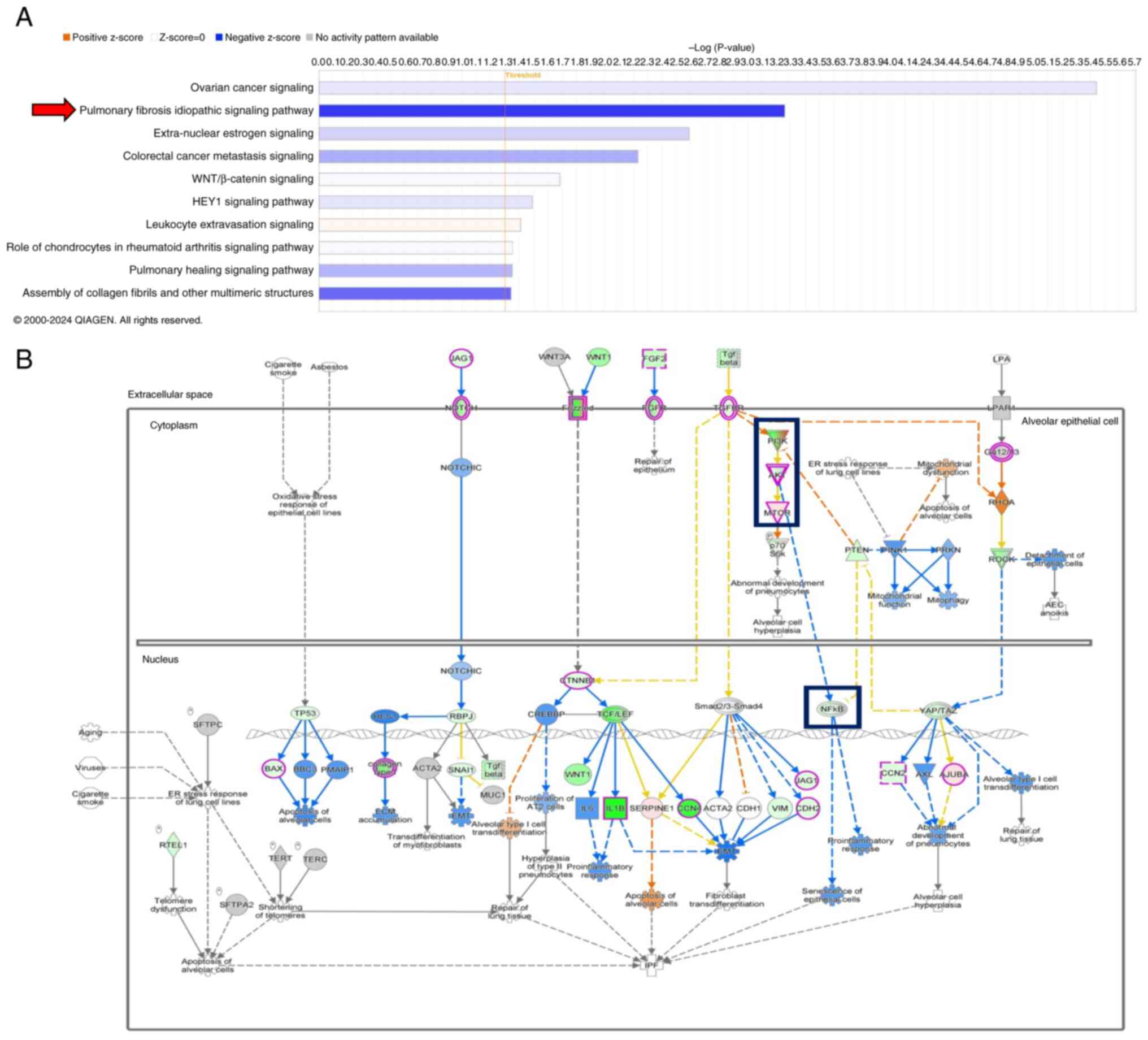 | Figure 5.Biological processes regulated by
antcin K. IPA pathway enrichment of RNA sequencing data comparing
control with JJ012 cells treated with antcin K. (A) Histogram of 10
canonical pathways. (B) Map of ‘pulmonary fibrosis idiopathic
signaling pathway’ enriched by IPA. IPA, Ingenuity Pathway
Analysis; AEC, alveolar epithelial cell; ACTA2, alpha smooth muscle
actin 2; CCL2, C-C motif chemokine ligand 2; CDKN1A (p21),
cyclin-dependent kinase inhibitor 1A; COL1A1, collagen type I alpha
1 chain; connective tissue growth factor; EDN1, endothelin 1; ER,
endoplasmic reticulum; FGF2, fibroblast growth factor 2; FN1,
fibronectin 1; FOXO3, forkhead box O3; HIF1A, hypoxia inducible
factor 1 subunit alpha; ITGA5, integrin subunit alpha 5; JAG1,
jagged canonical Notch ligand 1; MAPK1, mitogen-activated protein
kinase 1; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and
tensin homolog; RARA, retinoic acid receptor alpha; RBPJ,
recombination signal binding protein for immunoglobulin kappa J
region; RIDA, regulator of inflammatory and damage-associated
responses; SERPINE1 (PAI-1), serpin family E member 1; SNAI1, snail
family transcriptional repressor 1; SOX2, SRY-box transcription
factor 2; TERT, telomerase reverse transcriptase; TGFB1,
transforming growth factor beta 1; TNF, tumor necrosis factor;
TP53, tumor protein p53; VEGFA, vascular endothelial growth factor
A; VIM, vimentin. |
Discussion
The discovery of natural compounds and their
structural analogs has been beneficial to pharmacotherapy,
particularly in the treatment of cancer (41). Agents such as vinblastine, taxol
and camptothecin are useful in treating numerous types of cancer,
including ovarian, lung and breast cancer (42). The fungus A. cinnamomea has
been used in Taiwanese traditional medicine for centuries to treat
hypertension, cancer, liver disease and inflammatory conditions
(43). Antcin K, a functional
molecule derived from the fruiting bodies of A. cinnamomea,
has anti-inflammatory abilities that markedly decrease IL-6, IL-1β
and TNF-α production (44) and
mediates anti-inflammatory effects in arthritic illness (45). Antcin K inhibits proliferation and
motility in hepatoma cancer (27,28),
however, to the best of our knowledge, the present study is the
first to investigate the effects of antcin K on chondrosarcoma
motility. Antcin K inhibited chondrosarcoma MMP-7 generation, cell
migration and invasion. Antcin K also inhibited the PI3K, Akt, mTOR
and NF-κB signaling cascades. Han et al (44) reported that antcin K decreases
central neuroinflammation, thereby alleviating depression in mice
at doses of 5 and 15 mg/kg. Antcin K reduced cartilage degradation
in collagen-induced arthritic mice (25).
As triterpenoids progress from laboratory research
to clinical application, highlighted by the US Food and Drug
Administration (FDA) approval of omaveloxolone (46), and numerous clinical trials of
triterpenoids in different types of disease (47,48),
obtaining an understanding of their biological mechanisms in the
context of cancer is key. The present study and previous research
(28,49) implicate antcin K, a triterpenoid,
in carcinogenesis inhibition and suggest that it may be a potential
candidate for clinical trials.
Cancer cells undergo metastasis to colonize
additional locations (50). MMPs
serve a key role in a number of cancer metastatic processes.
Overexpressed and active MMPs facilitate the establishment of a
conducive microenvironment in tumor tissue (51). The identification of efficient MMP
inhibitors for therapeutic use has yielded encouraging results
(52,53). However, due to their poor
solubility, lack of potency and adverse clinical trial outcomes,
the FDA has not yet licensed any MMP inhibitors for use in cancer
treatment (54,55). The smallest enzyme in the MMP
family, MMP-7 serves a key role in promoting the progression of
numerous types of tumor such as gastric, pancreatic and colorectal
cancer (56). By contrast with
normal cartilage, MMP-7 is substantially expressed in the tissue of
patients with chondrosarcoma (11). The aforementioned study further
highlighted that MMP-7 regulates the migration and invasion of
chondrosarcoma cells (14). In the
present study, RNA-seq analysis revealed that antcin K treatment
had a more pronounced effect on downregulating MMP-7, compared with
other MMPs. Results from in vitro cell migration and
invasion assays demonstrated that antcin K markedly diminished
chondrosarcoma motility. Transfection with MMP-7 cDNA antagonized
antcin K-induced inhibition of cell motility, indicating that
antcin K inhibits MMP-7-dependent chondrosarcoma motility.
Several signaling pathways promote the motility,
angiogenesis and proliferation of chondrosarcoma cells (57). IPA demonstrated that the ‘pulmonary
fibrosis idiopathic signaling pathway’, which includes PI3K, Akt,
mTOR and NF-κB, is a prime candidate signaling pathway. Antcin K
stimulation diminished p85, Akt and mTOR phosphorylation.
Accumulating evidence indicates that the PI3K, Akt and mTOR
pathways serve key functions in chondrosarcoma metastasis (57–60).
First, endothelin-1 facilitates MMP-13 generation and
chondrosarcoma metastasis via the PI3K, Akt and mTOR pathway
(58). Secondly, the adipokine
adiponectin facilitates chondrosarcoma-associated angiogenesis via
the PI3K, Akt and mTOR pathway (59). Furthermore, nerve growth factor
controls PI3K, Akt and mTOR cascades, upregulating chondrosarcoma
motility (60). NF-κB mediates a
key role in chondrosarcoma metastasis (61,62)
and antcin K activation decreases expression of p-p65. NF-κB is a
key transcription factor regulating antcin K-regulated
chondrosarcoma motility, as evidenced by the fact that antcin K
eliminated NF-κB luciferase activity.
The present study had limitations, including lack of
experiments examining the inhibitory effects of antcin K on
chondrosarcoma metastasis in mice. The challenges of the synthesis
resulted in insufficient quantities of antcin K, limiting the
ability to conduct in vivo studies to explore its potential
effects on chondrosarcoma cell metastasis in animal models and
necessitating further research.
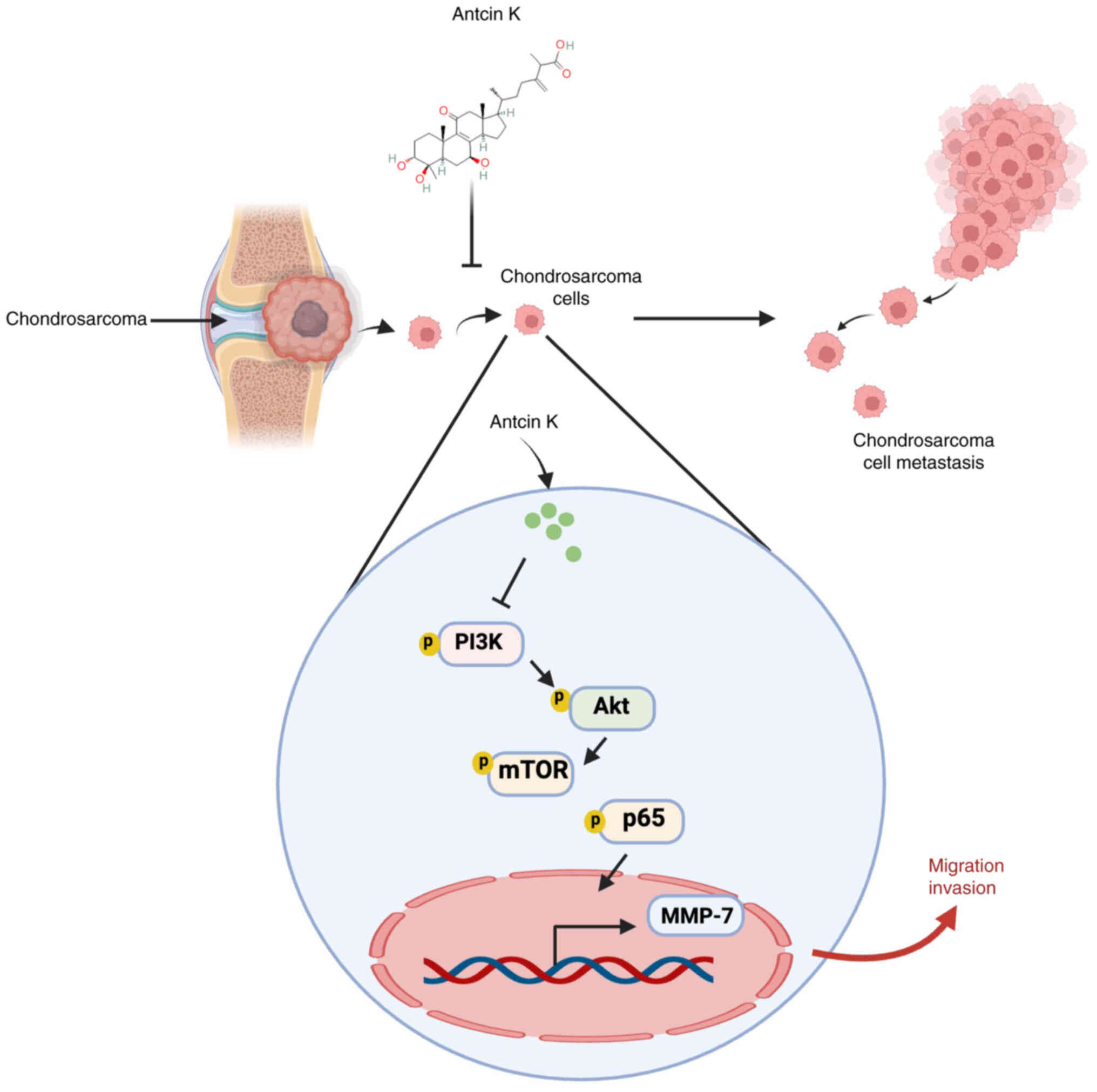
In conclusion, antcin K significantly suppressed
MMP-7 production and cell motility in chondrosarcoma by inhibiting
the PI3K, Akt, mTOR and NF-κB signaling pathways (Fig. 8). These findings demonstrated the
key effect of antcin K in metastatic chondrosarcoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Science and
Technology Council of Taiwan (grant no. NSTC
113-2320-B-039-049-MY3), China Medical University Hospital (grant
nos. DMR-113-070, DMR-113-200 and DMR-114-033) and China Medical
University under the Higher Education Sprout Project, Ministry of
Education, Taiwan (grant no. CMRC-CENTER-7).
Availability of data and materials
The data generated in the present study may be found
in the Gene Expression Omnibus database under accession number
GSE287361 or at the following URL: ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE287361.
Authors' contributions
YYL performed experiments, analyzed data and wrote
the manuscript. NBT and CYS analyzed data. YYW, HTC, YHK, CHT, YCF
and HCT designed the experiments. YHK performed experiments. CHT
conceived the study and revised the manuscript. YYL, NBT and CHT
confirm the authenticity of all raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Gazendam A, Popovic S, Parasu N and Ghert
M: Chondrosarcoma: A clinical review. J Clin Med. 12:25062023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quoniou R, Moreau E, Cachin F, Blavignac
C, Bortoli E, Chautard E and Peyrode C: Chondrosarcoma Co-Culture
3D model-an insight to evaluate drugs acting on TAMs. ACS Biomater
Sci Eng. 10:5832–5843. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pathmanapan S, Poon R, De Renshaw TB,
Nadesan P, Nakagawa M, Seesankar GA, Ho Loe AK, Zhang HH, Guinovart
JJ, Duran J, et al: Mutant IDH regulates glycogen metabolism from
early cartilage development to malignant chondrosarcoma formation.
Cell Rep. 42:1125782023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pennington Z, Ehresman J, Pittman PD,
Ahmed AK, Lubelski D, McCarthy EF, Goodwin CR and Sciubba DM:
Chondrosarcoma of the spine: A narrative review. Spine J.
1:2078–2096. 2021. View Article : Google Scholar
|
|
5
|
Nazeri E, Gouran Savadkoohi M,
Majidzadeh-A K and Esmaeili R: Chondrosarcoma: An overview of
clinical behavior, molecular mechanisms mediated drug resistance
and potential therapeutic targets. Crit Rev Oncol Hematol.
131:102–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skipar P, Dey M, Piątkowski J, Sulejczak
D, Rutkowski P and Czarnecka AM: MicroRNAs as prognostic biomarkers
and therapeutic targets in chondrosarcoma. Int J Mol Sci.
25:31762024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ducrot C, Dinart D, Reich M, Bonneau M,
Brunet M, Nannini S, Berchoud J, Bellio H, Cherrier G, Narciso B,
et al: 1970P Metastatic chondrosarcoma, patterns of care and
outcomes of patients in a real-life setting: The Metabone national
observational study. Ann Oncol. 34 (Suppl 2):S10522023. View Article : Google Scholar
|
|
8
|
Li KHC, Gulia A, Duffaud F and Jones RL:
Advancing systemic therapy in chondrosarcoma: New Horizons. Oncol
Ther. 13:1–9. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Majidpoor J and Mortezaee K: Steps in
metastasis: An updated review. Med Oncol. 38:32021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonzalez-Avila G, Sommer B, Mendoza-Posada
DA, Ramos C, Garcia-Hernandez AA and Falfan-Valencia R: Matrix
metalloproteinases participation in the metastatic process and
their diagnostic and therapeutic applications in cancer. Crit Rev
Oncol Hematol. 137:57–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X and Khalil RA: Matrix
metalloproteinases, vascular remodeling, and vascular disease. Adv
Pharmacol. 81:241–330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bassiouni W, Ali MAM and Schulz R:
Multifunctional intracellular matrix metalloproteinases:
Implications in disease. FEBS J. 288:7162–7182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L and Ke X: MMP7 as a potential
biomarker of colon cancer and its prognostic value by
bioinformatics analysis. Medicine (Baltimore). 100:e249532021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan PP, Yu X, Guo JJ, Wang Y, Wang T, Li
JY, Konstantopoulos K, Wang ZY and Wang P: By activating matrix
metalloproteinase-7, shear stress promotes chondrosarcoma cell
motility, invasion and lung colonization. Oncotarget. 6:9140–9159.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen BT, Lin CY, Chang TK, Fong YC,
Thadevoos LA, Lai CY, Huang YL, Tsai CH, Ko CY, Liu JF, et al:
Melatonin inhibits chondrosarcoma cell proliferation and metastasis
by enhancing miR-520f-3p production and suppressing MMP7
expression. J Pineal Res. 75:e128722023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Chen M, Yu H, Yuan G, Luo L, Xu X,
Xu Y, Sui X, Leung EL and Wu Q: The role and mechanisms of action
of natural compounds in the prevention and treatment of cancer and
cancer metastasis. Front Biosci (Landmark Ed). 27:1922022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen YF, Lu YH and Tsai HY: Crude extract
of Desmodium gangeticum process anticancer activity via arresting
cell cycle in G1 and modulating cell cycle-related protein
expression in A549 human lung carcinoma cells. BioMedicine
(Taipei). 12:31–39. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HP, Chen PC, Wang SW, Fong YC, Tsai
CH, Tsai FJ, Chung JG, Huang CY, Yang JS, Hsu YM, et al: Plumbagin
suppresses endothelial progenitor cell-related angiogenesis in
vitro and in vivo. J Funct Foods. 52:537–544. 2019. View Article : Google Scholar
|
|
20
|
MacDonald IJ, Lin CY, Kuo SJ, Su CM and
Tang CH: An update on current and future treatment options for
chondrosarcoma. Expert Rev Anticancer Ther. 19:773–786. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ganesan N, Baskaran R, Velmurugan BK and
Thanh NC: Antrodia cinnamomea-An updated minireview of its
bioactive components and biological activity. J Food Biochem.
43:e129362019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YY, Chou PY, Chien YC, Wu CH, Wu TS
and Sheu MJ: Ethanol extracts of fruiting bodies of Antrodia
cinnamomea exhibit anti-migration action in human adenocarcinoma
CL1-0 cells through the MAPK and PI3K/AKT signaling pathways.
Phytomedicine. 19:768–778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang TT, Wu SP, Chong KY, Ojcius DM, Ko
YF, Wu YH, Wu CY, Lu CC, Martel J, Young JD and Lai HC: The
medicinal fungus Antrodia cinnamomea suppresses inflammation by
inhibiting the NLRP3 inflammasome. J Ethnopharmacol. 155:154–164.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Achudhan D, Li-Yun Chang S, Liu SC, Lin
YY, Huang WC, Wu YC, Huang CC, Tsai CH, Ko CY, Kuo YH and Tang CH:
Antcin K inhibits VCAM-1-dependent monocyte adhesion in human
rheumatoid arthritis synovial fibroblasts. Food Nutr Res. 66:2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Achudhan D, Liu SC, Lin YY, Huang CC, Tsai
CH, Ko CY, Chiang IP, Kuo YH and Tang CH: Antcin K inhibits TNF-α,
IL-1β and IL-8 expression in synovial fibroblasts and ameliorates
cartilage degradation: Implications for the treatment of rheumatoid
arthritis. Front Immunol. 12:7909252021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Achudhan D, Liu SC, Lin YY, Lee HP, Wang
SW, Huang WC, Wu YC, Kuo YH and Tang CH: Antcin K inhibits
VEGF-dependent angiogenesis in human rheumatoid arthritis synovial
fibroblasts. J Food Biochem. 46:e140222022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai CI, Chu YL, Ho CT, Su YC, Kuo YH and
Sheen LY: Antcin K, an active triterpenoid from the fruiting bodies
of basswood cultivated Antrodia cinnamomea, induces mitochondria
and endoplasmic reticulum stress-mediated apoptosis in human
hepatoma cells. J Tradit Complement Med. 6:48–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang YL, Chu YL, Ho CT, Chung JG, Lai CI,
Su YC, Kuo YH and Sheen LY: Antcin K, an active triterpenoid from
the fruiting bodies of basswood-cultivated antrodia cinnamomea,
inhibits metastasis via suppression of integrin-mediated adhesion,
migration, and invasion in human hepatoma cells. J Agric Food Chem.
63:4561–4569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tzeng HE, Lin SL, Thadevoos LA, Ko CY, Liu
JF, Huang YW, Lin CY, Fong YC and Tang CH: The mir-423-5p/MMP-2
axis regulates the nerve growth factor-induced promotion of
chondrosarcoma metastasis. Cancers (Basel). 13:33472021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HP, Wang SW, Wu YC, Wu Y, Lin LW, Tsai
FJ, Yang JS, Li TM and Tang CH: Soya-cerebroside inhibits
VEGF-facilitated angiogenesis in endothelial progenitor cells. Food
Agr Immunol. 31:193–204. 2020. View Article : Google Scholar
|
|
31
|
Liu SC, Tsai CH, Wu TY, Liu SC, Tsai CH,
Wu TY, Tsai CH, Tsai FJ, Chung JG, Huang CY, et al:
Soya-cerebroside reduces IL-1β-induced MMP-1 production in
chondrocytes and inhibits cartilage degradation: Implications for
the treatment of osteoarthritis. Food Agric Immunol. 30:620–632.
2019. View Article : Google Scholar
|
|
32
|
Tran NB, Chang TK, Chi NDP, Lai KY, Chen
HT, Fong YC, Liaw CC and Tang CH: Ugonin inhibits chondrosarcoma
metastasis through suppressing cathepsin V via promoting
miR-4799-5p expression. Int J Biol Sci. 21:1144–1157. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee KT, Su CH, Liu SC, Chen BC, Chang JW,
Tsai CH, Huang WC, Hsu CJ, Chen WC, Wu YC and Tang CH:
Cordycerebroside A inhibits ICAM-1-dependent M1 monocyte adhesion
to osteoarthritis synovial fibroblasts. J Food Biochem.
46:e141082022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su CM, Tsai CH, Chen HT, Wu YS, Chang JW,
Yang SF and Tang CH: Melatonin improves muscle injury and
differentiation by increasing Pax7 expression. Int J Biol Sci.
19:1049–1062. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen CY, Su CM, Hsu CJ, Huang CC, Wang SW,
Liu SC, Chen WC, Fuh LJ and Tang CH: CCN1 promotes VEGF production
in osteoblasts and induces endothelial progenitor cell angiogenesis
by inhibiting miR-126 expression in rheumatoid arthritis. J Bone
Miner Res. 32:34–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang SW, Tai HC, Tang CH, Lin LW, Lin TH,
Chang AC, Chen PC, Chen YH, Wang PC, Lai YW and Chen SS: Melatonin
impedes prostate cancer metastasis by suppressing MMP-13
expression. J Cell Physiol. 236:3979–3990. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu SC, Law YY, Wu YY, Huang YL, Tsai CH,
Chen WC and Tang CH: Fibrosis factor CTGF facilitates
VCAM-1-dependent monocyte adhesion to osteoarthritis synovial
fibroblasts via the FAK and JNK pathways. Mol Med Rep. 31:1242025.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakagawa M, Nakatani F, Matsunaga H, Seki
T, Endo M, Ogawara Y, Machida Y, Katsumoto T, Yamagata K, Hattori
A, et al: Selective inhibition of mutant IDH1 by DS-1001b
ameliorates aberrant histone modifications and impairs tumor
activity in chondrosarcoma. Oncogene. 38:6835–6849. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Senthil Kumar KJ, Gokila Vani M, Chen CY,
Hsiao WW, Li J, Lin ZX, Chu FH, Yen GC and Wang SY: A mechanistic
and empirical review of antcins, a new class of phytosterols of
formosan fungi origin. J Food Drug Anal. 28:38–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao S, Yan L, Zhang H, Fan X, Jiao X and
Shao F: Identification of a metastasis-associated gene signature of
clear cell renal cell carcinoma. Front Genet. 11:6034552021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Atanasov AG, Zotchev SB and Dirsch VM;
International Natural Product Sciences Taskforce; Supuran CT, :
Natural products in drug discovery: Advances and opportunities. Nat
Rev Drug Discov. 20:200–216. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Atanasov AG, Waltenberger B,
Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L,
Schwaiger S, Heiss EH, et al: Discovery and resupply of
pharmacologically active plant-derived natural products: A review.
Biotechnol Adv. 33:1582–1614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang BB, Guan YY, Hu PF, Chen L, Xu GR,
Liu L and Cheung PCK: Production of bioactive metabolites by
submerged fermentation of the medicinal mushroom Antrodia
cinnamomea: Recent advances and future development. Crit Rev
Biotechnol. 39:541–554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han C, Pei H, Shen H, Zhai L, Yang Y, Li W
and Wang J: Antcin K targets NLRP3 to suppress neuroinflammation
and improve the neurological behaviors of mice with depression. Int
Immunopharmacol. 117:1099082023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tung YT, Tsai TC, Kuo YH, Yen CC, Sun JY,
Chang WH, Chen HL and Chen CM: Comparison of solid-state-cultured
and wood-cultured Antrodia camphorata in anti-inflammatory effects
using NF-ĸB/luciferase inducible transgenic mice. Phytomedicine.
21:1708–1716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moerland JA and Liby KT: The triterpenoid
CDDO-Methyl ester reduces tumor burden, reprograms the immune
microenvironment, and protects from chemotherapy-induced toxicity
in a preclinical mouse model of established lung cancer.
Antioxidants (Basel). 13:6212024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yadav VR, Prasad S, Sung B, Kannappan R
and Aggarwal BB: Targeting inflammatory pathways by triterpenoids
for prevention and treatment of cancer. Toxins (Basel).
2:2428–2466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bankar AA, Nagulwar VP, Kotagale NR and
Inamdar NN: Neuroprotective prospectives of triterpenoids. Explor
Neurosci. 3:231–254. 2024. View Article : Google Scholar
|
|
49
|
Tien AJ, Chien CY, Chen YH, Lin LC and
Chien CT: Fruiting bodies of antrodia cinnamomea and its active
triterpenoid, Antcin K, Ameliorates N-nitrosodiethylamine-induced
hepatic inflammation, fibrosis and carcinogenesis in rats. Am J
Chin Med. 45:173–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Suhail Y, Cain MP, Vanaja K, Kurywchak PA,
Levchenko A and Kalluri R: Kshitiz: Systems biology of cancer
metastasis. Cell Syst. 9:109–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang C, Jiang G and Gao X: Matrix
metalloproteinase-responsive drug delivery systems. Bioconjug Chem.
34:1349–1365. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fields GB: The rebirth of matrix
metalloproteinase inhibitors: Moving beyond the dogma. Cells.
8:9842019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rashid ZA and Bardaweel SK: Novel matrix
metalloproteinase-9 (MMP-9) inhibitors in cancer treatment. Int J
Mol Sci. 24:121332023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Almutairi S, Kalloush HM, Manoon NA and
Bardaweel SK: Matrix metalloproteinases inhibitors in cancer
treatment: An updated review (2013–2023). Molecules. 28:55672023.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liao HY, Da CM, Liao B and Zhang HH: Roles
of matrix metalloproteinase-7 (MMP-7) in cancer. Clin Biochem.
92:9–18. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Micaily I, Roche M, Ibrahim MY,
Martinez-Outschoorn U and Mallick AB: Metabolic pathways and
targets in chondrosarcoma. Front Oncol. 11:7722632021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu MH, Lo JF, Kuo CH, Lin JA, Lin YM, Chen
LM, Tsai FJ, Tsai CH, Huang CY and Tang CH: Endothelin-1 promotes
MMP-13 production and migration in human chondrosarcoma cells
through FAK/PI3K/Akt/mTOR pathways. J Cell Physiol. 227:3016–3026.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee HP, Lin CY, Shih JS, Fong YC, Wang SW,
Li TM and Tang CH: Adiponectin promotes VEGF-A-dependent
angiogenesis in human chondrosarcoma through PI3K, Akt, mTOR, and
HIF-α pathway. Oncotarget. 6:36746–36761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tzeng HE, Lin SL, Thadevoos LA, Lien MY,
Yang WH, Ko CY, Lin CY, Huang YW, Liu JF, Fong YC, et al: Nerve
growth factor promotes lysyl oxidase-dependent chondrosarcoma cell
metastasis by suppressing miR-149-5p synthesis. Cell Death Dis.
12:11012021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu Y, Li ZH, Zhang L and Lu SB: ADAM8
promotes chondrosarcoma cell migration and invasion by activating
the NF-κB/MMP-13 signaling axis. Anticancer Drugs. 30:e07902019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tzeng HE, Tang CH, Wu SH, Chen HT, Fong
YC, Lu YC, Chen WC, Huang HD, Lin CY and Wang SW: CCN6-mediated
MMP-9 activation enhances metastatic potential of human
chondrosarcoma. Cell Death Dis. 9:9552018. View Article : Google Scholar : PubMed/NCBI
|