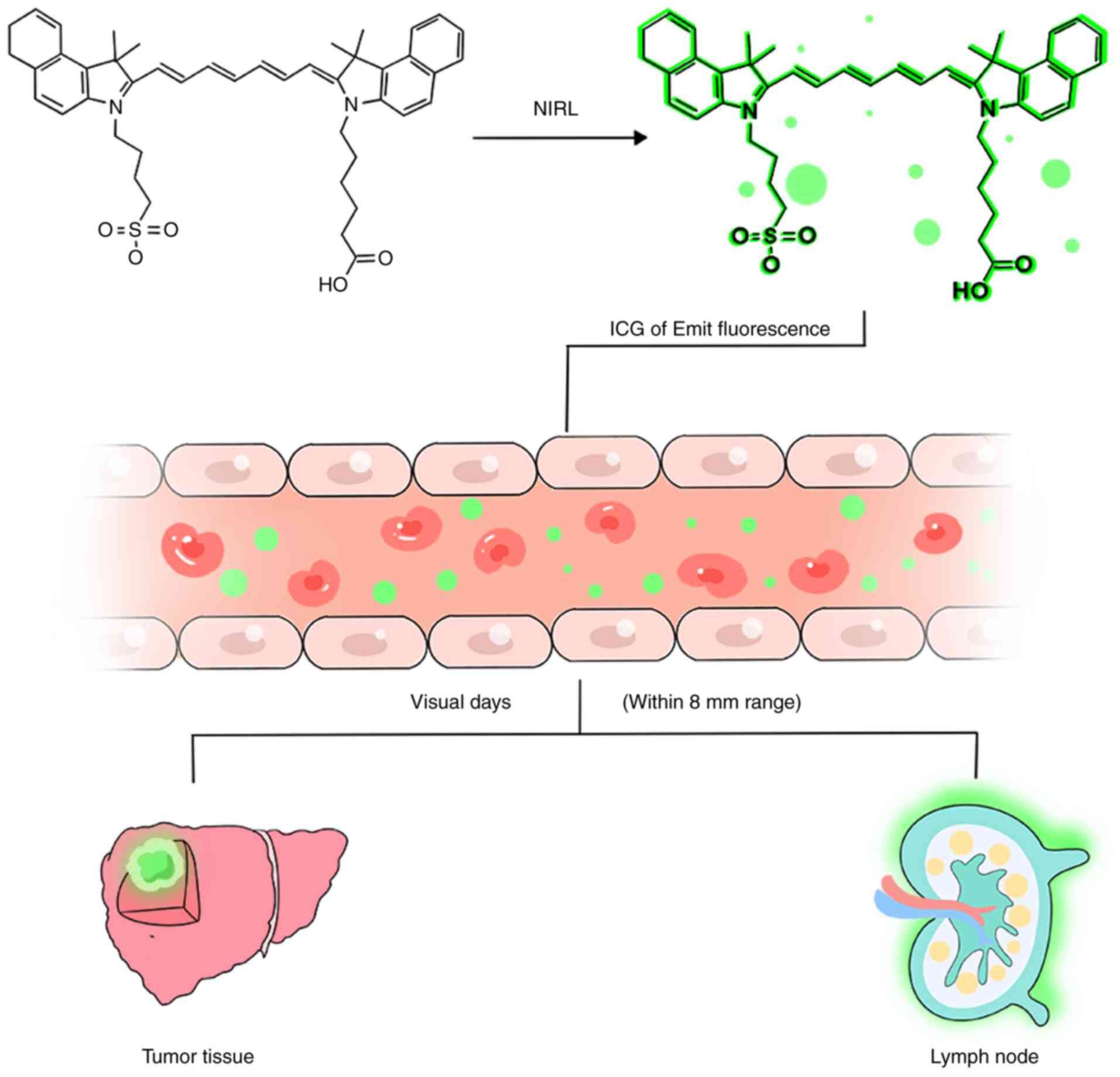
The development of new drugs is challenging, as it
requires lengthy research and development cycles, and incurs high
costs. This makes explorations of new clinical applications for
existing drugs advantageous. One such example is the use of
indocyanine green (ICG) in near-infrared light (NIRL) fluorescence
imaging, which involves repurposing an existing drug for innovative
applications. ICG is a water-soluble, relatively non-toxic iodide
dye with a molecular weight of 776 Da (1). After intravenous injection, ICG binds
to plasma proteins, remains in the vascular compartment and is
rapidly excreted into bile, resulting in rapid hepatic clearance
(2). These properties allow its
effective application even at lower doses. The United States Food
and Drug Administration approved the use of ICG in the medical
field in 1959 (3), and it has been
safely utilized since the mid-1950s. The established safety and
efficacy of ICG have paved the way for new applications in various
fields.
Primary liver cancer is the 6th most common cancer
worldwide and the second leading cause of cancer-related mortality,
and 80% of cases are attributed to hepatocellular carcinoma (HCC)
(16). Additionally, secondary
liver metastases are frequently observed in patients with common
types of cancer, including breast, lung, colorectal, pancreatic,
esophageal, gastric and small intestine cancer, among others
(17). In previous years, ICG NIRL
fluorescence imaging has been integrated into hepatobiliary surgery
to enhance the identification of HCC lesions during operations. In
one study, 273 out of 276 HCC lesions were successfully identified
using fluorescence imaging, with a sensitivity of 99%. However, 16
false-positive lesions were detected, primarily including large
regenerative nodules, heterogeneous hyperplastic nodules, bile duct
hyperplasia and necrosis, leading to a positive predictive value of
94% (18). The fluorescence
pattern of HCC is closely associated with its pathological
features; well- and moderately differentiated HCCs display complete
or partial fluorescence, whereas poorly differentiated HCCs, often
with microvascular invasion, predominantly show marginal
fluorescence (18–20). Furthermore, Abo et al
(21) demonstrated that among 12
patients with intrahepatic cholangiocarcinoma, 10 (83%) exhibited
ICG fluorescence. Of these, 60% displayed marginal staining and 30%
showed complete or partial staining. This study also revealed the
potential of ICG fluorescence imaging combined with portal vein
injections to assess the extent of portal vein tumor thrombosis in
patients with HCC while also identifying benign lesions, such as
hepatic hemangiomas, cholangiocarpal adenomas and cavernous
hemangiomas (21). Therefore, ICG
fluorescence imaging has been demonstrated to be a reliable tool
for surgical navigation, enhancing the safety and precision of HCC
resection.
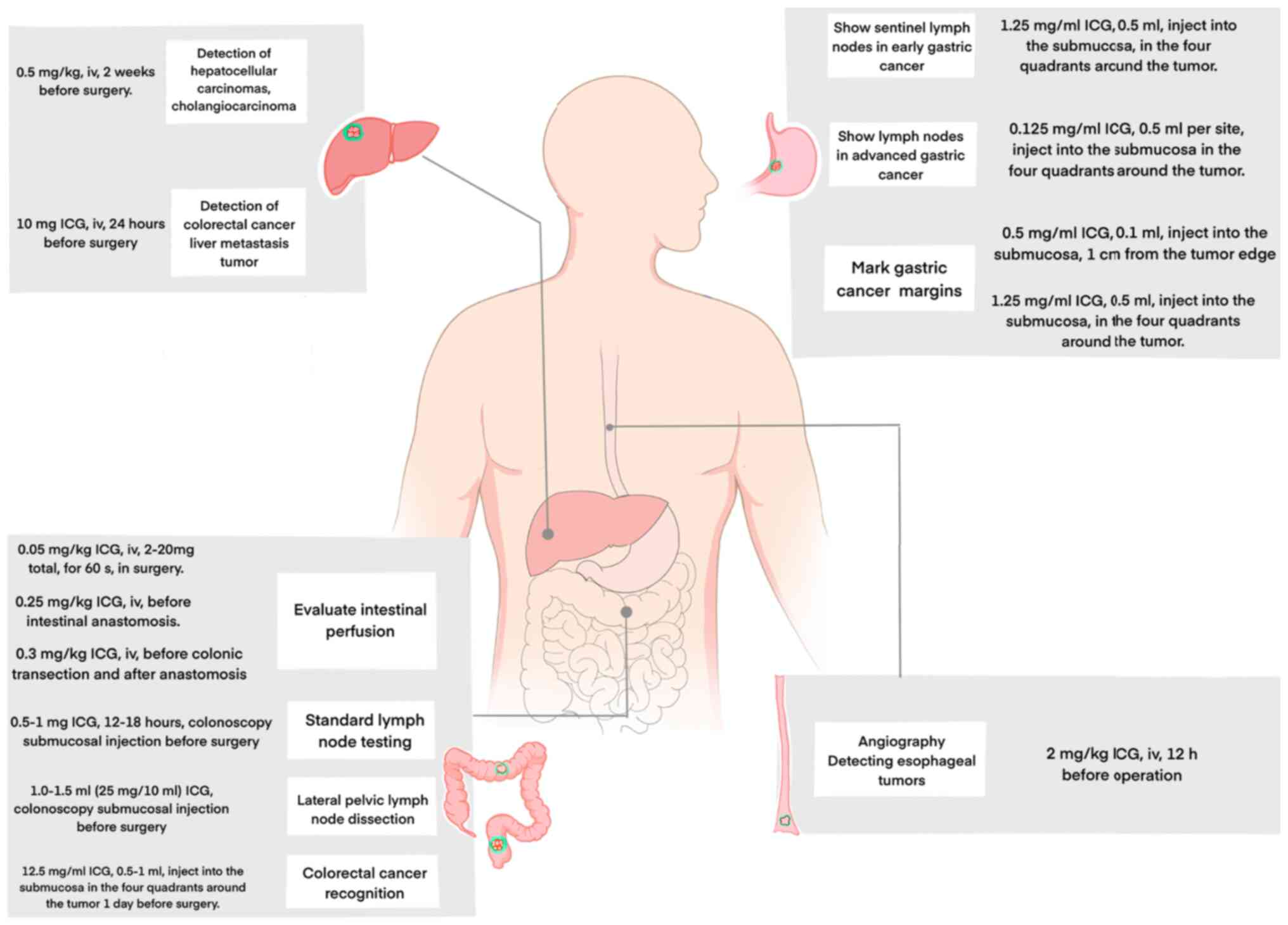
For injection protocols, previous studies have
suggested that administering ICG intravenously at a dose of 0.5
mg/kg body weight within 2 weeks prior to surgery enables the
effective intraoperative visualization of liver tumors (19,22)
(Table I; Fig. 2). In the surgical treatment of
colorectal cancer (CRC) liver metastasis, ICG fluorescence imaging
provides real-time feedback regarding tumor margins and yields
higher rates of complete tumor resection in patients who received a
single intravenous infusion of 10 mg of ICG 24 h before surgery
(23) (Fig. 2). This guideline is based on
protocols that were originally designed for preoperative liver
function assessments rather than direct tumor detection. In
patients with cirrhosis or compromised liver function due to
chemotherapy, the administration of ICG the day before surgery
should be avoided to minimize background liver signals and reduce
the likelihood of false positives (24). These injection protocols may need
to be tailored for specific clinical scenarios on the basis of
emerging evidence.
Despite the high sensitivity and positive predictive
value of ICG fluorescence imaging, false-positive rates remain a
concern, reaching up to 40% in certain cases (18,19).
The factors that contribute to high false-positive rates include
cirrhosis, dysplastic nodules, brief intervals between ICG
injection and surgery (<24 h), bile duct hyperplasia, necrosis,
cysts, hemangiomas and atypical non-malignant lesions. As a result,
new lesions that are detected by fluorescence should undergo
additional evaluation through examination, palpation or
intraoperative ultrasound (IOUS). In response to these
false-positive situations, nanoprobe fluorescence imaging is more
targeted, more feasible and safer when compared with ICG
fluorescence imaging (25).
Occasionally, ICG fluorescence imaging may also yield
false-negative results, particularly when the time between ICG
injection and surgery exceeds 24 days, requiring careful
consideration in clinical decision-making.
ICG NIRL fluorescence imaging improves
intraoperative visualization and the resection rate of liver
tumors, and it is a good navigation tool in the surgical treatment
of gastrointestinal and esophageal cancer, with improved
recognition and safety of tumor resection.
Gastric cancer is the 5th most prevalent malignancy
worldwide and a leading cause of cancer-related mortality due to
its advanced stage at diagnosis; gastric cancer is the third most
common cause of cancer-related mortality (26). Currently, surgical treatment
remains the cornerstone of treatment for both early and advanced
gastric cancer (26). In previous
years, laparoscopic surgery has been validated as a safe and
effective option for gastric cancer management in both Eastern
Europe and Western nations (27–39).
These findings suggest that minimally invasive approaches for
gastric cancer treatment may dominate future surgical trends.
Initially, ICG NIRL fluorescence imaging was used
for sentinel lymph node detection in early gastric cancer (40,41).
Its use has since expanded to include preoperative endoscopic
marking for tumor localization, ensuring negative surgical margins
(42) and facilitating
comprehensive lymph node mapping (43). Lymph node retrieval is key for
accurate staging, with most guidelines recommending the examination
of at least 16 regional nodes, whereas the retrieval of 30 or more
nodes is considered optimal (44,45).
Several studies have demonstrated that ICG NIRL fluorescence
imaging increases the efficiency of regional lymph node dissection
during laparoscopic surgery, increasing the yield of nodes at key
anatomical sites (45,46–49).
Compared with traditional lymphadenectomy methods, ICG-guided
laparoscopic lymphadenectomy is both safe and effective in
improving survival outcomes for patients with resectable gastric
cancer (50). Additionally, a
randomized controlled trial revealed that ICG markedly increases
the quality of lymph node dissection in patients with locally
advanced gastric cancer undergoing laparoscopic radical gastrectomy
following neoadjuvant chemotherapy (51). For early mucosal tumors without
ulceration or ulcer scarring, endoscopic mucosal resection (EMR) is
typically recommended. The decision to carry out additional radical
gastric cancer surgery with lymph node dissection is made on the
basis of the pathologic assessment of the EMR specimen,
particularly regarding infiltration depth and ulceration status
(52). While no reports have
specifically addressed the use of fluorescence imaging to guide
tumor localization during EMR, a previous study suggests that ICG
fluorescent lymphography-administered via endoscopic injection
around the postoperative scar-can allow effective visualization of
lymphatic vessels and assessment of the sensitivity and negative
predictive value for detecting lymph node metastasis (53). There is no standardized method in
terms of specific injections when ICG fluorescence imaging is used
to identify lymph nodes in gastric cancer. For identification of
sentinel lymph nodes in gastric cancer, 0.5–2.5 mg/ml of ICG
solution was endoscopically injected into 4–8 sites in the
submucosal layer surrounding the tumor (a total of 2–4 ml) during
surgery for gastric cancer (54).
During surgical treatment of advanced gastric cancer, 0.125 mg/ml
ICG solution was endoscopically injected into four sites in the
submucosal layer surrounding the tumor, 0.5 ml per site, during
surgery for gastric cancer (55)
(Fig. 2).
In addition to lymph node localization, ICG NIRL
fluorescence imaging has been demonstrated to be valuable for
marking tumor margins. In laparoscopic gastrectomy, ICG was
endoscopically injected into the submucosal layer of the stomach,
~1 cm from the tumor edge, to delineate early-stage cancer
boundaries. The recommended dose was 0.1 ml of 0.5 mg/ml ICG
(Fig. 2), which ensured clear
margins without excessive blurring (56). Despite the lack of a standardized
submucosal injection protocol, a study have utilized higher
concentrations (1.25 mg/ml), injecting 0.5 ml around the primary
tumor in four quadrants, equivalent to 2.5 mg of ICG (57) (Table
I) (Fig. 2).
While extensive research supports the role of
ICG-guided lymph node dissection during laparoscopic gastrectomy,
its practical advantages remain under debate, with certain studies
highlighting potential limitations (58,59),
but they have small sample sizes, necessitating larger, randomized
clinical trials. A previous single-center study published in The
Journal of the American Medical Association Surgery, which
increased the sample size to 133 cases, revealed that only 56.3% of
metastatic lymph nodes were detected by fluorescence, suggesting a
notable risk of false-negative results when ICG NIRL fluorescence
imaging was used for metastatic lymph node identification (60). These false-negative results may
occur due to extensive cancer cell infiltration or lymphatic
obstruction (61), resulting in
the failure of ICG to accumulate in positive lymph nodes. This may
also be the result of insufficient learning time for the operator
and incomplete histological evaluation of frozen sections. A small
prospective study by Shoji et al (62) revealed that when ICG was injected
around the primary tumor during surgery, followed by a one-step
nucleic acid amplification assay, the expression of the epithelial
protein CK19 could be rapidly determined. The detection rate of
sentinel lymph nodes using this method was 85%, with a
false-negative rate as low as 0% (62).
Esophageal cancer is associated with high morbidity
and mortality rates, surpassing several other malignancies in terms
of severity (63). Radical
esophagectomy remains the primary treatment option for early-stage
esophageal cancer. However, despite surgical intervention, ~80% of
patients experience tumor recurrence, with >40% of recurrences
attributed to lymph node metastasis (64). Thus, the precision of lymph node
dissection during radical esophagectomy is key for improving the
outcomes of patients. ICG NIRL fluorescence imaging has
demonstrated promise in identifying sentinel lymph nodes across
various types of cancer (65,66).
In esophageal cancer surgery, several studies have demonstrated the
feasibility of ICG NIRL fluorescence-guided lymphography, which has
achieved high detection rates for sentinel lymph nodes (67–69).
In addition to lymph node detection, recent research has revealed
that ICG NIRL fluorescence imaging can reduce the risk of
anastomotic leakage by allowing the assessment of blood flow during
surgery (70,71). This technique involves intravenous
injection of ICG (2 mg/kg) 12 h before surgical resection of the
esophagus (Fig. 2). Both animal
models and clinical studies investigating patients with esophageal
cancer have demonstrated the efficacy of this dosage in accurately
localizing tumors (72).
Importantly, no adverse reactions related to intravenous ICG
administration have been reported in these patients, highlighting
the safety of the procedure (Table
I).
CRC is the 3rd most commonly diagnosed cancer
worldwide and the second leading cause of cancer-related mortality
(73). Despite advances in
treatment, surgical resection remains the primary approach for
achieving a complete response (74). A considerable postoperative concern
is anastomotic leakage, which not only increases morbidity and
mortality rates but also negatively impacts long-term oncological
outcomes and reduces quality of life (75). Adequate blood supply to the
anastomotic site is an important factor influencing successful
healing. ICG NIRL fluorescence imaging has become a valuable tool
for evaluating intestinal perfusion during anastomosis, aiming to
reduce leakage rates (76–78). ICG NIRL fluorescence imaging
perfusion assessment has the advantages of safety, simplicity and
short adjustment time, and it is a tool that should be considered
for reducing the incidence of anastomotic leakage after colorectal
surgery (79). This method is
known for its safety, simplicity and rapid assessment, with typical
intraoperative ICG doses ranging from 2 to 20 mg (Fig. 2). Intestinal blood perfusion can
generally be evaluated within 60 sec following intravenous ICG
administration (80,81). For standard ICG preparation, 25 mg
of ICG was dissolved in 10 ml of distilled water, corresponding to
0.05 mg/kg (82) (Table I). The optimal distance between the
near infrared camera and the colon is 4–5 cm for accurate
visualization (83). A study by
Watanabe et al (84)
revealed that anastomotic fistula and reoperation rates were
markedly reduced when patients were given 0.25 mg/kg ICG (Fig. 2) intravenously prior to intestinal
anastomosis, and a study by De Nardi et al (85) demonstrated a reduction in
anastomotic fistula and reoperation rates when patients were given
0.3 mg/kg ICG intravenously before colonic transection and after
anastomosis (Fig. 2).
In addition, sentinel node identification using ICG
fluorescence imaging has been reported as a viable adjunct for use
in CRC surgery (86). This
approach enables the precise identification of metastatic and
lateral lymph nodes, as well as peritoneal metastases, supporting
complete lymph node dissection during cancer resection (87–89).
While effective, the accuracy of this technique heavily relies on
the expertise and experience of the surgeon. Ahn et al
(90) demonstrated favorable
results with submucosal injection of 0.5–1 mg of ICG for standard
lymph node testing for colonoscopy 12–18 h before surgery (90) (Fig.
2). Su et al (91) and
Kim et al (92)
demonstrated the effectiveness of a colonoscopy submucosal
injection of 1.0–1.5 ml (25 mg/10 ml) ICG for lateral pelvic lymph
node detection (metastasis of rectal cancer) before surgery
(91,92) (Fig.
2). Fluorescence imaging also holds promise for the management
of early-stage CRC, particularly in the context of minimally
invasive surgery. Accurate tumor localization without tactile
feedback is essential, particularly for small lesions or tumors in
resectable colon segments (93–95).
There is potential for ICG NIRL fluorescence imaging to facilitate
precise tumor resection in endoscopic procedures, although further
research is needed to validate its efficacy. Park et al
(96) revealed that 0.5–1 ml of
ICG at a concentration of 12.5 mg/ml can be injected into each of
the four sites around the tumor 1 day before surgery (96) (Table
I; Fig. 2). The field has also
seen innovations, such as ICG-liposome conjugates, which improve
tumor specificity and enhance visibility during surgery (97,98).
ICG NIRL fluorescence imaging has been extensively
studied not only in the surgical treatment of liver cancer and
gastrointestinal tumors but also in the surgical treatment of
gynecological tumors (99), breast
cancer (100) and brain tumors,
and it has also been studied in the context of transplantation
surgery (101). ICG NIRL
fluorescence imaging aids in lesion localization and portal vein
tumor thrombosis detection during liver cancer surgery. Its
applications in gastrointestinal oncology include tumor
identification, localization, lymph node navigation and blood
perfusion assessment, enabling more precise resections of tumors
and lymph nodes. This precision has contributed to more accurate
tumor staging, reduced postoperative complications and improved
patient outcomes. The localization of ICG in certain regions of
tumor tissue appears to be the result of increased tissue
permeability and retention, but is not tumor cell-specific
(102). While several specific
methods and dosages for ICG application have been explored in
small-scale clinical case studies, large-scale clinical trials are
still needed to establish standardized protocols for clinical
practice. Due to the penetrating ability of near infrared light,
fluorescence imaging can visualize tumors up to 8 mm from the
surface of the liver or 8 mm from the surface of the parenchymal
cut. For deeper liver lesions, IOUS can be used simultaneously
(19). However, ICG can only be
used to localize liver tumors, and some false-positive and
false-negative rates were observed in the present review;
additionally, ICG does not inhibit tumor growth. The combination of
ICG and nanotechnology markedly increases the photostability and
tumor-targeting ability of ICG and achieves liver tumor clearance
(103,104). In addition, ICG-based targeted
radiopharmaceutical therapy has also been proposed (105). In early gastric cancer, ICG can
be used to identify the location of the lesion as well as sentinel
lymph nodes and specimen lymph nodes, and it can aid in tumor
resection and complete lymph node dissection during surgery. In
advanced gastric cancer, the application of ICG can minimize the
extent of lymph node dissection. These applications and advantages
should be validated in more cases. The false-negative and
false-positive rates involved should also be emphasized. The
reasons for the occurrence of these false-negative and
false-positive results may be the obstruction of lymphatic vessels
caused by cancer cells and inappropriate injection doses;
furthermore, the targeting of ICG needs to be improved. ICG in
combination with other molecules, tracers or monoclonal antibodies
also has great potential to detect metastasis and can be detected
by a variety of diagnostic tools (magnetic resonance imaging; near
infrared and multimodal imaging using a variety of novel
fluorophores) (106). An
increasing number of devices are also being developed with the aim
of making fluorescence quantifiable, overcoming dual tracer methods
(107) or assessing perfusion
quality (108). ICG NIRL
fluorescence imaging perfusion evaluation has the advantages of
safety, simplicity and short adjustment time to reduce the
incidence of anastomotic fistula after CRC surgery. It can also
identify sentinel lymph nodes, accurately identify metastatic and
lateral lymph nodes as well as peritoneal metastases, support
complete lymph node clearance during cancer resection and locate
the tumor site to enable further precise resection. However, there
is still a lack of high-quality evidence from randomized controlled
trials for the present review. Since ICGs do not bind specifically
to tumor cells, further reduction of the resection margin distance
is not possible. Therefore, the study of tumor-targeted
fluorophores, which promote the precise localization of tumors, is
valuable and may be a solution to effectively shorten the margin
distance. Certain tumor-targeted fluorophores, such as SGM-101 and
IR-783, have achieved good results in clinical studies (109,110). In addition, quantification of the
fluorescence signal is challenging. The selection of appropriate
quantification parameters is a major issue and fluorescence
intensity may be affected by a variety of factors, such as ambient
light, the fluorescence emission source and the distance between
the camera and the colorectum (85,111).
Notably, minimally invasive treatment for early
gastrointestinal cancer is rapidly advancing. Considering the
effectiveness of ICG NIRL fluorescence imaging in laparoscopic
surgery, there is a possibility that this technology can be applied
to gastrointestinal endoscopic procedures, such as endoscopic
submucosal dissection and EMR. This would increase the precision of
surgical resection and minimize the risks of insufficient or
excessive excision. Such advances could considerably improve the
techniques that are used in the management of early
gastrointestinal cancer and further enhance the capabilities of
minimally invasive endoscopic procedures.
The authors would like to thank Professor Hui Dong
(Department of Medicine School of Medicine University of
California, San Diego University) for providing guidance in the
writing of the paper.
The present study was funded by the National Natural Science
Foundation of China (grant no. 81960507), the Basic Research
Projects of Science and Technology Department of Guizhou Province
[grant no. Qian Ke He-zk(2022)-646] and the 2023 Graduate Research
Fund Program B (grant no. ZYK248).
Not applicable.
YH contributed to the studyconception and manuscript
drafting. TW contributed to critical revisions of the intellectual
content. BT made contributions to the study conception and design.
All authors read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Schaafsma BE, Mieog JS, Hutteman M, van
der Vorst JR, Kuppen PJ, Löwik CW, Frangioni JV, van de Velde CJ
and Vahrmeijer AL: The clinical use of indocyanine green as a
near-infrared fluorescent contrast agent for Image-guided oncologic
surgery. J Surg Oncol. 104:323–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keller DS, Ishizawa T, Cohen R and Chand
M: Indocyanine green fluorescence imaging in colorectal surgery:
Overview, applications, and future directions. Lancet Gastroenterol
Hepatol. 2:757–766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boni L, David G, Mangano A, Dionigi G,
Rausei S, Spampatti S, Cassinotti E and Fingerhut A: Clinical
applications of indocyanine green (ICG) enhanced fluorescence in
laparoscopic surgery. Surg Endosc. 29:2046–2055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Gasperi A, Mazza E and Prosperi M:
Indocyanine green kinetics to assess liver function: Ready for a
clinical dynamic assessment in major liver surgery? World J
Hepatol. 8:355–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo S, Zhang E, Su Y, Cheng T and Shi C: A
review of NIR dyes in cancer targeting and imaging. Biomaterials.
32:7127–7138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daskalaki D, Fernandes E, Wang X, Bianco
FM, Elli EF, Ayloo S, Masrur M, Milone L and Giulianotti PC:
Indocyanine green (ICG) fluorescent cholangiography during robotic
cholecystectomy: Results of 184 consecutive cases in a single
institution. Surg Innov. 21:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakamoto E, Dias AR, Ramos M,
Safatle-Ribeiro AV, Zilberstein B and Ribeiro Junior U: Indocyanine
green and Near-Infrared fluorescence imaging in gastric cancer
precision surgical approach. Arq Gastroenterol. 58:569–570. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reuthebuch O, Kadner A, Lachat M, Kunzli
A, Schurr UP and Turina MI: Early bypass occlusion after deployment
of nitinol connector devices. J Thorac Cardiovasc Surg.
127:1421–1426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sekijima M, Tojimbara T, Sato S, Nakamura
M, Kawase T, Kai K, Urashima Y, Nakajima I, Fuchinoue S and Teraoka
S: An intraoperative fluorescent imaging system in organ
transplantation. Transplant Proc. 36:2188–2190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Desai ND, Miwa S, Kodama D, Koyama T,
Cohen G, Pelletier MP, Cohen EA, Christakis GT, Goldman BS and
Fremes SE: A randomized comparison of intraoperative indocyanine
green angiography and transit-time flow measurement to detect
technical errors in coronary bypass grafts. J Thorac Cardiovasc
Surg. 132:585–594. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang Y, Lee J, Kwon K and Choi C:
Application of novel dynamic optical imaging for evaluation of
peripheral tissue perfusion. Int J Cardiol. 145:e99–e101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuo WS, Chang YT, Cho KC, Chiu KC, Lien
CH, Yeh CS and Chen SJ: Gold nanomaterials conjugated with
indocyanine green for dual-modality photodynamic and photothermal
therapy. Biomaterials. 33:3270–3278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YW, Fu YY, Peng Q, Guo SS, Liu G, Li
J, Yang HH and Chen GN: Dye-enhanced graphene oxide for
photothermal therapy and photoacoustic imaging. J Mater Chem B.
1:5762–5767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kono Y, Ishizawa T, Tani K, Harada N,
Kaneko J, Saiura A, Bandai Y and Kokudo N: Techniques of
fluorescence cholangiography during laparoscopic cholecystectomy
for better delineation of the bile duct anatomy. Medicine
(Baltimore). 94:e10052015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baiocchi GL, Diana M and Boni L:
Indocyanine green-based fluorescence imaging in visceral and
hepatobiliary and pancreatic surgery: State of the art and future
directions. World J Gastroenterol. 24:2921–2930. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McGlynn KA, Petrick JL and London WT:
Global epidemiology of hepatocellular carcinoma: An emphasis on
demographic and regional variability. Clin Liver Dis. 19:223–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horn SR, Stoltzfus KC, Lehrer EJ, Dawson
LA, Tchelebi L, Gusani NJ, Sharma NK, Chen H, Trifiletti DM and
Zaorsky NG: Epidemiology of liver metastases. Cancer Epidemiol.
67:1017602020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishizawa T, Masuda K, Urano Y, Kawaguchi
Y, Satou S, Kaneko J, Hasegawa K, Shibahara J, Fukayama M, Tsuji S,
et al: Mechanistic background and clinical applications of
indocyanine green fluorescence imaging of hepatocellular carcinoma.
Ann Surg Oncol. 21:440–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishizawa T, Fukushima N, Shibahara J,
Masuda K, Tamura S, Aoki T, Hasegawa K, Beck Y, Fukayama M and
Kokudo N: Real-time identification of liver cancers by using
indocyanine green fluorescent imaging. Cancer. 115:2491–2504. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimada S, Ohtsubo S, Ogasawara K and
Kusano M: Macro- and microscopic findings of ICG fluorescence in
liver tumors. World J Surg Oncol. 13:1982015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abo T, Nanashima A, Tobinaga S, Hidaka S,
Taura N, Takagi K, Arai J, Miyaaki H, Shibata H and Nagayasu T:
Usefulness of intraoperative diagnosis of hepatic tumors located at
the liver surface and hepatic segmental visualization using
indocyanine green-photodynamic eye imaging. Eur J Surg Oncol.
41:257–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Terasawa M, Ishizawa T, Mise Y, Inoue Y,
Ito H, Takahashi Y and Saiura A: Applications of
fusion-fluorescence imaging using indocyanine green in laparoscopic
hepatectomy. Surg Endosc. 31:5111–5118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Achterberg FB, Bijlstra OD, Slooter MD,
Sibinga Mulder BG, Boonstra MC, Bouwense SA, Bosscha K, Coolsen
MME, Derksen WJM, Gerhards MF and Gobardhan PD: ICG-Fluorescence
imaging for margin assessment during minimally invasive colorectal
liver metastasis resection. JAMA Netw Open. 7:e2465482024.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Teh CSC, Ishizawa T, Aoki T,
Cavallucci D, Lee SY, Panganiban KM, Perini MV, Shah SR, Wang H, et
al: Consensus guidelines for the use of fluorescence imaging in
hepatobiliary surgery. Ann Surg. 274:97–106. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z, Fang C, Zhang Y, Su S, Li B, Liu
G, Hu Z and Tian J: NIR-II nano fluorescence image guided hepatic
carcinoma resection on cirrhotic patient. Photodiagnosis Photodyn
Ther. 40:1030982022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NCT and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim W, Kim HH, Han SU, Kim MC, Hyung WJ,
Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, et al: Decreased
morbidity of laparoscopic distal gastrectomy compared with open
distal gastrectomy for stage I gastric cancer: Short-term outcomes
from a multicenter randomized controlled trial (KLASS-01). Ann
Surg. 263:28–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J,
Xue Y, Suo J, Tao K, He X, et al: Morbidity and mortality of
laparoscopic versus open D2 distal gastrectomy for advanced gastric
cancer: A randomized controlled trial. J Clin Oncol. 34:1350–1357.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smyth EC, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D: Gastric cancer: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
27:v38–v49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Katai H, Mizusawa J, Katayama H, Takagi M,
Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K,
et al: Short-term surgical outcomes from a phase III study of
laparoscopy-assisted versus open distal gastrectomy with nodal
dissection for clinical stage IA/IB gastric cancer: Japan Clinical
Oncology Group Study JCOG0912. Gastric Cancer. 20:699–708. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He H, Li H, Su X, Li Z, Yu P, Huang H,
Huang C, Ye J, Li Y, Suo J, et al: Study on safety of laparoscopic
total gastrectomy for clinical stage I gastric cancer: The protocol
of the CLASS02-01 multicenter randomized controlled clinical trial.
BMC Cancer. 18:9442018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hyung WJ, Yang HK, Han SU, Lee YJ, Park
JM, Kim JJ, Kwon OK, Kong SH, Kim HI, Lee HJ, et al: A feasibility
study of laparoscopic total gastrectomy for clinical stage I
gastric cancer: A prospective multi-center phase II clinical trial,
KLASS 03. Gastric Cancern. 22:214–222. 2019. View Article : Google Scholar
|
|
33
|
Belia F, Biondi A, Agnes A, Santocchi P,
Laurino A, Lorenzon L, Pezzuto R, Tirelli F, Ferri L, D'Ugo D and
Persiani R: The use of indocyanine green (ICG) and Near-infrared
(NIR) Fluorescence-guided imaging in gastric cancer surgery: A
narrative review. Front Surg. 9:8807732022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katai H, Mizusawa J, Katayama H, Kunisaki
C, Sakuramoto S, Inaki N, Kinoshita T, Iwasaki Y, Misawa K,
Takiguchi N, et al: Single-arm confirmatory trial of
laparoscopy-assisted total or proximal gastrectomy with nodal
dissection for clinical stage I gastric cancer: Japan Clinical
Oncology Group study JCOG1401. Gastric Cancer. 22:999–1008. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee HJ, Hyung WJ, Yang HK, Han SU, Park
YK, An JY, Kim W, Kim HI, Kim HH, Ryu SW, et al: Short-term
outcomes of a multicenter randomized controlled trial comparing
laparoscopic distal gastrectomy with D2 lymphadenectomy to open
distal gastrectomy for locally advanced gastric cancer
(KLASS-02-RCT). Ann Surg. 270:983–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J,
Du X, Huang H, Hu J, Li G, et al: Morbidity and mortality of
laparoscopic vs open total gastrectomy for clinical stage i gastric
cancer: The CLASS02 multicenter randomized clinical trial. JAMA
Oncol. 6:1590–1597. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hyung WJ, Yang HK, Park YK, Lee HJ, An JY,
Kim W, Kim HI, Kim HH, Ryu SW, Hur H, et al: Long-Term outcomes of
laparoscopic distal gastrectomy for locally advanced gastric
cancer: The KLASS-02-RCT randomized clinical trial. J Clin Oncol.
38:3304–3313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2018 (5th edition).
Gastric Cancer. 24:1–21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van der Veen A, Brenkman HJF, Seesing MFJ,
Haverkamp L, Luyer MDP, Nieuwenhuijzen GAP, Stoot JHMB, Tegels JJW,
Wijnhoven BPL, Lagarde SM, et al: Laparoscopic versus open
gastrectomy for gastric cancer (LOGICA): A multicenter randomized
clinical trial. J Clin Oncol. 39:978–989. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yano K, Nimura H, Mitsumori N, Takahashi
N, Kashiwagi H and Yanaga K: The efficiency of micrometastasis by
sentinel node navigation surgery using indocyanine green and
infrared ray laparoscopy system for gastric cancer. Gastric Cancer.
15:287–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takeuchi H and Kitagawa Y: Sentinel node
navigation surgery in patients with early gastric cancer. Digestive
surgery. 30:104–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ushimaru Y, Omori T, Fujiwara Y,
Yanagimoto Y, Sugimura K, Yamamoto K, Moon JH, Miyata H, Ohue M and
Yano M: The feasibility and safety of preoperative fluorescence
marking with indocyanine green (ICG) in laparoscopic gastrectomy
for gastric cancer. J Gastrointest Surg. 23:468–476. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Romanzi A, Mancini R, Ioni L, Picconi T
and Pernazza G: ICG-NIR-guided lymph node dissection during robotic
subtotal gastrectomy for gastric cancer. A single-centre
experience. Int J Med Robot. 17:e22132021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smith DD, Schwarz RR and Schwarz RE:
Impact of total lymph node count on staging and survival after
gastrectomy for gastric cancer: Data from a large US-population
database. J Clin Oncol. 23:7114–7124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Son T, Hyung WJ, Lee JH, Kim YM, Kim HI,
An JY, Cheong JH and Noh SH: Clinical implication of an
insufficient number of examined lymph nodes after curative
resection for gastric cancer. Cancer. 118:4687–4693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kwon IG, Son T, Kim HI and Hyung WJ:
Fluorescent lymphography-guided lymphadenectomy during robotic
radical gastrectomy for gastric cancer. JAMA Surg. 154:150–158.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
An JY, Min JS, Hur H, Lee YJ, Cho GS, Park
YK, Jung MR, Park JH, Hyung WJ, Jeong SH, et al: Laparoscopic
sentinel node navigation surgery versus laparoscopic gastrectomy
with lymph node dissection for early gastric cancer. Br J Surg.
107:1429–1439. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lan YT, Huang KH, Chen PH, Liu CA, Lo SS,
Wu CW, Shyr YM and Fang WL: A pilot study of lymph node mapping
with indocyanine green in robotic gastrectomy for gastric cancer.
SAGE Open Med. 5:20503121177274442017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ma S, Xie YB, Zeng HM, Xu Q, Zhong YX, Liu
H, Ma FH, Zhao F, Li H, Li Y and Tian YT: Feasibility and efficacy
of indocyanine green used in laparoscopic gastrectomy for advanced
gastric cancer patients. Zhonghua Zhong Liu Za Zhi. 41:904–908.
2019.(In Chinese). PubMed/NCBI
|
|
50
|
Chen QY, Zhong Q, Liu ZY, Li P, Lin GT,
Zheng QL, Wang JB, Lin JX, Lu J, Cao LL, et al: Indocyanine green
fluorescence imaging-guided versus conventional laparoscopic
lymphadenectomy for gastric cancer: Long-term outcomes of a phase 3
randomised clinical trial. Nat Commun. 14:74132023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang ZN, Tang YH, Zhong Q, Li P, Xie JW,
Wang JB, Lin JX, Lu J, Cao LL, Lin M, et al: Assessment of
laparoscopic indocyanine green Tracer-guided lymphadenectomy after
neoadjuvant chemotherapy for locally advanced gastric cancer: A
randomized controlled trial. Ann Surg. 279:923–931. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shimada S, Yagi Y, Shiomori K, Honmyo U,
Hayashi N, Matsuo A, Marutsuka T and Ogawa M: Characterization of
early gastric cancer and proposal of the optimal therapeutic
strategy. Surgery. 129:714–719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roh CK, Choi S, Seo WJ, Cho M, Son T, Kim
HI and Hyung WJ: Indocyanine green fluorescence lymphography during
gastrectomy after initial endoscopic submucosal dissection for
early gastric cancer. Br J Surg. 107:712–719. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Miyashiro I, Kishi K, Yano M, Tanaka K,
Motoori M, Ohue M, Ohigashi H, Takenaka A, Tomita Y and Ishikawa O:
Laparoscopic detection of sentinel node in gastric cancer surgery
by indocyanine green fluorescence imaging. Surg Endosc.
25:1672–1676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lombardi PM, Mazzola M, Nicastro V,
Giacopuzzi S, Baiocchi GL, Castoro C, Rosati R, Fumagalli Romario
U, Bonavina L, Staderini F, et al: The iGreenGO Study: The clinical
role of indocyanine green imaging fluorescence in modifying the
Surgeon's conduct during the surgical treatment of advanced gastric
cancer-study protocol for an international multicenter prospective
study. Front Oncol. 12:8547542022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tanaka C, Kanda M, Funasaka K, Miyahara R,
Murotani K, Tanaka Y, Takeda S, Kobayashi D, Hirooka Y, Fujiwara M,
et al: Detection of indocyanine green fluorescence to determine
tumor location during laparoscopic gastrectomy for gastric cancer:
Results of a prospective study. Asian J Endosc Surg. 13:160–167.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen QY, Zhong Q, Li P, Xie JW, Liu ZY,
Huang XB, Lin GT, Wang JB, Lin JX, Lu J, et al: Comparison of
submucosal and subserosal approaches toward optimized indocyanine
green tracer-guided laparoscopic lymphadenectomy for patients with
gastric cancer (FUGES-019): A randomized controlled trial. BMC Med.
19:2762021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim TH, Kong SH, Park JH, Son YG, Huh YJ,
Suh YS, Lee HJ and Yang HK: Assessment of the completeness of lymph
node dissection using Near-infrared imaging with indocyanine green
in laparoscopic gastrectomy for gastric cancer. J Gastric Cancer.
18:161–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tajima Y, Murakami M, Yamazaki K, Masuda
Y, Kato M, Sato A, Goto S, Otsuka K, Kato T and Kusano M: Sentinel
node mapping guided by indocyanine green fluorescence imaging
during laparoscopic surgery in gastric cancer. Ann Surg Oncol.
17:1787–1793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen QY, Xie JW, Zhong Q, Wang JB, Lin JX,
Lu J, Cao LL, Lin M, Tu RH, Huang ZN, et al: Safety and efficacy of
indocyanine green Tracer-guided lymph node dissection during
laparoscopic radical gastrectomy in patients with gastric cancer: A
randomized clinical trial. JAMA Surg. 155:300–311. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Qian Y and Cai S: A safe and effective
surgical navigation technique in laparoscopic radical gastrectomy:
Indocyanine Green-mediated near-infrared fluorescent imaging.
Cancer Commun (Lond). 40:270–272. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shoji Y, Kumagai K, Kamiya S, Ida S,
Nunobe S, Ohashi M, Yoshimizu S, Horiuchi Y, Yoshio T, Ishiyama A,
et al: Prospective feasibility study for single-tracer sentinel
node mapping by ICG (indocyanine green) fluorescence and OSNA
(one-step nucleic acid amplification) assay in laparoscopic gastric
cancer surgery. Gastric Cancer. 22:873–880. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Uhlenhopp DJ, Then EO, Sunkara T and
Gaduputi V: Epidemiology of esophageal cancer: update in global
trends, etiology and risk factors. Clin J Gastroenterol.
13:1010–1021. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tachibana M, Kinugasa S, Shibakita M,
Tonomoto Y, Hattori S, Hyakudom R, Yoshimura H, Dhar DK and Nagasue
N: Surgical treatment of superficial esophageal cancer. Langenbecks
Arch Surg. 391:304–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nimura H, Narimiya N, Mitsumori N,
Yamazaki Y, Yanaga K and Urashima M: Infrared ray electronic
endoscopy combined with indocyanine green injection for detection
of sentinel nodes of patients with gastric cancer. Br J Surg.
91:575–579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Picchetto A, Seeliger B, La Rocca S,
Barberio M, D'Ambrosio G, Marescaux J and Diana M:
Fluorescence-guided detection of lymph node metastases of
gastrointestinal tumors. Chirurg. 90:891–898. 2019.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hachey KJ, Gilmore DM, Armstrong KW,
Harris SE, Hornick JL, Colson YL and Wee JO: Safety and feasibility
of Near-infrared image-guided lymphatic mapping of regional lymph
nodes in esophageal cancer. J Thorac Cardiovasc Surg. 152:546–554.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang X, Hu Y, Wu X, Liang M, Hu Z, Gan X,
Li D, Cao Q and Shan H: Near-infrared fluorescence imaging-guided
lymphatic mapping in thoracic esophageal cancer surgery. Surg
Endosc. 36:3994–4003. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Helminen O, Mrena J and Sihvo E:
Near-infrared image-guided lymphatic mapping in minimally invasive
oesophagectomy of distal oesophageal cancer. Eur J Cardiothorac
Surg. 52:952–957. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tamburini N, Chiozza M, Maniscalco P,
Resta G, Marino S, Quarantotto F, Anania G and Cavallesco G:
Application of indocyanine green enhanced fluorescence in
esophageal surgery: A mini review. Front Surg. 9:9618562022.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Koyanagi K, Ozawa S, Ninomiya Y, Yatabe K,
Higuchi T, Yamamoto M, Kanamori K and Tajima K: Indocyanine green
fluorescence imaging for evaluating blood flow in the reconstructed
conduit after esophageal cancer surgery. Surgery Today. 52:369–376.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rho J, Quan YH, Choi BH, Han KN, Kim BM,
Choi YH and Kim HK: Near-infrared fluorescent imaging with
indocyanine green in rabbit and patient specimens of esophageal
cancer. J Thorac Dis. 213:6314–6322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Romero-Zoghbi SE, Krumina E, López-Campos
F and Couñago F: Current and future perspectives in the management
and treatment of colorectal cancer. World J Clin Oncol.
16:1008072025. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Vallance A, Wexner S, Berho M, Cahill R,
Coleman M, Haboubi N, Heald RJ, Kennedy RH, Moran B, Mortensen N,
et al: A collaborative review of the current concepts and
challenges of anastomotic leaks in colorectal surgery. Colorectal
Dis. 19:O1–O12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rutegård M and Rutegård J: Anastomotic
leakage in rectal cancer surgery: The role of blood perfusion.
World J Gastrointest Surg. 7:289–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Iguchi K, Watanabe J, Suwa Y, Chida K,
Atsumi Y, Numata M, Sato T, Takeda K and Kunisaki C: The usefulness
of indocyanine green fluorescence imaging for intestinal perfusion
assessment of intracorporeal anastomosis in laparoscopic colon
cancer surgery. Int J Colorectal Dis. 38:72023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Peltrini R, Podda M, Castiglioni S, Di
Nuzzo MM, D'Ambra M, Lionetti R, Sodo M, Luglio G, Mucilli F, Di
Saverio S, et al: Intraoperative use of indocyanine green
fluorescence imaging in rectal cancer surgery: The state of the
art. World J Gastroenterol. 27:6374–6386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Maione F, Manigrasso M, Chini A, Vertaldi
S, Anoldo P, D'Amore A, Marello A, Sorrentino C, Cantore G, Maione
R, et al: The Role of indocyanine Near-infrared fluorescence in
colorectal surgery. Front Surg. 9:8864782022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
van Manen L, Handgraaf HJM, Diana M,
Dijkstra J, Ishizawa T, Vahrmeijer AL and Mieog JSD: A practical
guide for the use of indocyanine green and methylene blue in
fluorescence-guided abdominal surgery. J Surg Oncol. 118:283–300.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Watanabe J, Ishibe A, Suwa Y, Suwa H, Ota
M, Kunisaki C and Endo I: Indocyanine green fluorescence imaging to
reduce the risk of anastomotic leakage in laparoscopic low anterior
resection for rectal cancer: A propensity score-matched cohort
study. Surg Endosc. 34:202–208. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cahill RA, Anderson M, Wang LM, Lindsey I,
Cunningham C and Mortensen NJ: Near-infrared (NIR) laparoscopy for
intraoperative lymphatic road-mapping and sentinel node
identification during definitive surgical resection of early-stage
colorectal neoplasia. Surg Endosc. 26:197–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ahn HM, Son GM, Lee IY, Park SH, Kim NS
and Baek KR: Optimization of indocyanine green angiography for
colon perfusion during laparoscopic colorectal surgery. Colorectal
Dis. 23:1848–1859. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Watanabe J, Ishibe A, Ohya H, Suwa Y, Suwa
H, Kunisaki C and Endo I: Evaluating the effect of intraoperative
near-infrared observation on anastomotic leakage after stapled
side-to-side anastomosis in colon cancer surgery using propensity
score matching. Dis Colon Rectum. 64:1542–1550. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
De Nardi P, Elmore U, Maggi G, Maggiore R,
Boni L, Cassinotti E, Fumagalli U, Gardani M, De Pascale S, Parise
P, et al: Intraoperative angiography with indocyanine green to
assess anastomosis perfusion in patients undergoing laparoscopic
colorectal resection: Results of a multicenter randomized
controlled trial. Surg Endosc. 34:53–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Staniloaie D, Budin C, Ilco A, Vasile D,
Calinoiu AL, Rusu A, Iancu G, Ammar T, Georgescu CF, Tanasescu MD,
et al: In Vivo sentinel lymph node detection with indocyanine green
in colorectal cancer. Maedica. 17:264–270. 2022.PubMed/NCBI
|
|
87
|
Chand M and Dean M: Mapping the mesentery
using ICG. Clin Colon Rectal Surg. 35:338–341. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Watanabe J, Ota M, Suwa Y, Ishibe A, Masui
H and Nagahori K: Real-time indocyanine green fluorescence
imaging-guided complete mesocolic excision in laparoscopic flexural
colon cancer surgery. Dis Colon Rectum. 59:701–705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Park SY, Park JS, Kim HJ, Woo IT, Park IK
and Choi GS: Indocyanine green fluorescence Imaging-guided
laparoscopic surgery could achieve radical D3 dissection in
patients with advanced right-sided colon cancer. Dis Colon Rectum.
63:441–449. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ahn HM, Son GM, Lee IY, Shin DH, Kim TK,
Park SB and Kim HW: Optimal ICG dosage of preoperative colonoscopic
tattooing for fluorescence-guided laparoscopic colorectal surgery.
Surg Endosc. 36:1152–1163. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Su H, Xu Z, Bao M, Luo S, Liang J, Pei W,
Guan X, Liu Z, Jiang Z, Zhang M, et al: Lateral pelvic sentinel
lymph node biopsy using indocyanine green fluorescence navigation:
Can it be a powerful supplement tool for predicting the status of
lateral pelvic lymph nodes in advanced lower rectal cancer. Surg
Endosc. 37:4088–4096. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kim HJ, Choi GS, Park JS, Park SY, Cho SH,
Seo AN and Yoon GS: S122: Impact of fluorescence and 3D images to
completeness of lateral pelvic node dissection. Surg Endosc.
34:469–476. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nagata J, Fukunaga Y, Akiyoshi T, Konishi
T, Fujimoto Y, Nagayama S, Yamamoto N and Ueno M: Colonic marking
with Near-infrared, Light-emitting, Diode-activated indocyanine
green for laparoscopic colorectal surgery. Dis Colon Rectum.
59:e14–e18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zako T, Ito M, Hyodo H, Yoshimoto M,
Watanabe M, Takemura H, Kishimoto H, Kaneko K, Soga K and Maeda M:
Extra-luminal detection of assumed colonic tumor site by
near-infrared laparoscopy. Surg Endosc. 30:4153–4159. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Watanabe M, Murakami M, Ozawa Y, Yoshizawa
S, Matsui N and Aoki T: Intraoperative identification of colonic
tumor sites using a Near-Infrared fluorescence endoscopic imaging
system and indocyanine green. Dig Surg. 34:495–501. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Park JH, Moon HS, Kwon IS, Yun GY, Lee SH,
Park DH, Kim JS, Kang SH, Lee ES, Kim SH, et al: Usefulness of
colonic tattooing using indocyanine green in patients with
colorectal tumors. World J Clin Cases. 6:632–640. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Magdassi S, Bar-David S, Friedman-Levi Y,
Zigmond E, Varol C, Lahat G, Klausner J, Eyal S and Nizri E:
Intraoperative localization of rectal tumors using liposomal
indocyanine green. Surg Innov. 24:139–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bar-David S, Larush L, Goder N, Aizic A,
Zigmond E, Varol C, Klausner J, Magdassi S and Nizri E: Size and
lipid modification determine liposomal Indocyanine green
performance for tumor imaging in a model of rectal cancer. Sci Rep.
9:85662019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Koual M, Benoit L, Nguyen-Xuan HT,
Bentivegna E, Azaïs H and Bats AS: Diagnostic value of indocyanine
green fluorescence guided sentinel lymph node biopsy in vulvar
cancer: A systematic review. Gynecol Oncol. 161:436–441. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Goonawardena J, Yong C and Law M: Use of
indocyanine green fluorescence compared to radioisotope for
sentinel lymph node biopsy in Early-stage breast cancer: Systematic
review and meta-analysis. Am J Surg. 220:665–676. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gerken ALH, Nowak K, Meyer A, Weiss C,
Krüger B, Nawroth N, Karampinis I, Heller K, Apel H, Reissfelder C,
et al: Quantitative assessment of intraoperative laser fluorescence
angiography with indocyanine green predicts early graft function
after kidney transplantation. Ann Surg. 276:391–397. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Nguyen HN, Pertzborn D, Ziadat R, Ernst G,
Guntinas-Lichius O, Von Eggeling F and Hoffmann F: Indocyanine
green uptake by human tumor and non-tumor cell lines and tissue.
Biomed Rep. 21:1362024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jiang X, Du B, Huang Y, Yu M and Zheng J:
Cancer photothermal therapy with ICG-Conjugated gold nanoclusters.
Bioconjug Chem. 31:1522–1528. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tang W, Kang J, Yang L, Lin J, Song J,
Zhou D and Ye F: Thermosensitive nanocomposite components for
combined photothermal-photodynamic therapy in liver cancer
treatment. Colloids Surf B Biointerfaces. 226:1133172023.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Marker SC, Espinoza AF, King AP, Woodfield
SE, Patel RH, Baidoo K, Nix MN, Ciaramicoli LM, Chang YT, Escorcia
FE, et al: Development of iodinated indocyanine green analogs as a
strategy for targeted therapy of liver cancer. ACS Med Chem Lett.
14:1208–1215. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Debie P and Hernot S: Emerging fluorescent
molecular tracers to guide intra-operative surgical
decision-making. Front Pharmacol. 10:5102019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Okubo K, Uenosono Y, Arigami T, Matsushita
D, Yanagita S, Kijima T, Amatatsu M, Ishigami S, Maemura K and
Natsugoe S: Quantitative assessment of fluorescence intensity of
ICG in sentinel nodes in early gastric cancer. Gastric Cancer.
21:776–781. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Slooter MD, de Bruin DM, Eshuis WJ, Veelo
DP, van Dieren S, Gisbertz SS and van Berge Henegouwen MI:
Quantitative Fluorescence-guided perfusion assessment of the
gastric conduit to predict anastomotic complications after
esophagectomy. Dis Esophagus. 34:doaa1002021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Boogerd LSF, Hoogstins CES, Schaap DP,
Kusters M, Handgraaf HJM, van der Valk MJM, Hilling DE, Holman FA,
Peeters KCMJ, Mieog JSD, et al: Safety and effectiveness of
SGM-101, a fluorescent antibody targeting carcinoembryonic antigen,
for intraoperative detection of colorectal cancer: A
dose-escalation pilot study. Lancet Gastroenterol Hepatol.
3:181–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Park Y, Park MH and Hyun H:
Structure-inherent tumor-targeted IR-783 for near-infrared
fluorescence-guided photothermal therapy. Int J Mol Sci.
25:53092024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kawada K, Hasegawa S, Wada T, Takahashi R,
Hisamori S, Hida K and Sakai Y: Evaluation of intestinal perfusion
by ICG fluorescence imaging in laparoscopic colorectal surgery with
DST anastomosis. Surg Endosc. 31:1061–1069. 2017. View Article : Google Scholar : PubMed/NCBI
|