Introduction
Coronary heart diseases (CADs) and their various
complications, including cardiomegaly, angina pectoris and
myocardial infarction, are the proegumenal cause of human morbidity
and mortality worldwide (1).
Atherosclerosis (AS) is the pathological foundation of various
CADs. AS-induced coronary artery stenosis and insufficient blood
flow to the myocardium lead to cardiomyocyte ischemia and a
dysfunctional heart (2). Chronic
endothelial inflammation in the aorta is a crucial trigger for
atherosclerotic plaque formation (3). Endothelial BTB and CNC homology 1
deficiency could decrease the expression of intercellular cell
adhesion molecule-1 (ICAM-1) and vascular cell adhesion
molecule-1(VCAM-1), reduce plasma tumor necrosis factor-α (TNF-α)
and interleukin (IL)1β levels, ameliorating turbulent blood flow-
or high-fat-diet (HFD)-induced plaque formation in atherosclerotic
mice (4). In
gonadotropin-releasing hormone agonist-treated mice, blockade of
follicle-stimulating hormone decreases VCAM-1 levels and mitigates
endothelial inflammation, causing smaller atherosclerotic lesions
with increased plaque stabilities (5). Pyroptosis, a pro-inflammatory form of
programmed cell death, is crucial in amplifying protective immunity
against pathogen invasion (6). It
is characterized by activating inflammatory caspases
(caspase-1/4/15) and gasdermin (GSDM), causing cell swelling, pore
formation of plasma membranes and the robust release of IL-1β,
IL-18 and high-mobility group box 1 (7). Vascular endothelial cells (VECs) line
the inner surface of vessel walls. The injury and abnormal death of
VECs may decrease nitric oxide (NO) production, amplify adhesion
molecule expression and inflammation and induce dysfunctional
angiogenesis, leading to accelerated AS and a higher risk of
cardiovascular events (8). Yin
et al (9) observed that in
apoE−/− mice fed an HFD for 3 weeks, the activity of
caspase-1 in VECs was markedly increased. In caspase-1-deficient
ApoE−/− mice, endothelial activation, vascular
inflammation and the size of atheromatous plaques were decreased
considerably. Low-shear stress (LSS) can induce VEC pyroptosis
in vitro and in vivo and promote the development of
AS through the IKKε/STAT1/NLRP3 pathway (10). Trimethylamine N-oxide (TMAO), a
well-known pro-atherogenic factor, could upregulate
phosphatidylethanolamine acyltransferase and induce excessive
mitophagy, leading to human umbilical vein endothelial cell (HUVEC)
pyroptosis and inflammation (11).
These recent findings denote the critical negative role of VEC
inflammation and pyroptosis in atherogenesis.
Compared with western medicine, Chinese herbal
medicines have enormous advantages in improving the environment of
the immune system to gain long-lasting effects and prevent
potential adverse effects (12).
Chinese herbal medicines and active metabolites, such as
resveratrol (13), curcumin
(14), salidroside (15), quercetin (16) and berberine (17), possess multiple anti-atherogenic
activities. Eburicoic acid (Fig.
1A) is a triterpenoid derivative extracted from the fruiting
bodies of Antrodia cinnamomea. It is also an active
ingredient from Banxia Baizhu Tianma Decoction, a Chinese herbal
formula widely used to treat CADs. So far, research regarding the
biological effects of eburicoic acid in various diseases is still
preliminary. Deng et al (18) injected ICR mice with λ-carrageenan
(Carr), a seaweed polysaccharide, to induce the paw edema model.
They found that Carr-induced increase in serum levels of TNF-α and
IL-1β was markedly inhibited following the administration of
eburicoic acid. Tung et al (19) found that compared with normoxic
mice, treating hyperoxia mice with eburicoic acid decreases NF-κB,
IL-6, TNF-α, IL-1β and IL-8 levels in lung tissue. Su et al
(20) observed that treating human
hepatoma cells with eburicoic acid effectively decreases cell
viability by inducing endoplasmic reticulum stress-mediated
autophagy and reactive oxygen species (ROS) production.
Intraperitoneal injection of eburicoic acid into CCL4-treated mice
decreases hepatic levels of inducible nitric oxide synthase (iNOS)
and cyclooxygenase-2 (COX2) and increased the activities of several
antioxidant enzymes (21). Wang
et al (22) reported that
incubation of RAW264.7 macrophages with eburicoic acid decreases
the production of NO, iNOS, prostaglandin E2 (PGE2) and COX2 and
downregulates the secretion level of pro-inflammatory cytokines,
including TNF-α, IL-6 and IL-1β, by inhibiting the activation of
PI3K/Akt/mTOR/NF-κB pathways. Moreover, administering HFD-fed mice
with eburicoic acid markedly decreases blood glucose, triglycerides
(TG) and total cholesterol (TC) levels, ameliorating adipocyte
hypertrophy and hepatocytic ballooning (23). These findings reveal that eburicoic
acid exerts anti-inflammatory, anti-hyperlipidemic and
anti-diabetic effects and may protect against AS. The present study
was designed to investigate the effect of eburicoic acid on VEC
pyroptosis and atherogenesis, as well as the underlying mechanisms,
aiming at identifying eburicoic acid as a novel phytomedicine in
treating AS.
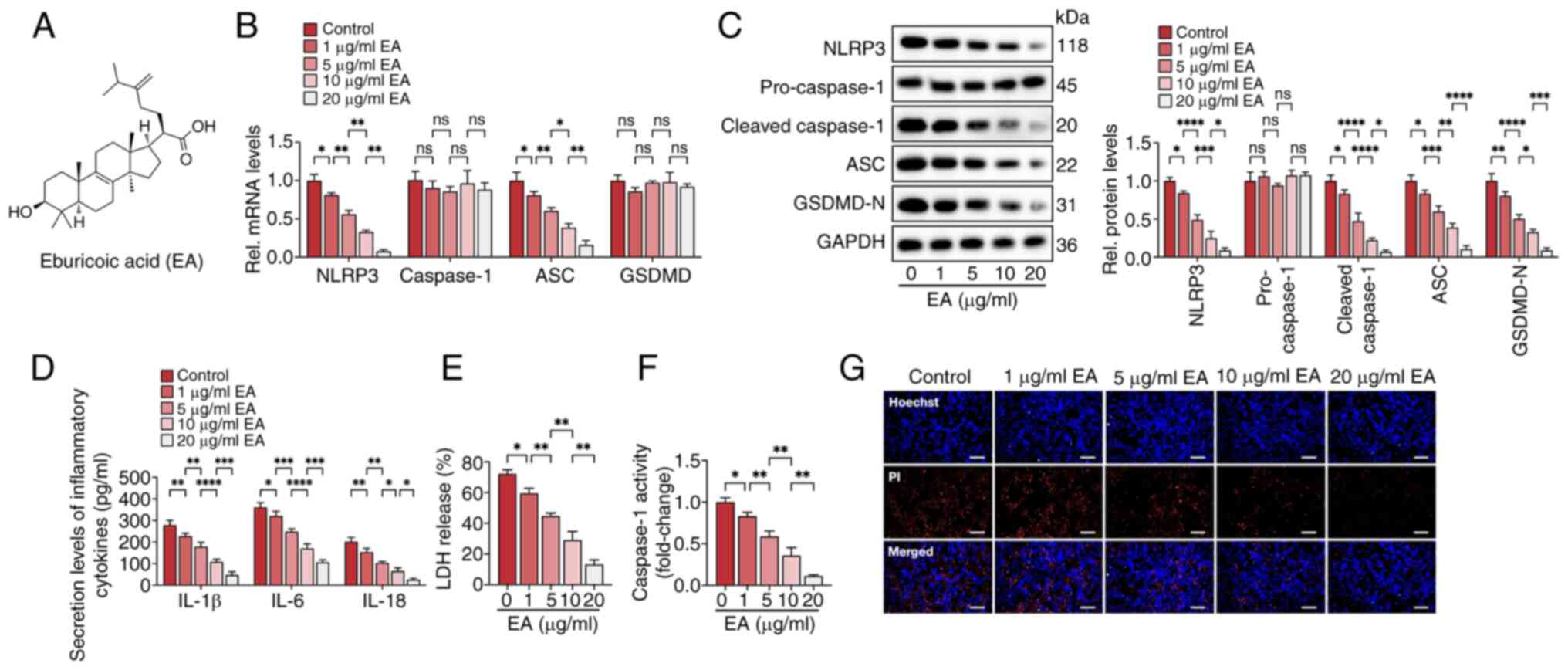 | Figure 1.Eburicoic acid inhibits NLRP3
inflammasome activation and pyroptosis in ox-LDL-treated HUVECs in
a dose-dependent manner. Cells were incubated with 100 µg/ml ox-LDL
for 24 h, followed by treatment with 1, 5, 10 and 20 µg/ml
eburicoic acid for 24 h. (A) Chemical structure of eburicoic acid;
(B) RT-qPCR analyses of NLRP3, caspase-1, ASC and GSDMD mRNA
levels. (C) western blotting analyses of NLRP3, pro-caspase-1,
cleaved caspase-1, ASC and GSDMD-N protein levels; (D) ELISA
Analyses of IL-1β, IL-6 and IL-18 secretion levels; (E)
Determination of LDH release using an LDH release assay kit; (F)
Spectrophotometry analysis of caspase-1 activity; (G)
Representative images of Hoechst/PI staining. Data are represented
as mean ± SD. ns, statistically insignificant, *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001. Scale bar, 20 µm. n=3.
EA, eburicoic acid; NLRP3, NLR family pyrin domain-containing
protein 3; ox-LDL, oxidized low-density lipoprotein; HUVECs, human
umbilical vascular endothelial cells; RT-qPCR, reverse
transcription-quantitative PCR; ASC, apoptosis-associated
speck-like protein containing CARD; GSDMD-N, N-terminal
gasdermin-D; LDH, lactate dehydrogenase. |
Materials and methods
Cell culture
HUVECs (ATCC; cat. no. CRL-1730) were cultured in
RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
11875093) containing 10% FBS (Procell Life Science & Technology
Co., Ltd.; cat. no. 164210), 20 µg/ml penicillin and 20 µg/ml
streptomycin. Cells were maintained in an incubator under a
humidified atmosphere of 5% CO2 and 95% air at 37°C.
They were seeded in 6- or 96-well plates with serum-free RPMI 1640
medium for at least 6 h to obtain the synchronization of growth
before the experiments commenced. Prior to other treatments, cells
were treated with 100 µg/ml oxidized low-density lipoprotein
(ox-LDL) (Yiyuan Biotechnology Co., Ltd.; cat. no. YB-002) for 24 h
to induce pyroptosis. Besides, cells were pre-incubated with 10 µM
DMNQ (ROS agonist; MedChemExpress; cat. no. HY-121026) for 6 h to
increase ROS production.
Small interfering RNA (siRNA)
transfection
siRNA targeting HO-1 (NCBI Gene ID: 3162),
NF-E2-related factor 2 (Nrf2; NCBI Gene ID: 4780) and scrambled
control siRNA were designed and synthesized by Shanghai GenePharma
Co., Ltd. using GenePharma RNAi designer V2.0 software. Prior to
transfection, cells were planted in 6-well plates
(2×106/well) and cultured in RPMI 1640 medium without
FBS or antibiotics for 12 h. siRNA-Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) transfection mixture,
including 125 µl Opti-MEM® Medium (Gibco; Thermo Fisher
Scientific, Inc.; cat. no. 31985-062), 100 pmol (5 µl) siRNA and 5
µl Lipofectamine® 2000 reagent (Thermo Fisher; cat. no.
11668019) for 48 h at 37°C. Western blotting was used to evaluate
the transfection efficiency. The siRNA sequences were as follows:
HO-1 siRNA, 5′-GGGUGAUAGAAGAGGCCAATT-3′; Nrf2 siRNA,
5′-CUAUCACUUUGCAAAGCUUUCAACC-3′; scrambled control siRNA,
5′-UUCUCCGAACGUGUCACGUTT-3′.
Reverse transcription-quantitative
(RT-q) PCR
After HUVECs (2×106/well) were subjected
to different treatments, the total RNAs were extracted using the
TRIzol® reagent (Thermo Fisher Scientific, Inc.; cat.
no. 15596018) according to the manufacturer's protocol. A Nanodrop
3000 spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
detect the purity of obtained RNAs. Genomic DNA (gDNA) elimination
and cDNA synthesis are performed using the PrimeScript RT Reagent
Kit (Takara Biotechnology Co., Ltd.; cat. no. RR047A). Briefly, 1
µg total RNA was transferred to a 0.2 ml EP tube, dissolved in 7 µl
RNase-Free dH2O, followed by incubation with 1 gDNA
Eraser and 2 µl 5XgDNA Eraser Buffer to remove contaminating gDNA.
Then, the EP tube was added with 4 µl 5× PrimeScript Buffer 2, 1 µl
PrimeScript RT Enzyme Mix1, 1 µl RT Primer Mix and 4 µl RNase Free
dH2O at 37°C for 15 min. This reaction was ended at 85°C
for 5 sec and cDNA was obtained. The cDNA was then subjected to PCR
amplification using TB Green® Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd.; cat. no. RR820A) on an ABI 7500 Fast
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) to detect the expression of target genes, including Nrf2,
HO-1, NLRP3, ASC, caspase-1 and GSDMD. The PCR reaction mixture
involved 2 µl cDNA, 10 µl TB Green Premix Ex Taq II (2X), 0.8 µl
forward primer (10 µM), 0.8 µl reverse primer (10 µM), 0.4 µl ROX
Reference Dye II (50X) and 6 µl dH2O. The PCR conditions
were 30 sec at 95°C for pre-denaturation, 40 cycles of 5 sec at
95°C and 30 sec at 60°C. Sangon Biotech Co., Ltd. designed and
synthesized all primers, the sequences of which are listed in
Table SI. The relative expression
of target genes was evaluated using the 2−ΔΔCq method
(24) and the GAPDH level was used
as an internal control. The PCR reactions were repeated three times
from three independent experiments.
Western blotting
After the different treatments of HUVECs and
apoE−/− mice, cells and aortic tissues were harvested
and the proteins were extracted using the mixture of RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd.; cat. no.
R0010), 1% PMSF (Beijing Solarbio Science & Technology Co.,
Ltd.; cat. no. P0100) and 1% protein phosphatase inhibitor (Beijing
Solarbio Science & Technology Co., Ltd.; cat. no. P1260). The
lysate solutions were transferred into a 1.5 ml EP tube and
centrifuged at 12,000 g for 5 min at 4°C. The supernatants were
carefully collected and 10 µl of the solution was used to quantify
protein concentrations using a BCA assay kit (Beijing Solarbio
Science & Technology Co., Ltd.; cat. no. PC0020). The rest of
the protein solutions were mixed with an SDS-loading buffer (5X) at
a ratio of 1:4 and boiled for 10 min for denaturation. The nuclear
and cytoplasmic proteins were extracted according to the
instructions of the nuclear and cytoplasmic protein extraction kit
(Wuhan Boster Biological Technology, Ltd.; cat. no. AR0106). Equal
amounts of protein were loaded onto 10% SDS-PAGE (~15 µl/lane)
fractionated by SDS-PAGE at a constant voltage of 110 V for 1.5 h,
followed by transfer into the 0.45 µm PVDF membranes (260 mA, 2 h).
The membranes were probed with primary antibodies (1:1,000) against
Kelch-like ECH-associated protein-1 (Keap1; Proteintech Group,
Inc.; cat. no. 10503-2-AP), Nrf2 (Proteintech Group, Inc.; cat. no.
80593-1-RR), HO-1 (Proteintech Group, Inc.; cat. no. 81281-1-RR),
NLRP3 (Proteintech Group, Inc.; cat. no. 27458-1-AP), ASC
(Proteintech Group, Inc.; cat. no. 10500-1-AP), caspase-1
(Proteintech Group, Inc.; cat. no. 22915-1-AP), caspase-3
(Proteintech Group, Inc.; cat. no. 82202-1-RR), caspase-8
(Proteintech Group, Inc.; cat. no. 13423-1-AP), Bcl-2 (Proteintech
Group, Inc.; cat. no. 12789-1-AP), Bax (Proteintech Group, Inc.;
cat. no. 50599-2-Ig), glutathione peroxidase 4 (GPX4; Proteintech
Group, Inc.; cat. no. 30388-1-AP), SLC7A11 (Proteintech Group,
Inc.; cat. no. 26864-1-AP), FPN (Proteintech Group, Inc.; cat. no.
26601-1-AP), ACSL4 (Proteintech Group, Inc.; cat. no. 22401-1-AP),
TLR4 (Proteintech Group, Inc.; cat. no. 19811-1-AP), GAPDH
(Proteintech Group, Inc.; cat. no. 10494-1-AP) and lamin B1
(Proteintech Group, Inc.; cat. no. 12987-1-AP) overnight at 4°C
with gentle shaking. Skimmed milk powder (5%) in TBS-T buffer
(0.05% Tween-20) diluted the antibodies. Afterwards, immunoblotted
membranes were rinsed in TBS-T solution (three times, 10 min/time)
and incubated with HRP-conjugated secondary antibodies (1:5,000,
Proteintech Group, Inc.; cat. no. 10500-1-AP) for 4 h at 4°C.
Following rinsed triple times in TBS-T, membranes were dried and
subjected to visualize gel blots using the Immobilon®
ECL UltraPlus Western HRP Substrate (Merck KGaA; cat. no.
WBULP-10ML). Image Pro Plus software 7.0 (Media Cybernetics, Inc.)
was used to assess the density of protein bands and analyze the
relative protein expression. GAPDH was used as the internal
standard for total and cytoplasmic proteins and lamin B1 was used
as the loading control for nuclear proteins.
ELISA assay
After the different treatments of HUVECs and
apoE−/− mice, the culture medium and serum samples were
collected to evaluate the secretion of pro-inflammatory cytokines.
The levels of human IL-1β (Wuhan Boster Biological Technology,
Ltd.; cat. no. EK0392), mouse IL-1β (Wuhan Boster Biological
Technology, Ltd.; cat. no. EK0394), human IL-6 (Wuhan Boster
Biological Technology, Ltd.; cat. no. EK0410), mouse IL-6 (Wuhan
Boster Biological Technology, Ltd.; cat. no. EK0411), human IL-18
(Wuhan Boster Biological Technology, Ltd.; cat. no. EK0864), mouse
IL-18 (Wuhan Boster Biological Technology, Ltd.; cat. no. EK0433)
and mouse TNF-α (Wuhan Boster Biological Technology, Ltd.; cat. no.
EK0527) were measured by corresponding commercial ELISA kits per
the manufacturer's instructions. Absorbance at 450 nm was monitored
using an iMark Microplate Absorbance Reader (Bio-Rad Laboratories,
Inc.).
Caspase-1 activity assay
Caspase-1 activity was assessed according to the
manual instructions within the caspase-1 activity assay kit
(Beyotime Institute of Biotechnology; cat. no. C1101). Cells were
seeded into 6-well plates at a density of 2×106/well.
When cells were 70–80% confluent, they were treated or transfected
with different agents. Afterward, the mediums were removed and
lysis buffer was added into each well (200 µl/well) for 30 min at
4°C. Cell debris was collected, transferred into a 1 ml EP tube and
centrifuged at 12,000 g for 5 min at 4°C. The supernatants were
harvested and protein concentrations were determined using a BCA
assay. The EP tube was then supplemented with 10 µl AcYVAD-pNA for
2 h at 37°C. Absorbance at 405 nm was recorded and the standard pNA
(yellow) curve was drawn to evaluate the caspase-1 activity. Data
were presented as fold change normalized to control.
Lactate dehydrogenase (LDH) release
assay
LDH release assay was applied to evaluate plasma
membrane damage using an LDH cytotoxicity assay kit (Beyotime
Institute of Biotechnology; cat. no. C0016). After treatment or
transfection, HUVECs were planted in 96-well plates
(1×104/well) with 100 µl RPMI complete medium. Each well
was added with 10 µl LDH release solution and cultured in a
humidified 5% CO2 incubator for 12 h. Through lysis and
centrifugation at 12,000 g for 5 min at 4°C, an aliquot of 50 µl
medium or cell lysates was mixed with 60 µl LDH working solution,
including 20 µl 1X INT solution, 20 µl enzyme solution and 20 µl
lactic acid solution, for 30 min at 4°C in the dark. Absorbance at
490 nm was monitored using a microplate spectrophotometer. The
percentage of LDH release was calculated as the absorbance of the
supernatant divided by the absorbance of the supernatant plus the
absorbance of the lysate, as previously described (25).
Hoechst/PI staining assay
Hoechst 33342/PI double staining assay detected
plasma membrane integrity. HUVECs were seeded in 6-well plates and
treated differently. Cells were washed with PBS twice and the
remaining medium was removed entirely. Following the manual
instructions (Beijing Solarbio Science & Technology Co., Ltd.;
cat. no. CA1120), each well was supplemented with 1 ml staining
buffer, 5 µl Hoechst solution and 5 µl PI solution with a gentle
shake. The plate was then incubated at 4°C for 30 min in the dark.
After rinsing in PBS, the staining solution was disposed of and the
images were captured using a fluorescent microscope.
Determination of intracellular ROS
levels
Per the manufacturer's guidance, cellular ROS
content was measured using a ROS assay kit (Beyotime Institute of
Biotechnology; cat. no. S0033M). Briefly, fluorescent probe DCFH-DA
was diluted into 10 µM using serum-free RPMI medium and stored in
the dark. HUVECs were seeded in 6-well plates
(2×106/well) and treated differently, followed by
incubation with 10 µM DCFH-DA (1 ml/well) at 37°C for 20 min in the
humidified 5% CO2 incubator. Then, each well was rinsed
with cold PBS triple times to remove redundant probes.
Representative images were taken with a fluorescent microscope.
Animal treatment
A total of 30 8-week-old male apoE−/−
mice on C57BL/6J background were bought from Nanjing Junke
Bioengineering Co., Ltd. Mice were housed at 24±2°C and 50±5%
humidity on a 12-h light/dark cycle with free access to water and
food. All mice were fed a high-fat diet (HFD; 21% fat, 0.3%
cholesterol) and randomized into the control and eburicoic acid
groups (n=15/group). Mice in the eburicoic acid group were
administered 10 mg/kg eburicoic acid (MedChemExpress; cat. no.
HY-122951), dissolved in 0.5% carboxymethyl cellulose (CMV) daily
by oral gavage. The control group was orally treated with an
equivalent amount of 0.5% CMV daily. At 12 weeks, mice were
anesthetized using 3% pentobarbital sodium (30 mg/kg, i.p.),
euthanized by cervical dislocation and dissected. The hearts,
aortas and blood were collected for further analysis. The Anhui
Medical University Ethics Committee approved all animal procedures
(approval no. LLSC20242407) per the Declaration of Helsinki.
Evaluation of atherosclerotic plaques
in the aortic roots
Freshly isolated hearts were rinsed in PBS, fixed in
4% paraformaldehyde at 4°C overnight and embedded in OCT compound
(Sakura Finetek USA, Inc.; cat. no. 4583) at −20°C overnight.
Serial 6-µm-thick cryosections were obtained throughout the three
aortic valves (eight sections/mouse). They were stained with Oil
Red O (Beijing Solarbio Science & Technology Co., Ltd.; cat.
no. G1261), hematoxylin and eosin (H&E, Beijing Solarbio
Science & Technology Co., Ltd.; cat. no. G1120) and Masson
(Beijing Solarbio Science & Technology Co., Ltd.; cat. no.
G1346) for 30 min at 37°C to detect lipidosis, plaque area and
collagen contents. Representative images were captured using an
inverted microscope (IX-81; Olympus Corporation). The
positive-staining area was calculated using Image Pro Plus software
7.0 (Media Cybernetics, Inc.) and normalized to the cross-sectional
luminal area.
Detection of serum levels of lipids,
pro-inflammatory cytokines and biochemical indices
Prior to sacrifice, blood samples (~1.5 ml/mouse)
were obtained from the retroorbital plexus under anesthesia and
kept in EDTA-coated tubes. After centrifugation at 5,000 g at 4°C
for 10 min, the supernatants (serum) were gathered into another EP
tube, followed by the detection of aspartate aminotransferase (AST;
cat. no. C010-2-1), alanine aminotransferase (ALT; cat. no.
C009-2-1), blood urea nitrogen (BUN; cat. no. C013-2-1), TC (cat.
no. A111-1-1), low-density lipoprotein cholesterol (LDL-C; cat. no.
A113-1-1), high-density lipoprotein protein cholesterol (HDL-C;
cat. no. A112-1-1) and TG (cat. no. A110-1-1) following the
manufactural instructions in the commercial kits (Nanjing Jiancheng
Bioengineering Institute). The serum levels of IL-1β, IL-6, IL-18
and TNF-α were detected using ELISA, as aforementioned.
Statistical analyses
All experiments were repeated at least three
independent times. Data were processed using GraphPad Prism 10.1
software (Dotmatics) and analyzed using unpaired Student's t-test
or one-way ANOVA followed by Tukey's test. Results were expressed
as the mean ± standard deviation (SD). P<0.05 was considered to
indicate a statistically significant difference.
Results
Eburicoic acid inhibits ox-LDL-induced
NLRP3 inflammasome activation and pyroptosis in HUVECs in a dose-
and time-dependent manner
The present study detected the effects of eburicoic
acid on pyroptosis and inflammation in HUVECs induced by ox-LDL.
HUVECs were maintained in a fresh serum-free medium for 6 h for
synchronization and treated with 100 µg/ml ox-LDL for 24 h. Then,
cells were incubated with a fresh serum-free medium containing
various concentrations of eburicoic acid (1, 5, 10 and 20 µg/ml)
for 24 h. RT-qPCR and western blotting showed that with increasing
dose, eburicoic acid markedly decreases pyroptosis-related factors'
mRNA and protein levels, including NLRP3, ASC, cleaved caspase-1
and GSDMD-N (Fig. 1B and C). The
secretion of pro-inflammatory cytokines (IL-1β, IL-6 and IL-18),
caspase-1 activity and LDH release were reduced after eburicoic
acid treatment (Fig. 1D-F).
Hoechst/PI staining showed that eburicoic acid improved the plasma
membrane integrity (Fig. 1G).
These effects of eburicoic acid were observed even at a low
concentration of 1 µg/ml, with the most substantial effect at 20
µg/ml. Then, HUVECs were treated with 10 µg/ml eburicoic acid for
6, 12, 24 and 48 h to investigate whether eburicoic acid can
repress inflammation and pyroptosis time-dependently. The results
showed that ox-LDL-induced pyroptosis indicator mRNA and protein
expression, secretion of pro-inflammatory factors, caspase-1
activity, LDH release and plasma membrane disruption were inhibited
in HUVECs after incubation with 10 µg/ml eburicoic acid for 6 h.
These effects were more pronounced as the incubation time was
prolonged, peaking at 48 h (Fig.
2). In the meantime, it was observed that eburicoic acid did
not affect the expression of apoptosis- and ferroptosis-related
proteins in ox-LDL-treated HUVECs (Figs. S1 and S2). These findings demonstrated that
eburicoic acid inhibited ox-LDL-induced NLRP3 inflammasome
activation and pyroptosis in a dose and time-dependent manner in
HUVECs.
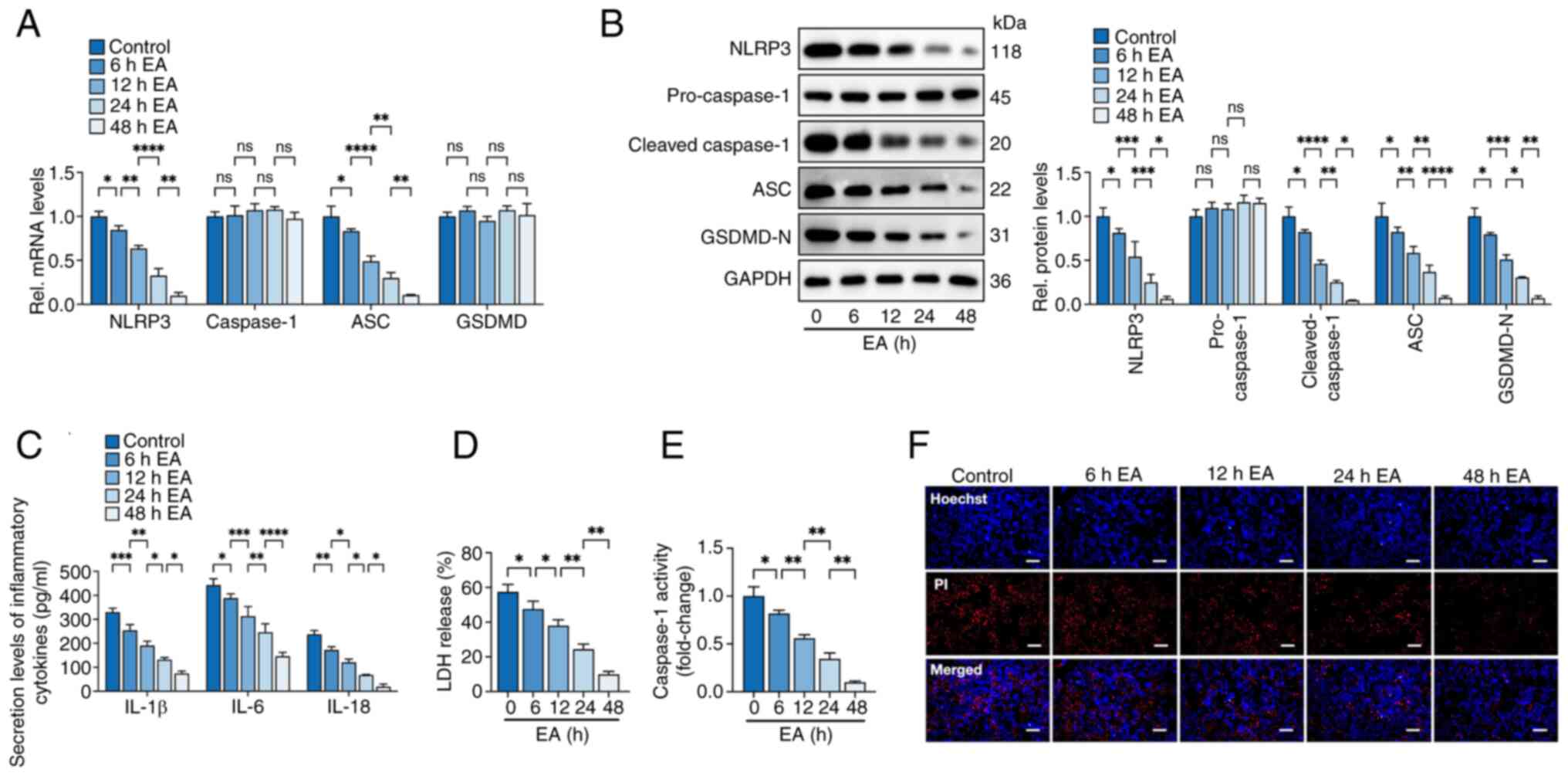 | Figure 2.Eburicoic acid inhibits NLRP3
inflammasome activation and pyroptosis in ox-LDL-treated HUVECs in
a time-dependent manner. Cells were incubated with 100 µg/ml ox-LDL
for 24 h, followed by treatment with 10 µg/ml eburicoic acid. (A)
RT-qPCR analyses of NLRP3, caspase-1, ASC and GSDMD mRNA levels;
(B) western blotting analyses of NLRP3, pro-caspase-1, cleaved
caspase-1, ASC and GSDMD-N protein levels; (C) ELISA Analyses of
IL-1β, IL-6 and IL-18 secretion levels; (D) Determination of LDH
release using an LDH release assay kit; (E) Spectrophotometry
analysis of caspase-1 activity; (F) Representative images of
Hoechst/PI staining. Data are represented as mean ± SD. ns,
statistically insignificant, *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. Scale bar, 20 µm. n=3. NLRP3, NLR
family pyrin domain-containing protein 3; ox-LDL, oxidized
low-density lipoprotein; HUVECs, human umbilical vascular
endothelial cells; RT-qPCR, reverse transcription-quantitative PCR;
ASC, apoptosis-associated speck-like protein containing CARD;
GSDMD-N, N-terminal gasdermin-D; IL, interleukin; LDH, lactate
dehydrogenase EA, eburicoic acid. |
Eburicoic acid inhibits ox-LDL-induced
pyroptosis by decreasing ROS production in HUVECs
Previous studies have noted that ROS accumulation is
closely related to NLRP3 inflammasome activation and pyroptosis
(26,27). The present study explored whether
eburicoic acid inhibited ox-LDL-induced macrophage pyroptosis by
decreasing ROS. Cells were treated with 100 µg/ml ox-LDL for 24 h
and incubated with various concentrations of eburicoic acid (1, 5,
10 and 20 µg/ml) for 24 h or 10 µg/ml for different durations (6,
12, 24 and 48 h). The results showed that eburicoic acid decreased
ROS production dose- and time-dependently (Fig. 3A). Then, cells were treated with 10
µg/ml eburicoic acid for 24 h, with or without 10 µM DMNQ (ROS
agonist) for 6 h. As reflected in Fig.
3B-G, the inhibitory effects of eburicoic acid on the
expression of pyroptosis-related factors, secretion of
pro-inflammatory factors, caspase-1 activity, LDH release and
plasma membrane injury were mainly reversed, suggesting that
eburicoic acid suppresses pyroptosis and inflammation by reducing
ROS generation in ox-LDL-treated HUVECs.
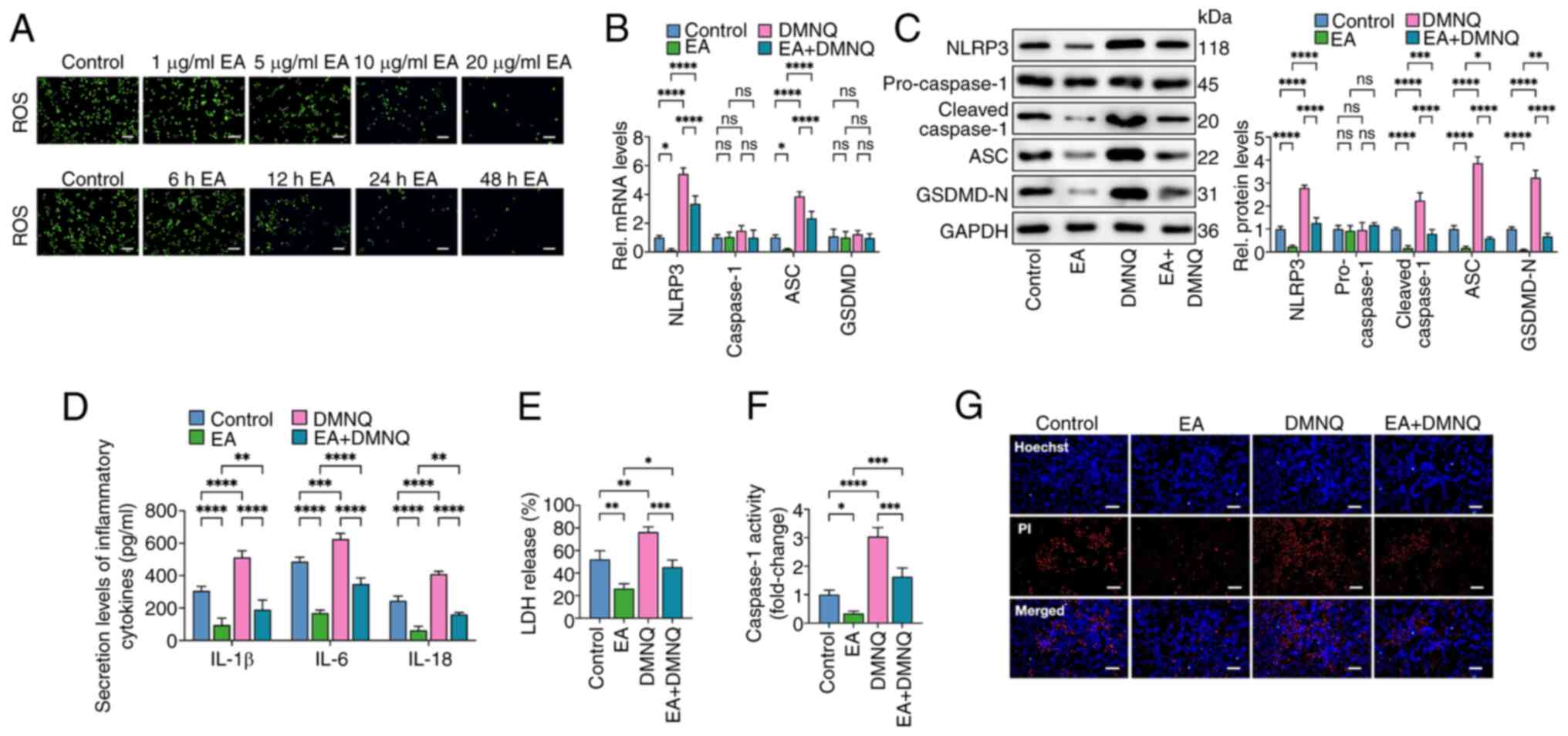 | Figure 3.Eburicoic acid inhibits NLRP3
inflammasome activation and pyroptosis in ox-LDL-treated HUVECs by
decreasing ROS production. (A) HUVECs were pre-treated with 100
µg/ml ox-LDL for 24 h and further incubated with 1, 5, 10 and 20
µg/ml eburicoic acid for 24 h or incubated with 10 µg/ml eburicoic
acid for 6, 12, 24 and 48 h, respectively. Intracellular ROS
production was detected using the peroxide-sensitive fluorescent
probe DCFH-DA. (B-G) Cells were pre-treated with 100 µg/ml ox-LDL
for 24 h. Then, cells were treated with 10 µg/ml eburicoic acid for
24 h, with or without co-incubation of 10 µM DMNQ (ROS agonist) for
6 h. (B) RT-qPCR analyses of NLRP3, caspase-1, ASC and GSDMD mRNA
levels; (C) western blotting analyses of NLRP3, pro-caspase-1,
cleaved caspase-1, ASC and GSDMD-N protein levels; (D) ELISA
Analyses of IL-1β, IL-6 and IL-18 secretion levels; (E)
Determination of LDH release using an LDH release assay kit; (F)
Spectrophotometry analysis of caspase-1 activity; (G)
Representative images of Hoechst/PI staining. Data are represented
as mean ± SD. ns, statistically insignificant, *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001. Scale bar, 20 µm. n=3.
NLRP3, NLR family pyrin domain-containing protein 3; ox-LDL,
oxidized low-density lipoprotein; HUVECs, human umbilical vascular
endothelial cells; ROS, reactive oxygen species; RT-qPCR, reverse
transcription-quantitative PCR; ASC, apoptosis-associated
speck-like protein containing CARD; GSDMD-N, N-terminal
gasdermin-D; IL, interleukin; LDH, lactate dehydrogenase EA,
eburicoic acid. |
Eburicoic acid inhibits ox-LDL-induced
NLRP3 inflammasome activation and pyroptosis in HUVECs via the
HO-1/ROS pathway
Heme oxygenase-1 (HO-1) is critical in inflammatory
responses, oxidative stress, iron metabolism and vascular
physiology. Increased HO-1 expression is associated with
facilitated ROS removal and cellular redox balance maintenance
(28,29). It was hypothesized that HO-1 was
involved in the suppressive effects of eburicoic acid on ROS
accumulation and pyroptosis of HUVECs. RT-qPCR and western blotting
showed that eburicoic acid treatment markedly increased the mRNA
and protein levels of HO-1 in a dose- and time-dependent manner
(Fig. 4A-D). Then, cells were
transfected with HO-1 siRNA to decrease HO-1 expression. As shown
in Fig. 4E, the protein levels of
HO-1 were diminished by nearly 85.4% following HO-1 siRNA
treatment, indicating an effective transfection. Moreover, HO-1
silencing markedly reversed the repressive effects of eburicoic
acid on ROS production, inflammation and pyroptosis in HUVECs
(Fig. 4F-K). These observations
suggested that eburicoic acid could suppress pyroptosis of HUVECs
via the HO-1/ROS pathway.
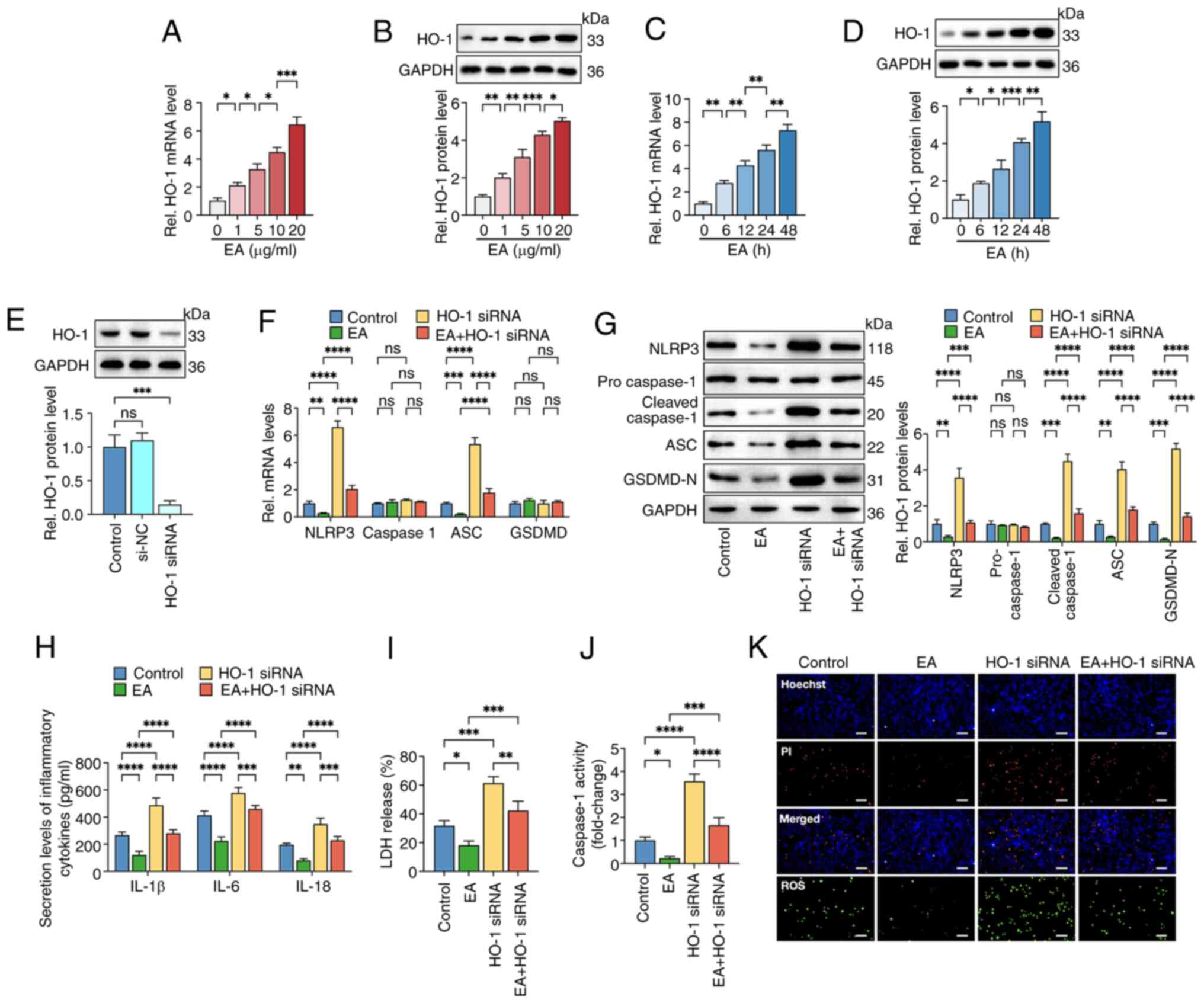 | Figure 4.Eburicoic acid inhibits NLRP3
inflammasome activation and pyroptosis in ox-LDL-treated HUVECs via
the HO-1/ROS pathway. HUVECs were pre-treated with 100 µg/ml ox-LDL
for 24 h and further incubated with (A and B) 1, 5, 10 and 20 µg/ml
eburicoic acid for 24 h or (C and D) incubated with 10 µg/ml
eburicoic acid 6, 12, 24 and 48 h, respectively. The mRNA and
protein levels of HO-1 were detected using RT-qPCR and western
blotting, respectively. (E) Cells were transfected with HO-1 siRNA
or scrambled control siRNA for 48 h. Western blotting was used to
detect HO-1 protein level. Cells were pre-treated with 100 µg/ml
ox-LDL for 24 h. Then, they were transfected with HO-1 siRNA for 48
h before treatment with 10 µg/ml eburicoic acid for 24 h. (F)
RT-qPCR analyses of NLRP3, caspase-1, ASC and GSDMD mRNA levels.
(G) Western blotting analyses of NLRP3, pro-caspase-1, cleaved
caspase-1, ASC and GSDMD-N protein levels. (H) ELISA Analyses of
IL-1β, IL-6 and IL-18 secretion levels. (I) Determination of LDH
release using an LDH release assay kit. (J) Spectrophotometry
analysis of caspase-1 activity. (K) Representative images of
Hoechst/PI staining and ROS production. Data are represented as
mean ± SD. ns, statistically insignificant, *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001. Scale bar, 20 µm. n=3.
NLRP3, NLR family pyrin domain-containing protein 3; ox-LDL,
oxidized low-density lipoprotein; HUVECs, human umbilical vascular
endothelial cells; HO-1, heme oxygenase-1; ROS, reactive oxygen
species; si, small interfering; ASC, apoptosis-associated
speck-like protein containing CARD; GSDMD-N, N-terminal
gasdermin-D; IL, interleukin; LDH, lactate dehydrogenase; ROS,
reactive oxygen species EA, eburicoic acid. |
Eburicoic acid ameliorates
inflammation and pyroptosis in ox-LDL-treated HUVECs via the
Keap1/Nrf2/HO-1/ROS pathway
Evidence has identified that HO-1 is a critical
functional effector of Nrf2-induced ROS clearance and
anti-oxidation. Under basal conditions, Nrf2 is mainly sequestered
in the cytoplasm by binding to Kelch-like ECH-associated protein-1
(Keap1) (30). Under oxidative and
electrophilic stress, Nrf2 is released from Keap1 and enters the
nucleus, where Nrf2 binds to the antioxidant response element and
promotes HO-1 expression (31,32).
Next, the present study detected whether eburicoic acid promoted
Nrf2 nuclear translocation in ox-LDL-treated HUVECs. Cells were
treated with 100 µg/ml ox-LDL for 24 h, followed by incubation with
various concentrations of eburicoic acid (1, 5, 10 and 20 µg/ml)
for 24 h or 10 µg/ml for different durations (6, 12, 24 and 48 h).
As shown in Fig. 5, eburicoic acid
markedly decreased cytoplasmic Keap1 and Nrf2 protein levels dose-
and time-dependently (Fig. 5A and
C). In contrast, it increased the nuclear and total Nrf2 levels
in a dose- and time-dependent manner (Fig 5B, D-H), indicating that eburicoic
acid could promote Nrf2 nuclear translocation. To further verify
the role of Nrf2 in the anti-pyroptosis activities of eburicoic
acid, cells were transfected with Nrf2 siRNA to reduce its
expression. The results showed that Nrf2 siRNA transduction
decreased the protein levels of Nrf2 by 69%. Importantly, eburicoic
acid-induced HO-1 enhancement, ROS clearance, anti-inflammation and
anti-pyroptosis effects were mainly compromised after Nrf2 silence
(Fig. 6), demonstrating that
eburicoic acid inhibits inflammation and pyroptosis in HUVECs via
the Keap1/Nrf2/HO-1/ROS pathway.
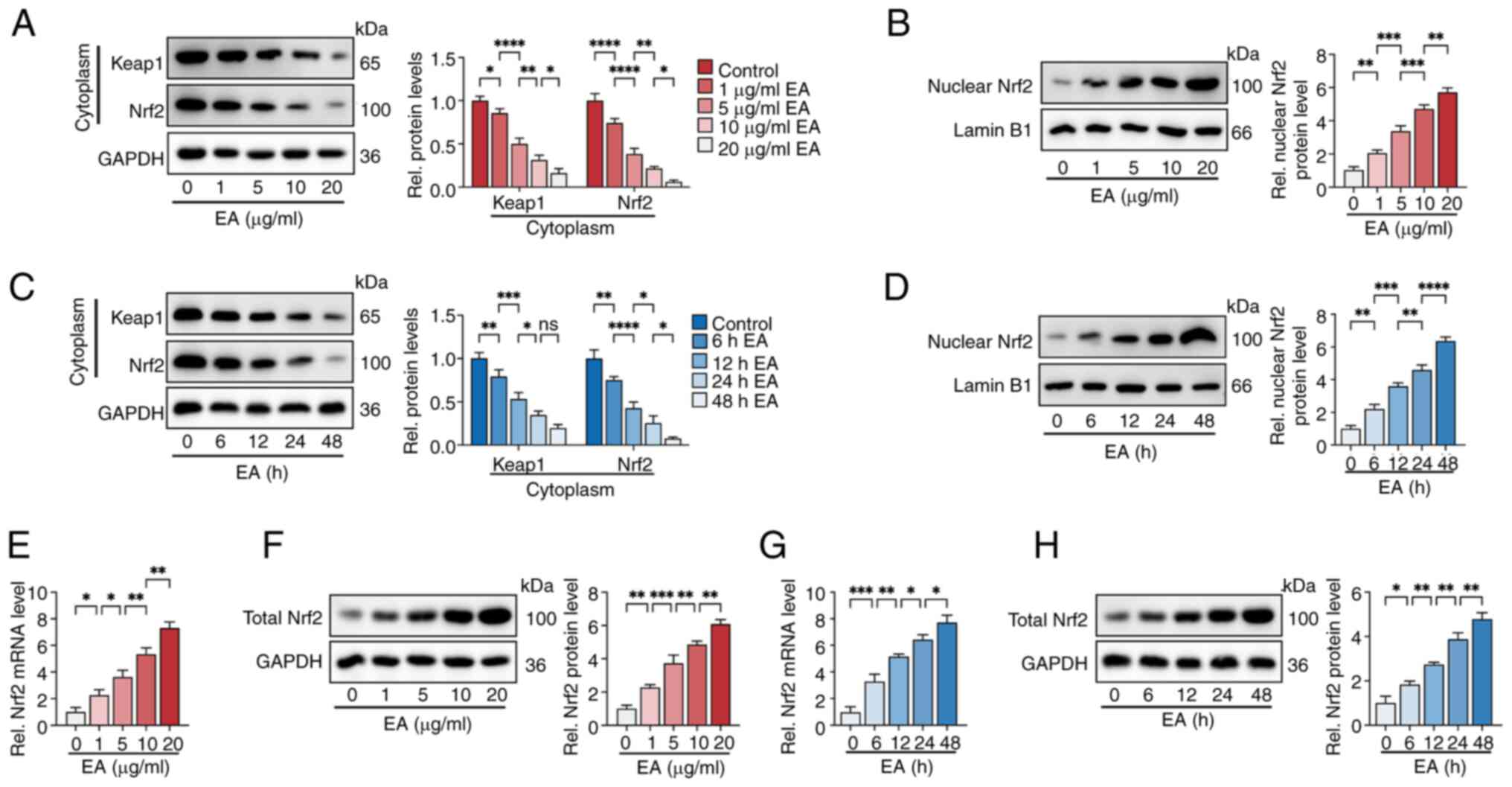 | Figure 5.Eburicoic acid promotes Nrf2 nuclear
translocation in ox-LDL-treated HUVECs. HUVECs were pre-treated
with 100 µg/ml ox-LDL for 24 h. (A and B) Cells were incubated with
1, 5, 10 and 20 µg/ml eburicoic acid for 24 h. The protein levels
of cytoplasmic Keap1 and Nrf2 and nuclear Nrf2 were detected using
western blotting. (C and D) Cells were incubated with 10 µg/ml
eburicoic acid for 6, 12, 24 and 48 h, respectively. The protein
levels of cytoplasmic Keap1 and Nrf2 and nuclear Nrf2 were detected
using western blotting. (E-H) Cells were incubated with various
doses of eburicoic acid for 24 h or 10 µg/ml eburicoic acid for
various durations. The mRNA and protein levels of total Nrf2 were
detected using RT-qPCR and western blotting, respectively. Data are
represented as mean ± SD. ns, statistically insignificant,
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Scale bar,
20 µm. n=3. Nrf2, NF-E2-related factor 2; ox-LDL, oxidized
low-density lipoprotein; HUVECs, human umbilical vascular
endothelial cells; Keap1, Kelch-like ECH-associated protein 1;
RT-qPCR, reverse transcription-quantitative PCR EA, eburicoic
acid. |
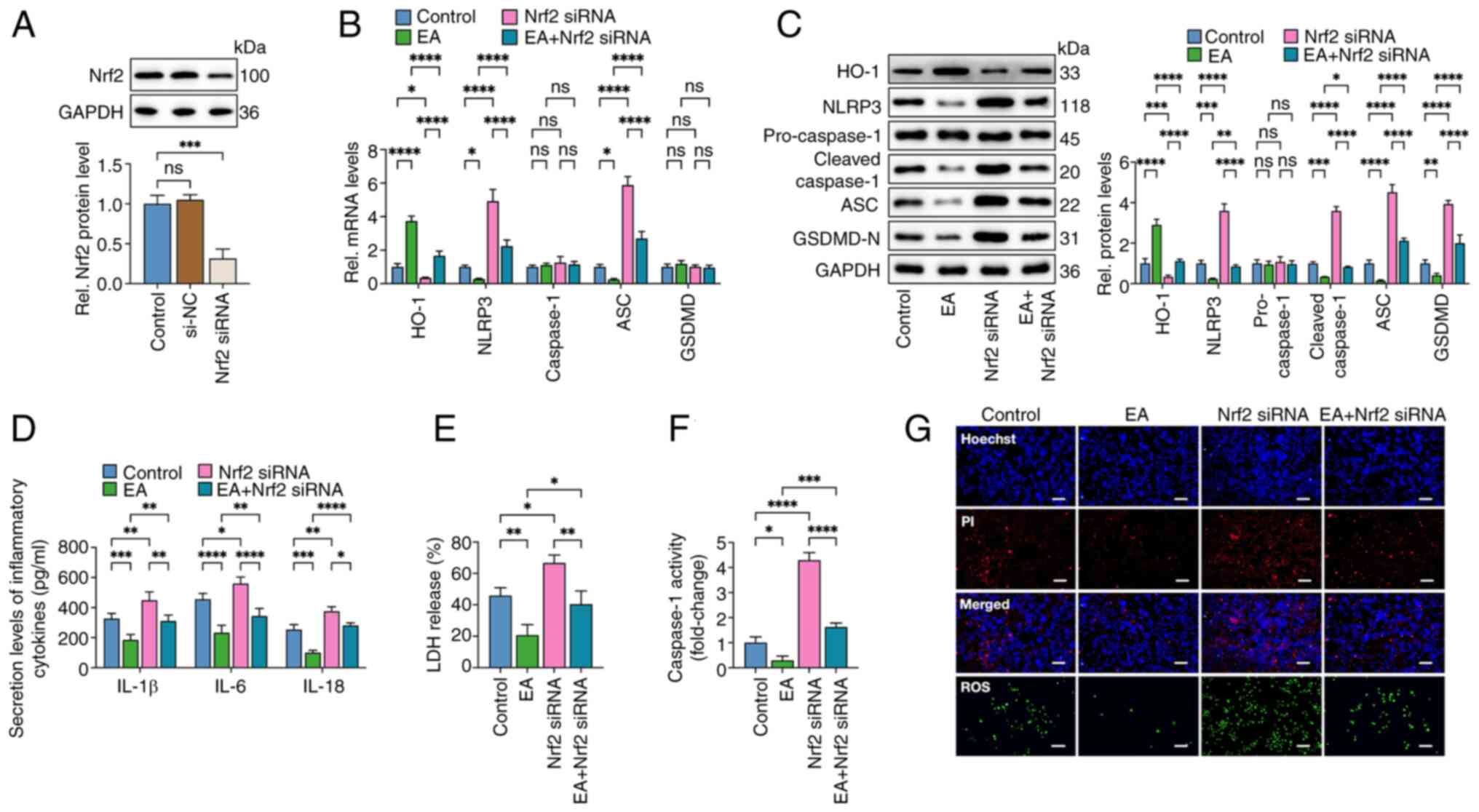 | Figure 6.Eburicoic acid inhibits NLRP3
inflammasome activation and pyroptosis in ox-LDL-treated HUVECs via
the Nrf2/HO-1/ROS pathway. (A) Cells were transfected with Nrf2
siRNA or scrambled control siRNA for 48 h. western blotting was
used to detect the Nrf2 protein level. (B-G) Cells were pre-treated
with 100 µg/ml ox-LDL for 24 h. Then, they were transfected with
Nrf2 siRNA for 48 h before treatment with 10 µg/ml eburicoic acid
for 24 h. (B) RT-qPCR analyses of HO-1, NLRP3, caspase-1, ASC and
GSDMD mRNA levels; (C) western blotting analyses of NLRP3,
pro-caspase-1, cleaved caspase-1, ASC and GSDMD-N protein levels;
(D) ELISA Analyses of IL-1β, IL-6 and IL-18 secretion levels; (E)
Determination of LDH release using an LDH release assay kit; (F)
Spectrophotometry analysis of caspase-1 activity; (G)
Representative images of Hoechst/PI staining and ROS production.
Data are represented as mean ± SD. ns, statistically insignificant,
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Scale bar,
20 µm. n=3. NLRP3, NLR family pyrin domain-containing protein 3;
ox-LDL, oxidized low-density lipoprotein; HUVECs, human umbilical
vascular endothelial cells; Nrf2, NF-E2-related factor 2; HO-1,
heme oxygenase-1; ROS, reactive oxygen species; si, small
interfering; ASC, apoptosis-associated speck-like protein
containing CARD; GSDMD-N, N-terminal gasdermin-D; LDH, lactate
dehydrogenase; ROS, reactive oxygen species; RT-qPCR, reverse
transcription-quantitative PCR EA, eburicoic acid. |
Eburicoic acid mitigates the
development of atherosclerotic plaques in vivo
At last, the present study explored the effects of
eburicoic acid on the development of atherosclerotic plaques in
HFD-fed apoE−/− mice. Mice were orally gavaged with 10
mg/kg eburicoic acid (dissolved in 0.5% CMV) or vehicle once daily.
The results showed that compared with the control group, the serum
levels of ALT, AST, BUN and serum creatinine (Scr) were not changed
in mice of the eburicoic acid group, suggesting that eburicoic acid
displays no apparent hepatotoxicity and nephrotoxicity (Fig. 7A-C). In addition, eburicoic acid
administration increased the protein levels of Nrf2 and HO-1and
decreased the protein levels of Keap1 and pyroptosis-related
factors (NLRP3, cleaved caspase-1, ASC and GSDMD-N) in the aortas
(Fig. 7D). Furthermore, eburicoic
acid treatment increased HDL-C levels and downregulated TC, LDL-C,
TG and pro-inflammatory cytokines (IL-1β, IL-6, TNF-α and IL-18)
levels in the serum (Fig. 7E and
F). Oil Red O, HE and Masson staining showed eburicoic acid
alleviated lipid accumulation, decreased lesion area and increased
collagen fiber content in atherosclerotic plaques (Fig. 7G). These findings suggested that
eburicoic acid retarded the progression of AS in HFD-fed
apoE−/− mice.
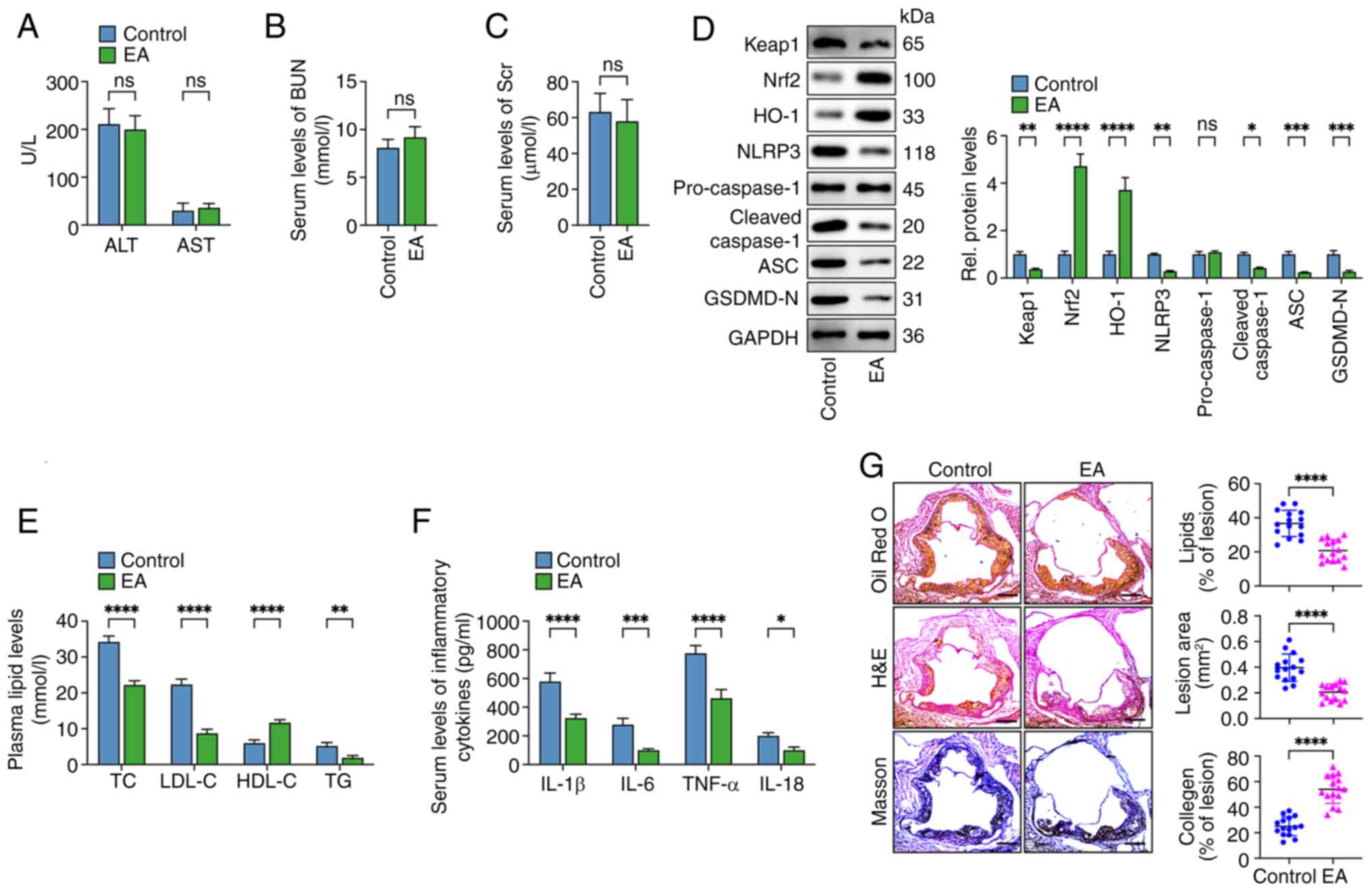 | Figure 7.Eburicoic acid mitigates the
progression of atherosclerotic plaques in HFD-fed
apoE−/− mice. apoE−/− mice (n=15/each group)
fed on an HFD were administered with 10 mg/kg eburicoic acid
(dissolved in 0.5% CMV) or vehicle only by oral gavage once daily.
(A-C) The AST, ALT, BUN and Scr levels were detected using the
respective commercial kits; (D) western blotting analyses of Keap1,
Nrf2, HO-1, NLRP3, cleaved caspase-1, ASC and GSDMD-N protein
levels in the aorta; n=3. (E) Assessment of plasma TC, TG, HDL-C
and LDL-C levels; n=3. (F) Determination of serum IL-1β, IL-6,
TNF-α and IL-18 levels using ELISA assay; n=3. (G) Oil Red O, HE
and Masson staining of cross-sections of the aortic root. Image Pro
software analyzed the lipid accumulation, lesion area and collagen
content within atherosclerotic plaques. n=15. Data are represented
as mean ± SD. ns, statistically insignificant, *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001. Scale bar, 100 µm.
HFD, high-fat-diet; AST, aspartate aminotransferase; ALT, alanine
aminotransferase; BUN, blood urea nitrogen; Scr, serum creatinine;
Keap1, Kelch-like ECH-associated protein 1; Nrf2, NF-E2-related
factor 2; HO-1, heme oxygenase-1; NLRP3, NLR family pyrin
domain-containing protein 3; TC, total cholesterol; TG,
triglycerides; HDL-C high-density lipoprotein cholesterol LDL-C,
low-density lipoprotein cholesterol IL, interleukin; EA, eburicoic
acid. |
Discussion
The present study, for the first time to the best of
the authors' knowledge, discovered that eburicoic acid inhibited
the activation of NLRP3 inflammasome activation, improved plasma
integrity, reduced pro-inflammatory cytokine secretion and
suppressed pyroptosis in HUVECs. Mechanistically speaking,
eburicoic acid facilitated the disassociation of Nrf2 from Keap1
and promoted Nrf2 nuclear translocation. Activation of the
Nrf2/HO-1 pathway-induced ROS reduction served as the upstream
signal for upregulating the expression of pyroptosis-related
indicators and secretion of pro-inflammatory cytokines. Finally,
the present study determined that eburicoic acid mitigated the
lipid accumulation in atherosclerotic plaques, increased plaque
stability and inhibited inflammatory response in apoE−/−
mice.
Chinese herbal medicines and their natural extracts
markedly affect inflammation and pyroptosis in VECs through various
mechanisms (33,34). Tongxinluo (TXL), one of the most
common traditional Chinese medicines, could decrease ROS
generation, caspase-1 activity and pro-inflammatory cytokines
secretion, exerting anti-pyroptosis effects in mouse aortic
endothelial cells (26). Moreover,
treatment of HFD-fed apoE−/− mice with TXL suppresses
caspase-1 activation in plaque endothelium, decreases the
expression of pyroptosis-related factors in the aorta and
diminishes atherosclerotic plaque size dose-dependently (26). Salvianolic acid A (SAA) can protect
the HUVECs against high glucose-induced pyroptosis in a pyruvate
kinase M2 (PKM2)/protein kinase R-dependent manner. Administering
SAA to HFD-fed apoE−/− mice markedly decreases the
expression of pyroptosis-related factors, including NLRP3, ASC and
GSDMD in the endothelium of the aortic sinus, alleviates plaque
lipid accumulation and increases fibrous content (35). Isoliquiritigenin can inhibit NLRP3
inflammasome activation, inflammation and pyroptosis in
TNF-α-treated HUVECs via the TNF receptor 1/sirtuin 6 (SIRT6)
pathway (36). Treatment of human
coronary artery endothelial cells with Zhilong Huoxue Tongyu
capsule markedly increases cell viability, decreases the expression
of pyroptosis-related genes, including NLRP3, ASC and caspase 1,
inhibits IL-1β and IL-18 secretion and alleviates pyroptosis
through the miR-30b-5p/NLRP3 pathway (37). Lycopene can protect endothelial
progenitor cells from ox-LDL-induced damage, downregulate the
expression level of pyroptosis indicators and production of
inflammatory factors, suppressing pyroptosis through the AMPK/mTOR
pathway (38). As a
multi-functional bioactive triterpenoid of Antrodia
cinnamomea, eburicoic acid can decrease oxidative injury and
inflammation in various cell types. Saba et al (39) reported that acetyl eburicoic acid
decreases NO production and the expression of iNOS, IL-1β, IL-6 and
TNF-α in LPS-treated RAW264.7 macrophages dose-dependently. Wang
et al (40) found that
eburicoic acid can directly inhibit the
H+/K+-ATPase and attenuate ethanol and
aspirin-induced gastric ulcers specifically by inhibiting gastric
acid. They inferred that the gastric-protecting effects of
eburicoic acid may be related to the reduction of
antioxidant-dependent mechanisms and inhibition of the inflammatory
process. Notably, Lin et al (41) treated streptozotocin -induced
diabetic mice with eburicoic acid and found that the plasma TG, TC
and glucose levels are markedly reduced, demonstrating the
anti-hyperlipidemic and anti-diabetic activities of eburicoic acid.
The present study observed that incubation of HUVECs with eburicoic
acid markedly decreased the expression of NLRP3, ASC, cleaved
caspase-1 and GSDMD-N, reduced caspase-1 activity, LDH release and
secretion of IL-1β, IL-6 and IL-18 and improved plasma integrity in
a dose- and time-dependent manner. These effects of eburicoic acid
on HUVECs were mainly compromised by adding ROS inducer DMNQ,
demonstrating that eburicoic acid suppressed NLRP3 inflammasome
activation and HUVEC pyroptosis by decreasing ROS content. In
HFD-fed apoE−/− mice, eburicoic acid diminished the
serum levels of IL-1β, IL-6 and TNF-α, improved plasma lipid
profile and decreased the expression of pyroptosis-associated
factors in aortic tissue, leading to smaller plaque size, less
lipid deposition and more fibrous content in the cross-section of
aortic roots, which was consistent with the previous studies.
In addition to inflammation, the massive deposition
of macrophage-derived foam cells in blood walls is another feature
of AS (42). Jin et al
(43) found that incubation of
ox-LDL-laden bone marrow-derived macrophages with VX-765, a
specific caspase-1 inhibitor, can suppress pyroptosis and inhibit
foam cell formation, suggesting a positive relationship between
pyroptosis and lipid accumulation in macrophages. Whether eburicoic
acid prevents foam cell formation by inhibiting macrophage
pyroptosis needs further investigation.
The Nrf2/HO-1 signaling pathway is indispensable in
oxidative stress response, anti-inflammation and antioxidation.
Extracts from herbal medicines exert endothelium-protective action
via activation of the Nrf2/HO-1 pathway (44). Dihydromyricetin can inhibit
palmitic acid-induced NLRP3 inflammasome activation and pyroptosis
in HUVECs by activating the Nrf2/HO-1/NQO1 pathway and decreasing
mitochondrial ROS production (45). Fisetin can increase HO-1 expression
dose- and time-dependently by activating Nrf2, PKC-δ and p38,
conferring cytoprotection in H2O2-treated
HUVECs (46). Astragaloside IV can
activate the Nrf2/HO-1 pathway, decrease ROS content, downregulate
TNF-α and IL-6 levels and improve oxidative damage in HUVECs with
ox-LDL intervention (47).
Ginsenoside Rg1 can markedly antagonize PM2.5-induced
HUVEC cell death, ROS accumulation and MDA production by promoting
Nrf2 nuclear translocation and HO-1 expression (48). Andrographolide can suppress
TNF-α-induced ICAM-1 expression and ROS generation by increasing
NADPH oxidase by stimulating the PI3K/Akt/Nrf2/HO-1 pathway in
eburicoic acid.hy926 endothelial-like cells (49). The present study found that
incubating HUVECs with eburicoic acid increased HO-1 expression in
a dose- and time-dependent manner. Silencing of HO-1 mainly
compromised eburicoic acid-induced inhibition of ROS accumulation,
NLRP3 inflammasome activation, inflammation and pyroptosis in
HUVECs. Furthermore, eburicoic acid markedly promoted the
dissociation of Nrf2 from the Keap1-Nrf2 complex and subsequent
nuclear translocation. Nrf2 knockdown markedly reversed HO-1
enhancement, ROS reduction and pyroptosis suppression induced by
eburicoic acid, demonstrating that eburicoic acid alleviates HUVEC
pyroptosis via the Keap1/Nrf2/HO-1/ROS pathway. Kong et al
(50) reported that TLR4, a
transmembrane protein, is an upstream positive regulator of the
Keap1/NRF2 pathway. The present study found that treating HUVECs
with eburicoic acid markedly increased the TLR4 protein level,
suggesting that eburicoic acid may activate the Keap1/Nrf2 pathway
by stimulating TLR4. Previous studies have shown that miRNAs are
critical regulators of Keap1/Nrf2 pathway and pyroptosis. Yao et
al (51) found that
Ginsenoside Rd could ameliorate neuronal pyroptosis and protect
against cerebral ischemia/reperfusion injury by upregulating
miR-139-5p, inhibiting FoxO1 expression and activating the
Keap1/Nrf2/ROS pathway. Xu et al (52) found that platelet-rich plasma can
attenuate intervertebral disc degeneration by delivering miR-141-3p
into the nucleus pulposus cells. Specifically, miR-141-3p can
target Keap1 3′UTR, which decreases Keap1 expression and, in turn,
promotes nuclear translocation of Nrf2, inhibiting oxidative stress
and pyroptosis of nucleus pulposus cells. Whether miRNAs, such as
miR-139-5p and miR-141-3p, are involved in the effects of eburicoic
acid on Keap1/Nrf2 pathway and pyroptosis in HUVECs warrants
further exploration.
In summary, the present study identified the
anti-atherogenic role of eburicoic acid and its relationship with
endothelial pyroptosis. Specifically, eburicoic acid can decrease
Keap1 expression and promote Nrf2 nuclear translocation, which in
turn increases HO-1 expression and represses ROS accumulation and
VEC pyroptosis, ultimately alleviating hyperlipidemia, inflammation
and the development of atheromatous plaques in vivo.
Eburicoic acid may be regarded as an effective phytomedicine for
preventing AS.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Anhui Provincial Natural
Science Foundation (grant no. 2008085MH239).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XHL and MQM conceived and designed the experiments.
MQM, CY, SYJ, YY and YYP performed experiments and analyzed data.
MQM, YY and XHL wrote and revised the manuscript. MQM and YYP
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Anhui
Medical University Ethics Committee (approval no.
LLSC20242407).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dibben GO, Faulkner J, Oldridge N, Rees K,
Thompson DR, Zwisler AD and Taylor RS: Exercise-based cardiac
rehabilitation for coronary heart disease: A meta-analysis. Eur
Heart J. 44:452–469. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan H, Ho SE, Xue C, Cui J, Johanson QS,
Sachs N, Ross LS, Li F, Solomon RA, Connolly ES Jr, et al:
Atherosclerosis is a smooth muscle Cell-Driven Tumor-like disease.
Circulation. 149:1885–1898. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xing Y and Lin X: Challenges and advances
in the management of inflammation in atherosclerosis. J Adv Res.
Jun 21–2024.doi: 10.1016/j.jare.2024.06.016.
|
|
4
|
Jia M, Li Q, Guo J, Shi W, Zhu L, Huang Y,
Li Y, Wang L, Ma S, Zhuang T, et al: Deletion of BACH1 attenuates
atherosclerosis by reducing endothelial inflammation. Circ Res.
130:1038–1055. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Q, Han J, Liang Z, Geng X, Du Y, Zhou
J, Yao W and Xu T: FSH is responsible for androgen deprivation
Therapy-associated atherosclerosis in mice by exaggerating
endothelial inflammation and monocyte adhesion. Arterioscler Thromb
Vasc Biol. 44:698–719. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oladapo A, Jackson T, Menolascino J and
Periyasamy P: Role of pyroptosis in the pathogenesis of various
neurological diseases. Brain Behav Immun. 117:428–446. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Volchuk A, Ye A, Chi L, Steinberg BE and
Goldenberg NM: Indirect regulation of HMGB1 release by gasdermin D.
Nat Commun. 11:45612020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y,
Mai J, Virtue A, Lopez-Pastrana J, Meng S, et al: Early
hyperlipidemia promotes endothelial activation via a
caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol.
35:804–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv Y, Jiang Z, Zhou W, Yang H, Jin G, Wang
D, Kong C, Qian Z, Gu Y, Chen S and Zhu L: Low-shear stress
promotes atherosclerosis via inducing endothelial cell pyroptosis
mediated by IKKε/STAT1/NLRP3 pathway. Inflammation. 47:1053–1066.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Yuan C, Qin W, Yu B, Wei D and Wu
P: TMAO promotes vascular endothelial cell pyroptosis via the
LPEAT-mitophagy pathway. Biochem Biophys Res Commun.
703:1496672024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song L, Zhang J, Lai R, Li Q, Ju J and Xu
H: Chinese herbal medicines and active metabolites: Potential
antioxidant treatments for atherosclerosis. Front Pharmacol.
12:6759992021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jing Y, Hu T, Yuan J, Liu Z, Tao M, Ou M,
Cheng X, Cheng W, Yi Y and Xiong Q: Resveratrol protects against
postmenopausal atherosclerosis progression through reducing PCSK9
expression via the regulation of the ERα-mediated signaling
pathway. Biochem Pharmacol. 211:1155412023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao S, Zhang W, Zhao Q, Zhou J, Wu Y, Liu
Y, Yuan Z and Wang L: Curcumin ameliorates atherosclerosis in
apolipoprotein E deficient asthmatic mice by regulating the balance
of Th2/Treg cells. Phytomedicine. 52:129–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xing SS, Yang J, Li WJ, Li J, Chen L, Yang
YT, Lei X, Li J, Wang K and Liu X: Salidroside decreases
atherosclerosis plaque formation via inhibiting endothelial cell
pyroptosis. Inflammation. 43:433–440. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao H, Jia Q, Yan L, Chen C, Xing S and
Shen D: Quercetin suppresses the progression of atherosclerosis by
regulating MST1-Mediated autophagy in ox-LDL-Induced RAW264.7
macrophage foam cells. Int J Mol Sci. 20:60932019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma SR, Tong Q, Lin Y, Pan LB, Fu J, Peng
R, Zhang XF, Zhao ZX, Li Y, Yu JB, et al: Berberine treats
atherosclerosis via a vitamine-like effect down-regulating
Choline-TMA-TMAO production pathway in gut microbiota. Signal
Transduct Target Ther. 7:2072022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng JS, Huang SS, Lin TH, Lee MM, Kuo CC,
Sung PJ, Hou WC, Huang GJ and Kuo YH: Analgesic and
anti-inflammatory bioactivities of eburicoic acid and
dehydroeburicoic acid isolated from Antrodia camphorata on
the inflammatory mediator expression in mice. J Agric Food Chem.
61:5064–5071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tung YT, Tsai TC, Kuo YH, Yen CC, Sun JY,
Chang WH, Chen HL and Chen CM: Comparison of solid-state-cultured
and wood-cultured Antrodia camphorata in anti-inflammatory
effects using NF-κB/luciferase inducible transgenic mice.
Phytomedicine. 21:1708–1716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su YC, Liu CT, Chu YL, Raghu R, Kuo YH and
Sheen LY: Eburicoic acid, an active triterpenoid from the fruiting
bodies of basswood cultivated antrodia cinnamomea, induces ER
Stress-mediated autophagy in human hepatoma cells. J Tradit
Complement Med. 2:312–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang GJ, Deng JS, Huang SS, Lee CY, Hou
WC, Wang SY, Sung PJ and Kuo YH: Hepatoprotective effects of
eburicoic acid and dehydroeburicoic acid from Antrodia
camphorata in a mouse model of acute hepatic injury. Food Chem.
141:3020–3027. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Zhang P, He H, Se X, Sun W, Chen
B, Zhang L, Yan X and Zou K: Eburicoic acid from Laetiporus
sulphureus (Bull.:Fr.) Murrill attenuates inflammatory responses
through inhibiting LPS-induced activation of PI3K/Akt/mTOR/NF-κB
pathways in RAW264.7 cells. Naunyn Schmiedebergs Arch Pharmacol.
390:845–856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin CH, Kuo YH and Shih CC: Eburicoic
acid, a triterpenoid compound from Antrodia camphorata,
displays antidiabetic and antihyperlipidemic effects in
Palmitate-treated C2C12 myotubes and in High-Fat Diet-Fed mice. Int
J Mol Sci. 18:23142017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andrews CS, Matsuyama S, Lee BC and Li JD:
Resveratrol suppresses NTHi-induced inflammation via up-regulation
of the negative regulator MyD88 short. Sci Rep. 6:344452016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang X, Ma C, Gao Y, Zheng Y, Li J, Zong
W and Zhang Q: Tongxinluo attenuates atherosclerosis by inhibiting
ROS/NLRP3/caspase-1-mediated endothelial cell pyroptosis. J
Ethnopharmacol. 304:1160112023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Zhang Y, Wang H, Zhang X, Chen Y
and Chen G: Microglial pyroptosis in hippocampus mediates
Sevolfurane-induced cognitive impairment in aged mice via ROS-NLRP3
inflammasome pathway. Int Immunopharmacol. 116:1097252023.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo P, Liu D, Zhang Q, Yang F, Wong YK,
Xia F, Zhang J, Chen J, Tian Y, Yang C, et al: Celastrol induces
ferroptosis in activated HSCs to ameliorate hepatic fibrosis via
targeting peroxiredoxins and HO-1. Acta Pharm Sin B. 12:2300–2314.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang W, Wang Y, Zhang C, Huang Y, Yu J,
Shi L, Zhang P, Yin Y, Li R and Tao K: Maresin1 protect against
ferroptosis-induced liver injury through ROS inhibition and
Nrf2/HO-1/GPX4 activation. Front Pharmacol. 13:8656892022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hong H, Lou S, Zheng F, Gao H, Wang N,
Tian S, Huang G and Zhao H: Hydnocarpin D attenuates
lipopolysaccharide-induced acute lung injury via MAPK/NF-κB and
Keap1/Nrf2/HO-1 pathway. Phytomedicine. 101:1541432022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen H, Fu J, Chen H, Hu Y, Soroka DN,
Prigge JR, Schmidt EE, Yan F, Major MB, Chen X and Sang S: Ginger
compound [6]-shogaol and its cysteine-conjugated metabolite (M2)
activate Nrf2 in colon epithelial cells in vitro and in vivo. Chem
Res Toxicol. 27:1575–1585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo RR, Yang J, Sun YL, Zhou BY, Zhou SX,
Zhang GX and Yang AX: Dexmedetomidine attenuates ferroptosis by
Keap1-Nrf2/HO-1 pathway in LPS-induced acute kidney injury. Naunyn
Schmiedebergs Arch Pharmacol. 397:7785–7796. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan Q, Li P, Liu S, Sun Y, Chen C, Long J,
Lin Y, Liang J, Wang H, Zhang L, et al: Dihydromyricetin treats
pulmonary hypertension by modulating CKLF1/CCR5 axis-induced
pulmonary vascular cell pyroptosis. Biomed Pharmacother.
180:1176142024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Guan X, Gao CL, Ruan W, Zhao S,
Kai G, Li F and Pang T: Medioresinol as a novel PGC-1α activator
prevents pyroptosis of endothelial cells in ischemic stroke through
PPARα-GOT1 axis. Pharmacol Res. 169:1056402021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu J, Chen H, Le Y, Guo J, Liu Z, Dou X
and Lu D: Salvianolic acid A regulates pyroptosis of endothelial
cells via directly targeting PKM2 and ameliorates diabetic
atherosclerosis. Front Pharmacol. 13:10092292022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He J, Deng Y, Ren L, Jin Z, Yang J, Yao F,
Liu Y, Zheng Z, Chen D, Wang B, et al: Isoliquiritigenin from
licorice flavonoids attenuates NLRP3-mediated pyroptosis by SIRT6
in vascular endothelial cells. J Ethnopharmacol. 303:1159522023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu M, Luo G, Liu T, Yang T, Wang R, Ren
W, Liu P, Lai X, Zhou H and Yang S: Zhilong huoxue tongyu capsule
alleviated the pyroptosis of vascular endothelial cells induced by
ox-LDL through miR-30b-5p/NLRP3. Evid Based Complement Alternat
Med. 2022:39813502022.PubMed/NCBI
|
|
38
|
Tan C, Chen J, Tu T, Chen L and Zou J:
Lycopene inhibits pyroptosis of endothelial progenitor cells
induced by ox-LDL through the AMPK/mTOR/NLRP3 pathway. Open Med
(Wars). 19:202409732024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saba E, Son Y, Jeon BR, Kim SE, Lee IK,
Yun BS and Rhee MH: Acetyl eburicoic acid from laetiporus
sulphureus var. miniatus suppresses inflammation in murine
macrophage RAW 264.7 cells. Mycobiology. 43:131–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang J, Sun W, Luo H, He H, Deng W, Zou K,
Liu C, Song J and Huang W: Protective effect of eburicoic acid of
the chicken of the woods mushroom, laetiporus sulphureus (Higher
Basidiomycetes), against gastric ulcers in mice. Int J Med
Mushrooms. 17:619–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin CH, Kuo YH and Shih CC: Antidiabetic
and hypolipidemic activities of eburicoic acid, a triterpenoid
compound from Antrodia camphorata, by regulation of Akt
phosphorylation, gluconeogenesis, and PPARα in
streptozotocin-induced diabetic mice. RSC Adv. 8:20462–20476. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
La Chica Lhoëst MT, Martinez A, Claudi L,
Garcia E, Benitez-Amaro A, Polishchuk A, Piñero J, Vilades D,
Guerra JM, Sanz F, et al: Mechanisms modulating foam cell formation
in the arterial intima: Exploring new therapeutic opportunities in
atherosclerosis. Front Cardiovasc Med. 11:13815202024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin Y, Liu Y, Xu L, Xiong Y, Peng Y, Ding
K, Zheng S, Yang N, Zhang Z, Li L, et al: Novel role for caspase 1
inhibitor VX765 in suppressing NLRP3 inflammasome assembly and
atherosclerosis via promoting mitophagy and efferocytosis. Cell
Death Dis. 13:5122022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Q, Liu J, Duan H, Li R, Peng W and
Wu C: Activation of Nrf2/HO-1 signaling: An important molecular
mechanism of herbal medicine in the treatment of atherosclerosis
via the protection of vascular endothelial cells from oxidative
stress. J Adv Res. 34:43–63. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu Q, Zhang T, Yi L, Zhou X and Mi M:
Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis
by activating the Nrf2 signaling pathway in vascular endothelial
cells. Biofactors. 44:123–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee SE, Jeong SI, Yang H, Park CS, Jin YH
and Park YS: Fisetin induces Nrf2-mediated HO-1 expression through
PKC-δ and p38 in human umbilical vein endothelial cells. J Cell
Biochem. 112:2352–2360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu Z, Li J and Zhang X: Astragaloside IV
protects against oxidized Low-density lipoprotein (ox-LDL)-induced
endothelial cell injury by reducing oxidative stress and
inflammation. Med Sci Monit. 25:2132–2140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li CP, Qin G, Shi RZ, Zhang MS and Lv JY:
Ginsenoside Rg1 reduces toxicity of PM(2.5) on human umbilical vein
endothelial cells by upregulating intracellular antioxidative
state. Environ Toxicol Pharmacol. 35:21–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu CY, Yang YC, Li CC, Liu KL, Lii CK and
Chen HW: Andrographolide inhibits TNFα-induced ICAM-1 expression
via suppression of NADPH oxidase activation and induction of HO-1
and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1
pathways in human endothelial cells. Biochemical Pharmacology.
91:40–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kong C, Yan X, Zhu Y, Zhu H, Luo Y, Liu P,
Ferrandon S, Kalady MF, Gao R, He J, et al: Fusobacterium nucleatum
promotes the development of colorectal cancer by activating a
cytochrome P450/Epoxyoctadecenoic acid axis via TLR4/Keap1/NRF2
signaling. Cancer Res. 81:4485–4498. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yao Y, Hu S, Zhang C, Zhou Q, Wang H, Yang
Y, Liu C and Ding H: Ginsenoside Rd attenuates cerebral
ischemia/reperfusion injury by exerting an anti-pyroptotic effect
via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int Immunopharmacol.
105:1085822022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu J, Xie G, Yang W and Wang W, Zuo Z and
Wang W: Platelet-rich plasma attenuates intervertebral disc
degeneration via delivering miR-141-3p-containing exosomes. Cell
Cycle. 20:1487–1499. 2021. View Article : Google Scholar : PubMed/NCBI
|





















