Introduction
Colorectal cancer (CRC) is the third most common
cancer, with significant health implications worldwide (1). The 5-year survival rate for patients
with CRC is ~90% when diagnosed at early and localized stages.
However, this rate decreases markedly to only 13.1% for those
diagnosed at later, metastatic stages (2). Currently, the treatment of colorectal
cancer includes various approaches such as surgical resection,
chemotherapy, radiotherapy, targeted therapy, and immunotherapy.
Surgery is the primary treatment, with curative intent for
early-stage patients (3,4). Combined surgery and chemoradiotherapy
are often used for metastatic disease, but complications and side
effects can be severe (5–9). To mitigate these challenges,
complementary approaches such as traditional Chinese herbal
medicines have been used in combination therapies to reduce the
adverse reactions of radiotherapy and chemotherapy, thereby
improving the quality of life for patients (10). Targeted therapies, such as
anti-VEGF and anti-EGFR drugs, have shown efficacy in improving
survival with fewer side effects compared to traditional
treatments. However, issues such as off-target effects and drug
resistance remain significant challenges (11,12).
Immunotherapy, particularly anti-programmed cell death 1 (PD-1) and
anti-cytotoxic T lymphocyte associated protein 4 agents, has
achieved remarkable success in colorectal cancer with high
microsatellite instability and mismatch-repair deficiency
(MSI-H/dMMR) but has limited effectiveness in patients with
microsatellite stable and proficient mismatch repair (MSS/pMMR)
(13–16). Overall, while progress has been
made in treating CRC, several challenges remain. Further research
into the disease mechanisms and the development of new therapeutic
targets and strategies are needed to improve patient outcomes and
quality of life.
The C-X-C motif chemokine ligand (CXCL) family plays
a crucial role in a variety of biological processes, including cell
migration, tumor development, angiogenesis, and several other
essential functions (17). These
chemokines are instrumental in the recruitment and activation of
immune cells such as neutrophils, monocytes, T-lymphocytes and
natural killer cells. They serve roles in both inflammatory and
homeostatic functions; inflammatory chemokines such as CXCL8 are
involved in directing leukocytes to sites of infection or injury,
whereas homeostatic chemokines such as CXCL12 maintain the baseline
migration of immune cells and are consistently expressed (18). CXCL9, a member of the CXC chemokine
family, plays a multifaceted role in the tumor microenvironment.
Expressed predominantly by tumor-associated macrophages and
dendritic cells (DCs), CXCL9 is instrumental in modulating
antitumor immunity (19). It is
particularly noted for its involvement in the recruitment and
positioning of CXCR3-expressing stem-like CD8 T cells, which are
crucial for responses to anti-PD(L)-1 treatment (20–22).
Previous studies have highlighted the diverse roles
of CXCL9 across various cancer types. For instance, in breast
cancer, CXCL9 has been shown to promote tumor progression by
enhancing the recruitment of immune cells and modulating the tumor
microenvironment (23–26). In melanoma, CXCL9 expression has
been associated with improved response to immunotherapy, suggesting
its potential as a predictive biomarker (27,28).
In lung cancer, CXCL9 has been implicated in both tumor suppression
and progression (29,30). These findings underscore the
complexity of CXCL9′s function and its potential as a therapeutic
target in multiple cancers.
Although the CXCL family is commonly associated with
promoting anti-tumour responses, there are a number of findings
demonstrating pro-oncogenic effects of the CXCL family, suggesting
a more complex role in tumour progression. In order to understand
the molecular function of CXCL9 in colorectal cancer, the present
study conducted bioinformatics analysis and in vitro
validation.
Materials and methods
Data acquisition
The Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/tcga) and the Gene Expression
Omnibus (GEO) repository (https://www.ncbi.nlm.nih.gov/geo/) were used to obtain
gene expression data. To obtain comprehensive gene expression data,
the present study used two major public databases: The Cancer
Genome Atlas (TCGA; http://www.cancer.gov/tcga) and the Gene Expression
Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/). TCGA is a landmark
cancer genomics program that has characterized over 20,000 primary
cancer and matched normal samples across 33 cancer types. The
present study specifically used the TCGA dataset for colorectal
adenocarcinoma (COAD), which provides rich genomic and
transcriptomic information, enabling the exploration of the
molecular underpinnings of colorectal cancer. Additionally, the
present study focused on the GSE41258 dataset from GEO, which
includes samples from patients with colonic neoplasms treated at
Memorial Sloan-Kettering Cancer Center between 1992 and 2004. This
dataset comprises 390 expression arrays from primary colon
adenocarcinomas, adenomas, metastases, and corresponding normal
mucosae. Data processing was performed using R (version 4.4.1,
http://www.R-project.org/).
Definition of the CXCL family
prognostic model
Least absolute shrinkage and selection operator
(LASSO) regression was used to create a prognostic signature using
the samples from the TCGA cohort. The regression coefficients (β)
from the CXCL family model were then combined with the relevant
gene expression levels to generate a multi-gene marker-based
predictive risk score. The TCGA data was used to train the risk
score model, which was built as follows: Risk score=expression
level of CXCL1 × (−0.097330812) + expression level of CXCL6 ×
(0.08298018) + expression level of CXCL8 × (0.009198228) +
expression level of CXCL9 × (−0.053426341) + expression level of
CXCL11 × (−0.10920036) + expression level of CXCL12 × (0.051584401)
+ expression level of CXCL14 × (−0.055678695). LASSO regression was
implemented with the glmnet package (version 4.1.2; http://CRAN.R-project.org/package=glmnet).
Cross-validation and model evaluation were carried out using the
ROCR package (version 1.0.11; http://CRAN.R-project.org/package=ROCR). ggplot2
(version 3.3.6; http://CRAN.R-project.org/package=ggplot2) was used
for data visualization.
Construction of protein-protein
interaction (PPI) networks
Using the STRING database, a PPI network map was
constructed encompassing the CXCL family and their interacting
proteins. The network was subsequently refined and visualized
within the Cytoscape (version 3.9.1; http://cytoscape.org/download) (31). Hub genes were identified employing
the Degree algorithm (32), where
the size, color, and spatial arrangement of the nodes within the
network corresponded to their respective Degree scores.
Survival analysis
The Kaplan-Meier Plotter (http://kmplot.com/) (33) was used to analyze the correlation
between gene expression levels and overall survival (OS),
Recurrence-Free Survival (RFS) and Post-progression Survival (PPS)
in colorectal cancer cohorts.
Gene set enrichment analysis
Gene Ontology (GO) and Kyoto Encyclopedia of Genes
and Genomes (KEGG) enrichment analyses were performed on the
related genes and co-expressed genes using the clusterProfiler
package (version 4.4.4; http://CRAN.R-project.org/package=clusterProfiler) to
identify overrepresented biological processes and pathways.
Immune infiltration analysis
For the immune infiltration analysis, the
single-sample gene set enrichment analysis (ssGSEA) algorithm
provided in the R package GSVA (version 1.46.0; http://bioconductor.org/packages/release/bioc/html/GSVA.html)
was used to calculate the correlation between CXCL9 and immune
cells. The Tumor Immune System Interaction Database (TISIDB)
(http://cis.hku.hk/TISIDB/) was employed
to analyze the chemokines and chemokine receptors related to CXCL9.
TISIDB is a comprehensive database that integrates multi-omics data
related to the interaction between tumors and the immune system. It
contains information from various sources, including genomics,
transcriptomics, proteomics and immunology. This database enables
researchers to explore the complex relationships between
tumor-associated genes, immune cell infiltration, immune-related
pathways, and therapeutic responses (34).
Prediction of m6A sites
Target RNA sequences were obtained from the National
Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov). The NCBI is a leading
bioinformatics hub under the U.S. National Library of Medicine,
offering extensive biological databases, such as GenBank, and
powerful analysis tools, such as BLAST, to support global
scientific research in genetics, genomics and molecular biology.
The prediction of m6A methylation sites was performed using the
sequence-based RNA adenosine methylation site predictor (SRAMP;
http://www.cuilab.cn/sramp) database.
SRAMP is a mammalian m6A sites predictor, which was constructed by
extracting and integrating sequence and predicted structural
features around m6A sites within a machine learning framework. It
shows promising performance in cross-validation tests on its
training dataset and in rigorous independent tests (35).
Cell culture
The human colorectal cancer cell line SW480, RKO and
the human kidney epithelial cell line 293T were procured from the
American Type Culture Collection. Cells were routinely cultured in
Dulbecco's Modified Eagle's Medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Zhejiang Tianhang Biotechnology Co., Ltd.), 1%
penicillin-streptomycin solution. Cells were maintained at 37°C in
a humidified incubator with 5% CO2.
Design and validation of siRNA
To initiate the present study, three distinct small
interfering (si)RNA oligonucleotides were designed specifically
targeting the coding sequence of ALKBH5, as well as a scrambled
negative control sequence (Table
SI). These siRNA sequences were synthesized by Beijing Tsingke
Biotech Co., Ltd. Subsequently, each siRNA (at a concentration of
50 nM) was transiently transfected into RKO cells cultured in
6-well plates using Lipofectamine 2000® (Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. 11668019) at 37°C for 24
h. This process was conducted to evaluate the knockdown efficiency
of each siRNA (Fig. S1A). Based
on the results, the siRNA sequence with the highest knockdown
efficiency (GAAAGGCTGTTGGCATCAATA) was selected to design shRNA
primers for further construction of the knockdown vector.
Construction of vectors
For the overexpression vector, the pCDH plasmid was
used as the backbone. For the knockdown vector, the pLKO.1 plasmid
was employed. The primers used for amplification and annealing were
designed based on the sequences provided in Table SI. These primers, along with the
empty vectors, were synthesized or purchased by Beijing Tsingke
Biotech Co., Ltd. The detailed procedure involved PCR amplification
of the target sequences or annealing of primers at high
temperatures, followed by restriction enzyme digestion of the
vectors. For the overexpression vector, the pCDH vector was
digested using EcoRI (NEB, R3101VVIAL) and BamHI (NEB, R3136VVIAL)
restriction enzymes, For the knockdown vector, the pLKO.1 vector
was digested using AgeI (NEB, R3552SVIAL) and EcoRI (NEB,
R3101VVIAL) restriction enzymes. Homologous recombination (2X
MultiF Seamless Assembly Mix, ABclonal, cat. no. RM20523) was
performed to integrate the target sequences into the vectors. The
ligation products were transformed into competent DH5α cells, which
were subsequently cultured overnight at 37°C. Single colonies were
selected and further verified by sequencing to ensure the
successful construction of the vectors.
Lentivirus packaging and
infection
To achieve efficient gene knockdown and
overexpression, a lentivirus vector system was employed. For
lentivirus packaging, 293T cells were co-transfected with a mixture
of the recombinant vector or the control vector, the pCMV–VSV-G
envelope vector, and the pCMV-Gag-Pol packaging vector (both from
Promega Corporation) at a 4:3:1 ratio (totaling 10 µg of DNA) using
the PEI reagent (Polyplus-transfection SA). The cells were then
incubated at 37°C for 72 h. Following incubation, the viral
supernatant was collected and filtered through a 0.22-µm filter to
remove cellular debris. The viral titer was determined using the
Lenti-Pac™ HIV qRT-PCR Titration Kit (GeneCopoeia,
Inc.). Subsequently, the lentivirus was used to infect RKO cells at
a multiplicity of infection of 5. After infection, puromycin was
applied to select stable RKO cell lines.
Reagents
3-Methyladenine (3-MA, MCE) was prepared in DMSO and
stored at −80°C until use. Actinomycin D was purchased from
MilliporeSigma. Stock solutions were prepared in sterile distilled
water at a concentration of 1 mg/ml and stored at −20°C until use.
3-MA was used at a final concentration of 1 mM. Actinomycin D was
used at a final concentration of 5 µg/ml.
Reverse transcription-quantitative
(RT-q) PCR
RNA extraction, cDNA synthesis and qPCR performed
according to the manufacturer's protocols. All experiments were
repeated at least three times. Total RNA was isolated from cellular
samples using the Ultrapure RNA Kit, a product of CWBio. Subsequent
to RNA extraction, the Reverse Hifair® III 1st Strand
cDNA Synthesis SuperMix for qPCR, manufactured by Shanghai Yeasen
Biotechnology Co., Ltd., was used to perform reverse transcription,
converting the isolated total RNA into complementary DNA (cDNA).
Following this, the 2X Universal Blue SYBR Green qPCR Master Mix
(with UDG), provided by Wuhan Servicebio Technology Co., Ltd., was
employed for qPCR, which was conducted on the CFX Connect Real-Time
PCR Detection System (Bio-Rad Laboratories, Inc.), under the
following thermal cycling conditions: Initial denaturation step at
95°C for 10 min, followed by 40 cycles consisting of denaturation
at 95°C for 10 sec, annealing at 60°C for 20 sec, and extension at
72°C for 15 sec. Relative gene expression was quantified using the
comparative Cq (2−ΔΔCq) method (36), with GAPDH serving as an endogenous
reference gene for normalization of the data. The primers used for
qPCR were shown in Table SII.
Cell proliferation assays
The Cell Counting Kit-8 (CCK-8) assay was employed
to quantitatively assess cell proliferation. Cells were seeded at a
density of 1×104 cells per well in 96-well plates and
incubate at 37°C with 5% CO2 for 12 h to allow for cell
adhesion. After cells attached to 96-well plate for 0–120 h, 10 µl
of the Cell Counting Kit-8 (Dalian Meilun Biology Technology Co.,
Ltd.) was added to each well and incubated for 1 h at 37°C.
Subsequently, the optical density of the samples was measured using
a spectrophotometer, with the wavelength set to 450 nm which is
indicative of viable cell count.
Transwell assay
The upper aspect of the Transwell membrane was
uniformly layered with Matrigel, followed by a 30-min incubation
period at 37°C to facilitate solidification. Subsequently, a
cellular suspension consisting of 1×105 cells in 100 µl
of serum-free medium was dispensed into the upper chamber of each
Transwell insert. Concurrently, the lower chamber was supplemented
with 600 µl of medium enriched with 10% serum to act as a
chemotactic agent. After being incubated for 20 h at 37°C, the
cells remaining at the upper surface of the membrane were removed
by a cotton swab. The cells that had successfully traversed to the
inferior surface of the membrane were then immobilized using a 4%
paraformaldehyde solution for a 15-min fixation at room temperature
and stained with 1X modified Giemsa stain (Beyotime Institute of
Biotechnology) for 45 min at room temperature. The migratory cells
on the underside of the membrane were subsequently captured using a
fluorescence microscope for documentation.
Cell wound healing assay
Once a confluent monolayer was established, the cell
monolayers were subjected to mechanical wounding via a sterile 200
µl micropipette tip to create a standardized wound. To eliminate
cellular debris and ensure accurate wound assessment, the wounded
monolayers were copiously rinsed with PBS multiple times.
Subsequently, the cells were maintained in a serum-free medium for
a duration of 72 h to simulate wound healing conditions. The
progression of in vitro wound closure was monitored using a
fluorescent microscope at designated time intervals. The in
vitro wound healing was recorded and assessed using the closure
rate, which was calculated as follows: [(Wound area at 0 h-Wound
area at × h)/Wound area at 0 h] ×100%.
Western blotting
Cells were subjected to lysis in a RIPA buffer,
which was complemented with Phenylmethanesulfonyl Fluoride (PMSF,
Beyotime Institute of Biotechnology). The resultant lysate was
centrifuged at 12,000 × g for a duration of 15 min at 4°C to
achieve clarification. Subsequently, the protein concentration
within the supernatant was ascertained using a BCA (Beyotime
Institute of Biotechnology; cat. no. P0012) assay. The
Omni-Easy™ One-step Color PAGE Gel Rapid Preparation Kit
(Epizym, Inc.; PG211 and PG213) was used to prepare 7.5 and 12.5%
gels. An aliquot of the protein lysate, containing 20 µg of total
protein, was resolved on SDS-PAGE and subsequently transferred onto
PVDF membrane (MilliporeSigma; cat. no. IPVH00010). The membranes
were pre-incubated with a blocking solution consisting of 5%
non-fat milk for 1 h at room temperature. Thereafter, the membranes
were incubated with the respective primary antibodies at 4°C
overnight. Following this incubation, the membranes were washed
thoroughly and then exposed to anti-rabbit/mouse/rat secondary
antibodies for 1 h at room temperature. The immunoreactive bands
were visualized using an ECL substrate (Biosharp Life Sciences;
cat. no. BL520A), and the membrane was detected using a
chemiluminescence imaging system (eBlot). Primary antibodies used
in this study were: Mouse anti-GAPDH (1:5,000; Proteintech Group,
Inc.; cat. no. 60004-1-Ig), rabbit anti-LC3B (1:1,000; ABclonal
Biotech Co., Ltd.; cat. no. A11282), rabbit anti-P62 (1:2,000;
ABclonal Biotech Co., Ltd.; cat. no. A19700), rabbit anti-CXCL9/MIG
(1:1,000; ABclonal Biotech Co., Ltd.; cat. no. A25183); and the
secondary antibodies were: HRP Goat Anti-Rat IgG (H+L; 1:10,000;
ABclonal Biotech Co., Ltd.; cat. no. AS028), HRP Goat Anti-Mouse
IgG (H+L; 1:10,000; ABclonal Biotech Co., Ltd.; cat. no. AS003) and
HRP Goat Anti-Rabbit IgG (H+L; 1:10,000; ABclonal Biotech Co.,
Ltd.; cat. no. AS014). The gray value was measured by ImageJ
software (version 1.54f; National Institutes of Health).
Methylated RNA immunoprecipitation
sequencing (MeRIP-seq)
Total RNA was extracted from cells, for both the IgG
group and the m6A group, 100 µg of total RNA was added, along with
an appropriate quantity of RNase inhibitor. Subsequently, 1 µl of
either IgG antibody (Proteintech Group, Inc.; cat. no. B900620; for
the IgG group) or m6A antibody (Proteintech Group, Inc.; cat. no.
68055-1-Ig; for the m6A group) was introduced. IP buffer was then
supplemented to adjust the volume to 200 µl. After thorough mixing,
the samples in both groups were incubated overnight at 4°C. Protein
A/G Magnetic beads are washed with IP buffer, and the premix is
added and incubated at 4°C for 3 h. The beads with bound RNA are
washed 4–5 times with IP buffer. After adding TRIzol®
(Thermo Fisher Scientific, Inc.) an RNA purification kit (CWBio.)
to was used to extract the RNA on the magnetic beads. The purified
m6A-enriched RNA were analyzed by RT-qPCR as aforementioned to
quantify specific m6A/modified RNAs.
Statistical analysis
All data analyses were conducted using GraphPad
Prism software, version 8.0.1 (Dotmatics). The experimental groups
were compared using the non-parametric two-tailed Student's t-test
to assess the statistical significance between two independent
samples. For multiple group comparisons, a one-way analysis of
variance was employed, followed by Tukey's post hoc test to correct
for multiple comparisons and to identify the source of significant
variance within the dataset. Each treatment condition for the
cellular samples was replicated a minimum of three times to ensure
the reproducibility and reliability of the experimental outcomes.
Data are presented as the mean ± standard error of the mean to
reflect the variability within the sample set. P-values are
reported for all statistical tests. P<0.05 was considered to
indicate a statistically significant difference. Specific P-values
are indicated in the figure legends and results sections
(*P<0.05, **P<0.01, ***P<0.001).
Results
Expression patterns and prognostic
models of CXCL family in COAD
Variance analysis indicated that the CXCL family
displayed significant differential expression in COAD, as presented
in Fig. 1A. A prognostic model of
CXCL family in COAD was constructed through LASSO regression, which
has good diagnostic value (Fig.
1B-E). COX regression analysis was performed on seven genes in
the prognostic model of CXCL family (Fig. 1F), and the association between
molecular and methylation genes with P<0.2 in univariate
regression was analyzed. The results showed that CXCL8, CXCL9, and
CXCL11 have an association with methylation genes (Fig. 1G). The PPI network with CXCL family
and their interacting genes shows that CXCL9 is the hub gene with
the highest degree score in the prognostic model genes of the CXCL
family (Fig. 1H).
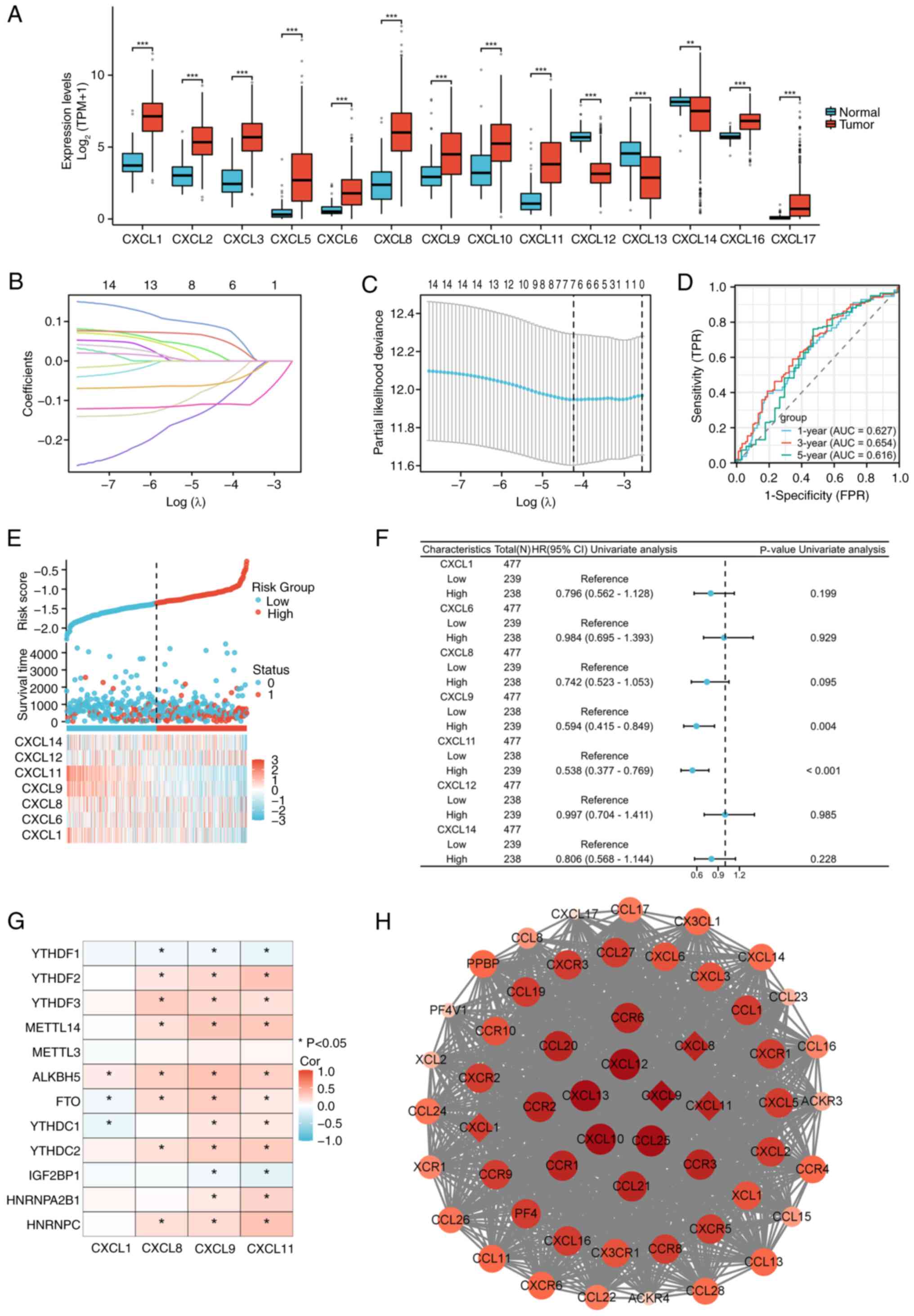 | Figure 1.Construction of a prognostic CXCL
family model for COAD. (A) Expression patterns of the CXCL family
in COAD. (B) Lasso coefficient spectrum of CXCL family genes. (C)
Cross-validation for selection of the tuning parameter in a
proportional hazards model. (D) ROC curves for the CXCL family risk
model in the TCGA-COAD cohort. (E) Distribution of risk scores,
survival status, and expression profiles of CXCL model genes in the
TCGA-COAD dataset (Sample size: 521). (F) Prognostic forest plot of
CXCL family risk model genes. (G) Relationship between prognostic
CXCL family genes and m6a-related genes. (H) PPI network diagram of
CXCL family and interacting genes, the top ten genes with the
highest degree scores were displayed in red and genes in CXCL
family risk model were marked with diamonds. *P<0.05,
**P<0.01, ***P<0.001. CXCL, C-X-C motif chemokine ligand;
COAD, colorectal adenocarcinoma; ROC, receiver operating
characteristic; TCGA, The Cancer Genome Atlas; PPI, protein-protein
interaction; AUC, area under the curve; Cor, correlation. |
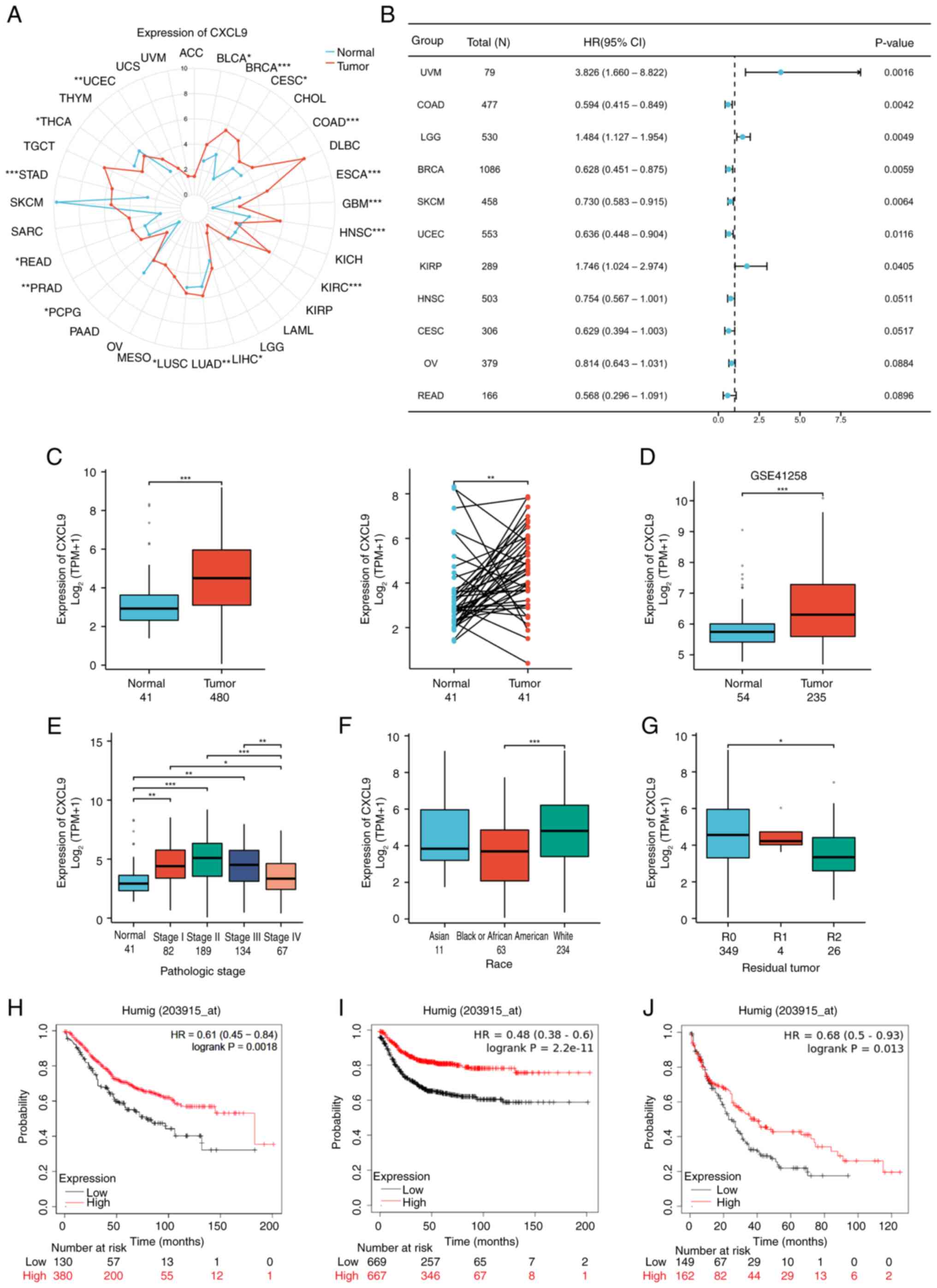
Expression of CXCL9 in pan-cancer and
COAD
Considering the significant role of CXCL9 in the
prognostic model of the CXCL family, the expression of CXCL9 in
cancer was further analyzed, particularly in COAD, as well as its
effect on the prognosis of COAD patients. Results showed that CXCL9
is highly expressed in most types of cancer (Fig. 2A) and is markedly associated with
patient PFI in cancers such as breast cancer (BRCA), COAD, KIRP,
LGG, SARC and UCEC (Fig. 2B). The
TCGA and GSE41258 dataset from GEO indicated that CXCL9 was highly
expressed in COAD (Fig. 2C and D).
An analysis of the relationship between CXCL9 expression and
various clinical indicators in the TCGA dataset revealed that CXCL9
expression exhibited a trend of first increasing and then
decreasing with the progression of COAD (Fig. 2E). Slight differences in CXCL9
expression were observed among different ethnicities and in
different residual tumor classifications (Fig. 2F and G). KMplot showed that
patients with elevated CXCL9 expression exhibited markedly improved
OS, RFS and PPS compared to those with lower expression levels
(Fig. 2H-J).
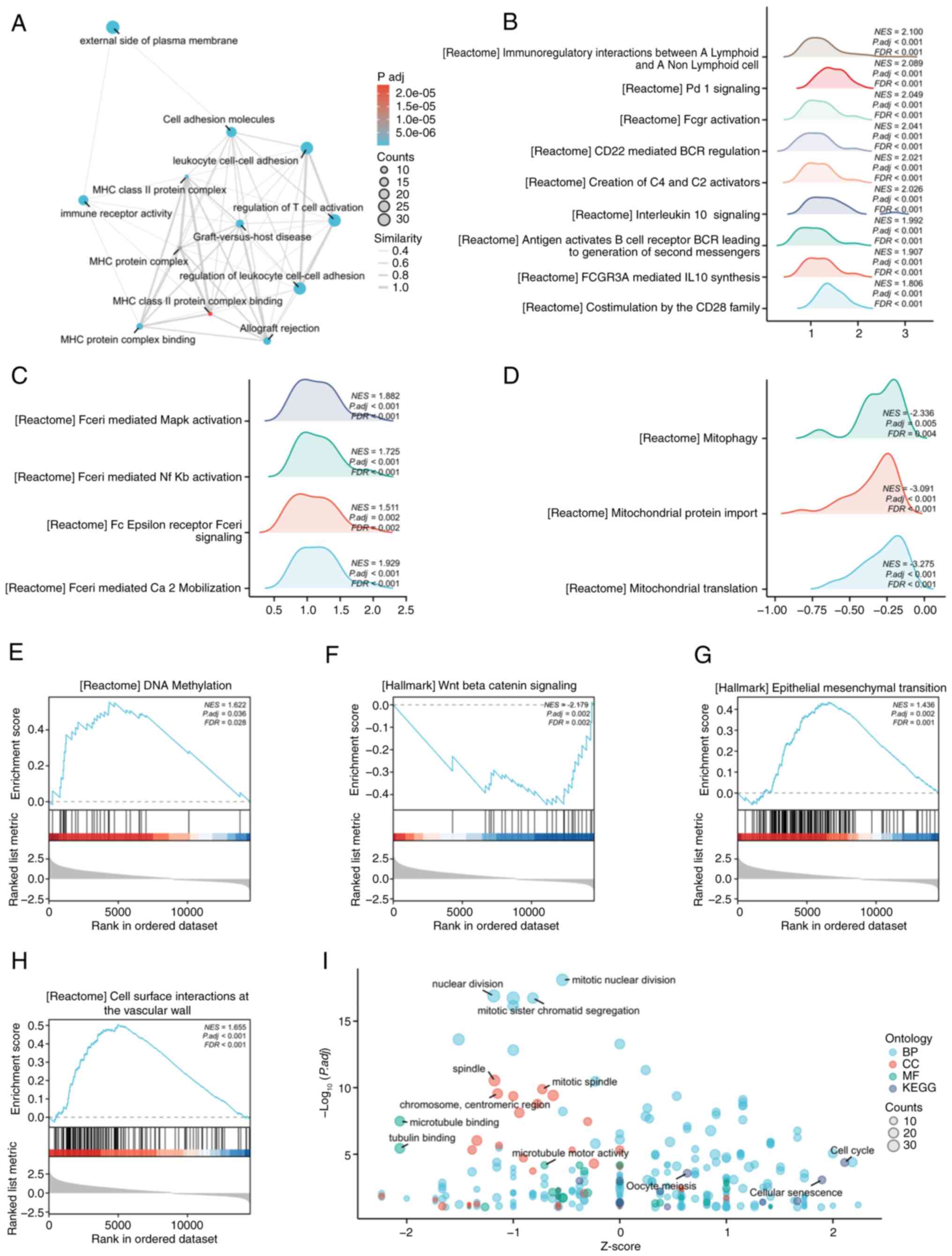
Molecular mechanisms and pathway
enrichment of CXCL9
To further clarify the molecular mechanisms
underlying the role of CXCL9 in COAD, the present study conducted
enrichment analyses using the GO, KEGG and GSEA. GO and KEGG
enrichment analysis was performed using the top 200 most-associated
genes. The EMAP plot revealed significant enrichment of CXCL9 and
its associated genes in pathways related to T cell activation and
leukocyte adhesion (Fig. 3A). In
GSEA enrichment analysis using co-expressed genes, numerous
pathways related to the functions of T cells and B cells were
identified, such as Co-stimulation by the CD28 family and CD22
mediated BCR regulation (Fig. 3B).
In addition, pathways related to Fc epsilon Receptor I (FcεRI) and
mitochondria related pathways as well as DNA methylation, and the
Wnt Beta catenin signaling pathway, cell surface interactions at
the vascular wall and others were also enriched (Fig. 3C-H). GO and KEGG combined with FC
bubble chart demonstrated a correlation with the cell cycle related
pathway (Fig. 3I).
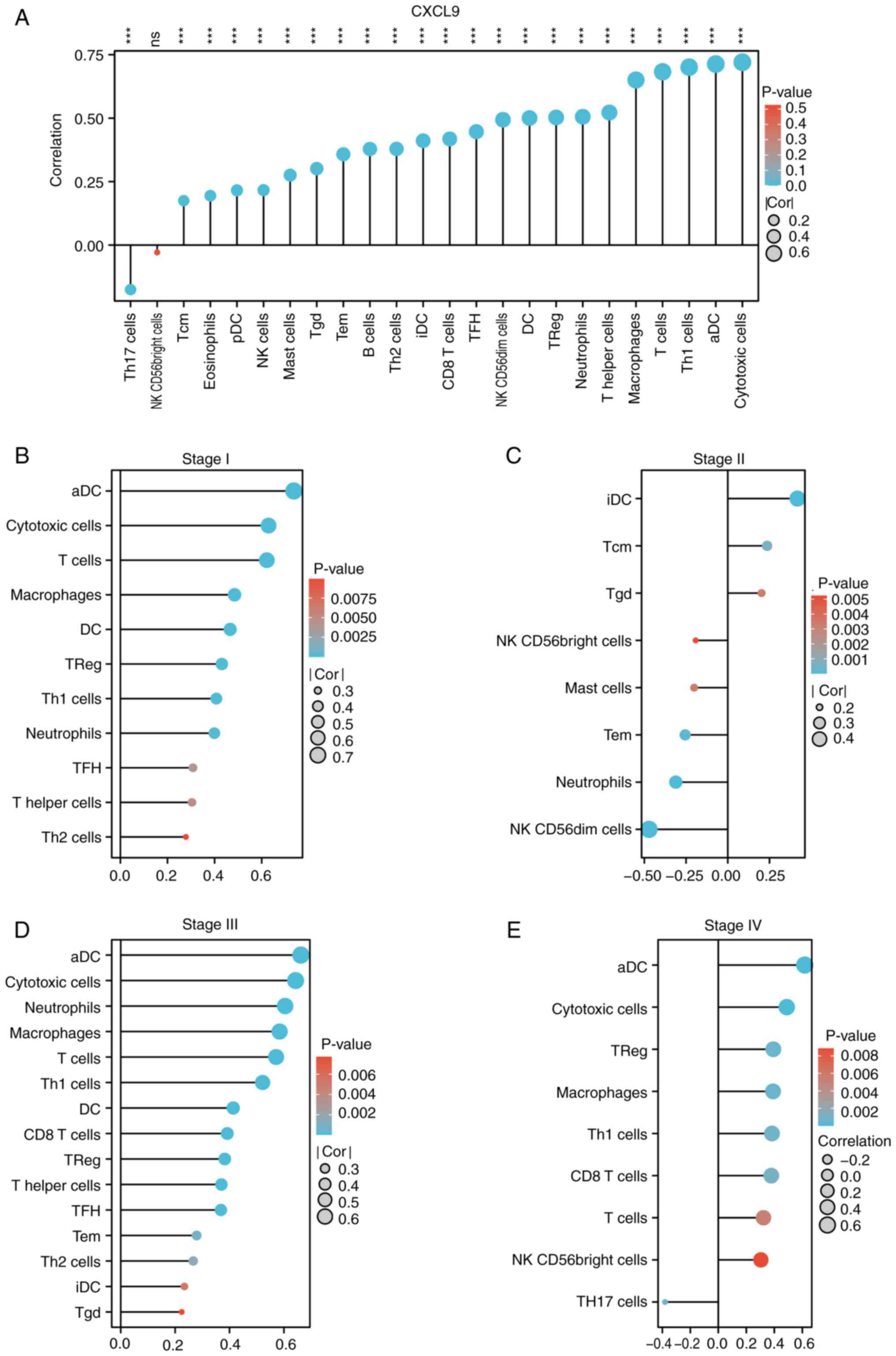
CXCL9 is associated with immune
infiltration and exhibits differential immune infiltration patterns
across various stages
Immune infiltration analysis indicated that CXCL9 in
CRC was positively associated with the majority of immune
infiltration cells, predominantly cytotoxic cells, activated
dendritic cells (aDC), Th1 cells, and T cells (Fig. 4A). The effect of CXCL9 on CRC
immune infiltration varies across different stages of cancer
progression. In Stage I, CXCL9 shows a positive correlation with
cytotoxic cells, aDC, and Th1 cells (Fig. 4B). In Stage III, it maintains a
positive correlation with cytotoxic cells, macrophages, and T cells
(Fig. 4D). In Stage IV, the
positive correlation was observed with cytotoxic cells, Th1 cells
and CD8 T cells (Fig. 4E).
However, a notable alteration in immune infiltration is observed in
Stage II, where CXCL9 exhibited a negative correlation with
numerous immune cells, including cytotoxic cells, aDC, macrophages,
Th1 cells, T cells, and neutrophils (Fig. 4C). The TISIDB analysis suggested
that CXCL9 in COAD was most closely associated with chemokines
CCL4, CCL5, CCL8, CCL18, CXCL10, CXCL11 and CXCL13, as well as
chemokine receptors CCR1, CCR2, CCR5, CCR8 and CXCR6 (Fig. S2A and B). Therefore, the
expression of these chemokine receptors in different stages of COAD
was analyzed and it was found that the expression patterns of CCR5,
CCR8 and CXCR6 were similar to CXCL9 (Fig. S2C).
CXCL9 is highly expressed in
colorectal cancer and markedly enhances the malignant traits of
colorectal cancer cells
Subsequent to bioinformatics analysis of CXCL9,
in vitro assays were performed to explore its potential role
in promoting tumor growth in colorectal cancer cells. In colon
cancer cell lines, the expression of the CXCL9 gene was examined
and it was found that its expression level was markedly higher in
RKO and SW480 cells compared to NCM460 (Fig. 5A). Through qPCR and western
blotting validation, cell lines with overexpression of CXCL9 were
successfully constructed (Fig.
5B-D).
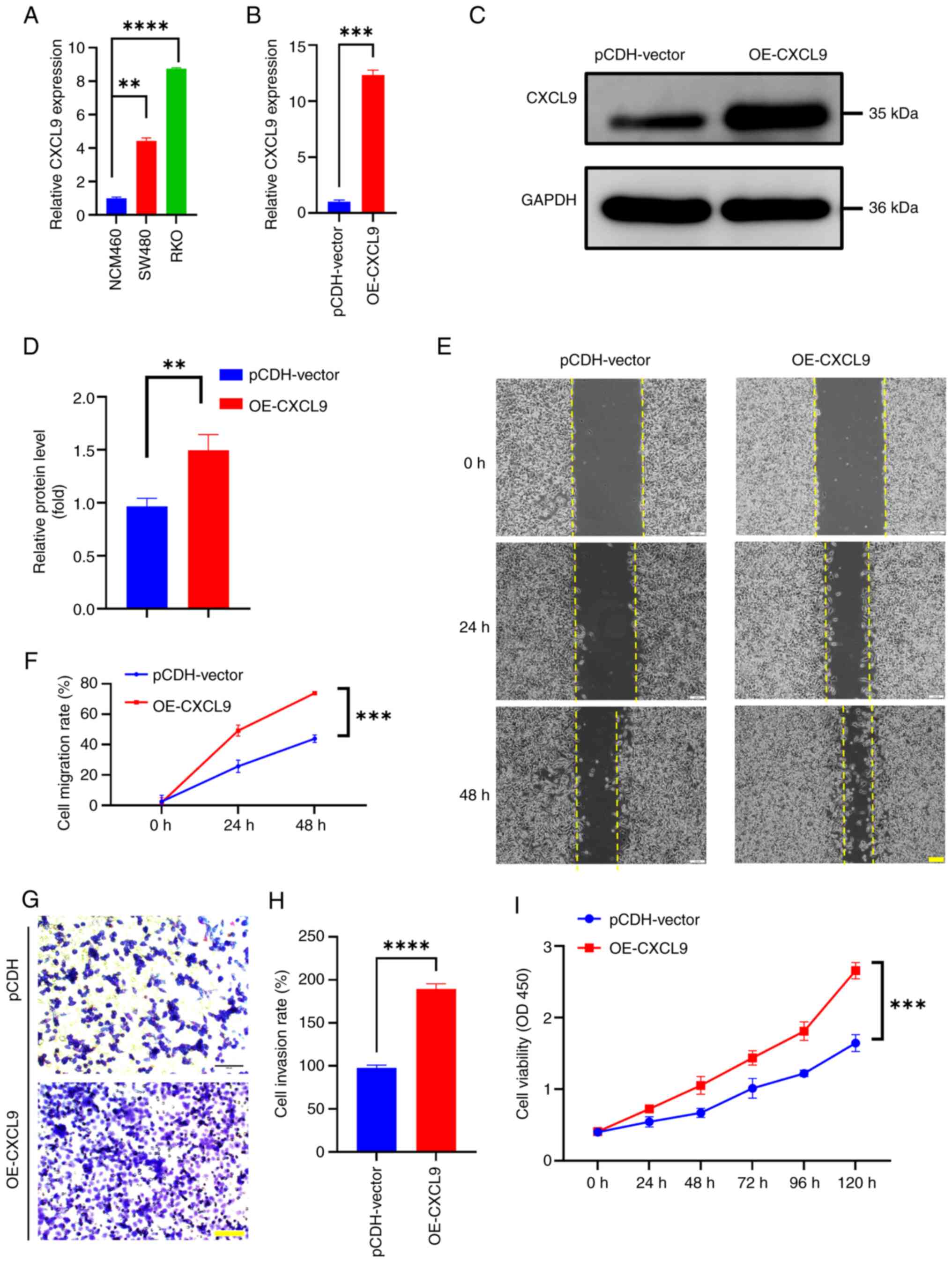 | Figure 5.CXCL9 is related to colorectal cancer
cell proliferation, migration and invasion. (A) Expression of CXCL9
in colon cancer cell lines (t-test of SW480 vs. NCM460: P=0.0013,
95% confidence interval: 2.904–3.964, η2=0.9974; t-test
of RKO vs. NCM460: P<0.0001, 95% confidence interval:
7.465–7.998, η2=0.9999). (B-D) Validation of stable cell
construction by quantitative PCR (t-test of OE-CXCL9 vs.
pCDH-vector: P=0.0008, 95% confidence interval: 9.961 to 12.71,
η2=0.9984) and western blotting (OE-CXCL9 vs.
pCDH-vector: P=0.0054, 95% confidence interval: 0.2612 to 0.7961,
η2=0.8828). (E and F) The effect of overexpressing CXCL9
on the migration ability of colon cancer cells (scale bar, 100 µm;
mutiple t-test of OE-CXCL9 vs. pCDH-vector: 24 h P<0.001,
difference=23.5070; 48 h P<0.001 difference=29.9395). (G and H)
The effect of overexpressing CXCL9 on the invasion ability of colon
cancer cells (scale bar, 100 µm; t-test of OE-CXCL9 vs.
pCDH-vector: P<0.0001, 95% confidence interval: 80.87 to 102.6,
η2=0.9928]. (I) The effect of overexpressing CXCL9 on
the proliferation ability of colon cancer cells. Data are expressed
as means ± SEM from at least three experiments (multiple t-test of
OE-CXCL9 vs. pCDH-vector: 24 h P=0.006, Difference=0.18; 48 h
P=0.001, Difference=0.3825; 72 h P=0.002, Difference=0.425; 96 h
P<001, Difference=0.59; 120 h P<001, Difference=1.0125).
**P<0.01, ***P<0.001, ****P<0.0001. CXCL, C-X-C motif
chemokine ligand; OE, overexpression. |
Wound healing assays were performed to assess the
effect of CXCL9 overexpression on cell migratory capacity. The
results demonstrated that cells overexpressing CXCL9 closed the
wound area more rapidly than control cells within 48 h (Fig. 5E and F). The invasive potential of
colorectal cancer cells with CXCL9 overexpression was further
evaluated through Transwell assays. Notably, the number of cells
that traversed the chamber was markedly higher when compared with
the control cohort (Fig. 5G and
H), indicating a significant increase in invasiveness
(P<0.01) attributed to the overexpression of CXCL9. CCK-8 assays
were utilized to measure the impact of CXCL9 overexpression on cell
proliferation. The absorbance at OD450, which reflects cell
viability, showed that cells with overexpressed CXCL9 exhibited a
higher proliferation rate during 0–120 h (Fig. 5I). These results indicated a
potential role of CXCL9 in the progression of colorectal
cancer.
Overexpression of CXCL9 can regulate
autophagy
The present study treated SW480 and RKO cells with
3-MA and found changes in the expression of the CXCL9 gene within
0–8 h (Fig. 6A and B). These
observations suggested a potential role of CXCL9 in autophagy
regulation Following CXCL9 overexpression, an increase in the RNA
levels of autophagy-related genes was observed, indicating a
potential involvement of CXCL9 in the regulation of autophagic flux
(Fig. 6C). Western blotting
results indicated that overexpression of CXCL9 can inhibit the
degradation of p62 and suppress the conversion of LC3-I to LC3-II,
thereby blocking autophagy flux (Fig.
6D).
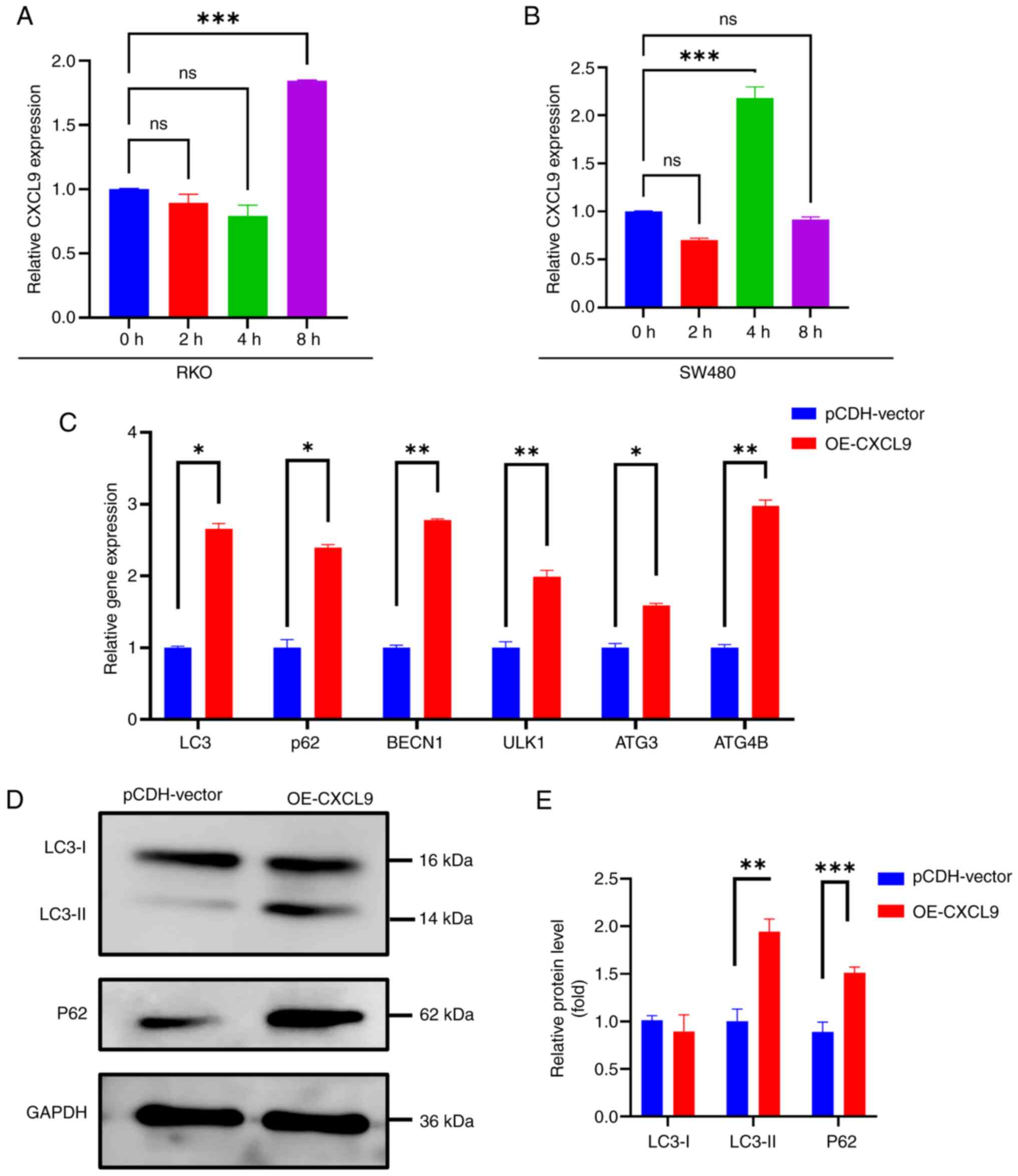 | Figure 6.CXCL9 is associated with autophagy.
Changes in the CXCL9 gene in colon cancer cells after the addition
of 3-MA: (A) RKO (One-way ANOVA of 8 h vs. 0 h: P=0.0009, 95%
confidence interval: 1.120–0.5686, η2=0.9836). (B) SW480
(One-way ANOVA of 4 h vs. 0 h: P=0.0004, 95% confidence interval:
1.491–0.8686, η2=0.9890). (C) The effect of CXCL9 gene
overexpression on the mRNA expression of autophagy-related genes
(multiple t-test of OE-CXCL9 vs. pCDH-vector: LC3 P=0.001,
Difference=1.65802; p62 P=0.004, Difference=1.38966; BECN1
P<0.001, Difference=1.78132; ULK1 P=0.008, Difference=0.98638;
ATG3 P=0.006, Difference=0.591265; ATG4B P=0.001,
Difference=1.97811). (D and E) The influence of CXCL9 gene
overexpression on the protein expression levels of LC3 and P62
(multiple t-test of OE-CXCL9 vs. pCDH-vector: LC3-II P<0.001,
Difference=0.942989; P62 P<001, Difference=0.623079).
*P<0.05, **P<0.01, ***P<0.001. ns; no significance. CXCL,
C-X-C motif chemokine ligand; OE, overexpression. |
Regulation of CXCL9 by the m6A Eraser
ALKBH5 in CRC
SRAMP identified a high-confidence m6A methylation
site within the CXCL9 gene (Fig. 7A
and B). This implied that CXCL9 may be subject to
post-transcriptional regulation through m6A modification, which
could have significant implications for its expression and function
in cellular processes. Through bioinformatics analysis, a strong
correlation between CXCL9 and the m6A eraser ALKBH5 was predicted
(Fig. 7C). Consequently, the
expression levels of CXCL9 in cell lines with ALKBH5 knockdown and
overexpression were examined. The results showed that the
expression of CXCL9 increased in response to ALKBH5 overexpression
and decreased following ALKBH5 knockdown (Fig. 7D and E). Actinomycin D chase
experiments revealed that the stability of CXCL9 was markedly
reduced in ALKBH5 knockdown cells compared to the control group,
while overexpression of ALKBH5 markedly enhanced the stability of
CXCL9 (Fig. 7F and G). The MeRIP
experiment demonstrated that the m6A level of CXCL9 decreased upon
overexpression of ALKBH5, while it increased upon the knockdown of
ALKBH5 (Fig. 7H and I). The
present study stably expressed a knockdown of ALKBH5 in RKO cells
and transiently transfected them with the OE-CXCL9 plasmid. The
results from wound healing, CCK8, and Transwell assays demonstrated
that CXCL9 can partially restore the effects of ALKBH5 knockdown on
colorectal cancer cells (Fig.
8).
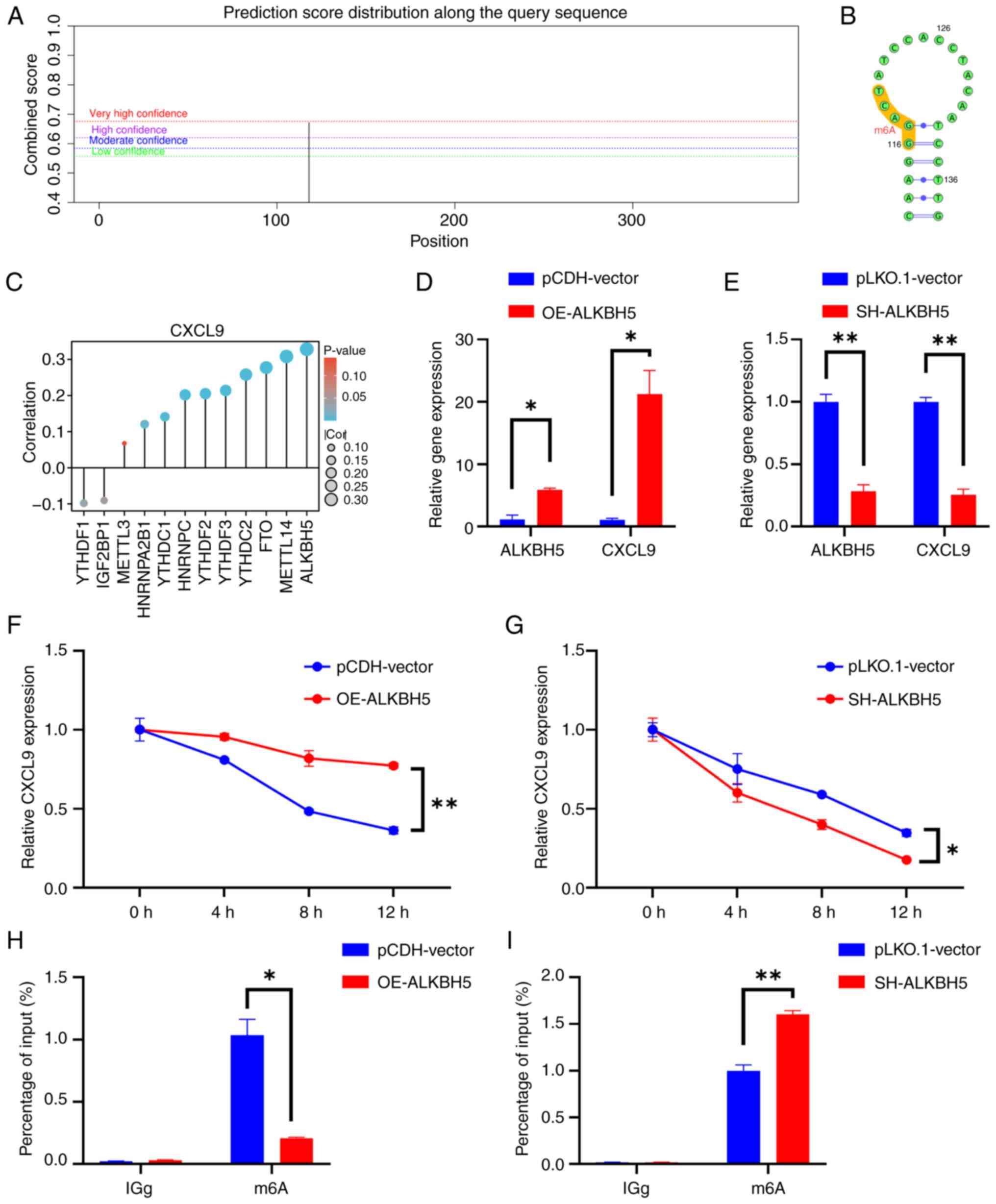 | Figure 7.ALKBH5 mediates the demethylation
modification of CXCL9 to enhance its stability. (A) SRAMP-predicted
m6A modification sites of CXCL9 and (B) the secondary structure of
RNA. (C) Correlation between CXCL9 and m6A-related gene expression.
(D and E) The effect of overexpression and knockdown of the m6A
eraser ALKBH5 on CXCL9 mRNA expression (t-test of OE-ALKBH5 vs.
pCDH-vector, ALKBH5: P=0.0126, 95% confidence interval:
2.425–7.054, η2=0.9749; CXCL9: P=0.0176, 95% confidence
interval: 8.512–31.87, η2=0.9651. t-test of SH-ALKBH5
vs. pLKO.1-vector: ALKBH5: P=0.0061, 95% confidence interval:
−0.9606 to −0.4765, η2=0.9879; CXCL9: P=0.0029, 95%
confidence interval: −0.9205 to −0.5724, η2=0.9942]. (F
and G) The impact of overexpression and knockdown of the m6A eraser
ALKBH5 on the stability of CXCL9 mRNA (multiple t-tests of
OE-ALKBH5 vs. pCDH-vector: 4 h P=0.011, Difference=0.145836; 8 h
P=0.011, Difference=0.334887; 12 h P=0.002, Difference=0.41087;
multiple t-tests of SH-ALKBH5 vs. pLKO.1-vector: 8 h P=0.016,
Difference=−0.190368; 12 h P=0.009, Difference=−0.170625). (H and
I) Effect of ALKBH5 overexpression/knockdown on m6a level of CXCL9
(Multiple t-test of OE-ALKBH5 vs. pCDH-vector, m6A: P=0.012,
Difference=−0.826860; multiple t-test of SH-ALKBH5 vs.
pLKO.1-vector, m6A: P=0.008, Difference=0.604586). *P<0.05,
**P<0.01. CXCL, C-X-C motif chemokine ligand; SRAMP,
sequence-based RNA adenosine methylation site predictor; OE,
overexpression; SH, short hairpin RNA. |
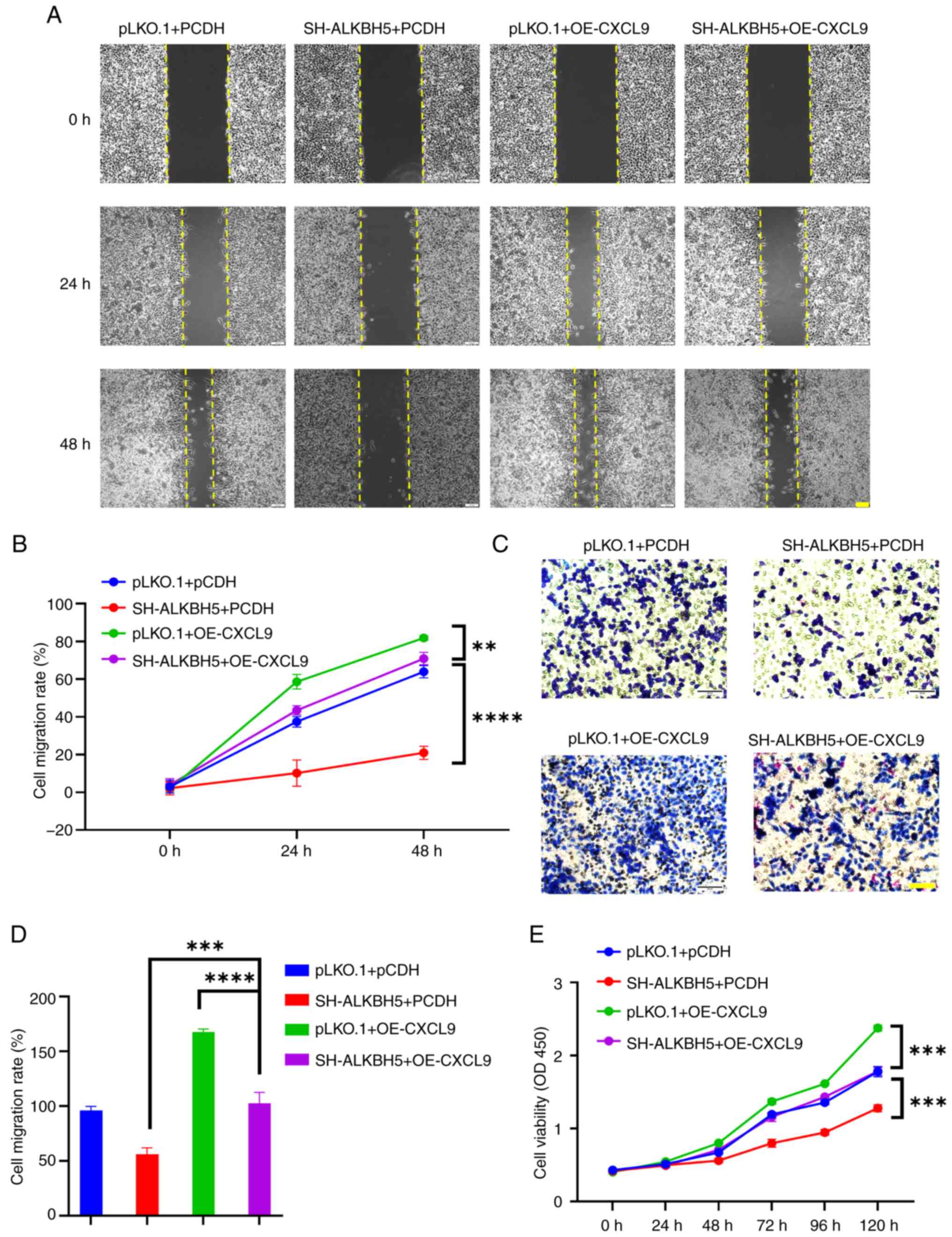 | Figure 8.Overexpression of CXCL9 can
counteract the effects of ALKBH5 knockdown on the development and
progression of colon cancer. (A and B) The scratch assay
demonstrated that overexpression of CXCL9 reversed the migration
inhibition caused by ALKBH5 knockdown in RKO cells (scale bar, 100
µm; two-way ANOVA of SH-ALKBH5+PCDH vs. SH-ALKBH5 + OE-CXCL9: 24 h
P<0.0001, 95% confidence interval: −41.23 to −25.34; 48 h
P<0.0001, 95% confidence interval: −57.96 to −42.06. of
pLKO.1+OE-CXCL9 vs. two-way ANOVA of SH-ALKBH5 + OE-CXCL9: 24 h
P=0.0001, 95% confidence interval: 7.226 to 23.12; 48 h P=0.0047,
95% confidence interval: 2.949 to 18.85). (C and D) Transwell assay
showed that overexpression of CXCL9 reversed the invasion
inhibition caused by ALKBH5 knockdown in RKO cells (scale bar, 100
µm; one-way ANOVA of SH-ALKBH5+PCDH vs. SH-ALKBH5 + OE-CXCL9:
P<0.0001, 95% confidence interval: −62.67 to −30.03; one-way
ANOVA of pLKO.1+OE-CXCL9 vs. SH-ALKBH5 + OE-CXCL9: P<0.0001, 95%
confidence interval: 48.87–81.50). (E) CCK-8 assay indicated that
overexpression of CXCL9 reversed the proliferation inhibition
caused by ALKBH5 knockdown in RKO cells (two-way ANOVA of SH-ALKBH5
+ PCDH vs. SH-ALKBH5 + OE-CXCL9 48 h P<0.0001, 95% confidence
interval: −0.2106 to −0.08369; 72 h P<0.0001, 95% confidence
interval: −0.4235 to −0.2966; 96 h P<0.0001, 95% confidence
interval: −0.5499 to −0.4230; 120 h P<0.0001, 95% confidence
interval: −0.5683 to −0.4414. Two-way ANOVA of pLKO.1 + OE-CXCL9
vs. SH-ALKBH5 + OE-CXCL9: 0 h P=0.8309, 95% confidence interval:
−0.08391 to 0.04296; 24 h P=0.165, 95% confidence interval:
−0.01294 to 0.1139; 48 h P=0.0016, 95% confidence interval: 0.02859
to 0.1555; 72 h P<0.0001, 95% confidence interval: 0.1452 to
0.2721; 96 h P<0.0001, 95% confidence interval: 0.1173 to
0.2442; 120 h P<0.0001, 95% confidence interval: 0.5292 to
0.6560). **P<0.01, ***P<0.001, ****P<0.0001. CXCL, C-X-C
motif chemokine ligand; OE, overexpression; SH, short hairpin
RNA. |
Discussion
As a subfamily of chemokines, the CXCL family not
only regulates the migration of leukocytes but also controls the
growth and development of tumors (17). Luo et al (37) demonstrated that the high-level
expression of the CXCL family can result in lymph node metastasis
and a higher tumor-node-metastasis stage in patients with CRC.
According to numerous studies, CXCL family members have been shown
to be involved in the occurrence and development of CRC closely
related to its role including regulation of immune cell
infiltration, tumor cell proliferation, invasion, metastasis and
angiogenesis (38–40). Given the significant effect of the
CXCL family on the progression of colorectal cancer, a prognostic
model integrating CXCL-associated biomarkers was established
utilizing data from TCGA. A comprehensive PPI network analysis of
CXCL family and their interacted genes identified CXCL9 as a
central node, which has a strong correlation with m6a methylation
underscoring its potential as a key regulatory gene in the
progression of COAD.
Bioinformatics analysis revealed significant
upregulation of CXCL9 in colorectal cancer, while survival analysis
demonstrated higher OS, RFS and PPS rates in patients with high
CXCL9 expression. the present study conducted an analysis of CXCL9
expression at different pathologic stages and the results showed a
trend of initial increase followed by decrease during cancer
progression. Furthermore, within the residual tumor classification,
the expression level of CXCL9 was highest in R0. The tumor
microenvironment characteristics of residual tumors differ,
potentially caused by CXCL9 expression and function. Low CXCL9
expression in R2 tumors may correlate with facilitating immune
evasion. Given the lower baseline expression of CXCL9 in Black or
African American individuals, treatment strategies are adjusted
based on ethnic-specific biomarkers such as CXCL9 expression levels
may be important.
The present study further investigated the mechanism
of action of CXCL9 in COAD. The result of GO, KEGG and GSEA
enrichment analysis demonstrated that co-expressed genes and
related genes of CXCL9 were markedly enriched not only in pathways
associated with immune response but also in other pathways closely
related to tumorigenesis and development. Therefore, it was
hypothesized that CXCL9 not only altered immune infiltration in
colorectal cancer by recruiting immune cells but also influenced
the initiation and progression of colorectal cancer itself.
The present study revealed a significant correlation
between CXCL9 and other chemokines within the same CXC family,
specifically CXCL10, CXCL11 and CXCL13. These chemokines are
characterized by their ability to attract immune cells, such as T
cells, natural killer (NK) cells, and monocytes, to sites of
inflammation or infection (41–44).
In the context of cancer, these chemokines are implicated in the
recruitment of immune cells to the tumor microenvironment, where
they can either promote or inhibit tumor growth depending on the
context (44–47). This high correlation indicated that
CXCL9 may play an important dual role in regulating tumor
environment and tumor occurrence and development.
The effect of CXCL9 on immune cell infiltration in
COAD varies across different stages of cancer progression. During
the majority of stages, CXCL9 positively influences the recruitment
of cytotoxic cells, aDC and T cells, which are critical for
mounting an effective anti-tumor immune response. However, in Stage
II, where CXCL9 is highest expressed, it exhibits an inverse
correlation with neutrophils and NK CD56 dim cells, suggesting a
shift in its role that may contribute to an immunosuppressive
environment conducive to tumor progression.
The PD-1/PD-L1 axis modulates the establishment and
persistence of immune tolerance within the tumor microenvironment.
The interaction between PD-1 and PD-L1 or PD-L2, plays a pivotal
role in regulating T cell activity, including their activation,
proliferation and the secretion of cytotoxic factors in cancer.
This interaction can lead to a diminishment in effective anti-tumor
immune reactions (48,49). GSEA enrichment analysis indicated a
positive correlation between CXCL9 expression and the PD-1
signaling pathway. When CXCL9 levels are elevated, the PD-1
signaling pathway is activated, which may suppress T cell cytotoxic
activity against cancer cells. In recent years, innovations in
immune checkpoint blockade therapies targeting the PD-1/PD-L1 axis
have yielded surprising therapeutic outcomes in the treatment of
CRC (50–52).
The aforementioned findings indicated that the
influence of CXCL9 on immune cells is stage-dependent and may
facilitate immune evasion during the progression of cancer,
particularly in Stage II. As a prognostic marker associated with
immune infiltration, CXCL9, when combined with PD-L1 therapy, could
potentially represent an effective treatment strategy for Stage II
CRC. In vitro experiments demonstrated that CXCL9
overexpression markedly contributed to the malignant
characteristics of tumor cells, including cell proliferation,
migration and invasion. Due to the enrichment of CXCL9 co-expressed
genes in the mitophagy, further experiments were conducted to
investigate the potential involvement of CXCL9 in autophagy.
Following the addition of the autophagy inhibitor 3-MA, alterations
in the expression of CXCL9 gene were observed. This suggested that
changes in CXCL9 expression may be part of cellular adaptation to
stress, as autophagy is a stress tolerance mechanism in cancer
(53). In cells overexpressing
CXCL9, the mRNA and protein expression levels of autophagy-related
markers was assessed. qPCR results indicated that ATG3 and ATG4B
were upregulated following CXCL9 overexpression, which can promote
the transition from LC3-I to LC3-II and facilitating autophagosome
formation (54). However, western
blotting results showed an accumulation of LC3-II and p62, which
suggested autophagy flow was blocked. The experimental findings
suggested that the overexpression of CXCL9 may lead to the
accumulation of autophagosomes and impair their degradation.
Coupled with the results from GSEA analysis showing
a negative correlation between mitophagy, mitochondrial protein
import and CXCL9 expression, it was hypothesized that the
overexpression of CXCL9 might disrupt mitophagy, thereby blocking
autophagic flux. GSEA results indicated that CXCL9 was positively
associated with the MAPK and NF-κB pathways, while it was
negatively associated with the Wnt signaling pathway. Additionally,
CXCL9 showed a negative correlation with pathways associated with
mitophagy. Studies have revealed that the MAPK pathway, when
inhibited, can lead to the activation of mitophagy through PINK1
and OPTN-dependent mechanisms, highlighting its complex role in
regulating mitochondrial autophagy (52,55,56).
NF-κB signaling is a key driver of mitochondrial dysfunction and
morphological changes, redox imbalance, and insulin signaling
disruption (57,58). Based on the in vitro
experiments, it was hypothesized that CXCL9 may inhibit mitophagy
by activating the MAPK and NF-κB pathways, thereby affecting
mitophagy from multiple angles.
The present study delved into the intricate
interplay between CXCL9 and ALKBH5. Existing studies have
demonstrated that ALKBH5 influences gene expression levels by
affecting the stability of its mRNA (59). Through a series of experiments, it
demonstrated that ALKBH5 can indeed influence the stability of
CXCL9 mRNA, thereby affecting its expression levels. Notably, the
overexpression of CXCL9 was found to partially attenuate the
tumor-suppressive effects observed upon ALKBH5 knockdown. This
observation suggested that CXCL9 functions as a downstream target
of ALKBH5-mediated methylation, highlighting a novel mechanistic
link between mRNA methylation and the regulation of chemokine
expression in cancer biology.
The present study suggested that CXCL9 plays a dual
role both in tumor progression and immune surveillance. On one
hand, elevated levels of CXCL9 have been observed in CC tissues and
correlate with advanced disease stage and tumor proliferation,
invasion, metastasis and autophagy. On the other hand, CXCL9 has
also been implicated in anti-tumor immunity within the CC
microenvironment. Additionally, CXCL9 expression has been
associated with improved overall survival and enhanced response to
immunotherapy in CC patients, highlighting its potential as a
predictive biomarker and therapeutic target. Furthermore, the
dysregulation of CXCL9 in CC has been attributed to various
molecular mechanisms, including m6a modifications. Understanding
these underlying mechanisms may provide insights into the
development of targeted therapies aimed at modulating CXCL9
expression and activity in CRC.
The present study has enhanced the understanding of
ALKBH5-mediated modification of CXCL9 and its role in CRC, as well
as its association with mitophagy. However, it has limitations.
Although it analyzed the expression of CXCL9 across CRC stages
using GEO and TCGA datasets, the absence of more comprehensive
datasets restricted an in-depth understanding of its dynamic
changes during disease progression. The expression of CXCL9 varies
among different ethnicities and residual tumor classifications,
which underscores the limitations of conducting experiments solely
in cell lines. To address these gaps, it is hoped to collect
additional patient samples in future experiments to confirm its
role in CRC and explore its regulatory mechanisms on mitophagy at
the in vivo level. The broader effect of CXCL9 on the tumor
microenvironment and immune response will also be investigated to
identify new therapeutic strategies for CRC. Further in vivo
studies and clinical validations are essential for a complete
understanding of the role of CXCL9 in tumor progression.
In conclusion, CXCL9 plays a multifaceted role in
CRC pathogenesis, influencing both tumor progression and anti-tumor
immunity. Further research is warranted to elucidate its precise
mechanisms of action and explore its therapeutic potential in CRC
management.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the 2024 Lincang City
Association for Science and Technology Society Capacity Service
Innovation and Development Project (grant no. 202404) and the Dali
University Education Teaching Reform Project (grant no.
JG09YX209).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GH was instrumental in the conceptualization and
design of the study, led the analysis of bioinformatics data, was
the primary author of the initial manuscript draft, and also
coordinated revisions with all co-authors and provided
comprehensive supervision and guidance throughout the project. SS
was actively involved in the experimental procedures, data
collection and preliminary data analysis, and also contributed to
the writing of the manuscript and was responsible for securing
partial funding for the research. MZ executed in vitro
experiments using cell lines and played a significant role in the
review of the manuscript. All authors read and approved the final
manuscript. GH and SS confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Geng Hu (https://orcid.org/0009-0008-7876-8139)
Shijun Shen (https://orcid.org/0000-0002-4532-9366)
Mingchao Zhu (https://orcid.org/0000-0001-5425-97803)
References
|
1
|
Morgan E, Arnold M, Gini A, Lorenzoni V,
Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N and Bray F:
Global burden of colorectal cancer in 2020 and 2040: Incidence and
mortality estimates from GLOBOCAN. Gut. 72:338–344. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Housini M, Dariya B, Ahmed N, Stevens A,
Fiadjoe H, Nagaraju GP and Basha R: Colorectal cancer: Genetic
alterations, novel biomarkers, current therapeutic strategies and
clinical trials. Gene. 892:1478572024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Potter JD, Slattery ML, Bostick RM and
Gapstur SM: Colon cancer: A review of the epidemiology. Epidemiol
Rev. 15:499–545. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, et
al: Colon cancer, version 2.2021, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 19:329–359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schlechter BL: Management of rectal
cancer. Hematol Oncol Clin North Am. 36:521–537. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schrag D, Shi Q, Weiser MR, Gollub MJ,
Saltz LB, Musher BL, Goldberg J, Al Baghdadi T, Goodman KA,
McWilliams RR, et al: Preoperative treatment of locally advanced
rectal cancer. N Engl J Med. 389:322–334. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niu SQ, Li RZ, Yuan Y, Xie WH, Wang QX,
Chang H, Lu ZH, Ding PR, Li LR, Wu XJ, et al: Neoadjuvant
chemoradiotherapy in patients with unresectable locally advanced
sigmoid colon cancer: Clinical feasibility and outcome. Radiat
Oncol. 16:932021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong MY, Li LY, Lou YM, Chi HY and Wu JJ:
Chinese herbal medicines for prevention and treatment of colorectal
cancer: From molecular mechanisms to potential clinical
applications. J Integr Med. 18:369–384. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Underwood PW, Ruff SM and Pawlik TM:
Update on targeted therapy and immunotherapy for metastatic
colorectal cancer. Cells. 13:2452024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chalabi M, Fanchi LF, Dijkstra KK, Van den
Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C,
Beets GL, Snaebjornsson P, et al: Neoadjuvant immunotherapy leads
to pathological responses in MMR-proficient and MMR-deficient
early-stage colon cancers. Nat Med. 26:566–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ieranò C, Righelli D, D'Alterio C,
Napolitano M, Portella L, Rea G, Auletta F, Santagata S, Trotta AM,
Guardascione G, et al: In PD-1+ human colon cancer cells NIVOLUMAB
promotes survival and could protect tumor cells from conventional
therapies. J Immunother Cancer. 10:e0040322022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki S, Kawakami H, Miike T and Yamamoto
S: Complete remission of colon cancer with ipilimumab monotherapy.
Intern Med. 60:957–958. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chalabi M, Verschoor YL, Tan PB, Balduzzi
S, Van Lent AU, Grootscholten C, Dokter S, Büller NV, Grotenhuis
BA, Kuhlmann K, et al: Neoadjuvant immunotherapy in locally
advanced mismatch repair-deficient colon cancer. N Engl J Med.
390:1949–1958. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou C, Gao Y, Ding P, Wu T and Ji G: The
role of CXCL family members in different diseases. Cell Death
Discov. 9:2122023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cambier S, Gouwy M and Proost P: The
chemokines CXCL8 and CXCL12: Molecular and functional properties,
role in disease and efforts towards pharmacological intervention.
Cell Mol Immunol. 20:217–251. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
House IG, Savas P, Lai J, Chen AXY, Oliver
AJ, Teo ZL, Todd KL, Henderson MA, Giuffrida L, Petley EV, et al:
Macrophage-derived CXCL9 and CXCL10 are required for antitumor
immune responses following immune checkpoint blockade. Clin Cancer
Res. 26:487–504. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mikucki ME, Fisher DT, Matsuzaki J,
Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Frelinger JG, Odunsi K,
Gajewski TF, et al: Non-redundant requirement for CXCR3 signalling
during tumoricidal T-cell trafficking across tumour vascular
checkpoints. Nat Commun. 6:74582015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tokunaga R, Zhang W, Naseem M, Puccini A,
Berger MD, Soni S, McSkane M, Baba H and Lenz HJ: CXCL9, CXCL10,
CXCL11/CXCR3 axis for immune activation-A target for novel cancer
therapy. Cancer Treat Rev. 63:40–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andersson As, Yang SC, Huang M, Zhu L, Kar
UK, Batra RK, Elashoff D, Strieter RM, Dubinett SM and Sharma S:
IL-7 promotes CXCR3 ligand-dependent T cell antitumor reactivity in
lung cancer. J Immunol. 182:6951–6958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu L, Sun S, Qu F, Sun M, Liu X, Sun Q,
Cheng L, Zheng Y and Su G: CXCL9 influences the tumor immune
microenvironment by stimulating JAK/STAT pathway in triple-negative
breast cancer. Cancer Immunol Immunother. 72:1479–1492. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Santana-Hernández S, Suarez-Olmos J,
Servitja S, Berenguer-Molins P, Costa-Garcia M, Comerma L, Rea A,
Perera-Bel J, Menendez S, Arpí O, et al: NK cell-triggered
CCL5/IFNγ-CXCL9/10 axis underlies the clinical efficacy of
neoadjuvant anti-HER2 antibodies in breast cancer. J Exp Clin
Cancer Res. 43:102024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bronger H, Karge A, Dreyer T, Zech D,
Kraeft S, Avril S, Kiechle M and Schmitt M: Induction of cathepsin
B by the CXCR3 chemokines CXCL9 and CXCL10 in human breast cancer
cells. Oncol Lett. 13:4224–4230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Wang Q, Guan Y, Sun Y, Wang X,
Lively K, Wang Y, Luo M, Kim JA, Murphy EA, et al: Breast cancer
cell-derived microRNA-155 suppresses tumor progression via
enhancing immune cell recruitment and antitumor function. J Clin
Invest. 132:e1572482022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Romano G, Paradiso F, Li P, Shukla P,
Barger LN, El Naggar O, Miller JP, Liang RJ, Helms TL, Lazar AJ, et
al: Microparticle-delivered Cxcl9 prolongs braf inhibitor efficacy
in melanoma. Cancer Immunol Res. 11:558–569. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui Y, Miao Y, Cao L, Guo L, Cui Y, Yan C,
Zeng Z, Xu M and Han T: Activation of melanocortin-1 receptor
signaling in melanoma cells impairs T cell infiltration to dampen
antitumor immunity. Nat Commun. 14:57402023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim RJ, Salehi-Rad R, Tran LM, Oh MS,
Dumitras C, Crosson WP, Li R, Patel TS, Man S, Yean CE, et al:
CXCL9/10-engineered dendritic cells promote T cell activation and
enhance immune checkpoint blockade for lung cancer. Cell Rep Med.
5:1014792024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woo SJ, Kim Y, Kang HJ, Jung H, Youn DH,
Hong Y, Lee JJ and Hong JY: Tuberculous pleural effusion-induced
Arg-1+ macrophage polarization contributes to lung
cancer progression via autophagy signaling. Respir Res. 25:1982024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Assenov Y, Ramírez F, Schelhorn SE,
Lengauer T and Albrecht M: Computing topological parameters of
biological networks. Bioinformatics. 24:282–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Győrffy B: Integrated analysis of public
datasets for the discovery and validation of survival-associated
genes in solid tumors. Innovation (Camb). 5:1006252024.PubMed/NCBI
|
|
34
|
Ru B, Wong CN, Tong Y, Zhong JY, Zhong
SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, et al: TISIDB: An
integrated repository portal for tumor-immune system interactions.
Bioinformatics. 35:4200–4202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou Y, Zeng P, Li YH, Zhang Z and Cui Q:
SRAMP: Prediction of mammalian N6-methyladenosine (m6A) sites based
on sequence-derived features. Nucleic Acids Res. 44:e912016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo X, Tai J, Zhao Y, Zhao P, Sun D and
Wang L: Associations of C-X-C motif chemokine ligands 1/2/8/13/14
with clinicopathological features and survival profile in patients
with colorectal cancer. Oncol Lett. 24:3482022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhuo C, Ruan Q, Zhao X, Shen Y and Lin R:
CXCL1 promotes colon cancer progression through activation of
NF-κB/P300 signaling pathway. Biol Direct. 17:342022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lepsenyi M, Algethami N, Al-Haidari AA,
Algaber A, Syk I, Rahman M and Thorlacius H: CXCL2-CXCR2 axis
mediates αV integrin-dependent peritoneal metastasis of colon
cancer cells. Clin Exp Metastasis. 38:401–410. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han B, Feng D, Yu X, Liu Y, Yang M, Luo F,
Zhou L and Liu F: MicroRNA-144 mediates chronic inflammation and
tumorigenesis in colorectal cancer progression via regulating C-X-C
motif chemokine ligand 11. Exp Ther Med. 16:1935–1943.
2018.PubMed/NCBI
|
|
41
|
Cao Y, Jiao N, Sun T, Ma Y, Zhang X, Chen
H, Hong J and Zhang Y: CXCL11 correlates with antitumor immunity
and an improved prognosis in colon cancer. Front Cell Dev Biol.
9:6462522021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Lu M, Yuan M, Ye J, Zhang W, Xu L,
Wu X, Hui B, Yang Y, Wei B, et al: CXCL10-armed oncolytic
adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance
anti-PD-1 therapy. Oncoimmunology. 11:21182102022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang B, Wang M, Ao D and Wei X:
CXCL13-CXCR5 axis: Regulation in inflammatory diseases and cancer.
Biochim Biophys Acta Rev Cancer. 1877:1887992022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hussain M, Liu J, Wang GZ and Zhou GB:
CXCL13 signaling in the tumor microenvironment. Adv Exp Med Biol.
1302:71–90. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu G, Sun J, Yang ZF, Zhou C, Zhou PY,
Guan RY, Sun BY, Wang ZT, Zhou J, Fan J, et al: Cancer-associated
fibroblast-derived CXCL11 modulates hepatocellular carcinoma cell
migration and tumor metastasis through the
circUBAP2/miR-4756/IFIT1/3 axis. Cell Death Dis. 12:2602021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang J, Ouyang X, Zhu W, Yi Q and Zhong J:
The Role of CXCL11 and its receptors in cancer: Prospective but
challenging clinical targets. Cancer Control.
31:107327482412411622024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu X, Sun A, Yu W, Hong C and Liu Z:
CXCL10 mediates breast cancer tamoxifen resistance and promotes
estrogen-dependent and independent proliferation. Mol Cell
Endocrinol. 512:1108662020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zuo H and Wan Y: Inhibition of myeloid
PD-L1 suppresses osteoclastogenesis and cancer bone metastasis.
Cancer Gene Ther. 29:1342–1354. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
50
|
Zhang Z, Zhang H, Cui L, Wang X, Wang D,
Liu Z, Zhang X and Tang Z: An MMAE-loaded PDL1 active targeting
nanomedicine for the precision treatment of colon cancer. Biomater
Sci. 11:5195–5204. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang S, Song Y, Cao K, Zhang L, Fang X,
Chen F, Feng S and Yan F: Photothermal therapy mediated by gold
nanocages composed of anti-PDL1 and galunisertib for improved
synergistic immunotherapy in colorectal cancer. Acta Biomater.
134:621–632. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gao Y, Zhao K, Huang Y, Zhang D, Luo N,
Peng X, Yang F, Xiao W, Wang M, Shi R and Miao H: Lanosterol
synthase deficiency promotes tumor progression by orchestrating
PDL1-dependent tumor immunosuppressive microenvironment. MedComm
(2020). 5:e5282024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Limagne E and Ghiringhelli F: Mitophagy: A
new actor in the efficacy of chemo-immunotherapy. Autophagy.
18:3033–3034. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang T, Gao T, Fujisawa M, Ohara T,
Sakaguchi M, Yoshimura T and Matsukawa A: SPRED2 is a novel
regulator of autophagy in hepatocellular carcinoma cells and normal
hepatocytes. Int J Mol Sci. 25:62692024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guido C, Whitaker-Menezes D, Lin Z,
Pestell RG, Howell A, Zimmers TA, Casimiro MC, Aquila S, Ando' S,
Martinez-Outschoorn UE, et al: Mitochondrial fission induces
glycolytic reprogramming in cancer-associated myofibroblasts,
driving stromal lactate production, and early tumor growth.
Oncotarget. 3:798–810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qiu Y, Wang J, Li H, Yang B, Wang J, He Q
and Weng Q: Emerging views of OPTN (optineurin) function in the
autophagic process associated with disease. Autophagy. 18:73–85.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu X, Li P, Huang Y, Li H, Liu X, Du Y,
Lin X, Chen D, Liu H and Zhou Y: M6A demethylase ALKBH5
regulates FOXO1 mRNA stability and chemoresistance in
triple-negative breast cancer. Redox Biol. 69:1029932024.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu M and Chen X: N6-methyladenosine
demethylase ALKBH5 promotes pyroptosis by modulating PTBP1 mRNA
stability in LPS-induced myocardial dysfunction. Acta Cardiol Sin.
40:312–321. 2024.PubMed/NCBI
|
|
59
|
Wang YY, Ye LH, Zhao AQ, Gao WR, Dai N,
Yin Y and Zhang X: M6A modification regulates tumor suppressor
DIRAS1 expression in cervical cancer cells. Cancer Biol Ther.
25:23066742024. View Article : Google Scholar : PubMed/NCBI
|