Alzheimer's disease (AD), the most prevalent form of
dementia in the elderly, leads to a gradual decline in cognitive
function. It is characterized by memory loss, language difficulty,
impaired judgment, mood swings and, in advanced stages, loss of
self-care ability (1).
Epidemiological data reveals that >50 million individuals
worldwide are affected by AD, with age-standardized prevalence of
dementia in patients aged >60 is ~5–7% worldwide, making it one
of the most expensive and fatal diseases globally (the absolute
numbers of deaths have increased by 39%) (2). China has the highest number of
patients with AD, with ~9.83 million individuals >60 years old
diagnosed with AD (3). While the
exact etiology of AD remains unclear, its development is linked to
a range of factors, including genetic predisposition, abnormal
protein aggregation, neurotransmitter imbalance and neuronal damage
(4–6). Chinese Food and Drug
Administration-approved treatments for AD include memantine,
rivastigmine, galantamine and donepezil (7). In China, treatment recommendations
for cognitive symptoms involve cholinesterase inhibitors, glutamate
receptor antagonists such as memantine and combination therapy with
both classes of drugs. Psychobehavioral symptoms are commonly
managed with medications such as atypical antipsychotics and
selective 5-hydroxytryptamine receptor agonists (8). The rise of artificial intelligence
has led to the increasing use of data mining techniques, with
complex network analysis based on graph theory offering a promising
approach for clinical application (9,10).
Topological indices expedite AD drug discovery by enabling rapid
computational screening of compounds. They highlight promising
candidates for further testing, bridging computational predictions
and therapeutic development. Their integration with multi-omics
data and machine learning holds promise for future breakthroughs in
understanding and treating AD. Ashraf et al (11) employed quantitative
structure-property association analysis to explore topological
indices and drug properties for AD treatment. The analysis
identified key structural features(such as topological indices)
associated with drug efficacy, providing valuable insight for the
design of more effective AD therapeutics (11). Despite the variety of mechanisms
through which current drugs operate, most approved treatments fail
to prevent the pathological progression of AD, and often exhibit
limited efficacy or notable side effects (12,13).
Thus, further research is key to improve understanding of the
underlying mechanisms of AD and develop more effective
therapies.
Ferroptosis, a distinct form of cell death driven by
iron-dependent lipid peroxidation, serves a key role in several
biological processes, including development, aging, immune
regulation and cancer (14–16).
Previous studies suggest that oxidative stress and iron overload
contribute to neuronal death in AD (17,18).
Iron, an essential trace element for the human body, is involved in
numerous physiological functions such as erythropoiesis, energy
metabolism, muscle function and cell cycle regulation (19). Elevated iron levels in the gray
matter of patients with AD have been documented (1), along with dysregulated iron
homeostasis and lipid peroxidation, hallmarks of ferroptosis that
are implicated in AD pathology (20). Therapeutic strategies targeting
ferroptosis to prevent or mitigate organ damage have gained
attention (21,22).
Ferroptosis typically results from disruptions in
iron metabolism, lipid peroxidation and decreased glutathione (GSH)
levels or inactivation of GSH peroxidase 4 (GPX4) (14). In ferroptotic cells, mitochondria
are smaller, with ruffled and reduced cristae and membrane rupture,
while non-ferroptotic cells exhibit swollen mitochondria. Vitamin
E, ferrostatin-1 (Fer-1) and liproxstatin-1 (Lip-1) inhibit
ferroptotic cell death without affecting other cell death pathways
(23). The molecular mechanisms
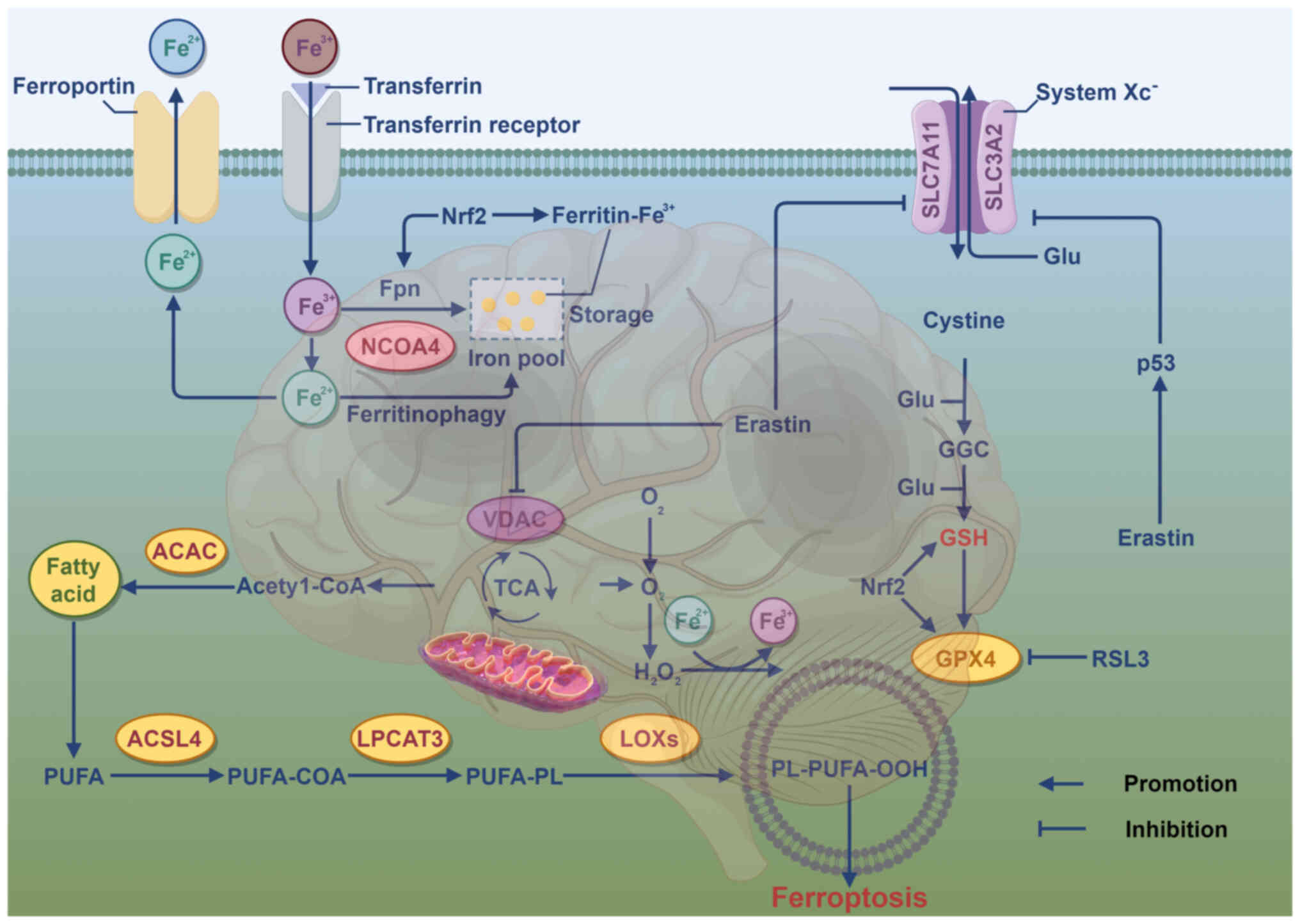
underlying ferroptosis primarily involve lipid, iron and amino acid
metabolism (Fig. 1). Lipid
metabolism is key for ferroptosis, which is driven by the
accumulation of lipid peroxides resulting from the oxidation of
polyunsaturated fatty acids (PUFAs) (24). Enzymes regulating lipid metabolism
serve a key role in ferroptosis during lipid peroxidation. Acyl-CoA
synthetase long-chain family member 4, a key enzyme in phospholipid
metabolism, facilitates the conversion of PUFAs, such as
arachidonoyl and adrenic acid, into PUFA-CoA (25). GSH, synthesized from glutamate,
cysteine and glycine via glutamine cysteine ligase and GSH
synthetase, serves as the primary antioxidant in mammalian cells.
During cellular transport, glutamate and cystine are exchanged
between cells through system Xc−, which is key for GSH
synthesis (26). Cysteine, due to
its limited intracellular availability, is the rate-limiting
precursor in GSH synthesis. System Xc−, consisting of
subunits solute carrier family 7 member 11 (SLC7A11) and SLC3A2,
exports glutamate when GSH is consumed in excess (27). Disruptions in iron metabolism lead
to pathological conditions, with transferrin-Fe3+
complex formation occurring when transferrin binds external
Fe3+ on the cell membrane, which is subsequently
internalized by transferrin receptor 1 (24). Divalent metal transporter 1
mediates release of Fe3+ ions from the six-transmembrane
epithelial antigen of prostate 3 endosome into the cytosol
(28). Ferritin releases
Fe2+ ions via ferroportin 1. In ferroptosis, free
Fe2+ interacts with hydrogen peroxide to generate highly
reactive lipid peroxides, resulting from the disruption of the
balance between ferrous iron absorption, depletion and recycling
(29).
Aβ plaque accumulation is a hallmark pathological
feature of AD, where abnormal Aβ aggregation disrupts synaptic
function and impairs memory (30,31).
Aβ, composed of 39–43 amino acids, is cleaved from amyloid
precursor protein (APP) (32). APP
is a highly conserved protein involved in synapse formation,
dendritic growth and neuronal migration (33). Iron facilitates the dissociation of
iron regulatory protein (IRP) 1 from the iron-responsive element
(IRE) (34). Elevated
intracellular iron levels disrupt the IRP/IRE signaling pathway,
leading to increased expression of APP (35,36)
and Aβ production. Additionally, Fe2+ binds to the
N-terminal domain of Aβ, destabilizing its helical structure and
promoting peptide aggregation by enhancing peptide-peptide
interactions (37). Concurrently,
τ hyperphosphorylation and its abnormal accumulation, coupled with
impaired clearance, lead to the formation of NFTs, further
compromising neuronal function. NFT formation is a key pathological
hallmark of AD (38–41). Dysregulated iron homeostasis has
been linked to τ hyperphosphorylation and NFT development (42). In the cortex and hippocampus of
patients with AD, NFTs accumulate in response to increased iron
levels (43). Excessive neuronal
iron promotes NFT formation via the activation of CDK5
(Cyclin-dependent kinase 5)/P25 complexes and GSK-3β (Glycogen
synthase kinase-3 beta) kinase pathways. Fe3+ also
induces hyperphosphorylated τ aggregation by binding to the
histidine residues of τ (44–46).
Microglia are essential components of the central
nervous system (CNS), serving key roles in energy metabolism,
synaptic plasticity and ion homeostasis. In addition, microglia
serve as resident immune cells, engaging in immune responses with
memory-like behavior and maintaining brain homeostasis. During
neurodegenerative processes, microglial activation is frequently
observed, with increasing evidence suggesting that iron overload
and disrupted iron homeostasis contribute to neurodegeneration in
AD (47,48). As such, microglia serve a key role
in neurological disorder. In response to infection or tissue
injury, microglia rapidly adapt to the local environment,
undergoing activation that can result in either beneficial or
harmful outcomes. Kroner et al (49) demonstrated that elevated iron
levels in microglia promote phagocytosis and drive a harmful M1
phenotype, which triggers the release of pro-inflammatory factors
such as TNF-α, IL-1β, IL-6 and nitric oxide (NO), causing neuronal
damage. Similarly, Rao et al (50) found that increased intracellular
iron disrupts the neuromelanin-iron complex in neurons, releasing
free iron ions that damage neurons and lead to neuromelanin
leakage. This leakage further activates microglia towards the M1
phenotype, promoting release of neurotoxic agents, including TNF-α
and IL-6 (50). Moreover, iron
accumulation in activated microglia contributes to iron deposition
within the CNS (51,52). In the M1 state, microglia express
inducible NO synthase (iNOS), which converts arginine into NO. The
resulting NO accumulation exacerbates glutamate-induced
neurotoxicity, contributing to neuronal ferroptosis (53).
Ferroptosis, an iron-dependent form of cell death
distinct from apoptosis and necrosis, is primarily triggered by
oxidative stress, a key pathological process in AD. The
accumulation of reactive oxygen species (ROS), a hallmark of
oxidative stress, serves a key role in initiating ferroptosis.
Oxidative stress affects numerous molecular pathways, including
inhibition of the cystine/glutamate antiporter system, decreased
expression of GPX4, disruption of iron homeostasis and lipid
peroxidation, which is a major driver of ferroptosis activation
(54). Excess lipid peroxide
accumulation in cells, a key feature of ferroptosis, results from a
free radical chain reaction. Oxygen radicals insert into the C-H
bonds of PUFAs, generating lipid hydroperoxides and elevating
levels of ROS, which induce ferroptosis (55). Malondialdehyde, a byproduct of
lipid peroxidation, is a marker for both ferroptosis and oxidative
stress (56). Furthermore,
oxidative stress impairs the antioxidant defense system by
decreasing expression of key enzymes such as GSH, catalase,
superoxide dismutase and GPX, thus accelerating ferroptosis
(56,57). Neuronal loss, a defining
characteristic of neurodegenerative disease, is associated with
cognitive decline in AD (58).
Elevated iron levels promote ROS production, depleting
intracellular GSH levels and accelerating lipid peroxidation. This
cascade leads to ferroptosis, contributing to neuronal death
(59). Bao et al (60) found downregulation of the
ferroptosis regulator GPX4 in both Fpnfl/fl/NEXcre
(NEX-Cre mice were mated with Fpn-floxed (Fpnfl/fl) mice to
generate conditional Fpnfl/fl/NEXcre mice.) and
APPswe/PS1dE9 (Carrying genetically modified mice with AD-related
mutations: a chimeric mouse/human APP with the Swedish mutation and
human PSEN1 lacking exon 9) mouse models compared with controls.
Additionally, mRNA expression of iron response element binding
protein 2, encodes a master regulator of iron metabolism), and CS
(citrate synthase, regulating the mitochondrial fatty acid
metabolism) was upregulated in both models, while ACSF2 ((acyl-CoA
synthetase family member 2, regulating the mitochondrial fatty acid
metabolism) was upregulated only in APPswe/PS1dE9 mice. These
findings suggest that ferroptosis is activated in the hippocampus
of both mouse models.
In cellular defense against oxidative stress, the
Keap1/Nrf2/ARE signaling pathway regulates the expression of
various proteins involved in detoxification and antioxidant
defense, positioning it as a potential target for AD treatment
(61). Nrf2, a transcription
factor that is highly responsive to oxidative stress, serves a key
role in mitigating lipid peroxidation and ferroptosis (62). Under physiological conditions,
Keap1 suppresses Nrf2 by facilitating its ubiquitination and
degradation via the ubiquitin-proteasome system. By contrast,
during oxidative stress, Nrf2 dissociates from Keap1, translocates
to the nucleus, forms a heterodimer with small musculoaponeurotic
fibrosarcoma oncogene homolog proteins and binds to ARE, thereby
enhancing the transcription of antioxidant genes (63–65).
Nrf2 regulates key components of anti-ferroptotic pathways,
positioning it as a central modulator of lipid peroxidation and
ferroptosis (62). In the nucleus,
Nrf2 induces the expression of cytoprotective genes that mitigate
ferroptosis by regulating iron metabolism and enhancing antioxidant
defenses. This includes the upregulation of ferritin heavy and
light chain, ferroportin, transferrin receptor and heme oxygenase-1
(HO-1), alongside increased production of NADPH, GSH and CoQ10
(coenzyme Q10) which counter lipid peroxidation and suppress
ferroptosis (66,67). Moreover, the detachment of the DLG
motif of Nrf2 from Keap1 prevents its ubiquitination and
degradation, thus strengthening antioxidant defenses and inhibiting
ferroptosis (68).
This process is initiated when unsaturated FAs in
cell membranes undergo catalytic lipid peroxidation, driven by
divalent iron or esteroxygenases, leading to cell death (69–71).
The p53 protein, a key human tumor suppressor, regulates the
expression of oncogenes and downstream signaling pathways,
contributing to biological effects (72–75):
Beyond its role in cancer, p53 is highly expressed in the brain,
where it influences dendritic growth, oxidative stress response,
apoptosis and autophagy, making p53 dysfunction and associated
pathways noteworthy in the pathogenesis of AD (76–78).
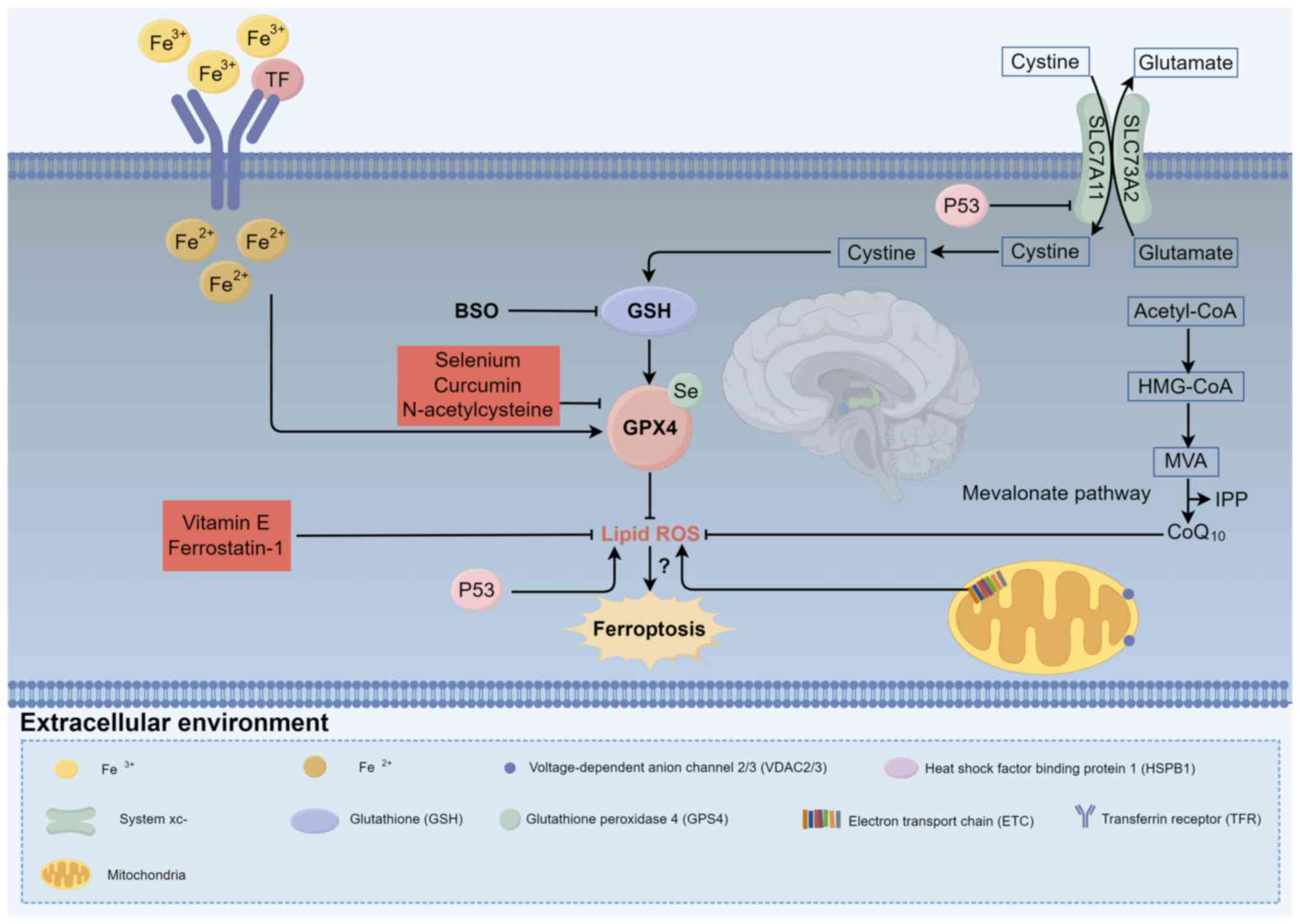
SLC7A11, a key ferroptosis regulator, is a transmembrane protein
that is part of the system Xc−, responsible for cystine
import into cells for cysteine synthesis and GSH production
(79,80). Downregulation of SLC7A11 disrupts
cysteine metabolism, leading to decreased intracellular cystine and
GSH levels, impairing GPX4 activity and triggering lipid peroxide
accumulation and ferroptosis (79,81,82).
Studies indicate that p53 binds the SLC7A11 promoter, suppressing
its expression and limiting GSH production, thereby promoting
ferroptosis (83,84). Aristolochic acid, mediated by p53,
may limit ferroptosis in liver cancer to enhance tumor growth. The
p53(3KR) mutant, lacking acetylation due to lysine-to-arginine
substitutions at three residues, decreases expression of SLC7A11
without affecting other p53 targets such as CDKN1A/p21 (involved in
cell cycle progression) or BAX (involved in apoptosis). By
contrast, the p53(4KR98) mutant, with an additional lysine 98
substitution, does not downregulate SLC7A11 (85–87).
In AD, excessive lipid peroxide accumulation is a
key initiator of ferroptosis, with elevated markers of lipid
peroxidation observed in neurons. Iron accumulation drives the
Fenton and Haber-Weiss reactions, generating ROS that induce lipid
peroxidation, leading to oxidative damage to subcellular structures
(88). GPX4 and GSH are key
regulators of ferroptosis. GSH, containing a thiol group derived
from cysteine, serves as a vital antioxidant, neutralizing ROS and
reactive nitrogen species, maintaining cellular redox balance and
detoxifying xenobiotics (89).
GPX4, a selenium (Se)-dependent enzyme, relies on a Se-containing
amino acid residue to execute its reductive function (90). It converts lipid hydroperoxides
into less harmful lipid alcohols, preventing oxidative damage
(91,92). The active site of GPX4,
selenocysteine, alternates between reduced and oxidized states to
sustain its catalytic activity. In the presence of peroxides, the
selenolate form of GPX4 is oxidized to selenic acid, which is
regenerated to its active form by two molecules of reduced GSH,
converting lipid hydroperoxides into non-toxic lipid alcohols and
generating oxidized GSH (16,92–94).
This highlights the key role of GPX4 synthesis and its associated
pathways in the regulation of ferroptosis.
Given the mechanisms underlying ferroptosis in AD,
researchers have focused on its inhibition as a potential
therapeutic strategy. Growing evidence suggests that targeting
ferroptosis may offer benefits for CNS disorders, driving the
development of effective inhibitors (24,95).
This approach presents promising therapeutic opportunities for AD
management (Table I; Fig. 2). As AD is characterized by iron
accumulation in brain cells, exacerbating oxidative damage and
cognitive decline (96–100), these inhibitors show therapeutic
promise. Vitamin E, Se, Fer-1, N-acetylcysteine (NAC) and curcumin
exhibit antioxidant and neuroprotective properties (101–131).
Vitamin E, an antioxidant in AD treatment, inhibits
ferroptosis primarily by preventing lipid peroxidation (101). It consists of two subclasses:
Tocotrienols and tocopherols, with four saturated analogs (α, β, γ
and δ) (132,133). These lipophilic compounds, along
with their derivatives, serve as radical-trapping antioxidants,
preventing the formation of phospholipid hydroperoxides. As the
dominant form in tissue, α-tocopherol exerts its antioxidant
effects by interrupting the chain reaction of lipid oxidation.
Specifically, the oxidative conversion of α-tocopherol produces
α-tocopherol quinone, which is reduced to α-tocopherol
hydroquinone. This reduction is key for inhibiting the enzymatic
activity of 15-lipoxygenase, primarily by converting its non-heme
Fe3+ to an inactive Fe2+ state. This
inhibition disrupts the ferroptotic signaling cascade, effectively
preventing lipid peroxidation and mitigating ferroptosis (106). α-tocopherol transfer protein
(TTP), which is abundant in the brain, regulates α-tocopherol
levels and distribution (107).
Vitamin E or TTP deficiency induce oxidative stress in the brain.
Studies show that patients with AD exhibit reduced vitamin E levels
in plasma, serum and cerebrospinal fluid, and those receiving
vitamin E supplementation experience slower cognitive decline and
decreased oxidative stress compared with placebo-treated
individuals (101,108). These findings suggest that
vitamin E deficiency contributes to neurodegeneration, while
supplementation may offer protection against ferroptotic
stress.
Se, an essential trace element involved in GPX4
synthesis, is incorporated into several proteins in the body
(109). Known for its antioxidant
properties, Se also serves a role in inhibiting ferroptosis
(102,110). Clinical studies suggest Se may
have potential in mitigating cognitive decline (111,134). In a mouse model of stroke,
intracerebroventricular sodium selenate treatment elevates GPX4
levels by activating transcription factors activating enhancer
binding protein 2γ and specificity protein 1, while also providing
protection against excitotoxicity and endoplasmic reticulum
stress-induced cell death independent of GPX4 (102). Decreased Se levels in the brains
of patients with AD are associated with disease progression. In
primary neuronal cultures, Se was shown to decrease Aβ production
by downregulating 4-hydroxy-2-nonenal-induced β-secretase
transcription, thus preventing Aβ-associated toxicity (102). Se-containing compounds inhibit
ferroptosis by upregulating GPX4. A clinical trial demonstrated
that oral sodium selenate supplementation increases brain Se levels
without notable side effects, and participants with improved Se
levels do not exhibit worsening Mini-Mental State Examination
scores over time (108). However,
Kryscio et al (108)
revealed that both Se and vitamin E have adverse effects on
progression of AD in male patients. Thus, current evidence does not
definitively support a potential therapeutic role of Se in AD, and
further clinical trials are needed to clarify its effects.
Fer-1, the first synthetic ferroptosis inhibitor,
has served as a pivotal reference compound (27). It effectively prevents oxidative
lipid damage and cell death in disease models, including
Huntington's disease, periventricular leukomalacia, renal
dysfunction, cerebral hemorrhage and cardiomyopathy (103,112,113). As a highly specific ferroptosis
inhibitor, Fer-1 surpasses phenolic antioxidants in its ability to
suppress ferroptosis, particularly by inhibiting lipid ROS
accumulation induced by erastin or RSL3 in HT-1080 human
fibrosarcoma cells) (27). The
anti-ferroptotic action of Fer-1 primarily arises from its capacity
to scavenge alkoxyl radicals and reactive species generated by
ferrous iron in lipid hydroperoxides. Additionally, it reduces
labile iron, as confirmed by calcein fluorescence assays, further
supporting its iron-complexing properties (114). In HT-22 (mouse hippocampal
neurons cells) cells, Fer-1 notably decreases both cytoplasmic and
lipid ROS levels, counteracts glutamate-induced suppression of GSH
and GPX activity and protects against oxidative toxicity.
Furthermore, Fer-1 mitigates oxidative stress by downregulating
prostaglandin endoperoxide synthase 2 while upregulating GPX4 and
Nrf2, thereby decreasing lipid peroxidation (115). Despite its documented efficacy
in vitro and in vivo in alleviating oxidative stress
and preventing iron deficiency, Fer-1 remains untested in clinical
trials (114). Further
investigation is required to assess its therapeutic potential,
safety and clinical effectiveness for treating AD.
NAC, a cysteine precursor, is used to treat
acetaminophen overdose and is listed as an essential medicine by
the World Health Organization (116). In addition to its role in
treating acute poisoning, NAC is recognized for its pro-neurogenic
and neuroprotective effects in neurodegenerative and psychiatric
disorder (120). NAC can cross
the blood-brain barrier and mitigate age-associated memory decline
(117,120). NAC may prevent ferroptosis by
activating Nrf2, which regulates the expression of
metallothioneins, ferritins and ferroportins, thereby preventing
iron accumulation. Other Nrf2-dependent genes, including GPX4, GSH
and NADPH synthesis genes, as well as epigenetic regulators of
lipid hydroperoxides, contribute to its ferroptosis-inhibitory
effects (117–119). In AD, NAC has potential as a GSH
precursor, enhancing antioxidant defenses (120). Rat and gerbil studies have
demonstrated that intraperitoneal NAC administration protects
against oxidative damage induced by acrolein, peroxynitrite,
hydroxyl radicals and 3-nitropropionic acid while increasing GSH
levels in the brain and synaptosomes (119–123).
Curcumin, a polyphenolic compound initially
identified as diferuloylmethane in turmeric rhizomes, has
demonstrated protective effects against oxidative damage resulting
from iron accumulation in cells (124). Curcumin treatment has been shown
to decrease expression of GPX4 while increasing levels of
heme-oxygenase (HO-1) and Nrf2 (124). As a key regulator of oxidative
stress, Nrf2 governs the expression of ARE-dependent genes
(62,125). Hirata et al (126) developed hybrid molecules that
combine the oxidized indole structure of neuroprotective agents
with the polyphenolic structure of curcumin, applying them to mouse
hippocampal HT22 cells. These hybrids exhibited superior
neuroprotective effects and lower cytotoxicity compared with
curcumin alone, activated ARE, chelated ferrous ions and scavenged
ROS, thereby protecting cells from oxidative stress and
ferroptosis, ultimately promoting neuronal survival (104,127,128).
Lip-1, a derivative of quinoxaline spirocyclic
compounds, is a potent ferroptosis inhibitor first identified in
2014 through small molecule compound library screening (129). Lip-1 primarily exerts its effects
by inhibiting lipid peroxidation. Li et al (130) demonstrated that Lip-1 alleviates
memory deficits in a mouse model of lipopolysaccharide
(LPS)-induced cognitive impairment. Moreover, Lip-1 reduced
microglial activation and the secretion of pro-inflammatory
cytokines such as IL-6 and TNF-α, while mitigating oxidative
stress, lipid peroxidation, mitochondrial damage and neuronal
injury following LPS exposure. Further studies have revealed that
Lip-1 not only prevents mitochondrial lipid peroxidation but also
restores the expression of key molecules involved in ferroptosis
regulation, including GSH, GPX4 and ferroptosis suppressor protein
1 (135,136). These findings suggest that GPX4
inhibition may induce ferroptosis in oligodendrocytes, with Lip-1
serving as a potent ferroptosis antagonist. Therefore, Lip-1 holds
promise as a therapeutic candidate for CNS disease (131).
Research on iron-lowering strategies in AD has been
limited, likely due to the predominant focus on amyloid-lowering
treatments, which have generally yielded unfavorable results
(137,138). Nevertheless, studies involving
iron chelators in cell and animal models of AD have demonstrated
promising outcomes (95,139). While iron metabolism and lipid
peroxidation trigger ferroptosis under pathological conditions, the
precise mechanisms remain incompletely understood and warrant
further investigation. Additionally, iron chelating agents,
including chloroiodohydroxyquine and its derivatives, as well as
antioxidants, have shown efficacy in animal models of AD, though
clinical trials are yet to be conducted (27,106,116). Ferroptosis may serve as a key
target pathway for advancing AD treatment strategies.
In conclusion, the present review summarized the
role of ferroptosis in the pathology of AD and how mechanisms such
as iron metabolism disorders, lipid peroxidation and GSH depletion
lead to neuronal damage, as well as the role of ferroptosis
inhibitors such as vitamin E, Se and Fer-1 as potential therapeutic
strategies. Keap1/Nrf2/ARE, p53/SLC7A11 and GSH/GPX4 signaling
pathways underlie the pathological mechanism of AD, positioning
them as a potential direction for future research.
Not applicable.
Funding: No funding was received.
Not applicable.
HZ and ZJ wrote and reviewed the manuscript. All
authors have read and approved the final version of the manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zhao D, Yang K, Guo H, Zeng J, Wang S, Xu
H, Ge A, Zeng L, Chen S and Ge J: Mechanisms of ferroptosis in
Alzheimer's disease and therapeutic effects of natural plant
products: A review. Biomed Pharmacother. 164:1143122023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Graff-Radford J, Yong KXX, Apostolova LG,
Bouwman FH, Carrillo M, Dickerson BC, Rabinovici GD, Schott JM,
Jones DT and Murray ME: New insights into atypical Alzheimer's
disease in the era of biomarkers. Lancet Neurol. 20:222–234. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Q, Sun J, Chen T, Song S, Hou Y, Feng
L, Fan C and Li M: Ferroptosis, pyroptosis, and cuproptosis in
Alzheimer's disease. ACS Chem Neurosci. 14:3564–3587. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lane DJR, Metselaar B, Greenough M, Bush
AI and Ayton SJ: Ferroptosis and NRF2: An emerging battlefield in
the neurodegeneration of Alzheimer's disease. Essays Biochem.
65:925–940. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Q, Wu W, Wen Y, Lu S and Zhao C:
Potential therapeutic natural compounds for the treatment of
Alzheimer's disease. Phytomedicine. 132:1558222024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nayak V, Patra S, Rout S, Jena AB, Sharma
R, Pattanaik KP, Singh J, Pandey SS, Singh RP, Majhi S, et al:
Regulation of neuroinflammation in Alzheimer's disease via
nanoparticle-loaded phytocompounds with anti-inflammatory and
autophagy-inducing properties. Phytomedicine. 122:1551502024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weintraub D, Aarsland D, Chaudhuri KR,
Dobkin RD, Leentjens AF, Rodriguez-Violante M and Schrag A: The
neuropsychiatry of Parkinson's disease: Advances and challenges.
Lancet Neurol. 21:89–102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Markus HS, van Der Flier WM, Smith EE,
Bath P, Biessels GJ, Briceno E, Brodtman A, Chabriat H, Chen C, de
Leeuw FE, et al: Framework for clinical trials in cerebral small
vessel disease (FINESSE): A Review. JAMA Neurol. 79:1187–1198.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu JB, Wang X and Cao J: The coherence
and properties analysis of balanced 2 p-Ary tree networks. IEEE
Trans Netw Sci Eng. 11:4719–4728. 2024. View Article : Google Scholar
|
|
10
|
Liu JB, Zhang X, Cao J and Chen L: Mean
first-passage time and robustness of complex cellular mobile
communication network. IEEE Trans Netw Sci Eng. 11:3066–3076. 2024.
View Article : Google Scholar
|
|
11
|
Ashraf T, Idrees N and Belay MB:
Regression analysis of topological indices for predicting efficacy
of Alzheimer's drugs. PLoS One. 19:e03094772024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Song X, Wang R, Xu X, Du Y, Chen G
and Mei J: Genome-wide mendelian randomization identifies
ferroptosis-related drug targets for Alzheimer's disease. J
Alzheimers Dis Rep. 8:1185–1197. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soni P, Ammal Kaidery N, Sharma SM,
Gazaryan I, Nikulin SV, Hushpulian DM and Thomas B: A critical
appraisal of ferroptosis in Alzheimer's and Parkinson's disease:
New insights into emerging mechanisms and therapeutic targets.
Front Pharmacol. 15:13907982024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei P, Ayton S and Bush AI: The essential
elements of Alzheimer's disease. J Biol Chem. 296:1001052021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yong YY, Yan L, Wang BD, Fan DS, Guo MS,
Yu L, Wu JM, Qin DL, Law BY, Wong VK, et al: Penthorum chinense
Pursh inhibits ferroptosis in cellular and Caenorhabditis elegans
models of Alzheimer's disease. Phytomedicine. 127:1554632024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andrews NC: Disorders of iron metabolism.
N Engl J Med. 341:1986–1995. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buijs M, Doan NT, van Rooden S, Versluis
MJ, van Lew B, Milles J, van der Grond J and van Buchem MA: In vivo
assessment of iron content of the cerebral cortex in healthy aging
using 7-Tesla T2*-weighted phase imaging. Neurobiol Aging.
53:20–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chu J, Li J, Sun L and Wei J: The role of
cellular defense systems of ferroptosis in Parkinson's disease and
Alzheimer's disease. Int J Mol Sci. 24:141082023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J and Wang JQ: Research mechanisms of
and pharmaceutical treatments for ferroptosis in liver diseases.
Biochimie. 180:149–157. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ajoolabady A, Aslkhodapasandhokmabad H,
Libby P, Tuomilehto J, Lip GYH, Penninger JM, Richardson DR, Tang
D, Zhou H, Wang S, et al: Ferritinophagy and ferroptosis in the
management of metabolic diseases. Trends Endocrinol Metab.
32:444–462. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jakaria M, Belaidi AA, Bush AI and Ayton
S: Ferroptosis as a mechanism of neurodegeneration in Alzheimer's
disease. J Neurochem. 159:804–825. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y,
Fang J, Xu S, Gao Y, Chen X, Sui X and Li G: The emerging role of
ferroptosis in inflammation. Biomed Pharmacother. 127:1101082020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Couto N, Wood J and Barber J: The role of
glutathione reductase and related enzymes on cellular redox
homoeostasis network. Free Radic Biol Med. 95:27–42. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu H, Chen Y, Jing L, Zhai C and Shen L:
The link between ferroptosis and cardiovascular diseases: A novel
target for treatment. Front Cardiovasc Med. 8:7109632021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bu ZQ, Yu HY, Wang J, He X, Cui YR, Feng
JC and Feng J: Emerging role of ferroptosis in the pathogenesis of
ischemic stroke: A new therapeutic target? ASN Neuro.
13:175909142110375052021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foley KE and Wilcock DM: Vascular
considerations for amyloid immunotherapy. Curr Neurol Neurosci Rep.
22:709–719. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Chen Z, Li B, Yao H, Zarka M, Welch
J, Sachdev P, Bridge W and Braidy N: Supplementation with
γ-glutamylcysteine (γ-GC) lessens oxidative stress, brain
inflammation and amyloid pathology and improves spatial memory in a
murine model of AD. Neurochem Int. 144:1049312021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen LL, Fan YG, Zhao LX, Zhang Q and Wang
ZY: The metal ion hypothesis of Alzheimer's disease and the
anti-neuroinflammatory effect of metal chelators. Bioorg Chem.
131:1063012023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Müller UC, Deller T and Korte M: Not just
amyloid: physiological functions of the amyloid precursor protein
family. Nat Rev Neurosci. 18:281–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou ZD and Tan EK: Iron regulatory
protein (IRP)-iron responsive element (IRE) signaling pathway in
human neurodegenerative diseases. Mol Neurodegener. 12:752017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goel P, Chakrabarti S, Goel K, Bhutani K,
Chopra T and Bali S: Neuronal cell death mechanisms in Alzheimer's
disease: An insight. Front Mol Neurosci. 15:9371332022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Fu J, Zhao Y, Liu Q, Yan X and Su
J: Iron and targeted iron therapy in Alzheimer's disease. Int J Mol
Sci. 24:163532023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boopathi S and Kolandaivel P: Fe2+ binding
on amyloid β-peptide promotes aggregation. Proteins. 84:1257–1274.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Faraji P, Kühn H and Ahmadian S: Multiple
roles of apolipoprotein E4 in oxidative lipid metabolism and
ferroptosis during the pathogenesis of Alzheimer's disease. J Mol
Neurosci. 74:622024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao Y and Tan L, Yu JT and Tan L: Tau in
Alzheimer's disease: Mechanisms and therapeutic strategies. Curr
Alzheimer Res. 15:283–300. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sinsky J, Pichlerova K and Hanes J: Tau
protein interaction partners and their roles in Alzheimer's disease
and other tauopathies. Int J Mol Sci. 22:92072021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cody KA, Langhough RE, Zammit MD, Clark L,
Chin N, Christian BT, Betthauser TJ and Johnson SC: Characterizing
brain tau and cognitive decline along the amyloid timeline in
Alzheimer's disease. Brain. 147:2144–2157. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang F, Wang J, Shen Y, Li H, Rausch WD
and Huang X: Iron dyshomeostasis and ferroptosis: A new Alzheimer's
disease hypothesis? Front Aging Neurosci. 14:8305692022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Spotorno N, Acosta-Cabronero J, Stomrud E,
Lampinen B, Strandberg OT, van Westen D and Hansson O: Relationship
between cortical iron and tau aggregation in Alzheimer's disease.
Brain. 143:1341–1349. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guo C, Wang P, Zhong ML, Wang T, Huang XS,
Li JY and Wang ZY: Deferoxamine inhibits iron induced hippocampal
tau phosphorylation in the Alzheimer transgenic mouse brain.
Neurochem Int. 62:165–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vossel KA, Xu JC, Fomenko V, Miyamoto T,
Suberbielle E, Knox JA, Ho K, Kim DH, Yu GQ and Mucke L: Tau
reduction prevents Aβ-induced axonal transport deficits by blocking
activation of GSK3β. J Cell Biol. 209:419–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim AC, Lim S and Kim YK: Metal ion
effects on Aβ and tau aggregation. Int J Mol Sci. 19:1282018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mills E, Dong XP, Wang F and Xu H:
Mechanisms of brain iron transport: Insight into neurodegeneration
and CNS disorders. Future Med Chem. 2:51–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Masaldan S, Bush AI, Devos D, Rolland AS
and Moreau C: Striking while the iron is hot: Iron metabolism and
ferroptosis in neurodegeneration. Free Radic Biol Med. 133:221–233.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kroner A, Greenhalgh AD, Zarruk JG, Passos
Dos Santos R, Gaestel M and David S: TNF and increased
intracellular iron alter macrophage polarization to a detrimental
M1 phenotype in the injured spinal cord. Neuron. 83:1098–1116.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rao KS, Hegde ML, Anitha S, Musicco M,
Zucca FA, Turro NJ and Zecca L: Amyloid β and neuromelanin-toxic or
protective molecules?: The cellular context makes the difference.
Prog Neurobiol. 78:364–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guo JJ, Yue F, Song DY, Bousset L, Liang
X, Tang J, Yuan L, Li W, Melki R, Tang Y, et al: Intranasal
administration of α-synuclein preformed fibrils triggers microglial
iron deposition in the substantia nigra of Macaca fascicularis.
Cell Death Dis. 12:812021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kenkhuis B, Somarakis A, de Haan L,
Dzyubachyk O, IJsselsteijn ME, de Miranda NFCC, Lelieveldt BPF,
Dijkstra J, van Roon-Mom WMC, Höllt T and van der Weerd L: Iron
loading is a prominent feature of activated microglia in
Alzheimer's disease patients. Acta Neuropathol Commun. 9:272021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang M, Tang G, Zhou C, Guo H, Hu Z, Hu Q
and Li G: Revisiting the intersection of microglial activation and
neuroinflammation in Alzheimer's disease from the perspective of
ferroptosis. Chem Biol Interact. 375:1103872023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li S, Wen P, Zhang D, Li D, Gao Q, Liu H
and Di Y: PGAM5 expression levels in heart failure and protection
ROS-induced oxidative stress and ferroptosis by Keap1/Nrf2. Clin
Exp Hypertens. 45:21625372023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Conrad M, Kagan VE, Bayir H, Pagnussat GC,
Head B, Traber MG and Stockwell BR: Regulation of lipid
peroxidation and ferroptosis in diverse species. Genes Dev.
32:602–619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Endale HT, Tesfaye W and Mengstie TA: ROS
induced lipid peroxidation and their role in ferroptosis. Front
Cell Dev Biol. 11:12260442023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hanseeuw BJ, Betensky RA, Jacobs HI,
Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT,
Mormino EC, et al: Association of amyloid and tau with cognition in
preclinical Alzheimer disease: A longitudinal study. JAMA Neurol.
76:915–924. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Maher P: Potentiation of glutathione loss
and nerve cell death by the transition metals iron and copper:
Implications for age-related neurodegenerative diseases. Free Radic
Biol Med. 115:92–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bao WD, Pang P, Zhou XT, Hu F, Xiong W,
Chen K, Wang J, Wang F, Xie D, Hu YZ, et al: Loss of ferroportin
induces memory impairment by promoting ferroptosis in Alzheimer's
disease. Cell Death Differ. 28:1548–1562. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tu W, Wang H, Li S, Liu Q and Sha H: The
anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE
signaling pathway in chronic diseases. Aging Dis. 10:637–651. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Baird L, Swift S, Llères D and
Dinkova-Kostova AT: Monitoring Keap1-Nrf2 interactions in single
live cells. Biotechnol Adv. 32:1133–1144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kumar A and Mittal R: Nrf2: A potential
therapeutic target for diabetic neuropathy. Inflammopharmacology.
25:393–402. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sun Y, Xia X, Basnet D, Zheng JC, Huang J
and Liu J: Mechanisms of ferroptosis and emerging links to the
pathology of neurodegenerative diseases. Front Aging Neurosci.
14:9041522022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shin CS, Mishra P, Watrous JD, Carelli V,
D'Aurelio M, Jain M and Chan DC: The glutamate/cystine xCT
antiporter antagonizes glutamine metabolism and reduces nutrient
flexibility. Nat Commun. 8:150742017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liang D, Minikes AM and Jiang X:
Ferroptosis at the intersection of lipid metabolism and cellular
signaling. Mol Cell. 82:2215–2227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ursini F and Maiorino M: Lipid
peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic
Biol Med. 152:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pope LE and Dixon SJ: Regulation of
ferroptosis by lipid metabolism. Trends Cell Biol. 33:1077–1087.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Finlay CA, Hinds PW and Levine AJ: The 53
proto-oncogene can act as a suppressor of transformation. Cell.
57:1083–1093. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kenzelmann Broz D and Attardi LD: In vivo
analysis of p53 tumor suppressor function using genetically
engineered mouse models. Carcinogenesis. 31:1311–1318. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kamada R, Toguchi Y, Nomura T, Imagawa T
and Sakaguchi K: Tetramer formation of tumor suppressor protein
p53: Structure, function, and applications. Biopolymers.
106:598–612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Joerger AC and Fersht AR: Structural
biology of the tumor suppressor p53. Annu Rev Biochem. 77:557–582.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li H, Zhang Z, Li H, Pan X and Wang Y: New
insights into the roles of p53 in central nervous system diseases.
Int J Neuropsychopharmacol. 26:465–473. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ohyagi Y, Asahara H, Chui DH, Tsuruta Y,
Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, et
al: Intracellular Abeta42 activates p53 promoter: A pathway to
neurodegeneration in Alzheimer's disease. FASEB J. 19:255–257.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Masaldan S, Belaidi AA, Ayton S and Bush
AI: Cellular senescence and iron dyshomeostasis in Alzheimer's
disease. Pharmaceuticals (Basel). 12:932019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang C, Liu H, Xu S, Deng Y, Xu B, Yang T
and Liu W: Ferroptosis and neurodegenerative diseases: Insights
into the regulatory roles of SLC7A11. Cell Mol Neurobiol.
43:2627–2642. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lee J and Roh JL: SLC7A11 as a gateway of
metabolic perturbation and ferroptosis vulnerability in cancer.
Antioxidants (Basel). 11:24442022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Iida Y, Okamoto-Katsuyama M, Maruoka S,
Mizumura K, Shimizu T, Shikano S, Hikichi M, Takahashi M, Tsuya K,
Okamoto S, et al: Effective ferroptotic small-cell lung cancer cell
death from SLC7A11 inhibition by sulforaphane. Oncol Lett.
21:712021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shin D, Lee J and Roh JL: Pioneering the
future of cancer therapy: Deciphering the p53-ferroptosis nexus for
precision medicine. Cancer Lett. 585:2166452024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hou CY, Suo YH, Lv P, Yuan HF, Zhao LN,
Wang YF, Zhang HH, Sun J, Sun LL, Lu W, et al: Aristolochic
acids-hijacked p53 promotes liver cancer cell growth by inhibiting
ferroptosis. Acta Pharmacol Sin. 46:208–221. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kang R, Kroemer G and Tang D: The tumor
suppressor protein p53 and the ferroptosis network. Free Radic Biol
Med. 133:162–168. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang SJ, Li D, Ou Y, Jiang L, Chen Y, Zhao
Y and Gu W: Acetylation is crucial for p53-mediated ferroptosis and
tumor suppression. Cell Rep. 17:366–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang Y, Wang M and Chang W: Iron
dyshomeostasis and ferroptosis in Alzheimer's disease: Molecular
mechanisms of cell death and novel therapeutic drugs and targets
for AD. Front Pharmacol. 13:9836232022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dwivedi D, Megha K, Mishra R and Mandal
PK: Glutathione in brain: Overview of its conformations, functions,
biochemical characteristics, quantitation and potential therapeutic
role in brain disorders. Neurochem Res. 45:1461–1480. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rohr-Udilova N, Sieghart W, Eferl R,
Stoiber D, Björkhem-Bergman L, Eriksson LC, Stolze K, Hayden H,
Keppler B, Sagmeister S, et al: Antagonistic effects of selenium
and lipid peroxides on growth control in early hepatocellular
carcinoma. Hepatology. 55:1112–1121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Imai H, Matsuoka M, Kumagai T, Sakamoto T
and Koumura T: Lipid peroxidation-dependent cell death regulated by
GPx4 and ferroptosis. Curr Top Microbiol Immunol. 403:143–170.
2017.PubMed/NCBI
|
|
92
|
Xu Y, Li K, Zhao Y, Zhou L, Liu Y and Zhao
J: Role of ferroptosis in stroke. Cell Mol Neurobiol. 43:205–222.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Magtanong L, Ko PJ and Dixon SJ: Emerging
roles for lipids in non-apoptotic cell death. Cell Death Differ.
23:1099–1109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lin KJ, Chen SD, Lin KL, Liou CW, Lan MY,
Chuang YC, Wang PW, Lee JJ, Wang FS, Lin HY, et al: Iron brain
menace: The involvement of ferroptosis in Parkinson disease. Cells.
11:38292022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shen W, Li C, Liu Q, Cai J, Wang Z, Pang
Y, Ning G, Yao X, Kong X and Feng S: Celastrol inhibits
oligodendrocyte and neuron ferroptosis to promote spinal cord
injury recovery. Phytomedicine. 128:1553802024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ward RJ, Zucca FA, Duyn JH, Crichton RR
and Zecca L: The role of iron in brain ageing and neurodegenerative
disorders. Lancet Neurol. 13:1045–1060. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi
AA and Lei P: Ferroptosis: Mechanisms and links with diseases.
Signal Transduct Target Ther. 6:492021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chaudhary S, Ashok A, McDonald D, Wise AS,
Kritikos AE, Rana NA, Harding CV and Singh N: Upregulation of local
hepcidin contributes to iron accumulation in Alzheimer's disease
brains. J Alzheimers Dis. 82:1487–1497. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li B, Xia M, Zorec R, Parpura V and
Verkhratsky A: Astrocytes in heavy metal neurotoxicity and
neurodegeneration. Brain research. 1752:1472342021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Villalón-García I, Povea-Cabello S,
Álvarez-Córdoba M, Talaverón-Rey M, Suárez-Rivero JM,
Suárez-Carrillo A, Munuera-Cabeza M, Reche-López D,
Cilleros-Holgado P, Piñero-Pérez R and Sánchez-Alcázar JA: Vicious
cycle of lipid peroxidation and iron accumulation in
neurodegeneration. Neural Regen Res. 18:1196–1202. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gugliandolo A, Bramanti P and Mazzon E:
Role of vitamin E in the treatment of Alzheimer's disease: Evidence
from animal models. Int J Mol Sci. 18:25042017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Alim I, Caulfield JT, Chen Y, Swarup V,
Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie
EJ, et al: Selenium drives a transcriptional adaptive program to
block ferroptosis and treat stroke. Cell. 177:1262–1279. e252019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li Q, Han X, Lan X, Gao Y, Wan J, Durham
F, Cheng T, Yang J, Wang Z, Jiang C, et al: Inhibition of neuronal
ferroptosis protects hemorrhagic brain. JCI insight. 2:e907772017.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ikawa T, Sato M, Oh-Hashi K, Furuta K and
Hirata Y: Oxindole-curcumin hybrid compound enhances the
transcription of γ-glutamylcysteine ligase. Eur J Pharmacol.
896:1738982021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Deepmala, Slattery J, Kumar N, Delhey L,
Berk M, Dean O, Spielholz C and Frye R: Clinical trials of
N-acetylcysteine in psychiatry and neurology: A systematic review.
Neurosci Biobehav Rev. 55:294–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hinman A, Holst CR, Latham JC, Bruegger
JJ, Ulas G, McCusker KP, Amagata A, Davis D, Hoff KG, Kahn-Kirby
AH, et al: Vitamin E hydroquinone is an endogenous regulator of
ferroptosis via redox control of 15-lipoxygenase. PLoS One.
13:e02013692018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ashraf A and So PW: Spotlight on
ferroptosis: Iron-dependent cell death in Alzheimer's disease.
Front Aging Neurosci. 12:1962020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kryscio RJ, Abner EL, Caban-Holt A, Lovell
M, Goodman P, Darke AK, Yee M, Crowley J and Schmitt FA:
Association of antioxidant supplement use and dementia in the
prevention of Alzheimer's disease by vitamin E and selenium trial
(PREADViSE). JAMA Neurol. 74:567–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Conrad M and Proneth B: Selenium: Tracing
another essential element of ferroptotic cell death. Cell Chem
Biol. 27:409–419. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ingold I, Berndt C, Schmitt S, Doll S,
Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T,
et al: Selenium utilization by GPX4 is required to prevent
hydroperoxide-induced ferroptosis. Cell. 172:409–422. e212018.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
R Cardoso B, Hare DJ, Lind M, McLean CA,
Volitakis I, Laws SM, Masters CL, Bush AI and Roberts BR: The APOE
ε4 allele is associated with lower selenium levels in the brain:
Implications for Alzheimer's disease. ACS Chem Neurosci.
8:1459–1464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Skouta R, Dixon SJ, Wang J, Dunn DE, Orman
M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A and
Stockwell BR: Ferrostatins inhibit oxidative lipid damage and cell
death in diverse disease models. J Am Chem Soc. 136:4551–4556.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Fang X, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Miotto G, Rossetto M, Di Paolo ML, Orian
L, Venerando R, Roveri A, Vučković AM, Bosello Travain V, Zaccarin
M, Zennaro L, et al: Insight into the mechanism of ferroptosis
inhibition by ferrostatin-1. Redox Biol. 28:1013282020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Asano M, Yamasaki K, Yamauchi T, Terui T
and Aiba S: Epidermal iron metabolism for iron salvage. J Dermatol
Sci. 87:101–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kalyanaraman B: NAC, NAC, Knockin' on
Heaven's door: Interpreting the mechanism of action of
N-acetylcysteine in tumor and immune cells. Redox Biol.
57:1024972022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M,
Buchfelder M and Savaskan N: Nrf2-Keap1 pathway promotes cell
proliferation and diminishes ferroptosis. Oncogenesis. 6:e3712017.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kerins MJ and Ooi A: The roles of NRF2 in
modulating cellular iron homeostasis. Antioxid Redox Signal.
29:1756–1773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Rojo de la Vega M, Chapman E and Zhang DD:
NRF2 and the Hallmarks of cancer. Cancer Cell. 34:21–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hara Y, McKeehan N, Dacks PA and Fillit
HM: Evaluation of the neuroprotective potential of N-acetylcysteine
for prevention and treatment of cognitive aging and dementia. J
Prev Alzheimers Dis. 4:201–206. 2017.PubMed/NCBI
|
|
121
|
Pocernich CB, La Fontaine M and
Butterfield DA: In-vivo glutathione elevation protects against
hydroxyl free radical-induced protein oxidation in rat brain.
Neurochem Int. 36:185–191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Koppal T, Drake J and Butterfield DA: In
vivo modulation of rodent glutathione and its role in
peroxynitrite-induced neocortical synaptosomal membrane protein
damage. Biochim Biophys Acta. 1453:407–411. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Pocernich CB, Cardin AL, Racine CL,
Lauderback CM and Butterfield DA: Glutathione elevation and its
protective role in acrolein-induced protein damage in synaptosomal
membranes: Relevance to brain lipid peroxidation in
neurodegenerative disease. Neurochem Int. 39:141–149. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Prasad S, Tyagi AK and Aggarwal BB: Recent
developments in delivery, bioavailability, absorption and
metabolism of curcumin: the golden pigment from golden spice.
Cancer Res Treat. 46:2–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wei Z, Shaohuan Q, Pinfang K and Chao S:
Curcumin attenuates ferroptosis-induced myocardial injury in
diabetic cardiomyopathy through the Nrf2 pathway. Cardiovasc Ther.
2022:31597172022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hirata Y, Ito Y, Takashima M, Yagyu K,
Oh-Hashi K, Suzuki H, Ono K, Furuta K and Sawada M: novel
oxindole-curcumin hybrid compound for antioxidative stress and
neuroprotection. ACS Chem Neurosci. 11:76–85. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hirata Y, Tsunekawa Y, Takahashi M,
Oh-Hashi K, Kawaguchi K, Hayazaki M, Watanabe M, Koga KI, Hattori
Y, Takemori H and Furuta K: Identification of novel neuroprotective
N, N-dimethylaniline derivatives that prevent oxytosis/ferroptosis
and localize to late endosomes and lysosomes. Free Radic Biol Med.
174:225–235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hirata Y, Okazaki R, Sato M, Oh-Hashi K,
Takemori H and Furuta K: Effect of ferroptosis inhibitors
oxindole-curcumin hybrid compound and N, N-dimethylaniline
derivatives on rotenone-induced oxidative stress. Eur J Pharmacol.
928:1751192022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li Y, Sun M, Cao F, Chen Y, Zhang L, Li H,
Cao J, Song J, Ma Y, Mi W and Zhang X: The ferroptosis inhibitor
liproxstatin-1 ameliorates LPS-induced cognitive impairment in
mice. Nutrients. 14:45992022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fan BY, Pang YL, Li WX, Zhao CX, Zhang Y,
Wang X, Ning GZ, Kong XH, Liu C, Yao X and Feng SQ: Liproxstatin-1
is an effective inhibitor of oligodendrocyte ferroptosis induced by
inhibition of glutathione peroxidase 4. Neural Regen Res.
16:561–566. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Singh VK, Beattie LA and Seed TM: Vitamin
E: Tocopherols and tocotrienols as potential radiation
countermeasures. J Radiat Res. 54:973–988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Angeli JPF, Shah R, Pratt DA and Conrad M:
Ferroptosis inhibition: mechanisms and opportunities. Trends
Pharmacol Sci. 38:489–498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang ZH, Chen C, Jia SZ, Cao XC, Liu M,
Tian J, Hoffmann PR, Xu HX, Ni JZ and Song GL: Selenium restores
synaptic deficits by modulating NMDA receptors and selenoprotein K
in an Alzheimer's disease model. Antioxid Redox Signal. 35:863–884.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Bao C, Liu C, Liu Q, Hua L, Hu J, Li Z and
Xu S: Liproxstatin-1 alleviates LPS/IL-13-induced bronchial
epithelial cell injury and neutrophilic asthma in mice by
inhibiting ferroptosis. Int Immunopharmacol. 109:1087702022.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Pei Z, Qin Y, Fu X, Yang F, Huo F, Liang
X, Wang S, Cui H, Lin P, Zhou G, et al: Inhibition of ferroptosis
and iron accumulation alleviates pulmonary fibrosis in a bleomycin
model. Redox Biol. 57:1025092022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Li Z, Lu Y, Zhen Y, Jin W, Ma X, Yuan Z,
Liu B, Zhou XL and Zhang L: Avicularin inhibits ferroptosis and
improves cognitive impairments in Alzheimer's disease by modulating
the NOX4/Nrf2 axis. Phytomedicine. 135:1562092024. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wu Y, Wei M, Wang M, Guo M, Yu H, Chen Y,
Xu T and Zhou Y: Schisandra total lignans ameliorate neuronal
ferroptosis in 3×Tg-AD mice via regulating NADK/NADPH/GSH pathway.
Phytomedicine. 140:1566122025. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Li X, Chen J, Feng W, Wang C, Chen M, Li
Y, Chen J, Liu X, Liu Q and Tian J: Berberine ameliorates iron
levels and ferroptosis in the brain of 3 × Tg-AD mice.
Phytomedicine. 118:1549622023. View Article : Google Scholar : PubMed/NCBI
|