Introduction
Most physiological processes are associated with
mechanical forces and cells can sense whether the mechanical forces
of the microenvironment have changed and can make proper adaptation
in response to the changes. Mechanotransduction is mediated by a
wide variety of mechanosensitive channel proteins found in cells.
Piezo type mechanosensitive ion channel component 1 (Piezo1) is a
novel mechanosensitive cation channel discovered by Coste et
al (1) in a mouse
neuroblastoma cell line in 2010. The human Piezo1 gene, Fam38a, is
located in chromosome 16q24.3 and contains 51 exons and 2,520 amino
acids (1). Piezo1 is a
transmembrane protein and the Piezo protein family has a unique
sequence that lacks sequence homology with any other known cation
channel protein families (2).
As a part of the cellular response to mechanical
forces, Piezo1 senses mechanical stress and triggers inflammation
(3). In contrast to Transient
Receptor Potential Vanilloid 4 (TRPV 4), which is activated in
response to mechanical loads at physiological levels, Piezo1 is
activated in response to supraphysiological mechanical deformation
(>50% cell deformation) and acts directly by physically
deforming and opening channels through increased cell membrane
tension (4,5). In addition, Piezo1 is able to respond
to a variety of mechanical stimuli and convert mechanical stimuli
into intracellular signaling cascades in multiple systems, such as
the circulatory and respiratory systems, which influence the
development of chronic inflammatory diseases (6). For example, both TRPV4 and Piezo1 are
involved in mediating deflection-gated currents in chondrocytes,
but TRPV4 cannot be effectively gated by pressure-induced membrane
stretch and only Piezo1 mediates stretch-activated currents
(7). This suggests that Piezo1
plays a more extensive role in the mechanotransduction of
inflammatory responses. The present study analysed how Piezo1
conducts mechanical stimuli to modulate inflammatory responses in
osteoarthritis, atherosclerosis, pulmonary inflammation,
periodontitis and Alzheimer's disease and summarizes agonists and
antagonists that modulate Piezo1 activity and give a further
exploration of their potentials for clinical treatment.
Basic structure and function of Piezo1
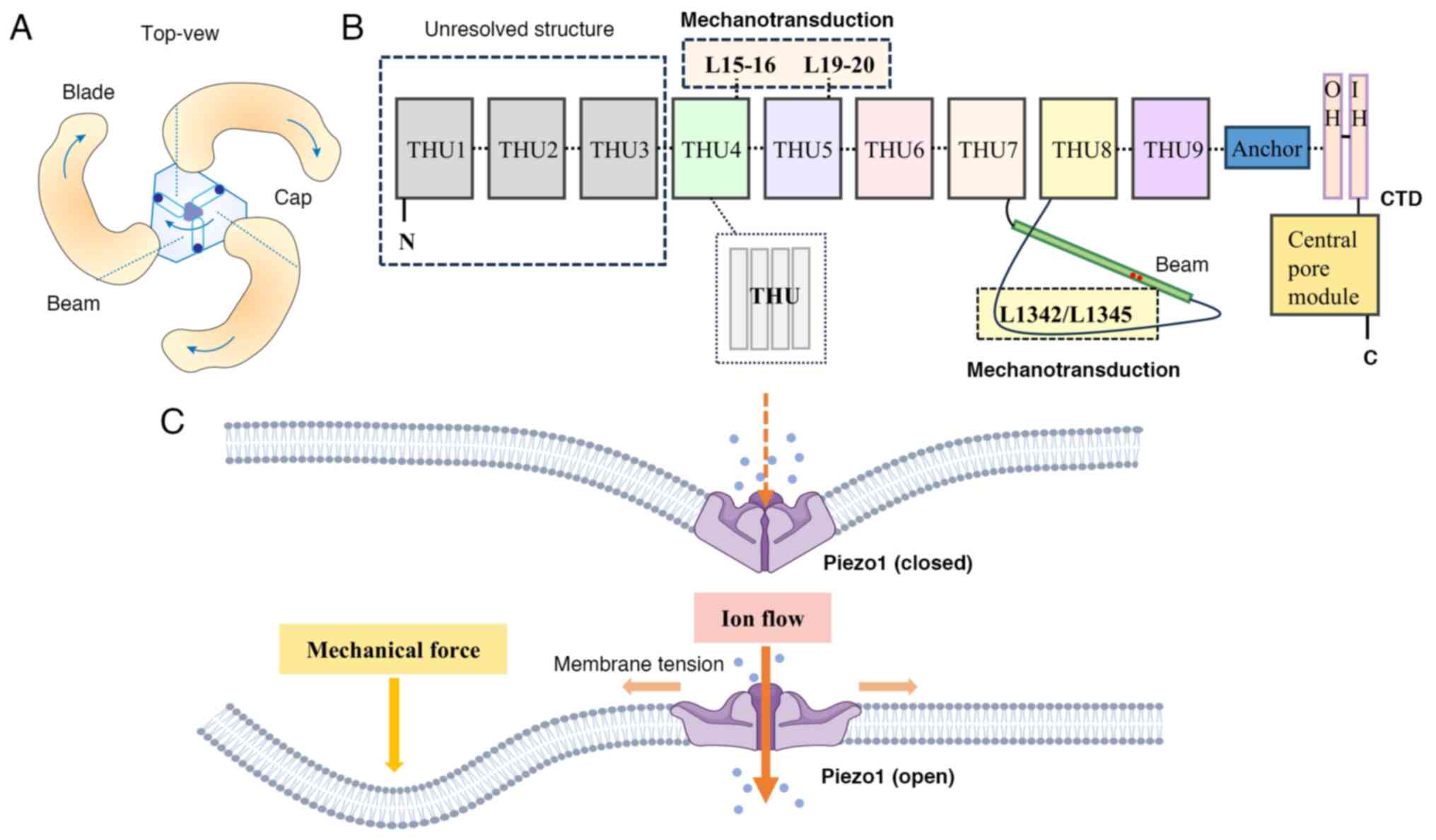
Using cryo-electron microscopy, researchers have
found that the structure of the Piezo1 protein is a three-bladed
propeller-like trimer consisting of a unique central pore domain
and three peripheral blade-like propellers (8) (Fig.
1A). Piezo1 is encapsulated in the lipid bilayer, where the
three propeller-like structural domains extend outward in the lipid
bilayer. Each blade-like propeller contains a unique 38
transmembrane α-helices, which can be divided into three parts
based on their structural and functional characteristics: the
N-terminal blade in the mechanosensory module, the C-terminal
ion-conducting pore module and the transactivation module
consisting of the anchor and the beam (9). Specifically speaking, the N-terminal
contains nine repetitive folded structures with four α-helices
(transmembrane α-helices 1–36) called transmembrane helical units
(THUs), which serve as the backbone of each blade (8). The remaining two α-helices (37 and
38) at the C-terminal, referred to as the inner helix (IH) and
outer helix (OH) (10), constitute
the C-terminal intracellular structural domain (CTD) with the
central pore module of Piezo1. At the top of the central pore,
there exists a cap of negatively charged residues consisting of the
C-terminal extracellular structural domains (CED) and deletion of
the cap structure or restriction of the movement of the cap
structure prevents the channel from opening, suggesting that the
conformational changes of the cap structure is necessary for Piezo1
to perform its function of mechanical gating (11). The anchor domain consists of three
helices (α1, α2 and α3) and serves as a bridge connecting THU9 and
OH-IH (12). The beam is located
on the inner surface of the cell and connects THU7 and THU8 to the
center pore domain, supporting the blade-like propeller (9). The beam structure contains a
convoluted helical motif LAQLKRQM (1341–1348) near the CTD, in
which mutating L1342 and L1345 decreases the mechanosensitivity of
Piezo1 and markedly reduces poking-induced currents, suggesting
that L1342 and L1345 are important for the mechanical activation of
Piezo1 (9). Further studies have
reveals that L1342 and L1345 acts as fulcrums to form a lever-like
structure that effectively amplifies distant mechanical stimuli and
ensures selective cation penetration (13) (Fig.
1B).
Piezo1 is mainly located on plasma membranes such as
endoplasmic reticulum, cytoplasmic compartment and nuclear envelope
near the nucleus (9). The
existence of Piezo1 enables cells to sense such mechanical forces
as radial force, membrane stretch, compression, shear stress,
matrix stiffness and osmotic pressure. When these mechanical forces
act on the cell membrane, Piezo1 is induced to shift from the
closed state to the open state, allowing the passage of cations
such as Ca2+, K+ and Na+ and
regulating cellular physiological activities such as protein
synthesis, secretion and cell migration, differentiation,
proliferation and apoptosis (5)
(Fig. 1C). When cells are
subjected to non-physiological mechanical stimuli that damage
tissues and induce inflammation, the progression of inflammation is
often accompanied by alterations in the mechanical forces of the
microenvironment. Piezo1, as a mechanosensitive channel, plays an
important role in the onset, development and prognosis of
inflammation. For example, in aortic stenosis, monocytes sense
shear stress and activate via Piezo1, adhering to endothelial cells
and leading to valve inflammation (14). Piezo1 on macrophages is stimulated
by cyclic hydrostatic pressure (CHP) to promote expression of
inflammatory factors and macrophage M1-type polarization,
exacerbating the inflammatory response in the lungs (15). In addition, inflammatory responses
around bone tissue are often accompanied by alterations in
osteoblast activity, leading to pathological changes in bone and
its surrounding structures. Piezo1 mediates mechanical load in
osteoblasts and coordinates osteoblast-osteoclast crosstalk in bone
to maintain bone mass in vivo (16). In osteoarthritis, Piezo1 induces
the chondrocyte apoptosis and the release of inflammatory factors
that destroy articular cartilage (17). In chronic inflammation, Piezo1
senses changes in microenvironmental homeostasis and regulates
cellular function and its dysfunction tends to accelerate the
development of chronic inflammation (Fig. 2).
Association of Piezo1 with chronic
inflammatory diseases
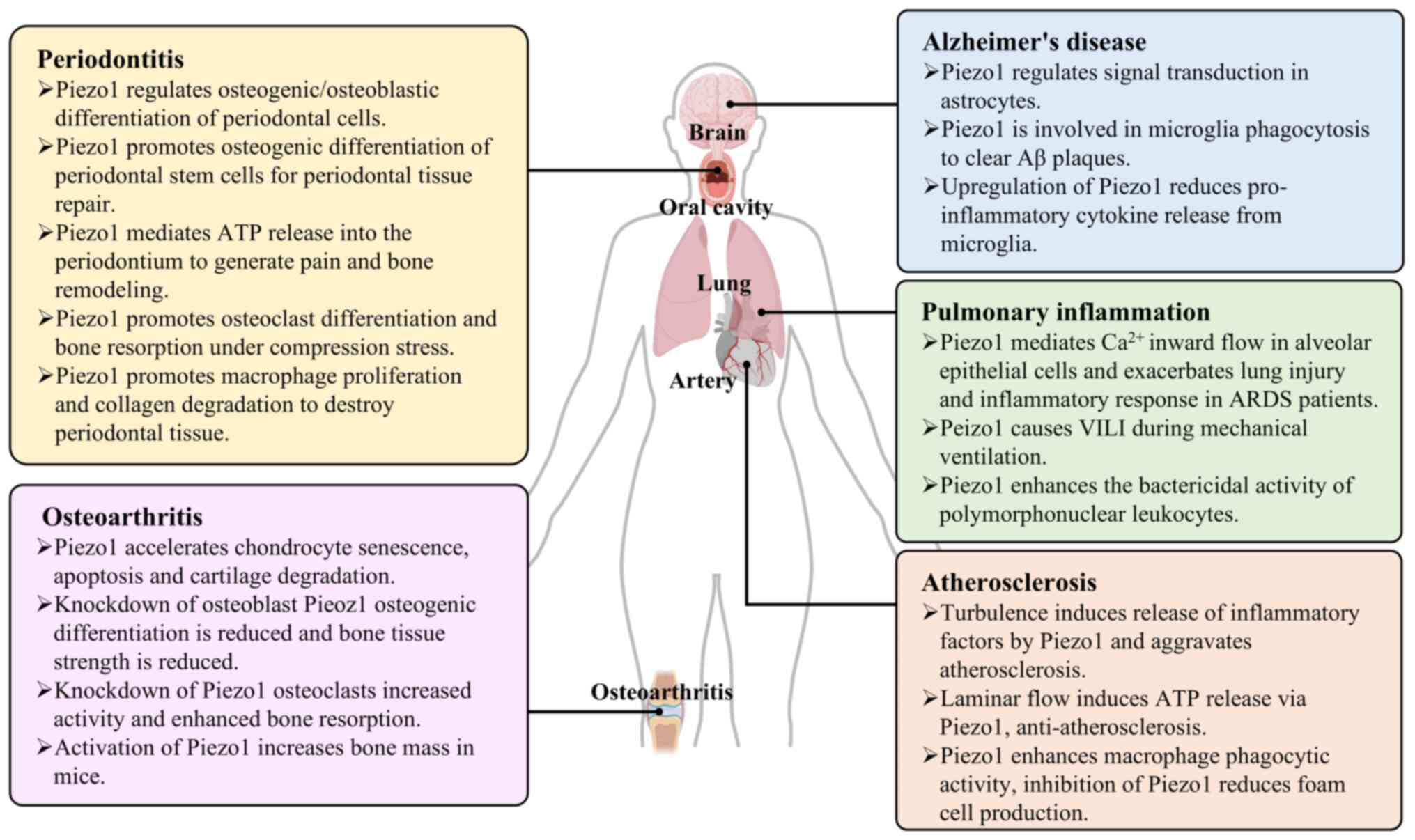
Osteoarthritis (OA)
OA is a common degenerative disease of the joints.
In addition to destroying articular cartilage, OA is now more
widely recognized as a lesion of the entire joint. Inflammatory
exudation leads to increased intra-articular mesenchymal fluid and
increased pressure in the joint cavity, initiating apoptosis and
the inflammatory program (18). A
study has shown that Piezo1 is expressed in chondrocytes,
osteoblasts and osteoclasts and regulates the onset and progression
of OA by mediating mechano-biological signaling (19).
Mechanical stress at physiologic level is the basis
for the normal functioning of bones and joints and excessive
mechanical loading of bones causes inflammation and degeneration.
The ionic homeostasis of internal environment is the basis for
chondrocytes to exercise normal functions. In OA, the expression of
Piezo1 in chondrocytes is upregulated under supraphysiological
levels of mechanical stimulation and Ca2+ signaling is
continuously enhanced, which ultimately leads to apoptosis
(20). Another study notes that
excessive apoptosis in chondrocytes under excessive mechanical
stress stimulation is mediated by Piezo1-mediated downstream
signaling molecules MAPK/ERK5 and MAPK/ERK1/2: This process
produces a large number of oxygen radicals and inflammatory
mediators (for example, IL-1b and TNF-α), which damage the new
chondrogenic tissues and blood vessels and further aggravate the
apoptotic death of chondrocytes, thus forming a vicious cycle
(17). The use of the Piezo1
inhibitor GsMTx4 delays the progression of osteoarthritis (17). The microRNA (miR)-155-5p is an mRNA
associated with cell proliferation, differentiation and
inflammatory responses. Activation of Piezo1 also leads to the
upregulation of miR-155-5p, which brings about the downregulation
of the downstream target gene GDF6 and accelerates chondrocyte
senescence and cartilage degradation and induces inflammatory
responses to disrupt bone and joint homeostasis (21). Reintroducing GDF6 into overloaded
chondrocytes reverses the negative effects of inflammation, such as
collagen loss and impaired chondrocyte proliferation (21).
Piezo1 is also involved in regulating the cellular
activities of osteoblasts and osteoclasts and modulating the
development of OA (22). In
osteoblasts, Piezo1 senses mechanical loads, which is important for
cell proliferation, migration, differentiation and bone formation.
An in vitro study has shown that under shear stress
stimulation, expression of Piezo1 increases, activating the
AKT/GSK-3β/β-catenin signaling pathway and promoting the expression
of the osteoblast gene Runx2 (23). The knockout of Piezo1 in osteoblast
lineage cells impairs osteogenesis, resulting in structural
disruption and reduced strength of bone (23). Meanwhile, Piezo1-deficient
osteoblastic cells are also able to increase the number and
activity of osteoblasts by regulating the Yes-associated protein
(YAP)-dependent expression of type II and type IX collagen, which
enhances bone resorption, leading to further bone loss and
spontaneous fractures (24). The
use of the Piezo1 agonist Yoda1 increases in vivo bone mass
and expression of bone formation markers in mice (25). Notably, in the absence of long-term
mechanical loading, bone mass and bone strength also rapidly
decrease. Piezo1-deficient mice are resistant to bone loss and
resorption caused by lack of mechanical loading (26).
These findings reveal the role of Piezo1 in
chondrocyte apoptosis and osteoblast-osteoclast crosstalk,
providing a potential therapeutic target for OA.
Atherosclerosis
Atherosclerosis (AS) is an inflammatory disease
caused by multiple factors such as obesity, hypertension, diabetes
and hyperlipidemia, and vascular endothelial cell injury is the
initiating factor of AS (27).
Subsequently, cholesterol and lipids in the blood are deposited
under the endothelial cells, attracting monocytes to aggregate and
then differentiate into macrophages, which phagocytose lipids to
convert into foam cells and secrete pro-inflammatory factors,
leading to inflammatory reactions (28). During this process, vascular
endothelial cells are continuously subjected to shear stress from
blood flow. A study has shown that the expression of Piezo1
increases markedly in carotid plaque tissues of AS mice and is
involved in several response processes in AS, such as vascular
endothelial cell injury and macrophage activation (29).
It has been found that Piezo1 is abundantly
expressed in vascular endothelial cells and has both injurious and
anti-injurious effects, depending on the type of blood flow
signaling to which the vascular endothelial cells are subjected
(30). Blood flow is categorized
into laminar and turbulent flow, with laminar flow leading to
nitric oxide (NO) formation and the endothelial barrier acting as a
protective shield against inflammation and turbulent flow leading
to vasoconstriction, endothelial barrier disruption and
atherosclerosis development (31).
Cells in turbulence are subjected to forces in random directions
that activate Piezo1, which induces inflammatory signaling through
integrin-associated PECAM-1/VE-calmodulin/VEGFR2 and PI3K-dependent
activation, further leading to FAK-dependent nuclear factor κB
(NF-κB) activation (32). The
activation of NF-κB promotes the leukocyte adhesion molecule
VCAM-1, ICAM-1 and chemokine CCL2 expression, thus promoting AS
development (33). By contrast,
vascular endothelial cells in continuous laminar flow are subjected
to shear forces only in the direction of the cytosolic long-axis,
which induces the release of ATP via Piezo1 and activates P2Y
purinoceptor 2 (P2Y2) receptors and Gq/G11-mediated
signaling. This in turn leads to the phosphorylation of protein
kinase B (AKT) and release endothelial nitric oxide synthase
(eNOS), thus demonstrating the anti-atherosclerotic process
(32,34). Meanwhile, a certain concentration
of oxidized low-density lipoprotein (ox-LDL) induces the expression
of Piezo1 in endothelial cells, activates YAP and transcriptional
coactivator with PDZ-binding motif (TAZ) and enhances JNK signaling
pathway to promote inflammation and AS progression (35). The pharmacological inhibition of
Piezo1 or the knockdown of the Piezo1 gene effectively reduces
atherosclerotic plaque formation and attenuates the atherosclerotic
inflammatory response in vascular endothelial cells (30).
Piezo1 has also been confirmed to be highly
expressed in monocytes (36).
Atherosclerotic plaques lead to arterial stenosis and increased
shear stress of blood flow, which promotes the activation of a
series of monocytes through Piezo1 and enhances macrophage
phagocytic activity, ox-LDL uptake and cytokine expression of
monocytes (37). The knockdown of
Piezo1 gene is able to reduce atherosclerotic plaque formation
(38). Transcatheter aortic valve
implantation (TAVI) is an effective treatment for aortic stenosis.
As the mechanism of the role of Piezo1 in the development of AS is
becoming clearer, researchers have found that TAVI reduces
Piezo1-mediated activation of monocyte and exerts an
anti-inflammatory effect (14). In
addition, Kaempferol inhibits foam cell formation and ameliorates
AS by inhibiting Piezo1 channels on macrophages and Ca2+
endocytosis to regulate MAPK/NF-κB and NFE2-related factor 2
(Nrf2)/heme oxygenase-1 (HO-1) downstream signaling (39).
In conclusion, Piezo1 promotes inflammatory
responses in vascular endothelial cells and monocyte activation in
AS and inhibition of Piezo1 can delay the progression of AS. Piezo1
has promising treatment prospects in AS researches.
Pulmonary inflammation and lung
injury
Mechanical stress plays a crucial role in the
development, functional maturation and pathogenesis of lung tissue.
For example, alveolar epithelial cells are predominantly exposed to
mechanical stress during respiration and vascular endothelial cells
are mainly exposed to shear stress, strain and hydrostatic
pressure, both of which play an important role in the perception of
mechanical stress in lung tissue (40). It has been shown that Piezo1 is
highly expressed in alveolar epithelial cells, endothelial cells
and monocyte macrophages in response to lung mechanical stress and
that it participates in pulmonary inflammation through multiple
mechanisms (41).
Piezo1 is one of the major ion channels mediating
Ca2+ endocytosis in alveolar epithelial cells. In acute
respiratory distress syndrome (ARDS), mechanical stress induces the
activation of Piezo1, which mediates apoptosis of type II alveolar
cells through the Bcl-2 pathway and induces abnormal secretion of
alveolar surface-active substances, thereby exacerbating lung
injury and inflammation in ARDS patients. The inhibition of Piezo1
attenuates these responses (42).
Piezo1 in human pulmonary microvascular endothelial cells
participates in the mechanism of ventilator-associated lung injury
(VILI) and cyclic stretch induces cell apoptosis in mechanical
ventilation therapy for ARDS by activating the RhoA/ROCK1 signaling
pathway (43). Blockade of Piezo1
reduces the concentrations of TNF-α, IL-1β and IL-6 and attenuates
the inflammatory response in lung tissues of rats with VILI
(43). In addition, Piezo1 can
induce the detachment of AREG protein from the cell surface, which
upregulates metalloproteinase ADAM10 and ADAM17 activity, further
breaking down intercellular junction proteins and causing secondary
damage to the lung endothelial barrier and VILI (44).
Piezo1 responds to CHP in the lungs, mediating
inflammatory responses in lung immune cells. The sensing of CHP
through Piezo1 in lung monocytes promotes the activation
Ca2+-activating protein-1 (AP-1) and transcription of
endothelin-1 (EDN1), leading to the stabilization of HIF1α, which
triggers the pro-inflammatory state during pulmonary infection
(45). However, the activation of
Piezo1 in polymorphonuclear leukocytes upregulates nicotinamide
adenine dinucleotide phosphate oxidase 4 which enhances
bactericidal activity and thus promotes the resolution of bacterial
pneumonia (46). Moreover, in
group 2 innate lymphoid cells (ILC2) in the lung, Piezo1 reduces
ILC2 oxidative metabolism, thereby inhibiting ILC2 mediated type 2
inflammation (47).
Therefore, Piezo1 is expected to be a therapeutic
target for lung inflammation and the use of Piezo1 modulators to
regulate the function of alveolar epithelial cells and monocyte
macrophages offers a possible option for the treatment of lung
inflammation.
Periodontitis
Periodontitis is an inflammatory and destructive
disease caused by plaque microorganisms on tooth-supporting
tissues, destroying periodontal ligament, alveolar bone and dental
bone and is the leading cause of tooth loss in adults. In addition
to oral bacteria, excessive mechanical forces such as occlusal
trauma promotes the progression of periodontitis and exacerbate
alveolar bone resorption (48).
Periodontal ligament cells (PDLCs) play an important
role in maintaining periodontal homeostasis and regulating
periodontal tissue remodeling. PDLCs are mechanically stimulated to
produce a variety of inflammatory factors including prostaglandins,
leukotrienes, IL-1, IL-6 and TNF-α (49). Piezo1 plays an important role in
the perception of mechanical stimuli in PDLCs. Piezo1 enhances the
expression of osteogenesis-related genes RUNX2 and OSX under
compressive stress and regulates PDLCs through the Wnt/β-catenin
pathway (50). Piezo1 is also
involved in compressive stress-induced osteoclast formation through
the NF-κB signaling pathway in PDLCs (51). A further study has shown that
compressive stress stimulates the expression of RANKL and decreases
the expression of osteoprotegerin (OPG) in PDLCs, thereby promoting
RANKL-mediated osteoclastogenesis by increasing the RANKL/OPG ratio
(50). Low-intensity pulsed
ultrasound is able to downregulate the expression of Piezo1 in
PDLCs and reduce alveolar bone resorption under compressive
stresses (52). It may be a
therapeutic tool to reduce bone resorption in periodontitis.
PDLCs contain a variety of cell types, including
periodontal ligament stem cells (PDLSCs) and periodontal ligament
fibroblasts (PDLFs), which are also important for maintaining
periodontal homeostasis. PDLSCs are capable of regenerating
osteoid-like and periodontal membrane-like tissues and promoting
periodontal tissue repair and have pro-periodontal regeneration
potential (53). A study
demonstrated by an in vitro mechanical tension stress cell
model shows that mechanical tension stress upregulates the
expression of Piezo1, activates the Notch1 signaling pathway,
increases the expression of osteogenic genes and promotes the
osteogenic differentiation of PDLSCs (54). PDLFs play an important role in
alveolar bone remodeling by increasing the formation of osteoclast
in response to inflammation-induced or mechanical force stimuli
(55). In vitro studies
have shown that PDLFs activate Piezo1 after mechanical stimulation,
which mediates Ca2+ endocytosis and releases ATP to the
periodontium (56). ATP activates
multiple receptors that produce pain and bone remodeling (57).
In addition, macrophages also play a crucial role in
the inflammatory response and alveolar bone resorption in
periodontitis. Appropriate mechanical stimulation induces
macrophage polarization toward M2 type through Piezo1-mediated p53
acetylation and deacetylation and releases TGF-β1 to promote
osteogenic differentiation of bone marrow mesenchymal stem cells
(58). However, under
non-physiological mechanical stimulation, macrophages express high
levels of Piezo1, which elicits an inflammatory response and
promotes osteoclast differentiation and bone resorption.
Specifically, high expression of Piezo1 increases the expression of
cell cycle protein D1 (Ccnd1), a potential downstream effector of
the AKT/GSK3β signaling pathway, promoting macrophage proliferation
(59). In addition to this,
macrophages in periodontitis tissues mediate the degradation of
collagen in gingival fibroblasts by Piezo1-mediated matrix
metalloproteinases (MMPs), thereby destroying periodontal tissues
(60).
In brief, Piezo1 regulates osteogenic/osteoclastic
differentiation of PDLCs and macrophages under mechanical
stimulation and is an important mechanotransduction channel in
periodontal destruction and alveolar bone resorption in
periodontitis.
Alzheimer's disease (AD)
AD is a progressive neurodegenerative disease in
which amyloid-β (Aβ) plaques are deposited in cells of normal brain
tissue leading to increased hardness of the brain matrix under the
microscope and ultimately causing necrosis of nerve cells and brain
tissue damage (61). Neuroglial
cells are another large group of cells in the nervous system
besides neurons, which are highly mechanosensitive during their
growth and respond rapidly to changes in the stiffness of the
surrounding environment through mechanosensitive ion channels
(62).
Astrocytes, the most abundant glial cell type in the
brain, exhibit a ‘reactive’ phenotype with increased intermediate
filament expression in response to amyloid plaque deposition
(63). In the brains of AD
patients, glial cells are the most abundant type of glial cell in
the brain and astrocytes are more reactive and release more
pro-inflammatory cytokines (64).
Peripheral infections and aging upregulate Piezo1 expression in
reactive astrocytes and this upregulation is not detected in non-AD
cells (62). Astrocytes upregulate
Piezo1 channels in response to lipopolysaccharide (LPS), attempting
to inhibit the release of pro-inflammatory cytokines and suppress
neuroinflammation (65). Piezo1
may potentially regulate signaling in reactive astrocytes, thereby
influencing astrocyte phenotype.
Microglia are phagocytic and scavenging, capable of
removing dying neurons and engulfing abnormal protein or lipid
plaques in neurodegenerative diseases. In AD, microglia
proliferate, activate and accumulate around Aβ plaques and invade
their nuclei to phagocytose and remove Aβ plaques (66). Through researchers have found that
on the one hand, microglia express an innate immune receptor, TLR4,
which is activated by Aβ plaques and infection-associated bacterial
LPS, while upregulating Piezo1 (67). Piezo1 synergizes with TLR signaling
to induce Ca2+ endocytosis and activate the
CaMKII-Mst1/2-Rac axis that exerts phagocytic and scavenging
effects (68). On the other hand,
Aβ plaques may directly upregulate Piezo1 in microglia, thereby
affecting the immunoreactivity of microglia. It has been
demonstrated that Piezo1 is highly expressed in the cell membrane
and nucleus of microglia derived from artificially induced
pluripotent stem cells. When Piezo1 is activated by the agonist
Yoda1, the functional phenotype of microglia is altered and its
migration, phagocytosis and lysosomal activity are enhanced,
thereby assisting the clearance of Aβ plaques from the body
(69). Aβ plaques stimulate
microglia to release a variety of pro-inflammatory cytokines,
leading to neuroinflammation, neuronal dysfunction and death. In
addition to its involvement in the clearance of Ab plaques by
microglia, Piezo1 is also involved in the inflammatory activation
of microglia. In LPS-induced upregulation of Piezo1, Piezo1 reduces
LPS induced pro-inflammatory cytokines TNF-α, IL-1β and IL-6
through the expression of JNK1 and mTOR signaling pathways
(70). Polyunsaturated fatty acid
ω3-PUFA upregulates miR-107 to inhibit LPS-induced Piezo1
activation and NF-κB p65 signaling pathway, providing a potential
treatment for neuroinflammation (71).
In summary, Piezo1 regulates signal transduction in
reactive astrocytes in the brains of AD patients, assists microglia
in phagocytosis to clear Aβ plaques and reduces the release of
inflammatory factors. It may hopefully become a novel drug target
for the treatment of AD in the future.
Mechanism of Piezo1 transduction of
inflammatory signaling
The concentration of free Ca2+ in the
cytoplasm is much lower than that in the extracellular and various
cellular stimuli increase the concentration of free Ca2+
in the cytoplasm (72). Based on
the localization of Piezo1 in cells, activation of Piezo1 not only
triggers the flow of extracellular Ca2+ to the
intracellular compartment, but also promotes the release of
Ca2+ from calcium stores, resulting in an increase in
intracellular Ca2+ concentration (65). As a second messenger,
Ca2+ plays direct and robust roles in a number of
biological processes, as an increasing number of studies have
reported over the last decades (73,74).
The enhancement of Ca2+ signaling induces inflammatory
responses and other cellular events through signaling cascades.
Ca2+ activates downstream effector molecules (such as
CaMK kinase family and transcription factor NFAT) by binding to
calmodulin (73). Ca2+
collaborates with calcium channels (such as TRP and Piezo) and
endoplasmic reticulum/mitochondrial storage systems to regulate key
physiological processes, such as cell proliferation, apoptosis and
immune response (74).
Piezo1-mediated Ca2+ signaling participates in the
inflammation through a variety of signaling pathways.
There are obvious differences in the role and
downstream mechanisms of Piezo1 in various tissue inflammations
(Table I). MAPK/ERK signaling
pathway is an important intracellular signaling pathway involved in
the regulation of various biological processes such as cell
proliferation, differentiation, migration and apoptosis. A study
has shown that Piezo1 regulates inflammatory responses by
activating the MAPK/ERK signaling pathway. For instance, in
chondrocytes, Piezo1 activation promotes apoptosis through the
classical MAPK/ERK1/2 signaling pathway (17). Specifically, mechanical stress
activates Piezo1, leading to an increase in intracellular
Ca2+ concentration. This in turn activates ERK1/2, which
ultimately causes changes in apoptosis-related genes Bcl-2, Bax and
caspase-3, leading to apoptosis.
 | Table I.Research on Piezo1 in chronic
inflammatory diseases. |
Table I.
Research on Piezo1 in chronic
inflammatory diseases.
| First author/s,
year | Disease | Factors activating
Piezo1 |
Animals/cells/tissues | Possible
mechanisms | Negative regulation
of Piezo1 | Results | (Refs.) |
|---|
| Li et al,
2016 | OA | Compressive
stress | Chondrocytes | Activates
MAPK/ERK1/2 signaling pathway, upregulates apoptosis related genes
Bcl-2, Bax, and caspase-3 in the nucleus | GsMTx4 | Reduces apoptosis
of chondrocytes | (17) |
| Qin et al,
2024 | OA | Compressive
stress |
Mice/chondrocytes/joint cartilage |
Piezo1/miR-155-5p/GDF6/ SMAD2/3 axis,
upregulates miR-155-5p and downregulates GDF6 | Dooku1 | Decreases
miR-155-5p expression, ameliorated OA deterioration | (21) |
| Albarrán-Juárez
et al, 2018 | AS | Oscillatory
flow | Mice/human
umbilical artery endothelial cells |
Gq/G11-mediated
PECAM-1/VE-calmodulin/VEGFR2 and PI3-kinase-dependent activation,
activate FAK-dependent NF-κB pathway | siRNA-mediated
knockdown | Reduces
inflammation and AS progression | (32) |
| Pourteymour et
al, 2024 | AS | Yoda1 | Mice/monocyte
differentiated macrophages | Mitochondrial DRP1
phosphorylation, mtROS production, mitochondrial fragmentation | siRNA-mediated
knockdown | Reduces the
expression of anti-inflammatory and anti-apoptotic molecules | (37) |
| Huang et al,
2021 | VILI | Cyclic stretch | Rats/human
pulmonary microvascular endothelial cells | Activates RhoA/ROCK
pathway | GsMTx4,
siRNA-mediated knockdown | Alleviates the
inflammatory reaction of lung tissue | (43) |
| Solis et al,
2019 | Bacterial
pneumonia | CHP | Mice/monocytes | Activates AP-1,
stabilizes HIF1, secrets EDN1 and CXCL2 | Knockout | Reduces pulmonary
inflammation | (45) |
| Mukhopadhyay et
al, 2024 | Bacterial
pneumonia | Tension |
Mice/polymorphonuclear leukocytes | HIF1α-dependent
expression of the NADPH oxidase isoform NOX4 gene | Knockout | Severe
infection | (46) |
| Hurrell et
al, 2024 | Allergic
asthma | Yoda1 | Mice/group 2 innate
lymphoid cells | KLF2-mediated
inhibition of NF-κB signaling and ILC2 cytokine secretion | Knockout | Severe
infection | (47) |
| Jiang et al,
2024 | Periodontitis | Compressive
force | Rats/PDLCs | Increases the
RANKL/OPG ratio; activates Wnt/β-catenin signaling pathway | GsMTx4,
siRNA-mediated knockdown | Reduces bone
resorption and osteogenesis | (50) |
| Jin et al,
2015 | Alveolar bone
injury | Compressive
force | Periodontal
ligament cells (PDLCs) | nuclear
translocation of NF-κB | GsMTx4 | Reduces mechanical
stress-induced osteoclastogenesis | (51) |
| Xu et al,
2022 | Periodontitis | Mechanical
tension |
Mice/macrophage | Activates
Piezo1-PI3K/AKT-Ccnd1 axis | GsMTx4 | Reduces macrophage
proliferation | (59) |
| Zhao et al,
2023 | Periodontitis | LPS | Macrophage | Generates more ROS
via Piezo1, causing oxidative stress and enhancing MMPs
secretion | GsMTx4 | Reduces
pro-inflammatory cytokines | (60) |
| Velasco-Estevez
et al, 2020 | AD | LPS | Astrocytes | Regulates
intracellular Ca2+ signaling | GsMTx4 | Enhances cell
migration | (65) |
| Jäntti et
al, 2022 | AD | Yoda1 | Mice/microglia | Mediates
Ca2+ influx, enhances lysosomal activity | None | None | (69) |
| Liu et al,
2021 | AD | LPS | Microglia | Inhibits JNK1 and
mTOR pathway | GsMTx4,
siRNA-mediated knockdown | Alleviates
high-glucose cytotoxicity and restores microglial immune
function | (70) |
| Liu et al,
2024 | AD | LPS |
Mice/astrocytes/microglia | Inhibits the
transduction of NF-κB p65 signaling | ω3-PUFA | Inhibits
LPS-induced activation of Piezo1 by upregulating miR-107, reduces
the inflammatory activation | (71) |
In addition, NF-κB signaling pathway is also a core
regulatory pathway of inflammatory response, which is involved in
the transcriptional regulation of various inflammatory factors.
Research has shown that Piezo1 regulated the inflammatory response
by activating the NF-κB signaling pathway. In endothelial cells,
Piezo1 activation promotes inflammatory responses through the NF-κB
signaling pathway (32).
Specifically, mechanical stress activates Piezo1 in endothelial
cells, resulting in elevated intracellular Ca2+
concentration, which in turn activates NF-κB and promotes the
release of inflammatory factors such as TNF-α, IL-1β and IL-6. This
plays an important role in the inflammation of atherosclerosis.
Apart from these two classic inflammatory pathways,
signaling pathways such as inflammasome NLRP3 (75), AKT/mTOR (70) and CaMKII-Mst1/2-Rac (68) are also involved in Piezo1-mediated
inflammatory response. Although the specific mechanisms and targets
of action vary in different diseases, the core mechanism is to
activate downstream signaling pathways by regulating intracellular
Ca2+ concentration, thereby regulating inflammatory
responses.
Piezo1 is a potential therapeutic
target
Since Piezo1 plays an important role in a number of
physiological and pathological processes, targeted modulation of
Piezo1 activity may become a novel strategy for the treatment of a
number of diseases. Although the specific ligand binding mechanism
of Piezo1 has not been fully elucidated, there are a number of
agonists and antagonists that have produced effects in vivo
and in vitro (Table
II).
 | Table II.Currently reported drugs that
regulate Piezo1. |
Table II.
Currently reported drugs that
regulate Piezo1.
| First author/s,
year | Category | Drugs | Selectivity | Mechanisms | Inadequacies | (Refs.) |
|---|
| Syeda et al,
2015 | Agonist | Yoda1 | Selective | Binds a hydrophobic
pocket located near residues 1961–2063, enhances a twist-tilt-twist
like opening motion of the arm, reduces the channel's mechanical
activation threshold | Poor solubility in
body fluids, cytotoxicity | (76) |
| Olsen et al,
2000 | Agonist | Jedi1/2 | Selective | Binds the
extracellular regions of the peripheral blade, leads to the
conformational change | Lacks in
vivo evidence for Piezo1 | (82) |
| Coste et al,
2012 | Antagonist | Ruthenium red | Nonselective | Binds two acidic
residues, E2495 and E2496, blocks Piezo1-mediated currents from the
extracellular side | Lacks in
vivo evidence for Piezo1 | (85) |
| Coste et al,
2010 | Antagonist | Gadolinium | Nonselective | Interferes with the
adjacent membrane lipids | The specific
mechanism is unclear and lacks in vivo evidence for
Piezo1 | (1) |
| Cox et al,
2019 | Antagonist | Aβ | Nonselective | Changes the
physical and mechanical properties of the membrane | The specific
mechanism is unclear and lacks in vivo evidence for
Piezo1 | (87) |
| Romero et
al, 2019 | Antagonist | Saturated fatty
acids | Nonselective | Inhibits Piezo1
currents by increasing the mechanical threshold required for
activation | The specific
mechanism is unclear and lacks in vivo evidence for
Piezo1 | (89) |
| Romero et
al, 2019 | Antagonist | Polyunsaturated
fatty acids | Nonselective | Modulates the
allosteric coupling between the CED and the inner pore helix;
alters the interaction between Piezo1 and other proteins | The specific
mechanism is unclear and lacks in vivo evidence for
Piezo1 | (89) |
| Gnanasambandam
et al, 2017 | Antagonist | GsMTx4 | Nonselective | Mediates area
expansion of the outer leaflet, transfers tension to the fixed-area
inner monolayer, potentiates TREK-1 channels | Non-specific
interactions with other cation channels | (93) |
| Evans et al,
2018 | Antagonist | Dooku1 | A selective
antagonist of Yoda1 | A competitive
inhibitor to Yoda1 | Poor solubility in
body fluids, not directly blocks the channels and the specific
mechanism is unclear | (77) |
| Wang et al,
2022 | Antagonist | Tubeimoside I | A selective
antagonist of Yoda1 | Competes with Yoda1
for Piezo1 in the Yoda1 binding sites | Effects depend on
the cell type and the concentration of Yoda1 | (102) |
| Pan et al,
2022 | Antagonist | Salvianolic acid
B | A selective
antagonist of Yoda1 | Competes with Yoda1
for Piezo1 in the Yoda1 binding sites, inhibits cationic
current | Does not show
selectivity in terms of cell types | (30) |
| Hong et al,
2023 | Antagonist | Jatrorrhizine | Selective | Inhibits
Yoda1-induced Piezo1 channel activation and the high expression of
endothelial mesenchymal transition related molecules caused by
Piezo1 activation | The role and
mechanism in different cardiovascular diseases and cell subtypes
are unclear | (103) |
| Wang et al,
2023 | Antagonist | Escin | Selective | Inhibits
Yoda1-evoked Ca2+ signals in ECs and mechanical
stretch-induced activation of NF-κB via Piezo1 | The role and
mechanism in different cell subtypes are unclear | (105) |
| Chu et al,
2024 | Antagonist | Kaempferol | Selective | Inhibits the Piezo1
channels and Ca2+ influx, and regulates the downstream
pathways of MAPK/NF-κB and Nrf2/HO-1, regulates scavenger receptor
CD36-mediated mitochondrial ROS production | The role and
mechanism in different cell subtypes are unclear | (39) |
Piezo1-specific agonists
Yoda1
Researchers screened millions of compounds and
discovered Yoda1, the first synthetic small molecule agonist
targeting Piezo1 (76). Structural
analysis of Yoda1 revealed that the (2.6-dichlorobenzyl)thioether
group and pyrazine and thiadiazol groups are important for its
agonism (77). Yoda1 binds to
Piezo1 and induces a conformational change in the channel that
opens the channel and effectively lowers the mechanical activation
threshold of Piezo1 (78). In some
animal experiments, Yoda1 can activate Piezo1 to regulate palatal
bone development (79), regulate
lung fibroblasts to improve airflow and also increases bone mass
and reduces bone loss in mice, making it a potential target for the
treatment of osteoporosis (80). A
recent study found that the association of Yoda1 with low-magnitude
high-frequency (LMHF) vibration synergistically promotes YAP
nuclear translocation and strengthens osteoblast responses to
mechanical stimuli, potentially enhancing the efficacy of LMHF
vibration in the treatment of osteoporosis (81). However, this finding needs to be
further validated in animal models and clinical trials in
vivo. Apart from the shortcomings of the experimental model,
Yoda1′s poor solubility and certain cytotoxicity have hampered
further clinical research (77).
Jedi1/2
Through high-throughput screening, researchers found
that Jedi1 and Jedi2 are specific chemical agonists of Piezo1 and
have no effect on Piezo2 (82).
Jedi1/2 might act through the extracellular regions of the
peripheral blade, which is formed by the large region containing
residues 1–2,190 (83).
Jedi1/2-induced currents activate more rapidly and decay more
markedly, whereas Yoda1 activation is slow and irreversible.
Jedi1/2 have improved solubility compared to Yoda1 (83). In addition, co-administration of
Jedi1 and Yoda1 produce a synergistic activation of Piezo1,
suggesting that the two agonists may activate Piezo1 through
different binding sites (83).
However, there is a lack of animal models or in vivo
toxicity assessments and follow-up studies are needed to verify
efficacy and safety.
Piezo1 antagonists
Ruthenium red (RR) and Gadolinium
(Gd3+)
RR is an inorganic polycationic dye that has been
found to block the binding of Piezo1. The acidic residues E2495 and
E2496 of Piezo1, which are located on the inner side of the cell,
may be the binding sites of RR (84,85).
Gd3+ is a trivalent lanthanide that inhibits
mechanosensitive ion channels (86). Gd3+ has been
shown to inhibit Piezo1-mediated mechanosensitive currents
(1), although the exact mechanism
is not clear. As RR and Gd3+ are non-specific
Piezo1 inhibitors, they have limited therapeutic applications and
are currently used to study Piezo1 function in cells and tissues
(5).
Aβ
As aforementioned earlier, Aβ plays an important
role in the pathogenesis of Alzheimer's disease and it is an
amphiphilic molecule capable of inhibiting the function of Piezo1
by altering membrane structure (87). A study has shown that the L- and
D-isomers of monomeric Aβ peptide do not differ in their inhibitory
effects on Piezo1, suggesting that Aβ peptide does not regulate the
activity of Piezo1 through direct contact with Piezo1, but rather
by modulating the cytoskeletal and membrane mechanical properties
(88).
Saturated and polyunsaturated fatty
acids
Piezo1 channels have three states, from closed to
open to inactivated and studies have shown that different fatty
acid types affect the different states of Piezo1 (89,90).
The saturated fatty acid margaric acid affects the Piezo1 channel
from closed to open by increasing the order and bending stiffness
of the lipid structure of the cell membrane so that greater
mechanical stimulation is required for the activation of Piezo1
(89). In addition, some
polyunsaturated fatty acids (PUFA), such as arachidonic acid (AA)
and eicosapentaenoic acid (EPA), can inhibit Piezo1 activity by
affecting the transition from opening to inactivation of Piezo1
(89). In osteoarthritis, ω3-PUFA
has a potential cartilage-protective effect by inhibiting
Piezo1/TRPV4 mechanical signaling and modulating membrane
properties and inflammatory responses, whereas ω6-PUFA may increase
the risk of membrane damage (90).
The balanced application of PUFA in vivo needs to be further
explored to optimize nutritional intervention strategies for
osteoarthritis.
GsMTx4
GsMTx4, extracted from the venom of the tarantula
spider Grammostola spatulata, is the first mechanosensitive
channel inhibitor discovered to block endogenous mechanically gated
channels (91). Subsequently,
GsMTx4 has been shown to reversibly block Piezo1 channel activity
(92,93). It is now considered that GsMTx4
also acts by altering local plasma membrane tension rather than by
direct contact with Piezo1 (94).
GsMTx4 has been widely used in physiological and pathological
studies of Piezo1. Studies have shown that GsMTx4 is able to treat
animal models of pulmonary hypertension (95), osteoarthritis (96) and cancer (97), but the development and clinical
trials of GsMTx4 as a Piezo1-targeted drug have been hampered by
its action on broad cationic mechanosensitive channels (94).
Dooku1
By replacing the pyrazin-2-yl thiadiazole of Yoda1
with a pyrrole-2-yl oxadiazole moiety, researchers discovered a new
Piezo1-selective antagonist acting through competitive inhibition
of Yoda1; Dooku1 (77). Several
studies on the pharmacological activity of Dooku1 have shown that
Dooku1 has potential therapeutic effects on a number of diseases.
For example, Dooku1 can prevent thrombosis and erythrocyte death
associated with sickle cell anemia by decreasing Piezo1-induced
Ca2+ efflux (98). In
addition, inhibition of Piezo1 by Dooku1 is also able to attenuate
aortic stenosis (99), regulate
brown adipocyte differentiation (100) and reduce neurological deficits
after cerebral hemorrhage (101).
However, similar to Yoda1, Dooku1 is poorly soluble in body fluids,
which to some extent prevents Dooku1 from functioning in
vivo.
Natural extracts
A number of substances extracted from natural herbs
also specifically antagonize Piezo1. Tubeimoside I is a
triterpenoid saponin extracted from the Chinese herbal medicine
Bolbostemmatis Rhizoma and is currently mostly used in the
treatment of a number of types of tumor diseases (102). A study has found that it also
competes with Yoda1 for the binding site and inhibits Yoda1 from
activating Piezo1 and that this inhibition is selective for Piezo1,
but not for other mechanosensitive channels (such as TRPC5, TRPM2
and TRPV4) (36). Salvianolic acid
B is a polyphenolic compound extracted from Danshen (Salvia
miltiorrhiza Bge.). Its mechanism of action is similar to that
of Tubeimoside I and it can play a role in the treatment of
atherosclerosis (30). The
protoberberine alkaloid jatrorrhizine, which is mainly derived from
Chinese plants such as Coptidis Rhizoma, used to be commonly used
as an anti-inflammatory drug. However, a recent study has found
that it can also inhibit Piezo1 activation mediated Ca2+
influx, making it a potential drug for treating vasculitis
(103). Escin is a mixture of
triterpenoid saponins isolated from extracts of the seeds of horse
chestnut (Aesculus hippocastanum L.), which is currently
used clinically for the treatment of chronic venous insufficiency
and postoperative edema (104). A
study has shown that Escin inhibits the expression of
Piezo1-induced inflammatory factors (such as IL-1β and IL-6) in
endothelial cells when endothelial cells are subjected to tensile
stress, playing an important role in the anti-inflammatory response
(105).
Challenges in drug development
Researchers face multiple challenges when developing
drugs targeting Piezo1 channels. First, drug selectivity is a key
issue. Since Piezo1 is widely expressed in a variety of tissues and
cell types, designing a compound that specifically acts on Piezo1
without affecting other ion channels or physiological processes is
a challenging task. For example, existing Piezo1 agonists such as
Yoda1 and Jedi1/2 have shown activation effects on Piezo1, but
their selectivity is not perfect and may interact with other
channels or receptors (76,83).
Second, the side effects of the drugs are also an important
consideration. Since Piezo1 plays an important role in normal
physiological functions, such as vascular development, blood
pressure regulation and erythrocyte volume control, any
interference with these functions may lead to undesirable side
effects. Therefore, when developing Piezo1 targeted drugs, it is
necessary to carefully evaluate their potential impact on
physiological processes and ensure a balance between therapeutic
efficacy and potential risks. Pharmacokinetic characterization is
also an important aspect in drug development. Understanding how
drugs are absorbed, distributed, metabolized and excreted in the
body is critical to ensuring their efficacy and safety. Currently,
pharmacokinetic studies on Piezo1 channels are inadequate, which
limits our understanding of how these drugs function in
vivo. Therefore, despite the great potential of Piezo1 as a
therapeutic target, multiple challenges such as drug selectivity,
side effects and pharmacokinetics still need to be overcome in
practical development.
Conclusion and perspectives
As a novel mechanosensitive cation channel, Piezo1
converts mechanical signals into biological signals to initiate
cascaded responses in cellular inflammatory upon imbalance of
external mechanical forces and changes in the local cellular
environment. Piezo1 further influences the development and
regression of inflammation with changes in local mechanical forces
during inflammation progression. Chronic inflammation is one that
progresses slowly for a long time. It is related to a number of
diseases in immune and cardiovascular systems, cancer and diabetes.
It is an inflammatory response that endangers the whole body. The
present study summarized the role and possible mechanisms of Piezo1
in several common chronic inflammatory diseases, specifically its
role in periodontal tissue inflammation and alveolar bone
destruction. In addition to the aforementioned diseases, Piezo1
also plays an important role in chronic cystitis (106) and Crohn's disease (75). Therefore, pharmacological
modulation of the activity of Piezo1 at the early stage of the
disease to inhibit the transduction of mechanical damage signals
delays the development of chronic inflammation and improve its
prognosis. Due to the wide range of Piezo1 downstream pathways,
targeting Piezo1 downstream pathways for the treatment of multiple
inflammatory diseases can be investigated in the future. For
example, in Crohn's disease Piezo1 exacerbates inflammation through
a calcium signaling-mitochondrial damage-NLRP3 pathway cascade,
while the NLRP3 pathway plays a role in a variety of chronic
inflammatory diseases such as obesity and Parkinson's disease
(75). Targeting Piezo1 may be
possible to treat patients with comorbid multiple inflammatory
diseases.
However, there are a number of challenges and
limitations in the design of drugs targeting Piezo1. A number of
agonists and antagonists have been identified to directly or
indirectly modulate Piezo1 activity, but these drugs are poorly
soluble and unstable, making them difficult to use in vivo.
Due to the wide range of biological functions of Piezo1 in multiple
tissues and organs, single activation or inhibition of Piezo1 may
produce side effects in addition to therapeutic effects. For
example, in AS, inhibition of Piezo1 is not the best treatment
because it leads to vasoconstriction and hypertension at the same
time (107). These shortcomings
prevent drugs that modulate the activity of Piezo1 from being used
in clinical therapy at present. The future direction of disease
treatment resides in researches into tissue-specific
Piezo1-targeted drugs that achieve ideal therapeutic effects while
avoiding potential side effects. In addition to drugs that
specifically regulate Piezo1 activity, the use of gene editing
techniques enables more precise regulation of Piezo1. A recent
study showed that using CRISPR to knock down Piezo1 in a high-grade
serous ovarian cancer model interrupted the cascade reaction caused
by increased stiffness and activation of Piezo1, slowing down
disease progression (108).
In summary, the relationship between Piezo1 and
inflammatory diseases is complex. The discovery of Piezo1 provides
a new therapeutic target for disease treatment and drugs that
regulate its activity have been widely used in basic researches.
The role and mechanism of Piezo1 in chronic inflammatory diseases,
as well as the development and application of drugs that target
Piezo1, may become the focus of future researches.
Acknowledgements
Not applicable.
Funding
National Key R&D Program of China (grant no. 2023YFC2506304)
and Sichuan Science and Technology Program (grant no. 2023YFS0032)
to JW; Fundamental Research Funds for the Central Universities,
Research and Develop Program, West China Hospital of Stomatology
Sichuan University (grant no. RD-02-202403) to CX; National Natural
Science Foundation of China (grant no. 82201073) and Research
Funding from West China School/Hospital of Stomatology Sichuan
University (grant no. RCDWJS2024-11) to XX.
Availability of data and materials
Not applicable.
Authors' contributions
JY was responsible for writing the original draft,
reviewing and editing, validation and conceptualization. CX, XX and
JW was responsible for writing the original draft. PS was
responsible for writing, reviewing and editing, supervision and
project administration. Data authentication is not applicable. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coste B, Mathur J, Schmidt M, Earley TJ,
Ranade S, Petrus MJ, Dubin AE and Patapoutian A: Piezo1 and Piezo2
are essential components of distinct mechanically activated cation
channels. Science. 330:55–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murthy SE, Dubin AE and Patapoutian A:
Piezos thrive under pressure: Mechanically activated ion channels
in health and disease. Nat Rev Mol Cell Biol. 18:771–783. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orsini EM, Perelas A, Southern BD, Grove
LM, Olman MA and Scheraga RG: Stretching the function of innate
immune cells. Front Immunol. 12:7673192021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nims RJ, Pferdehirt L, Ho NB, Savadipour
A, Lorentz J, Sohi S, Kassab J, Ross AK, O'Conor CJ, Liedtke WB, et
al: A synthetic mechanogenetic gene circuit for autonomous drug
delivery in engineered tissues. Sci Adv. 7:eabd98582021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bagriantsev SN, Gracheva EO and Gallagher
PG: Piezo proteins: Regulators of mechanosensation and other
cellular processes. J Biol Chem. 289:31673–31681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Richardson J, Kotevski A and Poole K: From
stretch to deflection: The importance of context in the activation
of mammalian, mechanically activated ion channels. FEBS J.
289:4447–4469. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Servin-Vences MR, Moroni M, Lewin GR and
Poole K: Direct measurement of TRPV4 and PIEZO1 activity reveals
multiple mechanotransduction pathways in chondrocytes. Elife.
6:e210742017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saotome K, Murthy SE, Kefauver JM, Whitwam
T, Patapoutian A and Ward AB: Structure of the mechanically
activated ion channel Piezo1. Nature. 554:481–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Q, Zhou H, Chi S, Wang Y, Wang J,
Geng J, Wu K, Liu W, Zhang T, Dong MQ, et al: Structure and
mechanogating mechanism of the Piezo1 channel. Nature. 554:487–492.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kefauver JM, Ward AB and Patapoutian A:
Discoveries in structure and physiology of mechanically activated
ion channels. Nature. 587:567–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lewis AH and Grandl J: Inactivation
kinetics and mechanical gating of piezo1 ion channels depend on
subdomains within the cap. Cell Rep. 30:870–880.e872. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Y, Yang X, Jiang J and Xiao B:
Structural designs and mechanogating mechanisms of the
mechanosensitive piezo channels. Trends Biochem Sci. 46:472–488.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Q, Zhou H, Li X and Xiao B: The
mechanosensitive piezo1 channel: A three-bladed propeller-like
structure and a lever-like mechanogating mechanism. FEBS J.
286:2461–2470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baratchi S, Zaldivia MTK, Wallert M,
Loseff-Silver J, Al-Aryahi S, Zamani J, Thurgood P, Salim A, Htun
NM, Stub D, et al: Transcatheter aortic valve implantation
represents an anti-inflammatory therapy via reduction of shear
stress-induced, piezo-1-mediated monocyte activation. Circulation.
142:1092–1105. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Atcha H, Jairaman A, Holt JR, Meli VS,
Nagalla RR, Veerasubramanian PK, Brumm KT, Lim HE, Othy S, Cahalan
MD, et al: Mechanically activated ion channel Piezo1 modulates
macrophage polarization and stiffness sensing. Nat Commun.
12:32562021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du Y, Xu B, Li Q, Peng C and Yang K: The
role of mechanically sensitive ion channel Piezo1 in bone
remodeling. Front Bioeng Biotechnol. 12:13421492024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li XF, Zhang Z, Li XD, Wang TB and Zhang
HN: Mechanism of the piezo1 protein-induced apoptosis of the
chondrocytes through the MAPK/ERK1/2 signal pathway. Zhonghua Yi
Xue Za Zhi. 96:2472–2477. 2016.(In Chinese). PubMed/NCBI
|
|
18
|
Amin AK, Huntley JS, Bush PG, Simpson AH
and Hall AC: Chondrocyte death in mechanically injured articular
cartilage-the influence of extracellular calcium. J Orthop Res.
27:778–784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugimoto A, Miyazaki A, Kawarabayashi K,
Shono M, Akazawa Y, Hasegawa T, Ueda-Yamaguchi K, Kitamura T,
Yoshizaki K, Fukumoto S and Iwamoto T: Piezo type mechanosensitive
ion channel component 1 functions as a regulator of the cell fate
determination of mesenchymal stem cells. Sci Rep. 7:176962017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee W, Leddy HA, Chen Y, Lee SH, Zelenski
NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, et al:
Synergy between piezo1 and piezo2 channels confers high-strain
mechanosensitivity to articular cartilage. Proc Natl Acad Sci USA.
111:E5114–E5122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin C, Feng Y, Yin Z, Wang C, Yin R, Li Y,
Chen K, Tao T, Zhang K, Jiang Y and Gui J: The
PIEZO1/miR-155-5p/GDF6/SMAD2/3 signaling axis is involved in
inducing the occurrence and progression of osteoarthritis under
excessive mechanical stress. Cell Signal. 118:1111422024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Huang Z and Bai L: Cell interplay in
osteoarthritis. Front Cell Dev Biol. 9:7204772021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song J, Liu L, Lv L, Hu S, Tariq A, Wang W
and Dang X: Fluid shear stress induces Runx-2 expression via
upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol Int.
44:1491–1502. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, You X, Lotinun S, Zhang L, Wu N
and Zou W: Mechanical sensing protein PIEZO1 regulates bone
homeostasis via osteoblast-osteoclast crosstalk. Nat Commun.
11:2822020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Han L, Nookaew I, Mannen E, Silva
MJ, Almeida M and Xiong J: Stimulation of Piezo1 by mechanical
signals promotes bone anabolism. Elife. 8:e496312019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong
Q, Zhang X, Zhou Y, Yadav PS, Lin J, et al: Piezo1/2 mediate
mechanotransduction essential for bone formation through concerted
activation of NFAT-YAP1-ß-catenin. Elife. 9:e527792020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jebari-Benslaiman S, Galicia-García U,
Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K,
Benito-Vicente A and Martín C: Pathophysiology of atherosclerosis.
Int J Mol Sci. 23:33462022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lanzer P, Hannan FM, Lanzer JD, Janzen J,
Raggi P, Furniss D, Schuchardt M, Thakker R, Fok PW, Saez-Rodriguez
J, et al: Medial arterial calcification: JACC state-of-the-art
review. J Am Coll Cardiol. 78:1145–1165. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Li Y, Ma X, Liu J, Wang X, Zhang
L, Li C, Li Y and Yang W: Ginsenoside Rg1-Notoginsenoside
R1-protocatechuic aldehyde reduces atherosclerosis and attenuates
low-shear stress-induced vascular endothelial cell dysfunction.
Front Pharmacol. 11:5882592020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan X, Wan R, Wang Y, Liu S, He Y, Deng B,
Luo S, Chen Y, Wen L, Hong T, et al: Inhibition of chemically and
mechanically activated piezo1 channels as a mechanism for
ameliorating atherosclerosis with salvianolic acid B. Br J
Pharmacol. 179:3778–3814. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lan Y, Lu J, Zhang S, Jie C, Chen C, Xiao
C, Qin C and Cheng D: Piezo1-mediated mechanotransduction
contributes to disturbed flow-induced atherosclerotic endothelial
inflammation. J Am Heart Assoc. 13:e0355582024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Albarrán-Juárez J, Iring A, Wang S, Joseph
S, Grimm M, Strilic B, Wettschureck N, Althoff TF and Offermanns S:
Piezo1 and G(q)/G(11) promote endothelial inflammation depending on
flow pattern and integrin activation. J Exp Med. 215:2655–2672.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feaver RE, Gelfand BD, Wang C, Schwartz MA
and Blackman BR: Atheroprone hemodynamics regulate fibronectin
deposition to create positive feedback that sustains endothelial
inflammation. Circ Res. 106:1703–1711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang S, Chennupati R, Kaur H, Iring A,
Wettschureck N and Offermanns S: Endothelial cation channel PIEZO1
controls blood pressure by mediating flow-induced ATP release. J
Clin Invest. 126:4527–4536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Wang D, Zhang C, Yang W, Li C, Gao
Z, Pei K and Li Y: Piezo1 mediates endothelial atherogenic
inflammatory responses via regulation of YAP/TAZ activation. Hum
Cell. 35:51–62. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu S, Pan X, Cheng W, Deng B, He Y, Zhang
L, Ning Y and Li J: Tubeimoside I antagonizes yoda1-evoked piezo1
channel activation. Front Pharmacol. 11:7682020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pourteymour S, Fan J, Majhi RK, Guo S, Sun
X, Huang Z, Liu Y, Winter H, Bäcklund A, Skenteris NT, et al:
PIEZO1 targeting in macrophages boosts phagocytic activity and foam
cell apoptosis in atherosclerosis. Cell Mol Life Sci. 81:3312024.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Atcha H, Kulkarni D, Meli VS,
Veerasubramanian PK, Wang Y, Cahalan MD, Pathak MM and Liu WF:
Piezo1-mediated mechanotransduction enhances macrophage oxidized
low-density lipoprotein uptake and atherogenesis. PNAS Nexus.
3:pgae4362024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chu T, Wang Y, Wang S, Li J, Li Z, Wei Z,
Li J and Bian Y: Kaempferol regulating macrophage foaming and
atherosclerosis through piezo1-mediated MAPK/NF-κB and Nrf2/HO-1
signaling pathway. J Adv Res. 17:S2090–S1232. 2024.
|
|
40
|
Lin C, Zheng X, Lin S, Zhang Y, Wu J and
Li Y: Mechanotransduction regulates the interplays between alveolar
epithelial and vascular endothelial cells in lung. Front Physiol.
13:8183942022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Diem K, Fauler M, Fois G, Hellmann A,
Winokurow N, Schumacher S, Kranz C and Frick M: Mechanical stretch
activates piezo1 in caveolae of alveolar type I cells to trigger
ATP release and paracrine stimulation of surfactant secretion from
alveolar type II cells. FASEB J. 34:12785–12804. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang GP, Xu J, Cao LL, Zeng YH, Chen BX,
Yang J, Zhang ZW and Kang Y: Piezo1 induced apoptosis of type II
pneumocytes during ARDS. Respir Res. 20:1182019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang JQ, Zhang H, Guo XW, Lu Y, Wang SN,
Cheng B, Dong SH, Lyu XL, Li FS and Li YW: Mechanically activated
calcium channel PIEZO1 modulates radiation-induced
epithelial-mesenchymal transition by forming a positive feedback
with TGF-β1. Front Mol Biosci. 8:7252752021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang L, Zhang Y, Lu D, Huang T, Yan K,
Yang W and Gao J: Mechanosensitive Piezo1 channel activation
promotes ventilator-induced lung injury via disruption of
endothelial junctions in ARDS rats. Biochem Biophys Res Commun.
556:79–86. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Solis AG, Bielecki P, Steach HR, Sharma L,
Harman CCD, Yun S, de Zoete MR, Warnock JN, To SDF, York AG, et al:
Mechanosensation of cyclical force by PIEZO1 is essential for
innate immunity. Nature. 573:69–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mukhopadhyay A, Tsukasaki Y, Chan WC, Le
JP, Kwok ML, Zhou J, Natarajan V, Mostafazadeh N, Maienschein-Cline
M, Papautsky I, et al: Trans-Endothelial neutrophil migration
activates bactericidal function via Piezo1 mechanosensing.
Immunity. 57:52–67.e10. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hurrell BP, Shen S, Li X, Sakano Y, Kazemi
MH, Quach C, Shafiei-Jahani P, Sakano K, Ghiasi H and Akbari O:
Piezo1 channels restrain ILC2s and regulate the development of
airway hyperreactivity. J Exp Med. 221:e202318352024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ríos CC, Campiño JI, Posada-López A,
Rodríguez-Medina C and Botero JE: Occlusal trauma is associated
with periodontitis: A retrospective case-control study. J
Periodontol. 92:1788–1794. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Grieve WG III, Johnson GK, Moore RN,
Reinhardt RA and DuBois LM: Prostaglandin E (PGE) and interleukin-1
beta (IL-1 beta) levels in gingival crevicular fluid during human
orthodontic tooth movement. Am J Orthod Dentofacial Orthop.
105:369–374. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang Y, Lin H, Chen Y, Lan Y, Wang H, Li
T, Hu Z and Zou S: Piezo1 contributes to alveolar bone remodeling
by activating β-catenin under compressive stress. Am J Orthod
Dentofacial Orthop. 165:458–470. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jin Y, Li J, Wang Y, Ye R, Feng X, Jing Z
and Zhao Z: Functional role of mechanosensitive ion channel Piezo1
in human periodontal ligament cells. Angle Orthod. 85:87–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zheng F, Wu T, Wang F, Li H, Tang H, Cui
X, Li C, Wang Y and Jiang J: Low-intensity pulsed ultrasound
promotes the osteogenesis of mechanical force-treated periodontal
ligament cells via Piezo1. Front Bioeng Biotechnol. 12:13474062024.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang L, Wang X, Ji N, Li HM and Cai SX:
Mechanisms of the mechanically activated ion channel Piezo1 protein
in mediating osteogenic differentiation of periodontal ligament
stem cells via the Notch signaling pathway. Hua Xi Kou Qiang Yi Xue
Za Zhi. 38:628–636. 2020.(In Chinese). PubMed/NCBI
|
|
55
|
Sokos D, Everts V and de Vries TJ: Role of
periodontal ligament fibroblasts in osteoclastogenesis: A review. J
Periodontal Res. 50:152–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Horie S, Nakatomi C, Ito-Sago M, Morii A,
Orimoto A, Ikeda H, Hsu CC, Naniwa M, Mizuhara M, Gunjigake K, et
al: PIEZO1 promotes ATP release from periodontal ligament cells
following compression force. Eur J Orthod. 45:565–574. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Agrawal A and Gartland A: P2X7 receptors:
Role in bone cell formation and function. J Mol Endocrinol.
54:R75–R88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cai G, Lu Y, Zhong W, Wang T, Li Y, Ruan
X, Chen H, Sun L, Guan Z, Li G, et al: Piezo1-mediated M2
macrophage mechanotransduction enhances bone formation through
secretion and activation of transforming growth factor-β1. Cell
Prolif. 56:e134402023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xu H, Guan J, Jin Z, Yin C, Wu S, Sun W,
Zhang H and Yan B: Mechanical force modulates macrophage
proliferation via Piezo1-AKT-Cyclin D1 axis. FASEB J.
36:e224232022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao T, Chu Z, Chu CH, Dong S, Li G, Wu J
and Tang C: Macrophages induce gingival destruction via
piezo1-mediated MMPs-degrading collagens in periodontitis. Front
Immunol. 14:11946622023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Velasco-Estevez M, Mampay M, Boutin H,
Chaney A, Warn P, Sharp A, Burgess E, Moeendarbary E, Dev KK and
Sheridan GK: Infection augments expression of mechanosensing piezo1
channels in amyloid plaque-reactive astrocytes. Front Aging
Neurosci. 10:3322018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Segel M, Neumann B, Hill MFE, Weber IP,
Viscomi C, Zhao C, Young A, Agley CC, Thompson AJ, Gonzalez GA, et
al: Niche stiffness underlies the ageing of central nervous system
progenitor cells. Nature. 573:130–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wilhelmsson U, Bushong EA, Price DL, Smarr
BL, Phung V, Terada M, Ellisman MH and Pekny M: Redefining the
concept of reactive astrocytes as cells that remain within their
unique domains upon reaction to injury. Proc Natl Acad Sci USA.
103:17513–17518. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu H, Hu J, Zheng Q, Feng X, Zhan F, Wang
X, Xu G and Hua F: Piezo1 channels as force sensors in mechanical
force-related chronic inflammation. Front Immunol. 13:8161492022.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Velasco-Estevez M, Rolle SO, Mampay M, Dev
KK and Sheridan GK: Piezo1 regulates calcium oscillations and
cytokine release from astrocytes. Glia. 68:145–160. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen Y and Colonna M: Microglia in
Alzheimer's disease at single-cell level. Are there common patterns
in humans and mice? J Exp Med. 218:e202027172021.PubMed/NCBI
|
|
67
|
Rodríguez-Gómez JA, Kavanagh E,
Engskog-Vlachos P, Engskog MKR, Herrera AJ, Espinosa-Oliva AM,
Joseph B, Hajji N, Venero JL and Burguillos MA: Microglia: Agents
of the CNS pro-inflammatory response. Cells. 9:17172020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Geng J, Shi Y, Zhang J, Yang B, Wang P,
Yuan W, Zhao H, Li J, Qin F, Hong L, et al: TLR4 signalling via
Piezo1 engages and enhances the macrophage mediated host response
during bacterial infection. Nat Commun. 12:35192021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jäntti H, Sitnikova V, Ishchenko Y,
Shakirzyanova A, Giudice L, Ugidos IF, Gómez-Budia M, Korvenlaita
N, Ohtonen S, Belaya I, et al: Microglial amyloid beta clearance is
driven by PIEZO1 channels. J Neuroinflammation. 19:1472022.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu H, Bian W, Yang D, Yang M and Luo H:
Inhibiting the Piezo1 channel protects microglia from acute
hyperglycaemia damage through the JNK1 and mTOR signalling
pathways. Life Sci. 264:1186672021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu H, Zhou L, Yi P, Zhan F, Zhou L, Dong
Y, Xiong Y, Hua F and Xu G: ω3-PUFA alleviates neuroinflammation by
upregulating miR-107 targeting PIEZO1/NFκB p65. Int
Immunopharmacol. 132:1119962024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kavalali ET: Neuronal Ca2+
signalling at rest and during spontaneous neurotransmission. J
Physiol. 598:1649–1654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu L, Lian W and Zhao L: Calcium signaling
in cancer progression and therapy. FEBS J. 288:6187–6205. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Veiga A, Abreu DS, Dias JD, Azenha P,
Barsanti S and Oliveira JF: Calcium-dependent signaling in
astrocytes: Downstream mechanisms and implications for cognition. J
Neurochem. 169:e700192025. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu Q, Wang D, Yang X, Ma F, Han W, Hu J
and Mei Q: The mechanosensitive ion channel PIEZO1 in intestinal
epithelial cells mediates inflammation through the NOD-Like
receptor 3 pathway in Crohn's disease. Inflamm Bowel Dis.
29:103–115. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Syeda R, Xu J, Dubin AE, Coste B, Mathur
J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, et al: Chemical
activation of the mechanotransduction channel piezo1. Elife.
4:e073692015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Evans EL, Cuthbertson K, Endesh N, Rode B,
Blythe NM, Hyman AJ, Hall SJ, Gaunt HJ, Ludlow MJ, Foster R and
Beech DJ: Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked
activation of piezo1 and aortic relaxation. Br J Pharmacol.
175:1744–1759. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Botello-Smith WM, Jiang W, Zhang H, Ozkan
AD, Lin YC, Pham CN, Lacroix JJ and Luo Y: A mechanism for the
activation of the mechanosensitive piezo1 channel by the small
molecule Yoda1. Nat Commun. 10:45032019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nie X, Abbasi Y and Chung MK: Piezo1 and
piezo2 collectively regulate jawbone development. Development.
151:dev2023862024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Steinecker-Frohnwieser B, Lohberger B,
Toegel S, Windhager R, Glanz V, Kratschmann C, Leithner A and Weigl
L: Activation of the mechanosensitive ion channels piezo1 and TRPV4
in primary human healthy and osteoarthritic chondrocytes exhibits
ion channel crosstalk and modulates gene expression. Int J Mol Sci.
24:78682023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lin CY, Sassi A, Wu Y, Seaman K, Tang W,
Song X, Bienenstock R, Yokota H, Sun Y, Geng F, et al:
Mechanotransduction pathways regulating YAP nuclear translocation
under Yoda1 and vibration in osteocytes. Bone. 190:1172832025.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Olsen BR, Reginato AM and Wang W: Bone
development. Ann Rev Cell Dev Biol. 16:191–220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang Y, Chi S, Guo H, Li G, Wang L, Zhao
Q, Rao Y, Zu L, He W and Xiao B: A lever-like transduction pathway
for long-distance chemical- and mechano-gating of the
mechanosensitive Piezo1 channel. Nat Commun. 9:13002018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi
P, Zhang M and Xiao B: Ion permeation and mechanotransduction
mechanisms of mechanosensitive piezo channels. Neuron.
89:1248–1263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Coste B, Xiao B, Santos JS, Syeda R,
Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, et al:
Piezo proteins are pore-forming subunits of mechanically activated
channels. Nature. 483:176–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Dhein S, Salameh A, Berkels R and Klaus W:
Dual mode of action of dihydropyridine calcium antagonists: A role
for nitric oxide. Drugs. 58:397–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cox CD and Gottlieb PA: Amphipathic
molecules modulate PIEZO1 activity. Biochem Soc Trans.
47:1833–1842. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Maneshi MM, Ziegler L, Sachs F, Hua SZ and
Gottlieb PA: Enantiomeric Aβ peptides inhibit the fluid shear
stress response of PIEZO1. Sci Rep. 8:142672018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Romero LO, Massey AE, Mata-Daboin AD,
Sierra-Valdez FJ, Chauhan SC, Cordero-Morales JF and Vásquez V:
Dietary fatty acids fine-tune Piezo1 mechanical response. Nat
Commun. 10:12002019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Marushack GK, Savadipour A, Tang R,
Garcia-Castorena JM, Rashidi N, Nims RJ, Harasymowicz NS, Kim YS
and Guilak F: Polyunsaturated fatty acids suppress PIEZO ion
channel mechanotransduction in articular chondrocytes. FASEB J.
39:e702902025. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ostrow KL, Mammoser A, Suchyna T, Sachs F,
Oswald R, Kubo S, Chino N and Gottlieb PA: cDNA sequence and in
vitro folding of GsMTx4, a specific peptide inhibitor of
mechanosensitive channels. Toxicon. 42:263–274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bae C, Sachs F and Gottlieb PA: The
mechanosensitive ion channel piezo1 is inhibited by the peptide
GsMTx4. Biochemistry. 50:6295–6300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gnanasambandam R, Ghatak C, Yasmann A,
Nishizawa K, Sachs F, Ladokhin AS, Sukharev SI and Suchyna TM:
GsMTx4: Mechanism of inhibiting mechanosensitive ion channels.
Biophys J. 112:31–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Suchyna TM: Piezo channels and GsMTx4: Two
milestones in our understanding of excitatory mechanosensitive
channels and their role in pathology. Prog Biophys Mol Biol.
130:244–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang Z, Chen J, Babicheva A, Jain PP,
Rodriguez M, Ayon RJ, Ravellette KS, Wu L, Balistrieri F, Tang H,
et al: Endothelial upregulation of mechanosensitive channel Piezo1
in pulmonary hypertension. Am J Physiol Cell Physiol.
321:C1010–C1027. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ren X, Zhuang H, Li B, Jiang F, Zhang Y
and Zhou P: Gsmtx4 alleviated osteoarthritis through
Piezo1/Calcineurin/NFAT1 signaling axis under excessive mechanical
strain. Int J Mol Sci. 24:40222023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kang H, Hong Z, Zhong M, Klomp J, Bayless
KJ, Mehta D, Karginov AV, Hu G and Malik AB: Piezo1 mediates
angiogenesis through activation of MT1-MMP signaling. Am J Physiol
Cell Physiol. 316:C92–C103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wadud R, Hannemann A, Rees DC, Brewin JN
and Gibson JS: Yoda1 and phosphatidylserine exposure in red cells
from patients with sickle cell anaemia. Sci Rep. 10:201102020.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhong G, Su S, Li J, Zhao H, Hu D, Chen J,
Li S, Lin Y, Wen L, Lin X, et al: Activation of Piezo1 promotes
osteogenic differentiation of aortic valve interstitial cell
through YAP-dependent glutaminolysis. Sci Adv. 9:eadg04782023.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kenmochi M, Kawarasaki S, Takizawa S,
Okamura K, Goto T and Uchida K: Involvement of mechano-sensitive
piezo1 channel in the differentiation of brown adipocytes. J
Physiol Sci. 72:132022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Qu J, Zong HF, Shan Y, Zhang SC, Guan WP,
Yang Y and Zhao HL: Piezo1 suppression reduces demyelination after
intracerebral hemorrhage. Neural Regen Res. 18:1750–1756.
2023.PubMed/NCBI
|
|
102
|
Wang CL, Gao MZ, Gao DM, Guo YH, Gao Z,
Gao XJ, Wang JQ and Qiao MQ: Tubeimoside-1: A review of its
antitumor effects, pharmacokinetics, toxicity, and targeting
preparations. Front Pharmacol. 13:9412702022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hong T, Pan X, Xu H, Zheng Z, Wen L, Li J
and Xia M: Jatrorrhizine inhibits Piezo1 activation and reduces
vascular inflammation in endothelial cells. Biomed Pharmacother.
163:1147552023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gallelli L: Escin: A review of its
anti-edematous, anti-inflammatory, and venotonic properties. Drug
Des Devel Ther. 13:3425–3437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang Y, Chu T, Pan X, Bian Y and Li J:
Escin ameliorates inflammation via inhibiting mechanical stretch
and chemically induced Piezo1 activation in vascular endothelial
cells. Eur J Pharmacol. 956:1759512023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liu Q, Long Z, Dong X, Zhang T, Zhao J,
Sun B, Zhu J, Li J, Wang Q, Yang Z, et al: Cyclophosphamide-induced
HCN1 channel upregulation in interstitial Cajal-like cells leads to
bladder hyperactivity in mice. Exp Mol Med. 49:e3192017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Iring A, Jin YJ, Albarrán-Juárez J,
Siragusa M, Wang S, Dancs PT, Nakayama A, Tonack S, Chen M, Künne
C, et al: Shear stress-induced endothelial adrenomedullin signaling
regulates vascular tone and blood pressure. J Clin Invest.
129:2775–2791. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Micek HM, Yang N, Dutta M, Rosenstock L,
Ma Y, Hielsberg C, McCord M, Notbohm J, McGregor S and Kreeger PK:
The role of piezo1 mechanotransduction in high-grade serous ovarian
cancer: Insights from an in vitro model of collective detachment.
Sci Adv. 10:eadl44632024. View Article : Google Scholar : PubMed/NCBI
|