Zinc (Zn) is an essential trace element that has a
role in prenatal and postnatal growth and development; notably, it
is involved in gene expression, DNA and RNA stabilization,
neurotransmission and apoptosis (1–3). Zn
serves an important role in the activity of several enzymes,
including dopamine β-hydroxylase, superoxide dismutase (SOD),
thymidylate synthase, DNA and RNA polymerases, matrix
metalloproteinases and N-acyl-D-aspartate deacylase (4,5). In
addition, Zn is an inhibitor of NMDA receptor (NMDAR) activity, and
it interacts with GABA and serotonin receptors (6,7); it
is found in high quantities in the hippocampus and limbic system in
glutamatergic neurons, and it can cause cognitive and memory
impairment (8,9). Sources of Zn include red meat,
oysters, crabs, nuts, beans and whole grains (7). Notably, Zn deficiency can result in
symptoms of depression, anxiety, growth restriction, loss of
appetite, impaired immune function, loss of smell, diarrhea and
hair loss as well as in acrodermatitis enteropathica specifically
in children (7,10–13).
However, increased concentrations of Zn can have a toxic outcome;
it has been reported that patients with Parkinson's disease (PD)
exhibit increased Zn concentrations in the substantia nigra, and
after continuous Zn administration for 12 weeks, rats have
demonstrated upregulation of COX-2 mRNA in the substantia nigra
(14). The important role of Zn in
neurodegenerative disorders, schizophrenia (SCH) and depression has
been established in several research studies (8,15–17).
It has been suggested that prenatal Zn deficiency,
as a result of maternal Zn insufficiency or fetal gene variants,
results in decreased brain volume in rodent models (20,21)
and increases the incidence of SCH. Other SCH risk genes (including
CACNA1G, SOBP, GRIA3, SRRM2, NR3C2, TRIO and RYR2) (22) and Zn deficiency after birth may
contribute to the occurrence of SCH. Within this context, a
decrease in brain Zn content has been reported in postmortem
samples from patients with early onset psychosis compared with that
in control samples (15,23). Furthermore, in a post-mortem study
of the brains of patients with SCH, ionic Zn staining within the
hippocampus was observed; Zn staining in the dentate gyrus was
shown to be more intense in female and older donors (24). In another post-mortem study, Zn
levels were revealed to be elevated in the hippocampus, and reduced
within the amygdala and caudate nucleus; notably, no significant
differences were reported in Zn levels between patients with SCH
and controls (25).
Dysfunction of Zn transferring molecules,
particularly of the SLC39 family responsible for transporting zinc
into the cytoplasm, may lead to irregular Zn concentrations and
have been implicated in SCH (17).
Genome-wide association studies have demonstrated common genetic
influences of the SLC39A8 gene for SCH and inflammatory bowel
disease (26,27). Variants of this gene have been
reported to be associated with metabolic abnormalities (lipid
levels, blood pressure and obesity) and SCH-associated inflammatory
indicators (a shift in gut microbiome composition and T-cell
immunity) (26,28–30).
One missense single nucleotide polymorphism of the
SLC39A8 gene, which encodes ZIP8, rs13107325, has been found to be
related to brain Zn homeostasis in psychosis (31). Li et al (32) demonstrated that cortical dendritic
spine density in SLC39A8-p.393T knock-in mice was significantly
diminished, and it has been proposed that abnormalities in
dendritic spines are associated with the development of SCH.
Furthermore, Tseng et al (33) reported that the missense variant
rs13107325 of gene SLC39A8 resulted in an elevated innate immune
response and glutamate receptor hypofunction. Moreover, mRNA
expression levels of the SLC39A12 gene, which encodes ZIP12, have
been reported to be increased in the dorsolateral prefrontal cortex
(PFC) of patients with SCH (34).
Perez-Becerril et al (35)
reported that allelic variants in SLC30A3 were also associated with
SCH in female participants. Regarding the ZNF family of genes,
ZNF804A has also been demonstrated to be associated with SCH
(36).
It has been reported that Zn serves an antioxidant
protective role in patients with diabetes and patients undergoing
hemodialysis, and it decreases inflammation, ameliorates
mucociliary clearance, inhibits ventilator-induced lung injury, and
regulates antiviral and antibacterial immunity in patients with
COVID-19, respiratory syncytial virus, common cold and pneumonia
(37–40). Moreover, Zn seems to be implicated
in chronic liver disease through Zn-supported metalloproteinase
enzymes (41). In addition, it has
been reported that an anti-aging telomerase-activating
nutraceutical preparation, containing vitamins D3 and C,
Centella asiatica extract and Zn, can exhibit anti-aging
properties on rat brains, by preserving or even enhancing telomere
size and action (42,43).
An extensive electronic search was conducted using
the databases included in the National Library of Medicine
(https://www.nlm.nih.gov/; accessed November 7,
2024), as well as PsycInfo (https://www.apa.org/pubs/databases/psycinfo; accessed
November 7, 2024) and Google Scholar (https://scholar.google.com/; accessed November 7,
2024), for studies that have investigated Zn levels (including in
serum and scalp hair) in patients with psychosis (such as SCH and
bipolar disorder). In addition, studies in which the therapeutic
effects of Zn in psychosis were demonstrated were searched for. In
addition, the Cochrane Library (https://www.cochranelibrary.com/; accessed November 7,
2024) was searched for the references of retrieved articles. The
exclusion criteria included studies on other psychiatric
conditions, depression, anxiety disorders, other neurological
conditions, drug abuse, and severe systematic or malignant
conditions. Two authors read the abstracts, and when agreement
could not be reached between the two, the senior author resolved
the matter. There were no limitations regarding study design, the
present narrative review includes meta-analyses, systematic
reviews, randomized controlled trials (RCTs) and open-label
studies; unpublished studies were not searched for.
A number of studies have estimated Zn levels in
patients with psychosis compared with in controls (Table I). Pfeiffer et al was the
first to report on reduced Zn serum level in patients with SCH
(4,54). Subsequently, Srinivasan et
al (55) reported that mean
serum Zn levels in patients with SCH were lower than those in the
control group, as assessed by atomic absorption spectrometry.
However, Gillin et al (56)
reported that patients with acute and chronic SCH, on or off
treatment with various major tranquillizers, did not exhibit
significant deviation from normal regarding concentrations of Zn in
serum, urine, gastric fluid or hair. Potkin et al (57) observed that cerebrospinal fluid Zn
concentrations did not differ significantly between drug-free
patients with SCH, patients with SCH on antipsychotics and
controls. By contrast, Vaddadi et al (58) reported that Zn serum levels in
patients with SCH under treatment with depot neuroleptics were
reduced compared with in controls, as assessed by atomic absorption
spectrometry.
Several studies have demonstrated the therapeutic
effects of Zn on psychosis. In a 6-week double blind
placebo-controlled study, Mortazavi et al (94) reported that patients with SCH
exhibited marked reductions in the PANSS subscale scores,
aggression risk subscale and PANSS total score when they received
risperidone treatment combined with Zn sulfate compared with those
receiving risperidone treatment plus a placebo.
It has been proposed that genetic susceptibility and
prenatal/perinatal risk issues (viral infection, LPS-induced
inflammation, malnutrition, hypoxia and maternal stress) may result
in the individual being more susceptible to environmental stressors
(childhood trauma, migration, substance abuse and urbanicity)
(107–112). Therefore, following trauma
(108,109), gene-environment interplay
(108) and epigenetic mechanisms
(110,111) may alter the expression of genes
implicated in neurodevelopment, the stress reaction and synaptic
transmission, and could increase the incidence of psychosis through
their influence on neurotransmitters, the immune response and
subsequent oxidative stress (112). Within this context, elevated
glucocorticoid signaling has been found to induce acceleration of
DNA methylation age, leading to hippocampal atrophy (110). Both DNA hypermethylation and
hypomethylation, non-coding of microRNAs and long-chain non-coding
RNAs, and histone modification are among the types of epigenetic
mechanisms that have been reported to be associated with SCH
(111). In addition, Zn
deficiency may result in oxidative stress and abnormal immune
response, which leads to cell apoptosis (113).
Notably, it has been indicated that early adversity
may alter the HPA axis, leading to an abnormal stress reaction
(114), and amplified sensitivity
to potential stressors in adolescence and adulthood (115–117), thus stimulating the incidence of
SCH symptoms through dopaminergic hyperactivity (118). In addition, extended contact with
stress and glucocorticoids may ensue a decrease in hippocampal
volume (119) and decreased
brain-derived neurotrophic factor levels, as detected in SCH
(120–125).
Individuals who experience metal dysregulation
during early placental nutrition are more susceptible to memory
disorders and psychotic symptomatology (79,80,126). Velthorst et al (79) reported that lower Zn levels (as
assessed with tooth biomarkers) in the final prenatal weeks were
associated with significantly elevated positive and general PANSS
scores. Notably, contact with inflammation throughout gestation may
have perpetuating behavioral and neuronal outcomes in children
(127). Placental inflammation
during fetal development has been suggested to account for
nutritional disruption and metal dyshomeostasis of the fetus
(128,129); in a previous study, fetal Zn
deficiency has been reported to induce epigenetic alterations in
the gene coding for the metal transporter, metallothionein-2, which
also regulates other metals (130). In addition, Tellez-Merlo et
al (131) revealed that
LPS-treated rats developed behavioral abnormalities along with
elevations in Zn and nitric oxide brain concentrations;
furthermore, post-pubertal neuronal hypertrophy was detected in the
PFC and basolateral amygdala, and decreased spine density in the
nucleus accumbens. In a study by Camacho-Abrego et al
(132), an increase in nitric
oxide, Zn and metallothionein levels was found in pre-pubertal rats
with neonatal ventral hippocampus lesions (an animal model of SCH),
particularly in the lesion. Post puberty, the observed changes were
considered to be the final result of the excitotoxic neonatal
ventral hippocampus lesions, resulting in lower levels of the
neuroprotective molecule metallothionein in the PFC, and an
increase in the levels of nitric oxide and Zn in the PFC, both of
which have an excitotoxic effect at high levels. In another study
by Savareh et al (103),
an animal model of SCH was used to demonstrate the beneficial
effect of Zn supplementation during pregnancy to protect against
LPS-induced inflammation in the hippocampus of adult rats. It has
been proposed that decreased Zn levels within the hippocampus may
result in the activation of the HPA axis (45), and the concurrent production of the
NMDAR agonist quinolinic acid; consequently, there is an elevation
in NMDAR activity, which results in increased glutamate release and
neurotoxicity. Decreased Zn levels in patients with SCH and bipolar
disorder who exhibit enduring oxidative stress do not permit
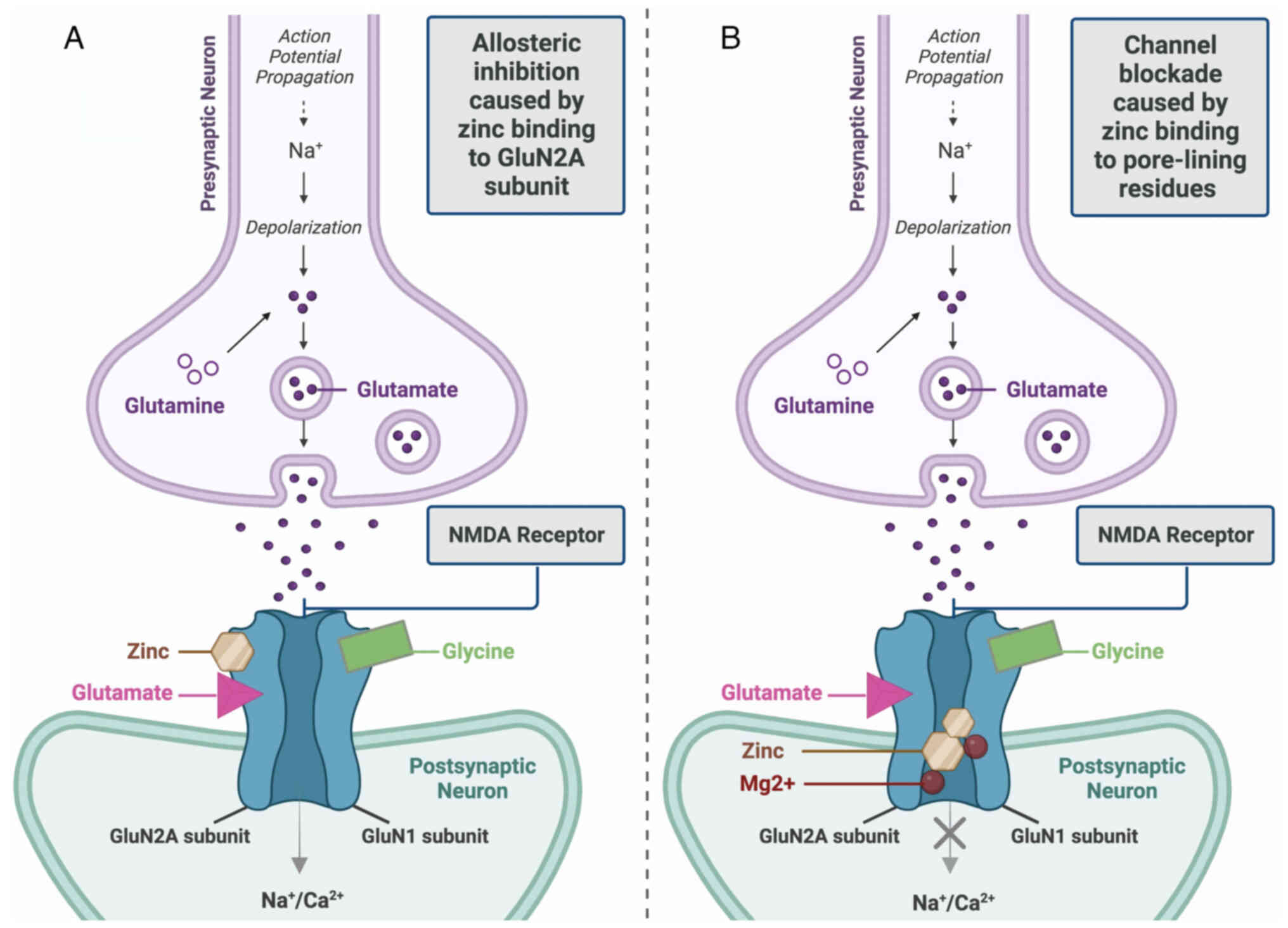
effective inhibition of the NMDAR (46). There are two ways that the
inhibitory effects of Zn on NMDARs unfold (Fig. 1). First, allosteric inhibition is
caused by Zn binding to the GluN2A subunit of the NMDAR, which
diminishes the possibility of the channel opening. Second,
low-affinity binding to pore-lining residues of NMDAR blocks the
channel.
The hypothesis of NMDAR hypofunction in SCH
originated from the observation that a sub-class of non-competitive
NMDAR antagonists, phencyclidine (PCP) and ketamine, induces
behaviors suggestive of all three symptoms of schizophrenia in
human subjects (positive, negative, and cognitive) (33,133,134) (Fig.
1). Functional NMDAR blockade appears to occur in cortical
GABAergic interneurons in both PCP/ketamine drug abuse and
anti-NMDAR encephalitis.
Zn is considered a possible diagnostic biomarker
associated with SCH since its altered homeostasis can contribute to
abnormal glutamatergic neurotransmission, inflammation,
neurodegeneration and autoimmune abnormalities. It has been
proposed by researchers that a number of patients with SCH could
benefit from the use of Zn alone (94), or in combination with vitamins C, E
and B6 (96,98,99).
Furthermore, studies have suggested that prenatal supplementation
of Zn during the gestation period may mitigate LPS-induced rat
models of maternal immune activation (101–104). Notably, in a number of animal
studies, Zn has been shown to exert an antipsychotic therapeutic
effect on rats and mice (100,105,106), and when supplemented in rats
during pregnancy it may mitigate LPS-induced abnormalities in
working memory, GAD67 mRNA levels, object recognition and
inflammation in the hippocampus (101–103). More studies are required to
determine whether Zn can also mitigate LPS-induced abnormalities in
humans.
Notably, there are just a few human RCTs exploring
the effect of Zn treatment on patients with psychosis. Mortazavi
et al (94) demonstrated an
increased antipsychotic efficacy (positive results regarding PANSS
subscale scores and aggression) of a combination of Zn and
risperidone in patients with psychosis vs. controls. Similar
results to those of Mortazavi et al (94) were reported by Tokdemir et
al (62); this previous study
reported that mean plasma Zn values were significantly lower in
criminal subjects with SCH vs. noncriminal subjects with SCH.
Furthermore, Walsh et al (135) reported that serum Cu/plasma Zn
concentration in young men with violent behavior was 1.40 compared
with 1.02 in noncriminal controls. Estimating Zn and Cu plasma
concentrations in patients with psychosis exhibiting aggression,
and treatment of these patients with Zn may prove helpful in the
mitigation of this symptom. However, there were limitations in the
study by Mortazavi et al (94): The sample size was small, side
effects were not noted in detail, there was a short follow-up
period, and plasma Zn concentrations were not available. In the
study by Russo and de Vito (98)
an improvement only in anxiety was observed in patients with SCH
following administration of Zn in combination with vitamins C, E,
and B6. Future studies that include an increased number of patients
from various countries, with a longer follow-up period than that
used in the previous study (94)
could provide more information regarding the therapeutic use of Zn
in psychosis. Furthermore, a specific treatment target for these
studies could be aggression in patients with psychosis, as it was
demonstrated in Mortazavi et al (94) and Tokdemir et al (62). In conclusion, Zn may help a number
of patients with psychosis by alleviating psychotic symptoms;
consequently, patients may demonstrate better adherence to
treatment (17), while the
quantity of psychotic drugs needed could be reduced leading to
fewer adverse effects.
Not applicable.
Funding: No funding was received.
Not applicable.
CT and ER contributed to the conception and design
of the review, and the acquisition, analysis or interpretation of
data that were included. CT and ER were also involved in the
drafting of the manuscript, and in revising it critically for
important intellectual content. MD, MIS, EA, MM, VZ, MP, NS and DAS
contributed to the design of the review. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Not applicable.
Not applicable.
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
|
1
|
Adamo AM and Oteiza PI: Zinc deficiency
and neurodevelopment: The case of neurons. Biofactors. 36:117–124.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joe P, Getz M, Redman S, Petrilli M, Kranz
TM, Ahmad S and Malaspina D: Serum zinc levels in acute psychiatric
patients: A case series. Psychiatry Res. 261:344–350. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stachowicz K: Regulation of COX-2
expression by selected trace elements and heavy metals: Health
implications, and changes in neuronal plasticity. A review. J Trace
Elem Med Biol. 79:1272262023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pfeiffer CC and Iliev V: A study of Zn
deficiency and copper excess in the schizophrenias. Pfeiffer CC:
Neurobiology of Trace Metals Zinc and Copper. Academic Press; New
York: pp. 141–165. 1972
|
|
5
|
Scassellati C, Bonvicini C, Benussi L,
Ghidoni R and Squitti R: Neurodevelopmental disorders: Metallomics
studies for the identification of potential biomarkers associated
to diagnosis and treatment. J Trace Elem Med Biol. 60:1264992020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakashima AS and Dyck RH: Zinc and
cortical plasticity. Brain Res Rev. 59:347–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Młyniec K, Davies CL, de Agüero Sánchez
IG, Pytka K, Budziszewska B and Nowak G: Essential elements in
depression and anxiety. Part I. Pharmacol Rep. 66:534–544. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawahara M, Tanaka KI and Kato-Negishi M:
Zinc, carnosine, and neurodegenerative diseases. Nutrients.
10:1472018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tamano H and Takeda A: Age-dependent
modification of intracellular Zn2+ buffering in the
hippocampus and its impact. Biol Pharm Bull. 42:1070–1075. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moynahan EJ: Letter: Zinc deficiency and
disturbances of mood and visual behaviour. Lancet. 1:911976.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prasad AS: Discovery of human zinc
deficiency: Its impact on human health and disease. Adv Nutr.
4:176–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Um P, Dickerman BA and Liu J:
Zinc, magnesium, selenium and depression: A review of the evidence,
potential mechanisms and implications. Nutrients. 10:5842018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teschke R: Aluminum, arsenic, beryllium,
cadmium, chromium, cobalt, copper, iron, lead, mercury, molybdenum,
nickel, platinum, thallium, titanium, vanadium, and zinc: Molecular
aspects in experimental liver injury. Int J Mol Sci. 23:122132022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chauhan AK, Mittra N, Patel DV and Singh
C: Cyclooxygenase-2 directs microglial activation-mediated
inflammation and oxidative stress leading to intrinsic apoptosis in
Zn-induced parkinsonism. Mol Neurobiol. 55:2162–2173. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimura K and Kumura J: Preliminary reports
on the metabolism of trace elements in neuro psychiatric diseases.
I. Zinc in schizophrenia. Proc Jap Acad Sci. 41:943–947. 1965.
View Article : Google Scholar
|
|
16
|
Grønli O, Kvamme JM, Friborg O and Wynn R:
Zinc deficiency is common in several psychiatric disorders. PLoS
One. 8:e827932013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrilli MA, Kranz TM, Kleinhaus K, Joe P,
Getz M, Johnson P, Chao MV and Malaspina D: The emerging role for
zinc in depression and psychosis. Front Pharmacol. 8:4142017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murray RM and Lewis SW: Is schizophrenia a
neurodevelopmental disorder? Br Med J (Clin Res Ed). 295:681–682.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weinberger DR: Implications of normal
brain development for the pathogenesis of schizophrenia. Arch Gen
Psychiatry. 44:660–669. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sandstead HH, Frederickson CJ and Penland
JG: History of zinc as related to brain function. J Nutr. 130 (2S
Suppl):496S–502S. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeda A and Tamano H: Insight into zinc
signaling from dietary zinc deficiency. Brain Res Rev. 62:33–44.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han S, Gilmartin M, Sheng W and Jin VX:
Integrating rare variant genetics and brain transcriptome data
implicates novel schizophrenia putative risk genes. Schizophr Res.
276:205–213. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McLardy T: Hippocampal zinc in chronic
alcoholism and schizophrenia. IRCS Med Sci. 2:10101973.
|
|
24
|
Adams CE, Demasters B and Freedman R:
Regional zinc staining in postmortem hippocampus from schizophrenic
patients. Schizophr Res. 18:71–77. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kornhuber J, Lange KW, Kruzik P, Rausch
WD, Gabriel E, Jellinger K and Riederer P: Iron, copper, zinc,
magnesium, and calcium in postmortem brain tissue from
schizophrenic patients. Biol Psychiatry. 36:31–34. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li D, Achkar JP, Haritunians T, Jacobs JP,
Hui KY, D'Amato M, Brand S, Radford-Smith G, Halfvarson J, Niess
JH, et al: A pleiotropic missense variant in SLC39A8 is associated
with Crohn's disease and human gut microbiome composition.
Gastroenterology. 151:724–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pickrell JK, Berisa T, Liu JZ, Ségurel L,
Tung JY and Hinds DA: Detection and interpretation of shared
genetic influences on 42 human traits. Nat Genet. 48:709–717. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marger L, Schubert CR and Bertrand D:
Zinc: An underappreciated modulatory factor of brain function.
Biochem Pharmacol. 91:426–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Theleritis C, Stefanou MI, Demetriou M,
Alevyzakis E, Triantafyllou K, Smyrnis N, Spandidos DA and Rizos E:
Association of gut dysbiosis with first-episode psychosis (review).
Mol Med Rep. 30:1302024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steiner J, Jacobs R, Panteli B, Brauner M,
Schiltz K, Bahn S, Herberth M, Westphal S, Gos T, Walter M, et al:
Acute schizophrenia is accompanied by reduced T cell and increased
B cell immunity. Eur Arch Psychiatry Clin Neurosci. 260:509–518.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carrera N, Arrojo M, Sanjuán J, Ramos-Ríos
R, Paz E, Suárez-Rama JJ, Páramo M, Agra S, Brenlla J, Martínez S,
et al: Association study of nonsynonymous single nucleotide
polymorphisms in schizophrenia. Biol Psychiatry. 71:169–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Ma C, Li Y, Chen R, Liu Y, Wan LP,
Xiong Q, Wang C, Huo Y, Dang X, et al: The schizophrenia-associated
missense variant rs13107325 regulates dendritic spine density.
Transl Psychiatry. 12:3612022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tseng WC, Reinhart V, Lanz TA, Weber ML,
Pang J, Le KXV, Bell RD, O'Donnell P and Buhl DL:
Schizophrenia-associated SLC39A8 polymorphism is a loss-of-function
allele altering glutamate receptor and innate immune signaling.
Transl Psychiatr. 11:1362021. View Article : Google Scholar
|
|
34
|
Scarr E, Udawela M, Greenough MA, Neo J,
Suk SM, Money TT, Upadhyay A, Bush AI, Everall IP, Thomas EA and
Dean B: Increased cortical expression of the zinc transporter
SLC39A12 suggests a breakdown in zinc cellular homeostasis as part
of the pathophysiology of schizophrenia. NPJ Schizophr.
2:160022016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Perez-Becerril C, Morris AG, Mortimer A,
McKenna PJ and de Belleroche J: Allelic variants in the zinc
transporter-3 gene, SLC30A3, a candidate gene identified from gene
expression studies, show gender-specific association with
schizophrenia. Eur Psychiatry. 29:172–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Y, Hu D, Liang J, Bao YP, Meng SQ, Lu
L and Shi J: Association between variants of zinc finger genes and
psychiatric disorders: Systematic review and meta-analysis.
Schizophr Res. 162:124–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lima VB, Sampaio Fde A, Bezerra DL, Moita
Neto JM and Marreiro Ddo N: Parameters of glycemic control and
their relationship with zinc concentrations in blood and with
superoxide dismutase enzyme activity in type 2 diabetes patients.
Arq Bras Endocrinol Metabol. 55:701–707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Noleto Magalhães RC, Guedes Borges de
Araujo C, Batista de Sousa Lima V, Machado Moita Neto J, do
Nascimento Nogueira N and do Nascimento Marreiro D: Nutritional
status of zinc and activity superoxide dismutase in chronic renal
patients undergoing hemodialysis. Nutr Hosp. 26:1456–1461.
2011.PubMed/NCBI
|
|
39
|
Marreiro DDN, Cruz KJC, Morais JBS,
Beserra JB, Severo JS and de Oliveira ARS: Zinc and oxidative
stress: Current mechanisms. Antioxidants (Basel). 6:242017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Skalny AV, Rink L, Ajsuvakova OP, Aschner
M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos
DA, Aaseth J, et al: Zinc and respiratory tract infections:
Perspectives for COVID-19 (review). Int J Mol Med. 46:17–26.
2020.PubMed/NCBI
|
|
41
|
Consolo M, Amoroso A, Spandidos DA and
Mazzarino MC: Matrix metalloproteinases and their inhibitors as
markers of inflammation and fibrosis in chronic liver disease
(review). Int J Mol Med. 24:143–152. 2009.PubMed/NCBI
|
|
42
|
Tsatsakis A, Renieri E, Tsoukalas D, Buga
AM, Sarandi E, Vakonaki E, Fragkiadaki P, Alegakis A, Nikitovic D,
Calina D, et al: A novel nutraceutical formulation increases
telomere length and activates telomerase activity in middle-aged
rats. Mol Med Rep. 28:2322023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsoukalas D, Buga AM, Docea AO, Sarandi E,
Mitrut R, Renieri E, Spandidos DA, Rogoveanu I, Cercelaru L,
Niculescu M, et al: Reversal of brain aging by targeting
telomerase: A nutraceutical approach. Int J Mol Med. 48:1992021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Santa Cruz EC, Madrid KC, Arruda MAZ and
Sussulini A: Association between trace elements in serum from
bipolar disorder and schizophrenia patients considering treatment
effects. J Trace Elem Med Biol. 59:1264672020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nowak G: Does interaction between zinc and
glutamate system play a significant role in the mechanism of
antidepressant action? Acta Pol Pharm. 58:73–75. 2001.PubMed/NCBI
|
|
46
|
Prakash A, Bharti K and Majeed AB: Zinc:
indications in brain disorders. Fundam Clin Pharmacol. 29:131–149.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Salim S: Oxidative stress and
psychological disorders. Curr Neuropharmacol. 12:140–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Akarsu S, Bolu A, Aydemir E, Znir SB, Kurt
YG, Znir S, Erdem M and Uzun Ö: The relationship between the number
of manic episodes and oxidative stress indicators in bipolar
disorder. Psychiatry Investig. 15:514–519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo CH, Chen PC, Yeh MS, Hsiung DY and
Wang CL: Cu/Zn ratios are associated with nutritional status,
oxidative stress, inflammation, and immune abnormalities in
patients on peritoneal dialysis. Clin Biochem. 44:275–280. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kunz M, Gama CS, Andreazza AC, Salvador M,
Ceresér KM, Gomes FA, Belmonte-de-Abreu PS, Berk M and Kapczinski
F: Elevated serum superoxide dismutase and thiobarbituric acid
reactive substances in different phases of bipolar disorder and in
schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry.
32:1677–1681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hendouei N, Farnia S, Mohseni F, Salehi A,
Bagheri M, Shadfar F, Barzegar F, Hoseini SD, Charati JY and Shaki
F: Alterations in oxidative stress markers and its correlation with
clinical findings in schizophrenic patients consuming perphenazine,
clozapine and risperidone. Biomed Pharmacother. 103:965–972. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Al-Hakeim HK, Al-Musawi AF, Al-Mulla A,
Al-Dujaili AH, Debnath M and Maes M: The
interleukin-6/interleukin-23/T helper 17-axis as a driver of
neuro-immune toxicity in the major neurocognitive psychosis or
deficit schizophrenia: A precision nomothetic psychiatry analysis.
PLoS One. 17:e02758392022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Al-Hakeim HK, Altufaili MF, Almulla AF,
Moustafa SR and Maes M: Increased lipid peroxidation and lowered
antioxidant defenses predict methamphetamine induced psychosis.
Cells. 11:36942022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pfeiffer CC and Bacchi D: Copper, zinc,
manganese, niacin and pyridoxine in the schizophrenias. Appl Nutr.
27:9–39. 1975.
|
|
55
|
Srinivasan DP, Marr S, Wareing RA and
Birch NJ: Magnesium Zn and copper in acute psychiatric patients.
Mag Bull. 4:45–48. 1982.
|
|
56
|
Gillin JC, Carpenter WT, Hambidge KM,
Wyatt RJ and Henkin RI: Zinc and copper in patients with
schizophrenia. Encephale. 8:435–444. 1982.PubMed/NCBI
|
|
57
|
Potkin SG, Shore D, Torrey EF, Weinberger
DR, Gillin JC, Henkin RI, Agarwal RP and Wyatt RJ: Cerebrospinal
fluid zinc concentrations in ex-heroin addicts and patients with
schizophrenia: Some preliminary observations. Biol Psychiatry.
17:1315–1322. 1982.PubMed/NCBI
|
|
58
|
Vaddadi KS, Gilleard CJ, Mindham RH and
Butler R: A controlled trial of prostaglandin E1 precursor in
chronic neuroleptic resistant schizophrenic patients.
Psychopharmacology (Berl). 88:362–367. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Craven C, Duggan PF, Buckley N and
Gaughran F: Serum zinc levels in patients with schizophrenia and
their mothers. Schizophr Res. 26:83–84. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Herrán A, García-Unzueta MT,
Fernández-González MD, Vázquez-Barquero JL, Alvarez C and Amado JA:
Higher levels of serum copper in schizophrenic patients treated
with depot neuroleptics. Psychiatry Res. 94:51–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Stanley PC and Wakwe VC: Toxic trace
metals in the mentally ill patients. Niger Postgrad Med J.
9:199–204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tokdemir M, Polat SA, Acik Y, Gursu F,
Cikim G and Deniz O: Blood zinc and copper concentrations in
criminal and noncriminal schizophrenic men. Arch Androl.
49:365–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nechifor M, Vaideanu C, Palamaru I, Borza
C and Mindreci I: The influence of some antipsychotics on
erythrocyte magnesium and plasma magnesium, calcium, copper and
zinc in patients with paranoid schizophrenia. J Am Coll Nutr.
23:549S–551S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yanik M, Kocyigit A, Tutkun H, Vural H and
Herken H: Plasma manganese, selenium, zinc, copper, and iron
concentrations in patients with schizophrenia. Biol Trace Elem Res.
98:109–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Farzin D, Mansouri N and Yazdani T:
Elevated plasma copper/zinc ratios in patients with schizophrenia.
Eur Neuropsychopharmacol. 16:S364–S365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Devi PU, Chinnaswamy P, Murugan S and
Selvi S: Plasma levels of trace elements in patients with different
symptoms of schizophrenia. Biosci Biotechnol Res Asia. 5:261–268.
2008.
|
|
67
|
Rahman A, Azad MAK, Hossain I, Qusar MMAS,
Bari W, Begum F, Huq SMI and Hasnat A: Zinc, manganese, calcium,
copper, and cadmium level in scalp hair samples of schizophrenic
patients. Biol Trace Elem Res. 127:102–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ghanem AEA, Ali EMM, El-Bakary AA, El
Morsi D, Elkanishi SMH, Saleh ES and El-Said H: Copper and Zinc
levels in hair of both schizophrenic and depressed. Mansoura J
Forensic Med Clin Toxicol. 17:89–102. 2009. View Article : Google Scholar
|
|
69
|
Arinola G, Idonije B, Akinlade K and
Ihenyen O: Essential trace metals and heavy metals in newly
diagnosed schizophrenic patients and those on anti-psychotic
medication. J Res Med Sci. 15:245–249. 2010.PubMed/NCBI
|
|
70
|
Kaya B, Akdağ N, Fadıllıoğlu E, Taycan SE,
Emre MH, Unal S, Sayal A, Erdoğan H and Polat R: Elements levels
and glucose-6-phosphate dehydrogenase activity in blood of patients
with schizophrenia. J Psychiatry Neurol Sci. 25:198–205. 2012.
|
|
71
|
Cai L, Chen T, Yang J, Zhou K, Yan X, Chen
W, Sun L, Li L, Qin S, Wang P, et al: Serum trace element
differences between schizophrenia patients and controls in the Han
Chinese population. Sci Rep. 5:150132015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Olabanji O, Ngila JC, Msagati TAM, Oluyemi
EA, Fatoye FO and Mamba BB: Effect of metal poisoning and the
implications of gender and age on the elemental composition in
patients with mental behavioural disorders. Afr J Biotechnol.
10:3585–3593. 2011.
|
|
73
|
Vidović B, Dorđević B, Milovanović S,
Škrivanj S, Pavlović Z, Stefanović A and Kotur-Stevuljević J:
Selenium, zinc, and copper plasma levels in patients with
schizophrenia: relationship with metabolic risk factors. Biol Trace
Elem Res. 156:22–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sharma SK, Sood S, Sharma A and Gupta ID:
Estimation of serum zinc and copper levels patients with
schizophrenia: A preliminary study. SL J Psychiatry. 5:14–17.
2013.
|
|
75
|
Asare G, Tetteh R, Amedonu E, Asiedu B and
Doku D: Toxicity, deficiency and dysmetabolism of trace elements in
Ghanaian clinically stable schizophrenics. Open Access Maced J Med
Sci. 2:293–298. 2014. View Article : Google Scholar
|
|
76
|
Nawaz R, Zahir E, Siddiqui S, Usmani A and
Shad KF: The role of trace metals and environmental factors in the
onset and progression of schizophrenia in Pakistani population.
World J Neurosci. 4:450–460. 2014. View Article : Google Scholar
|
|
77
|
Liu T, Lu QB, Yan L, Guo J, Feng F, Qiu J
and Wang J: Comparative study on serum levels of 10 trace elements
in schizophrenia. PLoS One. 10:e01336222015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lin T, Liu T, Lin Y, Yan L, Chen Z and
Wang J: Comparative study on serum levels of macro and trace
elements in schizophrenia based on supervised learning methods. J
Trace Elem Med Biol. 43:202–208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Velthorst E, Smith L, Bello G, Austin C,
Gennings C, Modabbernia A, Franke N, Frangou S, Wright R, de Haan
L, et al: New research strategy for measuring pre- and postnatal
metal dysregulation in psychotic disorders. Schizophr Bull.
43:1153–1157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Modabbernia A, Velthorst E, Gennings C, De
Haan L, Austin C, Sutterland A, Mollon J, Frangou S, Wright R,
Arora M and Reichenberg A: Early-life metal exposure and
schizophrenia: A proof-of-concept study using novel tooth-matrix
biomarkers. Eur Psychiatry. 36:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen X, Li Y, Zhang T, Yao Y, Shen C and
Xue Y: Association of serum trace elements with schizophrenia and
effects of antipsychotic treatment. Biol Trace Elem Res. 181:22–30.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li Z, Liu Y, Li X, Ju W, Wu G, Yang X, Fu
X and Gao X: Association of elements with schizophrenia and
intervention of selenium supplements. Biol Trace Elem Res.
183:16–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cao B, Yan L, Ma J, Jin M, Park C, Nozari
Y, Kazmierczak OP, Zuckerman H, Lee Y, Pan Z, et al: Comparison of
serum essential trace metals between patients with schizophrenia
and healthy controls. J Trace Elem Med Biol. 51:79–85. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ma J, Yan L, Guo T, Yang S, Liu Y, Xie Q,
Ni D and Wang J: Association between serum essential metal elements
and the risk of schizophrenia in China. Sci Rep. 10:108752020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
de Souza Pessôa G, de Jesus JR, Balbuena
TS and Arruda MAZ: Metallomics-based platforms for comparing the
human blood serum profiles between bipolar disorder and
schizophrenia patients. Rapid Commun Mass Spectrom. 34 (Suppl
3):e86982020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Uddin SMN, Sultana F, Uddin MG, Dewan SMR,
Hossain MK and Islam MS: Effect of antioxidant, malondialdehyde,
macro-mineral, and trace element serum concentrations in
Bangladeshi patients with schizophrenia: A case-control study.
Health Sci Rep. 4:e2912021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Awais MH, Aamir M, Bibi A, Ali S, Ahmed W
and Safdar SA: Association of trace metals in patients with
schizophrenia. J Coll Physicians Surg Pak. 32:193–196. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lotan A, Luza S, Opazo CM, Ayton S, Lane
DJR, Mancuso S, Pereira A, Sundram S, Weickert CS, Bousman C, et
al: Perturbed iron biology in the prefrontal cortex of people with
schizophrenia. Mol Psychiatry. 28:2058–2070. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dos Santos AB, Bezerra MA, Rocha ME,
Barreto GE and Kohlmeier KA: Higher zinc concentrations in hair of
Parkinson's disease are associated with psychotic complications and
depression. J Neural Transm (Vienna). 126:1291–1301. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tabata K, Miyashita M, Yamasaki S, Toriumi
K, Ando S, Suzuki K, Endo K, Morimoto Y, Tomita Y, Yamaguchi S, et
al: Hair zinc levels and psychosis risk among adolescents.
Schizophrenia (Heidelb). 8:1072022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Joe P, Petrilli M, Malaspina D and
Weissman J: Zinc in schizophrenia: A meta-analysis. Gen Hosp
Psychiatry. 53:19–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zaks N, Austin C, Arora M and Reichenberg
A: Reprint of: Elemental dysregulation in psychotic spectrum
disorders: A review and research synthesis. Schizophr Res.
247:33–40. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
da Paulsen Bda S, Cardoso SC, Stelling MP,
Cadilhe DV and Rehen SK: Valproate reverts zinc and potassium
imbalance in schizophrenia-derived reprogrammed cells. Schizophr
Res. 154:30–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mortazavi M, Farzin D, Zarhghami M,
Hosseini SH, Mansoori P and Nateghi G: Efficacy of zinc sulfate as
an add-on therapy to risperidone versus risperidone alone in
patients with schizophrenia: A double-blind randomized
placebo-controlled trial. Iran J Psychiatry Behav Sci. 9:e8532015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pfeiffer CC and Sohler A: Treatment of
pyroluric schizophrenia with large doses of pyridoxine and a
dietary supplement of zinc. J Orthomol Med. 3:292–300. 1974.
|
|
96
|
Grabrucker AM and Rowan Garner CC:
Brain-delivery of zinc-ions as potential treatment for neurological
diseases: Mini review. Drug Deliv Lett. 1:13–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Rohde J, Claussen MC, Kuechenhoff B,
Seifritz E and Schuepbach D: Combined symptomatology of psychosis,
pica syndrome, and hippocampal sclerosis: A case report. Int J Eat
Disord. 46:89–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Russo AJ and de Vito R: Decreased serum
hepatocyte growth factor (HGF) in individuals with schizophrenia
normalizes after zinc and B-6 therapy. Proteomics Insights.
3:71–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Russo A: Decreased serum hepatocyte growth
factor (HGF) in individuals with bipolar disorder normalizes after
zinc and anti-oxidant therapy. Nutr Metab Insights. 3:49–55. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Czerniak P and Haim DB: Phenothiazine
derivatives and brain zinc. Turnover radioactive isotope study.
Arch Neurol. 24:555–560. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Alizadeh F, Davoodian N, Kazemi H,
Ghasemi-Kasman M and Shaerzadeh F: Prenatal zinc supplementation
attenuates lipopolysaccharide-induced behavioral impairments in
maternal immune activation model. Behav Brain Res. 377:1122472020.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mousaviyan R, Davoodian N, Alizadeh F,
Ghasemi-Kasman M, Mousavi SA, Shaerzadeh F and Kazemi H: Zinc
supplementation during pregnancy alleviates
lipopolysaccharide-induced glial activation and inflammatory
markers expression in a rat model of maternal immune activation.
Biol Trace Elem Res. 199:4193–4204. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Savareh E, Davoodian N, Mousaviyan R,
Ghasemi-Kasman M, Atashabparvar A and Eftekhar E: Prenatal zinc
supplementation ameliorates hippocampal astrocytes activation and
inflammatory cytokines expression induced by lipopolysaccharide in
a rat model of maternal immune activation. Basic Clin Neurosci.
13:335–347. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Coyle P, Tran N, Fung JNT, Summers BL and
Rofe AM: Maternal dietary zinc supplementation prevents aberrant
behaviour in an object recognition task in mice offspring exposed
to LPS in early pregnancy. Behav Brain Res. 197:210–218. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Onaolapo OJ, Ademakinwa OQ, Olalekan TO
and Onaolapo AY: Ketamine-induced behavioural and brain oxidative
changes in mice: An assessment of possible beneficial effects of
zinc as mono- or adjunct therapy. Psychopharmacology (Berl).
234:2707–2725. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Joshi M, Akhtar M, Najmi AK, Khuroo AH and
Goswami D: Effect of zinc in animal models of anxiety, depression
and psychosis. Hum Exp Toxicol. 31:1237–1243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bayer TA, Falkai P and Maier W: Genetic
and non-genetic vulnerability factors in schizophrenia: The basis
of the ‘two hit hypothesis’. J Psychiatr Res. 33:543–548. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Giannopoulou I, Georgiades S, Stefanou MI,
Spandidos DA and Rizos E: Links between trauma and psychosis
(review). Exp Ther Med. 26:3862023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Morgan C, Charalambides M, Hutchinson G
and Murray RM: Migration, ethnicity, and psychosis: Toward a
sociodevelopmental model. Schizophr Bull. 36:655–664. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Davis EG, Humphreys KL, McEwen LM, Sacchet
MD, Camacho MC, MacIsaac JL, Lin DTS, Kobor MS and Gotlib IH:
Accelerated DNA methylation age in adolescent girls: Associations
with elevated diurnal cortisol and reduced hippocampal volume.
Transl Psychiatry. 7:e12232017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chen Q, Li D, Jin W, Shi Y, Li Z, Ma P,
Sun J, Chen S, Li P and Lin P: Research progress on the correlation
between epigenetics and schizophrenia. Front Neurosci.
15:6887272021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Alameda L, Rodriguez V, Carr E, Aas M,
Trotta G, Marino P, Vorontsova N, Herane-Vives A, Gadelrab R,
Spinazzola E, et al: A systematic review on mediators between
adversity and psychosis: Potential targets for treatment. Psychol
Med. 50:1966–1976. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Fraker PJ and King LE: Reprogramming of
the immune system during zinc deficiency. Annu Rev Nutr.
24:277–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Charmandari E, Kino T, Souvatzoglou E and
Chrousos GP: Pediatric stress: Hormonal mediators and human
development. Horm Res. 59:161–179. 2003.PubMed/NCBI
|
|
115
|
Lardinois M, Lataster T, Mengelers R, Van
Os J and Myin-Germeys I: Childhood trauma and increased stress
sensitivity in psychosis. Acta Psychiatr Scand. 123:28–35. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Walker EF, Brennan PA, Esterberg M,
Brasfield J, Pearce B and Compton MT: Longitudinal changes in
cortisol secretion and conversion to psychosis in at-risk youth. J
Abnorm Psychol. 119:401–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Walker EF, Trotman HD, Pearce BD,
Addington J, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH,
Perkins DO, Seidman LJ, et al: Cortisol levels and risk for
psychosis: Initial findings from the North American prodrome
longitudinal study. Biol Psychiatry. 74:410–417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sapolsky RM: Glucocorticoids and
hippocampal atrophy in neuropsychiatric disorders. Arch Gen
Psychiatry. 57:925–935. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Vita A, De Peri L, Silenzi C and Dieci M:
Brain morphology in first-episode schizophrenia: A meta-analysis of
quantitative magnetic resonance imaging studies. Schizophr Res.
82:75–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Thompson Ray M, Weickert CS, Wyatt E and
Webster MJ: Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in
the hippocampus of individuals with schizophrenia and mood
disorders. J Psychiatry Neurosci. 36:195–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Daskalakis NP, De Kloet ER, Yehuda R,
Malaspina D and Kranz TM: Early life stress effects on
glucocorticoid-BDNF interplay in the hippocampus. Front Mol
Neurosci. 8:682015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Rizos EN, Rontos I, Laskos E, Arsenis G,
Michalopoulou PG, Vasilopoulos D, Gournellis R and Lykouras L:
Investigation of serum BDNF levels in drug-naive patients with
schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry.
32:1308–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rizos EN, Papathanasiou M, Michalopoulou
PG, Mazioti A, Douzenis A, Kastania A, Nikolaidou P, Laskos E,
Vasilopoulou K and Lykouras L: Association of serum BDNF levels
with hippocampal volumes in first psychotic episode drug-naive
schizophrenic patients. Schizophr Res. 129:201–204. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Rizos EN, Michalopoulou PG, Siafakas N,
Stefanis N, Douzenis A, Rontos I, Laskos E, Kastania A, Zoumpourlis
V and Lykouras L: Association of serum brain-derived neurotrophic
factor and duration of untreated psychosis in first-episode
patients with schizophrenia. Neuropsychobiology. 62:87–90. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Theleritis C, Fisher HL, Shäfer I, Winters
L, Stahl D, Morgan C, Dazzan P, Breedvelt J, Sambath I, Vitoratou
S, et al: Brain derived neurotropic factor (BDNF) is associated
with childhood abuse but not cognitive domains in first episode
psychosis. Schizophr Res. 159:56–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Tsang BL, Holsted E, McDonald CM, Brown
KH, Black R, Mbuya MNN, Grant F, Rowe LA and Manger MS: Effects of
foods fortified with zinc, alone or cofortified with multiple
micronutrients, on health and functional outcomes: A systematic
review and meta-analysis. Adv Nutr. 12:1821–1837. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Flores G, Morales-Medina JC and Diaz A:
Neuronal and brain morphological changes in animal models of
schizophrenia. Behav Brain Res. 301:190–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bronson SL and Bale TL: Prenatal
stress-induced increases in placental inflammation and offspring
hyperactivity are male-specific and ameliorated by maternal
antiinflammatory treatment. Endocrinology. 155:2635–2646. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Walker CK, Ashwood P and Hertz-Picciotto
I: Preeclampsia, placental insufficiency, autism, and
antiphospholipid antibodies-reply. JAMA Pediatr. 169:606–607. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kurita H, Ohsako S, Hashimoto S, Yoshinaga
J and Tohyama C: Prenatal zinc deficiency-dependent epigenetic
alterations of mouse metallothionein-2 gene. J Nutr Biochem.
24:256–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tellez-Merlo G, Morales-Medina JC,
Camacho-Ábrego I, Juárez-Díaz I, Aguilar-Alonso P, de la Cruz F,
Iannitti T and Flores G: Prenatal immune challenge induces
behavioral deficits, neuronal remodeling, and increases brain
nitric oxide and zinc levels in the male rat offspring.
Neuroscience. 406:594–605. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Camacho-Abrego I, González-Cano SI,
Aguilar-Alonso P, Brambila E, de la Cruz F and Flores G: Changes in
nitric oxide, zinc and metallothionein levels in limbic regions at
pre-pubertal and post-pubertal ages presented in an animal model of
schizophrenia. J Chem Neuroanat. 111:1018892021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lee K, Mills Z, Cheung P, Cheyne JE and
Montgomery JM: The role of zinc and NMDA receptors in autism
spectrum disorders. Pharmaceuticals (Basel). 16:12022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Paz RD, Tardito S, Atzori M and Tseng KY:
Glutamatergic dysfunction in schizophrenia: From basic neuroscience
to clinical psychopharmacology. Eur Neuropsychopharmacol.
18:773–786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Walsh WJ, Isaacson HR, Rehman F and Hall
A: Elevated blood copper/zinc ratios in assaultive young males.
Physiol Behav. 62:327–329. 1997. View Article : Google Scholar : PubMed/NCBI
|