With the increasing standard of living, cancer has
become a major contributor to the global burden of disease, which
continues to grow worldwide, placing a serious economic burden on
individuals, families and society (1). According to the Global Agency for
Research on Cancer (GARC), 10 cancers accounted for two-thirds of
new cases and mortalities worldwide in 2022. It is estimated that
there will be >35 million new cancer cases in 2050, an increase
of ~77% over the 2022 projections (2,3). In
the face of the rapidly growing cancer burden, in addition to the
urgent need for improvements in public preventive measures, the
enhancement of cancer treatment strategies is equally crucial.
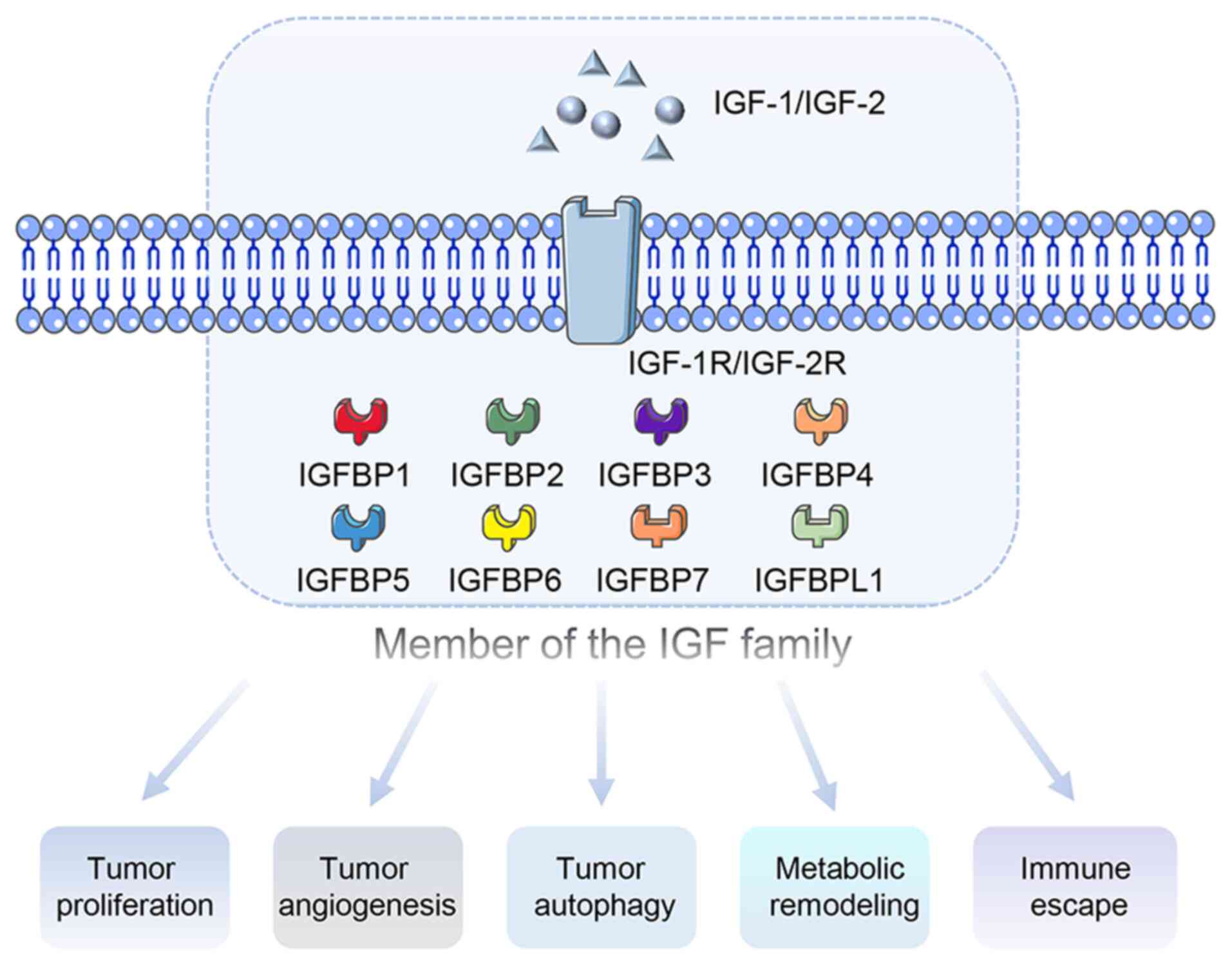
The IGF family consists mainly of two
low-molecular-weight protein (IGF-1 and IGF-2), the corresponding
receptors (IGF-1R and IGF-2R) and specific binding proteins
(4). In 1963, Froesch et al
(5) discovered the presence of a
certain active substance in serum that could not be completely
inhibited by insulin antibodies; this substance was subsequently
purified by two scientists, Rinderknecht and Humbel (6), and named IGF-1 and IGF-2. IGF-1 is a
small polypeptide consisting of 70 amino acids that is synthesized
and secreted into the bloodstream mainly by the liver and some
IGF-1 is acted upon by the kidneys or skeletal muscles in an
autocrine or paracrine manner in their own tissues or periphery
(7). The concentration of IGF1 in
serum is regulated by insulin-like growth factor-binding proteins
(IGFBPs). When IGFBPs are hydrolyzed by proteases, free IGF-1 binds
to IGF-1R on the cell membrane to mediate the corresponding
biological effects (8). In
addition, IGF-1 levels are affected by a number of factors, such as
age, nutritional status and the release of growth hormones
(9). IGF-2 is composed of 67 amino
acids and has growth-promoting activity similar to that of IGF-1,
but its expression pattern is not controlled by growth hormone
(10). IGF-2 is considered to play
a critical role in fetal growth and development. Deficiency of
IGF-1 inhibits the proliferation and protein synthesis of most
cells in the body, predisposing the individual to various diseases,
including metabolic bone disease, cardiovascular disease and
neurodegenerative diseases (11,12).
In addition to inhibiting brain development, reducing IGF-2 affects
cell metabolism and stem cell self-renewal (13,14).
IGFs are involved in multiple stages of cancer
development. IGF-1 serum levels are markedly higher in patients
with advanced gastric cancer than in those with early-stage disease
and are associated with a Helicobacter pylori positive
status (15). Moreover, IGF1R
activated by IGF-1 plays a role in epidermal growth factor receptor
(EGFR)-mediated primary or secondary resistance to colorectal
cancer by upregulating the PI3K/AKT signaling pathway (16). A prospective case-control study
suggests that low serum IGF-2 levels are strongly associated with
hepatocellular carcinoma risk (17). The IGFs system is involved in the
regulation of cancer progression through a variety of signaling
pathways, including the MAPK signaling pathway (18), the PI3K/AKT/mTOR signaling pathway
(19) and the NF-κB signaling
pathway (20). Therefore,
strategies targeting the IGF system may be potential candidates for
anticancer therapeutic options.
Tumor cells have a unique malignant biological
phenotype, that manifests as a continuous proliferation signal,
escape from growth inhibition, unlimited replication ability,
continuous angiogenesis, resistance to cell death, invasion and
metastasis, genomic instability and mutation, immune escape and
other biological phenomena (21).
The number of characteristics of cancer add to the complexity of
cancer treatment. This section provides insights into the possible
mechanisms by which IGFs influence the malignant biological
behavior of cancers, providing new strategies for cancer
therapy.
Continuous proliferation signals, invasion and
metastasis are the main characteristics of the malignant phenotype
of tumors (22). As an effective
mitogen of various cell types, IGF-1 regulates various biological
behaviors of tumor cells by binding to IGF-1R. Studies have shown
that interferon-induced transmembrane protein (IFITM) is positively
associated with gastric cancer progression, recurrence and
mortality (23,24). Knocking down IFITM inhibits the
proliferation, migration, invasion and epithelial mesenchymal
transformation of gastric cancer cells. Further mechanistic studies
suggest that IGF-1 induces IFITM2 expression through IGF-1R/STAT3
signal transduction, which ultimately affects tumor growth and
metastasis (25). In melanoma,
downregulation of IGF-1 reduces the dry character of melanoma
initiating cells, including the expression of dry markers (SOX2,
Oct-3/4, CD24 and CD133) and functional properties (melanosphere
formation, aldehyde dehydrogenase activity and side population) and
eventually inhibiting the proliferation and metastasis of tumor
cells (26). Moreover, in breast
cancer, IGF-1 mainly upregulates cysteine-rich 61 (Cyr61) by
activating the PI3K/AKT pathway and an increase in Cyr61 promotes
the growth and invasion of breast cancer cells (27). Notably, key transcription factors
such as SOX2, which promote and maintain the dry characteristics of
cancer cells, can upregulate the expression and autocrine activity
of IGF-2, and IGF-2 then activates the IGF-1R/AKT signaling pathway
to enhance the invasive and stemness characteristics of bladder
cancer, forming a vicious cycle (28). IGFBP-2, an important member of the
insulin-like growth factor binding protein family, is able to
regulate a variety of cell signaling pathways to influence tumor
progression. In oral cancer, IGFBP-2 promotes the upregulation of
matrix metalloproteinase (MMP)2 and MMP9 through the activation of
the PI3K/Akt/mTOR signaling pathway, which ultimately leads to the
proliferation, migration and invasion of cancer cells (29). In addition, IGFBP-1 can be
upregulated by H. pylori in a dose-dependent manner,
promoting malignant biological processes such as the proliferation,
migration and invasion of gastric cancer cells (30). Moreover, IGFBP-7 is also able to
regulate the proliferation and migration of cancer cells through
the JAK/PI3K signaling axis (31).
In conclusion, these findings suggest that targeting IGFs is
important for inhibiting cancer proliferation and
aggressiveness.
To meet the oxygen and nutrients requirements for
continuous proliferation, tumors grow in a variety of ways to
stimulate the formation of new blood vessels (32). Especially for solid tumors, new
blood vessels are the key link between tumor invasion and
metastasis, which also leads to difficulty in tumor treatment.
Previous studies have shown that IGFs play an important role in
endothelial cell physiology by promoting the expression of the
vasodilators NO, VEGF and hypoxia-inducible factor (HIF) (33,34).
IGF-1R is involved in most pathophysiological processes mediated by
IGFs, including proangiogenic effects. However, IGF-2 also promotes
angiogenesis through the insulin receptor (35). Studies have reported that the use
of bisphosphonates can delay bone metastasis in patients with
breast cancer and improve overall survival (36,37).
Mechanistic exploration suggested that pamidronate and clodronate
markedly inhibit IGF-1-induced HIF-1α protein accumulation and VEGF
expression in breast cancer cells via the PI-3K/AKT/mTOR signaling
pathway and ultimately eliminate IGF-1-induced tumor angiogenesis
in vivo and in vitro (38). In hypoxic epithelial ovarian
cancer, as a transcription factor of IGF-1, highly expressed
ELF3-mediated secretion of IGF-1 and VEGF promoted endothelial cell
proliferation, migration and tumor angiogenesis through activation
of the tyrosine kinase pathway, whereas ELF3 silencing attenuated
angiogenesis and tumorigenesis in a xenograft mouse model,
demonstrating the pro-vascular effect of IGF-1 (39). Moreover, a variety of IGFBPs are
also involved in angiogenesis (40,41).
In glioblastoma, proteomic results suggest that IGFBP-1 is a key
mediator of cancer cell secretion in response to the vascular
production-promoting factor macrophage colony-stimulating factor
(MCSF). When conditioned medium from cancer cells was added to
human umbilical vein endothelial cells (HUVECs), the silencing of
MCSF prevented blood vessel formation. Moreover, IGFBP-1 inhibition
in cancer cells also blocked angiogenesis in HUVECs treated with
conditioned medium (42).
Furthermore, IGF-1R is also involved in the proangiogenic effect of
IGFBP-2. IGFBP-2 causes the inactivation of protein tyrosine
phosphatase β (RPTP-β) by binding to the RPTP-β receptor and
subsequently inhibits the transcription of the tumor suppressor
gene PTEN. Inhibition of PTEN mediates the activation of the
IGF-I/PI3K/AKT signaling pathway, which in turn promotes vascular
smooth muscle proliferation and tumor angiogenesis (43). In addition, other types of IGFBPs
can promote or inhibit tumor angiogenesis (44,45).
However, the pro-vascular effects of IGFBP seem to be independent
of IGF, which provides a new direction for further exploration of
the relationship between the IGF system and tumor angiogenesis.
Autophagy plays a dual role in tumor growth. Early
autophagy inhibits cancer progression, but with the continuous
growth of tumors, autophagy provides nutrients and energy for tumor
survival (46). The basal level
autophagy flux is usually associated with tumor inhibition and it
is often observed in breast cancer, prostate cancer gastric cancer,
hepatocellular carcinoma and other types of cancer in which
decreased expression of the-autophagy-associated protein Beclin 1
leads to increased proliferation of tumor cells (47–49).
Moreover, deficiency of autophagy regulatory factors such as
autophagy-related 4C cysteine peptidase (ATG4C) is more likely to
cause cancer (50). However, a
number of RAS mutated cancer cells maintain their own growth and
metabolism through high levels of autophagy, including those of
colorectal cancer and pancreatic cancer (51). IGF signal transduction can activate
a number of intracellular kinases to activate and induce a series
of reactions, related to apoptosis, autophagy and proliferation
(52). In breast cancer cell lines
(MCF-7), activation of IGF/PI3K signaling enhances mitochondrial
homeostasis by increasing the number of new mitochondria and levels
of oxidative phosphorylation and promotes the degradation of
damaged mitochondria (mitochondrial autophagy) by increasing BNIP3,
a protective mechanism that ultimately influences the cancer
treatment response and evolution of the cancer phenotype (53). Moreover, the role of IGF-1
signaling in promoting autophagy has been demonstrated in breast
cancer, prostate cancer and osteosarcoma (54). Furthermore, in colorectal cancer,
IGF-2 is critical for cancer stem cell formation and IGF-2
preferentially interacts with insulin receptor isoform A rather
than with IGF-1R to accelerate autophagy and metabolic remodeling
in colorectal cancer (55).
IGFBP-3, one of the major members of the insulin-like growth factor
binding protein family, has growth inhibitory effects in
vitro (56). However, high
levels of IGFBP-3 in breast tumor tissues are associated with
increased xenograft growth in mice and poor prognosis.
Specifically, the binding of IGFBP3 to GRP78 increases autophagic
site formation and autophagic system flux, thereby promoting breast
cancer cell survival even under glucose starvation and hypoxic
conditions (57). By contrast,
IGFBP-3 has an oncogenic effect on ovarian cancer cells, reflecting
the heterogeneity of tumor tissues and the diverse features of
IGFBP-3 functions (58). The
pro-autophagic effect of IGFBPs is also reflected in processes such
as chemoresistance in hepatocellular carcinoma (59). These studies provide good prospects
for in-depth exploration of the regulatory role of the insulin
growth factor system in tumor autophagy.
The proposed Warburg effect revealed the important
role of metabolic reprogramming in cancer (60). To meet the increased demand for
energy and substance synthesis, tumor cells change their flux by
adjusting various metabolic pathways and targeting metabolic
pathways has gradually become a focus of tumor therapy research
(61). However, metabolic
adaptability and heterogeneity caused by tumor heterogeneity and
plasticity limit metabolic efficacy. IGFs, including IGF-1 and
IGF-2, are involved in cellular metabolic signaling and influence
glucose and cholesterol uptake and glycogen storage (62,63).
Circulating levels of IGF-1 and certain IGFBPs are critical for the
maintenance of glucose homeostasis (64). Studies have shown that chronic
hyperglycemic diets increase the risk of colon cancer, in part by
regulating the insulin/IGF-1 signaling axis by activating the
downstream PI3K/AKT/mTOR, Ras/MAPK signaling pathways, glucose
transporter proteins (GLUT1) and key enzymes of glycolysis (LDHA
and HK2), thereby affecting glucose uptake and aerobic glycolysis
in cancer cells (65). In breast
cancer, the PPP1R1B truncated subtype (t-Darpp) is upregulated in
trastuzumab resistant HER2+ breast cancer. t-Darpp activates
IGF-1R/AKT signaling through heterodimerization with EGFR and HER2,
promoting and stimulating glucose uptake, glycolysis and
trastuzumab resistance in SK-BR-3 cells. Pharmacological inhibition
and IGF-1R-targeted knockdown reverse the effects of t-Darpp on
metabolic remodeling and drug resistance in tumor cells (66). IGFBP family proteins can bind to
IGF-1 and IGF-2, thereby regulating the downstream conduction of
IGF signals. IGFBP-1 plays an important role in the regulation of
IGF-I signaling and influences a series of downstream biological
events such as cell proliferation, survival, movement and
metabolism (67). It is shown that
IGFBP1 expression and secretion were significantly elevated in
cancer cells, and secreted IGFBP-1 inhibits AKT1-mediated
phosphorylation of ser27 of mitochondrial superoxide dismutase 2
(SOD2), thereby increasing the activity of SOD2 antioxidant
enzymes. Increased SOD2 activity weakens the accumulation of
mitochondrial reactive oxygen species (ROS) in spatially
constrained cancer cells, thereby supporting the survival of tumor
cells in blood vessels in lung tissue and accelerating tumor
metastasis in mice (68). However,
studies of the IGF system in cancer metabolism are still
insufficient, not only the uptake and utilization of glucose, but
also the mechanism of changes in lipid and amino acid metabolism
still need to be explored.
The tumor microenvironment (TME) is composed mainly
of tumor cells, stromal cells, immune cells and the extracellular
matrix, which assume the functions of material exchange,
environmental stress and immune regulation (69). In the early stage of tumor
colonization or growth, activated immune cells contribute to a
tumor-suppressive inflammatory microenvironment that hinders tumor
development, whereas long-term persistent antigenic stimulation,
the activation of immunosuppressive cells and metabolic stress
prompt the tumor to escape from the surveillance of the immune
system and continue to grow, which is known as immune escape of the
tumor (70,71). Modification changes in the tumor
cells themselves and changes in the immune microenvironment lead to
complexity of the immune escape process. Remodeling the positive
immune microenvironment and stimulating or restoring the innate
tumor suppression ability of the immune system are crucial for
improving the malignant progression of tumors. IGF-1/IGF-1R was
found to be critical in regulating the activity of a number of
immune cells, including T cells. In a mouse model of hepatocellular
carcinoma, regulatory T cells (Tregs) with high IGF-1R expression
presented increased PI3K/AKT/mTOR signaling and were able to
produce more ATP, lactate and ROS, which contribute to enhanced
immunosuppressive effects (72).
Tumor-associated macrophages (TAMs), important immunosuppressive
cells in the microenvironment, are able to activate the
Gli2/IGF-2/ERK1/2 signaling axis to promote TGF-β secretion and
thus mediate the migration, invasion and EMT of hepatocellular
carcinoma cells (Huh-7 cells) (73). Moreover, M2-type TAMs, through the
secretion of IGF-1 and IGF-2, activate PI3K/AKT/mTOR signaling and
enhance the malignant proliferation and stemness characteristics of
cancer cells (74). In addition,
adipose tissue in the microenvironment is also capable of secreting
IGF-1 to support microenvironmental remodeling (75). IGF-1 serves as a key mediator that
communicates between the microenvironment and cancer cells and
targeting IGF-1 has become a critical part of the fight against
tumor immunosuppression. Nevertheless, the relationships between
IGFs and numerous microenvironmental components, such as NK cells,
neutrophils and even the microbiota, remain to be elucidated and
incorporating other targets or combination therapies to address the
individual and tissue heterogeneity of tumors is crucial. Fig. 1 shows the role of IGF family
members in tumor progression.
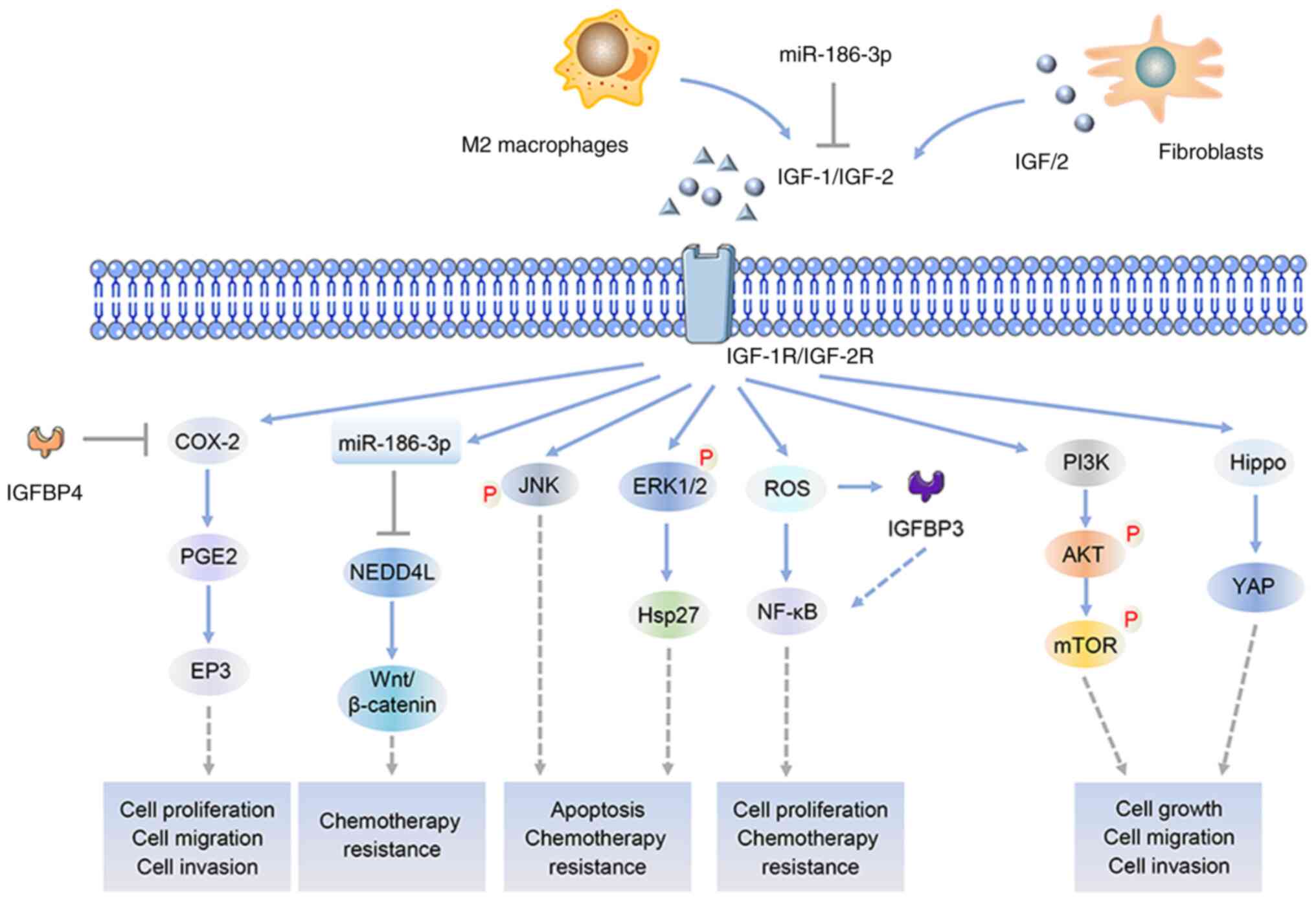
During the malignant progression of tumors, a
variety of signaling pathways are activated to participate in the
proliferation, migration, angiogenesis and metabolic remodeling of
tumor cells, among which IGFs play important regulatory roles
(76). This section summarizes the
key signaling pathways involved in the regulation of IGFs in tumor
progression.
In an oncogenic context, the IGF family regulates a
number of biological processes such as cancer cell proliferation,
apoptosis, metabolism and protein synthesis, which are closely
associated with the activation of PI3K/AKT signaling, which
subsequently promotes the transcription of downstream pro-oncogenic
target genes such as c-Myc and HIF-1α (77). In colorectal cancer, IGF-2 secreted
by cancer-associated fibroblasts binds to IGF-1R on cancer cells to
activate the PI3K/AKT/mTOR and Hippo-YAP1 signaling pathways to
promote cancer cell proliferation, migration and invasion; after
knockdown of IGF-1R or inhibition of IGF-1R with the IGF-1R
inhibitor picropodophyllin, the tumor-promoting effects are
reversed (78). In another study,
IGF-1 was shown to regulate glucose metabolism in cancer cells with
the involvement of kallikrein-related peptidase 10 (KLK10) and the
knockdown of KLK10 markedly inhibited glucose metabolism and
PI3K/Akt/mTOR signaling activation, a process that could be
reversed by IGF-1, suggesting that IGF-1 and KLK10 serve as
potential targets for regulating metabolic remodeling in colon
cancer (79). As an important
noncoding RNA, microRNA (miR)-186-3p is involved in the
proliferation, migration and apoptosis of a number of cancer cells,
especially cervical cancer cells. It can inhibit the activation of
PI3K/AKT signaling through the inverse regulation of IGF-1
expression and ultimately suppress the tumorigenesis of cervical
cancer cells (80). Moreover,
IGF-1 mediates the activation of the PI3K/AKT/mTOR pathway in
uterine smooth muscle tumors, gliomas and pancreatic cancer
(81–83). Numerous components of the
microenvironment are also involved in malignant tumor progression.
M2 macrophages, as infiltration-rich immunosuppressive cells, are
able to activate the PI3K/AKT/mTOR signaling axis by secreting
IGF-1 and IGF-2, which promotes thyroid cancer cell invasion and
the expression of stemness markers (Oct4, SOX2 and CD133),
exacerbating the immunosuppressive effects of cancer (74). IGFBP-like protein 1 (IGFBP-L1), a
key member of the IGFBP family, regulates its function by binding
to IGF (84). In esophageal
cancer, the methylation process of IGFBP-L1 was associated with
tumor size and TNM stage. Relevant in vivo and in
vitro experiments have confirmed that IGFBP-L1 methylation
exerts a pro-oncogenic effect by promoting PI3K/AKT phosphorylation
and IGFBP-L1 methylation has become a potential marker for the
early detection of esophageal cancer, as well as a predictive
marker for PI3K-targeted therapy in esophageal cancer (85).
NF-κB is a general term for a group of protein
complexes, including mainly subunits such as RelA (p65), RelB,
c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2), which play
important roles in cell proliferation, immune regulation and the
stress response (86). High-level
NF-κB activation is involved in tumorigenesis, angiogenesis,
microenvironmental remodeling and chemoresistance (87). Activation of the NF-κB pathway
under the influence of IGFs mediates the transcription of
downstream signals and a range of tumorigenic activities. IGF-1, as
a nutrient-responsive growth factor, activates NF-κB and the
expression of downstream genes (Ccdn1, Vegf, Birc5 and Ptgs2) to
promote the growth of pancreatic cancer in vitro and in
vivo (88). Furthermore, the
cross-talk between IGF-1 and ROS is involved in the occurrence and
development of a variety of cancers, including liver, cervical and
colorectal cancers. Further studies revealed that IGF-1 activates
the inflammatory signals NF-κB and NLRP3 in cancer cells and that
this activation depends on the accumulation of ROS and NOX2. The
inhibition of the IGF-1 receptor substrates IRS-1 and NOX2
effectively prevents the development of cancer-related inflammation
(89). IGFBP-3, a secreted
glycoprotein, can regulate the mitogenic activity of IGF-1R. Recent
studies have shown that high expression of IGFBP-3 can increase the
radiosensitivity of cancer cells and induce their apoptosis by
activating apoptosis-related proteins. Under irradiation, IGFBP-3
induces apoptosis and ROS production by activating NF-κB signaling
and ROS further promote IGFBP-3 mediated signaling activity. The
positive circuit of NF-κB activation and ROS production can
accumulate more ROS in irradiated OSCC cells and this positive
feedback regulation overcomes the pro-survival effect of NF-κB/IL-6
signaling (90). In another study,
IGFBP-3 was able to enhance etoposide-induced cell growth
inhibition by blocking the NF-κB signaling pathway in gastric
cancer cells, confirming that IGFBP-3 has become a key target and
marker for cancer therapy and further exploration of its in-depth
regulatory mechanisms is worthwhile (91).
MAPK is a key transmitter of signals from the cell
surface to the nucleus and can be activated by factors such as
cytokines, hormones, stressors and others to regulate cell growth,
differentiation, inflammation and other physiological and
pathological processes (92). In
the context of cancer progression, MAPK signals are involved in
various activities of cancer cells, including proliferation,
apoptosis and immune escape. However, simply targeting MAPK-related
signals has not been effective in treating cancer (93). The function of IGFs in the MAPK
signaling pathway provides a new direction in the fight against
cancer. IGF-1R is considered a potential cellular oncogene,
especially in breast cancer, where high expression with IGF-1R is a
driver of the malignant phenotype. Under continuous stimulation of
IGF-1, IGF-1R/MAPK/PI3K signaling is activated, leading to
resistance to estrogen tamoxifen and fluvestrant. Low doses of
tamoxifen act as agonists in IGF-1-stimulated breast cancer cells
and further increase IGF-1 expression. Key components involved in
the IGF-1/IGF-1R signaling network have become potential targets
for combined antiestrogen therapy (94). Ovarian cancer-associated antigen 66
(OVA66) was first shown to play a role in ovarian cancer by
reducing IGF-1R expression and downstream phosphorylation of
ERK1/2-Hsp27 signaling. In-depth mechanistic studies have shown
that OVA66 can interact with MDM2 to coregulate the activation of
the IGF-1R-ERK1/2 signaling pathway to promote tumorigenesis
(95). Research has shown that
type 2 diabetes (T2DM) is associated with an increased risk of
colon cancer, along with increased insulin and IGF-1. Insulin and
IGF-1 alone or in combination promoted the proliferation of MC38
colon cancer cells and reduced apoptosis. However, the use of
ERK1/2 or JNK inhibitors inhibited the growth of colon cancer cells
in vivo and in vitro, suggesting that the activation
of ERK1/2 and JNK signaling by insulin and IGF-1 is at least
partially responsible for the development of T2DM-associated colon
cancer (96). These studies
effectively confirmed the critical regulatory role of the
IGF-1/IGF-1R/MAPK signaling axis in malignant tumor
progression.
The Wnt signaling pathway is mainly mediated by the
activation of β-catenin and the sustained accumulation of β-catenin
into the nucleus initiates the transcription of target genes by
binding to T-cell factor (TCF)/lymphoid enhancer-binding factor
transcription factors (97). The
Wnt/β-catenin signaling pathway is closely related to stem cell
differentiation and organ regeneration. Studies have shown that the
activation of Wnt signaling in colorectal cancer is associated with
the loss of function of the tumor regulator APC and the involvement
of Wnt signaling has been reported in a variety of malignancies,
including breast and stomach cancer (98,99).
Nevertheless, targeting the Wnt pathway alone presents significant
challenges for cancer therapy, including poor drug response and
toxic side effects (100). As a
receptor for IGF-1 and IGF-2, IGF1R-mediated phosphorylation of Akt
and GSK3β promotes β-catenin stability and nuclear localization
(101). Moreover, the nuclear
localization of IGF-1R is mediated mainly by its C-terminal domain.
Following its nuclear localization, IGF-1R promotes TCF-mediated
β-catenin transcriptional activity, which has been confirmed in
hepatocellular carcinoma cells (102). In colorectal cancer cell lines
(HT-29 and SW620), knockdown of IGF-1R by small interfering RNA
resulted in a blockade of the downstream PI3K/Akt and typical Wnt
signaling pathways, which ultimately inhibited cancer cell
proliferation and promoted apoptosis (103). To identify IGF-1-mediated miRNA
regulatory networks that cause temozolomide (TMZ) to be insensitive
to glioblastoma multiforme treatment, on the basis of comprehensive
analysis of multiple databases and in vitro experiments,
Chen et al (104)
confirmed that IGF-1 upregulated miR-513a-5p signaling reduced the
sensitivity of glioma cells to TMZ by inhibiting the
NEDD4L-inactivated Wnt/β-catenin pathway. The mining of this
signaling pathway provides a feasible target for improving the drug
sensitivity of TMZ.
Cyclooxygenase-2 (COX-2) is a key regulatory
molecule that catalyzes the synthesis of arachidonic acid to
prostaglandin (PG), which is expressed in large quantities when
cells are stimulated by inflammation, accelerating the occurrence
of inflammatory storms (105).
COX-2 is highly expressed in most tumors and promotes tumor
proliferation, migration and angiogenesis (106). Moreover, the overexpression of
COX-2 is often closely associated with chemotherapy resistance and
immune escape processes in tumors, suggesting that COX-2 is an
attractive therapeutic target in tumors (107). Nevertheless, the regulation of
COX-2-related signaling pathways remains to be elucidated. The
IGF-1/IGF-1R system has been shown to be an important promoter of
tumor growth in various cancers, promoting tumor progression and
metastasis by regulating multiple signaling pathways, such as those
of PI3K/AKT and MAPK/ERK signaling (78,94).
Notably, COX-2 expression is also affected by the IGF-1/IGF-1R
signaling pathway (108). In a
study of colon cancer cells, COX-2 expression and PGE2 synthesis
were upregulated by the IGF-2/IGF-1R autocrine pathway and IGF-1R
blockade decreased COX-2 activity and inhibited tumor cell
proliferation and promoted apoptosis (103). Stoeltzing et al (109) reported that IGF-I selectively
upregulates COX-2 through the MAPK/(ERK1/2) pathway in pancreatic
cancer and that treatment with anti-IGF-IR antibodies can
effectively inhibit IGF-IR and MAPK/ERK activation and reduce COX-2
expression in parental cells. Furthermore, in a BK5.IGF-1 mouse
model of breast cancer, elevated levels of IGF-1 expression
activated the COX-2/PGE2/EP3 signaling pathway, accompanied by
increased VEGF expression and tumor angiogenesis (110). Celecoxib treatment resulted in a
45% reduction in mammary PGE2 levels and attenuated mast cell
influx and angiogenesis, suggesting that COX-2-selective inhibitors
may be useful in the prevention or treatment of breast cancer
associated with elevated human IGF-1 levels (110). As a member of the IGFBP family,
IGFBP-4 influences the inflammatory regulation of the tumor
microenvironment. In lung cancer tissues, the expression of IGFBP-4
was markedly lower than that in normal tissues adjacent to the
cancer, but the expression of COX-2 was greater in lung cancer
tissues. In addition to inhibiting the expression of COX-2 in lung
cancer cells, IGFBP-4 also inhibited the proliferation, migration
and metastasis of cancer cells through the modulation of the
PI3K/AKT, ERK and CREB pathways, which highlights the anticancer
value of IGFBP-4 (111). These
findings suggest that the regulation of the COX-2-related signaling
axis by the IGF system could be a potential target for cancer
therapy and deserves further exploration. Fig. 2 shows the key signaling pathways
involved in the regulation of IGFs in tumor progression.
The treatment of cancer is very complex and
IGF-related signals affect multiple processes of cancer cell
proliferation, invasion and metastasis. Moreover, IGFs also mediate
tumor resistance to chemotherapy and confer resistance to
immunotherapy (112). Therefore,
IGFs are promising targets for cancer treatment. This section
focusses on the application and related mechanisms of targeting
IGFs in cancer therapy.
Targeting the IGF-1R signaling pathway is considered
a potential breakthrough in anticancer therapy and a number of
small molecule inhibitors and monoclonal antibodies targeting
IGF-1R, such as BIIB-022, BMS-754807 and Teprotumumab, have been
developed and evaluated in clinical trials (113–115). However, owing to their limited
anticancer activity or drug toxicity, a number of clinical trials
targeting IGF-1R have been abandoned and few drugs have actually
entered clinical use. The unique advantages that natural compounds
have in cancer progression make targeting IGF-1R possible. For
example, the combination of curcumin and resveratrol inhibits NF-κB
activity by targeting IGF-1R signaling, ultimately leading to
apoptosis and cell cycle arrest in natural colon cancer cells,
suggesting that IGF-1R may be an anticancer approach (116). Quercetin, which is abundant in
fruits, vegetables, leaves and grains, can also inhibit skin cancer
proliferation by targeting IGF-1R (117). Epigallocatechin gallate, a
polyphenolic component of green tea, inhibits the progression of
various cancers, including glioma, breast and liver cancers, by
inhibiting IGF-1R via the phosphorylation of the tyrosine kinase
IGF-1R (118–120). Targeting circulating IGF-1 and
IGF-2 levels is another anticancer strategy. However, although
serum IGF levels are closely associated with tumor progression, IGF
also plays an important regulatory role in normal life activities,
such as maintaining skeletal muscle growth and development, islet
proliferation and cell metabolism. How to maximally inhibit tumor
growth without interfering with normal physiological activities is
a key issue in the development of IGF-1/2 inhibitors. For example,
MEDI-573 inhibits IGF-1R signaling and tumor growth in vivo
by neutralizing IGF-1 and IGF-2. MEDI-573 offers a potential
targeted treatment strategy for cancer (121). Numerous studies have shown that
IGFBPs can also affect the malignant biological behavior of tumors
in an IGF-independent manner. Therefore, targeting IGFBPs has also
become an IGFs-based cancer therapy (122,123). α1-Antitrypsin (AAT) is a member
of the serine protease inhibitor superfamily and has
anti-inflammatory and tissue protective effects. It can reduce
colitis and chronic ileitis by inhibiting cytokine production and
enhancing intestinal barrier function. In a mouse model of colon
cancer, the level of IGFBP-3 decreased markedly under the action of
serine protease and the use of AAT reversed this outcome, resulting
anticancer effects (124).
Several studies have shown that METTL3 plays important regulatory
roles in prostate cancer proliferation, migration, invasion,
apoptosis, drug resistance androgen-induced splicing and glycolipid
metabolism maintenance (125,126). The inhibitor STM2457 can reduce
the m6A level in cancer cells by inhibiting the IGFBP3/AKT
signaling axis, thus exerting anticancer effects in vitro
and in vivo (127). These
studies confirm that targeting IGFs is an important strategy for
cancer therapy.
For most cancers, chemotherapy is the mainstay of
late-stage intervention, a treatment strategy that helps improve
prognosis and overall survival. However, with the frequent
occurrence of drug resistance, chemotherapeutic agents have become
less effective in treating cancer (128,129). Therefore, researchers have begun
to explore the potential mechanisms of tumor drug resistance with
the aim of improving the efficacy of chemotherapy. Studies have
confirmed that IGFs play important roles in drug resistance in
tumors, including the IGF-1/IGF-2/IGF-1R signaling axis and the
IGFBP family members that reduce the susceptibility of cancers such
as lung and breast cancers to chemotherapy resistance (130,131). Therefore, targeting IGFs may help
solve the problem of tumor drug resistance. Her2-positive breast
cancer resistant to trastuzumab therapy severely affects prognosis.
In trastuzumab-resistant Her2-positive breast cancer cells, IGFBP-3
expression was reduced, leading to the inhibition of Wnt signaling
pathway release and increased Cullin7 expression mediated by
TCF7L2. Cullin7 was subsequently involved in the degradation of
IRS-1 in an mTOR/S6K-dependent manner to increase drug resistance.
Intervention with IGFBP-3 or Cullin7 partially restored trastuzumab
sensitivity in trastuzumab-resistant Her2-positive breast cancer
cells, which is important for selecting the optimal therapeutic
strategy for Her2-positive breast cancer (132). Tamoxifen, a selective estrogen
receptor modulator and antagonist of ERα in breast tissue, is a
commonly used adjuvant therapy for patients with ERα-positive
breast cancer (133). However,
tamoxifen resistance is becoming more common. Studies have shown
that tamoxifen resistance is associated with IGFBP-1 accumulation
and that the overexpression of IGFBP-1 promotes tamoxifen
resistance in breast cancer cells by activating the ERK pathway,
which can be reversed by knocking down IGFBP-1 (134). Moreover, in hepatocellular
carcinoma (HCC), antiangiogenic tyrosine kinase inhibitors (TKIs)
are effective therapeutic agents and the main therapeutic effect is
to induce severe hypoxia in the tumor microenvironment (TME)
through depletion of the vascular density of tumor. However,
patients with HCC often develop resistance to TKIs and this
resistance development is associated with increased IGFBP-1
expression, the aggregation mechanism manifested by TKI-induced
hypoxia increasing IGFBP-1 expression through activation of HIF-1α
and HIF-2α. Tumor-derived IGFBP-1 induces tumor angiogenesis by
activating integrin α5β1/focal adhesion kinase/extracellular
signal-regulated kinase signaling. The acquired resistance of tumor
cells to TKIs is partially reversed by inhibiting IGFBP-1 and the
combination of antiangiogenic TKIs and IGFBP-1 inhibitors may be a
promising therapeutic strategy for HCC (135). IGFBP-2 is a secreted protein that
prevents IGF-1/IGF-2 from binding to its receptor and it also
participates in the regulation of the TME in a macrophage-dependent
manner. IGFBP-2 is highly expressed in the blood of lung tumors and
patients with lung cancer and high levels of IGFBP-2 are associated
with poor survival and metastasis in patients with lung cancer.
In vitro and in vivo experiments have shown that
IGFBP-2 plays an important role in the acquisition of gefitinib
resistance. Mechanistically, IGFBP-2 can activate STAT3 to increase
the transcriptional activity of C-X-C motif ligand 1 (CXCL1),
thereby increasing the intracellular expression level of CXCL1,
which contributes to the survival of lung cancer cells in the
gefitinib environment (136). The
aforementioned results suggest the potential of IGFBP-2 as a
biomarker of gefitinib resistance and a potential target for
intervention. In addition, decreased survival of human lung cancer
cells is associated with increased IGFBP-3 expression. IGFBP-3
plays an anticancer role by exerting cytotoxic effects on cell
survival through a mechanism dependent on the interaction between
the glycosaminoglycan hyaluronic acid (HA) and CD44. Loss of
IGFBP-3 expression decreases the sensitivity of lung cancer cells
to cisplatin. Casein kinase 2 (CK2) is an antiapoptotic kinase that
maintains cell survival. Phosphorylation of IGFBP-3 by CK2 blocks
the binding of IGFBP-3 to HA, activates HA-CD44 signaling and leads
to reduced apoptosis, increased cell survival and cisplatin
resistance. Blocking CK2 and IGFBP-3 phosphorylation may be an
effective strategy to increase lung cancer susceptibility to
cisplatin (137). Therefore,
further exploration of the mechanism of IGFs in tumor drug
resistance is important for improving tumor sensitivity to
chemotherapy drugs.
Immunotherapy is another innovative cancer treatment
strategy following surgery, chemoradiotherapy and targeted therapy,
opening a new era of cancer treatment. This means that working on
cancer cells alone will not achieve the goal of completely
eliminating the tumor and new treatment strategies should consider
the TME, that is, the surrounding immune cell components. Some
inherent mechanisms of the tumor itself help tumor cells escape the
surveillance and killing effects of the immune system, so tumor
immune escape is also one of the bottlenecks to improving the
current therapeutic effect on tumors (138). How to solve the immune escape and
secondary drug resistance of tumor has become a difficult problem
for the wide application of tumor immunotherapy. Ovarian cancer is
the deadliest gynecological malignancy. Immune checkpoint
inhibitors have shown good therapeutic efficacy in most
malignancies, but have limited efficacy in patients with ovarian
cancer. The main reason is that the large amount of extracellular
matrix deposition in the ovarian cancer microenvironment leads to
tumor vascular collapse, reduced vascular perfusion, poor drug
delivery and blocked migration of cytotoxic T cells to the tumor
area (139). As a widely used
antihypertensive drug, losartan enhances vascular perfusion,
thereby enhancing drug delivery and intratumoral invasion of immune
effector cells and, on the other hand, enhances chemotherapy
sensitivity by inhibiting IGF-1 signaling to reshape ovarian cancer
and the microenvironment (140).
Several studies have shown that IGF-2 in the tumor microenvironment
is derived mainly from CAFs and that high levels of IGF-2 inhibit
the infiltration and cytotoxicity of CD8+ T cells, exacerbating the
immunosuppressive effect of tumors (78,141). Mechanistically, autocrine IGF-2
promotes self-activation by binding to the IGF-1 receptor (IGF-1R)
on CAFs and activating PI3K/AKT signaling, followed by the
secretion of various chemokines and cytokines (CCL5 and CXCL12) by
CAFs to influence the infiltration of T cell. Furthermore, CAFs
interact with T cells via the PD-1/PD-L1 and CD73/adenosine axes
and inhibit their activation, proliferation and effector responses.
Genetic inhibition or the targeted inhibitor of IGF-2, lincitinib,
markedly enhances the response to immune checkpoint blockade,
suggesting the potential of IGF-2 as a biomarker and therapeutic
target in immunotherapy (141).
In a mouse model of pancreatic cancer liver metastasis, the use of
IGF-IR inhibitor IGF-Trap reshaped the local immunosuppressive
microenvironment of liver tumors, reduced the recruitment of bone
marrow-derived suppressor cells, reversed innate immune cell
polarization and inhibited metastatic expansion. Moreover, when
IGF-1R was combined with an anti-PD-1 antibody, the growth of
experimental pancreatic ductal adenocarcinoma liver metastases was
inhibited and the response of T cell was further enhanced. These
results suggest that blocking IGFs has the dual effects of
reshaping the immune microenvironment and enhancing immunotherapy
(142). Current research confirms
that single-agent immunotherapy is not advantageous in cancer
treatment and that cotargeting IGFs offers a new therapeutic
strategy to improve the efficacy of immunotherapy.
Radiotherapy plays a pivotal role in controlling and
eradicating tumors as an adjuvant cancer treatment, either alone or
in combination with other modalities (surgery, chemotherapy,
immunotherapy and targeted therapy) (143). Despite the continuous progress in
radiation technology, which allows for more precise radiotherapy of
local tumor tissues while reducing the effect on normal tissues,
problems such as radioresistance and tumor recurrence remain major
challenges in the application of radiation therapy (144). Improving the sensitivity of tumor
tissues to radiotherapy is the main direction of current research.
Studies have shown that IGFs are closely related to radiation
response and tumor radiosensitivity. Among them, IGF/IGF-1R signals
can improve radiotherapy sensitivity by activating a series of
signal transduction events involved in DNA damage repair. In colon
cancer cells (HT-29 and SW480 cells), genetic knockout of IGF-1R or
the use of the IGF-1R inhibitor NVP-ADW742 enhanced the killing
effect of radiation on cancer cells, confirming that depletion of
IGF-1R improves radiosensitivity to colon cancer therapy (145,146). Antrocin is a sesquiterpene
lactone isolated from camphor that is used as a dietary supplement
for cancer prevention and liver protection. In addition, antrocin
has been shown to effectively antagonize a variety of cancers,
including breast, lung, liver and colon cancers (147,148). In prostate cancer, the
combination of antrocin and ionizing radiation (IR) synergistically
inhibits the proliferation of radioresistant prostate cancer cells
and induces apoptosis. Specifically, antrocin regulates the cell
cycle and apoptosis through the inhibition of β-catenin mediated by
IGF-1R, suggesting the potential value of antrocin as a potent
therapeutic agent to overcome radioresistance (149). Due to the heterogeneity of tumor
tissues, the challenges posed by radioresistance are increasingly
significant. An in-depth exploration of the important role of IGFs
in tumor radioresistance can provide potential therapeutic targets
for improving tumor radiosensitivity. The application of targeted
IGFs in cancer therapies and related mechanisms of action are
summarized in Table I.
Cancer is a major social, economic and public safety
issue in the 21st century and increasing morbidity and mortality
rates have placed great burdens on the worldwide population.
Despite advances in technology, the treatment and prevention of
cancer are still in their infancy. Recently, as the function of
IGFs has gradually been revealed, the important role that IGFs play
in cancer progression has brought new hope for cancer treatment.
The present review introduced the role of IGFs in cancer and their
molecular mechanism, focusing on the application of IGFs in current
cancer therapies, with the goal of providing a theoretical basis
for comprehensive cancer diagnosis and treatment.
Inhibition of IGF-1R signaling is considered a
promising strategy for inhibiting tumor growth and improving
survival rates in a variety of cancers. However, several drugs,
including teprotumumab and BIIB-022, which target IGF-1R, have not
shown good therapeutic effects in clinical trials, possibly due to
the lack of reliable IGF-1R inhibition biomarkers. Moreover,
IGF-related molecular mechanisms not only play a role in tumor
cells but also affect other nontumor cell components in the
microenvironment, such as fibroblasts, macrophages and T cells,
demonstrating that targeting IGFs alone cannot completely inhibit
tumor growth and progression. Furthermore, owing to the
heterogeneity and diversity of tumor cells themselves, the role of
IGFs is not necessarily only as a tumor suppressor, so the
development of related inhibitors must fully consider the impact on
the tumor and its surrounding environment. With the advance of
combination therapy, the combination of targeted IGFs and other
technologies for the treatment of tumors has shown advantages,
which not only increase the efficacy but also effectively prevent
the occurrence of drug resistance. Future research should focus on
exploring the use of combination therapy in cancer treatment.
Moreover, with the advancement of multiomics technology, the
importance of precision therapy and individualized treatment is
increasingly emphasized and multidimensional and different levels
of cancer-related mechanisms need to be explored to develop more
reliable inhibitors, which, combined with a more rational target
delivery mechanism, is the direction of cancer treatment in the
future. In conclusion, targeting IGFs can provide more therapeutic
options for cancer patients.
Not applicable.
The present study was supported by National Natural Science
Foundation of China (grant no. 82260555) and The First Hospital of
Lanzhou University Intra-Hospital Fund (grant no.
ldyyyn2020-09).
Not applicable.
DW prepared and wrote the original draft and was
responsible for supervision and project administration. SD prepared
and wrote the original draft and was responsible for visualization.
WZ prepared and wrote the original draft and was responsible for
supervision, project administration and funding acquisition. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jassim A, Rahrmann EP, Simons BD and
Gilbertson RJ: Cancers make their own luck: Theories of cancer
origins. Nat Rev Cancer. 23:710–724. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Forbes BE, Blyth AJ and Wit JM: Disorders
of IGFs and IGF-1R signaling pathways. Mol Cell Endocrinol.
518:1110352020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Froesch ER, Buergi H, Ramseier EB, Bally P
and Labhart A: antibody-suppressible and nonsuppressible
insulin-like activities in human serum and their physiologic
significance. an insulin assay with adipose tissue of increased
precision and specificity. J Clin Invest. 42:1816–1834. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rinderknecht E and Humbel RE: The amino
acid sequence of human insulin-like growth factor I and its
structural homology with proinsulin. J Biol Chem. 253:2769–2776.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Zhu Q, Cao D, Peng Q, Zhang X, Li
C, Zhang C, Zhou BO and Yue R: Bone marrow-derived IGF-1
orchestrates maintenance and regeneration of the adult skeleton.
Proc Natl Acad Sci USA. 120:e22037791202023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guan X, Yan Q, Wang D, Du G and Zhou J:
IGF-1 signaling regulates mitochondrial remodeling during myogenic
differentiation. Nutrients. 14:12492022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsushita M, Fujita K, Hatano K, De
Velasco MA, Uemura H and Nonomura N: Connecting the dots between
the gut-IGF-1-prostate axis: A role of IGF-1 in prostate
carcinogenesis. Front Endocrinol (Lausanne). 13:8523822022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
LeRoith D, Holly JMP and Forbes BE:
Insulin-like growth factors: Ligands, binding proteins and
receptors. Mol Metab. 52:1012452021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dixit M, Poudel SB and Yakar S: Effects of
GH/IGF axis on bone and cartilage. Mol Cell Endocrinol.
519:1110522021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vassilakos G, Lei H, Yang Y, Puglise J,
Matheny M, Durzynska J, Ozery M, Bennett K, Spradlin R, Bonanno H,
et al: Deletion of muscle IGF-I transiently impairs growth and
progressively disrupts glucose homeostasis in male mice. FASEB J.
33:181–194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alberini CM and Chen DY: Memory
enhancement: Consolidation, reconsolidation and insulin-like growth
factor 2. Trends Neurosci. 35:274–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alfares MN, Perks CM, Hamilton-Shield JP
and Holly JMP: Insulin-like growth factor-II in adipocyte
regulation: Depot-specific actions suggest a potential role
limiting excess visceral adiposity. Am J Physiol Endocrinol Metab.
315:E1098–E1107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghafari F, Alizadeh AM, Agah S, Irani S
and Mokhtare M: Insulin-like growth factor 1 serum levels in
different stages of gastric cancer and their association with
Helicobacter pylori status. Peptides. 158:1708922022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kasprzak A: Autophagy and the insulin-like
growth factor (IGF) system in colonic cells: Implications for
colorectal neoplasia. Int J Mol Sci. 24:36652023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adachi Y, Nojima M, Mori M, Himori R, Kubo
T, Akutsu N, Lin Y, Kurozawa Y, Wakai K and Tamakoshi A; Japan
Collaborative Cohort Study, : Insulin-like growth factor 2 and
incidence of liver cancer in a nested case-control study. Cancer
Epidemiol Biomarkers Prev. 30:2130–2135. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stefani C, Miricescu D, Stanescu-Spinu II,
Nica RI, Greabu M, Totan AR and Jinga M: Growth Factors,
PI3K/AKT/mTOR and MAPK Signaling Pathways in Colorectal Cancer
Pathogenesis: Where Are We Now? Int J Mol Sci. 22:102602021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng L, Li B, Xi Y, Cai M and Tian Z:
Aerobic exercise and resistance exercise alleviate skeletal muscle
atrophy through IGF-1/IGF-1R-PI3K/Akt pathway in mice with
myocardial infarction. Am J Physiol Cell Physiol. 322:C164–C176.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang WY, Tseng YT, Lee TY, Fu YC, Chang
WH, Lo WW, Lin CL and Lo YC: Triptolide prevents LPS-induced
skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating
protein synthesis/degradation pathway. Br J Pharmacol.
178:2998–3016. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Visser KE and Joyce JA: The evolving
tumor microenvironment: From cancer initiation to metastatic
outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paredes F, Williams HC and San Martin A:
Metabolic adaptation in hypoxia and cancer. Cancer Lett.
502:133–142. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Liu J, Tian Z, Zhang Z, Liu T, Chen
C, Tang X and Zhu J: Highly expressed IFITM10 is associated with
early diagnosis and T stage of gastric cancer. Transl Cancer Res.
10:382–392. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seo GS, Lee JK, Yu JI, Yun KJ, Chae SC and
Choi SC: Identification of the polymorphisms in IFITM3 gene and
their association in a Korean population with ulcerative colitis.
Exp Mol Med. 42:99–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu L, Zhou R, Yuan L, Wang S, Li X, Ma H,
Zhou M, Pan C, Zhang J, Huang N, et al: IGF1/IGF1R/STAT3
signaling-inducible IFITM2 promotes gastric cancer growth and
metastasis. Cancer Lett. 393:76–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Le Coz V, Zhu C, Devocelle A, Vazquez A,
Boucheix C, Azzi S, Gallerne C, Eid P, Lecourt S and Giron-Michel
J: IGF-1 contributes to the expansion of melanoma-initiating cells
through an epithelial-mesenchymal transition process. Oncotarget.
7:82511–82527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarkissyan S, Sarkissyan M, Wu Y, Cardenas
J, Koeffler HP and Vadgama JV: IGF-1 regulates Cyr61 induced breast
cancer cell proliferation and invasion. PLoS One. 9:e1035342014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiu YF, Wu CC, Kuo MH, Miao CC, Zheng MY,
Chen PY, Lin SC, Chang JL, Wang YH and Chou YT: Critical role of
SOX2-IGF2 signaling in aggressiveness of bladder cancer. Sci Rep.
10:82612020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsai YF, Chou HC, Liou MH, Liao EC, Cheng
CT, Chang SJ and Chan HL: Role of IGFBP-2 in oral cancer
metastasis. Biochim Biophys Acta Mol Basis Dis. 1867:1661432021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo C, Sun F, Zhu H, Ni Y, Fang J, Liu Y,
Shao S, Shen H and Hu J: Insulin-like growth factor binding
protein-1 (IGFBP-1) upregulated by Helicobacter pylori and is
associated with gastric cancer cells migration. Pathol Res Pract.
213:1029–1036. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mo W, Deng L, Cheng Y, Ge S and Wang J:
IGFBP7 regulates cell proliferation and migration through JAK/STAT
pathway in gastric cancer and is regulated by DNA and RNA
methylation. J Cell Mol Med. 28:e700802024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren F, Wu K, Yang Y, Yang Y, Wang Y and Li
J: Dandelion polysaccharide exerts anti-angiogenesis effect on
hepatocellular carcinoma by regulating VEGF/HIF-1α expression.
Front Pharmacol. 11:4602020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Higashi Y, Pandey A, Goodwin B and
Delafontaine P: Insulin-like growth factor-1 regulates glutathione
peroxidase expression and activity in vascular endothelial cells:
Implications for atheroprotective actions of insulin-like growth
factor-1. Biochim Biophys Acta. 1832:391–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bid HK, Zhan J, Phelps DA, Kurmasheva RT
and Houghton PJ: Potent inhibition of angiogenesis by the IGF-1
receptor-targeting antibody SCH717454 is reversed by IGF-2. Mol
Cancer Ther. 11:649–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glass K, Fines C, Coulter P, Jena L,
McCarthy HO and Buckley N: Development and characterization of a
peptide-bisphosphonate nanoparticle for the treatment of breast
cancer. Mol Pharm. 21:4970–4982. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishikawa T: Differences between zoledronic
acid and denosumab for breast cancer treatment. J Bone Miner Metab.
41:301–306. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang X, Zhang Q, Shi S, Yen Y, Li X, Zhang
Y, Zhou K and Le AD: Bisphosphonates suppress insulin-like growth
factor 1-induced angiogenesis via the HIF-1alpha/VEGF signaling
pathways in human breast cancer cells. Int J Cancer. 126:90–103.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seo SH, Hwang SY, Hwang S, Han S, Park H,
Lee YS, Rho SB and Kwon Y: Hypoxia-induced ELF3 promotes tumor
angiogenesis through IGF1/IGF1R. EMBO Rep. 23:e529772022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu X, He H, Zhang F, Hu X, Bi F, Li K, Yu
H, Zhao Y, Teng X, Li J, et al: m6A methylated EphA2 and VEGFA
through IGF2BP2/3 regulation promotes vasculogenic mimicry in
colorectal cancer via PI3K/AKT and ERK1/2 signaling. Cell Death
Dis. 13:4832022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Slater T, Haywood NJ, Matthews C, Cheema H
and Wheatcroft SB: Insulin-like growth factor binding proteins and
angiogenesis: From cancer to cardiovascular disease. Cytokine
Growth Factor Rev. 46:28–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nijaguna MB, Patil V, Urbach S, Shwetha
SD, Sravani K, Hegde AS, Chandramouli BA, Arivazhagan A, Marin P,
Santosh V and Somasundaram K: Glioblastoma-derived macrophage
colony-stimulating factor (MCSF) induces microglial release of
insulin-like growth factor-binding protein 1 (IGFBP1) to promote
angiogenesis. J Biol Chem. 290:23401–23415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen X, Xi G, Wai C and Clemmons DR: The
coordinate cellular response to insulin-like growth factor-I
(IGF-I) and insulin-like growth factor-binding protein-2 (IGFBP-2)
is regulated through vimentin binding to receptor tyrosine
phosphatase β (RPTPβ). J Biol Chem. 290:11578–11590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma YS, Shi BW, Guo JH, Liu JB, Yang XL,
Xin R, Shi Y, Zhang DD, Lu GX, Jia CY, et al: microRNA-320b
suppresses HNF4G and IGF2BP2 expression to inhibit angiogenesis and
tumor growth of lung cancer. Carcinogenesis. 42:762–771. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei LF, Weng XF, Huang XC, Peng YH, Guo HP
and Xu YW: IGFBP2 in cancer: Pathological role and clinical
significance (Review). Oncol Rep. 45:427–438. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer. 19:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li X, Yang KB, Chen W, Mai J, Wu XQ, Sun
T, Wu RY, Jiao L, Li DD, Ji J, et al: CUL3 (cullin 3)-mediated
ubiquitination and degradation of BECN1 (beclin 1) inhibit
autophagy and promote tumor progression. Autophagy. 17:4323–4340.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huangfu L, Wang X, Tian S, Chen J, Wang X,
Fan B, Yao Q, Wang G, Chen C, Han J, et al: Piceatannol enhances
Beclin-1 activity to suppress tumor progression and its combination
therapy strategy with everolimus in gastric cancer. Sci China Life
Sci. 66:298–312. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Filali-Mouncef Y, Hunter C, Roccio F,
Zagkou S, Dupont N, Primard C, Proikas-Cezanne T and Reggiori F:
The ménage à trois of autophagy, lipid droplets and liver disease.
Autophagy. 18:50–72. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wen ZP, Zeng WJ, Chen YH, Li H, Wang JY,
Cheng Q, Yu J, Zhou HH, Liu ZZ, Xiao J and Chen XP: Knockdown ATG4C
inhibits gliomas progression and promotes temozolomide
chemosensitivity by suppressing autophagic flux. J Exp Clin Cancer
Res. 38:2982019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Masliah-Planchon J, Garinet S and Pasmant
E: RAS-MAPK pathway epigenetic activation in cancer: miRNAs in
action. Oncotarget. 7:38892–38907. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen PC, Kuo YC, Chuong CM and Huang YH:
Niche modulation of IGF-1R signaling: its role in stem cell
pluripotency, cancer reprogramming and therapeutic applications.
Front Cell Dev Biol. 8:6259432021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lyons A, Coleman M, Riis S, Favre C,
O'Flanagan CH, Zhdanov AV, Papkovsky DB, Hursting SD and O'Connor
R: Insulin-like growth factor 1 signaling is essential for
mitochondrial biogenesis and mitophagy in cancer cells. J Biol
Chem. 292:16983–16998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Riis S, Murray JB and O'Connor R: IGF-1
signalling regulates mitochondria dynamics and turnover through a
conserved GSK-3β-Nrf2-BNIP3 pathway. Cells. 9:1472020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gao T, Liu X, He B, Pan Y and Wang S: IGF2
loss of imprinting enhances colorectal cancer stem cells
pluripotency by promoting tumor autophagy. Aging (Albany NY).
12:21236–21252. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cai Q, Dozmorov M and Oh Y:
IGFBP-3/IGFBP-3 receptor system as an anti-tumor and
anti-metastatic signaling in cancer. Cells. 9:12612020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Grkovic S, O'Reilly VC, Han S, Hong M,
Baxter RC and Firth SM: IGFBP-3 binds GRP78, stimulates autophagy
and promotes the survival of breast cancer cells exposed to adverse
microenvironments. Oncogene. 32:2412–2420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen X, Shao C, Liu J, Sun H, Yao B, Ma C,
Xu H and Zhu W: ULK2 suppresses ovarian cancer cell migration and
invasion by elevating IGFBP3. PeerJ. 12:e176282024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lin JC, Liu TP, Chen YB and Yang PM:
PF-429242 exhibits anticancer activity in hepatocellular carcinoma
cells via FOXO1-dependent autophagic cell death and
IGFBP1-dependent anti-survival signaling. Am J Cancer Res.
13:4125–4144. 2023.PubMed/NCBI
|
|
60
|
Xia P, Zhang H, Lu H, Xu K, Jiang X, Jiang
Y, Gongye X, Chen Z, Liu J, Chen X, et al: METTL5 stabilizes c-Myc
by facilitating USP5 translation to reprogram glucose metabolism
and promote hepatocellular carcinoma progression. Cancer Commun
(Lond). 43:338–364. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jing Z, Liu Q, He X, Jia Z, Xu Z, Yang B
and Liu P: NCAPD3 enhances Warburg effect through c-myc and E2F1
and promotes the occurrence and progression of colorectal cancer. J
Exp Clin Cancer Res. 41:1982022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Okuyama T, Kyohara M, Terauchi Y and
Shirakawa J: The roles of the IGF axis in the regulation of the
metabolism: interaction and difference between insulin receptor
signaling and IGF-I receptor signaling. Int J Mol Sci. 22:68172021.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ravera S, Puddu A, Bertola N, Verzola D,
Russo E, Maggi D and Panfoli I: IGF-1 signaling modulates oxidative
metabolism and stress resistance in ARPE-19 cells through PKM2
function. Int J Mol Sci. 25:136402024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Stanley TL, Fourman LT, Zheng I, McClure
CM, Feldpausch MN, Torriani M, Corey KE, Chung RT, Lee H, Kleiner
DE, et al: Relationship of IGF-1 and IGF-binding proteins to
disease severity and glycemia in nonalcoholic fatty liver disease.
J Clin Endocrinol Metab. 106:e520–e533. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kasprzak A: Insulin-Like growth factor 1
(IGF-1) signaling in glucose metabolism in colorectal cancer. Int J
Mol Sci. 22:64342021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lenz G, Hamilton A, Geng S, Hong T, Kalkum
M, Momand J, Kane SE and Huss JM: t-Darpp Activates IGF-1R
signaling to regulate glucose metabolism in trastuzumab-resistant
breast cancer cells. Clin Cancer Res. 24:1216–1226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lung Cancer Cohort Consortium (LC3), . The
blood proteome of imminent lung cancer diagnosis. Nat Commun.
14:30422023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cai G, Qi Y, Wei P, Gao H, Xu C, Zhao Y,
Qu X, Yao F and Yang W: IGFBP1 sustains cell survival during
spatially-confined migration and promotes tumor metastasis. Adv Sci
(Weinh). 10:e22065402023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Onkar SS, Carleton NM, Lucas PC, Bruno TC,
Lee AV, Vignali DAA and Oesterreich S: The great immune escape:
Understanding the divergent immune response in breast cancer
subtypes. Cancer Discov. 13:23–40. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Leuzzi G, Vasciaveo A, Taglialatela A,
Chen X, Firestone TM, Hickman AR, Mao W, Thakar T, Vaitsiankova A,
Huang JW, et al: SMARCAL1 is a dual regulator of innate immune
signaling and PD-L1 expression that promotes tumor immune evasion.
Cell. 187:861–881.e32. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
De Martino M, Rathmell JC, Galluzzi L and
Vanpouille-Box C: Cancer cell metabolism and antitumour immunity.
Nat Rev Immunol. 24:654–669. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huang Y, Huang L, Zhu J, Wu Y, Shi J and
Dai K: Differential expression of insulin-like growth factor type 1
receptor identifies heterogeneous intrahepatic regulatory T subsets
in mouse hepatocellular carcinoma. Clin Exp Immunol. 208:47–59.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu M, Zhong YB, Shao J, Zhang C and Shi
C: Tumor-associated macrophages promote human hepatoma Huh-7 cell
migration and invasion through the Gli2/IGF-II/ERK1/2 axis by
secreting TGF-β1. Cancer Biol Ther. 21:1041–1050. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lv J, Liu C, Chen FK, Feng ZP, Jia L, Liu
PJ, Yang ZX, Hou F and Deng ZY: M2-like tumour-associated
macrophage-secreted IGF promotes thyroid cancer stemness and
metastasis by activating the PI3K/AKT/mTOR pathway. Mol Med Rep.
24:6042021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Uehara H, Kobayashi T, Matsumoto M,
Watanabe S, Yoneda A and Bando Y: Adipose tissue:Critical
contributor to the development of prostate cancer. J Med Invest.
65:9–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Manzella L, Massimino M, Stella S, Tirrò
E, Pennisi MS, Martorana F, Motta G, Vitale SR, Puma A, Romano C,
et al: Activation of the IGF axis in thyroid cancer: Implications
for tumorigenesis and treatment. Int J Mol Sci. 20:32582019.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xie X, Zhu Y, Cheng H, Li H, Zhang Y, Wang
R, Li W and Wu F: BPA exposure enhances the metastatic aggression
of ovarian cancer through the ERα/AKT/mTOR/HIF-1α signaling axis.
Food Chem Toxicol. 176:1137922023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang J, Chen B, Li H, Wang Y, Liu X, Wong
KY, Chan WN, Chan AK, Cheung AH, Leung KT, et al: Cancer-associated
fibroblasts potentiate colorectal cancer progression by crosstalk
of the IGF2-IGF1R and Hippo-YAP1 signaling pathways. J Pathol.
259:205–219. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wei H, Dong C and Shen Z:
Kallikrein-related peptidase (KLK10) cessation blunts colorectal
cancer cell growth and glucose metabolism by regulating the
PI3K/Akt/mTOR pathway. Neoplasma. 67:889–897. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lu X, Song X, Hao X, Liu X, Zhang X, Yuan
N, Ma H and Zhang Z: miR-186-3p attenuates the tumorigenesis of
cervical cancer via targeting insulin-like growth factor 1 to
suppress PI3K-Akt signaling pathway. Bioengineered. 12:7079–7092.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang C, Sun Y, Cong S and Zhang F:
Insulin-like growth factor-1 promotes human uterine leiomyoma cell
proliferation via PI3K/AKT/mTOR pathway. Cells Tissues Organs.
212:194–202. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gao C, He XF, Xu QR, Xu YJ and Shen J:
Sevoflurane downregulates insulin-like growth factor-1 to inhibit
cell proliferation, invasion and trigger apoptosis in glioma
through the PI3K/AKT signaling pathway. Anticancer Drugs.
30:e07442019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rieder S, Michalski CW, Friess H and
Kleeff J: Insulin-like growth factor signaling as a therapeutic
target in pancreatic cancer. Anticancer Agents Med Chem.
11:427–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Guo C, Cho KS, Li Y, Tchedre K, Antolik C,
Ma J, Chew J, Utheim TP, Huang XA, Yu H, et al: IGFBPL1 regulates
axon growth through IGF-1-mediated signaling cascades. Sci Rep.
8:20542018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu Y, Zhang M, He T, Yang W, Wang L,
Zhang L and Guo M: Epigenetic silencing of IGFBPL1 promotes
esophageal cancer growth by activating PI3K-AKT signaling. Clin
Epigenetics. 12:222020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Guo Q, Jin Y, Chen X, Shen X, Lin M, Zeng
C, Zhou T and Zhang J: NF-κB in biology and targeted therapy: New
insights and translational implications. Signal Transduct Target
Ther. 9:532024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang L, Dou X, Zheng Z, Ye C, Lu TX,
Liang HL, Wang L, Weichselbaum RR and He C: YTHDF2/m6 A/NF-κB axis
controls anti-tumor immunity by regulating intratumoral Tregs. EMBO
J. 42:e1131262023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Harvey AE, Lashinger LM, Hays D, Harrison
LM, Lewis K, Fischer SM and Hursting SD: Calorie restriction
decreases murine and human pancreatic tumor cell growth, nuclear
factor-κB activation and inflammation-related gene expression in an
insulin-like growth factor-1-dependent manner. PLoS One.
9:e941512014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang C, An Y, Wang Y, Shen K, Wang X, Luan
W, Ma F, Ni L, Liu M and Yu L: Insulin-like growth factor-I
activates NFκB and NLRP3 inflammatory signalling via ROS in cancer
cells. Mol Cell Probes. 52:1015832020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wang SH, Chen YL, Hsiao JR, Tsai FY, Jiang
SS, Lee AY, Tsai HJ and Chen YW: Insulin-like growth factor binding
protein 3 promotes radiosensitivity of oral squamous cell carcinoma
cells via positive feedback on NF-κB/IL-6/ROS signaling. J Exp Clin
Cancer Res. 40:952021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kim MS and Lee DY: Insulin-like growth
factor binding protein-3 enhances etoposide-induced cell growth
inhibition by suppressing the NF-κB activity in gastric cancer
cells. Mol Cell Biochem. 403:107–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cao Y, Chen J, Ren G, Zhang Y, Tan X and
Yang L: Punicalagin prevents inflammation in LPS-Induced RAW264.7
macrophages by inhibiting FoxO3a/autophagy signaling pathway.
Nutrients. 11:27942019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Peluso I, Yarla NS, Ambra R, Pastore G and
Perry G: MAPK signalling pathway in cancers: Olive products as
cancer preventive and therapeutic agents. Semin Cancer Biol.
56:185–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang Y, Moerkens M, Ramaiahgari S, de
Bont H, Price L, Meerman J and van de Water B: Elevated
insulin-like growth factor 1 receptor signaling induces
antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling
routes. Breast Cancer Res. 13:R522011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rao W, Li H, Song F, Zhang R, Yin Q, Wang
Y, Xi Y and Ge H: OVA66 increases cell growth, invasion and
survival via regulation of IGF-1R-MAPK signaling in human cancer
cells. Carcinogenesis. 35:1573–1581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Teng JA, Wu SG, Chen JX, Li Q, Peng F, Zhu
Z, Qin J and He ZY: The activation of ERK1/2 and JNK MAPK signaling
by insulin/IGF-1 Is responsible for the development of colon cancer
with type 2 diabetes Mellitus. PLoS One. 11:e01498222016.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu
C, Wang C and Ye L: Wnt/β-catenin signaling in cancers and targeted
therapies. Signal Transduct Target Ther. 6:3072021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xue W, Yang L, Chen C, Ashrafizadeh M,
Tian Y and Sun R: Wnt/β-catenin-driven EMT regulation in human
cancers. Cell Mol Life Sci. 81:792024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chatterjee A, Paul S, Bisht B,
Bhattacharya S, Sivasubramaniam S and Paul MK: Advances in
targeting the WNT/β-catenin signaling pathway in cancer. Drug
Discov Today. 27:82–101. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hsieh CH, Cheng LH, Hsu HH, Ho TJ, Tu CC,
Lin YM, Chen MC, Tsai FJ, Hsieh YL and Huang CY: Apicidin-resistant
HA22T hepatocellular carcinoma cells strongly activated the
Wnt/β-catenin signaling pathway and MMP-2 expression via the
IGF-IR/PI3K/Akt signaling pathway enhancing cell metastatic effect.
Biosci Biotechnol Biochem. 77:2397–2404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jamwal G, Singh G, Dar MS, Singh P, Bano
N, Syed SH, Sandhu P, Akhter Y, Monga SP and Dar MJ: Identification
of a unique loss-of-function mutation in IGF1R and a crosstalk
between IGF1R and Wnt/β-catenin signaling pathways. Biochim Biophys
Acta Mol Cell Res. 1865:920–931. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang QY, Wang L, Song ZY and Qu XJ:
Knockdown of type I insulin-like growth factor receptor inhibits
human colorectal cancer cell growth and downstream PI3K/Akt,
WNT/β-catenin signal pathways. Biomed Pharmacother. 73:12–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chen KC, Chen PH, Ho KH, Shih CM, Chou CM,
Cheng CH and Lee CC: IGF-1-enhanced miR-513a-5p signaling
desensitizes glioma cells to temozolomide by targeting the
NEDD4L-inhibited Wnt/β-catenin pathway. PLoS One. 14:e02259132019.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hashemi Goradel N, Najafi M, Salehi E,
Farhood B and Mortezaee K: Cyclooxygenase-2 in cancer: A review. J
Cell Physiol. 234:5683–5699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Peng Y, Wang Y, Tang N, Sun D, Lan Y, Yu
Z, Zhao X, Feng L, Zhang B, Jin L, et al: Andrographolide inhibits
breast cancer through suppressing COX-2 expression and angiogenesis
via inactivation of p300 signaling and VEGF pathwa. J Exp Clin
Cancer Res. 37:2482018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Huang R, Yu J, Zhang B, Li X, Liu H and
Wang Y: Emerging COX-2 inhibitors-based nanotherapeutics for cancer
diagnosis and treatment. Biomaterials. 315:1229542025. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Song KH, Kang JH, Woo JK, Nam JS, Min HY,
Lee HY, Kim SY and Oh SH: The novel IGF-IR/Akt-dependent anticancer
activities of glucosamine. BMC Cancer. 14:312014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Stoeltzing O, Liu W, Fan F, Wagner C,
Stengel K, Somcio RJ, Reinmuth N, Parikh AA, Hicklin DJ and Ellis
LM: Regulation of cyclooxygenase-2 (COX-2) expression in human
pancreatic carcinoma cells by the insulin-like growth factor-I
receptor (IGF-IR) system. Cancer Lett. 258:291–300. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Tian J, Lambertz I, Berton TR, Rundhaug
JE, Kiguchi K, Shirley SH, Digiovanni J, Conti CJ, Fischer SM and
Fuchs-Young R: Transgenic insulin-like growth factor-1 stimulates
activation of COX-2 signaling in mammary glands. Mol Carcinog.
51:973–983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li W, Sun D, Lv Z, Wei Y, Zheng L, Zeng T
and Zhao J: Insulin-like growth factor binding protein-4 inhibits
cell growth, migration and invasion and downregulates COX-2
expression in A549 lung cancer cells. Cell Biol Int. 41:384–391.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Mengzhe G, Shanshan X, Di Z and Shihua W:
Editorial: The role of the IGF axis in tumorigenesis and cancer
treatment: From genes to metabolites. Front Endocrinol (Lausanne).
13:11239622023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang P, Mak VC and Cheung LW: Drugging
IGF-1R in cancer: New insights and emerging opportunities. Genes
Dis. 10:199–211. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Fuentes-Baile M, Ventero MP, Encinar JA,
García-Morales P, Poveda-Deltell M, Pérez-Valenciano E, Barberá VM,
Gallego-Plazas J, Rodríguez-Lescure Á, Martín-Nieto J and Saceda M:
Differential effects of IGF-1R small molecule tyrosine kinase
inhibitors BMS-754807 and OSI-906 on human cancer cell lines.
Cancers (Basel). 12:37172020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Stan MN and Krieger CC: The adverse
effects profile of teprotumumab. J Clin Endocrinol Metab.
108:e654–e662. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang J, Wen G, Sun L, Yuan W, Wang R,
Zeng Q, Zhang G and Yu B: Cryptotanshinone inhibits cellular
proliferation of human lung cancer cells through downregulation of
IGF-1R/PI3K/Akt signaling pathway. Oncol Rep. 40:2926–2934.
2018.PubMed/NCBI
|
|
117
|
Jung M, Bu SY, Tak KH, Park JE and Kim E:
Anticarcinogenic effect of quercetin by inhibition of insulin-like
growth factor (IGF)-1 signaling in mouse skin cancer. Nutr Res
Pract. 7:439–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Le CT, Leenders WPJ, Molenaar RJ and van
Noorden CJF: Effects of the green tea polyphenol
epigallocatechin-3-gallate on glioma: A critical evaluation of the
literature. Nutr Cancer. 70:317–333. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Liu F, Ye S, Zhao L and Niu Q: The role of
IGF/IGF-1R signaling in the regulation of cancer stem cells. Clin
Transl Oncol. 26:2924–2934. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ding F and Yang S:
Epigallocatechin-3-gallate inhibits proliferation and triggers
apoptosis in colon cancer via the hedgehog/phosphoinositide
3-kinase pathways. Can J Physiol Pharmacol. 99:910–920. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Gao J, Chesebrough JW, Cartlidge SA,
Ricketts SA, Incognito L, Veldman-Jones M, Blakey DC, Tabrizi M,
Jallal B, Trail PA, et al: Dual IGF-I/II-neutralizing antibody
MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer
Res. 71:1029–1040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kim JH, Choi DS, Lee OH, Oh SH, Lippman SM
and Lee HY: Antiangiogenic antitumor activities of IGFBP-3 are
mediated by IGF-independent suppression of Erk1/2 activation and
Egr-1-mediated transcriptional events. Blood. 118:2622–2631. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Gao S, Sun Y, Zhang X, Hu L, Liu Y, Chua
CY, Phillips LM, Ren H, Fleming JB, Wang H, et al: IGFBP2 Activates
the NF-κB pathway to drive epithelial-mesenchymal transition and
invasive character in pancreatic ductal adenocarcinoma. Cancer Res.
76:6543–6554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Cai Q, Kim M, Harada A, Idowu MO,
Sundaresan G, Zweit J and Oh Y: Alpha-1 Antitrypsin inhibits
tumorigenesis and progression of colitis-associated colon cancer
through suppression of inflammatory neutrophil-activated serine
proteases and IGFBP-3 proteolysis. Int J Mol Sci. 23:137372022.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wei X, Huo Y, Pi J, Gao Y, Rao S, He M,
Wei Q, Song P, Chen Y, Lu D, et al: METTL3 preferentially enhances
non-m6A translation of epigenetic factors and promotes
tumourigenesis. Nat Cell Biol. 24:1278–1290. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
He P, Liu X, Yu G, Wang Y, Wang S, Liu J
and An Y: METTL3 facilitates prostate cancer progression via
inducing HOXC6 m6A modification and stabilizing its expression
through IGF2BP2-dependent mechanisms. Mol Cell Biochem.
479:1707–1720. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Chen X, Wang M, Wang H, Yang J, Li X,
Zhang R, Ding X, Hou H, Zhou J and Wu M: METTL3 inhibitor
suppresses the progression of prostate cancer via IGFBP3/AKT
pathway and synergizes with PARP inhibitor. Biomed Pharmacother.
179:1173662024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Pomeroy AE, Schmidt EV, Sorger PK and
Palmer AC: Drug independence and the curability of cancer by
combination chemotherapy. Trends Cancer. 8:915–929. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Dias MP, Moser SC, Ganesan S and Jonkers
J: Understanding and overcoming resistance to PARP inhibitors in
cancer therapy. Nat Rev Clin Oncol. 18:773–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ravi P, Wang V, Fichorova RN, McGregor B,
Wei XX, Basaria S and Sweeney CJ: IGF-1 axis changes with ADT and
docetaxel in metastatic prostate cancer. Endocr Relat Cancer.
30:e2302412023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Codony-Servat J, Cuatrecasas M, Asensio E,
Montironi C, Martínez-Cardús A, Marín-Aguilera M, Horndler C,
Martínez-Balibrea E, Rubini M, Jares P, et al: Nuclear IGF-1R
predicts chemotherapy and targeted therapy resistance in metastatic
colorectal cancer. Br J Cancer. 117:1777–1786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Qiu N, He YF, Zhang SM, Zhan YT, Han GD,
Jiang M, He WX, Zhou J, Liang HL, Ao X, et al: Cullin7 enhances
resistance to trastuzumab therapy in Her2 positive breast cancer
via degrading IRS-1 and downregulating IGFBP-3 to activate the
PI3K/AKT pathway. Cancer Lett. 464:25–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Xu Z, Wang X, Sun W, Xu F, Kou H, Hu W,
Zhang Y, Jiang Q, Tang J and Xu Y: RelB-activated GPX4 inhibits
ferroptosis and confers tamoxifen resistance in breast cancer.
Redox Biol. 68:1029522023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zheng Y, Sowers JY and Houston KD: IGFBP-1
expression promotes tamoxifen resistance in breast cancer cells via
Erk pathway activation. Front Endocrinol (Lausanne). 11:2332020.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Suzuki H, Iwamoto H, Seki T, Nakamura T,
Masuda A, Sakaue T, Tanaka T, Imamura Y, Niizeki T, Nakano M, et
al: Tumor-derived insulin-like growth factor-binding protein-1
contributes to resistance of hepatocellular carcinoma to tyrosine
kinase inhibitors. Cancer Commun (Lond). 43:415–434. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lu H, Ai J, Zheng Y, Zhou W, Zhang L, Zhu
J, Zhang H and Wang S: IGFBP2/ITGA5 promotes gefitinib resistance
via activating STAT3/CXCL1 axis in non-small cell lung cancer. Cell
Death Dis. 15:4472024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Coleman KL, Chiaramonti M, Haddad B,
Ranzenberger R, Henning H, Al Khashali H, Ray R, Darweesh B,
Guthrie J, Heyl D and Evans HG: Phosphorylation of IGFBP-3 by
casein kinase 2 blocks its interaction with hyaluronan, enabling
HA-CD44 signaling leading to increased NSCLC cell survival and
cisplatin resistance. Cells. 12:4052023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chen X, Lu Q, Zhou H, Liu J, Nadorp B,
Lasry A, Sun Z, Lai B, Rona G, Zhang J, et al: A
membrane-associated MHC-I inhibitory axis for cancer immune
evasion. Cell. 186:3903–3920.e21. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yu L, Ding Y, Wan T, Deng T, Huang H and
Liu J: Significance of CD47 and Its association with tumor immune
microenvironment heterogeneity in ovarian cancer. Front Immunol.
12:7681152021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Sun Y, Yin Z, Li S, Wu L, Zhang Y, Zhao Y,
Gomes Dos Santos IL, Subudhi S, Lei P, Muzikansky A, et al:
Losartan rewires the tumor-immune microenvironment and suppresses
IGF-1 to overcome resistance to chemo-immunotherapy in ovarian
cancer. Br J Cancer. 131:1683–1693. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Song D, Wu Y, Li J, Liu J, Yi Z, Wang X,
Sun J, Li L, Wu Q, Chen Y, et al: Insulin-like growth factor 2
drives fibroblast-mediated tumor immunoevasion and confers
resistance to immunotherapy. J Clin Invest. 134:e1833662024.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Hashimoto M, Konda JD, Perrino S, Celia
Fernandez M, Lowy AM and Brodt P: Targeting the IGF-Axis
potentiates immunotherapy for pancreatic ductal adenocarcinoma
liver metastases by altering the immunosuppressive
microenvironment. Mol Cancer Ther. 20:2469–2482. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Pointer KB, Pitroda SP and Weichselbaum
RR: Radiotherapy and immunotherapy: Open questions and future
strategies. Trends Cancer. 8:9–20. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hessels AC, Langendijk JA, Gawryszuk A,
Heersters MAAM, van der Salm NLM, Tissing WJE, van der Weide HL and
Maduro JH: Review-Late toxicity of abdominal and pelvic
radiotherapy for childhood cancer. Radiother Oncol. 170:27–36.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zong R, Chen X, Feng J and Xu S: IGF-1R
depletion sensitizes colon cancer cell lines to radiotherapy.
Cancer Biomark. 32:199–206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Chen L, Zhu Z, Gao W, Jiang Q, Yu J and Fu
C: Systemic analysis of different colorectal cancer cell lines and
TCGA datasets identified IGF-1R/EGFR-PPAR-CASPASE axis as important
indicator for radiotherapy sensitivity. Gene. 627:484–490. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Chen JH, T H Wu A, T W Tzeng D, Huang CC,
Tzeng YM and Chao TY: Antrocin, a bioactive component from Antrodia
cinnamomea, suppresses breast carcinogenesis and stemness via
downregulation of β-catenin/Notch1/Akt signaling. Phytomedicine.
52:70–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Su YH, Wu JS, Dai YZ, Chen YT, Lin YX,
Tzeng YM and Liao JW: Anti-oxidant, anti-mutagenic activity and
safety evaluation of antrocin. Toxics. 11:5472023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Chen YA, Tzeng DTW, Huang YP, Lin CJ, Lo
UG, Wu CL, Lin H, Hsieh JT, Tang CH and Lai CH: Antrocin sensitizes
prostate cancer cells to radiotherapy through inhibiting PI3K/AKT
and MAPK signaling pathways. Cancers (Basel). 11:342018. View Article : Google Scholar : PubMed/NCBI
|