Introduction
Pancreatitis, characterized by inflammation of the
pancreas, is a severe and often life-threatening condition that
occurs in both acute and chronic forms. Acute pancreatitis (AP) is
frequently associated with reversible pancreatic injury, whereas
chronic pancreatitis (CP) is associated with persistent
inflammation, fibrosis and loss of pancreatic function (1,2). The
pathogenesis of pancreatitis involves complex interactions between
the pancreas, immune system and various metabolic and environmental
factors, such as alcohol consumption, gallstones and dyslipidemia
(3). When left untreated,
pancreatitis can progress to complications such as pancreatic
necrosis, pseudocysts, or pancreatic cancer, making timely
diagnosis and effective treatment critical (4). Despite substantial advancements in
understanding the pathophysiology of pancreatitis, clinical
management of this condition remains challenging, with a lack of
reliable biomarkers for early diagnosis and limited therapeutic
options to mitigate inflammation and tissue damage (5,6).
Exosomal miRNAs are small, non-coding RNA molecules
packaged into extracellular vesicles (EVs), such as exosomes, which
mediate intercellular communication by transferring genetic
material and modulating the function of recipient cells (7,8). A
number of miRNAs have been implicated in a wide range of
physiological and pathological processes, including inflammation,
immune modulation, cell survival and fibrosis, which are key
factors in pancreatitis (9,10).
Exosomal miRNAs can modulate various cellular responses in
pancreatitis, by influencing the behavior of various cell types,
such as pancreatic acinar cells (PACs), immune cells and pancreatic
stellate cells (PSCs). For example, exosomal miR-148a and
miR-551b-5p, which are upregulated in the peripheral blood of
patients with AP, promote activation of the IL-6/STAT3 pathways,
further amplifying autophagy impairment in PACs (11). Conversely, exosomal miR-130a-3p, a
profibrotic miRNA, plays a protective role by promoting PSC
activation and collagen formation via suppressing suppression of
peroxisome proliferator-activated receptor gamma (PPAR-γ) (12). Indeed, exosomal miRNA profiles
evolve throughout pancreatitis progression. During the acute phase
of pancreatitis, exosomal miRNAs such as miR-21 and miR-155 are
upregulated and associated with inflammatory responses and immune
cell activation. As pancreatitis progresses to the chronic stage,
the role of exosomal miRNAs in fibrosis becomes more prominent,
with miR-122 being involved in regulating collagen deposition and
tissue remodeling. Furthermore, the chronic stage may also see an
increased presence of exosomal miRNAs that modulate immune cell
infiltration, particularly miR-146a, which is involved in
controlling macrophage polarization (13). Therefore, understanding the role of
exosomal miRNAs in the pathogenesis of pancreatitis is crucial for
identifying novel biomarkers for early diagnosis and exploring
miRNA-based therapeutic strategies.
The present review summarized the pathogenesis of
pancreatitis and the biogenesis and functions of exosomal miRNAs.
It also focused on the mechanisms by which exosomal miRNAs modulate
cellular responses during pancreatitis, their potential as
biomarkers for early diagnosis and their emerging role as
therapeutic agents for the management of this disease.
Pathogenesis of pancreatitis
Pancreatitis, an inflammatory condition of the
pancreas, is classified as AP and CP. AP is typically a
self-limiting disease, whereas CP results from repeated episodes of
inflammation that culminates in irreversible damage, fibrosis and
functional impairment of the pancreas (14). The pathogenesis of pancreatitis
involves a complex interplay between PACs, inflammatory cells and
various molecular signaling pathways.
Pancreatitis is initiated by an insult to PACs,
resulting in the intracellular activation of digestive enzymes such
as trypsinogen that target the breakdown of pancreatic tissue and
initiate an inflammatory an inflammatory response that contributes
to both local and systemic complications (15). One of the most common causes of AP
is obstruction of the common bile duct by gallstones, which leads
to increased pancreatic duct pressure and premature activation of
digestive enzymes within PACs. This obstruction also facilitates
the reflux of bile acids into the pancreatic ducts, which can
directly injure acinar cells and further enhance inflammation
(16). Additionally, alcohol
induces the secretion and activation of pancreatic enzymes within
PACs. Alcohol metabolism in the liver produces toxic metabolites,
such as acetaldehyde, which promote oxidative stress, inflammation
and pancreatic tissue injury (17). Viral infections, such as mumps and
coxsackievirus, as well as hematological conditions, such as
hypertriglyceridemia, can also trigger pancreatitis. Elevated
triglyceride levels result in the formation of lipid droplets that
obstruct the pancreatic ducts and facilitate PAC injury and
inflammation (18).
The activation of digestive enzymes within PACs
initiates a cascade of inflammatory events. Persistent inflammation
can progress to severe forms in the presence of systemic
inflammation, ischemia and multi-organ failure (19). Upon injury, PACs release
pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, which
amplify the inflammatory response by recruiting and activating
immune cells, such as neutrophils and macrophages (20). These cytokines contribute to
systemic inflammation and trigger the development of sepsis and
systemic inflammatory response syndrome (21). Also, cytokine signaling promotes
endothelial activation, increases vascular permeability and
facilitates extravasation of immune cells into pancreatic tissue.
Infiltrating inflammatory cells release additional pro-inflammatory
mediators, such as reactive oxygen species (ROS), matrix
metalloproteinases and cytokines, thereby propagating local tissue
injury and contributing to systemic inflammation (22). As a key regulator of inflammation
in pancreatitis, the pyrin domain (PYD)-containing protein 3
(NLRP3) inflammasome can be activated by cellular stressors such as
ROS, calcium overload and ATP release, thus promoting the release
of IL-1β and IL-18, which amplifies the inflammatory cascade and
contributes to the tissue damage observed in both acute and chronic
pancreatitis (23). In chronic
pancreatitis, ongoing inflammation leads to the activation of PSCs,
which play a central role in fibrosis. Upon activation, PSCs
transform into myofibroblasts and produce excessive extracellular
matrix components, such as collagen, leading to pancreatic
fibrosis. This fibrotic process impairs pancreatic function and
disrupts normal tissue architecture (24).
The involvement of exosomal miRNAs in pancreatic
cell injury, inflammation, fibrosis and tissue remodeling processes
provides an additional layer of regulation, which influences
disease progression and resolution. Exosomal miRNAs are key
modulators of inflammation, fibrosis, PAC death and immune cell
function, making them attractive candidates for diagnostic and
therapeutic applications in pancreatitis. Exosomal miRNAs function
as a part of complex networks involving cytokines, chemokines and
other exosome-associated RNAs (25). The interplay between exosomal
miRNAs and factors such as extracellular matrix components, growth
factors and immune modulators contributes to the pathogenesis of
pancreatitis. Although animal models of pancreatitis, such as the
cerulein-induced model, have provided valuable insights into the
inflammatory and fibrotic stages of the disease, they fail to fully
mimic human pancreatitis owing to species differences in disease
progression and immune responses. Current models typically lack the
complexity of human tissue microenvironments, limiting their
ability to accurately reflect the role of exosomal miRNAs in
intercellular communication (26).
Thus, the development of more advanced models, such as organoid
cultures and humanized mouse models, is necessary to study the
interactions between exosomal miRNAs and pancreatic cells.
Overviews of exosomal miRNAs
miRNAs are small, non-coding RNA (ncRNA) molecules
that play crucial roles in the regulation of gene expression at the
post-transcriptional level and are involved in various
physiological and pathological processes, including inflammatory
responses, immune modulation and pancreatitis pathogenesis
(27). Exosomes, a subset of EVs,
have emerged as vital mediators of intercellular communication,
transporter of various bioactive molecules including miRNAs
(9). Exosomal miRNAs can transfer
genetic information and modulate gene expression in recipient
cells, influencing their function and behavior (10). Exosomal communication is vital for
the maintenance of cellular homeostasis and coordination of complex
biological responses.
Biogenesis of miRNAs
The biogenesis of miRNAs begins in the nucleus with
the transcription of miRNA genes, which are often located within
intergenic regions, although they can also be found within introns
of protein-coding genes or within the untranslated regions (UTRs)
of other ncRNA (28).
Transcription of miRNA genes is tightly regulated by various
factors that respond to cellular cues, including those involved in
inflammation, stress and immune responses (29). In pancreatitis, inflammatory
signals can influence the expression of specific miRNAs,
potentially altering disease progression and resolution (30).
The biogenesis of miRNAs is an intricate, multi-step
process involving both canonical and non-canonical pathways that
are modulated to ensure gene expression and cellular function
(Fig. 1). Initially, miRNAs are
transcribed into primary miRNAs (pri-miRNAs) by RNA polymerase II,
which produces a long primary transcript that is capped,
polyadenylated and spliced, similar to conventional mRNAs (31). Subsequently in the nucleus,
pri-miRNAs undergo crucial catalyzation by a microprocessor complex
consisting of Drosha, a ribonuclease III enzyme and its cofactor
DGCR8 (DiGeorge syndrome critical region 8) to generate a precursor
miRNA (pre-miRNA) (32). Drosha
cleaves the pri-miRNA in a sequence-dependent manner, excising a
60–70 nucleotide stem-loop structure from the primary transcript,
resulting in the formation of the pre-miRNA. Subsequently,
Exportin-5 recognizes the pre-miRNA's stem-loop structure and
facilitates its transport across the nuclear envelope and into the
cytoplasm for further processing. In the cytoplasm, pre-miRNAs
undergo a second processing step facilitated by another
ribonuclease III enzyme Dicer, which cleaves the pre-miRNA~22
nucleotides from the terminal loop, resulting in a double-stranded
RNA duplex composed of the mature miRNA (the guide strand) and its
complementary passenger strand (33). The miRNA duplexes are then loaded
onto argonaute (AGO) proteins to form the RNA-induced silencing
complex (RISC), wherein the guide strand retained is considered a
mature miRNA and the other strand is typically degraded (34). Mature miRNAs within the RISC
complex then bind to complementary sequences in the 3′UTRs of
target mRNAs, promoting mRNA degradation or translational
suppression, thereby post-transcriptionally regulating gene
expression (35).
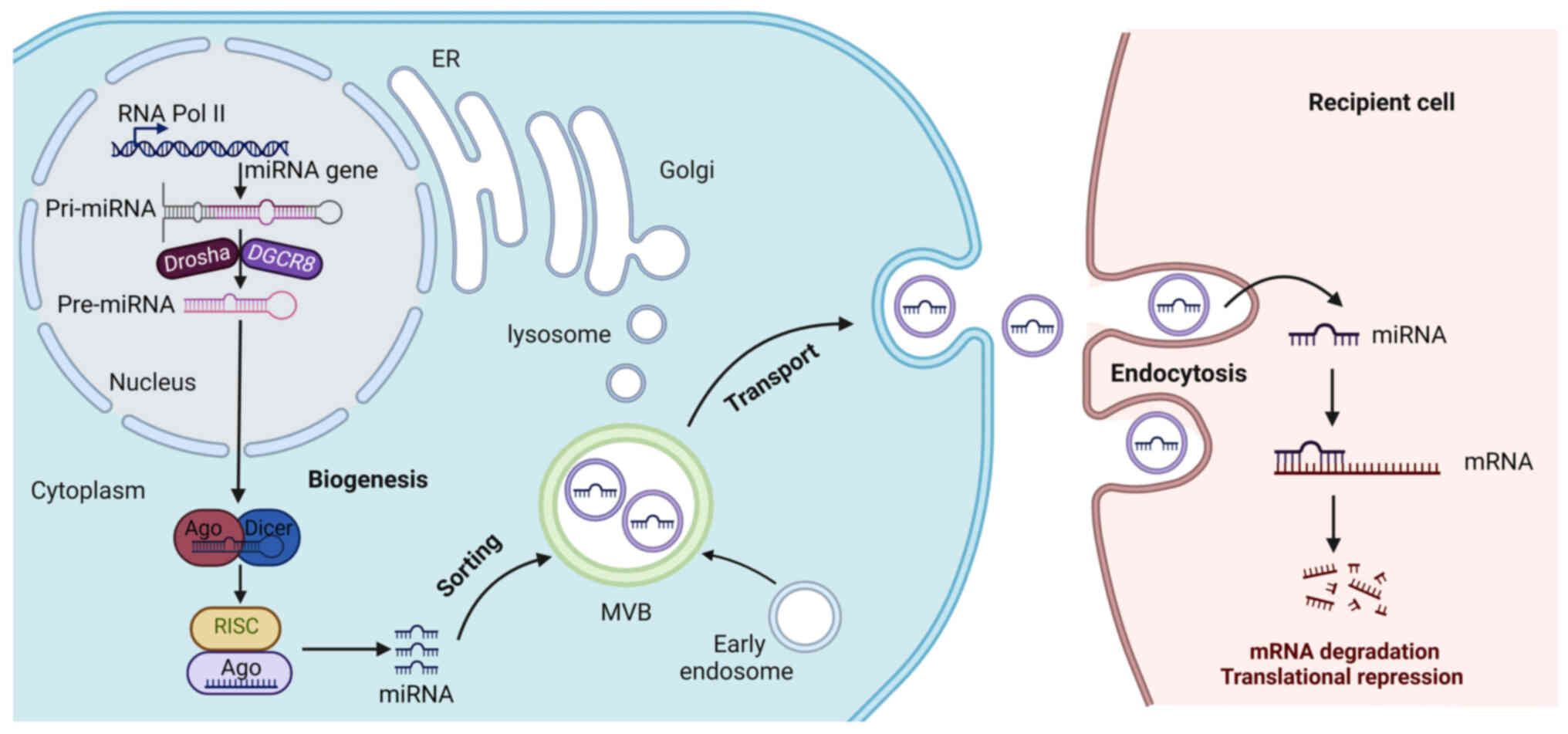 | Figure 1.Biogenesis, sorting and transport of
exosomal miRNAs. RNA polymerase II transcribes miRNA genes,
producing pri-miRNA, which is processed in the nucleus by Drasha
and DGCR8, generating pre-miRNA. Pre-miRNA is then transported to
the cytoplasm and is further processed in the cytoplasm by Dicer,
forming mature miRNA, which is incorporated into to silence the
target gene. Early endosomes are generated by endocytosis of parent
cell and then undergo the second invagination of the plasma
membrane, thus forming MVBs, which are subsequently either degraded
or released from the cell as exosomes. A number of proteins are
responsible for miRNA entry into the exosomes, such as
heterogeneous nuclear ribonucleoproteins. Subsequently, the
recipient cells internalize the exosomes attached to the cell
membrane through endocytosis and finally release their contents
into the cell. The released miRNA specifically binds to the 3′UTR
of the target mRNA and inhibits the expression of the target gene.
miRNA, microRNA; AGO, Argonaute; pre-miRNAs, precursor miRNAs;
pri-miRNAs, primary miRNAs; DGCR8, DiGeorge syndrome critical
region 8; MVBs, multivesicular bodies; UTRs, untranslated regions;
RISCs, RNA-induced silencing complexes;. |
Although the canonical pathway of miRNA biogenesis
is well-established, several non-canonical pathways also exist. For
example, mirtrons are a prominent class of Drosha-independent
miRNAs that are directly spliced from introns (36). Additionally, pre-miRNAs are
directly cleaved by ribonuclease 3B2, resulting in the generation
of mature miRNAs without the need for Dicer (37). Another non-canonical miRNA
biogenesis pathway involves alternative splicing of primary
transcripts. Exon-derived miRNAs, also called exonic miRNAs, are a
subset of miRNAs formed by the alternative splicing of pre-mRNA.
These miRNAs are produced from exon-exon junctions of coding genes
and are typically processed by Dicer without requiring the typical
Drosha cleavage step (38). These
alternative mechanisms enable cells to generate miRNAs through
distinct enzymatic processes, bypassing key steps in the canonical
pathway and providing additional layers of control over miRNA
expression and function. The biogenesis of miRNAs is not only
crucial for physiological processes but also participates in
disease pathogenesis. Understanding the detailed mechanisms of
miRNA biogenesis, including both canonical and non-canonical
pathways and the regulatory networks involved and provides valuable
insights into their roles in pancreatitis.
Exosomal miRNA sorting and
transport
The sorting of miRNAs into exosomes is a highly
selective and regulated process (Fig.
1). Exosome biogenesis begins in the multivesicular bodies
(MVBs), which are formed when the early endosomes mature and
invaginate, creating intraluminal vesicles (ILVs) that fuse the MVB
with the plasma membrane to generate exosomes (39). The endosomal sorting complex
required for transport (ESCRT) proteins, including TSG101 and Alix,
are involved in the budding of intraluminal vesicles within the
MVBs. The ESCRT machinery interacts with RNA-binding proteins to
enrich specific miRNAs in exosomes (40). For instance, heterogeneous nuclear
ribonucleoprotein A2B1 binds to miRNAs and facilitates their
interaction with the exosomal membrane, while Y-box binding protein
1 acts as an adaptor to facilitate the incorporation of miRNAs into
exosomes by interacting with both miRNAs and ESCRT proteins
(41,42). Rab GTPases are key regulators of
vesicular trafficking and several members of the Rab family,
including Rab27a and Rab27b, have been implicated in the packaging
of miRNAs into exosomes. These GTPases control the movement of MVBs
to the plasma membrane and their subsequent fusion with the
membrane to release exosomes (43,44).
Once miRNA-loaded exosomes are released into the extracellular
environment, they can be taken up by recipient cells through
several mechanisms, including endocytosis, phagocytosis, or direct
fusion with the plasma membrane (45). For instance, caveolin-mediated and
clathrin-mediated endocytosis are two prominent mechanisms by which
exosomes can enter target cells (46,47).
Also, exosomes can fuse directly with the plasma membrane of
recipient cells, releasing their cargo without internalization
(48). The transport of exosomal
miRNAs to distant tissues is particularly important in
pancreatitis, in which inflammatory signals and cellular stress may
elicit the secretion of exosomes containing miRNAs that modulate
immune responses and tissue damage.
Exosomal miRNA functions
Exosomal miRNAs mediate intercellular communication
under various physiological and pathological conditions. Once
inside the recipient cell, exosomal miRNAs are released from the
vesicles and participate in diverse cellular processes including
inflammation, immune modulation, tissue repair and fibrosis
(49,50). As messengers, exosomal miRNAs exert
profound effects on recipient cells by regulating gene expression
at the post-transcriptional level, often via translational
repression or mRNA degradation (51). In addition, exosomal miRNAs exert a
ligand-like function in recipient cells, where they act as direct
agonists of specific receptor families by interacting with
proteins, thereby affecting cellular processes and disease
progression (52). Thus, exosomal
miRNAs function as critical mediators of intercellular
communication, orchestrating a complex network of pro-inflammatory
and pro-fibrotic signals by regulating both local pancreatic cell
behaviors and systemic immune responses. Understanding the
biogenesis, sorting, transport and function of exosomal miRNAs is
essential for elucidating their roles in pancreatitis. Furthermore,
different exosome isolation techniques affect the purity and yield
of exosomes, which in turn affects downstream miRNA analysis. For
example, ultracentrifugation leads to contamination with protein
aggregates and other vesicles, which skew miRNA profiles (53). Precipitation-based methods, such as
ExoQuick, fail to isolate the purest exosomes (54). The emerging microfluidic
technologies offer the advantage of high precision and minimal
sample volume, but they are not yet widely available for routine
clinical use (55). However,
factors include variations in the source of exosomes, such as blood
and pancreatic fluid, differences in isolation protocols and the
presence of other biomolecules, such as proteins and lipids that
may interfere with miRNA quantification. Additionally, the dynamic
nature of exosomal miRNA release in response to environmental
stimuli such as inflammation or oxidative stress can lead to
fluctuations in miRNA levels, complicating their use as stable
biomarkers.
Effects of exosomal miRNAs on various cell
types during pancreatitis
Exosomal miRNAs are involved in pancreatitis by
influencing various pathological processes, such as inflammation,
pancreatic injury, fibrosis and external pancreatic organ injury.
Various cell types are affected by exosomal miRNAs, which regulate
the expression of genes related to various cellular processes and
signaling pathways in pancreatitis (Table I; Fig.
2). Exosomes are produced by various cells, including PACs and
immune cells and the miRNA cargo in these exosomes can vary based
on their tissue of origin. For example, exosomes derived from PACs
carry miRNAs that regulate digestive enzyme secretion and
inflammation, such as miR-503-322 (56). By contrast, exosomes from immune
cells such as macrophages and T cells carry miRNAs that modulate
the immune response in pancreatitis (57). Understanding the tissue-specific
profiles of exosomal miRNAs is important for developing targeted
diagnostic and therapeutic approaches. It should be noticed that
lncRNAs and circRNAs can act as sponges for miRNAs, thereby
altering their availability and functional impact on target genes.
For instance, the lncRNA MALAT1 has been shown to modulate the
effects of miR-181a-5 in macrophage polarization. The collective
activity of these RNAs within exosomes can influence the
inflammatory response, fibrosis and immune regulation in
pancreatitis, reflecting a more complex and integrated view of
disease mechanisms (58).
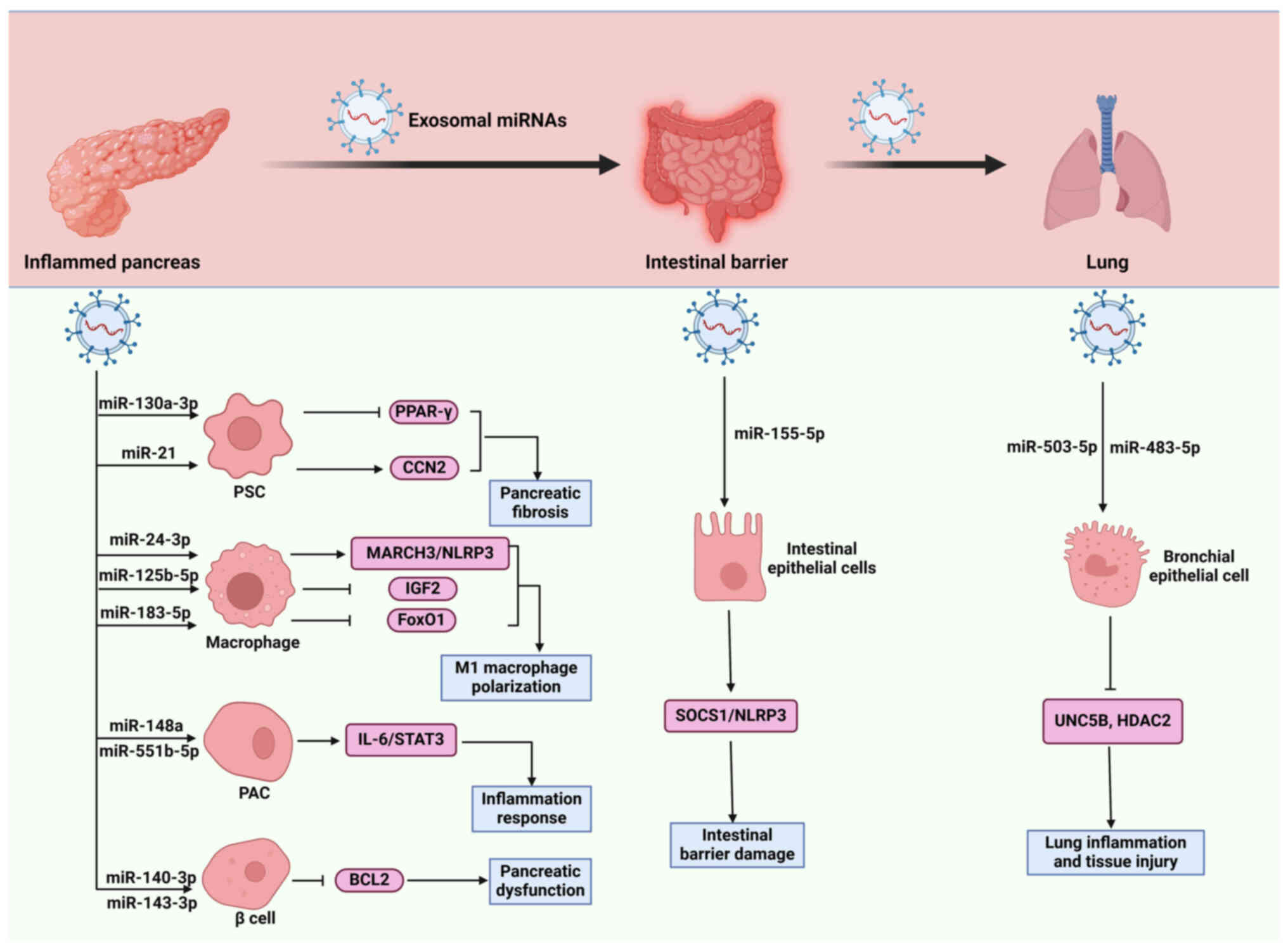 | Figure 2.Role of exosomal miRNAs in
pancreatitis progression. Exosomal miRNAs exert crucial roles in
promoting macrophage polarization, inflammatory responses,
pancreatic fibrosis, β cell injury, intestinal barrier damage and
lung injury by acting on various cell types, including macrophages,
PACs, PSCs, β cells, intestinal epithelial cells, bronchial
epithelial cells, thereby affecting the functions recipient cells
through regulating divers signaling pathways, such as MAPK,
IL-6/STAT3 and SOCS1/NLRP3. CCN2, connective tissue growth factor;
HDAC2, histone deacetylase-2; MARCH3, membrane-associated
RING-CH-type finger 3; miRNAs, microRNAs; NF-κB, nuclear factor-κB;
NLRP3, pyrin domain (PYD)-containing protein 3; PACs, pancreatic
acinar cells; PSCs, pancreatic stellate cells; PPAR-γ, peroxisome
proliferator-activated receptor gamma; SOCS1, suppressor of
cytokine signaling 1; TAB2, TAK1-binding protein 2; TRAF6, tumor
necrosis factor receptor-associated factor 6; UNC5B,
uncoordinated-5 homolog B; ⊥, inhibitory effect and → indicates a
promoting effect. |
 | Table I.Role of exosomal miRNAs in
pancreatitis. |
Table I.
Role of exosomal miRNAs in
pancreatitis.
| First author/s,
year | Exosomal
miRNAs | Expression | Origin | Targets | Recipient
cells | Effects | (Refs.) |
|---|
| Su et al
(2024) | miR-24-3p | UP | PACs | MARCH3/NLRP3 | Macrophages | Stimulate M1
macrophage polarization and pyroptosis | (69) |
| Zheng et al
(2023) | miR-125b-5p | UP | PACs | IGF2 | Macrophages | Trigger M1
macrophage polarization and PACs apoptosis | (68) |
| Tang et al
(2022) | miR-183-5p | UP | PACs | FoxO1 | Macrophages | Promote M1
macrophage polarization and reduce macrophage phagocytosis | (67) |
| Jimenez-Alesanco
et al, 2019 | miR-155 | UP | Undefined | Undefined | Macrophages | Induce
pro-inflammatory responses of macrophages | (63) |
| Zhao et al
(2016) | Undefined
miRNAs | Undefined | PACs | MAPK | Macrophages | Promote macrophage
activation and pro-inflammatory responses | (62) |
| Wei et al
(2024) | miR-148a,
miR-551b-5p | UP | PBMCs | IL-6/STAT3 | PACs | Mediate autophagy
damage and inflammatory responses | (11) |
| Wang et al
(2021) | miR-130a-3p | Up | PACs | PPAR-γ | PSCs | Enhance collagen
formation and pancreatic fibrosis | (12) |
| Charrier et
al (2014) | miR-21 | Up | PSCs | CCN2 | PSCs | Facilitate collagen
deposition and pancreatic fibrosis | (77) |
| Zhu et al
(2022) | miR-140-3p,
miR-143-3p | UP | PSCs | BCL2 | β cells | Decrease the cell
count and viability of β cell | (82) |
| Xiong et al
(2023) | miR-503-5p,
miR-483-5p | UP, DOWN | Undefined | UNC5B, HDAC2 | Bronchial
epithelial cells | Aggravate lung
inflammation and tissue injury | (86) |
| Shao et al,
2023 | miR-155-5p | UP | Undefined | SOCS1/NLRP3 | Intestinal
epithelial cells | Induce cell
pyroptosis and intestinal barrier damage | (88) |
Macrophages
Macrophage activation leads to an imbalance in
cytokine networks, promoting systemic inflammation and potentially
resulting in multiple organ failure in AP. Overactivated
macrophages produce excessive pro-inflammatory cytokines such as
TNF-α, IL-6 and IL-1β, which exacerbate the inflammatory response
and contribute to pancreatic endothelial barrier dysfunction and
tissue damage (59). During CP,
macrophages are activated by IL-4 and IL-13, which are secreted by
PSCs and drive acinar-to-ductal metaplasia through the activation
of nuclear factor-κB (NF-κB) and matrix metalloproteinases, thus
participating in extracellular matrix remodeling and fibrosis,
which have protective effects in pancreatitis but also contribute
to cancer pathogenesis (60,61).
Exosomal miRNAs derived from pancreatic cells
influence the inflammatory response of macrophages in pancreatitis
by modulating their activation and polarization. For instance, PACs
are known to release exosomes containing miRNAs that activate
macrophages and promote inflammatory responses through the tumor
necrosis factor receptor-associated factor 6/NF-κB signaling
cascade, with miRNA target genes primarily involved in
mitogen-activated protein kinases pathways, further mediating
macrophage-mediated tissue damage (62). In rats with taurocholate-induced
AP, plasma exosomes are enriched with miR-155, which harbors potent
pro-inflammatory activity on macrophages (63). Overexpressed miR-155 aggravates
impaired autophagy in caerulein-treated PACs by inhibiting the
expression of Rictor (64).
Silencing of miR-155 ameliorates pancreatic and lung damage in an
AP mouse model by blocking the accumulation of autophagosomes that
are unable to fuse with lysosomes and alleviates pancreatic
inflammation by targeting TAK1-binding protein 2 (65). Increased levels of miR-155 are
associated with severe AP and its expression increases with disease
progression. Further mechanistic evaluation revealed that miR-155
increases the Th17-mediated inflammatory response by targeting
suppressor of cytokine signaling 1 (SOCS1) (66). These findings demonstrate the
pro-inflammatory role of miR-155, which can be loaded with exosomes
to facilitate macrophage activation and inflammatory damage to the
pancreas. In addition, elevated miR-183-5p levels in serum EVs are
related to AP severity. Mechanistically, miR-183-5p is elevated in
injured PACs and transported by EVs to macrophages where miR-183-5p
induces M1 macrophage polarization through the downregulation of
FoxO1 and the release of inflammatory cytokines, thereby
aggravating AP-related injuries (67). In activated PACs and AP pancreatic
tissue, exosomal miR-125b-5p is upregulated to promote M1
macrophage polarization and inhibit M2 macrophage polarization,
resulting in massive production of pro-inflammatory factors and ROS
accumulation by inhibiting insulin-like growth factor 2 expression
and further activating the PI3K/AKT signaling pathway, thus
exacerbating AP (68). Similarly,
exosomal miR-24-3p derived from cerulein-treated PACs promotes
peritoneal macrophage M1 polarization and pyroptosis by inhibiting
membrane-associated RING-CH-type finger 3 (MARCH3) expression and
reducing NLRP3 ubiquitination, which contributes to ROS production,
inflammation and apoptosis in PACs (69).
Collectively, exosomal miRNAs affect macrophage
behavior and shape the inflammatory milieu that is associated with
pancreatitis. Although exosomal miRNAs from pancreatic cells
predominantly promote inflammatory responses in macrophages, there
is potential for therapeutic intervention. For instance, targeting
specific exosomal miRNAs could mitigate the inflammatory effects
and offer new avenues for treating pancreatitis. Additionally,
understanding the exact role of exosomal miRNA-regulated
macrophages in inflammation and tissue repair could provide
insights into balancing these processes to improve clinical
outcomes.
PACs
PACs are responsible for synthesizing and secreting
digestive enzymes and the dysfunction of PACs initiates a cascade
of events, including enzyme activation and inflammatory signaling,
leading to inflammation and tissue damage (70). Pathological calcium overload
triggers enzyme activation and cellular necrosis. It also elicits
mitochondrial dysfunction and impairs ATP production, exacerbating
cellular stress and injury, suggesting that calcium dysregulation
is a key factor in the pathogenesis of pancreatitis (19). Hence, PAC injury and dysfunction
are central to the pathogenesis of pancreatitis.
Exosomal miRNAs are considered to regulate PAC
function, thus affecting the progression of pancreatitis. It has
been reported that peripheral blood mononuclear cell-derived
exosomal miR-148a and miR-551b-5p are highly expressed in patients
with AP compared with healthy individuals. Mechanistic
investigation reveals that overexpression of miR-148a in PACs
decreases the secretion of IL-1β and IL-18 to mediate autophagy
impairment through the IL-6/STAT3 signaling pathway (11). Moreover, miR-148a-3p deletion
reduces inflammatory infiltration and protects against cell
necrosis, amylase and lipase activity in AP by inducing phosphatase
with tensin homology (PTEN) expression (71). These findings indicate that
exosomal miR-148a functions as a detrimental factor in accelerating
the progression of pancreatitis. Additionally, miR-551-5p is
increased in the serum of both patients with mild and severe AP and
upregulated miR-551-5p is involved in the regulation of
inflammatory responses (72).
Serum miR-551-5p levels can be useful for the assessment of
pancreatic injury in the acute phase of AP and can also predict AP
severity (73). These results
imply that overexpressed miR-551-5p in the serum of patients with
pancreatitis can be loaded by exosomes, which further disrupts PAC
function and contributes to disease development.
Overall, exosomal miRNAs are pivotal in modulating
PAC function in pancreatitis; however, their roles remain elusive.
Future studies should explore the intricate network of miRNA
interactions in the exosomal milieu and how these interactions
influence PAC behavior. Understanding the synergistic or
antagonistic effects of different miRNAs within exosomes may reveal
new therapeutic avenues.
PSCs
When activated, PSCs transform from a quiescent
state to a highly active phenotype that contributes to the fibrotic
and inflammatory environment characteristic of pancreatitis. This
transformation is influenced by various factors, including alcohol
metabolites, cytokines and oxidative stress, which collectively
exacerbate the disease progression (24). Activated PSCs contribute to the
inflammatory milieu in pancreatitis by interacting with immune
cells and producing cytokines and chemokines that recruit and
activate immune cells, further amplifying inflammation (60). PSCs exhibit unique gene expression
profiles associated with the progression from acute to CP,
including genes involved in extracellular matrix remodeling and
inflammation (74). Although PSCs
are primarily associated with fibrogenesis and inflammation, they
have been shown to have immunomodulatory functions, potentially
preventing autoimmune pancreatitis by regulating immune cell
recruitment (75). PSCs play a
central role in the pathophysiology of AP, including fibrogenesis
and inflammation. Understanding the regulatory roles of exosomal
miRNAs in PSCs offers potential therapeutic targets for mitigating
the fibrotic and inflammatory responses in pancreatitis.
Exosomal miRNAs are implicated in the modulation of
PSC activation and proliferation during pancreatitis. Exosomal
miR-130a-3p, derived from PACs, can promote PSC activation and
collagen formation by suppressing PPAR-γ in PSCs (12). Knockdown of miR-130a-3p alleviates
pancreatic fibrosis, accompanied by decreased serum levels of
hyaluronic acid and β-amylase and increased C-peptide levels,
suggesting improved pancreatic function (12). Likewise, let-7 miRNA, which is
enriched in exosomes from intact acinar cells, has been identified
as a suppressor of PSC activation. Let-7 mediates the anti-fibrotic
effects of acinar cells by modulating the expression of key
signaling molecules involved in PSC activation (76). Additionally, in a murine model of
alcoholic CP, exosomal miR-21 derived from activated PSCs promotes
the expression of connective tissue growth factor; in turn, this
provokes a phenotypic and functional transition from quiescent PSC
to activated myofibroblasts, thereby producing and depositing
collagen at high levels (77).
During AP, miR-21 is upregulated to facilitate the inflammatory
response and induce pancreatitis-associated lung injury by
upregulating protein inhibitor of activated Stat3 and
downregulating amphoterin (HMGB1) expression (78). Following resveratrol treatment, the
expression of miR-21 in activated PSCs is reduced, which attenuates
ROS-induced activation, invasion, migration and glycolysis of PSCs
by upregulating the PTEN protein level (79). These results indicate that exosomal
miR-21 represents novel aspects in PSC activation and fibrogenic
regulation during pancreatitis.
In conclusion, exosomal miRNAs modulate PSC
activation and proliferation, thus affecting inflammatory responses
and fibrosis during pancreatitis. Exosomal miRNAs are involved in
complex networks of cellular communication that extend beyond PSCs
and influence pancreatitis progression. Clarifying the broader
mechanisms underlying the role of exosomal miRNAs in pancreatitis
is crucial to validate their therapeutic utility. It can be
hypothesized that miRNAs associated with exosomes from PSCs could
have distinct functional roles compared with those derived from
acinar or ductal cells. In addition, exosomal miRNAs may interact
with other exosomal secretions to form regulatory networks that
influence pancreatic inflammation and fibrosis.
β cells
Pancreatic β cells, primarily responsible for
insulin production, are affected by inflammatory processes,
oxidative stress and immune-mediated damage, leading to their
dysfunction and eventual loss, which are critical factors in the
progression of pancreatitis as they result in impaired insulin
secretion and glucose regulation (80). These cells are involved in the
inflammatory response during pancreatitis through their interaction
with cytokines and the subsequent cellular stress responses. During
AP, EVs derived from M1 macrophages encapsulate inflammatory
mitochondria and subsequently penetrate pancreatic β cells, causing
lipid peroxidation and mitochondrial disruption (81). Moreover, fragments of mitochondrial
DNA are released into the cytosol, activating the STING pathway and
ultimately inducing apoptosis of β cells, which aggravates
inflammatory responses and pancreatitis progression (81). Notably, exosomal miRNAs have been
shown to modulate β cells and affect disease development. In
transforming growth factor-β1-treated PSCs, exosomal miR-140-3p and
miR-143-3p are delivered into β cells, where they increase the
cleaved caspase-3 levels and induce cell death via repressing the
expression of B-cell lymphoma 2 (82). However, downregulated miR-140-3p
has been discovered in patients with severe AP and acute lung
injury (83). Consistently,
overexpression of miR-143-3p in β cells reverses high
glucose-induced cell apoptosis and impairments in cell viability
and insulin secretion, as well as attenuates proinflammatory
cytokine production (84). These
findings suggest that miR-140-3p and miR-143-3p exert protective
effects against pancreatitis.
In summary, the role of exosomal miRNAs is
context-dependent. As key regulators of β cell differentiation and
function, dysregulation of exosomal miRNAs can affect pancreatitis
and various forms of diabetes mellitus. Understanding the specific
roles of exosomal miRNAs in β cells can provide insights into novel
therapeutic strategies for preserving β cell function and treating
pancreatitis and diabetes-related complications.
Other cells
Pancreatitis triggers a systemic inflammatory
response characterized by the release of pro-inflammatory cytokines
and chemokines, which lead to the activation of immune cells like
neutrophils and macrophages, thereby contributing to endothelial
dysfunction, capillary leakage and increased pulmonary vascular
permeability, pulmonary edema and tissue injury (85). Notably, miRNA transcriptomics
analysis shows miR-483-5p and miR-503-5p derived from EVs of severe
AP-associated lung injury patients, can promote inflammatory
responses and pulmonary injury by targeting histone deacetylase-2
(HDAC2) and uncoordinated-5 homolog B (UNC5B), respectively
(86). In addition, the systemic
inflammatory response in pancreatitis activates the gut-associated
lymphoid tissue and increases cytokine production, along with the
hydrolysis of pancreatic enzymes, which contribute to intestinal
mucosal injury, leading to conditions such as ileus, ischemia and
microbial dysbiosis (87).
miR-155-5p is enriched in circulating exosomes from severe AP rats
and can be delivered into intestinal epithelial cells, where it
inhibits SOCS1 to activate NLRP3 inflammasome-mediated pyroptosis,
leading to intestinal barrier damage (88). In addition, blocking exosome
release with GW4869 attenuates intestinal injury (88). Similarly, downregulating the
expression of miR-155-5p by stellate ganglion block can lessen the
production of pro-inflammatory cytokines and alleviate lung tissue
damage and edema via the SOCS5/JAK2/STAT3 axis in severe AP rats
(89).
In summary, exosomal miRNAs contribute to the
pathogenesis of pancreatitis-related lung and gut injury by
modulating inflammatory responses, gut barrier integrity,
endothelial function and immune cell recruitment. Their roles as
mediators of inter-organ communication suggest that targeting
exosomal miRNAs may offer a novel therapeutic approach to mitigate
the severity of pancreatitis. However, further research is needed
to fully elucidate the precise mechanisms by which exosomal miRNAs
affect the function of distant organs.
Exosomal miRNAs as biomarkers for
pancreatitis
Exosomal miRNAs have emerged as promising biomarkers
in the diagnosis and prognosis of various diseases, due to their
stability and presence in body fluids, which makes them accessible
for non-invasive testing (90–92).
The potential of exosomal miRNAs as diagnostic and prognostic tools
to offer insights into disease mechanisms and progression has been
increasingly recognized. These miRNAs serve as potential biomarkers
for the diagnosis of pancreatitis. They exhibit distinct expression
patterns in pancreatitis patients compared with healthy individuals
and their levels are related to disease severity. For instance,
three previously unreported exosomal miRNAs from plasma of patients
with severe AP are named as Novel1, Novel2 and Novel3, which are
remarkably different from healthy participants, providing
classification of patients with severe AP from healthy populations;
moreover, animal experiments reveal that complement component 3 is
a target gene of Novel3 and may serve as early diagnostic biomarker
of severe AP (93). In addition,
seven candidate signature exosomal miRNAs derived from blood of
severe AP are identified as diagnostic biomarkers, which including
miR-603, miR-548ad-5p, miR-122-5p, miR-4477a, miR-192-5p,
miR-215-5p and miR-583 (94).
Compared with healthy individuals, miR-579-3p was decreased in EVs
from patients with CP and CP narcotic users, but is enriched within
EV in pre-diabetic CP patients compared with non-diabetic patients
with CP. These findings suggest that plasma EV miR-579-3p serve as
basis for assessing pancreatic health (95). In addition, exosomal miRNA profiles
can help differentiate various disease phenotypes, allowing for
more tailored treatment approaches. For example, in type 1
autoimmune pancreatitis, circulating EVs show altered miRNA
expression patterns with elevated miR-21-5p when compared with
those in healthy controls and CP. Further studies unveiled that
miR-21-5p is highly expressed in pancreatic inflammatory cells,
suggesting that EV miR-21-5p derived from inflammatory cells might
be involved in the progression of type 1 autoimmune pancreatitis
(96). In addition, the diagnostic
potential of exosomal miRNAs extends beyond pancreatitis to other
complications, such as patients with AP, where exosomal miR-4265,
miR-1208, miR-3127-5p are verified to have the early predictive
value for persistent organ failure. Increased levels of these
exosomal miRNAs indicate urgent need for prolonged hospitalization,
elevated mortality rate and thus unfavorable prognosis (97).
It can be inferred that exosomal miRNAs possess the
potential to function as biomarkers for the early diagnosis,
phenotypic characterization and prognostic assessment of
pancreatitis (Table II).
Consequently, it is imperative that ongoing investigations are
directed towards the validation of the diagnostic efficacy of
exosomal miRNAs in more extensive and heterogeneous patient
populations. Furthermore, efforts should be made to standardize
method for exosome isolation and miRNA analysis, thereby ensuring
consistency and precision within clinical environments. Advances in
technology, exemplified by high-throughput sequencing and digital
PCR, are being used to enhance the sensitivity and specificity of
exosomal miRNA detection. Moreover, the integration of exosomal
miRNA profiling with other omics technologies, such as proteomics
and metabolomics, has the potential to yield a more comprehensive
understanding of the pathogenesis of pancreatitis and to identify
novel biomarkers. In addition, the development of point-of-care
diagnostic instruments for the rapid detection of exosomal miRNAs
could transform the management of pancreatitis by facilitating
real-time monitoring and enabling personalized therapeutic
adjustments. Exosomal miRNAs exhibit good performance in the early
diagnosis of pancreatitis. Serum levels of amylase and lipase, the
most common markers for AP, typically increase only after
significant pancreatic damage has occurred (98). By contrast, exosomal miRNAs reflect
subtle changes in the cellular environment, including inflammation
and stress responses, even before clinical symptoms or conventional
biomarkers become detectable. For example, miR-503-322 is
upregulated in the early stages of pancreatitis, providing a high
sensitivity for disease detection than enzyme-based assays
(56). While exosomal miRNAs, such
as miR-579-3p and miR-21-5p, have shown high specificity for
pancreatitis, their sensitivity may be limited, especially in the
early stages of the disease when miRNA levels can be low. To
improve both sensitivity and specificity, several studies have
employed panels of miRNAs rather than focusing on a single
biomarker. For instance, combining miR-4265 with miR-1208 and
miR-3127-5p has been shown to improve their performance in the
early diagnosis of pancreatitis (97). In addition, exosomal miRNAs have
significant potential for prognostication in pancreatitis. For
example, the elevation of miR-216a has been associated with the
severity of AP and subsequent complications, such as pancreatic
necrosis and acute lung injury. Elevated levels of miR-216a in
exosomes reflect the degree of inflammation and tissue injury,
offering an early indicator of disease severity (99). Traditional techniques such as
RT-qPCR are highly sensitive but are limited by their inability to
capture a broad spectrum of miRNAs. Newer technologies, such as
next-generation sequencing, allow for high-throughput analysis and
the identification of novel miRNAs, but they face challenges
related to cost, complexity and bioinformatics analysis. Recent
developments in digital PCR and microfluidic-based technologies,
however, show promise for achieving higher sensitivity and
specificity with minimal sample volume. For instance, advancements
in liquid biopsy technology, such as the development of exosomal
miRNA detection using surface acoustic wave sensor, have improved
the detection sensitivity in clinical settings (100). These cutting-edge technologies
enable the detection of low-abundance miRNAs and improve the
reproducibility of miRNA profiling.
 | Table II.Exosomal miRs act as biomarkers in
pancreatitis. |
Table II.
Exosomal miRs act as biomarkers in
pancreatitis.
| First author/s,
year | Exosomal miR | Source | Cohort | Clinical
implication | (Refs.) |
|---|
| Galgaro et
al, 2021 | Novel1, Novel2 and
Novel3 | Plasma | SAP (n=4) and
healthy control (n=4) | Act as an early
diagnostic biomarker of SAP | (103) |
| Xie et al
(2020) | miR-603,
miR-548ad-5p, miR-122-5p, miR-4477a, miR-192-5p, miR-215-5p and
miR-583 | Serum | SAP (n=7), MSAP
(n=7), MAP (n=8) and control (n=7) | Are related to
severity of AP and act as biomarkers for SAP diagnosis | (104) |
| Pang et al
(2023) | miR-579-3p | Plasma | CP (n=15) and
healthy control (n=10) | Serve as diagnostic
biomarker of CP | (105) |
| Oveili et al
(2023) | miR-21-5p | Serum | AIP (n=10), CP
(n=10) and healthy control (n=10) | Discriminate AIP
from CP and healthy control | (106) |
| Li et al
(2024) | miR-4265, miR-1208
and miR-3127-5p | Serum | APs with (n=5) or
without (n=5) persistent organ failure | Early predict
persistent organ failure in patients with AP | (107) |
Exosomal miRNA-based potential therapies in
pancreatitis
Mesenchymal stem cells (MSCs) are inhabit nearly all
post-natal organs and tissues, including, but not limited to, bone
marrow, adipose tissue, umbilical cord and placenta (101,102). Under suitable conditions, MSCs
can differentiate into osteoblasts, adipocytes and chondroblasts,
rendering them as promising candidates for therapeutic applications
due to their remarkable plasticity (103,104). Exosomes derived from MSCs play a
pivotal role in mediating the therapeutic efficacy of MSCs. These
exosomes facilitate tissue repair and regeneration by transferring
growth factors and microRNAs that promote cellular proliferation
and differentiation (105,106).
In AP, bone marrow MSC-derived exosomal miR-181a-5p can inhibit PSC
cell apoptosis and promoting M2 macrophage polarization by
downregulating the expression of zinc finger E-box binding homeobox
2 and further increasing receptor for activated C-kinase 1
expression, alleviating AP injury (107). Moreover, in sodium taurocholate
and caerulein-induced severe AP rat models, miR-181a-5p derived
from bone marrow MSCs mitigates severe AP and reduces inflammatory
responses and PSC cell apoptosis by inhibiting the PTEN/AKT/TGF-β1
signaling pathway (108). Also,
miR-29a-3p within MSC-derived EVs can be transferred into
cardiomyocytes, where it reduces the production of inflammatory
cytokines and attenuates severe AP-induced myocardial injury by
inhibiting the expression of HMGB1 to downregulate TLR4 expression
and further inactivating the AKT signaling pathway (109).
A number of drugs exert the therapeutic effect in
pancreatitis via regulate exosomal miRNAs. For example, melatonin
prevents M1 macrophage polarization and thus reduces the secretion
of inflammatory EV miRNAs and thereby decreasing inflammatory
EV-mediated β cell failure and apoptosis (57). Further mechanistic studies unveiled
that melatonin downregulates the transcription of specific miRNAs
and reduces miRNA transport into EVs by inactivating the NF-κB
pathway (57). In addition,
emodin, an anthraquinone derivative with anti-inflammatory,
antioxidant, anti-fibrotic properties, is abundantly present in
Chinese herbal medicines such as Polygoni cuspidati rhizoma et
radix and Polygoni multiflori root (110). Intriguingly, in rats with severe
AP, emodin treatment ameliorates pancreatic and lung injury and
inflammation by elevating the expression of exosomal miR-29a-3p
derived from bronchoalveolar lavage fluid (111).
However, as yet, no clinical trial has estimated
exosomal miRNA-based therapeutic strategies in pancreatitis. The
exploration of exosomal miRNAs as therapeutic targets in
pancreatitis has unveiled a wealth of promising avenues for
research and clinical application. Continued research into the
biological roles of exosomal miRNAs, their clinical applications
and the development of targeted therapies holds promise for
transforming the landscape of pancreatitis treatment. In addition,
an interdisciplinary approach that bridges fundamental research
with clinical application will be crucial in unlocking the full
potential of exosome-derived miRNAs in the fight against
pancreatitis. A key challenge lies in the standardization of
exosome isolation protocols, as current techniques, such as
ultracentrifugation and immunoaffinity capture, can yield variable
results, affecting the reproducibility of findings. To address this
issue, regulatory bodies like the US FDA have initiated efforts to
establish standardized guidelines for exosome-based diagnostics and
therapeutics. Moreover, rigorous validation of miRNA biomarkers in
large, multi-center clinical trials is essential to ensure their
clinical utility. Additionally, the integration of exosomal
miRNA-based therapies into clinical practice must navigate
regulatory approval pathways that involve safety and efficacy
trials. One promising approach involves the use of engineered
exosomes as vehicles for miRNA delivery, which could improve
tissue-specific targeting and reduce off-target effects.
Exosome-based therapeutics could be designed to carry miRNAs that
either inhibit pro-inflammatory pathways or promote tissue repair
during pancreatitis.
Future directions
Exosomal miRNAs are increasingly recognized as
critical mediators of intercellular communication that influence
inflammation, fibrosis and tissue repair during pancreatitis. They
have been implicated in several critical processes, including PAC
functional modulation, macrophage activation and PSC-mediated
fibrotic response (11,12,62).
Dysregulation of exosomal miRNA profiles correlates with the
severity and progression of both acute and CP, providing strong
evidence for their utility as diagnostic and prognostic biomarkers.
Some exosomal miRNAs, such as miR-24-3p and miR-130a-3p, are
implicated in promoting inflammation and fibrosis in pancreatitis,
while others like miR-503-5p play a protective role in tissue
repair and resolution of inflammation (12,69,86).
These discrepancies may stem from differences in study design, such
as the timing of exosomal miRNA analysis during disease
progression, or the use of distinct animal models. Thus, these
conflicting findings highlight the complications in targeting
miRNAs for therapeutic purposes. Indeed, exosomal miRNAs are known
to regulate multiple target genes and cellular processes and their
effects can vary based on several factors, including the timing of
expression, the cellular context and the presence of other
molecular players (77,82,88).
These factors complicate the development of exosomal miRNA-based
therapies, as the therapeutic targeting of miRNAs could have
unintended consequences depending on the disease stage and the
specific cellular environment. One of the primary challenges is the
potential for off-target effects, which underscore the need for
precise delivery systems and methods to control miRNA activity
in vivo. Another significant challenge is the effective
delivery of exosomal miRNAs. The ability to selectively target
pancreas, while avoiding systemic distribution, is critical to
minimizing side effects and achieving therapeutic efficacy.
Furthermore, the differential expression of specific exosomal
miRNAs across different stages of pancreatitis could lead to the
identification of novel biomarkers that are not only specific to
pancreatitis but also sensitive enough to detect early disease
onset, which is often clinically challenging. In addition, exosomal
miRNAs have been demonstrated to modulate inflammation and
fibrosis, both of which are central to the pathogenesis of
pancreatitis. By either inhibiting pro-inflammatory miRNAs or
restoring the levels of anti-inflammatory miRNAs, researchers may
reduce the extent of tissue damage and promote healing in the
pancreas.
Despite promising findings, challenges remain to
fully harness the potential of exosomal miRNAs for clinical
application. One of the primary obstacles is the efficient and
standardized isolation of exosomes from biofluids, particularly
from complex samples such as blood, urine and pancreatic juice. The
heterogeneous nature of exosomes, derived from various cell types,
complicates the identification of disease-specific miRNA signatures
(56–58). Current advanced isolation
techniques have limitations in terms of purity, yield and
reproducibility (53–55). Therefore, the development of more
reliable and scalable methods for isolating and characterizing
exosomes will be crucial for the clinical implementation of
exosomal miRNA-based diagnostics. Another critical issue pertains
to the efficient delivery of exosomal miRNAs for therapeutic
purposes. While exosomes can naturally transport miRNAs across cell
membranes, their use as therapeutic agents requires further
refinement (10,13,102). Ensuring that miRNA mimics or
inhibitors are delivered to the appropriate tissues in sufficient
concentrations, without off-target effects, is a significant
hurdle. Moreover, the immunogenicity and potential toxicity of
exosomal preparations must be carefully evaluated before clinical
applications. Progress in engineering exosomes for specific
targeting, improving the stability of miRNAs during circulation and
overcoming cellular barriers to miRNA uptake will be essential to
fully realize their therapeutic potential (50,91,106). There is also great potential in
utilizing exosomal miRNAs as part of a broader multi-omics approach
to studying pancreatitis. Incorporating transcriptomic, proteomic
and metabolomic data with exosomal miRNA profiles could lead to the
identification of novel molecular pathways involved in disease
progression. Such integrated approaches will be crucial for
identifying therapeutic targets, as well as for improving
diagnostic accuracy and patient stratification. One promising
avenue is investigating the role of exosomal miRNAs in the
regulation of pancreatic cell fate, such as apoptosis and
autophagy. Using single-cell RNA sequencing technologies to profile
the exosomal miRNA cargo at a high resolution, can link miRNA
expression to specific cell types involved in pancreatitis.
Additionally, integrating spatial transcriptomics with exosomal
miRNA analysis could provide insights into the tissue-specific
functions of these miRNAs during pancreatitis (10–13).
Furthermore, methods such as ultracentrifugation
remain the gold standard for exosome isolation but are
time-consuming and costly, limiting their use in routine clinical
practice. Newer techniques such as size-exclusion chromatography
and microfluidic devices have shown promise for improving
cost-effectiveness and scalability (54,55).
Additionally, for miRNA detection, technologies like RT-qPCR and
next-generation sequencing are highly sensitive but may not be
cost-effective for large-scale clinical use (53). A key point is the need for
integrated platforms that combine exosome isolation and miRNA
profiling, reducing the overall cost and improving throughput.
Variations in exosome isolation methods, sample preparation
protocols and miRNA detection techniques can lead to discrepancies
in results. It is proposed that future researches should focus on
developing consensus guidelines for exosome-based research, similar
to those in other fields like genomics and proteomics, to improve
the consistency of results across studies (102,106). In addition, researchers should
take interdisciplinary research opportunities for integrating
exosomal miRNA research with emerging technologies. For example,
artificial intelligence and machine learning revolutionize the
analysis of miRNA profiles by identifying hidden patterns in large
datasets and predicting miRNA interactions with their targets
(10). Single-cell RNA sequencing
could be used in tandem with exosomal miRNA profiling to
investigate how individual cells within the pancreas respond to
specific miRNAs during disease progression (13). Spatial transcriptomics also offer a
way to map the localization of exosomal miRNAs within tissue
architecture, providing key insights into how these miRNAs
influence cellular communication in the pancreatic microenvironment
(50).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LW wrote the manuscript and designed the figures.
JZ, ZH, XK, CL, YX and MY revised the manuscript. KW conceived the
topic and revised the manuscript. All authors read and approved the
final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AP
|
acute pancreatitis
|
|
AGO
|
argonaute
|
|
CP
|
chronic pancreatitis
|
|
CCN2
|
connective tissue growth factor
|
|
DGCR8
|
DiGeorge syndrome critical region
8
|
|
ESCRT
|
endosomal sorting complex required for
transport
|
|
EVs
|
extracellular vesicles
|
|
HDAC2
|
histone deacetylase-2
|
|
HMGB1
|
amphoterin
|
|
IGF2
|
insulin-like growth factor 2
|
|
ILVs
|
intraluminal vesicles
|
|
miRNAs
|
microRNAs
|
|
MSCs
|
mesenchymal stem cells
|
|
MVBs
|
multivesicular bodies
|
|
NLRP3
|
pyrin domain (PYD)-containing protein
3
|
|
PACs
|
pancreatic acinar cells
|
|
PSCs
|
pancreatic stellate cells
|
|
PPAR-γ
|
peroxisome proliferator-activated
receptor gamma
|
|
ROS
|
reactive oxygen species
|
|
RISC
|
RNA-induced silencing complex
|
|
SOCS1
|
suppressor of cytokine signaling 1
|
|
UNC5B
|
uncoordinated-5 homolog B
|
|
UTRs
|
untranslated regions
|
References
|
1
|
Trikudanathan G, Yazici C, Evans Phillips
A and Forsmark CE: Diagnosis and management of acute pancreatitis.
Gastroenterology. 167:673–688. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hines OJ and Pandol SJ: Management of
chronic pancreatitis. BMJ. 384:e0709202024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saluja A, Dudeja V, Dawra R and Sah RP:
Early Intra-acinar events in pathogenesis of pancreatitis.
Gastroenterology. 156:1979–1993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Capurso G, Tacelli M, Vanella G, Ponz de
Leon Pisani R, Dell'Anna G, Abati M, Mele R, Lauri G, Panaitescu A,
Nunziata R, et al: Managing complications of chronic pancreatitis:
A guide for the gastroenterologist. Expert Rev Gastroenterol
Hepatol. 17:1267–1283. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szatmary P, Grammatikopoulos T, Cai W,
Huang W, Mukherjee R, Halloran C, Beyer G and Sutton R: Acute
pancreatitis: Diagnosis and treatment. Drugs. 82:1251–1276. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barreto SG, Habtezion A, Gukovskaya A,
Lugea A, Jeon C, Yadav D, Hegyi P, Venglovecz V, Sutton R and
Pandol SJ: Critical thresholds: Key to unlocking the door to the
prevention and specific treatments for acute pancreatitis. Gut.
70:194–203. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Sui S and Goel A: Extracellular
vesicles associated microRNAs: Their biology and clinical
significance as biomarkers in gastrointestinal cancers. Semin
Cancer Biol. 99:5–23. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bayat M and Sadri Nahand J: Exosomal
miRNAs: The tumor's trojan horse in selective metastasis. Mol
Cancer. 23:1672024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghafouri-Fard S, Shoorei H, Dong P,
Poornajaf Y, Hussen BM, Taheri M and Akbari Dilmaghani N: Emerging
functions and clinical applications of exosomal microRNAs in
diseases. Noncoding RNA Res. 8:350–362. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Lv D, Yang H, Lu Y and Jia Y: A
review on the current literature regarding the value of exosome
miRNAs in various diseases. Ann Med. 55:22329932023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei H, Zhao H, Cheng D, Zhu Z, Xia Z, Lu
D, Yu J, Dong R and Yue J: miR-148a and miR-551b-5p regulate
inflammatory responses via regulating autophagy in acute
pancreatitis. Int Immunopharmacol. 127:1114382024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Wang H, Jing Q, Yang Y, Xue D, Hao
C and Zhang W: Regulation of pancreatic fibrosis by acinar
Cell-derived exosomal miR-130a-3p via targeting of stellate cell
PPAR-γ. J Inflamm Res. 14:461–477. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia YC, Ding YX, Mei WT, Wang YT, Zheng Z,
Qu YX, Liang K, Li J, Cao F and Li F: Extracellular vesicles and
pancreatitis: Mechanisms, status and perspectives. Int J Biol Sci.
17:549–561. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mihoc T, Latcu SC, Secasan CC, Dema V,
Cumpanas AA, Selaru M, Pirvu CA, Valceanu AP, Zara F, Dumitru CS,
et al: Pancreatic morphology, immunology, and the pathogenesis of
acute pancreatitis. Biomedicines. 12:26272024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zaman S and Gorelick F: Acute
pancreatitis: Pathogenesis and emerging therapies. J Pancreatol.
7:10–20. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mederos MA, Reber HA and Girgis MD: Acute
pancreatitis: A review. JAMA. 325:382–390. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Ni HM, Chao X, Ma X, Kolodecik T,
De Lisle R, Ballabio A, Pacher P and Ding WX: Critical role of
TFEB-mediated lysosomal biogenesis in Alcohol-induced pancreatitis
in mice and humans. Cell Mol Gastroenterol Hepatol. 10:59–81. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu M, Zhou X, Zippi M, Goyal H, Basharat
Z, Jagielski M and Hong W: Comprehensive review on the pathogenesis
of Hypertriglyceridaemia-associated acute pancreatitis. Ann Med.
55:22659392023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Gao J, Wen L, Huang K, Liu H, Zeng
L, Zeng Z, Liu Y and Mo Z: Ion channels in acinar cells in acute
pancreatitis: Crosstalk of calcium, iron, and copper signals. Front
Immunol. 15:14442722024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
An J, Jiang T, Qi L and Xie K: Acinar
cells and the development of pancreatic fibrosis. Cytokine Growth
Factor Rev. 71-72:40–53. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ge P, Luo Y, Okoye CS and Chen H, Liu J,
Zhang G, Xu C and Chen H: Intestinal barrier damage, systemic
inflammatory response syndrome, and acute lung injury: A
troublesome trio for acute pancreatitis. Biomed Pharmacother.
132:1107702020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang H, Yang Y, Zhu L, Zhao X, Li J, Tang
W and Wan M: Role of neutrophil extracellular traps in inflammatory
evolution in severe acute pancreatitis. Chin Med J (Engl).
135:2773–2784. 2022.PubMed/NCBI
|
|
23
|
Papantoniou K, Aggeletopoulou I,
Michailides C, Pastras P and Triantos C: Understanding the role of
NLRP3 inflammasome in acute pancreatitis. Biology (Basel).
13:9452024.PubMed/NCBI
|
|
24
|
Kong F, Pan Y and Wu D: Activation and
regulation of pancreatic stellate cells in chronic pancreatic
fibrosis: A potential therapeutic approach for chronic
pancreatitis. Biomedicines. 12:1082024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nail HM, Chiu CC, Leung CH, Ahmed MMM and
Wang HD: Exosomal miRNA-mediated intercellular communications and
immunomodulatory effects in tumor microenvironments. J Biomed Sci.
30:692023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melzer MK and Kleger A: Acute
pancreatitis: Murine model systems unravel disease-modifying genes
with potential implications for diagnostics and patient
stratification. United European Gastroenterol J. 10:618–619. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patel HR, Diaz Almanzar VM, LaComb JF, Ju
J and Bialkowska AB: The role of MicroRNAs in pancreatitis
development and progression. Int J Mol Sci. 24:10572023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim H, Lee YY and Kim VN: The biogenesis
and regulation of animal microRNAs. Nat Rev Mol Cell Biol.
26:276–296. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shang R, Lee S, Senavirathne G and Lai EC:
microRNAs in action: Biogenesis, function and regulation. Nat Rev
Genet. 24:816–833. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Huang Q, Luo C, Wen Y, Liu R, Sun
H and Tang L: MicroRNAs in acute pancreatitis: From pathogenesis to
novel diagnosis and therapy. J Cell Physiol. 235:1948–1961. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Correia de Sousa M, Gjorgjieva M, Dolicka
D, Sobolewski C and Foti M: Deciphering miRNAs' Action through
miRNA Editing. Int J Mol Sci. 20:62492019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Treiber T, Treiber N and Meister G:
Regulation of microRNA biogenesis and its crosstalk with other
cellular pathways. Nat Rev Mol Cell Biol. 20:5–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu K, He J, Pu W and Peng Y: The role of
Exportin-5 in MicroRNA biogenesis and cancer. Genomics Proteomics
Bioinformatics. 16:120–126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hynes C and Kakumani PK: Regulatory role
of RNA-binding proteins in microRNA biogenesis. Front Mol Biosci.
11:13748432024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rani V and Sengar RS: Biogenesis and
mechanisms of microRNA-mediated gene regulation. Biotechnol Bioeng.
119:685–692. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Komatsu S, Kitai H and Suzuki HI: Network
regulation of microRNA biogenesis and target interaction. Cells.
12:3062023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cánovas-Márquez JT, Falk S, Nicolás FE,
Padmanabhan S, Zapata-Pérez R, Sánchez-Ferrer Á, Navarro E and
Garre V: A ribonuclease III involved in virulence of Mucorales
fungi has evolved to cut exclusively single-stranded RNA. Nucleic
Acids Res. 49:5294–5307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Slezak-Prochazka I, Kluiver J, de Jong D,
Kortman G, Halsema N, Poppema S, Kroesen BJ and van den Berg A:
Cellular localization and processing of primary transcripts of
exonic microRNAs. PLoS One. 8:e766472013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arya SB, Collie SP and Parent CA: The
ins-and-outs of exosome biogenesis, secretion, and internalization.
Trends Cell Biol. 34:90–108. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wozniak AL, Adams A, King KE, Dunn W,
Christenson LK, Hung WT and Weinman SA: The RNA binding protein
FMR1 controls selective exosomal miRNA cargo loading during
inflammation. J Cell Biol. 219:e2019120742020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Villarroya-Beltri C, Gutiérrez-Vázquez C,
Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N,
Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M and
Sánchez-Madrid F: Sumoylated hnRNPA2B1 controls the sorting of
miRNAs into exosomes through binding to specific motifs. Nat
Commun. 4:29802013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sonoda Y, Kano F and Murata M:
Applications of cell resealing to reconstitute microRNA loading to
extracellular vesicles. Sci Rep. 11:29002021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jaé N, McEwan DG, Manavski Y, Boon RA and
Dimmeler S: Rab7a and Rab27b control secretion of endothelial
microRNA through extracellular vesicles. FEBS Lett. 589:3182–3188.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ostrowski M, Carmo NB, Krumeich S, Fanget
I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et
al: Rab27a and Rab27b control different steps of the exosome
secretion pathway. Nat Cell Biol. 12:19–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Payandeh Z, Tangruksa B, Synnergren J,
Heydarkhan-Hagvall S, Nordin JZ, Andaloussi SE, Borén J, Wiseman J,
Bohlooly YM, Lindfors L and Valadi H: Extracellular vesicles
transport RNA between cells: Unraveling their dual role in
diagnostics and therapeutics. Mol Aspects Med. 99:1013022024.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Robinson H, Ruelcke JE, Lewis A, Bond CS,
Fox AH, Bharti V, Wani S, Cloonan N, Lai A, Margolin D, et al:
Caveolin-1-driven membrane remodelling regulates hnRNPK-mediated
exosomal microRNA sorting in cancer. Clin Transl Med. 11:e3812021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun H, Bhandari K, Burrola S, Wu J and
Ding WQ: Pancreatic ductal Cell-derived extracellular vesicles are
effective drug carriers to enhance Paclitaxel's efficacy in
pancreatic cancer cells through Clathrin-mediated endocytosis. Int
J Mol Sci. 23:47732022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Groot M and Lee H: Sorting mechanisms for
MicroRNAs into extracellular vesicles and their associated
diseases. Cells. 9:10442020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Minhua Q, Bingzheng F, Zhiran X, Yingying
Z, Yuwei Y, Ting Z, Jibing C and Hongjun G: Exosomal-microRNAs
improve islet cell survival and function in islet transplantation.
Curr Stem Cell Res Ther. 19:669–677. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park EJ, Shimaoka M and Kiyono H:
Functional flexibility of exosomes and micrornas of intestinal
epithelial cells in affecting inflammation. Front Mol Biosci.
9:8544872022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
He K, Yang T, Yu J, Zang X, Jiang S, Xu S,
Liu J, Xu Z, Wang W and Hong S: Dermatophagoides farinae microRNAs
released to external environments via exosomes regulate
inflammation-related gene expression in human bronchial epithelial
cells. Front Immunol. 14:13032652023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Isaac R, Reis FCG, Ying W and Olefsky JM:
Exosomes as mediators of intercellular crosstalk in metabolism.
Cell Metab. 33:1744–1762. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Otahal A, Kuten-Pella O, Kramer K,
Neubauer M, Lacza Z, Nehrer S and De Luna A: Functional repertoire
of EV-associated miRNA profiles after lipoprotein depletion via
ultracentrifugation and size exclusion chromatography from
autologous blood products. Sci Rep. 11:58232021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gemoll T, Rozanova S, Roder C, Hartwig S,
Kalthoff H, Lehr S, ElSharawy A and Habermann JK: Protein profiling
of serum extracellular vesicles reveals qualitative and
quantitative differences after differential ultracentrifugation and
exoquickTM isolation. J Clin Med. 9:14292020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rekker K, Saare M, Roost AM, Kubo AL,
Zarovni N, Chiesi A, Salumets A and Peters M: Comparison of serum
exosome isolation methods for microRNA profiling. Clin Biochem.
47:135–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu K, Lv T, He L, Tang W, Zhang Y, Xiao
X, Li Y, Chang X, Wang S, Pandol SJ, et al: Endocrine-exocrine
miR-503-322 drives aging-associated pancreatitis via targeting
MKNK1 in acinar cells. Nat Commun. 16:26132025. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shao Y, Wu W, Fan F, Liu H, Ming Y, Liao
W, Bai C and Gao Y: Extracellular vesicle content changes induced
by melatonin promote functional recovery of pancreatic beta cells
in acute pancreatitis. J Inflamm Res. 16:6397–6413. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu J, Niu Z, Zhang R, Peng Z, Wang L, Liu
Z, Gao Y, Pei H and Pan L: MALAT1 shuttled by extracellular
vesicles promotes M1 polarization of macrophages to induce acute
pancreatitis via miR-181a-5p/HMGB1 axis. J Cell Mol Med.
25:9241–9254. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ryu S and Lee EK: The pivotal role of
macrophages in the pathogenesis of pancreatic diseases. Int J Mol
Sci. 25:57652024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Iyer S, Enman M, Sahay P and Dudeja V:
Novel therapeutics to treat chronic pancreatitis: Targeting
pancreatic stellate cells and macrophages. Expert Rev Gastroenterol
Hepatol. 18:171–183. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xiang H, Yu H, Zhou Q, Wu Y, Ren J, Zhao
Z, Tao X and Dong D: Macrophages: A rising star in immunotherapy
for chronic pancreatitis. Pharmacol Res. 185:1065082022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhao Y, Wang H, Lu M, Qiao X, Sun B, Zhang
W and Xue D: Pancreatic acinar cells employ miRNAs as mediators of
intercellular communication to participate in the regulation of
Pancreatitis-associated macrophage activation. Mediators Inflamm.
2016:63404572016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jimenez-Alesanco A, Marcuello M,
Pastor-Jimenez M, Lopez-Puerto L, Bonjoch L, Gironella M, Carrascal
M, Abian J, de-Madaria E and Closa D: Acute pancreatitis promotes
the generation of two different exosome populations. Sci Rep.
9:198872019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang X, Chu J, Sun H, Zhao D, Ma B, Xue
D, Zhang W and Li Z: MiR-155 aggravates impaired autophagy of
pancreatic acinar cells through targeting Rictor. Acta Biochim
Biophys Sin (Shanghai). 52:192–199. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wan J, Yang X, Ren Y, Li X, Zhu Y, Haddock
AN, Ji B, Xia L and Lu N: Inhibition of mir-155 reduces impaired
autophagy and improves prognosis in an experimental pancreatitis
mouse model. Cell Death Dis. 10:3032019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang D, Tang M, Zong P, Liu H, Zhang T,
Liu Y and Zhao Y: MiRNA-155 regulates the Th17/Treg ratio by
targeting SOCS1 in severe acute pancreatitis. Front Physiol.
9:6862018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tang DS, Cao F, Yan CS, Cui JT, Guo XY,
Cheng L, Li L, Li YL, Ma JM, Fang K, et al: Acinar Cell-derived
extracellular vesicle MiRNA-183-5p aggravates acute pancreatitis by
promoting M1 macrophage polarization through downregulation of
FoxO1. Front Immunol. 13:8692072022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zheng Z, Cao F, Ding YX, Lu JD, Fu YQ, Liu
L, Guo YL, Liu S, Sun HC, Cui YQ and Li F: Acinous cell
AR42J-derived exosome miR125b-5p promotes acute pancreatitis
exacerbation by inhibiting M2 macrophage polarization via PI3K/AKT
signaling pathway. World J Gastrointest Surg. 15:600–620. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Su XJ, Chen Y, Zhang QC, Peng XB, Liu YP,
Wang L and Du YQ: Exosomes derived from cerulein-stimulated
pancreatic acinar cells mediate peritoneal macrophage M1
polarization and pyroptosis via an miR-24-3p/MARCH3/NLRP3 axis in
acute pancreatitis. Pancreas. 53:e641–e651. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen F, Xu K, Han Y, Ding J, Ren J, Wang
Y, Ma Z and Cao F: Mitochondrial dysfunction in pancreatic acinar
cells: Mechanisms and therapeutic strategies in acute pancreatitis.
Front Immunol. 15:15030872024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cai SW, Han Y and Wang GP: miR-148a-3p
exhaustion inhibits necrosis by regulating PTEN in acute
pancreatitis. Int J Clin Exp Pathol. 11:5647–5657. 2018.PubMed/NCBI
|
|
72
|
Zhang Y, Yan L and Han W: Elevated level
of miR-551b-5p is associated with inflammation and disease
progression in patients with severe acute pancreatitis. Ther Apher
Dial. 22:649–655. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kusnierz-Cabala B, Nowak E, Sporek M,
Kowalik A, Kuzniewski M, Enguita FJ and Stepien E: Serum levels of
unique miR-551-5p and endothelial-specific miR-126a-5p allow
discrimination of patients in the early phase of acute
pancreatitis. Pancreatology. 15:344–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu C, Yin L, Chen Z, Waldron RT, Lugea A,
Lin Y, Zhai X, Wen L, Han YP, Pandol SJ, et al: The unique
pancreatic stellate cell gene expression signatures are associated
with the progression from acute to chronic pancreatitis. Comput
Struct Biotechnol J. 19:6375–6385. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chan LK, Tsesmelis M, Gerstenlauer M,
Leithäuser F, Kleger A, Frick LD, Maier HJ and Wirth T: Functional
IKK/NF-κB signaling in pancreatic stellate cells is essential to
prevent autoimmune pancreatitis. Commun Biol. 5:5092022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhao Y, Feng Y, Sun F, Li L, Chen J, Song
Y, Zhu W, Hu X, Li Z, Kong F, et al: Optimized rAAV8 targeting
acinar KLF4 ameliorates fibrosis in chronic pancreatitis via
exosomes-enriched let-7s suppressing pancreatic stellate cells
activation. Mol Ther. 32:2624–2640. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Charrier A, Chen R, Chen L, Kemper S,
Hattori T, Takigawa M and Brigstock DR: Connective tissue growth
factor (CCN2) and microRNA-21 are components of a positive feedback
loop in pancreatic stellate cells (PSC) during chronic pancreatitis
and are exported in PSC-derived exosomes. J Cell Commun Signal.
8:147–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li X, Lin Z, Wang L, Liu Q, Cao Z, Huang
Z, Zhong M, Peng S, Zhang Y, Li Y and Ma X: RNA-Seq analyses of the
role of miR-21 in acute pancreatitis. Cell Physiol Biochem.
51:2198–2211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yan B, Cheng L, Jiang Z, Chen K, Zhou C,
Sun L, Cao J, Qian W, Li J, Shan T, et al: Resveratrol inhibits
ROS-Promoted activation and glycolysis of pancreatic stellate cells
via suppression of miR-21. Oxid Med Cell Longev. 2018:13469582018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ciccarelli G, Di Giuseppe G, Soldovieri L,
Quero G, Nista EC, Brunetti M, Cinti F, Moffa S, Capece U, Tondolo
V, et al: Beta-cell function and glucose metabolism in patients
with chronic pancreatitis. Eur J Intern Med. 128:112–118. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gao Y, Mi N, Wu W, Zhao Y, Fan F, Liao W,
Ming Y, Guan W and Bai C: Transfer of inflammatory mitochondria via
extracellular vesicles from M1 macrophages induces ferroptosis of
pancreatic beta cells in acute pancreatitis. J Extracell Vesicles.
13:e124102024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhu X, Liu D, Li G, Zhi M, Sun J, Qi L, Li
J, Pandol SJ and Li L: Exosomal miR-140-3p and miR-143-3p from
TGF-β1-treated pancreatic stellate cells target BCL2 mRNA to
increase β-cell apoptosis. Mol Cell Endocrinol. 551:1116532022.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lu XG, Kang X, Zhan LB, Kang LM, Fan ZW
and Bai LZ: Circulating miRNAs as biomarkers for severe acute
pancreatitis associated with acute lung injury. World J
Gastroenterol. 23:7440–7449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu C, Feng H, Zhang L, Guo Y, Ma J and
Yang L: MicroRNA-143-3p levels are reduced in the peripheral blood
of patients with gestational diabetes mellitus and influences
pancreatic β-cell function and viability. Exp Ther Med. 25:812023.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu Q, Zhu X and Guo S: From pancreas to
lungs: The role of immune cells in severe acute pancreatitis and
acute lung injury. Immun Inflamm Dis. 12:e13512024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xiong Y, Chen X, Yang X, Zhang H, Li X,
Wang Z, Feng S, Wen W and Xiong X: miRNA transcriptomics analysis
shows miR-483-5p and miR-503-5p targeted miRNA in extracellular
vesicles from severe acute pancreatitis-associated lung injury
patients. Int Immunopharmacol. 125:1110752023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li F, Wang Z, Cao Y, Pei B, Luo X, Liu J,
Ge P, Luo Y, Ma S and Chen H: Intestinal mucosal immune barrier: A
powerful firewall against severe acute pancreatitis-associated
acute lung injury via the Gut-lung axis. J Inflamm Res.
17:2173–2193. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shao Y, Li Y, Jiang Y, Li H, Wang J and
Zhang D: Circulating exosomal miR-155-5p contributes to severe
acute pancreatitis-associated intestinal barrier injury by
targeting SOCS1 to activate NLRP3 inflammasome-mediated pyroptosis.
FASEB J. 37:e230032023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang L, Yuan N, Li Y, Ma Q, Zhou Y, Qiao
Z, Li S, Liu C, Zhang L, Yuan M and Sun J: Stellate ganglion block
relieves acute lung injury induced by severe acute pancreatitis via
the miR-155-5p/SOCS5/JAK2/STAT3 axis. Eur J Med Res. 27:2312022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Balaraman AK, Moglad E, Afzal M, Babu MA,
Goyal K, Roopashree R, Kaur I, Kumar S, Kumar M, Chauhan AS, et al:
Liquid biopsies and exosomal ncRNA: Transforming pancreatic cancer
diagnostics and therapeutics. Clin Chim Acta. 567:1201052025.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xu C, Jiang C, Li Z, Gao H, Xian J, Guo W,
He D, Peng X, Zhou D and Li D: Exosome nanovesicles: Biomarkers and
new strategies for treatment of human diseases. MedComm (2020).
5:e6602024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Preethi KA, Selvakumar SC, Ross K,
Jayaraman S, Tusubira D and Sekar D: Liquid biopsy: Exosomal
microRNAs as novel diagnostic and prognostic biomarkers in cancer.
Mol Cancer. 21:542022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xu Y, Sun Y, Yin R, Dong T, Song K, Fang
Y, Liu G, Shen B and Li H: Differential expression of plasma
exosomal microRNA in severe acute pancreatitis. Front Pharmacol.
13:9809302022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Qu Y, Ding Y, Lu J, Jia Y, Bian C, Guo Y,
Zheng Z, Mei W, Cao F and Li F: Identification of key microRNAs in
exosomes derived from patients with the severe acute pancreatitis.
Asian J Surg. 46:337–347. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Desai CS, Khan A, Bellio MA, Willis ML,
Mahung C, Ma X, Baldwin X, Williams BM, Baron TH, Coleman LG, et
al: Characterization of extracellular vesicle miRNA identified in
peripheral blood of chronic pancreatitis patients. Mol Cell
Biochem. 476:4331–4341. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Nakamaru K, Tomiyama T, Kobayashi S,
Ikemune M, Tsukuda S, Ito T, Tanaka T, Yamaguchi T, Ando Y, Ikeura
T, et al: Extracellular vesicles microRNA analysis in type 1
autoimmune pancreatitis: Increased expression of microRNA-21.
Pancreatology. 20:318–324. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li L, Zhang Q, Feng Y, Kong F, Sun F, Xie
P, Zhao J, Yu H, Zhou J, Wu S, et al: A novel serum exosomal miRNA
signature in the early prediction of persistent organ failure in
patients with acute pancreatitis. Ann Surg. Feb 7–2024.(Epub ahead
of print) doi: 10.1097/SLA.0000000000006229. View Article : Google Scholar
|
|
98
|
Zerem E, Kurtcehajic A, Kunosic S, Zerem
Malkocevic D and Zerem O: Current trends in acute pancreatitis:
Diagnostic and therapeutic challenges. World J Gastroenterol.
29:2747–2763. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhu H, Zhou X, Sun X, Fu C, Li G, Dong X,
Kong X, Su X and Du Y: Serum exosomal miR-216a contributes to acute
pancreatitis-associated acute lung injury by enhancing endothelial
cell vascular permeability via downregulating LAMC1. Pancreas. Feb
13–2025.(Epub ahead of print). doi: 10.1097/MPA.0000000000002467.
View Article : Google Scholar
|
|
100
|
Han SB and Lee SS: Simultaneous detection
of exosomal microRNAs isolated from cancer cells using surface
acoustic wave sensor array with high sensitivity and
reproducibility. Micromachines (Basel). 15:2492024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Trigo CM, Rodrigues JS, Camoes SP, Sola S
and Miranda JP: Mesenchymal stem cell secretome for regenerative
medicine: Where do we stand? J Adv Res. 70:103–124. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rahimian S, Mirkazemi K, Nejad AK and
Doroudian M: Exosome-based advances in pancreatic cancer: The
potential of mesenchymal stem cells. Crit Rev Oncol Hematol.
207:1045942025. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Galgaro BC, Beckenkamp LR, van den MNM,
Korb VG, Naasani LIS, Roszek K and Wink MR: The adenosinergic
pathway in mesenchymal stem cell fate and functions. Med Res Rev.
41:2316–2349. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Xie Q, Liu R, Jiang J, Peng J, Yang C,
Zhang W, Wang S and Song J: What is the impact of human umbilical
cord mesenchymal stem cell transplantation on clinical treatment?
Stem Cell Res Ther. 11:5192020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Pang K, Kong F and Wu D: Prospect of
mesenchymal Stem-cell-conditioned medium in the treatment of acute
pancreatitis: A systematic review. Biomedicines. 11:23432023.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Oveili E, Vafaei S, Bazavar H, Eslami Y,
Mamaghanizadeh E, Yasamineh S and Gholizadeh O: The potential use
of mesenchymal stem cells-derived exosomes as microRNAs delivery
systems in different diseases. Cell Commun Signal. 21:202023.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li H, Du R, Xiang A, Liu Y, Guan M and He
H: Bone marrow mesenchymal stem cell-derived exosomal miR-181a-5p
promotes M2 macrophage polarization to alleviate acute pancreatitis
through ZEB2-mediated RACK1 ubiquitination. FASEB J. 38:e700422024.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li HY, He HC, Song JF, Du YF, Guan M and
Wu CY: Bone marrow-derived mesenchymal stem cells repair severe
acute pancreatitis by secreting miR-181a-5p to target
PTEN/Akt/TGF-β1 signaling. Cell Signal. 66:1094362020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ren S, Pan L, Yang L, Niu Z, Wang L, Feng
H and Yuan M: miR-29a-3p transferred by mesenchymal stem
cells-derived extracellular vesicles protects against myocardial
injury after severe acute pancreatitis. Life Sci. 272:1191892021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sharifi-Rad J, Herrera-Bravo J, Kamiloglu
S, Petroni K, Mishra AP, Monserrat-Mesquida M, Sureda A, Martorell
M, Aidarbekovna DS, Yessimsiitova Z, et al: Recent advances in the
therapeutic potential of emodin for human health. Biomed
Pharmacother. 154:1135552022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yang Q, Luo Y, Ge P, Lan B, Liu J, Wen H,
Cao Y, Sun Z, Zhang G, Yuan H, et al: Emodin ameliorates severe
acute Pancreatitis-associated acute lung injury in rats by
modulating Exosome-specific miRNA expression profiles. Int J
Nanomedicine. 18:6743–6761. 2023. View Article : Google Scholar : PubMed/NCBI
|
















