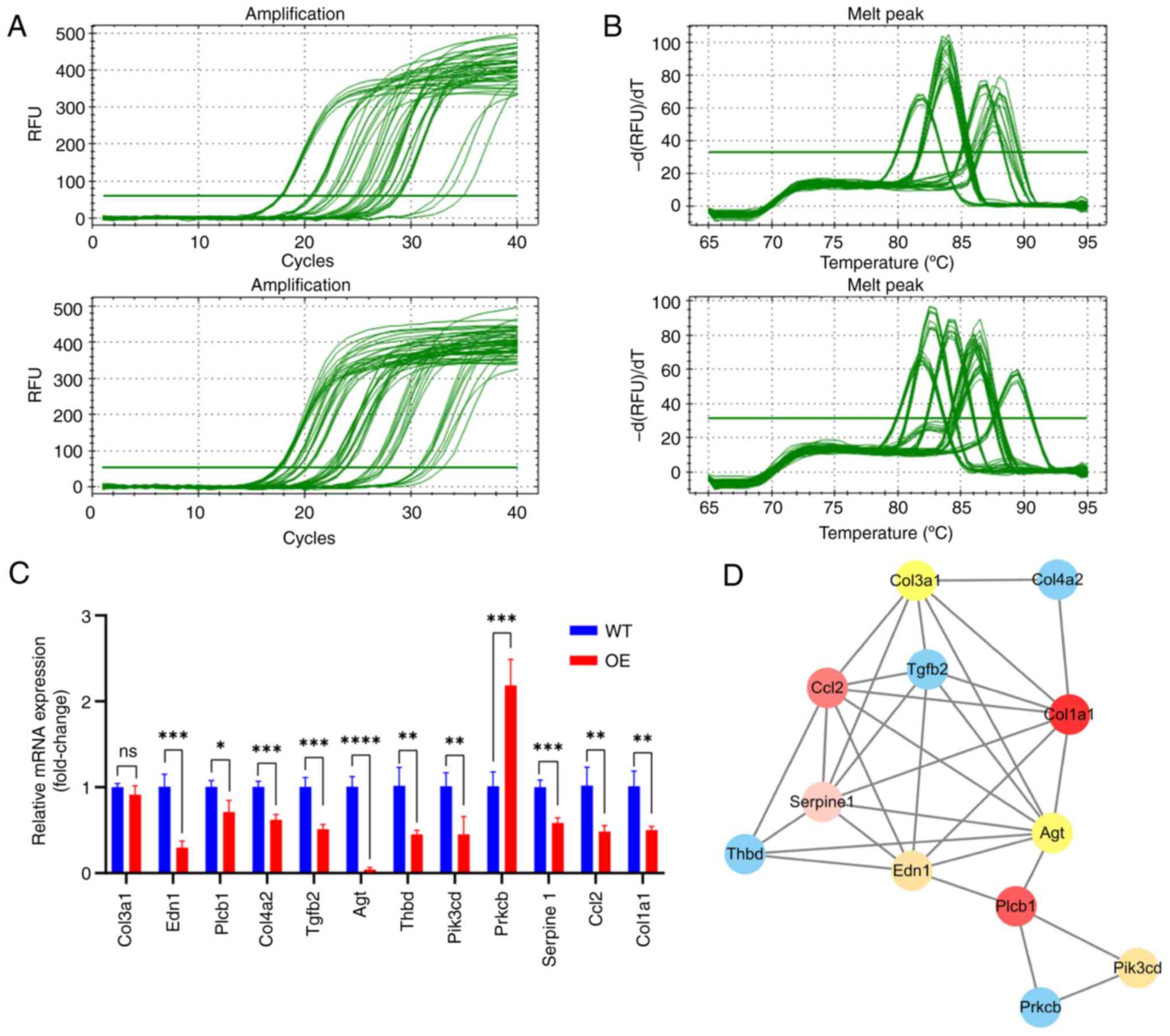
|
1
|
Ogurtsova K, da Rocha Fernandes JD, Huang
Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE and
Makaroff LE: IDF diabetes atlas: Global estimates for the
prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract.
128:40–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng C and Zhang Y, Lang X and Zhang Y:
Role of mitochondrial metabolic disorder and immune infiltration in
diabetic cardiomyopathy: New insights from bioinformatics analysis.
J Transl Med. 21:662023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuentes-Antrás J, Picatoste B, Ramírez E,
Egido J, Tuñón J and Lorenzo Ó: Targeting metabolic disturbance in
the diabetic heart. Cardiovasc Diabetol. 14:172015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan Q, Sun Y, Yang F, Yan D, Shen M, Jin
Z, Zhan L, Liu G, Yang L, Zhou Q, et al: CircRNA DICAR as a novel
endogenous regulator for diabetic cardiomyopathy and diabetic
pyroptosis of cardiomyocytes. Signal Transduct Target Ther.
8:992023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan Y, Zhang Z, Zheng C, Wintergerst KA,
Keller BB and Cai L: Mechanisms of diabetic cardiomyopathy and
potential therapeutic strategies: Preclinical and clinical
evidence. Nat Rev Cardiol. 17:585–607. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murtaza G, Virk HUH, Khalid M, Lavie CJ,
Ventura H, Mukherjee D, Ramu V, Bhogal S, Kumar G, Shanmugasundaram
M and Paul TK: Diabetic cardiomyopathy-A comprehensive updated
review. Prog Cardiovasc Dis. 62:315–326. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia G, Hill MA and Sowers JR: Diabetic
cardiomyopathy: An update of mechanisms contributing to this
clinical entity. Circ Res. 122:624–638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dillmann WH: Diabetic cardiomyopathy. Circ
Res. 124:1160–1162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia G, Whaley-Connell A and Sowers JR:
Diabetic cardiomyopathy: A hyperglycaemia- and
insulin-resistance-induced heart disease. Diabetologia. 61:21–28.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adeghate E and Singh J: Structural changes
in the myocardium during diabetes-induced cardiomyopathy. Heart
Fail Rev. 19:15–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song K, Liang D, Xiao D, Kang A and Ren Y:
Role of bariatric surgery in improving diabetic cardiomyopathy:
Molecular mechanisms and therapeutic perspectives (Review). Mol Med
Rep. 30:1992024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia G, DeMarco VG and Sowers JR: Insulin
resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat
Rev Endocrinol. 12:144–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leary S, Anthony R, Gwaltney-Brant S,
Cartner S, Dewell R, Webb P, Plummer P, Hoenig DE, Moyer W, Smith
SA, et al: AVMA guidelines for the depopulation of animals.
2019.
|
|
14
|
Leek JT, Johnson WE, Parker HS, Jaffe AE
and Storey JD: The sva package for removing batch effects and other
unwanted variation in high-throughput experiments. Bioinformatics.
28:882–883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao P, Cao M, Jiang X, Wang X, Zhang G,
Tang X, Yang C, Komuro I, Ge J, Li L and Zou Y: Cannabinoid
receptor 2-centric molecular feedback loop drives necroptosis in
diabetic heart injuries. Circulation. 147:158–174. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun HJ, Xiong SP, Wu ZY, Cao L, Zhu MY,
Moore PK and Bian JS: Induction of caveolin-3/eNOS complex by
nitroxyl (HNO) ameliorates diabetic cardiomyopathy. Redox Biol.
32:1014932020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu L, Wei J, Zhang Y, Wang Z, Tang J, Tang
J, Gao Y, Zhang X, Li Y, Liu Y, et al: ANGPTL8 is a negative
regulator in pathological cardiac hypertrophy. Cell Death Dis.
13:6212022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Y, Wang J, Zhao T, Sun M, Xu M, Che S,
Pan Z, Wu C and Shen L: Polystyrenenanoplastics lead to ferroptosis
in the lungs. J Adv Res. 56:31–41. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
An Z, Hu T, Lv Y, Li P and Liu L: Targeted
amino acid and related amines analysis based on iTRAQ®-LC-MS/MS for
discovering potential hepatotoxicity biomarkers. J Pharm Biomed
Anal. 178:1128122020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen W, Gong L, Guo Z, Wang W, Zhang H,
Liu X, Yu S, Xiong L and Luo J: A novel integrated method for
large-scale detection, identification, and quantification of widely
targeted metabolites: Application in the study of rice
metabolomics. Mol Plant. 6:1769–1780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaludercic N and Di Lisa F: Mitochondrial
ROS formation in the pathogenesis of diabetic cardiomyopathy. Front
Cardiovasc Med. 7:122020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sitte N, Huber M, Grune T, Ladhoff A,
Doecke WD, Von Zglinicki T and Davies KJ: Proteasome inhibition by
lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J.
14:1490–1498. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Farhangkhoee H, Khan ZA, Mukherjee S,
Cukiernik M, Barbin YP, Karmazyn M and Chakrabarti S: Heme
oxygenase in diabetes-induced oxidative stress in the heart. J Mol
Cell Cardiol. 35:1439–1448. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu R, Duan T, Yu L, Tang Y, Liu S, Wang C
and Fang WJ: Acid sphingomyelinase promotes diabetic cardiomyopathy
via NADPH oxidase 4 mediated apoptosis. Cardiovasc Diabetol.
22:252023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiang H, Jin S, Tan F, Xu Y, Lu Y and Wu
T: Physiological functions and therapeutic applications of neutral
sphingomyelinase and acid sphingomyelinase. Biomed Pharmacother.
139:1116102021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imgrund S, Hartmann D, Farwanah H,
Eckhardt M, Sandhoff R, Degen J, Gieselmann V, Sandhoff K and
Willecke K: Adult ceramide synthase 2 (CERS2)-deficient mice
exhibit myelin sheath defects, cerebellar degeneration, and
hepatocarcinomas. J Biol Chem. 284:33549–33560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cutler RG and Mattson MP: Sphingomyelin
and ceramide as regulators of development and lifespan. Mech Ageing
Dev. 122:895–908. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzuki R, Murakami C, Dilimulati K,
Atsuta-Tsunoda K, Kawai T and Sakane F: Human sphingomyelin
synthase 1 generates diacylglycerol in the presence and absence of
ceramide via multiple enzymatic activities. FEBS Lett.
597:2672–2686. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Wu Y, Zhao J, Wang H, Tan J, Yang M,
Li Y, Deng S, Gao S, Li H, et al: Distinct cardiac energy
metabolism and oxidative stress adaptations between obese and
non-obese type 2 diabetes mellitus. Theranostics. 10:2675–2695.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Da Silva D, Ausina P, Alencar EM, Coelho
WS, Zancan P and Sola-Penna M: Metformin reverses hexokinase and
phosphofructokinase downregulation and intracellular distribution
in the heart of diabetic mice. IUBMB Life. 64:766–774. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Palsgaard J, Brøns C, Friedrichsen M,
Dominguez H, Jensen M, Storgaard H, Spohr C, Torp-Pedersen C, Borup
R, De Meyts P and Vaag A: Gene expression in skeletal muscle
biopsies from people with type 2 diabetes and relatives:
Differential regulation of insulin signaling pathways. PLoS One.
4:e65752009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Osawa H, Sutherland C, Robey RB, Printz RL
and Granner DK: Analysis of the signaling pathway involved in the
regulation of hexokinase II gene transcription by insulin. J Biol
Chem. 271:16690–16694. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye G, Donthi RV, Metreveli NS and Epstein
PN: Overexpression of hexokinase protects hypoxic and diabetic
cardiomyocytes by increasing ATP generation. Cardiovasc Toxicol.
5:293–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang C, Tanizawa H, Hill C, Havas A, Zhang
Q, Liao L, Hao X, Lei X, Wang L, Nie H, et al: METTL3-mediated
chromatin contacts promote stress granule phase separation through
metabolic reprogramming during senescence. Nat Commun. 15:54102024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Banani SF, Lee HO, Hyman AA and Rosen MK:
Biomolecular condensates: Organizers of cellular biochemistry. Nat
Rev Mol Cell Biol. 18:285–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alberti S and Hyman AA: Biomolecular
condensates at the nexus of cellular stress, protein aggregation
disease and ageing. Nat Rev Mol Cell Biol. 22:196–213. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang H, Ji X, Li P, Liu C, Lou J, Wang Z,
Wen W, Xiao Y, Zhang M and Zhu X: Liquid-liquid phase separation in
biology: Mechanisms, physiological functions and human diseases.
Sci China Life Sci. 63:953–985. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nott TJ, Craggs TD and Baldwin AJ:
Membraneless organelles can melt nucleic acid duplexes and act as
biomolecular filters. Nat Chem. 8:569–575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Riback JA, Katanski CD, Kear-Scott JL,
Pilipenko EV, Rojek AE, Sosnick TR and Drummond DA:
Stress-Triggered phase separation is an adaptive, evolutionarily
tuned response. Cell. 168:1028–1040.e19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mo Y, Feng Y, Huang W, Tan N, Li X, Jie M,
Feng T, Jiang H and Jiang L: Liquid-liquid phase separation in
cardiovascular diseases. Cells. 11:30402022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bayeva M, Sawicki KT and Ardehali H:
Taking diabetes to heart-deregulation of myocardial lipid
metabolism in diabetic cardiomyopathy. J Am Heart Assoc.
2:e0004332013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barbé-Tuana F, Funchal G, Schmitz CRR,
Maurmann RM and Bauer ME: The interplay between immunosenescence
and age-related diseases. Semin Immunopathol. 42:545–557. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Henson SM and Aksentijevic D: Senescence
and type 2 diabetic cardiomyopathy: How young can you die of old
age? Front Pharmacol. 12:7165172021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shen CY, Lu CH, Wu CH, Li KJ, Kuo YM,
Hsieh SC and Yu CL: The development of maillard reaction, and
advanced glycation end product (AGE)-receptor for AGE (RAGE)
signaling inhibitors as novel therapeutic strategies for patients
with AGE-related diseases. Molecules. 25:55912020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bodiga VL, Eda SR and Bodiga S: Advanced
glycation end products: Role in pathology of diabetic
cardiomyopathy. Heart Fail Rev. 19:49–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nielsen JM, Kristiansen SB, Nørregaard R,
Andersen CL, Denner L, Nielsen TT, Flyvbjerg A and Bøtker HE:
Blockage of receptor for advanced glycation end products prevents
development of cardiac dysfunction in db/db type 2 diabetic mice.
Eur J Heart Fail. 11:638–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chong CR, Clarke K and Levelt E: Metabolic
remodeling in diabetic cardiomyopathy. Cardiovasc Res. 113:422–430.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Heather LC, Gopal K, Srnic N and Ussher
JR: Redefining diabetic cardiomyopathy: Perturbations in substrate
metabolism at the heart of its pathology. Diabetes. 73:659–670.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Loghmani H and Conway EM: Exploring
traditional and nontraditional roles for thrombomodulin. Blood.
132:148–158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Isermann B, Vinnikov IA, Madhusudhan T,
Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh
J, et al: Activated protein C protects against diabetic nephropathy
by inhibiting endothelial and podocyte apoptosis. Nat Med.
13:1349–1358. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
55
|
Herzog C, Lorenz A, Gillmann HJ, Chowdhury
A, Larmann J, Harendza T, Echtermeyer F, Müller M, Schmitz M,
Stypmann J, et al: Thrombomodulin's lectin-like domain reduces
myocardial damage by interfering with HMGB1-mediated TLR2
signalling. Cardiovasc Res. 101:400–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen S, Khan ZA, Karmazyn M and
Chakrabarti S: Role of endothelin-1, sodium hydrogen exchanger-1
and mitogen activated protein kinase (MAPK) activation in
glucose-induced cardiomyocyte hypertrophy. Diabetes Metab Res Rev.
23:356–367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu M, Zhou Y, Pei D and Gao S: Unveiling
the role of AGT in lipid metabolism and regulated cell death in
colon cancer. Neoplasia. 54:1010092024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu F, Zhang L, Wang L and Zhang D: AGT may
serve as a prognostic biomarker and correlated with immune
infiltration in gastric cancer. Int J Gen Med. 15:1865–1878. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cassis LA, Police SB, Yiannikouris F and
Thatcher SE: Local adipose tissue renin-angiotensin system. Curr
Hypertens Rep. 10:93–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang W, Zhang S, Xu L, Feng Y, Wu X, Zhang
M, Yu Z and Zhou X: Involvement of circHIPK3 in the pathogenesis of
diabetic cardiomyopathy in mice. Diabetologia. 64:681–692. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yurchenco PD: Basement membranes: Cell
scaffoldings and signaling platforms. Cold Spring Harb Perspect
Biol. 3:a0049112011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lu Y, Huo H, Liang F, Xue J, Fang L, Miao
Y, Shen L and He B: Role of pericytes in cardiomyopathy-associated
myocardial infarction revealed by multiple single-cell sequencing
analysis. Biomedicines. 11:28962023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fazio A, Evangelisti C, Cappellini A,
Mongiorgi S, Koufi FD, Neri I, Marvi MV, Russo M, Ghigo A, Manzoli
L, et al: Emerging roles of phospholipase C beta isozymes as
potential biomarkers in cardiac disorders. Int J Mol Sci.
24:130962023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Woodcock EA, Grubb DR, Filtz TM, Marasco
S, Luo J, McLeod-Dryden TJ, Kaye DM, Sadoshima J, Du XJ, Wong C, et
al: Selective activation of the ‘b’ splice variant of phospholipase
Cbeta1 in chronically dilated human and mouse atria. J Mol Cell
Cardiol. 47:676–683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bernal-Lopez MR, Llorente-Cortes V,
Calleja F, Lopez-Carmona D, Mayas MD, Gomez-Huelgas R, Badimon L
and Tinahones FJ: Effect of different degrees of impaired glucose
metabolism on the expression of inflammatory markers in monocytes
of patients with atherosclerosis. Acta Diabetol. 50:553–562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yue Y, Meng K, Pu Y and Zhang X:
Transforming growth factor beta (TGF-β) mediates cardiac fibrosis
and induces diabetic cardiomyopathy. Diabetes Res Clin Pract.
133:124–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tuersuntuoheti M, Zhou L, Li J, Yang S,
Zhou S and Gong H: Investigation of crucial genes and mitochondrial
function impairment in diabetic cardiomyopathy. Gene.
923:1485632024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Du X, Li X, Chen L, Zhang M, Lei L, Gao W,
Shi Z, Dong Y, Wang Z, Li X and Liu G: Hepatic miR-125b inhibits
insulin signaling pathway by targeting PIK3CD. J Cell Physiol.
233:6052–6066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Koya D and King GL: Protein kinase C
activation and the development of diabetic complications. Diabetes.
47:859–866. 1998. View Article : Google Scholar : PubMed/NCBI
|