Continuous bone formation and absorption are key
processes in maintaining bone health. In adolescence, the rate of
novel bone formation in the body is higher than that of old bone
degradation, and thus, bone mass increases. However, after the age
of 20 years, this process slows and the majority of individuals
reach peak bone mass at the age of 30 years (1,2).
Osteoblasts and osteoclasts serve key roles in bone remodeling in
the bone microenvironment. The balanced regulation of osteogenic
and adipogenic differentiation of bone marrow mesenchymal stem
cells is involved in the formation of novel bone mass (3); these are the primary components of
the bone microenvironment. Osteoporosis is a chronic systemic
endocrine and metabolic disorder. Primary osteoporosis caused by
aging or sex hormone deficiency, and secondary osteoporosis caused
by hyperthyroidism, diabetes, obesity, Cushing's syndrome,
anorexia, rheumatoid arthritis (RA) or adverse drug effects have
similar potential mechanisms, namely, an imbalance of bone
remodeling such that the loss of bone mass exceeds the formation of
new bone (4). Low bone mineral
density (LBMD), including osteoporosis and low bone mass, has
becoming a serious public health concern. Global deaths and
disability-adjusted life years attributable to LBMD increased from
207,367 and 8,588,936 in 1990 to 437,884 and 16,647 466 in 2019,
with a raise of 111.16% and 93.82%, respectively (5). Furthermore, osteoporosis can increase
hospitalization rates due to associated secondary complications,
There are more than 8.9 million osteoporotic fractures worldwide.
In other words, an osteoporotic fracture occurs every three seconds
(6). Osteoporosis has become a
notable public health problem and markedly increased healthcare
expenditure. Studying the pathological mechanism of osteoporosis
may facilitate decreased expenditure and improved quality of life
of older adults.
Due to the large amount of energy required by the
internal bone environment to maintain bone homeostasis (7), research on energy metabolism
processes in the osteoporosis microenvironment has increased
(8,9). Energy metabolism includes pathways
that produce energy in the form of adenosine triphosphate (ATP)
from nutrients, such as carbohydrate, fat and protein. Both
anabolic and catabolic pathways are catalyzed by enzymes that
require cofactors and ATP for activation (10). In addition to enzymatic activity,
proteins [combinations of >20 amino acids (AAs)] serve as
functional molecules (such as cell components, receptors,
cytoskeleton and growth factors) in cells, extracellular matrices
and circulatory systems. AAs needed to produce them are derived
from dietary and/or cellular protein degradation, as well as
synthesis through metabolic pathways, such as glycogen production
and the tricarboxylic acid (TCA) cycle (also known as the citric
acid cycle). The TCA cycle is dependent on carbohydrate and fatty
acid metabolic pathways that are key for bone homeostasis (11,12).
AAs are basic components of collagen (the primary component of the
bone matrix) (13,14) and other bone-associated proteins
(such as osteocalcin and alkaline phosphatase) (15,16).
Therefore, AA metabolism disorders lead to a variety of
pathologies, including those affecting the bone tissue (17–19).
There are nine essential AAs (EAAs): Histidine, lysine, tryptophan
(Trp), phenylalanine (Phe), methionine, threonine, isoleucine
(Ile), leucine (Leu) and valine (Val). These AAs, including
branched-chain amino acids (BCAAs) with aliphatic side chains and
branched-chain structures, have a notable impact on bone formation
and degradation (20,21).
An imbalance in AA acid metabolism is a key driver
of the development of osteoporosis, which can aggravate bone loss
by affecting bone cell energy supplies, epigenetic modification and
the immune microenvironment. The balance of bone remodeling is
restored by intervening in specific AA metabolic pathways, thereby
decreasing bone loss (22–24). Although previous studies have
revealed the regulatory role of energy metabolism (such as glucose
and fatty acid metabolism) in bone homeostasis (25,26),
a knowledge gap remains regarding the effects of AA metabolism on
osteoporosis. To the best of our knowledge, the specific mechanism
by which AA categories (such as BC and aromatic AAs) affect bone
mineral density (BMD) and quality by regulating the function of key
cells in the bone microenvironment has not been systematically
elucidated, and the clinical transformation potential of AA
metabolism intervention strategies need to be explored. The purpose
of the present review is to address the limitations of traditional
single-mechanism research by integrating metabolomics, genetics and
cell biology evidence, combined with Mendelian randomization (MR)
and computer simulation technology, to summarize the differential
regulatory networks of AA categories (BCAA, aromatic AAs and
glutamine) in osteoblasts, osteoclasts and bone marrow mesenchymal
stem cells (BMSCs), systematically analyze the multiple mechanisms
of AA metabolism in osteoporosis and ways in which it affects bone
homeostasis by regulating the bone microenvironment and evaluate
the potential therapeutic value of metabolic intervention. The
present review aimed to provide understanding of the metabolic
heterogeneity of the bone microenvironment and a scientific
foundation for the development of novel metabolic therapies.
However, in a previous study, older adult
individuals were divided into two groups. One group received 0.8
citrulline and 1.6 g Leu twice daily for 20 weeks, and the other
group received a placebo. Both groups exercised independently. The
results revealed no difference in BMD or bone area between the
groups (29). The lack of a
significant positive effect of citrulline and Leu combined with
exercise may have been due to the small sample size, insufficient
dose, the short intervention time, limited AA intake in the control
group, limited increase in plasma Leu levels, a low basic body mass
index (BMI), insufficient dietary control or low exercise
intensity. These factors may have masked the potential effects of
citrulline and Leu on body composition and physical activity. In
another study, Leu-rich whey protein drinks were provided to
individuals aged between 60 and 90 years of age for 16 weeks
(30). Although the aforementioned
study revealed that protein supplementation and resistance training
were beneficial for certain cardiovascular metabolic indicators
(such as low-density lipoprotein levels), the overall intervention
effect was limited, potentially due to insufficient time, low
exercise intensity, poor protein compliance and limited statistical
power (30). Therefore, further
research is needed on intestinal and osteoporosis
microenvironments. Future studies should optimize the intervention
design to verify the effect of proteins on the metabolic health of
older individuals. The intake of proteins and AAs in the daily diet
should be controlled and the study time should be prolonged to
observe long-term effects to evaluate the role of AAs in older
individuals with low BMI.
AAs containing a benzene ring, such as Phe, tyrosine
and Trp, are aromatic. Phe and Trp are EAAs for human nutrition. As
Phe is structurally similar to tyrosine, it can be converted into
tyrosine by hydroxylation in the liver and kidney. Aromatic AAs
stimulate anabolic metabolic pathways associated with bone
remodeling under physiological conditions and have a positive
effect on bone mass maintenance in vivo (20,31).
Studies have revealed that in the environment of osteoporosis, Trp,
an EAA for BMD, is damaged, and its metabolic pathway may be
abnormal, which affects bone health (32,33).
Kim et al (34) and Apalset
et al (35) revealed that
levels of kynurenine markedly increase in individuals with reduced
hip bone mass. Kynurenine is a key intermediate of Trp metabolism
in humans. Apalset et al (35) revealed a negative association
between the kynurenine/Trp ratio and hip BMD. Ling et al
(36) used targeted metabolomic
techniques to analyze fecal samples from patients with osteoporosis
and revealed that lumbar spine tyrosine and femoral neck Trp levels
are higher compared with the normal group. However, by contrast
with the results reported by Apalset et al (35), there were differences in the levels
of Trp metabolism (35), which may
be due to the different metabolites of bone loss analyzed and
sample sources. Therefore, further research is needed to explore
the association between different tissue, sample biomarkers and the
pathogenesis of osteoporosis, especially the association between
osteoporosis epidemic sites and characteristic metabolites.
Future research should focus on exploring the deeper
reasons for sex differences. Wang et al (43) used mass spectrometry (MS)
technology to analyze serum samples from patients with osteoporosis
and revealed that arginine, glutamine, histidine and serine levels
in males, and glycine and hydroxyproline (t4-OH-Pro) levels in
postmenopausal patients are associated with BMD. Glutamine can
regulate bone metabolism by osteoclasts and trigger the bone
resorption mediated by glutamate receptors on osteoblasts via
conversion to glutamate (44). Sex
differences in amino acid metabolism may be due to heterogeneity in
hormone regulation, metabolic pathways and physiological
characteristics (45). In the
future, it is necessary to promote accurate diagnosis and develop
sex-specific intervention strategies for osteoporosis through
multicenter large-scale research, mechanism exploration and
multi-omics integration.
Generally, an imbalance in AA metabolism is a
notable characteristic in patients with osteoporosis (56). Osteoporotic cells are primarily
composed of osteoblasts, osteoclasts and bone-marrow-derived stem
cells. Therefore, it is important to study the metabolism of AAs in
the osteoporotic microenvironment.
Osteocytes are the main regulators of bone
homeostasis, which is achieved by the regulation of bone formation
by osteoblasts and bone absorption by osteoclasts (57). Recent studies have revealed that a
lack of EAAs can lead to the phosphorylation of MAPK, which leads
to cell cycle arrest, thereby inhibiting cell proliferation and
osteogenic differentiation (58–60).
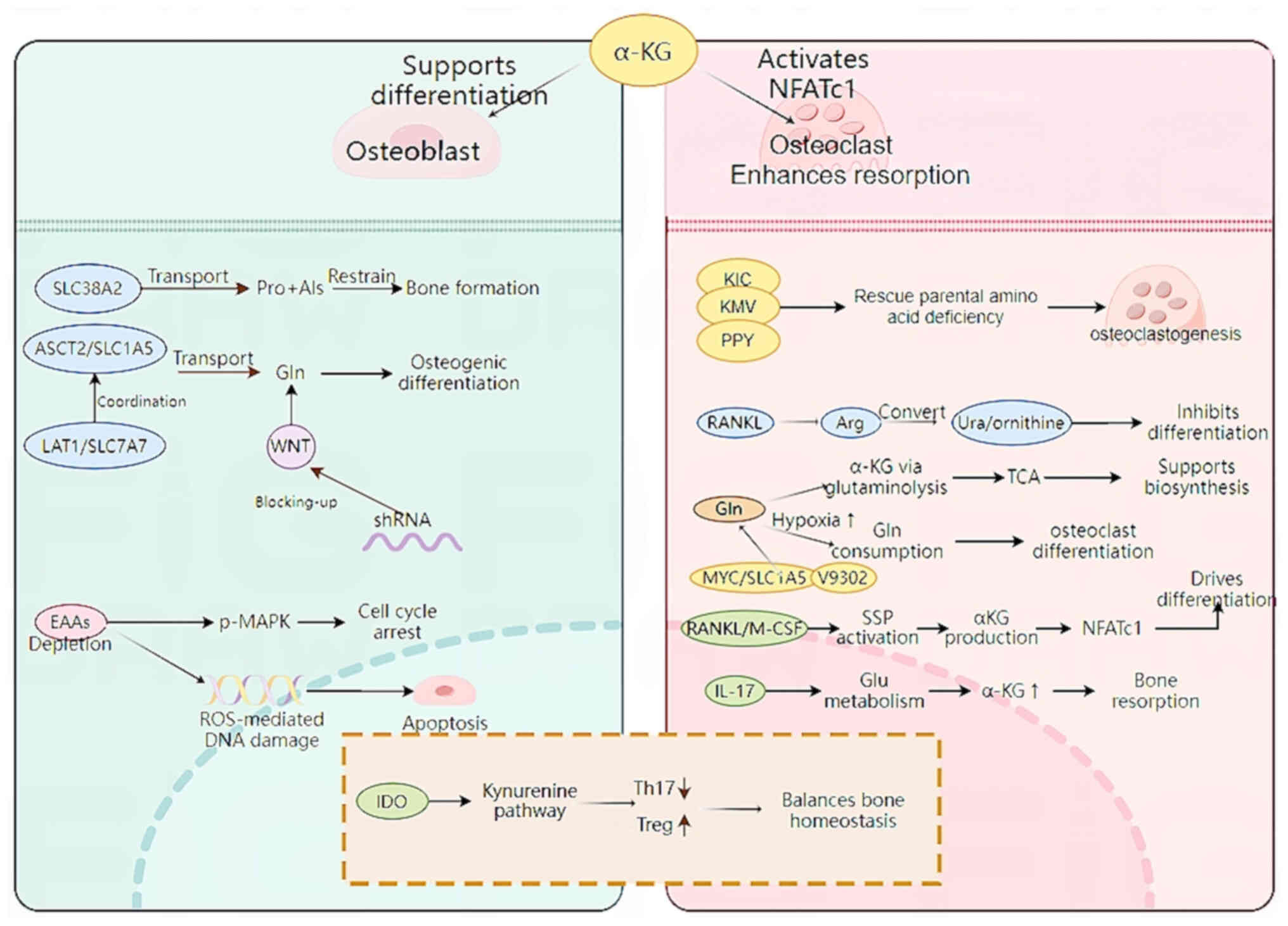
In addition, the lack of EAAs induces reactive oxygen
species-mediated DNA damage and apoptosis (60), confirming the role of EAAs in
osteoblasts.
In addition to the effect of EAAs on osteoblasts,
the neutral AA solute carrier family 38 member 2 (SLC38A2) provides
proline and alanine to osteoblasts. In mice, ablation of SLC38A2
results in a decreased bone mass, highlighting the role of
SLC38A2-mediated proline and alanine intake for postpartum bone
formation and bone homeostasis (61). In addition, genetic and
metabolomics studies have demonstrated that the AA transporter
cysteine transporter 2 (SLC1A5, encoded by Slc1a5) provide
glutamine and asparagine, thereby regulating protein synthesis and
osteoblast differentiation (62,63).
AA transporters, system γ(+)-L transporter γ(+)-L transporter 1 [γ
(+)-LAT1] and SLC1A5 (encoded by SlC7A7 and SlC1A5, respectively),
are the primary transporters of glutamine in response to Wnt and
SLC1A5 mediates the majority of glutamine intake (64). Using short hairpin RNAs targeting
SlC7A7 or SlC1A5 decreases Wnt-induced glutamine intake, thereby
preventing osteoblast differentiation (64). The mechanism of AA metabolism in
osteoblasts is shown in Fig. 1. In
summary, the aforementioned studies demonstrated the key role of
EAAs and associated transport genes in osteoblasts and revealed the
potential for targeting AA metabolism to interfere with bone
formation and resorption (Table
I).
During osteoclast development, metabolic pathways
change, especially AA metabolism, and this serves a key role in the
regulation of osteoclast formation. EAAs α-ketoisocaproate,
α-keto-β-methylvalerate and phenylpyruvate, the intermediates of
Leu, Ile and Phe metabolism, respectively (4), serve a key role in the formation of
osteoclasts. These amino acids can effectively alleviate the
inhibition of osteoclast formation because of a lack of parental
AAs (65). Osteoclasts contain a
large number of intracellular proteins involved in lysine
decomposition, which stimulate the biosynthesis of tyrosine, Phe
and Trp (66). Osteoclasts are
rich in intracellular proteins involved in lysine degradation,
which activates the biosynthesis of tyrosine, Phe and Trp (67). Recent studies have revealed that
receptor activator of nuclear factor-κB ligand (RANKL)-induced
osteoclast formation is primarily dependent on the presence of
extracellular arginine (68,69).
RANKL-induced proteins are antagonized by recombinant arginase 1,
which metabolizes arginine to urea and ornithine. Excessive
arginine intake can restore osteoclastogenesis by supplementing
arginine-succinic acid and citrulline, but direct supplementation
of TCA intermediates, such as α-ketoglutarate (αKG), has no effect
(65). The effect of arginine
deficiency on osteoclast formation is not affected by mTORC1
activity or the inhibition of overall transcription and translation
(67). In patients with RA and
pre-RA, L-arginine effectively inhibits the progression of
arthritis and bone loss, and can directly block TNFα-induced
osteoclastogenesis in mice and humans (8).
Osteoclast differentiation is synergistically
affected by macrophage colony-stimulating factor and RANKL, which
activate signaling pathways and interact with each other to
regulate the expression and function of the key transcriptional
regulator NFATc1. Early in the process of osteoclast formation, αKG
produced by RANKL-induced serine synthesis pathway activation
regulates the expression of Nfatc1 by epigenetics, thereby
promoting osteoclast differentiation (70). In addition to facilitating protein
synthesis, glutamine is an important energy source and a carbon and
nitrogen donor for the synthesis of AAs, nucleotides, glutathione
and aminohexose. It is converted to αKG via glutamine
decomposition, enters the TCA cycle, and is converted to citric
acid (71,72). During osteoclast differentiation,
the expression of Na+-dependent glutamine neutral AA
transporter B increases, which serves a key role in the later stage
of differentiation (73). In
addition, Tsumura et al (74) revealed that a hypoxic environment
can stimulate osteoclasts to consume glutamine. The inhibition of
MYC can effectively prevent osteoclast differentiation and function
and inhibit the expression of SLC1A5 and glutaminase (GLS).
Therefore, glutamine uptake is key for osteoclast development and
bone resorption (75). Although
glutamine metabolism may indirectly support energy supply by
providing intermediates of the TCA cycle, its primary role is to
promote the synthesis of biomolecules that accelerate osteoclast
differentiation and functional maturation (44). During the development of
osteoporosis, osteoclasts require ATP to exert their bone
resorption function. Glu and its downstream metabolite αKG are
involved in the IL-17-mediated pathway in vivo to aggravate
ovariectomy-induced bone loss, which can be inhibited by V9302 (an
SLC1A5 inhibitors), thereby interfering with osteoclast
differentiation and bone resorption (76).
An imbalance of osteoblast and osteoclast activity
is a key factor in AA metabolism (77). Specific T cell subsets, such as
regulatory (Treg) and helper T cells 17 (Th17) are involved in the
imbalance between osteoblast and osteoclast activity (78). Indoleamine 2,3-dioxygenase, a
rate-limiting enzyme of Trp catabolism, serves a role in the
kynurenine pathway and may be a key protein in regulating the
balance ratio of Th17/Treg cells (79). Its metabolites inhibit Th17 cell
differentiation and promote Treg cells production, thereby
affecting the balance between Th17 and Treg cells (80). In summary, amino acid metabolism is
associated with osteoclasts (Fig.
1; Table I).
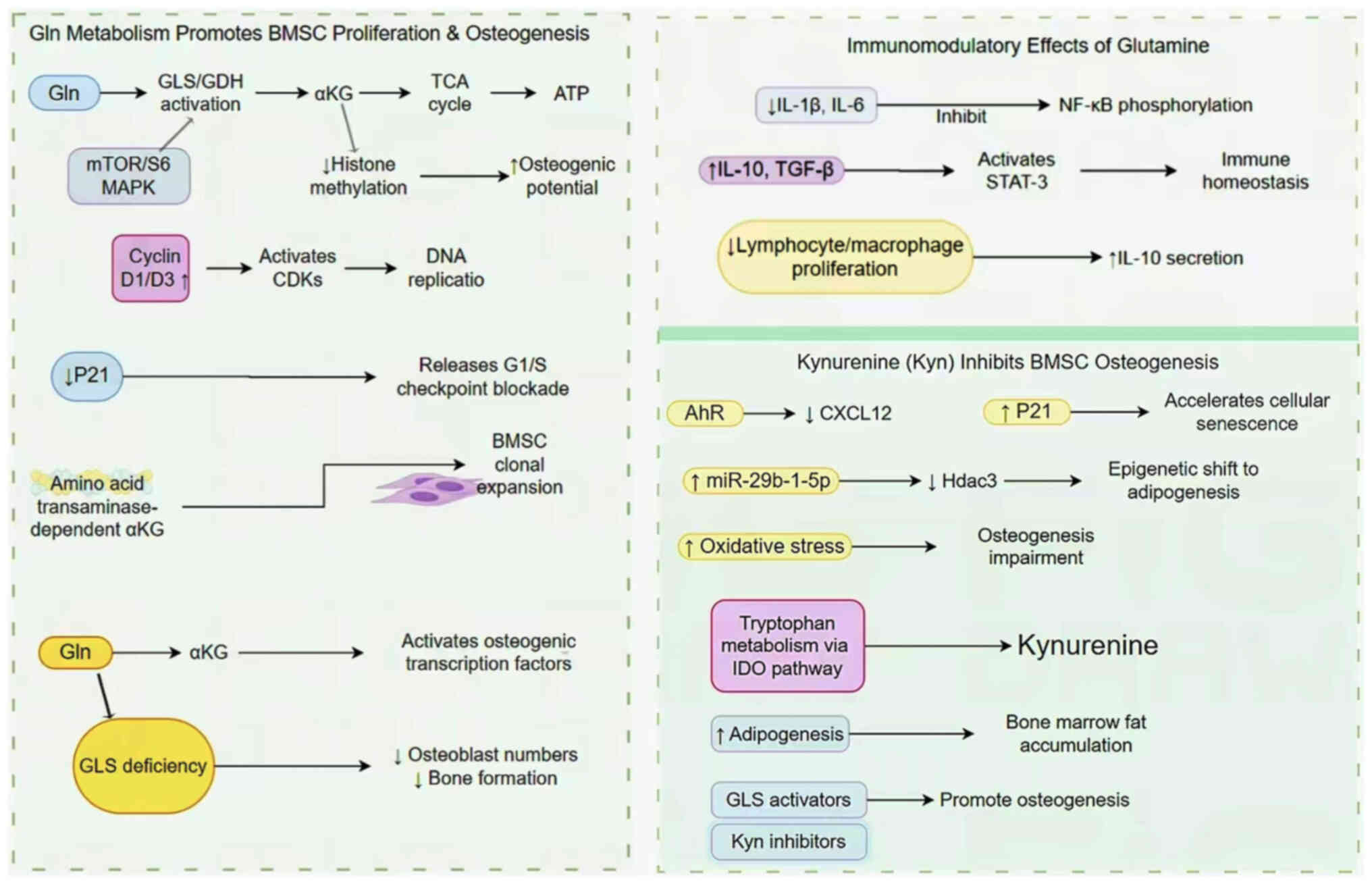
BMSCs are progenitor cells with potential to
differentiate into bone, fat and cartilage lineages (88). Previous studies have revealed that
human and mouse BMSCs consume large amounts of glutamine during
differentiation (71,89). During this process, glutamine
metabolism produces ATP through the TCA cycle to meet the energy
needs of physiological functions (72). In addition, GLS has also been
demonstrated to promote the differentiation of BMSCs into
osteoblasts (90). However, when
BMSCs lack GLS, there is a decrease in the total number of
osteoblasts and bone formation ability (89). Yu et al (72) also revealed that the proliferation
and colony expansion of BMSCs is dependent on the production of AA
transaminase-dependent αKG, which explains the adverse effects of
decreased GLS activity on the proliferation of BMSCs. Other studies
have revealed that the glutamine metabolite αKG improves the
osteogenic potential of BMSCs by decreasing histone methylation
(91,92). These results suggest that glutamine
and αKG may promote the osteogenic differentiation of BMSCs.
Therefore, in-depth study on glutamine metabolism in BMSCs may
provide novel strategies and methods for the treatment of bone
loss.
In BMSCs, the glutamine concentration directly
affects immune characteristics. High concentrations of glutamine
effectively inhibit inflammation, potentially by decreasing
activity of pro-inflammatory cytokines, such as IL-1β and IL-6, and
increasing the expression levels of the anti-inflammatory cytokines
IL-10 and transforming growth factor-β (93). The mechanism mainly involves the
regulation of cytokines by decreasing the levels of phosphorylated
NF-κB and signal transduction and activator 3 (STAT-3) (94). In particular, IL-10, an important
anti-inflammatory cytokine that can hinder the activity of NF-κB
and regulate cytokine production (95). Studies (96–98)
have shown that glutamine concentration has a regulatory effect on
IL-10 expression. Adequate glutamine supply upregulates IL-10
expression and enhances its anti-inflammatory function; glutamine
deficiency leads to decreased IL-10 expression and impairs its
inhibitory effect on the NF-κB signaling pathway, thereby
exacerbating the inflammatory response (99,100). IL-10 can also activate STAT-3,
thereby decreasing the levels of pro-inflammatory cytokines
(101,102). In addition, proliferation of
lymphocytes and macrophages in BMSCs decreases following glutamine
exposure and the secretion of IL-10 increases (103). At 4 weeks following the
intraperitoneal injection of kynurenine (10 mg/kg) in adult mice,
the osteogenic differentiation ability of BMSCs decreases
considerably, accompanied by the deterioration of osteoblast
bioenergetics and productivity (104). In vitro studies have
revealed that human pluripotent stem cells stimulated by kynurenine
have altered microRNA (miRNA or miR) expression levels, resulting
in increased oxidative stress and the inhibition of osteogenic
differentiation (105,106). The C-X-C motif chemokine ligand
12 (CXCL12) protein, a necessary mediator of the osteogenic
differentiation of BMSCs, is also notably affected by kynurenine.
Kynurenine decreases its expression through the AhR signaling
pathway (107). Kynurenine also
increases the levels of P21 and cell death (108). In addition, it causes BMSCs to
develop mainly in the direction of adipogenesis by increasing the
expression level of miR-29b-1-5p and downregulating the levels of
histone deacetylase-3 (Hdac3) and CXCL12, creating a toxic bone
marrow environment, thus exacerbating bone loss and increasing the
fracture risk (109). The
mechanism of AA metabolism in BMSCs is shown in Fig. 2. In summary, kynurenine regulates
the levels of miRNAs, proteins and activity of metabolic pathways
to affect the osteogenic differentiation ability of BMSCs (Table II).
Osteoporosis is a metabolic disease characterized by
an imbalance in bone remodeling. Its pathology is caused by
dysfunction of osteoblasts, osteoclasts and BMSCs. Recent studies
have demonstrated that AA metabolism serves a key role in the
occurrence and development of osteoporosis by regulating energy
supply, signal transduction, epigenetic modification and immune
homeostasis of the bone microenvironment (20,44,110). The present review summarized the
differential regulatory networks of types of AA, such as BCAAs,
aromatic AAs and glutamine, in bone homeostasis and the mechanisms
underlying their effects on osteoporosis by interfering with the
functions of osteoblasts, osteoclasts and bone marrow mesenchymal
stem cells, as well as application prospects of research methods
based on metabolomics and genetics for promoting the development of
precise treatment.
Although several studies have revealed the role of
AA metabolism in osteoporosis, they have limitations (9,20,56,111). First, the specific regulatory
mechanisms of AAs in bone metabolism have not yet been fully
elucidated. For example, to the best of our knowledge, a systematic
analysis of the roles of BCAAs, aromatic AAs and glutamine in
different bone cells (osteoblasts, osteoclasts and BMSCs) is
lacking (112). In addition, the
specific regulatory network of AA metabolism in the bone
microenvironment is complex and is affected by numerous factors,
including gene expression, signaling pathways, the immune
microenvironment and energy metabolism (21). However, the majority of studies are
limited to exploring a single mechanism and do not reveal the
overall regulatory model (20,56).
Although observational and genetic association studies have
revealed an association between AA metabolism and the occurrence
and development of osteoporosis, randomized controlled trials based
on metabolic interventions are limited (9,27).
For example, there is a lack of long-term intervention data
demonstrating that BCAA supplementation can improve BMD and
decrease fracture risk (113). In
addition, certain studies have problems such as a small sample
size, a short intervention time and insufficient dietary control,
resulting in inconsistent results and affecting its clinical
transformation value (114,115). In addition, individual
differences such as sex, age, genetic background and lifestyle may
lead to differences in AA metabolism patterns. For example, female
patients are more affected by changes in estrogen levels, whereas
male patients may be more affected by androgens and other metabolic
pathways (116). Therefore,
future studies should consider individual factors to improve the
applicability of the results.
In future, the mechanisms of AA metabolism in
osteoporosis should be elucidated. In addition, new animal models
of osteoporosis should be developed using specific metabolic
markers. This may provide a novel perspective for the direct visual
analysis of bone cell metabolic changes to develop drug, prevention
and treatment concepts. The effects of AA metabolism on the energy
supply and epigenetic modification of bone cells should be
explored. In clinical practice, a larger-scale, long-term follow-up
clinical intervention trial should be designed to evaluate the
effects of AA supplementation on BMD, fracture risk and quality of
life in patients with osteoporosis and optimize the clinical
transformation of AA metabolism intervention strategies. In
addition, the application of individualized medicine should be
strengthened by combining genomic and metabolomic data to explore
the differences in the responses of individuals to AA metabolism
interventions. Artificial intelligence and machine-learning
techniques can be used in combination with big data analysis to
establish predictive models to assess the risk of individual AA
metabolism patterns in osteoporosis and provide personalized
nutrition intervention recommendations. In recent years, more
evidence has revealed that the gut microbiota serve a key role in
the development of osteoporosis (117,118). It is of clinical value to explore
the interactions between AA metabolism and intestinal microecology.
Specifically, RNA sequencing, metagenomic and metabolomic analyses
should be used to study the composition of the intestinal flora and
its association with AA metabolism in different groups of patients
with osteoporosis. In addition, the effects of probiotics,
prebiotics and specific AA supplementation on BMD should be
evaluated to explore the regulation of the intestinal microecology
as a novel strategy for the treatment of osteoporosis.
Not applicable.
The present study was supported by National College Students'
Innovation and Entrepreneurship Training Project (grant no.
S202310541024), Guiding Science and Technology Plan Project of
Changsha City in 2022 (grant no. kzd22005), Hunan Provincial
Natural Science Foundation Project in 2025 (grant nos. 2025JJ90029,
2025JJ90014), Hunan University of Traditional Chinese Medicine
Graduate Innovation Project (grant no. 2024CX042) and Hunan
University of Traditional Chinese Medicine 2024 ‘Yifang’ Graduate
Student Innovation Project (grant no. 2024YF10) and Undergraduate
Fund of Hunan University of Traditional Chinese Medicine
(2024BKS0402024BKS007).
Not applicable.
CZ, JZ and QL wrote the manuscript. ML, JT, SP, XD
and YG performed the literature review. RL, HL and GZ designed the
study. CZ and JZ edited the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Weaver CM, Gordon CM, Janz KF, Kalkwarf
HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC and Zemel BS: The
National Osteoporosis Foundation's position statement on peak bone
mass development and lifestyle factors: A systematic review and
implementation recommendations. Osteoporos Int. 27:1281–1386. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rozenberg S, Bruyère O, Bergmann P,
Cavalier E, Gielen E, Goemaere S, Kaufman JM, Lapauw B, Laurent MR,
De Schepper J and Body JJ: How to manage osteoporosis before the
age of 50. Maturitas. 138:14–25. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Y, Xie L, Wang M, Xiong Q, Guo Y, Liang
Y, Li J, Sheng R, Deng P, Wang Y, et al: Mettl3-mediated mA RNA
methylation regulates the fate of bone marrow mesenchymal stem
cells and osteoporosis. Nat Commun. 9:47722018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lademann F, Tsourdi E, Hofbauer LC and
Rauner M: Thyroid hormone actions and bone remodeling-the role of
the wnt signaling pathway. Exp Clin Endocrinol Diabetes.
128:450–454. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen Y, Huang X, Wu J, Lin X, Zhou X, Zhu
Z, Pan X, Xu J, Qiao J, Zhang T, et al: The Global Burden of
osteoporosis, low bone mass, and its related fracture in 204
countries and territories, 1990–2019. Front Endocrinol (Lausanne).
13:8822412022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnston CB and Dagar M: Osteoporosis in
older adults. Med Clin North Am. 104:873–884. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Confavreux CB, Levine RL and Karsenty G: A
paradigm of integrative physiology, the crosstalk between bone and
energy metabolisms. Mol Cell Endocrinol. 310:21–29. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao S, Li Y, Song R, Meng X, Fuchs M,
Liang C, Kachler K, Meng X, Wen J, Schlötzer-Schrehardt U, et al:
L-arginine metabolism inhibits arthritis and inflammatory bone
loss. Ann Rheum Dis. 83:72–87. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panahi N, Fahimfar N, Roshani S, Arjmand
B, Gharibzadeh S, Shafiee G, Migliavacca E, Breuille D, Feige JN,
Grzywinski Y, et al: Association of amino acid metabolites with
osteoporosis, a metabolomic approach: Bushehr elderly health
program. Metabolomics. 18:632022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilson MP, Plecko B, Mills PB and Clayton
PT: Disorders affecting vitamin B6 metabolism. J Inherit Metab Dis.
42:629–646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Montalvany-Antonucci CC, Duffles LF, de
Arruda JAA, Zicker MC, de Oliveira S, Macari S, Garlet GP, Madeira
MFM, Fukada SY, Andrade I Jr, et al: Short-chain fatty acids and
FFAR2 as suppressors of bone resorption. Bone. 125:112–121. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lucas S, Omata Y, Hofmann J, Böttcher M,
Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B,
Krönke G, et al: Short-chain fatty acids regulate systemic bone
mass and protect from pathological bone loss. Nat Commun. 9:552018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prockop DJ and Kivirikko KI: Collagens:
Molecular biology, diseases, and potentials for therapy. Annu Rev
Biochem. 64:403–434. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shoulders MD and Raines RT: Collagen
structure and stability. Annu Rev Biochem. 78:929–958. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whyte MP: Physiological role of alkaline
phosphatase explored in hypophosphatasia. Ann N Y Acad Sci.
1192:190–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heaney RP and Layman DK: Amount and type
of protein influences bone health. Am J Clin Nutr. 87:1567S–1570S.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding KH, Cain M, Davis M, Bergson C,
McGee-Lawrence M, Perkins C, Hardigan T, Shi X, Zhong Q, Xu J, et
al: Amino acids as signaling molecules modulating bone turnover.
Bone. 115:15–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Long F: Energy metabolism and bone. Bone.
115:12018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dirckx N, Moorer MC, Clemens TL and Riddle
RC: The role of osteoblasts in energy homeostasis. Nat Rev
Endocrinol. 15:651–665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv Z, Shi W and Zhang Q: Role of essential
amino acids in age-induced bone loss. Int J Mol Sci. 23:112812022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Devignes CS, Carmeliet G and Stegen S:
Amino acid metabolism in skeletal cells. Bone Rep. 17:1016202022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu F, Li W, Yang X, Na L, Chen L and Liu
G: The roles of epigenetics regulation in bone metabolism and
osteoporosis. Front Cell Dev Biol. 8:6193012021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Huang X, Zhang X and Chen Z:
Metabolism-epigenetic interaction-based bone and dental
regeneration: From impacts and mechanisms to treatment potential.
Bone. 192:1173822025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang L, Chu Z, Liu M, Zou Q, Li J, Liu Q,
Wang Y, Wang T, Xiang J and Wang B: Amino acid metabolism in immune
cells: Essential regulators of the effector functions, and
promising opportunities to enhance cancer immunotherapy. J Hematol
Oncol. 16:592023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karner CM and Long F: Glucose metabolism
in bone. Bone. 115:2–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alekos NS, Moorer MC and Riddle RC: Dual
effects of lipid metabolism on osteoblast function. Front
Endocrinol (Lausanne). 11:5781942020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui Z, Feng H, He B, He J and Tian Y:
Relationship between serum amino acid levels and bone mineral
density: A mendelian randomization study. Front Endocrinol
(Lausanne). 12:7635382021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao M, Mu Y, Su X, Zheng L, Zhang S, Chen
H, Xu S, Ma J, Ouyang R, Li W, et al: Association between
Branched-Chain Amino Acid Intake and Physical Function among
Chinese Community-Dwelling Elderly Residents. Nutrients.
14:43672022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim M, Isoda H and Okura T: Effect of
Citrulline and leucine intake with exercises on body composition,
physical activity, and amino acid concentration in older women: A
Randomized double-blind placebo-controlled study. Foods.
10:31172021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kirk B, Mooney K, Vogrin S, Jackson M,
Duque G, Khaiyat O and Amirabdollahian F: Leucine-enriched whey
protein supplementation, resistance-based exercise, and
cardiometabolic health in older adults: A randomized controlled
trial. J Cachexia Sarcopenia Muscle. 12:2022–2033. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Refaey ME, Zhong Q, Ding KH, Shi XM, Xu J,
Bollag WB, Hill WD, Chutkan N, Robbins R, Nadeau H, et al: Impact
of dietary aromatic amino acids on osteoclastic activity. Calcif
Tissue Int. 95:174–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Michalowska M, Znorko B, Kaminski T,
Oksztulska-Kolanek E and Pawlak D: New insights into tryptophan and
its metabolites in the regulation of bone metabolism. J Physiol
Pharmacol. 66:779–791. 2015.PubMed/NCBI
|
|
33
|
Akinsuyi OS and Roesch LFW: Meta-analysis
reveals compositional and functional microbial changes associated
with osteoporosis. Microbiol Spectr. 11:e00322232023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim BJ, Hamrick MW, Yoo HJ, Lee SH, Kim
SJ, Koh JM and Isales CM: The detrimental effects of kynurenine, a
tryptophan metabolite, on human bone metabolism. J Clin Endocrinol
Metab. 104:2334–2342. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Apalset EM, Gjesdal CG, Ueland PM, Midttun
Ø, Ulvik A, Eide GE, Meyer K and Tell GS: Interferon
(IFN)-γ-mediated inflammation and the kynurenine pathway in
relation to bone mineral density: The Hordaland health study. Clin
Exp Immunol. 176:452–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ling CW, Miao Z, Xiao ML, Zhou H, Jiang Z,
Fu Y, Xiong F, Zuo LS, Liu YP, Wu YY, et al: The association of gut
microbiota with osteoporosis is mediated by amino acid metabolism:
Multiomics in a large cohort. J Clin Endocrinol Metab.
106:e3852–e3864. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyamoto K, Hirayama A, Sato Y, Ikeda S,
Maruyama M, Soga T, Tomita M, Nakamura M, Matsumoto M, Yoshimura N
and Miyamoto T: A metabolomic profile predictive of new
osteoporosis or sarcopenia development. Metabolites. 11:2782021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Xu H, Li GH, Long MT, Cheung CL,
Vasan RS, Hsu YH, Kiel DP and Liu CT: Metabolomics insights into
osteoporosis through association with bone mineral density. J Bone
Miner Res. 36:729–738. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eriksson AL, Friedrich N, Karlsson MK,
Ljunggren Ö, Lorentzon M, Nethander M, Wallaschofski H, Mellström D
and Ohlsson C: Serum glycine levels are associated with cortical
bone properties and fracture risk in men. J Clin Endocrinol Metab.
106:e5021–e5029. 2021.PubMed/NCBI
|
|
40
|
Jennings A, MacGregor A, Spector T and
Cassidy A: Amino acid intakes are associated with bone mineral
density and prevalence of low bone mass in women: Evidence from
discordant monozygotic twins. J Bone Miner Res. 31:326–335. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim MH, Kim HM and Jeong HJ: Estrogen-like
osteoprotective effects of glycine in in vitro and in vivo models
of menopause. Amino Acids. 48:791–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Lin Q, Cui Y, Wang H, Wang P, Yang
L, Ye Q, Zhang R and Zhu X: Glycine acts through estrogen receptor
alpha to mediate estrogen receptor signaling, stimulating
osteogenesis and attenuating adipogenesis in ovariectomized rats.
Mol Nutr Food Res. 66:e21008572022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, Yan D, Zhao A, Hou X, Zheng X,
Chen P, Bao Y and Jia W, Hu C, Zhang ZL and Jia W: Discovery of
potential biomarkers for osteoporosis using LC-MS/MS metabolomic
methods. Osteoporos Int. 30:1491–1499. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu G, Yu Y, Ren Y, Tower RJ, Zhang GF and
Karner CM: Glutaminolysis provides nucleotides and amino acids to
regulate osteoclast differentiation in mice. EMBO Rep.
25:4515–4541. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lamont LS, McCullough AJ and Kalhan SC:
Gender differences in the regulation of amino acid metabolism. J
Appl Physiol (1985). 95:1259–1265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang YW, Song PR, Wang SC, Liu H, Shi ZM
and Su JC: Diets intervene osteoporosis via gut-bone axis. Gut
Microbes. 16:22954322024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mann ER, Lam YK and Uhlig HH: Short-chain
fatty acids: Linking diet, the microbiome and immunity. Nat Rev
Immunol. 24:577–595. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Palacios-González B, Ramírez-Salazar EG,
Rivera-Paredez B, Quiterio M, Flores YN, Macias-Kauffer L,
Moran-Ramos S, Denova-Gutiérrez E, Ibarra-González I, Vela-Amieva
M, et al: A Multi-Omic Analysis for Low Bone Mineral Density in
Postmenopausal Women Suggests a RELATIONSHIP between Diet,
Metabolites, and Microbiota. Microorganisms. 8:16302020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Palacios-González B, León-Reyes G,
Rivera-Paredez B, Ibarra-González I, Vela-Amieva M, Flores YN,
Canizales-Quinteros S, Salmerón J and Velázquez-Cruz R: Serum
metabolite profile associated with sex-dependent visceral adiposity
index and low bone mineral density in a mexican population.
Metabolites. 11:6042021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao J, Xu K, Liu H, Liu G, Bai M, Peng C,
Li T and Yin Y: Impact of the gut microbiota on intestinal immunity
mediated by tryptophan metabolism. Front Cell Infect Microbiol.
8:132018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ye Q, Xi X, Fan D, Cao X, Wang Q, Wang X,
Zhang M, Wang B, Tao Q, Xiao C, et al: Polycyclic aromatic
hydrocarbons in bone homeostasis. Biomed Pharmacother.
146:1125472022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang L, Wang Z, Luo P, Bai S, Chen Y and
Chen W: Dietary zinc glycine supplementation improves tibia quality
of meat ducks by modulating the intestinal barrier and bone
resorption. Biol Trace Elem Res. 201:888–903. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gao W: Effects of lactobacillus on
glucolipids metabolism and intestinal flora in type 2 diabetic mice
fed with high-glucose and high-fat diet (master's thesis). Shanxi
Normal University; 2018, (In Chinese).
|
|
54
|
Amar J, Chabo C, Waget A, Klopp P, Vachoux
C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S,
et al: Intestinal mucosal adherence and translocation of commensal
bacteria at the early onset of type 2 diabetes: Molecular
mechanisms and probiotic treatment. EMBO Mol Med. 3:559–572. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Imerb N, Thonusin C, Chattipakorn N and
Chattipakorn SC: Aging, obese-insulin resistance, and bone
remodeling. Mech Ageing Dev. 191:1113352020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lau KT, Krishnamoorthy S, Sing CW and
Cheung CL: Metabolomics of osteoporosis in humans: A systematic
review. Curr Osteoporos Rep. 21:278–288. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kitaura H, Marahleh A, Ohori F, Noguchi T,
Shen WR, Qi J, Nara Y, Pramusita A, Kinjo R and Mizoguchi I:
Osteocyte-related cytokines regulate osteoclast formation and bone
resorption. Int J Mol Sci. 21:51692020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li R, Kato H, Nakata T, Yamawaki I,
Yamauchi N, Imai K, Taguchi Y and Umeda M: Essential amino acid
starvation induces cell cycle arrest, autophagy, and inhibits
osteogenic differentiation in murine osteoblast. Biochem Biophys
Res Commun. 672:168–176. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rong Y, Darnell AM, Sapp KM, Vander Heiden
MG and Spencer SL: Cells use multiple mechanisms for cell-cycle
arrest upon withdrawal of individual amino acids. Cell Rep.
42:1135392023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li R, Kato H, Fumimoto C, Nakamura Y,
Yoshimura K, Minagawa E, Omatsu K, Ogata C, Taguchi Y and Umeda M:
Essential amino acid starvation-induced oxidative stress causes DNA
damage and apoptosis in murine osteoblast-like cells. Int J Mol
Sci. 24:153142023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shen L, Yu Y and Karner CM: SLC38A2
provides proline and alanine to regulate postnatal bone mass
accrual in mice. Front Physiol. 13:9926792022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sharma D, Yu Y, Shen L, Zhang GF and
Karner CM: SLC1A5 provides glutamine and asparagine necessary for
bone development in mice. Elife. 10:e715952021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jiménez JA, Lawlor ER and Lyssiotis CA:
Amino acid metabolism in primary bone sarcomas. Front Oncol.
12:10013182022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shen L, Sharma D, Yu Y, Long F and Karner
CM: Biphasic regulation of glutamine consumption by WNT during
osteoblast differentiation. J Cell Sci.
134:jcs2516452021.PubMed/NCBI
|
|
65
|
Nie C, He T, Zhang W, Zhang G and Ma X:
Branched chain amino acids: beyond nutrition metabolism. Int J Mol
Sci. 19:9542018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Brunner JS, Vulliard L, Hofmann M, Kieler
M, Lercher A, Vogel A, Russier M, Brüggenthies JB, Kerndl M,
Saferding V, et al: Environmental arginine controls multinuclear
giant cell metabolism and formation. Nat Commun. 11:4312020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bordbar A, Mo ML, Nakayasu ES,
Schrimpe-Rutledge AC, Kim YM, Metz TO, Jones MB, Frank BC, Smith
RD, Peterson SN, et al: Model-driven multi-omic data analysis
elucidates metabolic immunomodulators of macrophage activation. Mol
Syst Biol. 8:5582012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Onuora S: L-arginine inhibits arthritis
and bone loss by reprogramming osteoclast metabolism. Nat Rev
Rheumatol. 19:7602023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shen Y, Wang H, Xie H, Zhang J, Ma Q, Wang
S, Yuan P, Xue H, Hong H, Fan S, et al: l-arginine promotes
angio-osteogenesis to enhance oxidative stress-inhibited bone
formation by ameliorating mitophagy. J Orthop Translat. 46:53–64.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Stegen S, Moermans K, Stockmans I,
Thienpont B and Carmeliet G: The serine synthesis pathway drives
osteoclast differentiation through epigenetic regulation of NFATc1
expression. Nat Metab. 6:141–152. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhou T, Yang Y, Chen Q and Xie L:
Glutamine metabolism is essential for stemness of bone marrow
mesenchymal stem cells and bone homeostasis. Stem Cells Int.
2019:89289342019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yu Y, Newman H, Shen L, Sharma D, Hu G,
Mirando AJ, Zhang H, Knudsen E, Zhang GF, Hilton MJ and Karner CM:
Glutamine metabolism regulates proliferation and lineage allocation
in skeletal stem cells. Cell Metab. 29:966–978.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gao P, Tchernyshyov I, Chang TC, Lee YS,
Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT and
Dang CV: c-Myc suppression of miR-23a/b enhances mitochondrial
glutaminase expression and glutamine metabolism. Nature.
458:762–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tsumura H, Shindo M, Ito M, Igarashi A,
Takeda K, Matsumoto K, Ohkura T, Miyado K, Sugiyama F, Umezawa A
and Ito Y: Relationships between Slc1a5 and osteoclastogenesis.
Comp Med. 71:285–294. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Indo Y, Takeshita S, Ishii KA, Hoshii T,
Aburatani H, Hirao A and Ikeda K: Metabolic regulation of
osteoclast differentiation and function. J Bone Miner Res.
28:2392–2399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Peng R, Dong Y, Zheng M, Kang H, Wang P,
Zhu M, Song K, Wu W and Li F: IL-17 promotes osteoclast-induced
bone loss by regulating glutamine-dependent energy metabolism. Cell
Death Dis. 15:1112024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM
and Xie C: Osteoblast-osteoclast interactions. Connect Tissue Res.
59:99–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang W, Dang K, Huai Y and Qian A:
Osteoimmunology: The regulatory roles of T lymphocytes in
osteoporosis. Front Endocrinol (Lausanne). 11:4652020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mellor AL and Munn DH: IDO expression by
dendritic cells: Tolerance and tryptophan catabolism. Nat Rev
Immunol. 4:762–774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zara C, Severino A, Flego D, Ruggio A,
Pedicino D, Giglio AF, Trotta F, Lucci C, D'Amario D, Vinci R, et
al: Indoleamine 2,3-Dioxygenase (IDO) enzyme links innate immunity
and altered T-cell differentiation in Non-ST segment elevation
acute coronary syndrome. Int J Mol Sci. 19:632017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Eagle H, Oyama VI, Levy M, Horton CL and
Fleischman R: The growth response of mammalian cells in tissue
culture to L-glutamine and L-glutamic acid. J Biol Chem.
218:607–616. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Colombo SL, Palacios-Callender M, Frakich
N, Carcamo S, Kovacs I, Tudzarova S and Moncada S: Molecular basis
for the differential use of glucose and glutamine in cell
proliferation as revealed by synchronized HeLa cells. Proc Natl
Acad Sci USA. 108:21069–21074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ahn E, Kumar P, Mukha D, Tzur A and Shlomi
T: Temporal fluxomics reveals oscillations in TCA cycle flux
throughout the mammalian cell cycle. Mol Syst Biol. 13:9532017.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Malakar P, Singha D, Choudhury D and
Shukla S: Glutamine regulates the cellular proliferation and cell
cycle progression by modulating the mTOR mediated protein levels of
β-TrCP. Cell Cycle. 22:1937–1950. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Minchenko DO, Hubenya OV, Terletsky BM,
Moenner M and Minchenko OH: Effect of glutamine or glucose
deprivation on the expression of cyclin and cyclin-dependent kinase
genes in glioma cell line U87 and its subline with suppressed
activity of signaling enzyme of endoplasmic reticulum-nuclei-1. Ukr
Biokhim Zh (1999). 83:18–29. 2011.PubMed/NCBI
|
|
86
|
Yuan L, Sheng X, Willson AK, Roque DR,
Stine JE, Guo H, Jones HM, Zhou C and Bae-Jump VL: Glutamine
promotes ovarian cancer cell proliferation through the mTOR/S6
pathway. Endocr Relat Cancer. 22:577–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kim B, Li J, Jang C and Arany Z: Glutamine
fuels proliferation but not migration of endothelial cells. EMBO J.
36:2321–2333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen Q, Shou P, Zheng C, Jiang M, Cao G,
Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al: Fate decision of
mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death
Differ. 23:1128–1139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ning K, Liu S, Yang B, Wang R, Man G, Wang
DE and Xu H: Update on the effects of energy metabolism in bone
marrow mesenchymal stem cells differentiation. Mol Metab.
58:1014502022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Skerry TM: The role of glutamate in the
regulation of bone mass and architecture. J Musculoskelet Neuronal
Interact. 8:166–173. 2008.PubMed/NCBI
|
|
91
|
Wang Y, Deng P, Liu Y, Wu Y, Chen Y, Guo
Y, Zhang S, Zheng X, Zhou L, Liu W, et al: Alpha-ketoglutarate
ameliorates age-related osteoporosis via regulating histone
methylations. Nat Commun. 11:55962020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fan M, Shi H, Yao H, Wang W, Zhang Y,
Jiang C and Lin R: Glutamate regulates gliosis of BMSCs to promote
ENS regeneration through α-KG and H3K9/H3K27 demethylation. Stem
Cell Res Ther. 13:2552022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Qian D, Wei G, Xu C, He Z, Hua J, Li J, Hu
Q, Lin S, Gong J, Meng H, et al: Bone marrow-derived mesenchymal
stem cells (BMSCs) repair acute necrotized pancreatitis by
secreting microRNA-9 to target the NF-κB1/p50 gene in rats. Sci
Rep. 7:5812017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ganesan R and Rasool M: Interleukin 17
regulates SHP-2 and IL-17RA/STAT-3 dependent Cyr61, IL-23 and
GM-CSF expression and RANKL mediated osteoclastogenesis by
fibroblast-like synoviocytes in rheumatoid arthritis. Mol Immunol.
91:134–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Saraiva M, Vieira P and O'Garra A: Biology
and therapeutic potential of interleukin-10. J Exp Med.
217:e201904182020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Mielle J, Morel J, Elhmioui J, Combe B,
Macia L, Dardalhon V, Taylor N, Audo R and Daien C: Glutamine
promotes the generation of B10+ cells via the mTOR/GSK3 pathway.
Eur J Immunol. 52:418–430. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu JQ, Geng XR, Hu TY, Mo LH, Luo XQ, Qiu
SY, Liu DB, Liu ZG, Shao JB, Liu ZQ and Yang PC: Glutaminolysis is
required in maintaining immune regulatory functions in B cells.
Mucosal Immunol. 15:268–278. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Coëffier M, Marion R, Ducrotté P and
Déchelotte P: Modulating effect of glutamine on IL-1beta-induced
cytokine production by human gut. Clin Nutr. 22:407–413. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Santos AC, Correia CA, de Oliveira DC,
Nogueira-Pedro A, Borelli P and Fock RA: Intravenous glutamine
administration modulates TNF-α/IL-10 ratio and attenuates NFkB
phosphorylation in a protein malnutrition model. Inflammation.
39:1883–1891. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
da Silva Lima F, Rogero MM, Ramos MC,
Borelli P and Fock RA: Modulation of the nuclear factor-kappa B
(NF-κB) signalling pathway by glutamine in peritoneal macrophages
of a murine model of protein malnutrition. Eur J Nutr.
52:1343–1351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sun Y, Ma J, Li D, Li P, Zhou X, Li Y, He
Z, Qin L, Liang L and Luo X: Interleukin-10 inhibits interleukin-1β
production and inflammasome activation of microglia in epileptic
seizures. J Neuroinflammation. 16:662019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Levy DE and Lee CK: What does Stat3 do? J
Clin Invest. 109:1143–1148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Dos Santos GG, Hastreiter AA, Sartori T,
Borelli P and Fock RA: L-Glutamine in vitro modulates some
immunomodulatory properties of bone marrow mesenchymal stem cells.
Stem Cell Rev Rep. 13:482–490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Refaey ME, McGee-Lawrence ME, Fulzele S,
Kennedy EJ, Bollag WB, Elsalanty M, Zhong Q, Ding KH, Bendzunas NG,
Shi XM, et al: Kynurenine, a tryptophan metabolite that accumulates
with age, induces bone loss. J Bone Miner Res. 32:2182–2193. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dalton S, Smith K, Singh K, Kaiser H,
Kolhe R, Mondal AK, Khayrullin A, Isales CM, Hamrick MW, Hill WD
and Fulzele S: Accumulation of kynurenine elevates oxidative stress
and alters microRNA profile in human bone marrow stromal cells. Exp
Gerontol. 130:1108002020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sas K, Szabó E and Vécsei L: Mitochondria,
oxidative stress and the kynurenine system, with a focus on ageing
and neuroprotection. Molecules. 23:1912018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Elmansi AM, Hussein KA, Herrero SM,
Periyasamy-Thandavan S, Aguilar-Pérez A, Kondrikova G, Kondrikov D,
Eisa NH, Pierce JL, Kaiser H, et al: Age-related increase of
kynurenine enhances miR29b-1-5p to decrease both CXCL12 signaling
and the epigenetic enzyme Hdac3 in bone marrow stromal cells. Bone
Rep. 12:1002702020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kondrikov D, Elmansi A, Bragg RT, Mobley
T, Barrett T, Eisa N, Kondrikova G, Schoeinlein P, Aguilar-Perez A,
Shi XM, et al: Kynurenine inhibits autophagy and promotes
senescence in aged bone marrow mesenchymal stem cells through the
aryl hydrocarbon receptor pathway. Exp Gerontol. 130:1108052020.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Anaya JM, Bollag WB, Hamrick MW and Isales
CM: The role of tryptophan metabolites in musculoskeletal stem cell
aging. Int J Mol Sci. 21:66702020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sautchuk R Jr and Eliseev RA: Cell energy
metabolism and bone formation. Bone Rep. 16:1015942022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li S, Tian Q, Zheng L and Zhou Y:
Functional amino acids in the regulation of bone and its diseases.
Mol Nutr Food Res. 68:e24000942024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ledesma-Colunga MG, Passin V, Lademann F,
Hofbauer LC and Rauner M: Novel insights into osteoclast energy
metabolism. Curr Osteoporos Rep. 21:660–669. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Carbone L, Bůžková P, Fink HA, Robbins JA,
Barzilay JI, Elam RE, Isales C, Connelly MA and Mukamal KJ: Plasma
levels of branched chain amino acids, incident hip fractures, and
bone mineral density of the hip and spine. J Clin Endocrinol Metab.
108:e1358–e1364. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Su Y, Elshorbagy A, Turner C, Refsum H,
Chan R and Kwok T: Circulating amino acids are associated with bone
mineral density decline and ten-year major osteoporotic fracture
risk in older community-dwelling adults. Bone. 129:1150822019.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liang B, Shi X, Wang X, Ma C, Leslie WD,
Lix LM, Shi X, Kan B and Yang S: Association between amino acids
and recent osteoporotic fracture: A matched incident case-control
study. Front Nutr. 11:13609592024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang YY, Xie N, Sun XD, Nice EC, Liou YC,
Huang C, Zhu H and Shen Z: Insights and implications of sexual
dimorphism in osteoporosis. Bone Res. 12:82024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Guan Z, Luo L, Liu S, Guan Z, Zhang Q, Li
X and Tao K: The role of depletion of gut microbiota in
osteoporosis and osteoarthritis: A narrative review. Front
Endocrinol (Lausanne). 13:8474012022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hao L, Yan Y, Huang G and Li H: From gut
to bone: deciphering the impact of gut microbiota on osteoporosis
pathogenesis and management. Front Cell Infect Microbiol.
14:14167392024. View Article : Google Scholar : PubMed/NCBI
|