Chimeric antigen receptor (CAR) T cell therapy
serves an important role in cancer treatment (1) and CAR T cells have been approved for
treating hematological malignancy (2). CAR T cells may cause lethal toxicity
through several mechanisms (3–5) and
cytokine release syndrome (CRS) is the most common toxic effect
(6). Its symptoms often mimic
those of influenza, including fever, headache, malaise, myalgia,
rigor and anorexia (1). However,
some severe cases progress to life-threatening symptoms, such as
hypotension, hypoxia, pulmonary edema, pleural effusion and
tachycardia (7). CAR T
cell-associated hemophagocytic lymphohistiocytosis (CARHLH), which
carries a high risk of lethality, shares similar clinical
manifestations with CRS, such as fever, multiorgan dysfunction,
hyperferritinemia and hypercytokinemia (8,9).
A retrospective analysis by the European Society for
Blood and Marrow Transplantation (EBMT) included 201 patients
receiving CAR-T therapy from 114 centers across 24 countries, with
a CARHLH incidence rate of 3.48% between 2016 and 2018 (10). A retrospective study of 59 patients
with relapsed/refractory B cell acute lymphoblastic
leukemia/lymphoblastic lymphoma treated with CD22 CAR T cells
reported that 52 patients (88.1%) developed CRS, of whom 21 (40.4%)
developed CARHLH (all following CRS onset), including six of 12
patients with severe (grade3/4) CRS (11). Another study on 27 patients with
relapsed and/or refractory CD19-positive acute lymphoblastic
leukemia treated with CD19 CAR T cells reported that 11 (40%) had
CRS and four (14.8%) had CRS and CARHLH (12); all patients with CARHLH either
concurrently or previously experienced CRS. Typically, CRS resolves
or is fully resolved prior to the onset of CARHLH (11,13).
The progression of CRS and CARHLH is connected, making it
challenging to distinguish between these (11,13).
However, previous studies have indicated that the
pathogenesis, treatment and prognosis of these diseases differ
(11,13,14).
Patients suspected of having CARHLH should be diagnosed as soon as
possible and promptly receive effective treatment to control
disease progression and improve outcomes (1,5,10,11,15–18).
Therefore, early identification and prompt treatment are essential
for improving patient prognosis.
Genetic or acquired immunoregulatory defects
imbalance the regulatory pathways responsible for the natural
termination of immune or inflammatory responses, leading to
sustained and excessive activation of T and antigen-presenting
cells. Abnormal NK cell function is also observed in patients with
HLH (19). NK cells regulate the
initial responses of antigen-presenting cells to pathogens and
attenuate signals communicated by antigen-driven T cells. NK cells
also serve a role in the contraction of immune responses by culling
activated T cells and histiocytes during the later stages of
antigen-driven activation (26).
The abnormal function of CTLs and NK cells may lead to continuous
activation and proliferation of macrophages triggered by antigens.
Activated macrophages persistently proliferate and release
cytokines that promote the recruitment and expansion of lymphocytes
and other inflammatory cells. This can lead to organ damage and
systemic manifestations of hypercytokinemia. In addition, activated
macrophages non-selectively phagocytose hematopoietic components,
resulting in observable hemophagocytosis in the bone marrow, lymph
nodes and spleen (27).
HLH is a rare condition: The incidence of HLH
(including pHLH and sHLH) in individuals aged <18 years in the
United States is 1/100,000 (28).
In Sweden, the incidence of familial HLH (fHLH) in children is
1.2/1,000,000 (20), whereas that
of sHLH in children is 0.19/100,000 (29). HLH is a life-threatening disease. A
Swedish study of 32 children with fHLH, with a median survival of
only 2–3 months (20). However,
mortality rates differ between subtypes, with malignancy-associated
HLH having the worst prognosis (17,18).
The 2-month survival rate for malignancy-associated HLH was 52%
overall from 1997 to 2018 in Sweden (29).
CAR T cell therapy is based on the isolation of T
cells from peripheral blood, which are genetically engineered to
express CARs. These cells are expanded in vitro and then
re-infused into patients with cancer. CAR T cells undergo
non-specific expansion in vivo and simulate immune responses
against cancer cells (30). This
modification enables the modified T cells to recognize and attack
tumor cells independently of major histocompatibility complex (MHC)
engagement (2,31–33).
CAR T cell therapy targets tumor cells independently of antigen
processing and presentation, making them resistant to tumor escape
mechanisms associated with MHC loss. Furthermore, this approach can
effectively recognize antigens such as glycolipids, proteins with
abnormal glycosylation and conformational epitopes (34,35).
CAR T cell therapy has notable benefits in the treatment of various
malignancies (33,36–38).
The response rates of patients with relapsed or refractory B
non-Hodgkin's lymphoma is 52–82% (38,39).
In patients with relapsed or refractory B cell ALL, the rate of
complete remission is 90%, with a 2-year overall survival rate of
73% and an event-free survival rate of 49.5% (40). The objective and complete remission
rates in patients with relapsed or refractory multiple myeloma are
85 and 45%, respectively. The median progression-free survival is
11.8 months (41).
However, CAR T cells may damage normal tissue by
cross-reacting with antigens that are not expressed in tumor cells
(3,4). CAR-targeted tumor-associated antigens
may be expressed in normal tissue, leading to CAR T cell-mediated
damage to normal tissue cells (5).
CAR T cells can cause complications such as CRS and neurotoxicity.
Additionally, both the malignancies and the chemotherapy that
patients may receive before CAR T cell infusion can impair immune
function, thereby increasing the risk of infection (42). CAR T cell therapy has the potential
to trigger life-threatening toxicity, such as CARHLH (5).
CRS is the most frequent adverse effect associated
with CAR T cell therapy. A meta-analysis of 2,592 patients from 84
studies who received CAR T cell therapy reported that the all-grade
CRS rate is 77%, while the grade ≥3 CRS rate is 29% (43). This is a systemic inflammatory
response triggered by the release of pro-inflammatory cytokines
from activated CAR T, ruptured cancer and other immune cells. The
interplay between CAR T and cancer cells triggers the activation of
immune cells, such as macrophages and endothelial cells, which
amplifies the production of cytokines (such as IL-6, IFN-γ, IL-8
and IL-10), leading to a cytokine storm (44).
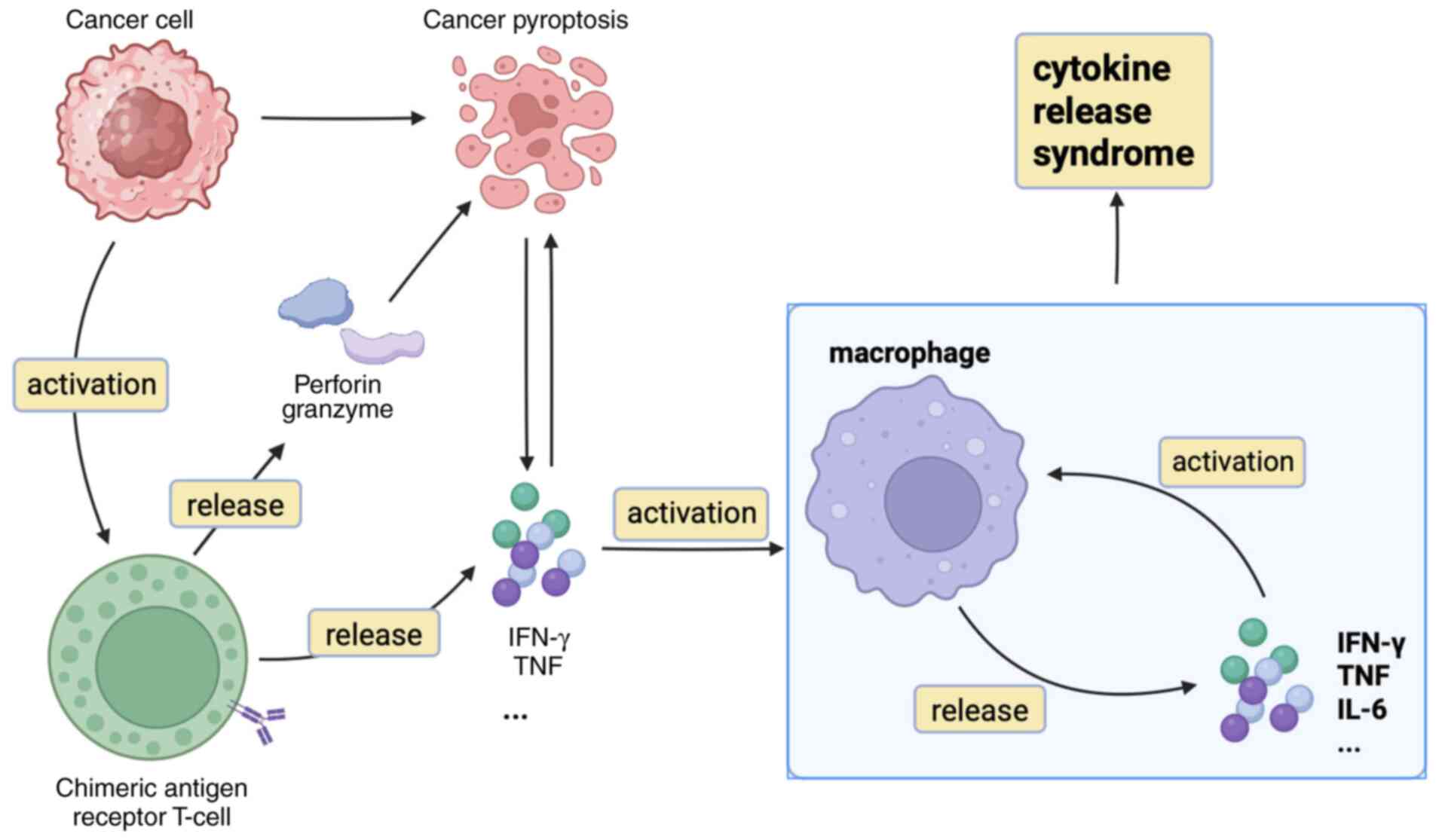
Furthermore, cytokines, perforin and granzyme from
CAR T cells induce pyroptosis in tumor cells. Pyroptosis is a form
of programmed cell death distinct from apoptosis, marked by cell
swelling, lysis and the release of cellular contents and
proinflammatory factors (Fig. 1)
(45). The majority of CRS cases
develop within the first 14 days of infusion and persist for 7 or 8
days (7,39,46,47).
In most cases, CRS resolves naturally without intervention, and
patients typically require only symptomatic care (1,5,7,15,16,48,49).
The initial symptoms include fever, headache, malaise, myalgias,
and rigors (1). However, some
severe cases progress to life-threatening symptoms, such as
hypotension, hypoxia, pulmonary edema, pleural effusion and
tachycardia (7). These symptoms
may lead to multiorgan failure and require intensive care
management (1,7). A meta-analysis by Lei et al
(43) reported a CRS mortality
rate <1%.
To the best of our knowledge, there is a lack of
large studies investigating CARHLH (19). CARHLH is rapidly becoming a fatal
condition. In a clinical study (56), 100 patients with relapsed or
refractory large B cell lymphoma received CAR T cell therapy.
CARHLH occurred in five cases, of which two were treated with
anakinra (a recombinant human IL-1 receptor antagonist), etoposide
and corticosteroids but succumbed to CARHLH on days 15 and 71 of
treatment (56). A multicenter
clinical trial (38) of 101
patients treated with CD19 CAR T cell therapy observed that 94
patients (93%) developed CRS, and one patient developed CRS
progressed and succumbed to CARHLH. By contrast, the CRS
manifestations in the other patients resolved after treatment, with
one patient succumbing to cardiac arrest (38). A retrospective review (57) of 105 patients with relapsed or
refractory large B cell lymphoma who received CAR T cell therapy
reported that six patients (5.7%) developed CARHLH. The
progression-free and overall survival rates of patients who
developed CARHLH were markedly worse than those of patients without
HLH (57). Hines et al
(12) conducted a cohort study of
27 patients with relapsed or refractory CD19 acute lymphoblastic
leukemia. Following CAR T cell therapy, four patients (14.8%)
developed CARHLH, one succumbed to CARHLH and three succumbed to
leukemia, with a median survival of 44.5 days. The aforementioned
study revealed that patients who developed CARHLH experienced worse
outcomes, a greater incidence of non-response to CAR T cell therapy
and markedly decreased overall survival (12).
Although CARHLH and CRS have similar clinical
manifestations, there are differences in their pathogenesis.
Patients with CARHLH have more severe and persistent clinical
manifestations; therefore, early recognition and rational treatment
are key (11).
Diagnosis of CRS is primarily based on clinical
symptoms. The American Society for Transplantation and Cellular
Therapy (ASTCT) defines CRS following immunotherapy as ‘a
supraphysiologic response following any immune therapy that results
in the activation or engagement of endogenous or infused T cells
and/or other immune effector cells. Symptoms can be progressive,
must include fever at the onset, and may include hypotension,
capillary leak (hypoxia) and end organ dysfunction’ (58). CRS should be suspected if ≥1 of the
following four manifestations occurs within 3 weeks of CAR T cell
infusion: Fever (temperature ≥38°C), hypotension (systolic blood
pressure <90 mmHg), hypoxia (arterial oxygen saturation <90%)
or organ toxicity (48).
Laboratory tests are key for identifying other diseases, such as
sepsis and tumor lysis syndrome, because the clinical
manifestations of CRS are non-specific. If CRS is suspected, a
thorough evaluation of the patient should be conducted, and
treatment should be initiated accordingly (1). The ASTCT consensus provides grading
criteria for CRS based on clinical manifestation (Table I) (58).
To the best of our knowledge, there are currently no
standardized diagnostic criteria for CARHLH, although both the
guidelines released by the ASTCT in 2023 and the previous HLH
diagnostic criteria are used in conjunction with the clinical
manifestations of patients to diagnose CARHLH (50,59,60).
The diagnosis should be accompanied by close clinical observation
and repeated laboratory tests. The HLH-2004 criteria, H-score,
Takagi criteria and MD Anderson criteria are used to help diagnose
CARHLH (Table II) (9,10,48,61–63).
The 2023 ASTCT expert recommendation on CARHLH/IEC-HS use the term
‘immune effector cell-associated HLH-like syndrome (IEC-HS)’ to
describe CARHLH and describe the definition of IEC-HS. The
guideline recommends a diagnosis of CARHLH based on clinical
manifestations, with a considerable elevation in serum ferritin
levels being the primary criterion for diagnosis (Table II) (9). ASTCT proposed a grading schema for
this condition (Table III). The
MD Anderson criteria (10) are the
diagnostic standards for CARHLH. However, their clinical
application has not yet become widespread (10). The HLH-2004 criteria are currently
the accepted diagnostic protocol for HLH and are used to diagnose
CARHLH (10,61). The H-score identifies patients with
HLH by assigning points based on clinical manifestation (62), whereas the Takagi criteria diagnose
HLH based on a combination of pathological and clinical
characteristics (63). The
HLH-2004 protocol, H-score and Takagi criteria were not developed
specifically for CARHLH, and therefore have limitations in
diagnosing CARHLH. A study (14)
of 20 adult and 16 pediatric HLH patients from Belgium. The study
found that the H-score demonstrated better sensitivity and
specificity than the HLH-2004 protocol in the early diagnosis of
the disease (14). For diagnosing
HLH in adults, the H-score is more effective than the traditional
HLH-2004 criteria. However, the sensitivity and specificity of the
H-score decrease in the diagnosis of deteriorating diseases
(14).
Early recognition of CARHLH in patients with CRS and
the timely initiation of effective treatment are critical for
patient prognosis. The ASTCT expert recommendations state that
patients with HLH-like toxicities experience either a preceding or
concurrent CRS and suggest the use of markedly elevated ferritin
levels as the primary criterion for diagnosing CARHLH (9). Ferritin is the most frequently used
laboratory test to distinguish CRS and CARHLH; however, the
threshold value is not yet standardized (9,64).
Elevated ferritin levels may indicate CARHLH; however, low ferritin
levels have a negative predictive value (10). Zu et al (44) retrospectively studied 99 patients
treated with B cell maturation antigen (BCMA). Serum parameter
monitoring revealed elevated ferritin, aspartate aminotransferase
and lactate dehydrogenase (LDH) levels in patients with CARHLH and
CRS, as well as a marked peak difference between the two groups;
elevated parameters in patients with CARHLH persisted for an
extended duration (44). Similar
patterns were observed during monitoring of cytokines. Compared
with patients with CRS, the levels of IFN-γ, granzyme B, IL-1a,
IL-1b, IL-10, IL-12, IL-33 and chemokine are markedly increased in
patients with CARHLH (11,44).
According to MD Anderson Cancer Center, patients
should be hospitalized and closely monitored for ≥7 days following
CAR T cell infusion (48). The
treatment protocol should be formulated based on the overall
clinical condition of the patient (15). Management of ASTCT grade 1 CRS
primarily involves supportive measures including rehydration,
antipyretics, oxygenation and elevation of blood pressure. The
option of anti-cytokine treatment may be evaluated in patients with
moderate-to-severe CRS (15,48,58).
IL-6 is a key factor in the pathogenesis of CRS, and its expression
is associated with the severity of CRS following CAR T cell
therapy. Furthermore, anti-IL-6 therapy does not affect the
expansion or efficacy of CAR T cells (38,48,65,66).
Consequently, anti-IL-6 is the preferred treatment option for
patients with severe CRS (48,66).
Numerous case reports demonstrate the efficacy of tocilizumab (an
IL-6 monoclonal antibody) in managing CAR T cell-associated CRS
(50,67,68).
The Society for Immunotherapy of Cancer (SITC) and American Society
of Clinical Oncology (ASCO) (69,70)
recommend the use of anti-cytokine therapy and corticosteroids in
CRS (Table IV). Compared with
SITC, ASCO is more proactive in the use of anti-cytokine therapy
and corticosteroids (69,70).
The current first-line treatment regimen for HLH
consists of etoposide, cyclosporine A and dexamethasone, as well as
intrathecal methotrexate and dexamethasone and is indicated for
most types of HLH. Due to the toxic side effects of chemotherapy,
maximum supportive treatment should be provided during the
treatment period. Bone marrow transplantation is the sole curative
option for treating pHLH, malignancy-associated HLH and
Epstein-Barr virus-related HLH (19). Disease remission prior to
transplantation is key for patient prognosis (71). Biological agent-targeted therapies
are a promising treatment option: In a clinical study of 34
patients with pHLH (27 treated and seven primary), 8-week treatment
with emapalumab (an IFN-γ monoclonal antibody) had effective rates
of 63 and 65%, respectively (72).
Ge et al (73) included 21
patients with pHLH (12 treated and nine primary) using a
ruxolitinib (a JAK inhibitor)-based regimen, with a 1-year overall
survival rate of 90.5%; all patients achieved objective remission
within the first 8 weeks, with 19 (90.5%) achieving complete
remission.
The treatment of CARHLH has not yet been
standardized. The ASTCT CRS guidelines published in 2019 (58) suggest that there is no need to
distinguish CARHLH from CRS. However, there is an increasing
consensus that the early identification of CARHLH and prompt
treatment are essential for improving patient outcomes (58,69,70).
A case report described two patients with CARHLH: A patient who
received timely diagnosis and treatment experienced improvement in
CARHLH after therapy. By contrast, a patient who did not receive
prompt medical attention, quickly succumbed from multi-organ
failure (68). The MD Anderson
Cancer Center recommends patients with suspected CARHLH initially
be considered as having CRS and should be treated with
corticosteroids and anti-IL-6 therapy (48).
A retrospective study by the EBMT reported that the
most frequently used combination regimen for the treatment of
CARHLH is corticosteroids combined with chemotherapy, followed by
corticosteroids combined with chemotherapy and monoclonal
antibodies or anti-cytokine drugs and other treatment (10). A retrospective clinical study
analyzed the clinical data of patients with hematological
malignancy who received CD22 CAR T cell therapy and reported that
during the active phase of CARHLH, HLH-related cytokine levels are
persistently elevated, including IFN-γ, IL-1 and IL-6(11). These cytokines are key contributors
to disease progression (11).
Rejeski (74) proposed that
patients with CARHLH be treated with anakinra in combination with
high-dose corticosteroids. Refractory patients can be treated with
ruxolitinib and emapalumab therapy (74). Porter et al (64) reported that three patients with
CD19 CARHLH demonstrated gradual improvement in laboratory
manifestations after treatment with anakinra and/or ruxolitinib.
Repeat PET-CT scans in two patients revealed complete remission,
but relapse or progression of the disease was observed (64). A clinical study analyzed data from
58 patients who received CD22+ CAR T cell therapy: CRS
was observed in 50 patients and HLH-like toxicity was identified in
19 patients (38%). Notably, the toxic manifestations improved in
all patients following administration of anakinra or the addition
of corticosteroids (13). In one
patient, all abnormal laboratory parameters returned to normal
following 1 month of treatment with anakinra (13).
SITC guidelines suggest that tocilizumab treatment
should be promptly initiated in patients with suspectedCARHLH.
Anakinra and corticosteroids may be considered for
tocilizumab-refractory HLH/MAS-like toxicity (69). Corticosteroids have side effects,
including hypertension, metabolic derangement, fractures and
increased risk of infection (75).
According to the ASCO guidelines (70), corticosteroids and IL-6 antagonist
therapy should be used to manage CARHLH with grade ≥3 organ
toxicity. Anakinra is administered to patients with refractory
CARHLH (74). Tocilizumab
demonstrates limited therapeutic effectiveness in adult patients
with sHLH and may elevate the risk of infection-related
complications when compared with conventional treatment regimens
(76). ASTCT expert
recommendations for CARHLH suggest that anakinra and/or
corticosteroids are first-line agents (Table V). Anakinra is an IL-1 receptor
antagonist; clinical trials of anakinra for sHLH have demonstrated
benefit without compromising the efficacy of CAR T cells (77–79).
Common toxicities associated with anakinra include infection,
headaches and fever. For patients needing further intervention to
manage CARHLH, particularly patients with worsening inflammatory
markers or signs of progressive end-organ damage, second-line
treatments such as ruxolitinib may be considered as a therapeutic
option (80). However, ruxolitinib
may increase the risk of infection and exacerbate cytopenia and
there is still a lack of research and clinical experience (80). Etoposide and emapalumab may serve
as alternative treatment options for patients with life-threatening
refractory CARHLH (9). Etoposide
is the preferred T cell-depleting agent owing to the large amount
of data available in both pHLH and sHLH (81–84).
The side effects of etoposide include dose-limiting
myelosuppression, particularly neutropenia, which may increase the
risk of infection (85). Although
IFN-γ serves a key role in the development of CARHLH, research on
anti-IFN therapy for the treatment of CARHLH is limited (11,86,87).
Antagonizing IFN-γ may not affect the efficacy of CAR T cells
(86). The application of
emapalumab for treating adults with CARHLH is largely speculative.
Although emapalumab has been approved by the US Food and Drug
Administration for the treatment of pHLH and yields good results,
there is debate regarding its efficacy and safety in the treatment
of CARHLH (88,89). Currently, targeted therapy is the
primary treatment approach for CARHLH. The pathophysiological
mechanisms underlying CARHLH are under investigation, and future
research may uncover novel mechanisms, facilitating development of
targeted therapies based on new molecular targets (19,90).
Owing to limited literature on the management of CARHLH and a lack
of prospective clinical trials for CARHLH, there is no standardized
and unified treatment for CARHLH. However, current guidelines and
expert consensus suggest that anti-cytokine therapy serves an
important role in the treatment of CARHLH.
CARHLH and CRS exhibit distinct pathogenic
mechanisms, although their clinical manifestations are overlapping,
with all patients with CARHLH presenting concurrent or prior CRS
(8,9,11).
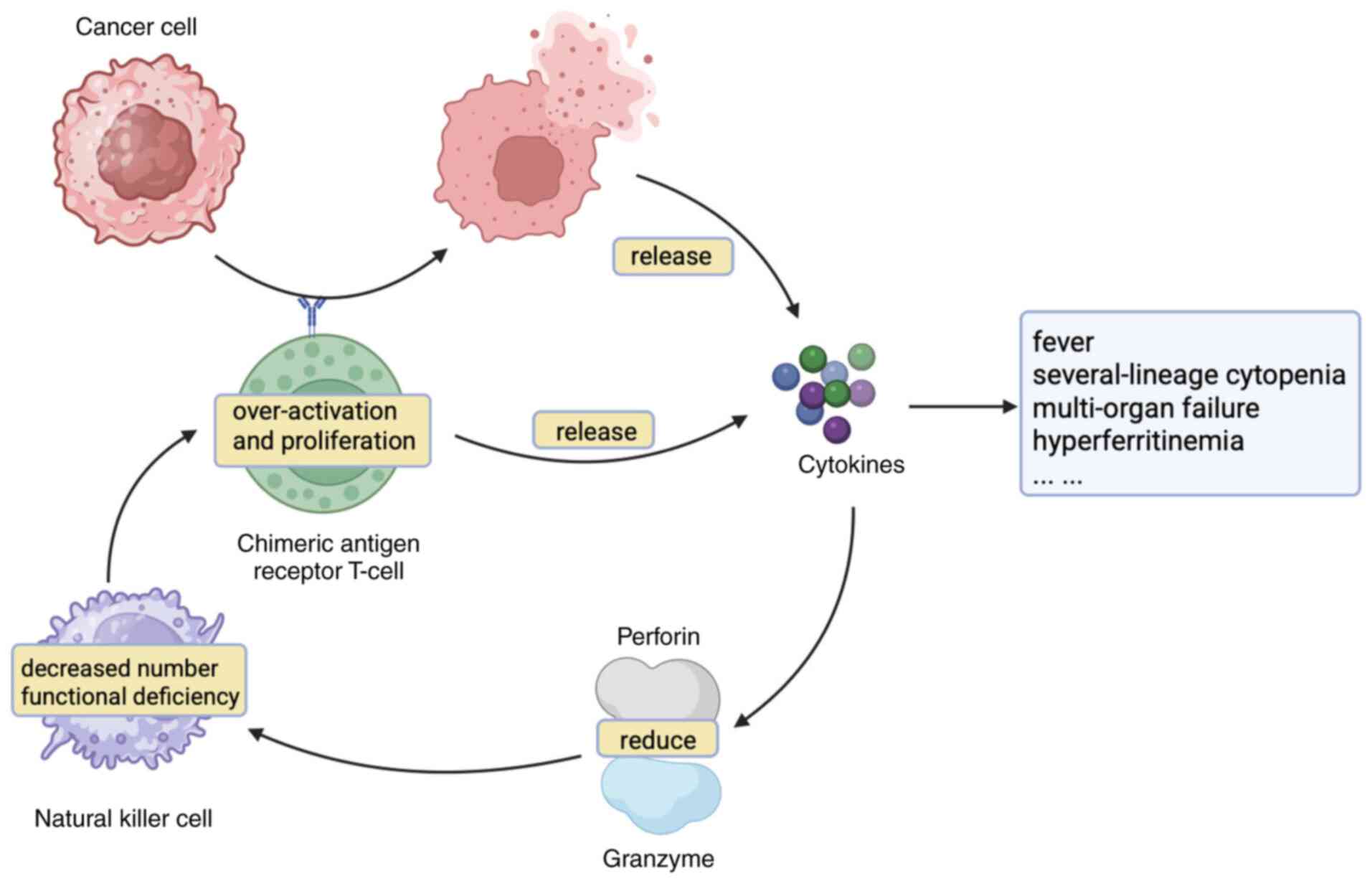
The pathogenesis of CARHLH is still being explored. Excessive CAR T
cell expansion, NK cell decrease and delayed T cell contraction are
the potential mechanisms of action (11,51–53).
There are no standardized diagnostic or treatment criteria for
CARHLH. ASCO guidelines recommend tocilizumab (an IL-6 monoclonal
antibody) for CRS treatment (58).
The timing of targeted therapy is determined by the CRS grade
(58). During the diagnostic
workup for CARHLH, it is key to differentiate whether sHLH is
driven by CAR T therapy (59).
Regular monitoring of CAR T cell expansion dynamics can aid this
distinction (59). Patients at a
high risk of HLH can be identified early using indicators such as
ferritin and HLH-associated cytokine levels (9). Patients with suspected CARHLH should
receive CARHLH treatment as soon as possible (9). Currently, there is no unified
therapeutic regimen for CARHLH. The 2023 ASTCT expert consensus
recommends anakinra and corticosteroids as first-line agents, while
ruxolitinib, etoposide and emapalumab may serve as alternative
therapies for refractory cases (58). Corticosteroids, anti-IL-1 and
anti-IL-6 therapies are the most frequently used, followed by
etoposide (12,50,59,67,68,91–93).
Given the abnormal CAR T cell proliferation during CARHLH
progression, T cell-targeted therapy could be considered in rapidly
progressing or life-threatening scenarios when first- and
second-line treatments fail. However, research and clinical
experience remain scarce, necessitating further investigation.
In conclusion, the specific pathogenesis of CARHLH
is unclear, and the diagnostic and treatment criteria need to be
improved and standardized. CARHLH is a rare disease, and the
mechanisms of its occurrence and development are not fully
understood. Larger multicenter studies need to be conducted or
insights should be extrapolated from other sHLH cases to develop
diagnosis, treatment and prediction models and systematic
understanding of CARHLH.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 82170122).
Not applicable.
JH, CF, LH and YW wrote the manuscript. CF, LH and
YW revised the manuscript. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Shimabukuro-Vornhagen A, Böll B,
Schellongowski P, Valade S, Metaxa V, Azoulay E and von
Bergwelt-Baildon M: Critical care management of chimeric antigen
receptor T-cell therapy recipients. CA Cancer J Clin. 72:78–93.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin H, Cheng J, Mu W, Zhou J and Zhu L:
Advances in universal CAR-T cell therapy. Front Immunol.
12:7448232021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cameron BJ, Gerry AB, Dukes J, Harper JV,
Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, et al:
Identification of a Titin-derived HLA-A1-presented peptide as a
cross-reactive target for engineered MAGE A3-directed T cells. Sci
Transl Med. 5:197ra1032013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgan RA, Yang JC, Kitano M, Dudley ME,
Laurencot CM and Rosenberg SA: Case report of a serious adverse
event following the administration of T cells transduced with a
chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brudno JN and Kochenderfer JN: Toxicities
of chimeric antigen receptor T cells: Recognition and management.
Blood. 127:3321–3330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Owusu KA, Schiffer M and Perreault S:
Chimeric antigen receptor T cells: Toxicity and management
considerations. AACN Adv Crit Care. 33:301–307. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freyer CW and Porter DL: Cytokine release
syndrome and neurotoxicity following CAR T-cell therapy for
hematologic malignancies. J Allergy Clin Immunol. 146:940–948.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Major A, Collins J, Craney C, Heitman AK,
Bauer E, Zerante E, Stock W, Bishop MR and Jasielec J: Management
of hemophagocytic lymphohistiocytosis (HLH) associated with
chimeric antigen receptor T-cell (CAR-T) therapy using
anti-cytokine therapy: An illustrative case and review of the
literature. Leuk Lymphoma. 62:1765–1769. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hines MR, Knight TE, McNerney KO, Leick
MB, Jain T, Ahmed S, Frigault MJ, Hill JA, Jain MD, Johnson WT, et
al: Immune effector cell-associated hemophagocytic
lymphohistiocytosis-like syndrome. Transplant Cell Ther.
29:438.e1–438.e16. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sandler RD, Tattersall RS, Schoemans H,
Greco R, Badoglio M, Labopin M, Alexander T, Kirgizov K, Rovira M,
Saif M, et al: Diagnosis and management of secondary HLH/MAS
following HSCT and CAR-T cell therapy in adults; A review of the
literature and a survey of practice within EBMT centres on behalf
of the autoimmune diseases working party (ADWP) and transplant
complications working party (TCWP). Front Immunol. 11:5242020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lichtenstein DA, Schischlik F, Shao L,
Steinberg SM, Yates B, Wang HW, Wang Y, Inglefield J, Dulau-Florea
A, Ceppi F, et al: Characterization of HLH-like manifestations as a
CRS variant in patients receiving CD22 CAR T cells. Blood.
138:2469–2484. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hines MR, Keenan C, Maron Alfaro G, Cheng
C, Zhou Y, Sharma A, Hurley C, Nichols KE, Gottschalk S, Triplett
BM and Talleur AC: Hemophagocytic lymphohistiocytosis-like toxicity
(carHLH) after CD19-specific CAR T-cell therapy. Br J Haematol.
194:701–707. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shah NN, Highfill SL, Shalabi H, Yates B,
Jin J, Wolters PL, Ombrello A, Steinberg SM, Martin S, Delbrook C,
et al: CD4/CD8 T-cell selection affects chimeric antigen receptor
(CAR) T-cell potency and toxicity: updated results from a phase I
anti-CD22 CAR T-cell trial. J Clin Oncol. 38:1938–1950. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Debaugnies F, Mahadeb B, Ferster A,
Meuleman N, Rozen L, Demulder A and Corazza F: Performances of the
H-score for diagnosis of hemophagocytic lymphohistiocytosis in
adult and pediatric patients. Am J Clin Pathol. 145:862–870. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schubert ML, Schmitt M, Wang L, Ramos CA,
Jordan K, Müller-Tidow C and Dreger P: Side-effect management of
chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol.
32:34–48. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayden PJ, Roddie C, Bader P, Basak GW,
Bonig H, Bonini C, Chabannon C, Ciceri F, Corbacioglu S, Ellard R,
et al: Management of adults and children receiving CAR T-cell
therapy: 2021 Best practice recommendations of the European society
for blood and marrow transplantation (EBMT) and the joint
accreditation committee of ISCT and EBMT (JACIE) and the European
haematology association (EHA). Ann Oncol. 33:259–275. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ponnatt TS, Lilley CM and Mirza KM:
Hemophagocytic lymphohistiocytosis. Arch Pathol Lab Med.
146:507–519. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schram AM, Comstock P, Campo M, Gorovets
D, Mullally A, Bodio K, Arnason J and Berliner N: Haemophagocytic
lymphohistiocytosis in adults: A multicentre case series over 7
years. Br J Haematol. 172:412–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Henter JI: Hemophagocytic
lymphohistiocytosis. N Engl J Med. 392:584–598. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Henter JI, Elinder G, Söder O and Ost A:
Incidence in Sweden and clinical features of familial
hemophagocytic lymphohistiocytosis. Acta Paediatr Scand.
80:428–435. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ravelli A, Minoia F, Davi S, Horne A,
Bovis F, Pistorio A, Aricò M, Avcin T, Behrens EM, De Benedetti F,
et al: 2016 Classification criteria for macrophage activation
syndrome complicating systemic juvenile idiopathic arthritis: A
European league against rheumatism/American college of
rheumatology/paediatric rheumatology international trials
organisation collaborative initiative. Ann Rheum Dis. 75:481–489.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramos-Casals M, Brito-Zerón P,
López-Guillermo A, Khamashta MA and Bosch X: Adult haemophagocytic
syndrome. Lancet. 383:1503–1516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He L, Yang C and Wang Y: Biological
therapies for hemophagocytic lymphohistiocytosis: Current knowledge
and future perspectives. Expert Opin Biol Ther. 23:1005–1013. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Janka GE and Lehmberg K: Hemophagocytic
syndromes-an update. Blood Rev. 28:135–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Griffin G, Shenoi S and Hughes GC:
Hemophagocytic lymphohistiocytosis: An update on pathogenesis,
diagnosis, and therapy. Best Pract Res Clin Rheumatol.
34:1015152020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Filipovich A, McClain K and Grom A:
Histiocytic disorders: Recent insights into pathophysiology and
practical guidelines. Biol Blood Marrow Transplant. 16 (1
Suppl):S82–S89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arceci RJ: When T cells and macrophages do
not talk: The hemophagocytic syndromes. Curr Opin Hematol.
15:359–367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niece JA, Rogers ZR, Ahmad N, Langevin AM
and McClain KL: Hemophagocytic lymphohistiocytosis in Texas:
Observations on ethnicity and race. Pediatr Blood Cancer.
54:424–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Löfstedt A, Jädersten M, Meeths M and
Henter JI: Malignancy-associated hemophagocytic lymphohistiocytosis
in Sweden: Incidence, clinical characteristics, and survival.
Blood. 143:233–242. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh S, Khasbage S, Kaur RJ, Sidhu JK and
Bhandari B: Chimeric antigen receptor T cell: A cancer
immunotherapy. Indian J Pharmacol. 54:226–233. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chong EA, Ruella M and Schuster SJ;
Lymphoma Program Investigators at the University of Pennsylvania, :
Five-year outcomes for refractory B-cell lymphomas with CAR T-cell
therapy. N Engl J Med. 384:673–674. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cappell KM, Sherry RM, Yang JC, Goff SL,
Vanasse DA, McIntyre L, Rosenberg SA and Kochenderfer JN: Long-term
follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J
Clin Oncol. 38:3805–3815. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feins S, Kong W, Williams EF, Milone MC
and Fraietta JA: An introduction to chimeric antigen receptor (CAR)
T-cell immunotherapy for human cancer. Am J Hematol. 94((S1)):
S3–S9. 2019.PubMed/NCBI
|
|
35
|
Stone JD and Kranz DM: Role of T cell
receptor affinity in the efficacy and specificity of adoptive T
cell therapies. Front Immunol. 4:2442013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berdeja JG, Madduri D, Usmani SZ,
Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M,
Lesokhin A, et al: Ciltacabtagene autoleucel, a B-cell maturation
antigen-directed chimeric antigen receptor T-cell therapy in
patients with relapsed or refractory multiple myeloma
(CARTITUDE-1): A phase 1b/2 open-label study: A phase 1b/2
open-label study. Lancet. 398:314–324. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang T, Tang Y, Cai J, Wan X, Hu S, Lu X,
Xie Z, Qiao X, Jiang H, Shao J, et al: Coadministration of CD19-
and CD22-directed chimeric antigen receptor T-cell therapy in
childhood B-cell acute lymphoblastic leukemia: A single-arm,
multicenter, phase II trial. J Clin Oncol. 41:1670–1683. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene ciloleucel CAR T-cell therapy in
refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schuster SJ, Bishop MR, Tam CS, Waller EK,
Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin
JR, et al: Tisagenlecleucel in adult relapsed or refractory diffuse
large B-cell lymphoma. N Engl J Med. 380:45–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Frey NV, Shaw PA, Hexner EO, Pequignot E,
Gill S, Luger SM, Mangan JK, Loren AW, Perl AE, Maude SL, et al:
Optimizing chimeric antigen receptor T-cell therapy for adults with
acute lymphoblastic leukemia. J Clin Oncol. 38:415–422. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Raje N, Berdeja J, Lin Y, Siegel D,
Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A,
et al: Anti-BCMA CAR T-cell therapy bb2121 in relapsed or
refractory multiple myeloma. N Engl J Med. 380:1726–1737. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bupha-Intr O, Haeusler G, Chee L, Thursky
K, Slavin M and Teh B: CAR-T cell therapy and infection: A review.
Expert Rev Anti Infect Ther. 19:749–758. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lei W, Xie M, Jiang Q, Xu N, Li P, Liang
A, Young KH and Qian W: Treatment-related adverse events of
chimeric antigen receptor T-cell (CAR T) in clinical trials: A
systematic review and meta-analysis. Cancers (Basel). 13:39122021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zu C, Wu S, Zhang M, Wei G, Xu H, Cui J,
Chang AH, Huang H and Hu Y: A distinct cytokine network
distinguishes chimeric antigen receptor T cell (CAR-T)-associated
hemophagocytic lymphohistiocytosis-like toxicity (carHLH) from
severe cytokine release syndrome following CAR-T therapy.
Cytotherapy. 25:1167–1175. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Y, Fang Y, Chen X, Wang Z, Liang X,
Zhang T, Liu M, Zhou N, Lv J, Tang K, et al: Gasdermin E-mediated
target cell pyroptosis by CAR T cells triggers cytokine release
syndrome. Sci Immunol. 5:eaax79692020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maude SL, Laetsch TW, Buechner J, Rives S,
Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers
GD, et al: Tisagenlecleucel in children and young adults with
B-cell lymphoblastic leukemia. N Engl J Med. 378:439–448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brudno JN and Kochenderfer JN: Current
understanding and management of CAR T cell-associated toxicities.
Nat Rev Clin Oncol. 21:501–521. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Neelapu SS, Tummala S, Kebriaei P, Wierda
W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et
al: Chimeric antigen receptor T-cell therapy-assessment and
management of toxicities. Nat Rev Clin Oncol. 15:47–62. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fajgenbaum DC and June CH: Cytokine storm.
N Engl J Med. 383:2255–2273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yan W, Xiong Y, Lv R, Du C, Yu T, Zhang S,
Sui W, Deng S, Xiao J, Xu Y, et al: Uncommon biphasic CAR-T
expansion induces hemophagocytic lymphohistiocytosis-like syndrome
and fatal multiple infections following BCMA CAR-T cell therapy: A
case report. J Immunother Cancer. 12:e0100802024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vandenhaute J, Wouters CH and Matthys P:
Natural killer cells in systemic autoinflammatory diseases: A focus
on systemic juvenile idiopathic arthritis and macrophage activation
syndrome. Front Immunol. 10:30892020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Weiss ES, Girard-Guyonvarc'h C, Holzinger
D, de Jesus AA, Tariq Z, Picarsic J, Schiffrin EJ, Foell D, Grom
AA, Ammann S, et al: Interleukin-18 diagnostically distinguishes
and pathogenically promotes human and murine macrophage activation
syndrome. Blood. 131:1442–1455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kaplanski G: Interleukin-18: Biological
properties and role in disease pathogenesis. Immunol Rev.
281:138–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Miao L, Zhang Z, Ren Z and Li Y: Reactions
related to CAR-T cell therapy. Front Immunol. 12:6632012021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nichols KE and Hines MR: NK cells:
Energized yet exhausted in adult HLH. Blood. 136:524–525. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Strati P, Ahmed S, Kebriaei P, Nastoupil
LJ, Claussen CM, Watson G, Horowitz SB, Brown ART, Do B, Rodriguez
MA, et al: Clinical efficacy of anakinra to mitigate CAR T-cell
therapy-associated toxicity in large B-cell lymphoma. Blood Adv.
4:3123–3127. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ahmed S, Furqan F, Strati P, Westin J,
Fayad LE, Hagemeister FB, Lee HJ, Iyer SP, Nair R, Nastoupil LJ, et
al: Haemophagocytic lymphohistiocytosis (HLH) in patients with
large B-cell lymphoma treated with standard of care (SOC)
axicabtagene ciloleucel (Axi-cel). J Clin Oncol. 38 (15
Suppl):S80572020. View Article : Google Scholar
|
|
58
|
Lee DW, Santomasso BD, Locke FL, Ghobadi
A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, et
al: ASTCT consensus grading for cytokine release syndrome and
neurologic toxicity associated with immune effector cells. Biol
Blood Marrow Transplant. 25:625–638. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Meireles AM, Iacoboni G, Moço LM, Ramos I,
Brás G, Azevedo J, Rodrigues Â, Moreira C and Mariz M:
Hemophagocytic lymphohistiocytosis in a patient with Epstein-Barr
virus-positive diffuse large B-cell lymphoma treated with chimeric
antigen receptor T-cell therapy. Immunotherapy. 16:1105–1111. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Khurana A, Rosenthal AC, Mohty R, Gaddam
M, Bansal R, Hathcock MA, Nedved AN, Durani U, Iqbal M, Wang Y, et
al: Chimeric antigen receptor T-cell therapy associated
hemophagocytic lymphohistiocytosis syndrome: Clinical presentation,
outcomes, and management. Blood Cancer J. 14:1362024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Henter JI, Horne A, Aricó M, Egeler RM,
Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski
J and Janka G: HLH-2004: Diagnostic and therapeutic guidelines for
hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer.
48:124–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fardet L, Galicier L, Lambotte O, Marzac
C, Aumont C, Chahwan D, Coppo P and Hejblum G: Development and
validation of the HScore, a score for the diagnosis of reactive
hemophagocytic syndrome. Arthritis Rheumatol. 66:2613–2620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Takagi S, Masuoka K, Uchida N, Ishiwata K,
Araoka H, Tsuji M, Yamamoto H, Kato D, Matsuhashi Y, Kusumi E, et
al: High incidence of haemophagocytic syndrome following umbilical
cord blood transplantation for adults. Br J Haematol. 147:543–553.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Porter TJ, Lazarevic A, Ziggas JE, Fuchs
E, Kim K, Byrnes H, Luznik L, Bolaños-Meade J, Ali SA, Shah NN, et
al: Hyperinflammatory syndrome resembling haemophagocytic
lymphohistiocytosis following axicabtagene ciloleucel and
brexucabtagene autoleucel. Br J Haematol. 199:720–727. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Turtle CJ, Hanafi LA, Berger C, Hudecek M,
Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, et
al: Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of
CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T
cells. Sci Transl Med. 8:355ra1162016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Y, Zhou F, Wu Z, Li Y, Li C, Du M,
Luo W, Kou H, Lu C and Mei H: Timing of tocilizumab administration
under the guidance of IL-6 in CAR-T therapy for R/R acute
lymphoblastic leukemia. Front Immunol. 13:9149592022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
He J, Xu N, Zhou H, Zhou Y, Wu D, Zhao R,
Lin T, Xu J, Cao R, Li P and Liu Q: Case report: Chimeric antigen
receptor T cells induced late severe cytokine release syndrome.
Front Oncol. 12:8939282022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sigha OB, Mbono Betoko R, Nkoro GA, Fossi
Happi M, Ekoube CE, Kelbaba BB, Mandeng Ma Linwa E and Kouotou EA:
Bart's syndrome associated with a disorder of sexual
differentiation: An atypical presentation in a Cameroonian newborn.
Clin Case Rep. 10:e052342022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Maus MV, Alexander S, Bishop MR, Brudno
JN, Callahan C, Davila ML, Diamonte C, Dietrich J, Fitzgerald JC,
Frigault MJ, et al: Society for Immunotherapy of Cancer (SITC)
clinical practice guideline on immune effector cell-related adverse
events. J Immunother Cancer. 8:e0015112020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Santomasso BD, Nastoupil LJ, Adkins S,
Lacchetti C, Schneider BJ, Anadkat M, Atkins MB, Brassil KJ,
Caterino JM, Chau I, et al: Management of immune-related adverse
events in patients treated with chimeric antigen receptor T-cell
therapy: ASCO guideline. J Clin Oncol. 39:3978–3992. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Henter JI, Aricò M, Egeler RM, Elinder G,
Favara BE, Filipovich AH, Gadner H, Imashuku S, Janka-Schaub G,
Komp D, et al: HLH-94: A treatment protocol for hemophagocytic
lymphohistiocytosis. HLH study group of the histiocyte society. Med
Pediatr Oncol. 28:342–347. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Locatelli F, Jordan MB, Allen C, Cesaro S,
Rizzari C, Rao A, Degar B, Garrington TP, Sevilla J, Putti MC, et
al: Emapalumab in children with primary hemophagocytic
lymphohistiocytosis. N Engl J Med. 382:1811–1822. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ge J, Zhang Q, Ma H, Wang D, Zhao Y, Zhu
T, Wang W, Zhou C, Wei A, Lian H, et al: Ruxolitinib-based regimen
in children with primary hemophagocytic lymphohistiocytosis.
Haematologica. 109:458–465. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rejeski K, Subklewe M, Aljurf M, Bachy E,
Balduzzi A, Barba P, Bruno B, Benjamin R, Carrabba MG, Chabannon C,
et al: Immune effector cell-associated hematotoxicity: EHA/EBMT
consensus grading and best practice recommendations. Blood.
142:865–877. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Curtis JR, Westfall AO, Allison J, Bijlsma
JW, Freeman A, George V, Kovac SH, Spettell CM and Saag KG:
Population-based assessment of adverse events associated with
long-term glucocorticoid use. Arthritis Rheum. 55:420–426. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kim JY, Kim M, Park JK, Lee EB, Park JW
and Hong J: Limited efficacy of tocilizumab in adult patients with
secondary hemophagocytic lymphohistiocytosis: A retrospective
cohort study. Orphanet J Rare Dis. 17:3632022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Park JH, Nath K, Devlin SM, Sauter CS,
Palomba ML, Shah G, Dahi P, Lin RJ, Scordo M, Perales MA, et al:
CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or
refractory lymphoma: Phase 2 trial interim results. Nat Med.
29:1710–1717. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wohlfarth P, Agis H, Gualdoni GA, Weber J,
Staudinger T, Schellongowski P and Robak O: Interleukin 1 receptor
antagonist anakinra, intravenous immunoglobulin, and
corticosteroids in the management of critically ill adult patients
with hemophagocytic lymphohistiocytosis. J Intensive Care Med.
34:723–731. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Diorio C, Vatsayan A, Talleur AC, Annesley
C, Jaroscak JJ, Shalabi H, Ombrello AK, Hudspeth M, Maude SL,
Gardner RA and Shah NN: Anakinra utilization in refractory
pediatric CAR T-cell associated toxicities. Blood Adv. 6:3398–3403.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Abedin S, McKenna E, Chhabra S, Pasquini
M, Shah NN, Jerkins J, Baim A, Runaas L, Longo W, Drobyski W, et
al: Efficacy, toxicity, and infectious complications in
ruxolitinib-treated patients with corticosteroid-refractory
graft-versus-host disease after hematopoietic cell transplantation.
Biol Blood Marrow Transplant. 25:1689–1694. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bracaglia C, de Graaf K, Pires Marafon D,
Guilhot F, Ferlin W, Prencipe G, Caiello I, Davì S, Schulert G,
Ravelli A, et al: Elevated circulating levels of interferon-γ and
interferon-γ-induced chemokines characterise patients with
macrophage activation syndrome complicating systemic juvenile
idiopathic arthritis. Ann Rheum Dis. 76:166–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Horne A, von Bahr Greenwood T, Chiang SCC,
Meeths M, Björklund C, Ekelund M, Erensjö P, Berg S, Hagelberg S,
Bryceson YT, et al: Efficacy of moderately dosed etoposide in
macrophage activation syndrome-hemophagocytic lymphohistiocytosis.
J Rheumatol. 48:1596–1602. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zondag TCE, Lika A and van Laar JAM: The
role of etoposide in the treatment of adult patients with
hemophagocytic lymphohistiocytosis. Exp Hematol Oncol. 12:22023.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Song Y, Wang J, Wang Y, Wu L and Wang Z:
Requirement for containing etoposide in the initial treatment of
lymphoma associated hemophagocytic lymphohistiocytosis. Cancer Biol
Ther. 22:598–606. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Henter JI, von Bahr Greenwood T and
Bergsten E: Emapalumab in primary hemophagocytic
lymphohistiocytosis. N Engl J Med. 383:596–598. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bailey SR, Vatsa S, Larson RC, Bouffard
AA, Scarfò I, Kann MC, Berger TR, Leick MB, Wehrli M, Schmidts A,
et al: Blockade or deletion of IFNγ reduces macrophage activation
without compromising CAR T-cell function in hematologic
malignancies. Blood Cancer Discov. 3:136–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
McNerney KO, DiNofia AM, Teachey DT, Grupp
SA and Maude SL: Potential role of IFNγ inhibition in refractory
cytokine release syndrome associated with CAR T-cell therapy. Blood
Cancer Discov. 3:90–94. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Verkamp B, Jodele S, Sabulski A, Marsh RA,
Kieser P and Jordan MB: Emapalumab therapy for hemophagocytic
lymphohistiocytosis before reduced-intensity transplantation
improves chimerism. Blood. 144:2625–2636. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Garonzi C, Chinello M and Cesaro S:
Emapalumab for adult and pediatric patients with hemophagocytic
lymphohistiocytosis. Expert Rev Clin Pharmacol. 14:527–534. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wu Y, Sun X, Kang K, Yang Y, Li H, Zhao A
and Niu T: Hemophagocytic lymphohistiocytosis: Current treatment
advances, emerging targeted therapy and underlying mechanisms. J
Hematol Oncol. 17:1062024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Roddie C, Lekakis LJ, Marzolini MAV,
Ramakrishnan A, Zhang Y, Hu Y, Peddareddigari VGR, Khokhar N, Chen
R, Basilico S, et al: Dual targeting of CD19 and CD22 with
bicistronic CAR-T cells in patients with relapsed/refractory large
B-cell lymphoma. Blood. 141:2470–2482. 2023.PubMed/NCBI
|
|
92
|
Schultz LM, Jeyakumar N, Kramer AM, Sahaf
B, Srinagesh H, Shiraz P, Agarwal N, Hamilton M, Erickson C, Jacobs
A, et al: CD22 CAR T cells demonstrate high response rates and
safety in pediatric and adult B-ALL: Phase 1b results. Leukemia.
38:963–968. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Masih KE, Ligon JA, Yates B, Shalabi H,
Little L, Islam Z, Ombrello AK, Inglefield J, Nussenblatt V, Manion
M, et al: Consequences of hemophagocytic lymphohistiocytosis-like
cytokine release syndrome toxicities and concurrent bacteremia.
Pediatr Blood Cancer. 68:e292472021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Service USDoHaH, Health NIo and Institute
NC: National Cancer Institute, . Common terminology criteria for
adverse events (CTCAE). Version 5.0.2017.
|