Introduction
Cervical cancer (CC) ranks as the second leading
cause of cancer-associated mortalities in developing nations and
the 10th in more affluent countries, highlighting a global
disparity in incidence rate (1,2).
Human papilloma virus (HPV) strains 16 and 18 are responsible for
~70% of CC cases worldwide (3,4). The
genomes of HPV16 and HPV18 contain six early and two late open
reading frames and a substantial non-coding regulatory region
(5). The early genes of HPV18 are
key for viral replication and pathogenesis. In infected cells, the
viral oncoproteins E6 and E7 drive malignant cell proliferation and
other cancer-associated processes by inhibiting apoptosis (6). E6 binds p53 and Bcl-2, inhibiting
apoptosis and enabling cells to bypass cell cycle protective
checkpoints, thus promoting uncontrolled cell division (7). E7 targets retinoblastoma protein for
ubiquitination, causing premature entry into the S phase and
bypassing the G1-S checkpoint, which leads to unchecked cellular
proliferation (8).
In CC, CD8+ T lymphocytes serve a key
role in identifying and eliminating cancerous cells. These
cytotoxic T cells predominantly activate the major
histocompatibility complex (MHC) class I pathway, revealing a key
role in the cell-mediated immune response against HPV-infected
cells (9). CD8+ T
lymphocytes recognise peptide-MHCs (p-MHC) on infected cells,
triggering the release of cytotoxic chemicals that kill the
HPV-infected cells (10). However,
when patients test positive for high-risk HPV or exhibit abnormal
cytological results, identifying those at a higher risk of
developing high-grade cervical lesions or cancer without treatment
remains a challenge (11).
Insufficient screening among marginalised or hard-to-reach
populations, such as immigrants or rural dwellers, increases the
risk of undetected precancerous lesions and cancer, while excessive
screening in wealthier communities leads to unnecessary treatment
and associated risks and complications (12). Enhancing the efficacy of less
frequent screening protocols is a practical strategy to expand CC
screening availability for under-screened populations, potentially
reducing both incidence and mortality rates.
To determine the specific viral proteins presented
by naturally occurring human MHC class I molecules, identification
of the unique epitopes that different alleles of human MHC class I
molecules present to CD8+ T cells from the E6 and E7
oncoproteins is necessary. Studies have demonstrated the widespread
presence of human leukocyte antigens (HLAs) A1, A2, A11, A24, B7
and B44, which belong to the HLA class I family, across the
population (13–16). HPV-infected cervical cells,
especially those undergoing precancerous or malignant
transformation, exhibit upregulation of the E6 and E7 oncoproteins.
This increase in expression facilitates the detection of these
viral oncoproteins, which are used for diagnosing precancerous and
cancerous lesions (17).
Phage display technology has advanced identification
of optimal peptide or protein sequences, surpassing traditional
methods such as ribosome and yeast display technologies), and
providing deeper insight into molecular evolution (18). The effective presentation of
antibody fragments on the primary coat protein of filamentous
bacteriophages has enabled the generation of diverse antibody
libraries, advancing therapeutic interventions for various diseases
such as pemphigus foliaceus and paraneoplastic pemphigus) diseases
(19). To produce effective T cell
receptor (TCR)-like antibodies, T cells must recognise a pure,
recombinant p-MHC in its native conformation (20). Obtaining a purified recombinant
MHC/peptide complex folded in its native conformation, as
recognised by T cells (21), is
key for the successful production of TCR-like antibodies using
either conventional hybridoma or phage display technology. These
recombinant MHC/peptide complexes can be efficiently produced in
relatively high quantities, facilitating antibody isolation. The
generation process involves expressing the extracellular domains of
the human leukocyte antigen (HLA) heavy chain and β2-microglobulin
as inclusion bodies in E. coli, followed by in vitro
refolding in the presence of the desired HLA-restricted peptide
(22). The resulting peptide/MHC
complexes exhibit high purity, exist in a monomeric form, and adopt
the correct conformation, as confirmed by structural (23), and functional studies (24). Furthermore, these complexes can be
biotinylated in a site-specific manner, a feature that enhances
their utility in the in vitro selection of TCR-like
antibodies and subsequent specificity characterisation (20). This approach holds promise as both
a precise diagnostic tool and powerful immunotherapeutic strategy
for combating HPV 18-induced cervical cancer.
Materials and methods
This present study followed a structured sequence of
protocols that combined bioinformatics analyses with experimental
laboratory procedures, as illustrated in the flowchart (Fig. S1).
Generation of HPV18 (E6&E7)
p-MHC-A24 complex
Bioinformatics analysis generation of HPV18
p-MHC-A24 complexes
As described by Yazdani et al (25), FASTA sequences from the National
Centre for Biotechnology Information (NCBI) database (ncbi.com)
were used to find peptides derived from the oncoproteins of HPV18,
E6 and E7 (25). Amino acid
sequences associated with HPV18 oncoproteins E6 and E7 were
obtained using the accession nos. AGM34425.1 and AGM34461.1,
respectively. The SYFPEITHI algorithm (Ver. 1.0) (syfpeithi.de) was
used to determine how well the HPV18 peptides bound HLA-A24, as
previously described (26). The
selection of 9-mer peptides was predicated upon their binding
scores derived from these SYFPEITHI algorithms. The peptides were
synthesized by Elabscience Bionovation, Inc.
Transformation and evaluation of
HLA-A24 and β-2-microglobulin (β2m) vectors
Vector transformation using the heat-shock approach
was performed as described by Chang et al (27), with some adjustments. Independent
transfection of the HLA-A24 and β2m chains was performed on
competent cells of the bacterial strain BL21 (DE3) pLysS. A total
of 200 µl of β2m-transformant culture was plated on a pre-prepared
2-YT agar plate supplemented with 100 µg/ml carbenicillin and 34
µg/ml chloramphenicol (Nacalai Tesque, Japan), 2% glucose, and 200
µl of HLA-A-24 transformant culture was plated on the pre-prepared
2-YT agar plate supplemented with 100 µg/ml carbenicillin and 2%
glucose, and they were also incubated at 37°C overnight the next
day. Plates were screened, and single colonies selected from each
culture were inoculated into 10 ml of 2-YT broth (Sigma Aldrich,
USA) containing the same antibiotics, followed by overnight
incubation at 37°C. Subsequently, plasmid extraction was performed
(27). HLA-A24 and β2m chains
sequences were validated using Sanger sequencing method) conducted
by 1st BASE, using T7 vector primers (T7 promoter and T7 terminator
primers; Table SI). This involved
comparing the sequences of the HLA-A24 and β2m regions with their
original maps using SnapGene software V 1.1.3 (snapgene.com).
Furthermore, the molecular sizes of the HLA-A24 and β2m chains were
verified using PCR combined with gel electrophoresis (1% agarose
gel containing 0.5 µg/ml ethidium bromide and Generuler™
1 kb Plus ladder; Thermo Scientific, Inc.), employing DreamTaq
Green PCR Master Mix (2X) with T7 vector promoter and terminator
primers, following the manufacturer's instructions (Tables SII and SIII) (28). The results were captured using the
Syngene Gene Flash Gel Imaging System, followed by quantification
using ImageJ software V 1.54 g. Extraction of plasmids containing
the HLA-A24 and β2m constructs was performed as previously
described using Qiaprep Spin Plasmid Miniprep Kit; Qiagen, Germany)
(29).
Generation of HPV16 P-MHC-A24
complexes
HLA-A24 and β2m protein extraction was performed as
described by Rodenko et al (30) and stored at 4°C for future use. The
proteins) underwent examination using SDS-PAGE assay to ascertain
the molecular weight of the separated proteins as described by
Manns (31). SDS-resolving gel
with a concentration of 10% was used to analyse HLA-A24, whereas a
gel with a concentration of 15% was used to examine β2m. The
molecular weights of the proteins were established by comparison
with a conventional protein ladder (31), briefly the concentrations of the
extracted proteins were measured using the Bradford microplate
assay. Subsequently, 15 µg/lane HLA-A24 and β2m proteins, along
with the appropriate volume (5 µl) of Protein Marker (PM2610 Excel
Band ladder; SMOBIO Technology, Inc.), was loaded onto the
corresponding SDS gel and electrophoresed at 110 V for 70 min. The
results were then captured using the FluorChem Q Gel Imaging
System, followed by quantification analysis of the SDS gel
electrophoresis bands using ImageJ software (version 1.54 g;
National Institutes of Health). The refolding of HPV18 E6 and E7
p-MHC complexes containing HLA-A24 was performed as described by
Luimstra et al (32), with
some adjustments. Specifically, the proteins (HLA-A24, B2M and the
relevant HPV18 peptide) were added to 10 ml cold dilution buffer
for each complex (Tables SIV and
SV). The complexes were incubated
at 4°C for 10 days, covered with aluminium foil and agitated for 30
sec daily. Highly concentrated refolded MHC complexes were
recovered using ultrafiltration Amicon 4 ml spin columns with a
molecular weight cut-off of 30 kDa. These complexes were stored in
microcentrifuge tubes, which were covered with aluminium foil at a
temperature of 4°C until they were used again.
Evaluation of generated p-MHC-A24
complex stabilization by HLA ELISA
The method of Rodenko et al (30), was modified to include six wells
containing 1X PBS and refolded β2M as controls. Each well was
coated with 2 µg/ml streptavidin in 100 µl 1X PBS and incubated at
4°C overnight. The wells were rinsed three times with 1X PBS-T (300
µl/well; 0.1% Tween-20). Blocking was performed using 300 µl 2% BSA
(Nacalai Tesque) at room temperature for 1 h. Subsequently, the
wells were washed three times with 1X PBS-T before adding 50 µg/ml
each p-MHC complex in 100 µl 1X PBS. Positive control wells were
supplemented with 50 µg/ml refolded β2m in 100 µl 1X PBS, while
negative control wells received 100 µl 1X PBS. The plate was
incubated at room temperature for 1 h at 15 × g shaking. Wells were
rinsed three times with 1X PBS-T and 100 µl anti-β2m HRP (1:3,000;
Cat. No. GTX40576; GeneTex, Inc.) was added to each well. Following
1 h incubation at room temperature with stirring at 15 × g shaking,
wells were rinsed three times with 1X PBS-T. Finally, 100 µl
2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) (5 mg
tablet; Thermo Scientific, USA) solution was added to each well at
room temperature for 10–15 min, during which the colour development
was monitored. An optical plate reader was used to measure the
absorbance at 405 nm.
Human DAB library screening for
TCR-like antibody discovery
Preparation and purification of biopanning
phages
Preparation and purification of the KM13 helper
phage were performed as described by Lee et al (33). Precipitation of the helper phage
was performed using a 20% polyethylene glycol-NaCl solution
(Table SVI). Colony counts and
bacterial titers were calculated as follows: Colony-forming units
(c.f.u.)=colony count × dilution factor ×100. The DAB library was
generated and purified using the same procedure as that for the
helper phage. The resulting DAB library was stored at 4°C for
future use (33).
Biopanning of HPV18 P-MHC-A24
complexes against human DAB library
A traditional biopanning assay for HPV18 E6 and E7
P-MHC-A24 complexes was performed using the DAB phagemid library,
following the procedure outlined by Lee et al (33), with modification as described by
Dass et al (34). All ELISA
experiments used PBS-T and 2% BSA as the washing and blocking
solutions, respectively. The secondary antibody, anti-M13 HRP (cat.
no GE27-9421-01; Sigma Aldrich, USA), was used at a 1:5,000
dilution in 2% BSA buffer for 1 h at room temperature). ABTS (5
mg/tablet; Cat. No 11204521001; Sigma Aldrich, USA) was used as the
detection reagent (34). Plasmid
purification of specific positive monoclonal clones identified by
comparative ELISA was performed according to the manufacturer's
instructions (Qiagen, Inc.; Table
SVII). Sanger sequencing analysis of all positive clone
plasmids was conducted by 1st BASE using M13 vector primers
(Table SI). Final analysis of
sequencing results was performed using ImMunoGeneTics information
system®/vquest databases (imgt.org) and VBASE2
(vbase2.org), as described by Dass et al (35).
Purification of monoclonal soluble DAB
protein
Successful integration of validated, sequenced
plasmids into competent Escherichia coli BL21(DE3) pLysS
cells (Table SVIII) was performed
as described by Chang et al (27). The expression of single-domain
TCR-like antibodies was performed as described by Dass et al
(34) with modifications (250 ml
2-YT broth medium). Intracellular extraction of soluble,
domain-specific antibody fragments was performed as described by
Falgenhauer et al (36),
with adjustments. Specifically, a lysozyme buffer solution was
prepared by dissolving lysozyme (Sigma Aldrich; Merck KGaA) in 1X
PBS to a final concentration of 300 µg/ml, total volume 15 ml. The
resulting pellets were homogenised in 15 ml lysozyme buffer, cooled
on ice for 1 h and subjected to ultrasonication for 5 min (10/10
sec on/off) at 3.5V. The mixture underwent two rounds of
centrifugation at 10,000 × g at 4°C for 30 min. The supernatant was
collected and stored at 4°C for further analysis. Samples were
analysed by SDS-PAGE followed by western blotting to confirm the
presence of TCR-specific antibody proteins. Briefly, the
concentrations of the extracted TCR-like antibody proteins were
measured using the Bradford microplate assay. Subsequently, 15
µg/lane crude TCR-like antibody protein and 5 µl/lane Protein
Marker (cat. no. PM2610 ExcelBand ladder; SMOBIO Technology, Inc.)
were loaded onto 15% SDS gel and subjected to electrophoresis under
the conditions of 110 V for 60 min. The TCR-like antibody proteins
(primary antibodies) were then transferred onto PVDF membranes.
Following a 1-h blocking step at room temperature with 2% BSA
(Nacalai Tesque, Japan) dissolved in PBS, the membranes were washed
three times with PBS-T buffer and then incubated with an anti-c-Myc
HRP (9E10) secondary antibody (1:5,000; Cat. No. NC9638329; Santa
Cruz Biotechnology, USA) for 1 h at room temperature. The secondary
antibody used in this study specifically detects c-Myc-tagged
TCR-like antibody proteins. After incubation, the membranes were
washed 3 times again with PBS-T buffer. Protein bands were
visualised using the Peroxidase Stain DAB Kit (Brown Stain Kit,
Cat. No. 25985-50; Nacalai Tesque, Japan), 10 min incubation at
room temperature. The molecular weights of the detected proteins
were determined by comparing them with the Protein Marker (PM2610
ExcelBand ladder) using ImageJ software (version 1.54 g; National
Institutes of Health). Purification of TCR-like antibody proteins
was performed as described by Rouet et al (37), using Protein A high-performance
(HP) column according to the manufacturer's instructions (Cytiva),
with adjustments. To increase the amount of purified antibody, the
pH of the binding/wash buffer (20 mM sodium phosphate) was
optimized to 6.8 and the elution buffer (100 mM citric acid) was
set at pH 2.8. A binding buffer pH of 6.8 allowed the protein to
retain activity and improved its interaction with Protein A.
Similarly, a pH of 2.8 in the elution buffer facilitated complete
dissociation from Protein A, improving both protein release and
concentration (38). Since buffers
stored at 4°C exhibited a pH change of 0.4, they were freshly
prepared at room temperature. Before sample introduction (15 ml),
the column was washed with 5 ml binding solution. Next the sample
was passed through the column followed by two washes (5 and 2 ml,
respectively), waste fractions were collected in tubes labelled W1
and W2. The elution solution was applied in runs of 5 and 2 ml,
with eluted fractions collected in tubes E1 and E2. The soluble
TCR-like antibodies were subsequently concentrated using a HiTrap
Protein A HP column (10 kDa a molecular weight cut-off) and
analysed by SDS-PAGE as previously described.
Exploring the ability of TCR-like
antibodies to bind corresponding p-MHC-A24 complexes
A systematic evaluation of the ability of soluble,
purified TCR-like antibodies to selectively bind their target was
conducted using TCR-ELISA and western blotting, with adjustments
based on the approach developed by Dass et al (34). To each well of a 96-well microtiter
plate, 100 µl purified TCR-like antibody (targeted and
non-targeted) diluted in 1X PBS at a concentration of 10 µg/ml was
added. The background control was 1X PBS, while the negative
control used the non-target 16 kDa p-MHC-A24 complex. The plates
were incubated overnight at 4°C. The wells were rinsed three times
with PBS-T, followed by blocking with 2% BSA for 1 h, 80 × g at
room temperature. Following another washing step (3 times with
PBS-T), 100 µl HPV18 p-MHC-A24 complexes in 1X PBS at a
concentration of 10 µg/ml was added to the wells containing the
antibodies and incubated for 1 h, 80 × g at room temperature.
Following three washes with PBS-T), 100 µl streptavidin-HRP,
(1:5,000 in 2% BSA; cat. No. 21130; Thermo Fisher Scientific, USA)
was added to each well and incubated 1 h, 80 × g at room
temperature. A final 3 times wash with PBS-T was performed and 100
µl ABTS (5 mg/tablet; Cat. No 11204521001; Sigma Aldrich, USA)
substrate was added to each well. The plates were placed in the
dark, the colour development was monitored, and absorbance was
measured at 405 nm after 15 min at room temperature. Western blot
analysis of antibody-antigen interactions was performed as
described by Dass et al (34), with adjustments. The concentrations
of the purified TCR-like antibody proteins were measured using the
Sigma Bradford microplate assay. Subsequently, 15 µg/lane each
purified TCR-like antibody protein and 5 µl of Protein Marker
(PM2610 ExcelBand ladder; SMOBIO Technology, Inc.) were loaded onto
a 15% SDS gel (two gels). The first gel was stained overnight with
Coomassie Brilliant Blue, while the second gel included purified
TCR-like antibody proteins (primary antibodies) was transferred
directly onto a PVDF membrane using a wet transfer system (100 V
for 60 min in 1X cold Towbin buffer) for western blot analysis.
Following 1 h blocking step at room temperature with 2% BSA
(Nacalai Tesque, Japan) dissolved in PBS, then incubated 1 h at
room temperature with 10 µg/ml of the HPV18 p-MHC-A24 complexes in
1X PBS (5 ml total) under gentle agitation. The membranes were
washed three times with PBS-T buffer and then incubated with
streptavidin-HRP diluted in 2% BSA (1:5,000; Cat. No. 21130; Thermo
Scientific, USA) for 1 h at room temperature. The streptavidin-HRP
reagent specifically detects biotin-tagged HLA-A24 chain protein.
After incubation, the membranes were washed three more times with
PBS-T buffer. Protein bands were visualised using the Peroxidase
Stain DAB Kit (Brown Stain Kit, Cat. No. 25985-50; Nacalai Tesque,
Japan), 10 min incubation at room temperature. The molecular
weights of the detected protein bands were determined by comparing
them with the Protein Marker (PM2610 ExcelBand ladder) using ImageJ
software (version 1.54 g; National Institutes of Health).
Statistical analysis
A parametric one-way ANOVA was conducted using
GraphPad Prism 10 software (Dotmatics), followed by Tukey's post
hoc test to analyse the results. P<0.05 was considered to
indicate a statistically significant difference. Results are
presented as the mean ± standard deviation from three independent
experiments. ELISA was performed using sextuplicate wells for each
sample and control.
Results
Bioinformatics analysis for generation
of HPV18 P-MHC-A24 complexes
Using the NCBI FASTA database, peptides derived from
the E6 and E7 oncoproteins of human papillomavirus type 18 (HPV18)
were identified. The affinity of these peptides for binding to
MHC-A24 was assessed using the SYFPEITHI algorithm. The binding
scores for each peptide sequence were notably high. The synthesis
of the selected peptides achieved a purity of 98% (Table I).
 | Table I.Binding scores of HPV18 E6 and E7
oncoproteins to HLA-A24 using the National Centre for Biotechnology
Information database and SYFPEITHI method. |
Table I.
Binding scores of HPV18 E6 and E7
oncoproteins to HLA-A24 using the National Centre for Biotechnology
Information database and SYFPEITHI method.
| HPV
oncoprotein | Peptide
sequence | SYFPEITHI
score | Purity, % |
|---|
| HPV18 E6 | V Y C K T V L E
L | 24 | 98 |
| HPV18 E7 | V D L L C H E Q
L | 16 | 98 |
Transformation and evaluation of
HLA-A24 and β2m vectors
The sequence integrity of the HLA-A24 and β2m chains
was verified through analysis of sequencing data (Fig. S2, Fig. S3, Fig. S4, Fig. S5, Fig. S6, Fig. S7). These data were compared with
the original vector maps using SnapGene software (Table SIX). The results showed complete
match with the original sequence (Figs. 1 and 2). The sizes of the synthesised HLA-A24
and β2m chains were largely in accordance with their corresponding
sizes as indicated in the vector system map. Specifically, the
HLA-A24 chain was 1,144 bp, while the β2m chain was 495 bp
(Fig. 3A).
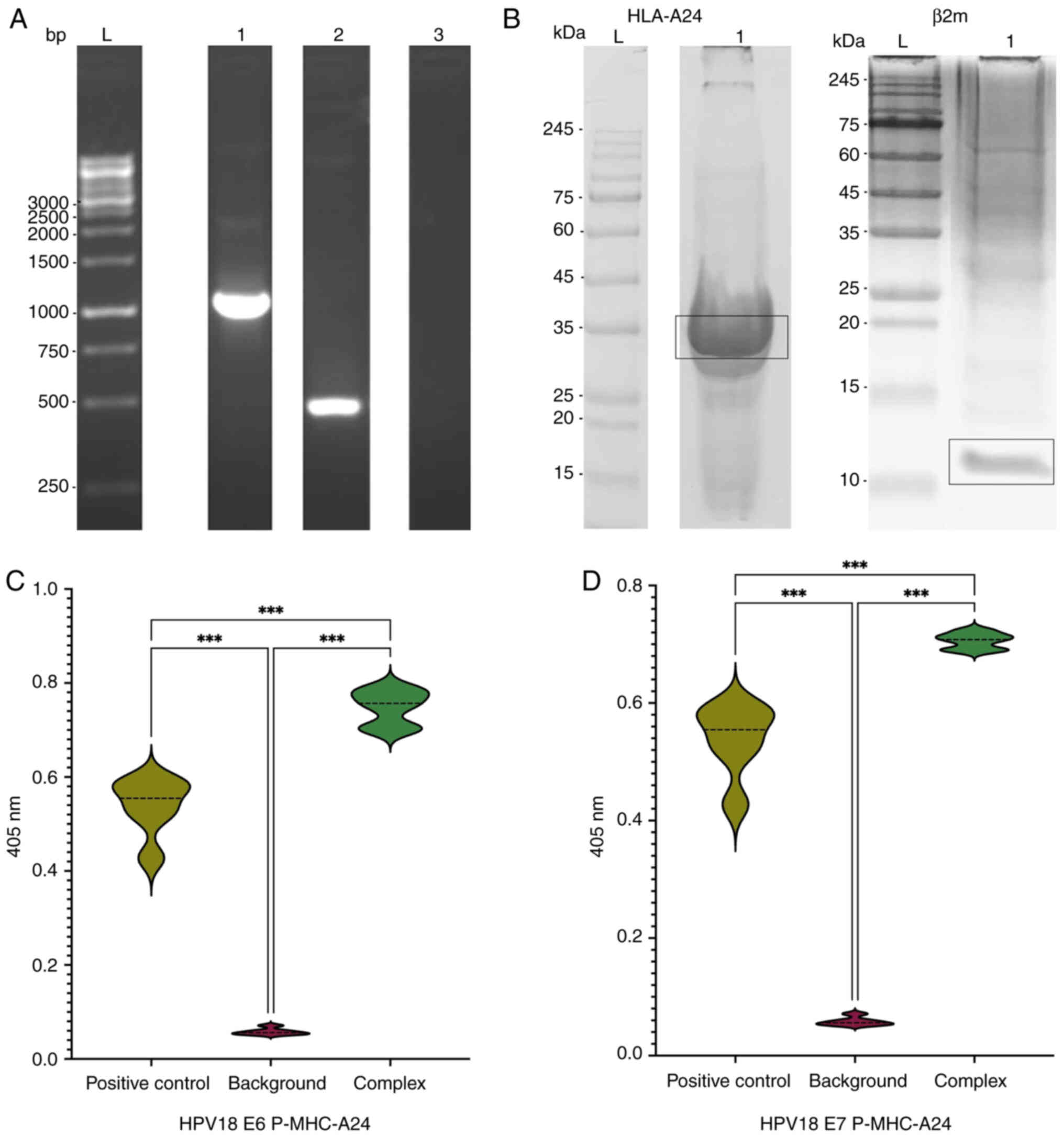 | Figure 3.Evaluation of P-MHC-A2402 complex
components. (A) Agarose gel electrophoresis visualizing the PCR
amplification of HLA-A24 and β2m chains. L, 1 KB plus DNA ladder;
1, HLA-A24 chain at ~1,144 bp; 2, β2m chain at ~495 bp; 3, negative
control (ddH2O). (B) SDS-PAGE analysis of inclusion body
fractions of heavy and light chains. L, pre-stained protein ladder
(9–245 kDa); 1, HLA-A24 fraction band at ~35 kDa or β2m fraction
band at ~12 kDa. HLA-ELISA analysis of the stability of the
generated p-MHC complex. The OD 405 nm results indicate successful
formation of the HPV18 (C) E6 and (D) E7 p-MHC-A24 complexes.
***P<0.001. HLA, human leukocyte antigen; β2m,
β-2-microglobulin; OD, optical density. |
Generation of HPV18 p-MHC-A24
complexes
Quantification of proteins from both chain fractions
was performed using the Bradford assay for each chain. SDS-PAGE was
employed to examine the HLA-A24 and β2M protein fractions for the
determination of target protein sizes. HLA-A24 chains had the
expected protein size of ~35 kDa (Fig.
3B), whereas the β2M chain was ~12 kDa (Fig. 3B).
Evaluation of generated p-MHC
stabilisation by HLA ELISA
ELISA indicated increased levels of p-MHC complexes
compared with both the negative and positive controls, validating
the development of stable p-MHC complexes. ELISA yielded values of
0.0586 for the negative control and 0.5291 for the positive
control. The corresponding values for the E6 p-MHC and the E7 p-MHC
complexes were 0.7481and 0.7059, respectively (Fig. 3C and D). This indicated successful
formation of stable p-MHC complexes through refolding of the HPV18
peptides with the β2M light chain and the HLA-A24 heavy chains.
Biopanning of HPV18 p-MHC-A24
complexes against human DAB library
After preparing and purifying the biopanning phages,
the titer of the KM13 helper phage was 5×1012 c.f.u. The
size of the main antibody library was confirmed by titration to be
4×1012 c.f.u. Biopanning of HPV18 p-MHC-A24 complexes
was carried out using a library of human DABs. E6 and E7 p-MHC-A24
exhibited enrichment ratios of 1.6×104 and
4.5×105, respectively (data not shown). These values
suggest an enrichment of >50%.
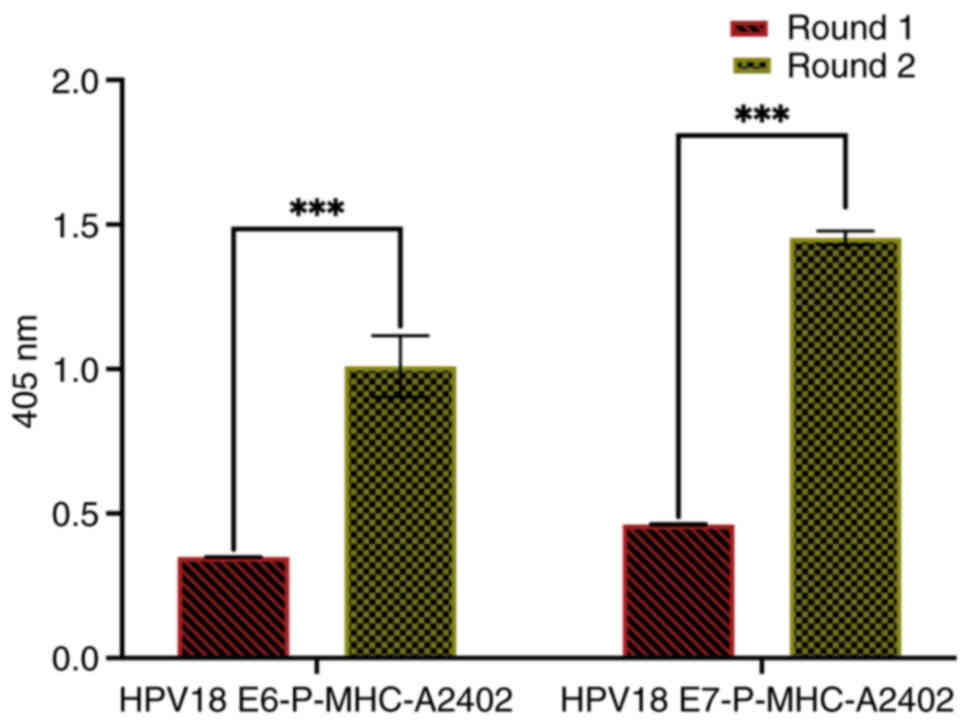
Polyclonal antibody phage ELISA
Polyclonal ELISA revealed HPV18 E6 p-MHC-A24 values
of 0.349 and 0.984 in the first and second rounds, respectively,
and HPV18 E7 p-MHC-A24 values of 0.462 and 1.445 in the first and
second rounds, respectively (Fig.
4). Based on these results, the phages from the second round
were used for monoclonal analysis of each target.
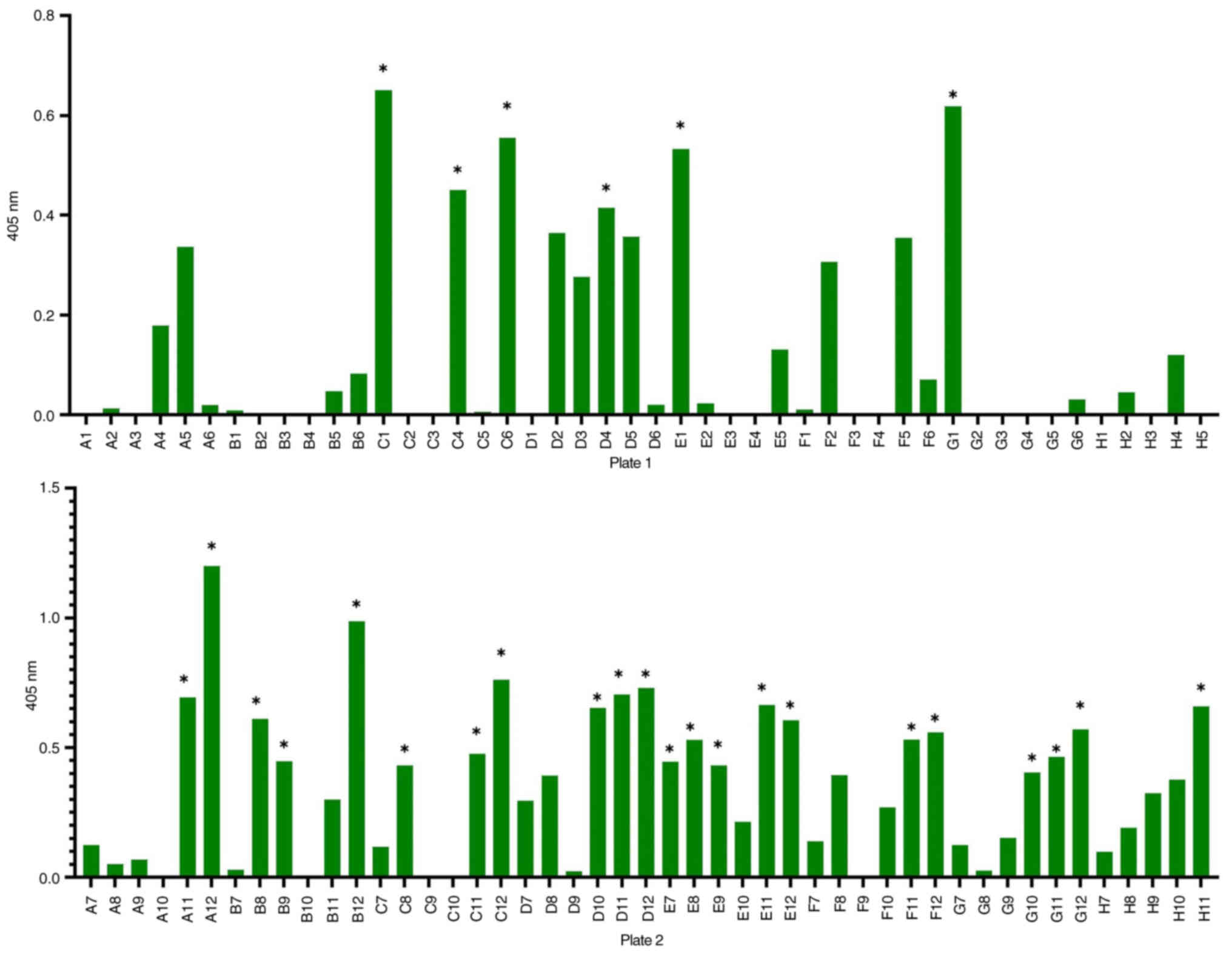
Monoclonal antibody phage ELISA
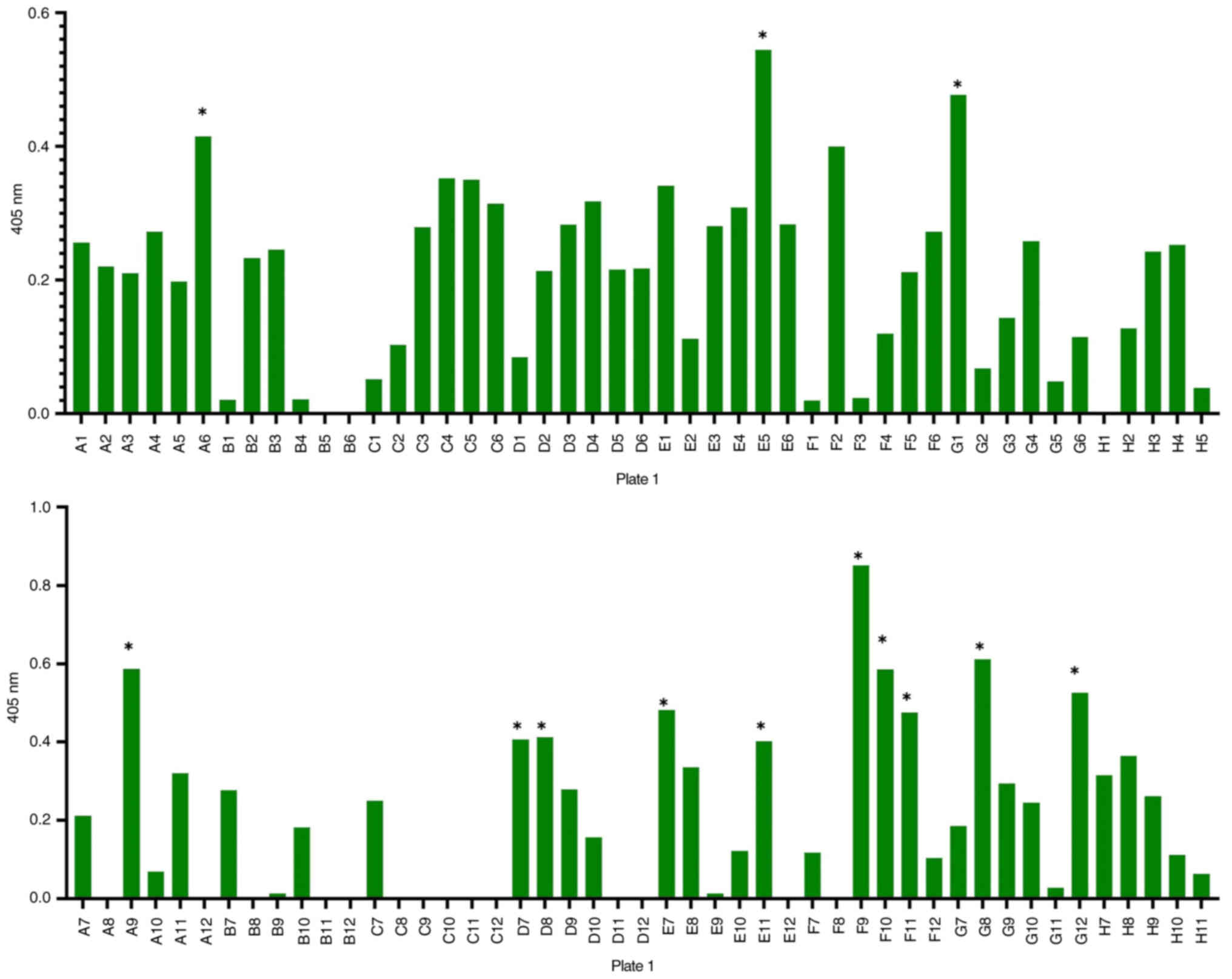
A total of 94 clones were analysed in the monoclonal
antibody assay. Of these, 28 clones were verified as positive for
the HPV18 complex E6 p-MHC-A24 (Fig.
5), while 14 clones tested positive for the HPV18 complex E7
p-MHC-A24 (Fig. 6). To select
clones that demonstrated high binding affinity, which will be
selected for further analysis in future steps, the results with
values <0.4 nm was excluded after subtracting background values,
which were uniformly low for all clones.
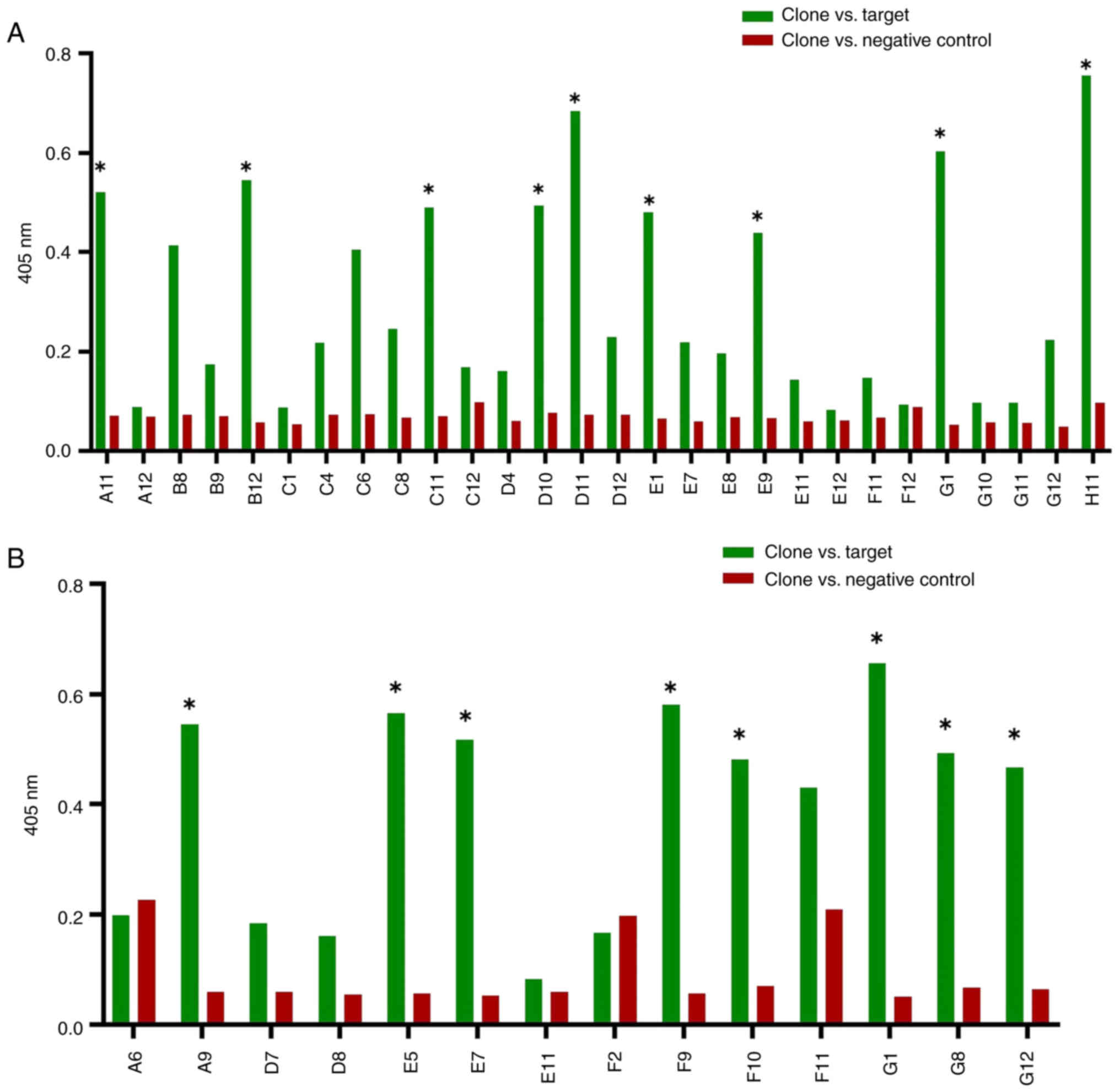
Comparative ELISA
Prior to sequencing analysis, comparative ELISA was
conducted to evaluate positive clones against target and non-target
HLA-A24 peptide complexes and decrease false positives. A total of
nine clones (A11, B12, C11, D10, D11, E1, E9, G1 and H11) tested
positive for the HPV18 E6 p-MHC-A24 complex (Fig. 7A) and eight (A9, E5, E7, F9, F10,
G1, G8 and G12) tested positive for the HPV18 E7 P-MHC-A24 complex
(Fig. 7B). Results with values
<0.4 nm were excluded after adjusting for background value, as
the background values for all clones were uniformly low.
Antibody sequencing
Sequence analysis identified three positive clones
against HPV18 p-MHC complexes, each presenting a complete sequence
without frameshift mutations or stop codons, as confirmed by VBASE2
and IMGT/VQUEST testing. H11 clone, corresponding to the HPV18 E6
p-MHC-A24 complex, was designated as H11/Ab, while E5 and G8
clones, corresponding to the HPV18 E7 P-MHC-A24 complex, were
designated E5/Ab and G8/Ab, respectively. Analysis confirmed the
absence of stop codons and in-frame junctions in these positive
clones. The TCR structure, including the variable, diversity (D),
and joining (J) gene segments, was characterized. These
diversification mechanisms contribute to the vast TCR repertoire
and are responsible for encoding the complementarity-determining
regions (CDRs), with each TCR containing three CDRs (CDR1, CDR2 and
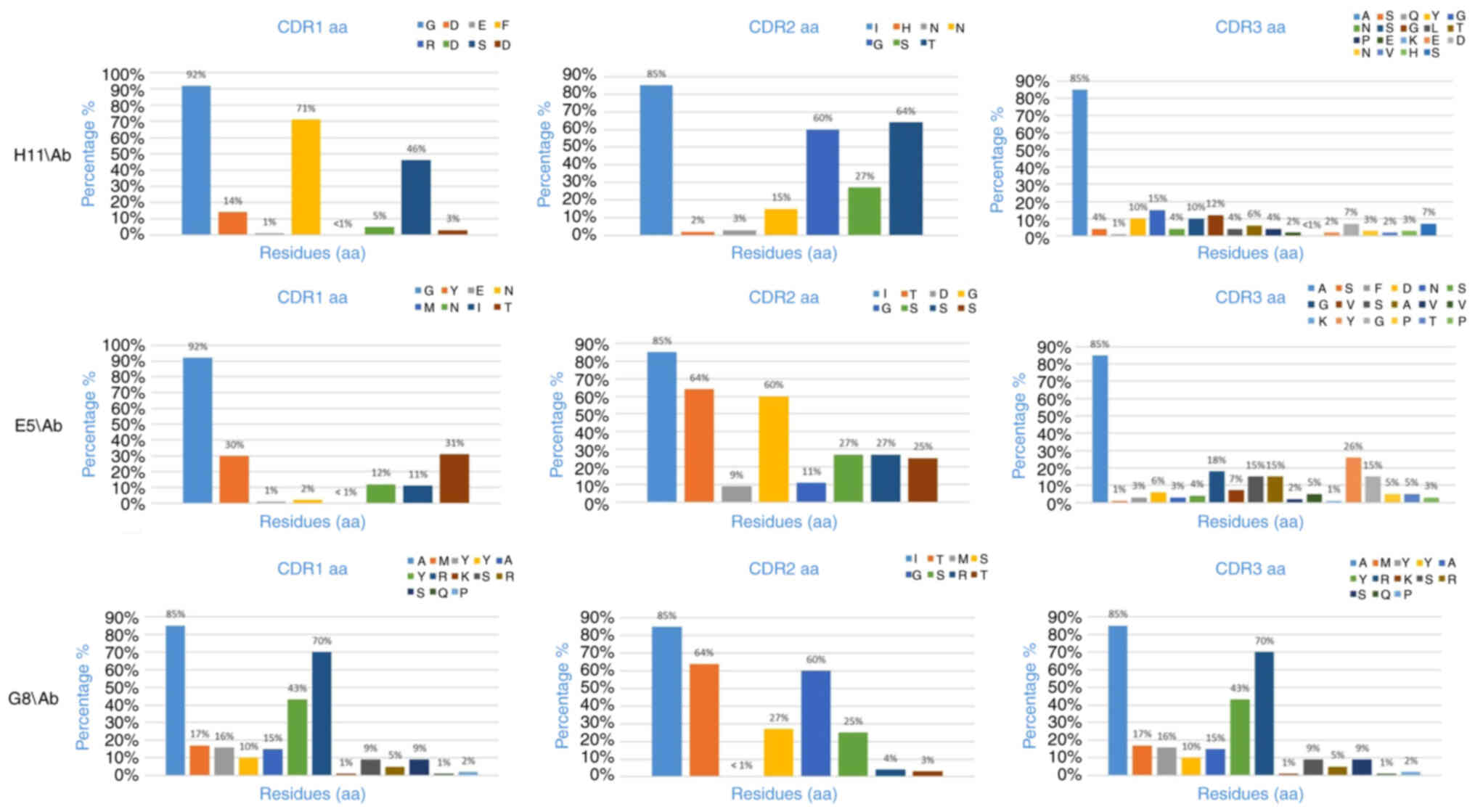
CDR3). Among these, CDR3 exhibits the highest variability (Table II). According to the IMGT, the
H11/Ab clone comprised 195 amino acids, while the E5/Ab and G8/Ab
clones contained 183 and 160 amino acids, respectively,
encompassing all complementarity determining regions (CDRs) and
frame sections. Sequence analysis using the IMGT numbering tool
identified the positions and lengths of CDR segments in the clones
(Table III). The amino acid
frequencies within the CDRs of these clones were calculated based
on IMGT numbering (Fig. 8). The
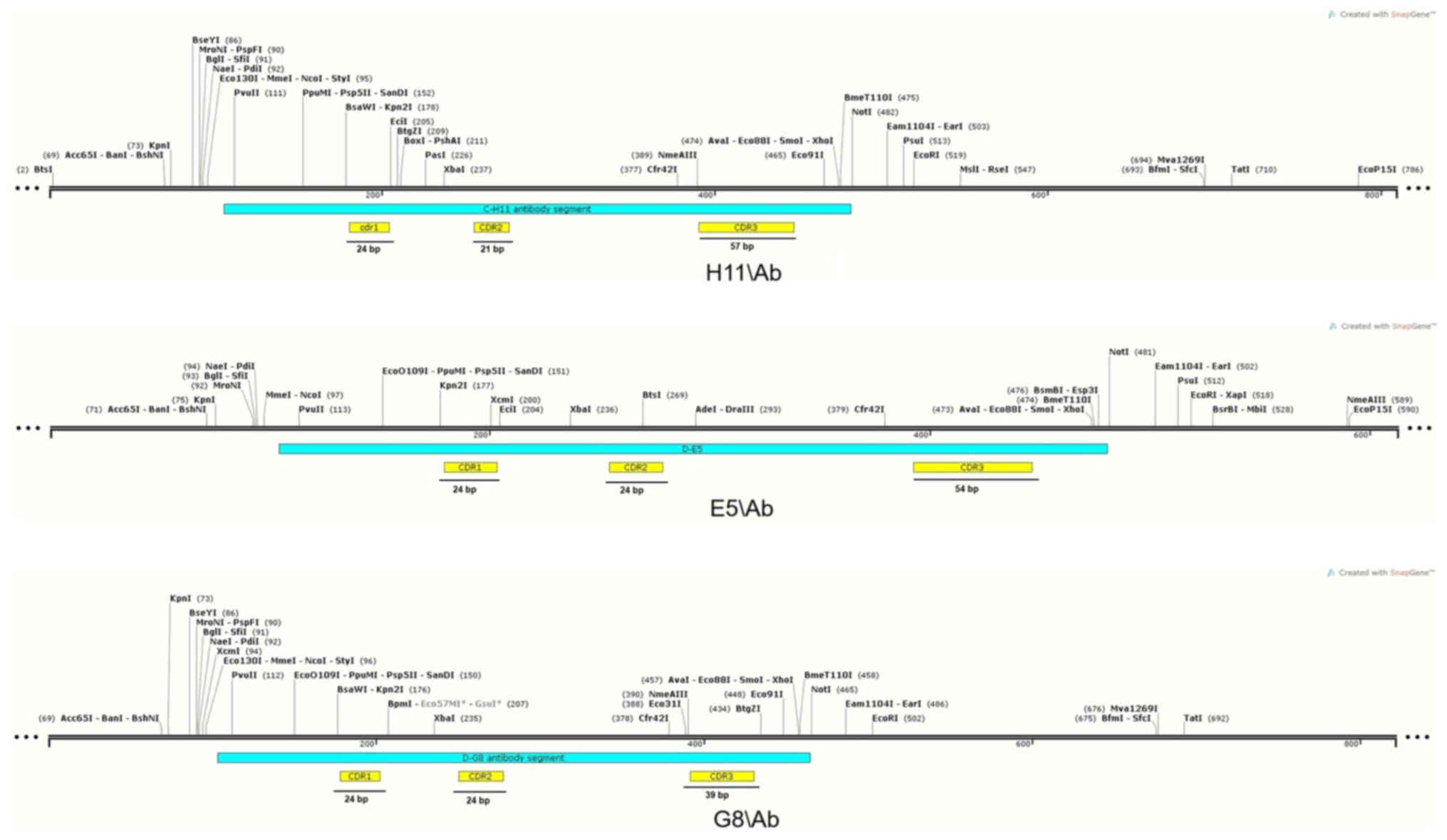
genetic maps of TCR-like antibodies were generated using sequencing
data (Fig. S8, Fig. S9, Fig. S10). The length of the CDRs varied
between antibodies. CDR1 regions in the H11/Ab, E5/Ab and G8/Ab
TCR-like antibodies were each 24 bp in length. The CDR2 regions
measured 21 in H11/Ab and 24 bp in E5/Ab and G8/Ab. Meanwhile, the
lengths of the CDR3 regions were 57 in H11/Ab, 54 in E5/Ab and 39
bp in G8/Ab (Fig. 9). These
sequencing results (which confirmed the full lengths of the CDR
segments in the selected clones) support further investigation into
the soluble form of these clones, to exploration the binding
ability of TCR-like antibodies to their corresponding P-MHC-A24
complexes using TCR-like antibody ELISA and Western blot
techniques.
 | Table II.VBASE2 and IMGT-vquest analysis of
TCRs. |
Table II.
VBASE2 and IMGT-vquest analysis of
TCRs.
| Antibody clone | V-gene family | D segment | J segment |
|---|
| H11\Ab | IGHV3 | IGHD3-22*01 | IGHJ1*01 |
| E5\Ab | IGHV3 | IGHD2-15*01 | IGHJ5*02 |
| G8\Ab | IGHV3 | IGHD3-9*01 | IGHJ4*02 |
 | Table III.Region sequence for positive
TCRs. |
Table III.
Region sequence for positive
TCRs.
| A, H11/Ab |
|---|
|
|---|
| Region | Residue | Length, amino
acids |
|---|
| Leader | 1-34 | 34 |
| HFR1 | 3-60 | 26 |
| CDR-1 | 61-68 | 8 |
| HFR2 | 6-85 | 17 |
| CDR-2 | 86-92 | 7 |
| HFR3 | 93-130 | 38 |
| CDR-3 | 131-149 | 19 |
| HFR4 | 150-160 | 11 |
| Tail | 160-195 | 35 |
|
| B,
E5/Ab |
|
| Region | Residue | Length, amino
acids |
|
| Leader | 1-34 | 34 |
| HFR1 | 35-59 | 25 |
| CDR-1 | 60-67 | 8 |
| HFR2 | 68-84 | 17 |
| CDR-2 | 85-92 | 8 |
| HFR3 | 93-130 | 38 |
| CDR-3 | 131-148 | 18 |
| HFR4 | 149-159 | 11 |
| Tail | 159-183 | 24 |
|
| C,
G8/Ab |
|
| Region | Residue | Length, amino
acids |
|
| Leader | 1-8 | 8 |
| HFR1 | 9-33 | 25 |
| CDR-1 | 34-41 | 8 |
| HFR2 | 42-58 | 17 |
| CDR-2 | 59-66 | 8 |
| HFR3 | 67-104 | 38 |
| CDR-3 | 105-117 | 13 |
| HFR4 | 118-128 | 11 |
| Tail | 128-160 | 32 |
Purification of soluble TCR-like
antibodies
Prior to purifying TCR-like antibodies, the
expression of target proteins was assessed using western blot
analysis. The expected size of the DAB, ~15 kDa, was confirmed by
western blot analysis with anti-c-Myc HRP (Fig. 10B). DABs were isolated using
protein A affinity chromatography and SDS-PAGE was employed to
confirm the successful isolation of each specific target. SDS-PAGE
analysis identified the expected single DAB band (~15 kDa) in all
the specific antibodies (Fig.
10A).
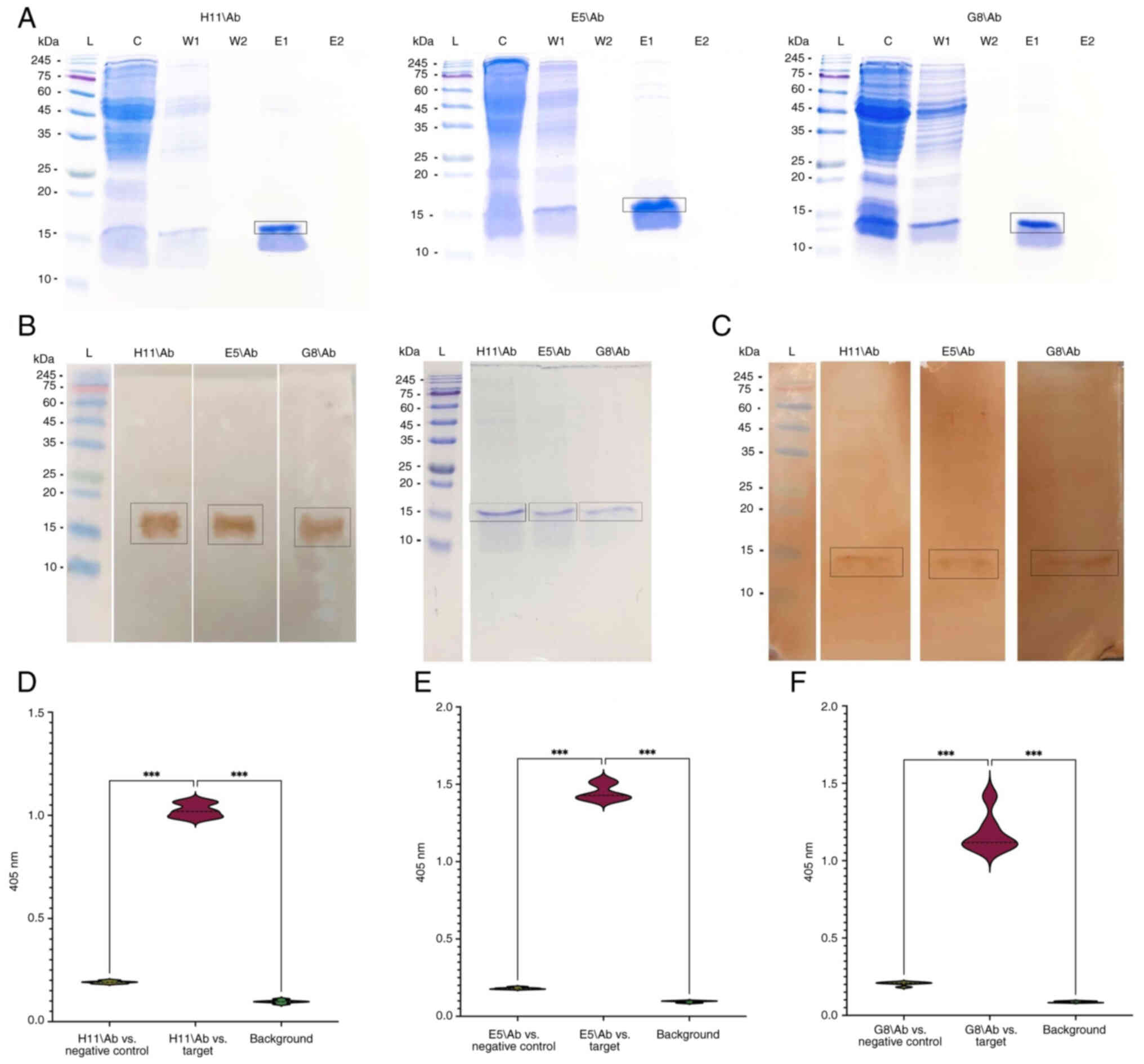 | Figure 10.Investigation of TCR-like domain
antibodies in soluble form. (A) SDS-PAGE analysis of purified
soluble fractions of H11/Ab, E5/Ab, and A12/Ab. L, pre-stained
protein ladder; C, crude antibody sample; W1, concentrated flow
from the first column wash; W2, concentrated flow from the second
column wash; E1, concentrated flow from the first elution (purified
soluble antibody fractions); E2, concentrated flow from the second
elution. (B) Western blot detection of soluble TCR-like domain
antibody proteins in crude samples. (C) Western blot analysis to
assess the binding capacities of H11/Ab, E5/Ab and G8/Ab TCR-like
domain antibodies with the respective HPV18 P-MHC complexes. ELISA
was conducted to evaluate the binding capacities of (D) H11/Ab, (E)
E5/Ab and (F) A12/Ab TCR-like antibodies against their respective
p-MHC-A24 complex. ***P<0.001. HPV, human papilloma virus;
p-MHC, peptide-major histocompatibility complex; TCR, T cell
receptor. |
Ability of TCR-like antibodies to bind
corresponding p-MHC-A24 complexes
Western blot analysis revealed a 15 kDa band
associated with all TCR-like antibodies, including H11/Ab, E5/Ab
and G8/Ab (Fig. 10C). These
results confirmed the strong binding affinity of the antibodies
towards their specific targets, thereby demonstrating the
functional capability of the TCR in the soluble antibodies.
Additionally, ELISA of the TCR DABs demonstrated interactions with
the target p-MHC complexes. In the presence of a negative control
(non-target p-MHC complex), the optical densities for H11/Ab, E5/Ab
and G8/Ab were 1.024, 1.440 and 1.17 nm, respectively, at 405 nm
(Fig. 10D).
Discussion
The present study aimed to design TCR-like
antibodies that selectively target the HPV18 E6 and E7 oncoprotein
peptides using DAB) library, driven by the demonstrated efficacy of
TCR-like antibodies in treating various conditions, including
malignancy, viral infections and autoimmune disorders (39). In targeting cervical cancer,
peptides derived from the HPV 18 oncoprotein E6 and E7 serve as
antigenic targets for TCR-like antibody generation against cervical
carcinogenesis (Fig. S11)
(40). The development of these
antibodies focused on the primary p-MHC complex, which presents the
HPV18 E6 and E7 oncoprotein peptides for immune recognition.
Peptide selection was based on their binding affinity to HLA-A24.
As a result, the TCR-like antibodies in the present study may serve
as promising candidates for the early detection of latent CC
(41).
HPV18 E6 and E7 oncoprotein peptides were selected
as specific targets for TCR-like antibodies based on their
predicted affinity for HLA-A24 using SYFPEITHI computational
methods. SYFPEITHI predictions are derived from known sequences and
natural ligands, with an emphasis on common amino acids located at
anchor sites (42). The scoring
system assigns values to amino acids based on their roles as
anchors or preferred residues. Ideal anchors receive a score of 10,
atypical anchors score 6–8 points, auxiliary anchors 4–6 points and
preferred residues 1–4 points. Amino acids that reduce binding
affinity are assigned scores ranging from −1 to −3 (43).
HPV18 p-MHC-A24 complexes were generated by
refolding α chains and incorporating β2m light chain residues and
HPV18 peptides in a cold buffer solution. Efficient refolding of
HLA-A24 was observed with the selected peptides when using the
optimal ligand (30). ELISA
confirmed the successful formation of each p-MHC complex. Anti-β2m
HRP was used instead of streptavidin HRP due to the high
selectivity of anti-β2m HRP for the β2m light chain, which prevents
detection of unbound heavy chains and ensures maximal accuracy
(35).
Monoclonal antibodies were selected using the
antibody phage display technique, based on biopanning analysis of
HPV18 p-MHC-A24 complexes against a library of antibodies targeting
the human domain. Effective phage enrichment was achieved after two
rounds of selection, eliminating the need for further rounds
(44). Significant ELISA results
confirmed successful phage retrieval and packaging, facilitated by
a precise trypsin-mediated elution. Trypsin was used to remove
background contamination from the trypsin-sensitive helper phage
protein III due to its ability to cleave the c-Myc tag located
between the antibody fragment and the phage coat protein (protein
III) (33). The screening for
monoclonal antibodies involved the selection of 94 clones with low
background values. To ensure the production of highly specific
antibodies targeting HPV18 p-MHC-A24 complexes, comparative ELISA
was conducted on all clones. Sequencing data were analyzed using
the IMGT tool to align the core set with the CDR and framework
region placements, confirming that the length of each region fell
within the expected range.
The binding affinity of TCR-like antibodies to
target complexes was evaluated using western blotting and ELISA. In
the western blot analysis, a notable interaction between the
TCR-like antibodies and targets was demonstrated on the membrane
surface, following the detection of biotinylated HLA-A24 heavy
chain within the p-MHC complex using HRP-conjugated streptavidin.
These findings confirmed the high affinity of the TCR-like
antibodies for their target complexes. To assess their selectivity,
two controls were employed in the ELISA screenings. The first
control, targeting non-p-MHC-A24 complexes, ensured the TCR-like
antibodies did not bind unrelated p-MHC segments. Additionally,
background control was used to verify the absence of non-specific
binding to the p-MHC complex. TCR-like DABs exhibited no
non-specific binding to unrelated p-MHC complexes or unintended
interaction, demonstrating a high level of selectivity for their
target p-MHC complexes.
TCR-like antibodies serve a role in identifying
cancer-associated proteins, as demonstrated in previous clinical
studies (45–48). The development of TCR-like
antibodies has gained substantial attention (49–52).
Increasingly, studies have explored their potential medical
applications, aiming to harness these antibodies for immunotherapy
and diagnosis of various types of disease as melanoma and prostate
and Breast cancer (48,50,53–56).
This may advance TCR-cancer therapy research, particularly if these
antibodies exhibit enhanced efficacy against antigenic targets
(57,58). Recent advancements in
new-generation immunotherapy have potential application in cancer
treatment (59–66).
The ability of TCR-like antibodies to recognize
tumor-specific or -associated antigens, several of which are
intracellular and presented on MHC molecules (40,46),
provides a foundation for effective immunotherapy of CC (67). These antigens derive from
tumor-specific and oncogenic viral proteins or neoantigens, which
are processed by the cellular proteasome into shorter antigenic
peptide sequences. These peptides are transported into the
endoplasmic reticulum, where they bind MHC class I molecules. The
resulting p-MHC complex is transferred via the Golgi apparatus and
displayed on the cell surface (21,68,69).
TCRs recognize intracellular CC antigenic peptides presented on MHC
class I molecules of cancer cells, thereby eliciting immune
responses, including phagocytosis, antibody-dependent cellular
cytotoxicity (ADCC), complement-dependent cytotoxicity and other
immune components. When combined with immunotoxins, TCR-like
antibody fusion molecules or chemotherapy, these antibodies
contribute to a therapeutic strategy for CC (67). Unlike T cell-based immunotherapy,
TCR-like antibodies are unaffected by T cell depletion, overcoming
a key limitation and enhancing therapeutic efficacy (34).
TCR-like antibodies have traditionally been
challenging to generate. However, new advances, including improved
antigen production methods, have facilitated the development of
TCR-like antibodies targeting an expanding repertoire of tumor and
viral antigens (39,70–72).
These antigens include Wilms' tumor gene1 (WT1), α-fetoprotein
(AFP), preferentially expressed antigen in melanoma (PRAME),
NY-ESO-1, MAGEA1, telomerase reverse transcriptase (hTERT), TCRγ
alternate reading frame protein (TARP), tyrosinase, hCG β, p53, p68
MIF, proteinase 3, MAGE3 and Epstein Barr virus (EBV) proteins
(50). The primary methods for
isolating TCR-like antibodies are immunization followed by
hybridoma and in vitro screening through phage display.
Additionally, single B cell sorting and cloning offer an
alternative approach for TCR-like antibody isolation, further
broadening the potential for development (73). TCR-like antibody fusion molecules
also show promise for enhancing cancer immunotherapy. These
antibodies can be paired with single-chain variable fragment (scFv)
dimers, known as diabodies or tandem scFvs, which increase affinity
by doubling interactions between tumor and effector immune cells,
thus enhancing cytotoxicity and overall therapeutic effectiveness
(74). Another promising approach
involves developing chimeric antigen receptors (CARs) incorporating
TCR-like antibodies. CARs enable cytotoxic T lymphocytes to
selectively destroy tumor cells. Designed with the intracellular
domain of the CD3 protein essential for T cell activation, TCR-like
antibody CARs specifically recognize p-MHC molecules on cancer
cells, triggering T cell-mediated tumor destruction (75). Furthermore, priority should be
given to developing faster, more cost-effective and widely
accessible diagnostic methods at the population level, leveraging
the high potential of TCR-like antibodies to selectively diagnose
cancer antigens with precision.
Discovery of CC biomarkers has unveiled new
opportunities for diagnostic and therapeutic advances. These
biomarkers not only improve histopathological screening accuracy
but also enhance risk assessment, enable improved monitoring of
high-risk and post-treatment patients and support personalized
treatment planning (76–78). Additionally, certain biomarkers
serve as key antigenic targets for TCR-like antibody development
(78). For example, HPV
oncoproteins E6 and E7, which are key drivers of cervical
carcinogenesis, are ideal candidates. The E6 oncoprotein helps
cells proliferate by blocking the p53 tumor suppressor, which
normally stops cells from dying and the cell cycle from stopping.
Meanwhile, the E7 oncoprotein inactivates the retinoblastoma (Rb)
tumour suppressor, contributing to cell immortalization (79). Another distinctive target is
cancer-testis (CT) antigens, which are normally restricted to
testis germ cells in healthy individuals but are upregulated in
several types of malignancy, including CC and ovarian, breast,
renal and colorectal cancer (80).
The differential expression of CT antigens between healthy and
cancerous cells makes them valuable for diagnosis and as
therapeutic targets for TCR-like antibody generation. CT antigens
in CC include sperm-associated antigen (SPAG), B cell
receptor-associated protein 31, MAGE family proteins (A3, A4, A6
and A12), GAGE-3/6 (tumor-associated antigens), PRAME and LAGE-1
(53,81,82).
TCR-like human IgG1 antibody (Pr20) targeting the PRAME peptide ALY
mediates ADCC against leukemia cells in vivo, validating the
potential of CT antigens for CC immunotherapy (83). Similar results have been observed
with other TCR-like antibodies targeting tumor antigens expressed
in CC, emphasizing the potential of using the same antigenic
targets for therapeutic antibody generation (84–87).
Contrary to earlier hypotheses, previous analysis of patient
samples of CC suggests that HPV is not responsible for MHC class I
downregulation (88). An analysis
of >800 CC datasets from The Cancer Genome Atlas previously
revealed that MHC class I expression in HPV-positive CC cells is
comparable with that in HPV-negative tumors and non-cancerous cells
(89).
Despite the availability of conventional diagnostic
methods, the incidence of CC continues to rise (90): The numbers of estimated cervical
cancer cases and deaths are projected to rise by 56.8 and 80.7%,
respectively, by 2050. Furthermore, the anticipated increase in
early-onset CC is predominantly observed in countries with a low
Human Development Index), whereas a decline in disease burden is
expected in countries with a high Human Development Index HDI)
(90). The Visual Inspection with
Acetic Acid (VIA) test and Pap smear are commonly used procedures
for CC screening (91). However,
these methods have limited effectiveness, particularly in
resource-poor regions (92).
Comparative analyses of Pap smears and VIA tests reveal that VIA
has sensitivity of ~80 and an accuracy of 63.4, whereas the Pap
smear demonstrates a sensitivity of ~50 and an accuracy of 69.9%.
While VIA offers greater sensitivity, its overall accuracy remains
limited in CC screening. The Pap smear requires the visual
identification of cellular changes near the optical resolution
limit, making it challenging to detect small and localized
precancerous lesions (93).
Additionally, the number of diagnostic pre-malignant cells in a
specimen may be minimal and ~1,000 fields of view must be examined
under 10× magnification to analyze the entire sample. The time
required for this varies depending on sample complexity but
generally ranges from 5 to 10 min/sample. Fatigue-associated
guidelines of American Society of Cytopathology Workload recommend
that cytotechnicians work <7 h/day (94). Additionally, the molecular methods,
such as PCR assays for HPV diagnosis, selectively amplify DNA
targets but present additional challenges (95). HPV molecular testing identifies
viral genes rather than oncoproteins, making it incapable of
determining the stage of CC. For example, HPV DNA detection in CC
specimens has sensitivity of 37.9% (96). In resource-limited settings, test
specificity is key, as follow-up procedures for false-positive
results increase costs and may lead to unnecessary treatment. Low
specificity contributes to overtreatment, placing strain on
healthcare systems (95). While
therapeutic vaccines for CC offer a multifaceted approach, they are
associated with limitations. These include potential toxicity in
immunocompromised patients, the development of neutralizing
antibodies that diminish efficacy with repeated doses, HLA
restrictions that impede universal vaccination and the need for
booster components to enhance low-grade immunoglobulin responses
(40). Although HPV vaccines
effectively decrease levels of HPV-associated cancer precursors and
minimize the need for therapeutic interventions, they are linked to
increased risks of severe nervous system disorder (97,98),
and other adverse effects (99–101). The benefits of HPV vaccination
remain uncertain when weighed against these risks, as several
studies have prioritized efficacy over safety (102,103). Furthermore, limited access to
comprehensive clinical study reports and trial data prevents
thorough assessment of potential adverse effects (104). Moreover, current vaccines
primarily target HPV strains 16 and 18, which account for ~70% of
CC cases, providing only partial protection (105). The high cost of vaccines
restricts their use in public health programs in low-income
countries. This highlights the need for innovative approaches to
detect precursor lesions and address CC (104). Given these challenges, there is
need for novel strategies for early detection of CC. Diagnostic
techniques that detect intracellular markers using TCR-like
antibodies offer a promising approach for the early and accurate
diagnosis of CC.
TCR-like antibodies hold promise for the future of
CC immunotherapy. However, numerous challenges must be addressed to
optimize their effectiveness in treatment. One key challenge is the
HLA restriction of epitopes, as TCR-like antibodies are specific to
particular types of HLA (106).
This specificity means that the antibodies are only beneficial for
individuals with certain types of HLA. Moreover, prevalence of HLA
types varies based on factors such as geographical location and
disease susceptibility (107).
For example, the HLA-A2 gene is prevalent globally, while HLA-A11
and HLA-A24 are concentrated in Asian populations (108,109). Additionally, certain HLA genes,
such as HLA-DQB10602 and HLA-DRB11501, are associated with
susceptibility to HPV infection (110). These factors highlight the
importance of selecting HLAs during TCR-like antibody development
to maximize their applicability. Previous studies have explored
multiple HLAs, including HLA-A2, HLA-A11, and HLA-A24, to ensure
broader population coverage (111–113). A second challenge of TCR-like
antibodies, particularly in cancer therapy, is the suppression of
antigen presentation on HLA molecules by tumors (114). Various strategies have been
proposed to optimize the use of TCR-like antibodies. These includes
using cytokines such as IFNγ to increase the expression of
proteasome activators as low molecular mass polypeptides (LMP2,
LMP7), multicatalytic endopeptidase complex (MECL-1), transporter
associated with antigen processing complex (TAP1/TAP2) and MHC
heavy chains, with TNFα helping to stabilize and enhance MHC
functionality (115). LMP2, LMP7
and MECL-1 facilitate protein degradation and peptide generation
for cytotoxic T cell presentation (116), while TAP1/TAP2 transport foreign
peptides to the endoplasmic reticulum for binding with MHC I. The
resulting p-MHC I complexes are then presented on the cell surface,
initiating an immune response (117). Another strategy involves
administering low-dose chemotherapeutic agents, ionizing radiation
or topotecan, all of which increase MHC class I expression in
breast cancer cells (118). A
third strategy involves using pathway inhibitors, such as MEK
inhibitors (targeting the MAPK pathway) and erlotinib (targeting
the EGFR pathway), to enhance MHC class I expression in esophageal
and gastric cancer (119). In
cases with low p-MHC presentation, TCR-like antibody fusion
molecules offer a promising solution. For example, in a mouse
xenograft model with minimal HLA-A antigen presentation activated
humoral immune responses targeting the cancer-testis antigen SPAG9
(120). Similarly, a TCR-like
antibody conjugated with a therapeutic agent shows cytotoxic
activity against breast and colon cancer cells, even under low
target MHC density, validating the efficacy of TCR-like antibodies
in conditions of reduced antigen presentation (121).
A third challenge of TCR-like antibodies is unknown
specificity of tumour-infiltrating lymphocytes (TILs). Adoptive T
cell transfer is a therapeutic strategy using ex
vivo-expanded TILs), which effectively induce tumor regression
following reinfusion into patients with cancer (122). Despite response rates of 20–50%
(123), widespread use is limited
by lengthy production times and low commercial viability (124). Furthermore, the unknown
specificity of TILs complicate outcome predictions, fostering
interest in therapies such as CAR T cells (125) or T cells engineered with TCRs
targeting known cancer antigens (126). TCR-based gene therapy has gained
feasibility through studies demonstrating redirected T cell
specificity via αβTCR gene transduction, enabling both antiviral
and antitumor responses (127–129). Trials with DMF4 TCRs (130) and DMF5 TCRs (131) targeting HLA-A0201 MART-1 melanoma
peptides have showed that high-affinity TCRs enhance efficacy but
can cause autotoxicity (132).
The aforementioned studies indicate that the success of TCR-based
gene therapy relies on the transfer of T cells expressing
high-affinity TCRs. This is supported by research showing an
association between TCR binding affinity and functional responses,
underscoring the importance of affinity optimization to enhance
therapeutic efficacy (133). As a
result, strategies to design high-affinity TCRs for broader
application have been developed as deep mutational scan (134) and Yeast display strategy
(135).
A fourth challenge is cross-reactivity. Clinical
trials have evaluated the safety and efficacy of adoptive T cell
transfer using TCR-transduced T cells (136–139). While this approach holds
potential for treating cancer with ‘off-the-shelf’ T cell products
applicable to patients sharing the same HLA haplotype, the
development of such therapy is hindered by immunotoxicity risks.
Two key trials have highlighted the need for caution in TCR-based
therapy. In the first, a high-avidity TCR targeting a
MAGE-A3-derived peptide in HLA-A0201 transgenic mice achieved
clinical responses in five of nine mice. However, three mice
experienced neurological toxicity, and two fatalities occurred
(140) due to TCR
cross-recognition of a related brain-expressed MAGE-A12 peptide,
differing by a single residue (141). In the second trial, a TCR
targeting a MAGE-A3-derived peptide presented by HLA-A0101
(142), originally derived from a
vaccinated patient (143), and
engineered to enhance its affinity (144), was administered to two patients
with myeloma and melanoma. Both patients suffered cardiac arrest
and died shortly after receiving the T cell infusion; despite a 55%
sequence overlap with the MAGE-A3 peptide, the TCR cross-reacted
with titin, a protein expressed in contracting cardiomyocytes
(145). Numerous strategies have
been developed to enhance the identification of TCR
cross-reactivity including combinatorial peptide libraries, yeast
display and DNA barcode-labeled MHC multimers. These approaches aim
to identify specific amino acids within peptide sequences that are
key for TCR recognition. By integrating this information with human
proteome and HLA presentation data, these strategies provide a
predictive framework to identify potential cross-reactivity risk
(146).
The combinatorial peptide library method is a
valuable tool for determining the amino acid requirements for TCR
recognition (147), offering
insight into the specific interactions between TCRs and peptides
(148). Although effective for
detailed functional analysis, this approach requires large
quantities of TCR-expressing cells and does not yield a relative
ranking of interactions. To complement this method, smaller-scale
assays may serve as an early-stage selection criterion to identify
TCRs that may cross-react with endogenous peptides, enabling a more
efficient and targeted assessment of TCR specificity (146). The yeast display system focuses
on direct p-MHC-TCR interactions, providing a more accurate
depiction of TCR binding degeneracy compared with combinatorial
peptide libraries and DNA barcode-labeled MHC multimer strategies
(149). This technique, which
uses random peptide sequences and unbiased screening, identifies
TCR-specific peptide targets, including those with previously
unknown specificity (146).
However, this method has limitations, such as the inability to
equally display all peptide sequence positions and cover all
peptide variants. Additionally, its applicability is restricted to
a limited number of MHC molecules and specialized laboratories
(150). Strategies that use
cellular systems, such as T cell clones or TCR-transduced T cells,
bear similarities to the yeast display system and offer notable
advantages. Methods such as signaling and antigen-presenting
bifunctional receptors and trogocytosis-based systems have
effectiveness in antigen discovery and evaluating the breadth of
TCR recognition (151). These
approaches hold potential for identifying TCR-specific antigens and
assessing cross-recognition risk, making them promising tools for
advancing T cell therapy (146).
TCR fingerprinting, which uses DNA barcode-labelled MHC multimers,
is a powerful technique for determining TCR amino acid preferences
for specific peptide sequences (152). This method generates libraries of
peptide variants and analyzes the binding hierarchy of TCRs,
enabling the creation of a positional scoring matrix to determine
TCR specificity (153). While
this approach offers flexibility, sensitivity and ease of
implementation, it has limitations, such as costs of peptide
synthesis and the necessity of a pre-established peptide target for
library construction. Furthermore, the method may not identify
novel p-MHC targets for TCRs with unknown specificity and single
amino acid substitutions might not capture all potential TCR
interactions (154). Despite
these constraints, TCR fingerprinting has shown promise in
identifying TCRs with lower cross-recognition risk, particularly
for mutation-derived neoepitopes, establishing it as a valuable
first-selection criterion for clinical applications (154). Combining experimental approaches
with structural data and in silico modelling may provide
more comprehensive understanding of TCR binding degeneracy and
cross-recognition potential (142). Translating molecular interaction
points of TCRs into cross-recognition potential is another
promising strategy. Understanding the molecular interaction points
of a TCR can identify cross-recognized peptides derived from
endogenous proteins, offering a tool to assess the
cross-recognition potential of a TCR before clinical application.
This approach could decrease risk of severe side effects associated
with TCR therapy. To evaluate this risk, insights from analysis of
TCR interactions with p-MHC should be explored in silico
using tools such as Find Individual Motif Occurrences (155) or Scan Prosite (156), which identify peptide sequences
in the human proteome that align with the molecular interaction
points of a TCR and are therefore at risk of cross-recognition
(146). Despite these challenges,
ongoing advancements and refinements offer viable solutions. He
et al (157) demonstrated
that a Fab-immunotoxin containing Pseudomonas exotoxin could
recognize the melanoma antigenic peptide MART-1 presented by the
HLA-A2*01 molecule, inducing cell death in human melanoma cells
(157). Further validation of
TCR-like antibody-immunotoxins came with the development of another
Fab-immunotoxin targeting TCR gamma alternative reading frame
protein, an antigenic protein associated with breast and prostate
cancer, which effectively induced cell cytotoxicity (158). Additionally, two TCR-mimic
antibody derivatives conjugated with the cytotoxic agent monomethyl
auristatin E (MMAE), ESK-MMAE, and Q2L-MMAE, were designed to
target WT1) oncoprotein (19).
TCR-like antibodies represent a novel and promising class of
therapeutic tool, holding potential for improving diagnosis and
immunotherapy, particularly for CC (40).
The present study identified three TCR-like
antibodies through a systematic screening process targeting the
HPV18 (E6 and E7) oncoprotein antigens presented by MHC-A24.
Analysis of the soluble forms of TCR-like antibodies revealed
notable binding affinity for their respective HPV18 targets. The
findings of the present study may contribute to the enhancement and
optimization of early detection methods and immunotherapeutic
strategies for CC.
Future studies should investigate all HPV variants
associated with CC, specifically HPV 16 and 18. Integrating a range
of HLAs, including A2, A11 and A24, which are part of the MHC class
I family may facilitate applicability of this diagnostic tool to
the global population, regardless of individual HLA variations.
Antibodies should also be tested on cancer cell line models, such
as CaSki, HeLa, SiHa, ME180 and C-33A cells, before proceeding to
the clinical evaluation phase.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Ministry of Higher Education,
Malaysia, Higher Education Centre of Excellence (grant no.
A305-KR-AKH002-0004401005-0000).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BAS performed experiments, analyzed data and wrote
the manuscript. SAD edited the manuscript, conceived the study and
designed the experiments. RSR analyzed data and edited the
manuscript. GJT and VB conceived the study and designed the
experiments. All authors have read and approved the final
manuscript. BS and VB confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CC
|
cervical cancer
|
|
HPV
|
human papilloma virus
|
|
TCR
|
T cell receptor
|
|
HLA-A24
|
human leukocyte antigen within the
HLA-A serotype group
|
|
p-MHC
|
peptide-major histocompatibility
complex
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
NCBI
|
National Centre for Biotechnology
Information
|
|
β2m
|
β-2-microglobulin
|
|
IMGT
|
ImMunoGeneTics information
system®
|
References
|
1
|
World Health Organization (WHO), . Global
strategy to accelerate the elimination of cervical cancer as a
public health problem. WHO; Geneva: 2020
|
|
2
|
Wang M, Huang K, Wong MCS, Huang J, Jin Y
and Zheng ZJ: Global cervical cancer incidence by histological
subtype and implications for screening methods. J Epidemiol Glob
Health. 14:94–101. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al: Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boni SP, Tenet V, Horo A, Heideman DAM,
Bleeker MCG, Tanon A, Mian B, Mohenou ID, Ekouevi DK, Gheit T, et
al: High-risk human papillomavirus distribution according to human
immunodeficiency virus status among women with cervical cancer in
Abidjan, Côte d'Ivoire, 2018 to 2020. Int J Cancer. 154:962–968.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Li M, Yuan F, Jiang J and Zhang
X: The difference of transcriptome of HPV-infected patients
contributes more to the occurrence of cervical cancer than the
mutations of E6 and E7 genes in HPV16. Medicine (Baltimore).
103:e368222024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jabbar SF, Abrams L, Glick A and Lambert
PF: Persistence of high-grade cervical dysplasia and cervical
cancer requires the continuous expression of the human
papillomavirus type 16 E7 oncogene. Cancer Res. 69:4407–4414. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pflaum J, Schlosser S and Müller M: p53
family and cellular stress responses in cancer. Front Oncol.
4:2852014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boyer SN, Wazer DE and Band V: E7 protein
of human papilloma virus-16 induces degradation of retinoblastoma
protein through the ubiquitin-proteasome pathway. Cancer Res.
56:4620–4624. 1996.PubMed/NCBI
|
|
9
|
Bahmani B, Amini-Bayat Z, Ranjbar MM,
Bakhtiari N and Zarnani AH: HPV16-E7 protein T cell epitope
prediction and global therapeutic peptide vaccine design based on
human leukocyte antigen frequency: An in-silico study. Int J Pept
Res Ther. 27:365–378. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koliopoulos G, Nyaga VN, Santesso N,
Bryant A, Martin-Hirsch PP, Mustafa RA, Schünemann H, Paraskevaidis
E and Arbyn M: Cytology versus HPV testing for cervical cancer
screening in the general population. Cochrane Database Syst Rev.
8:CD0085872017.PubMed/NCBI
|
|
11
|
Cortés-Alaguero C, González-Mirasol E,
Morales-Roselló J and Poblet-Martinez E: Do clinical data and human
papilloma virus genotype influence spontaneous regression in grade
I cervical intraepithelial neoplasia? J Turk Ger Gynecol Assoc.
18:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sroczynski G, Esteban E, Widschwendter A,
Oberaigner W, Borena W, von Laer D, Hackl M, Endel G and Siebert U:
Reducing overtreatment associated with overdiagnosis in cervical
cancer screening-A model-based benefit-harm analysis for Austria.
Int J Cancer. 147:1131–1142. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng S, Xing D, Ferrall L, Tsai YC, Hung
CF and Wu TC: Identification of human MHC-I HPV18 E6/E7-specific
CD8 + T cell epitopes and generation of an HPV18 E6/E7-expressing
adenosquamous carcinoma in HLA-A2 transgenic mice. J Biomed Sci.
29:802022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Middleton D, Menchaca L, Rood H and
Komerofsky R: New allele frequency database. http://www.allelefrequencies.netTissue Antigens.
61:403–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanchez-Mazas A and Nunes JM: PGAE HLA
Consortium of the 18th International HLA and Immunogenetics
Workshop: The most frequent HLA alleles around the world: A
fundamental synopsis. Best Pract Res Clin Haematol. 37:1015592024.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arrieta-Bolaños E, Hernández-Zaragoza DI
and Barquera R: An HLA map of the world: A comparison of HLA
frequencies in 200 worldwide populations reveals diverse patterns
for class I and class II. Front Genet. 14:8664072023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferrera A, Valladares W, Cabrera Y, de la
Luz Hernandez M, Darragh T, Baena A, Almonte M and Herrero R:
Performance of an HPV 16/18 E6 oncoprotein test for detection of
cervical precancer and cancer. Int J Cancer. 145:2042–2050. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagano K and Tsutsumi Y: Phage display
technology as a powerful platform for antibody drug discovery.
Viruses. 13:1782021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hammers CM and Stanley JR: Antibody phage
display: Technique and applications. J Invest Dermatol. 134:1–5.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen M and Reiter Y: T-cell receptor-like
antibodies: Targeting the intracellular proteome therapeutic
potential and clinical applications. Antibodies. 2:517–534. 2013.
View Article : Google Scholar
|
|
21
|
Andersen PS, Stryhn A, Hansen BE, Fugger
L, Engberg J and Buus S: A recombinant antibody with the
antigen-specific, major histocompatibility complex-restricted
specificity of T cells. Proc Natl Acad Sci USA. 93:1820–1824. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Altman JD, Moss PA, Goulder PJ, Barouch
DH, McHeyzer-Williams MG, Bell JI, McMichael AJ and Davis MM:
Phenotypic analysis of antigen-specific T lymphocytes. Science.
274:94–96. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garboczi DN, Hung DT and Wiley DC:
HLA-A2-peptide complexes: Refolding and crystallization of
molecules expressed in Escherichia coli and complexed with
single antigenic peptides. Proc Natl Acad Sci USA. 89:3429–3433.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denkberg G, Cohen CJ and Reiter Y:
Critical role for CD8 in binding of MHC tetramers to TCR: CD8
antibodies block specific binding of human tumor-specific
MHC-peptide tetramers to TCR. J Immunol. 167:270–276. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yazdani Z, Rafiei A, Valadan R, Ashrafi H,
Pasandi M and Kardan M: Designing a potent L1 protein-based HPV
peptide vaccine: A bioinformatics approach. Comput Biol Chem.
85:1072092020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rammensee H, Bachmann J, Emmerich NP,
Bachor OA and Stevanović S SYFPEITHI: Database for MHC ligands and
peptide motifs. Immunogenetics. 50:213–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang AY, Chau V, Landas JA and Pang Y:
Preparation of calcium competent Escherichia coli and
heat-shock transformation. JEMI Methods. 1:22–25. 2017.
|
|
28
|
McConkey VL: DNA-barcoding for the
inference of larval community structure of non-biting midges
(Chironomidae) from the River Stour, Kent (unpublished thesis).
Canterbury Christ Church University; 2017
|
|
29
|
Figueroa-Bossi N, Balbontín R and Bossi L:
Preparing plasmid DNA from bacteria. Cold Spring Harb Protoc.
2022.Pdb.prot107852. 2022. View Article : Google Scholar
|
|
30
|
Rodenko B, Toebes M, Hadrup SR, Van Esch
WJE, Molenaar AM, Schumacher TNM and Ovaa H: Generation of
peptide-MHC class I complexes through UV-mediated ligand exchange.
Nat Protoc. 1:1120–1132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manns JM: SDS-polyacrylamide gel
electrophoresis (SDS-PAGE) of proteins. Curr Protoc Microbiol.
22:A.3M.1–A.3M.13. 2011.
|
|
32
|
Luimstra JJ, Franken KMCL, Garstka MA,
Drijfhout JW, Neefjes J and Ovaa H: Production and thermal exchange
of conditional peptide-MHC I multimers. Curr Protoc Immunol.
126:e852019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee CMY, Iorno N, Sierro F and Christ D:
Selection of human antibody fragments by phage display. Nat Protoc.
2:3001–3008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dass SA, Norazmi MN, Dominguez AA, San
Miguel MESG and Tye GJ: Generation of a T cell receptor (TCR)-like
single domain antibody (sDAb) against a mycobacterium tuberculosis
(Mtb) heat shock protein (HSP) 16kDa antigen presented by Human
Leukocyte Antigen (HLA)-A*02. Mol Immunol. 101:189–196. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dass SA, Norazmi MN, Acosta A, Sarmiento
ME and Tye GJ: TCR-like domain antibody against mycobacterium
tuberculosis (Mtb) heat shock protein antigen presented by HLA-A*11
and HLA-A*24. Int J Biol Macromol. 155:305–314. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Falgenhauer E, von Schönberg S, Meng C,
Mückl A, Vogele K, Emslander Q, Ludwig C and Simmel FC: Evaluation
of an E. coli cell extract prepared by lysozyme-assisted
sonication via gene expression, phage assembly and proteomics.
Chembiochem. 22:2805–2813. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rouet R, Lowe D, Dudgeon K, Roome B,
Schofield P, Langley D, Andrews J, Whitfeld P, Jermutus L and
Christ D: Expression of high-affinity human antibody fragments in
bacteria. Nat Protoc. 7:364–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao WB, Shen Y, Liu WH, Li YM, Jin SJ, Xu
YC, Pan LQ, Zhou Z and Chen SQ: Soluble expression of Fc-fused T
cell receptors allows yielding novel bispecific T cell engagers.
Biomedicines. 9:7902021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dahan R and Reiter Y: T-cell-receptor-like
antibodies-generation, function and applications. Expert Rev Mol
Med. 14:e62012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dass SA, Selva Rajan R, Tye GJ and
Balakrishnan V: The potential applications of T cell receptor
(TCR)-like antibody in cervical cancer immunotherapy. Hum Vaccin
Immunother. 17:2981–2994. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan LK, Mohd-Farid B, Salsabil S, Heselynn
H, Wahinuddin S, Lau S, Gun SC, Nor-Suhaila S, Eashwary M,
Mohd-Shahrir MS, et al: HLA-A, -B, -C, -DRB1 and -DQB1 alleles and
haplotypes in 951 Southeast Asia Malays from Peninsular Malaysia.
Hum Immunol. 77:818–819. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schuler MM, Nastke MD and Stevanović S:
SYFPEITHI: Database for searching and T-cell epitope prediction.
Immunoinformatics: Predicting immunogenicity in silico. 75–93.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rammensee H, Bachmann J, Emmerich NP,
Bachor OA and Stevanović S: SYFPEITHI: Database for MHC ligands and
peptide motifs. Immunogenetics. 50:213–319. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lim BN, Chin CF, Choong YS, Ismail A and
Lim TS: Generation of a naïve human single chain variable fragment
(scFv) library for the identification of monoclonal scFv against
Salmonella Typhi Hemolysin E antigen. Toxicon. 117:94–101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dang E, Yang S, Song C, Jiang D, Li Z, Fan
W, Sun Y, Tao L, Wang J, Liu T, et al: BAP31, a newly defined
cancer/testis antigen, regulates proliferation, migration, and
invasion to promote cervical cancer progression. Cell Death Dis.
9:7912018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheng J, Zhao L, Zhang Y, Qin Y, Guan Y,
Zhang T, Liu C and Zhou J: Understanding the mechanisms of
resistance to CAR T-cell therapy in malignancies. Front Oncol.
9:12372019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dao T, Yan S, Veomett N, Pankov D, Zhou L,
Korontsvit T, Scott A, Whitten J, Maslak P, Casey E, et al:
Targeting the intracellular WT1 oncogene product with a therapeutic
human antibody. Sci Transl Med. 5:176ra332013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chames P, Hufton SE, Coulie PG,
Uchanska-Ziegler B and Hoogenboom HR: Direct selection of a human
antibody fragment directed against the tumor T-cell epitope
HLA-A1-MAGE-A1 from a nonimmunized phage-Fab library. Proc Natl
Acad Sci USA. 97:7969–7974. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Neethling FA, Ramakrishna V, Keler T,
Buchli R, Woodburn T and Weidanz JA: Assessing vaccine potency
using TCRmimic antibodies. Vaccine. 26:3092–3102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li D, Bentley C, Anderson A, Wiblin S,
Cleary KL, Koustoulidou S, Hassanali T, Yates J, Greig J, Nordkamp
MO, et al: Development of a T-cell receptor mimic antibody against
wild-type p53 for cancer immunotherapy. Cancer Res. 77:2699–2711.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Weidanz JA, Nguyen T, Woodburn T,
Neethling FA, Chiriva-Internati M, Hildebrand WH and Lustgarten J:
Levels of specific peptide-HLA class I complex predicts tumor cell
susceptibility to CTL killing. J Immunol. 177:5088–5097. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Epel M, Carmi I, Soueid-Baumgarten S, Oh
S, Bera T, Pastan I, Berzofsky J and Reiter Y: Targeting TARP, a
novel breast and prostate tumor-associated antigen, with T cell
receptor-like human recombinant antibodies. Eur J Immunol.
38:1706–1720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wittman VP, Woodburn D, Nguyen T,
Neethling FA, Wright S and Weidanz JA: Antibody targeting to a
class I MHC-peptide epitope promotes tumor cell death. J Immunol.
177:4187–4195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cohen CJ, Hoffmann N, Farago M, Hoogenboom
HR, Eisenbach L and Reiter Y: Direct detection and quantitation of
a distinct T-cell epitope derived from tumor-specific epithelial
cell-associated mucin using human recombinant antibodies endowed
with the antigen-specific, major histocompatibility
complex-restricted specificity of T cells. Cancer Res.
62:5835–5844. 2002.PubMed/NCBI
|
|
55
|
Lev A, Denkberg G, Cohen CJ, Tzukerman M,
Skorecki KL, Chames P, Hoogenboom HR and Reiter Y: Isolation and
characterization of human recombinant antibodies endowed with the
antigen-specific, major histocompatibility complex-restricted
specificity of T cells directed toward the widely expressed tumor
T-cell epitopes of the telomerase catalytic subunit. Cancer Res.
62:3184–3194. 2002.PubMed/NCBI
|
|
56
|
Willemsen R, Debets R, Hart E, Hoogenboom
H, Bolhuis R and Chames P: A phage display selected fab fragment
with MHC class I-restricted specificity for MAGE-A1 allows for
retargeting of primary human T lymphocytes. Gene Ther. 8:1601–1608.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chang AY, Dao T, Gejman RS, Jarvis CA,
Scott A, Dubrovsky L, Mathias MD, Korontsvit T, Zakhaleva V, Curcio
M, et al: A therapeutic T cell receptor mimic antibody targets
tumor-associated PRAME peptide/HLA-I antigens. J Clin Invest.
127:2705–2718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sastry KS, Too CT, Kaur K, Gehring AJ, Low
L, Javiad A, Pollicino T, Li L, Kennedy PT, Lopatin U, et al:
Targeting hepatitis B virus-infected cells with a T-cell
receptor-like antibody. J Virol. 85:1935–1942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Desai I, Thakur S and Pagariya P: Current
advances in immunotherapy for cancer. Oral Oncol Rep.
12:1006522024. View Article : Google Scholar
|
|
60
|
Zhao Y, Zhang L, Fang W, Yang Y, Huang Y,
Zou W, Wang Z, Ding M, Peng Y, Xiao S, et al: SI-B001 plus
chemotherapy in patients with locally advanced or metastatic
EGFR/ALK wild-type non-small cell lung cancer: A phase II,
multicenter, open-label study. J Clin Oncol. 41 (16
Suppl):S90252023. View Article : Google Scholar
|
|
61
|
Yang Y, Zhao Y, Zhou T, Chen G, Huang Y,
Liu F, Liu Z, Qu S, Lei Y, Chen X, et al: A phase Ib study of
SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β,
in patients with recurrent or metastatic nasopharyngeal carcinoma
(RM-NPC). J Clin Oncol. 40 (16 Suppl):S60242022. View Article : Google Scholar
|
|
62
|
Lesokhin AM, Tomasson MH, Arnulf B, Bahlis
NJ, Miles Prince H, Niesvizky R, Rodrίguez-Otero P, Martinez-Lopez
J, Koehne G, Touzeau C, et al: Elranatamab in relapsed or
refractory multiple myeloma: Phase 2 MagnetisMM-3 trial results.
Nat Med. 29:2259–2267. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Singh AK, Dadey DY, Rau MJ, Fitzpatrick J,
Shah HK, Saikia M, Townsend R, Thotala D, Hallahan DE and Kapoor V:
Blocking the functional domain of TIP1 by antibodies sensitizes
cancer to radiation therapy. Biomed Pharmacother. 166:1153412023.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Modi S, Jacot W, Yamashita T, Sohn J,
Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Chae YS, Lee KS, et al:
Trastuzumab deruxtecan (T-DXd) versus treatment of physician's
choice (TPC) in patients (pts) with HER2-low unresectable and/or
metastatic breast cancer (mBC): Results of DESTINY-Breast04, a
randomized, phase 3 study. J Clin Oncol. 40 (17 Suppl):LBA32022.
View Article : Google Scholar
|
|
65
|
Äärelä A, Räsänen K, Holm P, Salo H
and Virta P: Synthesis of site-specific
antibody-[60]fullerene-oligonucleotide conjugates for cellular
targeting. ACS Appl Bio Mater. 6:3189–3198. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kosuge H, Nagatoishi S, Kiyoshi M,
Ishii-Watabe A, Terao Y, Ide T and Tsumoto K: Biophysical
characterization of the contribution of the Fab region to the
igG-fcγRIIIa interaction. Biochemistry. 62:262–269. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rosenberg SA, Restifo NP, Yang JC, Morgan
RA and Dudley ME: Adoptive cell transfer: A clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sela-Culang I, Kunik V and Ofran Y: The
structural basis of antibody-antigen recognition. Front Immunol.
4:3022013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Blum JS, Wearsch PA and Cresswell P:
Pathways of antigen processing. Annu Rev Immunol. 31:443–473. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Welsh RM, Selin LK and Szomolanyi-Tsuda E:
Immunological memory to viral infections. Annu Rev Immunol.
22:711–743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
de Haard HJ, van Neer N, Reurs A, Hufton
SE, Roovers RC, Henderikx P, de Bruïne AP, Arends JW and Hoogenboom
HR: A large non-immunized human Fab fragment phage library that
permits rapid isolation and kinetic analysis of high affinity
antibodies. J Biol Chem. 274:18218–18230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sergeeva A, Alatrash G, He H, Ruisaard K,
Lu S, Wygant J, McIntyre BW, Ma Q, Li D, St John L, et al: An
anti-PR1/HLA-A2 T-cell receptor-like antibody mediates
complement-dependent cytotoxicity against acute myeloid leukemia
progenitor cells. Blood. 117:4262–4272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Duan Z and Ho M: T-cell receptor mimic
antibodies for cancer immunotherapy. Mol Cancer Ther. 20:1533–1541.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Doran SL, Stevanović S, Adhikary S,
Gartner JJ, Jia L, Kwong MLM, Faquin WC, Hewitt SM, Sherry RM, Yang
JC, et al: T-cell receptor gene therapy for human
papillomavirus-associated epithelial cancers: A first-in-human,
phase I/II study. J Clin Oncol. 37:2759–2768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Turunen L, Takkinen K, Söderlund H and
Pulli T: Automated panning and screening procedure on microplates
for antibody generation from phage display libraries. J Biomol
Screen. 14:282–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Welters MJP, Kenter GG, Piersma SJ, Vloon
APG, Löwik MJG, Berends-van Der Meer DMA, Drijfhout JW, Valentijn
AR, Wafelman AR, Oostendorp J, et al: Induction of tumor-specific
CD4+ and CD8+ T-cell immunity in cervical cancer patients by a
human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin
Cancer Res. 14:178–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kenter GG, Welters MJP, Valentijn ARPM,
Lowik MJG, Berends-van der Meer DMA, Vloon APG, Essahsah F, Fathers
LM, Offringa R, Drijfhout JW, et al: Vaccination against HPV-16
oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med.
361:1838–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Vigneron N: Human tumor antigens and
cancer immunotherapy. Biomed Res Int. 2015:9485012015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yim EK and Park JS: The role of HPV E6 and
E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer
Res Treat. 37:319–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wieczorek M, Abualrous ET, Sticht J,
Álvaro-Benito M, Stolzenberg S, Noé F and Freund C: Major
histocompatibility complex (MHC) class I and MHC class II proteins:
Conformational plasticity in antigen presentation. Front Immunol.
8:2922017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Van Erp EA, Luytjes W, Ferwerda G and Van
Kasteren PB: Fc-mediated antibody effector functions during
respiratory syncytial virus infection and disease. Front Immunol.
10:5482019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sergeeva A, He H, Ruisaard K, St John L,
Alatrash G, Clise-Dwyer K, Li D, Patenia R, Hong R, Sukhumalchandra
P, et al: Activity of 8F4, a T-cell receptor-like anti-PR1/HLA-A2
antibody, against primary human AML in vivo. Leukemia.
30:1475–1484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Dubrovsky L, Dao T, Gejman RS, Brea EJ,
Chang AY, Oh CY, Casey E, Pankov D and Scheinberg DA: T cell
receptor mimic antibodies for cancer therapy. Oncoimmunology.
5:e10498032015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ataie N, Xiang J, Cheng N, Brea EJ, Lu W,
Scheinberg DA, Liu C and Ng HL: Structure of a TCR-mimic antibody
with target predicts pharmacogenetics. J Mol Biol. 428:194–205.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Aruna G: Immunotoxins: A review of their
use in cancer treatment. J Stem Cells Regen Med. 1:31–36. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kreitman RJ: Immunotoxins for targeted
cancer therapy. AAPS J. 8:E532–E551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Klechevsky E, Gallegos M, Denkberg G,
Palucka K, Banchereau J, Cohen C and Reiter Y: Antitumor activity
of immunotoxins with T-cell receptor-like specificity against human
melanoma xenografts. Cancer Res. 68:6360–6367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shen Y, Li YM, Zhou JJ, Zhou Z, Xu YC,
Zhao WB and Chen SQ: The antitumor activity of TCR-mimic
antibody-drug conjugates (TCRm-ADCs) targeting the intracellular
wilms tumor 1 (WT1) oncoprotein. Int J Mol Sci. 20:39122019.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kurosawa N, Wakata Y, Ida K, Midorikawa A
and Isobe M: High throughput development of TCR-mimic antibody that
targets survivin-2B80-88/HLA-A*A24 and its application
in a bispecific T-cell engager. Sci Rep. 9:98272019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wu J, Jin Q, Zhang Y, Ji Y, Li J, Liu X,
Duan H, Feng Z, Liu Y, Zhang Y, et al: Global burden of cervical
cancer: Current estimates, temporal trend and future projections
based on the GLOBOCAN 2022. Journal of the National Cancer Center.
2025. View Article : Google Scholar
|
|
91
|
Romli R, Shahabudin S, Saddki N and
Mokhtar N: Effectiveness of a health education program to improve
knowledge and attitude towards cervical cancer and pap smear: A
controlled community trial in Malaysia. Asian Pac J Cancer Prev.
21:853–859. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Catarino R, Petignat P, Dongui G and
Vassilakos P: Cervical cancer screening in developing countries at
a crossroad: Emerging technologies and policy choices. World J Clin
Oncol. 6:281–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Huy NVQ, Tam LM, Tram NVQ, Thuan DC, Vinh
TQ, Thanh CN and Chuang L: The value of visual inspection with
acetic acid and Pap smear in cervical cancer screening program in
low resource settings-a population-based study. Gynecol Oncol Rep.
24:18–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Elsheikh TM, Austin RM, Chhieng DF, Miller
FS, Moriarty AT and Renshaw AA; American Society of Cytopathology,
: American society of cytopathology workload recommendations for
automated pap test screening: Developed by the productivity and
quality assurance in the era of automated screening task force.
Diagn Cytopathol. 41:174–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kumar A, Suri V and Dabral A: Role of
conventional pap smear in current times. J Colposcopy Low Genit
Tract Pathol. 2:60–64. 2024. View Article : Google Scholar
|
|
96
|
Cocuzza CE, Martinelli M, Sina F, Piana A,
Sotgiu G, Dell'Anna T and Musumeci R: Human papillomavirus DNA
detection in plasma and cervical samples of women with a recent
history of low grade or precancerous cervical dysplasia. PLoS One.
12:e01885922017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Blitshteyn S and Brook J: Postural
tachycardia syndrome (POTS) with anti-NMDA receptor antibodies
after human papillomavirus vaccination. Immunol Res. 65:282–284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Brinth LS, Pors K, Theibel AC and Mehlsen
J: Orthostatic intolerance and postural tachycardia syndrome as
suspected adverse effects of vaccination against human papilloma
virus. Vaccine. 33:2602–2605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Palmieri B, Poddighe D, Vadala M, Laurino
C, Carnovale C and Clementi E: Severe somatoform and dysautonomic
syndromes after HPV vaccination: case series and review of
literature. Immunol Res. 65:106–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ojha RP, Jackson BE, Tota JE,
Offutt-Powell TN, Singh KP and Bae S: Guillain-Barre syndrome
following quadrivalent human papillomavirus vaccination among
vaccine-eligible individuals in the United States. Hum Vaccin
Immunother. 10:232–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Little DT and Ward HRG: Premature ovarian
failure 3 years after menarche in a 16-year-old girl following
human papillomavirus vaccination. BMJ Case Rep.
2012:bcr20120068792012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jørgensen L, Gøtzsche PC and Jefferson T:
Benefits and harms of the human papillomavirus (HPV) vaccines:
Systematic review with meta-analyses of trial data from clinical
study reports. Syst Rev. 9:432020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jefferson T and Jørgensen L: Human
papillomavirus vaccines, complex regional pain syndrome, postural
orthostatic tachycardia syndrome, and autonomic dysfunction-a
review of the regulatory evidence from the European medicines
agency. Indian J Med Ethics. 2:30–37. 2017.PubMed/NCBI
|
|
104
|
Brotherton JML: Impact of HPV vaccination:
Achievements and future challenges. Papillomavirus Res. 7:138–140.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Abbas K, Yoo KJ, Prem K and Jit M: Equity
impact of HPV vaccination on lifetime projections of cervical
cancer burden among cohorts in 84 countries by global, regional,
and income levels, 2010–22: A modelling study. EClinicalMedicine.
70:1025242024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Spaans VM, Trietsch MD, Peters AAW, Osse
M, Ter Haar N, Fleuren GJ and Jordanova ES: Precise classification
of cervical carcinomas combined with somatic mutation profiling
contributes to predicting disease outcome. PLoS One.
10:e01336702015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Medhasi S and Chantratita N: Human
leukocyte antigen (HLA) system: Genetics and association with
bacterial and viral infections. J Immunol Res. 2022:97103762022.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jiang W, Xiang L, Pei X, He T, Shen X, Wu
X and Yang H: Mutational analysis of KRAS and its clinical
implications in cervical cancer patients. J Gynecol Oncol.
29:e42018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Shen Y, Wei X, Jin S, Wu Y, Zhao W, Xu Y,
Pan L, Zhou Z and Chen S: TCR-mimic antibody-drug conjugates
targeting intracellular tumor-specific mutant antigen KRAS G12V
mutation. Asian J Pharm Sci. 15:777–785. 2020.PubMed/NCBI
|
|
110
|
Skora AD, Douglass J, Hwang MS, Tam AJ,
Blosser RL, Gabelli SB, Cao J, Diaz LA Jr, Papadopoulos N, Kinzler
KW, et al: Generation of MANAbodies specific to HLA-restricted
epitopes encoded by somatically mutated genes. Proc Natl Acad Sci
USA. 112:9967–9972. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nakamura H, Taguchi A, Kawana K, Baba S,
Kawata A, Yoshida M, Fujimoto A, Ogishima J, Sato M, Inoue T, et
al: Therapeutic significance of targeting survivin in cervical
cancer and possibility of combination therapy with TRAIL.
Oncotarget. 9:134512018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Mora MJ, de los Ángeles Bayas-Rea R, Mejía
L, Cruz C, Guerra S, Calle P, Sandoval DM, Galarza JM and
Zapata-Mena S: Identification of human leukocyte antigen in
precancerous and cancerous cervical lesions from Ecuadorian women.
Infect Genet Evol. 105:1053652022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Xiong C, Huang L, Kou H, Wang C, Zeng X,
Sun H, Liu S, Wu B, Li J, Wang X, et al: Identification of novel
HLA-A*11:01-restricted HPV16 E6/E7 epitopes and T-cell receptors
for HPV-related cancer immunotherapy. J Immunother Cancer.
10:e0047902022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Vranic S, Cyprian FS, Akhtar S and Al
Moustafa AE: The role of epstein-barr virus in cervical cancer: A
brief update. Front Oncol. 8:1132018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang G, Wang L, Cui H, Wang X, Zhang G,
Ma J, Han H, He W, Wang W, Zhao Y, et al: Anti-melanoma activity of
T cells redirected with a TCR-like chimeric antigen receptor. Sci
Rep. 4:35712014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Liu Q, Hu W, Zhang YL, Hu SP, Zhang Z, He
XJ and Cai XH: Anti-viral immune response in the lung and thymus:
Molecular characterization and expression analysis of
immunoproteasome subunits LMP2, LMP7 and MECL-1 in pigs. Biochem
Biophys Res Commun. 502:472–478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ambagala APN, Solheim JC and Srikumaran S:
Viral interference with MHC class I antigen presentation pathway:
The battle continues. Vet Immunol Immunopathol. 107:1–15. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yim EK and Park JS: Biomarkers in cervical
cancer. Biomark Insights. 1:215–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wentzensen N and von Knebel Doeberitz M:
Biomarkers in cervical cancer screening. Dis Markers. 23:315–330.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Suri A, Saini S, Sinha A, Agarwal S, Verma
A, Parashar D, Singh S, Gupta N and Jagadish N: Cancer testis
antigens: A new paradigm for cancer therapy. Oncoimmunology.
1:1194–1196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Garg M, Kanojia D, Salhan S, Suri S, Gupta
A, Lohiya NK and Suri A: Sperm-associated antigen 9 is a biomarker
for early cervical carcinoma. Cancer. 115:2671–2683. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Rosenberg SA, Packard BS, Aebersold PM,
Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp
CA, et al: Use of tumor-infiltrating lymphocytes and interleukin-2
in the immunotherapy of patients with metastatic melanoma. A
preliminary report. N Engl J Med. 319:1676–1680. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ellebaek E, Iversen TZ, Junker N, Donia M,
Engell-Noerregaard L, Met Ö, Hölmich LR, Andersen RS, Hadrup SR,
Andersen MH, et al: Adoptive cell therapy with autologous tumor
infiltrating lymphocytes and low-dose Interleukin-2 in metastatic
melanoma patients. J Transl Med. 10:1692012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Svane IM and Verdegaal EM: Achievements
and challenges of adoptive T cell therapy with tumor-infiltrating
or blood-derived lymphocytes for metastatic melanoma: What is
needed to achieve standard of care? Cancer Immunol Immunother.
63:1081–1091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
June CH, O'Connor RS, Kawalekar OU,
Ghassemi S and Milone MC: CAR T cell immunotherapy for human
cancer. Science. 359:1361–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kessels HW, Wolkers MC, van den Boom MD,
van den Valk MA and Schumacher TN: Immunotherapy through TCR gene
transfer. Nat Immunol. 2:957–961. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hughes MS, Yu YYL, Dudley ME, Zheng Z,
Robbins PF, Li Y, Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA
and Morgan RA: Transfer of a TCR gene derived from a patient with a
marked antitumor response conveys highly active T-cell effector
functions. Hum Gene Ther. 16:457–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Marcu-Malina V, Heijhuurs S, van Buuren M,
Hartkamp L, Strand S, Sebestyen Z, Scholten K, Martens A and Kuball
J: Redirecting αβ T cells against cancer cells by transfer of a
broadly tumor-reactive γδT-cell receptor. Blood. 118:50–59. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Bovolenta ER, García-Cuesta EM, Horndler
L, Ponomarenko J, Schamel WW, Mellado M, Castro M, Abia D and van
Santen HM: A set point in the selection of the αβTCR T cell
repertoire imposed by pre-TCR signaling strength. Proc Natl Acad
Sci USA. 119:e22019071192022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Morgan RA, Dudley ME, Wunderlich JR,
Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US,
Restifo NP, et al: Cancer regression in patients after transfer of
genetically engineered lymphocytes. Science. 314:126–129. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Johnson LA, Morgan RA, Dudley ME, Cassard
L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich
JR, et al: Gene therapy with human and mouse T-cell receptors
mediates cancer regression and targets normal tissues expressing
cognate antigen. Blood. 114:535–546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Borbulevych OY, Santhanagopolan SM,
Hossain M and Baker BM: TCRs used in cancer gene therapy
cross-react with MART-1/Melan-A tumor antigens via distinct
mechanisms. J Immunol. 187:2453–2463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhong S, Malecek K, Johnson LA, Yu Z,
Vega-Saenz de Miera E, Darvishian F, McGary K, Huang K, Boyer J,
Corse E, et al: T-cell receptor affinity and avidity defines
antitumor response and autoimmunity in T-cell immunotherapy. Proc
Natl Acad Sci USA. 110:6973–6978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Harris DT, Wang N, Riley TP, Anderson SD,
Singh NK, Procko E, Baker BM and Kranz DM: Deep mutational scans as
a guide to engineering high affinity T cell receptor interactions
with peptide-bound major histocompatibility complex. J Biol Chem.
291:24566–24578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Richman SA, Healan SJ, Weber KS,
Donermeyer DL, Dossett ML, Greenberg PD, Allen PM and Kranz DM:
Development of a novel strategy for engineering high-affinity
proteins by yeast display. Protein Eng Des Sel. 19:255–264. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kageyama S, Ikeda H, Miyahara Y, Imai N,
Ishihara M, Saito K, Sugino S, Ueda S, Ishikawa T, Kokura S, et al:
Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced
lymphocytes in patients with recurrent esophageal cancer. Clin
Cancer Res. 21:2268–2277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Robbins PF, Morgan RA, Feldman SA, Yang
JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ,
Mackall CL, et al: Tumor regression in patients with metastatic
synovial cell sarcoma and melanoma using genetically engineered
lymphocytes reactive with NY-ESO-1. J Clin Oncol. 29:917–924. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Brentjens RJ, Rivière I, Park JH, Davila
ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda
O, et al: Safety and persistence of adoptively transferred
autologous CD19-targeted T cells in patients with relapsed or
chemotherapy refractory B-cell leukemias. Blood. 118:4817–4828.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Morgan RA, Chinnasamy N, Abate-Daga D,
Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry
RM, et al: Cancer regression and neurological toxicity following
anti-MAGE-A3 TCR gene therapy. Jo J Immunother. 36:133–151. 2013.
View Article : Google Scholar
|
|
141
|
Van den Berg JH, Gomez-Eerland R, Van de
Wiel B, Hulshoff L, Van den Broek D, Bins A, Tan HL, Harper JV,
Hassan NJ, Jakobsen BK, et al: Case report of a fatal serious
adverse event upon administration of T cells transduced with a
MART-1-specific T-cell receptor. Mol Ther. 23:1541–1550. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Cameron BJ, Gerry AB, Dukes J, Harper JV,
Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, et al:
Identification of a Titin-derived HLA-A1-presented peptide as a
cross-reactive target for engineered MAGE A3-directed T cells. Sci
Transl Med. 5:197ra1032013. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Connerotte T, Van Pel A, Godelaine D,
Tartour E, Schuler-Thurner B, Lucas S, Thielemans K, Schuler G and
Coulie PG: Functions of Anti-MAGE T-cells induced in melanoma
patients under different vaccination modalities. Cancer Res.
68:3931–3940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Robbins PF, Li YF, El-Gamil M, Zhao Y,
Wargo JA, Zheng Z, Xu H, Morgan RA, Feldman SA, Johnson LA, et al:
Single and dual amino acid substitutions in TCR CDRs can enhance
antigen-specific T cell functions. J Immunol. 180:6116–6131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Linette GP, Stadtmauer EA, Maus MV,
Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM,
Cimino PJ, et al: Cardiovascular toxicity and titin
cross-reactivity of affinity-enhanced T cells in myeloma and
melanoma. Blood. 122:863–871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Bentzen AK and Hadrup SR: T-cell-receptor
cross-recognition and strategies to select safe T-cell receptors
for clinical translation. Immunooncol Technol. 2:1–10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Bijen HM, van der Steen DM, Hagedoorn RS,
Wouters AK, Wooldridge L, Falkenburg JHF and Heemskerk MHM:
Preclinical strategies to identify off-target toxicity of
high-affinity TCRs. Mol Ther. 26:1206–1214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wooldridge L, Laugel B, Ekeruche J,
Clement M, van den Berg HA, Price DA and Sewell AK: CD8 controls T
cell cross-reactivity. J Immunol. 185:4625–4632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Birnbaum ME, Mendoza JL, Sethi DK, Dong S,
Glanville J, Dobbins J, Ozkan E, Davis MM, Wucherpfennig KW and
Garcia KC: Deconstructing the peptide-MHC specificity of T cell
recognition. Cell. 157:1073–1087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Gejman RS, Klatt MG, Chang A, Jones HF, Oh
CY, Chandran SS, Korontsvit T, Zakahleva V, Dao T, Klebanoff CA and
Scheinberg DA: Prospective identification of cross-reactive human
peptide-MHC ligands for T cell receptor based therapies. BioRxiv.
2670472018.
|
|
151
|
Joglekar AV, Leonard MT, Jeppson JD, Swift
M, Li G, Wong S, Peng S, Zaretsky JM, Heath JR, Ribas A, et al: T
cell antigen discovery via signaling and antigen-presenting
bifunctional receptors. Nat Methods. 16:191–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Bentzen AK and Hadrup SR: Evolution of
MHC-based technologies used for detection of antigen-responsive T
cells. Cancer Immunol Immunother. 66:657–666. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Riley TP, Hellman LM, Gee MH, Mendoza JL,
Alonso JA, Foley KC, Nishimura MI, Vander Kooi CW, Garcia KC and
Baker BM: T cell receptor cross-reactivity expanded by dramatic
peptide-MHC adaptability. Nat Chem Biol. 14:934–942. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zhang SQ, Ma KY, Schonnesen AA, Zhang M,
He C, Sun E, Williams CM, Jia W and Jiang N: High-throughput
determination of the antigen specificities of T cell receptors in
single cells. Nat Biotechnol. 36:1156–1159. 2018. View Article : Google Scholar
|
|
155
|
de Castro E, Sigrist CJA, Gattiker A,
Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A and Hulo
N: ScanProsite: Detection of PROSITE signature matches and
ProRule-associated functional and structural residues in proteins.
Nucleic Acids Res. 34((Web Server Issue)): W362–W365. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Ohkura N, Kitagawa Y and Sakaguchi S:
Development and maintenance of regulatory T cells. Immunity.
38:414–423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
He Q, Liu Z, Liu Z, Lai Y, Zhou X and Weng
J: TCR-like antibodies in cancer immunotherapy. J Hematol Oncol.
12:992019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Høydahl LS, Frick R, Sandlie I and Løset
GÅ: Targeting the MHC ligandome by use of TCR-like antibodies.
Antibodies (Basel). 8:322019. View Article : Google Scholar : PubMed/NCBI
|