Surgery is a fundamental approach to disease
treatment and prevention, carrying out a vital role in enhancing
the quality of life of patients and driving economic growth. The
continuous advancements in surgical and anesthetic techniques have
led to a steady increase in global surgical procedures, with annual
operations now at >300 million (1). However, this growing surgical volume
also brings heightened concerns regarding postoperative
complications, making their prevention, management and prognosis
key priorities for medical professionals. Among these,
perioperative neurocognitive disorders are among the most prevalent
neurological complications (2),
with an incidence rate of 50% (3),
adversely affecting both short-term recovery and long-term health,
particularly in elderly patients (4). With the aging Chinese population, the
number of elderly individuals undergoing anesthesia and surgery is
steadily increasing. Currently, elderly patients account for more
than 30% of all surgical cases in China (5), which has further intensified concerns
about perioperative neurocognitive disorders and highlighted the
necessity for effective management strategies.
In 2018, the Perioperative Cognition Nomenclature
Working Group, composed of multidisciplinary experts, redefined
delirium as an acute and transient brain dysfunction characterized
by attention deficits, memory impairment, fluctuations in
consciousness levels, disorganized thinking, sleep cycle
disturbances and mood disorders (6). Among its various subtypes,
postoperative delirium (POD) is specifically defined as an acute
episode that meets the Diagnostic and Statistical Manual of Mental
Disorders (DSM), 5th edition criteria for delirium and occurs
within 1 week after surgery or before hospital discharge (7). POD exhibits a time-related pattern,
with its occurrence mainly observed between postoperative days 1
and 3 (6). Notably, identifying
and diagnosing POD in clinical practice is challenging for several
reasons. First, 50% of POD cases present as low-activity types,
characterized by quietness, silence, slow movements, drowsiness and
reduced interaction. These atypical clinical symptoms are often
overlooked by healthcare staff. Second, the lack of formal
assessment strategies exacerbates the difficulty in diagnosis. The
DSM is considered the gold standard for diagnosing POD, but its
clinical application is limited by resource and time constraints
(8). As a result, clinicians may
opt for shorter assessment tools, such as the Intensive Care Unit
Confusion Assessment Method (9) or
the Nursing Delirium Screening Scale (10), although this often comes at the
cost of sensitivity (11–13). Prioritizing high specificity over
sensitivity can lead to a subset of patients with delirium being
missed. While POD is difficult to recognize and diagnose, its
consequences are severe, encompassing increased complication rates,
impaired postoperative recovery, prolonged hospital stays,
heightened healthcare costs, greater risk of hospital readmission
and elevated mortality (14).
Furthermore, accumulating evidence suggests that POD, as a hallmark
of acute postoperative cognitive dysfunction, serves as a precursor
to long-term cognitive impairment (15–17).
Specifically, POD has been associated with persistent brain
dysfunction, including progressive cognitive decline and an
increased risk of dementia. These findings underscore the need for
early identification and effective management strategies to
mitigate the long-term impact of POD on cognitive health.
By contrast, postoperative cognitive decline (POCD)
typically emerges at the end of the first postoperative week and
does not affect the level of consciousness of the patient. This
distinction was highlighted by Glumac et al (18), who demonstrated that POCD and POD
present temporally and clinically distinct phenomena.
Differentiating between the two is key as they differ in onset,
pathophysiology, clinical course, prognosis, and management
approaches. POD is characterized by an acute onset, fluctuating
course, and potential reversibility with prompt intervention,
whereas POCD presents more insidiously, may persist for weeks or
months, and currently lacks standardized treatment protocols.
Failure to distinguish between them may result in misdiagnosis,
inappropriate management, and inaccurate interpretation of clinical
or research findings. Therefore, the aim of the present review was
to summarize advances in understanding the pathogenesis and
signaling pathways of POD, and to explore current strategies for
its prevention and treatment.
The present review was conducted to summarize the
current evidence on the pathogenesis, signaling pathways and
strategies for the prevention and treatment of POD. A comprehensive
literature search was carried out using the PubMed database
(pubmed.ncbi.nlm.nih.gov/) for studies published up to January
2025. The search strategy included the following key words:
‘Postoperative delirium’, ‘POD’, ‘cognition disorders’,
‘pathogenesis’, ‘signal transduction’, ‘prevention’ and
‘treatment’. Boolean operators (AND and OR) were used to refine the
search. Only articles published in English were considered. Both
clinical studies and animal studies were included if they
contributed to understanding the mechanisms or management of POD.
The initial search and selection of studies were independently
conducted by three authors (RHB, LL and XMD). The final list of
included studies was reviewed and approved by WQL.
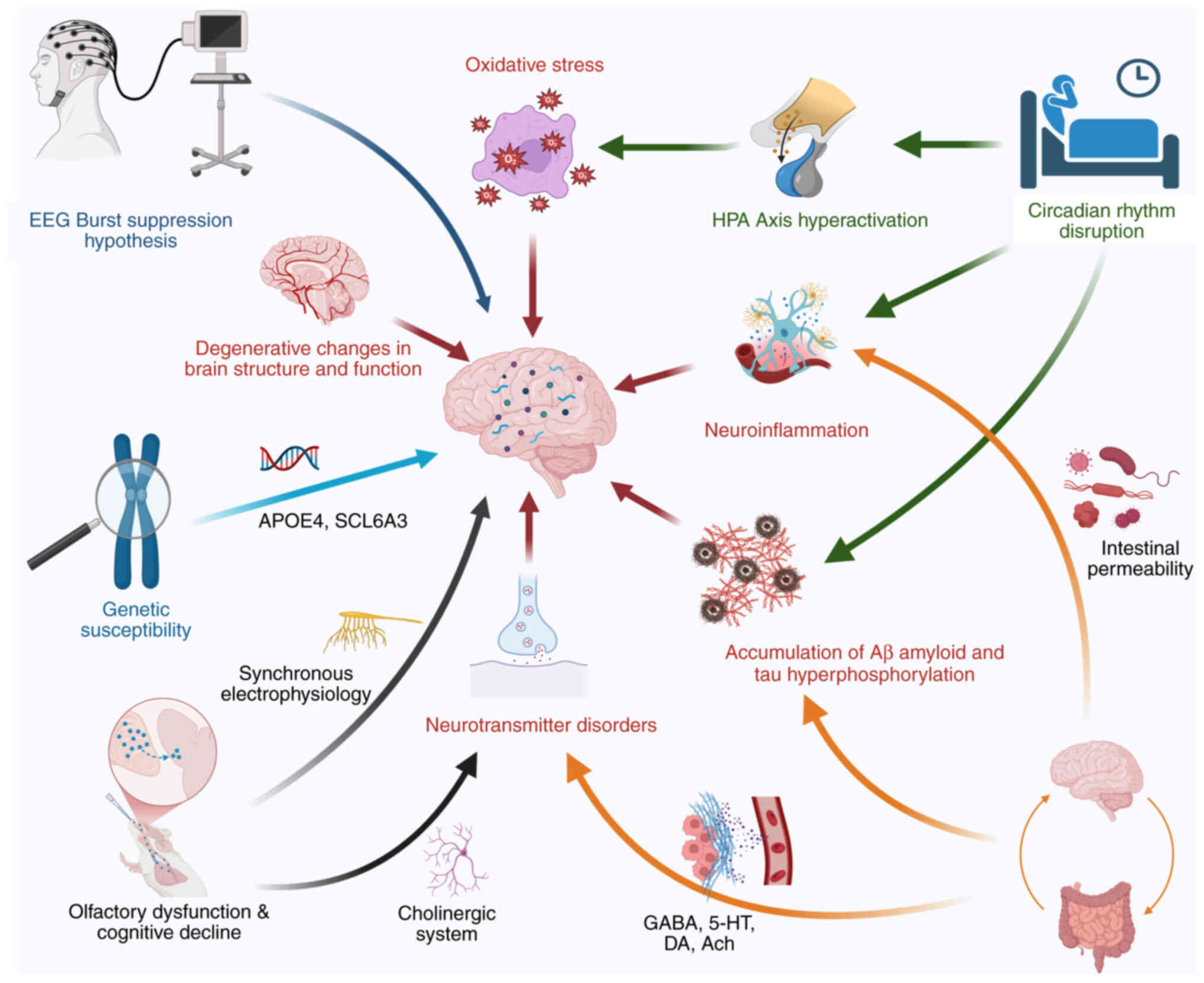
The occurrence of POD results from multiple
interacting factors and is influenced by preoperative,
intraoperative and postoperative conditions. Despite extensive
research, the underlying mechanisms of POD remain incompletely
understood. Numerous studies suggest that its pathogenesis may
involve neurodegenerative changes, neuroinflammation, sleep
disturbances, neurotransmitter imbalances, β-amyloid (Aβ)
deposition and excessive tau protein phosphorylation (19–22).
In addition to these mechanisms, emerging evidence indicates that
gut microbiota can regulate cognitive function via the
microbiota-gut-brain axis (23).
Furthermore, sensory impairments, including deficits in vision,
hearing and olfaction, have also been associated with postoperative
neurocognitive disorders (24,25).
Notably, these mechanisms intersect, interact and collectively
contribute to the development of POD (Fig. 1).
Brains are highly energy-demanding organs that
require sufficient energy to sustain normal functional activities
(26). A decline in brain
metabolism has been implicated in the development of POD (27). Specifically, during delirium
episodes, cortical glucose metabolism is markedly reduced, with
partial recovery observed as delirium symptoms subside. In support
of this, Caplan et al (28)
observed increased anaerobic metabolism in the brains of patients
with delirium, characterized by elevated cerebrospinal fluid
lactate levels and decreased neuron-specific enolase
concentrations, suggesting metabolic stress. Furthermore, with
aging, pathological and physiological changes occur in cerebral
blood vessels, leading to impaired brain perfusion and vascular
reactivity (29,30). Multiple studies have demonstrated
that reduced cerebral perfusion may contribute to cognitive and
learning impairments. For instance, using computed tomography,
Yokota et al (31) and Aa
et al (32) identified
notably decreased cerebral perfusion in patients with delirium,
which returned to normal following symptom resolution.
Consistently, numerous studies have confirmed that individuals with
preoperative cognitive impairment or diminished cognitive reserve,
such as those with dementia or mild cognitive impairment, are at a
higher risk of developing POD after surgery (33–35).
Extracellular Aβ plaques, tau hyperphosphorylation
and neurofibrillary tangles (NFTs) formed by tau aggregation are
major pathological hallmarks of neurodegenerative diseases,
particularly Alzheimer's disease (AD) (42). Studies suggest that POD shares a
similar pathological basis with AD (43,44).
Aβ protein readily aggregates in brain tissue, forming highly toxic
oligomers that induce neuronal death and synaptic damage. Moreover,
Aβ oligomers disrupt central nervous system insulin signaling,
thereby interfering with brain energy metabolism and further
exacerbating neurodegeneration (45). Tau protein, a
microtubule-associated protein, plays a key role in microtubule
assembly, stabilization and axonal transport; it is essential for
regulating neuronal growth, development and signal transmission
(42,44). However, surgery and anesthesia have
been revealed to induce tau hyperphosphorylation, leading to
structural and functional abnormalities (46,47).
Hyperphosphorylated tau proteins further aggregate and precipitate,
forming NFTs, which are sensitive biomarkers of axonal injury in
the central nervous system (48,49).
Notably, elevated levels of NFTs in cerebrospinal fluid and blood
have been associated with POD (50). Aβ and abnormally phosphorylated tau
proteins act synergistically to influence the onset and progression
of POD. Aβ oligomers promote tau phosphorylation by increasing
GSK-3β activity. Additionally, both of them collectively activate
microglia and astrocytes, triggering the release of various
inflammatory cytokines (51–53).
These inflammatory mediators further accelerate tau
hyperphosphorylation and Aβ oligomerization, ultimately leading to
neuronal damage and the development of POD.
Sleep is key for neurodevelopment and the
maintenance of brain function. Studies suggest that perioperative
sleep disturbances are associated with the occurrence of POD
(54–56). Various perioperative factors,
including trauma, anesthesia, stress, pain and inflammation, can
disrupt the sleep-wake regulatory system and circadian rhythm,
further contributing to postoperative neurocognitive dysfunction.
Multiple studies have demonstrated that patients with delirium
exhibit a pronounced reduction in the rapid eye movement phase of
sleep, increased sleep fragmentation and heightened wakefulness
(57–59). Sleep disturbances lead to
hyperactivation of the hypothalamic-pituitary-adrenal axis,
resulting in excessive cortisol secretion. Elevated cortisol levels
inhibit neuronal glucose uptake, making neurons metabolically
vulnerable to oxidative stress. This process contributes to
neuronal loss, alterations in dendritic spine density and changes
in synaptic number, morphology and function, ultimately leading to
cognitive impairment (56,60,61).
Additionally, sleep deprivation increases the release of
inflammatory cytokines, activates microglia and exacerbates
neuroinflammatory responses (62,63).
Fultz et al (64) further
revealed that disruptions in the sleep-wake cycle dysregulate
aquaporin-4 expression levels, impairing the glymphatic system and
hindering cerebrospinal fluid clearance of Aβ protein. These
pathological changes suggest that disturbances in the sleep-wake
cycle contribute to POD development through multiple interrelated
pathways. Notably, in patients of postoperative cardiac surgery,
sleep deprivation consistently precedes the onset of delirium.
Furthermore, patients in the ICU experiencing sleep deprivation are
markedly more likely to develop delirium compared with those with
adequate sleep (65).
Olfactory signals are transmitted via the olfactory
filaments, olfactory bulb and olfactory tract to the primary
olfactory cortex, from where they further project to brain regions,
including the insula, hypothalamus and hippocampus (66). Notably, certain olfactory centers
and the hippocampus exhibit synchronized electrophysiological
activity and directly participate in memory processes, highlighting
the functional interplay between olfaction and cognition (67). Emerging evidence suggests that
anesthesia and surgery can impair both olfactory and cognitive
functions. Zhang et al (24) revealed that anesthesia and surgery
lead to deficits in olfaction and cognition in mice, while
olfactory stimulation reverses these effects by restoring the
expression of olfactory marker protein 13 and growth-associated
protein 43, and by preventing the reduction of hippocampal synaptic
markers postsynaptic density protein (PSD)-95 and synaptophysin.
Clinical studies further support this finding. Kamath et al
(68) discovered that preoperative
olfactory dysfunction is associated with both the incidence and
severity of POD, suggesting that olfactory assessment could serve
as a valuable preoperative screening tool for identifying high-risk
patients with POD. Similarly, in the study by Yang et al
(69), 14 out of 21 (66.67%)
patients with preoperative olfactory dysfunction exhibited
postoperative cognitive dysfunction, providing additional evidence
of the association between olfactory function and cognitive
performance. Mechanistically, research has indicated that olfactory
and cognitive processing centers share overlapping neural pathways,
with the cholinergic system carrying out a central role in both
olfactory transmission and cognitive function. Dopamine and
acetylcholine serve as key neurotransmitters regulating both
cognition and olfaction. Given these interconnections, olfactory
impairment may contribute to the development of POD by disrupting
shared neurochemical and neural circuits (70,71).
Previous studies have revealed that alterations in
gut microbiota are associated with abnormal cognitive behaviors.
The gut microbiota carries out a key role in regulating neural
functions in the brain through multiple pathways, including immune
modulation and neuroendocrine regulation, thereby influencing
cognitive processes (72–74). Anesthesia and surgery induce gut
microbiota dysbiosis, which is primarily characterized by a notable
reduction in microbial abundance and diversity, particularly with
aging (75). This dysbiosis
exacerbates systemic inflammation, increases gut permeability and
subsequently compromises the integrity of the BBB, ultimately
leading to disruptions in brain immune homeostasis. Furthermore,
gut microbiota can produce Aβ protein, which, despite having a
different primary structure from brain-derived Aβ, shares a highly
similar tertiary structure. This structural similarity suggests
that gut microbiota dysbiosis may contribute to the abnormal
deposition of Aβ and tau proteins, potentially triggering
cross-immune reactions and excessive activation of pro-inflammatory
signaling pathways in the brain (76). In addition to its role in protein
aggregation, gut microbiota dysbiosis also alters neurotransmitter
levels, including γ-aminobutyric acid, 5-hydroxytryptamine,
dopamine and acetylcholine, thereby affecting central nervous
system function (74,77). Notably, disruptions in gut
microbiota composition can induce neuropsychiatric symptoms such as
anxiety and depression, which may accelerate cognitive decline by
further impairing neural function (78,79).
EEG burst suppression is a common neurophysiological
phenomenon observed during clinical anesthesia, characterized by
alternating high-amplitude burst activity and periods of
isoelectric suppression on EEG. Notably, the occurrence of POD is
associated with the depth of general anesthesia, as excessive
anesthetic depth increases the likelihood of EEG burst suppression,
which in turn elevates the risk of POD (80). Clinical studies have demonstrated
this association (81–83). In a study of spinal fixation
surgery under total intravenous anesthesia, 78 patients (69.6%)
exhibited intraoperative burst suppression (BS), while 10 patients
(8.9%) developed POD. All cases of POD occurred in patients who
experienced intraoperative BS, and prolonged BS duration was
observed in these individuals (84). Further research has indicated a
quantitative relationship between BS duration and risk of POD, with
each additional minute of intraoperative BS doubling the likelihood
of developing POD. Given this evidence, intraoperative BS has been
proposed as a potential predictor of POD, highlighting the
importance of anesthetic depth monitoring to mitigate the risk of
POD (85).
In recent years, the genetic hypothesis of delirium
susceptibility has emerged as a promising research direction.
Studies have identified associations between POD and several
genetic factors, including APOE4, the dopamine transporter gene
SCL6A3, the dopamine receptor 2 gene, the glucocorticoid receptor,
the melatonin receptor and mitochondrial DNA haplotypes (86–88).
Additionally, two long intergenic non-coding RNA genes with
potential functional implications have been revealed, further
expanding the understanding of the genetic basis of POD (89).
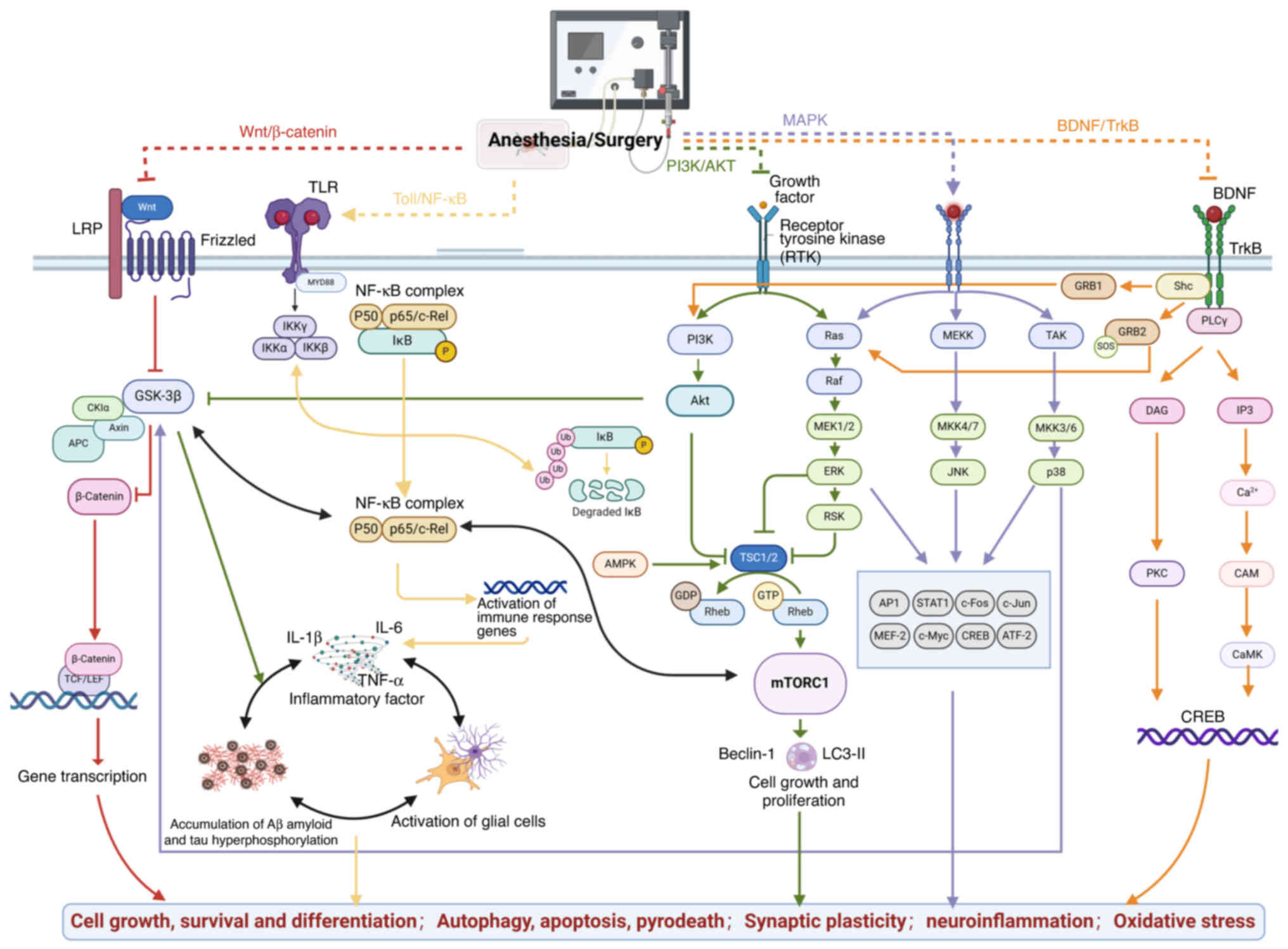
Given the multifaceted pathogenesis of POD, numerous
interconnected signaling pathways are implicated, including the
Wnt/β-catenin, PI3K/AKT, BDNF/tropomyosin receptor kinase B (TrkB),
toll-like receptor (TLR) and NF-κB pathways (90) (Fig.
2).
The Wnt signaling pathway consists of one canonical
and two non-canonical pathways, which enhance synaptic plasticity,
promote neuronal survival and regulate cell death, thereby carrying
out a key role in brain function (91). Notably, studies have revealed that
the canonical Wnt/β-catenin pathway is involved in the pathogenesis
of POD. In a study on Sprague-Dawley rats, exposure to 3%
sevoflurane for 6 h resulted in cognitive impairment,
downregulation of β-catenin and phosphorylated GSK-3β in the
hippocampus, increased levels of TNF-α and IL-1, and structural
damage to hippocampal neurons. These findings suggest that
sevoflurane suppresses Wnt/β-catenin signaling, activates
inflammatory responses and induces hippocampal injury, ultimately
contributing to POD development (92). Notably, this damage was reversed
upon exogenous administration of lithium chloride, an activator of
the Wnt/β-catenin pathway. Further supporting the role of
Wnt/β-catenin signaling in POD, a study on endothelial cells have
shown that exposure to 3% sevoflurane for 6 h downregulates
Wnt/β-catenin activity, leading to reduced Annexin A1 expression,
disruption of the BBB and subsequent POD onset (93). Similarly, in transgenic mice,
overexpression of DKK-1 inhibits the Wnt/β-catenin pathway,
promoting excessive tau protein phosphorylation and cognitive
impairment. Conversely, activation of the Wnt/β-catenin pathway
suppresses inflammatory responses and improves cognitive function,
suggesting its potential as a therapeutic target for POD prevention
and treatment (94).
Key roles are conducted by the PI3K/AKT signaling
pathway, including cell growth, proliferation, differentiation and
apoptosis. PI3K functions as a key intracellular signaling
molecule, while AKT serves as its primary downstream protein
kinase. Upon activation, PI3K further phosphorylates AKT, which in
turn further phosphorylates key downstream targets, including
GSK-3β, mTOR, endothelial nitric oxide synthase, FoxO3a and NF-κB,
thereby regulating physiological processes such as cell growth,
proliferation, cell cycle progression and glucose metabolism
(95,96).
GSK-3 is a key substrate of AKT and exists in two
isoforms in the brain: GSK-3α and GSK-3β. Among them, GSK-3β is
widely expressed in the central nervous system and is implicated in
the pathological mechanisms underlying cognitive decline in
neurodegenerative diseases (96).
GSK-3β is a key kinase responsible for tau phosphorylation. Both
animal and clinical studies have demonstrated a positive
association between the abnormal upregulation of GSK-3β, tau
phosphorylation and the accumulation of toxic tau aggregates
(97–99). Notably, increased levels of GSK-3β
and phosphorylated tau have been observed in rodent brain tissues
following anesthesia and surgery, further supporting its role in
postoperative neurocognitive impairment. Beyond tau pathology,
GSK-3β activation has been revealed to contribute to Aβ formation
and accumulation in the brain by regulating amyloid precursor
protein cleavage (51).
Additionally, it can induce Aβ pathology by disrupting insulin
signaling pathways (100).
Studies have proposed a feedback loop between Aβ and GSK-3β
activation, where sustained interactions in specific pathways may
exacerbate tau hyperphosphorylation and neurotoxicity (101–103). GSK-3β is also involved in
neuroinflammation. The activation of GSK-3β promotes the production
of IL-1, IL-6 and TNF-α, activates the JNK, STAT3/5, and NF-κB
signaling pathways, and regulates microglial migration,
contributing to inflammatory responses in the brain (104). Furthermore, GSK-3β has been
implicated in the suppression of adult hippocampal neurogenesis.
The excessive activation of GSK-3β impairs long-term potentiation
and enhances N-methyl-D-aspartate receptor-dependent
long-term depression, thereby disrupting synaptic plasticity,
memory formation and neurogenesis. These mechanisms collectively
suggest that GSK-3β overactivation may play a key role in the
pathogenesis of POD (51).
mTOR is an atypical serine/threonine kinase that
plays a key role in cell growth, proliferation, protein synthesis
and autophagy. As a major downstream target of the PI3K/AKT
signaling pathway, mTOR serves as the principal negative regulator
of autophagy, a process essential for cell survival, development,
division and homeostasis (105).
Autophagy has a dual role in neural function. First, it facilitates
the clearance and degradation of Aβ protein. In both cellular and
animal models, autophagy activators, such as mTOR inhibitors, have
been revealed to reduce tau hyperphosphorylation and the misfolding
of other aggregated proteins by promoting the autophagic
degradation of NFTs and Aβ plaques (106). Secondly, autophagy is involved in
synaptic plasticity and neurotransmission, highlighting its broader
role in cognitive function.
The PI3K/AKT signaling pathway has been extensively
studied in neurological diseases, with numerous associated pathways
being explored. In addition to those previously discussed, other
pathways, such as PI3K/AKT/Nrf2, PI3K/AKT/CREB and PI3K/AKT/MAPK,
have been identified. These interconnected pathways regulate a
range of neurophysiological and pathological processes, including
mitochondrial function restoration, abnormal protein clearance,
cerebrovascular regeneration and synaptic plasticity enhancement
(111–113).
BDNF is the most abundant neurotrophic factor in the
brain, playing a key role in regions responsible for learning,
memory and higher cognitive functions, such as the hippocampus,
cerebral cortex and basal forebrain (114). Dysregulation of BDNF and its
downstream pathways may lead to abnormal neuronal differentiation,
synaptic loss and cognitive dysfunction, suggesting that the
BDNF/TrkB signaling pathway may be involved in the pathogenesis of
POD. Qiu et al (115)
demonstrated that anesthesia and surgery induce microglial
activation, IL-1β release and BDNF downregulation in the
hippocampus, resulting in hippocampus-dependent cognitive
impairment in aged mice. Subsequent studies have also reported a
notable reduction in total TrkB expression (114,116,117). Similarly, Fan et al
(118) revealed that surgery
reduces BDNF expression and neurogenesis while also decreasing
phosphorylated/activated TrkB and ERK expression. However, this
study observed no significant impact on total TrkB expression
levels. This discrepancy may be attributed to differences in
surgical procedures, mouse age and tissue collection time points.
In addition to its role in neurogenesis, the BDNF/TrkB pathway is
also key for synaptic plasticity and neuronal growth. Notably, the
BDNF/proBDNF ratio carries out a key role in regulating synaptic
plasticity (119). Jia et
al (117) revealed that
exposure to 3% sevoflurane markedly inhibited the proliferation of
neural stem cells, immature neurons and newly formed neurons, which
was accompanied by reduced BDNF and TrkB protein expression.
Furthermore, studies have suggested that the BDNF/TrkB signaling
pathway is involved in Aβ aggregation and tau protein
phosphorylation (119–121). Activation of this pathway has
been revealed to reduce tau phosphorylation levels and enhance
learning and memory abilities, highlighting its potential as a
therapeutic target for neurodegenerative diseases and POD.
TLR signaling involves at least two distinct
pathways: The MyD88-dependent and MyD88-independent pathways
(122). The MyD88-dependent
pathway is the primary TLR signaling cascade, transmitting signals
through two major activation routes: The MAPK and NF-κB pathways.
Both pathways regulate the transcription of inflammatory factors,
leading to excessive cytokine release, which ultimately affects the
central nervous system and contributes to cognitive dysfunction
(123). Among the TLR family,
TLR4 is highly expressed in microglial cells and serves as a key
receptor for microglial activation and function (122). Lu et al (124) subjected rats to tibial fracture
surgery and observed increased expression of S100A8 and S100A9,
along with hippocampal TLR4/MyD88 activation. This pro-inflammatory
response was associated with the onset of POD. Similarly, in aged
rats undergoing splenectomy, TLR4 activation was detected,
accompanied by elevated levels of inflammatory mediators such as
IL-6 and IL-1β, which triggered central neuroinflammation (125). Beyond TLR4, other TLR family
members have also been implicated in surgery-induced
neuroinflammation and cognitive dysfunction. Lin et al
(126) conducted anesthesia and
surgery on both TLR2-knockout and wild-type mice, assessing their
learning and memory abilities. The findings suggested that TLR2
contributes to surgery-induced neuroinflammation and cognitive
impairment. A study by Yang et al (127) demonstrated that inhibition of the
TLR2/TLR4 signaling pathway suppresses hippocampal
neuroinflammatory cytokines and alleviates postoperative cognitive
dysfunction in rats. Chen et al (128) revealed that extracellular
RNAs-TLR3 carries out a role in learning and memory deficits
following nephrectomy in mice. Additionally, a recent study
indicated that TLR7 is involved in anesthesia- and surgery-induced
cognitive dysfunction (129).
Collectively, these findings suggest that TLR signaling pathways
carry out a key role in the development of POD, highlighting their
potential as therapeutic targets for intervention.
The MAPK signaling pathway mediates specific
biological functions such as cell proliferation, differentiation
and survival, encompassing the p38 MAPK pathway, the JNK pathway
and the ERK pathway (130).
p38 MAPK, also known as a stress-activated protein
kinase, regulates key cellular processes, including cell
proliferation, differentiation, survival and stress-induced
apoptosis. Evidence suggests that its dysregulation carries out a
key role in cognitive impairment and neuroinflammation (131). Lv et al (132) demonstrated that mice subjected to
exploratory laparotomy exhibited cognitive deficits, with both
pathological analysis and western blotting results consistently
demonstrating increased phosphorylated (p)-p38 expression.
Similarly, Song et al (131) conducted a study on neonates
exposed to sevoflurane and found a time-dependent upregulation of
p-p38 and p-p65, as well as the p-p38/p38 and p-p65/p65 ratios.
Beyond its involvement in neuroinflammatory responses, p38 MAPK
directly modulates GSK-3β activity, leading to increased GSK-3β
kinase function, which in turn promotes tau hyperphosphorylation
and impairs synaptic plasticity (133). Studies have demonstrated that
activation of the p38 MAPK signaling pathway contributes to the
onset of POD (131–133). Conversely, inhibition of p-p38
expression has been revealed to mitigate POD symptoms (134).
The JNK pathway has a key role in transmitting
extracellular signals to the nucleus and is involved in various
biological processes, including cytokine regulation and inhibition
of protein synthesis (135). JNK
is classified into three isoforms: JNK1, JNK2 and JNK3. Among them,
JNK3 is highly expressed and activated in the brain tissue and
cerebrospinal fluid of patients with delirium, with a notable
association with the rate of cognitive decline (136). In a study by Li et al
(137), exposure to isoflurane
led to increased expression of p-JNK and p-c-Jun, suggesting that
JNK pathway activation contributes to isoflurane-induced
neuroapoptosis in the developing brain. Similarly, Bi et al
(138) revealed that sevoflurane
activates the JNK/c-Jun/AP-1 signaling pathway, which in turn
upregulates the apoptotic factor connexin 43, leading to neuronal
apoptosis. Yang et al (139) noted that JNK signaling may have a
key role in the reduced survival rate of fetal neural stem cells
induced by sevoflurane. Notably, inhibition of the JNK pathway has
been revealed to mitigate neuronal apoptosis and exert
neuroprotective effects (140).
ERK is a key regulator of pro-inflammatory
microglial activation, and its signaling pathway carries out a key
role in reducing oxidative stress and exerting neuroprotective
effects in POD (141). The ERK
phosphorylation status is associated with anesthesia-induced
neurotoxicity. Yufune et al (142) observed that oxidative
stress-mediated inhibition of ERK phosphorylation serves as a
fundamental mechanism underlying sevoflurane-induced neurotoxicity.
Numerous studies have demonstrated that anesthetics such as
sevoflurane, ketamine and propofol suppress the ERK1/2 signaling
pathway, leading to neuronal apoptosis in the developing brain
(143–145). Conversely, certain studies have
confirmed that restoring ERK phosphorylation can counteract
anesthetic-induced neuronal apoptosis (143–146). Lithium,
N-stearoyl-L-tyrosine, the phosphodiesterase-4 inhibitor,
roflumilast, and electroacupuncture pretreatment have all been
demonstrated to attenuate anesthetic-induced neuronal apoptosis and
improve cognitive function by upregulating ERK signaling. Several
studies have underscored the key role of the ERK signaling pathway
in neuronal growth, survival and synaptic plasticity (147,148).
NF-κB is a transcription factor that has a key role
in various physiological processes, including the immune response,
cell proliferation and growth, synaptic plasticity and cell
survival. NF-κB activation may also be associated with
neuroinflammation and cognitive impairment, particularly in the
context of surgery and anesthesia. Surgical procedures and
anesthetic exposure trigger the release of endogenous factors such
as high mobility group box 1 and TNF-α, which activate NF-κB
translocation into the nucleus. This activation promotes the
transcription of target genes, leading to the release of
inflammatory mediators and inducing a neuroinflammatory response
(149,150). Additionally, key contributors to
neuroinflammation, including neutrophils, macrophages, T cells and
glial cells, further amplify this inflammatory cascade via NF-κB
signaling (149). Notably,
activation of the NF-κB signaling pathway has been observed in
various animal models of POD, reinforcing its role in postoperative
neuroinflammation. Liu et al (151) observed a marked increase in
p-NF-κB and p65 levels following exploratory laparotomy in mice,
which was accompanied by a reduction in the BBB-associated
proteins, zonula occludens protein-1, occludin and claudin-5, in
the hippocampus. These findings indicate that NF-κB activation
compromises BBB integrity, thereby contributing to POD
pathogenesis. NF-κB activation is also associated with various
forms of neuronal death. Li et al (152) and Wang et al (153) demonstrated that surgery and
anesthesia induce neuronal apoptosis via NF-κB signaling
activation, while Dai et al (154) reported that repeated sevoflurane
exposure in neonatal mice leads to NF-κB-mediated neuronal
pyroptosis. This process disrupts neuronal architecture and
connectivity, ultimately impairing cognitive function. Given its
key role in neuroinflammation and neuronal damage, targeting NF-κB
signaling inhibition may serve as a promising therapeutic approach
for POD treatment (155,156).
In addition to the aforementioned pathways, several
other signaling cascades have been implicated in the pathogenesis
of POD, including the NLRP3 inflammasome, JAK/STAT, Notch and
AMP-activated protein kinase pathways (157). Rather than acting in isolation,
these pathways engage in extensive crosstalk and dynamic
interactions, collectively shaping the onset and progression of
POD. For instance, activation of the Wnt/β-catenin pathway enhances
PI3K/AKT signaling, which in turn upregulates the BDNF/TrkB pathway
which is key for promoting neuronal survival, synaptic plasticity
and overall neuroprotection (158). Conversely, pro-inflammatory
signaling routes such as TLR and NF-κB may antagonize these
protective mechanisms. TLR activation facilitates NF-κB nuclear
translocation and the induction of pro-inflammatory cytokines,
thereby compromising BBB integrity and suppressing the BDNF
expression levels, both of which impair synaptic function (159). Moreover, dysregulated PI3K/AKT
signaling may further amplify NF-κB activity, while NF-κB can also
be activated downstream of MAPK signaling, establishing a positive
feedback loop that exacerbates neuroinflammation (160). These antagonistic interactions
between neuroprotective and inflammatory pathways reflect a dynamic
imbalance in signal transduction, which may underlie the molecular
pathology of POD. A deeper understanding of the interactions among
these signaling pathways may provide a theoretical basis for
elucidating the molecular mechanisms of POD and for developing
multi-target therapeutic strategies.
Although the incidence of POD is high, studies
indicate that up to 40% of cases are preventable and the majority
of patients experience recovery once the underlying causative
factors are addressed (161,162). Therefore, early prediction,
identification and diagnosis in clinical practice, combined with
timely and effective interventions, are essential for reducing the
incidence of POD, particularly in elderly patients.
Several studies support the use of
non-pharmacological approaches for the prevention of POD (69,163–176). Among these, cognitive training is
a particularly effective intervention that enhances preoperative
cognitive reserve and has been demonstrated to reduce the incidence
of POD in elderly patients (163–165). With technological advancements,
more accessible computer-based cognitive training programs have
emerged, further facilitating their clinical application. In
addition to cognitive training, regular physical exercise has been
shown to markedly reduce the risk of delirium (166–168). This protective effect is
potentially mediated by mechanisms such as increased skeletal
muscle mass, elevated BDNF levels, enhanced angiogenesis and
improved cerebral blood flow. Sleep regulation is another key
component of POD prevention. Non-pharmacological strategies to
improve sleep quality, such as using earplugs and eye masks,
dimming lights and reducing nighttime nursing activities, have been
demonstrated to decrease both the incidence and severity of POD
(169,170). Similarly, multisensory
stimulation, including music therapy, olfactory training and
environmental enrichment, has been revealed to reduce the risk of
POD in elderly patients (69,171–173),. Multicomponent interventions,
such as Hospital Elder Life Program (HELP), are regarded as the
most effective strategy for delirium prevention (174–176). A meta-analysis involving 3,605
patients with delirium demonstrated that HELP-based interventions
reduce the likelihood of delirium by 53% (177). Additionally, several other
approaches have demonstrated efficacy in preventing and managing
POD. These include transcutaneous acupoint electrical stimulation,
non-invasive brain stimulation, comprehensive geriatric assessment
and delirium-specialized hospital units (178). These interventions provide
diverse strategies for addressing POD and may contribute to
improved patient outcomes (Table
I).
At present, the prevention of POD in China
primarily relies on pharmacological interventions; however,
reliable supporting evidence remains limited (179–194). Previous studies have suggested
that antipsychotic drugs are the first-line treatment for POD due
to their sedative, antiemetic, anxiolytic and sleep-improving
properties, but they may also lead to considerable extrapyramidal
side effects (179–181). The role of benzodiazepines in POD
has been debated. While they were once considered an independent
risk factor, recent studies have revealed no clear association
between benzodiazepine use and increased POD risk (182,183). Moreover, some short-acting
benzodiazepines, such as remimazolam and midazolam, may even reduce
the incidence of POD (184).
Dexmedetomidine is a first-line agent for preventing POD and ICU
delirium, offering sedative, analgesic, anxiolytic, sympatholytic
and cardiovascular stabilizing effects (185–187). Additionally, randomized
controlled trials (RCTs) have demonstrated the potential benefits
of esketamine (188–190), melatonin (191,192), NSAIDs (193,194) and glucocorticoids (195,196) in improving POD outcomes. In
recent years, intranasal insulin administration has emerged as a
potential strategy for reducing POD incidence (56,197). However, the effects of
pharmacological interventions on POD remain controversial. A
standardized protocol for drug timing and dosage has yet to be
established, necessitating further validation through multicenter,
large-sample, high-quality clinical trials (Table II).
Anesthesia and surgery serve a key role in the
development of POD, and appropriate perioperative anesthesia
management can help mitigate this risk (198–209). Regional blockade combined with
general anesthesia has been shown to attenuate endocrine and
surgical stress responses, reduce opioid consumption and improve
postoperative pain control, making it an effective strategy for POD
prevention (198–200). Maintaining an optimal depth of
anesthesia through intraoperative EEG and bispectral index
monitoring can help prevent burst suppression and minimize
anesthetic exposure, thereby potentially reducing the incidence of
POD (83,201). Additionally, adopting
lung-protective ventilation strategies (202,203), optimizing blood pressure
regulation (204,205) and monitoring regional cerebral
oxygen saturation (206) can
enhance cerebral blood flow, improve brain perfusion and ensure
adequate oxygenation, further lowering the risk of POD. Other
effective perioperative anesthesia management strategies for POD
prevention include temperature monitoring, multimodal analgesia and
goal-directed fluid therapy (207–209). However, current research on
anesthesia management remains largely focused on single-modality
interventions. There is an urgent need to explore comprehensive,
multimodal approaches and to develop standardized clinical
anesthesia management pathways to optimize POD prevention and
patient outcomes (Table
III).
The present review has several limitations. First,
it primarily focuses on studies published in the English language,
with data limited to a specific academic database. While this
database is comprehensive, it may have overlooked relevant studies
published in other languages or those not included in the searched
database. Second, due to limited data on some interventions, the
present review includes several retrospective studies, which may be
subject to recall bias and other confounding factors. Additionally,
some interventions showed contradictory results, and more
large-scale RCTs with standardized methods may be needed for
further validation. Another limitation is that the studies included
in the present review focus on specific populations, which may
limit the applicability of the findings to other patient groups.
Moreover, different studies employed various diagnostic criteria
and assessment methods, which could impact the consistency and
reliability of the results.
With the widespread adoption of enhanced recovery
after surgery protocols and the growing demand for perioperative
comfort care, there has been increasing attention on postoperative
complications. POD is one of the most common and concerning
complications, but its pathogenesis remains unclear, and effective
preventive and therapeutic strategies are still lacking. The
present review provides a comprehensive and innovative analysis of
the mechanisms underlying POD, including the associated signaling
pathways, while summarizing preventive and therapeutic strategies
across three key areas: Pharmacological interventions,
non-pharmacological interventions and anesthesia management.
However, several key issues need to be addressed in
future research. First, robust preclinical models are needed to
effectively replicate human postoperative POD conditions, enabling
further exploration of the mechanisms that trigger POD and its
downstream effects. Understanding how various interventions
influence these processes is essential for developing more targeted
and effective treatments. Second, standardized inclusion criteria
and diagnostic methods must be established to enhance the
comparability of therapeutic efficacy and ensure the consistency
and reliability of research findings. Furthermore, the potential
role of neuroimaging and biomarkers in diagnosing POD should be
thoroughly investigated. Third, although some interventions have
demonstrated promising results, controversies remain regarding
their effectiveness. To resolve these uncertainties, large-scale,
multicenter RCTs are required to validate their efficacy.
Additionally, clinical practice should integrate multimodal
interventions that combine pharmacological treatments with
non-pharmacological approaches, rather than focusing exclusively on
pharmacological interventions or single-treatment strategies. In
summary, closing these research gaps will enhance the understanding
of POD and facilitate the development of more effective strategies
for its prevention and management.
Not applicable.
The present review was funded by the Major Project of the
General Hospital of Western Theater Command (grant no.
2021-XZYG-A10), the Youth Incubation Project of the General
Hospital of Western Theater Command (grant no. 2021-XZYG-C25) and
the Clinical Independent Innovation Project of the General Hospital
of Western Theater Command (grant no. 2024-YGLC-B12).
Not applicable.
WQL designed the study and wrote the manuscript. QS
and JZZ created the figures using BioRender (https://www.biorender.com/). RHB, LL and XMD
conducted the literature search. QQH and GG revised the manuscript.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Feng H, Zhang Z, Lyu W, Kong X, Li J, Zhou
H and Wei P: The effects of appropriate perioperative exercise on
perioperative neurocognitive disorders: A narrative review. Mol
Neurobiol. 61:4663–4676. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang X, Li J, Yang B, Lei C and Dong H:
Efficacy of sleep interventions on postoperative delirium: A
systematic review and meta-analysis of randomiszed controlled
trials. APS. 1:292023. View Article : Google Scholar
|
|
3
|
Ishizawa Y: Preoperative cognitive
optimization and postoperative cognitive outcomes: A narrative
review. Clin Interv Aging. 20:395–402. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen M, Liang H, Zhao Y, Liao R, Fang J,
Lin L, Tan P, Xu Y, Chen S, Chen H and Wei L: The perioperative
frailty index derived from the Chinese hospital information system:
A validation study. BMC Geriatr. 24:9572024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dilmen OK, Meco BC, Evered LA and Radtke
FM: Postoperative neurocognitive disorders: A clinical guide. J
Clin Anesth. 92:1113202024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evered L, Silbert B, Knopman DS, Scott DA,
DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M and Eckenhoff
RG; Nomenclature Consensus Working Group, : Recommendations for the
nomenclature of cognitive change associated with anaesthesia and
surgery-2018. Br J Anaesth. 121:1005–1012. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chinese Society of Geriatric Medicine and
Anesthesiology Branch, . Expert Consensus on the Prevention and
Treatment of Postoperative Delirium in Elderly Patients.
International Journal of Anesthesia and Resuscitation. 44:1–27.
2023.
|
|
8
|
Hughes CG, Boncyk CS, Culley DJ, Fleisher
LA, Leung JM, McDonagh DL, Gan TJ, McEvoy MD and Miller TE;
Perioperative Quality Initiative (POQI) 6 Workgroup, : American
society for enhanced recovery and perioperative quality initiative
joint consensus statement on postoperative delirium prevention.
Anesth Analg. 130:1572–1590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ely EW, Margolin R, Francis J, May L,
Truman B, Dittus R, Speroff T, Gautam S, Bernard GR and Inouye SK:
Evaluation of delirium in critically ill patients: Validation of
the confusion assessment method for the intensive care unit
(CAM-ICU). Crit Care Med. 29:1370–1379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaudreau JD, Gagnon P, Harel F, Tremblay A
and Roy MA: Fast, systematic, and continuous delirium assessment in
hospitalized patients: The nursing delirium screening scale. J Pain
Symptom Manage. 29:368–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hargrave A, Bastiaens J, Bourgeois JA,
Neuhaus J, Josephson SA, Chinn J, Lee M, Leung J and Douglas V:
Validation of a nurse-based delirium-screening tool for
hospitalized patients. Psychosomatics. 58:594–603. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neufeld KJ, Leoutsakos JS, Sieber FE,
Joshi D, Wanamaker BL, Rios-Robles J and Needham DM: Evaluation of
two delirium screening tools for detecting post-operative delirium
in the elderly. Br J Anaesth. 111:612–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gusmao-Flores D, Salluh JIF, Chalhub RÁ
and Quarantini LC: The confusion assessment method for the
intensive care unit (CAM-ICU) and intensive care delirium screening
checklist (ICDSC) for the diagnosis of delirium: A systematic
review and meta-analysis of clinical studies. Crit Care.
16:R1152012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Qiu Y, Zhang Z, Zhao Y and Ding
Y: Current perspectives on postoperative cognitive dysfunction in
geriatric patients: Insights from clinical practice. Front Med
(Lausanne). 11:14666812024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sprung J, Roberts RO, Weingarten TN,
Cavalcante AN, Knopman DS, Petersen RC, Hanson AC, Schroeder DR and
Warner DO: Postoperative delirium in elderly patients is associated
with subsequent cognitive impairment. Br J Anaesth. 119:316–323.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldberg TE, Chen C, Wang Y, Jung E,
Swanson A, Ing C, Garcia PS, Whittington RA and Moitra V:
Association of delirium with long-term cognitive decline: A
meta-analysis. JAMA Neurol. 77:1373–1381. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kunicki ZJ, Ngo LH, Marcantonio ER, Tommet
D, Feng Y, Fong TG, Schmitt EM, Travison TG, Jones RN and Inouye
SK: Six-year cognitive trajectory in older adults following major
surgery and delirium. JAMA Intern Med. 183:442–450. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glumac S, Kardum G and Karanovic N:
Postoperative cognitive decline after cardiac surgery: A narrative
review of current knowledge in 2019. Med Sci Monit. 25:3262–3270.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang AC, Stevens MY, Chen MB, Lee DP,
Stähli D, Gate D, Contrepois K, Chen W, Iram T, Zhang L, et al:
Physiological blood-brain transport is impaired with age by a shift
in transcytosis. Nature. 583:425–430. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Subramaniyan S and Terrando N:
Neuroinflammation and perioperative neurocognitive disorders.
Anesth Analg. 128:781–788. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin X, Tang J, Liu C, Li X, Cao X, Wang B,
Dong R, Xu W, Yu X, Wang M and Bi Y: Cerebrospinal fluid
cholinergic biomarkers are associated with postoperative delirium
in elderly patients undergoing total hip/knee replacement: A
prospective cohort study. BMC Anesthesiol. 20:2462020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu J, Liang F, Bai P, Liu C, Xu M, Sun Z,
Tian W, Dong Y, Zhang Y, Quan Q, et al: Blood tau-PT217 contributes
to the anesthesia/surgery-induced delirium-like behavior in aged
mice. Alzheimers Dement. 19:4110–4126. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rogers GB, Keating DJ, Young RL, Wong ML,
Licinio J and Wesselingh S: From gut dysbiosis to altered brain
function and mental illness: Mechanisms and pathways. Mol
Psychiatry. 21:738–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang C, Han Y, Liu X, Tan H, Dong Y,
Zhang Y, Liang F, Zheng H, Crosby G, Culley DJ, et al: Odor
enrichment attenuates the anesthesia/surgery-induced cognitive
impairment. Ann Surg. 277:e1387–e1396. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vance DE, Del Bene VA, Kamath V, Frank JS,
Billings R, Cho DY, Byun JY, Jacob A, Anderson JN, Visscher K, et
al: Does olfactory training improve brain function and cognition? A
systematic review. Neuropsychol Rev. 34:1–37. 2023.
|
|
26
|
Nogueira-de-Almeida CA, Zotarelli-Filho
IJ, Nogueirade-Almeida ME, Souza CG, Kemp VL and Ramos WS:
Neuronutrients and central nervous system: A systematic review.
Cent Nerv Syst Agents Med Chem. 23:1–12. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kashyap B, Hanson LR and Frey II WH:
Intranasal insulin: a treatment strategy for addiction.
Neurotherapeutics. 17:105–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caplan GA, Kvelde T, Lai C, Yap SL, Lin C
and Hill MA: Cerebrospinal fluid in long-lasting delirium compared
with alzheimer's dementia. J Gerontol A Biol Sci Med Sci.
65:1130–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Safar ME: Arterial aging-hemodynamic
changes and therapeutic options. Nat Rev Cardiol. 7:442–449. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang QQ, Lei N, Li SN, Wei Y, Yuan LB and
Gong G: Progress in intranasal insulin administration and
postoperative delirium. J Clin Anesthesiol. 38:101–104. 2022.
|
|
31
|
Yokota H, Ogawa S, Kurokawa A and Yamamoto
Y: Regional cerebral blood flow in delirium patients. Psychiatry
Clin Neurosci. 57:337–339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akintola AA, van Opstal AM, Westendorp RG,
Postmus I, van der Grond J and van Heemst D: Effect of intranasally
administered insulin on cerebral blood flow and perfusion; a
randomized experiment in young and older adults. Aging (Albany NY).
9:790–802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oh TK and Song IA: Preoperative cognitive
function and surgical outcomes under general anesthesia among older
patients. J Clin Anesth. 104:1118522025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang Y, Fang P, Shang Z, Zhu W, Gao S and
Liu X: Cognitive training in surgical patients: A systematic review
and meta-analysis. APS. 1:182023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiu X, Wang L, Wen X, Meng Q, Qi J, Li C,
Yin H, Ling F, Yuhan Q, Zhang W and Zhang Y: Effect of different
durations of preoperative computerised cognitive training on
postoperative delirium in older patients undergoing cardiac
surgery: A study protocol for a prospective, randomised controlled
trial. BMJ Open. 14:e0881632024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alam A, Hana Z, Jin Z, Suen KC and Ma D:
Surgery, neuroinflammation and cognitive impairment. EBioMedicine.
37:547–556. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brabazon F, Bermudez S, Shaughness M,
Khayrullina G and Byrnes KR: The effects of insulin on the
inflammatory activity of BV2 microglia. PLoS One. 13:e02018782018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Wang D, Yin K, Liu Y, Lu H, Zhao H
and Xing M: Lycopene attenuates oxidative stress, inflammation, and
apoptosis by modulating Nrf2/NF-B balance in
sulfamethoxazole-induced neurotoxicity in grass carp
(ctenopharyngodon idella). Fish Shellfish Immunol. 121:322–331.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu H, Guo T, Zhang Y, Liu D, Hou L, Ma C
and Xing M: Endoplasmic reticulum stress-induced NLRP3 inflammasome
activation as a novel mechanism of polystyrene microplastics
(PS-MPs)-induced pulmonary inflammation in chickens. J Zhejiang
Univ Sci B. 25:233–243. 2024.(In English, Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sabolová G, Kočan L, Rabajdová M,
Rapčanová S and Vašková J: Association of inflammation, oxidative
stress, and deteriorated cognitive functions in patients after
cardiac surgery. Vessel Plus. 8:272024.
|
|
41
|
Glumac S, Kardum G, Sodic L, Supe-Domic D
and Karanovic N: Effects of dexamethasone on early cognitive
decline after cardiac surgery: A randomised controlled trial. Eur J
Anaesthesiol. 34:776–784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sinsky J, Pichlerova K and Hanes J: Tau
protein interaction partners and their roles in Alzheimer's disease
and other tauopathies. Int J Mol Sci. 22:92072021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tao G, Zhang J, Zhang L, Dong Y, Yu B,
Crosby G, Culley DJ, Zhang Y and Xie Z: Sevoflurane induces tau
phosphorylation and glycogen synthase kinase 3 activation in young
mice. Anesthesiology. 121:510–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Whittington RA, Bretteville A, Dickler MF
and Planel E: Anesthesia and tau pathology. Prog
Neuropsychopharmacol Biol Psychiatry. 47:147–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tu S, Okamoto S, Lipton SA and Xu H:
Oligomeric a-induced synaptic dysfunction in Alzheimer's disease.
Mol Neurodegener. 9:482014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu C, Zhang C, Chen L, Liu X, Wu J, Sun
Y, Liu J and Chen C: Lingo1 in the hippocampus contributes to
cognitive dysfunction after anesthesia and surgery in aged mice.
Int J Biol Sci. 21:595–613. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peng L, Fang X, Xu F, Liu S, Qian Y, Gong
X, Zhao X, Ma Z, Xia T and Gu X: Amelioration of hippocampal
insulin resistance reduces tau hyperphosphorylation and cognitive
decline induced by isoflurane in mice. Front Aging Neurosci.
13:6865062021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu J, Li C, Yao J, Zhang L, Zhao X, Lv X,
Liu Z, Miao C, Wang Y, Jiang H, et al: Clinical biomarkers of
perioperative neurocognitive disorder: Initiation and
recommendation. Sci China Life Sci. 22:10.1007/s11427–024-2797-x.
2025.
|
|
49
|
Wang S, Greene R, Song Y, Chan C, Lindroth
H, Khan S, Rios G, Sanders RD and Khan B: Postoperative delirium
and its relationship with biomarkers for dementia: A meta-analysis.
Int Psychogeriatr. 34:377–390. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fong TG, Vasunilashorn SM, Ngo L,
Libermann TA, Dillon ST, Schmitt EM, Pascual-Leone A, Arnold SE,
Jones RN, Marcantonio ER, et al: Association of plasma
neurofilament light with postoperative delirium. Ann Neurol.
88:984–994. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lauretti E, Dincer O and Praticò D:
Glycogen synthase kinase-3 signaling in Alzheimer's disease.
Biochim Biophys Acta Mol Cell Res. 1867:1186642020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ahn EH and Park JB: Molecular mechanisms
of Alzheimer's disease induced by amyloid- and tau phosphorylation
along with RhoA activity: Perspective of RhoA/rho-associated
protein kinase inhibitors for neuronal therapy. Cells. 14:892025.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang H, Wei W, Zhao M, Ma L, Jiang X, Pei
H, Cao Y and Li H: Interaction between A and Tau in the
pathogenesis of Alzheimer's disease. Int J Biol Sci. 17:2181–2192.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Zhang X, Jiang M, Zhang Y, Wang C,
Sun Y, Shi Z and Wang B: Impact of preoperative sleep disturbances
on postoperative delirium in patients with intracranial tumors: A
prospective, observational, cohort study. Nat Sci Sleep.
15:1093–1105. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guo H, Li LH, Lv XH, Su FZ, Chen J, Xiao
F, Shi M and Xie YB: Association between preoperative sleep
disturbance and postoperative delirium in elderly: A retrospective
cohort study. Nat Sci Sleep. 16:389–400. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang Q, Wu X, Lei N, Chen X, Yu S, Dai X,
Shi Q, Gong G and Shu HF: Effects of intranasal insulin
pretreatment on preoperative sleep quality and postoperative
delirium in patients undergoing valve replacement for rheumatic
heart disease. Nat Sci Sleep. 16:613–623. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Telias I and Wilcox ME: Sleep and
circadian rhythm in critical illness. Crit Care. 23:822019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin Y, Xu S, Peng Y, Li S, Huang X and
Chen L: Preoperative slow-wave sleep is associated with
postoperative delirium after heart valve surgery: A prospective
pilot study. J Sleep Res. 32:e139202023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dulko E, Jedrusiak M, Osuru HP, Atluri N,
Illendula M, Davis EM, Beenhakker MP and Lunardi N: Sleep
fragmentation, electroencephalographic slowing, and circadian
disarray in a mouse model for intensive care unit delirium. Anesth
Analg. 137:209–220. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li S, Zhou H, Yu Y, Lyu H, Mou T, Shi G,
Hu S, Huang M, Hu J and Xu Y: Effect of repetitive transcranial
magnetic stimulation on the cognitive impairment induced by sleep
deprivation: A randomized trial. Sleep Med. 77:270–278. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sic A, Cvetkovic K, Manchanda E and
Knezevic NN: Neurobiological implications of chronic stress and
metabolic dysregulation in inflammatory bowel diseases. Diseases.
12:2202024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sugama S and Kakinuma Y: Stress and brain
immunity: Microglial homeostasis through
hypothalamus-pituitary-adrenal gland axis and sympathetic nervous
system. Brain Behav Immun Health. 7:1001112020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sharan P and Vellapandian C:
Hypothalamic-pituitary-adrenal (HPA) axis: Unveiling the potential
mechanisms involved in stress-induced alzheimer's disease and
depression. Cureus. 16:e675952024.PubMed/NCBI
|
|
64
|
Fultz NE, Bonmassar G, Setsompop K,
Stickgold RA, Rosen BR, Polimeni JR and Lewis LD: Coupled
electrophysiological, hemodynamic, and cerebrospinal fluid
oscillations in human sleep. Science. 366:628–631. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Figueroa-Ramos MI, Arroyo-Novoa CM, Lee
KA, Padilla G and Puntillo KA: Sleep and delirium in ICU patients:
A review of mechanisms and manifestations. Intensive Care Med.
35:781–795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Han SA, Kim JK, Cho DY, Patel ZM and Rhee
CS: The olfactory system: Basic anatomy and physiology for general
otorhinolaryngologists. Clin Exp Otorhinolaryngol. 16:308–316.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Marin C, Vilas D, Langdon C, Alobid I,
López-Chacón M, Haehner A, Hummel T and Mullol J: Olfactory
dysfunction in neurodegenerative diseases. Curr Allergy Asthma Rep.
18:422018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kamath V, Yanek LR, Neufeld KJ, Lewis A,
Aziz H, Le LM, Tian J, Moghekar A, Hogue CW Jr and Brown CH IV:
Poor olfaction prior to cardiac surgery: Association with
cognition, plasma neurofilament light, and post-operative delirium.
Int J Geriatr Psychiatry. 39:e60662024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang Y, Chen J, Wen Q, Jin G, Liu F, Yu L
and He J: Effects of preoperative neoadjuvant chemotherapy on
postoperative delirium in patients with gynecological tumor
surgery: An observational study. J Cancer Res Clin Oncol.
150:4972024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liang H and Wang HR: Research progress on
the correlation between olfactory disorders and cognitive
impairment based on the olfactory-brain connection pathway.
Liaoning J Tradit Chin Med. 52:199–202. 2025.(In Chinese).
|
|
71
|
Pasquini J, Brooks DJ and Pavese N: The
cholinergic brain in Parkinson's disease. Mov Disord Clin Pract.
8:1012–1026. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gao Q, Sun TP and Wu GY: Research progress
on the pathophysiological mechanisms of postoperative delirium in
elderly patients. Med Res Educ. 39:8–13. 2022.
|
|
73
|
Mayer EA, Tillisch K and Gupta A:
Gut/brain axis and the microbiota. J Clin Invest. 125:926–938.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen Y, Xu J and Chen Y: Regulation of
neurotransmitters by the gut microbiota and effects on cognition in
neurological disorders. Nutrients. 13:20992021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liufu N, Liu L, Shen S, Jiang Z, Dong Y,
Wang Y, Culley D, Crosby G, Cao M, Shen Y, et al: Anesthesia and
surgery induce age-dependent changes in behaviors and microbiota.
Aging (Albany NY). 12:1965–1986. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lu J, Hou W, Gao S, Zhang Y and Zong Y:
The role of gut microbiota-gut-brain axis in perioperative
neurocognitive dysfunction. Front Pharmacol. 13:8797452022.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Qu S, Yu Z, Zhou Y, Wang S, Jia M, Chen T
and Zhang X: Gut microbiota modulates neurotransmitter and
gut-brain signaling. Microbiol Res. 287:1278582024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Simpson CA, Diaz-Arteche C, Eliby D,
Schwartz OS, Simmons JG and Cowan CSM: The gut microbiota in
anxiety and depression-a systematic review. Clin Psychol Rev.
83:1019432021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kolobaric A, Andreescu C, Jašarević E,
Hong CH, Roh HW, Cheong JY, Kim YK, Shin TS, Kang CS, Kwon CO, et
al: Gut microbiome predicts cognitive function and depressive
symptoms in late life. Mol Psychiatry. 29:3064–3075. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ma K and Bebawy JF:
Electroencephalographic burst-suppression, perioperative
neuroprotection, postoperative cognitive function, and mortality: A
focused narrative review of the literature. Anesth Analg.
135:79–90. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen YC, Hung IY, Hung KC, Chang YJ, Chu
CC, Chen JY, Ho CH and Yu CH: Incidence change of postoperative
delirium after implementation of processed electroencephalography
monitoring during surgery: A retrospective evaluation study. BMC
Anesthesiol. 23:3302023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Al-Qudah AM, Sivaguru S, Anetakis K,
Crammond DJ, Balzer JR, Thirumala PD, Subramaniam K, Sadhasivam S
and Shandal V: Role of intraoperative electroencephalography in
predicting postoperative delirium in patients undergoing
cardiovascular surgeries. Clin Neurophysiol. 164:40–46. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Evered LA, Chan MTV, Han R, Chu MHM, Cheng
BP, Scott DA, Pryor KO, Sessler DI, Veselis R, Frampton C, et al:
Anaesthetic depth and delirium after major surgery: A randomised
clinical trial. Br J Anaesth. 127:704–712. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lele AV, Furman M, Myers J, Kinney G,
Sharma D and Hecker J: Inadvertent burst suppression during total
intravenous anesthesia in 112 consecutive patients undergoing
spinal instrumentation surgery: A retrospective observational
quality improvement project. J Neurosurg Anesthesiol. 34:300–305.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li RX, Jiang ZS and Gu WD: Research
progress on burst suppression during general anesthesia and
perioperative neurocognitive dysfunction. Geriatr Med Health Care.
29:833–836. 2023.
|
|
86
|
Vasunilashorn SM, Ngo LH, Inouye SK, Fong
TG, Jones RN, Dillon ST, Libermann TA, O'Connor M, Arnold SE, Xie Z
and Marcantonio ER: Apolipoprotein E genotype and the association
between C-reactive protein and postoperative delirium: Importance
of gene-protein interactions. Alzheimers Dement. 16:572–580. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sepulveda E, Adamis D, Franco JG, Meagher
D, Aranda S and Vilella E: The complex interaction of genetics and
delirium: A systematic review and meta-analysis. Eur Arch
Psychiatry Clin Neurosci. 271:929–939. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
van Munster BC, Yazdanpanah M, Tanck MWT,
de Rooij SE, van de Giessen E, Sijbrands EJ, Zwinderman AH and
Korevaar JC: Genetic polymorphisms in the DRD2, DRD3, and SLC6A3
gene in elderly patients with delirium. Am J Med Genet B
Neuropsychiatr Genet. 153B:38–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang SQ and Cao J: Research progress on
postoperative delirium in elderly orthopedic patients. Chongqing
Med. 52:3182–3187. 2023.(In Chinese).
|
|
90
|
Zhang M and Yin Y: Dual roles of
anesthetics in postoperative cognitive dysfunction: Regulation of
microglial activation through inflammatory signaling pathways.
Front Immunol. 14:11023122023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chae WJ and Bothwell ALM: Canonical and
non-canonical wnt signaling in immune cells. Trends Immunol.
39:830–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chang X, Yang WQ, Han CF, Zhao XL and Chen
Y: Mechanism of the Wnt/-catenin signaling pathway in
sevoflurane-induced postoperative cognitive dysfunction. J Integr
Tradit Chin West Med Cardiovasc Dis. 22:75–78. 2024.(In
Chinese).
|
|
93
|
Hu N, Wang C, Zheng Y, Ao J, Zhang C, Xie
K, Li Y, Wang H, Yu Y and Wang G: The role of the
wnt/-catenin-annexin A1 pathway in the process of
sevoflurane-induced cognitive dysfunction. J Neurochem.
137:240–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Killick R, Ribe EM, Al-Shawi R, Malik B,
Hooper C, Fernandes C, Dobson R, Nolan PM, Lourdusamy A, Furney S,
et al: Clusterin regulates -amyloid toxicity via dickkopf-1-driven
induction of the wnt-PCP-JNK pathway. Mol Psychiatry. 19:88–98.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lu H, Yin K, Su H, Wang D, Zhang Y, Hou L,
Li JB, Wang Y and Xing M: Polystyrene microplastics induce
autophagy and apoptosis in birds lungs via PTEN/PI3K/AKT/mTOR.
Environ Toxicol. 38:78–89. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zeng GH, Zhu T, Zou L and Zhou J: Research
progress on the PI3K/Akt-related signaling pathway in the
pathophysiological mechanisms of neural cells. Chin J Pathophysiol.
40:1529–1535. 2024.(In Chinese).
|
|
97
|
Ochalek A, Mihalik B, Avci HX,
Chandrasekaran A, Téglási A, Bock I, Giudice ML, Táncos Z, Molnár
K, László L, et al: Neurons derived from sporadic Alzheimer's
disease iPSCs reveal elevated TAU hyperphosphorylation, increased
amyloid levels, and GSK3B activation. Alzheimers Res Ther.
9:902017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhou Q, Li S, Li M, Ke D, Wang Q, Yang Y,
Liu GP, Wang XC, Liu E and Wang JZ: Human tau accumulation promotes
glycogen synthase kinase-3 acetylation and thus upregulates the
kinase: A vicious cycle in Alzheimer neurodegeneration.
EBioMedicine. 78:1039702022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hampel H, Ewers M, Bürger K, Annas P,
Mörtberg A, Bogstedt A, Frölich L, Schröder J, Schönknecht P, Riepe
MW, et al: Lithium trial in Alzheimer's disease: A randomized,
single-blind, placebo-controlled, multicenter 10-week study. J Clin
Psychiatry. 70:922–931. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yamamoto N, Ishikuro R, Tanida M, Suzuki
K, Ikeda-Matsuo Y and Sobue K: Insulin-signaling pathway regulates
the degradation of amyloid-protein via astrocytes. Neuroscience.
385:227–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rekha A, Afzal M, Babu MA, Menon SV,
Nathiya D, Supriya S, Mishra SB, Gupta S, Goyal K, Rana M, et al:
GSK-3 dysregulation in aging: Implications for tau pathology and
Alzheimer's disease progression. Mol Cell Neurosci. 133:1040052025.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jolivalt CG, Lee CA, Beiswenger KK, Smith
JL, Orlov M, Torrance MA and Masliah E: Defective insulin signaling
pathway and increased glycogen synthase kinase-3 activity in the
brain of diabetic mice: Parallels with Alzheimer's disease and
correction by insulin. J Neurosci Res. 86:3265–3274. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Llorens-Martín M, Jurado J, Hernández F
and Avila J: GSK-3, a pivotal kinase in alzheimer disease. Front
Mol Neurosci. 7:462014.PubMed/NCBI
|
|
104
|
Gianferrara T, Cescon E, Grieco I,
Spalluto G and Federico S: Glycogen synthase kinase 3 involvement
in neuroinflammation and neurodegenerative diseases. Curr Med Chem.
29:4631–4697. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Guo M, Wang Y, Zhao H, Wang D, Yin K, Liu
Y, Li B and Xing M: Zinc antagonizes common carp (cyprinus carpio)
intestinal arsenic poisoning through PI3K/AKT/mTOR signaling
cascade and MAPK pathway. Aquat Toxicol. 240:1059862021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gao S, Zhang S, Zhou H, Tao X, Ni Y, Pei
D, Kang S, Yan W and Lu J: Role of mTOR-regulated autophagy in
synaptic plasticity related proteins downregulation and the
reference memory deficits induced by anesthesia/surgery in aged
mice. Front Aging Neurosci. 13:6285412021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Glaviano A, Foo ASC, Lam HY, Yap KCH,
Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al:
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies
in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chen M, Han Y, Que B, Zhou R, Gan J and
Dong X: Prophylactic effects of sub-anesthesia ketamine on
cognitive decline, neuroinflammation, and oxidative stress in
elderly mice. Am J Alzheimers Dis Other Demen.
37:153331752211415312022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Cheng LL, Tian Y, Tan YW and Wang D:
Effect of dexmedetomidine on hippocampal neuronal autophagy in rats
with postoperative neurocognitive dysfunction after hepatectomy via
the PI3K/Akt/mTOR signaling pathway. West Med. 33:793–798.
8032021.
|
|
111
|
Brasil FB, Gobbo RC, de Almeida FJ,
Luckachaki MD, Dall'Oglio EL and de Oliveira MR: The signaling
pathway PI3K/Akt/Nrf2/HO-1 plays a role in the mitochondrial
protection promoted by astaxanthin in the SH-SY5Y cells exposed to
hydrogen peroxide. Neurochem Int. 146:1050242021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Deng Q, Liang JQ, Gao CH, Ge X, Zheng JJ
and Zhang QP: Effects of electroacupuncture on the PI3K/Akt/CREB
signaling pathway and hippocampal neuronal apoptosis in diabetic
cognitive impairment rats. Zhen Ci Yan Jiu. 49:265–273. 2024.(In
English, Chinese). PubMed/NCBI
|
|
113
|
Ma RJ, Kannan M, Xia Q, Zhang SS, Tu PF,
Liu KC and Zhang Y: Kunxian capsule extract inhibits angiogenesis
in zebrafish embryos via PI3K/AKT-MAPK-VEGF pathway. Chin J Integr
Med. 29:137–145. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yang YR: Role and mechanism of the
BDNF/TrkB signaling pathway in sevoflurane-induced cognitive
dysfunction in aged rats. Inner Mongolia Medical University;
2022
|
|
115
|
Qiu LL, Ji MH, Zhang H and Yang JJ, Sun
XR, Tang H, Wang J, Liu WX and Yang JJ: NADPH oxidase 2-derived
reactive oxygen species in the hippocampus might contribute to
microglial activation in postoperative cognitive dysfunction in
aged mice. Brain Behav Immun. 51:109–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Qiu LL, Pan W, Luo D, Zhang GF, Zhou ZQ,
Sun XY, Yang JJ and Ji MH: Dysregulation of BDNF/TrkB signaling
mediated by NMDAR/Ca2+/calpain might contribute to postoperative
cognitive dysfunction in aging mice. J Neuroinflammation.
17:232020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Jia J, Zhu J, Yang Q, Wang Y, Zhang Z and
Chen C: The role of histone acetylation in the sevoflurane-induced
inhibition of neurogenesis in the hippocampi of young mice.
Neuroscience. 432:73–83. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Fan D, Li J, Zheng B, Hua L and Zuo Z:
Enriched environment attenuates surgery-induced impairment of
learning, memory, and neurogenesis possibly by preserving BDNF
expression. Mol Neurobiol. 53:344–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Pisani A, Paciello F, Del Vecchio V,
Malesci R, De Corso E, Cantone E and Fetoni AR: The role of BDNF as
a biomarker in cognitive and sensory neurodegeneration. J Pers Med.
13:6522023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gao L, Zhang Y, Sterling K and Song W:
Brain-derived neurotrophic factor in Alzheimer's disease and its
pharmaceutical potential. Transl Neurodegener. 11:42022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Jiao SS, Shen LL, Zhu C, Bu XL, Liu YH,
Liu CH, Yao XQ, Zhang LL, Zhou HD, Walker DG, et al: Brain-derived
neurotrophic factor protects against tau-related neurodegeneration
of Alzheimer's disease. Transl Psychiatry. 6:e9072016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wu L, Xian X, Xu G, Tan Z, Dong F, Zhang M
and Zhang F: Toll-like receptor 4: A promising therapeutic target
for Alzheimer's disease. Mediators Inflamm. 2022:79241992022.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Duan T, Du Y, Xing C, Wang HY and Wang RF:
Toll-like receptor signaling and its role in cell-mediated
immunity. Front Immunol. 13:8127742022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lu SM, Yu CJ, Liu YH, Dong HQ, Zhang X,
Zhang SS, Hu LQ, Zhang F, Qian YN and Gui B: S100A8 contributes to
postoperative cognitive dysfunction in mice undergoing tibial
fracture surgery by activating the TLR4/MyD88 pathway. Brain Behav
Immun. 44:221–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wang Y, He H, Li D, Zhu W, Duan K, Le Y,
Liao Y and Ou Y: The role of the TLR4 signaling pathway in
cognitive deficits following surgery in aged rats. Mol Med Rep.
7:1137–1142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lin F, Shan W, Zheng Y, Pan L and Zuo Z:
Toll-like receptor 2 activation and upregulation by high mobility
group box l contribute to postoperative neuroinflammation and
cognitive dysfunction in mice. J Neurochem. 158:328–341. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yang C, Sun S, Zhang Q, Guo J, Wu T, Liu
Y, Yang M, Zhang Y and Peng Y: Exosomes of antler mesenchymal stem
cells improve postoperative cognitive dysfunction in
cardiopulmonary bypass rats through inhibiting the TLR2/TLR4
signaling pathway. Stem Cells Int. 2020:21345652020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chen C, Gao R, Li M, Wang Q, Chen H, Zhang
S, Mao X, Behensky A, Zhang Z, Gan L, et al: Extracellular
RNAs-TLR3 signaling contributes to cognitive decline in a mouse
model of postoperative cognitive dysfunction. Brain Behav Immun.
80:439–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Deng L, Gao R, Chen H, Jiao B, Zhang C,
Wei L, Yan C, Ye-Lehmann S, Zhu T and Chen C: Let-7b-TLR7 signaling
axis contributes to the anesthesia/surgery-induced cognitive
impairment. Mol Neurobiol. 61:1818–1832. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Xie XY, Meng Y, Qiao ML, Wang YN and Yao
WW: Research progress on traditional Chinese medicine intervention
in Alzheimer's disease based on signaling pathways. Chin Med.
19:128–132. 2024.(In Chinese). PubMed/NCBI
|
|
131
|
Song H, Xun S, He H, Duan C and Li Q:
Compound porcine cerebroside and ganglioside injection (CPCGI)
attenuates sevoflurane-induced nerve cell injury by regulating the
phosphorylation of p38 MAP kinase (p38MAPK)/nuclear factor kappa B
(NF-B) pathway. Med Sci Monit. 26:e9196002020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lv JM, Gao YL, Wang LY, Li BD, Shan YL, Wu
ZQ, Lu QM, Peng HY, Zhou TT, Li XM and Zhang LM: Inhibition of the
P38 MAPK/NLRP3 pathway mitigates cognitive dysfunction and mood
alterations in aged mice after abdominal surgery plus sevoflurane.
Brain Res Bull. 217:1110592024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Bikkavilli RK, Feigin ME and Malbon CC:
p38 mitogen-activated protein kinase regulates canonical
Wnt-catenin signaling by inactivation of GSK3. J Cell Sci.
121:3598–3607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chen Y, Li L, Zhang J, Cui H, Wang J, Wang
C, Shi M and Fan H: Dexmedetomidine alleviates
lipopolysaccharide-induced hippocampal neuronal apoptosis via
inhibiting the p38 MAPK/c-myc/CLIC4 signaling pathway in rats. Mol
Neurobiol. 58:5533–5547. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Sabapathy K: Role of the JNK pathway in
human diseases. Prog Mol Biol Transl Sci. 106:145–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rehfeldt SC, Majolo F, Goettert MI and
Laufer S: c-Jun N-Terminal kinase inhibitors as potential leads for
new therapeutics for Alzheimer's diseases. Int J Mol Sci.
21:96772020. View Article : Google Scholar
|
|
137
|
Li Y, Wang F, Liu C, Zeng M, Han X, Luo T,
Jiang W, Xu J and Wang H: JNK pathway may be involved in
isoflurane-induced apoptosis in the hippocampi of neonatal rats.
Neurosci Lett. 545:17–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bi C, Cai Q, Shan Y, Yang F, Sun S, Wu X
and Liu H: Sevoflurane induces neurotoxicity in the developing rat
hippocampus by upregulating connexin 43 via the JNK/c-Jun/AP-1
pathway. Biomed Pharmacother. 108:1469–1476. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yang Z, Lv J, Li X, Meng Q, Yang Q, Ma W,
Li Y and Ke ZJ: Sevoflurane decreases self-renewal capacity and
causes c-Jun N-terminal kinase-mediated damage of rat fetal neural
stem cells. Sci Rep. 7:463042017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wang LY, Tang ZJ and Han YZ:
Neuroprotective effects of caffeic acid phenethyl ester against
sevoflurane-induced neuronal degeneration in the hippocampus of
neonatal rats involve MAPK and PI3K/Akt signaling pathways. Mol Med
Rep. 14:3403–3412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhao X, Li S, Gaur U and Zheng W:
Artemisinin improved neuronal functions in alzheimer's disease
animal model 3×tg mice and neuronal cells via stimulating the
ERK/CREB signaling pathway. Aging Dis. 11:801–819. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yufune S, Satoh Y, Akai R, Yoshinaga Y,
Kobayashi Y, Endo S and Kazama T: Suppression of ERK
phosphorylation through oxidative stress is involved in the
mechanism underlying sevoflurane-induced toxicity in the developing
brain. Sci Rep. 6:218592016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Peng S, Yan HZ, Liu PR, Shi XW, Liu CL,
Liu Q and Zhang Y: Phosphodiesterase 4 inhibitor roflumilast
protects rat hippocampal neurons from sevoflurane induced injury
via modulation of MEK/ERK signaling pathway. Cell Physiol Biochem.
45:2329–2337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Straiko MMW, Young C, Cattano D, Creeley
CE, Wang H, Smith DJ, Johnson SA, Li ES and Olney JW: Lithium
protects against anesthesia-induced developmental neuroapoptosis.
Anesthesiology. 110:862–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang WY, Yang R, Hu SF, Wang H, Ma ZW and
Lu Y: N-stearoyl-L-tyrosine ameliorates sevoflurane induced
neuroapoptosis via MEK/ERK1/2 MAPK signaling pathway in the
developing brain. Neurosci Lett. 541:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Huang H, Li Y, Wang X, Zhang Q, Zhao J and
Wang Q: Electroacupuncture pretreatment protects against
anesthesia/surgery-induced cognitive decline by activating CREB via
the ERK/MAPK pathway in the hippocampal CA1 region in aged rats.
Aging (Albany NY). 15:11227–11243. 2023.PubMed/NCBI
|
|
147
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Albert-Gascó H, Ros-Bernal F,
Castillo-Gómez E and Olucha-Bordonau FE: MAP/ERK signaling in
developing cognitive and emotional function and its effect on
pathological and neurodegenerative processes. Int J Mol Sci.
21:44712020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Huang X, Ying J, Yang D, Fang P, Wang X,
Zhou B, Zhang L, Fang Y, Yu W, Liu X, et al: The mechanisms of
sevoflurane-induced neuroinflammation. Front Aging Neurosci.
13:7177452021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Yang D, Su J, Chen Y and Chen G: The NF-B
pathway: Key players in neurocognitive functions and related
disorders. Eur J Pharmacol. 984:1770382024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Liu P, Gao Q, Guan L, Hu Y, Jiang J, Gao
T, Sheng W, Xue X, Qiao H and Li T: Atorvastatin attenuates
surgery-induced BBB disruption and cognitive impairment partly by
suppressing NF-B pathway and NLRP3 inflammasome activation in aged
mice. Acta Biochim Biophys Sin (Shanghai). 53:528–537. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Li ZQ, Rong XY, Liu YJ, Ni C, Tian XS, Mo
N, Chui DH and Guo XY: Activation of the canonical nuclear factor-B
pathway is involved in isoflurane-induced hippocampal interleukin-1
elevation and the resultant cognitive deficits in aged rats.
Biochem Biophys Res Commun. 438:628–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wang J, Xin Y, Chu T, Liu C and Xu A:
Dexmedetomidine attenuates perioperative neurocognitive disorders
by suppressing hippocampal neuroinflammation and HMGB1/RAGE/NF-B
signaling pathway. Biomed Pharmacother. 150:1130062022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Dai J, Li X, Wang C, Gu S, Dai L, Zhang J,
Fan Y and Wu J: Repeated neonatal sevoflurane induced
neurocognitive impairment through NF-B-mediated pyroptosis. J
Neuroinflammation. 18:1802021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tian Y, Guo S, Wu X, Ma L and Zhao X:
Minocycline alleviates sevoflurane-induced cognitive impairment in
aged rats. Cell Mol Neurobiol. 35:585–594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Sun L, Yong Y, Wei P, Wang Y, Li H, Zhou
Y, Ruan W, Li X and Song J: Electroacupuncture ameliorates
postoperative cognitive dysfunction and associated
neuroinflammation via NLRP3 signal inhibition in aged mice. CNS
Neurosci Ther. 28:390–400. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Zand H, Morshedzadeh N and Naghashian F:
Signaling pathways linking inflammation to insulin resistance.
Diabetes Metab Syndr. 11:S307–S309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Fleming-de-Moraes CD, Rocha MR, Tessmann
JW, de Araujo WM and Morgado-Diaz JA: Crosstalk between PI3K/Akt
and Wnt/-catenin pathways promote colorectal cancer progression
regardless of mutational status. Cancer Biol Ther. 23:1–13. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Muscat SM, Deems NP, Butler MJ, Scaria EA,
Bettes MN, Cleary SP, Bockbrader RH, Maier SF and Barrientos RM:
Selective TLR4 antagonism prevents and reverses morphine-induced
persistent postoperative cognitive dysfunction, dysregulation of
synaptic elements, and impaired BDNF signaling in aged male rats. J
Neurosci. 43:155–172. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Guo Q, Jin Y, Chen X, Ye X, Shen X, Lin M,
Zeng C, Zhou T and Zhang J: NF-B in biology and targeted therapy:
New insights and translational implications. Signal Transduct
Target Ther. 9:532024. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Rubin FH, Neal K, Fenlon K, Hassan S and
Inouye SK: Sustainability and scalability of the hospital elder
life program at a community hospital. J Am Geriatr Soc. 59:359–365.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
American Geriatrics Society Expert Panel
on Postoperative Delirium in Older Adults, . American geriatrics
society abstracted clinical practice guideline for postoperative
delirium in older adults. J Am Geriatr Soc. 63:142–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Humeidan ML, Reyes JPC, Mavarez-Martinez
A, Roeth C, Nguyen CM, Sheridan E, Zuleta-Alarcon A, Otey A,
Abdel-Rasoul M and Bergese SD: Effect of cognitive prehabilitation
on the incidence of postoperative delirium among older adults
undergoing major noncardiac surgery: The neurobics randomized
clinical trial. JAMA Surg. 156:148–156. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Jiang Y, Xie Y, Fang P, Shang Z, Chen L,
Zhou J, Yang C, Zhu W, Hao X, Ding J, et al: Cognitive training for
reduction of delirium in patients undergoing cardiac surgery: A
randomized clinical trial. JAMA Netw Open. 7:e2473612024.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Saleh AJ, Tang GX, Hadi SM, Yan L, Chen
MH, Duan KM, Tong J and Ouyang W: Preoperative cognitive
intervention reduces cognitive dysfunction in elderly patients
after gastrointestinal surgery: A randomized controlled trial. Med
Sci Monit. 21:798–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Ogawa M, Izawa KP, Satomi-Kobayashi S,
Kitamura A, Tsuboi Y, Komaki K, Ono R, Sakai Y, Tanaka H and Okita
Y: Preoperative exercise capacity is associated with the prevalence
of postoperative delirium in elective cardiac surgery. Aging Clin
Exp Res. 30:27–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Yanagisawa T, Tatematsu N, Horiuchi M,
Migitaka S, Yasuda S, Itatsu K, Kubota T and Sugiura H:
Preoperative low physical activity is a predictor of postoperative
delirium in patients with gastrointestinal cancer: A retrospective
study. Asian Pac J Cancer Prev. 23:1753–1759. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Janssen TL, Steyerberg EW, Langenberg JCM,
de Lepper CCHAVH, Wielders D, Seerden TCJ, de Lange DC, Wijsman JH,
Ho GH, Gobardhan PD, et al: Multimodal prehabilitation to reduce
the incidence of delirium and other adverse events in elderly
patients undergoing elective major abdominal surgery: An
uncontrolled before-and-after study. PLoS One. 14:e02181522019.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Shorofi SA, Dadashian P, Arbon P and
Moosazadeh M: The efficacy of earplugs and eye masks for delirium
severity and sleep quality in patients undergoing coronary artery
bypass grafting in cardiac intensive care units: A single-blind,
randomised controlled trial. Aust Crit Care. 37:74–83. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Kamdar BB, King LM, Collop NA, Sakamuri S,
Colantuoni E, Neufeld KJ, Bienvenu OJ, Rowden AM, Touradji P,
Brower RG and Needham DM: The effect of a quality improvement
intervention on perceived sleep quality and cognition in a medical
ICU. Crit Care Med. 41:800–809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Kappen PR, Mos MI, Jeekel J, Dirven CMF,
Kushner SA, Osse RJ, Coesmans M, Poley MJ, van Schie MS, van der
Holt B, et al: Music to prevent deliriUm during neuroSurgerY
(MUSYC): A single-centre, prospective randomised controlled trial.
BMJ Open. 13:e0699572023. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Han S, Cai Z, Cao L, Li J and Huang L:
Effects of Chinese traditional five-element music intervention on
postoperative delirium and sleep quality in elderly patients after
non-cardiac surgery: A randomized controlled trial. Perioper Med
(Lond). 13:472024. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Zhu F, Bai Y and Mo L: Effects of enriched
environment on perioperative psychological status and cognitive
function in patients with esophageal cancer. Chin J Mod Med.
28:96–100. 2018.(In Chinese).
|
|
174
|
Inouye SK, Bogardus ST, Charpentier PA,
Leo-Summers L, Acampora D, Holford TR and Cooney LM Jr:
multicomponent intervention to prevent delirium in hospitalized
older patients. N Engl J Med. 340:669–676. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Chen CCH, Li HC, Liang JT, Lai IR, Purnomo
JDT, Yang YT, Lin BR, Huang J, Yang CY, Tien YW, et al: Effect of a
modified hospital elder life program on delirium and length of
hospital stay in patients undergoing abdominal surgery: A cluster
randomized clinical trial. JAMA Surg. 152:827–834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Wang YY, Yue JR, Xie DM, Carter P, Li QL,
Gartaganis SL, Chen J and Inouye SK: Effect of the tailored,
family-involved hospital elder life program on postoperative
delirium and function in older adults: A randomized clinical trial.
JAMA Intern Med. 180:17–25. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Hshieh TT, Yang T, Gartaganis SL, Yue J
and Inouye SK: Hospital elder life program: Systematic review and
meta-analysis of effectiveness. Am J Geriatr Psychiatry.
26:1015–1033. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Zhao L, Guo Y, Zhou X, Mao W, Zhu H, Chen
L, Liu X, Zhang L, Xie Y and Li L: The research progress of
perioperative non-pharmacological interventions on postoperative
cognitive dysfunction: a narrative review. Front Neurol.
15:13698212024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Wang W, Li HL, Wang DX, Zhu X, Li SL, Yao
GQ, Chen KS, Gu XE and Zhu SN: Haloperidol prophylaxis decreases
delirium incidence in elderly patients after noncardiac surgery: A
randomized controlled trial*. Crit Care Med. 40:731–739. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Hakim SM, Othman AI and Naoum DO: Early
treatment with risperidone for subsyndromal delirium after on-pump
cardiac surgery in the elderly: A randomized trial. Anesthesiology.
116:987–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
van den Boogaard M, Slooter AJC,
Brüggemann RJM, Schoonhoven L, Beishuizen A, Vermeijden JW,
Pretorius D, de Koning J, Simons KS, Dennesen PJW, et al: Effect of
haloperidol on survival among critically ill adults with a high
risk of delirium. JAMA. 319:680–691. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Li H, Liu C, Yang Y, Wu QP, Xu JM, Wang
DF, Sun JJ, Mao MM, Lou JS, Liu YH, et al: Effect of intraoperative
midazolam on postoperative delirium in older surgical patients: A
prospective, multicenter cohort study. Anesthesiology. 142:268–277.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Kim J, Lee S, Yoo BH, Lim YH and Jun IJ:
The effects of remimazolam and inhalational anesthetics on the
incidence of postoperative hyperactive delirium in geriatric
patients undergoing hip or femur surgery under general anesthesia:
A retrospective observational study. Medicina (Kaunas). 61:3362025.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Cai YH, Zhong JW, Ma HY, Szmuk P, Wang CY,
Wang Z, Zhang XL, Dong LQ and Liu HC: Effect of remimazolam on
emergence delirium in children undergoing laparoscopic surgery: A
double-blinded randomized trial. Anesthesiology. 141:500–510. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Li S, Li R, Li M, Cui Q, Zhang X, Ma T,
Wang D, Zeng M, Li H, Bao Z, et al: Dexmedetomidine administration
during brain tumour resection for prevention of postoperative
delirium: A randomised trial. Br J Anaesth. 130:e307–e316. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
van Norden J, Spies CD, Borchers F,
Mertens M, Kurth J, Heidgen J, Pohrt A and Mueller A: The effect of
peri-operative dexmedetomidine on the incidence of postoperative
delirium in cardiac and non-cardiac surgical patients: A
randomised, double-blind placebo-controlled trial. Anaesthesia.
76:1342–1351. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang
DX, Zhu X, Zhu SN, Maze M and Ma D: Dexmedetomidine for prevention
of delirium in elderly patients after non-cardiac surgery: A
randomised, double-blind, placebo-controlled trial. Lancet.
388:1893–1902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Liu J, Wang T, Song J and Cao L: Effect of
esketamine on postoperative analgesia and postoperative delirium in
elderly patients undergoing gastrointestinal surgery. BMC
Anesthesiol. 24:462024. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zhang CL, Yan Y, Zhang Y, Bai HL, Zhuang
Q, Song NN, Feng CJ, Xie LJ, Wang SY, Li XH, et al: Effects of
esketamine combined with dexmedetomidine on postoperative delirium
and quality of recovery in elderly patients undergoing
thoracoscopic radical lung cancer surgery: A randomized controlled
trial. CNS Spectr. 20:1–10. 2024.
|
|
190
|
Xiong X, Shao Y, Chen D, Chen B, Lan X and
Shi J: Effect of esketamine on postoperative delirium in patients
undergoing cardiac valve replacement with cardiopulmonary bypass: A
randomized controlled trial. Anesth Analg. 139:743–753. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Fazel MR, Mofidian S, Mahdian M, Akbari H
and Razavizadeh MR: The effect of melatonin on prevention of
postoperative delirium after lower limb fracture surgery in elderly
patients: A randomized double blind clinical trial. Int J Burns
Trauma. 12:161–167. 2022.PubMed/NCBI
|
|
192
|
Elbakry AEA, El-Desoky IM, Saafan AG and
Elsersy HE: The impact of melatonin on postoperative delirium in
geriatric patients after colorectal surgery: A randomized
placebo-controlled trial. Minerva Anestesiol. 90:509–519. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Wang JH, Liu T, Bai Y, Chen YQ, Cui YH,
Gao XY and Guo JR: The effect of parecoxib sodium on postoperative
delirium in elderly patients with hip arthroplasty. Front
Pharmacol. 14:9479822023. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Shen L, Chen J, Yang X, Hu J, Gao W, Chai
X and Wang D: Flurbiprofen used in one-lung ventilation improves
intraoperative regional cerebral oxygen saturation and reduces the
incidence of postoperative delirium. Front Psychiatry.
13:8896372022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Huang JW, Yang YF, Gao XS and Xu ZH: A
single preoperative low-dose dexamethasone may reduce the incidence
and severity of postoperative delirium in the geriatric
intertrochanteric fracture patients with internal fixation surgery:
An exploratory analysis of a randomized, placebo-controlled trial.
J Orthop Surg Res. 18:4412023. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Xiang XB, Chen H, Wu YL, Wang K, Yue X and
Cheng XQ: The effect of preoperative methylprednisolone on
postoperative delirium in older patients undergoing
gastrointestinal surgery: A randomized, double-blind,
placebo-controlled trial. J Gerontol A Biol Sci Med Sci.
77:517–523. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Huang Q, Shi Q, Yi X, Zeng J, Dai X, Lin
L, Yang Y, Wu X and Gong G: Effect of repeated intranasal
administration of different doses of insulin on postoperative
delirium, serum and a protein in elderly patients undergoing
radical esophageal cancer surgery. Neuropsychiatr Dis Treat.
19:1017–1026. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Choi HR, Kim S, Song IA and Oh TK:
Anesthetic technique and incidence of delirium after total knee or
hip arthroplasty: A nationwide cohort study. BMC Anesthesiol.
24:4332024. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Zhao L and Qiu D: Ultrasound-guided
femoral nerve block reduced the incidence of postoperative delirium
after total knee arthroplasty: A double-blind, randomized study.
Medicine (Baltimore). 103:e405492024. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Lim EJ, Koh WU, Kim H, Kim HJ, Shon HC and
Kim JW: Regional nerve block decreases the incidence of
postoperative delirium in elderly hip fracture. J Clin Med.
10:35862021. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Radtke FM, Franck M, Lendner J, Krüger S,
Wernecke KD and Spies CD: Monitoring depth of anaesthesia in a
randomized trial decreases the rate of postoperative delirium but
not postoperative cognitive dysfunction. Br J Anaesth. 110 (Suppl
1):i98–i105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Wang J, Zhu L, Li Y, Yin C, Hou Z and Wang
Q: The potential role of lung-protective ventilation in preventing
postoperative delirium in elderly patients undergoing prone spinal
surgery: A preliminary study. Med Sci Monit.
26:e9265262020.PubMed/NCBI
|
|
203
|
Song J, Shao YM, Zhang GH, Fan BQ, Tao WH,
Liu XF, Huang XC and Hu XW: Examining the impact of permissibility
hypercapnia on postoperative delirium among elderly patients
undergoing thoracoscopic-laparoscopic esophagectomy: A
single-center investigative study. Shock. 62:319–326. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Xu X, Hu X, Wu Y, Li Y, Zhang Y, Zhang M
and Yang Q: Effects of different BP management strategies on
postoperative delirium in elderly patients undergoing hip
replacement: A single center randomized controlled trial. J Clin
Anesth. 62:1097302020. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Brown CH, Neufeld KJ, Tian J, Probert J,
LaFlam A, Max L, Hori D, Nomura Y, Mandal K, Brady K, et al: Effect
of targeting mean arterial pressure during cardiopulmonary bypass
by monitoring cerebral autoregulation on postsurgical delirium
among older patients: A nested randomized clinical trial. JAMA
Surg. 154:819–826. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Wang JY, Li M, Wang P and Fang P:
Goal-directed therapy based on rScO2 monitoring in elderly patients
with one-lung ventilation: A randomized trial on perioperative
inflammation and postoperative delirium. Trials. 23:6872022.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Wang DD, Li Y, Hu XW, Zhang MC, Xu XM and
Tang J: Comparison of restrictive fluid therapy with goal-directed
fluid therapy for postoperative delirium in patients undergoing
spine surgery: A randomized controlled trial. Perioper Med (Lond).
10:482021. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Wei W, Zheng X, Gu Y, Fu W, Tang C and Yao
Y: Effect of general anesthesia with thoracic paravertebral block
on postoperative delirium in elderly patients undergoing
thoracoscopic lobectomy: A randomized-controlled trial. BMC
Anesthesiol. 22:12022. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Mu DL, Zhang DZ, Wang DX, Wang G, Li CJ,
Meng ZT, Li YW, Liu C and Li XY: Parecoxib supplementation to
morphine analgesia decreases incidence of delirium in elderly
patients after hip or knee replacement surgery: A randomized
controlled trial. Anesth Analg. 124:1992–2000. 2017. View Article : Google Scholar : PubMed/NCBI
|