Introduction
As the first line of defense against infections and
malignancies, human natural killer (NK) cells are a critical
component of innate immune system. Unlike T cells, which require
priming, NK cells can eliminate virus-infected cells and
transformed cells through immune mechanisms (1). Various NK cell sources have been
investigated in clinical trials for cellular immunotherapy
(2). Induced pluripotent stem cel
-derived NK cells (IPSC-NK) have emerged as a research hotspot in
tumor immunotherapy due to their scalability, standardized
manufacturing potential and genetic engineering capabilities.
Compared with other immunotherapies, NK cells demonstrate improved
cytotoxic activity and are less sensitive to tumor immune escape
strategies, making them a promising approach for cancer treatment
(3).
NK cells do not require human leukocyte antigen
(HLA) compatibility, reducing the risk of complications such as
graft-versus-host disease (GVHD) and cytokine release syndrome
(CRS), even when administered as allogeneic cells (4). Their favorable safety profile,
coupled with notable anti-tumor capabilities, positions NK cells as
a compelling cellular candidate for the application of chimeric
antigen receptor (CAR) technology (5). This enables the strategic redirection
of their cytotoxic capabilities towards precise targets (6,7).
Despite these advantages compared with other
immunotherapies, NK cell-based therapies face several challenges,
including immunosuppressive tumor microenvironments, limitations in
cell manufacturing and insufficient therapeutic persistence.
IPSC-NK cells have emerged as an attractive alternative to overcome
these limitations (8). IPSC-NK
cells facilitate the mass production of homogeneous NK cells, which
can be stored for later use. Moreover, both viral and non-viral
methods can be used to effectively genetically modify these cells
to meet the needs of different cancer treatments (9). Therefore, in biomedical research and
clinical applications, iPSCs hold great promise for translational
research and clinical applications (10). As the ‘off the shelf’ product,
iPSC-NK cells possess strong drug-forming characteristics and
represent a promising strategy in cancer immunotherapy. This
present review examined the immunotherapy and therapeutic
perspective of NK cells derived from iPSCs.
Biological properties of NK cells
Unlike T and B cells, NK cells lack genetically
rearranged antigen receptors, allowing them to directly eliminate
target cells without prior sensitization (11). NK cells are positioned as promising
candidates for cancer adoptive cell therapy because of this unique
feature, as well as their ‘off-the-shelf’ availability and low risk
of GVHD (12,13). NK cells are a type of lymphocyte
that play a crucial role in the innate immune system. Their
development begins in the bone marrow, where hematopoietic stem
cells differentiate into common lymphoid progenitors. These
progenitors further differentiate into NK cell precursors by
downregulating CD34 and upregulating CD56, with IL2RB (CD122)
expression marking the entry into the NK cell lineage (14). The precursors migrate to lymph
nodes, where cytokines from stromal and dendritic cells facilitate
their maturation into functional NK cells. This process is
characterized by the differential expression of genes, including
CD34, KIT, KLRB1, CD244 and interleukin (IL)-15R (15–17).
During this process, NK cells start expressing receptors that
enable them to recognize and bind to target cells, such as infected
or cancerous cells.
NK cells are broadly categorized into
CD56bright and CD56dim NK subsets (Fig. 1). The CD56dim subset
constitutes ~90% of peripheral blood NK cells and plays a primary
role in direct target cells elimination through the release of
perforin and granzymes. These cells exhibit the strongest cytotoxic
activity and antibody-dependent cell-mediated cytotoxicity (ADCC)
capabilities (18–20).
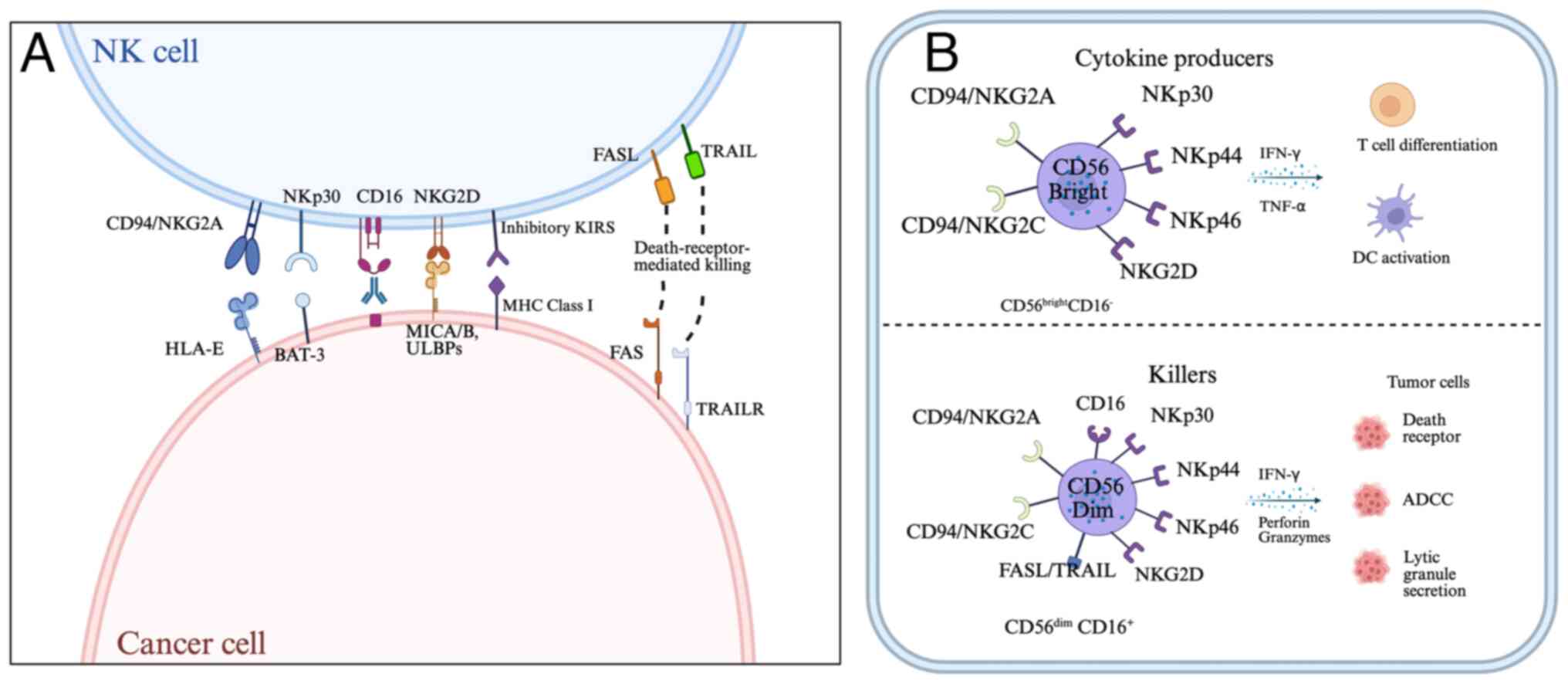 | Figure 1.The function of NK cells is governed
by a range of activating and inhibitory receptors. (A) The major NK
cell receptors and their respective ligands are depicted on the
left. (B) CD56bright cells function as cytokine
producers, while cytolytic activity was associated with the
CD56dim subset. NK, natural killer; HLA-E, major
histocompatibility complex, class I, E; FASL, fas ligand; TRAIL,
tumor necrosis factor-related apoptosis inducing ligand; TRAILR,
tumor necrosis factor related apoptosis-inducing ligand receptor;
MICA/B, MHC class I chain-related protein A/B; KIRS, killer cell
Inhibitory receptors; DC, dendritic cells; ADCC, antibody-dependent
cell-mediated cytotoxicity. |
By contrast, CD56bright cells, which
represent 2–10% of peripheral blood NK cells, have less cytotoxic
activity but play a crucial role in immunomodulation. They can
interact with dendritic cells (DCs) and T cells to maintain the
balance and effectiveness of the immune system (21). Activated NK cells secrete cytokines
such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α and
granulocyte-macrophage colony-stimulating factor to enhance
cytotoxic T cell responses and macrophage antigen presentation. NK
cells can be activated by cytokines secreted by DCs, such as IL-12
and IL-18 (22,23).
CD56bright cells also exert
anti-proliferative, anti-angiogenic and pro-apoptotic effects on
cancer cells while recruiting DCs to the tumor microenvironment via
chemokines such as C-C motif chemokine ligand 5, recombinant
chemokine C-Motif ligand (XCL) 1 and XCL2, thereby promoting
anti-tumor immunity (24).
Furthermore, CD56bright cells express chemokine
receptors such as C-C chemokine receptor type (CCR) 7, CCR5 and
CXCR4, which allow them to migrate and localize to secondary
lymphoid organs. They also express CD62L-selectin, which interacts
with high endothelial venues to facilitate homing and retention in
lymphoid tissues (17,25,26).
This characteristic underscores the crucial role of NK cells in
immune regulation and cytokine-mediated immune responses (25).
Adoptive NK cell therapy
The unique biological properties of NK cells provide
a strong rationale for their application in adoptive immunotherapy.
NK cell activation and inhibition are governed by a range of
activating and inhibitory receptors (Table I) (13). Whether NK cells are activated or
inhibited depends on how signals from these receptors interact
(27). The capacity of NK cells to
detect and eliminate cancer cells through mechanisms such as MHC-I
downregulation recognition is a defining characteristic of their
activity (28,29). This recognition is facilitated by
the presence of killer cell immunoglobulin-like receptors (KIRs) on
NK cells, which are sensitive to the absence of major
histocompatibility complex (MHC) class I molecules (30). Inhibitory KIRs contain immune
receptor tyrosine-based inhibitory motifs, which mediate inhibitory
signals, while activating KIRs associate with immunoreceptor
tyrosine-based activation motifs, transmitting activating signals
(31).
 | Table I.Human NK cell receptors and
function. |
Table I.
Human NK cell receptors and
function.
| Receptor | Function | Ligands on
tumor |
|---|
| NKG2D | Activation | MIC-A/B, Rael, H60,
ULBP |
| CD94/,NKG2C | Activation | HLA-E |
| NKP30 | Activation | HLA-EB7-H6, BAT3,
CMVPP65 |
| NKP44 | Activation | Viral HA |
| NKP46 | Activation | Viral HA |
| CD160 | Activation | HLA-C |
| KIR2DS1 | Activation | HLA-C |
| CD94/NKG2A | Inhibition | HLA-E |
| CD96 | Inhibition | CD155 |
| LAIR1 | Inhibition | Collagen |
| KLRG1 | Inhibition | E-Cad |
| PD-1 | Inhibition | PD-L1/2 |
| SIGLEC-3/7/9 | Inhibition | Sialic acid |
| NKR-PIA | Inhibition | CLEC2D |
| TIGIT | Inhibition | CD155 |
| iKIRs | Inhibition | HLA-I |
| ILT2 | Inhibition | HLA-C |
| CD96 | Inhibition | CD155 |
When KIRs on NK cells encounter and bind to MHC
class I molecules on the surface of healthy cells, inhibitory
signals are transmitted, preventing the NK cells from attacking
these healthy cells (32,33). However, in the case of aberrant
expression of MHC class I molecules, the inhibitory receptors on NK
cells are unable to recognize the altered MHC class I molecules,
resulting in an inability to transmit inhibitory signals (Fig. 2) (34). As a result, when the activating
signal outweigh inhibitory signals, NK cells receive an activating
signal and proceed to recognize and eliminate the tumor cells. For
example, when tumor cells downregulate MHC class I molecule
expression, inhibitory signals on NK cells are lifted, releasing
the ‘missing-self’ restraint and activating their cytotoxic
functions. Simultaneously, MHC-I-deficient tumor cells may
upregulate stress-induced molecules, which bind to activating
receptors on NK cells, further amplifying cytotoxic signaling
(35). This mechanism enables NK
cells to target tumors that evade T cell-mediated immune
surveillance, forming a complementary anti-tumor immune defense
(36). human leukocyte antigen
(HLA)-E-related peptides transmit inhibitory signals by interacting
with NK inhibitory receptors (37).
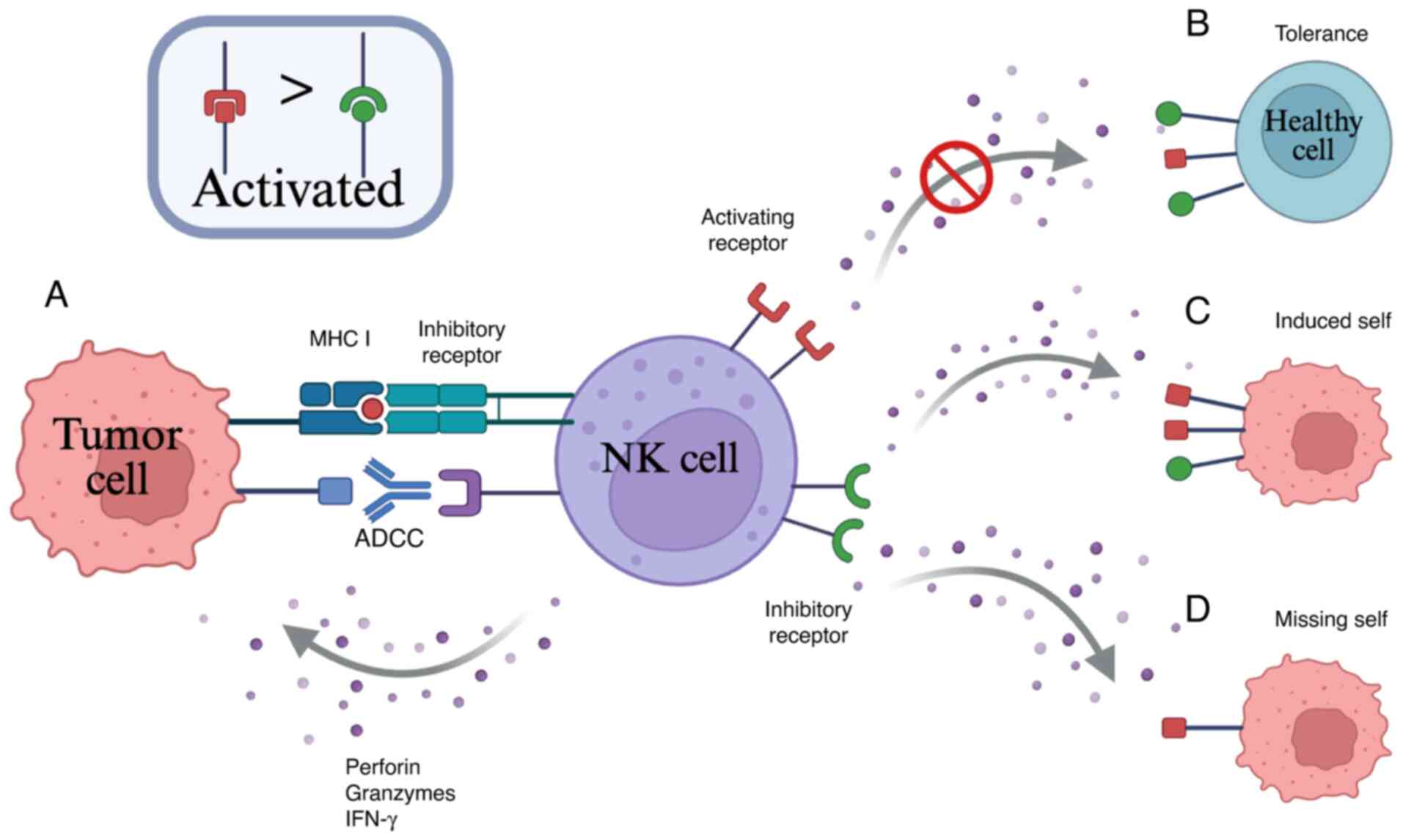 | Figure 2.Schematic of NK cell functions. (A)
Tumor cell targeting can occur in NK cells via ADCC and MHC-I. (B)
In normal cells, MHC class I molecules can engage inhibitory
receptors on NK cells, outnumbering and overriding activating
receptors, thereby preventing NK-mediated cytotoxicity. (C) In
malignant cells, NK cells recognize and respond to target cells
through their activating and inhibitory receptors, resulting in
NK-mediated cytotoxicity. (D) In tumor cells with downregulation or
lack expression of MHC class I molecules, NK cytotoxicity can still
be induced through the upregulation of stress-induced activation
ligands, which bind to activating receptors on NK cells. NK,
natural killer; ADCC, antibody-dependent cell-mediated
cytotoxicity; IFN, interferon; MHC-I, major histocompatibility
complex class I. |
For instance, NKG2A dimerizes with CD94 on the cell
surface and binds to HLA-E, which is crucial for tumors to resist
immune cell activity. In hepatocellular carcinoma (HCC) tissues,
the surface expression of NKG2A is increased in NK cells, together
with the expression of its corresponding ligand, HLA-E (38,39).
Analysis of gene expression data in tumor samples reveals a
significant correlation between HLA-E expression levels within
tumor tissues and expression levels of NKG2A and CD94 on the
surface of NK cells (40). Unlike
T cell activation, which requires dual signaling, the activation of
NK cells is determined solely by the regulation of surface
receptors and is not constrained by MHC. As the tumor progresses,
NK cells are rapidly activated, executing their functions ahead of
T cells (41,42).
Additionally, NK cells can eliminate tumor cells
through ADCC, mediated by Fc γ receptors, which engage antibodies
to induce target cell death (43–45).
The most important activation receptor in this process is CD16, an
antibody receptor that binds immunoglobulin Fc, working alongside
other key activation receptors such as NKG2D and natural
cytotoxicity receptor (NCR) (46,47).
A regulatory mechanism for NK cell cytotoxicity is also provided by
the binding of ligands on target cell surfaces to NCR family
receptors, including NKp30, NKp44, NKp46 and NKG2D (48,49).
For subsequent gene editing of iPSC-NK cells, it is
crucial to understand the characteristics and functions of
activating and inhibitory receptors on the surface of NK cells. By
exploiting gene editing technology, the expression of receptors on
the surface of NK cells can be precisely regulated, thereby
optimizing the balance between their activating and inhibitory
signals. This approach can enhance the anti-tumor and anti-viral
capabilities of NK cells while reducing the risk of potential
immune overreaction.
Sourcing NK cells
Due to the limited antitumor effect of autologous NK
cells, researchers have shifted their focus from autologous to
allogeneic NK cell therapy (50).
Over the past decade, various NK cell sources have been
investigated in clinical trials for cellular immunotherapy,
including peripheral blood, umbilical cord blood and NK-92
(Fig. 3). NK cells sourced from
peripheral blood are easy to acquire and handle. Each of these
sources has distinct advantages. Umbilical cord blood provides a
readily available source with weaker allogenic reactions and a low
risk of viral transmission (51).
Meanwhile, NK-92 cells, an infinitely proliferating cell line,
exhibit high cytotoxicity and stable immune properties (52). However, each of these sources has
drawbacks. Peripheral blood and umbilical cord blood-derived NK
cells are inconvenient to store and subject to donor variability.
NK-92 cells, while highly cytotoxic, pose potential carcinogenic
risks, lack CD16 and NKp44 and require irradiation for
inactivation, which markedly reduces their proliferative capacity
and cytotoxicity. The absence of CD16 also hampers their ability to
perform ADCC (53,54).
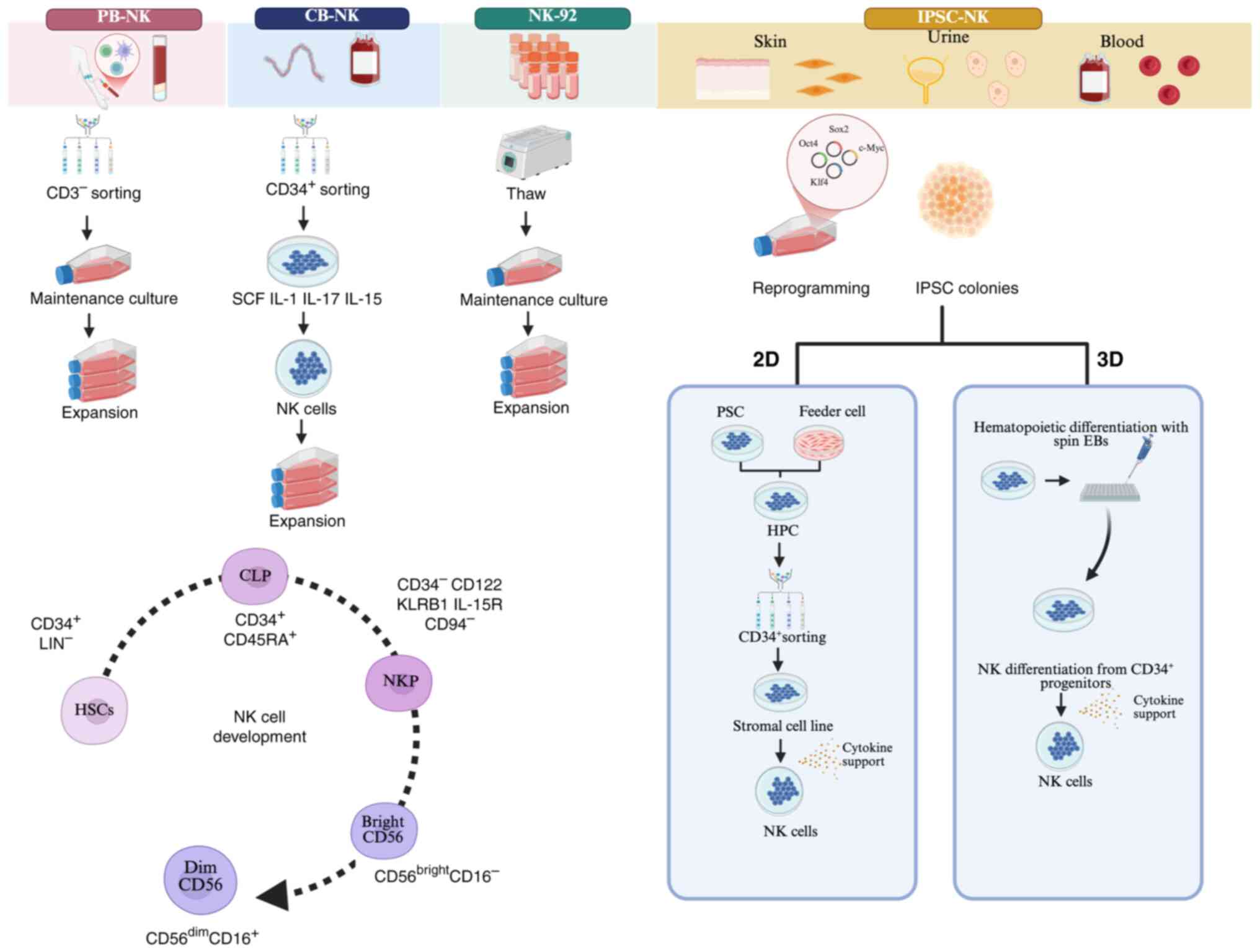 | Figure 3.Different sources of NK cells and the
development of NK cells. Various NK cell sources have been
investigated in clinical trials for cellular immunotherapy, such as
peripheral blood, umbilical cord blood and NK-92 cells. In the
schematic representation of NK cell differentiation,
CD34+ cells differentiate into NK cells through the
sequential acquisition of receptors/markers and functional
properties. Three main subsets can be identified: NK cell
precursors, CD56bright NK cells and CD56dim
NK cells. The process of iPSC-NK development begins with the
introduction of reprogramming factors into somatic cells, primarily
fibroblasts, to induce their differentiation into pluripotent stem
cells. These pluripotent stem cells are then guided to
differentiate into CD34+ HPCs using a combination of
small molecules and cytokines, or through co-culture with
irradiated stromal feeder cell lines. Next, the HPCs differentiate
into iPSC-NK cells following the addition of NK cell initiating
cytokines. In the 3D protocol, the differentiation of HPCs occurs
in a suspension culture thorough EB formation or a spin-EB
approach. The two methods utilize spin EBs to generate
CD34+ cells, which are HPCs. Over a period of 3–5 weeks,
mature and functional NK cells are developed through this process.
NK, natural killer; HPCs, hematopoietic progenitor cells; iPSC-NK,
induced pluripotent stem cell-derived NK cells; EB, spin embryoid
body. PB, peripheral blood; CB, cord blood. |
To address these limitations, IPSC-NK cells have
emerged as a promising alternative, which can be used as a
standardized, ‘off-the-shelf’ allogeneic cell therapy for treating
diverse malignancies and amenable to genome editing (55). Furthermore, modified iPSC-NK cells
have shown high target specificity, persistence and immune
activation potential in cancer treatment (8,56).
Development and differentiation of
iPSC-derived NK cells
The development of iPSC technology began in 2006.
The approach was pioneered by Shinya Yamanaka, who developed a
method to generate iPSCs by introducing four transcription factors
(OCT3/4, SOX2, KLF4 and c-Myc) into somatic cells (57–59).
IPSCs show characteristics identical to embryonic stem cells (ESCs)
in terms of morphology, proliferation, gene expression and the
ability to form teratomas.
IPSC-NK cell therapy is a novel cancer immunotherapy
approach based on iPSC technology. In 2024, Kiran et al
(60) developed a method to induce
and sustain transgene-free human iPSCs, enabling efficient and
uniform amplification using reprogramming factors such as SOX2,
OCT4, KLF4, MYC, NANOG, LIN28 and SV40 T antigen in a feeder-free
system. This method showed an effective performance against
malignant brain rhomboid tumor cells. To effectively mitigate the
risk of residual exogenous genes, technologies focus on employing
non-integrating vectors and small molecule compounds to achieve
gene silencing or post-reprogramming removal in iPSCs (61). The differentiation of iPSC into
mature NK is typically divided into three stages. Initially, to
promote the differentiation of iPSCs into hematopoietic progenitor
cells (HPCs), they are co-cultured with a combination of small
molecules and cytokines, or irradiated stromal cell lines.
Subsequently, CD34+ HPCs are isolated and enriched
before being directed towards NK cell differentiation using
specific cytokines (IL-3, IL-7, IL-15, SCF and FLT3L) or through
co-culture with a second stromal cell line. In the final stage,
iPSC-NK cells co-cultured with irradiated and engineered feeder
cells to further expand their population (62,63).
In addition, researchers formulated a technique for
spin embryoid body (EB) protocol, which has further enhanced the
efficiency of iPSC differentiation (64,65).
The differentiation of iPSCs into NK cells occurs through a
multi-stage process: First, iPSCs are co-cultured with small
molecules and induction media, which promotes their differentiation
into HPCs. This stage typically takes 12 days to produce
CD34+ HPCs. Second, lymphoid induction media and
differentiation factors are used to induce lymphoid progenitor
cells. The third stage involves guiding the differentiation of
iPSCs into NK cells using specific NK induction media. The fourth
and fifth stages involve the maturation process of NK cells
(Fig. 3).
Zhang et al (66) confirmed the successful
differentiation process from iPSC to iPSC-NK cells using EBs and
analyzed the temporal changes in the expression of key genes
through bulk RNA-seq analysis. Their findings revealed that while
iPSC-NK cells share transcriptomic similarities with PBMC-derived
NK cells, they maintained unique phenotypes characteristics.
Pluripotency genes are highly expressed at the iPSC stage but
gradually decreased with differentiation, becoming barely
detectable at the EB stage. Hematopoietic-related genes were
expressed at the EB stage and gradually increased during the
differentiation process. Gene's specific to NK cells, such as GZMB,
PRF1 and IL2RB, were gradually expressed from the early stage of
differentiation and peaked in the late stage. The expression of
some NK cell-specific markers (such as NKG2D, NKp46 and NKp30)
began at the early stage of in the process of EB differentiation
into iPSC-NK cells. During the middle stage, there was an increase
in the expression of GATA2, the HSC regulator and key regulator of
NK cell maturation. Simultaneously, the expression of mature NK
cell-specific markers (such as natural killer cell cytotoxicity
receptors and activation receptors) gradually increased. Compared
with traditional 2D culture, EB-based iPSC-NK cells improved
imitate the in vivo microenvironment. combining
Clinical-grade iPSC-NK manufacturing is made possible by the cost
reduction that results from combining EB with bioreactor technology
(66).
Utilization of iPSC-derived NK cell in
cancer treatment
In recent years, iPSC-NK cells have emerged as a
novel direction in the field of immunotherapy. These cells open new
possibilities for treating various diseases, particularly cancer,
by harnessing the advantages of iPSCs and NK cells. They have
demonstrated significant clinical benefits in cancer treatment, as
well as in autologous and allogeneic transplantation, infectious
diseases, antiviral therapy and autoimmune diseases (66,67).
Currently, antibodies are extensively utilized in
numerous cancer treatment modalities. However, treatment with
antibodies alone might not be enough to increase the immune
response because certain tumor patients (both treated and
untreated) have severe lymphopenia (68). As a result, the infusion of a
substantial quantity of NK cells is often required in the most
clinical trials involving NK cells, ranging from
5×106−1×108 cells/kg (8). One major advantage of iPSC-NK cells
is their ability to undergo genetic modification and
cryopreservation after differentiation, facilitating the production
of homogeneous functional NK cells at a clinical scale (69). A study showed that differentiated
iPSC-NK cells exhibit remarkable 3,000-fold expansion when Compared
with primary NK cells Over 200 doses, each containing
>1×109 cells, can be produced by utilizing this cell
production scale (70). Markedly,
iPSC-NK cells retain the characteristic NK cell phenotype,
including the presence of activating receptors such as NKG2D,
NKp44, NKp46 and DNAM-1 (66).
Furthermore, a study analyzing NK cell populations
from peripheral blood, umbilical cord blood and iPSC-NK cells found
that these different sources exhibit relatively similar expression
patterns of cell surface antigens, as well as activation and
inhibitory receptors. Notably, unlike CB-NK and PB-NK cells,
iPSC-NK cells show variable expression of killer
immunoglobulin-like receptors (KIRs). However, their cytotoxic
activity against tumor targets is not markedly impaired by this
mechanism. In fact, Compared with PB-NK cells, iPSC-NK cells
produced more IFN-γ and TNF-α, thereby enhancing their function
(65).
In November 2018, the FDA approved FT500, the first
clinical trial for iPSC-NK cell immunotherapy (56). Recent clinical trials have shown
that iPSC-NK cells boost anti-tumor cytotoxicity and promote T cell
activation and homing (71).
Higher cytotoxicity against lung carcinoma cells, hepatocyte
carcinoma cells, ovarian adenocarcinoma cells, melanoma cells and
myeloid leukemia cells is demonstrated by their ability to
recognize and lyse HLA-I downregulated tumor cells. This is
particularly beneficial for solid tumor patients receiving
anti-programmed death-1 (PD-1) or anti- programmed death ligand 1
(PD-L1) antibody therapy (72).
Moreover, iPSC-NK cells may be cryopreserved and retained for
subsequent repeated infusions.
A study comparing fresh, frozen and thawed PB-NK
cells showed that among patients who received cell therapy, frozen
cells did not proliferate and exhibited reduced cytotoxicity after
thawing. By contrast, iPSC-NK cells can be cryopreserved at high
densities. For example, both the FT596 and FT396 clinical trials
used a density of 1.1×108 for cryopreservation and the
thawed cells still demonstrated high recovery rates and
cytotoxicity (73). The method of
producing cGMP-grade NK cells from iPSC lines is a significant
advance. This approach not only improves NK cell production
efficiency but also preserves their functional integrity.
Additionally, iPSC-NK cells serve as a powerful tool for cancer
treatment, serving as a cell seed bank (62,74).
While NK cells exhibit advantages compared with other
immunotherapies, several limitations for solid tumors should also
be overcome. The following sections introduce the strategies to
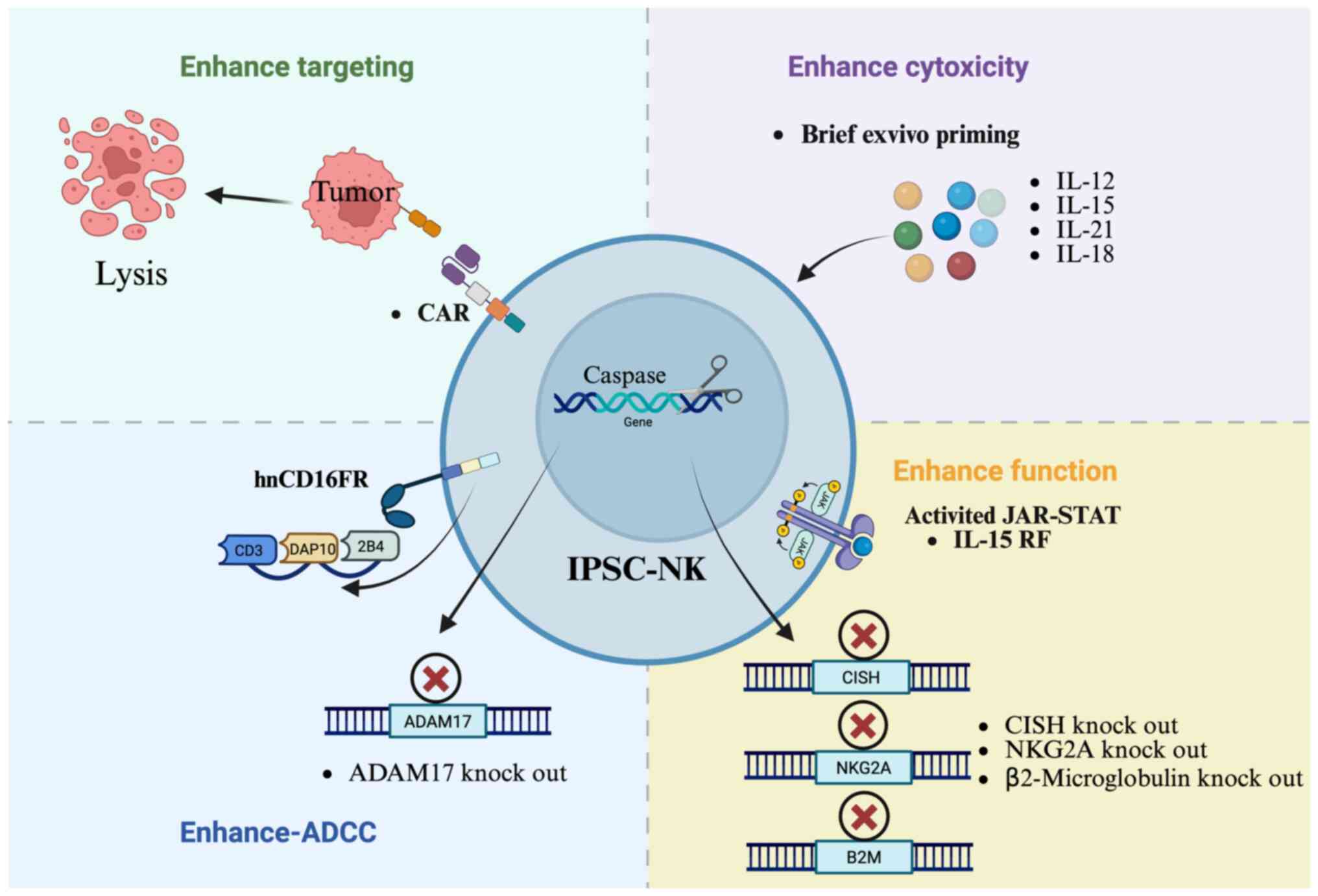
enhance the function of iPSC-NK cells (Fig. 4).
Effects of cytokines and chemokines on
iPSC-derived NK cells
IL-15 plays a pivotal role in the immune system,
particularly in the development, maintenance and function of NK
cells (75,76). IL-15 induces the proliferation of
NK cells through binding to its receptor and activation of
downstream signaling pathways, such as JAK-STAT and PI3K/AKT
(77,78). Research has shown that culturing NK
cells with a combination of IL-15, IL-18 and IL-12 enhances their
targeting and killing of tumor cells both in vivo and in
vitro (79). For iPSC-NK
cells, Chen et al (80)
developed a TALEN-based workflow to knock in IL-15, which enhanced
the cellular function and persistence of iPSC-NK cells (80). A study by Kim et al
(45) demonstrate that
NK-exosIL-15/21 (natural killer cell-derived exosomes loaded with
IL-15 and IL-21) enhances cytotoxicity and apoptotic activity in
Hep3B cells. This effect was achieved by activating specific
pro-apoptotic proteins, including Bax, cleaved caspase-3, cleaved
poly ADP-ribose polymerase, perforin and granzyme B. Additionally,
the treatment inhibited the anti-apoptotic protein Bcl-2, which
prevents apoptosis and promotes cell survival.
Furthermore, a platform has been developed using
CISH knock out in iPSC-NK cells to enhance JAK-STAT signaling
through IL-15. Through improved metabolic fitness, which is
characterized by mTOR signaling, this alteration directly
contributes to enhanced NK function (11). These mechanisms still need to be
deeply explored to optimize treatment strategies and translate
pre-clinical findings into more effective clinical therapies for
cancer patients (81).
Increased CD16 receptors on iPSC-derived NK
cells
CD16 is a receptor involved in ADCC (82). When CD16a recognizes IgG-coated
targets, NK cells release various cytotoxic molecules to mediate
the death of the target cells (83,84).
The cleavage of CD16a by ADAM17 (a disintegrin and
metalloproteinase) is one of the mechanisms underlying its
shedding. Activated NK cells exhibit a loss of CD16, which is
cleaved by the ADAM17, and the homing receptor CD62L (84,85).
Therefore, a strategy to improve cellular ADCC is to target ADAM17
to prevent CD16a shedding (85,86).
Research has found that using CRISPR/Cas9 technology
to knock out ADAM17 can lead to improved production of IFN-γ and
enhanced NK cell activity both in vitro and in vivo
(87). Clinical trials have
modified iPSC-NK cells to express a novel high-affinity 158V,
non-cleavable CD16 Fc downregulation and enhance their binding
ability with monoclonal antibodies (19). Meng et al (86) developed a novel hnCD16 fusion
receptor, which consist of the extracellular domain of hnCD16, NK
cell-specific co-stimulatory molecules (2B4 and DAP10) and the
intracellular domain of CD3ζ. In vitro, iPSC-NK cells with
hnCD16 fusion receptor demonstrate a marked increase in cytokine
secretion against tumor cells compared with the control group.
Additionally, it has been demonstrated that the anti-tumor
capability of iPSC-NK cells can be markedly enhanced by combing
high-affinity, non-cleavable CD16 fusion receptor
(hnCD16FR)-iPSC-NK cells with CD20 monoclonal antibodies (88).
Increased other receptors on iPSC-derived NK
cells to enhance functions
CD38 is a transmembrane protein primarily
responsible for catalyzing the activity of molecules such as
nicotinamide adenine dinucleotide and nicotinamide mononucleotide
(89). Of CD38, ~90% is located on
the cell membrane. To enhance the cytotoxic efficacy of NK cells, a
strategic approach involves suppressing CD38 activity through
genetic ablation (54,90).
Research has shown that knocking out CD38 and
introducing the CD16-158V receptor in NK cells results in stronger
ADCC effects and improved anti-tumor activity, particularly in
multiple myeloma (90). NKG2A is
an inhibitory receptor that binds to HLA-E on NK cells. Qin et
al (68) focus on delating the
expression of NKG2A by knocking out the NKG2A receptor gene in
iPSC-NK cells, demonstrating excellent killing ability against
tumors that highly express HLA-E.
T cell immune receptor with immunoglobulin and ITIM
domains (TIGIT) and CD73 are key molecules in immune inhibitory
pathways, often highly expressed in the tumor microenvironment,
thereby suppressing immune cell function (68). Targeting TIGIT and CD73 alleviates
immune suppression, enhancing anti-tumor immune responses. In
glioblastoma therapy, inhibiting the TIGIT and CD73 pathways helps
restore the activity of NK cells and T cells, leading to more
effective tumor cell killing (91,92).
Lupo et al (93) used the
synNotch system to program iPSC-NK cells to express molecules
capable of disrupting TIGIT and CD73 activity, showing a good
performance in disrupting the immunosuppressive network in
glioblastoma (93). Advances in
gene editing technology have enabled the reduction of
immunosuppression by inactivating HLA genes. This is achieved by
the deletion of their common component, β-2 microglobulin (B2M),
using CRISPR-Cas9 (94).
By employing gene editing techniques, it is possible
to generate cell lines with a consistent genetic background. This
approach minimizes phenotypic variations arising from genetic
disparities, thereby enhancing the precision and reliability of
disease research.
Increased targeting on iPSC-derived NK cells
to enhance functions
Currently, researchers are improving the targeting
capabilities of iPSC-NK cells by modifying them with CAR. A classic
CAR consists of an extracellular recognition domain, such as a
single-chain variable fragment (scFv) that recognizes
tumor-specific antigens, along with a transmembrane domain and an
intracellular signaling domain (10,95).
The remarkable clinical efficacy of CAR-NK cells has sparked
significant enthusiasm among researchers worldwide. As a result,
clinical trials utilizing CAR-NK cells derived from diverse sources
are currently underway at a rapid pace (Table II) (96). However, genetic modification of
primary NK cells presents several challenges. The freeze-thaw
process further complicates these challenges by compromising both
the viability and anti-tumor capabilities of these cells (78). Additionally, the expansion and
persistence of CAR-NK cells are limited by metabolic exhaustion and
insufficient cytokine support within the tumor microenvironment
(79).
 | Table II.CAR-NK cell clinical trials. |
Table II.
CAR-NK cell clinical trials.
| Number of NCT | Start year | Stage | Tumors | Target | NK source | Locations |
|---|
| NCT06307054 | 2024/2/28 | Phase 1 | Relapsed Adult AML.
Refractory AML | CLL-1 | PB-NK | Shanghai General
Hospital, Shanghai Jiao Tong University School of Medicine |
| NCT06242249 | 2024/1/27 | Phase1/Phase 2 | Multiple Myeloma,
Refractory | BCMA | PB-NK | Shahid Beheshti
University of Medical Sciences |
| NCT06201247 | 2023/12/30 | Early Phase 1 | Acute Myeloid
Leukemia, in Relapse; Acute Myeloid Leukemia Refractory | CD123 | PB-NK | Peking University
People's Hospital |
| NCT06182735 | 2023/12/6 | Phase 1 | Renal Cell
carcinoma | CD70 | PB-NK | Fudan
University |
| NCT06066424 | 2023/9/27 | Phase 1 | Solid Tumors | TROP2 | PB-NK | M.D. Anderson
Cancer Center |
| NCT06045091 | 2023/9/13 | Early Phase 1 | Multiple Myeloma;
Plasma Cell Leukemia | BCMA | PB-NK | Shanghai Changzheng
Hospital |
| NCT06027853 | 2023/8/30 | Phase 1 | Acute
Non-Lymphoblastic Leukemia; Myeloid Leukemia; Acute Myeloid
Leukemia; Acute Graft Versus Host Disease | CLL1 | iPSC-NK | Zhejiang
University |
| NCT06006403 | 2023/8/17 | Phase 1/Phase
2 | AML; Blastic
Plasmacytoid Dendritic Cell Neoplasm; relapse Leukemia; Refractory
Leukemia | CD123 | PB-NK | Chongqing Precision
Biotech Co., Ltd |
| NCT05987696 | 2023/7/24 | Phase 1 | AML, Adult; Minimal
Residual Disease | CLL1/CD33 | iPSC-NK | Institute of
Hematology & Blood Diseases Hospital, China |
| NCT05922930 | 2023/6/20 | Phase 1/Phase
2 | Pancreatic Cancer;
Ovarian cancer; Adenocarcinoma | TROP2 | CB-NK | M.D. Anderson
Cancer Center |
| NCT05856643 | 2023/5/3 | Early Phase 1 | Ovarian Epithelial
Carcinoma | SZ-011 | PB-NK | Shantou University
Medical College |
| NCT05845502 | 2023/4/25 | Not Applicable | Advanced
Hepatocellular Carcinoma | SZ003 |
| Shantou University
Medical College |
| NCT05842707 | 2023/3/25 | Phase 1/Phase
2 | Refractory or
Relapsed B-cell Non-Hodgkin lymphoma | CD19/CD70 | CB-NK | Shanghai Tongji
Hospital, Tongji University School of Medicine |
| NCT05776355 | 2023/3/8 | Not Applicable | Ovarian Cancer | NKG2D | PB-NK | Hangzhou Cheetah
Cell Therapeutics Co., Ltd |
| NCT05739227 | 2023/2/11 | Early Phase 1 | Acute lymphoblastic
Leukemia; B-cell Lymphoma; Chronic Lymphocytic Leukemia | CD19 | PB-NK | Xuzhou Medical
University |
| NCT05747586 | 2023/1/30 | Not Applicable | Multiple Myeloma in
Relapse; Multiple Myeloma, Refractory | BCMA | PB-NK | Zhejiang
University |
| NCT05734898 | 2023/1/30 | Not Applicable | Acute
Non-Lymphoblastic leukemia; Acute Myeloid Leukemia; | NKG2D | PB-NK | Zhejiang
University |
| ChiCTR23000766 | 2023/9/21 | Early Phase 1 | AML | NKG2D | PB-NK | Tongji Hospital,
Tongji Medical College, Huazhong University of Science and
Technology |
By contrast, iPSC-NK cells offer several advantages,
including a low requirement for seed cells, scalability,
cost-effectiveness and the potential for autologous supply with
minimal immunogenicity. Furthermore, these cells can be
cryopreserved long-term, ensuring immediate availability for
critically patients (9,76,77).
Consequently, the development of CAR-iPSC-NK cells has emerged as a
promising strategy for generating readily available allogeneic
lymphocytes that can specifically target and combat malignant
tumors. At present, CAR-iPSC-NK cells have evolved into a promising
strategy for producing allogeneic lymphocytes to specifically
target and combat certain malignant tumors (97,98).
The potential of CAR-iPSC-NK cells was first reported in June 2018,
when researchers introduced a CAR construct (scFv-NKG2D-2B4-CD3ζ)
into iPSC-NK cells. The results demonstrated that CAR-iPSC-NK cells
exhibited antigen-specific cell killing and increased expression of
the granule marker CD107a (9).
In vivo experiments further show that mice
treated with CAR-iPSC-NK cells experienced fewer side effects and
much higher survival rates than treated with CAR-T cells. This
study provided the first evidence that CAR-targeted NK cell therapy
a viable option for treating refractory malignancies when chimeric
antigen receptors are combined with the clinical-scale NK cells
from iPSCs (9). Karvouni et
al (99) developed a specific
CAR targeting GPRC5D, which is highly expressed in Multiple myeloma
(MM). They conducted a series of gene edits on iPSC-NK cells,
including the incorporation of IL15/IL15RF fusion protein, hnCD16
and the knockout of CD38. These findings indicated that CAR-iPSC-NK
cells a demonstrated strong cytotoxicity and tumor-clearing
capabilities in allogeneic MM transplants (99). In vivo results indicated
that two out of five mice achieved complete tumor clearance by day
80, highlighting the synergistic effect of CAR and hnCD16 (100–102).
Wang et al (103) developed 70-CAR-iPSC-NK cells,
incorporating four gene edits which exhibited robust cytotoxicity
against a wide range of tumors. The efficacy in precisely targeting
lymphoma and renal cancer was further substantiated through
xenograft models. The study also found that allogeneic reactive T
cells expressed high levels of CD70, which 70-CAR-iPSC-NK cells
effectively targeted and cleared, enhancing their survival and
persistence (103). This finding
underscores the importance of selecting an appropriate CAR
construct to maximize the anti-tumor efficacy of CAR-iPSC-NK cells.
The importance of CAR selection is further emphasized in ovarian
cancer xenograft model. Compared with PB-NK cells, iPSC -derived NK
cells and T-CAR-expressing iPSC-NK cells
(scFv-CD28-CD28-CD137-CD3ζ,), NK-CAR expressing-iPSC-NK
(scFv-NKG2D-2B4-CD3ζ) cells demonstrated markedly improved tumor
suppression and prolonged survival (9).
Additionally, studies have reported remarkable
anti-tumor performance of iPSC-derived NK cells expressing various
CAR constructs, such as EGFR-CAR, CD19-CAR and CD33-CAR (104–106). By carefully selecting the CAR
construct and employing metabolic engineering and gene-editing
strategies, the functionality and targeting capabilities of
CAR-iPSC-NK cells can be optimized.
Advances in clinical studies of iPSC-derived
NK cells
IPSC-NK cells are currently undergoing clinical
trials to evaluate their potential in treating various diseases. In
order to provide data support for further clinical applications,
these trials aim to determine the safety, efficacy and feasibility
of iPSC-NK cells (Table III).
The fastest-progressing clinical projects for iPSC-NK thus far
primarily include those associated with Fate Therapeutics (FT596,
FT576) and Century Therapeutics (CNTY-101).
 | Table III.Ongoing clinical trials with iPSC-NK
cells. |
Table III.
Ongoing clinical trials with iPSC-NK
cells.
| Company | iPSC platform | Product | Indications | Phase |
|---|
| Fate
Therapeutics | iNK | FT576 | Multiple
Myeloma | Phase I |
|
| iNK | FT522 | B-Cell
Lymphoma | Phase I |
| Century | iNK | CYTN-101 | B-Cell
Lymphoma | Phase I |
|
| iNK | CYTN-104 | Acute Myeloid
Leukemia | Preclinical |
|
| iNK | CYTN-106 | Multiple
Myeloma | Preclinical |
| Nuwacell | iNK | NCR300 | Myelodysplastic
syndromes | Phase I |
|
| iNK | NCR301 | Myelodysplastic
syndrome | Preclinical |
|
| iNK | NCR305 | Solid tumor | Preclinical |
| Cytovia
Therapeutics | iNK | CYT-103 | Hepatocellular
carcinoma | Preclinical |
|
| iNK | CYT-303 | Hepatocellular
Carcinoma | Preclinical |
|
|
| CYT-150 |
|
|
|
| CAR-iNK | CYT-503 | Hepatocellular
Carcinoma | Preclinical |
|
| CAR-iNK | CYT-538 | Multiple
Myeloma | Preclinical |
|
| CAR-iNK | CYT-501 | Glioblastoma
Multiforme | Preclinical |
| Hebecell | iNK | HC101 | Acute Myeloid
Leukemia | Preclinical |
In February 2025, the first clinical trial of an
iPSC-derived CAR-NK cells product was completed. FT596 (trial no.
NCT04245722) received Investigational New Drug approval from the US
FDA (107). This product includes
a CD19 CAR, a high-affinity, non-cleavable CD16 Fc receptor and an
interleukin-15-interleukin-15 receptor fusion (58,108,109). In the Phase I trial, FT596
demonstrated a complete Remission (CR) rate of 85% (17/20 patients)
in relapsed follicular lymphoma, with a median duration of response
of ~16.9 months. Among patients previously treated with CD19 CAR-T
therapy, the CR rate reached 30%. Furthermore, the study found that
the proportion of PD1+CD8+ T cells in the
tumor was positively associated with efficacy. This suggests that
FT596 may exert an auxiliary anti-tumor effect by activating
endogenous T cells, achieving a dual combined action of NK cells
and T cells (107).
FT522 (NCT05950334) is a further optimized version
of FT596. In order to expand the range of indications and reduce
dependence on preconditioning chemotherapy, it incorporates an
allo-defense receptor targeting 4-1BB function. Peripheral B
lymphocytes were rapidly and profoundly reduced in patients
receiving FT522 therapy in Phase 1 clinical trials. FT522
demonstrated enhanced persistence in comparison to the CAR-iPSC-NK
cell product of the preceding generation, FT596 (110).
FT576 (NCT05182073) is an ‘off-the-shelf’ NK cell
therapy derived from iPSC lines. It has been engineered with a CAR
targeting B-cell maturation antigen (BCMA) and an IL-15 receptor
fusion protein. In an evaluation involving nine patients, the
following observations were made: No dose-limiting toxicities, no
grade CRS, no immune-related neurotoxicity, no cases of GvHD.
Notably, one patient who had previously undergone five lines of
treatment achieved a very good partial response after the second
administration of FT576 monotherapy, accompanied by a significant
decrease in soluble BCMA (102).
These findings indicated iPSC-NK cells have promising safety and
efficacy for treating solid tumor, offering therapeutic benefits
even in patients with extensive prior treatments (100–102).
CNTY-101 represents a pioneering cell therapy
product candidate with six precise gene edits (111). These edits include: The
incorporation of a CD19-CAR for targeted cell recognition,
insertion of transgenes encoding HLA-E protein to disrupt B2M,
insertion of transgenes encoding the extracellular and
transmembrane domains of EGFR, implementation of Allo-Evasion™
technology (Century Therapeutics) to enhance compatibility across
diverse patient populations, abnormal cells can be rapidly
eliminated through the integration of suicide genes or
drug-inducible switches to rapidly eliminate abnormal cells when
toxicity induced by cellular therapies (94). These modifications enhance the
cytotoxic effects of CNTY-101, even after over 15 successive rounds
of in vitro killing. In vivo studies have shown
significant effects, with fresh cells demonstrated a notable 91%
reduction in tumor growth and cryopreserved cells showing a
substantial 76% reduction. These results underscore the exceptional
potential of CNTY-101, which is attributed to its enhanced
properties and efficacy in precisely targeting and suppressing
tumor growth in preclinical settings (112–114).
Therefore, CAR-iPSC-NK cells can directly enhance
anti-tumor activity by improving ADCC and cytokine secretion. The
clinical trials of iPSC-NK cells aim to validate their potential in
treating cancer and autoimmune diseases, providing scientific
evidence for their widespread application as an innovative cell
therapy (3,115).
Challenges of iPSC-derived NK cells for
clinical application
The performance of iPSC-NK cells in treating
malignancies has generated great interest in their application.
However, several obstacles must be tackled before their successful
use. First, quality challenges cannot be overlooked. The
preparation and differentiation of iPSCs require strict control of
various conditions to ensure the final cell products are consistent
and of high quality (71).
Heterogeneity in differentiation protocols may yield
iPSC-NK cells with divergent phenotypes and functional profiles
(116). For example, compared
with feeder-free differentiation strategies, a lymphoid-based
differentiation strategy using OP9 cells may produce more mature
and potent iPSC-NK cells (117).
However, due to technical limitations and the complexity of cell
biological characteristics, it is difficult to completely avoid
issues such as cell variation and contamination in practical
operation. Researchers should evaluate the long-term safety of cell
therapy and closely monitor patients for adverse reactions and side
effects when applying these cells to cancer patients (118). This will include monitoring
immune responses, tumor formation and other potential risks
associated with cell therapy. To enhance the efficacy and safety of
iPSC-NK cells in the tumor microenvironment, future research needs
to further explore how to optimize their preparation and
modification methods.
Second, the tumor microenvironment plays a crucial
role in enabling tumor cells to evade NK cell immune surveillance
(10,119). The migration and infiltration
abilities of iPSC-NK cells are crucial for targeting tumors.
However, the dense stroma, high interstitial fluid pressure and
abnormal vascular structures in the tumor microenvironment can
impede these processes (116,120). Lactic acid in the tumor
microenvironment suppresses NK cell cytotoxicity by inhibiting mTOR
signaling (121–123).
To counteract this effect, strategies such as
engineering iPSC-NK cells with lactate dehydrogenase overexpression
have been employed (124,125). Enhancing the migration and
infiltration abilities of iPSC-NK cells to enable them to reach the
tumor site more effectively is an important research direction.
Moreover, immunosuppressive factors in the tumor microenvironment
also negatively impact the activity of iPSC-NK cells (126,127). The proliferation, activation and
effector functions of iPSC-NK cells can be inhibited by these
factors, which include regulatory T cells, myeloid-derived
suppressor cells and immunosuppressive molecules (127,128). Thus, it is imperative to overcome
these immunosuppressive factors to improve the survival rate and
antitumor activity of iPSC-NK cells in the tumor
microenvironment.
Tumor heterogeneity poses a significant challenge
for iPSC-NK cells cell therapy. Genetic and epigenetic
heterogeneity among tumor cells drive the emergence of diverse
cellular subpopulations within tumors, resulting in varied
responses to treatment and uncertainty in therapeutic efficacy.
Studies have indicated that NK cells do not persist well in
vivo, which limits the durability of their response against
tumors (129–131). Therefore, enhancing the
persistence of iPSC-NK cells is crucial for their therapeutic
efficacy in vivo.
In summary, although iPSC-NK cells have the
potential to recognize and kill tumor cells, several challenges
must be addressed for their clinical application: Enhancing in
vivo persistence, improving tumor targeting, overcoming the
effects of the tumor microenvironment, optimizing differentiation
strategies, achieving scalable production and quality control and
managing immunogenicity and toxicity issues. Improving the
specificity and targeting efficiency in the complex tumor
microenvironment will aid in advancing the development and
application of iPSC-NK cells therapies.
Discussion and future perspectives
To date, only Fate Therapeutics has completed a
phase I clinical trial using iPSC-NK cells to treat cancer
(107). Partial clinical trial
data indicate that both genetically modified and unmodified iPSC-NK
cells have demonstrated good safety and tolerability, with no
severe adverse events related to NK cells (58,73,109). It has been reported that
unmodified iPSC-NK cells show therapeutic effects against various
types of tumors, especially in solid tumors (62). Meanwhile, genetically modified
iPSC-NK cells have displayed favorable overall response rates and
complete response rates in treating relapsed or refractory
lymphomas (110). The development
of CAR-iPSC-NK cells has brought new hope to the field of cancer
therapy (6,88,132).
Despite these promising results, potential risks
associated with iPSC-NK cells remain. Hypoxia and the accumulation
of metabolites are two factors that may affect the metabolic
adaptability and function of CAR-NK cells. For example, the
activation receptors of CAR-NK cells may be inhibited in hypoxic
environments, leading to a reduction in their cytotoxicity.
Additionally, NK cells can enhance their utilization of glucose to
promote glycolysis and mitochondrial oxidative phosphorylation.
This metabolic adaptation provides sufficient energy and
biosynthetic precursors, thereby supporting their proliferation and
cytotoxic functions. (133,134).
The rapid utilization of glucose by tumor cells may
also induce metabolic reprogramming in NK cells, thereby affecting
their effector functions (123).
The specificity and durability of CAR-iPSC derived NK cells have
been further enhanced by recent advances in CRISPR-Cas9-based gene
editing technology. For example, Shankar et al (135) used non-viral CRISPR gene editing
technology on iPSC-NK cells to successfully insert CAR to GD2 while
knocking out the KLRC1 gene. This modification markedly enhanced
the ability to kill solid tumors. The improvements enhance the NK
cells to proliferate by prolonging their survival in vivo
and alleviating inhibitory signals. This improvement may be partly
attributed to modifications in CAR that influence cellular
metabolism. Therefore, further in-depth research is needed to
explore strategies for overcoming the immunosuppressive effects of
metabolites through CAR-iPSC-NK cells.
In the context of combination therapies,
integrating iPSC-NK cells with monoclonal antibodies targeting
tumor antigens may further enhance tumor recognition and
eradication. Existing studies show that iPSC-NK cells and
PD-1/PD-L1 inhibitors can synergistically enhance anti-tumor
efficacy by overcoming immunosuppression in the tumor
microenvironment, ultimately improving anti-tumor immune responses
(136,137). Currently, clinical trials
employing iPSC-NK cells in combination with monoclonal antibodies
mainly include FT596 and CD19t-haNK (NCT06334991). CD19t-haNK is a
clinical trial evaluating CAR-NK as a monotherapy or in combination
with rituximab for treating relapsed/refractory CD19 and CD20
B-cell non-Hodgkin lymphoma (138). The first patients were
successfully dosed in October 2024, with additional data expected
in subsequent phases.
As reprogramming factors such as Oct3/4, Sox2, Klf4
and c-Myc are closely associated with tumorigenesis, their use in
iPSC generation raises concerns. Notably, c-Myc mutations are
frequent in human cancers, further highlighting the need for
caution in iPSC-based therapies. To fully unlock the potential of
iPSC-NK cells therapies in oncology, rigorous safety measures and
technological advancements are critical for addressing these risks
(139). Therefore, meticulous
control of the iPSC cell amplification process is necessary. This
includes selecting iPSC lines that have already demonstrated
stability and safety in preclinical studies and employing
gene-editing techniques to repair or correct potential
mutation-causing sites to enhance iPSC stability. For example, to
replace oncogenic factors, Ding et al (140) employed transient mRNA delivery or
small molecules.
Meanwhile, single-cell RNA sequencing technology
can markedly contribute to resolving the heterogeneity during the
differentiation process of iPSCs. By identifying functional subsets
of NK cells, it can optimize the differentiation strategy of
iPSC-NK cells (42,117). This technology has been used to
analyze gene expression differences in iPSC-NK cells within the
tumor microenvironment, shedding light on their interaction
mechanisms with tumor cells, immunosuppressive cells and stromal
cells. Additionally, single-cell sequencing has the potential to
identify new therapeutic targets, thereby advancing iPSC-NK cells
therapy in cancer treatment and immunotherapy (141). Using single-cell sequencing
technology help detect abnormal genomic mutations, thereby reducing
potential iPSC-related risks.
IPSC-NK cells are emerging as a novel and promising
cell therapy tool in multiple ongoing clinical studies for solid
tumor. They have demonstrated safety and potential efficacy in
several reported clinical studies. However, some challenges remain
in the clinical application of iPSC-NK cells, such as tumor
microenvironment, differentiation strategies and quality control.
Thus, more preclinical and clinical studies are required to push
the iPSC-NK therapy into clinical applications. The establishment
of allogeneic iPSC-NK cells as a next-generation immunotherapy,
particularly in oncology, will be strongly supported by overcoming
these hurdles.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
XW, CS, YL, NW, KX, QQ and ZX wrote and revised the
manuscript. ZX and CS designed and supervised the study. YL and NW
reviewed the references. QQ and KX provided supervision, reviewing
and editing of the final manuscript. Data authentication is not
applicable. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Not applicable.
Glossary
Abbreviations
Abbreviations:
|
NK
|
natural killer
|
|
IPSC
|
induced pluripotent stem cell
|
|
CAR
|
chimeric antigen receptor
|
|
CR
|
complete response
|
|
CRS
|
cytokine release syndrome
|
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
DCs
|
dendritic cells
|
|
IFN
|
interferon
|
|
TNF
|
tumor necrosis factor
|
|
XCL
|
recombinant chemokine C-motif
ligand
|
|
CCR
|
C-C chemokine receptor
|
|
MHC
|
major histocompatibility complex
|
|
HLA
|
human leukocyte antigen
|
|
NCR
|
natural cytotoxicity receptor
|
|
KIR
|
killer cell immunoglobulin-like
receptor
|
|
EB
|
spin embryoid body
|
|
HPCs
|
hematopoietic progenitor cells
|
|
KIRs
|
killer immunoglobulin-like
receptors
|
|
PD-1
|
anti- programmed death-1
|
|
PD-L1
|
Programmed cell death ligand 1
|
|
ADAM17
|
a disintegrin and
metalloproteinase
|
|
CD16 non-cleavable
|
hnCD16
|
|
TIGIT
|
T cell immune receptor with
immunoglobulin and ITIM domains
|
|
B2M
|
β-2 macroglobulin
|
|
scFv
|
single-chain variable fragment
|
|
MM
|
Multiple myeloma
|
References
|
1
|
Bald T, Krummel MF, Smyth MJ and Barry KC:
The NK cell-cancer cycle: Advances and new challenges in NK
cell-based immunotherapies. Nat Immunol. 21:835–847. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang D, Sun Z, Zhu X, Zheng X, Zhou Y, Lu
Y, Yan P, Wang H, Liu H, Jin J, et al: GARP-mediated active
TGF-beta1 induces bone marrow NK cell dysfunction in AML patients
with early relapse post-allo-HSCT. Blood. 140:2788–2804. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Terren I, Orrantia A, Vitalle J,
Astarloa-Pando G, Zenarruzabeitia O and Borrego F: Modulating NK
cell metabolism for cancer immunotherapy. Semin Hematol.
57:213–224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hernani R, Benzaquen A and Solano C:
Toxicities following CAR-T therapy for hematological malignancies.
Cancer Treat Rev. 111:1024792022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mansour AG, Teng KY, Li Z, Zhu Z, Chen H,
Tian L, Ali A, Zhang J, Lu T, Ma S, et al: Off-the-shelf
CAR-engineered natural killer cells targeting FLT3 enhance killing
of acute myeloid leukemia. Blood Adv. 7:6225–6239. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu X and Matosevic S: Gene-edited and
CAR-NK cells: Opportunities and challenges with engineering of NK
cells for immunotherapy. Mol Ther Oncolytics. 27:224–238. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raneros AB, Lopez-Larrea C and
Suarez-Alvarez B: Acute myeloid leukemia and NK cells: Two warriors
confront each other. Oncoimmunology. 8:e15396172019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woan KV, Kim H, Bjordahl R, Davis ZB,
Gaidarova S, Goulding J, Hancock B, Mahmood S, Abujarour R, Wang H,
et al: Harnessing features of adaptive NK cells to generate
iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell.
28:2062–2075.e2065. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Hermanson DL, Moriarity BS and
Kaufman DS: Human iPSC-Derived natural killer cells engineered with
chimeric antigen receptors enhance anti-tumor activity. Cell Stem
Cell. 23:181–192.e185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin X, Sun Y, Dong X, Liu Z, Sugimura R
and Xie G: IPSC-derived CAR-NK cells for cancer immunotherapy.
Biomed Pharmacother. 165:1151232023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu H, Blum RH, Bernareggi D, Ask EH, Wu
Z, Hoel HJ, Meng Z, Wu C, Guan KL, Malmberg KJ and Kaufman DS:
Metabolic reprograming via deletion of CISH in human iPSC-derived
NK cells promotes in vivo persistence and enhances anti-tumor
activity. Cell Stem Cell. 27:224–237.e226. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farahzadi R, Valipour B, Anakok OF, Fathi
E and Montazersaheb S: The effects of encapsulation on NK cell
differentiation potency of C-kit+ hematopoietic stem cells via
identifying cytokine profiles. Transpl Immunol. 77:1017972023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mace EM: Human natural killer cells: Form,
function, and development. J Allergy Clin Immunol. 151:371–385.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galat Y, Du Y, Perepitchka M, Li XN,
Balyasnikova IV, Tse WT, Dambaeva S, Schneiderman S, Iannaccone PM,
Becher O, et al: In vitro vascular differentiation system
efficiently produces natural killer cells for cancer
immunotherapies. Oncoimmunology. 12:22406702023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vacca P, Vitale C, Montaldo E, Conte R,
Cantoni C, Fulcheri E, Darretta V, Moretta L and Mingari MC: CD34+
hematopoietic precursors are present in human decidua and
differentiate into natural killer cells upon interaction with
stromal cells. Proc Natl Acad Sci USA. 108:2402–2407. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
López-Botet M, De Maria A, Muntasell A,
Della Chiesa M and Vilches C: Adaptive NK cell response to human
cytomegalovirus: Facts and open issues. Semin Immunol.
65:1017062023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Panjwani MK, Grassmann S, Sottile R, Le
Luduec JB, Kontopoulos T, van der Ploeg K, Sun JC and Hsu KC:
Single-cell profiling aligns CD56bright and cytomegalovirus-induced
adaptive natural killer cells to a naïve-memory relationship. Front
Immunol. 15:14994922024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu M, Liu J, Zhang X, Xiao Y, Jiang G and
Huang X: Activation status of CD56 dim natural killer cells is
associated with disease activity of patients with systemic lupus
erythematosus. Clin Rheumatol. 40:1103–1112. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oboshi W, Watanabe T, Matsuyama Y, Kobara
A, Yukimasa N, Ueno I, Aki K, Tada T and Hosoi E: The influence of
NK cell-mediated ADCC: Structure and expression of the CD16
molecule differ among FcγRIIIa-V158F genotypes in healthy Japanese
subjects. Hum Immunol. 77:165–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Federicis D, Capuano C, Ciuti D,
Molfetta R, Galandrini R and Palmieri G: Nutrient transporter
pattern in CD56dim NK cells: CD16 (FcγRIIIA)-dependent modulation
and association with memory NK cell functional profile. Front
Immunol. 15:14777762024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wagner JA, Rosario M, Romee R,
Berrien-Elliott MM, Schneider SE, Leong JW, Sullivan RP, Jewell BA,
Becker-Hapak M, Schappe T, et al: CD56 bright NK cells exhibit
potent antitumor responses following IL-15 priming. J Clin Invest.
127:4042–4058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Enomoto Y, Li P, Jenkins LM, Anastasakis
D, Lyons GC, Hafner M and Leonard WJ: Cytokine-enhanced cytolytic
activity of exosomes from NK Cells. Cancer Gene Ther. 29:734–749.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lachota M, Zielniok K, Palacios D, Kanaya
M, Penna L, Hoel HJ, Wiiger MT, Kveberg L, Hautz W, Zagożdżon R and
Malmberg KJ: Mapping the chemotactic landscape in NK cells reveals
subset-specific synergistic migratory responses to dual chemokine
receptor ligation. EBioMedicine. 96:1048112023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toffoli E, van Vliet A, Forbes C, Arns AJ,
Verheul HWM, Tuynman J, van der Vliet HJ, Spanholtz J and de Gruijl
TD: Allogeneic NK cells induce the in vitro activation of
monocyte-derived and conventional type-2 dendritic cells and
trigger an inflammatory response under cancer-associated
conditions. Clin Exp Immunol. 216:159–171. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodriguez-Mogeda C, van Ansenwoude CM, van
der Molen L, Strijbis EM, Mebius RE and de Vries HE: The role of
CD56bright NK cells in neurodegenerative disorders. J
Neuroinflammation. 21:482024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van de Donk N, Richardson PG and Malavasi
F: CD38 antibodies in multiple myeloma: Back to the future. Blood.
131:13–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wagner JA and Fehniger TA: Human adaptive
natural killer cells: Beyond NKG2C. Trends Immunol. 37:351–353.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zappa E, Vitali A, Anders K, Molenaar JJ,
Wienke J and Künkele A: Adoptive cell therapy in pediatric
extracranial solid tumors: Current approaches and future
challenges. Eur J Cancer. 194:1133472023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taylor BC and Balko JM: Mechanisms of
MHC-I downregulation and role in immunotherapy response. Front
Immunol. 13:8448662022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shreeve N, Depierreux D, Hawkes D,
Traherne JA, Sovio U, Huhn O, Jayaraman J, Horowitz A, Ghadially H,
Perry JRB, et al: The CD94/NKG2A inhibitory receptor educates
uterine NK cells to optimize pregnancy outcomes in humans and mice.
Immunity. 54:1231–1244.e1234. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pollock NR, Harrison GF and Norman PJ:
Immunogenomics of killer cell immunoglobulin-like receptor (KIR)
and HLA class I: Coevolution and consequences for human health. J
Allergy Clin Immunol Pract. 10:1763–1775. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rascle P, Woolley G, Jost S, Manickam C
and Reeves RK: NK cell education: Physiological and pathological
influences. Front Immunol. 14:10871552023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neo SY, Jing X, Tong L, Tong D, Gao J,
Chen Z, De Los Santos MC, Burduli N, De Souza Ferreira S, Wagner
AK, et al: Tumor MHC class I expression alters cancer-associated
myelopoiesis driven by host NK cells. J Immunother Cancer.
10:e0053082022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen X, Lu Q, Zhou H, Liu J, Nadorp B,
Lasry A, Sun Z, Lai B, Rona G, Zhang J, et al: A
membrane-associated MHC-I inhibitory axis for cancer immune
evasion. Cell. 186:3903–3920.e3921. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toyoda H, Kuramasu A, Hosonuma M, Murayama
M, Narikawa Y, Isobe J, Baba Y, Tajima K, Funayama E, Shida M, et
al: MHC class I polypeptide-related sequence B shedding modulates
pancreatic tumor immunity via the activation of NKG2DLow
T cells. Sci Rep. 14:234012024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harkus U, Wankell M, Palamuthusingam P,
McFarlane C and Hebbard L: Immune checkpoint inhibitors in HCC:
Cellular, molecular and systemic data. Semin Cancer Biol.
86:799–815. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eugene J, Jouand N, Ducoin K, Dansette D,
Oger R, Deleine C, Leveque E, Meurette G, Podevin J, Matysiak T, et
al: The inhibitory receptor CD94/NKG2A on CD8(+) tumor-infiltrating
lymphocytes in colorectal cancer: A promising new druggable immune
checkpoint in the context of HLAE/β2m overexpression. Mod Pathol.
33:468–482. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun C, Xu J, Huang Q, Huang M, Wen H,
Zhang C, Wang J, Song J, Zheng M, Sun H, et al: High NKG2A
expression contributes to NK cell exhaustion and predicts a poor
prognosis of patients with liver cancer. Oncoimmunology.
6:e12645622017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mahgoub S, Abosalem H, Emara M, Kotb N,
Maged A and Soror S: Restoring NK cells functionality via cytokine
activation enhances cetuximab-mediated NK-cell ADCC: A promising
therapeutic tool for HCC patients. Mol Immunol. 137:221–227. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hò GGT, Celik AA, Huyton T, Hiemisch W,
Blasczyk R, Simper GS and Bade-Doeding C: NKG2A/CD94 is a new
immune receptor for HLA-G and distinguishes amino acid differences
in the HLA-G heavy chain. Int J Mol Sci. 21:43622020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laskowski TJ, Biederstadt A and Rezvani K:
Natural killer cells in antitumour adoptive cell immunotherapy. Nat
Rev Cancer. 22:557–575. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bibby JA, Agarwal D, Freiwald T, Kunz N,
Merle NS, West EE, Singh P, Larochelle A, Chinian F, Mukherjee S,
et al: Systematic single-cell pathway analysis to characterize
early T cell activation. Cell Rep. 41:1116972022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wong JKM, Dolcetti R, Rhee H, Simpson F
and Souza-Fonseca-Guimaraes F: Weaponizing natural killer cells for
solid cancer immunotherapy. Trends Cancer. 9:111–121. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dixon KJ, Wu J and Walcheck B: Engineering
anti-tumor monoclonal antibodies and Fc receptors to enhance ADCC
by human NK cells. Cancers (Basel). 13:3122021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim IY, Kim HY, Song HW, Park JO, Choi YH
and Choi E: Functional enhancement of exosomes derived from NK
cells by IL-15 and IL-21 synergy against hepatocellular carcinoma
cells: The cytotoxicity and apoptosis in vitro study. Heliyon.
9:e169622023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bottino C, Castriconi R, Moretta L and
Moretta A: Cellular ligands of activating NK receptors. Trends
Immunol. 26:221–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang R, Liu Q, Zhou S, He H, Zhao M and
Ma W: Engineering CAR-NK cells targeting CD33 with concomitant
extracellular secretion of anti-CD16 antibody revealed superior
antitumor effects toward myeloid leukemia. Cancer Lett.
558:2161032023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Raulet DH: Roles of the NKG2D
immunoreceptor and its ligands. Nat Rev Immunol. 3:781–790. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kohlhapp FJ, O'Sullivan JA, Moore TV,
Zloza A and Guevara-Patino JA: NKG2D signaling shifts the balance
of CD8 T cells from single cytokine- to polycytokine-producing
effector cells. Mol Immunol. 155:1–6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Corvino D, Kumar A and Bald T: Plasticity
of NK cells in cancer. Front Immunol. 13:8883132022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Asl KD, Rafat A, Mazloumi Z, Valipour B,
Movassaghpour A, Talebi M, Mahdavi M, Nasrabadi HT and Charoudeh
HN: Cord blood stem cell-generated KIR(+)NK cells effectively
target leukemia cell lines. Hum Immunol. 84:98–105. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Klingemann H: The NK-92 cell line-30 years
later: Its impact on natural killer cell research and treatment of
cancer. Cytotherapy. 25:451–457. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
García Aponte OF, Kozma B, Egger D, Kasper
C and Herwig C: Kinetics of NK-92 growth and functionality in
pseudo-static cultures. Biochemical Engineering J. 196:1089292023.
View Article : Google Scholar
|
|
54
|
Clara JA, Levy ER, Reger R, Barisic S,
Chen L, Cherkasova E, Chakraborty M, Allan DSJ and Childs R:
High-affinity CD16 integration into a CRISPR/Cas9-edited CD38 locus
augments CD38-directed antitumor activity of primary human natural
killer cells. J Immunother Cancer. 10:e0038042022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Crow D: Could iPSCs enable ‘Off-the-Shelf’
cell therapy? Cell. 177:1667–1669. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Naama M and Buganim Y: Human trophoblast
stem cell-state acquisition from pluripotent stem cells and somatic
cells. Curr Opin Genet Dev. 81:1020842023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Goodridge JP, Mahmood S, Zhu H, Gaidarova
S, Blum R, Bjordahl R, Cichocki F, Chu H, Bonello G, Lee T, et al:
FT596: Translation of first-of-kind multi-antigen targeted
off-the-shelf CAR-NK cell with engineered persistence for the
treatment of B cell malignancies. Blood. 134:3012019. View Article : Google Scholar
|
|
59
|
Valamehr B, Robinson M, Abujarour R,
Rezner B, Vranceanu F, Le T, Medcalf A, Lee TT, Fitch M, Robbins D
and Flynn P: Platform for induction and maintenance of
transgene-free hiPSCs resembling ground state pluripotent stem
cells. Stem Cell Reports. 2:366–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kiran S, Xue Y, Sarker DB, Li Y and Sang
QXA: Feeder-free differentiation of human iPSCs into natural killer
cells with cytotoxic potential against malignant brain rhabdoid
tumor cells. Bioact Mater. 36:301–316. 2024.PubMed/NCBI
|
|
61
|
Lv Y, Rao Z, Liu L, Jia J, Wu C, Xu J, Du
Y, Liu Y, Liu B, Shi J, et al: The efficient generation of
functional human hepatocytes from chemically induced pluripotent
stem cells. Cell Prolif. 57:e135402024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lupo KB, Moon JI, Chambers AM and
Matosevic S: Differentiation of natural killer cells from induced
pluripotent stem cells under defined, serum- and feeder-free
conditions. Cytotherapy. 23:939–952. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Denman CJ, Senyukov VV, Somanchi SS,
Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN,
Huls MH, et al: Membrane-bound IL-21 promotes sustained ex vivo
proliferation of human natural killer cells. PLoS One.
7:e302642012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Knorr DA, Ni Z, Hermanson D, Hexum MK,
Bendzick L, Cooper LJ, Lee DA and Kaufman DS: Clinical-scale
derivation of natural killer cells from human pluripotent stem
cells for cancer therapy. Stem Cells Transl Med. 2:274–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Qiao W, Dong P, Chen H and Zhang J:
Advances in induced pluripotent stem cell-derived natural killer
cell therapy. Cells. 13:19762024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang L, Weiskittel TM, Zhu Y, Xue D,
Zhang H, Shen Y, Yu H, Li J, Hou L, Guo H, et al: Comparative
dissection of transcriptional landscapes of human iPSC-NK
differentiation and NK cell development. Life Med. 3:lnae0322024.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Thangaraj JL, Coffey M, Lopez E and
Kaufman DS: Disruption of TGF-β signaling pathway is required to
mediate effective killing of hepatocellular carcinoma by human
iPSC-derived NK cells. Cell Stem Cell. 31:1327–1343.e1325. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Qin Y, Cui Q, Sun G, Chao J, Wang C, Chen
X, Ye P, Zhou T, Jeyachandran AV, Sun O, et al: Developing enhanced
immunotherapy using NKG2A knockout human pluripotent stem
cell-derived NK cells. Cell Rep. 43:1148672024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Goldenson BH, Hor P and Kaufman DS:
iPSC-derived natural killer cell therapies-expansion and targeting.
Front Immunol. 13:8411072022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bernareggi D, Gonsalves C, Schabla M,
Gárate-Carrillo A, El-Kalay M, Kaufman DS, Hollingsworth R and Zhu
H: 336 A novel method for efficient cGMP production of natural
killer cells from clonal master induced pluripotent stem cells for
next generation, off-the-shelf cancer immunotherapy. Regular and
Young Investigator Award Abstracts. A354. 2022. View Article : Google Scholar
|
|
71
|
Maddineni S, Silberstein JL and Sunwoo JB:
Emerging NK cell therapies for cancer and the promise of next
generation engineering of iPSC-derived NK cells. J Immunother
Cancer. 10:e0046932022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Patel M, Park D, Tarantolo S, Dowlati A,
Olson D, Kaneko Y, Tang M, Soukharev S, Takizawa M, Okada Y, et al:
754 A phase 1/2 study of ASP1570 in participants with locally
advanced or metastatic solid tumors who have progressed on, or are
ineligible for, all available standard therapies. Regular and Young
Investigator Award Abstracts. A786. 2022. View Article : Google Scholar
|
|
73
|
Bachanova V, Ghobadi A, Patel K, Park JH,
Flinn IW, Shah P, Wong C, Bickers C, Szabo P, Wong L, et al: Safety
and efficacy of FT596, a first-in-class, multi-antigen targeted,
off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in
relapsed/refractory B-Cell lymphoma. Blood. 138:823. 2021.
View Article : Google Scholar
|
|
74
|
Tang SY, Zha S, Du Z, Zeng J, Zhu D, Luo Y
and Wang S: Targeted integration of EpCAM-specific CAR in human
induced pluripotent stem cells and their differentiation into NK
cells. Stem Cell Res Ther. 12:5802021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ma S, Caligiuri MA and Yu J: Harnessing
IL-15 signaling to potentiate NK cell-mediated cancer
immunotherapy. Trends Immunol. 43:833–847. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Vahidi S, Touchaei AZ and Samadani AA:
IL-15 as a key regulator in NK cell-mediated immunotherapy for
cancer: From bench to bedside. Int Immunopharmacol. 133:1121562024.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Seo IH, Eun HS, Kim JK, Lee H, Jeong S,
Choi SJ, Lee J, Lee BS, Kim SH, Rou WS, et al: IL-15 enhances
CCR5-mediated migration of memory CD8(+) T cells by upregulating
CCR5 expression in the absence of TCR stimulation. Cell Rep.
36:1094382021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Valipour B, Abedelahi A, Naderali E,
Velaei K, Movassaghpour A, Talebi M, Montazersaheb S, Karimipour M,
Darabi M, Chavoshi H, et al: Cord blood stem cell derived CD16(+)
NK cells eradicated acute lymphoblastic leukemia cells using with
anti-CD47 antibody. Life Sci. 242:1172232020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Quatrini L, Vacca P, Tumino N, Besi F, Di
Pace AL, Scordamaglia F, Martini S, Munari E, Mingari MC, Ugolini S
and Moretta L: Glucocorticoids and the cytokines IL-12, IL-15, and
IL-18 present in the tumor microenvironment induce PD-1 expression
on human natural killer cells. J Allergy Clin Immunol. 147:349–360.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen AP, Gao P, Lin L, Ashok P, He H, Ma
C, Zou DL, Allain V, Boyne A, Juillerat A, et al: An improved
approach to generate IL-15+/+/TGFβR2−/−
iPSC-derived natural killer cells using TALEN. Cell Rep Methods.
4:1008572024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fares J, Davis ZB, Rechberger JS, Toll SA,
Schwartz JD, Daniels DJ, Miller JS and Khatua S: Advances in NK
cell therapy for brain tumors. NPJ Precis Oncol. 7:172023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kim J, Phan MTT, Hwang I, Park J and Cho
D: Comparison of the different anti-CD16 antibody clones in the
activation and expansion of peripheral blood NK cells. Sci Rep.
13:94932023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Capuano C, Pighi C, Battella S, De
Federicis D, Galandrini R and Palmieri G: Harnessing CD16-mediated
NK cell functions to enhance therapeutic efficacy of
tumor-targeting mAbs. Cancers (Basel). 13:25002021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Romee R, Foley B, Lenvik T, Wang Y, Zhang
B, Ankarlo D, Luo X, Cooley S, Verneris M, Walcheck B and Miller J:
NK cell CD16 surface expression and function is regulated by a
disintegrin and metalloprotease-17 (ADAM17). Blood. 121:3599–3608.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang Y, Wu J, Newton R, Bahaie NS, Long C
and Walcheck B: ADAM17 cleaves CD16b (FcγRIIIb) in human
neutrophils. Biochim Biophys Acta. 1833:680–685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Meng F, Zhang S, Xie J, Zhou Y, Wu Q, Lu
B, Zhou S, Zhao X and Li Y: Leveraging CD16 fusion receptors to
remodel the immune response for enhancing anti-tumor immunotherapy
in iPSC-derived NK cells. J Hematol Oncol. 16:622023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yamamoto K, Blum R and Kaufman DS:
ADAM17-deficient pluripotent stem cell-derived natural killer cells
possess improved antibody-dependent cellular cytotoxicity and
antitumor activity. Blood. 136:22020. View Article : Google Scholar
|
|
88
|
Biederstädt A and Rezvani K: Engineering
the next generation of CAR-NK immunotherapies. Int J Hematol.
114:554–571. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gao L, Du X, Li J and Qin FXF: Evolving
roles of CD38 metabolism in solid tumour microenvironment. Br J
Cancer. 128:492–504. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Stikvoort A, van der Schans J, Sarkar S,
Poels R, Ruiter R, Naik J, Yuan H, de Bruijn JD, van de Donk NWCJ,
Zweegman S, et al: CD38-specific chimeric antigen receptor
expressing natural killer KHYG-1 cells: A proof of concept for an
‘Off the Shelf’ therapy for multiple myeloma. Hemasphere.
5:e5962021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Seiffert M: TIGIT: An immune checkpoint
beyond T cells in chronic lymphocytic leukemia. Haematologica.
108:1979–1981. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Neo SY, Yang Y, Record J, Ma R, Chen X,
Chen Z, Tobin NP, Blake E, Seitz C, Thomas R, et al: CD73 immune
checkpoint defines regulatory NK cells within the tumor
microenvironment. J Clin Invest. 130:1185–1198. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lupo KB, Yao X, Borde S, Wang J,
Torregrosa-Allen S, Elzey BD, Utturkar S, Lanman NA, McIntosh M and
Matosevic S: synNotch-programmed iPSC-derived NK cells usurp TIGIT
and CD73 activities for glioblastoma therapy. Nat Commun.
15:19092024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Reiser J, Chan SR, Mathavan K, Sillitti D,
Mottershead C, Mattson B, Pache M, Gutierrez A, Scoon W, Zhu Y, et
al: FT555: Off-the-Shelf CAR-NK cell therapy co-targeting GPRC5D
and CD38 for the treatment of multiple myeloma. Blood.
140:4560–4561. 2022. View Article : Google Scholar
|
|
95
|
Vahidian F, Khosroshahi LM, Akbarzadeh M,
Jahanban-Esfahlan A, Baghbanzadeh A, Ali-Hassanzadeh M and
Safarzadeh E: The tricks for fighting against cancer using CAR NK
cells: A review. Mol Cell Probes. 63:1018172022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Marofi F, Abdul-Rasheed OF, Rahman HS,
Budi HS, Jalil AT, Yumashev AV, Hassanzadeh A, Yazdanifar M,
Motavalli R, Chartrand MS, et al: CAR-NK cell in cancer
immunotherapy; A promising frontier. Cancer Sci. 112:3427–3436.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cichocki F, Bjordahl R, Gaidarova S,
Mahmood S, Abujarour R, Wang H, Tuininga K, Felices M, Davis ZB,
Bendzick L, et al: iPSC-derived NK cells maintain high cytotoxicity
and enhance in vivo tumor control in concert with T cells and
anti-PD-1 therapy. Sci Transl Med. 12:eaaz56182020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Karagiannis P and Kim SI: iPSC-derived
natural killer cells for cancer immunotherapy. Mol Cells.
44:541–548. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Karvouni M, Vidal-Manrique M, Susek KH,
Hussain A, Gilljam M, Zhang Y, Gray JD, Lund J, Kaufmann G,
Ljunggren HG, et al: Challenges in alphaCD38-chimeric antigen
receptor (CAR)-expressing natural killer (NK) cell-based
immunotherapy in multiple myeloma: Harnessing the CD38dim phenotype
of cytokine-stimulated NK cells as a strategy to prevent
fratricide. Cytotherapy. 25:763–772. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bjordahl R, Gaidarova S, Goodridge JP,
Mahmood S, Bonello G, Robinson M, Ruller C, Pribadi M, Lee T,
Abujarour R, et al: FT576: A novel multiplexed engineered
off-the-shelf natural killer cell immunotherapy for the
dual-targeting of CD38 and Bcma for the treatment of multiple
myeloma. Blood. 134:32142019. View Article : Google Scholar
|
|
101
|
Goodridge JP, Bjordahl R, Mahmood S,
Reiser J, Gaidarova S, Blum R, Cichocki F, Chu H, Bonello G, Lee T,
et al: FT576: Multi-Specific Off-the-shelf CAR-NK cell therapy
engineered for enhanced persistence, avoidance of self-fratricide
and optimized mab combination therapy to prevent antigenic escape
and elicit a deep and durable response in multiple myeloma. Blood.
136:4–5. 2020. View Article : Google Scholar
|
|
102
|
Dhakal B, Berdeja JG, Gregory T, Ly T,
Bickers C, Zong X, Wong L, Goodridge JP, Cooley S, Valamehr B, et
al: Interim phase I clinical data of FT576 as monotherapy and in
combination with daratumumab in subjects with relapsed/refractory
multiple myeloma. Blood. 140:4586–4587. 2022. View Article : Google Scholar
|
|
103
|
Wang L, Wang Y, He X, Mo Z, Zhao M, Liang
X, Hu K, Wang K, Yue Y, Mo G, et al: CD70-targeted iPSC-derived
CAR-NK cells display potent function against tumors and
alloreactive T cells. Cell Rep Med. 6:1018892025. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yu M, Mansour AG, Teng KY, Sun G, Shi Y
and Caligiuri MA: iPSC-derived natural killer cells expressing
EGFR-CAR against glioblastoma. Cancer Research. 80:33132020.
View Article : Google Scholar
|
|
105
|
Wang Y, Wang L, Shao M, He X, Yue Y, Zhou
Y, Yang L, Huang H and Hu Y: Off-the-Shelf, multiplexed-engineered
iPSC-derived CD33 CAR-NK cells for treatment of acute myeloid
leukemia. Blood. 140:126852022. View Article : Google Scholar
|
|
106
|
Shapiro RM and Romee R: iPSC-derived CD19
CAR NK cells for relapsed or refractory lymphoma. Lancet.
405:98–99. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ghobadi A, Bachanova V, Patel K, Park JH,
Flinn I, Riedell PA, Bachier C, Diefenbach CS, Wong C, Bickers C,
et al: Induced pluripotent stem-cell-derived CD19-directed chimeric
antigen receptor natural killer cells in B-cell lymphoma: A phase
1, first-in-human trial. Lancet. 405:127–136. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Merino A, Maakaron J and Bachanova V:
Advances in NK cell therapy for hematologic malignancies: NK
source, persistence and tumor targeting. Blood Rev. 60:1010732023.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bachanova V, Cayci Z, Lewis D, Maakaron
JE, Janakiram M, Bartz A, Payne S, Wong C, Cooley S, Valamehr B, et
al: Initial clinical activity of FT596, a first-in-class,
multi-antigen targeted, off-the-shelf, iPSC-Derived CD19 CAR NK
cell therapy in Relapsed/Refractory B-Cell lymphoma. Blood.
136:82020. View Article : Google Scholar
|
|
110
|
Bachanova V, Deol A, Al-Juhaishi TMS,
Lulla PD, Byrne MT, Wong C, Bickers C, Greene T, Wong L, Villa B,
et al: Safety and efficacy of FT522, a first-in-class,
multi-antigen targeted, off-the-shelf, iPSC-Derived CD19 CAR NK
cell therapy with alloimmune defense receptor (ADR) in
Relapsed/Refractory B-Cell lymphoma. Blood. 144:65432024.
View Article : Google Scholar
|
|
111
|
Patel K, Namburi S, Latif T and Oluwole
OO: Interim results from the ELiPSE-1 study: A phase 1,
multicenter, open-label study of CNTY-101 in subjects with relapsed
or refractory CD19-positive B-cell malignancies. J Clin Oncol.
42:70232024. View Article : Google Scholar
|
|
112
|
Borges L, Wallet MA, Bullaughey CL, Naso
MF, Gurung B, Keating S, Carton JM, Wheeler JC, Campion L, Mendonca
M, et al: Development of multi-engineered iPSC-derived CAR-NK cells
for the treatment of B-cell malignancies. Blood. 138:17292021.
View Article : Google Scholar
|
|
113
|
Sermer D, Elavalakanar P, Abramson JS,
Palomba ML, Salles G and Arnason J: Targeting CD19 for diffuse
large B cell lymphoma in the era of CARs: Other modes of
transportation. Blood Rev. 57:1010022023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ramachandran I, Rothman S, Clausi M,
McFadden K, Salantes B, Jih G, Brigman T, Kelly S, Hall MS, Yee S,
et al: Multiple doses of Cnty-101, an iPSC-derived allogeneic CD19
targeting CAR-NK product, are safe and result in tumor
microenvironment changes associated with response: A case study.
Blood. 142:16542023. View Article : Google Scholar
|
|
115
|
Terren I, Orrantia A, Vitalle J,
Zenarruzabeitia O and Borrego F: NK cell metabolism and tumor
microenvironment. Front Immunol. 10:22782019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Xu Z, Yang J, Xin X, Liu C, Li L, Mei X
and Li M: Merits and challenges of iPSC-derived organoids for
clinical applications. Front Cell Dev Biol. 11:11889052023.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Huyghe M, Desterke C, Imeri J, Belliard N,
Chaker D, Oudrirhi N, Bezerra H, Turhan AG, Bennaceur-Griscelli A
and Griscelli F: Comparative analysis of iPSC-derived NK cells from
two differentiation strategies reveals distinct signatures and
cytotoxic activities. Front Immunol. 15:14637362024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Nianias A and Themeli M: Induced
pluripotent stem cell (iPSC)-derived lymphocytes for adoptive cell
immunotherapy: recent advances and challenges. Curr Hematol Malig
Rep. 14:261–268. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhou Y, Cheng L, Liu L and Li X: NK cells
are never alone: Crosstalk and communication in tumour
microenvironments. Mol Cancer. 22:342023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Linke JA, Munn LL and Jain RK: Compressive
stresses in cancer: Characterization and implications for tumour
progression and treatment. Nat Rev Cancer. 24:768–791. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Xie D, Zhu S and Bai L: Lactic acid in
tumor microenvironments causes dysfunction of NKT cells by
interfering with mTOR signaling. Sci China Life Sci. 59:1290–1296.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Gao Y, Zhou H, Liu G, Wu J, Yuan Y and
Shang A: Tumor microenvironment: lactic acid promotes tumor
development. J Immunol Res. 2022:31193752022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Miao L, Lu C, Zhang B, Li H, Zhao X, Chen
H, Liu Y and Cui X: Advances in metabolic reprogramming of NK cells
in the tumor microenvironment on the impact of NK therapy. J Transl
Med. 22:2292024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lozada JR, Zhang B, Miller JS and Cichocki
F: NK cells from human cytomegalovirus-seropositive individuals
have a distinct metabolic profile that correlates with elevated
mTOR signaling. J Immunol. 211:539–550. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Osuna-Espinoza KY and Rosas-Taraco AG:
Metabolism of NK cells during viral infections. Front Immunol.
14:10641012023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yin Y, Feng W, Chen J, Chen X, Wang G,
Wang S, Xu X, Nie Y, Fan D, Wu K and Xia L: Immunosuppressive tumor
microenvironment in the progression, metastasis, and therapy of
hepatocellular carcinoma: From bench to bedside. Exp Hematol Oncol.
13:722024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang Q, Shao X, Zhang Y, Zhu M, Wang FXC,
Mu J, Li J, Yao H and Chen K: Role of tumor microenvironment in
cancer progression and therapeutic strategy. Cancer Med.
12:11149–11165. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Arner EN and Rathmell JC: Metabolic
programming and immune suppression in the tumor microenvironment.
Cancer Cell. 41:421–433. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tong L, Jimenez-Cortegana C, Tay AHM,
Wickstrom S, Galluzzi L and Lundqvist A: NK cells and solid tumors:
Therapeutic potential and persisting obstacles. Mol Cancer.
21:2062022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Vivier E, Rebuffet L, Narni-Mancinelli E,
Cornen S, Igarashi RY and Fantin VR: Natural killer cell therapies.
Nature. 626:727–736. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tarannum M, Romee R and Shapiro RM:
Innovative strategies to improve the clinical application of NK
cell-based immunotherapy. Front Immunol. 13:8591772022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Carreira-Santos S, Lopez-Sejas N,
Gonzalez-Sanchez M, Sánchez-Hernández E, Pera A, Hassouneh F, Durán
E, Solana R, Casado JG and Tarazona R: Enhanced expression of
natural cytotoxicity receptors on cytokine-induced memory-like
natural killer cells correlates with effector function. Front
Immunol. 14:12564042023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wang Z, Guan D, Wang S, Chai LYA, Xu S and
Lam KP: Glycolysis and oxidative phosphorylation play critical
roles in natural killer cell receptor-mediated natural killer cell
functions. Front Immunol. 11:2022020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Poznanski SM, Barra NG, Ashkar AA and
Schertzer JD: Immunometabolism of T cells and NK cells: Metabolic
control of effector and regulatory function. Inflamm Res.
67:813–828. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Shankar K, Zingler-Hoslet I, Tabima DM,
Zima S, Shi L, Gimse K, Forsberg MH, Katta V, Davis SZ, Maldonado
D, et al: Virus-free CRISPR knockin of a chimeric antigen receptor
into KLRC1 generates potent GD2-specific natural killer cells. Mol
Ther. 33:1014–1030. 2024. View Article : Google Scholar
|
|
136
|
Hsu J, Hodgins JJ, Marathe M, Nicolai CJ,
Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph
HE, Thompson TW, et al: Contribution of NK cells to immunotherapy
mediated by PD-1/PD-L1 blockade. J Clin Invest. 128:4654–4668.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Clerico M, Ragaini S and Cavallo F:
Non-Hodgkin lymphoma treated with anti-CD20 antibody-based
immunochemotherapy. Resistance to Anti-Cd20 Antibodies and
Approaches for their Reversal Elsevier. 103–122. 2024. View Article : Google Scholar
|
|
139
|
Hoffman B and Liebermann DA: Apoptotic
signaling by c-MYC. Oncogene. 27:6462–6472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ding S: Therapeutic reprogramming toward
regenerative medicine. Chem Rev. 125:1805–1822. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Agostini A, Orlacchio A, Carbone C and
Guerriero I: Understanding tricky cellular and molecular
interactions in pancreatic tumor microenvironment: New food for
thought. Front Immunol. 13:8762912022. View Article : Google Scholar : PubMed/NCBI
|