Introduction
Bone is one of the few tissues that heals without
forming a fibrous scar (1). The
term ‘bone fracture’ is used to describe the destruction of bone
continuity or breakdown of the bone structural integrity (2). Bone heals over a period of time
(1); however, open fractures are
vulnerable to complications, such as malunion, delayed union,
non-union and refracture (3,4). In
total, ~5–10% of fractures will show delayed healing (5). At present, the incidence of traumatic
fracture continues to increase, current estimates for traumatic
fractures are 23.3% for men and 11.2% for women (6), and this markedly affects the quality
of life of patients (7,8). Fracture healing is a dynamic process.
The initial stage includes the hematoma, which generates an
inflammatory environment. Next, endochondral ossification, removal
and calcification of the endochondral cartilage indicate the
fracture healing has reached the middle to late stage (9). Then, the chronic remodeling concludes
the healing process (9).
The ubiquitin-proteasome system is a major pathway
of protein post-translational modification, mediated by E1, E2 and
E3 ubiquitin ligases, in addition to deubiquitinating enzymes
(10). Ubiquitination is a
reversible process; whereby deubiquitinating enzymes remove
ubiquitin from poly-ubiquitin chains and targeted proteins
(11). Otubain (OTUB)2 is a member
of the OTUB superfamily of deubiquitinylases that inhibits the
ubiquitination of substrates and enhance protein stability
(12,13). Dysregulation of the OTUB protein
family may lead to bone dysplasia (13). Stanišić et al (14) reported that OTUB1 weakens the
stability of estrogen receptor α (14), which serves an essential role in
skeletal formation (15). Thus,
OTUB proteins may act as a vital part in the pathophysiology of
bone development and homeostasis, which refers to the balance
between osteoblast-mediated bone formation and osteoclast-mediated
bone resorption (16). Results of
the previous study revealed that osteoblast activity and
osteogenesis were enhanced during fracture healing. In addition,
osteoclast function and bone resorption were reduced, promoting the
formation of an external callus used to stabilize bone fragments
(17). Li et al (18) reported that OTUB2 knockdown
reverses Shh- or Smo-induced upregulation of runt related
transcription factor 2 (RUNX2), bone morphogenetic protein 2 and
tissue nonspecific alkaline phosphatase (TNAP), key regulators of
bone formation (18). Thus, OTUB2
may exhibit potential in osteogenesis and bone fracture healing
(18). Results of a previous study
demonstrate that fracture healing may also be mediated by the
ubiquitination of genes associated with osteogenesis (19).
The present study aimed to investigate the effects
of OTUB2 on bone fracture healing both in vivo and in
vitro. A model of open fracture was established in the femora
of Sprague Dawley rats, as previously described (4). Notably, bone callus formation
requires the osteoblastic differentiation of bone marrow
mesenchymal stem cells (BMSCs) (20,21)
and previous studies demonstrate that this differentiation promotes
bone fracture healing (22,23).
Thus, BMSCs were obtained from the rat marrow cavity of femurs and
tibiae and subsequently subjected to osteogenic differentiation
in vitro (24). The
functions of OTUB2 in bone fracture healing, histological damage,
bone formation and mineralization were further explored.
Materials and methods
Animal experiments
Animal experiments were approved by the Laboratory
Animal Ethical and Welfare Committee of Hebei Medical University
(Hebei, China; approval no. IACUC-Hebmu-2021007), following The
Guideline for the Care and Use of Laboratory Animals (25).
Bioinformatics analysis
Proteins that interact with OTUB2 were analyzed
using the STRING database (https://cn.string-db.org/). Gene Ontology (GO)
enrichment analysis of proteins that interacted with OTUB2 was
carried out using the DAVID database (https://david.ncifcrf.gov/home.jsp).
Lentivirus preparation and
infection
A 2nd generation lentiviral vector system was used.
For OTUB2 overexpression, OTUB2 cDNA was amplified and subcloned
into the lentivirus vector (LV), pLVX–IRES-Puro (Unibio) and
referred to as LV-OTUB2. pLVX–IRES-Puro with no cDNA insertion was
used as the negative control (NC) for LV-OTUB2 and referred to as
LV-NC. For TNF-receptor associated factor 3 (TRAF3) knockdown,
TRAF3 short hairpin RNA (shRNA) was synthetized and subcloned into
pLVX-shRNA1 (Unibio). shNC, a non-targeting shRNA, was subcloned
into pLVX-shRNA1 and used as the NC for LV-shTRAF3. Subsequently,
pLVX-OTUB2/pLVX-shTRAF3/NC, pSPAX2 and pMD2.G vectors were mixed at
a ratio of 4:3:1 (14.0:10.5:3.5 µg in a 10 cm culture flask) and
transfected into 293T cells (iCell Bioscience). Following
transfection, recombinant virus-containing supernatants were
collected and filtered using a 0.45-µm membrane. In the logarithmic
phase of growth (multiplicity of infection, 20) BMSCs were infected
with LV-NC, LV-OTUB2, LV-shNC or LV-shTRAF3, as previously
described (26). Sequences of
shTRAF3 and shNC were as follows: shTRAF3,
5′-ccgCGAAGACAGTGGAGGACAAGTttcaagagaACTTGTCCTCCACTGTCTTCGttttt-3′;
and shNC,
5′-ccgTTCTCCGAACGTGTCACGTttcaagagaACGTGACACGTTCGGAGAAttttt-3′.
Cells were further subjected to the osteogenic differentiation at
72 h post-transfection.
Fracture model
Male Sprague Dawley rats (age, 12 weeks; weight,
410±10 g) were obtained from Liaoning Changsheng Biotechnology.
Rats were housed with food and water ad libitum in a
humidity (50±10%) and temperature-controlled (22±1°C) environment
for a week of acclimation under a 12/12 h light/dark cycle. The
fracture model was established as previously described (4), Briefly, animals were subjected to
inhalation of isoflurane for anesthesia, using an induction rate of
5% and a maintenance rate of 2%, as previously described (27). An incision was made on the middle
lateral thigh of rats, followed by the blunt dissection of muscle
around the femur. The femur was cut and a sterile Kirschner wire
was inserted to stabilize the fracture. Subsequently, rats were
randomly divided into three groups: i) Control; ii) LV-NC; and iii)
LV-OTUB2. Rats in the LV-NC and LV-OTUB2 groups were injected with
1×108 transducing units/ml (50 µl/injection) of LV-NC or
LV-OTUB2 at the fracture site and this was repeated once a week for
2, 4 or 8 weeks. Fractures of rats in the control group were left
untreated. Rats were sacrificed with 70% vol/min CO2 at
each time point unless they met a prior humane endpoint. All
animals were evaluated, weighted and scored daily with set criteria
as previously described (28,29).
Briefly, any finding listed as a humane endpoint, a score of 3 in
two or more categories and a total score >9 led to the animal
being euthanised. The categories including general appearance,
activity, hydration, respiration, ambulation, surgical
complications and weight loss. Following euthanasia, cardiac and
respiratory arrest, as well as fixed and dilated pupils were
observed in the rats for ~10 min before the animals were confirmed
as deceased. A total of 162 rats were used in the present study.
Among them, 36 rats [18 rats for western blotting; 18 rats for
X-radiography and reverse transcription-quantitative PCR (RT-qPCR);
n=6 rats/group] were used at 2 weeks post fracture. A total of 54
rats (18 rats for western blotting; 18 rats for X-radiography and
RT-qPCR; 18 rats for pathological staining; n=6 rats/group) were
used at 4 weeks post fracture. A total of 72 rats (18 rats for
western blotting; 18 rats for X-radiography and RT-qPCR; 18 rats
for micro-CT and pathological staining; 18 rats for TNAP activity
detection; n=6 rats/group) were used at 8 weeks post fracture.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from the callus tissue of
the femur of rats using TRIpure lysis solution (BioTeke
Corporation) according to the manufacturer's protocols.
Subsequently, total RNA was reverse transcribed into cDNA using the
BeyoRT II kit (Beyotime Institute of Biotechnology) according to
the manufacturer's instructions. Briefly, total RNA, oligo (dT),
reaction buffer, RNase inhibitor, dNTP Mix and BeyoRT II M-MLV
reverse transcriptase were used to form a reaction system.
According to the manufacturers' instructions, the system was
incubated at 42°C for 50 min, followed by incubation at 80°C for 10
min. Subsequently, cDNA was amplified using 2X Taq PCR MasterMix
and SYBR Green PCR Master Mix (Beijing Solarbio Science &
Technology Co., Ltd.) in accordance with manufacturer's
instructions. The reaction system included 1 µl cDNA, 1 µl primer,
0.3 µl SYBR Green, 10 µl 2X Taq PCR MasterMix and 7.7 µl
ddH2O. The thermocycling conditions used were as
follows: 94°C for 5 min, followed by 40 cycles of 94°C for 15 sec,
60°C for 25 sec and 72°C for 30 sec. The relative expression of
targeted genes was quantified using six independent measurements
and the 2−ΔΔCq method (30). β-actin was used as the internal
reference gene. Primer sequences are listed in Table I. The experiments were repeated
thrice.
 | Table I.Sequences of primers used in reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used in reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′-3′) |
|---|
| Otubain 2 | F:
TCAATCCGAAAGACCAAA |
|
| R:
TGTGAGGAGGCGTAAGAA |
| Tissue nonspecific
alkaline phosphatase | F:
AGTCCGTGGGCATCGTG |
|
| R:
CCTCTGGCGGCATCTCA |
| Runt related
transcription factor 2 | F:
CCATAACGGTCTTCACAAATC |
|
| R:
GAGGCGGTCAGAGAACAAACT |
|
Osteoprotegerin | F:
TCCCTTGCCCTGACTAC |
|
| R:
CCTGAGAAGAACCCATCC |
| Receptor activator
of nuclear factor-κB ligand | F:
CATCGGGTTCCCATAAAG |
|
| R:
GAAGCAAATGTTGGCGTA |
| β-actin | F:
TGGCACCACACTTTCTACAATGAGC |
|
| R:
GGGTCATCTTTTCACGGTTGG |
Western blotting analysis
Total protein was extracted from BMSCs or the callus
tissue in the femur of rats using Radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology) mixed with
phenylmethanesulfonyl fluoride (100:1; Beyotime Institute of
Biotechnology). Samples were centrifuged at 10,000 × g for 5 min at
4°C and supernatants were collected. Total protein was quantified
using a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. A total of
20 µg of protein was separated by SDS-PAGE s with a 5% stacking gel
and a 10% running gel. Separated proteins were transferred onto
PVDF membranes and blocked with 5% skimmed milk diluted in
tris-buffered saline tween-20 for 1 h at room temperature.
Following blocking, membranes were incubated with the following
primary antibodies at 4°C overnight: Polyclonal rabbit anti-OTUB2
(1:500; Sabbiotech), polyclonal rabbit anti-TNAP (1:1,000; ABclonal
Biotech, Co., Ltd.), polyclonal rabbit anti-RUNX2 (1:500;
Proteintech Group, Inc.), polyclonal rabbit anti-osteoprotegerin
(OPG; 1:1,000; Affinity Biosciences, Ltd.), polyclonal rabbit
anti-receptor activator of nuclear factor-κB ligand (RANKL; 1:500;
Affinity Biosciences, Ltd.), polyclonal rabbit anti-TRAF3 (1:1,000;
ABclonal Biotech, Co., Ltd.), polyclonal rabbit anti-Flag (ABclonal
Biotech, Co., Ltd.), polyclonal rabbit anti-ubiquitin (Proteintech
Group, Inc.) and monoclonal mouse anti-β-actin (1:1,000; Santa Cruz
Biotechnology, Inc.). Following primary incubation, membranes were
incubated with the following secondary antibodies at 37°C for 45
min: Goat anti-rabbit horseradish peroxidase-conjugated IgG
(1:5,000; Beyotime Institute of Biotechnology) and goat anti-mouse
horseradish peroxidase-conjugated IgG (1:5,000; Beyotime Institute
of Biotechnology). Protein bands were visualized using ECL reagent
(Beyotime Institute of Biotechnology) on the WD-9413B gel imaging
system (Liuyi Biotechnology). Six independent measurements were
obtained using callus tissue samples and three independent
measurements were obtained using BMSC samples. The band densities
were quantified with the Gel-Pro-Analyzer software (version 4.0;
Media Cybernetics, Inc.).
X-radiography and micro-computed
tomography (CT) analysis
Fractured femurs were imaged using the CSM-2R X-ray
apparatus (Softex) at 2-, 4- and 8-weeks post-fracture.
Radiographic fracture healing was scored from Grade 1–6, as
previously described (3). Briefly,
fracture healing was scored as follows: i) Grade 1, no
calcification; ii) Grade 2, patchy calcification; iii) Grade 3,
calcification takes on the appearance of a callus; iv) Grade 4,
callus bridging across the fracture gap; v) Grade 5, continuity of
bone trabeculae; and vi) Grade 6, remodeling to normal bone.
Fractured femurs were scanned using the Quantum GX
micro-CT imaging system (PerkinElmer, Inc.). Briefly, fractured
femurs were scanned at 90 kV and 88 µA using a 50-µm voxel. Using
the fracture line as the center, 50 slices were measured between
the distal and proximal edges. The callus was segmented from the
background as a region of interest. Subsequently, bone volume
fraction (BV/TV) and bone mineral density (BMD) were auto-obtained
using micro-CT analysis and 3D images were captured (31,32).
Histological analysis
Tissues were fixed in 10% formalin solution at 4°C
for 48 h. The fixed tissues were rinsed in running water for 4 h at
room temperature. Samples were dehydrated in gradient ethanol
(50–100 %), embedded in paraffin and then cut into 5 µm sections
using a microtome (cat. no. RM 2235; Leica Microsystems, Inc.).
Paraffin-embedded sections of fracture callus were deparaffinized
in xylene and rehydrated in an ethanol gradient with distilled
water. Formation of callus was examined using H&E staining
(33). Cartilaginous ossification
was analyzed using safranine O-fast green staining. Briefly,
sections were stained with safranine O for 5 min at room
temperature, followed by rinsing with ethanol gradient. Sections
were subsequently stained with fast green for 1 min at room
temperature and imaged using a BX53 microscope in bright-field mode
(Olympus Corporation).
Isolation and osteogenic
differentiation of BMSCs
Isolation and osteogenic differentiation of BMSCs
were performed as previously described (24). Briefly, femurs and tibiae were
collected from rats and muscles surrounding the bone were removed.
The marrow cavity was flushed with DMEM (Wuhan Servicebio
Technology Co., Ltd.) for cell harvesting and cells were
centrifuged at 300 × g for 7 min at room temperature. Subsequently,
cells were cultured in DMEM supplemented with 10% fetal bovine
serum (Sijiqing Biological Engineering Materials Co., Ltd.) at 37°C
in an incubator with 5% CO2. BMSCs were cultured until
the third passage was reached and used in subsequent experiments
after ~2 weeks. BMSC phenotype was confirmed via flow cytometry
with surface antigens.
Osteogenic differentiation was performed at 72 h
post-transfection, as previously described (34). Briefly, cell medium was removed and
replaced with medium for inducing osteogenic differentiation [100
nM dexamethasone (MilliporeSigma), 50 µM vitamin C (Sinopharm
Chemical Reagent Co., Ltd.) and 10 mM β-sodium glycerophosphate
(MilliporeSigma)]. Differentiation medium was changed every 2 days.
At 3 days post-induction, total protein was extracted for western
blotting analysis (35). At 7 days
post-induction, TNAP activity was detected and at 14 days
post-induction, Alizarin Red S staining was performed to assess
calcium deposition, as previously described (35,36).
Flow cytometry
Following centrifugation at 300 × g for 7 min at
4°C, BMSCs were rinsed and suspended with 100 µl phosphate buffer
saline. In total, 1×106 cells were incubated with 0.25
µg fluorescein isothiocyanate (FITC)-conjugated anti-CD45
(MultiSciences Biotech), 1 µg FITC-conjugated anti-CD29 (BioLegend,
Inc.), 0.06 µg FITC-conjugated anti-CD90 (BioLegend, Inc.) and 0.25
µg PE-Cyanine 7-conjugated anti-CD31 (Thermo Scientific, Inc.) at
4°C in the dark. Subsequently, cells were analyzed via NovoCyte
flow cytometry (ACEA Biosciences), using three independent
measurements. Data was analyzed using NovoExpress software (version
1.4.1; Agilent Technologies, Inc.).
Alizarin Red S staining
Cells were fixed with 4% paraformaldehyde at room
temperature for 15 min and stained with Alizarin Red for 30 min at
room temperature. Images were captured using a microscope (IX53l;
Olympus Corporation). Calcium deposits were quantified following
the addition of 10% cetylpyridinium chloride (Shanghai Macklin
Biochemical Co., Ltd.) for 15 min at room temperature. Absorbance
was measured at a wavelength of 570 nm (37).
Detection of TNAP
The levels of TNAP were assessed using the TNAP
Detection Kit (Wanleibio Co., Ltd.) according to the manufacturer's
instructions. Briefly, ultrasonic cell disruption (300 W;
ultrasonication for 3 sec with an interval of 30 sec repeated 5
times) was carried out in an ice bath and cells were subsequently
centrifuged at 421 × g for 10 min at 4°C. In total, three
independent supernatant samples were used for the detection of TNAP
levels.
Callus tissues were homogenized with normal saline
(1:9; g/v). The tissue homogenate was centrifuged at 421 × g at
room temperature for 10 min. The resulting supernatant was obtained
for the detection of TNAP levels. A total of six independent
supernatant samples were used for the detection of TNAP levels.
Co-immunoprecipitation
Wild-type fragments of OTUB2 cDNA, wild-type TRAF3
cDNA and OTUB2 fragments with a point mutation at C51S were
synthetized by General Biotech (Anhui) Co., Ltd. Subsequently,
wild-type OTUB2 fragments and OTUB2 fragments with a point mutation
were inserted into pcDNA3.1(+)_myc-His A vectors (General Biotech
(Anhui) Co., Ltd.). Wild-type fragments of TRAF3 were inserted into
p3×FLAG-CMV-10 vectors (General Biotech (Anhui) Co., Ltd.). All
constructs were verified using enzyme digestion and sequencing.
Subsequently, Lipofectamine® 3000 was used to
transiently transfect OTUB2-Myc/OTUB2-Myc (C51S) and TRAF3-Flag
plasmids into 293T cells. One day prior to transfection, cells were
cultured in 6 well-plates until 80% confluence was reached. In
addition, cells were starved for 2 h prior to transfection and
subsequently incubated with OptiMEM containing 5 µl
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), 5 µl P3000 reagent and 2 µg plasmids. Following
48 h of transfection, 293T cells were lysed with
radioimmunoprecipitation assay buffer mixed with
phenylmethanesulfonyl fluoride (100:1), followed by mixing in
liquid nitrogen. Cells were centrifuged at 10,000 × g for 5 min at
4°C and the supernatant was collected and subjected to
immunoprecipitation using a co-immunoprecipitation kit (cat. no.
26149; Thermo Fisher Scientific, Inc.). Briefly, 20 µl of resin
slurry was used to pre-clear the lysates, which were further
incubated with 1 µg of immobilized antibodies (Flag-tag or
Myc-tag). Following elution and centrifugation (1,000 × g for 5 min
at 4°C) to obtain the precipitates, proteins were used for
subsequent western blot analysis.
Molecular docking analysis
OTUB2 and TRAF3 sequences were obtained from the
National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/) and crystal structures
were obtained via homology modeling on the SWISS-MODEL database
(https://swissmodel.expasy.org/).
Interactions between proteins were forecasted using the GRAMM
database (https://gramm.compbio.ku.edu/request) and a graphic
representation of the protein-protein interaction was established
using PyMOL software (version 2.0; Schrodinger). Grey was
indicative of the OTUB2 protein, purple was indicative of the TRAF3
protein and stick structures of red and blue were indicative of
binding. Notably, the yellow dotted line was indicative of a
hydrogen bond.
Statistical analysis
Data are presented as the mean ± standard deviation,
or as box and whisker plots, with the ‘box’ depicting the median,
1st quartile and 3rd quartile and the ‘whisker’ depicting the
standard deviation. Data were analyzed using GraphPad Prism
software (version, 8.0; GraphPad; Dotmatics). In vitro
experiments were independently repeated three times and in
vivo experiments were independently repeated six times (6
rats/group). Differences between multiple groups were analyzed
using one-way ANOVA followed by Tukey's post hoc test and
comparisons between two groups were analyzed using an unpaired
Student's t-test. Effect size was calculated to determine the power
of the analysis, due to the small sample size included in the
present study, as previously described (38). The sample size of animals was
chosen according to the results of power analysis. The
priori/post-hoc power analysis was carried out using G*power
3.1.9.7 software (Franz Faul). It was found appropriate to complete
the study with at least 6 samples for each group (α err probe was
0.05; effect size was 0.9; power was 0.8). Previous studies
reported that Student's t-tests are suitable when sample size is
small and effect size is large (39,40).
In addition, bone fracture healing scores were analyzed using a
Kruskal-Wallis test followed by Dunn's post hoc analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
OTUB2 accelerates bone fracture
healing
To investigate the potential effects of OTUB2 on
bone fracture healing, a fracture model was established through the
injection of lentivirus targeting OTUB2 around the fracture sites
of rats (Fig. 1A). At 2-, 4- and
8-weeks post-fracture, mRNA and protein expression levels of OTUB2
were elevated in the fracture callus of rats in the LV-OTUB2 group
(Fig. 1B and C). The effects of
OTUB2 overexpression were subsequently investigated using
X-radiography and micro-CT analysis. Results of the present study
revealed that the speed of fracture healing was increased and
fracture healing remodeling was improved in the LV-OTUB2 group at 8
weeks post-fracture, when compared with the LV-NC group (Fig. 1D and E). In addition, the elevated
callus area was observed at the fracture sites (Fig. 1D). Results of the present study
also revealed that specimens exhibited severe loss and erosion of
the femur following bone fracture; however, this was alleviated
following OTUB2 overexpression. Representative micro-CT 3D images
of fractured femur are displayed in Fig. 1F. In addition, quantification of
the micro-CT 3D analysis demonstrated that BMD and BV/TV were
increased following OTUB2 overexpression, indicative of accelerated
bone fracture healing and bone formation (Fig. 1G). These results highlighted that
OTUB2 may promote bone fracture healing.
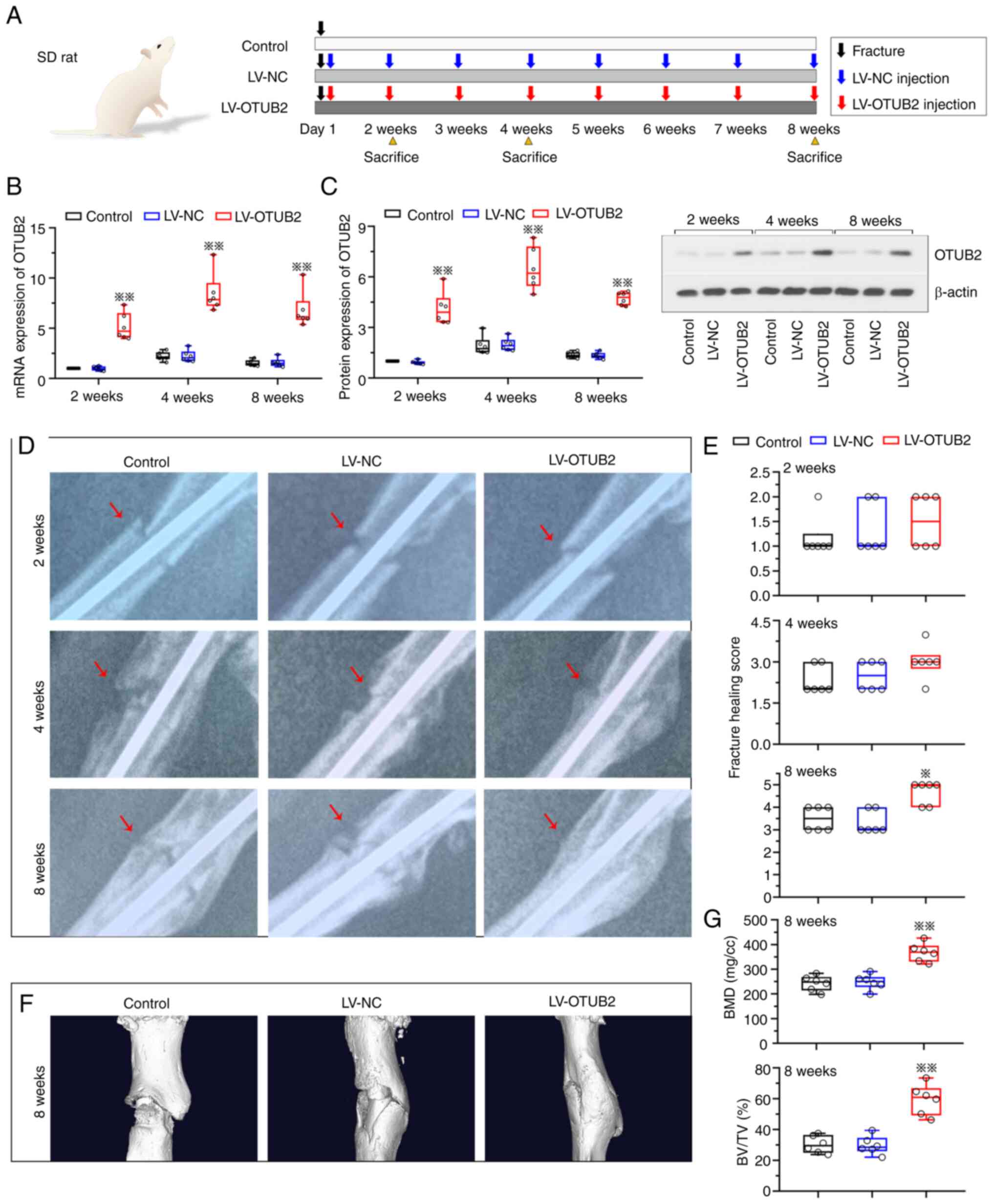 | Figure 1.OTUB2 enhances the bone fracture
healing. (A) Schematic diagram of the rat fracture model, which was
established by cutting the femur. The rats were further injected
with LV-NC or LV-OTUB2 around the fracture site. The image of SD
rat was from SciDraw platform (10.5281/zenodo.7368720). (B) mRNA
and (C) protein levels of OTUB2 in bony callus of mice at week 2, 4
and 8 post-fracture were evaluated by reverse
transcription-quantitative PCR and western blotting, respectively.
(D) Representative radiographs of femurs. (E) Fracture healing
score. (F) Micro-CT images of rat femora at week 8. (G) BMD and
BV/TV. Data shown as box- and whiskers plot, with the box depicting
the median and the 25 and 75th quartiles and the whisker revealing
the standard deviation in B, C and G. n=6; *P<0.05, **P<0.01
vs. LV-NC. OTUB2, otubain 2; LV, lentivirus vector; NC, negative
control; SD, Sprague Dawley; CT, computed tomography; BMD, bone
mineral density; BV/TV, bone volume fraction. |
OTUB2 regulates bone callus formation,
cartilaginous ossification and bone remodeling
The potential effects of OTUB2 on bone callus
formation and cartilaginous ossification were further investigated
in the present study. Results of the H&E staining analysis
demonstrated that OTUB2 overexpression was associated with improved
recovery at the fracture sites, with the formation of woven bone at
4- and 8-weeks post-fracture, compared with the LV-NC group
(Fig. 2A). In addition, OTUB2
overexpression promoted soft callus formation at 4- and 8-weeks
post-fracture. OTUB2 overexpression was associated with the
domination of woven bone at the fracture sites at 8 weeks
post-fracture and this was close to union (Fig. 2A). Results of the safranine O-fast
green staining analysis revealed that the cartilage area of rats in
the OTUB2 overexpression group was reduced at 4- and 8-weeks
post-fracture, compared with the LV-NC group (Fig. 2B). At week 8, a few calcified
cartilages and a limited number of uncalcified cartilages were
observed in the fracture calluses of rats in the OTUB2
overexpression group (Fig. 2B),
with ossification of the cartilage callus. Collectively, these data
suggested that OTUB2 may potentiate fracture callus formation and
cartilaginous ossification.
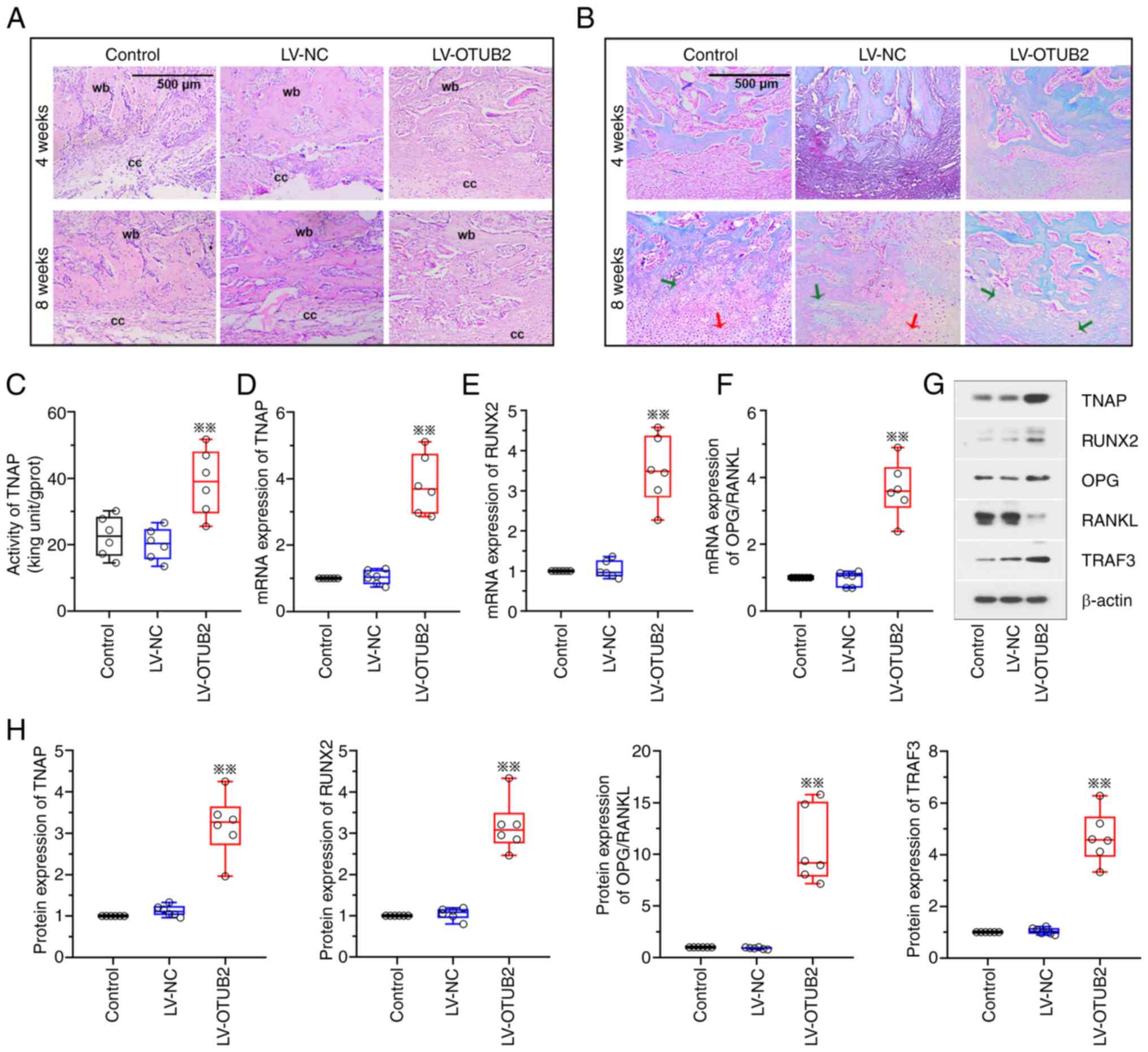 | Figure 2.OTUB2 regulates the bony callus
formation, cartilage calcification and bone remodeling. A rat
fracture model was established by cutting the femur and the rats
were further injected with LV-NC or LV-OTUB2 around the fracture
site. (A) H&E staining of the fracture callus sections. Scale
bar, 500 µm. (B) Safranine O-fast green staining of the fracture
callus sections. Scale bar, 500 µm. Red arrow indicates uncalcified
cartilage and green arrow indicates calcified cartilage. (C) TNAP
activities. (D-F) The mRNA expression of TNAP, RUNX2 and the ratio
between OPG and RANKL. (G and H) Protein levels of TNAP, RUNX2,
OPG, RANKL and TRAF3. Data was shown as box- and whiskers plot,
with the box depicting the median and the 25 and 75th quartiles and
the whisker revealing the standard deviation. n=6; **P<0.01 vs.
LV-NC. OTUB2, otubain 2; LV, lentivirus vector; NC, negative
control; wb, woven bone; cc, cartilage callus; TNAP, alkaline
phosphatase; RUNX2, runt related transcription factor 2; OPG
osteoprotegerin; RANKL, receptor activator of nuclear factor-kappa
B ligand; TRAF3, TNF-receptor associated factor 3. |
Bone formation and mineralization, cartilage
maturation and bone mass play vital roles in the bone remodeling of
fracture. Thus, genes and proteins associated with bone fracture
were investigated at 2 weeks post-fracture. Results of the present
study revealed that OTUB2 overexpression was associated with
increased TNAP activity (Fig. 2C).
In addition, OTUB2 overexpression was associated with increased
TNAP and RUNX2 mRNA expression levels and an increase in the ratio
between OPG and RANKL expression (Fig.
2D-F). Notably, results of the western blot analysis were
comparable with those obtained using RT-qPCR (Fig. 2G and H).
OTUB2 facilitates the osteogenic
differentiation and mineralization of BMSCs
Results of the present study revealed that OTUB2
exhibited protective effects on bone fracture healing in
vivo. Thus, it was hypothesized that OTUB2 may play a similar
role in vitro. In the present study, a model of osteogenic
differentiation was established using BMSCs (Fig. 3A). BMSCs were isolated from rats
and the phenotype was identified using flow cytometry with surface
antigens. Results of the present study exhibited positivity for
antigens CD29 and CD90 and negativity for antigens CD45 and CD31,
indicative of a high cell purity (Fig.
3B). Notably, OTUB2 expression was increased in BMSCs infected
with LV-OTUB2 (Fig. 3C). Results
of the Alizarin Red S staining analysis revealed that OTUB2
potentiated the mineralization of BMSCs, with an increased number
of calcified nodules that were bright red in color (Fig. 3D and E). In addition, OTUB2
overexpression was associated with increased TNAP expression levels
(Fig. 3F), increased RUNX2 and OPG
expression levels and reduced RANKL expression levels (Fig. 3G and H). Collectively, these
results suggested that OTUB2 may facilitate the osteogenic
differentiation and mineralization of BMSCs.
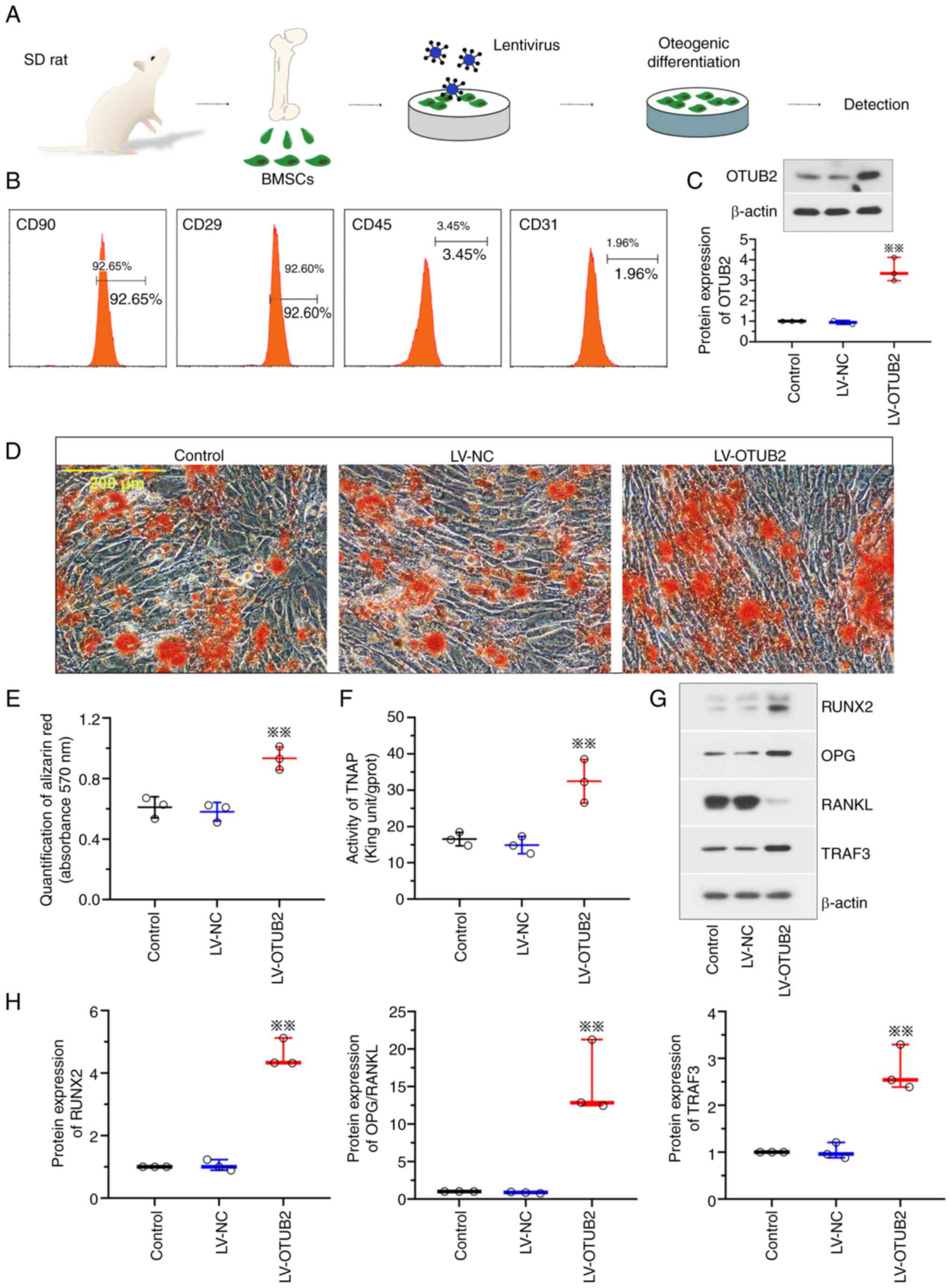 | Figure 3.OTUB2 facilitates the osteogenic
differentiation and bone mineralization. (A) A schematic diagram of
the model of osteogenic differentiation of BMSCs in vitro.
BMSCs were harvested from marrow cavity of rat femurs and tibiae
and then subjected to LV-OTUB2/LV-NC infection and osteogenic
differentiation. The image of SD rat was from SciDraw platform
(10.5281/zenodo.7368720). (B) BMSCs surface antigen identification
by flow cytometry. (C) Protein expression of OTUB2 in BMSCs was
detected by western blotting. (D) Alizarin Red S staining of BMSCs.
Scale bar, 200 µm. (E) Quantification of Alizarin Red S staining by
the measurement of absorbance at 570 nm. (F) TNAP activities of
BMSCs. (G and H) Protein levels of RUNX2, OPG, RANKL and TRAF3.
Data was shown as mean ± standard deviation. n=6; **P<0.01 vs.
LV-NC. OTUB2, otubain 2; BMSCs, bone marrow mesenchymal stem cells;
LV, lentivirus vector; NC, negative control; SD, Sprague Dawley;
TNAP, alkaline phosphatase; RUNX2, runt related transcription
factor 2; OPG osteoprotegerin; RANKL, receptor activator of nuclear
factor-kappa B ligand; TRAF3, TNF-receptor associated factor 3. |
OTUB2 deubiquitinates the TRAF3
protein
The present study aimed to determine the specific
mechanisms underlying the OTUB2-induced increases in osteogenesis
and mineralization. Notably, deubiquitinase OTUB2 interacts with
substrate proteins to remove covalently-attached ubiquitin, thereby
controlling substrate abundance. Thus, it was hypothesized that the
protective role of OTUB2 may be mediated by the stability of
downstream substrates and proteins that interacted with OTUB2 were
analyzed using the STRING database. As displayed in Fig. 4A, results of the present study
revealed 10 proteins that interacted with OTUB2. To further
investigate the specific functions of these proteins, GO enrichment
analysis was performed (Fig. 4B).
Results of the present study revealed that the OTUB2-interacting
proteins were enriched in ‘protein deubiquitination’,
‘ubiquitin-dependent protein catabolic process’ and ‘protein
modification by small protein conjugated or removal’. These
enriched terms provided direction for further investigation of the
mechanisms downstream of OTUB2 and TRAF3, a protein enriched in
‘regulation of protein polyubiquitination’ was selected for
subsequent analyses (Fig. 4C). As
a member of the TRAF family, TRAF3 plays a role in promoting bone
formation and remodeling (41).
Thus, it was hypothesized that the protective effects of OTUB2 in
fracture healing and the osteogenic differentiation of BMSCs may be
associated with TRAF3. In the present study, co-immunoprecipitation
analysis was used to verify the physical interaction between OTUB2
and TRAF3 in 293T cells (Fig. 4D and
E). Results of the protein molecular docking analysis revealed
a hydrogen bond between OTUB2 and the TRAF3 protein, indicative of
binding (Fig. 4F). In addition,
results of the present study revealed that OTUB2 reduced the
ubiquitination of TRAF3 (Fig. 4G).
Subsequently, HEK293 cells expressing inactive enzyme mutant OTUB2
C51S were generated and results of the present study revealed that
the OTUB2 mutation enhanced the ubiquitination of TRAF3 (Fig. 4H). These results highlighted that
OTUB2 may repress TRAF3 ubiquitination and this is dependent on the
deubiquitinase activity.
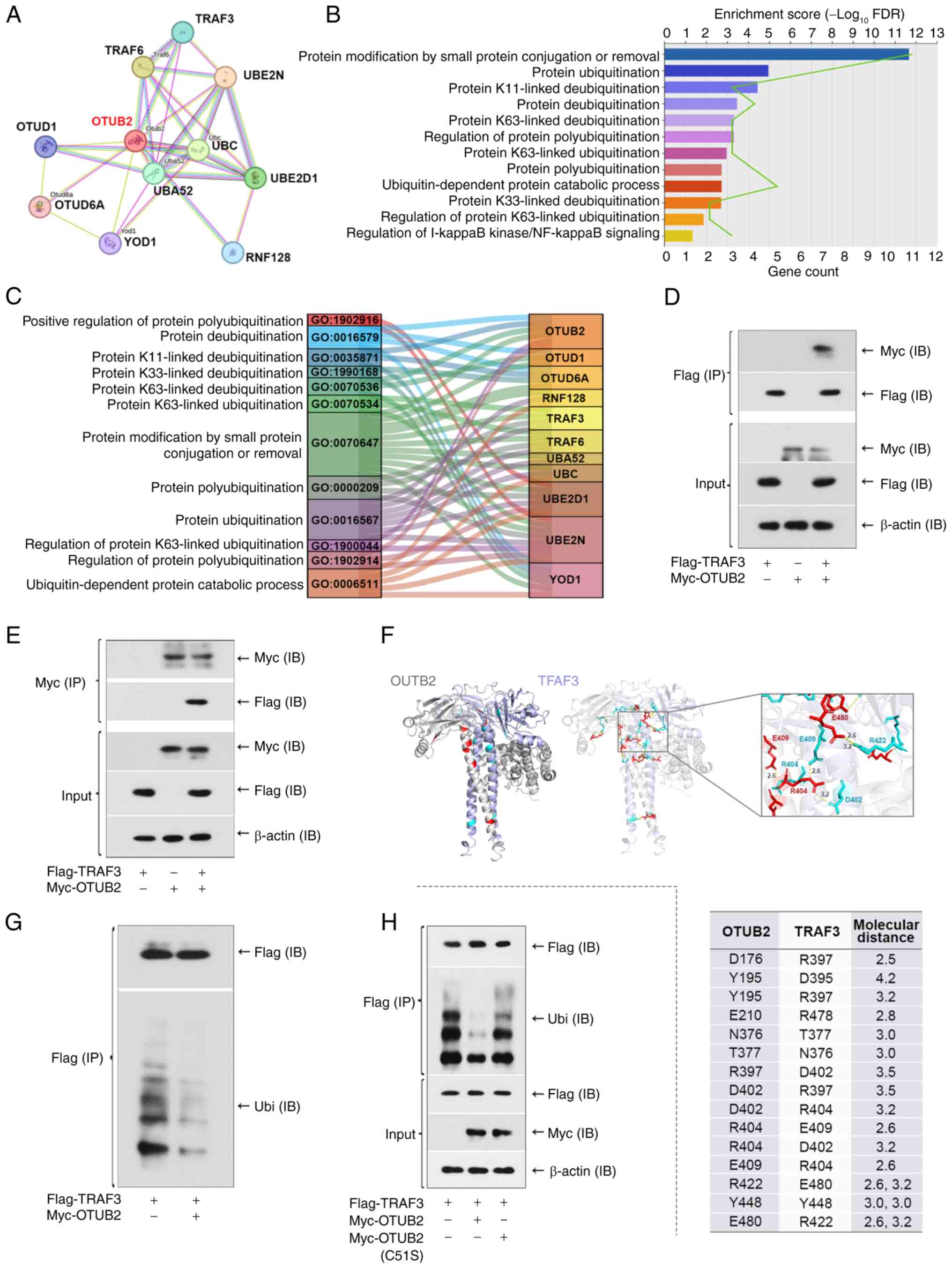 | Figure 4.OTUB2 deubiquitinates TRAF3 protein.
(A) Interacted proteins of OTUB2 were analyzed by STRING platform
(https://cn.string-db.org/). (B) GO
analysis of the OTUB2-inertacted proteins. (C) Sankey diagram of
the GO items with the related proteins. (D) Interaction of OTUB2
and TRAF3 was evaluated by Co-immunoprecipitation. 293T cells
expressing TRAF3-Flag, OTUB2-Myc or both were lysed. The protein
extract was immunoprecipitated with anti-Flag antibodies, followed
by western blotting using anti-Flag or anti-Myc antibodies. (E)
Protein extract was immunoprecipitated with anti-Myc antibodies,
followed by western blotting using anti-Flag or anti-Myc
antibodies. (F) Molecular docking model of OTUB2 and TRAF3. (G)
Anti-Flag immunoprecipitation was subjected to western blotting
with anti-Ubi or anti-Flag antibodies, showing the deubiquitination
of TRAF3 by OTUB2. (H) 293T cells expressing TRAF3-Flag,
OTUB2-Myc/OTUB2-Myc (C51S) or both were lysed. Protein extract was
immunoprecipitated with anti-Flag antibodies, followed by western
blotting using anti-Flag, anti-Ubi or anti-Myc antibodies. n=3.
OTUB2, otubain 2; TRAF3, TNF-receptor associated factor 3; GO, Gene
Ontology; Ubi, ubiquitin. |
TRAF3 knockdown represses the
osteogenic differentiation and mineralization of BMSCs
As displayed in Fig.
2G and 3G, TRAF3 protein
expression levels were increased following OTUB2 overexpression
in vivo and in vitro. As a downstream factor of
OTUB2, the role of TRAF3 attracted our interest. It was found that
TNAP activity was reduced following TRAF3 knockdown (Fig. 5A) and the mineralization of BMSCs
was markedly repressed (Fig. 5B and
C). Results of the present study also revealed that TRAF3
knockdown reduced the expression levels of RUNX2 and OPG and
promoted RANKL expression (Fig. 5D and
E). Collectively, these results demonstrated that TRAF3
knockdown may reduce the osteogenic differentiation of BMSCs.
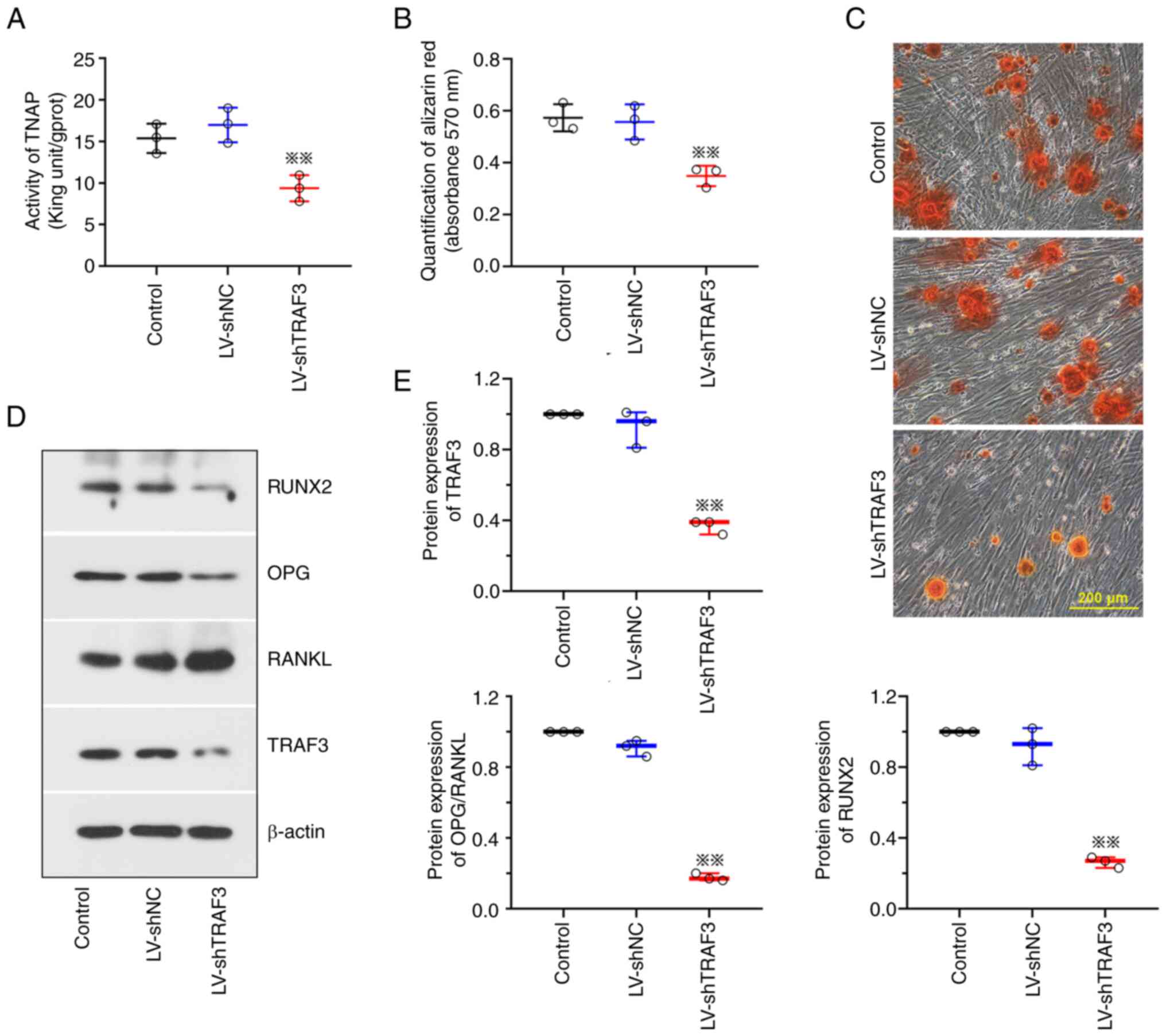 | Figure 5.Downregulation of TRAF3 repressed the
osteogenesis of BMSCs. BMSCs were harvested from marrow cavity of
rat femurs and tibiae and then subjected to LV-shTRAF3/LV-shNC
infection and osteogenic differentiation. (A) TNAP activities of
BMSCs. (B) Quantification of the Alizarin Red S staining by the
measurement of absorbance at 570 nm. (C) Alizarin Red S staining of
BMSCs. Scale bar, 200 µm. (D and E) Protein levels of RUNX2, OPG,
RANKL and TRAF3. Data was shown as mean ± standard deviation. n=3.
**P<0.01 vs. LV-shNC. TRAF3, TNF-receptor associated factor 3;
BMSCs, bone marrow mesenchymal stem cells; LV, lentivirus vector;
sh, short hairpin; NC, negative control; TNAP, alkaline
phosphatase; RUNX2, runt related transcription factor 2; OPG
osteoprotegerin; RANKL, receptor activator of nuclear factor-kappa
B ligand. |
OTUB2 promotes the osteogenic
differentiation and mineralization of BMSCs through increased TRAF3
expression
In the present study, TRAF3 expression was reduced
following transfection with LV-shTRAF3 (Fig. 6A). Results of the present study
revealed that TNAP expression levels were reduced and the
mineralization of BMSCs with OTUB2 overexpression was repressed
following TRAF3 downregulation (Fig.
6B-D). Collectively, these results demonstrated that OTUB2 may
promote the osteogenic differentiation and mineralization of BMSCs
through upregulation of TRAF3 (Fig.
6E).
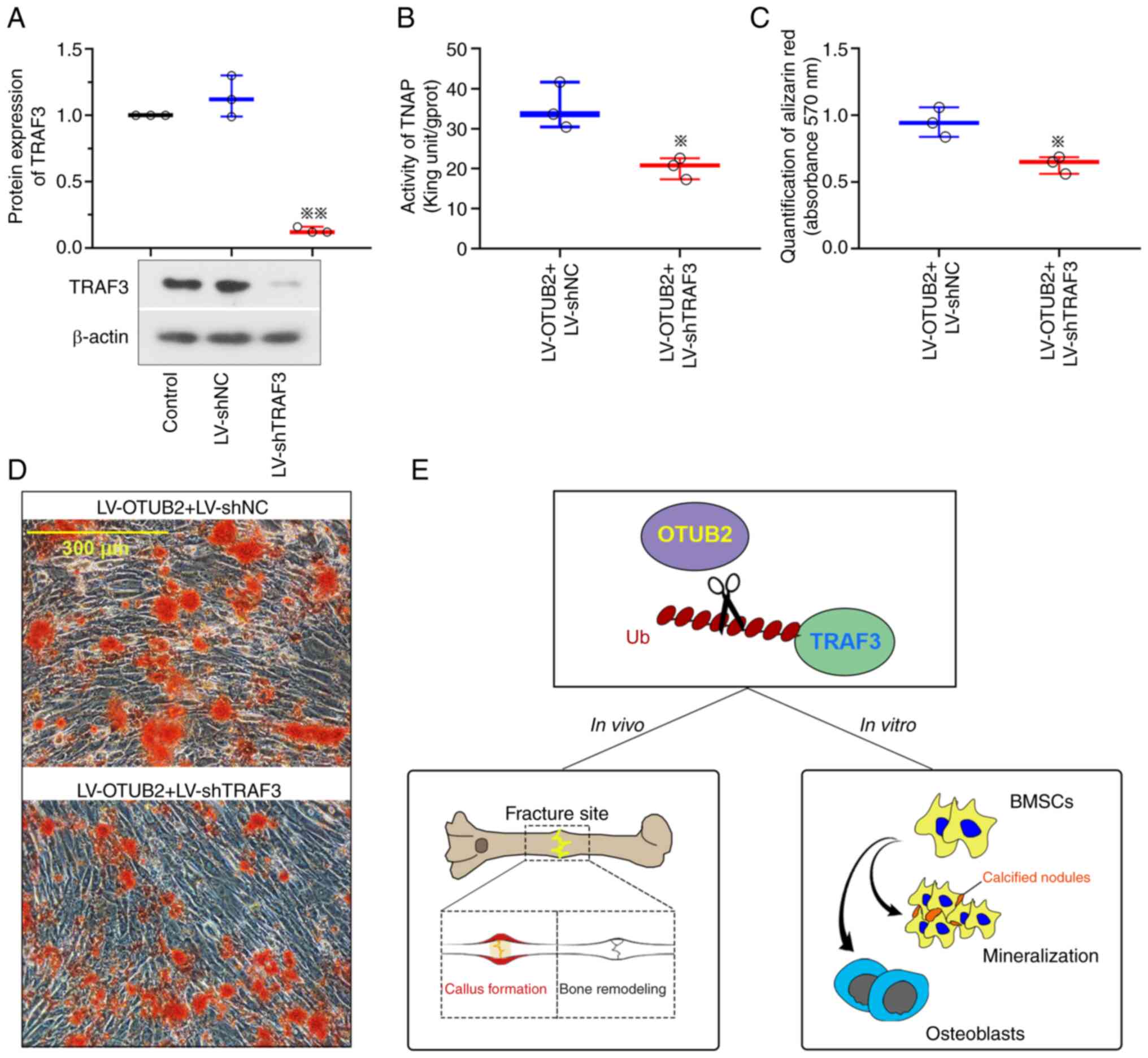 | Figure 6.OTUB2 promoted osteogenesis of BMSCs
by the deubiquitination of TRAF3 protein. (A) Protein levels of
TRAF3 in BMSCs, which were infected with LV-shTRAF3 and subjected
to osteogenic differentiation. (B) Activities of TNAP in BMSCs
co-infected with LV-OTUB2 and LV-shTRAF3. (C) Quantification of the
Alizarin Red S staining by the measurement of absorbance at 570 nm.
(D) Alizarin Red S staining of BMSCs. Scale bar, 300 µm. (E)
Schematic diagram of the potential underlying mechanism of OTUB2 on
promoting bone fracture healing. Data was shown as mean ± standard
deviation. n=3; *P<0.05, **P<0.01 vs. LV-shNC or LV-OTUB2 +
LV-shNC. OTUB2, otubain 2; BMSCs, bone marrow mesenchymal stem
cells; TRAF3, TNF-receptor associated factor 3; LV, lentivirus
vector; sh, short hairpin; TNAP, alkaline phosphatase; NC, negative
control; Ub, ubiquitin. |
Discussion
At present, the increasing incidence of traumatic
fracture markedly affects the quality of life of patients. Notably,
elderly patients who have experienced a fracture exhibit other
comorbidities, including pulmonary embolism, infection and heart
failure, which may lead to an increased risk of mortality (42). Results of the present study
highlighted that further investigations are required to determine
the specific mechanisms underlying fracture. To the to the best of
the authors' knowledge, the present study was the first to
demonstrate the protective effects of OTUB2 in bone fracture
healing. Notably, OTUB2 may serve a role in facilitating bone
callus formation, cartilaginous ossification and bone remodeling,
both in vitro and in vivo. Results of the present
study revealed that OTUB2 facilitated the osteogenic
differentiation and mineralization of BMSCs and this was mediated
by the deubiquitination of the TRAF3 protein.
Bone formation and mineralization, cartilage
maturation and bone mass serve a vital role in bone remodeling of
fracture, thus, genes and proteins associated with bone fracture
were investigated. Notably, OPG and RANKL are expressed by
osteoblasts and RANKL regulates osteoclastogenesis through binding
to RANK secreted by osteoclasts (43). On the other hand, OPG is a decoy
receptor of RANKL that protects cells from osteoclast formation
(43). Thus, the ratio between
OPG/RANKL is crucial in determining bone mass. Results of the
present study revealed that OTUB2 enhanced the OPG/RANKL ratio both
in vivo and in vitro, leading to an increase in bone
mass and ossification.
Fracture healing is a complex and long-term process
involving callus formation and multiple dynamic stages. The initial
stage involves a hematoma, which generates an inflammatory
environment. In addition, middle-to-late-stage fracture healing
involves endochondral ossification and removal and calcification of
the endochondral cartilage. The final stage of bone fracture
healing involves chronic remodeling (9). Results of the pathological analysis
demonstrated that a few calcified cartilages and minimal levels of
uncalcified cartilage existed in the fracture calluses of rats with
OTUB2 overexpression at 8 weeks post-fracture. Notably, the
cartilage callus had undergone ossification and woven bone had
formed. Comparable results were obtained using in vitro
experiments. In addition, bone formation depends on the amount and
activity of osteoblasts during bone remodeling, which are
differentiated from osteoprogenitor cells and BMSCs. The osteogenic
differentiation of BMSCs involves pre-osteoblasts, osteoblasts,
mature osteoblasts and the deposition and mineralization of the
extracellular matrix (44).
Several factors have been found to regulate BMSC osteogenic
differentiation. Of these, RUNX2 serves an essential role. The
onset of osteogenic differentiation is characterized by the
increased expression of the RUNX2 protein (45,46).
Results of a previous study reveal that RUNX2 induces TNAP activity
(45), which promotes bone
mineralization (47). Notably, the
loss of OTUB2 may inhibit TNAP activity and reduce RUNX2 expression
during the osteogenesis of BMSCs (18). These results are comparable with
those obtained in the present study. The present study found that
OTUB2 may promote the osteogenic differentiation of BMSCs via the
facilitation of TNAP activity and upregulation of RUNX2
expression.
Results of the present study revealed that OTUB2
overexpression accelerates fracture healing in vivo and
promotes the osteogenic differentiation of BMSCs in vitro.
As a downstream protein of OTUB2, it is possible that TRAF3 may
exert a similar role in bone repair and formation. As a member of
the TRAF family, TRAF3 serves a crucial role in the development of
an immune response (48,49). Results of previous studies revealed
that TRAF3 may promote bone formation and bone remodeling and
reduce bone destruction (41,50).
In addition, Yao et al (51) reveal that TRAF3 knockdown in
myeloid cells inhibits bone formation in a mouse osteoporosis
model. A previous study demonstrates that TRAF3 knockdown in
mesenchymal progenitor cells leads to the early onset of
osteoporosis in mice, due to decreased bone formation and enhanced
bone destruction. Collectively, these results demonstrate that
TRAF3 positively regulates the differentiation of mesenchymal
progenitor cells into osteoblasts and promotes osteogenesis
(49). In addition, TRAF3
overexpression facilitates the osteogenic differentiation and
suppresses the adipocytic differentiation of rat BMSCs (50). Results of previous studies also
demonstrate that increased TRAF3 expression mediates the inhibition
of osteoclastogenesis (52–54).
Collectively, these results highlight the therapeutic potential of
TRAF3 in bone-regulated diseases. Results of the present study
revealed that downregulation of TRAF3 repressed the osteogenic
differentiation and mineralization of BMSCs, which are the key
process in bone healing. Based on results of the OTUB2-induced
deubiquitination of TRAF3, it was hypothesized that the protective
role of OTUB2 was at least partly mediated by the deubiquitination
and accumulation of TRAF3. It is possible that TRAF3 might also act
as a part in bone fracture healing. Lack of verifying experiments
is a limitation of the present study.
Results of the co-immunoprecipitation analysis
demonstrated that OTUB2 interacted with TRAF3. Deubiquitinases
possess ubiquitin-binding sites that guide the ubiquitin C terminus
and the scissile bond into the active site for hydrolysis (55). Thus, it was hypothesized that the
interaction between TRAF3 and OTUB2 may be associated with the
deubiquitination of TRAF3. Results of the present study revealed
that OTUB2 overexpression reduced the ubiquitination of TRAF3. To
further determine whether OTUB2 directly deubiquitinates TRAF3,
HEK293 cells expressing inactive enzyme mutant OTUB2 C51S were
generated. Results of the co-immunoprecipitation analysis revealed
that the OTUB2 mutation reversed the OTUB2 wild-type mediated
deubiquitination of TRAF3, indicating that OTUB2 repressed TRAF3
ubiquitination and this was dependent on its deubiquitinase
activity. Thus, results of the present study revealed that OTUB2
may induce the deubiquitination of TRAF3, leading to the
accumulation of TRAF3 in cells. Notably, these results are
comparable with those of a previous study (56). In addition, results of the present
study revealed that TRAF3 knockdown inhibited OTUB2-mediated
osteogenic differentiation. Thus, OTUB2-mediated TRAF3
deubiquitination may serve a vital role in the process of bone
healing.
In conclusion, results of the present study revealed
that OTUB2 may promote bone fracture healing through the
deubiquitination of TRAF3. Thus, OTUB2 may exhibit potential as a
novel therapeutic target in the treatment of fracture and the use
of OTUB2 in clinical practice may improve patient outcomes.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Key R&D Program of the
China Ministry of Science and Technology (grant no. 2024YFC2510600)
and Natural Science Foundation of Hebei Province (grant no.
H2022206432).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LZ, JG, SF, YuZ, HW, HM, WC, YiZ and ZH conceived
and designed the research. LZ performed experiments, wrote the
manuscript and obtained funding. JG and SF performed experiments
and bioinformatics analysis. YuZ and HW performed data acquisition,
analysis and interpretation. HM, WC and YiZ conducted statistical
analysis and provided substantial intellectual input during the
drafting and revision of the manuscript. ZH oversaw the research
program, obtained funding and reviewed the manuscript. LZ and ZH
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by Laboratory
Animal Ethical and Welfare Committee of Hebei Medical University
(approval no. IACUC-Hebmu-2021007) following The Guideline for the
Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BMSCs
|
bone marrow mesenchymal stem cells
|
|
OTUB2
|
otubain 2
|
|
OPG
|
osteoprotegerin
|
|
RANKL
|
receptor activator of nuclear
factor-kappa B ligand
|
|
RUNX2
|
runt related transcription factor
2
|
|
TNAP
|
tissue-nonspecific alkaline
phosphatase
|
|
TRAF3
|
TNF-receptor associated factor 3
|
References
|
1
|
Marsell R and Einhorn TA: The biology of
fracture healing. Injury. 42:551–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh V: Medicinal plants and bone
healing. Natl J Maxillofac Surg. 8:4–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quirk BJ, Sannagowdara K, Buchmann EV,
Jensen ES, Gregg DC and Whelan HT: Effect of near-infrared light on
in vitro cellular ATP production of osteoblasts and fibroblasts and
on fracture healing with intramedullary fixation. J Clin Orthop
Trauma. 7:234–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng TL, Schindeler A and Little DG:
BMP-2 delivered via sucrose acetate isobutyrate (SAIB) improves
bone repair in a rat open fracture model. J Orthop Res.
34:1168–1176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Praemer A, Furner SE and Rice DP:
Musculoskeletal conditions in the United States. Am Acad Orthop
Surg. 22:1–199. 1976.
|
|
6
|
Antapur P, Mahomed N and Gandhi R:
Fractures in the elderly: When is hip replacement a necessity? Clin
Interv Aging. 6:1–7. 2011.PubMed/NCBI
|
|
7
|
Giannoudis PV, Einhorn TA and Marsh D:
Fracture healing: The diamond concept. Injury. 38 (Suppl 4):S3–S6.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Jin L, Guo J, Bao K, Hu J, Zhang
Y, Hou Z and Zhang L: Chronic intermittent hypobaric hypoxia
enhances bone fracture healing. Front Endocrinol (Lausanne).
11:5826702021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saul D and Khosla S: Fracture healing in
the setting of endocrine diseases, aging and cellular senescence.
Endocr Rev. 43:984–1002. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park J, Cho J and Song EJ:
Ubiquitin-proteasome system (UPS) as a target for anticancer
treatment. Arch Pharm Res. 43:1144–1161. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li M, Chen D, Shiloh A, Luo J, Nikolaev
AY, Qin J and Gu W: Deubiquitination of p53 by HAUSP is an
important pathway for p53 stabilization. Nature. 416:648–653. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ouyang S, Zeng Z, Liu Z, Zhang Z, Sun J,
Wang X, Ma M, Ye X, Yu J and Kang W: OTUB2 regulates KRT80
stability via deubiquitination and promotes tumour proliferation in
gastric cancer. Cell Death Discov. 8:452022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu Q, Fu Y, Li L, Liu CH and Zhang L: The
functions and regulation of Otubains in protein homeostasis and
diseases. Ageing Res Rev. 67:1013032021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stanišić V, Malovannaya A, Qin J, Lonard
DM and O'Malley BW: OTU Domain-containing ubiquitin
aldehyde-binding protein 1 (OTUB1) deubiquitinates estrogen
receptor (ER) alpha and affects ERalpha transcriptional activity. J
Biol Chem. 284:16135–16145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Almeida M, Laurent MR, Dubois V, Claessens
F, O'Brien CA, Bouillon R, Vanderschueren D and Manolagas SC:
Estrogens and androgens in skeletal physiology and pathophysiology.
Physiol Rev. 97:135–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JM, Lin C, Stavre Z, Greenblatt MB and
Shim JH: Osteoblast-osteoclast communication and bone homeostasis.
Cells. 9:20732020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jähn K, Saito H, Taipaleenmäki H, Gasser
A, Hort N, Feyerabend F, Schlüter H, Rueger JM, Lehmann W,
Willumeit-Römer R and Hesse E: Intramedullary Mg2Ag nails augment
callus formation during fracture healing in mice. Acta Biomater.
36:350–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li XY, Mao XF, Tang XQ, Han QQ, Jiang LX,
Qiu YM, Dai J and Wang YX: Regulation of Gli2 stability by
deubiquitinase OTUB2. Biochem Biophys Res Commun. 505:113–118.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, Zhang J, Li Z and Jia G: Bone
marrow mesenchymal stem cell-derived exosomal miR-25 regulates the
ubiquitination and degradation of Runx2 by SMURF1 to promote
fracture healing in mice. Front Med (Lausanne). 7:5775782020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang F, Guo J, Wang Y, Hu Y, Zhang H, Chen
J, Jing Y, Cao L, Chen X and Su J: Loss of Bcl-3 delays bone
fracture healing through activating NF-κB signaling in mesenchymal
stem cells. J Orthop Translat. 35:72–80. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arthur A and Gronthos S: Clinical
application of bone marrow mesenchymal stem/stromal cells to repair
skeletal tissue. Int J Mol Sci. 21:97592020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Wang C, Gou W, Xu X, Wang Y, Wang
A, Xu W, Guo Q, Liu S, Lu Q, et al: The optimal time to inject bone
mesenchymal stem cells for fracture healing in a murine model. Stem
Cell Res Ther. 9:2722018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Shi K, Ditzel N, Qiu W, Figeac F,
Nielsen LHD, Tencerova M, Kowal JM, Ding M, Andreasen CM, et al:
KIAA1199 deficiency enhances skeletal stem cell differentiation to
osteoblasts and promotes bone regeneration. Nat Commun.
14:20162023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jing Z, Qiong Z, Yonggang W and Yanping L:
Rat bone marrow mesenchymal stem cells improve regeneration of thin
endometrium in rat. Fertil Steril. 101:587–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Research Counci: Committee for
the Update of the Guide for the Care and Use of Laboratory Animals,
. Guide for the Care and Use of Laboratory Animals. 8th. National
Academies Press; Washington, DC: 2011
|
|
26
|
Song JL, Zheng W, Chen W, Qian Y, Ouyang
YM and Fan CY: Lentivirus-mediated microRNA-124 gene-modified bone
marrow mesenchymal stem cell transplantation promotes the repair of
spinal cord injury in rats. Exp Mol Med. 49:e3322017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chrastil J, Sampson C, Jones KB and
Higgins TF: Postoperative opioid administration inhibits bone
healing in an animal model. Clin Orthop Relat Res. 471:4076–4081.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kelly LS, Munley JA, Pons EE, Kannan KB,
Whitley EM, Bible LE, Efron PA and Mohr AM: A rat model of
multicompartmental traumatic injury and hemorrhagic shock induces
bone marrow dysfunction and profound anemia. Animal Model Exp Med.
7:367–376. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Munley JA, Kelly LS, Park G, Gillies GS,
Pons EE, Kannan KB, Whitley EM, Bible LE, Efron PA, Nagpal R and
Mohr AM: Multicompartmental traumatic injury induces sex-specific
alterations in the gut microbiome. J Trauma Acute Care Surg.
95:30–38. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang W, Ding P, Li G, Lu E and Zhao Z:
Hydroxyapatite nanoparticles facilitate osteoblast differentiation
and bone formation within sagittal suture during expansion in rats.
Drug Des Devel Ther. 15:905–917. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tseng JC, Meganck J, Peterson JD and
Hopkinton M: Quantum GX microCT Imaging System: Features and
Performance. PerkinElmer; Hopkington, MA: 2015
|
|
33
|
Ishikawa M, Ito H, Kitaori T, Murata K,
Shibuya H, Furu M, Yoshitomi H, Fujii T, Yamamoto K and Matsuda S:
MCP/CCR2 signaling is essential for recruitment of mesenchymal
progenitor cells during the early phase of fracture healing. PLoS
One. 9:e1049542014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferrin I, Beloqui I, Zabaleta L, Salcedo
JM, Trigueros C and Martin AG: Isolation, culture, and expansion of
mesenchymal stem cells. Methods Mol Biol. 1590:177–190. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Y, Xu L, Huang S, Hou Y, Liu Y, Chan
KM, Pan XH and Li G: mir-21 overexpressing mesenchymal stem cells
accelerate fracture healing in a rat closed femur fracture model.
Biomed Res Int. 2015:4123272015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Y, Xu J, Xu L, Zhang J, Chan K, Pan X
and Li G: MiR-503 promotes bone formation in distraction
osteogenesis through suppressing Smurf1 expression. Sci Rep.
7:4092017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Samuel S, Ahmad RE, Ramasamy TS,
Karunanithi P, Naveen SV, Murali MR, Abbas AA and Kamarul T:
Platelet-rich concentrate in serum free medium enhances osteogenic
differentiation of bone marrow-derived human mesenchymal stromal
cells. PeerJ. 4:e23472016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Faul F, Erdfelder E, Lang AG and Buchner
A: G*Power 3: A flexible statistical power analysis program for the
social, behavioral, and biomedical sciences. Behav Res Methods.
39:175–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De Winter JCF: Using the Student's t-test
with extremely small sample sizes. Pract Assess Res Eval.
18:102013.
|
|
40
|
Sullivan GM and Feinn R: Using effect
size-or why the P-value is not enough. J Grad Med Educ. 4:279–282.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Ayoub A, Xiu Y, Yin X, Sanders JO,
Mesfin A, Xing L, Yao Z and Boyce BF: TGFβ-induced degradation of
TRAF3 in mesenchymal progenitor cells causes age-related
osteoporosis. Nat Commun. 10:27952019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hashimoto K, Shinyashiki Y, Ohtani K,
Kakinoki R and Akagi M: How proximal femur fracture patients aged
65 and older fare in survival and cause of death 5+ years after
surgery: A long-term follow-up. Medicine (Baltimore).
102:e338632023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Boyce BF and Xing L: The RANKL/RANK/OPG
pathway. Curr Osteoporos Rep. 5:98–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma C, Gao J, Liang J, Dai W, Wang Z, Xia
M, Chen T, Huang S, Na J, Xu L, et al: HDAC6 inactivates Runx2
promoter to block osteogenesis of bone marrow stromal cells in
age-related bone loss of mice. Stem Cell Res Ther. 12:4842021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fujita T, Azuma Y, Fukuyama R, Hattori Y,
Yoshida C, Koida M, Ogita K and Komori T: Runx2 induces osteoblast
and chondrocyte differentiation and enhances their migration by
coupling with PI3K-Akt signaling. J Cell Biol. 166:85–95. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: A transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haarhaus M, Cianciolo G, Barbuto S, La
Manna G, Gasperoni L, Tripepi G, Plebani M, Fusaro M and Magnusson
P: Alkaline phosphatase: An old friend as treatment target for
cardiovascular and mineral bone disorders in chronic kidney
disease. Nutrients. 14:21242022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chang JH, Hu H, Jin J, Puebla-Osorio N,
Xiao Y, Gilbert BE, Brink R, Ullrich SE and Sun SC: TRAF3 regulates
the effector function of regulatory T cells and humoral immune
responses. J Exp Med. 211:137–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yi Z, Lin WW, Stunz LL and Bishop GA:
Roles for TNF-receptor associated factor 3 (TRAF3) in lymphocyte
functions. Cytokine Growth Factor Rev. 25:147–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang D, Cai G, Wang H and He J: TRAF3, a
target of MicroRNA-363-3p, suppresses senescence and regulates the
balance between osteoblastic and adipocytic differentiation of rat
bone marrow-derived mesenchymal stem cells. Stem Cells Dev.
29:737–745. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yao Z, Ayoub A, Srinivasan V, Wu J, Tang
C, Duan R, Milosavljevic A, Xing L, Ebetino FH, Frontier AJ and
Boyce BF: Hydroxychloroquine and a low antiresorptive activity
bisphosphonate conjugate prevent and reverse ovariectomy-induced
bone loss in mice through dual antiresorptive and anabolic effects.
Bone Res. 12:522024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yao Z, Lei W, Duan R, Li Y, Luo L and
Boyce BF: RANKL cytokine enhances TNF-induced osteoclastogenesis
independently of TNF receptor associated factor (TRAF) 6 by
degrading TRAF3 in osteoclast precursors. J Biol Chem.
292:10169–10179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Boyce BF, Li J, Xing L and Yao Z: Bone
remodeling and the role of TRAF3 in osteoclastic bone resorption.
Front Immunol. 9:22632018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xiu Y, Xu H, Zhao C, Li J, Morita Y, Yao
Z, Xing L and Boyce BF: Chloroquine reduces osteoclastogenesis in
murine osteoporosis by preventing TRAF3 degradation. J Clin Invest.
124:297–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mevissen TET and Komander D: Mechanisms of
deubiquitinase specificity and regulation. Annu Rev Biochem.
86:159–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li S, Zheng H, Mao AP, Zhong B, Li Y, Liu
Y, Gao Y, Ran Y, Tien P and Shu HB: Regulation of virus-triggered
signaling by OTUB1- and OTUB2-mediated deubiquitination of TRAF3
and TRAF6. J Biol Chem. 285:4291–4297. 2010. View Article : Google Scholar : PubMed/NCBI
|




















