Introduction
Non-alcoholic fatty liver disease (NAFLD) has
reached alarming proportions, affecting ~40% of the world
population (1,2). NAFLD is currently the main cause of
cirrhosis, hepatocellular carcinoma (HCC) and liver transplantation
in the western world (3,4). Therefore, understanding the
pathogenesis of NAFLD is crucial to establish an improved
prevention and management of the disease.
An excess of fat accumulation in the liver
characterizes NAFLD. The disease progresses through different
stages, beginning with simple steatosis, with the accumulation of
lipid droplets within hepatocytes. This may evolve to non-alcoholic
steatohepatitis (NASH), a more severe stage marked by inflammation,
that might lead to fibrosis and cirrhosis (3,4).
Between 2–5% of the cases with steatosis may develop NASH, fibrosis
and chronic liver disease (2,4). The
mechanisms behind the progression from simple steatosis to NASH
remain unclear.
The risk factors for NAFLD and NASH include obesity,
insulin resistance, diabetes and dyslipidemia, among others. The
excess of lipid accumulation and dysregulation of lipid metabolism
may unfold lipotoxic pathways (5).
In this context, the formation of lipid droplets may serve as a
protective mechanism by sequestering lipids and preventing
lipotoxicity (6–8). Triacylglycerides (TAG) and
cholesterol esters (CE) form the neutral core of lipid droplets.
Conversely, charged intermediaries such as free fatty acids (FFA),
diacylglycerols (DAG) and free cholesterol (Chol) are not able to
enter the neutral core of lipid droplets. Thus, to be included into
the droplets they must first be incorporated into TAG and CE
(9–11). The inclusion of these lipid
intermediaries in lipid droplets avoids its toxic effects such as
inflammation and disruption of membrane dynamics. For instance, FFA
can activate the Toll-like 4 (TLR4) receptors (12) and DAG can promote the activity of
protein kinase C (PKC) (13). In
the same manner, high levels of Chol may alter the fluidity of cell
membranes (14,15), promoting endoplasmic reticulum (ER)
stress and inflammation (16,17).
Therefore, the esterification of Chol to form CE is also essential
to avoid lipotoxicity (18).
The analysis of sex differences is crucial for
understanding NAFLD and NASH. Whereas the prevalence of NAFLD is
markedly higher in men than in women, the disease becomes more
frequent in women following menopause. This is probably due to the
higher prevalence of obesity and metabolic syndrome (MS) at this
stage of life (19–21). Although the mechanisms of this sex
difference remain unknown, the lack of estrogen may play an
essential role in the increased incidence of NASH and NAFLD after
menopause (22).
The present study aimed to evaluate the role of
menopause in the pathogenesis of liver damage in the context of
obesity and insulin resistance. Therefore, the present study used a
well-established murine model of obesity, including males and
females, where a subgroup was ovariectomized to mimic
menopause.
Materials and methods
Animal model
The present study utilized a phenotypic mouse model
of obesity and insulin resistance, described in our previous
publication (21). A total of 79
C57BL/6J mice (57 females and 22 males; age, ~6 weeks; weight,
16–20 g), were acquired from Charles River Laboratories, Inc. These
mice were then randomized to receive either a standard diet (SD) or
a high fat diet (HFD) for six months. For males, 10 animals were
assigned to the SD group and 12 to the HFD group. For females, 25
animals were assigned to the SD group (8 of which were
ovariectomized: SD-OVX) and 32 to the HFD group (12 of which were
ovariectomized: HFD-OVX). Animals were housed in cages at a
constant temperature of 22°C under a 12-h light/dark cycle and 50%
relative humidity in the animal facility at the University of La
Laguna (Tenerife, Spain). Ovariectomies were performed at 2 months
of age, after diet-randomization. During the follow-up period,
animals underwent intraperitoneal glucose tolerance test and
insulin tolerance test during the follow-up period (21). The sample size was determined based
on previous studies (21,23) using similar models. To effectively
implement the 3Rs principles without compromising statistical
power, 8–12 mice were initially included per group to mitigate
potential losses during follow-up. Since female mice underwent a
surgical procedure, more female mice were included at baseline than
male to prevent a reduction in number due to surgical or
post-surgical complications. All animals were euthanized by an
overdose of sodium pentobarbital (100 mg/kg) and death was
confirmed by cervical dislocation method. Animal care was performed
in accordance with institutional guidelines in compliance with
Spanish (Real Decreto 53/2013, February 1. BOE, February 8, 2013,
n: 34, p. 11370–11421) and international laws and policies
(Directive 2010/63/EU of the European Parliament and of the Council
of 22 September 2010 on the protection of animals used for
scientific purposes). All procedures were approved by the
Institutional Animal Care and Use Committee (Comité de Ética de la
Investigación y de Bienestar Animal) of University of La Laguna
(approval number CEIBA2021-3107).
Diet composition
The HFD provides 60% of the calories from fat (cat.
no. D12492; Research Diets Inc.). This HFD diet had 6-times more
total lipid content than the SD. Furthermore, the fatty acid (FA)
content was 7-fold higher in the HFD compared with SD (Table SI). The details of the diets have
been described in a previous article (23).
Liver histology
Slides from liver tissue in paraffin were stained
with hematoxylin-eosin and Sirius Red to evaluate steatosis,
ballooning, inflammation and fibrosis. A pathologist specialized in
liver diseases (SGH) evaluated all samples following the ‘SAF
index’ published by Bedossa in 2012 (24).
Steatosis
The percentage of hepatocytes with lipid vacuoles
was classified as S0 (minimal or non-existent): <5%; S1 (mild):
5–33%; S2 (moderate): 34–65%; and S3 (intense): >65%.
Lobular necroinflammatory activity and
ballooning
Lobular inflammation was classified in 3 grades: I0,
minimal lobular necroinflammation; I1, two lobular inflammatory
foci in one field; I2, globular activity with multiple clusters in
one field. Lobular inflammation was detected under a light
microscope (magnification, ×200; 20× objective). The pathologist
examined a fraction of the liver and chose a field representing the
sample. Hepatocyte ballooning was classified in 3 grades; B0: no
ballooning, B1 when the hepatocytes had rounded edges while
maintaining their size and B2 when the size of the cell is twice
the size of a normal hepatocyte.
Liver fibrosis
Fibrosis in liver tissue was determined using
Picrosirius Red staining. Liver sections (3 µm) were deparaffinized
in xylene and rehydrated through a graded series of alcohol
concentrations (100, 90 and 70%). The sections were covered in
Picrosirius Red solution and incubated at room temperature for 60
min. Following incubation, the slides were washed with 0.5% acetic
acid solution and 100% ethanol. Finally, tissues were dehydrated
and mounted. The slides were observed, and images were captured
under a digital light microscope (magnification, ×400; 40×
objective). The area of positively red-stained collagen was
quantified using ImageJ version 1.52k software (National Institutes
of Health).
Serum analysis
Serum TAG and Chol were measured using an enzymatic
colorimetric test. Hepatic aspartate (AST; cat. no. ACN 8687; Roche
Diagnostics) and alanine aminotransferase (AST; cat. no. ACN 8685;
Roche Diagnostics) transaminases were measured by
spectrophotometric analysis. All analyzes were performed in a Cobas
c711 Module (Roche Diagnostics).
Inflammation markers in liver
tissue
SDS-PAGE and western blotting of liver proteins were
performed as follows: Liver homogenates were prepared with RIPA
buffer and disaggregated using the System Polytron PT 1200 E
(Kinematica AG), followed by sonication for 3 min at 80% amplitude
with a cycle of 15 sec on/off at a frequency of 20 kHz and 4°C
using a Qsonica Q800R3 sonicator (Thermo Fisher Scientific, Inc.).
Homogenates were then centrifuged at 14,000 × g for 10 min at 4°C.
Supernatants were collected and protein concentration was
determined by using a BCA protein assay kit (MilliporeSigma). Gels
(10–12% acrylamide) were loaded with 50 µg of total protein per
sample, then transferred to nitrocellulose membranes
(Whatman-Protran; Merck KGaA). Membranes were blocked with 5%
non-fat milk for 1 h at room temperature and incubated overnight
with the respective primary antibody at 4°C. The primary antibodies
and dilutions used were TNF-α (1:1,000; BS2081R; BIOSS), IL-1β
(1:500; P420B; Invitrogen, MA, USA) and NF-κβ p-65 (1:500; ab16502;
Abcam). Membranes were washed in TBS-0.1% Tween and then incubated
with horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:5,000; cat. no. ab6721; Abcam) for 1 h at room
temperature. Finally, membranes were developed with Clarity ECL
Western Blotting Substrate (Bio-Rad Laboratories, Inc.) and images
acquired using ImageQuant LAS 4000 mini digital imaging system
(General Electric). α-Tubulin or β-Actin were used as controls.
Lipid analysis of liver tissue
Total lipid content
Lipids were extracted with chloroform/methanol (2:1
v/v) from a wedge of fresh liver tissue (~150/200 mg) following the
Folch method adapted by Christie (25). Further details of the lipid
extraction method were published previously (23).
Lipid classes
Aliquots of 30 µg from total lipid extracts were
used for the analysis. Lipid classes were separated by
high-performance thin-layer chromatography (HPTLC) in a
single-dimensional double-development following the Olsen and
Henderson method (26). Further
details of the method were published previously (23).
Fatty acid methyl esters (FAMEs) from
total lipids
1 mg of the total lipid extract was subjected to
acid-catalyzed transmethylation using toluene and sulfuric acid in
methanol, and was incubated for 16 h at 50°C to obtain the fatty
acid methyl esters (FAMEs) profile. Subsequently, FAMEs were
extracted and purified by thin-layer chromatography (TLC) and
stored under a 100% nitrogen atmosphere to prevent sample
oxidation. Finally, FAMEs were analyzed by gas chromatography-mass
spectrometry (GC-MS; Agilent 7890A/7010B; Agilent Technologies,
Inc.) and identification was performed according to retention times
by library matching with NIST v.2.2 (Agilent Technologies, Inc.).
Quantification was performed using the selected ion-monitoring mode
and the results are expressed as relative percentage areas. Further
details of the method are provided in a previously published
article (23).
Composition of FAMEs in lipid
classes
Aliquots of 2–4 mg of lipid extracts were loaded
into TLC 20×20 cm silica plates. Lipid classes were isolated in a
single-dimensional double-development following the Olsen and
Henderson method (26). The lipid
classes (LCs) were stained through spraying with
dichlorofluorescein. Then, the samples were visualized under UV
light and scraped from the silica plate. Finally, the LCs in the
silica were transmethylated at 50°C for 16 h to obtain FAMEs and
the four major LCs were selected: Phosphatidylcholine (PC),
phosphatidylethanolamine (PE), phosphatidylinositol (PI) and TAG.
The quantification of the samples was performed by GC-MS following
the aforementioned method. Further details can be found elsewhere
(23).
Statistical analysis
Quantitative variables are expressed as mean ±
standard deviation. For males, continuous variables between groups
were compared with unpaired Student's t-test, or non-parametric
Mann-Whitney test if the conditions of normality were not met. For
females, two-way ANOVA was performed, followed by Tukey's multiple
comparisons test, Tamhane's T2 test or Dunnett's T3 test (if
variables were not homoscedastic) as post-hoc tests. Pairwise
comparisons were performed using Mann-Whitney test with Bonferroni
correction if variables were not normally distributed. Fisher's
exact test was used to compare proportions between groups. Sample
size was calculated based on previous studies (21,23)
using similar obesity and ovariectomized models in which 6 to 10
cases should reach the end of the study. The present used SPSS
Statistics 20 (IBM Corp.) and GraphPad Prism 9 (Dotmatics) for
statistical analyzes and data plots, respectively. P<0.05 was
considered to indicate a statistically significant difference.
Results
Obesity and metabolic syndrome
The model was reported previously (21). Briefly, i) all mice on HFD
developed obesity and MS, ii) males reached higher weight than
females and iii) only obese males and obese ovariectomized females
developed hyperglycemia and insulin resistance (21).
Liver histology
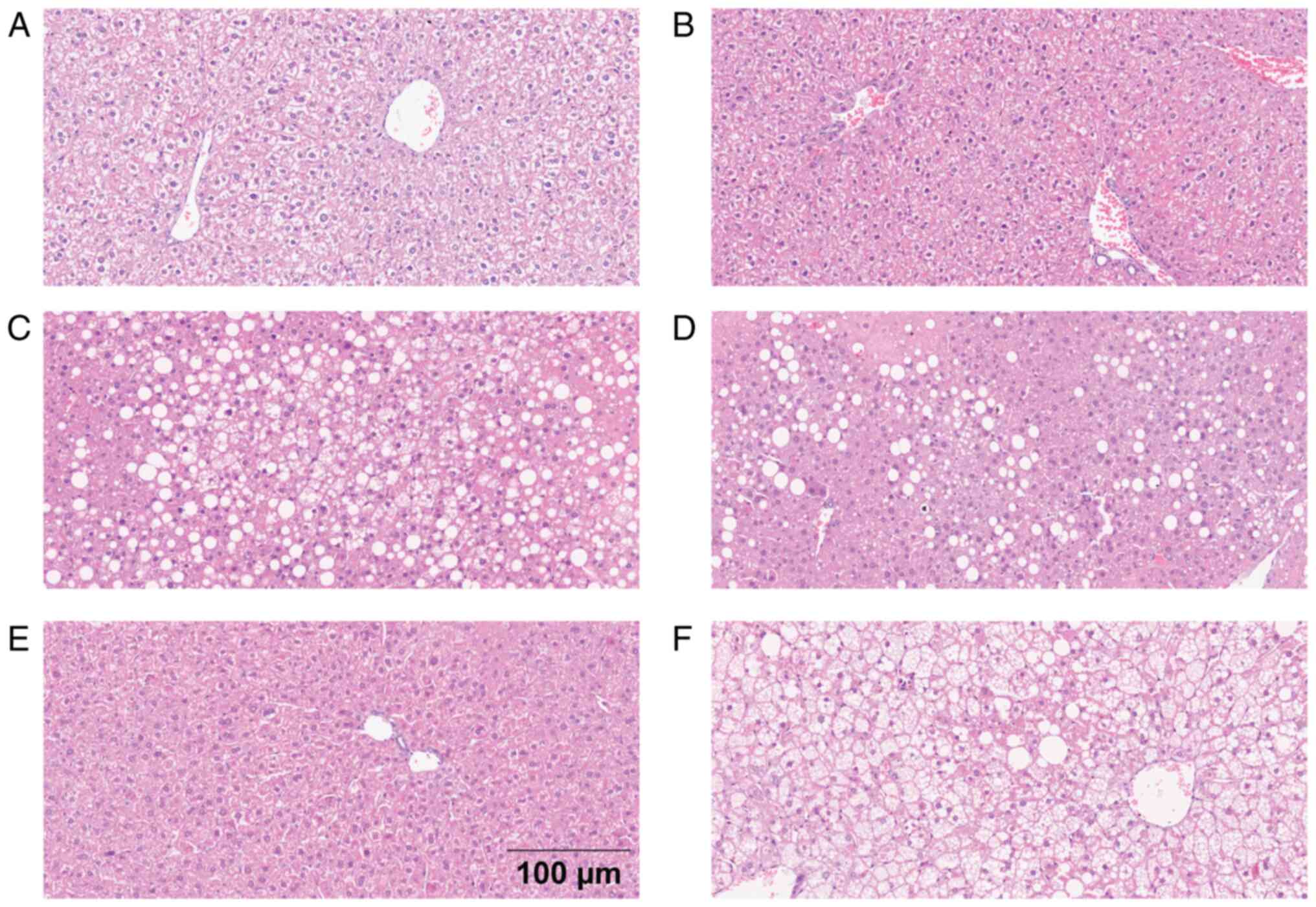
Females
Of the animals on HFD-OVX and HFD, 70 and 100%
showed steatosis, respectively (P>0.05), classified as severe
(27 and 57%), moderate (27 and 15%) or mild steatosis (13 and 29%).
Animals on HFD-OVX and HFD developed hepatocyte ballooning (67 and
85%), maintaining their size (60 and 57% in B1 stage) or increasing
twice or more the hepatocyte size (7 and 28% in B2 stage,
respectively; Table SII; Fig. 1). The HFD-OVX mice showed a mild
increase in collagen staining compared with SD-OVX (1.28 vs. 0.99%;
P=0.0109; Fig. S1). SD-OVX
animals presented lower collagen staining in comparison with SD
animals (0.99 vs. 1.61%; P=0.0106; Fig. S1). Despite the statistical
differences between groups, the observed fibrotic area was minimal,
representing only 1–2% of the total (Fig. S1). No differences were found
between SD and SD-OVX whereas animals on HFD and HFD-OVX had higher
steatosis and ballooning than those on SD and SD-OVX, respectively
(Table SII).
Males
Most animals on HFD developed steatosis (94%,
P<0.0001) with the following distribution: Severe (53%),
moderate (29%) and mild (12%), and ballooning (94%; P<0.0001)
where ~50% maintained the size of the hepatocyte and the other 50%
doubled the size at least (Fig.
1). Male animals on HFD presented an average of 1.10% of area
with positive collagen staining, while male animals in SD an
average of 0.77% (P=0.0124; Fig.
S1). Despite the statistical differences between groups, the
observed fibrotic area was minimal, representing only 1–2% of the
total (Fig. S1). Finally, animals
had comparable low levels of necroinflammation between groups
(Table SII).
Inflammation markers in liver
tissue
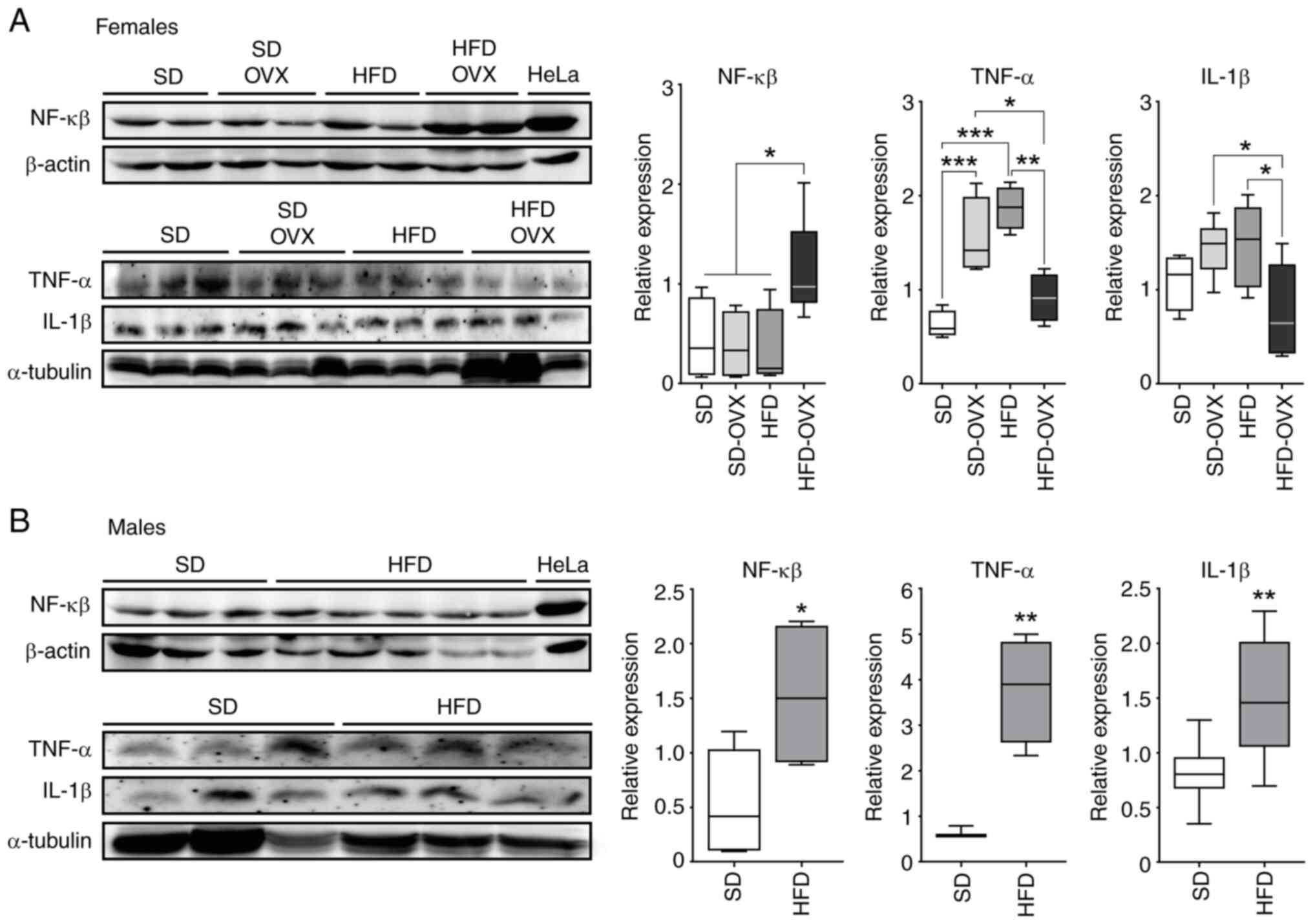
Females
The HFD-OVX group showed upregulation of NF-κβ p-65
compared with HFD (P=0.013), SD-OVX (P=0.008) and SD (P=0.049)
groups. The HFD-OVX also showed lower TNF-a compared with HFD
(P=0.002) and SD-OVX (P=0.041). HFD-OVX mice showed lower levels of
IL-1B compared with HFD (P=0.048) and SD-OVX (P=0.032). Finally,
both HFD and SD-OVX animals had an increase in TNF-α compared with
SD group (P<0.001, Fig.
2A).
Males
The HFD group showed higher levels of NF-κβ p-65
(P=0.032), TNF-α (P=0.005) and IL-1β compared with controls
(P=0.006, Fig. 2B).
Serum analysis
Females
The HFD-OVX showed higher levels of AST (P=0.002)
and ALT (P=0.024) compared with the SD, SD-OVX and HFD groups
(Fig. 3A). HFD and HFD-OVX both
showed higher total Chol levels compared with controls (Fig. 3B). No differences were observed in
the TAG levels among groups.
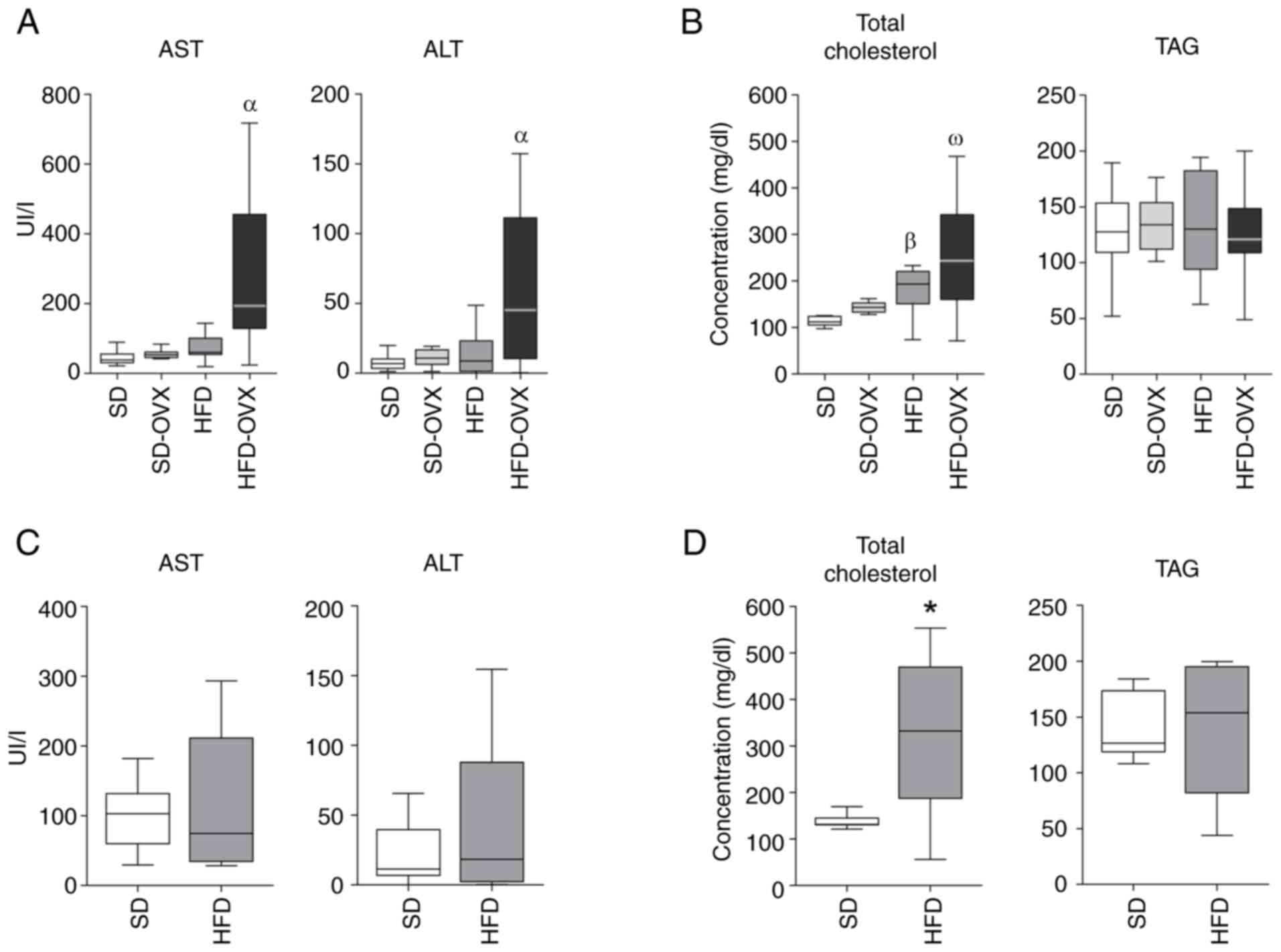 | Figure 3.Serum biochemical analysis. (A) Serum
transaminases in females. (B) Serum Chol and TAG levels in females.
(C) Serum transaminases in males. (D) Serum Chol and TAG levels in
males. Boxplots indicate the median as the horizontal line, the top
and bottom of the box show the upper and lower quartiles, maximum
and minimum values are displayed as whiskers. Female significance:
αHFD-OVX vs. HFD, SD and SD-OVX, P<0.05.
βHFD vs. SD, P<0.05, ωHFD-OVX vs. SD-OVX
and SD, P<0.05. Male significance: *HFD vs. SD, P<0.05. Chol,
free cholesterol; TAG, triglyceride AST, aspartate
aminotransferase; ALT, alanine aminotransferase; SD, standard diet;
HFD, high-fat diet; OVX, ovariectomized. |
Males
HFD and SD groups had comparable levels of AST, ALT
and TAG (Fig. 3C and D). The HFD
group displayed higher levels of serum total Chol (P=0.041;
Fig. 3D).
Liver lipidomics
Total hepatic lipid content in females
HFD-OVX had higher total lipid content than SD-OVX
(9.36±3.38 vs. 4.59±0.71; P<0.001) but lower than the HFD group
(9.36±3.38 vs. 13.28±5.04; P=0.027; Fig. 4A; Table SIII).
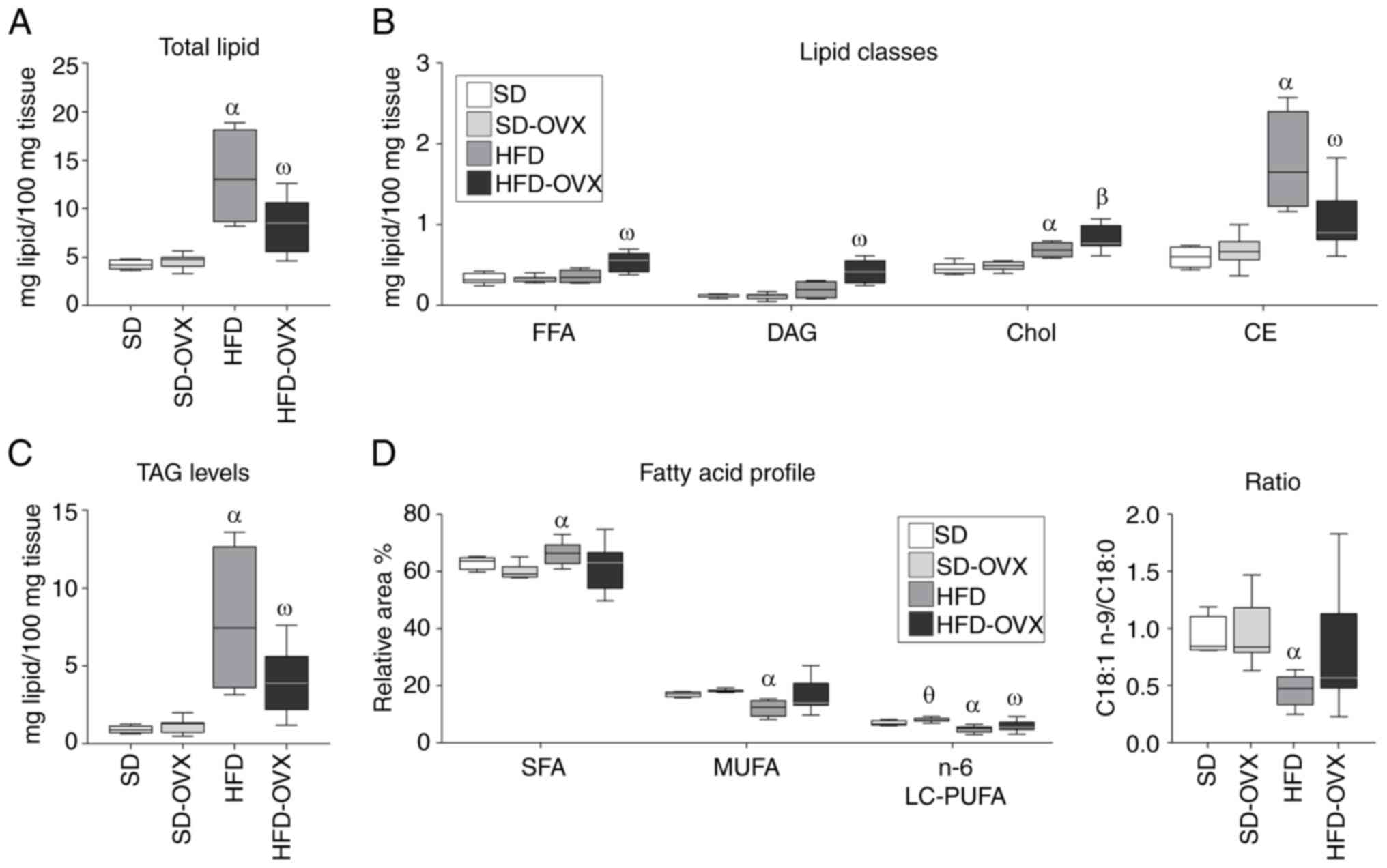 | Figure 4.Lipidomic profile of liver tissue in
female mice. (A) Total lipid content. (B) Lipid classes profile.
(C) TAG levels. (D) Fatty acid profile of females as indicated in
the figure. Boxplots show the median as the horizontal line, the
top and bottom of the box show the upper and lower quartiles,
maximum and minimum values are displayed as whiskers. Statistical
significance: αHFD vs. SD, P<0.05,
βHFD-OVX vs. SD-OVX and SD, P<0.05.
ωHFD-OVX vs. SD, SD-OVX and HFD, P<0.05.
θSD-OVX vs. SD, P<0.05. TAG, triglycerides; FFA, free
fatty acid; DAG, diglycerides; Chol, free cholesterol; CE,
cholesterol esters; SD, standard diet; HFD, high-fat diet; OVX,
ovariectomized. |
Total hepatic lipid content in
males
The HFD group showed higher total lipids compared
with the SD (13.92±6.14 vs. 4.82±0.74, P<0.001; Fig. 5A; Table SIII). Values correspond to the
mean mg total lipid/100 mg fresh tissue ± SD.
 | Figure 5.Lipidomic profile of liver tissue in
male mice. (A) Total lipid content. (B) Lipid classes profile. (C)
TAG levels. (D) Fatty acid profile as indicated in the figure.
Boxplots show the median as the horizontal line, the top and bottom
of the box show the upper and lower quartiles, maximum and minimum
values are displayed as whiskers. *P<0.05; ***P<0.001. TAG,
triglycerides; FFA, free fatty acid; DAG, diglycerides; Chol, free
cholesterol; CE, cholesterol esters; SD, standard diet; HFD,
high-fat diet; OVX, ovariectomized; SFAs, saturated fatty acids;
MUFAs, monounsaturated fatty acids; LC-PUFAs, long-chain
polyunsaturated fatty acids. |
Hepatic lipid classes profile in
females
HFD-OVX showed higher levels of FFA (0.54±0.12 vs.
0.36±0.08; P=0.044) and DAG (0.42±0.14 vs. 0.20±0.11; P=0.014) but
lower CE (1.06±0.35 vs. 1.76±0.62; P=0.004) and TAG (3.95±2.02 vs.
7.91±4.77; P=0.045) compared with the HFD group (Fig. 4B and C; Table SIII). Animals on HFD showed higher
levels of Chol (0.69±0.10 vs. 0.42±0.07; P=0.002), CE (1.76±0.62
vs. 0.57±0.12; P=0.002) and TAG (7.91±4.77 vs. 0.92±0.23; P=0.006)
compared with the SD group.
Hepatic lipid classes profile in
females
The HFD group showed increased FFA compared with SD
group (0.86±0.21 vs. 0.38±0.10, P<0.001), DAG (0.55±0.16 vs.
0.13±0.04, P<0.001), TAG (9.06±4.93 vs. 1.02±0.35, P<0.001),
Chol (0.95±0.17 vs. 0.57±0.09, P<0.001) and CE (0.96±0.44 vs.
0.41±0.12, P<0.05) (Fig. 5B and
C; Table SIII).
FAMEs profile of total lipids
Females
The HFD-OVX had lower 16:0 but higher 18:2 n-6 and
18:3 n-6 compared with the SD group (Fig. 4D; Table SIV). The HFD group showed lower
levels of monounsaturated fatty acids (MUFA), omega-6 long-chain
polyunsaturated fatty acids (n-6 LC-PUFA) and 18:1 n-9/18:0 ratio
compared with animals on SD (Table
SIV). Finally, the total FAMEs profile of the HFD group showed
lower 18:1 and 20:4 n-6 (arachidonic acid; ARA) but higher 18:0,
18:2 n-6, 18:3 n-6 and 18:3 n-3 compared with the SD group
(Fig. 4D; Table SIV).
Males
The FA profile showed that the HFD group had lower
SFA (53.36±5.83 vs. 59.64±2.13; P<0.001) and n-6 LC-PUFA
(4.98±1.95 vs. 8.71±1.98; P<0.001) but also higher MUFA
(25.17±4.68 vs. 17.37±4.77, P<0.001) and 18:1 n-9/18:0 ratio
(2.88±1.44 vs. 1.12±0.47; P<0.001) compared with SD (Fig. 5D; Table SIV). The HFD group also showed
lower 18:0, 20:4 n-6 and 22:6 n-3 (docosahexaenoic acid; DHA), and
higher 14:0, 18:1, 20:1, 18:2 n-6, 18:3 n-6, 20:2 n-6, and 18:3 n-3
compared with the SD group (Fig.
5D; Table SIV).
FAMEs profile of lipid classes
Females
HFD-OVX showed lower 16:0 in PC and higher 18:0
dimethyl acetal (DMA) in PE compared with all other groups. The HFD
group had lower 20:3 n-6 in PC, PI and PE) 16:1 and 18:1 (in PC and
PE), and higher 18:0 (in PC and PE) and 22:6 n-3 (in PC) compared
with the SD (Tables SV, SVI and SVII). HFD and HFD-OVX groups both had in
TAG a lower SFA and MUFA (14:0, 16:1 and 18:1) and higher
polyunsaturated (PUFAs) profile (20:3 n-6, 20:4 n-6, 22:4 n-6, 22:5
n-6 and 22:6 n-3) compared with SD and SD-OVX (Table SVIII).
Males
The HFD had lower 20:1 (in PC, PE and PI), 16:0,
16:1, 18:2 n-6 and 22:5 n-3 (in PC and PE) and higher 18:0, 20:4
n-6, 22:6 n-3 (in PC and PE) and 18:0 DMA (in PE) compared with SD
(Table SV, SVI and SVII). In TAG, the HFD group had lower
SFAs and MUFAs (14:0, 16:0, 16:1) and higher PUFAs (18:3 n-3, 20:2
n-6, 20:4 n-6, 22:4 n-6 and 22:6 n-3) compared with SD (Table SVIII).
Discussion
The main finding of the present study was that
ovariectomy in obese female mice caused severe hepatic lipotoxicity
and liver damage. Only obese males and obese-ovariectomized females
developed insulin resistance (21)
and impaired lipid droplet formation with a consequent accumulation
of toxic lipid intermediaries (FFA, DAG and Chol). These markers of
hepatic lipotoxicity were associated with inflammation in both
groups. Notably, only obese-ovariectomized females had signs of
liver injury in serum analysis, evidenced by higher transaminases
levels. These findings suggest that the lack of estrogen may lead
to detrimental effects in the context of obesity and insulin
resistance, thus potentially promoting chronic liver damage.
The present study used a well-established model of
obesity and metabolic syndrome to investigate the effects of these
conditions on hepatic lipid metabolism. Furthermore, to examine the
role of estrogen in the progression of liver damage, the present
study performed ovariectomy on a subgroup of female mice (21,23).
As expected, obese female (both ovariectomized or not) and male
mice exhibited liver steatosis, hepatocyte ballooning, mild
fibrosis and expression of pro-inflammatory markers. Importantly,
while chronic changes (especially fibrosis) were minimal, severe
steatosis and ballooning were the most significant histological
markers of damage in obese males. This suggested that the model
reflected early-state damage, consistent with previous findings in
preclinical and clinical models (27,28).
Notably, sex markedly influenced the effect of obesity on the
hepatic profile. Compared with obese males, obese female mice
exhibited a lower degree of steatosis, hepatocyte ballooning and
expression of inflammatory markers. Again, chronic fibrotic changes
were minimal and the most important histological marker in
ovariectomized obese females was a trend towards reduced steatosis.
These findings in males suggested that the current model reflected
an early stage of damage, thus, longer-term models might be
necessary to fully characterize chronic damage progression.
Similarly, the hepatic lipid metabolism was less affected in
females, as they displayed high levels of Chol and CE whereas obese
males had elevated Chol, CE, DAG and FFA. The hepatic accumulation
of DAG and FFA is associated with the development of obesity,
insulin resistance and NAFLD (29,30).
Markedly, ovariectomy in obese female mice resulted in lower
hepatic TAG and CE and higher levels of cytotoxic FFA, DAG and
Chol. These differences reflected the protective role of estrogen
on regulating the packaging of FFA and DAG into TAG to form lipid
droplets in the liver (31).
In the context of lipotoxicity, the accumulation of
lipids, particularly FFA and DAG, can lead to changes in the
phospholipid profile, contributing to inflammation, cell
dysfunction, and organ damage. The present study observed a
significant reduction in total phospholipids in obese mice,
representing only 20% of total lipids, almost twice the value
obtained in lean animals. The low proportion of phospholipids in
relation to neutral lipids might impair membrane fluidity and cell
functioning. Furthermore, inflammation in liver has been associated
with alterations in phosphatidylserine and PE levels (32). The present study found a mild
increment in these phospholipids in HFD-OVX mice as well as a
markedly elevated Chol/PC ratio, which has been associated with
liver injury (33). Altogether,
these findings may indicate that the loss of estrogen in the
context of obesity and insulin resistance may induce hepatic
lipotoxicity, promoting inflammation and chronic liver damage.
However, more studies are needed to clarify this pathway.
The complex interplay between insulin and estrogen
may be key to understanding the results obtained in the current
study, as they have a profound impact on lipid metabolism. On one
hand, insulin modulates lipid metabolism in the hepatocyte by i)
promoting lipogenesis from glucose and other metabolites (34), ii) mediating the packing of FFA and
DAG into TAG (35), iii) promoting
the esterification of Chol (36),
and iv) regulating the correct lipid droplet formation via SREBP1
and mTORC1 (37). Given the
toxicity of FFA, DAG, and Chol (11,12,38–41),
insulin's promotion of lipogenesis and lipid droplet formation can
be considered ‘cell protective’ in the context of lipid
accumulation (8,35,42).
However, obesity and MS can eventually induce insulin resistance in
the hepatocytes. Consequently, reduced insulin responsiveness may
impair lipid droplet synthesis and elevate FFA, DAG and Chol
levels, thereby increasing hepatic toxicity, as observed in our
model.
On the other hand, several studies have found that
estrogen promotes insulin sensitivity and glucose uptake in muscle
and liver (43,44). Estrogen and insulin can both
regulate lipogenesis via E2-induced activation of PI3K-Akt-Foxo1
(45). Therefore, these hormones
may facilitate neutral lipid droplet formation that protects from
toxic intermediaries in obesity (46). This may explain the impaired
hepatic lipid deposition associated with liver injury observed in
obese ovariectomized mice (Fig.
S2). Clinical trials have shown that estrogen replacement
improved insulin sensitivity and glucose levels in diabetic
postmenopausal women (47–49). Following ovariectomy in obese
female mice, estrogen supplementation reduced DAG accumulation in
the liver (44). In male mice with
obesity, estrogen administration prevented diet-induced hepatic
insulin resistance, hyperglycemia and the elevation in DAG levels
(50). Furthermore, a study has
shown that estrogen supplementation improved peripheral insulin
sensitivity and increased TAG levels in the liver of obese turkeys
(51). Notably, the metabolic
changes induced by ovariectomy depend on the nutritional status. In
murine models, ovariectomy in obese mice can result in a decreased
expression of genes related to lipid metabolism in adipose tissue,
liver and skeletal muscle, contributing to increased fat
accumulation as well as toxic lipids intermediaries (52,53).
By contrast, in non-obese mice, these effects are less pronounced,
suggesting that obesity amplifies the metabolic alterations induced
by estrogen deficiency (54). In
humans, the transition to menopause, which involves a natural
decline in estrogen levels, has been associated with increased
central adiposity and metabolic alterations, indicating parallels
to findings in animal models (55,56).
Lipid droplet formation is an essential metabolic
mechanism to circumvent hepatic lipotoxicity in conditions of
excessive energy intake (57).
Sequestration of FFA and DAG into inert TAG and CE within lipid
droplets is crucial for mitigating hepatic lipotoxicity. FFA, DAG
and Chol cannot readily enter lipid droplets due to their polar
groups (57). Thus, TAG and CE
serve to sequester these cytotoxic intermediaries to form the
neutral core of lipid droplets (58). Limiting lipid droplet formation can
be deleterious for hepatocytes, as elevated levels of FFA, DAG and
Chol are harmful to these cells (10–13,40).
For instance, FFA can induce lipid peroxidation and the activation
of TLR4 (12,59). DAG can induce inflammation and
impaired autophagy via PKC activation (11,13).
Furthermore, Chol can activate the unfolded protein response and
promote ER stress (14,15). Notably, increased levels of FFA,
DAG and Chol can promote the activation of NF-κβ and the initiation
of inflammatory pathways, in accordance with the present results
(53,60–62).
Differences in the hepatic FA profile between obese
and lean mice are related to differences in the dietary FA
composition. However, dysregulations of the metabolic pathway
involved in the synthesis/utilization of FAs were pronounced in
mice fed with HFD. On one hand, the present study found sexual
dimorphism in the distribution of FA in the liver of female and
male mice: Obese females (irrespective of ovariectomy) had higher
SFAs, lower MUFAs and lower n-6 LC-PUFAs whereas obese males showed
lower SFAs, higher MUFAs and lower n-6 LC-PUFAs. Taking into
account that SFAs are more toxic than MUFAs (5), the FA profile of males may indicate a
greater capacity to convert these SFAs into more inert MUFAs, as a
protective pathway against SFA-induced toxicity. These differences
might indicate sex-specific mechanisms to compensate metabolic
insults that should be further explored in future experiments.
Additionally, ARA (20:4 n-6) and consequently ARA/DHA ratio were
depleted in hepatic total lipids of all obese animals. As ARA is
the main precursor of proinflammatory prostanoids, this reduction
could be related to its increased utilization for the synthesis of
prostaglandins, thromboxanes and leukotrienes (63). Consistently, obese ovariectomized
females had the highest levels of DMAs in PE (chiefly 18:0 DMA, see
Table SVI). DMAs are precursors
for de novo biosynthesis of plasmalogens which are receiving
increasing attention due to their antioxidant role and their
involvement in mammalian anti-inflammatory responses (64). This aligns well with the higher
proportion of essential PUFAs, such as EPA and DHA in hepatic TAG
of obese mice that might be attributed to the described ability of
plasmalogens to reduce oxidative degradation (65).
NAFLD is associated with high SFA and Chol levels
that inhibit desaturase activity (66,67).
Notably, the present study revealed a decreased product 18C
precursor ratios for both n-6 and n-3 pathways in hepatic total
lipids, suggesting that delta-6 and delta-5-desaturase activities,
which catalyze the conversion of shorter chain precursors linoleic
acid (18:2 n-6) and linolenic acid (18:3 n-3) into their longer and
more unsaturated counterparts, might be impaired. Consistently, the
present study observed a decreased 18:1 n-9 proportion and 18:1
n-9/18:0 ratio in obese female mice; probably linked to the
proinflammatory status of this group. Chena et al (68) demonstrated the protective mechanism
of oleic acid (18:1 n-9) against SFA-induced hepatocyte
lipotoxicity in primary hepatocytes of rats with NASH, including
apoptosis, oxidative stress, mitochondrial dysfunction,
inflammation and fibrosis. Thus, an impairment of stearoyl-CoA
desaturase (delta-9 desaturase) involved in the conversion of 18:0
to 18:1 n-9 could be responsible for this reduction. The connection
between the observed lipid profile with the hepatic desaturase
activities must be addressed in future studies.
Several therapeutic strategies that involve
regulation of lipid metabolism and inflammation are being explored
for NAFLD (69–75), including the use of SIRT1
activators (70), AMPK modulators
(71), gut microbiota
interventions (72) and hormone
replacement therapy in the context of menopause (73). These approaches aim to reduce
hepatic lipotoxicity, improve insulin sensitivity, and ameliorate
inflammation. Among them, SIRT1 activation has gained attention for
its role in suppressing NF-κβ signaling and mitigating disease
progression (70). In the present
study, NF-κβ upregulation in obese ovariectomized mice, along with
increased transaminases, suggested an impairment in SIRT1 pathway,
and highlights the need to explore its therapeutic potential in
menopause-related liver injury. Finally, it is worth considering
that the alterations in inflammation and lipid metabolism are
related to cardiovascular disease, the main cause of death in
patients with NASH and NAFLD (74,75).
An important strength of the present study is that
it is among the few studies that analyze the interplay between
estrogen deficiency and obesity in mice. Most previous studies have
focused solely on males. Thus, sex-based comparisons may provide
valuable insights that pave the way for more efficient treatments
of metabolic disorders. One straightforward interpretation of the
present results is that the lack of sex hormones might predispose
females to more severe damage, mirroring the situation in males.
Furthermore, the comparison revealed that obese females did not
experience the same detrimental effects as obese males, which can
be attributed to the protective effect of estrogen. These results
demonstrated that ovariectomy generally worsened the hepatic
profile in obese females. However, the present study has
limitations. The first pertains to the applicability of
pre-clinical models to humans. As mouse lipid metabolism differs
from human metabolism, these findings should be confirmed in large
animal models and human studies. The duration of the experiment is
relatively short, and this may explain the mild fibrosis and
moderate variations in the hepatic fatty acid profile observed in
the model. Chronic animal models (small and large) are expensive,
which may compromise their viability in research projects. In any
case, larger periods of follow up could improve our understanding
about the impact of menopause in MS and liver disease.
In conclusion, ovariectomy in obese animals
exacerbated hepatic lipotoxicity, accelerating the progression of
liver injury. This suggested that estrogen loss may contribute to
the onset of hepatic damage in the context of obesity and insulin
resistance. Furthermore, estrogen may exert its protective effect
through the regulation of lipid droplet formation when insulin
action is impaired. Further experiments are needed to unveil
mechanistic insights, which may open therapeutic opportunities.
Additional research in sex differences regarding liver disease may
also contribute to achieve an improved understanding of the NAFLD
and NASH pathogenesis.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was funded by the Instituto de Salud Carlos III
(STT received grant no. PFIS-FI20/00147; EP received grant no.
PI19/01756 and AERR, who is a recipient of a contract from the Sara
Borrell program, received grant no. CD21/00142). This research was
also funded by the SENEFRO foundation (Sociedad Española de
Nefrología) and European Commission, HORIZON-WIDERA-2021-ACCESS-03
through Diabetes Obesity and the Kidney project (grant no.
PN:101079207).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AERR, EP and MHG designed, discussed and supervised
the study; AERR, EP, AAA, JDG, SLL, BAP, AHB, MIH, SGH, LDM and STT
performed the experiments, data analyses and result plotting; AAA,
JDG, JAPP, NGAG and CRG collaborated in the data interpretation,
drafting and revising the manuscript; AERR, EP, MHG, AAA and JDG
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Animal care was performed in accordance with
institutional guidelines in compliance with Spanish (Real Decreto
53/2013, February 1. BOE, February 8, 2013, n: 34, p. 11370-11421)
and international laws and policies (Directive 2010/63/EU of the
European Parliament and of the Council of 22 September 2010 on the
protection of animals used for scientific purposes) and were
approved by the Institutional Animal Care and Use Committee (Comité
de Ética de la Investigación y de Bienestar Animal of University of
La Laguna, Spain). The ethics committee approval number was
CEIBA2021-3107.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CE
|
cholesteryl esters
|
|
Chol
|
free cholesterol
|
|
DAG
|
diacylglycerol
|
|
FA
|
fatty acids
|
|
FAMEs
|
fatty acid methyl esters
|
|
FFA
|
free fatty acids
|
|
GC
|
gas chromatography
|
|
HFD
|
high-fat diet
|
|
HFD-OVX
|
ovariectomized animals on high-fat
diet
|
|
IR
|
insulin resistance
|
|
LC-PUFAs
|
long-chain polyunsaturated fatty
acids
|
|
MS
|
metabolic syndrome
|
|
MUFAs
|
monounsaturated fatty acids
|
|
n-3
|
omega-3 fatty acids
|
|
n-6
|
omega-6 fatty acids
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
NASH
|
non-alcoholic steatohepatitis
disease
|
|
PC
|
phosphatidylcholine
|
|
PE
|
phosphatidylethanolamine
|
|
PI
|
phosphatidylinositol
|
|
PUFAs
|
polyunsaturated fatty acids
|
|
SD
|
standard diet
|
|
SD-OVX
|
ovariectomized animals on standard
diet
|
|
SFAs
|
saturated fatty acids
|
|
TAG
|
triacylglycerol
|
|
TLC
|
thin-layer chromatography
|
References
|
1
|
Boutari C and Mantzoros CS: A 2022 update
on the epidemiology of obesity and a call to action: As its twin
COVID-19 pandemic appears to be receding, the obesity and
dysmetabolism pandemic continues to rage on. Metabolism.
133:1552172022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of nonalcoholic fatty
liver disease-Meta-analytic assessment of prevalence, incidence,
and outcomes. Hepatology. 64:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noureddin M, Vipani A, Bresee C, Todo T,
Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, et al:
NASH leading cause of liver transplant in women: Updated analysis
of indications for liver transplant and ethnic and gender
variances. Am J Gastroenterol. 113:1649–1659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baffy G, Brunt EM and Caldwell SH:
Hepatocellular carcinoma in non-alcoholic fatty liver disease: An
emerging menace. J Hepatol. 56:1384–1391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geng Y, Faber KN, de Meijer VE, Blokzijl H
and Moshage H: How does hepatic lipid accumulation lead to
lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int.
15:21–35. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bugianesi E, Moscatiello S, Ciaravella MF
and Marchesini G: Insulin resistance in nonalcoholic fatty liver
disease. Curr Pharm Des. 16:1941–1951. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rada P, Gonzalez-Rodriguez A,
Garcia-Monzon C and Valverde AM: Understanding lipotoxicity in
NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis.
11:8022020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Listenberger LL, Han X, Lewis SE, Cases S,
Farese RV Jr, Ory DS and Schaffer JE: Triglyceride accumulation
protects against fatty Acid-induced lipotoxicity. Proc Natl Acad
Sci USA. 100:3077–3082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tripathy D, Mohanty P, Dhindsa S, Syed T,
Ghanim H, Aljada A and Dandona P: Elevation of free fatty acids
induces inflammation and impairs vascular reactivity in healthy
subjects. Diabetes. 52:2882–2887. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hommelberg PP, Plat J, Langen RC, Schols
AM and Mensink RP: Fatty Acid-induced NF-kappaB activation and
insulin resistance in skeletal muscle are chain length dependent.
Am J Physiol Endocrinol Metab. 296:E114–E120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Yang SG, He CW, Zhang ZT, Liang Y,
Li H, Zhu J, Su X, Gong Q and Xie Z: Excess diacylglycerol at the
endoplasmic reticulum disrupts endomembrane homeostasis and
autophagy. BMC Biol. 18:1072020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi H, Kokoeva MV, Inouye K, Tzameli I,
Yin H and Flier JS: TLR4 links innate immunity and fatty
acid-induced insulin resistance. J Clin Invest. 116:3015–3025.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gassaway BM, Petersen MC, Surovtseva YV,
Barber KW, Sheetz JB, Aerni HR, Merkel JS, Samuel VT, Shulman GI
and Rinehart J: PKCε contributes to lipid-induced insulin
resistance through cross talk with p70S6K and through previously
unknown regulators of insulin signaling. Proc Natl Acad Sci USA.
115:E8996–E9005. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tabas I: Free cholesterol-induced
cytotoxicity a possible contributing factor to macrophage foam cell
necrosis in advanced atherosclerotic lesions. Trends Cardiovasc
Med. 7:256–263. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tabas I: Consequences of cellular
cholesterol accumulation: Basic concepts and physiological
implications. J Clin Invest. 110:905–911. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song Y, Liu J, Zhao K, Gao L and Zhao J:
Cholesterol-induced toxicity: An integrated view of the role of
cholesterol in multiple diseases. Cell Metab. 33:1911–1925. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong FJ, Wu JH, Sun SY and Zhou JQ: The
endoplasmic reticulum stress/autophagy pathway is involved in
cholesterol-induced pancreatic beta-cell injury. Sci Rep.
7:447462017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gan LT, Van Rooyen DM, Koina ME, McCuskey
RS, Teoh NC and Farrell GC: Hepatocyte free cholesterol
lipotoxicity results from JNK1-mediated mitochondrial injury and is
HMGB1 and TLR4-dependent. J Hepatol. 61:1376–1384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pu D, Tan R, Yu Q and Wu J: Metabolic
syndrome in menopause and associated factors: A meta-analysis.
Climacteric. 20:583–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Janssen I, Powell LH, Crawford S, Lasley B
and Sutton-Tyrrell K: Menopause and the metabolic syndrome: The
Study of Women's Health Across the Nation. Arch Intern Med.
168:1568–1575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodriguez-Rodriguez AE, Donate-Correa J,
Luis-Lima S, Diaz-Martin L, Rodriguez-Gonzalez C, Perez-Perez JA,
Acosta-González NG, Fumero C, Navarro-Díaz M, López-Álvarez D, et
al: Obesity and metabolic syndrome induce hyperfiltration,
glomerulomegaly, and albuminuria in obese ovariectomized female
mice and obese male mice. Menopause. 28:1296–1306. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stevenson JC: Metabolic effects of hormone
replacement therapy. J Br Menopause Soc. 10:157–161. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Afonso-Ali A, Porrini E, Teixido-Trujillo
S, Perez-Perez JA, Luis-Lima S, Acosta-Gonzalez NG, Sosa-Paz I,
Díaz-Martín L, Rodríguez-González C and Rodríguez-Rodríguez AE: The
role of gender differences and menopause in Obesity-related renal
disease, renal inflammation and lipotoxicity. Int J Mol Sci.
24:129842023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bedossa P, Poitou C, Veyrie N, Bouillot
JL, Basdevant A, Paradis V, Tordjman J and Clement K:
Histopathological algorithm and scoring system for evaluation of
liver lesions in morbidly obese patients. Hepatology. 56:1751–1759.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Christie WW: Gas Chromatography and
Lipids: A practical guide. Oily Press; 1989
|
|
26
|
Olsen RE and Henderson RJ: The rapid
analysis of neutral and polar marine lipids using
double-development HPTLC and scanning densitometry. J Exp Mar Bio
Ecol. 129:189–197. 1989. View Article : Google Scholar
|
|
27
|
Echeverria F, Valenzuela R, Bustamante A,
Alvarez D, Ortiz M, Espinosa A, Illesca P, Gonzalez-Mañan D and
Videla LA: High-fat diet induces mouse liver steatosis with a
concomitant decline in energy metabolism: Attenuation by
eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation
and the additive effects upon EPA and HT co-administration. Food
Funct. 10:6170–6183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sanyal AJ, Williams SA, Lavine JE,
Neuschwander-Tetri BA, Alexander L, Ostroff R, Biegel H, Kowdley
KV, Chalasani N, Dasarathy S, et al: Defining the serum proteomic
signature of hepatic steatosis, inflammation, ballooning and
fibrosis in non-alcoholic fatty liver disease. J Hepatol.
78:693–703. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teng X and Wang Y: Diacylglycerols as
lipid mediators in metabolic diseases: Insights from their role in
insulin resistance. Mol Metabolism. 21:86–98. 2019.
|
|
30
|
Bays HE and Tressler JA: Lipotoxicity: A
review of the relationship between obesity, type 2 diabetes, and
non-alcoholic fatty liver disease. J Clin Endocrinol Metabolism.
95:415–425. 2010.
|
|
31
|
Palmisano BT, Zhu L and Stafford JM: Role
of estrogens in the regulation of liver lipid metabolism.
Homeostasis, Diabetes and Obesity, Advances in Experimental
Medicine and Biology 1043. Springer International Publishing AG;
pp. 227–256. 2017
|
|
32
|
Shama S, Jang H, Wang X, Zhang Y, Shahin
NN, Motawi TK, Kim S, Gawrieh S and Liu W:
Phosphatidylethanolamines are associated with nonalcoholic fatty
liver disease (NAFLD) in obese adults and induce liver cell
metabolic perturbations and hepatic stellate cell activation. Int J
Mol Sci. 24:10342023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Puri P, BaillieR A, Wiest MM, Mirshahi F,
Choudhury J, Cheung O, Sargeant C, Contos MJ and Sanyal AJ: A
lipidomic analysis of nonalcoholic fatty liver disease. Hepatology.
46:1081–1090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haas JT, Miao J, Chanda D, Wang Y, Zhao E,
Haas ME, Hirschey M, Vaitheesvaran B, Farese RV Jr, Kurland IJ, et
al: Hepatic insulin signaling is required for obesity-dependent
expression of SREBP-1c mRNA but not for feeding-dependent
expression. Cell Metab. 15:873–884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Czech MP, Tencerova M, Pedersen DJ and
Aouadi M: Insulin signaling mechanisms for triacylglycerol storage.
Diabetologia. 56:949–964. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
O'Rourke L, Gronning LM, Yeaman SJ and
Shepherd PR: Glucose-dependent regulation of cholesterol ester
metabolism in macrophages by insulin and leptin. J Biol Chem.
277:42557–42562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laplante M and Sabatini DM: An emerging
role of mTOR in lipid biosynthesis. Curr Biol. 19:R1046–R1052.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chitraju C, Mejhert N, Haas JT,
Diaz-Ramirez LG, Grueter CA, Imbriglio JE, Pinto S, Koliwad SK,
Walther TC and Farese RV Jr: Triglyceride synthesis by DGAT1
protects adipocytes from Lipid-induced ER stress during lipolysis.
Cell Metab. 26:407–18.e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kellner-Weibel G, Jerome WG, Small DM,
Warner GJ, Stoltenborg JK, Kearney MA, Corjay MH, Phillips MC and
Rothblat GH: Effects of intracellular free cholesterol accumulation
on macrophage viability: A model for foam cell death. Arterioscler
Thromb Vasc Biol. 18:423–4231. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ly LD, Xu S, Choi SK, Ha CM, Thoudam T,
Cha SK, Wiederkehr A, Wollheim CB, Lee IK and Park KS: Oxidative
stress and calcium dysregulation by palmitate in type 2 diabetes.
Exp Mol Med. 49:e2912017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Warner GJ, Stoudt G, Bamberger M, Johnson
WJ and Rothblat GH: Cell toxicity induced by inhibition of acyl
coenzyme A: Cholesterol acyltransferase and accumulation of
unesterified cholesterol. J Biol Chem. 270:5772–5778. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bosma M, Dapito DH, Drosatos-Tampakaki Z,
Huiping-Son N, Huang LS, Kersten S, Drosatos K and Goldberg IJ:
Sequestration of fatty acids in triglycerides prevents endoplasmic
reticulum stress in an in vitro model of cardiomyocyte
lipotoxicity. Biochim Biophys Acta. 1841:1648–1655. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gupte AA, Pownall HJ and Hamilton DJ:
Estrogen: An emerging regulator of insulin action and mitochondrial
function. J Diabetes Res. 2015:9165852015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu L, Brown WC, Cai Q, Krust A, Chambon
P, McGuinness OP and Stafford JM: Estrogen treatment after
ovariectomy protects against fatty liver and may improve
pathway-selective insulin resistance. Diabetes. 62:424–434. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan H, Yang W, Zhou F, Li X, Pan Q, Shen
Z, Han G, Newell-Fugate A, Tian Y, Majeti R, et al: Estrogen
improves insulin sensitivity and suppresses gluconeogenesis via the
transcription factor foxo1. Diabetes. 68:291–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
De Paoli M, Zakharia A and Werstuck GH:
The role of estrogen in insulin resistance: A review of clinical
and preclinical data. Am J Pathol. 191:1490–1498. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cuadros JL, Fernandez-Alonso AM, Chedraui
P, Cuadros AM, Sabatel RM and Perez-Lopez FR: Metabolic and
hormonal parameters in post-menopausal women 10 years after
transdermal oestradiol treatment, alone or combined to micronized
oral progesterone. Gynecol Endocrinol. 27:156–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Os I, Os A, Abdelnoor M, Larsen A,
Birkeland K and Westheim A: Insulin sensitivity in women with
coronary heart disease during hormone replacement therapy. J Womens
Health (Larchmt). 14:137–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Soranna L, Cucinelli F, Perri C, Muzj G,
Giuliani M, Villa P and Lanzone A: Individual effect of E2 and
dydrogesterone on insulin sensitivity in post-menopausal women. J
Endocrinol Invest. 25:547–550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu L, Martinez MN, Emfinger CH, Palmisano
BT and Stafford JM: Estrogen signaling prevents diet-induced
hepatic insulin resistance in male mice with obesity. Am J Physiol
Endocrinol Metab. 306:E1188–E1197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dashti N, Kelley JL, Thayer RH and Ontko
JA: Concurrent inductions of avian hepatic lipogenesis, plasma
lipids, and plasma apolipoprotein B by estrogen. J Lipid Res.
24:368–380. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rogers NH, Perfield JW II, Strissel KJ,
Obin MS and Greenberg AS: Reduced energy expenditure and increased
inflammation are early events in the development of
ovariectomy-induced obesity. Endocrinology. 150:2161–2168. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ludgero-Correia A Jr, Aguila MB,
Mandarim-de-Lacerda CA and Faria TS: Effects of high-fat diet on
plasma lipids, adiposity, and inflammatory markers in
ovariectomized C57BL/6 mice. Nutrition. 28:316–323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kamei Y, Suzuki M, Miyazaki H,
Tsuboyama-Kasaoka N, Wu J, Ishimi Y and Ezaki O: Ovariectomy in
mice decreases lipid metabolism-related gene expression in adipose
tissue and skeletal muscle with increased body fat. J Nutr Sci
Vitaminol (Tokyo). 51:110–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ozbey N, Sencer E, Molvalilar S and Orhan
Y: Body fat distribution and cardiovascular disease risk factors in
pre- and postmenopausal obese women with similar BMI. Endocr J.
49:503–509. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ambikairajah A, Walsh E and Cherbuin N:
Lipid profile differences during menopause: A review with
meta-analysis. Menopause. 26:1327–1333. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zadoorian A, Du X and Yang H: Lipid
droplet biogenesis and functions in health and disease. Nat Rev
Endocrinol. 19:443–459. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Olzmann JA and Carvalho P: Dynamics and
functions of lipid droplets. Nat Rev Mol Cell Biol. 20:137–155.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nosadini R and Tonolo G: Role of oxidized
low density lipoproteins and free fatty acids in the pathogenesis
of glomerulopathy and tubulointerstitial lesions in type 2
diabetes. Nutr Metab Cardiovasc Dis. 21:79–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shen T, Li X, Jin B, Loor JJ, Aboragah A,
Ju L, Fang Z, Yu H, Chen M, Zhu Y, et al: Free fatty acids impair
autophagic activity and activate nuclear factor kappa B signaling
and NLR family pyrin domain containing 3 inflammasome in calf
hepatocytes. J Dairy Sci. 104:11973–1182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li Y, Schwabe RF, DeVries-Seimon T, Yao
PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA
and Tabas I: Free cholesterol-loaded macrophages are an abundant
source of tumor necrosis factor-alpha and interleukin-6: Model of
NF-kappaB- and map kinase-dependent inflammation in advanced
atherosclerosis. J Biol Chem. 280:21763–21772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cheng J, Montecalvo A and Kane LP:
Regulation of NF-κB induction by TCR/CD28. Immunol Res. 50:113–117.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Di Marzo V: Arachidonic acid and
eicosanoids as targets and effectors in second messenger
interactions. Prostaglandins Leukot Essent Fatty Acids. 53:239–254.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Brien M, Berthiaume L, Rudkowska I, Julien
P and Bilodeau JF: Placental dimethyl acetal fatty acid derivatives
are elevated in preeclampsia. Placenta. 51:82–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bozelli JC Jr, Azher S and Epand RM:
Plasmalogens and chronic inflammatory diseases. Front Physiol.
12:7308292021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Brenner RR: Nutritional and hormonal
factors influencing desaturation of essential fatty acids. Prog
Lipid Res. 20:41–47. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cook HW: The influence of trans-acids on
desaturation and elongation of fatty acids in developing brain.
Lipids. 16:920–926. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chena X, Lia L, Liua X, Luoa R, Liaob G,
Lia L, Liua J, Chenga J, Lua Y and Chen Y: Oleic acid protects
saturated fatty acid mediated lipotoxicity in hepatocytes and rat
of non-alcoholic steatohepatitis. Life Sci. 203:291–304. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sun J, Jin X and Li Y: Current strategies
for nonalcoholic fatty liver disease treatment (Review). Int J Mol
Med. 54:882024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tian C, Huang R and Xiang M: SIRT1:
Harnessing multiple pathways to hinder NAFLD. Pharmacol Res.
203:1071552024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Smith BK, Marcinko K, Desjardins EM, Lally
JS, Ford RJ and Steinberg GR: Treatment of nonalcoholic fatty liver
disease: Role of AMPK. Am J Physiol Endocrinol Metab.
311:E730–E740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu Q, Liu S, Chen L, Zhao Z, Du S, Dong
Q, Xin Y and Xuan S: Role and effective therapeutic target of gut
microbiota in NAFLD/NASH. Exp Ther Med. 18:1935–1944.
2019.PubMed/NCBI
|
|
73
|
Kim SE, Min JS, Lee S, Lee DY and Choi D:
Different effects of menopausal hormone therapy on non-alcoholic
fatty liver disease based on the route of estrogen administration.
Sci Rep. 13:154612023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Keskin M, Hayıroğlu Mİ, Uzun AO, Güvenç
TS, Şahin S and Kozan Ö: Effect of nonalcoholic fatty liver disease
on in-hospital and long-term outcomes in patients with ST-segment
elevation myocardial infarction. Am J Cardiol. 120:1720–1726. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Şaylık F, Çınar T and Hayıroğlu Mİ: Effect
of the obesity paradox on mortality in patients with acute coronary
syndrome: A comprehensive meta-analysis of the literature. Balkan
Med J. 40:93–103. 2023. View Article : Google Scholar : PubMed/NCBI
|