Introduction
The cellular prion protein (PrPc) has
been studied for its diverse roles across various biological
systems, such as providing neuroprotection through the regulation
of copper and NMDA receptors, as well as maintaining stem cell
health by influencing Wnt/β-catenin signaling pathways (1–6),
with particular emphasis on unraveling its mechanisms of action
within the nervous system (7,8).
Notably, PrPc has been revealed to interact with
amyloid-β peptides in Alzheimer's disease and α-synuclein in
Parkinson's disease (9,10). Furthermore, PrPc can
modulate neuroendocrine signaling pathways, especially pathways
that are associated with the regulation of gonadotropin, revealing
a possible role in reproductive physiology (11,12).
PrPc is involved in oocyte maturation (13). Furthermore, in ovariectomized ewes,
the expression of PrPc in ovine uteroplacental tissues
was shown to increase when the ewes were treated with estrogen and
during the early stage of pregnancy (14). However, the roles of
PrPc, including how it regulates ovarian reserve
maintenance and the dynamics of follicular recruitment, have not
been fully elucidated.
Recent advances in reproductive endocrinology
demonstrate the need to clarify the non-steroidogenic mechanisms
that govern follicular homeostasis, with a particular focus on
mechanisms involving anti-Müllerian hormone (AMH)-mediated
signaling (15–17). As AMH levels are closely linked to
ovarian reserve and are recognized as the most sensitive clinical
biomarker for diminished ovarian reserve (DOR) syndromes, it is
crucial to clarify the regulatory mechanisms involved (18,19).
We hypothesized that PrPc may act as a novel type of
modulator for AMH-dependent follicular recruitment, potentially
through mechanisms that are different from the regulation of
classical steroid hormones. To test this hypothesis, two
complementary models were used, the in vitro mouse ovarian
granulosa cells (mGCs) with prion protein gene (PRNP)
knockdown and overexpression (OE) and the in vivo PRNP
knockout (KO) and wild-type (WT) models. The specific effects of
PrPc on AMH secretion were compared with those on
progesterone (P4) and estradiol (E2) production. The
present study aimed to analyzed the effects of maintaining the
follicular pool through histomorphometric analysis and reproductive
longevity via litter size tracking, while also examining
neuroendocrine adaptations in the HPG axis using vaginal cytology,
serum FSH quantification, and ovarian reserve evaluation through
follicle counting.
Materials and methods
Cell culture
mGCs were procured from Haixing Biosciences Co.,
Ltd. (cat. no. ORCM050). The cells were grown in high-glucose DMEM
(cat. no. 12,100; Beijing Solarbio Science & Technology Co.,
Ltd.), which included 100 U/ml penicillin and 100 µg/ml
streptomycin as standard components, with 10% heat-inactivated FBS
(cat. no. FSS500; Shanghai ExCell Biology, Inc.). mGCs were
maintained at 37°C in an atmosphere with 5% CO2, and the
culture medium was replaced every 2 days. To preserve physiological
relevance, cell passage was restricted to early generations
(P2-P3), thereby minimizing cellular dedifferentiation.
Additionally, follicle stimulating hormone receptor (FSHR)
expression levels were periodically confirmed by
immunofluorescence, ≥80% of cells exhibited positive FSHR staining
(Fig. S1A).
Immunofluorescence
Cell coverslips were prepared by seeding
1×105 cells in 1 ml of medium and incubating them
overnight at 37°C with 5% CO2 until they reached 70–80%
confluency. After removing the medium, the cells were washed with
ice-cold PBS and fixed using 4% paraformaldehyde (PFA) for 30 min
at room temperature. Following additional washes with PBS, the
cells were permeabilized with 0.5% Triton™ X-100 for 15
min and then blocked with 10% goat serum (cat. no. S9070; Beijing
Solarbio Science & Technology Co., Ltd.) for 60 min at room
temperature. The coverslips were then incubated overnight at 4°C
with a rabbit anti-FSHR antibody diluted to 1:200 (cat. no.
22665-1-AP; Proteintech Group, Inc.). After washing, the cells were
stained with an Alexa Fluor® 488-conjugated secondary
antibody at a dilution of 1:500 (cat. no. SA00006-2; Proteintech
Group, Inc.) for 2 h at room temperature. Finally, the coverslips
were mounted on slides using DAPI antifade medium (cat. no. S2110;
Beijing Solarbio Science & Technology Co., Ltd.), and the
samples were imaged for analysis using an inverted platform
(Primovert LED; ZEISS, Germany).
Transfection
Recombinant lentiviruses, rLV-short hairpin
(sh)RNA-Puro-mPRNP and rLV-mPRNP−3flag-ZsGreen-Puro
were procured from Haixing Biosciences Co., Ltd. Empty vectors were
used as negative controls (NCs). The target sequences of the
PRNP shRNAs were as follows: mPRNP sh-1,
5′-GGACAACCTCATGGTGGTAGT-3′; mPRNP sh-2,
5′-GCGTCAATATCACCATCAAGC-3′; and mPRNP sh-3,
5′-GCCTATTACGACGGGAGAAGA-3′. To achieve stable silencing and OE of
the PRNP gene, the cells were seeded into 6-well plates at a
density of 2.5×105 cells per well, and cultured
overnight until they reached a confluence of 40–50%. On the
subsequent day, lentiviral transfection of the cells was performed
following the protocol supplied by the manufacturer. Lentiviral
vectors that encode EGFP reporters were generated using a
third-generation system in 293T cells (cat. no. CL-0005; Wuhan
Pricella Biotechnology Co., Ltd.). The cells were transfected at
70% confluence with 3 µg pLVX-shRNA2-Puro-mPrnp or
pLVX-mPrnp-3flag-ZsGreen-Puro transfer plasmid, 2 µg of psPAX2, and
1 µg of pMD2.G in a ratio of 3:2:1 using PEI in Opti-MEM. After 48
h of incubation at 37°C with 5% CO2, fluorescence
screening indicated a packaging efficiency of over 85%. To obtain
stably transfected mGCs, lentiviral transduction was performed at
the optimized multiplicity of infection (MOI) of 80, followed by
selection with 3 µg/ml of puromycin for 72 h (cat. no. PS1224;
Beijing Puxitang Biotechnology Co., Ltd.), and 1 µg/ml thereafter
for culture maintenance. After 72 h of transduction, the infected
mGCs were collected for subsequent experiments. Stable
PrPc-knockdown mGCs were established through lentiviral
shRNA transduction. Following this, these cells were infected with
a lentivirus designed to overexpress PrPc, allowing for
both the knockdown and overexpression of PrPc within the
same cellular environment.
Reverse transcription quantitative PCR
(RT-qPCR)
Total RNA was extracted from mGCs following the
manufacturer's instructions (cat. no. UE-MN-MS-RNA-10; Suzhou
UElandy Biotechnology Co., Ltd.). Quantity and purity of RNA were
determined using a NanoDrop™ 2000 spectrophotometer
(Thermo Scientific). The RNA concentrations were measured at A260,
while the purity was evaluated by calculating the A260/A280 ratios.
Subsequently, RT was performed following the manufacturer's
instructions for the RevertAid™ Master Mix (cat. no.
M1632; Thermo Fisher Scientific, Inc.). qPCR was carried out on a
CFX Connect fluorescent Real-Time PCR System (Bio-Rad Laboratories,
Inc.), using the Universal SYBR Green qPCR Supermix (cat. no.
S2024L; US Everbright, Inc.). The thermocycling conditions were as
follows: 95°C for 5 min, followed by 45 cycles of 95°C for 5 sec
and 60°C for 30 sec. The PRNP primer sequences (cat. no.
MQP094390; GeneCopoeia, Inc.) were as follows: PRNP forward
(F), 5′-GGCCCATGATCCATTTTGGC-3′ and reverse (R),
5′-TGCTGTACTGATCCACTGGC-3′. Additionally, the following primer
sequences were used for β-actin (Wuhan Servicebio Technology Co.,
Ltd.): β-actin F, 5′-GACTTTGTACATTGTTTTG-3′ and R,
5′-TGCACTTTTATTGGTCTCA-3′, which was selected as the internal
control for normalization. The 2−ΔΔCq method was
employed for quantification of the data (20). qPCR was selected for its superior
sensitivity in detecting transcript-level changes (detection
threshold ≤10 copies/µl) compared with conventional PCR (21,22),
with SYBR Green chemistry providing cost-effective quantification
of PRNP expression dynamics.
Western blotting (WB)
mGCs and ovarian tissue were lysed on ice for 30 min
in RIPA buffer supplemented with protease inhibitors (cat. no.
R0010; Beijing Solarbio Science & Technology Co., Ltd.). The
total protein content was determined using a BCA Protein
Quantification Kit (cat. no. C0050; TargetMol Chemicals Inc.).
Following protein quantification, 25 µg total protein/lane in each
sample were separated via 10% SWE Rapid High Resolution Running
Buffer (cat. no. G2081-1L; Wuhan Servicebio Technology Co., Ltd.).
Subsequently, the separated proteins were transferred onto PVDF
membranes (cat. no. IPVH00010; MilliporeSigma).
To block non-specific binding, the PVDF membranes
were incubated in a solution of 5% non-fat dried milk (cat. no.
S10191; Beijing Puxitang Biotechnology Co., Ltd.) in Tris-buffered
saline containing 0.1% Tween 20 (TBST; cat. no. T1082; Beijing
Solarbio Science & Technology Co., Ltd.) for 2 h at room
temperature. After blocking, the membranes were incubated overnight
at 4°C with the following primary antibodies: Anti-CD230 (Prion;
cat. no. 808001; BioLegend, Inc.) and anti-β-actin at a 1:5,000
dilution (cat. no 20536-1-AP; Proteintech Group, Inc.).
After the overnight incubation, the membranes were
washed three times with TBST and incubated for 2 h at room
temperature with the following secondary antibodies: HRP-conjugated
goat anti-rabbit IgG at a 1:1,000 dilution (SA00001-2; Proteintech
Group, Inc.) and HRP-conjugated goat anti-mouse IgG at a 1:10,000
dilution (SA00001-1; Proteintech Group, Inc.).
Finally, an enhanced chemiluminescence detection
reagent (cat. no. S6009L; Suzhou UE Landi Biotechnology Co., Ltd.)
was used to visualize the immunoreactive bands on ChemiDoc MP
(Bio-Rad Laboratories, Inc.). Densitometry of the WB bands was
performed using ImageJ 1.48V software (National Institutes of
Health).
Apoptosis assay
Following the instructions of the Annexin V/PI
detection Kit (cat. no. Y6026S; Suzhou UE Landy Biotechnology Co.,
Ltd.), 1×105 mGCs were digested with 0.25%
trypsinization solution (without EDTA)(cat. no. T1350; Beijing
Solarbio Science & Technology Co., Ltd.), then enzymatically
neutralized and resuspended in cold staining buffer, then
resuspended in a staining buffer, which was prepared by adding 5 µl
each Annexin V storage solution and 5 µl PI storage solution.
Subsequently, the cells were incubated in the dark at 4°C for 20
min and analyzed using a NovoCyte D3000 flow cytometer (Agilent
Technologies, Inc.) Flow cytometry data were analyzed using De Novo
FCS Express™ 6 software (Agilent Technologies, Inc.).
Annexin V/PI dual staining was employed to differentiate early
apoptotic (Annexin V+/PI−) from late
apoptotic/necrotic (Annexin V+/PI+)
populations, enabling stage-specific analysis of mGC death
pathways, the total percentage of apoptotic cells reflects the
overall measurement of both early and late apoptotic
populations.
Cell cycle assay
For cell cycle analysis, 5×105 mGCs were
harvested by trypsinization at 72 h post-infection. The cells were
then washed with cold PBS and fixed in 70% ice-cold ethanol at 4°C
overnight. Next, mGCs were incubated with RNase A and PI in a 0.5
ml reaction mixture (cat. no. C6031S; Suzhou UE Landy Biotechnology
Co., Ltd.) at 37°C for 30 min in a dark chamber. Cell cycle
distribution was measured using a NovoCyte D3000 flow cytometer and
analyzed with De Novo FCS Express™ 6 software (Agilent
Technologies, Inc.).
ELISA for hormone measurements
mGC culture medium containing 10% FBS and mouse
serum collected by centrifugation at 3,000 × g for 5 min at 4°C
were used to measure the concentrations of E2 (cat. no.
SEKM-0286; Beijing Solarbio Science & Technology Co., Ltd.),
AMH (cat. no SEKM-0310; Beijing Solarbio Science & Technology
Co., Ltd.), P4 (cat. no. SEKSM-0002; Beijing Solarbio Science &
Technology Co., Ltd.), FSH (cat. no. KE1425; ImmunoWay
Biotechnology Company) and luteinizing hormone (LH; cat. no.
KE1421; ImmunoWay Biotechnology Company), following the
manufacturer's instructions.
Animals
A total of 24 mice were used, comprising FVB-PRNP-/-
and FVB wild-type (WT) mice, with each group consisting of 12
females. At the start of the experiment, the mice were 7 weeks old,
and body weight was of 19.1–22.9 g (mean ± SD: 20.82±1.13 g). PRNP
KO mice with an FVB genetic background were provided by Professor
Wen-Quan Zou (Jiangxi Academy of Clinical Medical Sciences of the
First Affiliated Hospital of Nanchang University) and Professor Li
Cui (Department of Neurology, First Hospital of Jilin University).
FVB WT mice, sourced from GemPharmatech Co. Ltd., were utilized as
controls in the present study. All experimental protocols were
approved by the Institutional Animal Care and Use Committee (IACUC)
of the First Affiliated Hospital of Nanchang University (approval
no. CDYFY-IACUC-202310QR030; Nanchang, China) in accordance with
the National Institutes of Health Guidelines for animal welfare. To
maintain optimal health and experimental integrity, both the KO and
WT mice were housed in individually ventilated cages under specific
pathogen-free (SPF) conditions, maintaining a temperature of 22±1°C
and humidity levels of 55±10%. They were kept on a 12:12-h
light/dark cycle. The animals had unrestricted access to autoclaved
standard rodent chow and reverse-osmosis purified water. These
carefully controlled housing conditions minimized the potential
influence of external pathogens on the experimental outcomes,
ensuring that any observed differences between the KO and WT groups
could be more accurately attributed to the genetic modifications
rather than environmental factors. Breeding pairs were formed by
housing WT and KO female mice (n=6 per group) with proven-fertility
WT males at 10 weeks of age under specific pathogen-free
conditions. After confirming the presence of copulatory plugs,
which indicated successful mating and was designated as Gestational
Day 0, the dams were individually housed in ventilated cages.
Inhalant anesthesia was induced with 5% isoflurane (cat. no.
R510-22-16; RWD Life Science Co., Ltd.) in oxygen at 1 l/min flow
rate, and maintained for 5 min until the pedal withdrawal reflex
ceased and the respiratory rate stabilized (40–60 breaths/min).
Cervical dislocation was performed with simultaneous isoflurane
overdose to ensure humane euthanasia. Death validation included
confirmation of apnea for >3 min and absence of the corneal
reflex. Terminal whole blood samples (~0.3 ml) were obtained via
retro-orbital venous plexus puncture within 2 min post-mortem to
ensure coagulation integrity. Ovarian tissues were dissected, fixed
in 4% paraformaldehyde at 4°C for 24–48 h, then processed for
paraffin embedding and hematoxylin & eosin (H&E)
staining.
H&E staining
H&E staining was performed in accordance with a
standard protocol (23,24). Specifically, ovarian tissues were
fixed in 4% paraformaldehyde (w/v in 0.1 M PBS, pH 7.4) at 4°C for
a duration of 24 to 48 h, 5-µm-thick paraffin sections underwent
three 5-min immersions in xylene. This was followed by a
rehydration process through a graded series of ethanol
concentrations: 100, 95, 80, and 70% (v/v), with each step lasting
2 min, ultimately concluding in distilled water. Next, the sections
were stained with H&E (cat. no. L11021604l; Nanchang Yulu
Experimental Equipment Co., Ltd.). Sections were stained with
Mayer's hematoxylin at room temperature (23±2°C) for a duration of
3 min, and this was followed by staining with eosin Y alcoholic
solution at the same temperature for 2 min. Ovarian follicles were
quantified using systematic random sampling in every sixth serial
section with a thickness of 5 µm. A manual counting method was
employed, examining 30 non-overlapping fields per ovary at a
magnification of 200× using a light microscope (Beijing Sunny
Instruments Co., Ltd.). Follicles were identified across various
stages, from primordial to antral, in accordance with the Amsterdam
Consensus Criteria (25,26).
Estrous cycle staging
Estrous cycle staging was performed through daily
vaginal smear cytology between 9:00-10:00 a.m. Vaginal lavage
samples were obtained using 10 µl sterile PBS, placed onto
poly-L-lysine coated slides, and fixed in 4% paraformaldehyde at
4°C for 15 min. Hematoxylin (3 min) and eosin (30 sec) staining at
room temperature, adhering to standard protocols.
Statistical analysis
Data are presented as the mean ± SD based on a
minimum of three independent experiments. Prior to analysis,
normality was assessed using Shapiro-Wilk test (α=0.05) and the
homogeneity of variance was confirmed via Brown-Forsythe test.
Parametric tests were applied when assumptions were satisfied.
Statistical analyses were carried out using GraphPad Prism 9.5
software (Dotmatics). For the comparison between two groups, a
two-tailed unpaired Student's t-test was used, with Cohen's d
effect sizes and 95% confidence intervals automatically calculated
by the software. For the comparison of >2 groups, one-way ANOVA
with Tukey's post hoc test (for all pairwise comparisons) or
Dunnett's post hoc test (for comparisons vs. the control group) was
applied, with partial η2 effect sizes and family-wise
error rate control at α=0.05. P<0.05 was considered to indicate
a statistically significant difference.
Results
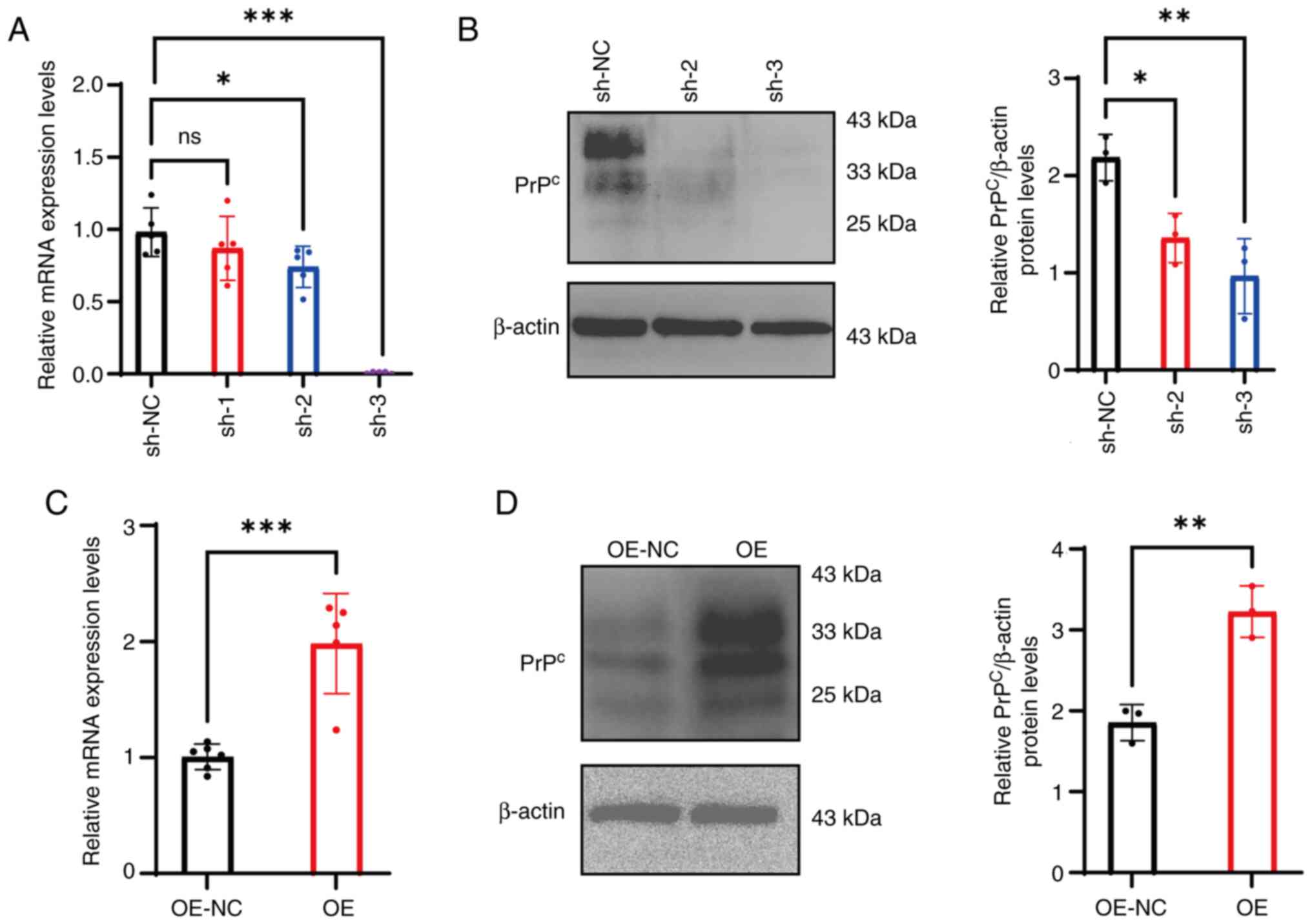
Validation of PrPc
knockdown and OE efficiency
To knock down PrPc in mGCs, the following
lentivirus-based vectors were used: mPRNP sh-1, sh-2 and
sh-3. Compared with those of mGCs infected with the NC vector
(sh-NC), the mRNA expression levels of PRNP in the sh-1
group exhibited no significant change (P>0.05). However,
infections with sh-2 and sh-3 resulted in a marked reduction in
mRNA expression (sh-2:P<0.05; Sh-3:P<0.001), respectively,
confirming successful knockdown of the PRNP gene (Fig. 1A). Supporting these findings, WB
analysis revealed that the gray values of the PrPc
protein bands in cells transfected with sh-2 and sh-3 were
significantly reduced compared with those in the sh-NC group
(P<0.05; Fig. 1B). Based on
these results, sh-2 and sh-3 were selected as the most efficient
shRNAs for subsequent experiments.
In the PrPc OE group, the mRNA expression
levels of PRNP were significantly increased compared with
those in the OE-NC group (P<0.001; Fig. 1C), indicating that the OE vector
effectively increased the transcription of the PRNP gene.
Correspondingly, the expression levels of the PrPc
protein in the OE group was significantly increased compared with
that in the OE-NC group (P<0.01; Fig. 1D), aligning with the observed trend
in mRNA expression levels.
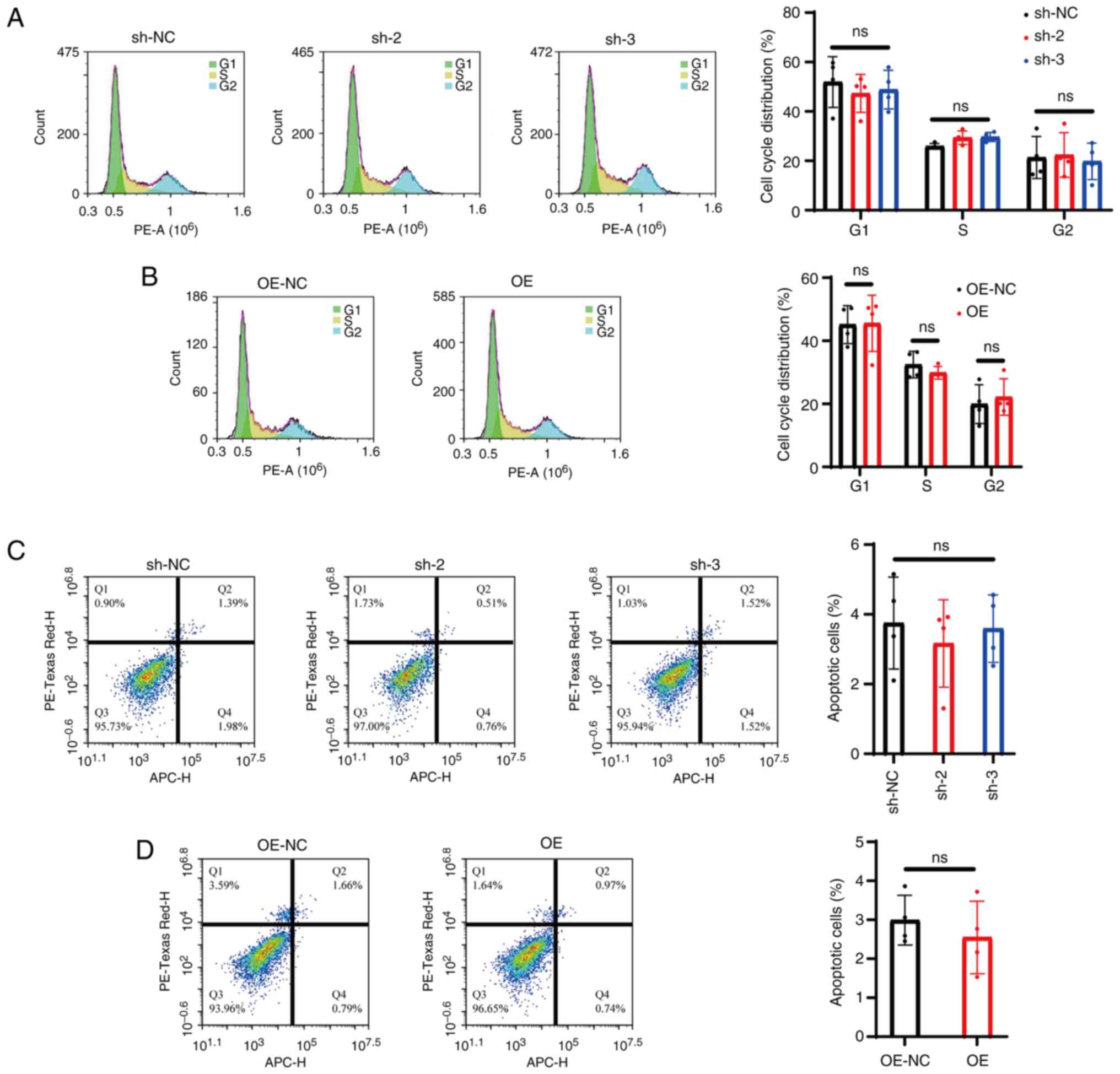
PrPc does not alter mGC
cycle progression or apoptosis
Flow cytometry analysis was carried out to assess
cell cycle distribution in mGCs. The results revealed no
significant differences in the proportions of cells in the
G1, S and G2 phases among the sh-2, sh-3 and
sh-NC groups (P>0.05; Fig. 2A).
Likewise, no notable changes were observed in the distribution of
cells across these phases in the OE group compared with OE-NC
(P>0.05; Fig. 2B).
Analysis of apoptosis using the Annexin V/PI double
staining method by flow cytometry revealed that, under normal
culture conditions, the apoptosis rate of mGCs was consistent
between the sh-NC and OE-NC groups. Moreover, sh-2, sh-3 and sh-NC
did not exhibit any significant differences in the proportion of
apoptotic cells (P<0.05; Fig.
2C). Similarly, the OE group demonstrated no notable variation
in the proportion of apoptotic cells compared with OE-NC
(P<0.05; Fig. 2D).
PrPc knockdown reduces AMH
secretion while preserving P4 and E2 production
in mGCs
AMH levels averaged 56.10±3.73 ng/ml in the sh-NC
group, while they significantly decreased to 42.27±7.51 and
42.7±6.85 ng/ml in the sh-2 and sh-3 groups respectively
(P<0.05; Fig. 3A). However,
PrPc expression was restored following knockdown in mGCs
infected both with the PRNP knockdown and OE vectors (sh-2 +
OE and sh-3 + OE; Fig. S1B), and
AMH levels increased compared with those in the sh-2 and sh-3
groups (P<0.05; Fig. 3A). By
contrast, the average AMH expression levels in the OE group was
46.96±4.22 ng/ml, exhibiting no significant difference compared
with that in the OE-NC group (P>0.05; Fig. 3A).
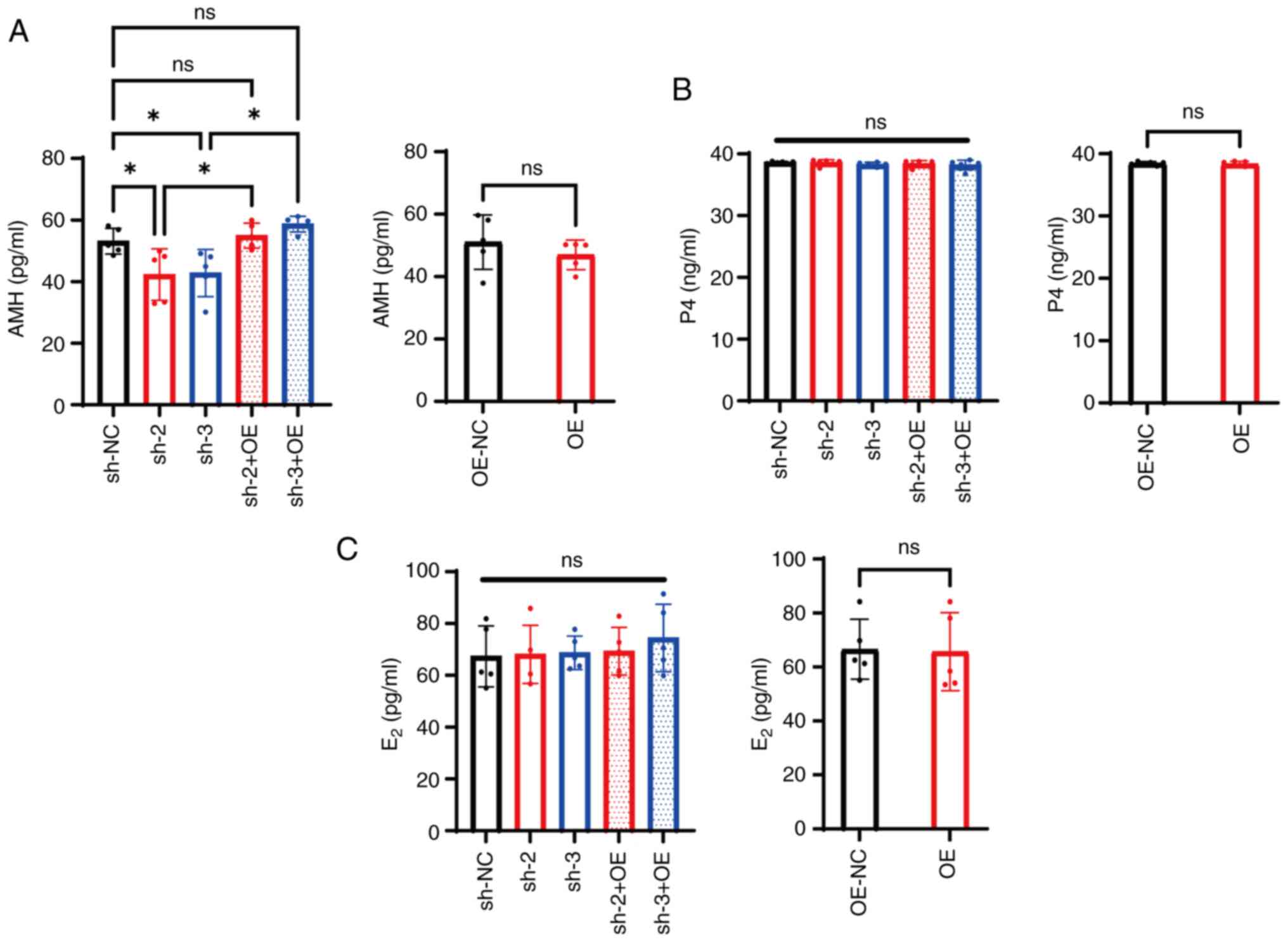 | Figure 3.Cellular prion protein knockdown
specifically reduces AMH secretion in mGCs. (A) AMH, (B) P4 and (C)
E2 levels in the culture medium of mGCs transfected with
different shRNAs, OE vectors or their combination. *P<0.05. ns,
non-significant; AMH, anti-Müllerian hormone; mGCs, mouse granulosa
cells; P4, progesterone; E2, estradiol; sh, short
hairpin; OE, overexpression; NC, negative control. |
The average P4 level was 38.61±0.15 ng/ml in the
sh-NC group, and 38.57±0.43 and 38.24±0.37 ng/ml in the sh-2 and
sh-3 groups, respectively, with the latter showing no significant
difference compared with the control group (P>0.05; Fig. 3B). Furthermore, compared with the
sh-2 and sh-3 groups, the sh-2 + OE and sh-3 + OE groups did not
exhibit significant changes in P4 levels (P>0.05; Fig. 3B). Similarly, the average P4 level
in the OE group was 38.33±0.40 ng/ml, which was comparable with
that in the OE-NC group (P>0.05; Fig. 3B).
The average E2 level in the sh-NC group
was 67.33±10.54 pg/ml, while the sh-2 and sh-3 groups exhibited
values of 68.11±10.07 and 68.74±5.81 pg/ml, respectively, both
showing no significant differences compared with sh-NC (P>0.05;
Fig. 3C). Similarly, compared with
the sh-2 and sh-3 groups, no notable changes in E2
expression levels were observed in the sh-2 + OE and sh-3 + OE
groups (P>0.05; Fig. 3C).
Likewise, the average E2 level in the OE group was
65.67±11.67 pg/ml, which was not significantly different compared
with that in the OE-NC group (P>0.05; Fig. 3C).
PrPc expression levels do
not affect the classification count of ovaries
Ovaries from WT and KO mice were isolated and WB was
carried out to assess PrPc protein expression levels.
The results indicated that PrPc levels in the ovaries of
KO mice were reduced compared with those in the WT group (Fig. 4A) confirming the effectiveness of
the KO.
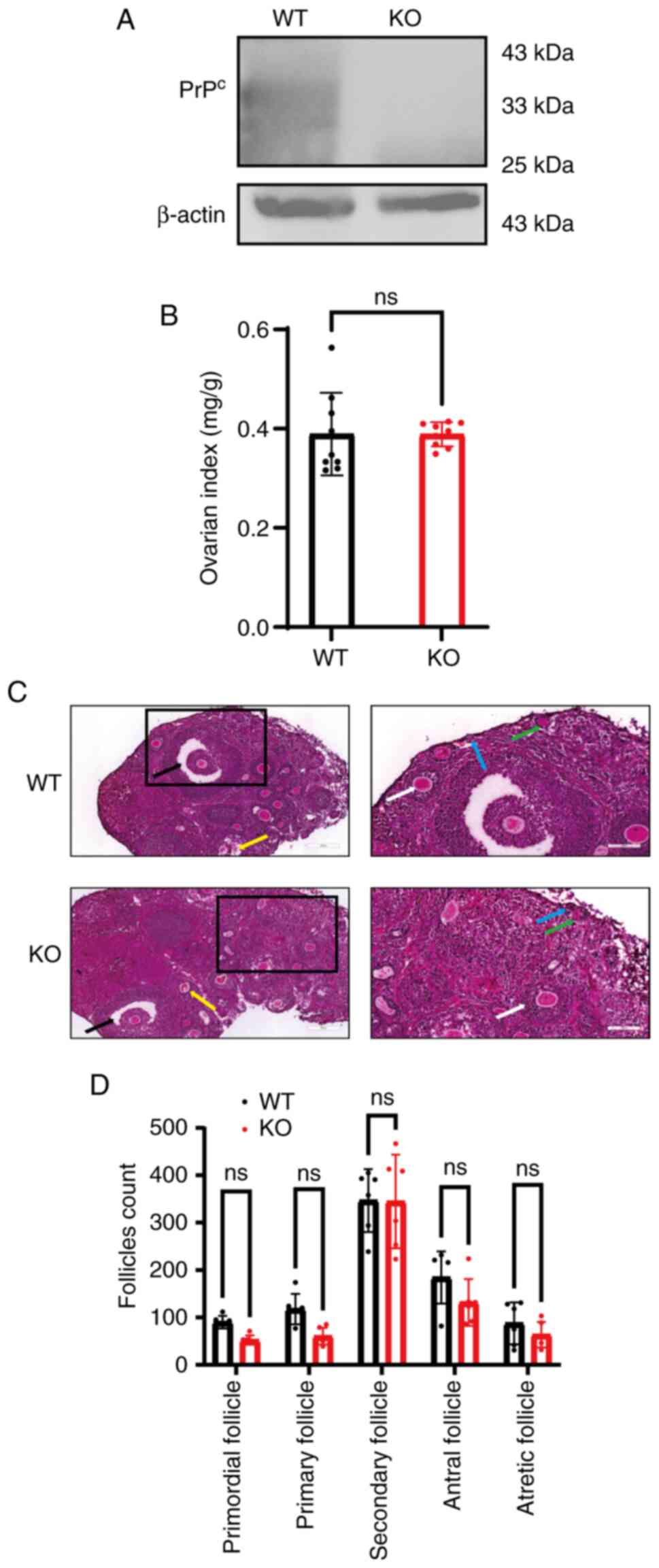 | Figure 4.Prion protein gene KO mice exhibit
normal ovarian morphology and follicular development. (A)
PrPc protein expression in the ovaries of WT and KO
mice. (B) Ratio of bilateral ovary weight to body weight showed no
differences between groups. (C) H&E staining on mouse ovaries
(scale bars, 200 µm for overviews and 100 µm for higher mag). The
stages of follicular development are clearly indicated by
color-coded arrows: Blue represents primordial follicles, which
consist of a single layer of squamous granulosa cells; green
denotes primary follicles, characterized by cuboidal granulosa
cells; white signifies secondary follicles, identifiable by having
≥3 layers of granulosa cells and the early formation of an antrum;
black marks antral follicles, distinguished by the presence of a
fluid-filled cavity; and yellow indicates atretic follicles, which
display pyknotic granulosa cell nuclei and fragmentation of the
zona pellucida. (D) Follicle counts across developmental stages
were comparable between WT and KO mice. ns, non-significant; WT,
wild-type; KO, knockout; PrPc, cellular prion
protein. |
At 7 weeks of age, ovarian tissue was collected, and
the wet weight index of ovaries was measured. The WT group had an
ovarian weight of 0.390±0.08 mg/g, while the KO group exhibited a
weight of 0.389±0.02 mg/g, with no statistically significant
difference between the groups (P>0.05; Fig. 4B).
H&E staining revealed the pathological and
morphological characteristics of the ovaries in each group
(Fig. 4C). Although the KO group
exhibited a slight reduction in the number of primordial and
primary follicles, this difference was not statistically
significant (P>0.05; Fig. 4D).
Similarly, there were no significant differences in the numbers of
secondary, antral or atretic follicles between the two groups
(P>0.05; Fig. 4D).
PRNP deficiency elevates circulating
FSH levels with unaltered AMH, LH and E2 levels
FSH levels in KO mice were significantly increased
at 6.64±0.68 ng/ml compared with 4.52±1.18 ng/ml in the WT group
(P<0.01; Fig. 5A). By contrast,
E2 levels were not significantly different between the
two groups (P>0.05; Fig. 5B).
Similarly, AMH and LH levels were also not significantly different
between WT and KO mice (P>0.05; Fig. 5C and D).
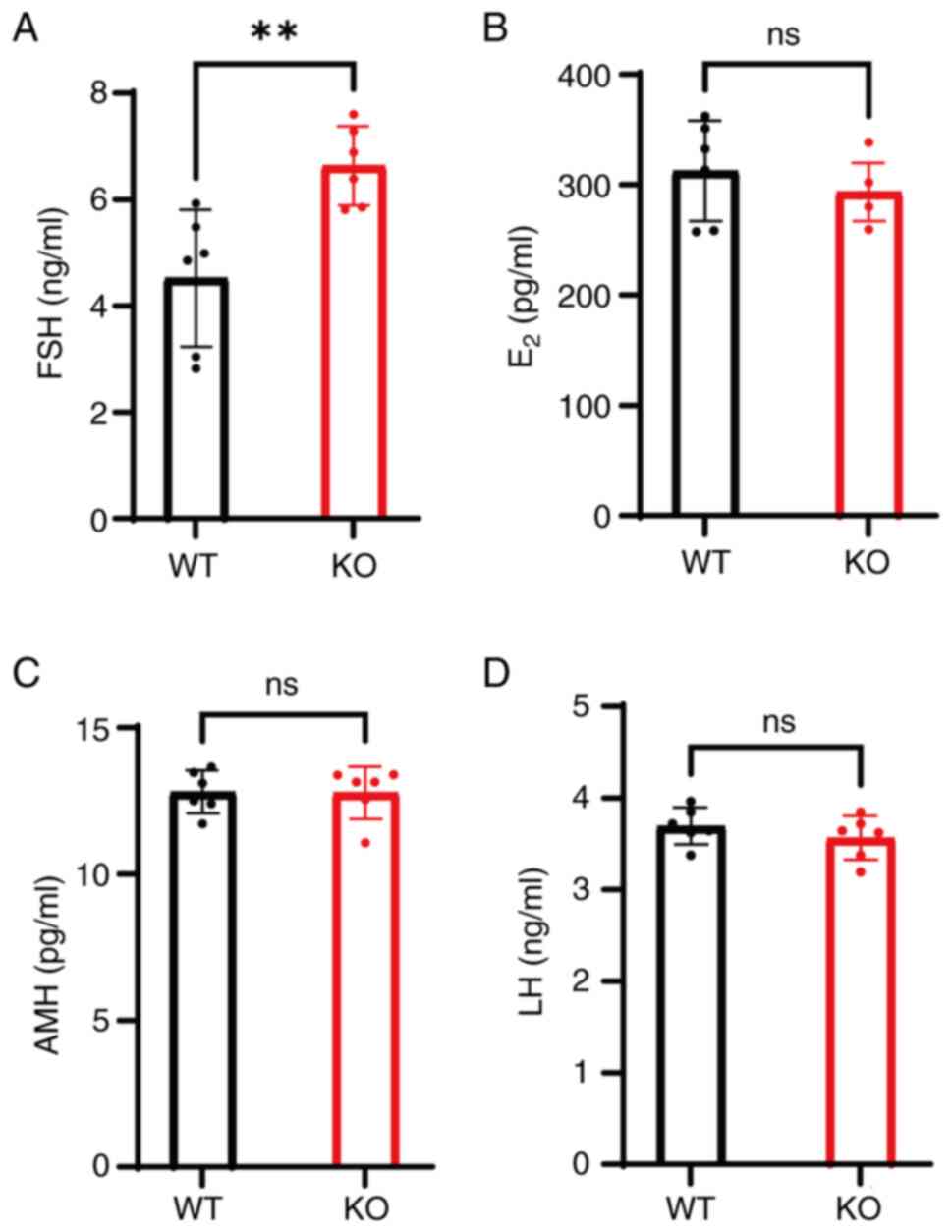 | Figure 5.KO mice exhibit elevated FSH levels
but normal E2, AMH and LH levels. Serum hormone levels
of (A) FSH, (B) E2, (C) AMH and (D) LH in WT and KO
mice. **P<0.01. ns, non-significant; FSH, follicle-stimulating
hormone; LH, luteinizing hormone; E2, estradiol; AMH,
anti-Müllerian hormone; WT, wild-type; KO, knockout. |
PRNP KO mice exhibit normal
reproductive performance
WT and KO mice exhibited a standard estrous cycle,
typically spanning 4–5 days per cycle and encompassing four
distinct phases: Proestrus, estrus, metestrus and diestrus
(Fig. 6A). In the proestrus stage
(P), the smears are primarily made up of nucleated epithelial
cells, which are characterized by their polygonal shape, large
spherical nuclei, and abundant cytoplasm. As the cycle progresses
to estrus (E), there is a notable increase in keratinized
epithelial cells, which display a distinct eosinophilic staining in
their cytoplasm. This is followed by metestrus (M), where the
number of keratinized cells begins to decline, and nucleated
epithelial cells become more present, accompanied by a few clusters
of leukocytes. Finally, in the diestrus stage, the cytological
composition shifts to predominantly leukocytes, with few scattered
nucleated epithelial cells present (27). Each stage in the WT mice was
clearly defined, with smooth and consistent transitions between
phases. Similarly, KO mice exhibited no notable alterations in
cycle duration, with neither a marked prolongation nor a noticeable
shortening of the cycle observed (Fig.
6B).
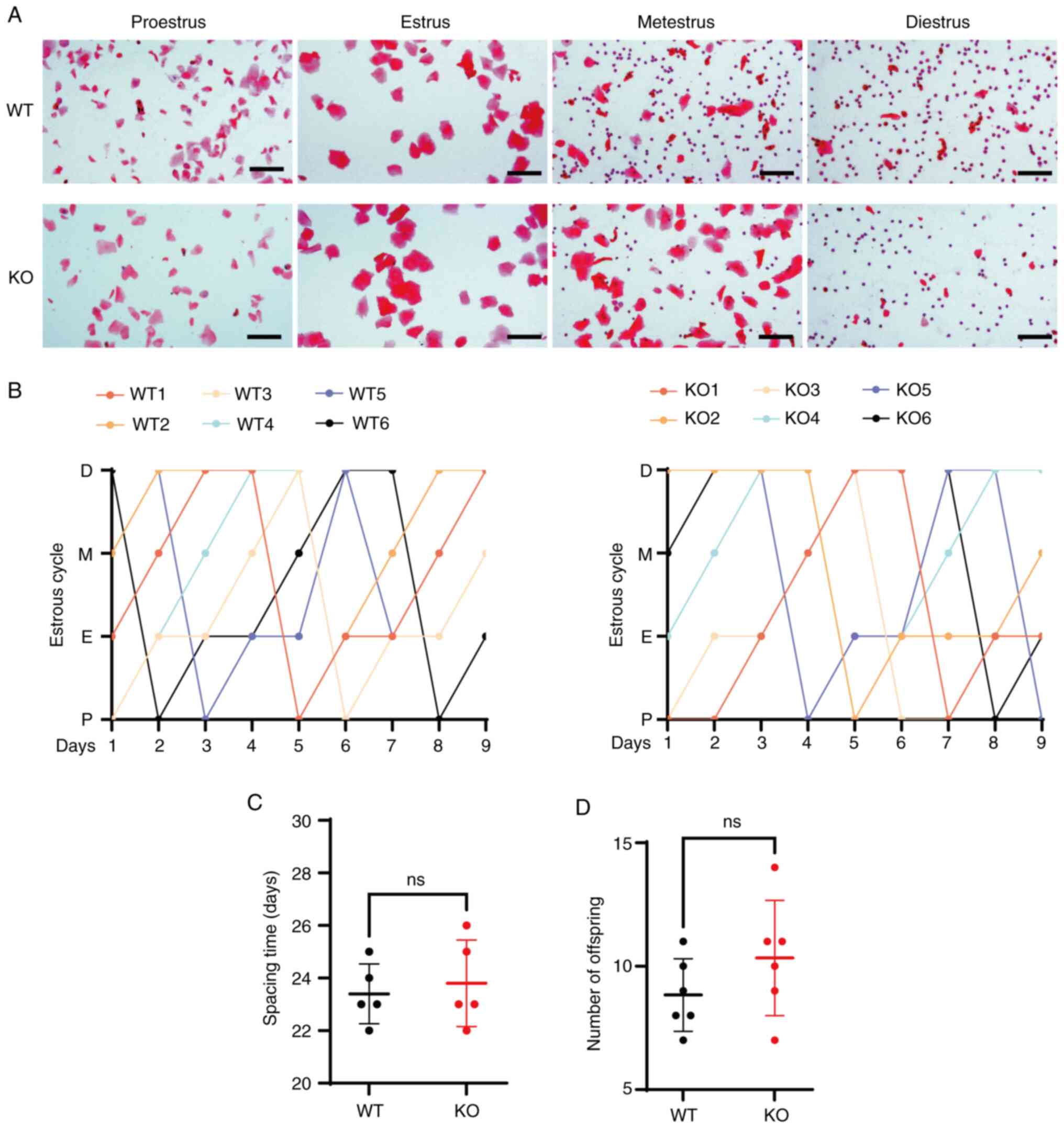 | Figure 6.KO mice exhibit regular estrous cycle
and fertility. (A) Vaginal secretion smears stained with H&E
stain during estrous cycle phases: Proestrus, estrus, metestrus,
diestrus (scale bar, 50 µm). (B) Regular estrous cycle patterns in
mice spanning 9 consecutive days. (C) Interval from mating,
following co-housing, to parturition. (D) Number of offspring at
birth. ns, non-significant; WT, wild-type; KO, knockout; P,
proestrus; E, estrus; M, metestrus; D, diestrus. |
No statistically significant differences were
observed in the litter intervals between the two groups (P>0.05;
Fig. 6C). The average litter size
remained consistent at 8–10 pups, and the mothers exhibited normal
nurturing abilities. The offspring survival rate was high, with no
notable differences detected between the groups (P>0.05;
Fig. 6D).
Discussion
Previous studies have predominantly explored the
role of PrPc within the nervous system (28–31).
However, there is little research on the function of
PrPc in non-neuronal tissues, especially in endocrine
regulation. The present in vitro study revealed that
PrPc can selectively affect the secretion of AMH, while
no significant changes in AMH, P4, E2, folliculogenesis
and the reproductive cyclicity in mice. These findings broaden the
functional range of PrPc beyond its typical roles in
neuroprotection and synaptic plasticity (32,33),
indicating that PrPc may be a potential niche regulator
of ovarian reserve biomarkers.
The present study revealed that alterations in the
expression of PrPc in mGCs did not significantly
influence the cell cycle or apoptosis, suggesting that
PrPc may not regulate cellular homeostasis in mGCs.
PrPc may not directly affect the signaling molecules
involved in key cell cycle transitions, such as those between the
G1/S and G2/M phases. However, its role
varies significantly in other systems. In neural environments,
PrPc acts as an accelerator of apoptosis during
proteotoxic stress by impairing the ESCRT-0/AMPAR axis. In renal
settings, it promotes regeneration through the activation of the
PI3K/Akt-mTOR pathway. In cardiac contexts, PrPc is
crucial for structural recovery, although it does not influence
functional recovery. Additionally, in oncogenic environments, it
serves as a key regulator of cell fate by modulating the dynamics
between p53 and MDM2 during endoplasmic reticulum stress (34–36).
Additionally, a previous study has highlighted the presence of
compensatory mechanisms among members of the prion protein family,
particularly PrPc and Shadoo, which aid in maintaining
cell cycle progression and suppressing apoptosis (37).
The levels of AMH, which is considered a marker of
the ovarian reserve function and is primarily secreted by GCs
(15), were decreased when
PrPc was knocked down. The effect of PrPc
knockdown may be associated with disrupted TGF-β/bone morphogenetic
protein (BMP)-SMAD superfamily signaling pathways (38). AMH transcription is strictly
controlled by SMAD1/5/8 complexes, which are activated by BMP
ligands (39). The present
findings are consistent with mechanistic framework proposed by Puig
et al (40), which suggests
that PrPc plays a role in modulating the trafficking of
FSHR and bone morphogenetic protein receptors (BMPR-II). Regarding
the secretion of P4 and E2, no significant changes were
observed after PrPc knockdown or overexpression, and the
AMH-specific effect contrasts those of classical steroidogenic
regulators, such as PPAR-γ agonists, miR-335-5p, retinoic acid, and
artemisinins, which alter multiple hormonal axes (41–44).
It may be hypothesized that PrPc stabilizes BMP receptor
clusters on GCs, which activates SMAD phosphorylation and
subsequent AMH synthesis. This hypothesis can be tested by mapping
the interactions between PrPc and BMP receptor 2 and by
quantifying the levels of phosphorylated-SMAD1/5 in PrPc
knockdown models.
The results of the present study indicating that
PrPc is dispensable for murine ovarian function
contrasts with its reported roles in neuronal survival,
underscoring profound tissue specificity (45). Although PrPc deletion in
neurons induces apoptosis via Bcl-2 suppression, GCs may remain
viable after PrPc depletion through compensatory
mechanisms involving the Shadoo protein (46).
Despite minor changes, no statistically significant
differences were observed in the ovary weight and the numbers of
primordial and primary follicles in the PrPc KO mice
compared with the WT group. Furthermore, there seemed to be no
evident changes in the estrous cycle, litter interval or the number
of pups per litter between the two groups. This suggests that
PrPc may not be critical in maintaining the structure
and reproductive activity of the ovaries. Vigorous compensatory
mechanisms in the organism may have abolished the effect of
PrPc deficiency on ovarian function.
Furthermore, the lack of PrPc does not
seem to interfere with the estrous cycle or the reproductive
functions, potentially because the hormonal regulatory pathways
within the hypothalamic-pituitary-ovarian axis maintain normal
reproductive rhythms and functions (47). Nonetheless, it cannot be ruled out
that, under chemotherapy-induced ovarian damage or under
hyperandrogenic conditions mimicking polycystic ovary syndrome
(PCOS), PrPc may have a key impact on reproductive
functions.
The mouse model used in the present study may not
capture all the physiological and pathological intricacies of the
human ovary. There are marked differences in the structural,
functional and hormonal regulation, as well as the reproductive
cycle between mouse and human ovaries. For example,
folliculogenesis in humans is a process that spans 90 to 120 days,
which is significantly longer than the 14 to 21-day cycle observed
in mice. In humans, primordial follicles can remain dormant for
several decades, a situation that can contribute to oxidative
stress within the ovarian environment. Additionally, humans
typically exhibit a single-wave, monovulatory pattern for the
selection of the dominant follicle, in contrast to mice, which
display multi-wave, polyovulatory cycles (48). In addition, there is an apparent
limitation to the in vitro cell culture model used in the
present study, since cultured cells may lose some of their
physiological characteristics (49,50)
and be affected by microenvironmental conditions (51,52),
which may result in findings that do not represent the in
vivo settings. Murine ovaries lack the protracted
folliculogenesis and hormonal complexity of humans. Furthermore,
environmental endocrine disruptors such as phthalates (53), which potently alter human AMH
levels, were not examined in the present study.
PRNP KO mice seem to have phenotypic
resilience, but this does not entirely invalidate their clinical
relevance. Under stress, there can be a compensatory failure and
PrPc may have a key effect on ovarian function. In mice,
Shadoo-mediated compensatory mechanisms may take place (54), whilst in humans these mechanisms
may not occur. When humans face prolonged oxidative stress, for
example during chemotherapy or when there is age-related follicular
depletion, Shadoo-mediated redundancy may not be sufficient. The
observation of elevated (FSH) levels in PRNP knockout (KO) mice
aligns with the clinical characteristic of increased
early-follicular phase FSH levels seen in patients diagnosed with
diminished ovarian reserve (DOR), as defined by established
international diagnostic criteria (55). Additionally, PrPc
expression in human follicular fluid may be associated with AMH
levels or in vitro fertilization outcomes; therefore, cohort
studies examining the association between PrPc
concentrations in human follicular fluid and key reproductive
parameters is warranted are warranted.
The anti-apoptotic role of PrPc in glioma
cells via interaction with PRKC Apoptosis WT1 Regulator (PAWR)
raises the possibility that PrPc may protect GCs from
chemotherapy-specific observations in glioma models (56). This hypothesis aligns with clinical
observations that the ovarian reserve declines post-chemotherapy
(57–59), it was hypothesized that
PrPc expression critically regulates
chemotherapy-induced ovarian reserve depletion by modulating
granulosa cell survival pathways. Inhibiting PrPc-PAWR
interactions may sensitize ovarian cancer cells to chemotherapy
while preserving normal ovarian tissue. Conversely, enhancing
PrPc activity could protect ovarian follicles during
oncotherapy.
In conclusion, the present study revealed that
PrPc selectively disrupted AMH secretion in mGCs without
affecting the levels of P4 and E2 or the reproductive
cycle. Unlike its anti-apoptotic roles in neurons or glioma, the
role of PrPc in AMH regulation was independent of cell
survival. It was hypothesized that: Murine ovaries may exhibit
compensatory mechanisms via Shadoo in the absence of
PrPc, while human cells exposed to prolonged stress,
such as chemotherapy, may rely more on PrPc for the
regulation of AMH levels and cell survival. Clinically,
PrPc could refine ovarian reserve diagnostics or inspire
fertility preservation strategies. Furthermore, targeting the
PrPc-BMP-SMAD interactions may also address AMH
dysregulation in polycystic ovary syndrome or ovarian
insufficiency.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Wen-Quan
Zou (Jiangxi Academy of Clinical Medical Sciences of the First
Affiliated Hospital of Nanchang University, Nanching, China), for
technical discussion. The authors would also like to thank
Professor Li Cui (Department of Neurology, First Hospital of Jilin
University, Changchun. China) for their donation of FVB-PRNP
tm1/ILAS congenic mice.
Funding
The present study was partially supported by the National
Natural Science Foundation of China (grant no. 82260295).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CT interpreted data and revised the manuscript. QC
and HW, who made contributions to the study's conceptualization and
data validation. In vivo investigations, including surgical
procedures and H&E staining, were carried out by QC, JH, and
YW. QC, FL, TD and XY performed experiments. QY contributed to
pathological assessments and the drafting of the manuscript. QC and
HW confirm the authenticity of all the raw data. All authors
participated in data analysis, manuscript review and they
collectively assume accountability for the published work. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental procedures in the present study
were approved by the Institutional Animal Care and Use Committee of
The First Affiliated Hospital of Nanchang University (approval no.
CDYFY-IACUC-202310QR030; Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ermonval M, Petit D, Le Duc A, Kellermann
O and Gallet PF: Glycosylation-related genes are variably expressed
depending on the differentiation state of a bioaminergic neuronal
cell line: Implication for the cellular prion protein. Glycoconj J.
26:477–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu W, Nessler S, Hemmer B, Eagar TN, Kane
LP, Leliveld SR, Müller-Schiffmann A, Gocke AR, Lovett-Racke A, Ben
LH, et al: Pharmacological prion protein silencing accelerates
central nervous system autoimmune disease via T cell receptor
signalling. Brain. 133:375–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wulf MA, Senatore A and Aguzzi A: The
biological function of the cellular prion protein: An update. BMC
Biol. 15:342017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watts JC, Bourkas MEC and Arshad H: The
function of the cellular prion protein in health and disease. Acta
Neuropathol. 135:159–178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sigurdson CJ, Bartz JC and Glatzel M:
Cellular and molecular mechanisms of prion disease. Annu Rev
Pathol. 14:497–516. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribes JM, Patel MP, Halim HA, Berretta A,
Tooze SA and Klöhn PC: Prion protein conversion at two distinct
cellular sites precedes fibrillisation. Nat Commun. 14:83542023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salvesen Ø, Tatzelt J and Tranulis MA: The
prion protein in neuroimmune crosstalk. Neurochem Int.
130:1043352019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dematteis G, Restelli E, Vanella VV,
Manfredi M, Marengo E, Corazzari M, Genazzani AA, Chiesa R, Lim D
and Tapella L: Calcineurin controls cellular prion protein
expression in mouse astrocytes. Cells. 11:6092022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goedert M: NEURODEGENERATION. Alzheimer's
and Parkinson's diseases: The prion concept in relation to
assembled Aβ, tau, and α-synuclein. Science. 349:12555552015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stoner A, Fu L, Nicholson L, Zheng C,
Toyonaga T, Spurrier J, Laird W, Cai Z and Strittmatter SM:
Neuronal transcriptome, tau and synapse loss in Alzheimer's
knock-in mice require prion protein. Alzheimers Res Ther.
15:2012023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Evoniuk JM, Johnson ML, Borowicz PP, Caton
JS, Vonnahme KA, Reynolds LP, Taylor JB, Stoltenow CL, O'Rourke KI
and Redmer DA: Effects of nutrition and genotype on prion protein
(PrPC) gene expression in the fetal and maternal sheep placenta.
Placenta. 29:422–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moldenhauer LM, Jin M, Wilson JJ, Green
ES, Sharkey DJ, Salkeld MD, Bristow TC, Hull ML, Dekker GA and
Robertson SA: Regulatory T cell proportion and phenotype are
altered in women using oral contraception. Endocrinology.
163:bqac0982022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Memon S, Li G, Xiong H, Wang L, Liu XY,
Yuan M, Deng W and Xi D: Deletion/insertion polymorphisms of the
prion protein gene (PRNP) in gayal (Bos frontalis). J Genet.
97:1131–1138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson ML, Grazul-Bilska AT, Reynolds LP
and Redmer DA: Prion (PrPC) expression in ovine uteroplacental
tissues increases after estrogen treatment of ovariectomized ewes
and during early pregnancy. Reproduction. 148:1–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buratini J, Dellaqua TT, Dal Canto M, La
Marca A, Carone D, Mignini Renzini M and Webb R: The putative roles
of FSH and AMH in the regulation of oocyte developmental
competence: From fertility prognosis to mechanisms underlying
age-related subfertility. Hum Reprod Update. 28:232–254. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suarez-Henriques P, Miranda E,
Silva-Chaves C, Cardoso-Leite R, Guilermo-Ferreira R, Katiki LM and
Louvandini H: Exploring AMH levels, homeostasis parameters, and
ovarian primordial follicle activation in pubertal infected sheep
on a high-protein diet. Res Vet Sci. 169:1051582024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith ER, Ye D, Luo S, Xu IRL and Xu XX:
AMH regulates a mosaic population of AMHR2-positive cells in the
ovarian surface epithelium. J Biol Chem. 300:1078972024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiang Y, Jiang L, Gou J, Sun Y, Zhang D,
Xin X, Song Z and Huang J: Chronic unpredictable mild
stress-induced mouse ovarian insufficiency by interrupting lipid
homeostasis in the ovary. Front Cell Dev Biol. 10:9336742022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rios JS, Greenwood EA, Pavone MEG, Cedars
MI, Legro RS, Diamond MP, Santoro N, Sun F, Robinson RD, Christman
G, et al: Associations between anti-mullerian hormone and
cardiometabolic health in reproductive age women are explained by
body mass index. J Clin Endocrinol Metab. 105:e555–e563. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Remans T, Keunen E, Bex GJ, Smeets K,
Vangronsveld J and Cuypers A: Reliable gene expression analysis by
reverse transcription-quantitative PCR: Reporting and minimizing
the uncertainty in data accuracy. Plant Cell. 26:3829–3837. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hatakeyama D, Chikamoto N, Fujimoto K,
Kitahashi T and Ito E: Comparison between relative and absolute
quantitative real-time PCR applied to single-cell analyses:
Transcriptional levels in a key neuron for long-term memory in the
pond snail. PLoS One. 17:e02790172022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salido J, Vallez N, González-López L,
Deniz O and Bueno G: Comparison of deep learning models for digital
H&E staining from unpaired label-free multispectral microscopy
images. Comput Methods Programs Biomed. 235:1075282023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asaf MZ, Salam AA, Khan S, Musolff N,
Akram MU and Rao B: E-Staining DermaRepo: H&E whole slide image
staining dataset. Data Brief. 57:1109972024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Egbert JR, Fahey PG, Reimer J, Owen CM,
Evsikov AV, Nikolaev VO, Griesbeck O, Ray RS, Tolias AS and Jaffe
LA: Follicle-stimulating hormone and luteinizing hormone increase
Ca2+ in the granulosa cells of mouse ovarian follicles†. Biol
Reprod. 101:433–444. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SJ, Kim TE and Jee BC: Impact of
imatinib administration on the mouse ovarian follicle count and
levels of intra-ovarian proteins related to follicular quality.
Clin Exp Reprod Med. 49:93–100. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wall EG, Desai R, Khant Aung Z, Yeo SH,
Grattan DR, Handelsman DJ and Herbison AE: Unexpected plasma
gonadal steroid and prolactin levels across the mouse estrous
cycle. Endocrinology. 164:bqad0702023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abrams J, Arhar T, Mok SA, Taylor IR,
Kampmann M and Gestwicki JE: Functional genomics screen identifies
proteostasis targets that modulate prion protein (PrP) stability.
Cell Stress Chaperones. 26:443–452. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmitt-Ulms G, Mehrabian M, Williams D
and Ehsani S: The IDIP framework for assessing protein function and
its application to the prion protein. Biol Rev Camb Philos Soc.
96:1907–1932. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawaya MR, Hughes MP, Rodriguez JA, Riek R
and Eisenberg DS: The expanding amyloid family: Structure,
stability, function, and pathogenesis. Cell. 184:4857–4873. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawrence JA, Aguilar-Calvo P, Ojeda-Juárez
D, Khuu H, Soldau K, Pizzo DP, Wang J, Malik A, Shay TF, Sullivan
EE, et al: Diminished neuronal ESCRT-0 function exacerbates AMPA
receptor derangement and accelerates prion-induced
neurodegeneration. J Neurosci. 43:3970–3984. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lidón L, Vergara C, Ferrer I, Hernández F,
Ávila J, Del Rio JA and Gavín R: Tau protein as a new regulator of
cellular prion protein transcription. Mol Neurobiol. 57:4170–4186.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ribeiro LW, Pietri M, Ardila-Osorio H,
Baudry A, Boudet-Devaud F, Bizingre C, Arellano-Anaya ZE, Haeberlé
AM, Gadot N, Boland S, et al: Titanium dioxide and carbon black
nanoparticles disrupt neuronal homeostasis via excessive activation
of cellular prion protein signaling. Part Fibre Toxicol. 19:482022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang CC, Sung PH, Chen KH, Chai HT, Chiang
JY, Ko SF, Lee FY and Yip HK: Valsartan- and melatonin-supported
adipose-derived mesenchymal stem cells preserve renal function in
chronic kidney disease rat through upregulation of prion protein
participated in promoting PI3K-Akt-mTOR signaling and cell
proliferation. Biomed Pharmacother. 146:1125512022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sheu JJ, Chai HT, Chiang JY, Sung PH, Chen
YL and Yip HK: Cellular prion protein is essential for myocardial
regeneration but not the recovery of left ventricular function from
apical ballooning. Biomedicines. 10:1672022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tuğrul B, Balcan E, Öztel Z, Çöllü F and
Gürcü B: Prion protein-dependent regulation of p53-MDM2 crosstalk
during endoplasmic reticulum stress and doxorubicin treatments
might be essential for cell fate in human breast cancer cell line,
MCF-7. Exp Cell Res. 429:1136562023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pimenta JMBGA, Pires VMR, Nolasco S,
Castelo-Branco P, Marques CC, Apolónio J, Azevedo R, Fernandes MT,
Lopes-da-Costa L, Prates J and Pereira RMLN: Post-transcriptional
silencing of Bos taurus prion family genes and its impact on
granulosa cell steroidogenesis. Biochem Biophys Res Commun.
598:95–99. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hart KN, Stocker WA, Nagykery NG, Walton
KL, Harrison CA, Donahoe PK, Pépin D and Thompson TB: Structure of
AMH bound to AMHR2 provides insight into a unique signaling pair in
the TGF-β family. Proc Natl Acad Sci USA. 118:e21048091182021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spector I, Derech-Haim S, Boustanai I,
Safrai M and Meirow D: Anti-Müllerian hormone signaling in the
ovary involves stromal fibroblasts: A study in humans and mice
provides novel insights into the role of ovarian stroma. Hum
Reprod. 39:2551–2564. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Puig B, Altmeppen HC, Linsenmeier L,
Chakroun K, Wegwitz F, Piontek UK, Tatzelt J, Bate C, Magnus T and
Glatzel M: GPI-anchor signal sequence influences PrPC sorting,
shedding and signalling, and impacts on different pathomechanistic
aspects of prion disease in mice. PLoS Pathog. 15:e10075202019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suriyakalaa U, Ramachandran R,
Doulathunnisa JA, Aseervatham SB, Sankarganesh D, Kamalakkannan S,
Kadalmani B, Angayarkanni J, Akbarsha MA and Achiraman S:
Upregulation of Cyp19a1 and PPAR-γ in ovarian steroidogenic pathway
by Ficus religiosa: A potential cure for polycystic ovary syndrome.
J Ethnopharmacol. 267:1135402021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang S, Liu Y, Wang M, Ponikwicka-Tyszko
D, Ma W, Krentowska A, Kowalska I, Huhtaniemi I, Wolczynski S,
Rahman NA and Li X: Role and mechanism of miR-335-5p in the
pathogenesis and treatment of polycystic ovary syndrome. Transl
Res. 252:64–78. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cai S, Chen M, Xue B, Zhu Z, Wang X, Li J,
Wang H and Zeng X, Qiao S and Zeng X: Retinoic acid enhances
ovarian steroidogenesis by regulating granulosa cell proliferation
and MESP2/STAR/CYP11A1 pathway. J Adv Res. 58:163–173. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Jiang JJ, Du SY, Mu LS, Fan JJ, Hu
JC, Ye Y, Ding M, Zhou WY, Yu QH, et al: Artemisinins ameliorate
polycystic ovarian syndrome by mediating LONP1-CYP11A1 interaction.
Science. 384:eadk53822024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Doeppner TR, Kaltwasser B, Schlechter J,
Jaschke J, Kilic E, Bähr M, Hermann DM and Weise J: Cellular prion
protein promotes post-ischemic neuronal survival, angioneurogenesis
and enhances neural progenitor cell homing via proteasome
inhibition. Cell Death Dis. 6:e20242015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Passet B, Castille J, Makhzami S, Truchet
S, Vaiman A, Floriot S, Moazami-Goudarzi K, Vilotte M, Gaillard AL,
Helary L, et al: The Prion-like protein Shadoo is involved in mouse
embryonic and mammary development and differentiation. Sci Rep.
10:67652020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lonardo MS, Cacciapuoti N, Guida B, Di
Lorenzo M, Chiurazzi M, Damiano S and Menale C:
Hypothalamic-ovarian axis and adiposity relationship in polycystic
ovary syndrome: Physiopathology and therapeutic options for the
management of metabolic and inflammatory aspects. Curr Obes Rep.
13:51–70. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Telfer EE, Grosbois J, Odey YL, Rosario R
and Anderson RA: Making a good egg: Human oocyte health, aging, and
in vitro development. Physiol Rev. 103:2623–2677. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park SU, Walsh L and Berkowitz KM:
Mechanisms of ovarian aging. Reproduction. 162:R19–R33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Amadei G, Handford CE, Qiu C, De Jonghe J,
Greenfeld H, Tran M, Martin BK, Chen DY, Aguilera-Castrejon A,
Hanna JH, et al: Embryo model completes gastrulation to neurulation
and organogenesis. Nature. 610:143–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chap BS, Rayroux N, Grimm AJ, Ghisoni E
and Dangaj Laniti D: Crosstalk of T cells within the ovarian cancer
microenvironment. Trends Cancer. 10:1116–1130. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schoutrop E, Moyano-Galceran L, Lheureux
S, Mattsson J, Lehti K, Dahlstrand H and Magalhaes I: Molecular,
cellular and systemic aspects of epithelial ovarian cancer and its
tumor microenvironment. Semin Cancer Biol. 86:207–223. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mariana M, Castelo-Branco M, Soares AM and
Cairrao E: Phthalates' exposure leads to an increasing concern on
cardiovascular health. J Hazard Mater. 457:1316802023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pepe A, Avolio R, Matassa DS, Esposito F,
Nitsch L, Zurzolo C, Paladino S and Sarnataro D: Regulation of
sub-compartmental targeting and folding properties of the
Prion-like protein Shadoo. Sci Rep. 7:37312017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Steiner AZ, Pritchard D, Stanczyk FZ,
Kesner JS, Meadows JW, Herring AH and Baird DD: Association between
biomarkers of ovarian reserve and infertility among older women of
reproductive age. JAMA. 318:1367–1376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhuang D, Liu Y, Mao Y, Gao L, Zhang H,
Luan S, Huang F and Li Q: TMZ-induced PrPc/par-4 interaction
promotes the survival of human glioma cells. Int J Cancer.
130:309–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Spears N, Lopes F, Stefansdottir A, Rossi
V, De Felici M, Anderson RA and Klinger FG: Ovarian damage from
chemotherapy and current approaches to its protection. Hum Reprod
Update. 25:673–693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Park HS, Seok J, Cetin E, Ghasroldasht MM,
Liakath Ali F, Mohammed H, Alkelani H and Al-Hendy A: Fertility
protection: A novel approach using pretreatment with mesenchymal
stem cell exosomes to prevent chemotherapy-induced ovarian damage
in a mouse model. Am J Obstet Gynecol. 231:111.e1–111.e18. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guo Y, Xue L, Tang W, Xiong J, Chen D, Dai
Y, Wu C, Wei S, Dai J, Wu M and Wang S: Ovarian microenvironment:
Challenges and opportunities in protecting against
chemotherapy-associated ovarian damage. Hum Reprod Update.
30:614–647. 2024. View Article : Google Scholar : PubMed/NCBI
|