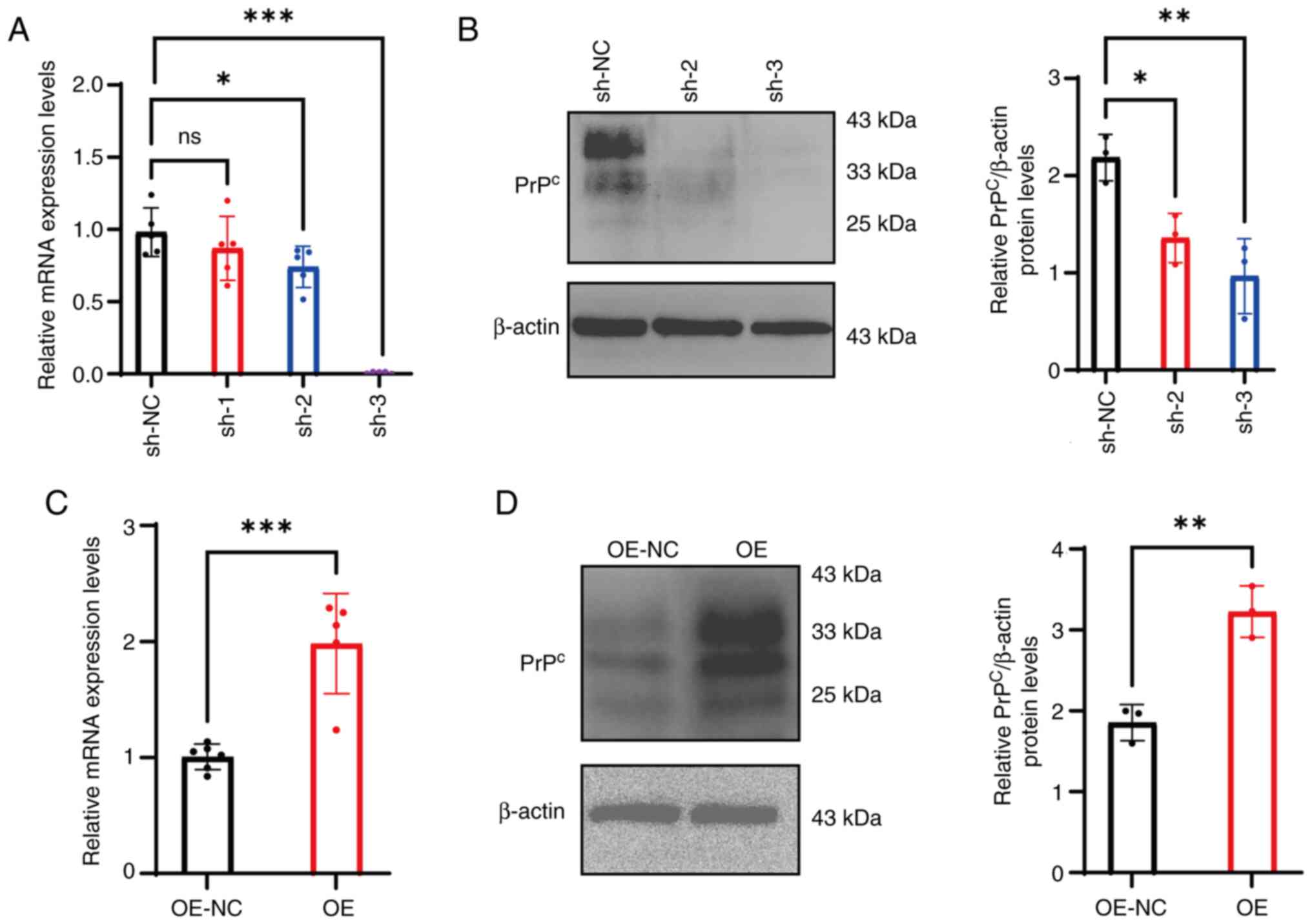
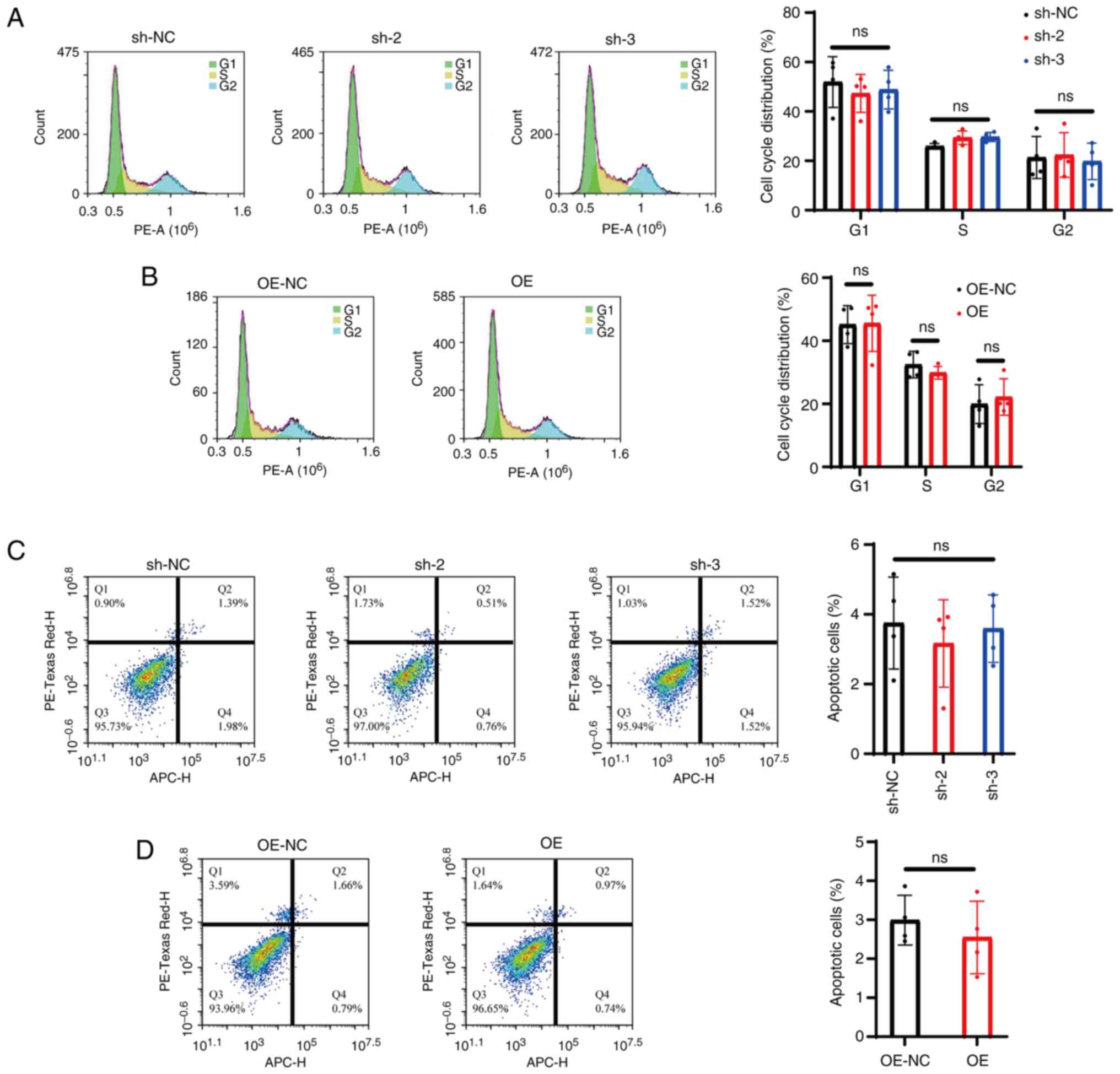
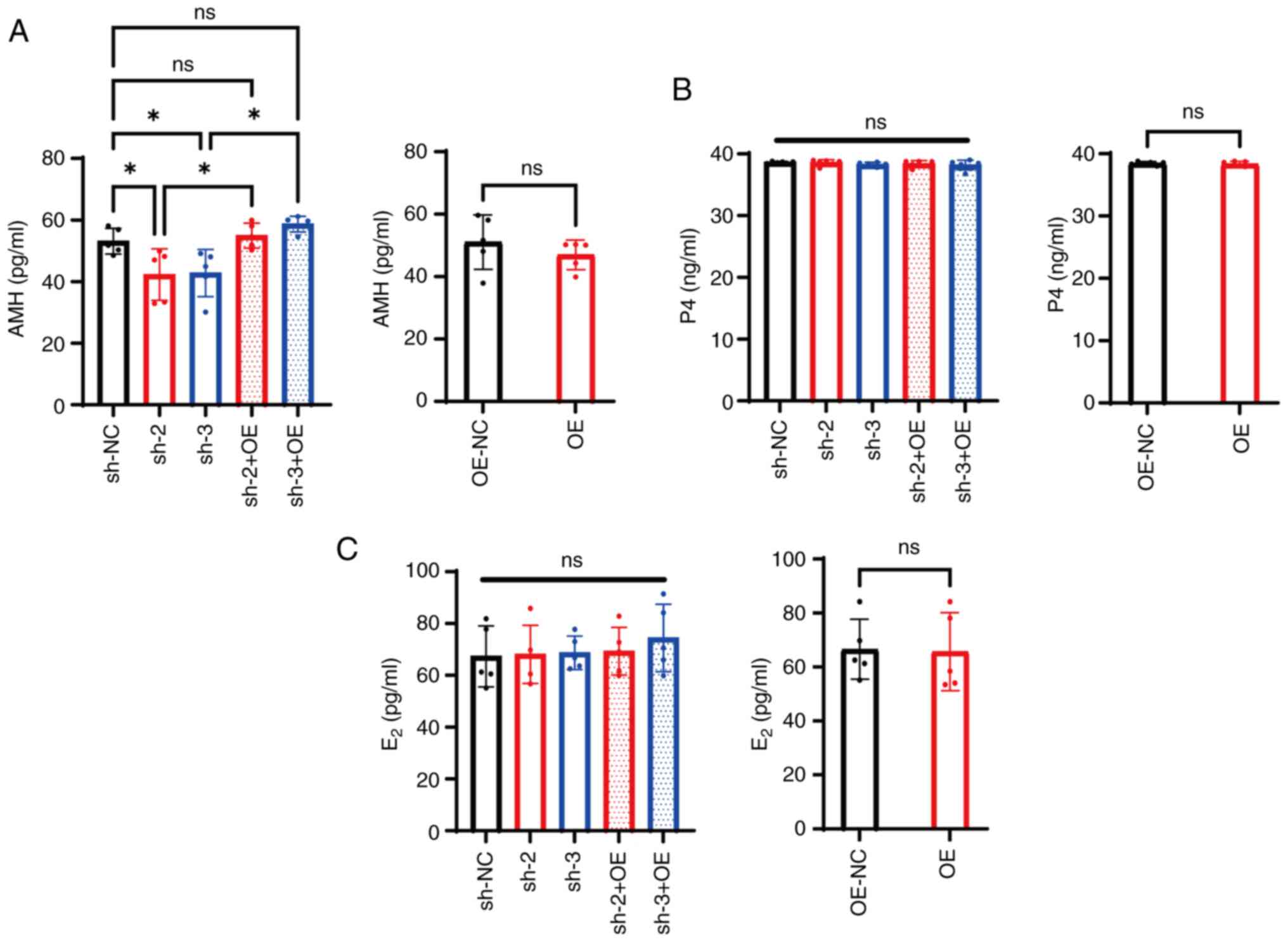
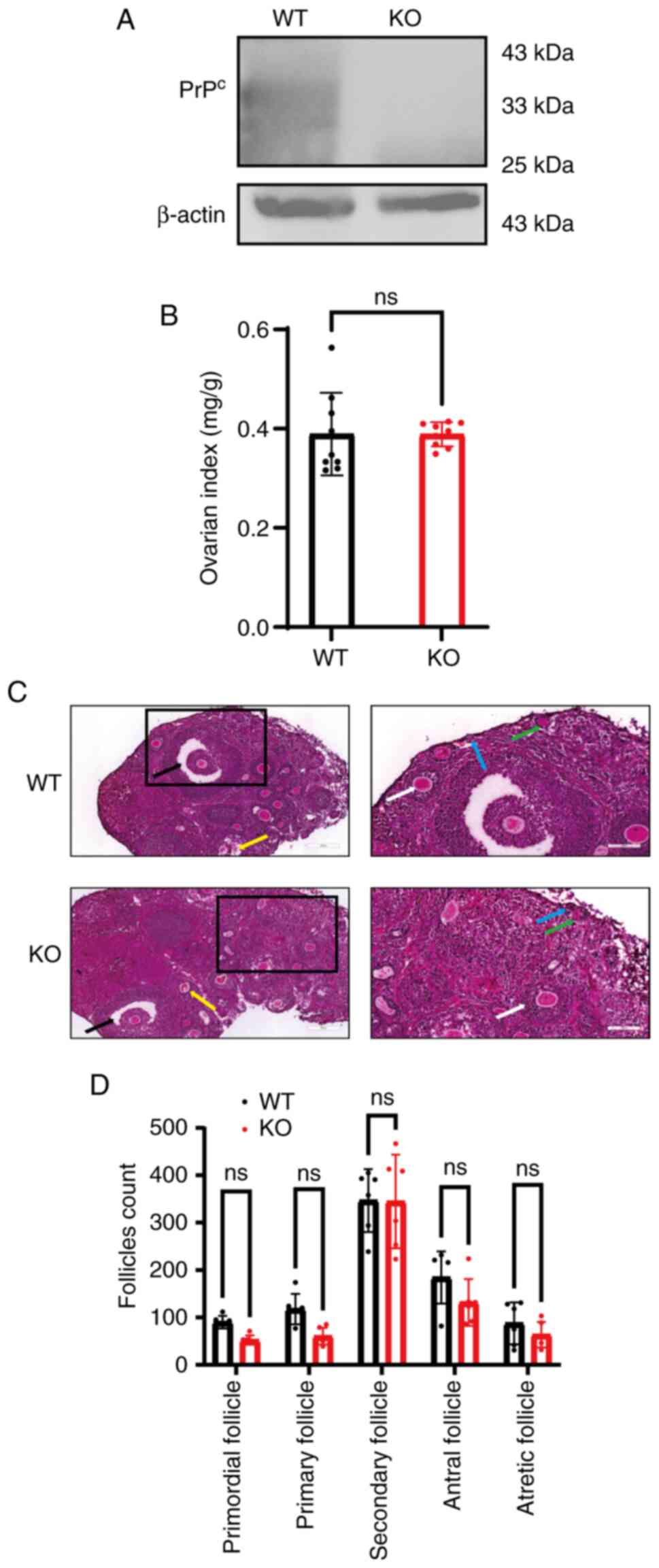
|
1
|
Ermonval M, Petit D, Le Duc A, Kellermann
O and Gallet PF: Glycosylation-related genes are variably expressed
depending on the differentiation state of a bioaminergic neuronal
cell line: Implication for the cellular prion protein. Glycoconj J.
26:477–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu W, Nessler S, Hemmer B, Eagar TN, Kane
LP, Leliveld SR, Müller-Schiffmann A, Gocke AR, Lovett-Racke A, Ben
LH, et al: Pharmacological prion protein silencing accelerates
central nervous system autoimmune disease via T cell receptor
signalling. Brain. 133:375–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wulf MA, Senatore A and Aguzzi A: The
biological function of the cellular prion protein: An update. BMC
Biol. 15:342017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watts JC, Bourkas MEC and Arshad H: The
function of the cellular prion protein in health and disease. Acta
Neuropathol. 135:159–178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sigurdson CJ, Bartz JC and Glatzel M:
Cellular and molecular mechanisms of prion disease. Annu Rev
Pathol. 14:497–516. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribes JM, Patel MP, Halim HA, Berretta A,
Tooze SA and Klöhn PC: Prion protein conversion at two distinct
cellular sites precedes fibrillisation. Nat Commun. 14:83542023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salvesen Ø, Tatzelt J and Tranulis MA: The
prion protein in neuroimmune crosstalk. Neurochem Int.
130:1043352019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dematteis G, Restelli E, Vanella VV,
Manfredi M, Marengo E, Corazzari M, Genazzani AA, Chiesa R, Lim D
and Tapella L: Calcineurin controls cellular prion protein
expression in mouse astrocytes. Cells. 11:6092022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goedert M: NEURODEGENERATION. Alzheimer's
and Parkinson's diseases: The prion concept in relation to
assembled Aβ, tau, and α-synuclein. Science. 349:12555552015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stoner A, Fu L, Nicholson L, Zheng C,
Toyonaga T, Spurrier J, Laird W, Cai Z and Strittmatter SM:
Neuronal transcriptome, tau and synapse loss in Alzheimer's
knock-in mice require prion protein. Alzheimers Res Ther.
15:2012023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Evoniuk JM, Johnson ML, Borowicz PP, Caton
JS, Vonnahme KA, Reynolds LP, Taylor JB, Stoltenow CL, O'Rourke KI
and Redmer DA: Effects of nutrition and genotype on prion protein
(PrPC) gene expression in the fetal and maternal sheep placenta.
Placenta. 29:422–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moldenhauer LM, Jin M, Wilson JJ, Green
ES, Sharkey DJ, Salkeld MD, Bristow TC, Hull ML, Dekker GA and
Robertson SA: Regulatory T cell proportion and phenotype are
altered in women using oral contraception. Endocrinology.
163:bqac0982022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Memon S, Li G, Xiong H, Wang L, Liu XY,
Yuan M, Deng W and Xi D: Deletion/insertion polymorphisms of the
prion protein gene (PRNP) in gayal (Bos frontalis). J Genet.
97:1131–1138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson ML, Grazul-Bilska AT, Reynolds LP
and Redmer DA: Prion (PrPC) expression in ovine uteroplacental
tissues increases after estrogen treatment of ovariectomized ewes
and during early pregnancy. Reproduction. 148:1–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buratini J, Dellaqua TT, Dal Canto M, La
Marca A, Carone D, Mignini Renzini M and Webb R: The putative roles
of FSH and AMH in the regulation of oocyte developmental
competence: From fertility prognosis to mechanisms underlying
age-related subfertility. Hum Reprod Update. 28:232–254. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suarez-Henriques P, Miranda E,
Silva-Chaves C, Cardoso-Leite R, Guilermo-Ferreira R, Katiki LM and
Louvandini H: Exploring AMH levels, homeostasis parameters, and
ovarian primordial follicle activation in pubertal infected sheep
on a high-protein diet. Res Vet Sci. 169:1051582024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith ER, Ye D, Luo S, Xu IRL and Xu XX:
AMH regulates a mosaic population of AMHR2-positive cells in the
ovarian surface epithelium. J Biol Chem. 300:1078972024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiang Y, Jiang L, Gou J, Sun Y, Zhang D,
Xin X, Song Z and Huang J: Chronic unpredictable mild
stress-induced mouse ovarian insufficiency by interrupting lipid
homeostasis in the ovary. Front Cell Dev Biol. 10:9336742022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rios JS, Greenwood EA, Pavone MEG, Cedars
MI, Legro RS, Diamond MP, Santoro N, Sun F, Robinson RD, Christman
G, et al: Associations between anti-mullerian hormone and
cardiometabolic health in reproductive age women are explained by
body mass index. J Clin Endocrinol Metab. 105:e555–e563. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Remans T, Keunen E, Bex GJ, Smeets K,
Vangronsveld J and Cuypers A: Reliable gene expression analysis by
reverse transcription-quantitative PCR: Reporting and minimizing
the uncertainty in data accuracy. Plant Cell. 26:3829–3837. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hatakeyama D, Chikamoto N, Fujimoto K,
Kitahashi T and Ito E: Comparison between relative and absolute
quantitative real-time PCR applied to single-cell analyses:
Transcriptional levels in a key neuron for long-term memory in the
pond snail. PLoS One. 17:e02790172022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salido J, Vallez N, González-López L,
Deniz O and Bueno G: Comparison of deep learning models for digital
H&E staining from unpaired label-free multispectral microscopy
images. Comput Methods Programs Biomed. 235:1075282023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asaf MZ, Salam AA, Khan S, Musolff N,
Akram MU and Rao B: E-Staining DermaRepo: H&E whole slide image
staining dataset. Data Brief. 57:1109972024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Egbert JR, Fahey PG, Reimer J, Owen CM,
Evsikov AV, Nikolaev VO, Griesbeck O, Ray RS, Tolias AS and Jaffe
LA: Follicle-stimulating hormone and luteinizing hormone increase
Ca2+ in the granulosa cells of mouse ovarian follicles†. Biol
Reprod. 101:433–444. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SJ, Kim TE and Jee BC: Impact of
imatinib administration on the mouse ovarian follicle count and
levels of intra-ovarian proteins related to follicular quality.
Clin Exp Reprod Med. 49:93–100. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wall EG, Desai R, Khant Aung Z, Yeo SH,
Grattan DR, Handelsman DJ and Herbison AE: Unexpected plasma
gonadal steroid and prolactin levels across the mouse estrous
cycle. Endocrinology. 164:bqad0702023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abrams J, Arhar T, Mok SA, Taylor IR,
Kampmann M and Gestwicki JE: Functional genomics screen identifies
proteostasis targets that modulate prion protein (PrP) stability.
Cell Stress Chaperones. 26:443–452. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmitt-Ulms G, Mehrabian M, Williams D
and Ehsani S: The IDIP framework for assessing protein function and
its application to the prion protein. Biol Rev Camb Philos Soc.
96:1907–1932. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawaya MR, Hughes MP, Rodriguez JA, Riek R
and Eisenberg DS: The expanding amyloid family: Structure,
stability, function, and pathogenesis. Cell. 184:4857–4873. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawrence JA, Aguilar-Calvo P, Ojeda-Juárez
D, Khuu H, Soldau K, Pizzo DP, Wang J, Malik A, Shay TF, Sullivan
EE, et al: Diminished neuronal ESCRT-0 function exacerbates AMPA
receptor derangement and accelerates prion-induced
neurodegeneration. J Neurosci. 43:3970–3984. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lidón L, Vergara C, Ferrer I, Hernández F,
Ávila J, Del Rio JA and Gavín R: Tau protein as a new regulator of
cellular prion protein transcription. Mol Neurobiol. 57:4170–4186.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ribeiro LW, Pietri M, Ardila-Osorio H,
Baudry A, Boudet-Devaud F, Bizingre C, Arellano-Anaya ZE, Haeberlé
AM, Gadot N, Boland S, et al: Titanium dioxide and carbon black
nanoparticles disrupt neuronal homeostasis via excessive activation
of cellular prion protein signaling. Part Fibre Toxicol. 19:482022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang CC, Sung PH, Chen KH, Chai HT, Chiang
JY, Ko SF, Lee FY and Yip HK: Valsartan- and melatonin-supported
adipose-derived mesenchymal stem cells preserve renal function in
chronic kidney disease rat through upregulation of prion protein
participated in promoting PI3K-Akt-mTOR signaling and cell
proliferation. Biomed Pharmacother. 146:1125512022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sheu JJ, Chai HT, Chiang JY, Sung PH, Chen
YL and Yip HK: Cellular prion protein is essential for myocardial
regeneration but not the recovery of left ventricular function from
apical ballooning. Biomedicines. 10:1672022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tuğrul B, Balcan E, Öztel Z, Çöllü F and
Gürcü B: Prion protein-dependent regulation of p53-MDM2 crosstalk
during endoplasmic reticulum stress and doxorubicin treatments
might be essential for cell fate in human breast cancer cell line,
MCF-7. Exp Cell Res. 429:1136562023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pimenta JMBGA, Pires VMR, Nolasco S,
Castelo-Branco P, Marques CC, Apolónio J, Azevedo R, Fernandes MT,
Lopes-da-Costa L, Prates J and Pereira RMLN: Post-transcriptional
silencing of Bos taurus prion family genes and its impact on
granulosa cell steroidogenesis. Biochem Biophys Res Commun.
598:95–99. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hart KN, Stocker WA, Nagykery NG, Walton
KL, Harrison CA, Donahoe PK, Pépin D and Thompson TB: Structure of
AMH bound to AMHR2 provides insight into a unique signaling pair in
the TGF-β family. Proc Natl Acad Sci USA. 118:e21048091182021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spector I, Derech-Haim S, Boustanai I,
Safrai M and Meirow D: Anti-Müllerian hormone signaling in the
ovary involves stromal fibroblasts: A study in humans and mice
provides novel insights into the role of ovarian stroma. Hum
Reprod. 39:2551–2564. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Puig B, Altmeppen HC, Linsenmeier L,
Chakroun K, Wegwitz F, Piontek UK, Tatzelt J, Bate C, Magnus T and
Glatzel M: GPI-anchor signal sequence influences PrPC sorting,
shedding and signalling, and impacts on different pathomechanistic
aspects of prion disease in mice. PLoS Pathog. 15:e10075202019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suriyakalaa U, Ramachandran R,
Doulathunnisa JA, Aseervatham SB, Sankarganesh D, Kamalakkannan S,
Kadalmani B, Angayarkanni J, Akbarsha MA and Achiraman S:
Upregulation of Cyp19a1 and PPAR-γ in ovarian steroidogenic pathway
by Ficus religiosa: A potential cure for polycystic ovary syndrome.
J Ethnopharmacol. 267:1135402021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang S, Liu Y, Wang M, Ponikwicka-Tyszko
D, Ma W, Krentowska A, Kowalska I, Huhtaniemi I, Wolczynski S,
Rahman NA and Li X: Role and mechanism of miR-335-5p in the
pathogenesis and treatment of polycystic ovary syndrome. Transl
Res. 252:64–78. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cai S, Chen M, Xue B, Zhu Z, Wang X, Li J,
Wang H and Zeng X, Qiao S and Zeng X: Retinoic acid enhances
ovarian steroidogenesis by regulating granulosa cell proliferation
and MESP2/STAR/CYP11A1 pathway. J Adv Res. 58:163–173. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Jiang JJ, Du SY, Mu LS, Fan JJ, Hu
JC, Ye Y, Ding M, Zhou WY, Yu QH, et al: Artemisinins ameliorate
polycystic ovarian syndrome by mediating LONP1-CYP11A1 interaction.
Science. 384:eadk53822024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Doeppner TR, Kaltwasser B, Schlechter J,
Jaschke J, Kilic E, Bähr M, Hermann DM and Weise J: Cellular prion
protein promotes post-ischemic neuronal survival, angioneurogenesis
and enhances neural progenitor cell homing via proteasome
inhibition. Cell Death Dis. 6:e20242015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Passet B, Castille J, Makhzami S, Truchet
S, Vaiman A, Floriot S, Moazami-Goudarzi K, Vilotte M, Gaillard AL,
Helary L, et al: The Prion-like protein Shadoo is involved in mouse
embryonic and mammary development and differentiation. Sci Rep.
10:67652020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lonardo MS, Cacciapuoti N, Guida B, Di
Lorenzo M, Chiurazzi M, Damiano S and Menale C:
Hypothalamic-ovarian axis and adiposity relationship in polycystic
ovary syndrome: Physiopathology and therapeutic options for the
management of metabolic and inflammatory aspects. Curr Obes Rep.
13:51–70. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Telfer EE, Grosbois J, Odey YL, Rosario R
and Anderson RA: Making a good egg: Human oocyte health, aging, and
in vitro development. Physiol Rev. 103:2623–2677. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park SU, Walsh L and Berkowitz KM:
Mechanisms of ovarian aging. Reproduction. 162:R19–R33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Amadei G, Handford CE, Qiu C, De Jonghe J,
Greenfeld H, Tran M, Martin BK, Chen DY, Aguilera-Castrejon A,
Hanna JH, et al: Embryo model completes gastrulation to neurulation
and organogenesis. Nature. 610:143–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chap BS, Rayroux N, Grimm AJ, Ghisoni E
and Dangaj Laniti D: Crosstalk of T cells within the ovarian cancer
microenvironment. Trends Cancer. 10:1116–1130. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schoutrop E, Moyano-Galceran L, Lheureux
S, Mattsson J, Lehti K, Dahlstrand H and Magalhaes I: Molecular,
cellular and systemic aspects of epithelial ovarian cancer and its
tumor microenvironment. Semin Cancer Biol. 86:207–223. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mariana M, Castelo-Branco M, Soares AM and
Cairrao E: Phthalates' exposure leads to an increasing concern on
cardiovascular health. J Hazard Mater. 457:1316802023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pepe A, Avolio R, Matassa DS, Esposito F,
Nitsch L, Zurzolo C, Paladino S and Sarnataro D: Regulation of
sub-compartmental targeting and folding properties of the
Prion-like protein Shadoo. Sci Rep. 7:37312017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Steiner AZ, Pritchard D, Stanczyk FZ,
Kesner JS, Meadows JW, Herring AH and Baird DD: Association between
biomarkers of ovarian reserve and infertility among older women of
reproductive age. JAMA. 318:1367–1376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhuang D, Liu Y, Mao Y, Gao L, Zhang H,
Luan S, Huang F and Li Q: TMZ-induced PrPc/par-4 interaction
promotes the survival of human glioma cells. Int J Cancer.
130:309–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Spears N, Lopes F, Stefansdottir A, Rossi
V, De Felici M, Anderson RA and Klinger FG: Ovarian damage from
chemotherapy and current approaches to its protection. Hum Reprod
Update. 25:673–693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Park HS, Seok J, Cetin E, Ghasroldasht MM,
Liakath Ali F, Mohammed H, Alkelani H and Al-Hendy A: Fertility
protection: A novel approach using pretreatment with mesenchymal
stem cell exosomes to prevent chemotherapy-induced ovarian damage
in a mouse model. Am J Obstet Gynecol. 231:111.e1–111.e18. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guo Y, Xue L, Tang W, Xiong J, Chen D, Dai
Y, Wu C, Wei S, Dai J, Wu M and Wang S: Ovarian microenvironment:
Challenges and opportunities in protecting against
chemotherapy-associated ovarian damage. Hum Reprod Update.
30:614–647. 2024. View Article : Google Scholar : PubMed/NCBI
|