Introduction
As a prominent global health concern, infertility
affects 8–12% of couples in the reproductive age group (between 15
and 49 years, with 50% being due to male-infertility-associated
factors associated with abnormal semen parameters (1,2).
Male infertility can be caused by congenital hypoplasia and
varicocele (3). Its pathogenesis
is usually caused by genetic and environmental factors (4). Studies have focused on key genes and
signaling pathways that regulate male reproduction (5–8), but
there is still controversy regarding this issue.
Sirtuins (SIRTs), highly conserved nicotinamide
adenine dinucleotide (NAD)-dependent deacetylases, are involved in
gene regulation, metabolism, aging and cancer (9). In mammals, seven types of SIRT, 1–7,
have been identified with distinct structures, cellular
localization and tissue expression (10). SIRTs are highly expressed in
mammalian testicular tissue; to the best of our knowledge, few
studies have evaluated the role of SIRTs in male reproductive
function (11–13). SIRT2 has been implicated in
regulating cell cycle progression and apoptosis in germ cells
during spermatogenesis (14,15).
Dysfunction of SIRT3 is associated with impaired sperm motility and
increased sperm DNA damage, highlighting its role in maintaining
sperm function (11,16,17).
Loss of SIRT6 in mice results in an elevated number of apoptotic
spermatids (18).
SIRT5 is a NAD-dependent desuccinylase, demalonylase
and deacetylase protein (19).
SIRT5 participates in a number of biological processes, including
the urea cycle, fatty acid oxidation and amino acid metabolism
(20). It also serves a key role
in cellular antioxidant defense mechanisms by activating
mitochondrial superoxide dismutase 2 (21,22).
In addition, via regulation of DNA repair proteins, such as
ATP-dependent DNA helicase 2 subunit Ku70-like protein, SIRT5
contributes to the maintenance of genomic integrity and protection
against DNA damage-induced cellular dysfunction (23). The multifaceted functions of SIRT5
underscore its role in cell physiology, including maintaining
metabolic homeostasis (22),
regulating stress responses (24)
and ensuring genomic stability (23). However, the specific functions and
mechanisms of SIRT5 in spermatogenic cells remain to be
explored.
The present study aimed to investigate the effects
of SIRT5 on GC-2 spd cells and its underlying mechanisms to
contribute to the development of more effective therapeutic
strategies for asthenospermia.
Materials and methods
Human semen sample collection
The present human study was approved by the Ethics
Committee of Shenzhen Ethics Review Committee on Biomedical
Research (Shenzhen, China) [(2023) approval no. 001]. All
participants provided written informed consent to participate. The
subjects were patients who were treated in Peking University
Shenzhen Hospital from July 2023 to January 2024. The present study
included 25 male patients aged between 22 and 45 years with
asthenozoospermia (sperm concentration, ≥15 million cells/ml;
progressive motility, <32%; total motility, <40%;
morphologically normal forms, ≥4%) and 25 participants with normal
semen parameters. Semen samples were collected by masturbation
following 3–7 days of abstinence. Samples were evaluated by
computer-assisted semen analysis (version SSA-II, Suijia Software
Co., Ltd) system. Inclusion criteria were as follows: i) Patients
aged between 20 and 45 years with complete clinical data and ii) no
syphilis, hepatitis, acquired immune deficiency syndrome or other
infectious disease. Exclusion criteria were as follows: i) Patients
with genitourinary tract infection; ii) patients with a history of
recent use of drugs that interfere with sperm or semen quality and
iii) malignant tumor or abnormal liver and kidney function.
Database mining
To compare the expression of SIRT5 among
asthenozoospermic, asthenoteratozoospermic and normal sperm from
fertile individuals, mRNA expression data was retrieved from the
GEO database (accession no. GSE160749)
(ncbi.nlm.nih.gov/geo/geo2r/?acc=GSE160749). In addition, the
protein expression of SIRT5 in testicular cells was queried using
the Human Protein Atlas (HPA) database
(proteinatlas.org/ENSG00000124523-SIRT5/single+cell+type).
Animals
A single male C57BL/6 J wildtype mouse (age, 2
months; weight, 22 g) was obtained from the Nanjing University
Experimental Animal Institute (Nanjing, China). The mouse was
housed in a specific-pathogen-free animal facility (22°C, 55%
humidity) with a 12/12-h light/dark cycle and free access to
standard food and water. Health and behavior were monitored every
day. The duration of the experiment was 20 weeks. Humane endpoints
were as follows: Complete anorexia or signs of depression
accompanied by hypothermia (body temperature <37°C) without
anesthesia or sedation. The mouse was euthanized by intraperitoneal
injection of an overdose of 1% pentobarbital sodium (150 mg/kg).
Animal death was confirmed by respiratory and cardiac arrest and
pupil dilation was observed for ≥10 min. All animals were treated
according to the Guide for the Care and Use of Laboratory Animals
of the Institute of Laboratory Animal Resources for the National
Research Council. The present study was approved by the Ethics
Committee of Shenzhen Peking University-Hong Kong University of
Science and Technology Medical Center (approval no. 2021-007).
Immunofluorescence staining
After the mouse was euthanized, the testicles were
dissected and removed. Testes were fixed with 4% paraformaldehyde
for 15–20 h at room temperature and dehydrated using a fully
automatic tissue dehydrator (Excelsior AS; Thermo Fisher
Scientific, Inc.). The samples were embedded in paraffin using an
embedding machine (HistoStar; Thermo Fisher Scientific, Inc.), and
sectioned to 3–5 µm thickness. Sections were deparaffinized in
xylene at room temperature, rehydrated using descending ethanol
concentrations, immersed in 0.01 M citrate buffer (Wuhan Servicebio
Technology Co., Ltd.) at 100°C for 10 min to facilitate antigen
retrieval, and cooled for 2–3 h at room temperature. Sections were
washed three times for 5 min each at room temperature with PBS
(Gibco; Thermo Fisher Scientific, Inc.). Sections were blocked with
5% bovine serum albumin (BSA; Merck KGaA) at room temperature for
1h. Sections were incubated with SIRT5 (1:100; Cat. No. 15122-1-AP,
Proteintech Group, Inc.) and γH2AX (1:100; ab26350, Abcam) in a
humid environment at 4°C overnight. Sections were washed in PBS and
incubated with Alexa Fluor secondary antibody (1:100; Invitrogen;
cat. # A-11008 and Cat. # A32742, Thermo Fisher Scientific, Inc.)
for 1 h at room temperature and treated with
VECTASHIELD® antifading mounting agent containing DAPI
(Vector Laboratories, Inc.) for 10 min at room temperature.
Sections were observed under a confocal microscope (STELLARIS 5;
Leica GmbH).
To observe the mitochondrial staining, the GC-2 spd
cells were cultivated in glass coverslips for 24 h and stained with
Mito-Tracker red (Thermo Fisher Scientific, Inc.) for 30 min, all
at 37°C. Cells were blocked in 5% BSA for 1 h and incubated with
SIRT5 (1:100) for 1 h at room temperature and sealed with DAPI,
then observed under the confocal laser scanning microscope.
Cell culture
GC-2 spd cell line was procured from the American
Type Culture Collection. Cells were maintained in a
25-cm2 petri dish with DMEM containing 10% fetal bovine
serum (both Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin-streptomycin. Cells were maintained at 37°C with 5%
CO2.
Plasmid transfection
GC-2 spd cells at 60–70%. GC-2 spd cells were
transfected with 3 µg/ml pCDNA3.1-SIRT5 plasmid (Sangon Biotech Co.
Ltd.) using LipofectamineTM 3000 Transfection Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and incubated in 5%
CO2 at 37°C for 8 h to overexpress (OE) SIRT5. Cells
transfected with 3 µg/ml pCDNA3.1 plasmid (Sangon Biotech Co. Ltd.)
were used as a control. Cells were treated in a 5% CO2
incubator at 37°C for 48 h before subsequent experiments.
Small interfering (si)RNA transfection. SIRT5 and
negative control siRNAs were synthesized by Suzhou GenePharma. The
antisense sequences (5′→3′) were as follows: Negative control,
ACGUGACACGUUCGGAGAATT; SIRT5 siRNA-1, CCAGUUGUGUUGUAGACGATT and
SIRT5 siRNA-2, GGCUCGUCCAAGUUCAAAUTT. When the cell density reached
~30% confluency, 1 µg/ml SIRT5 and control siRNAs were transfected
into GC-2 spd cells. The cells were incubated at 37°C in 5%
CO2 for 48 h before subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from sperm samples and
1×106 GC-2 spd cells using SteadyPure RNA extraction kit
(Hunan Accurate Bio-Medical Technology Co., Ltd.). According to the
manufacturer's protocol, the extracted RNA was reverse-transcribed
into cDNA using Evo M-MLV RT Mix Tracking kit with gDNA Clean for
qPCR (Hunan Accurate Bio-Medical Technology Co., Ltd.). qPCR was
carried out using SYBR Green Premix Pro Taq HS qPCR kit (Hunan
Accurate Bio-Medical Technology Co., Ltd.). The following
thermocycling conditions were used: 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec and 60°C for 30 sec. The housekeeping gene
GAPDH was used as an internal control for normalization. RT-qPCR
was performed out using a two-step process. The 2−ΔΔCq
method was employed to evaluate the relative expression of the
target genes (25). The primers
are listed in Table I.
 | Table I.Primer sequences used in reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used in reverse
transcription-quantitative PCR.
| Target | Forward, 5′→3′ | Reverse, 5′→3′ |
|---|
| SIRT5 (human) |
GGCACTTCCTCTGTGGTG |
CGTAGCTGGGGTGGTCT |
| SIRT5 (mouse) |
AAGCACATAGCCATCATCTC |
CCCTCCGGTAGTGGTAAA |
| GAPDH (human) |
CCACTCCTCCACCTTTGACG |
CTGGTGGTCCAGGGGTCTTA |
| GAPDH (mouse) |
AGGTCGGTGTGAACGGATTTG |
GGGGTCGTTGATGGCAACA |
Western blotting
Western blotting was performed as previously
described (17). The antibodies
were as follows: Anti-β-actin (1:10,000; cat. No. 66009-1-IG,
Proteintech Group, Inc.), anti-SIRT5 (1:5,000; Cat. No. 15122-1-AP,
Proteintech Group, Inc.), anti-PI3K (1:1,000; Cat. # 13666, Cell
Signaling Technology, Inc.), anti-AKT (1:1,000; Cat. no. 9272, Cell
Signaling Technology, Inc.,), anti-phosphorylated (p-)AKT (1:1,000;
Cat. # 4060, Cell Signaling Technology, Inc.), anti-Bax (1:1,000;
Santa Cruz Biotechnology, Inc.) and anti-Bcl-2 (1:1,000; Santa Cruz
Biotechnology, Inc.). The secondary antibodies were anti-rabbit
(1:2,000; Cat. #7074, Cell Signaling Technology, Inc.). and
anti-mouse IgG (1:2,000; Cat. #7076, Cell Signaling Technology,
Inc.).
Cell Counting Kit-8 (CCK-8)
CCK-8 assay (Dojindo Molecular Technologies Inc.)
was performed to evaluate cellular viability. Transfected GC-2 spd
cells were seeded into a 96-well plate at a density of 3,000
cells/well. A volume of 10 µl CCK-8 (Beyotime Institute of
Biotechnology) was added to the DMEM and incubated at 37°C for 2 h.
Subsequently, the optical density at 450 nm was measured every 24 h
using a Multiskan Go plate reader (Beckman Coulter, Inc.).
EdU assay
An EdU incorporation assay was carried out with the
EdU cell proliferation kit (cat. no. C0078L, Beyotime Institute of
Biotechnology). Sterilized slides were placed in a 24-well plate
and transfected cells were seeded into each well at a density of
30%. After culturing with 50 µmol/l EdU reagent for 2 h at room
temperature, slides were washed with PBS and fixed with 4%
paraformaldehyde for 20 min at room temperature (Beyotime Institute
of Biotechnology). After staining the nucleus with DAPI, the cells
were imaged and photographed with a confocal laser scanning
microscope.
Wound healing assay
Cell migration was detected using a wound healing
assay. 5×105 transfected cells were plated in a 6-well
plate and cultured to 90% confluency. Subsequently, monolayers were
scratched with a 200 µl pipette tip and washed with PBS. The medium
was replaced with a serum-free DMEM and images were captured 0 and
24 h by a Lecia DMi8 fluorescence microscope. All cells were grown
at 37°C in a 5% CO2 incubator.
Apoptosis assay
An apoptosis assay was performed using Annexin V,
633 Apoptosis Detection kit (Dojindo Laboratories, Inc.). In brief,
after digestion with trypsin, the cells were centrifuged at 700 × g
for 2 min at 4°C, washed twice with PBS and resuspended in binding
buffer at a final density of 1×106 cells/ml. Annexin
V-633 and PI (5 µl each) were added to 100 µl cell suspension. The
cell suspension was mixed and incubated for 15 min at room
temperature in the dark. A total of 200 µl binding buffer was added
and cells were measured by flow cytometry using Calibur (BD Accuri
C6 Plus; BD Biosciences). Data were analyzed using BD Accuri C6
Plus software (version 1.0.264.21; BD Biosciences) and the
apoptotic rate was calculated as the percentage of early (Annexin
V-FITC) + late apoptotic (Annexin V-FITC and PI) cells.
Co-immunoprecipitation
A total of 1×107 GC-2 cells were lysed in
1 ml NP-40 buffer (Beyotime Institute of Biotechnology) containing
protease inhibitor cocktail, centrifuged at 12,000 g for 10 min at
4°C, and the supernatant was removed for use. 1 ml lysates were
incubated with PI3K (1:100; Cat. # 13666, Cell Signaling
Technology, Inc.), FLAG (1:200; Cat. No. 20543-1-AP, Wuhan Sanying
Biotechnology) or mouse IgG (1:100; sc-515946, Santa Cruz
Biotechnology, Inc.) antibody for 12 h at 4°C and incubated with 20
µl Pierce Protein A/G Magnetic Beads (Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. The beads were washed with PBST
(0.1% Tween-20) and incubated at 95°C for 10 min in 1× loading
buffer (Beyotime Institute of Biotechnology). The beads were
separated using a magnetic rack and the supernatant was used for
Western blotting, as aforementioned.
Statistical analysis
Data analysis was performed using GraphPad Prism 8
(Dotmatics). The results from three experiments are reported as
mean ± standard deviation. Student's unpaired t-test was employed
for comparisons between two independent groups, while one-way ANOVA
followed by the Tukey's post hoc test was used to assess
significance between >2 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
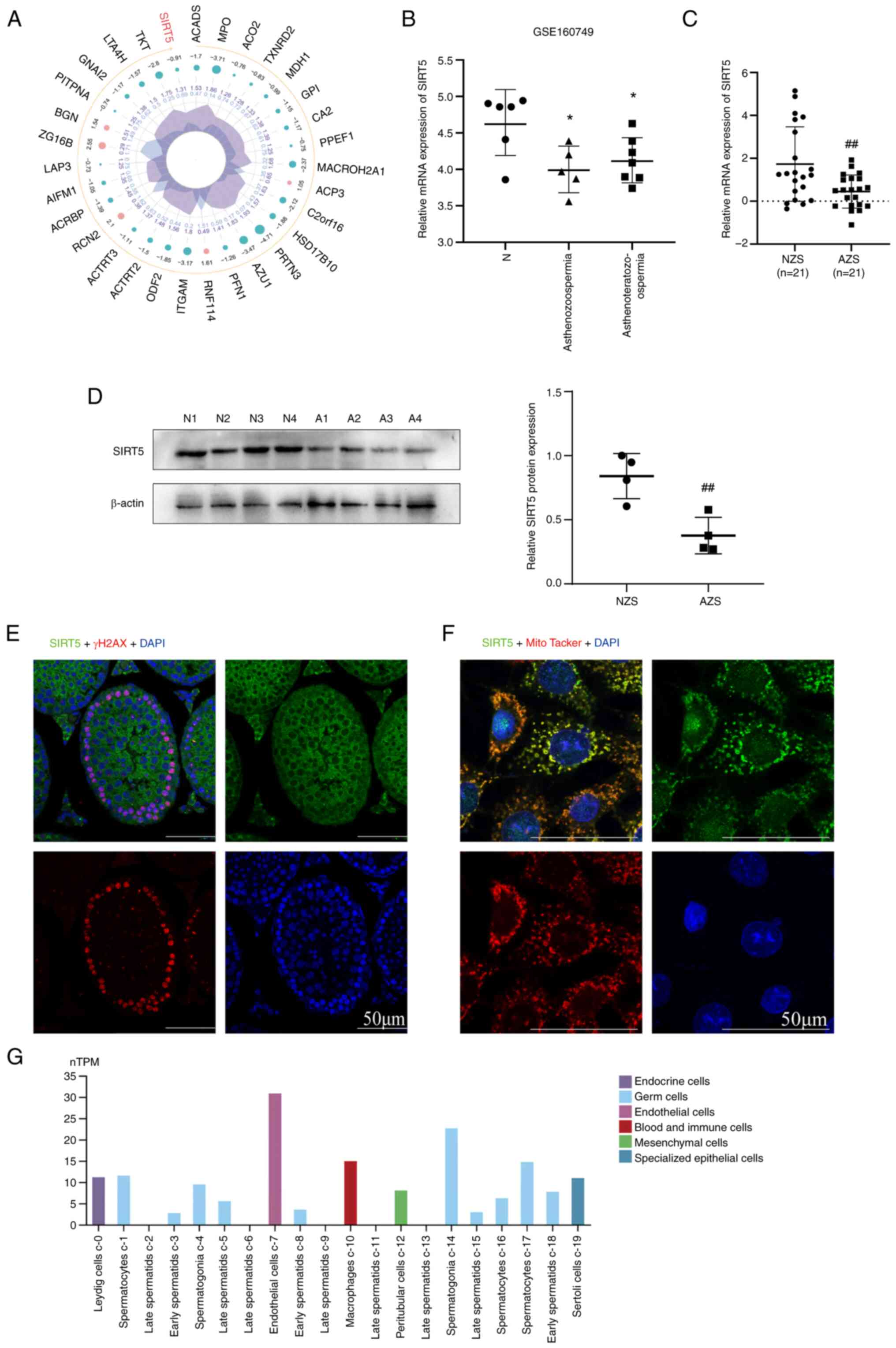
SIRT5 is downregulated in
asthenozoospermia and asthenoteratozoospermia
In our previous study, proteomic analysis revealed
that SIRT5 was downregulated in asthenozoospermia compared with
normal sperm samples (Fig. 1A)
(26). In addition, the expression
levels of SIRT5 were assessed in asthenozoospermia,
asthenoteratozoospermia and normal sperm from fertile men by
downloading the data from the GEO database (GSE160749). SIRT5
expression was significantly downregulated in asthenozoospermia and
asthenoteratozoospermia spermatozoa compared with normal (Fig. 1B). We obtained samples from
clinical patients and the basic semen parameters in normal and
asthenozoospermic patients are described in Table II. Furthermore, expression of
SIRT5 was assessed in normal and asthenozoospermia sperm samples,
which revealed that its mRNA and protein expression was
significantly downregulated in asthenozoospermia sperm samples
(Fig. 1C and D).
 | Table II.Basic semen parameters in patients
with asthenozoospermia and healthy controls. |
Table II.
Basic semen parameters in patients
with asthenozoospermia and healthy controls.
| Variable | Control |
Asthenozoospermia | P-value |
|---|
| Age, years | 31.44±3.98 | 32.60±3.42 | 0.4594 |
| Sperm count,
×106 sperm/ml | 94.72±21.05 | 80.20±40.31 | 0.1169 |
| Volume, ml | 4.28±1.17 | 4.30±1.07 | 0.9499 |
| Total motility,
% | 77.24±5.72 | 26.40±9.05 | <0.0001 |
| Progressive
motility, % | 66.72±7.04 | 13.44±6.16 | <0.0001 |
SIRT5 is expressed in the testis and
primarily expressed in mitochondria in spermatocytes
To determine the SIRT5 expression in the testicular
tissue, immunofluorescence analysis was conducted using testicular
tissue from a 2-month-old wild-type mouse. Analysis revealed
widespread expression of SIRT5 in testicular tissue, including its
presence in spermatocytes (Fig.
1E). This was congruent with the expression profile of SIRT5 in
the HPA database (Fig. 1G). In the
mouse spermatocyte cell line GC-2 spd, SIRT5 was mainly localized
to mitochondria (Fig. 1F).
SIRT5 promotes the proliferation of
GC-2 spd cells
Both gain- and loss-of-function assays were
performed to further elucidate the biological function of SIRT5 in
GC-2 spd cells. GC-2 spd cells were infected with lentivirus
harboring pLV-SIRT5 or siRNAs for SIRT5 knockdown. The efficacy of
SIRT5 modulation in GC-2 spd cells was evaluated by RT-qPCR and
western blot analysis (Fig. 2A-D).
CCK-8 assays revealed the viability of OE-SIRT5 GC-2 spd cells
increased compared with the vector control group (Fig. 2E). Conversely, knockdown of SIRT5
in GC-2 spd cells decreased viability (Fig. 2E). EdU assay revealed that SIRT5
knockdown inhibited cell proliferation while OE-SIRT5 increased
cell proliferation (Fig. 2F).
These results suggested that SIRT5 promoted the proliferation of
GC-2 spd cells.
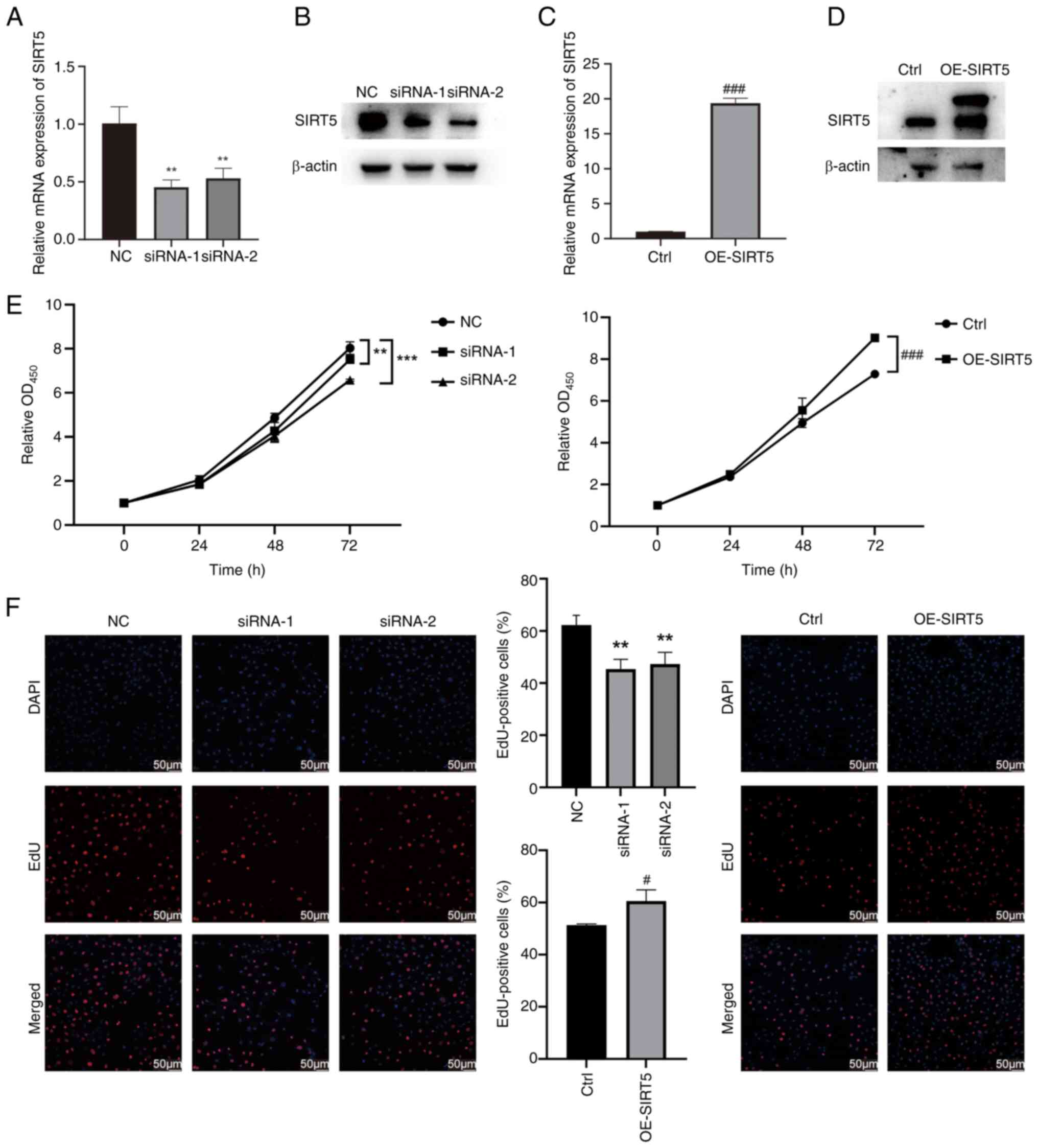 | Figure 2.Knockdown of SIRT5 inhibits
proliferation of GC-2 spd cells. Following transfection with
siRNAs, SIRT5 expression was suppressed in GC-2 spd cells at both
(A) transcriptional and (B) translational levels. After infection
with OE-SIRT5 lentivirus, SIRT5 expression was markedly increased
in GC-2 spd cells at both (C) transcriptional and (D) translational
levels. (E) Viability of GC-2 spd cells treated with siRNAs or
lentivirus. (F) EdU assays revealed the proliferation capacity of
GC-2 spd cells treated with siRNAs or lentivirus. **P<0.01,
***P<0.001 vs. NC; #P<0.05,
###P<0.001 vs. Ctrl. Ctrl, control; si, small
interfering; NC, negative control; OD, optical density; SIRT5,
sirtuin 5; OE, overexpression. |
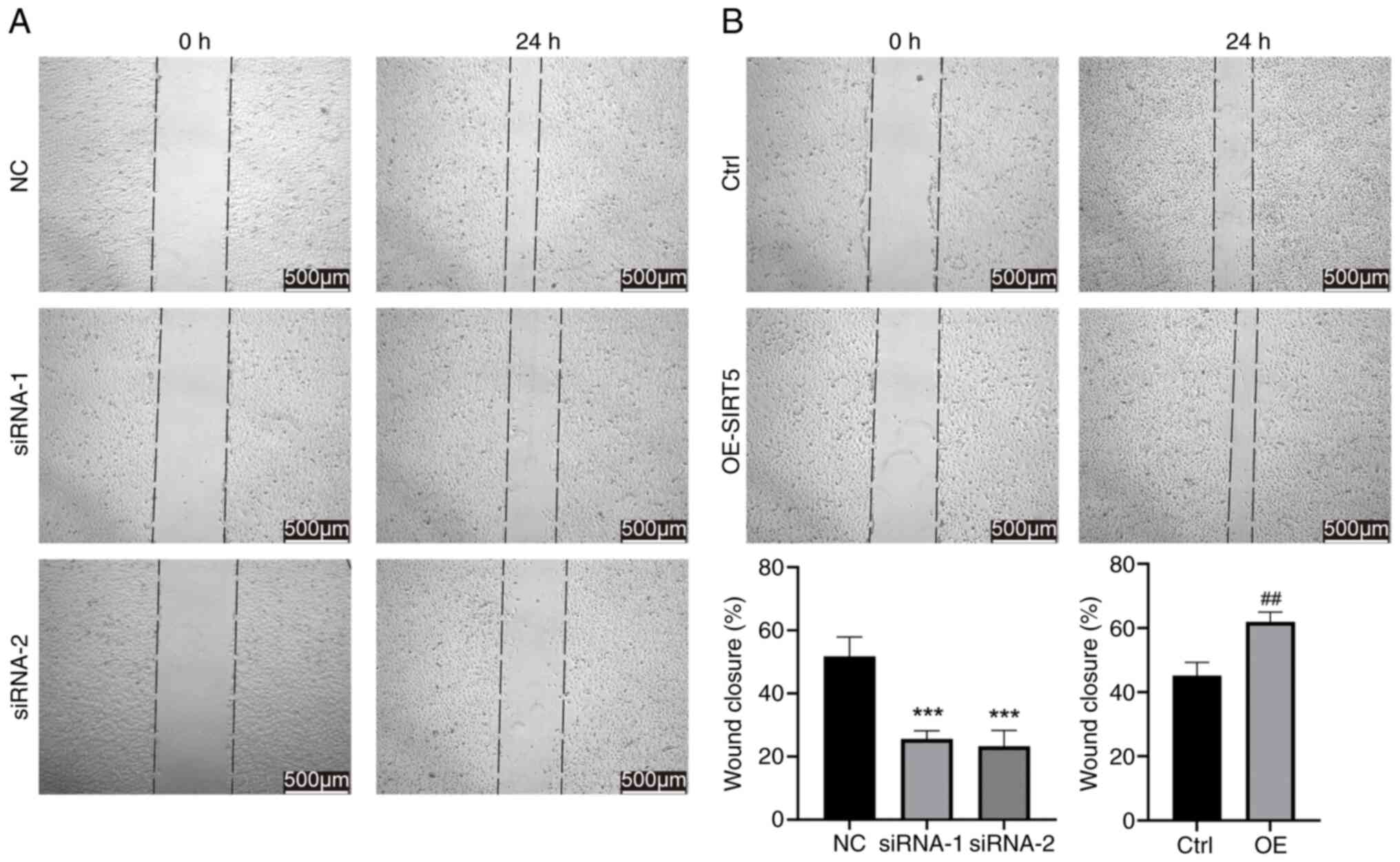
SIRT5 facilitates the migration of
GC-2 spd cells
Wound healing assay was performed to investigate the
effects of SIRT5 on the motility of GC-2 spd cells. Knockdown of
SIRT5 significantly impaired the migratory capacities of GC-2 spd
cells (Fig. 3A), whereas OE
increased migration (Fig. 3B).
SIRT5 inhibits apoptosis of GC-2 spd
cells
To determine the effects of SIRT5 on apoptosis in
GC-2 spd cells, flow cytometry was used. Notably, the proportion of
apoptotic cells was significantly elevated in the SIRT5 knockdown
compared with the control group (Fig.
4A). Conversely, the proportion of apoptotic cells with
OE-SIRT5 significantly decreased (Fig.
4B).
SIRT5 promotes the proliferation of
GC-2 spd cells via the PI3K/AKT pathway
To clarify the molecular mechanism underlying
regulation of proliferation and apoptosis by SIRT5 in GC-2 spd
cells, western blotting was performed. Knockdown of SIRT5
suppressed the expression of Bcl-2 and p-AKT, but promoted the
expression of Bax (Fig. 5A and B).
Conversely, OE-SIRT5 markedly enhanced the expression of Bcl-2 and
p-AKT, while decreasing expression of Bax (Fig. 5A and B). To explore how SIRT5
regulates the PI3K/AKT pathway, the binding of SIRT5 to PI3K was
assayed using co-immunoprecipitation (Fig. 5C). SIRT5 could bind with PI3K,
which might inhibit the phosphorylation of PI3K/AKT signaling
members.
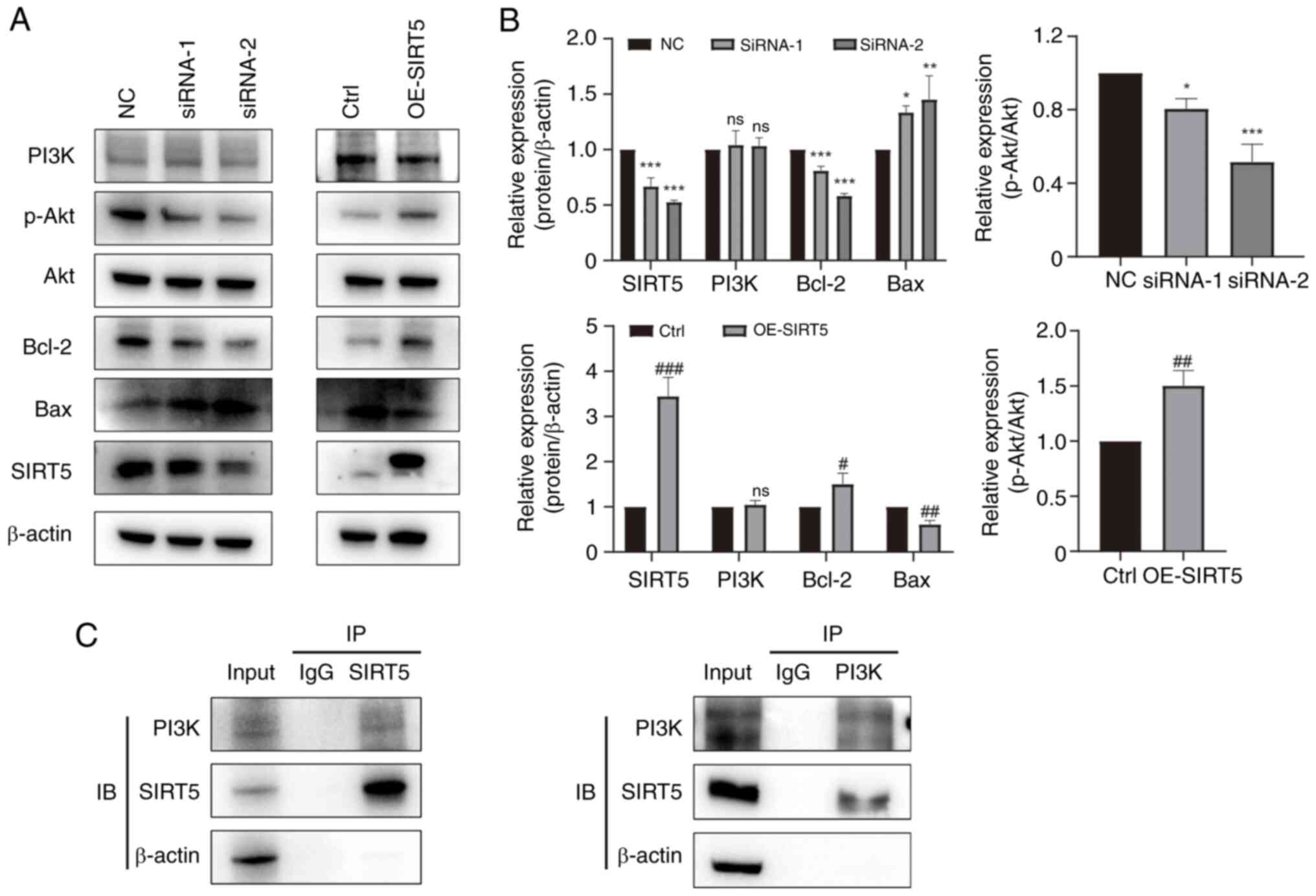 | Figure 5.SIRT5 regulates the PI3K/AKT
signaling pathway in GC-2 spd cells. (A) Western blot analysis of
PI3K, p-AKT, AKT, Bcl-2, Bax and SIRT5 in GC-2 spd cells treated
with siRNA or lentivirus. (B) Semi-quantitative analysis of western
blot results. (C) Co-IP using a PI3K and FLAG antibody on GC-2 spd
cells. *P<0.05, **P<0.01, ***P<0.001 vs. NC;
#P<0.05, ##P<0.01,
###P<0.001 vs. Ctrl. p-, phosphorylated; SIRT5,
sirtuin 5; Ctrl, control; NC, negative control; ns, not
significant; siRNA, small interfering RNA; OE, overexpression; IB,
immunoblot; IP, immunoprecipitation. |
Discussion
The causes of male infertility are complicated, and
its pathogenesis is unclear (27).
The process of spermatogenesis is regulated by factors including
energy metabolism, signaling pathways, and REDOX processes, to
ensure normal viability and fertilization ability (28,29).
Our previous proteomic analysis revealed that SIRT5 is
significantly downregulated in asthenozoospermic sperm (26); this finding was validated in human
sperm samples. However, the specific function of SIRT5 in male germ
cells has not been validated. To the best of our knowledge, the
present study is the first to demonstrate the role of SIRT5 in
inhibiting apoptosis and promoting sperm cell proliferation and
migration in vitro. The present study revealed that SIRT5
was involved in proliferation of GC-2 spd cells by regulating the
PI3K/AKT pathway.
Both the aforementioned proteomics results and
analysis of data from the GEO database revealed that SIRT5 was
significantly downregulated in asthenozoospermia compared with
normal motile sperm, which was also confirmed by RT-qPCR in human
sperm samples. These findings aligned with those reported in
previous studies (30,31). SIRT5 was expressed in the testis
and mainly localized in the mitochondria in the mouse spermatogenic
cell line. Loss of SIRT5 leads to mitochondrial membrane potential
defects and oxidative stress damage (32–34),
which can interfere with sperm motility (35,36).
Therefore, it was hypothesized that SIRT5 has an important role in
maintaining the normal movement of mature sperm.
The present study revealed that knockdown of SIRT5
decreased viability and proliferation and increased apoptosis of
GC-2 spd mouse spermatocytes. Previous research has reported that
SIRT5 participates in cellular processes through its regulation of
the PI3K/AKT pathway (37). This
pathway serves a key role in governing spermatogonial stem cell
self-renewal and spermatogonial proliferation (38). Therefore, we explored the
relationship between SIRT5 and the PI3K/AKT pathway and found that
the interaction between SIRT5 and PI3K was responsible for
inhibiting the phosphorylation of the PI3K/AKT signaling pathway
members. Studies have revealed that the PI3K/AKT signaling pathway
inhibits sperm apoptosis, accompanied by a decrease in the
expression of Bax (39,40), which is consistent with the
findings of the present study. In addition, inhibiting the PI3K/AKT
signaling pathway decreases the expression of premeiotic and
meiotic markers in the testes of postnatal and adult mice (41), whereas activation of PI3K/AKT
signaling promotes meiotic entry (42). Meiosis is a complex process that
begins in the primary spermatocyte and requires several proteins
and enzymes (43). Abnormal
meiosis has a marked effect on both sperm count and quality,
increasing the risk of infertility (44,45).
Therefore, it was hypothesized that SIRT5 may be an important gene
affecting male infertility via the regulation of PI3K/AKT
pathway.
SIRT5 has low deacetylation activity but strong
desuccinylation, demalonylation and deglutarylation activities
(18,19). For example, SIRT5 regulates the
malonylation of GAPDH and succinylation of pyruvate kinase M2
(46,47) and isocitrate dehydrogenase 2
(48) to alter their activity,
thereby participating in the regulation of glycolysis and the
tricarboxylic acid cycle. It was hypothesized that altered SIRT5
expression may cause disturbances in energy metabolism and
homeostasis via these metabolic changes, which in turn would affect
germ cell proliferation and apoptosis. Especially in mature sperm,
ATP is primarily produced through glycolysis to maintain sperm
motility. Therefore, it was hypothesized that changes in SIRT5
expression in sperm would exert an effect on sperm motility by
influencing the glycolytic pathway (49). In addition, SIRT5 interacts with
PI3K, therefore, SIRT5 may affect the PI3K/AKT pathway through the
regulation of the protein modifications. However, further studies
are needed to clarify the role of SIRT5 post-translational
modification in regulation of spermatogenesis and sperm
viability.
The present study revealed that SIRT5 is associated
with sperm motility, and served an important role in maintaining
the viability and proliferation of GC-2 cells and decreasing
apoptosis. The results of the present study are important for a
comprehensive understanding of the mechanism and treatment of male
infertility. However, the lack of in vivo data to assess how
SIRT5 affects infertility warrants further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by Guangdong Basic and Applied
Basic Research Foundation (grant no. 2025A1515012758), Shenzhen
Postdoctoral Research Start-Up Fund and Science Technology, the
Innovation Commission of Shenzhen Municipality (grant no.
JCYJ20200109140212277), the Sanming Project of Medicine in Shenzhen
(grant no. SZSM202111011) and the Shenzhen Key Medical Discipline
Construction Fund (grant no. SZXK051)..
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HX performed experiments and data analysis. HX, SC
and MW constructed figures, data interpretation, and wrote the
manuscript. HX, TZ and BY designed, supervised the study, and
confirmed the authenticity of all the raw data. TZ and BY provided
financial support. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments strictly followed the ‘Helsinki
Declaration’ and obtained ethical approval from the Ethics
Committee of Shenzhen Ethics Review Committee on Biomedical
Research (Shenzhen, China) [approval no. (2023) 001]. All patients
signed written informed consent. All animal protocols were in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals and approved by the Ethics
Committee of Shenzhen Peking University-Hong Kong University of
Science and Technology Medical Center (Shenzhen, China) (approval
no. 2021-007).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NAD
|
nicotinamide adenine dinucleotide
|
|
GEO
|
Gene Expression Omnibus
|
|
HPA
|
Human Protein Atlas
|
|
siRNA
|
small interfering RNA
|
|
RT-q
|
reverse transcription-quantitative
|
|
CCK-8
|
Cell Counting Kit-8
|
|
SIRT
|
sirtuin
|
References
|
1
|
Agarwal A, Baskaran S, Parekh N, Cho CL,
Henkel R, Vij S, Arafa M, Panner Selvam MK and Shah R: Male
infertility. Lancet. 397:319–333. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu S, Yu H, Liu Y, Liu X, Zhang Y, Bu C,
Yuan S, Chen Z, Xie G, Li W, et al: Chromodomain Protein CDYL Acts
as a Crotonyl-CoA Hydratase to regulate histone crotonylation and
spermatogenesis. Mol Cell. 67:853–866.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esteves SC and Agarwal A: Afterword to
varicocele and male infertility: Current concepts and future
perspectives. Asian J Androl. 18:319–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maurya S, Kesari KK, Roychoudhury S,
Kolleboyina J, Jha NK, Jha SK, Sharma A, Kumar A, Rathi B and Kumar
D: Metabolic dysregulation and sperm motility in male infertility.
Adv Exp Med Biol. 1358:257–273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu C, Tang D, Shao Z, Geng H, Gao Y, Li K,
Tan Q, Wang G, Wang C, Wu H, et al: Homozygous SPAG6 variants can
induce nonsyndromic asthenoteratozoospermia with severe MMAF.
Reprod Biol Endocrinol. 20:412022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuang BJ, Xu SY, Dong L, Zhang PH, Zhuang
BL, Huang XP, Li GS, You YD, Chen D, Yu XJ and Chang DG: Novel
DNAH1 mutation loci lead to multiple morphological abnormalities of
the sperm flagella and literature review. World J Mens Health.
40:551–560. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akbari A, Pipitone GB, Anvar Z, Jaafarinia
M, Ferrari M, Carrera P and Totonchi M: ADCY10 frameshift variant
leading to severe recessive asthenozoospermia and segregating with
absorptive hypercalciuria. Hum Reprod. 34:1155–1164. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao N, Hu C, Xia B, He Y, Huang J, Yuan Z,
Deng J and Duan P: The Activated AMPK/mTORC2 signaling pathway
associated with oxidative stress in seminal plasma contributes to
idiopathic asthenozoospermia. Oxid Med Cell Longev.
2022:42404902022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mei Z, Zhang X, Yi J, Huang J, He J and
Tao Y: Sirtuins in metabolism, DNA repair and cancer. J Exp Clin
Cancer Res. 35:1822016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kratz EM, Sołkiewicz K, Kubis-Kubiak A and
Piwowar A: Sirtuins as important factors in pathological states and
the role of their molecular activity modulators. Int J Mol Sci.
22:6302021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dhillon VS, Shahid M, Deo P and Fenech M:
Reduced SIRT1 and SIRT3 and lower antioxidant capacity of seminal
plasma is associated with shorter sperm telomere length in
oligospermic men. Int J Mol Sci. 25:7182024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iniesta-Cuerda M, Havránková J, Řimnáčová
H, García-Álvarez O and Nevoral J: Male SIRT1 insufficiency leads
to sperm with decreased ability to hyperactivate and fertilize.
Reprod Domest Anim. 57 (Suppl 5):S72–S77. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye F, Wu L, Li H, Peng X, Xu Y, Li W, Wei
Y, Chen F, Zhang J and Liu Q: SIRT1/PGC-1α is involved in
arsenic-induced male reproductive damage through mitochondrial
dysfunction, which is blocked by the antioxidative effect of zinc.
Environ Pollut. 320:1210842023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng YQ, Liu X, Zuo N, Yu MB, Bian WM, Han
BQ, Sun ZY, De Felici M, Shen W and Li L: NAD(+) precursors promote
the restoration of spermatogenesis in busulfan-treated mice through
inhibiting Sirt2-regulated ferroptosis. Theranostics. 14:2622–2636.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Hou X, Ma R, Moley K, Schedl T
and Wang Q: Sirt2 functions in spindle organization and chromosome
alignment in mouse oocyte meiosis. FASEB J. 28:1435–1445. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nasiri A, Vaisi-Raygani A, Rahimi Z,
Bakhtiari M, Bahrehmand F, Kiani A, Mozafari H and Pourmotabbed T:
Evaluation of the relationship among the levels of SIRT1 and SIRT3
with oxidative stress and DNA fragmentation in
asthenoteratozoospermic men. Int J Fertil Steril. 15:135–140.
2021.PubMed/NCBI
|
|
17
|
Wang Z, Zhu C, Song Y, Chen X, Zheng J, He
L, Liu X and Chen Z: SIRT3 inhibition suppresses hypoxia-inducible
factor 1α signaling and alleviates hypoxia-induced apoptosis of
type B spermatogonia GC-2 cells. FEBS Open Bio. 13:154–163. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei H, Khawar MB, Tang W, Wang L, Wang L,
Liu C, Jiang H and Li W: Sirt6 is required for spermatogenesis in
mice. Aging (Albany NY). 12:17099–17113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang
H, Kim J, Woo J, Kim JH, Choi BH, et al: Sirt5 is a NAD-dependent
protein lysine demalonylase and desuccinylase. Science.
334:806–809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park J, Chen Y, Tishkoff DX, Peng C, Tan
M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, et al:
SIRT5-mediated lysine desuccinylation impacts diverse metabolic
pathways. Mol Cell. 50:919–930. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greene KS, Lukey MJ, Wang X, Blank B,
Druso JE, Lin MJ, Stalnecker CA, Zhang C, Negrón Abril Y, Erickson
JW, et al: SIRT5 stabilizes mitochondrial glutaminase and supports
breast cancer tumorigenesis. Proc Natl Acad Sci USA.
116:26625–26632. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ji Z, Liu GH and Qu J: Mitochondrial
sirtuins, metabolism, and aging. J Genet Genomics. 49:287–298.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang HL, Chen Y, Wang YQ, Tao EW, Tan J,
Liu QQ, Li CM, Tong XM, Gao QY, Hong J, et al: Sirtuin5 protects
colorectal cancer from DNA damage by keeping nucleotide
availability. Nat Commun. 13:61212022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen XF, Tian MX, Sun RQ, Zhang ML, Zhou
LS, Jin L, Chen LL, Zhou WJ, Duan KL, Chen YJ, et al: SIRT5
inhibits peroxisomal ACOX1 to prevent oxidative damage and is
downregulated in liver cancer. EMBO Rep. 19:e451242018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J, Liu Q, Yu B, Han B and Yang B:
4D-quantitative proteomics signature of asthenozoospermia and
identification of extracellular matrix protein 1 as a novel
biomarker for sperm motility. Mol Omics. 18:83–91. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eisenberg ML, Esteves SC, Lamb DJ,
Hotaling JM, Giwercman A, Hwang K and Cheng YS: Male infertility.
Nat Rev Dis Primers. 9:492023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Kretser DM, Loveland KL, Meinhardt A,
Simorangkir D and Wreford N: Spermatogenesis. Hum Reprod. 13 (Suppl
1):1–8. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo J, Nie X, Giebler M, Mlcochova H, Wang
Y, Grow EJ; DonorConnect, ; Kim R, Tharmalingam M, Matilionyte G,
et al: The dynamic transcriptional cell atlas of testis development
during human puberty. Cell Stem Cell. 26:262–276.e4. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Janiszewska E, Kokot I, Kmieciak A,
Stelmasiak Z, Gilowska I, Faundez R and Kratz EM: The association
between clusterin sialylation degree and levels of
oxidative-antioxidant balance markers in seminal plasmas and blood
sera of male partners with abnormal sperm parameters. Int J Mol
Sci. 23:105982022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Emidio G, Falone S, Artini PG,
Amicarelli F, D'Alessandro AM and Tatone C: Mitochondrial sirtuins
in reproduction. Antioxidants (Basel). 10:10472021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L, Peltier R, Zhang M, Song D, Huang
H, Chen G, Chen Y, Zhou F, Hao Q, Bian L, et al:
Desuccinylation-triggered peptide self-assembly: Live cell imaging
of SIRT5 activity and mitochondrial activity modulation. J Am Chem
Soc. 142:18150–18159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boylston JA, Sun J, Chen Y, Gucek M, Sack
MN and Murphy E: Characterization of the cardiac succinylome and
its role in ischemia-reperfusion injury. J Mol Cell Cardiol.
88:73–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen H and Ong C: Detection of oxidative
DNA damage in human sperm and its association with sperm function
and male infertility. Free Radic Biol Med. 28:529–536. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kurkowska W, Bogacz A, Janiszewska M,
Gabryś E, Tiszler M, Bellanti F, Kasperczyk S, Machoń-Grecka A,
Dobrakowski M and Kasperczyk A: Oxidative stress is associated with
reduced sperm motility in normal semen. Am J Mens Health.
14:15579883209397312020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gill K, Machałowski T, Harasny P,
Grabowska M, Duchnik E and Piasecka M: Low human sperm motility
coexists with sperm nuclear DNA damage and oxidative stress in
semen. Andrology. 12:1154–1169. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi SY, Jeon JM, Na AY, Kwon OK, Bang IH,
Ha YS, Bae EJ, Park BH, Lee EH, Kwon TG, et al: SIRT5 directly
inhibits the PI3K/AKT pathway in prostate cancer cell lines. Cancer
Genomics Proteomics. 19:50–59. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen KQ, Wei BH, Hao SL and Yang WX: The
PI3K/AKT signaling pathway: How does it regulate development of
Sertoli cells and spermatogenic cells? Histol Histopathol.
37:621–636. 2022.PubMed/NCBI
|
|
39
|
Ding N, Zhang Y, Huang M, Liu J, Wang C,
Zhang C, Cao J, Zhang Q and Jiang L: Circ-CREBBP inhibits sperm
apoptosis via the PI3K-Akt signaling pathway by sponging miR-10384
and miR-143-3p. Commun Biol. 5:13392022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu Y, Fan Y, Fan W, Jing J, Xue K, Zhang
X, Ye B, Ji Y, Liu Y and Ding Z: RNASET2 impairs the sperm motility
via PKA/PI3K/calcium signal pathways. Reproduction. 155:383–392.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sahin P, Gungor-Ordueri NE and
Celik-Ozenci C: Inhibition of mTOR pathway decreases the expression
of pre-meiotic and meiotic markers throughout postnatal development
and in adult testes in mice. Andrologia. 50:May 10–2017.(Epub ahead
of print).
|
|
42
|
Deng CY, Lv M, Luo BH, Zhao SZ, Mo ZC and
Xie YJ: The Role of the PI3K/AKT/mTOR signalling pathway in male
reproduction. Curr Mol Med. 21:539–548. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ishiguro KI: Mechanisms of meiosis
initiation and meiotic prophase progression during spermatogenesis.
Mol Aspects Med. 97:1012822024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Y, Wu Y and Zhang S: Impact of
bisphenol-A on the spliceosome and meiosis of sperm in the testis
of adolescent mice. BMC Vet Res. 18:2782022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao J, Lu P, Wan C, Huang Y, Cui M, Yang
X, Hu Y, Zheng Y, Dong J, Wang M, et al: Cell-fate transition and
determination analysis of mouse male germ cells throughout
development. Nat Commun. 12:68392021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang F, Wang K, Xu W, Zhao S, Ye D, Wang
Y, Xu Y, Zhou L, Chu Y, Zhang C, et al: SIRT5 desuccinylates and
activates pyruvate kinase M2 to block macrophage IL-1β production
and to prevent DSS-induced colitis in mice. Cell Rep. 19:2331–2344.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Christofk HR, Vander Heiden MG, Wu N,
Asara JM and Cantley LC: Pyruvate kinase M2 is a
phosphotyrosine-binding protein. Nature. 452:181–186. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou L, Wang F, Sun R, Chen X, Zhang M, Xu
Q, Wang Y, Wang S, Xiong Y, Guan KL, et al: SIRT5 promotes IDH2
desuccinylation and G6PD deglutarylation to enhance cellular
antioxidant defense. EMBO Rep. 17:811–822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ford WC: Glycolysis and sperm motility:
Does a spoonful of sugar help the flagellum go round? Hum Reprod
Update. 12:269–274. 2006. View Article : Google Scholar : PubMed/NCBI
|