Introduction
Bronchopulmonary dysplasia (BPD) is a critical
respiratory disease with notable implications for the survival and
long-term prognosis of preterm infants (1,2). It
is particularly prevalent among severely preterm infants
(gestational age <28 weeks) and infants of very low birth weight
(VLBW; birth weight <1,000 g) (3). In recent years, with advancements in
perinatal and neonatal medicine, there has been a marked increase
in the survival rate of preterm infants but the incidence of BPD
has increased accordingly. Notably, BPD is associated with elevated
mortality in the neonatal period, where surviving infants will
frequently develop persistent airway hyperresponsiveness and
abnormal lung function (4). These
sequelae contribute to a poor long-term prognosis, imposing burdens
on the quality of life of affected children.
The prevailing perspective on the etiology of BPD
attributes its development to a number of factors, such as
premature lung structural immaturity, elevated oxygen exposure,
mechanical ventilation-induced injury and infection (1,5).
However, emerging evidence has highlighted the important role of
intestinal dysbacteriosis in the pathogenesis and progression of
BPD. Gut microecology, defined as the microbial community of the
intestinal flora, comprises both symbiotic and pathogenic
microorganisms residing in the tissues and intestines. Dysbiosis of
the intestinal flora has been associated with immune dysregulation
and is associated with multisystemic diseases, including obesity,
diabetes, atherosclerosis and non-alcoholic fatty liver disease
(6,7). The lung microenvironment is likely to
be affected by changes in the gut microbiota, since microbiomes
also exist in the upper and lower respiratory tract. This notion is
supported by clinical observations where respiratory diseases also
present with concomitant gastrointestinal symptoms, whereas
gastrointestinal disorders are frequently accompanied by
respiratory manifestations (8,9).
Patients with influenza virus infection may exhibit
gastrointestinal symptoms (8),
whilst ~50% patients with inflammatory bowel disease, which is
characterized by alterations in gut microbiota composition, have
been reported to exhibit impaired lung function (9). This bidirectional crosstalk between
the intestines and lungs is referred to as the gut-lung axis, which
may provide novel insights into the pathophysiology of BPD.
The gut-lung axis has been shown to serve a notable
role in respiratory disease. Gut microecology evolves with age,
where infants with BPD are more prone to early-life gut dysbiosis
(10). A previous study showed
that in vaginally delivered preterm infants, the BPD group exhibits
increased relative abundance of Escherichia/Shigella and
decreased levels of Klebsiella and Salmonella in
their gut flora compared with those in the non-BPD group (10). Chen et al (11) documented that the intestinal flora
diversity in preterm infants with BPD was markedly lower compared
with that in controls at postnatal day 28. The gut-lung axis may
influence BPD development through immune cell migration, mucosal
immune disruption and alterations in gut-lung microenvironmental
cytokines.
Previous studies have explored alterations in the
intestinal flora of patients with BPD (10,11).
However, these studies remain fragmented, where a gap exists
between the theoretical framework of the gut-lung axis and the
clinical management of BPD. To address this, there is a pressing
need to synthesize existing research on the gut-lung axis and its
relationship with BPD, thereby enhancing strategies for disease
prognosis management. The present review systematically
consolidates advances in understanding the role of the gut-lung
axis in BPD pathogenesis, aiming to provide a robust theoretical
foundation for the development of targeted interventions.
Bidirectional communication in the gut-lung
axis
Similarities between the respiratory and
intestinal tracts
Embryonic developmental homology
During early embryonic development, the endoderm
gives rise to the pro-intestinal tube, which subsequently
differentiates into the foregut, midgut and hindgut. The foregut
further develops into the respiratory tract and upper digestive
tract, including the esophagus, stomach and duodenum, whilst the
midgut and hindgut form the remaining intestinal tract. This shared
embryonic origin from the endoderm-derived pro-intestinal tube
establishes the homology between the respiratory and intestinal
tracts (12–14).
Similar signaling pathways and
transcription factor regulatory networks
During development, the formation of the respiratory
and intestinal tracts is orchestrated by analogous signaling
pathways and transcription factors. The Wnt, Bmp and Fgf signaling
pathways are essential for modulating cell proliferation,
differentiation and migration, thereby ensuring the normal
development of the respiratory and intestinal tracts (15–17).
In addition, various transcription factors, such as Nkx2.1 and
Sox9, are pivotal in driving the development of both systems
(18–20). These factors regulate the
expression of key genes involved in organ morphogenesis,
underscoring their critical role in the coordinated development of
the respiratory and intestinal tracts. Collectively, these
signaling pathways and transcription factors highlight the shared
regulatory mechanisms between these two systems, providing insights
into their interrelated functions and potential shared
pathologies.
Structural and functional parallels
The respiratory and intestinal tracts exhibit
notable structural and functional parallels. Structurally, the
mucosa of both tracts belongs to the mucosal immune system and
comprises epithelium and lamina propria. They both feature a
luminal structure consisting of endoderm-derived epithelial cells,
which undergo comparable morphogenetic processes during
development, such as the establishment of epithelial cell polarity
and the formation of intercellular junctions (13). Functionally, both tracts are
essential for maintaining ventilation and material exchange to
support the normal physiological functions of the body, whilst also
being capable of producing secretory immunoglobulin A. These
structural and functional similarities underscore their homology in
embryonic histogenesis (21).
The extensive similarities between the respiratory
and intestinal tracts not only enhance the comprehension of their
interplay during physiological development, but also establish the
biological foundation for bidirectional communication within the
gut-lung axis. While acknowledging that structural homology alone
does not guarantee communication, we contend that the anatomical
and developmental parallels between respiratory and intestinal
tracts create a permissive biological framework for bidirectional
crosstalk. These parallels further offer insights into the
mechanisms underlying related diseases, providing a framework for
exploring pathophysiological connections between these systems
(22).
Intestinal microbiota: A foundation of
bidirectional communication in the gut-lung axis
The gut-lung axis displays dynamic bidirectional
crosstalk between the intestinal and pulmonary systems (21). Lung diseases have been previously
shown to impact intestinal function and vice versa (21,23–28).
Amongst the key elements facilitating this interaction is the
bacterial flora, which serves as a critical link between these two
systems (29). Accumulating
evidence has indicated that commensal bacteria residing in the gut
and lungs are indispensable for the development and maintenance of
immune homeostasis (30,31). These microbiota begin to colonize
newborns not only at birth but also during fetal development. A
previous study identified overlapping gut and lung microbiota in
human fetuses as early as 10–18 weeks of gestation (32). It has also been reported that gut
and lung microbiota can translocate between these organs through
fluid circulation, where metabolites derived from gut microbiota
can influence lung physiology through various immune-mediated
pathways (such as immune cell migration, mucosal immune disruption
and alterations in gut-lung microenvironmental cytokines) (33). The gut-lung axis therefore provides
a framework for understanding the interplay between local
microbiota and distal immune mechanisms, offering novel
perspectives on disease pathogenesis and progression. This
conceptual model not only improves the comprehension of the
physiological connections between the gut and lungs, but also
highlights potential therapeutic targets for disorders affecting
these interconnected systems.
Characteristics of intestinal microbiota in
preterm infants
The gut microbiota in early human life is dynamic,
gradually stabilizing to resemble adult profiles after the age of
2–3 years. The precise timing of the colonization of the gut by
bacteria remains controversial. It has previously been considered
that the fetus and placenta are sterile, but advances in testing
techniques (such as 16S ribosomal DNA-based and whole-genome
shotgun metagenomic studies) have enabled the identification of a
unique placental microbiome niche that is comprised of
non-pathogenic commensal microbiota (such as Firmicutes,
Tenericutes, Proteobacteria, Bacteroidetes and Fusobacteria
phyla) (34). Collado et
al (35) also previously
identified a unique bacterial community in the amniotic fluid
dominated by Proteobacteria and successfully cultured bacteria from
the placentas of healthy pregnant women. The observation that the
amniotic fluid and placenta have their own microbiota suggests the
potential for fetal colonization by bacteria during the
intrauterine environment, which may contribute to the maturation of
early immune cells (36). However,
contamination could not be ruled out as one of the causes of the
aforementioned phenomena (37,38).
Bacteria can be detected in the fetal stool of
preterm infants after birth (39).
Compared with healthy term infants, preterm infants tended to have
more Staphylococcaceae and delayed colonization by
Bifidobacteriaceae in their feces (40). The intestinal microbiota of preterm
infants does not have a specific ‘phenotype’ and develops in a
manner similar to that of healthy term infants, from
Gram-positive cocci to Enterobacteriaceae and then to
Bifidobacteriaceae (39,40).
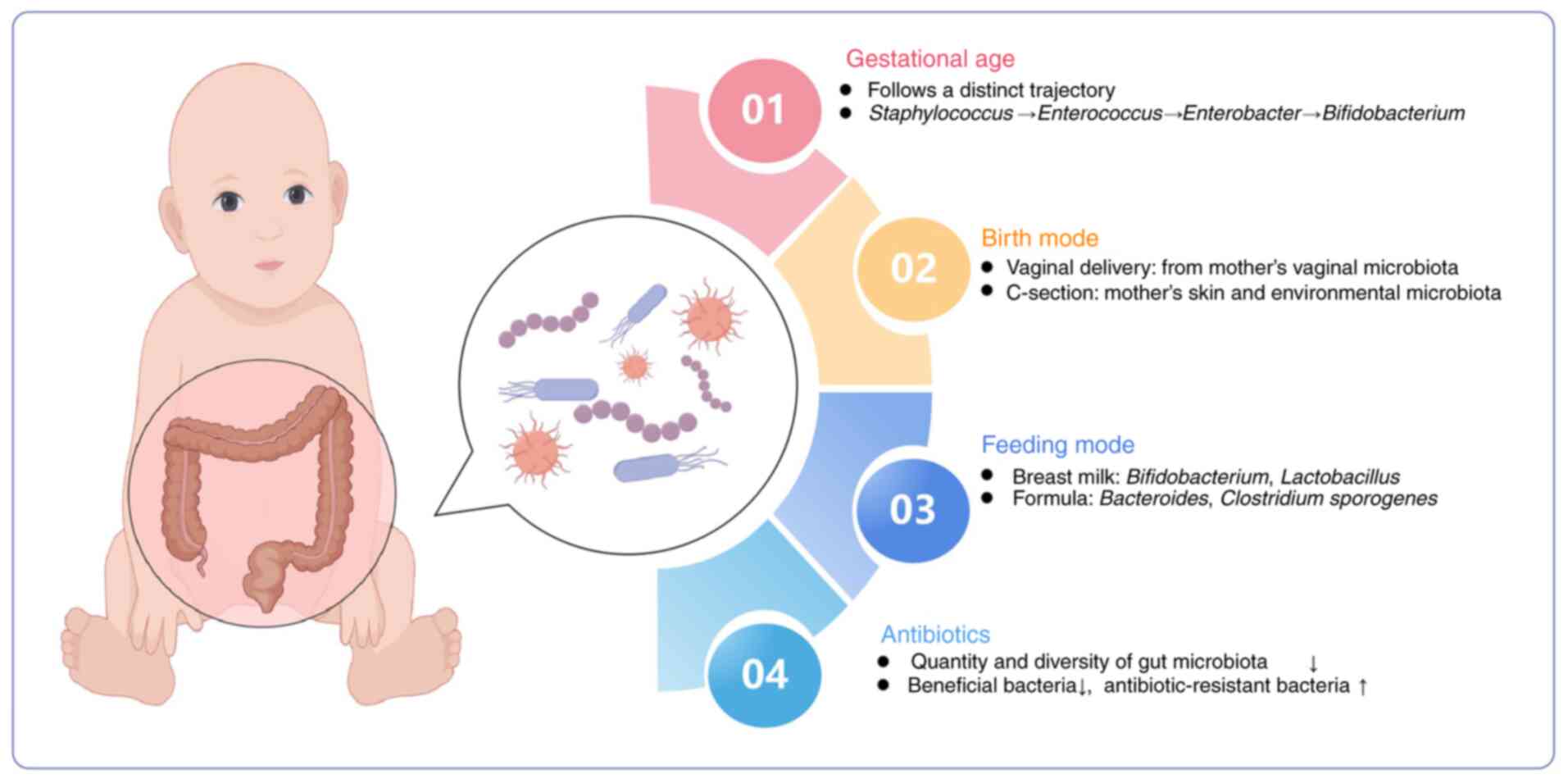
The development of intestinal microbes in preterm infants is
influenced by key perinatal factors, including gestational age,
delivery mode, feeding mode and the profile of antibiotics use
(Fig. 1).
Gestational age
In preterm neonates, gut microbiota development is
predominantly determined by gestational age. A previous study has
indicated that the gut microbiota of premature infants residing in
a tightly controlled microbial environment progresses through a
choreographed succession of bacterial classes from Bacilli
to Gammaproteobacteria to Clostridia (41). Antibiotics, vaginal vs. caesarian
birth, diet and age of the infants when sampled influence the pace,
but not the sequence of progression (41). Korpela et al (42) delineated four phases of gut
microbiota maturation in preterm infants, where the first three
phases are dominated by Staphylococcus, Enterococcus and
Enterobacter, then the fourth phase is characterized by the
prevalence of Bifidobacterium. This progression is closely
associated with postmenstrual age. Compared with term infants,
preterm neonates exhibit reduced gut microbiota diversity, increase
in conditionally pathogenic bacteria and delayed colonization by
beneficial species, such as Bifidobacteriaceae and
Bacteroidetes (43).
Birth mode
The intestinal microbiota of preterm infants
delivered vaginally is primarily sourced from the vaginal
microbiota of the mother, including Lactobacillus,
Prevotella and Sneathiella spp (44). By contrast, the gut microbiota of
preterm infants born through cesarean section (C-section) more
closely resembles the skin of the mother and the environmental
microbiota, which is predominantly comprised of Staphylococcus,
Corynebacterium and Propionibacterium spp. Notably, the
colonization of beneficial bacteria, such as Lactobacillus,
Bifidobacterium and Bacteroides spp., is delayed in
infants delivered by C-section (44,45).
This disparity in gut microbiota composition between infants
delivered vaginally and by C-section diminishes at 4 and 12 months
after birth (45).
Feeding mode
Feeding practices notably influence the gut
microbiota composition of preterm infants, with breastfed infants
exhibiting greater microbial diversity. Breast milk is rich in
diverse microbiota and human milk oligosaccharides (HMOs), which
promote the growth of Bifidobacterium (46). Consequently, the gut of breastfed
preterm infants is predominantly colonized by
Bifidobacterium and Lactobacillus, whereas
Bacteroides spp. and Clostridium sporogenes are more
prevalent in formula-fed infants (46). In particular, pasteurized donor
breastmilk has been shown to assist preterm infants in developing a
gut microbiota profile resembling that of breastfed healthy
neonates, accelerating the acquisition of microbial diversity
(47).
Antibiotics
Antibiotics can exert a profound impact on the gut
microbiota. Preterm infants frequently receive antibiotics for
extended durations during the perinatal period, longer than their
term counterparts. This prolonged exposure can markedly reduce the
quantity and diversity of gut microbiota, leading to a depletion of
beneficial bacteria (48). In
addition, extended antibiotic use is associated with increased
colonization by potentially pathogenic bacteria, such as
Escherichia coli, Shigella, Enterobacteriaceae and
Enterococcus, which may contribute to the emergence of
antibiotic-resistant bacteria (49).
Gut microbiota is integral to the health of preterm
infants, where it serves a key role in maintaining intestinal
immune homeostasis. A balanced symbiotic relationship between
commensal microbiota and the host is essential for establishing
effective immune defense and immune tolerance. Conversely,
dysbiosis of the gut microbiota can disrupt this delicate balance,
leading to immune system dysfunction and increasing the risk of
various morbidities in preterm infants (50).
The cause-and-effect of intestinal
microecology on BPD
BPD induces alterations in intestinal
microenvironment
BPD is a multifactorial and heterogeneous disease,
the pathogenesis of which is rooted in repeated injury to immature
lungs and aberrant repair processes triggered by a number of
factors, such as infection, mechanical ventilation and hyperoxia
(1,4). Maintaining a healthy gut microbiota
is crucial for immune homeostasis in preterm infants. Furthermore,
emerging evidence has indicated that both pulmonary and gut
microbiota are altered in infants with BPD (10,51).
Clinical studies have demonstrated that in preterm infants, the
progression of BPD is associated with dynamic changes in the
Proteobacteria and Firmicutes phyla and the Lactobacillus
genus within the lung microbiota, where that pulmonary dysbiosis
may exacerbate BPD severity (52,53).
Specifically, preterm infants with severe BPD exhibit more
pronounced changes in the respiratory microbiota, characterized by
reduced Staphylococcus and increased Ureaplasma
during early postnatal colonization (54,55).
The interaction between lung and gut microbiota is
bidirectional, with alterations in one able to influence the other.
This interplay is mediated through shared immune signaling
pathways, including cytokine signaling and Toll-like receptor (TLR)
activation, in addition to neuroendocrine pathways, such as the
hypothalamic-pituitary-adrenal axis and vagus nerve-mediated
communication, which collectively impact respiratory and
gastrointestinal health. Previous studies have shown associations
between gut dysbiosis and changes in lung microbiota composition,
with reciprocal effects of lung microbiota perturbations on gut
health (56,57). Chen et al (11) divided preterm infants with a
gestational age of 26–32 weeks into a BPD and non-BPD group and
demonstrated that gut microbiota diversity was significantly
reduced in the BPD group compared with non-BPD group at postnatal
day 28. Furthermore, other clinical studies reported lower Shannon
diversity of the gut microbiota indices in infants with BPD, which
documented that the relative abundance of Proteobacteria was
elevated in the gut microbiota of the BPD group whereas the
abundance of the Firmicutes phylum was decreased, between postnatal
days 14 and 28 (54,58). To address confounding factors, such
as preterm birth and delivery mode, Lal et al (59) investigated the gut microbiota of
vaginally delivered preterm infants and revealed that BPD was
associated with increased relative abundance of Escherichia
coli and Shigella, with decreased levels of
Klebsiella and Salmonella in the gut microbiota
(10). These findings collectively
support the existence of intestinal dysbiosis in BPD, highlighting
the gut-lung axis as a potential therapeutic target for mitigating
disease progression.
Gut microecological disorders
contribute to BPD progression through the gut-lung axis
Infants with BPD exhibit disruptions in both the
respiratory and gut microbiota. This can influence BPD progression
by both regulating immune-related gene expression and inducing
systemic and intrapulmonary inflammatory responses (60). In a BPD mouse model,
antibiotic-induced intestinal commensal disruption during the
perinatal period promotes a more severe BPD phenotype,
characterized by increased lung fibrosis, vascular remodeling,
alveolar inflammation and higher rates of morbidity and mortality.
This was attributed to the disruption of intestinal commensal
bacterial colonization, highlighting the gut-pulmonary axis as a
key factor in BPD development (61).
Intestinal microecology disorders can promote BPD
progression through the gut-lung axis and the mechanisms may
include the following: i) Intestinal barrier disruption, where gut
dysbiosis can compromise the intestinal barrier, increasing
intestinal permeability to allow bacterial translocation to the
lungs. Additionally, dysbiosis-generated metabolites may trigger
immune cell activation, leading to local or systemic inflammation
and metabolic disturbances (62).
ii) Amplification of pulmonary inflammation, where gut dysbiosis
can exacerbate lung injury by promoting pulmonary inflammation. A
previous study indicated that TLRs can recognize
lipopolysaccharides (LPS) from gut bacteria, upregulating IL-1β
expression to activate NF-κB in a rodent model. This initiates an
inflammatory cascade to exacerbates lung injury (63). iii) Metabolic dysregulation, where
gut dysbiosis influences BPD development through various metabolic
pathways. Li et al (64)
determined that 129 differentiated metabolites were changed in
patients with BPD via metabolomics analysis, and a correlation
analysis revealed a remarkable relationship between gut microbiota
and metabolites. Other studies have shown that oral administration
of Lactobacillus plantarum L168 may play a protective role
in BPD by regulating the systemic metabolome, such as glutathione
metabolism and arachidonic acid metabolism (65). iv) Association with growth
restriction. Gut dysbiosis is strongly associated with growth
restriction and BPD in preterm infants. Given the critical role of
the gut microbiota in the extrauterine growth of VLBW infants and
the protective effects of nutritional support against BPD (66), modulating gut microbiota to improve
nutritional status may reduce BPD risk in preterm infants.
Compared with term infants, preterm infants have a
reduced diversity of gut microbial communities, where factors such
as exposure to hyperoxia, antibiotic exposure and nosocomial
infections, further disrupt microbial homeostasis. These
disruptions compromise the gut barrier, trigger inflammatory
responses, metabolic disorders and malnutrition, all of which
further impair lung tissue repair through the gut-lung axis,
ultimately accelerating the progression of BPD (53).
Unique mechanism of the gut-lung axis
in BPD
The core pathological feature of BPD in preterm
infants is the developmental arrest of immature lungs due to
injuries, such as hyperoxia and mechanical ventilation (1). The gut-lung axis may contribute to
BPD through the following mechanisms: i) Early disruption of gut
microbiota diversity, where both the gut and lungs of preterm
infants are immature. Prolonged hyperoxia inhibits the growth of
beneficial bacteria, such as Lactobacillus, increasing
pathogen abundance and causing dysbiosis. This dysbiosis can
intensify pulmonary inflammation and alveolar developmental
disorders by altering metabolites, including short-chain fatty
acids (SCFAs), trimethylamine N-oxide (TMAO), volatile organic
compounds (VOCs) and butyrate, as soon as impairing immune
regulation (67,68). BPD-related dysbiosis is
characterized by reduced biodiversity and the absence of specific
bacteria (such as Lactobacillus) (11,58).
By contrast, necrotizing enterocolitis (NEC)-associated lung injury
involves a complete gut microbiota collapse, with endotoxins
(including LPS) entering the circulation and triggering systemic
inflammation (69). ii)
Hyperoxia-induced microbiota-inflammation interactions. Hyperoxia
exposure induces alveolar epithelial cells damage in BPD,
simultaneously alters gut microbiota composition and promotes
bacterial translocation to the lungs. This process activates immune
and inflammatory pathways, releasing cytokines (such as IL22, IL6
and IL17) that inhibit alveolar development (60,61).
NEC-related lung injury, however, results from systemic
inflammation and sepsis caused by acute intestinal necrosis, rather
than the direct effects of hyperoxia (69,70).
Immunological modulation of the gut-lung
axis in BPD progression
Gut microbiota and its metabolites
mediate the pulmonary immune response through the gut-lung
axis
The neonatal immune response consists of both innate
and adaptive components. In preterm infants with an immature immune
system, airway and gut microbiota serve a crucial role in immune
development. The ‘intestinal microbiota translocation’ theory
posits that gut microbiota or bacterial products can directly
transfer to the lungs through the gut-lung axis, disrupting
pulmonary microecological balance. Additionally, gut microbial
metabolites entering the pulmonary circulation through systemic
circulation can stimulate lung immune cells, activating
inflammatory responses and inducing lung injury (71). Furthermore, these metabolites,
including SCFAs, can enter the bone marrow through the bloodstream,
promoting hematopoiesis and stimulating hematopoietic stem cell
differentiation (31). These cells
then migrate to the respiratory tract, modulating lung inflammation
in conjunction with the aforementioned mechanisms.
Gut microbiota
The gut barrier is a critical defensive system
within the gastrointestinal tract, preventing pathogen and
endotoxin invasion to avert inflammation and bacterial
translocation. Numerous factors, such as gestational age, feeding
practices and antibiotic use, can all disrupt the intestinal
microecological balance, affecting gut barrier maturation in
preterm infants (10). In neonates
with NEC, intestinal mucosal macrophages and immune cells clear the
majority of bacteria. However, surviving bacteria or their
inflammatory fragments can reach the lungs, activating alveolar
macrophages and causing lung injury (72). Gut-derived bacterial products,
including bacterial fragments and metabolites, can enter the
pulmonary circulation through the systemic circulation to stimulate
lung immune cells (such as macrophages, T cells and neutrophils) to
trigger inflammation (29).
Dickson et al (73)
previously detected live gut bacteria in the lungs of septic mouse
models and in the bronchoalveolar lavage fluid of patients with
acute respiratory distress syndrome, suggesting that local or
systemic inflammation can mediate gut microbiota translocation,
disrupting lung microecological homeostasis. Intestinal
microecological imbalances can also increase gut permeability,
potentially allowing bacterial products to directly transfer to the
lungs and exacerbate pulmonary inflammation. Notably, recent
studies have drawn attention to the impact of strain-specific
microbiota (65,74). Shen et al (65) observed reduced lung injury and
improved lung development in BPD rats exposed to
Lactiplantibacillus plantarum L168. These results suggested
that L168 may improve BPD through downregulation of the
TLR4/NF-κB/Chemokine (C-C motif) ligand 4 pathway (65). By contrast, although supplementing
Lactobacillus reuteri DSM17938 has been shown to improve
intestinal health, it has no notable therapeutic effects on BPD
(75). The differences between the
two suggest strain-specific microbiota effects.
Microbiota metabolites
Alterations in gut flora composition can impact
pulmonary immune function, with bacterial metabolites serving a key
role in gut-lung immune communication and potentially contributing
to BPD progression. SCFAs are essential gut microbiota metabolites
that exhibit anti-inflammatory properties by reducing immune cell
migration and adhesion and increasing anti-inflammatory cytokine
release. SCFAs can traverse the gut-lung axis to modulate pulmonary
immunity, with two primary immunomodulatory mechanisms suggested:
i) Driving anti-inflammatory responses through downstream effector
molecules; and ii) inhibiting histone deacetylase (HDAC) activity
to regulate immune responses. SCFAs can also promote
anti-inflammatory responses in extraintestinal organs, mitigating
airway inflammation. Low fecal acetic acid levels in preterm
infants have been associated with higher BPD incidence, whereas
acetic acid supplementation could reduce inflammation in mice with
hyperoxia-exposed BPD, decrease the proportion of
Escherichia-Shigella, increase the abundance of
Ruminococcus and attenuate lung injury, potentially
preventing neonatal BPD (76).
Other gut microbiota metabolites, such as TMAO and
VOCs, can also influence BPD pathology by reducing airway
inflammation and restoring epithelial function. Altered urinary
metabolites, including lactate, taurine, TMAO, inositol and
gluconate, have all been observed in infants with BPD (77). Specifically, alanine and betaine
levels are elevated whereas TMAO, lactate and glycine levels are
reduced in patients with BPD compared with those in non-BPD
controls (78). Notably, gut
microbiota can alter TMAO levels, and TMAO can activate
inflammation and induce oxidative stress, thereby modulating BPD
susceptibility (79). Fecal VOCs,
produced by intestinal flora during polysaccharide fermentation,
can influence lung function by altering the air-liquid interface
properties of pulmonary surfactants, stimulating pro-inflammatory
factor production and exacerbating oxidative stress. These effects
suggest a potential link between VOCs and BPD development (80). Previous studies have shown
associations between fecal VOC changes and BPD severity, where VOCs
have demonstrated potential for early BPD diagnosis and prediction
within the first 2 weeks of birth (81,82).
Furthermore, it has been shown that butyrate can enhance gene
expression (such as p16INK4a, p14ARF, and p15INK4b) by inhibiting
HDACs, thereby promoting histone acetylation and a more relaxed
chromatin structure (83,84). SCFAs can also influence DNA
methylation patterns by modulating DNA methyltransferases,
chromatin remodeling complexes, microRNAs and transcription factors
(85,86). However, the exact role of these
epigenetic mechanisms in BPD pathogenesis remains to be fully
clarified.
Gut microenvironment alters the levels
of pulmonary inflammation through the lung-gut axis
It has been previously demonstrated that the gut
microenvironment serves a crucial role in modulating pulmonary
immunity through the gut-lung axis. Gut microenvironment disruption
can lead to increased cytokines and inflammatory mediators, which
then spill over into the systemic circulation, driving lung
inflammation, disrupting the lung microenvironment and worsening
BPD. This process involves alterations in key inflammatory
pathways, including changes in IL-17, IL-22, IL-6 and TNF-α
signaling (87). Metabolites from
gut microbiota, such as polysaccharide A, α-galactosylceramide and
tryptophan metabolites, can activate proinflammatory factors,
including IL-17 and IL-22, triggering local or systemic
inflammation and immune dysfunction. Aberrant signaling from these
metabolites can alter the developing lungs, contributing to lung
disease (88). In neonatal mice
with BPD, T helper (Th)17 inflammatory responses are heightened in
the lung tissue, where neutralizing IL-17 with specific antibodies
can alleviate BPD-associated lung injury (89). Additionally, Lactobacillus
species degrade tryptophan to produce IL-22, which suppresses
immune responses and promotes regulatory T-cell development, in
turn protecting both gut and lung tissues (81). FMT has also shown promise in
mitigating acute lung injury in rats by reducing inflammatory cell
infiltration and lung interstitial exudation through downregulating
TNF-α, IL-1β, IL-6 and TGF-β expression (90). These findings underscore the
influence of the gut microbiota on inflammatory mediator levels and
its subsequent impact on pulmonary pathology.
Immune cell migration-mediated mucosal
immunoregulatory mechanisms in the gut-lung axis
A primary communication pathway within the gut-lung
axis involves the migration of immune cells between the gut and
lungs, activating mucosal immunoregulatory mechanisms and
influencing BPD progression (91).
Certain intestinal mucosal immune cells, such as CD4+
and CD8+ cells, can reside in both the gut and lungs,
suggesting that mucosal immunity may constitute the immune network
between the lungs and the intestines (29). In BPD, lung inflammation involves
immune cell infiltration, some of which originate from the gut and
are activated by intestinal immune responses before migrating to
the lungs (91). Innate lymphoid
cells (ILCs) and dendritic cells (DCs) migrating along the gut-lung
axis may serve a notable role in BPD pathogenesis (92,93).
ILCs are widely distributed in the mucosa of the
intestine and respiratory tract, where they regulate immunity and
mucosal barrier homeostasis through cytokine secretion (92,94).
Gut ILCs can enter the bloodstream through the mesenteric lymphatic
system and migrate to the lungs, contributing to epithelial repair
(71). In neonatal mice with BPD,
type 2 ILCs (ILC2) numbers are elevated in the lung tissue and are
associated with alveolar differentiation arrest (92,94).
ILC2 depletion by anti-CD90.2 antibodies has been reported to
alleviate BPD-related lung injury (94). ILC2 can migrate from the intestine
to the lungs under the induction of IL-25 and participate in the
immune inflammatory response of the lungs (90). This was demonstrated in a previous
study where connecting the circulatory systems of two mice and
injecting IL-25 into one led to ILC2 migration from the gut of the
treated mouse to the lungs of both, exacerbating lung inflammation
(63). This suggests that
inflammatory stimulus may drive ILC2 recruitment to the lungs
through the gut-lung axis, impacting BPD pathogenesis, but the
exact molecular mechanisms require further investigation.
Type 3 ILCs (ILC3), a critical component of mucosal
immunity, serve an essential role in defending against lung
infections in preterm infants. A previous study showed that the
level of ILC3 expression is notably elevated in the lung tissue of
mice with BPD, promoting Th17 inflammatory responses and
exacerbating lung injury (89). In
preterm infants with NEC, gut mucosal DCs can recognize intestinal
dysbiosis, triggering ILC3 migration to the lungs of neonatal mice.
This disrupts pulmonary innate immunity and impairs type II
alveolar epithelial cell secretion of surfactants, leading to lung
injury (88). Gray et al
(93) previously demonstrated that
intestinal DCs can drive ILC3 migration to the lungs. Disrupting
postnatal bacterial colonization or selectively deleting DCs blocks
IL-22 and ILC3 migration, increasing susceptibility to
Streptococcus pneumoniae in neonatal mice (93). This highlights the role of
commensal gut bacteria in orchestrating pulmonary mucosal immunity,
crucial for lung defense system maturation. Disrupting ILC3
homeostasis in neonatal lungs may impair the pulmonary mucosal
defense system, raising the risk of respiratory infections and
inflammatory diseases, worsening BPD incidence and severity. Novel
insights into mucosal immunoregulation may offer promising avenues
for understanding BPD development, emphasizing the complex gut-lung
axis interactions. In conclusion, the present review discussed the
immunological modulation of the lung-gut axis in BPD (Fig. 2).
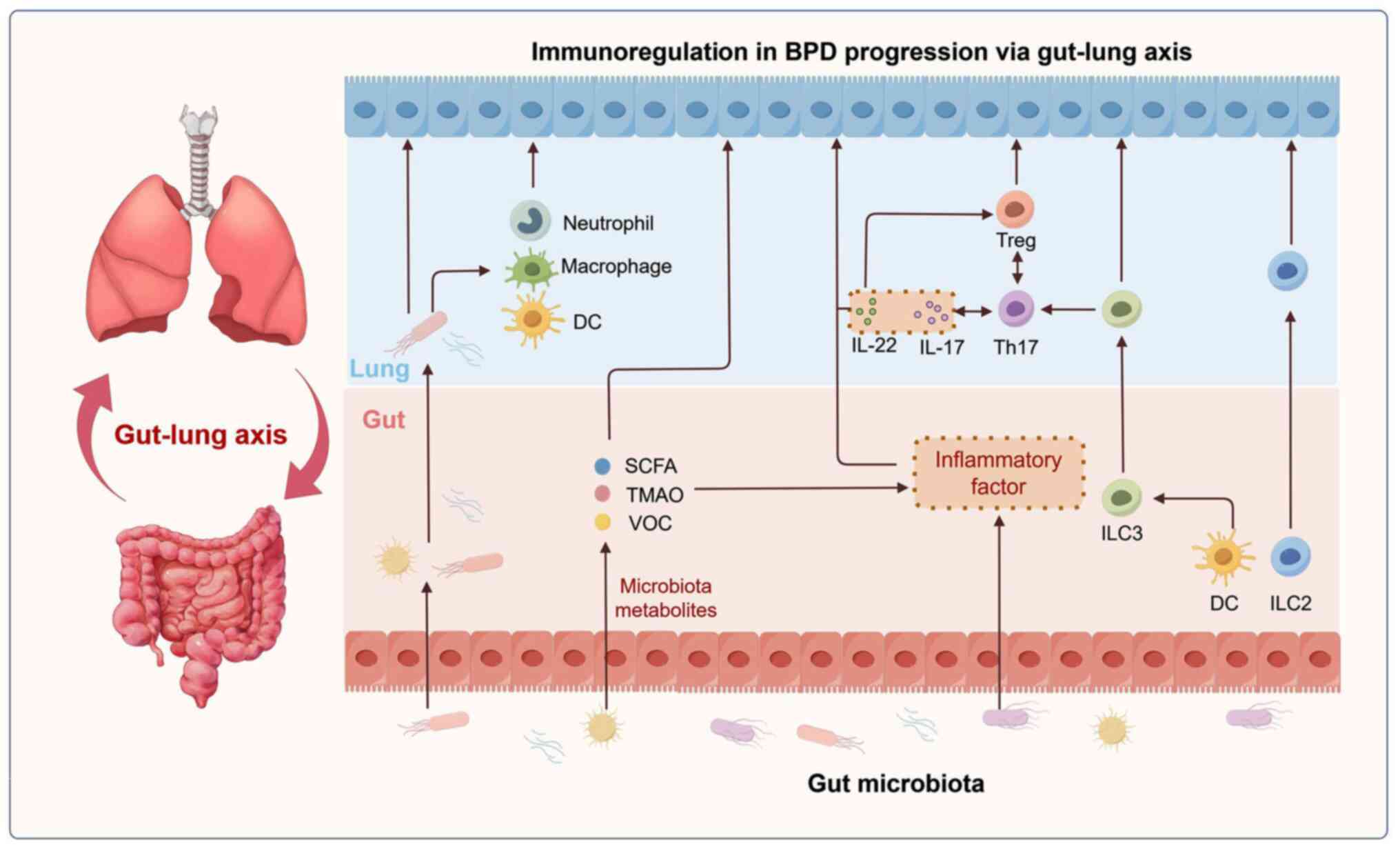 | Figure 2.Mechanism of the gut-lung axis in the
progression of BPD. The mechanism underlying immunological
modulation of the gut-lung axis in BPD includes: i) Gut microbiota
and its metabolites mediate the pulmonary immune response; ii) gut
microenvironment alters the level of pulmonary inflammation; and
iii) immune cell migration-mediated mucosal immunoregulatory
mechanisms. Figure drawn with Figdraw software 2.0 (ID: IRWIA45788,
www.figdraw.com; provided by Home for
Researchers, China). BPD, bronchopulmonary dysplasia; DC, dendritic
cell; ICL2, type 2 ILCs; ILC3, type 3 ILCs; ICLs, innate lymphoid
cells; SCFA, short-chain fatty acid; Th, T helper; TMAO,
trimethylamine N-oxide; Treg, regulatory T cells; VOC, volatile
organic compound. |
Emerging therapeutic approaches for BPD via
the gut-lung axis
The treatment implications of the gut microbiome in
BPD remain under investigation. However, several potential
treatment implications have emerged (Fig. 3). Probiotics offer a potential
approach to restoring the balance of gut microbiota, which in turn
can have a positive impact on lung health. Accumulating evidence
has suggested that specific probiotics may confer protection
against BPD and other respiratory conditions (e.g., asthma, COPD,
cystic fibrosis, lung cancer and respiratory infection). These
probiotics, such as Filamentous bacteria, Limosilactobacillus
reuteri and Bifidobacterium bifidum, achieve their
beneficial effects through immune modulation, reduction of
inflammation and the preservation of barrier integrity in both the
gut and lungs (94–96). Qu et al (97) previously investigated the effects
of Clostridium butyricum supplementation on extremely
preterm infants and found that probiotic-treated infants had lower
rates of BPD and invasive mechanical ventilation use, suggesting
that probiotics may be a notable factor influencing BPD risk (odds
ratio=0.034; 95% CI, 0.012–0.096). However, a meta-analysis by
Villamor-Martínez et al (98) found no significant effect of
probiotic supplementation on BPD risk. These contradictory results
should be interpreted cautiously and may stem from methodological
differences in randomized controlled trials, such as variations in
enrollment criteria, timing and dosing regimens. Additionally,
different probiotic strains, doses and formulations may yield
varying results. Future research should focus on optimizing
probiotic usage, including strain selection, dosing, timing and
administration methods, to understand their potential in BPD
management.
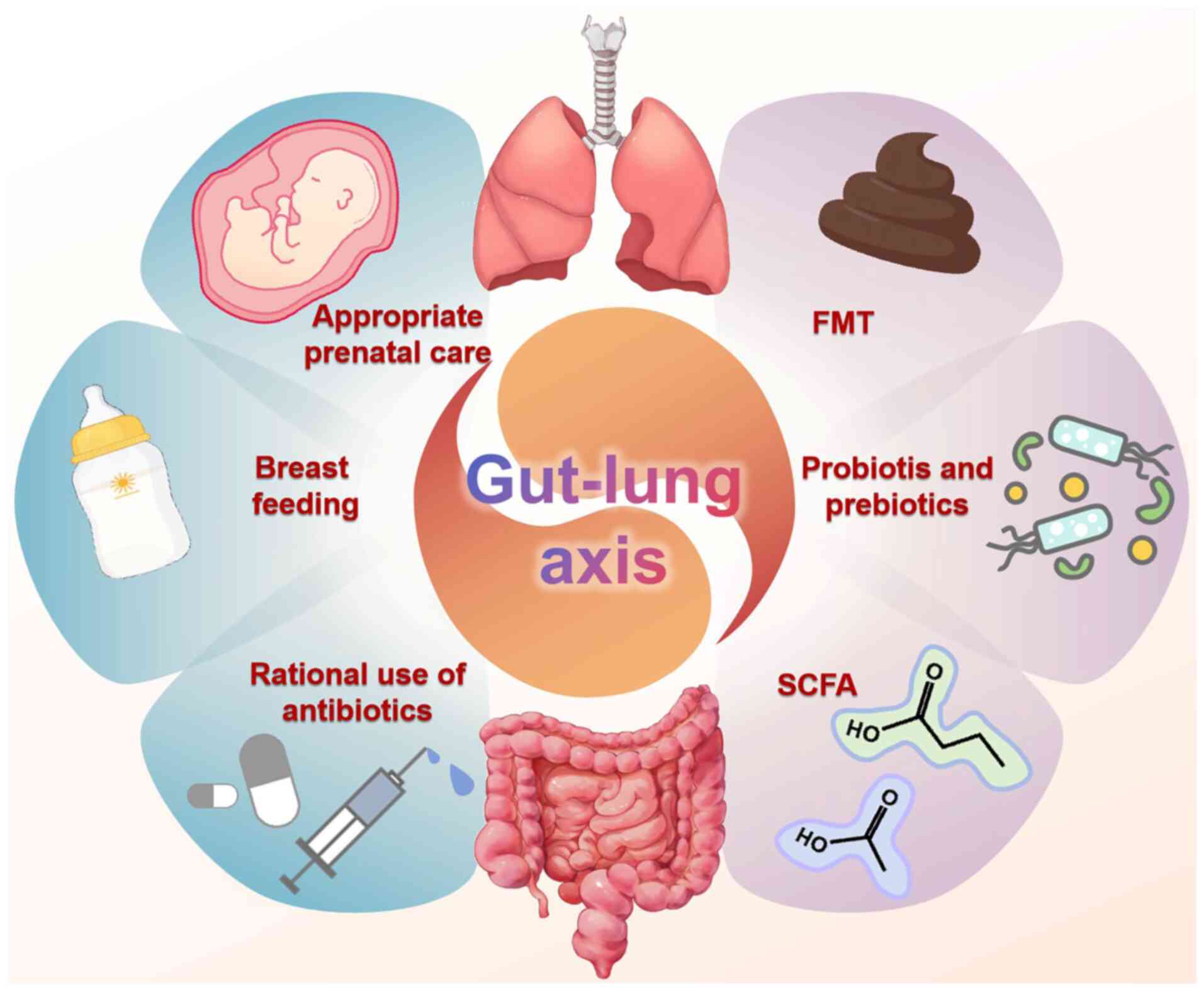 | Figure 3.Emerging therapeutic approaches for
BPD via the gut-lung axis. Several potential treatment implications
of the gut microbiome in BPD have emerged, including probiotics and
prebiotics, FMT, SCFA supplementation, avoiding premature birth,
breast feeding and the rational use of antibiotics. Figure drawn
with Figdraw software 2.0 (ID: TWPSObfadf, Obfadfwww.figdraw.com;
provided by Home for Researchers, China). BPD, bronchopulmonary
dysplasia; FMT, fecal microbiota transplantation; SCFA, short-chain
fatty acid. |
In addition to probiotics, prebiotics present
another avenue for influencing the gut microbiota. These
indigestible fibers can promote the growth of beneficial bacteria
in the gut (99). By incorporating
prebiotics (e.g. fructans, galactooligosaccharides,
xylooligosaccharides, chitooligosaccharides, lactulose, resistant
starch, polyphenols) as supplements, it is possible to affect
changes in the microbiota and their metabolites within both the
intestinal and pulmonary environments, potentially offering a novel
intervention strategy (98).
Furthermore, the direct administration of SCFAs has demonstrated
efficacy in regulating immune responses, preserving barrier
function and mitigating inflammatory processes in the gut and lungs
(99,100). SCFAs can be delivered through
various routes, including intranasal administration, in conjunction
with milk or through drinking water (98,99).
However, it is important to note that these findings have primarily
been observed in animal models and therefore require further
validation in clinical settings.
FMT represents another emerging therapeutic approach
that may influence lung health, by enhancing gut microbiota
diversity, reducing inflammation and modulating immune responses
(101). To the best of our
knowledge, FMT has not yet been investigated in the context of BPD,
which presents a promising area for future research. The
implementation of antibiotic stewardship programs in neonatal
intensive care units is therefore crucial at present. By optimizing
antibiotic use and minimizing unnecessary exposure, such programs
can help preserve the integrity of the gut microbiome and
potentially reduce the risk of BPD (102).
Gestational age is considered a pivotal factor in
influencing both microbiota composition and lung development.
Consequently, strengthening perinatal clinical care may be one of
the important means to prevent premature birth. For cases of
inevitable preterm birth, perinatal management strategies focused
on lung protection for preterm infants are particularly vital.
Breast milk has been shown to decrease the incidence of BPD, due to
its rich nutritional content and bioactive components, including
its microbial composition, exosomes and HMOs (46). Current optimal feeding strategies
emphasize the importance of promoting breastfeeding and fortifying
formula milk with HMOs (46).
Conclusions and prospects
The gut-lung axis, a bidirectional communication
pathway between the intestines and lungs, serves a pivotal role in
shaping the progression of BPD through alterations in the gut
microbiota and the immune microenvironment. Disruptions in
intestinal microecology are closely associated with pulmonary
pathogenesis. The gut-lung axis can influence the progression of
BPD through a range of mechanisms, such as bacterial translocation,
microbial metabolite exchange, inflammatory cytokine spillover and
immune cell migration. The present review delved into the role of
the gut-lung axis in BPD pathogenesis, including the bidirectional
communication of the gut-lung axis, the interplay between BPD and
gut microenvironmental changes and the immune regulatory mechanisms
of the gut-lung axis.
Emerging evidence from animal models and human
studies has suggested that FMT and probiotics may offer promising
therapeutic or preventive strategies for BPD. Additionally,
analyzing the microbial composition and metabolite profiles of
infants may enhance diagnostic accuracy and prognostic predictions
of BPD. However, research into the relationship between gut
microbiota and BPD remains in its infancy, with both progress and
limitations. Key limitations include: i) Small and heterogeneous
clinical samples, such that the majority of studies have small
sample sizes, where preterm infants vary greatly in gestational
age, birth weight and treatments (such as the duration of
mechanical ventilation), making results difficult to generalize
(11,52). ii) Limited microbiota analysis
methods, since the majority of existing studies use 16S rRNA
sequencing, which cannot detect strain-level differences or direct
metabolic effects (53,103). iii) Lack of longitudinal studies,
since the majority of previous studies focused on short-term
postnatal microbiota changes (52,104). However, the pathological process
of BPD can last months, where long-term microbiota dynamics data
remain scarce. A number of previous animal studies have explored
the relationship between gut microbiota and BPD. However, animal
experiments have difficulties in extrapolation of findings from
animal studies to humans, specifically regarding model
construction, physiological differences, microbiota complexity and
clinical transformation, as follows: i) Physiological differences,
where animal models differ physiologically from human preterm
infants, particularly in developmental staging and immune system
maturity (105,106). ii) Species-specific gut
microbiota, where gut microbiota composition and metabolites vary
by species. Differences exist in colonization patterns, strain
functions, metabolic pathways and pathogen virulence (107,108). iii) Clinical translation
barriers, where challenges remain in clinical translation, such as
determining administration methods and assessing safety. Although
FMT has shown promise in animal studies, its safety in
immunocompromised preterm infants requires further evaluation.
Research on the relationship between gut microbiota
in preterm infants and BPD remains in its early stages. The present
study reviewed the role of the lung-gut axis in BPD in recent years
through a qualitative synthesis. However, the cited evidence has
limitations, as follows: i) Uncontrolled confounders, such that the
majority of animal models failed to simulate concurrent nutritional
deprivation in preterm infants; ii) measurement bias, where 16S
rRNA sequencing in the vast majority of clinical studies could not
resolve strain-specific functions (such as commensal vs. pathogenic
E. coli); and iii) Generalizability, since the majority of
included cohorts were single-center with limited ethnic diversity.
By contrast, a meta-analysis would provide quantitative estimates
of the gut-lung axis mechanisms in BPD and strengthen the evidence
synthesis. However, a meta-analysis of the studies may have the
following methodological and scope-related limitations: i) The
available clinical studies exhibited substantial heterogeneity in
patient populations [including varying gestational ages (24–32
weeks), birth weights (500–1,500 g)], intervention protocols
(probiotic strains, dosing regimens) and outcome measures (BPD
diagnostic criteria ranging from National Institutes of Health 2001
to 2018 consensuses). Such heterogeneity would compromise the
validity of pooled effect estimates. ii) A meta-analysis is
typically suited for interventional trials with dichotomous
outcomes (such as BPD incidence), whereas the present review
focused on mechanistic pathways (such as immune cell migration,
SCFA-induced epigenetic regulation) primarily derived from animal
and in vitro studies, which cannot be meaningfully
quantified by statistical pooling. iii) Critical data required for
meta-analysis (including standard deviations of microbial abundance
and exact cytokine levels) are rarely reported in mechanistic
studies. Future large-scale, standardized cohorts are needed to
enable meta-analyses of gut microbiota signatures in BPD.
The present review systematically consolidated
advances in understanding the role of the gut-lung axis in BPD
pathogenesis and demonstrates advantages over similar reviews in
the following key areas (93,109): i) Focused mechanistic depth on
the gut-lung axis. The present study systematically focused on the
bidirectional causal relationship between intestinal dysbiosis and
the progression of BPD. In addition, by comparing the gut-lung axis
mechanisms in BPD with those in NEC-related lung injury, the
BPD-specific mechanisms were highlighted, which is rarely presented
in similar reviews (93,109). ii) Integration of developmental
immunology. The present review established the fundamental
biological basis of the gut-lung axis by detailing the shared
embryonic origin (endoderm), signaling pathways (Wnt/Bmp/Fgf) and
transcription factors (Nkx2.1/Sox9). The review also clarified the
immunomodulatory role of the gut-lung axis in BPD, a mechanism that
is rarely covered in similar reviews (59,109). iii) Critical appraisal of
therapeutics. The current review discussed the contradictory
clinical evidence of probiotics, avoiding overgeneralization.
Furthermore, the possibility of emerging therapeutic applications
(FMT, SCFA supplementation and prebiotics) was explored, whilst
acknowledging the translation gap from animals to humans. iv)
Methodological rigor and transparency. The present review clearly
outlined the shortcomings of the existing clinical studies (such as
small heterogeneous cohorts, limitations of 16S rRNA sequencing and
lack of longitudinal data). Furthermore, it was clearly explained
as to why the performance of a meta-analysis was inappropriate
(heterogeneity, mechanism focus and missing data), enhancing
academic credibility. In the future, further elucidation of the
role of the gut-lung axis in BPD is essential to uncover novel
patho-mechanistic insights and therapeutic targets. The present
study may facilitate a more integrated approach to BPD prevention,
management and prognosis, ultimately improving the quality of life
for affected patients.
Acknowledgements
Not applicable.
Funding
The present review was supported by the Graduate Student
Research and Creative Projects of Jiangsu Province (grant no.
KYCX21-3402) and the Excellent PhD Engineering Program of
Children's Hospital of Nanjing Medical University (grant no.
BSYC2024004).
Availability of data and materials
Not applicable.
Authors' contributions
YZ was involved in the conception of the study, the
formulation of overarching study aims, manuscript preparation and
article revision. RDD contributed by preparing the manuscript,
specifically its critical review, commentary and revision. Data
authentication is not applicable. Both authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dankhara N, Holla I, Ramarao S and
Kalikkot Thekkeveedu R: Bronchopulmonary dysplasia: Pathogenesis
and pathophysiology. J Clin Med. 12:42072023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Collaco JM and McGrath-Morrow SA:
Long-term outcomes of infants with severe BPD. Semin Perinatol.
48:1518912024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilfillan M, Bhandari A and Bhandari V:
Diagnosis and management of bronchopulmonary dysplasia. BMJ.
375:n19742021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shukla VV and Ambalavanan N: Recent
advances in bronchopulmonary dysplasia. Indian J Pediatr.
88:690–695. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Yuan L, Jiang S, Gu X, Lei X, Hu
L, Xiao T, Zhu Y, Dang D, Li W, et al: Synergistic effects of
achieving perinatal interventions on bronchopulmonary dysplasia in
preterm infants. Eur J Pediatr. 183:1711–1721. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu D, Huang Y, Kong Y, Tao T and Zhu X:
Gut microecology: Why our microbes could be key to our health.
Biomed Pharmacother. 131:1107842020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Zheng Y, Yang M, Wang L, Xu Y, You
S, Mao N, Fan J and Ren S: Gut microecology: Effective targets for
natural products to modulate uric acid metabolism. Front Pharmacol.
15:14467762024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L,
Guo F, Zhang X, Luo R, Huang C, et al: Alterations of the gut
microbiota in patients with coronavirus disease 2019 or H1N1
influenza. Clin Infect Dis. 71:2669–2678. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raftery AL, Tsantikos E, Harris NL and
Hibbs ML: Links between inflammatory bowel disease and chronic
obstructive pulmonary disease. Front Immunol. 11:21442020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryan FJ, Drew DP, Douglas C, Leong LEX,
Moldovan M, Lynn M, Fink N, Sribnaia A, Penttila I, McPhee AJ, et
al: Changes in the composition of the gut microbiota and the blood
transcriptome in preterm infants at less than 29 weeks gestation
diagnosed with bronchopulmonary dysplasia. mSystems. 4:e00484–19.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen SM, Lin CP and Jan MS: Early gut
microbiota changes in preterm infants with bronchopulmonary
dysplasia: A pilot Case-control study. Am J Perinatol.
38:1142–1149. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao L, Song W and Chen YG:
Mesenchymal-epithelial interaction regulates gastrointestinal tract
development in mouse embryos. Cell Rep. 40:1110532022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Batki J, Hetzel S, Schifferl D, Bolondi A,
Walther M, Wittler L, Grosswendt S, Herrmann BG and Meissner A:
Extraembryonic gut endoderm cells undergo programmed cell death
during development. Nat Cell Biol. 26:868–877. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang Y and Li X: Metabolic and epigenetic
regulation of endoderm differentiation. Trends Cell Biol.
32:151–164. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aros CJ, Pantoja CJ and Gomperts BN: Wnt
signaling in lung development, regeneration, and disease
progression. Commun Biol. 4:6012021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aspal M and Zemans RL: Mechanisms of
ATII-to-ATI cell differentiation during lung regeneration. Int J
Mol Sci. 21:31882020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Wang J, Ding B, Jiang Z, Yu F, Li
D, Sun W, Wang L, Xu H and Hu S: Feedback delivery of BMP 7 on the
pathological oxidative stress via smart hyaluronic acid hydrogel
potentiated the repairing of the gut epithelial integrity. Int J
Biol Macromol. 282:1367942024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herriges MJ, Tischfield DJ, Cui Z, Morley
MP, Han Y, Babu A, Li S, Lu M, Cendan I, Garcia BA, et al: The
NANCI-Nkx2.1 gene duplex buffers Nkx2.1 expression to maintain lung
development and homeostasis. Genes Dev. 31:889–903. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chelladurai P, Kuenne C, Bourgeois A,
Günther S, Valasarajan C, Cherian AV, Rottier RJ, Romanet C,
Weigert A, Boucherat O, et al: Epigenetic reactivation of
transcriptional programs orchestrating fetal lung development in
human pulmonary hypertension. Sci Transl Med. 14:eabe54072022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dolmatov IY, Kalacheva NV, Tkacheva ES,
Shulga AP, Zavalnaya EG, Shamshurina EV, Girich AS, Boyko AV and
Eliseikina MG: Expression of Piwi, MMP, TIMP, and Sox during Gut
Regeneration in Holothurian Eupentacta fraudatrix (Holothuroidea,
Dendrochirotida). Genes (Basel). 12:12922021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Budden KF, Gellatly SL, Wood DL, Cooper
MA, Morrison M, Hugenholtz P and Hansbro PM: Emerging pathogenic
links between microbiota and the gut-lung axis. Nat Rev Microbiol.
15:55–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thursby E and Juge N: Introduction to the
human gut microbiota. Biochem J. 474:1823–1836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Keely S, Talley NJ and Hansbro PM:
Pulmonary-intestinal cross-talk in mucosal inflammatory disease.
Mucosal Immunol. 5:7–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keely S and Hansbro PM: Lung-gut cross
talk: A potential mechanism for intestinal dysfunction in patients
with COPD. Chest. 145:199–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu G, Mateer SW, Hsu A, Goggins BJ, Tay
H, Mathe A, Fan K, Neal R, Bruce J, Burns G, et al: Platelet
activating factor receptor regulates Colitis-induced pulmonary
inflammation through the NLRP3 inflammasome. Mucosal Immunol.
12:862–873. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fricker M, Goggins BJ, Mateer S, Jones B,
Kim RY, Gellatly SL, Jarnicki AG, Powell N, Oliver BG,
Radford-Smith G, et al: Chronic cigarette smoke exposure induces
systemic hypoxia that drives intestinal dysfunction. JCI Insight.
3:e940402018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mateer SW, Maltby S, Marks E, Foster PS,
Horvat JC, Hansbro PM and Keely S: Potential mechanisms regulating
pulmonary pathology in inflammatory bowel disease. J Leukoc Biol.
98:727–737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mateer SW, Mathe A, Bruce J, Liu G, Maltby
S, Fricker M, Goggins BJ, Tay HL, Marks E, Burns G, et al: IL-6
drives Neutrophil-mediated pulmonary inflammation associated with
bacteremia in murine models of colitis. Am J Pathol. 188:1625–1639.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anand S and Mande SS: Diet, microbiota and
gut-lung connection. Front Microbiol. 9:21472018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marsland BJ, Trompette A and Gollwitzer
ES: The Gut-lung axis in respiratory disease. Ann Am Thorac Soc. 12
(Suppl 2):S150–S56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dang AT and Marsland BJ: Microbes,
metabolites, and the gut-lung axis. Mucosal Immunol. 12:843–850.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Al Alam D, Danopoulos S, Grubbs B, Ali N,
MacAogain M, Chotirmall SH, Warburton D, Gaggar A, Ambalavanan N
and Lal CV: human fetal lungs harbor a microbiome signature. Am J
Respir Crit Care Med. 201:1002–1006. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chakradhar S: A curious connection:
Teasing apart the link between gut microbes and lung disease. Nat
Med. 23:402–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aagaard K, Ma J, Antony KM, Ganu R,
Petrosino J and Versalovic J: The placenta harbors a unique
microbiome. Sci Transl Med. 6:237–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Collado MC, Rautava S, Aakko J, Isolauri E
and Salminen S: Human gut colonisation may be initiated in utero by
distinct microbial communities in the placenta and amniotic fluid.
Sci Rep. 6:231292016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ortiz Moyano R, Raya Tonetti F, Tomokiyo
M, Kanmani P, Vizoso-Pinto MG, Kim H, Quilodrán-Vega S, Melnikov V,
Alvarez S, Takahashi H, et al: The ability of respiratory commensal
bacteria to beneficially modulate the lung innate immune response
is a strain dependent characteristic. Microorganisms. 8:7272020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leiby JS, McCormick K, Sherrill-Mix S,
Clarke EL, Kessler LR, Taylor LJ, Hofstaedter CE, Roche AM, Mattei
LM, Bittinger K, et al: Lack of detection of a human placenta
microbiome in samples from preterm and term deliveries. Microbiome.
6:1962018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Goffau MC, Lager S, Sovio U, Gaccioli
F, Cook E, Peacock SJ, Parkhill J, Charnock-Jones DS and Smith GCS:
Human placenta has no microbiome but can contain potential
pathogens. Nature. 572:329–334. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tauchi H, Yahagi K, Yamauchi T, Hara T,
Yamaoka R, Tsukuda N, Watanabe Y, Tajima S, Ochi F, Iwata H, et al:
Gut microbiota development of preterm infants hospitalised in
intensive care units. Benef Microbes. 10:641–651. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guittar J, Shade A and Litchman E:
Trait-based community assembly and succession of the infant gut
microbiome. Nat Commun. 10:5122019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
La Rosa PS, Warner BB, Zhou Y, Weinstock
GM, Sodergren E, Hall-Moore CM, Stevens HJ, Bennett WE Jr, Shaikh
N, Linneman LA, et al: Patterned progression of bacterial
populations in the premature infant gut. Proc Natl Acad Sci USA.
111:12522–12527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Korpela K, Blakstad EW, Moltu SJ, Strømmen
K, Nakstad B, Rønnestad AE, Brække K, Iversen PO, Drevon CA and de
Vos W: Intestinal microbiota development and gestational age in
preterm neonates. Sci Rep. 8:24532018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bresesti I, Salvatore S, Valetti G, Baj A,
Giaroni C and Agosti M: The Microbiota-gut axis in premature
infants: Physio-pathological implications. Cells. 11:3792022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stewart CJ, Ajami NJ, O'Brien JL,
Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H,
Metcalf GA, et al: Temporal development of the gut microbiome in
early childhood from the TEDDY study. Nature. 562:583–588. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bäckhed F, Roswall J, Peng Y, Feng Q, Jia
H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al:
Dynamics and stabilization of the human gut microbiome during the
first year of life. Cell Host Microbe. 17:690–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lyons KE, Ryan CA, Dempsey EM, Ross RP and
Stanton C: Breast milk, a source of beneficial microbes and
associated benefits for infant health. Nutrients. 12:10392020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Quigley M, Embleton ND and McGuire W:
Formula versus donor breast milk for feeding preterm or low birth
weight infants. Cochrane Database Syst Rev.
6:CD0029712018.PubMed/NCBI
|
|
48
|
Aguilar-Lopez M, Dinsmoor AM, Ho TTB and
Donovan SM: A systematic review of the factors influencing
microbial colonization of the preterm infant gut. Gut Microbes.
13:1–33. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ramirez J, Guarner F, Bustos Fernandez L,
Maruy A, Sdepanian VL and Cohen H: Antibiotics as major disruptors
of gut microbiota. Front Cell Infect Microbiol. 10:5729122020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kalbermatter C, Fernandez Trigo N,
Christensen S and Ganal-Vonarburg SC: Maternal microbiota, early
life colonization and breast milk drive immune development in the
newborn. Front Immunol. 12:6830222021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Colombo SFG, Nava C, Castoldi F, Fabiano
V, Meneghin F, Lista G and Cavigioli F: Preterm Infants' Airway
microbiome: A scoping review of the current evidence. Nutrients.
16:4652024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lohmann P, Luna RA, Hollister EB, Devaraj
S, Mistretta TA, Welty SE and Versalovic J: The airway microbiome
of intubated premature infants: Characteristics and changes that
predict the development of bronchopulmonary dysplasia. Pediatr Res.
76:294–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pammi M, Lal CV, Wagner BD, Mourani PM,
Lohmann P, Luna RA, Sisson A, Shivanna B, Hollister EB, Abman SH,
et al: Airway microbiome and development of bronchopulmonary
dysplasia in preterm infants: A systematic review. J Pediatr.
204:126–133.e2. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun T, Yu H and Fu J: Respiratory tract
microecology and bronchopulmonary dysplasia in preterm infants.
Front Pediatr. 9:7625452021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen X, Huang X, Lin Y, Lin B and Yang C,
Huang Z and Yang C: Association of Ureaplasma infection pattern and
azithromycin treatment effect with bronchopulmonary dysplasia in
Ureaplasma positive infants: A cohort study. BMC Pulm Med.
23:2292023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chung KF and Adcock IM: Multifaceted
mechanisms in COPD: Inflammation, immunity, and tissue repair and
destruction. Eur Respir J. 31:1334–1356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Song Z, Meng Y, Fricker M, Li X, Tian H,
Tan Y and Qin L: The role of gut-lung axis in COPD: Pathogenesis,
immune response, and prospective treatment. Heliyon. 10:e306122024.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Z, Jiang J, Li Z and Wan W: The
change of cytokines and gut microbiome in preterm infants for
bronchopulmonary dysplasia. Front Microbiol. 13:8048872022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lal CV, Kandasamy J, Dolma K, Ramani M,
Kumar R, Wilson L, Aghai Z, Barnes S, Blalock JE, Gaggar A, et al:
Early airway microbial metagenomic and metabolomic signatures are
associated with development of severe bronchopulmonary dysplasia.
Am J Physiol Lung Cell Mol Physiol. 315:L810–L815. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang K and Dong W: Perspectives on
probiotics and bronchopulmonary dysplasia. Front Pediatr.
8:5702472020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Willis KA, Siefker DT, Aziz MM, White CT,
Mussarat N, Gomes CK, Bajwa A, Pierre JF, Cormier SA and Talati AJ:
Perinatal maternal antibiotic exposure augments lung injury in
offspring in experimental bronchopulmonary dysplasia. Am J Physiol
Lung Cell Mol Physiol. 318:L407–L418. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Enaud R, Prevel R, Ciarlo E, Beaufils F,
Wieërs G, Guery B and Delhaes L: The Gut-lung axis in health and
respiratory diseases: A place for inter-organ and inter-kingdom
crosstalks. Front Cell Infect Microbiol. 10:92020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wedgwood S, Gerard K, Halloran K,
Hanhauser A, Monacelli S, Warford C, Thai PN, Chiamvimonvat N,
Lakshminrusimha S, Steinhorn RH and Underwood MA: Intestinal
dysbiosis and the developing lung: The role of toll-like receptor 4
in the gut-lung axis. Front Immunol. 11:3572020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li Y, He L, Zhao Q and Bo T: Microbial and
metabolic profiles of bronchopulmonary dysplasia and therapeutic
effects of potential probiotics Limosilactobacillus reuteri and
Bifidobacterium bifidum. J Appl Microbiol. 133:908–921. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shen X, Yang Z, Wang Q, Chen X, Zhu Q, Liu
Z, Patel N, Liu X and Mo X: Lactobacillus plantarum L168 improves
hyperoxia-induced pulmonary inflammation and hypoalveolarization in
a rat model of bronchopulmonary dysplasia. NPJ Biofilms
Microbiomes. 10:442024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Underwood MA, Lakshminrusimha S, Steinhorn
RH and Wedgwood S: Malnutrition, poor post-natal growth, intestinal
dysbiosis and the developing lung. J Perinatol. 41:1797–1810. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ding J, Xu J, Wu H, Li M, Xiao Y, Fu J,
Zhu X, Wu N, Sun Q and Liu Y: The cross-talk between the metabolome
and microbiome in a double-hit neonatal rat model of
bronchopulmonary dysplasia. Genomics. 117:1109692025. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Thatrimontrichai A, Praditaukrit M,
Maneenil G, Dissaneevate S, Singkhamanan K and Surachat K:
Characterization of gut microbiota in very low birth weight infants
with versus without bronchopulmonary dysplasia. Clin Exp Pediatr.
68:503–511. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sodhi CP, Gonzalez Salazar AJ, Kovler ML,
Fulton WB, Yamaguchi Y, Ishiyama A, Wang S, Prindle T Jr, Vurma M,
Das T, et al: The administration of a pre-digested fat-enriched
formula prevents necrotising enterocolitis-induced lung injury in
mice. Br J Nutr. 128:1050–1063. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jia H, Sodhi CP, Yamaguchi Y, Lu P, Martin
LY, Good M, Zhou Q, Sung J, Fulton WB, Nino DF, et al: Pulmonary
epithelial TLR4 activation leads to lung injury in neonatal
necrotizing enterocolitis. J Immunol. 197:859–871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tan JY, Tang YC and Huang J: Gut
microbiota and lung injury. Adv Exp Med Biol. 1238:55–72. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Samuelson DR, Welsh DA and Shellito JE:
Regulation of lung immunity and host defense by the intestinal
microbiota. Front Microbiol. 6:10852015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dickson RP, Singer BH, Newstead MW,
Falkowski NR, Erb-Downward JR, Standiford TJ and Huffnagle GB:
Enrichment of the lung microbiome with gut bacteria in sepsis and
the acute respiratory distress syndrome. Nat Microbiol.
1:161132016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Moreno-Villares JM, Andrade-Platas D,
Soria-López M, Colomé-Rivero G, Catalan Lamban A, Martinez-Figueroa
MG, Espadaler-Mazo J and Valverde-Molina J: Comparative efficacy of
probiotic mixture Bifidobacterium longum KABP042 plus Pediococcus
pentosaceus KABP041 vs. Limosilactobacillus reuteri DSM17938 in the
management of infant colic: A randomized clinical trial. Eur J
Pediatr. 183:5371–5381. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Spreckels JE, Wejryd E, Marchini G,
Jonsson B, de Vries DH, Jenmalm MC, Landberg E, Sverremark-Ekström
E, Martí M and Abrahamsson T: Lactobacillus reuteri
Colonisation of extremely preterm infants in a randomised
Placebo-controlled trial. Microorganisms. 9:9152021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang Q, Ran X, He Y, Ai Q and Shi Y:
Acetate downregulates the activation of NLRP3 inflammasomes and
attenuates lung injury in neonatal mice with bronchopulmonary
dysplasia. Front Pediat. 8:5951572021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Fanos V, Pintus MC, Lussu M, Atzori L,
Noto A, Stronati M, Guimaraes H, Marcialis MA, Rocha G, Moretti C,
et al: Urinary metabolomics of bronchopulmonary dysplasia (BPD):
Preliminary data at birth suggest it is a congenital disease. J
Matern Fetal Neonatal Med. 27 (Suppl 2):S39–S45. 2014. View Article : Google Scholar
|
|
78
|
Pintus MC, Lussu M, Dessì A, Pintus R,
Noto A, Masile V, Marcialis MA, Puddu M, Fanos V and Atzori L:
Urinary 1H-NMR metabolomics in the first week of life
can anticipate BPD diagnosis. Oxid Med Cell Longev.
2018:76206712018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Piersigilli F and Bhandari V: Metabolomics
of bronchopulmonary dysplasia. Clin Chim Acta. 500:109–114. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhao Q, Li Y, Chai X, Xu L, Zhang L, Ning
P, Huang J and Tian S: Interaction of inhalable volatile organic
compounds and pulmonary surfactant: Potential hazards of VOCs
exposure to lung. J Hazard Mater. 369:512–520. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Berkhout DJC, Niemarkt HJ, Benninga MA,
Budding AE, van Kaam AH, Kramer BW, Pantophlet CM, van Weissenbruch
MM, de Boer NKH and de Meij TGJ: Development of severe
bronchopulmonary dysplasia is associated with alterations in fecal
volatile organic compounds. Pediatr Res. 83:412–419. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wright H, Bannaga AS, Iriarte R, Mahmoud M
and Arasaradnam RP: Utility of volatile organic compounds as a
diagnostic tool in preterm infants. Pediatr Res. 89:263–268. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Furusawa Y, Obata Y, Fukuda S, Endo TA,
Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K and Kato T:
Commensal microbe-derived butyrate induces the differentiation of
colonic regulatory T cells. Nature. 504:446–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Torow N, Hand TW and Hornef MW: Programmed
and environmental determinants driving neonatal mucosal immune
development. Immunity. 56:485–499. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jugder BE, Kamareddine L and Watnick PI:
Microbiota-derived acetate activates intestinal innate immunity via
the Tip60 histone acetyltransferase complex. Immunity.
54:1683–1697. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Woo V and Alenghat T: Epigenetic
regulation by gut microbiota. Gut Microbes. 14:20224072022.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Weiss GA and Hennet T: Mechanisms and
consequences of intestinal dysbiosis. Cell Mol Life Sci.
74:2959–2977. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
McDermott AJ and Huffnagle GB: The
microbiome and regulation of mucosal immunity. Immunology.
142:24–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Cai J, Lu H, Su Z, Mi L, Xu S and Xue Z:
Dynamic Changes of NCR-type 3 innate lymphoid cells and their role
in mice with bronchopulmonary dysplasia. Inflammation. 45:497–508.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li B, Yin GF, Wang YL, Tan YM, Huang CL
and Fan XM: Impact of fecal microbiota transplantation on
TGF-β1/Smads/ERK signaling pathway of endotoxic acute lung injury
in rats. 3 Biotech. 10:522020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tirone C, Pezza L, Paladini A, Tana M,
Aurilia C, Lio A, D'Ippolito S, Tersigni C, Posteraro B,
Sanguinetti M, et al: Gut and lung microbiota in preterm infants:
Immunological modulation and implication in neonatal outcomes.
Front Immunol. 10:29102019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yao HC, Zhu Y, Lu HY, Ju HM, Xu SQ, Qiao Y
and Wei SJ: Type 2 innate lymphoid cell-derived amphiregulin
regulates type II alveolar epithelial cell transdifferentiation in
a mouse model of bronchopulmonary dysplasia. Int Immunopharmacol.
122:1106722023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gray J, Oehrle K, Worthen G, Alenghat T,
Whitsett J and Deshmukh H: Intestinal commensal bacteria mediate
lung mucosal immunity and promote resistance of newborn mice to
infection. Sci Transl Med. 9:eaaf94122017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhu Y, Mi L, Lu H, Ju H, Hao X and Xu S:
ILC2 regulates hyperoxia-induced lung injury via an enhanced Th17
cell response in the BPD mouse model. BMC Pulm Med. 23:1882023.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Willis KA and Ambalavanan N: Necrotizing
enterocolitis and the Gut-lung axis. Semin Perinatol.
45:1514542021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ngo VL, Lieber CM, Kang HJ, Sakamoto K,
Kuczma M, Plemper RK and Gewirtz AT: Intestinal microbiota
programming of alveolar macrophages influences severity of
respiratory viral infection. Cell Host Microbe. 32:335–348. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Qu Y, Guo S, Liu Y, Wang G and Wu H:
Association between probiotics and bronchopulmonary dysplasia in
preterm infants. Sci Rep. 11:170602021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Villamor-Martínez E, Pierro M, Cavallaro
G, Mosca F, Kramer B and Villamor E: Probiotic supplementation in
preterm infants does not affect the risk of bronchopulmonary
dysplasia: A Meta-analysis of randomized controlled trials.
Nutrients. 9:11972017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yoo S, Jung SC, Kwak K and Kim JS: The
role of prebiotics in modulating gut microbiota: Implications for
human health. Int J Mol Sci. 25:48342024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Antunes KH, Singanayagam A, Williams L,
Faiez TS, Farias A, Jackson MM, Faizi FK, Aniscenko J, Kebadze T,
Chander Veerati P, et al: Airway-delivered short-chain fatty acid
acetate boosts antiviral immunity during rhinovirus infection. J
Allergy Clin Immunol. 151:447–457. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ito T, Nakanishi Y, Shibata R, Sato N,
Jinnohara T, Suzuki S, Suda W, Hattori M, Kimura I, Nakano T, et
al: The propionate-GPR41 axis in infancy protects from subsequent
bronchial asthma onset. Gut Microbes. 15:22065072023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lee SH, Lee JH and Lee SW: Application of
Microbiome-based therapies in chronic respiratory diseases. J
Microbiol. 62:201–216. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Menegolla MP, Silveira RC, Görgen ARH,
Gandolfi FE and Procianoy RS: Antibiotics and beyond: Unraveling
the dynamics of bronchopulmonary dysplasia in very preterm infants.
Pediatr Pulmonol. 59:3260–3267. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Martin I, Silverberg M, Abdelgawad A,
Tanaka K, Halloran BA, Nicola T, Myers ED, Desai JP, White CT,
Karabayir I, et al: The fungal microbiota modulate neonatal
oxygen-induced lung injury. Microbiome. 13:242025. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dorshkind K and Crooks G: Layered immune
system development in mice and humans. Immunol Rev. 315:5–10. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Loering S, Cameron GJM, Starkey MR and
Hansbro PM: Lung development and emerging roles for type 2
immunity. J Pathol. 247:686–696. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Moeller AH and Sanders JG: Roles of the
gut microbiota in the adaptive evolution of mammalian species.
Philos Trans R Soc Lond B Biol Sci. 75:201905972020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sprockett DD, Price JD, Juritsch AF,
Schmaltz RJ, Real MVF, Goldman SL, Sheehan M, Ramer-Tait AE and
Moeller AH: Home-site advantage for host species-specific gut
microbiota. Sci Adv. 9:54992023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Gao Y, Wang K, Lin Z, Cai S, Peng A, He L,
Qi H, Jin Z and Qian X: The emerging roles of microbiome and
short-chain fatty acids in the pathogenesis of bronchopulmonary
dysplasia. Front Cell Infect Microbiol. 14:14346872024. View Article : Google Scholar : PubMed/NCBI
|