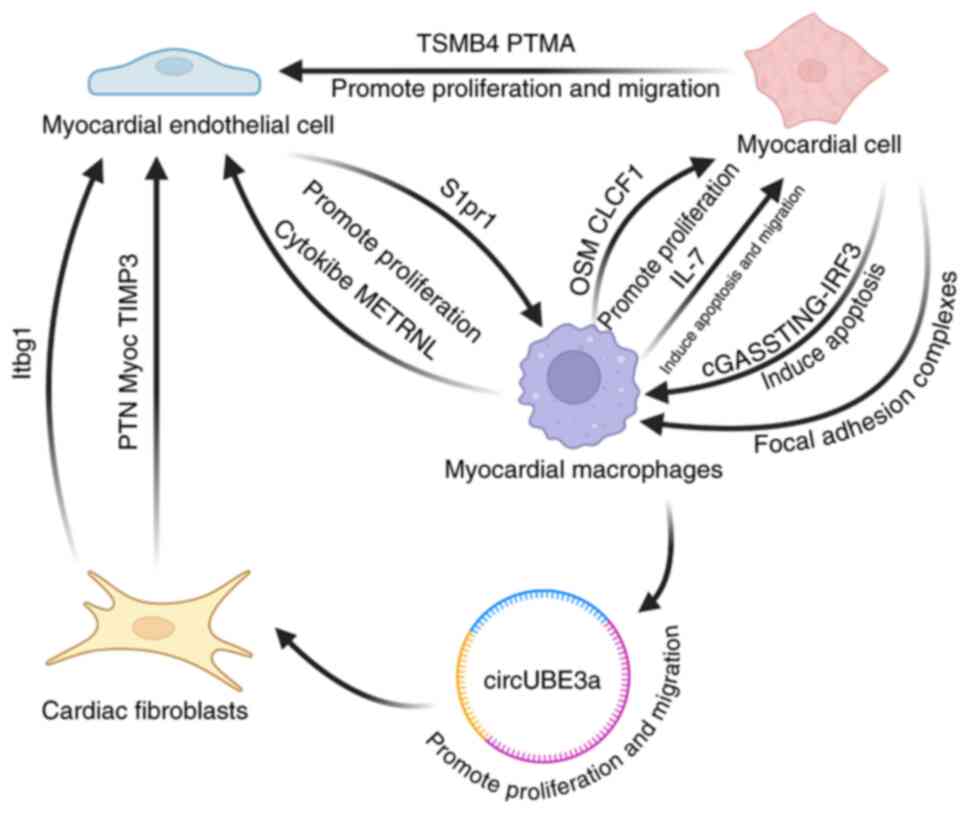
|
1
|
Heusch G: Myocardial ischaemia-reperfusion
injury and cardioprotection in perspective. Nat Rev Cardiol.
17:773–789. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Bijl P, Abou R, Goedemans L, Gersh
BJ, Holmes DR Jr, Ajmone Marsan N, Delgado V and Bax JJ: Left
ventricular post-infarct remodeling: Implications for systolic
function improvement and outcomes in the modern era. JACC Heart
Fail. 8:131–140. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Xu J, Wu M, Kang L and Xu B: The
effector cells and cellular mediators of immune system involved in
cardiac inflammation and fibrosis after myocardial infarction. J
Cell Physiol. 235:8996–9004. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng GXY, Terry JM, Belgrader P, Ryvkin
P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, McDermott GP, Zhu J,
et al: Massively parallel digital transcriptional profiling of
single cells. Nat Commun. 8:140492017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao Y, Hao S, Andersen-Nissen E, Mauck WM
III, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, et al:
Integrated analysis of multimodal single-cell data. Cell.
184:3573–3587.e29. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rondini EA and Granneman JG: Single cell
approaches to address adipose tissue stromal cell heterogeneity.
Biochem J. 477:583–600. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mackay IM, Arden KE and Nitsche A:
Real-time PCR in virology. Nucleic Acids Res. 30:1292–1305. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalisky T, Blainey P and Quake SR: Genomic
analysis at the single-cell level. Annu Rev Genet. 45:431–445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van der Leun AM, Thommen DS and Schumacher
TN: CD8+ T cell states in human cancer: Insights from
single-cell analysis. Nat Rev Cancer. 20:218–232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stewart BJ, Ferdinand JR and Clatworthy
MR: Using single-cell technologies to map the human immune
system-implications for nephrology. Nat Rev Nephrol. 16:112–128.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zaragosi LE, Deprez M and Barbry P: Using
single-cell RNA sequencing to unravel cell lineage relationships in
the respiratory tract. Biochem Soc Trans. 48:327–336. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ziegenhain C, Vieth B, Parekh S, Reinius
B, Guillaumet-Adkins A, Smets M, Leonhardt H, Heyn H, Hellmann I
and Enard W: Comparative analysis of single-cell RNA sequencing
methods. Mol Cell. 65:631–643.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao S: Data analysis in single-cell
transcriptome sequencing. Methods Mol Biol. 1754:311–326. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saliba AE, Westermann AJ, Gorski SA and
Vogel J: Single-cell RNA-seq: Advances and future challenges.
Nucleic Acids Res. 42:8845–8860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang F, Barbacioru C, Wang Y, Nordman E,
Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, et al: mRNA-Seq
whole-transcriptome analysis of a single cell. Nat Methods.
6:377–382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou Y, Song L, Zhu P, Zhang B, Tao Y, Xu
X, Li F, Wu K, Liang J, Shao D, et al: Single-cell exome sequencing
and monoclonal evolution of a JAK2-negative myeloproliferative
neoplasm. Cell. 148:873–885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Picelli S, Faridani OR, Björklund AK,
Winberg G, Sagasser S and Sandberg R: Full-length RNA-seq from
single cells using Smart-seq2. Nat Protoc. 9:171–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macosko EZ, Basu A, Satija R, Nemesh J,
Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck
EM, et al: Highly parallel genome-wide expression profiling of
individual cells using nanoliter droplets. Cell. 161:1202–1214.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klein CA, Seidl S, Petat-Dutter K, Offner
S, Geigl JB, Schmidt-Kittler O, Wendler N, Passlick B, Huber RM,
Schlimok G, et al: Combined transcriptome and genome analysis of
single micrometastatic cells. Nat Biotechnol. 20:387–392. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasagawa Y, Nikaido I, Hayashi T, Danno H,
Uno KD, Imai T and Ueda HR: Quartz-Seq: A highly reproducible and
sensitive single-cell RNA sequencing method, reveals non-genetic
gene-expression heterogeneity. Genome Biol. 14:R312013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jaitin DA, Kenigsberg E, Keren-Shaul H,
Elefant N, Paul F, Zaretsky I, Mildner A, Cohen N, Jung S, Tanay A
and Amit I: Massively parallel single-cell RNA-seq for marker-free
decomposition of tissues into cell types. Science. 343:776–779.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keren-Shaul H, Kenigsberg E, Jaitin DA,
David E, Paul F, Tanay A and Amit I: MARS-seq2.0: An experimental
and analytical pipeline for indexed sorting combined with
single-cell RNA sequencing. Nat Protoc. 14:1841–1862. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Li T, Liu F, Chen Y, Yao J, Li Z,
Huang Y and Wang J: Comparative analysis of droplet-based
ultra-high-throughput single-cell RNA-Seq systems. Mol Cell.
73:130–142.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yekelchyk M, Guenther S, Preussner J and
Braun T: Mono- and multi-nucleated ventricular cardiomyocytes
constitute a transcriptionally homogenous cell population. Basic
Res Cardiol. 114:362019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Habib N, Avraham-Davidi I, Basu A, Burks
T, Shekhar K, Hofree M, Choudhury SR, Aguet F, Gelfand E, Ardlie K,
et al: Massively parallel single-nucleus RNA-seq with DroNc-seq.
Nat Methods. 14:955–958. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fischer J and Ayers T: Single nucleus
RNA-sequencing: How it's done, applications and limitations. Emerg
Top Life Sci. 5:687–690. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Selewa A, Dohn R, Eckart H, Lozano S, Xie
B, Gauchat E, Elorbany R, Rhodes K, Burnett J, Gilad Y, et al:
Systematic comparison of high-throughput single-cell and
single-nucleus transcriptomes during cardiomyocyte differentiation.
Sci Rep. 10:15352020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu P, Liu J, Zhao J, Wilkins BJ, Lupino K,
Wu H and Pei L: Single-nucleus transcriptomic survey of cell
diversity and functional maturation in postnatal mammalian hearts.
Genes Dev. 32:1344–1357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Litviňuková M, Talavera-López C, Maatz H,
Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Heinig M,
Lee M, et al: Cells of the adult human heart. Nature. 588:466–472.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mikhailov AT and Torrado M: The enigmatic
role of the ankyrin repeat domain 1 gene in heart development and
disease. Int J Dev Biol. 52:811–821. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kwapiszewska G, Wygrecka M, Marsh LM,
Schmitt S, Trösser R, Wilhelm J, Helmus K, Eul B, Zakrzewicz A,
Ghofrani HA, et al: Fhl-1, a new key protein in pulmonary
hypertension. Circulation. 118:1183–1194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Geng T, Liu Y, Xu Y, Jiang Y, Zhang N,
Wang Z, Carmichael GG, Taylor HS, Li D and Huang Y: H19 lncRNA
promotes skeletal muscle insulin sensitivity in part by targeting
AMPK. Diabetes. 67:2183–2198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee JH, Protze SI, Laksman Z, Backx PH and
Keller GM: Human pluripotent stem cell-derived atrial and
ventricular cardiomyocytes develop from distinct mesoderm
populations. Cell Stem Cell. 21:179–194.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui M, Wang Z, Chen K, Shah AM, Tan W,
Duan L, Sanchez-Ortiz E, Li H, Xu L, Liu N, et al: Dynamic
transcriptional responses to injury of regenerative and
non-regenerative cardiomyocytes revealed by single-nucleus RNA
sequencing. Dev Cell. 53:102–116.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kretzschmar K, Post Y, Bannier-Hélaouët M,
Mattiotti A, Drost J, Basak O, Li VSW, van den Born M, Gunst QD,
Versteeg D, et al: Profiling proliferative cells and their progeny
in damaged murine hearts. Proc Natl Acad Sci USA.
115:E12245–E12254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Lui KO and Zhou B: Reassessing
endothelial-to-mesenchymal transition in cardiovascular diseases.
Nat Rev Cardiol. 15:445–456. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Libby P, Pasterkamp G, Crea F and Jang IK:
Reassessing the mechanisms of acute coronary syndromes. Circ Res.
124:150–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tani H, Sadahiro T, Yamada Y, Isomi M,
Yamakawa H, Fujita R, Abe Y, Akiyama T, Nakano K, Kuze Y, et al:
Direct reprogramming improves cardiac function and reverses
fibrosis in chronic myocardial infarction. Circulation.
147:223–238. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Z, Cui M, Shah AM, Tan W, Liu N,
Bassel-Duby R and Olson EN: Cell-type-specific gene regulatory
networks underlying murine neonatal heart regeneration at
single-cell resolution. Cell Rep. 33:1084722020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kalucka J, de Rooij LPMH, Goveia J,
Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA,
Veys K, et al: Single-cell transcriptome atlas of murine
endothelial cells. Cell. 180:764–779.e20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang H, Pu W, Li G, Huang X, He L, Tian
X, Liu Q, Zhang L, Wu SM, Sucov HM and Zhou B: Endocardium
minimally contributes to coronary endothelium in the embryonic
ventricular free walls. Circ Res. 118:1880–1893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Artap S, Manderfield LJ, Smith CL,
Poleshko A, Aghajanian H, See K, Li L, Jain R and Epstein JA:
Endocardial Hippo signaling regulates myocardial growth and
cardiogenesis. Dev Biol. 440:22–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Farbehi N, Patrick R, Dorison A, Xaymardan
M, Janbandhu V, Wystub-Lis K, Ho JW, Nordon RE and Harvey RP:
Single-cell expression profiling reveals dynamic flux of cardiac
stromal, vascular and immune cells in health and injury. Elife.
8:e438822019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tombor LS, John D, Glaser SF, Luxán G,
Forte E, Furtado M, Rosenthal N, Baumgarten N, Schulz MH, Wittig J,
et al: Single cell sequencing reveals endothelial plasticity with
transient mesenchymal activation after myocardial infarction. Nat
Commun. 12:6812021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garikipati VNS, Verma SK, Cheng Z, Liang
D, Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, et
al: Circular RNA CircFndc3b modulates cardiac repair after
myocardial infarction via FUS/VEGF-A axis. Nat Commun. 10:43172019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pei J, Cai L, Wang F, Xu C, Pei S, Guo H,
Sun X, Chun J, Cong X, Zhu W, et al: LPA2 contributes to
vascular endothelium homeostasis and cardiac remodeling after
myocardial infarction. Circ Res. 131:388–403. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kanisicak O, Khalil H, Ivey MJ, Karch J,
Maliken BD, Correll RN, Brody MJ, J Lin SC, Aronow BJ, Tallquist MD
and Molkentin JD: Genetic lineage tracing defines myofibroblast
origin and function in the injured heart. Nat Commun. 7:122602016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fu X, Khalil H, Kanisicak O, Boyer JG,
Vagnozzi RJ, Maliken BD, Sargent MA, Prasad V, Valiente-Alandi I,
Blaxall BC and Molkentin JD: Specialized fibroblast differentiated
states underlie scar formation in the infarcted mouse heart. J Clin
Invest. 128:2127–2143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tallquist MD and Molkentin JD: Redefining
the identity of cardiac fibroblasts. Nat Rev Cardiol. 14:484–491.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ivey MJ, Kuwabara JT, Pai JT, Moore RE,
Sun Z and Tallquist MD: Resident fibroblast expansion during
cardiac growth and remodeling. J Mol Cell Cardiol. 114:161–174.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhuang L, Lu L, Zhang R, Chen K and Yan X:
Comprehensive integration of single-cell transcriptional profiling
reveals the heterogeneities of non-cardiomyocytes in healthy and
ischemic hearts. Front Cardiovasc Med. 7:6151612020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ruiz-Villalba A, Romero JP, Hernández SC,
Vilas-Zornoza A, Fortelny N, Castro-Labrador L, San Martin-Uriz P,
Lorenzo-Vivas E, García-Olloqui P, Palacio M, et al: Single-Cell
RNA sequencing analysis reveals a crucial role for CTHRC1 (collagen
triple helix repeat containing 1) cardiac fibroblasts after
myocardial infarction. Circulation. 142:1831–1847. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Forte E, Skelly DA, Chen M, Daigle S,
Morelli KA, Hon O, Philip VM, Costa MW, Rosenthal NA and Furtado
MB: Dynamic interstitial cell response during myocardial infarction
predicts resilience to rupture in genetically diverse mice. Cell
Rep. 30:3149–3163.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rodriguez P, Sassi Y, Troncone L, Benard
L, Ishikawa K, Gordon RE, Lamas S, Laborda J, Hajjar RJ and Lebeche
D: Deletion of delta-like 1 homologue accelerates
fibroblast-myofibroblast differentiation and induces myocardial
fibrosis. Eur Heart J. 40:967–978. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Z, Cui M, Shah AM, Ye W, Tan W, Min
YL, Botten GA, Shelton JM, Liu N, Bassel-Duby R and Olson EN:
Mechanistic basis of neonatal heart regeneration revealed by
transcriptome and histone modification profiling. Proc Natl Acad
Sci USA. 116:18455–18465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Horn MA and Trafford AW: Aging and the
cardiac collagen matrix: Novel mediators of fibrotic remodelling. J
Mol Cell Cardiol. 93:175–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
González A, Schelbert EB, Díez J and
Butler J: Myocardial interstitial fibrosis in heart failure:
Biological and translational perspectives. J Am Coll Cardiol.
71:1696–1706. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang Y, Lee M, Yu G, Lee H, Han X and Kim
D: CTHRC1 activates pro-tumorigenic signaling pathways in
hepatocellular carcinoma. Oncotarget. 8:105238–105250. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Binks AP, Beyer M, Miller R and LeClair
RJ: Cthrc1 lowers pulmonary collagen associated with
bleomycin-induced fibrosis and protects lung function. Physiol Rep.
5:e131152017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Stohn JP, Perreault NG, Wang Q, Liaw L and
Lindner V: Cthrc1, a novel circulating hormone regulating
metabolism. PLoS One. 7:e471422012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
LeClair RJ, Durmus T, Wang Q, Pyagay P,
Terzic A and Lindner V: Cthrc1 is a novel inhibitor of transforming
growth factor-beta signaling and neointimal lesion formation. Circ
Res. 100:826–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Janbandhu V, Tallapragada V, Patrick R, Li
Y, Abeygunawardena D, Humphreys DT, Martin EMMA, Ward AO, Contreras
O, Farbehi N, et al: Hif-1a suppresses ROS-induced proliferation of
cardiac fibroblasts following myocardial infarction. Cell Stem
Cell. 29:281–297.e12. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhuang L, Wang Y, Chen Z, Li Z, Wang Z,
Jia K, Zhao J, Zhang H, Xie H, Lu L, et al: Global characteristics
and dynamics of single immune cells after myocardial infarction. J
Am Heart Assoc. 11:e0272282022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Aibar S, González-Blas CB, Moerman T,
Huynh-Thu VA, Imrichova H, Hulselmans G, Rambow F, Marine JC,
Geurts P, Aerts J, et al: SCENIC: Single-cell regulatory network
inference and clustering. Nat Methods. 14:1083–1086. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
King KR, Aguirre AD, Ye YX, Sun Y, Roh JD,
Ng RP Jr, Kohler RH, Arlauckas SP, Iwamoto Y, Savol A, et al: IRF3
and type I interferons fuel a fatal response to myocardial
infarction. Nat Med. 23:1481–1487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dick SA, Macklin JA, Nejat S, Momen A,
Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh
S, Aronoff L, et al: Self-renewing resident cardiac macrophages
limit adverse remodeling following myocardial infarction. Nat
Immunol. 20:29–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xu Y, Jiang K, Su F, Deng R, Cheng Z, Wang
D, Yu Y and Xiang Y: A transient wave of Bhlhe41+
resident macrophages enables remodeling of the developing infarcted
myocardium. Cell Rep. 42:1131742023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Qian J, Gao Y, Lai Y, Ye Z, Yao Y, Ding K,
Tong J, Lin H, Zhu G, Yu Y, et al: Single-cell RNA sequencing of
peripheral blood mononuclear cells from acute myocardial
infarction. Front Immunol. 13:9088152022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Leistner DM, Kränkel N, Meteva D,
Abdelwahed YS, Seppelt C, Stähli BE, Rai H, Skurk C, Lauten A,
Mochmann HC, et al: Differential immunological signature at the
culprit site distinguishes acute coronary syndrome with intact from
acute coronary syndrome with ruptured fibrous cap: Results from the
prospective translational OPTICO-ACS study. Eur Heart J.
41:3549–3560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Vergallo R and Crea F: Atherosclerotic
plaque healing. N Engl J Med. 383:846–857. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Heinrichs M, Ashour D, Siegel J, Büchner
L, Wedekind G, Heinze KG, Arampatzi P, Saliba AE, Cochain C,
Hofmann U, et al: The healing myocardium mobilizes a distinct
B-cell subset through a CXCL13-CXCR5-dependent mechanism.
Cardiovasc Res. 117:2664–2676. 2021.PubMed/NCBI
|
|
72
|
Zhang Y, Kanter EM and Yamada KA:
Remodeling of cardiac fibroblasts following myocardial infarction
results in increased gap junction intercellular communication.
Cardiovasc Pathol. 19:e233–e240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li Y, Feng J, Song S, Li H, Yang H, Zhou
B, Li Y, Yue Z, Lian H, Liu L, et al: gp130 controls cardiomyocyte
proliferation and heart regeneration. Circulation. 142:967–982.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yan M, Yang Y, Zhou Y, Yu C, Li R, Gong W
and Zheng J: Interleukin-7 aggravates myocardial
ischaemia/reperfusion injury by regulating macrophage infiltration
and polarization. J Cell Mol Med. 25:9939–9952. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wong NR, Mohan J, Kopecky BJ, Guo S, Du L,
Leid J, Feng G, Lokshina I, Dmytrenko O, Luehmann H, et al:
Resident cardiac macrophages mediate adaptive myocardial
remodeling. Immunity. 54:2072–2088.e7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hu S, Gao Y, Gao R, Wang Y, Qu Y, Yang J,
Wei X, Zhang F and Ge J: The selective STING inhibitor H-151
preserves myocardial function and ameliorates cardiac fibrosis in
murine myocardial infarction. Int Immunopharmacol. 107:1086582022.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gladka MM, Kohela A, Molenaar B, Versteeg
D, Kooijman L, Monshouwer-Kloots J, Kremer V, Vos HR, Huibers MMH,
Haigh JJ, et al: Cardiomyocytes stimulate angiogenesis after
ischemic injury in a ZEB2-dependent manner. Nat Commun. 12:842021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kuang Y, Li X, Liu X, Wei L, Chen X, Liu
J, Zhuang T, Pi J, Wang Y, Zhu C, et al: Vascular endothelial S1pr1
ameliorates adverse cardiac remodelling via stimulating reparative
macrophage proliferation after myocardial infarction. Cardiovasc
Res. 117:585–599. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Alonso-Herranz L, Sahún-Español Á, Paredes
A, Gonzalo P, Gkontra P, Núñez V, Clemente C, Cedenilla M,
Villalba-Orero M, Inserte J, et al: Macrophages promote
endothelial-to-mesenchymal transition via MT1-MMP/TGFβ1 after
myocardial infarction. Elife. 9:e579202020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Reboll MR, Klede S, Taft MH, Cai CL, Field
LJ, Lavine KJ, Koenig AL, Fleischauer J, Meyer J, Schambach A, et
al: Meteorin-like promotes heart repair through endothelial KIT
receptor tyrosine kinase. Science. 376:1343–1347. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ramilowski JA, Goldberg T, Harshbarger J,
Kloppmann E, Lizio M, Satagopam VP, Itoh M, Kawaji H, Carninci P,
Rost B and Forrest AR: A draft network of ligand-receptor-mediated
multicellular signalling in human. Nat Commun. 6:78662015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45(D1): D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang Y, Li C, Zhao R, Qiu Z, Shen C, Wang
Z, Liu W, Zhang W, Ge J and Shi B: CircUbe3a from M2
macrophage-derived small extracellular vesicles mediates myocardial
fibrosis after acute myocardial infarction. Theranostics.
11:6315–6333. 2021. View Article : Google Scholar : PubMed/NCBI
|















![Endothelial cell cluster typing and
marker genes for each cluster. The high expression of capillary
marker Gpihbp1 in VEC1 and VEC2, as well as the enrichment of
macrovascular genes such as Plvap and Vwf in VEC3, indicate that
they are related to the maintenance of vascular activity and
angiogenesis. Endothelial cells have multiple functions in blood
vessels, including vascular stability, blood flow regulation,
material exchange and immune response. Following myocardial
infarction, endothelial cells have the functions of repair,
vascular regeneration and remodeling, as well as coagulation. (A)
Endothelial cell clusters of ventricular tissue origin and marker
genes. (B) Endothelial cell clusters of TIP origin and marker
genes. TIP, cardiac interstitial cell population; ScRNA-seq,
single-cell RNA sequencing; VEC1-3, venous endothelial cells (VEC1
highly expresses Gpihbp1, VEC2 cluster highly expresses Cxcl1 and
Icam1, VEC3 highly expresses Plvap and Vwf); Art.EC, arterial
endothelial cells (enriched with artery-related genes such as
Cxcl12); Endo, metabolism-related endothelial cells (specifically
expressing metabolic genes such as H19 and Cpe); Pro.EC,
proliferative active endothelial cells [highly expressing
proliferation genes Hmgb2 and Birc5]; VEC1, microvascular
endothelial cells [expressing Ly6a (encoding SCA1) and vascular
transcription factor Sox17]; VEC2, arterial endothelial cells
(involved in NOTCH signaling pathways, such as Sox17, Hey1); VEC3,
venous endothelial cells.](/article_images/mmr/32/6/mmr-32-06-13680-g01.jpg)
![T and B cell clusters typing and
marker genes for each cluster [MZ marker genes related literature].
High expression of pro-inflammatory factor IL-1β, chemokine Ccl6
and transcription factor Irf5/Fosb in effector T cells regulate the
enrichment of immune regulatory pathways (such as Sp1) in T cells;
co-activation of the dual-chemokine receptor Cxcr5/Ccr7 and the
tissue repair factor Tgfb1 in hB cells; and upregulation of the
cell cycle gene Trp53/Cdc27 in Cyc B cells indicates that they are
respectively involved in inflammatory drive, immunosuppression,
repair chemotaxis and proliferation responses. (A) T cell clusters
typing and marker genes for each cluster. (B) Cell clusters typing
and marker genes for each cluster. ScRNA-seq, single-cell RNA
sequencing; Naive T cells, immune response preparatory T cells;
effector T cells, pro-inflammatory injury type T cells (expressing
Ccl6 and IL-1β); regulatory T cells, immunosuppressive T cells
(rich in Sp1 regulators); NK cells, direct killer T cells; B1
cells, natural immune barrier type B cells; B2 cells, lymphoid
tissue localization type B cells; marginal zone B cells (MZ), rapid
antibody-responsive B cells; germinal center B cells (GC),
antibody-affinity mature B cells; hB, heart-associated B cells,
repair of core-type B cells (expressing Tgfb1, Cd69, Cxcr5 and
Ccr7); CD74+, antigen-presenting type B fine (expressing
Cd74+); IFNR cells, interferon-responsive B cells; Cyc
cells, proliferation type B cells (expressing Trp53, Cdc27, Mrto4,
Nhp2, Ranbp1 and Ncl Gnl3).](/article_images/mmr/32/6/mmr-32-06-13680-g04.jpg)