Introduction
Insulin resistance represents a fundamental
pathophysiological mechanism in the development of type 2 diabetes
mellitus (T2DM), characterized by a reduced response of tissue to
insulin signaling. Research has demonstrated that intestinal immune
system dysregulation, which manifests as persistent inflammation,
serves a pivotal role in mediating T2DM-associated insulin
resistance (1). Experimental
evidence has indicated that aberrant activation of T helper (Th)17
cells triggers intestinal inflammation and precipitates autoimmune
disorders, whereas regulatory T cells (Tregs) exert
immunosuppressive functions to modulate inflammatory processes
(2). Furthermore, perturbation of
the homeostatic balance between Tregs and Th17 cells has emerged as
a critical determinant in the pathogenesis of insulin resistance,
as evidenced by elevated IL-17 levels and enhanced pro-inflammatory
cytokine secretion (3). Multiple
studies have established that Tregs and Th17 cells are essential
mediators in maintaining intestinal immune homeostasis, with their
dynamic equilibrium being crucial for proper immune function
(4–6).
Pseudostellaria heterophylla (Miq.) Pax ex
Pax et Hoffm, formerly known as TaiZiShen, was initially described
in the Chinese book Ben Cao Cong Xin, and is often used to treat
diabetes, chronic obstructive pneumonia, cardiomyocyte injury,
immune deficiency and other diseases (7–9).
This herb has been included in the Chinese Pharmacopoeia primarily
due to its medicinal value (7).
Polysaccharides (molecular weight: 52-210 kDa), such
as PF40, are the primary active ingredient of Pseudostellaria
heterophylla (10). Structural
elucidation of PF40 by UV spectroscopy, Fourier transform infrared
spectroscopy and nuclear magnetic resonance has confirmed the
absence of protein and nucleic acid impurities, as well as the
presence of characteristic α-pyranose configurations (11). Notably, Congo red assay and
scanning probe microscopy analyses have revealed that PF40 adopts a
triple-helical conformation and a multi-branched molecular
structure, features known to enhance the biological stability and
functional activity of polysaccharides. Compared with other
plant-derived polysaccharides, PF40 displays superior thermal
stability and is predominantly amorphous in physical state, as
evidenced by X-ray diffraction analysis. These properties not only
support its suitability for pharmaceutical processing but also
enhance its in vivo functionality (11). A radioisotope tracing study
previously demonstrated that PF40 exhibits targeted accumulation in
the small intestine following oral administration, suggesting a
high degree of intestinal bioavailability and potential for
interaction with gut-associated lymphoid tissues (12). This gut-enrichment behavior may
explain its previously observed ability to modulate intestinal
immune homeostasis, restore Th17 cell/Treg balance, and improve
mucosal barrier integrity in diabetic models (9,11).
Together, these unique structural and biopharmaceutical features
distinguish PF40 from other polysaccharides and support its
candidacy as a novel immunomodulatory agent for the treatment of
metabolic diseases.
Previous studies have shown that PF40 has favorable
pharmacological activity in improving insulin resistance and
reducing fasting glucose in a rat model of T2DM (10–12).
In our previous study, it was revealed that PF40 can correct
imbalances in Treg/Th17 cells in the jejunal tissue (11). Th17 cells and Tregs, alongside Th1
and Th2 cells, constitute crucial subpopulations of CD4+
T lymphocytes that serve key roles in maintaining intestinal
microbial balance and overall metabolic homeostasis (13,14).
Studies have shown that in states of insulin resistance,
pro-inflammatory CD4+ T cells, such as Th1 and Th17
cells, are markedly activated in the gut, whereas anti-inflammatory
Tregs are relatively deficient, exacerbating intestinal
inflammatory responses (15,16).
Additionally, impairment of the intestinal barrier and alterations
in gut microbiota composition further amplify the expansion of
inflammatory CD4+ T cells, thereby promoting the
progression of systemic insulin resistance (17,18).
Interfering with the balance of intestinal CD4+ T cell
subpopulations may offer a novel approach for preventing and
treating insulin resistance. Previous research has suggested that
enhancing Treg activity or suppressing the uncontrolled
proliferation of Th1 and Th17 cells can notably improve insulin
sensitivity and alleviate symptoms of metabolic disorders (19,20).
However, understanding regarding how the CD4+ T-cell
subset balance influences insulin resistance, and the detailed
molecular mechanisms involved, remains limited.
Our previous study established the therapeutic
efficacy of PF40 in rat models of T2DM, where dose-response
analyses were conducted, and it was demonstrated that PF40 could
markedly improve insulin sensitivity and metabolic parameters
(10). Notably, when combined with
metformin, PF40 exerts a synergistic effect, resulting in superior
glycemic control and reduced systemic inflammation compared with
either treatment alone (10,11).
These findings underscore the potential of PF40 as an effective
immunometabolic modulator. Building on this evidence, the present
study focuses on identifying the cellular and molecular mechanisms
by which PF40 influences hepatocellular insulin signaling via
intestinal CD4+ T cells.
The present study aimed to evaluate the activity of
differentially treated CD4+ T cells in ameliorating
insulin resistance using an in vitro cell model. Particular
focus was given to their effects on antioxidant activity,
apoptosis, glucose uptake and energy metabolism in
insulin-resistant (IR) cells, alongside their influence on the
insulin receptor substrate-1 (IRS-1)/PI3K/AKT signaling pathway. By
elucidating the roles and underlying regulatory mechanisms of
differentially treated CD4+ T cells, the current study
may provide theoretical support for developing interventional
strategies against insulin resistance and advance immunotherapeutic
approaches for metabolic diseases.
Materials and methods
Chemicals and materials
Pseudostellaria heterophylla was obtained
from a Good Agricultural Practice-certified cultivation base in
Zherong (Fujian Zheshen Biotechnology Co., Ltd), and PF40 was
prepared as described previously (10). The total sugar content was measured
using the anthrone-sulfuric acid method as described previously
(21), and the protein content of
PF40 was determined using a Bradford assay with BSA (Beyotime
Biotechnology) as the standard (22). The average molecular weight of PF40
was characterized by high-performance gel permeation chromatography
(23). The monosaccharide
composition was analyzed using pre-column derivatization with
1-phenyl-3-methyl-5-pyrazolone followed by high-performance liquid
chromatography (HPLC) (24). BNL
CL.2 cells were obtained from Procell Life Science & Technology
Co., Ltd. Other materials, including fetal bovine serum (FBS), DMEM
and trypsin (used for cell passaging), were obtained from Gibco
(Thermo Fisher Scientific, Inc.). DMSO was sourced from
MilliporeSigma. The glucose assay kit (cat. no. S0201S) was
acquired from Shanghai Beyotime Biotechnology. The phosphorylated
(p)-PI3K (p85-Tyr607; cat. no. AF3241) polyclonal antibody was
purchased from Affinity biosciences Co., Ltd. PI3K (cat. no.
A4992), IRS-1 (cat. no. A16902), p-IRS-1 (Ser307; cat. no. AP0552),
AKT (cat. no. A17909) and p-AKT (S473; cat. no. AP1208) polyclonal
antibodies were obtained from ABclonal Biotech Co., Ltd.
Intervention and purification of
CD4+ T cells
The T2DM rat model was established as described
previously (11). Briefly, 60 male
Sprague Dawley rats (4 weeks, 180-200 g, SPF conditions) were
obtained from Shanghai Institutes for Biological Sciences Shanghai
Laboratory Animal Center (certificate no. SCXK 2017-0005) and
housed under standard conditions with free access to food and water
and a 12 h light/12 h dark cycle. Except for the normal control
group, all other rats were intraperitoneally injected with freshly
prepared STZ solution (0.021 mol/l in pH 4.5 sodium citrate buffer,
0.5 ml/100 g bodyweight) after overnight fasting with free access
to water to induce diabetes. After 3 days, rats with fasting blood
glucose ≥16.7 mmol/l were considered to have T2DM. A total of 38
rats were successfully rendered diabetic. These T2DM rats were
randomly assigned to four groups: model (n=10), PF40 [1.5 g/kg body
weight (bw); n=10], metformin (Merck KGaA; 135 mg/kg bw; n=9) and
PF40 + metformin (PF40, 1.5 g/kg bw + metformin 135 mg/kg bw; n=9).
Animals received their assigned treatments via oral gavage once
daily for a duration of 4 weeks. The normal control group received
an equivalent volume of saline. Each intervention lasted 4 weeks in
total. Subsequently, the rats were euthanized in a small animal
anesthesia machine using 10% isoflurane for 30 min, with death
confirmed by the absence of a heartbeat. Jejunal lymphocytes were
isolated as described previously (25). Briefly, jejunal tissue was rapidly
excised and rinsed with cold PBS to remove feces. The tissue was
then minced and digested in a solution containing 50 mg/ml
deoxyribonuclease I and 30 mg/ml liberase for 30 min at 37°C to
facilitate lymphocyte release. The digested tissue was filtered
through a 70-µm cell mesh to obtain a single-cell suspension,
washed with PBS and resuspended in 40% Percoll solution. This
suspension was layered over 70% Percoll solution and centrifuged at
800 × g for 15 min at 4°C. Lymphocytes were collected from the
interface between the two layers. CD4+ T cells were then
purified from the lymphocyte population using the MagniSort™
CD4+ T Cell Enrichment Kit (Thermo Fisher Scientific,
Inc.; cat. no. 8804-6820-74) according to the manufacturer's
protocol. Cell purity was assessed using a NovoCyte flow cytometer
(NovoCyte; Agilent Technologies, Inc.) and analyzed with
NovoExpress software (version 1.4.1; Agilent Technologies, Inc.).
Samples in which CD4+ T cells accounted for at least 80%
of the population were used for further analysis. CD4+ T
cells were labeled based on the interventions the mice received as
follows: Control (Con-T), model (Mod-T), PF40 (P-T), metformin
(M-T), and combined PF40 and metformin (PM-T). Cells were cultured
in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin at
37°C in a humidified incubator with 5% CO2.
Establishment of the insulin
resistance model
BNL CL.2 cells were seeded in culture flasks and
maintained in low-glucose DMEM supplemented with 10% FBS and 1%
penicillin-streptomycin at 37°C in a humidified incubator with 5%
CO2 and 95% humidity. The culture medium was changed
every 48 h. Once the cells reached 80-90% confluence, the culture
medium was replaced with DMEM containing varying concentrations of
insulin (1×10−6, 1×10−7, 1×10−8
and 1×10−9 mol/l; Sigma-Aldrich; Merck KGaA; cat. no.
Y0001717) and incubated at 37°C for 24 h to identify the optimal
insulin concentration that could induce insulin resistance. Based
on glucose measurements in the culture medium, the concentration of
1×10−7 mol/l was identified as the ideal concentration
for inducing insulin resistance. Subsequently, cells were treated
with 1×10−7 mol/l insulin for 24, 36, 48 and 60 h to
determine the optimal duration for stable induction of insulin
resistance. Untreated cells were used as a control group and each
experimental condition included six replicates. After treatment,
the supernatant was collected, and the glucose concentration in the
culture medium was measured using a glucose assay kit using the
o-toluidine method (Beyotime Biotechnology). The glucose
consumption was calculated based on the difference between the
initial glucose concentration and the post-treatment concentration,
enabling assessment of the response of cells to insulin and
identification of the optimal concentration and treatment duration
for stable insulin resistance induction.
Co-culture of IR-BNL CL.2 cells with
CD4+ T cells
The co-culture model was established using the
Transwell insert system, with CD4+ T cells seeded in the
apical medium and IR-BNL CL.2 cells seeded in the basolateral
medium (26,27), using inserts with 6 wells and a
pore size of 0.4 µm. Purified CD4+ T cells were
co-cultured with IR-BNL CL.2 cells at various CD4+ T
cells to IR-BNL CL.2 cell ratios: 1:1, 2:1, 5:1, 10:1, 20:1 and
40:1, to determine the optimal ratio for the co-culture system.
Phenol red-free DMEM (Thermo Fisher Scientific, Inc.) was used to
avoid color interference. Both CD4+ T cells and IR-BNL
CL.2 cells were cultured in this medium, supplemented with 10% FBS,
and incubated at 37°C with 5% CO2 for 24 h. After 24 h,
10 µl CCK-8 solution (Dojindo Laboratories, Inc.) was added to each
well, followed by a 2-h incubation at 37°C. Subsequently,
absorbance was measured at 450 nm. The optimal ratio of
CD4+ T cells to IR-BNL CL.2 cells was determined based
on changes in IR-BNL CL.2 cell viability across different
co-culture ratios.
Glucose consumption assay
CD4+ T cells from different treatment
groups were co-cultured with IR-BNL CL.2 cells at the optimal cell
ratio in phenol red-free DMEM for 24 h. At the end of the
co-culture period, the supernatant was collected and glucose
concentration was measured according to the instructions provided
with the glucose assay kit (Beyotime Biotechnology). Glucose
consumption was calculated as the difference between the initial
glucose concentration and the glucose concentration in the
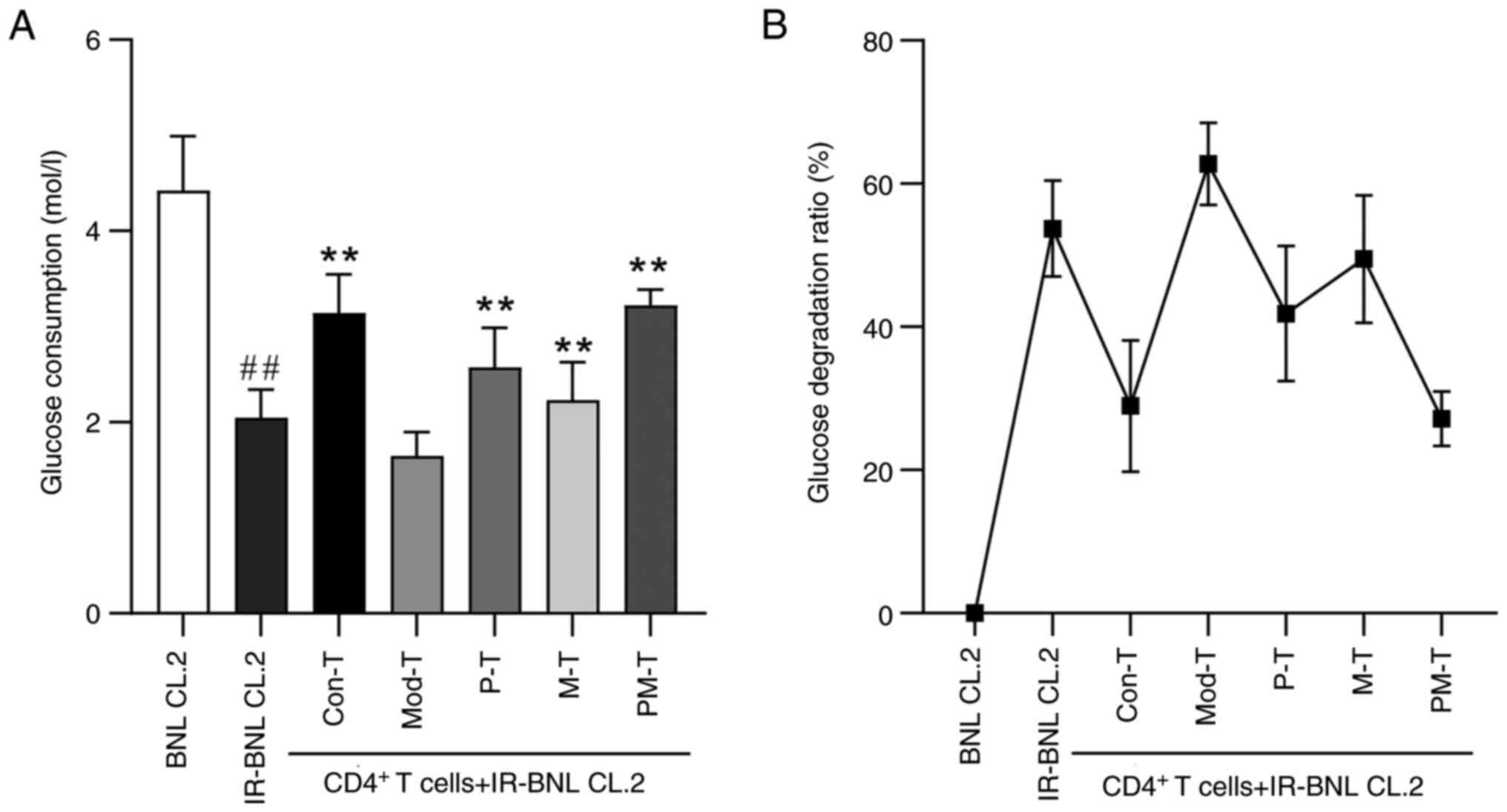
supernatant after 24 h. The glucose degradation ratio was
calculated as the percentage decrease in glucose consumption in the
experimental group relative to the BNL CL.2 control group.
Specifically, it was determined by subtracting the glucose
consumption of the experimental group from that of the control
group, dividing this difference by the glucose consumption of the
control group, and multiplying by 100.
Superoxide dismutase (SOD) and
malondialdehyde (MDA) assays
Following co-culture with CD4+ T cells
under the respective treatment conditions [the control (Con-T),
model (Mod-T), PF40 (P-T), metformin (M-T) or PF40 + metformin
(PM-T) groups], IR-BNL CL.2 cells from each treatment group were
collected and washed three times with cold PBS. Cells were then
lysed with an appropriate volume of RIPA lysis buffer (Thermo
Fisher Scientific, Inc.) and incubating on ice for 15 min. The
lysate was centrifuged at 12,000 × g for 10 min at 4°C and the
supernatant was collected for subsequent analysis. Protein
concentrations were quantified using a BCA protein assay kit (cat.
no. A045-4-2; Nanjing Jiancheng Bioengineering Institute) according
to the manufacturer's protocol. The activity of SOD and levels of
MDA were measured using respective commercial assay kits according
to the manufacturer's protocol (SOD Assay Kit, cat. no. A001-3-2;
MDA Assay Kit, cat. no. A003-1-2; both from Nanjing Jiancheng
Bioengineering Institute).
Reactive oxygen species (ROS)
assay
Treated IR-BNL CL.2 cells, following co-culture with
CD4+ T cells under the respective experimental
conditions (Con-T, Mod-T, P-T, M-T or PM-T), were collected and
seeded into black, opaque-bottom 96-well plates for ROS detection.
Cells were incubated for 24 h at 37°C with 5% CO2.
Following adherence, the cells were washed twice with serum-free
medium, and each well was loaded with 100 µM DCFDA in 1X buffer
(cat. no. S0035S; Beyotime Biotechnology). After a 45-min
incubation at 37°C, the DCFDA solution was removed and the cells
were washed twice with 1X PBS to minimize non-specific staining.
The plate was then analyzed using a flow cytometer (NovoCyte), and
data were collected and analyzed using NovoExpress software
(version 1.4.1) according to the DCFDA kit protocol to quantify ROS
levels (28).
Apoptosis assay
To evaluate apoptosis, IR-BNL CL.2 cells were
collected and washed twice with cold PBS after co-culture with
CD4+ T cells under the indicated treatment conditions
(Con-T, Mod-T, P-T, M-T or PM-T). Cells were resuspended in 1X
binding buffer, and 5 µl Alexa Fluor™ 488 Annexin V and 1 µl 100
µg/ml propidium iodide were added as per the manufacturer's
instructions (cat. no. A10788; Thermo Fisher Scientific, Inc.). The
mixture was incubated in the dark at room temperature for 20 min to
protect against photobleaching. Following incubation, the samples
were analyzed immediately on a flow cytometer (NovoCyte), and data
were collected and analyzed using NovoExpress software (version
1.4.1) to determine the proportions of live and apoptotic cells
across treatment groups (29).
Western blot analysis
IR-BNL CL.2 cells were collected and washed three
times with cold PBS following co-culture with CD4+ T
cells under the indicated treatment conditions (Con-T, Mod-T, P-T,
M-T or PM-T). Cells were then lysed with RIPA lysis buffer
containing protease inhibitors and lysates were incubated on ice
for 30 min, vortexing every 10 min, to ensure complete lysis.
Lysates were centrifuged at 12,000 × g for 10 min at 4°C and the
supernatant was collected for protein analysis. Protein
concentrations were quantified using a BCA assay kit, and equal
amounts of protein (30 µg/lane) from each sample were denatured at
95°C for 5 min before being separated by SDS-PAGE on 10% gels.
Following electrophoresis, the proteins were transferred onto PVDF
membranes at 4°C. Membranes were blocked with 5% BSA at room
temperature for 1 h to minimize non-specific binding, and were then
incubated overnight at 4°C with rabbit monoclonal primary
antibodies targeting p-PI3K (1:1,000), PI3K (1:1,000), p-AKT
(1:500), AKT (1:500), p-IRS-1 (1:1,000), IRS-1 (1:1,000) and
β-actin (1:5,000; cat. no. AC026; ABclonal Biotech Co., Ltd.). The
following day, the membranes were washed three times with TBS-T
buffer (TBS containing 0.1% Tween-20; 5 min each) to remove unbound
antibodies, followed by incubation with HRP-conjugated goat
anti-rabbit IgG (1:5,000; cat. no. 7074; Cell Signaling Technology,
Inc.) for 1 h at room temperature. After washing, protein bands
were visualized using a Pierce™ Fast Western kit (Thermo Fisher
Scientific, Inc.) and images were captured. Band intensities were
semi-quantified using Image Lab software (version 6.1.0; Bio-Rad
Laboratories, Inc.).
Targeted energy metabolomics analysis
by ultra performance liquid chromatography tandem mass pectrometry
(UPLC-MS/MS)
IR-BNL CL.2 cells were submitted to Wekemo Tech Co
(https://www.bioincloud.tech/) for
metabolomics analysis via UPLC-MS/MS. Briefly, 50 mg cell sample
was mixed with 1,000 µl water/methanol/acetonitrile (1:2:2, v/v),
vortexed for 25 min, and centrifuged at 14,000 × g for 15 min at
4°C. The supernatant was dried using a vacuum centrifuge, and the
residue was reconstituted in 100 µl 50% acetonitrile. The sample
was centrifuged again at 14,000 × g for 15 min at 4°C before
undergoing UPLC-MS/MS analysis at Wekemo Tech Co. The conditions
for mass spectrometry and chromatography are described in detail in
the Supplementary materials.
Statistical analysis and
visualization
Experimental data are presented as the mean ±
standard deviation. Baseline data across treatment groups were
analyzed using unpaired Student's t-test for comparisons between
two groups or a one-way ANOVA for comparisons between multiple
groups. Following one-way ANOVA, Tukey's post hoc multiple
comparisons test was performed to determine significant differences
between individual groups. P<0.05 was considered to indicate a
statistically significant difference. Targeted energy metabolomics
data analysis and visualization were performed on the Wekemo
Bioincloud platform (https://bioincloud.tech/) using default parameters. To
further explore metabolic differences between groups, principal
component analysis (PCA), orthogonal partial least squares
discriminant analysis (OPLS-DA) and redundancy analysis (RDA) were
conducted on the same platform. Unless otherwise specified, all
analyses were carried out with the standard settings provided by
Wekemo Bioincloud.
Results
Physicochemical characterization of
PF40
To investigate the physicochemical properties of
PF40, a series of compositional and structural analyses were
conducted. The polysaccharide yield from Pseudostellaria
heterophylla was 18.63±1.21%. The total carbohydrate content of
PF40 was determined to be 79.85±3.54%, while the protein content
was relatively low at 0.12±0.01%, indicating a high degree of
polysaccharide purity. Gel permeation chromatography revealed that
PF40 exhibited a molecular weight distribution ranging from 52.6 to
212 kDa (Fig. S1), suggesting a
heterogeneous macromolecular population. Monosaccharide composition
was analyzed by HPLC after acid hydrolysis. The results showed that
glucose was the predominant monosaccharide in PF40 (84.06 mol%),
followed by a minor proportion of galactose (2.50 mol%). These
findings were further supported by co-chromatography with standard
monosaccharides and peak area comparison, as shown in the
chromatograms. Fig. S2A presents
the chromatograms of glucose and galactose standards, while
Fig. S2B shows the monosaccharide
composition analysis of PF40. These results indicated that PF40 is
a polysaccharide mainly composed of glucose with a small amount of
galactose.
Establishment of an IR-BNL CL.2 cell
model
To establish an IR cell model, BNL CL.2 cells were
treated with a high concentration of insulin. To determine the
optimal insulin concentration, BNL CL.2 cells were exposed to
insulin at concentrations ranging from 1×10−6 to
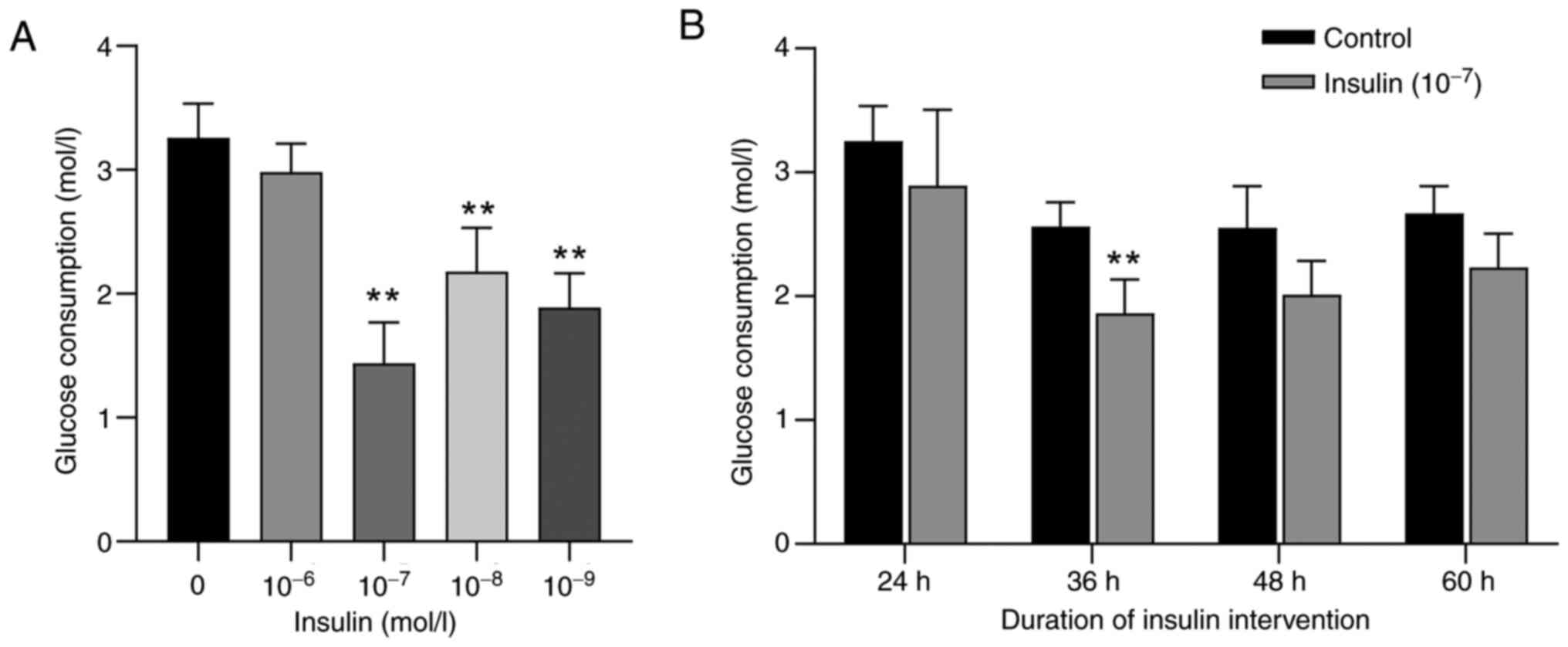
1×10−9 mol/l. As shown in Fig. 1A, compared with in the control
group, the 1×10−7 to 1×10−9 mol/l
insulin-treated groups exhibited significantly inhibited glucose
consumption in BNL CL.2 cells (P<0.01). The 1×10−7
mol/l insulin treatment exhibited the most pronounced inhibitory
effect, reducing glucose consumption to 1.44±0.32 mol/l, which
represents a 55.8% decrease from the control group (3.26±0.28
mol/l) (P<0.01). Therefore, 1×10−7 mol/l was selected
as the optimal insulin concentration. Subsequently, the effects of
varying exposure durations of 1×10−7 mol/l insulin on
glucose consumption in cells was examined (Fig. 1B). Following treatment with
1×10−7 mol/l insulin for 24, 36, 48 and 60 h, glucose
consumption decreased by 11.08, 27.11, 21.18 and 16.48%,
respectively, compared with that in the control group, with a
significant reduction observed at 36 h. These results indicated
that treatment with 1×10−7 mol/l insulin for 36 h
represented the optimal conditions for inducing insulin resistance
in BNL CL.2 cells.
Notably, a non-linear relationship between insulin
concentration and glucose consumption was observed in BNL CL.2
cells (Fig. 1). While low
concentrations of insulin significantly promoted glucose uptake,
higher concentrations did not further enhance glucose consumption
and even showed a plateau or decline in effect. This may be due to
insulin receptor desensitization, or negative feedback regulation
induced by prolonged or excessive insulin exposure. At high doses,
insulin can trigger signaling attenuation through mechanisms such
as increased serine phosphorylation of IRS-1, thereby impairing
downstream PI3K/AKT pathway activation and reducing glucose
utilization efficiency. This phenomenon is consistent with
previously reported insulin-induced insulin resistance in
hepatocyte models (30).
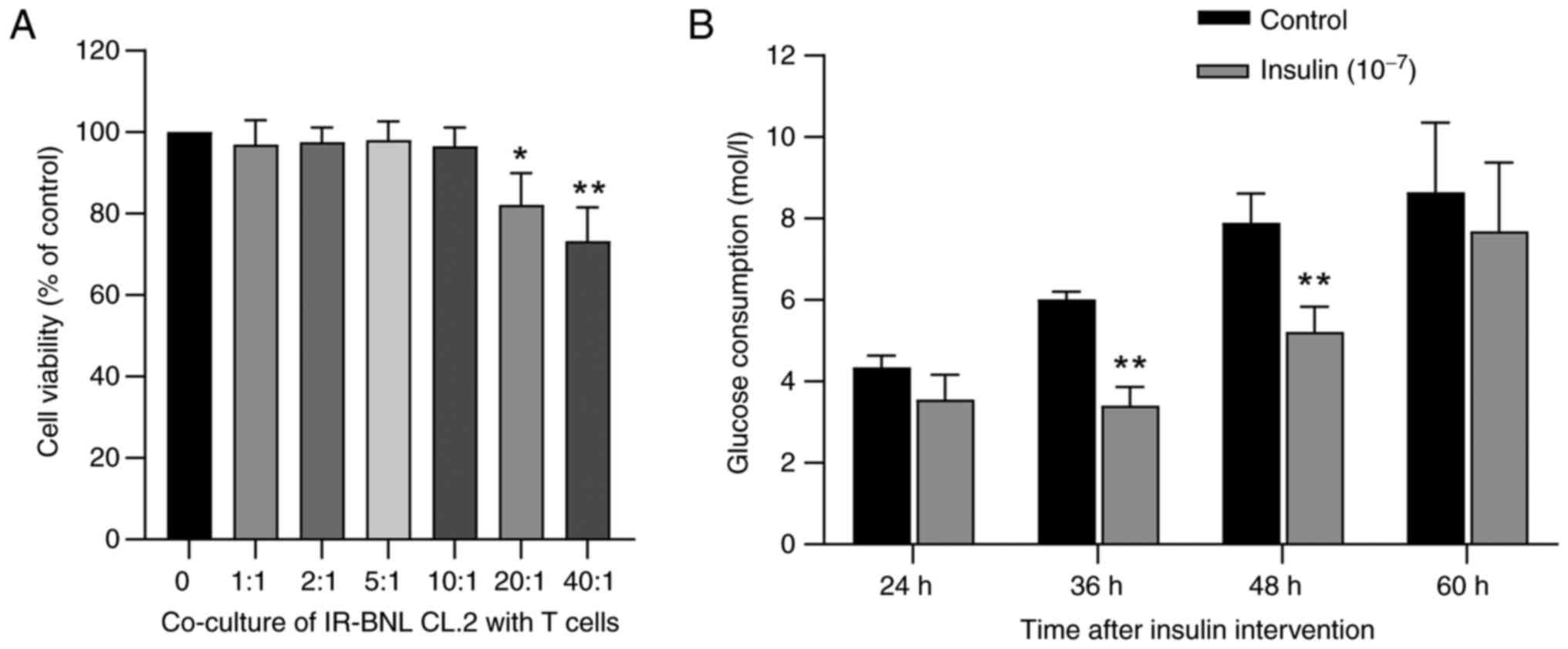
Stability of the IR-BNL CL.2
model
To evaluate the stability of the insulin resistance
cell model, IR-BNL CL.2 cells were continuously monitored following
insulin treatment. As shown in Fig.
2B, compared with that in the control group, glucose
consumption in cells pretreated with 1×10−7 mol/l
insulin was significantly reduced at 36 and 48 h after insulin
removal (P<0.01). Notably, at the final observation point of 60
h, although glucose consumption in the insulin-treated group
remained lower than that in the control group, this difference was
no longer statistically significant (P>0.05). This result
suggested that the insulin resistance model remains stable for up
to 48 h after insulin withdrawal but may begin to undergo partial
self-repair by 60 h. Thus, subsequent experiments were performed
within a 48-h time period following the establishment of the
model.
Biocompatibility of T cells with
IR-BNL CL.2
Isolated CD4+ T cells (Fig. S3) were co-cultured with IR-BNL
CL.2 cells at varying ratios (1:1, 2:1, 5:1, 10:1, 20:1 and 40:1)
to assess the biocompatibility of the co-culture system. A CCK-8
assay was used to evaluate the viability of IR-BNL CL.2 cells
(Fig. 2A). When the ratio of
CD4+ T cells was ≤10:1 there was no significant impact
on IR-BNL CL.2 cell viability (P>0.05). However, at ratios of
20:1 and 40:1, the viability of IR-BNL CL.2 cells was significantly
reduced (both P<0.01; 82.11 and 73.26% of the control group,
respectively). Therefore, a CD4+ T cell to IR-BNL CL.2
cell ratio of 10:1 was selected for subsequent experiments.
Glucose consumption of IR-BNL CL.2
cells in response to different CD4+ T-cell
treatments
CD4+ T cells subjected to various
treatments were co-incubated with IR-BNL CL.2 cells to assess their
impact on glucose consumption in the IR-BNL CL.2 cells. As shown in
Fig. 3, glucose consumption was
significantly reduced in IR-BNL CL.2 cells compared with that in
normal BNL CL.2 cells (P<0.05), confirming the successful
establishment of the IR model. In the co-culture experiments,
glucose consumption in IR-BNL CL.2 cells was markedly lower in the
Mod-T group than in the Con-T group (P<0.01). Notably,
co-culture of IR-BNL CL.2 cells with P-T, M-T or PM-T significantly
improved glucose metabolism compared with the Mod-T group. The PM-T
group showed the most pronounced effect, with glucose consumption
nearly doubled compared with that in the Mod-T group
(P<0.01).
Oxidative stress of IR-BNL CL.2 cells
in response to different CD4+ T-cell treatments
To evaluate the oxidative stress status in IR-BNL
CL.2 cells across different treatment groups, MDA levels and SOD
activity were measured. MDA, a primary product of lipid
peroxidation, reflects the extent of cellular oxidative damage,
whereas SOD, a key antioxidant enzyme, indicates cellular
antioxidant capacity (31). As
shown in Fig. 4, MDA levels in
IR-BNL CL.2 cells were significantly elevated in the Mod-T group
compared with those in the Con-T group (P<0.01), whereas SOD
activity was significantly reduced (P<0.01). Among the treatment
groups, both the P-T and PM-T groups significantly lowered MDA
levels (P<0.05), and although the M-T group also showed a trend
toward reducing MDA, the difference was not statistically
significant compared with the Mod-T group (P>0.05). All
treatment groups exhibited notable improvements in SOD activity
(P<0.01), with the PM-T group showing the most significant
effect, nearly restoring SOD activity to control levels.
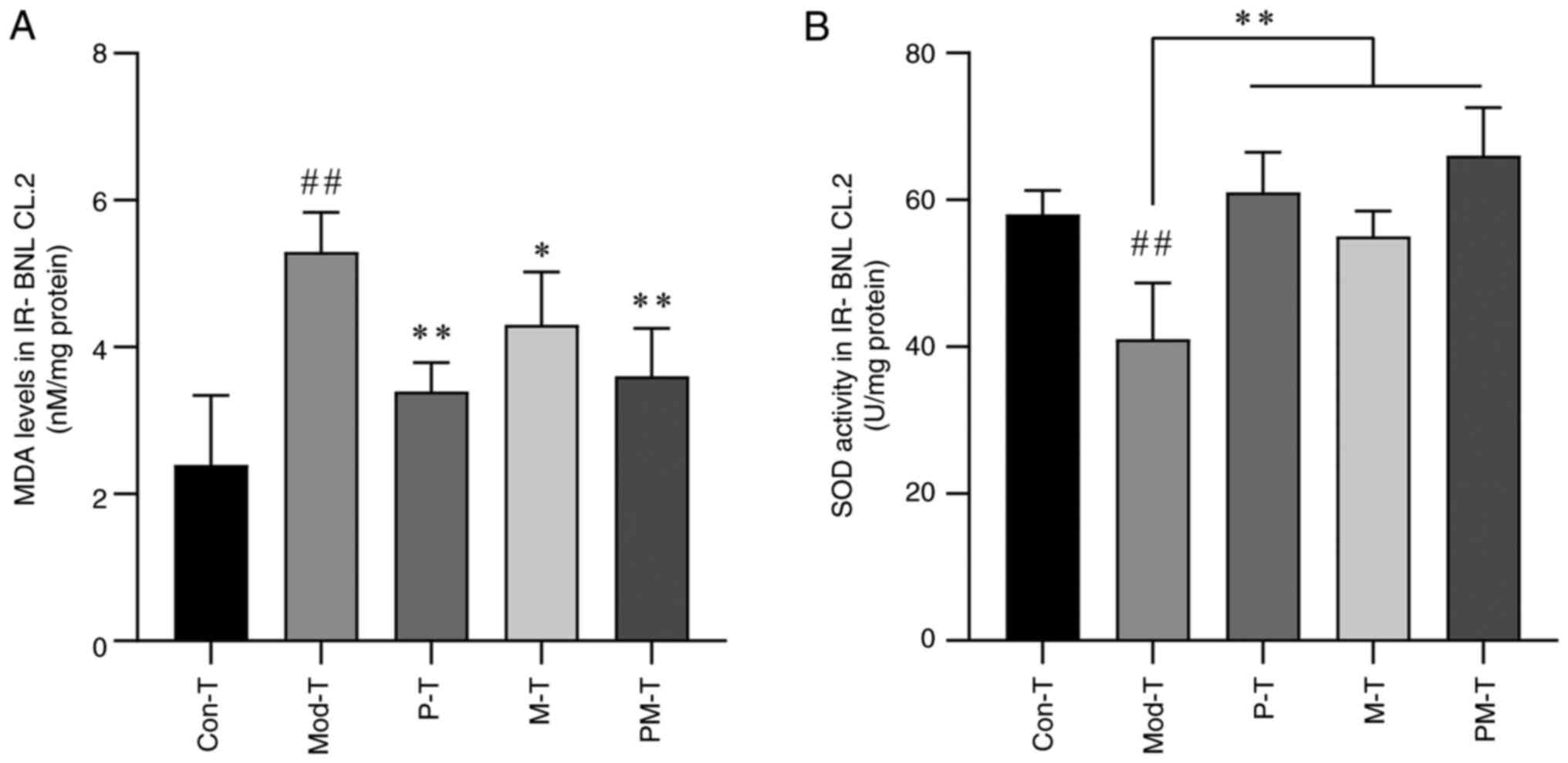 | Figure 4.Effects of differently treated
CD4+ T cells on (A) MDA and (B) SOD levels in IR-BNL
CL.2 cells. Data are presented as the mean ± SD, n=5.
##P<0.01 vs. Con-T group; *P<0.05, **P<0.01 vs.
Mod-T group. MDA, malondialdehyde; SOD, superoxide dismutase;
IR-BNL CL.2, insulin-resistant BNL CL.2; Con-T, control; Mod-T,
model; P-T, PF40; M-T, metformin; PM-T, combined PF40 and
metformin. |
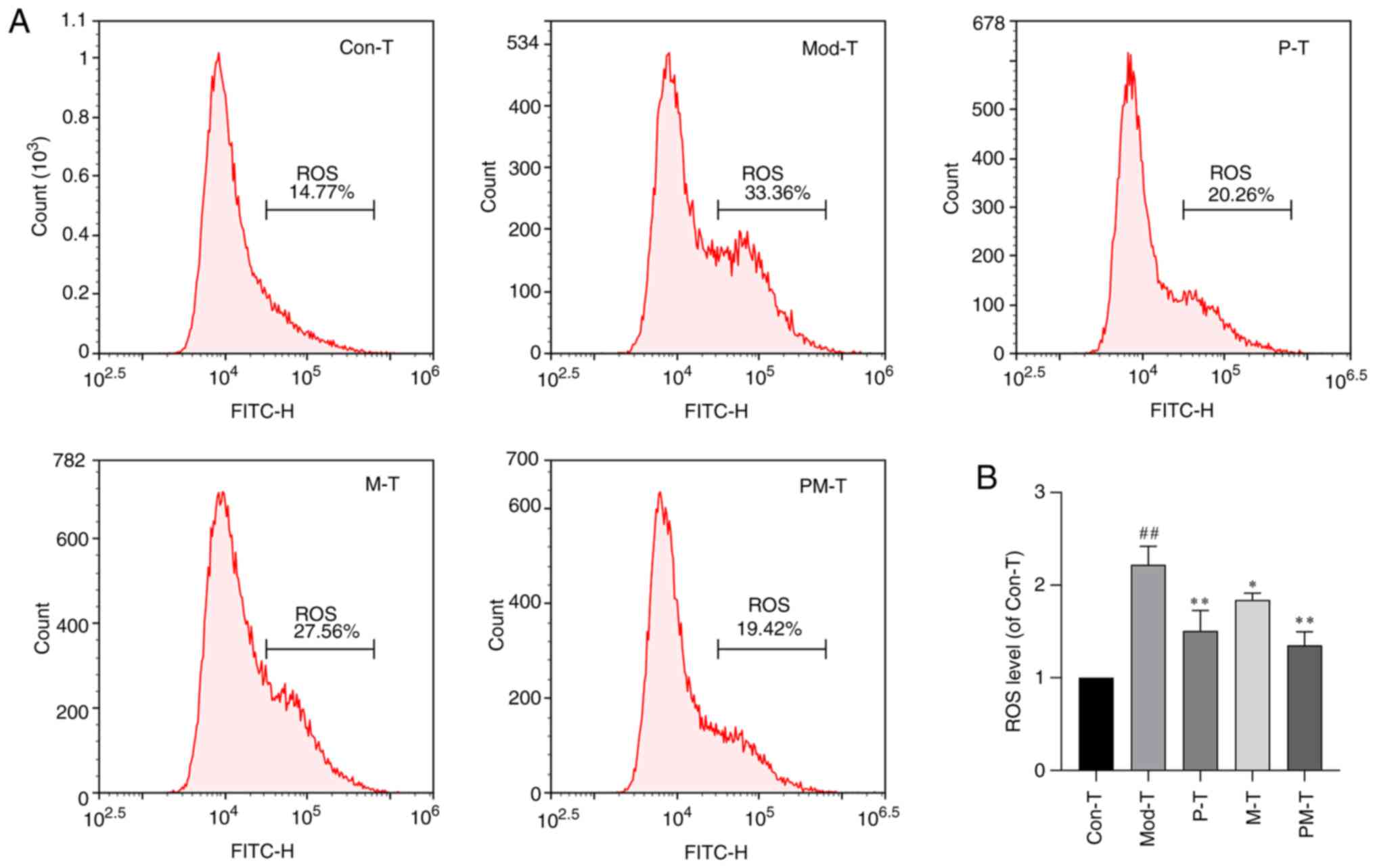
Subsequently, intracellular ROS levels in IR-BNL
CL.2 cells co-cultured with CD4+ T cells from various
treatment groups were assessed using flow cytometry. As shown in
Fig. 5, ROS levels were
significantly elevated in the Mod-T group compared with those in
the Con-T group (P<0.01), reaching approximately double that of
the control levels. Both the P-T and M-T treatment groups
significantly reduced ROS levels (P<0.05). Furthermore, the
combined treatment group (PM-T) exhibited the strongest inhibitory
effect on ROS levels, restoring them to close to the control levels
(P<0.01).
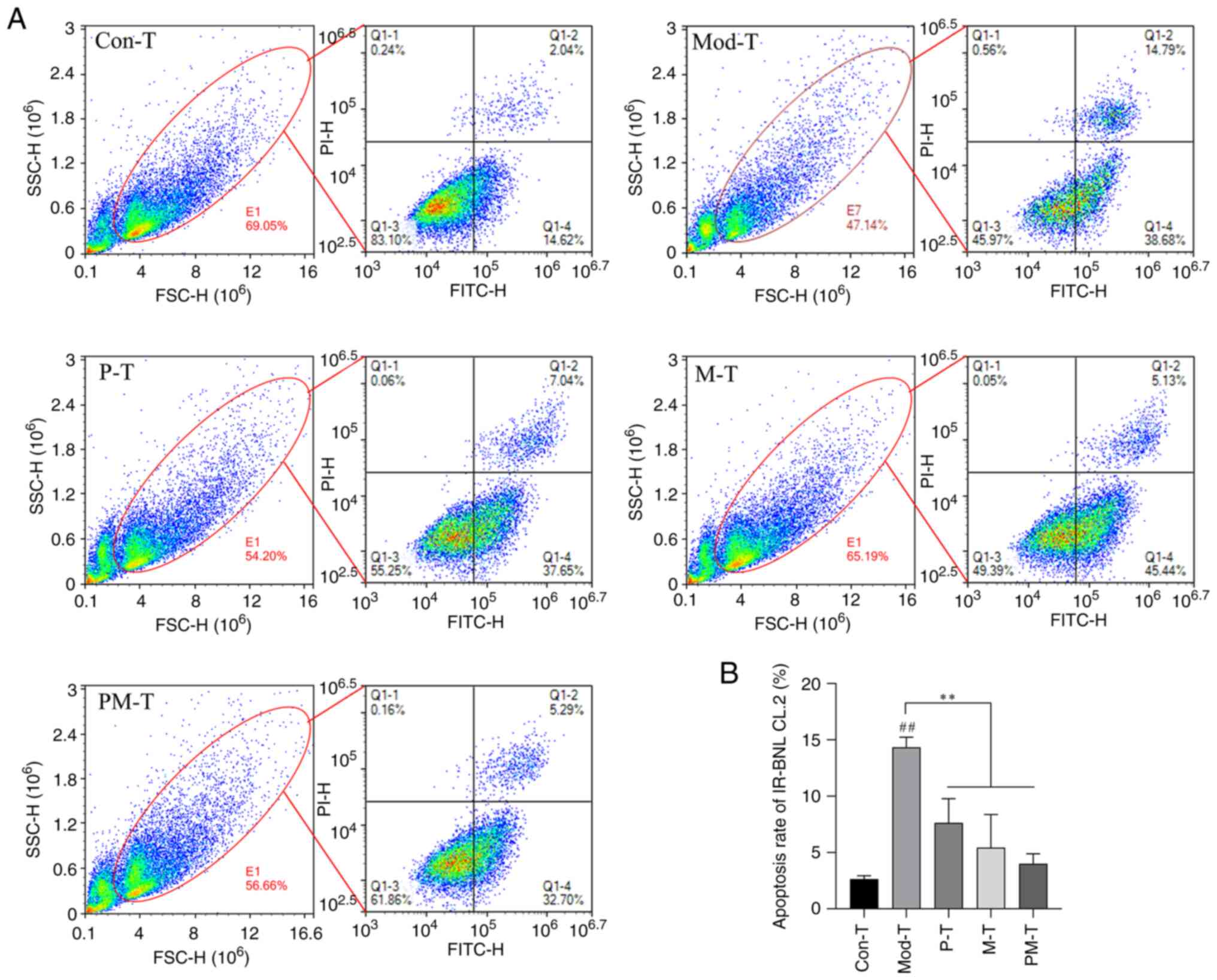
Apoptosis of IR-BNL CL.2 cells in
response to different CD4+ T-cells treatments
FSC (forward scatter) and SSC (side scatter) plots
were used to assess cell populations, where FSC reflects cell size
and SSC indicates cell granularity or internal complexity. To
investigate the impact of CD4+ T cells from different
treatment groups on IR-BNL CL.2 cell apoptosis, the apoptotic rate
of IR-BNL CL.2 cells in the co-culture system was measured using
flow cytometry (Fig. 6). The
results showed that the Mod-T group exhibited significantly
increased apoptosis of IR-BNL CL.2 cells compared with that in the
Con-T group (P<0.01). This finding indicated that
CD4+ T cells in a T2DM state may exacerbate insulin
resistance by promoting the apoptosis of IR-BNL CL.2 cells.
Following P-T or M-T treatment, the apoptosis rates in IR-BNL CL.2
cells were significantly reduced (P<0.01). Notably, the PM-T
group showed the most pronounced protective effect, reducing the
rate of apoptosis to close to that of control levels (P<0.01).
These findings suggested that PF40 and metformin may mitigate
apoptosis in IR-BNL CL.2 cells through functional modulation of
CD4+ T cells, with a potential synergistic effect.
Expression of IRS-1/PI3K/AKT and
phosphorylated proteins in IR-BNL CL.2 cells
The expression of insulin signaling markers in
IR-BNL CL.2 cells was assessed using western blot analysis
(Fig. 7). The results indicated
that in the Mod-T group IR-BNL CL.2 cells exhibited an impaired
insulin signaling cascade, as evidenced by increased
phosphorylation of IRS-1Ser307 (P<0.01), and
significantly decreased phosphorylation of
PI3Kp58-Tyr607 and AKTS473 (both P<0.01),
compared with that in the Con-T group. By contrast, the PM-T group
showed a marked reduction in p-IRS-1Ser307 (P<0.01),
and a significant increases in p-PI3Kp58-Tyr607 and
p-AKTS473 levels (P<0.01) compared with in the Mod-T
group. These findings suggested that P-T or M-T may activate the
PI3K/AKT signaling pathway by reducing p-IRS-1Ser307
expression, effectively increasing insulin sensitivity and
improving insulin resistance.
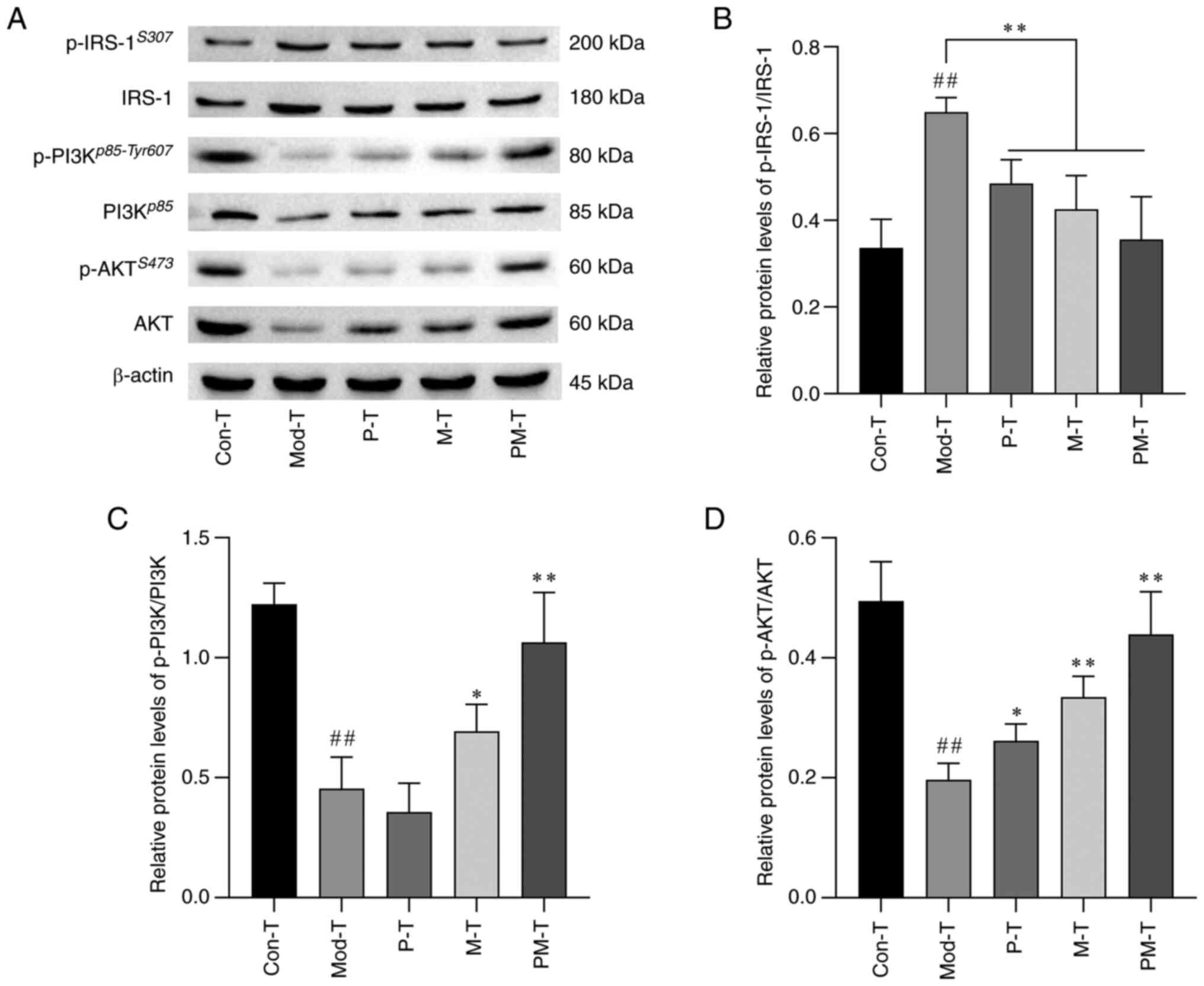 | Figure 7.Effects of differently treated
CD4+ T cells on the IRS-1/PI3K/AKT signaling pathway in
IR-BNL CL.2 cells. (A) Representative western blot images showing
protein and p-protein expression levels of IRS-1, PI3K and AKT
across the different treatment groups. Relative protein expression
levels of (B) p-IRS-1/IRS-1, (C) p-PI3K/PI3K, and (D) p-AKT/AKT.
Data are presented as the mean ± SD, n=3. ##P<0.01
vs. Con-T group; *P<0.05, **P<0.01 vs. Mod-T group. Con-T,
control; Mod-T, model; P-T, PF40; M-T, metformin; PM-T, combined
PF40 and metformin; IRS-1, insulin receptor substrate-1; p-,
phosphorylated. |
Energy metabolism in IR-BNL CL.2 cells
in response to different CD4+ T-cell treatments
UPLC-MS/MS was used to investigate changes in energy
metabolism-related metabolites in IR-BNL CL.2 cells. To streamline
data analysis, the Con-T, Mod-T and PM-T groups were selected. To
explore metabolic differences between samples, PCA and OPLS-DA were
performed (Fig. 8A-C). The PCA
score plot indicated a clear separation trend among the three
groups on PC1 (57.9%) and PC2 (12.1%). The OPLS-DA analysis between
the Con-T and Mod-T groups showed that the predictive component T
score and orthogonal component T score explained 42.1 and 25.3% of
the variance, respectively. In the OPLS-DA analysis between the
Mod-T and PM-T treatment groups, the predictive component T score
and orthogonal component T score explained 69.3 and 9.6% of the
variance, respectively. Both OPLS-DA score plots displayed good
group separation, indicating significant metabolic alterations due
to insulin resistance modeling, while PM-T treatment effectively
modulated these metabolic abnormalities.
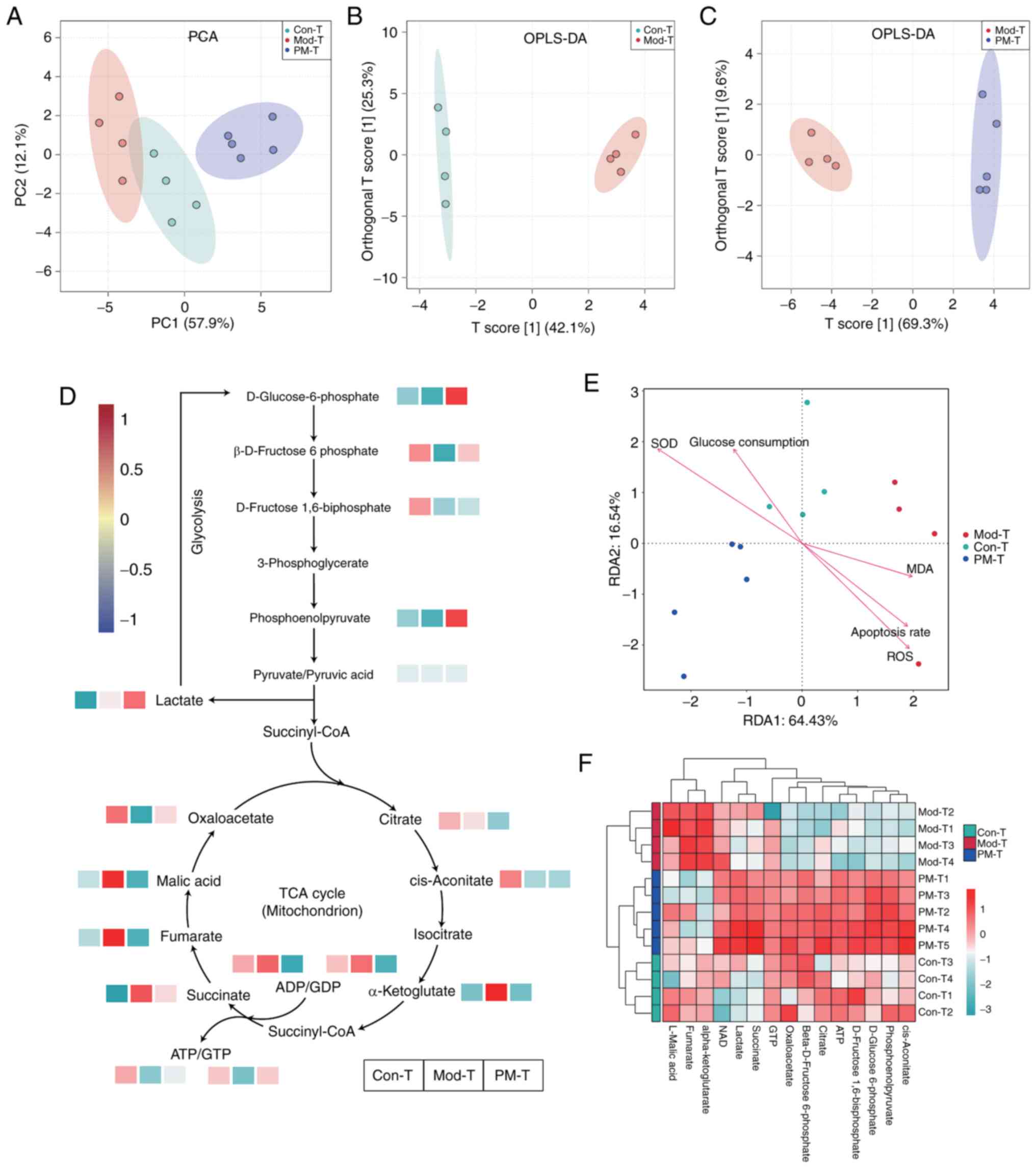 | Figure 8.Targeted energy metabolomics analysis
of the different treatment groups. (A) PCA of metabolites in the
different treatment groups. OPLS-DA was performed between the (B)
Con-T and Mod-T groups, and the (C) Mod-T and PM-T groups. (D) Heat
maps showing the relative content of key glycolysis products across
different treatment groups. (E) Redundancy analysis. Data are
presented as the mean ± SD, n=4 or 5. (F) Heat map showing the
relative content of key TCA cycle products across different
treatment groups. Mod-T. Con-T, control; Mod-T, model; P-T, PF40;
M-T, metformin; PM-T, combined PF40 and metformin; PCA, principal
component analysis; OPLS-DA, orthogonal partial least squares
discriminant analysis; TCA, tricarboxylic acid. |
As shown in Fig. 8D and
F, heatmaps illustrated the levels of key metabolites involved
in the tricarboxylic acid (TCA) cycle and glycolysis pathways
within IR-BNL CL.2 cells. Compared with in the Con-T group, the
Mod-T group exhibited significant changes in key metabolites within
the TCA cycle and glycolysis. In the TCA cycle, α-ketoglutarate,
succinate, fumarate and malic acid levels were markedly increased,
while oxaloacetate levels were markedly decreased. In the
glycolysis pathway, levels of D-glucose-6-phosphate and
β-D-fructose-6-phosphate were substantially reduced in the Mod-T
group. These results suggested that the Mod-T group may compensate
for impaired glycolysis by upregulating key metabolites in the TCA
cycle to maintain cellular energy balance. The PM-T treatment group
an opposing metabolic pattern was detected compared with that in
the Mod-T group, particularly regarding levels of α-ketoglutarate
and D-glucose-6-phosphate, suggesting that PM-T exerts therapeutic
effects by modulating the metabolic balance between the TCA cycle
and glycolysis pathways.
RDA showed that the first and second axes explained
64.43 and 16.54% of the total variance, respectively (Fig. 8E). The RDA ordination plot revealed
clear separation among the Con-T, Mod-T and PM-T groups. RDA
revealed that the Mod-T group was positively associated with ROS
and apoptosis rate, whereas the PM-T group was positively
associated with SOD activity and negatively with MDA levels,
indicating that PM-T treatment improves oxidative stress by
enhancing antioxidant capacity and reducing lipid peroxidation
damage.
Discussion
In the present study, an IR-BNL CL.2 cell model was
established and co-incubated with intestinal CD4+ T
cells treated with PF40, metformin or both. The results showed that
P-T reduced oxidative stress in IR-BNL CL.2 cells by lowering ROS
and MDA levels, while enhancing SOD activity, which reflected
improved antioxidant defense. Oxidative stress is a known factor in
insulin resistance, primarily by affecting glucose metabolism and
the insulin signaling pathways, thereby accelerating the
progression of insulin resistance (31). By mitigating oxidative stress, P-T
co-culture restored the redox balance and improved insulin
sensitivity. The findings of the present study emphasized that
CD4+ T cells may serve a critical role in alleviating
insulin resistance in IR-BNL CL.2 cells. This aligns with
accumulating evidence that immune cells, particularly
CD4+ T lymphocytes, notably impact metabolic activity
and inflammation in the context of insulin resistance (32,33).
The liver is a key organ involved in glucose
metabolism and a primary target in insulin resistance (34,35).
Establishing a stable and reliable IR cell model is crucial for
screening anti-insulin resistance drugs and studying their
mechanisms of action. Murine embryonic liver cells are widely used
as model cells to investigate hepatic insulin resistance, and
insulin serves as an inducer for developing IR cell models
(36,37). In developing the IR-BNL CL.2 cell
model, a marked reduction in glucose consumption in response to
optimal induction conditions was observed (1×10−7 mol/l
insulin for 36 h), and model stability was confirmed over 48 h.
Impaired glucose consumption and elevated oxidative stress levels
in IR-BNL CL.2 cells are consistent with previous findings,
validating the stability and reliability of the model (36,38).
When co-cultured with P-T cells, glucose uptake was restored in
IR-BNL CL.2 cells. The present study also investigated the
biocompatibility of CD4+ T cells with IR-BNL CL.2 cells
at specific co-culture ratios, and the results showed that the
optimal non-cytotoxic ratio for co-culturing CD4+ T
cells with IR-BNL CL.2 cells was 10:1. The establishment of this
co-culture system provides a basis for further exploration of the
mechanisms by which CD4+ T cells modulate insulin
resistance in IR-BNL CL.2 cells.
In the current study, the murine hepatocyte-derived
BNL CL.2 cell line was selected to establish an insulin resistance
model for co-culture with CD4+ T cells isolated from
PF40-treated rats with T2DM. This choice was made to maintain
taxonomic proximity within the Rodentia order and to minimize
potential immune incompatibility or non-specific activation that
could result from co-culturing rodent-derived immune cells with
more distantly related species. While BNL CL.2 cells are embryonic
in origin and do not fully recapitulate the metabolic complexity of
human hepatocytes, they are widely used in studies of hepatic
insulin signaling (39), oxidative
stress (40) and energy metabolism
(41). Nevertheless, the
limitations of this model in mimicking human pathophysiology should
be acknowledged, and future studies incorporating humanized
hepatocyte-immune co-culture systems or liver organoids will be
necessary to validate and extend the findings in a translational
context. Moreover, the T cell-to-hepatocyte ratio employed in the
co-culture system (10:1) exceeds physiological levels typically
found in vivo. This elevated ratio was selected based on the
biocompatibility testing of different CD4+ T
cell/IR-hepatocyte ratios, to ensure that CD4+ T cells
could exert sufficient paracrine and immunomodulatory effects on IR
hepatocytes without inducing excessive cytotoxicity. While this
setup enhances the detection of immunometabolic interactions in
vitro, it may not fully replicate the cellular microenvironment
of the gut-liver axis under physiological or pathological
conditions. Therefore, the data should be interpreted as
proof-of-concept findings, and future studies employing more
physiologically relevant cell ratios or three-dimensional
co-culture models are warranted to confirm the observed
effects.
SOD is a critical antioxidant enzyme that
effectively scavenges superoxide anions, thereby reducing oxidative
stress-induced cellular damage. In the pathophysiology of T2DM,
prolonged hyperglycemic conditions lead to a marked elevation in
intracellular ROS levels, exacerbating oxidative stress, which
further exacerbates insulin resistance and cellular injury
(42,43). P-T intervention significantly
increased SOD activity in IR-BNL CL.2 cells in the present study,
suggesting that P-T alleviated oxidative stress by enhancing
cellular antioxidant capacity. Furthermore, the observed reduction
in MDA levels supports the antioxidant role of P-T. As a marker of
lipid peroxidation, decreased MDA levels indicate that P-T may
reduce lipid peroxidation under IR conditions, thereby lowering the
risk of oxidative membrane damage. Lipid peroxidation, a
consequence of oxidative stress, destabilizes cell membranes,
increases permeability and impairs cell function (44). Compared with in the Mod-T group,
the P-T group exhibited a significant reduction in MDA levels,
suggesting that P-T may serve a crucial role in inhibiting lipid
peroxidation and preserving membrane integrity.
Oxidative stress is one of the primary inducers of
cellular apoptosis, with ROS acting as reactive molecules that
cause oxidative damage to membrane lipids, proteins and DNA under
oxidative stress, ultimately triggering apoptotic pathways
(45). In the present study, P-T
treatment significantly reduced ROS levels in IR-BNL CL.2 cells,
along with a decrease in the lipid peroxidation marker MDA. This
finding indicated that P-T not only exhibits antioxidant properties
but may also inhibit the progression of oxidative damage by
reducing ROS. Apoptosis assays of IR-BNL CL.2 cells further
validated these findings, with Mod-T showing substantial apoptosis
relative to the Con-T group, whereas P-T treatment significantly
reduced apoptotic cell counts. Thus, P-T may prevent apoptosis
pathway activation by lowering oxidative stress, which is crucial
for mitigating insulin resistance-associated cellular injury.
To further elucidate the potential molecular
mechanisms by which P-T influenced glucose metabolism, the
expression of insulin signaling-related markers in IR-BNL CL.2
cells was examined. Upon binding to its receptor, insulin promotes
glucose uptake through a series of signaling cascades, resulting in
the phosphorylation of IRS proteins at multiple tyrosine residues,
which subsequently activate downstream molecules such as PI3K and
AKT (46). The PI3K/AKT pathway is
a primary regulator of metabolic functions and glucose uptake
induced by insulin (46–48). Activated AKT serves a central role
in regulating various downstream pathways, including those involved
in cell proliferation, protein synthesis and glucose metabolism.
Phosphorylation of AKT further activates GLUT4, enhancing glucose
uptake in peripheral tissues. IRS-1, a major IRS subtype, is
closely associated with glucose homeostasis in the liver; however,
phosphorylation of IRS-1 at the Ser307 residue significantly
diminishes its activity, which is linked to the inhibition of
insulin signaling (49,50). Western blot analysis showed that in
the Mod-T group, levels of p-IRS-1Ser307 were markedly
elevated, whereas PI3Kp85-Tyr607 and
p-AKTSer473 levels were notably reduced, indicating
impairment of the insulin signaling cascade. Treatment with P-T or
M-T significantly reduced p-IRS-1Ser307 levels, enhanced
PI3Kp85-Tyr607 expression, and increased AKT
phosphorylation, thereby promoting glucose uptake.
Phosphorylation of AKT has a pivotal role in glucose
uptake and utilization by activating key glycolytic enzymes, such
as glucokinase, which facilitates glucose phosphorylation, an
essential step for entry into glycolysis and the TCA cycle
(51,52). During a state of insulin
resistance, reduced AKT phosphorylation may lead to decreased
glucokinase activity, directly lowering the levels of
glucose-6-phosphate (53). In the
present study, reduced glucose-6-phosphate levels were observed in
the Mod-T group. This reduction disrupted the TCA cycle, which
could impair glycolytic input, resulting in decreased ATP
production efficiency and subsequent ROS accumulation. In the
IR-BNL CL.2 model group, elevated ROS levels and reduced TCA cycle
intermediates suggested mitochondrial stress, compromising cellular
bioenergetics and promoting apoptosis. This increase in apoptosis
aligned with the results from RDA, where elevated ROS and lipid
peroxidation were associated with higher apoptotic rates. Thus,
disruption of the glucokinase-AKT pathway highlights links among
glucose metabolism disorders, oxidative stress and cell
survival.
The present study used targeted energy metabolomics
analysis to reveal differences in energy metabolism pathways in
IR-BNL CL.2 cells among the different treatment groups. In the
Mod-T group, a notable increase in multiple key metabolites within
the TCA cycle was observed, particularly α-ketoglutarate,
succinate, fumarate and malic acid, which suggested the presence of
a compensatory regulatory mechanism in response to metabolic
dysregulation. Studies have shown that the accumulation of TCA
cycle metabolites is often associated with mitochondrial
dysfunction (54,55). Concurrently, the significant
reduction of glycolytic intermediates (such as
D-glucose-6-phosphate and β-D-fructose-6-phosphate) in the Mod-T
group suggested that glycolysis may be suppressed. This metabolic
shift aligns with previously reported characteristics of metabolic
disease models (56). Notably, the
PM-T treatment group exhibited metabolic features that were the
opposite of those in the Mod-T group. Following PM-T treatment, the
levels of glycolytic intermediates, such as D-glucose-6-phosphate
and phosphoenolpyruvate, were increased, and TCA cycle metabolites
approached normal levels. This finding suggested that PM-T may
improve cellular energy metabolism by modulating the metabolic
balance between the TCA cycle and glycolysis pathways.
RDA provided further biochemical evidence supporting
the role of PM-T in ameliorating oxidative stress. The separation
between the Mod-T and PM-T groups on the RDA plot, along with the
positive association between the Mod-T group and elevated ROS
levels and apoptosis rates, indicated that Mod-T-induced metabolic
stress may be associated with increased oxidative damage. However,
PM-T treatment showed a strong positive association with SOD
activity and a negative association with MDA content, indicating
enhanced antioxidant defense capacity and reduced lipid
peroxidation in treated cells. This transition in oxidative status
may represent a key mechanism by which PM-T reduces cellular stress
and prevents apoptosis. Collectively, PM-T may exert its protective
effects through rebalancing energy metabolism and enhancing
antioxidant responses, potentially alleviating functional
impairments in IR cells.
The combined treatment of CD4+ T cells
with PF40 and metformin demonstrated synergistic effects on
antioxidant activity and apoptosis inhibition. Compared with M-T,
the PM-T group exhibited markedly enhanced antioxidant capacity and
reduced ROS levels, which may be attributed to the complementary
mechanisms of PF40 and metformin. PF40 primarily reduces oxidative
stress through its antioxidant properties, whereas metformin
improves cellular metabolism by modulating the insulin signaling
pathway (10,57). Thus, their combined application may
alleviate insulin resistance and improve cell status through
several different mechanisms. Although metformin is effective in
reducing insulin resistance, adjunct therapy with natural
antioxidants may further enhance therapeutic outcomes and
potentially reduce side effects associated with metformin
monotherapy (57,58). It has been reported that mushroom
polysaccharide NAP-3 can enhance the efficacy of metformin in lipid
and glucose metabolism in mice with T2DM by reshaping the
homeostasis of the gut microbiota community (59). The Astragalus polysaccharide
APS-D1 has also been shown to reduce the required dosage of
metformin by enriching Staphylococcus lentus, which promotes
fatty acid oxidation and inhibits gluconeogenesis (60).
In the present study, the effects of differentially
treated intestinal immune cell subsets on insulin resistance were
investigated. The results revealed that modulating the homeostasis
of CD4+ T cells in the gut immune system may notably
improve insulin signaling and glucose metabolism in an IR cell
model. This aligns with previous studies highlighting the role of
gut immune cells in metabolic regulation, particularly the
exacerbation of IR by T-cell alterations in inflammatory
environments (61,62). Targeting intestinal immune cells
may present a novel approach for the prevention and treatment of
insulin resistance and metabolic diseases (63). However, the CD4+ T-cell
population in the present study likely includes various subsets,
such as Th1, Th2 and Th17 cells, and Tregs. The specific roles of
these subsets in this process remain unclear. To further elucidate
this mechanism, future studies should employ techniques such as
single-cell sequencing to better understand the contributions of
these immune cell subsets in improving insulin resistance and
reducing apoptosis.
In our previous study, the regulatory effects of
PF40 on CD4+ T-cell subsets, particularly in the balance
between Tregs and Th17 cells were determined (11). PF40 may alleviate immune system
overactivation and reduce insulin resistance by enhancing the
function of Tregs. Tregs serve a critical role in maintaining
immune tolerance and suppressing excessive immune responses,
whereas Th17 cells are typically associated with inflammatory
responses and the development of insulin resistance (3,4). By
modulating the Treg/Th17 balance, PF40 may inhibit Th17 cell
expansion and reduce the inflammation induced by these cells,
thereby improving insulin signaling. Specifically, PF40 may enhance
the secretion of factors such as IL-10 by Tregs, while suppressing
the secretion of IL-17A by Th17 cells, thereby regulating the
immune microenvironment and mitigating insulin resistance caused by
immune activation (11). In the
present study, it was observed that P-T cells suppressed oxidative
stress responses and activated the IRS-1/PI3K/AKT signaling
pathway. These findings not only provide evidence for the potential
mechanisms through which PF40 maintains immune balance but also
highlight its promising application prospects in the treatment of
insulin resistance.
While the current study provides experimental data
on the role of PF40 in alleviating insulin resistance, its
limitations must be considered. First, the present study primarily
relied on in vitro models, and although the IR-BNL CL.2 cell
model has been widely validated in insulin resistance research, it
differs from in vivo environments, particularly regarding
the dynamic changes in immune cells and tissue-specific responses.
Therefore, future studies should consider validating these findings
in animal models to enhance the biological relevance of the
results. Second, since the study did not delve deeply into the
specific mechanisms of CD4+ T-cell subsets, future
research could employ single-cell sequencing technologies or other
high-throughput analysis methods to explore the roles and
interactions of different T-cell subsets in insulin resistance.
These studies would contribute to a more comprehensive
understanding of PF40-mediated modulation of the immune system and
provide novel insights and strategies for the treatment of insulin
resistance and related metabolic diseases. Finally, although the
combined treatment of PF40 and metformin indicated potential
synergistic effects, the absence of formal synergy calculations
represents a limitation of the present study, and further
quantitative analyses are warranted to substantiate this
interaction.
The present study provides evidence that P-T cells
are conducive to improving immune activation-induced insulin
resistance. However, the precise mechanisms by which PF40 regulates
CD4+ T-cell subset differentiation remain to be
elucidated. It is well known that polysaccharides are characterized
by their high molecular weight and low bioavailability. Our
previous research identified a water-soluble pectic polysaccharide
isolated from Pseudostellaria heterophylla that can be
absorbed through the intestinal mucosa and enter the systemic
circulation (12). Emerging
evidence has suggested that fermentation of Porphyra
haitanensis polysaccharides by Lactiplantibacillus
plantarum markedly reduces their particle size and enhances the
anti-allergic effects both in vitro and in vivo
(21). Fucoidan has also been
shown to markedly modulate the composition and functional output of
the gut microbiota (64,65). Notably, this microbial remodeling
appears to be a key mechanism underpinning the biological activity
of polysaccharides. The reshaped microbiota ferments these complex
carbohydrates, leading to the production of critical metabolites,
particularly short-chain fatty acids (SCFAs), such as acetate,
propionate and butyrate (66).
These SCFAs act as essential signaling molecules and energy
substrates, serving pivotal roles in the differentiation of
intestinal immune cells (67).
In conclusion, the present study demonstrated that
P-T cells significantly enhanced SOD activity, reduced MDA and ROS
levels, and lowered oxidative stress, thereby reducing apoptosis in
the IR-BNL CL.2 cell model. Additionally, these T cells alleviated
insulin resistance by activating the IRS-1/PI3K/AKT signaling
pathway, increasing glucose uptake in IR-BNL CL.2 cells, and
optimizing the TCA cycle and glycolysis. CD4+ T
lymphocytes in intestinal tissue serve a critical role in
PF40-mediated metabolic regulation and are key effector cells in
the action of PF40 in improving insulin resistance. Targeted
modulation of intestinal immune cell homeostasis may represent a
promising strategy for the prevention and treatment of insulin
resistance.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Bianhong Zhang
(Fujian Agriculture and Forestry University, Fuzhou, China) for
their help in data visualization of targeted energy metabolism. The
authors would also like to thank Dr Ye Liu (Fujian Agriculture and
Forestry University, Fuzhou, China) for assisting in the detection
of targeted energy metabolism.
Funding
This study was funded by the National Natural Science Foundation
of China (grant nos. 82405034, 81872994 and 81903945), a major
scientific and technological innovation project of Fujian
University of Traditional Chinese Medicine (grant no. XJB2022001),
the Key Laboratory of Traditional Chinese Medicine in Medical
Institutions of Fujian Province (Fujian University of Traditional
Chinese Medicine), the Construction Project of Quality Evaluation
System for Pseudostellaria heterophylla in Zherong County
Pharmaceutical Development Center (2024), and the Science and
Technology Innovation Special Fund of Fujian Agriculture and
Forestry University (grant no. CXZX2020037A).
Availability of data and materials
The metabolomics data generated in the present
study may be found in the OMIX, China National Center for
Bioinformation/Beijing Institute of Genomics, Chinese Academy of
Sciences database under accession number OMIX010841 or at the
following URL: https://ngdc.cncb.ac.cn/omix/release/OMIX010841.
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
YK and YL conceived and designed the experiments.
YH, LZ, JC and WP analyzed and interpreted the data. YK wrote the
manuscript. WL contributed to statistical analysis and manuscript
revision, and JH contributed to experimental design and manuscript
revision. YK and YL confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee at Fujian Academy of Chinese Medical
Sciences (Fuzhou, China; approval no. FJATCM-IAEC2020002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Garg SS, Kushwaha K, Dubey R and Gupta J:
Association between obesity, inflammation and insulin resistance:
Insights into signaling pathways and therapeutic interventions.
Diabetes Res Clin Pract. 200:1106912023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guzmán-Flores J, Ramírez-Emiliano J,
Pérez-Vázquez V and López-Briones S: Th17 and regulatory T cells in
patients with different time of progression of type 2 diabetes
mellitus. Cent Eur J Immunol. 45:29–36. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiernan K and MacIver NJ: A novel
mechanism for Th17 inflammation in human type 2 diabetes mellitus.
Trends Endocrinol Metab. 31:1–2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Gang X, Yang S, Cui M, Sun L, Li
Z and Wang G: The alterations in and the role of the Th17/Treg
balance in metabolic diseases. Front Immunol. 12:6783552021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan JB, Luo MM, Chen ZY and He BH: The
function and role of the Th17/Treg cell balance in inflammatory
bowel disease. J Immunol Res. 2020:88135582020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Z, Zhang J, Duan T, Yang J and Yang Y:
Trefoil factor 3 can stimulate Th17 cell response in the
development of type 2 diabetes mellitus. Sci Rep. 14:103402024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu DJ, Shakerian F, Zhao J and Li SP:
Chemistry, pharmacology and analysis of Pseudostellaria
heterophylla: A mini-review. Chin Med. 14:212019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu F, Yang H, Lin SD, Zhao L, Jiang C,
Chen ZB, Liu YY, Kan YJ, Hu J and Pang WS: Cyclic peptide extracts
derived from pseudostellaria heterophylla ameliorates COPD via
regulation of the TLR4/MyD88 pathway proteins. Front Pharmacol.
11:8502020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kan Y, Liu Y, Huang Y, Zhao L, Jiang C,
Zhu Y, Pang Z, Hu J, Pang W and Lin W: The regulatory effects of
Pseudostellaria heterophylla polysaccharide on immune function and
gut flora in immunosuppressed mice. Food Sci Nutr. 10:3828–3841.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu J, Pang W, Chen J, Bai S, Zheng Z and
Wu X: Hypoglycemic effect of polysaccharides with different
molecular weight of Pseudostellaria heterophylla. BMC Complement
Altern Med. 13:2672013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Kan Y, Huang Y, Jiang C, Zhao L, Hu
J and Pang W: Physicochemical characteristics and antidiabetic
properties of the polysaccharides from pseudostellaria
heterophylla. Molecules. 27:37192022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Pang W, Kan Y, Zhao L, He Z, Shi
W, Yan B, Chen H and Hu J: Structure of a pectic polysaccharide
from pseudostellaria heterophylla and stimulating insulin secretion
of INS-1 cell and distributing in rats by oral. Int J Biol
Macromol. 106:456–463. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brucklacher-Waldert V, Carr EJ, Linterman
MA and Veldhoen M: Cellular plasticity of CD4+ T cells in the
intestine. Front Immunol. 5:4882014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiménez JM, Contreras-Riquelme JS, Vidal
PM, Prado C, Bastías M, Meneses C, Martín AJM, Perez-Acle T and
Pacheco R: Identification of master regulator genes controlling
pathogenic CD4+ T cell fate in inflammatory bowel disease through
transcriptional network analysis. Sci Rep. 14:105532024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tao L, Liu H and Gong Y: Role and
mechanism of the Th17/Treg cell balance in the development and
progression of insulin resistance. Mol Cell Biochem. 459:183–188.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang M, Chen F, Wang J, Zeng Z, Yang Q and
Shao S: Th17 and Treg lymphocytes in obesity and type 2 diabetic
patients. Clin Immunol. 197:77–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Monteiro-Sepulveda M, Touch S, Mendes-Sá
C, André S, Poitou C, Allatif O, Cotillard A, Fohrer-Ting H, Hubert
EL, Remark R, et al: Jejunal T cell inflammation in human obesity
correlates with decreased enterocyte insulin signaling. Cell Metab.
22:113–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garidou L, Pomié C, Klopp P, Waget A,
Charpentier J, Aloulou M, Giry A, Serino M, Stenman L, Lahtinen S,
et al: The gut microbiota regulates intestinal CD4 T cells
expressing RORγt and controls metabolic disease. Cell Metab.
22:100–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zi C, He L, Yao H, Ren Y, He T and Gao Y:
Changes of Th17 cells, regulatory T cells, Treg/Th17, IL-17 and
IL-10 in patients with type 2 diabetes mellitus: A systematic
review and meta-analysis. Endocrine. 76:263–272. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei Y, Jing J, Peng Z, Liu X and Wang X:
Acacetin ameliorates insulin resistance in obesity mice through
regulating Treg/Th17 balance via MiR-23b-3p/NEU1 axis. BMC Endocr
Disorders. 21:572021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng Z, Wang SH, Li LY, Zhou YC and Zhang
CY: Fermented Porphyra haitanensis polysaccharides inhibit the
degranulation of mast cell and passive cutaneous anaphylaxis. Food
& Medicine Homology. 3:94200862025.
|
|
22
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai M, Xing H, Tian B, Xu J, Li Z, Zhu H,
Yang K and Sun P: Characteristics and antifatigue activity of
graded polysaccharides from Ganoderma lucidum separated by cascade
membrane technology. Carbohydr Polym. 269:1183292021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nataraj A, Govindan S, Ramani P, Subbaiah
KA, Sathianarayanan S, Venkidasamy B, Thiruvengadam M, Rebezov M,
Shariati MA, Lorenzo JM and Pateiro M: Antioxidant, anti-tumour,
and anticoagulant activities of polysaccharide from calocybe indica
(APK2). Antioxidants (Basel). 11:16942022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim E, Tran M, Sun Y and Huh JR: Isolation
and analyses of lamina propria lymphocytes from mouse intestines.
STAR Protoc. 3:1013662022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu X, Yu Q, Hou K, Ding X, Chen Y, Xie J,
Nie S and Xie M: Regulatory effects of Ganoderma atrum
polysaccharides on LPS-induced inflammatory macrophages model and
intestinal-like Caco-2/macrophages co-culture inflammation model.
Food Chem Toxicol. 140:1113212020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
da Rocha GH, Müller C, Przybylski-Wartner
S, Schaller H, Riemschneider S and Lehmann J: AhR-induced
anti-inflammatory effects on a Caco-2/THP-1 co-culture model of
intestinal inflammation are mediated by PPARγ. Int J Mol Sci.
25:130722024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Xiang D, Jin W, Zhao G, Li H, Xie B
and Gu X: Timosaponin B-II alleviates osteoarthritis-related
inflammation and extracellular matrix degradation through
inhibition of mitogen-activated protein kinases and nuclear
factor-κB pathways in vitro. Bioengineered. 13:3450–3461. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Huang S, Li S, Li X, Ling Y, Wang
X, Zhang S, Zhou D and Yin W: Rosa sterilis juice alleviated breast
cancer by triggering the mitochondrial apoptosis pathway and
suppressing the Jak2/Stat3 pathway. Nutrients. 16:27842024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan X, Tao J, Zhou Y, Hou Y, Wang Y, Gu D,
Su Y, Jang Y and Li S: Investigations on the effects of
ginsenoside-Rg1 on glucose uptake and metabolism in insulin
resistant HepG2 cells. Eur J Pharmacol. 843:277–284. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tangvarasittichai S: Oxidative stress,
insulin resistance, dyslipidemia and type 2 diabetes mellitus.
World J Diabetes. 6:456–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu L, Hu J, Wang Y, Lei H and Xu D: The
role and research progress of the balance and interaction between
regulatory T cells and other immune cells in obesity with insulin
resistance. Adipocyte. 10:66–79. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu H and Ballantyne CM: Metabolic
inflammation and insulin resistance in obesity. Circ Res.
126:1549–1564. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakurai Y, Kubota N, Yamauchi T and
Kadowaki T: Role of insulin resistance in MAFLD. Int J Mol Sci.
22:41562021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanase DM, Gosav EM, Costea CF, Ciocoiu M,
Lacatusu CM, Maranduca MA, Ouatu A and Floria M: The intricate
relationship between type 2 diabetes mellitus (T2DM), insulin
resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J
Diabetes Res. 2020:39201962020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schafer A, Neschen S, Kahle M, Sarioglu H,
Gaisbauer T, Imhof A, Adamski J, Hauck SM and Ueffing M: The
epoxyeicosatrienoic acid pathway enhances hepatic insulin signaling
and is repressed in insulin-resistant mouse liver. Mol Cell
Proteomics. 14:2764–2774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou X, Wang LL, Tang WJ and Tang B:
Astragaloside IV inhibits protein tyrosine phosphatase 1B and
improves insulin resistance in insulin-resistant HepG2 cells and
triglyceride accumulation in oleic acid (OA)-treated HepG2 cells. J
Ethnopharmacol. 268:1135562021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhong RF, Liu CJ, Hao KX, Fan XD and Jiang
JG: Polysaccharides from Flos Sophorae Immaturus ameliorates
insulin resistance in IR-HepG2 cells by co-regulating signaling
pathways of AMPK and IRS-1/PI3K/AKT. Int J Biol Macromol.
280:1360882024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang S, Wu X, Wang Y, Zou J and Zhao X:
The potential DPP-4 inhibitors from Xiao-Ke-An improve the
glucolipid metabolism via the activation of AKT/GSK-3β pathway. Eur
J Pharmacol. 882:1732722020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim S, Yoon D, Lee YH, Lee J, Kim ND, Kim
S and Jung YS: Transformation of liver cells by
3-methylcholanthrene potentiates oxidative stress via the
downregulation of glutathione synthesis. Int J Mol Med.
40:2011–2017. 2017.PubMed/NCBI
|
|
41
|
Jobgen WS and Wu G: L-Arginine increases
AMPK phosphorylation and the oxidation of energy substrates in
hepatocytes, skeletal muscle cells, and adipocytes. Amino Acids.
54:1553–1568. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ding W, Yang X, Lai K, Jiang Y and Liu Y:
The potential of therapeutic strategies targeting mitochondrial
biogenesis for the treatment of insulin resistance and type 2
diabetes mellitus. Arch Pharm Res. 47:219–248. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roden M, Petersen KF and Shulman GI:
Insulin resistance in type 2 diabetes. Textbook of Diabetes Wiley.
238–249. 2024. View Article : Google Scholar
|
|
44
|
Masenga SK, Kabwe LS, Chakulya M and
Kirabo A: Mechanisms of oxidative stress in metabolic syndrome. Int
J Mol Sci. 24:78982023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen H, Li J, Zhang Y, Zhang W, Li X, Tang
H, Liu Y, Li T, He H, Du B, et al: Bisphenol F suppresses
insulin-stimulated glucose metabolism in adipocytes by inhibiting
IRS-1/PI3K/AKT pathway. Ecotoxicol Environ Saf. 231:1132012022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao SL, Liu D, Ding LQ, Liu GK, Yao T, Wu
LL, Li G, Cao SJ, Qiu F and Kang N: Schisandra chinensis lignans
improve insulin resistance by targeting TLR4 and activating
IRS-1/PI3K/AKT and NF-κB signaling pathways. Int Immunopharmacol.
142:1130692024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang L, Guo Z, Huang M, Zeng X and Huang
H: Triiodothyronine (T3) promotes browning of white adipose through
inhibition of the PI3K/AKT signalling pathway. Sci Rep.
14:203702024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Báez AM, Ayala G, Pedroza-Saavedra A,
González-Sánchez HM and Amparan LC: Phosphorylation codes in IRS-1
and IRS-2 are associated with the activation/inhibition of insulin
canonical signaling pathways. Curr Issues Mol Biol. 46:634–649.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Woo JR, Bae SH, Wales TE, Engen JR, Lee J,
Jang H and Park S: The serine phosphorylations in the IRS-1 PIR
domain abrogate IRS-1 and IR interaction. Proc Nat Acad Sci USA.
121:e24017161212024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lien EC, Lyssiotis CA and Cantley LC:
Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer.
Metabolism in Cancer. 207:39–72. 2016. View Article : Google Scholar
|
|
52
|
Fontana F, Giannitti G, Marchesi S and
Limonta P: The PI3K/Akt pathway and glucose metabolism: A dangerous
liaison in cancer. Int J Biol Sci. 20:3113–3125. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Park J, Rho HK, Kim KH, Choe SS, Lee YS
and Kim JB: Overexpression of Glucose-6-Phosphate dehydrogenase is
associated with lipid dysregulation and insulin resistance in
obesity. Mol Cell Biol. 25:5146–5157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Martínez-Reyes I and Chandel NS:
Mitochondrial TCA cycle metabolites control physiology and disease.
Nat Commun. 11:1022020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Choi I, Son H and Baek JH: Tricarboxylic
Acid (TCA) cycle intermediates: Regulators of immune responses.
Life (Basel). 11:692021.PubMed/NCBI
|
|
56
|
Zhang X, Jia Y, Yuan Z, Wen Y, Zhang Y,
Ren J, Ji P, Yao W, Hua Y and Wei Y: Sheng Mai San ameliorated heat
stress-induced liver injury via regulating energy metabolism and
AMPK/Drp1-dependent autophagy process. Phytomedicine.
97:1539202022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tian JL, Si X, Shu C, Wang YH, Tan H, Zang
ZH, Zhang WJ, Xie X, Chen Y and Li B: Synergistic effects of
combined anthocyanin and metformin treatment for hyperglycemia in
vitro and in vivo. J Agric Food Chem. 70:1182–1195. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Namvarjah F, Shokri-Afra H,
Moradi-Sardareh H, Khorzoughi RB, Pasalar P, Panahi G and Meshkani
R: Chlorogenic acid improves anti-lipogenic activity of metformin
by positive regulating of AMPK signaling in HepG2 cells. Cell
Biochem Biophys. 80:537–545. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun L, Jiang J, Zeng Y, Zhu J, Wang S,
Huang D and Cao C: Polysaccharide NAP-3 synergistically enhances
the efficiency of metformin in type 2 diabetes via Bile Acid/GLP-1
axis through gut microbiota remodeling. J Agric Food Chem.
72:21077–21088. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Long J, Li M, Yao C, Ma W, Liu H and Yan
D: Structural characterization of Astragalus polysaccharide-D1 and
its improvement of low-dose metformin effect by enriching
Staphylococcus lentus. Int J Biol Macromol. 272:1328602024.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Toubal A, Kiaf B, Beaudoin L, Cagninacci
L, Rhimi M, Fruchet B, da Silva J, Corbett AJ, Simoni Y, Lantz O,
et al: Mucosal-associated invariant T cells promote inflammation
and intestinal dysbiosis leading to metabolic dysfunction during
obesity. Nat Commun. 11:37552020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Khan S, Luck H, Winer S and Winer DA:
Emerging concepts in intestinal immune control of obesity-related
metabolic disease. Nat Commun. 12:25982021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Riedel S, Pheiffer C, Johnson R, Louw J
and Muller CJF: Intestinal barrier function and immune homeostasis
are missing links in obesity and type 2 diabetes development. Front
Endocrinol (Lausanne). 12:8335442021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang YX, Chen XL, Zhou K, Wang LL, Zhong
YZ, Peng J, Ge BS, Ho CT and Lu CY: Fucoidan dose-dependently
alleviated hyperuricemia and modulated gut microbiota in mice. Food
& Medicine Homology. 3:94200952025.
|
|
65
|
Qin W, Wei B, Ren P, Chang Y, Xue C and
Tang Q: Fucoidan from Apostichopus japonicus enhances intestinal
barrier function and promotes intestinal immunity via regulating
the gut microbiota and tryptophan metabolism. Int J Biol Macromol.
301:1399292025. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Y, Ji W, Qin H, Chen Z, Zhou Y, Zhou
Z, Wang J and Wang K: Astragalus polysaccharides alleviate
DSS-induced ulcerative colitis in mice by restoring SCFA production
and regulating Th17/Treg cell homeostasis in a microbiota-dependent
manner. Carbohydr Polym. 349:1228292025. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang W, Yu T, Huang X, Bilotta AJ, Xu L,
Lu Y, Sun J, Pan F, Zhou J, Zhang W, et al: Intestinal
microbiota-derived short-chain fatty acids regulation of immune
cell IL-22 production and gut immunity. Nat Commun. 11:44572020.
View Article : Google Scholar : PubMed/NCBI
|