Introduction
Sepsis is a life-threatening condition associated
with multi-organ dysfunction caused by the dysregulated host
response to infection (1).
According to 2017 statistics, there were 48.9 million cases of
sepsis and 11 million cases of sepsis-related mortality worldwide,
and sepsis accounted for ~20% of all global deaths (2). Sepsis-induced multi-organ dysfunction
refers not only to peripheral organ damage, but also includes
central nervous system complications, which are associated with the
common symptom of cognitive dysfunction, which negatively affects
patient survival (3).
Sepsis-associated encephalopathy (SAE) is a type of acute brain
dysfunction caused by sepsis, which frequently causes confusion to
progress to delirium and even coma (4). In total, 70% of patients with sepsis
can develop SAE. The development of encephalopathy increases the
likelihood of mortality in patients with sepsis (5), the mechanisms underlying the
association between the two remain to be fully elucidated. Notably,
it is considered that the more severe the encephalopathy, the
higher the mortality rate (6). In
addition, 10% of sepsis survivors continue to suffer from chronic
cognitive dysfunction within 3 years after hospital discharge
(7), and SAE increases the risk of
suicide within 2 years after recovery (8). Survivors of sepsis often exhibit
long-term cognitive deficits and psychological disorders that
seriously affect their quality of life and morbidity (9). Therefore, the early recognition and
diagnosis of SAE, in addition to its effective treatment, have
important research value and social significance.
Currently, numerous pathogenic mechanisms of SAE
have been documented, including blood-brain barrier (BBB) damage,
neurotransmitter disorders, oxidative stress, neuroinflammation and
neuronal apoptosis, all of which interact in a complex manner to
aggravate cognitive impairment (10). However, due to its highly complex
pathophysiological characteristics, specific treatment regimens
remain elusive. Therefore, an in-depth study of the
pathophysiological mechanisms of SAE, and an exploration of novel
methods to effectively prevent and treat SAE have become research
hotspots in this field. Microglia-mediated neuroinflammation is a
key factor in the pathogenesis of SAE (11). Subsequently, inflammatory signals,
such as TNF-α and IL-1β, enter the brain in both neurological and
humoral manners following sepsis, resulting in the activation of
glial cells and the release of substantial quantities of
inflammatory factors such as IL-6, TNF-α and IL-1β. This in turn
leads to the further exacerbation of inflammation, disruption of
BBB integrity and neuronal cell death (12). Consequently, modulation of
microglia activity has emerged as a promising therapeutic approach
for the management of SAE.
Mitophagy is a form of selective autophagy that
removes aged or damaged mitochondria in response to a stimulus.
Mitophagy induces the clearance of damaged mitochondria through an
autophagic mechanism, thereby contributing to mitochondrial quality
control, and the maintenance of cellular and mitochondrial
homeostasis in inflammatory diseases (13,14).
Mitochondrial autophagy mainly consists of PTEN-induced kinase 1
(PINK1)/Parkin-dependent and PINK1/Parkin-independent [such as
Bcl-2-interacting protein 3 (BNIP3)/BNIP3-like (NIX) and FUN14
domain-containing protein 1] autophagic pathways (15). In sepsis, mitochondria are
particularly susceptible to damage, after which the inner
mitochondrial membrane potential is reduced, damaged mitochondria
are enclosed into autophagosomes and ultimately degraded by fusion
with lysosomes, thereby facilitating the recovery of
sepsis-affected organ function (16,17).
During the early stages of sepsis, mitophagy is enhanced, such that
mitochondrial danger-associated molecular patterns and reactive
oxygen species production is reduced, and mitochondrial mass is
controlled, thereby ameliorating sepsis-induced abnormalities
(18). Mitophagy may therefore be
a novel potential target for sepsis therapy.
Transient receptor potential vanilloid 1 (TRPV1) is
a member of the non-selective cation channel family that can be
activated by pH, noxious heat, capsaicin and vanilloid compounds
(19). TRPV1 expression is widely
distributed in the peripheral and central nervous systems (20). Capsaicin has been reported to exert
neuroprotective effects. It has been shown to reduce oxidative
stress and behavior impairment, reduce the aggregation of misfolded
proteins and improve mitochondrial function (21,22).
Capsaicin, which is the principal active ingredient in chili
peppers, has garnered attention due to its potential for the
treatment of sepsis. Capsaicin has been observed to protect against
sepsis-induced liver, lung, kidney and cardiac injury by
attenuating mitochondrial dysfunction, apoptosis and pyroptosis,
inhibiting the NF-κB pathway, suppressing oxidative stress and
inflammation, and modulating autophagy (23–26).
Capsaicin has also been demonstrated to directly inhibit the
pyruvate kinase isozymes M1/M2/lactate dehydrogenase A-mediated
Warburg effect and to reduce glycolytic metabolism in inflammatory
macrophages, thereby attenuating the excessive inflammatory
response in a septic setting (27). In the central nervous system,
targeting TRPV1-mediated autophagy may alleviate multiple
disease-related symptoms, such as Alzheimer's disease,
ischemia-reperfusion injury and Parkinson's disease (28–30).
In vitro, inhibition of TRPV1 has been shown to promote
mitophagy and regulate mitochondrial function in multiple myeloma
cell lines (31). By contrast,
capsaicin has been observed to induce mitochondrial loss and
activate mitophagy in PC12 cells in a dose-dependent manner
(32), suggesting that
TRPV1-mediated mitophagy can affect mitochondrial structure and
function. However, in vivo, the role of TRPV1-mediated
mitophagy and whether it is involved in SAE remains unknown.
Consequently, the present study aimed to investigate the potential
role of TRPV1-mediated mitophagy in SAE.
Materials and methods
Animals
Male C57BL/6 mice (age, 8–10 weeks; weight, 20–25 g)
were obtained from GemPharmatech Co., Ltd. All mice were housed in
a cage under a 12-h dark/light cycle at a room temperature of
23±1°C and 55% humidity; and all mice had free access to food and
water. Following the entrustment of the management and operation of
the animal facility to China Technology Industry Holdings
(Shenzhen) Co., Ltd., all experiments were approved by the
Committee on the Animal Research Ethics of China Technology
Industry Holdings (Shenzhen) Co., Ltd. (Shenzhen, China; approval
no. 202200105) The present study was conducted in accordance with
the ARRIVE guidelines (33). A
total of 148 C57BL/6 male mice were used in the present study and
were randomly divided into the following three experimental groups:
Sham group (no ligation and puncture; n=38), cecal ligation and
puncture (CLP) group (n=55) and CLP combined with capsaicin group
(n=55). Capsaicin (cat. no. HY-10448; MedChemExpress) was dissolved
in a solution containing 10% DMSO (v/v), 40% PEG300 (v/v), 5%
Tween-80 (v/v) and 45% saline (v/v). A subcutaneous (s.c.)
injection of capsaicin at 1 mg/kg was administered once 1 h before
CLP surgery or three times at different time points (1 h, 1 day and
2 days before CLP surgery). Mice in the sham group were injected
with an equal amount of saline vehicle. The total duration of the
animal study was 19 days. Mice were then sacrificed after the
Morris water maze (MWM) experiments, and hippocampal tissue was
removed for western blotting and immunofluorescence analyses. The
health of the mice was monitored daily throughout the experiment,
and mice were euthanized if they reached any of the following
humane endpoints: Weight loss >20%, persistent refusal to eat or
drink for >24 h, and signs of respiratory distress. The mice
were confirmed dead by an absence of pain responses, complete
cessation of cardiac and respiratory activity, and pupil
dilation.
CLP model
Mice were subjected to CLP according to a previously
described method (34). Briefly,
following anesthesia of the mice with isoflurane (2% induction,
1.5% maintenance) fur was removed from the abdomen and it was
sterilized with 75% alcohol. An incision of ~1.5 cm was then made
along the midline of the abdomen, where the cecum was isolated and
removed using blunt dissecting forceps, leaving the remaining small
and large intestines in the abdominal cavity. The procedure was
conducted with the utmost care to avoid any disruption or damage to
the mesenteric vessels. Subsequently, the cecum was ligated at a
point 50% of its length from the tip, where a 21G needle was used
to puncture the cecum at a point midway between the ligation and
the tip. The puncturing of blood vessels was avoided. Following the
removal of the needle, a minimal quantity of feces was expressed to
confirm patency. It was imperative that the cecum be repositioned
within the abdominal cavity in such a way that the feces did not
spread from the cecum to the edge of the abdominal wall. The
abdominal cavity was then closed layer by layer using sutures. To
compensate for the loss of body fluids, saline (5 ml/100 g body
weight; s.c.) was injected. Additionally, buprenorphine HCl (0.05
mg/kg body weight; s.c.) was administered for postoperative
analgesia after completion of the surgery. Thereafter, the animal
was placed back into the cage. If a mouse exhibited prolonged
lethargy and/or did not eat or drink for 24 consecutive hours, they
were evaluated and if it was determined that they would die of
sepsis, they were euthanized. Mice were euthanized by deep
anesthesia with sodium pentobarbital [120 mg/kg, intraperitoneal
(i.p.)] followed by cervical dislocation. The researchers made
every effort to minimize the number and suffering of the animals
for all experiments. Survival rates were 100% in the sham group and
~53% in the sepsis group, which is consistent with previous studies
(16,35).
Novel object recognition test
(NORT)
NORT is a tool used to assess the learning and
memory abilities of experimental animals (16). To evaluate the exploration drive of
mice towards novel objects, the animals were placed in boxes
(50×50×50 cm) within an open field experimental setup. The boxes
were made of white polyethylene walls and a white polyethylene
floor. Before and after each trial, the boxes and objects were
cleaned with ethanol to remove any residual odors or substances; it
was important to ensure that no traces of feces or odor remained
from the previous mouse. Over the course of 2 consecutive days, the
mice were allowed to acclimate to the empty boxes for 10 min each
day. Subsequently, the mice were placed in boxes containing two
different objects (A and B) for 5 min each. The number of times
each object was explored was recorded, representing the sample
phase. This process was repeated every 2 h. During this period, the
mice were returned to their cages while the objects that had been
explored the fewest times were replaced with a different object
(C). If a mouse stayed in the corner and did not explore objects,
it was removed, 3 mice in CLP group and 4 mice in CLP + capsaicin
group were removed. After 2 h, the mice were released into the
boxes and allowed to explore freely for 5 min, before the number of
times each object was explored was recorded, representing the
acquisition phase. The discrimination index was calculated as the
number of times a novel object was explored divided by the total
number of explorations.
MWM
This task was performed according to a previously
described method (36). The MWM
consisted of a circular pool (diameter, 100 cm; height, 38 cm) and
a removable circular platform (diameter, 6 cm); the pool was
divided into four quadrants with the circular platform fixed in the
third quadrant. Each quadrant had a clear pattern on the wall of
the pool and the temperature of the pool was controlled at 21±1°C.
The entire process included a training and testing phase, the
training phase was further divided into the platform visibility and
platform hiding period.
The initial stage was the platform visibility
period, which lasted for 1 day. During this stage, the platform was
situated at a height of 1 cm above the water surface. The mice were
trained four times per day, with each training session lasting for
1 min. The mice were gently guided into the water from the midpoint
of the four quadrants facing the pool wall and allowed to explore
freely for 1 min. The mice were permitted to locate the platform
and remain there for 15 sec. In the event that the mice were unable
to find the platform within 1 min, they were assisted in locating
it and were allowed to remain on it for 15 sec. At the conclusion
of the training period, the mice were dried with paper towels and
transferred to the pre-prepared warm and dry cages.
The second phase was the platform-hiding period,
which lasted for 4 days. During this period, the water surface was
maintained at a height of 1 cm above the platform, with the
remaining steps consistent with those employed during the platform
visibility period.
The third stage was the test phase, which lasted for
1 day. During this stage, the platform was removed and the mice
were placed at the midpoint of the edge of the opposite quadrant of
the pool and allowed to explore freely for 1 min. The number of
times the mice crossed the platform and the residence time in the
quadrant where the platform was located were analyzed using the
software (EthoVision XT 17.5; Noldus Information Technology
BV).
Cell culture and treatment
BV2 cells were obtained from Shenzhen University
(Shenzhen, China) and were incubated in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 2% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin, (100 U/ml; Gibco;
Thermo Fisher Scientific, Inc.). Lipopolysaccharide (LPS) was
purchased from Sigma-Aldrich; Merck KGaA. Capsaicin was dissolved
in DMSO. LPS was dissolved in PBS. BV2 cells were categorized into
three groups: Ctrl, LPS and capsaicin + LPS. The cells were
cultured in the presence or absence of LPS (1 µg/ml) for 12 h at
37°C, then further treated with capsaicin (10 µM) for 24 h at
37°C.
Immunofluorescence staining
For brain section staining, three groups (Sham, CLP
+ Veh and CLP + capsaicin) of mice (6 mice per group) were
anesthetized with sodium pentobarbital (65 mg/kg; i.p.), and
perfused transcardially with cold 4% paraformaldehyde. Next, the
whole brain was extracted, and post-fixed with 4% formaldehyde
overnight at 4°C, before being dehydrated with 30% sucrose
solution. The brain tissues were sectioned coronally at 30 µm with
a cryostat (Leica Microsystems GmbH). Subsequently, the sections
were permeabilized with 0.1% Triton X-100 in PBS and blocked with
freshly prepared blocking solution [3% donkey serum (cat. no.
SL050; Beijing Solarbio Science & Technology Co., Ltd.) and
0.2% Triton X-100 in PBS] for 1 h at room temperature, followed by
incubation with the primary antibody, anti-ionized calcium-binding
adapter molecule 1 [Iba1; cat. no. ab5076; research resource
identifier (RRID), AB_2224402; 1:500; Abcam], overnight at 4°C.
After washing with PBS, secondary antibody conjugated with Alexa
Flour 488 (cat. no. A-11055; 1:500; Thermo Fisher Scientific, Inc.)
was added for incubation for 2 h at room temperature. Slices were
counterstained with DAPI (cat. no. C1005; Beyotime Institute of
Biotechnology) for 5 min at room temperature and mounted with
Vectashield Antifade mounting medium (cat. no. H1000-10; Vector
Laboratories, Inc.). Immunofluorescence images were captured by
confocal microscopy (LSM 800; Carl Zeiss AG) and the experiments
were repeated three times. The numbers and area of soma on
microglia were quantified using ImageJ 1.54g software (National
Institutes of Health).
Western blotting
Hippocampal tissues (~30 mg) were homogenized in
RIPA buffer (Beyotime Institute of Biotechnology) containing
protease and phosphatase inhibitors (Roche Diagnostics GmbH) on ice
and stored at −80°C until use. The protein concentrations were
quantified using a BCA kit (cat. no. 23225; Thermo Fisher
Scientific, Inc.) (16).
Subsequently, 30 µg protein was separated on 7.5, 10 and 15% gels
by SDS-PAGE and transferred onto PVDF membranes (Merck KGaA). The
membranes were blocked with 5% non-fat milk for 1 h at room
temperature, before being incubated with primary antibodies
overnight at 4°C. After washing with TBS-0.1% Tween-20, the
membranes were incubated with the corresponding HRP-conjugated
secondary antibodies (anti-rabbit; cat. no. 7074S; 1:1,000;
anti-mouse; cat. no. 7076S; 1:1,000; Cell Signaling Technology,
Inc.) at room temperature for 1 h. The bands were detected with a
chemiluminescent reagent (MilliporeSigma). The experiments were
repeated three times. The following primary antibodies were used:
TRPV1 (cat. no. NB100-1617; RRID: AB_10002124; 1:1,000;
Bio-Techne), LC3B (cat. no. T55992; RRID: AB_2929010; 1:500; Abmart
Pharmaceutical Technology Co., Ltd.), p62 (cat. no. 23214; RRID:
AB_2798858; 1:500; Cell Signaling Technology, Inc.), Parkin (cat.
no. A11172; RRID: AB_2758446; 1:500; ABclonal Biotech Co., Ltd.),
PINK1 (cat. no. A7131; RRID: AB_2767686; 1:500; ABclonal Biotech
Co., Ltd.), pro-caspase 3 (cat. no. ET1608-64; RRID: AB_3069820;
1:500; HUABIO), cleaved caspase 3 (cat. no. BF0711; RRID:
AB_2846190; 1:500; Affinity Biosciences), nucleotide-binding
oligomerization domain, leucine rich repeat and CARD
domain-containing 4 (NLRC4; cat. no. A7382; RRID: AB_2767914;
1:500; ABclonal Biotech Co., Ltd.), BNIP3 (cat. no. 68091-1-Ig;
RRID: AB_2918828; 1:2,000; Proteintech Group, Inc.), NIX (cat. no.
12986-1-AP; RRID: AB_2877901; 1:1,000; Proteintech Group, Inc.),
β-actin (cat. no. 4970S; RRID: AB_2223172; 1:3,000, Cell Signaling
Technology, Inc.) and β-tubulin (cat. no. 2148S; RRID: AB_823664;
1:3,000, Cell Signaling Technology, Inc.).
ELISA
The hippocampal tissues were collected and stored at
−80°C until needed. The levels of TNF-α were measured using an
ELISA kit (cat. no. E-EL-M3063; Elabscience Bionovation Inc.)
according to the manufacturer's instructions.
Hematoxylin and eosin (H&E)
staining
A total of six mice per group were anesthetized with
sodium pentobarbital (65 mg/kg; i.p.), and perfused transcardially
with 4% paraformaldehyde in PBS resulting in mortality. Brain
tissue was extracted and post-fixed with 4% paraformaldehyde for 24
h at 4°C and embedded in paraffin. The brain tissues were then
sliced into 8-µm sections. The Hematoxylin-Eosin Stain kit (cat.
no. G1076; Wuhan Servicebio Technology Co., Ltd.) was used for
H&E staining according to the manufacturer's protocol. All
images were captured using an ECLIPSE E100 microscope (Nikon
Corporation). Hippocampal neuronal damage was evaluated by a
researcher blinded to each group, based on the following
observations (37): Grade 0, no
damage to any hippocampal subregion; grade 1, scattered neurons
were damaged in the CA1 subregion; grade 2, moderate numbers of
damaged neurons in the CA1 subregion; grade 3, severe damage to
pyramidal cells in the CA1 subregion; and grade 4, extensive cell
damage in all hippocampal regions.
Transmission electron microscopy
(TEM)
Hippocampal tissues were fixed with 2.5%
glutaraldehyde for 24 h at 4°C (16). After washing with 0.1 M PBS (pH
7.4) three times for 15 min each, the tissues were post-fixed with
1% OsO4 in 0.1 M PBS (pH 7.4) for 2 h at room temperature, followed
by dehydration with ethanol and embedding in Acetone-EMBed 812
resin for penetration for 2–4 h at 37°C. After polymerization, the
semi-thin slices were used for positioning, and then cut into
60–80-nm ultra-thin sections. The sections were then stained with
2% uranium acetate and 2.6% lead citrate for 8 min each at room
temperature before being observed under TEM (HT7800; Hitachi,
Ltd).
Autophagic flux measurements
BV2 cells were plated into 24-well plates at a
density of 1×105 cells/well and were cultured overnight.
Subsequently, the cells were infected with LC3-GFP-mCherry
lentivirus (MOI, 40; OBiO Technology (Shanghai) Corp., Ltd.) for 72
h at 37°C (38). Following a
change of medium, the virus-transfected cells were randomly divided
into the Ctrl, LPS and LPS + capsaicin groups. LPS (1 µg/ml) was
added to the LPS and LPS + capsaicin groups for 12 h. The Ctrl
groups were given the same volume of saline. The cells were
incubated with 10 µM capsaicin for 24 h and were then fixed with 4%
paraformaldehyde for 30 min at room temperature and stained with
DAPI (1 µg/ml) for 15 min at room temperature. Immunofluorescence
images were captured using a confocal microscope (LSM 800; Carl
Zeiss AG), with yellow fluorescence representing autophagosomes and
red fluorescence representing autophagolysosomes. The experiments
were repeated three times.
Statistical analysis
With the exception of the neuronal damage scores,
all data are presented as the mean ± standard deviation and
analyzed using GraphPad Prism 9.0 software (Dotmatics). Neuronal
damage scores are presented as median values with interquartile
ranges. Statistical significance was estimated using unpaired
Student's t-test or one-way ANOVA followed by Bonferroni post hoc
test. To assess neuronal damage score, Kruskal-Wallis test followed
by Dunn's multiple comparisons test was used. The Kaplan-Meier
method was used for survival analysis, and differences were
analyzed using the log-rank (Mantel-Cox) test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Capsaicin ameliorates cognitive
deficits in mice with sepsis
Initially, TRPV1 expression was measured in the
hippocampus of mice with CLP-induced sepsis, and the expression
levels of TRPV1 were significantly reduced in mice in the CLP group
compared with those in the sham group (Fig. 1A), suggesting that TRPV1 expression
in the hippocampus may be associated with CLP-induced SAE. Next,
the possible effect of capsaicin, a TRPV1 agonist, on the learning
and memory of mice with sepsis was assessed. Specifically, NORT and
MWM test were conducted to assess cognitive function. The s.c.
administration of capsaicin (1 mg/kg) 1 h before surgery
significantly improved the survival rate of mice (Fig. 1B). According to NORT, the
recognition index was significantly reduced after CLP surgery
compared with that in the sham group; however, a single application
of capsaicin did not ameliorate the recognition index compared with
that in mice from the CLP group (Fig.
1C). Consistent with the NORT results, MWM experiments
demonstrated that there was no significant difference in the time
spent in the platform quadrant between the CLP group and the sham
group. Moreover, mice in the capsaicin-treated group did not
exhibit improvements in learning and memory (Fig. 1D).
![Effects of subcutaneous CAP
administration on mouse survival rate and cognitive function after
CLP surgery. (A) Western blot analysis and semi-quantitative
analysis of TRPV1 protein expression levels in hippocampal tissues
after CLP surgery (n=6 mice/group; unpaired Student's t-test;
t(10)=3.221;
P<0.01). (B) Survival rate of sham (n=10), CLP + Veh (n=15) and
CLP + 1 mg/kg CAP (n=15) mice were monitored for 7 days. Log-rank
Mantel-Cox test, P=0.035. (C) Recognition index of the novel
objective in the novel object recognition test [Sham (n=8), CLP
(n=6) and CLP + CAP (n=7) mice; one-way ANOVA with Bonferroni post
hoc test; F(2,18) =3.670; P=0.046]. (D) Time
spent in the target quadrant in the Morris water maze test phase
[Sham (n=8), CLP (n=10) and CLP + CAP (n=12) mice]. Data are
presented as the mean ± standard deviation. *P<0.05 and
**P<0.01 vs. sham. CAP, capsaicin; CLP, cecal ligation and
puncture; TRPV1, transient receptor potential vanilloid 1; Veh,
vehicle; CAP-1*1, capsaicin 1 mg/kg * 1 application.](/article_images/mmr/32/6/mmr-32-06-13686-g00.jpg) | Figure 1.Effects of subcutaneous CAP
administration on mouse survival rate and cognitive function after
CLP surgery. (A) Western blot analysis and semi-quantitative
analysis of TRPV1 protein expression levels in hippocampal tissues
after CLP surgery (n=6 mice/group; unpaired Student's t-test;
t(10)=3.221;
P<0.01). (B) Survival rate of sham (n=10), CLP + Veh (n=15) and
CLP + 1 mg/kg CAP (n=15) mice were monitored for 7 days. Log-rank
Mantel-Cox test, P=0.035. (C) Recognition index of the novel
objective in the novel object recognition test [Sham (n=8), CLP
(n=6) and CLP + CAP (n=7) mice; one-way ANOVA with Bonferroni post
hoc test; F(2,18) =3.670; P=0.046]. (D) Time
spent in the target quadrant in the Morris water maze test phase
[Sham (n=8), CLP (n=10) and CLP + CAP (n=12) mice]. Data are
presented as the mean ± standard deviation. *P<0.05 and
**P<0.01 vs. sham. CAP, capsaicin; CLP, cecal ligation and
puncture; TRPV1, transient receptor potential vanilloid 1; Veh,
vehicle; CAP-1*1, capsaicin 1 mg/kg * 1 application. |
The effects of s.c. capsaicin administration for 2
days, 1 day and 1 h before CLP surgery on at 1 mg/kg were then
assessed on cognitive and memory deficits by NORT and MWM
experiments. Similar with single administration, 3 days of
application also ameliorated cognitive deficits in mice with
sepsis. Specifically, the results of NORT indicated that mice with
sepsis exhibited a diminished recognition index compared with those
in the sham group, whereas capsaicin application significantly
reversed this in the CLP mouse model (Fig. 2A). Mice in the CLP group also had a
longer escape latency compared with those in the sham group during
the training phase of MWM (Fig. 2B and
D). However, the mice that underwent CLP and were treated with
capsaicin displayed a significantly reduced escape latency compared
with that in the CLP group. In addition, during the testing phase
of MWM, mice in the CLP group spent less time in the target
quadrant, whereas capsaicin treatment markedly increased the time
in this quadrant (Fig. 2C and D).
Notably, there was no significant difference in the swim speed
among the three treatment groups (Fig.
2E). Taken together, these results suggested that longer-term
capsaicin application may ameliorate learning and memory deficits
in mice with sepsis, and this dose (1 mg/kg; 3 times) was
subsequently used in the following studies.
![CAP-induced protection in mice with
sepsis. (A) Recognition index of the novel object in the novel
object recognition test [Sham (n=10), CLP (n=12) and CLP + CAP
(n=11) mice; n=10-12 mice/group; one-way ANOVA with Bonferroni post
hoc test; F(2,30)=12.45; P=0.0001]. (B) Escape
latency in the MWM during the training phase [Sham (n=10), CLP
(n=15) and CLP + CAP (n=14) mice]. (C) Time spent in the target
quadrant in the MWM during the test phase (one-way ANOVA with
Bonferroni post hoc test; F(2,35)=8.925; P=0.0007). (D)
Representative swimming traces in the MWM during the learning and
test phase. (E) Average swimming speed in the MWM during the test
phase. Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and ***P<0.001. CAP, capsaicin; CLP,
cecal ligation and puncture; MWM, Morris water maze; Veh, vehicle;
CAP-1*3, capsaicin 1 mg/kg * 3 application.](/article_images/mmr/32/6/mmr-32-06-13686-g01.jpg) | Figure 2.CAP-induced protection in mice with
sepsis. (A) Recognition index of the novel object in the novel
object recognition test [Sham (n=10), CLP (n=12) and CLP + CAP
(n=11) mice; n=10-12 mice/group; one-way ANOVA with Bonferroni post
hoc test; F(2,30)=12.45; P=0.0001]. (B) Escape
latency in the MWM during the training phase [Sham (n=10), CLP
(n=15) and CLP + CAP (n=14) mice]. (C) Time spent in the target
quadrant in the MWM during the test phase (one-way ANOVA with
Bonferroni post hoc test; F(2,35)=8.925; P=0.0007). (D)
Representative swimming traces in the MWM during the learning and
test phase. (E) Average swimming speed in the MWM during the test
phase. Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and ***P<0.001. CAP, capsaicin; CLP,
cecal ligation and puncture; MWM, Morris water maze; Veh, vehicle;
CAP-1*3, capsaicin 1 mg/kg * 3 application. |
Capsaicin application suppresses
neuroinflammation and neuronal apoptosis in mice following CLP
Subsequently, the extent of microglial activation
was assessed. Immunofluorescence staining revealed a notable
activation of microglia in the mouse hippocampal tissue following
CLP surgery (Fig. 3A-C). The
number of Iba1-positive microglia was significantly increased
compared with that in the sham group, whereas this was reversed by
capsaicin application. The levels of TNF-α were detected by ELISA
and were revealed to be elevated in mice with sepsis compared with
those in the sham group mice; however, this was significantly
reversed by capsaicin treatment (Fig.
3D). In addition, the protein expression levels of NLRC4 in the
hippocampus were detected and were significantly increased in mice
in the CLP group compared with those in the sham-operated group;
however, capsaicin treatment reversed this increase (Fig. 3E and F).
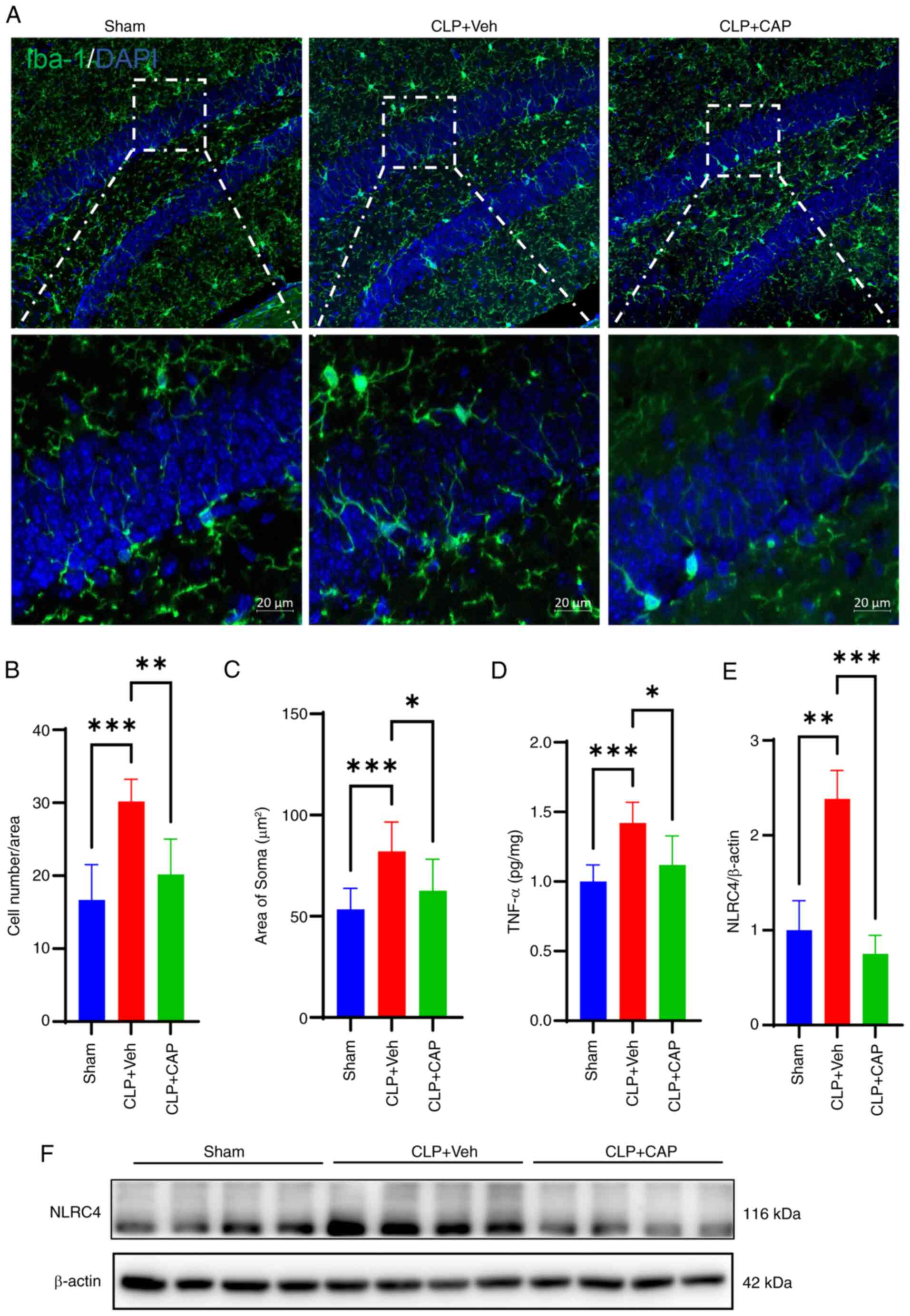 | Figure 3.CAP inhibits CLP-induced microglia
activation and level of pro-inflammatory factors. (A)
Immunofluorescence images of Iba1 (green) fluorescent signals.
Scale bars, 10 µm. (B) Quantification of microglia cell numbers
(n=6 mice/group) (one-way ANOVA with Bonferroni post hoc test;
F(2,15)=15.72; P=0.0002). (C)
Quantification of the microglial cell area (n=6 mice/group)
(one-way ANOVA with Bonferroni post hoc test; F(2,24)=10.48; P=0.0005). (D) Levels of
TNF-α in the hippocampus (n=6 mice/group) (one-way ANOVA with
Bonferroni post hoc test; F(2,15)=10.73; P=0.0013). (E) Relative
density of NLRC4 expression. (n=8 mice/group) (one-way ANOVA with
Bonferroni post hoc test; F(2,21)=10.37; P=0.0007). (F) NLRC4
expression was detected by western blotting. Data are presented as
the mean ± standard deviation. *P<0.05, **P<0.01 and
***P<0.001. CAP, capsaicin; CLP, cecal ligation and puncture;
Iba1, ionized calcium-binding adapter molecule 1; NLRC4,
nucleotide-binding oligomerization domain, leucine rich repeat and
CARD domain-containing 4; Veh, vehicle. |
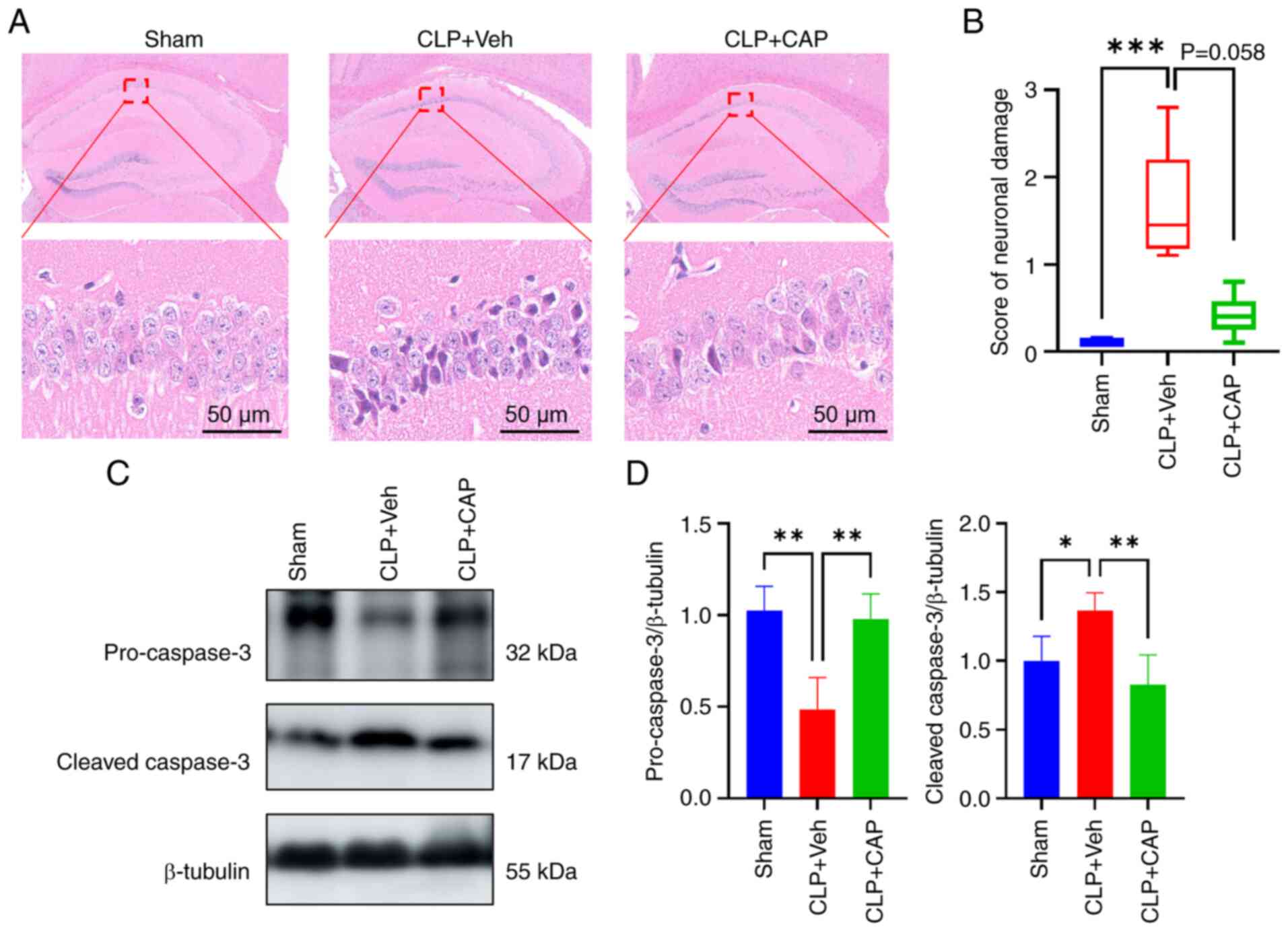
To detect the effects of capsaicin on sepsis-induced
neuronal damage, H&E staining was performed. The cells in the
CA1 region of the hippocampus of mice in the sham-operated group
exhibited intact and regular morphology, with clearly delineated
boundaries (Fig. 4A). However, the
cells in the CLP mice exhibited atrophy and profound staining.
Administration of capsaicin resulted in a reduction in the number
of abnormal neurons and a decrease in neuronal damage scores
(Fig. 4A and B). Next, the protein
levels of pro-caspase 3 and cleaved caspase 3 were examined. The
expression levels of pro-caspase 3 and cleaved caspase 3 were
markedly reduced and enhanced in in the CLP group, respectively,
when compared with those in the sham group; however these findings
were markedly reserved by capsaicin application (Fig. 4C and D).
Capsaicin application promotes
mitophagy through activating BNIP3/NIX-mediated mitophagy
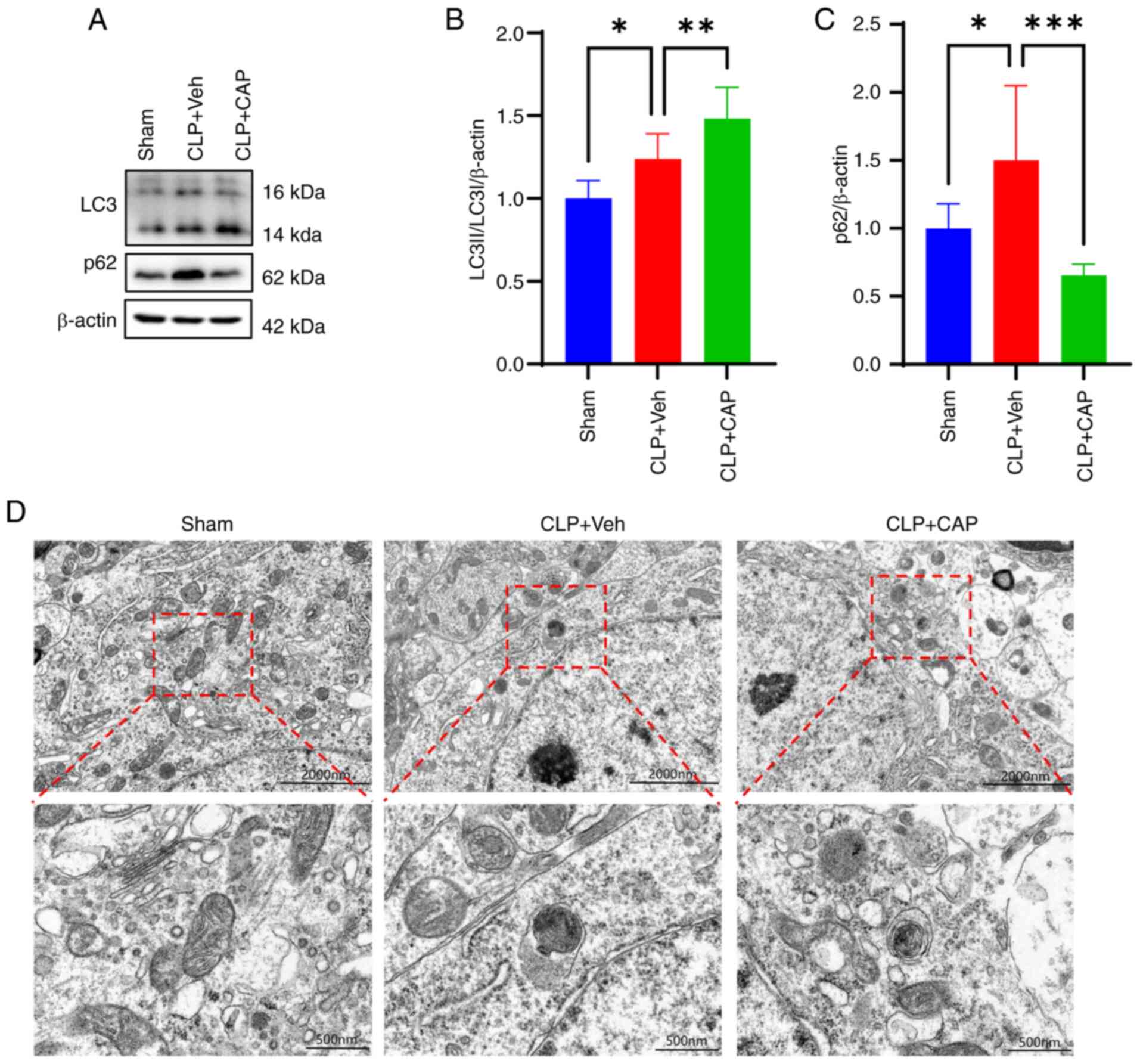
The expression levels of LC3 and p62 were next
assessed by western blotting. The results showed that the ratio of
LC3-II/LC3-I and p62 expression were significantly increased in the
CLP group compared with those in the sham group, whereas capsaicin
treatment markedly enhanced the ratio of LC3-II/LC3-I and decreased
p62 expression (Fig. 5A-C). TEM
was next used to further investigate the degree of mitophagy,
which. showed that the number of autophagic vesicles was increased
in the mitochondria of the CLP group, whereas the number of
autophagic vesicles was further increased in the CLP + capsaicin
group compared with that in the CLP group (Fig. 5D).
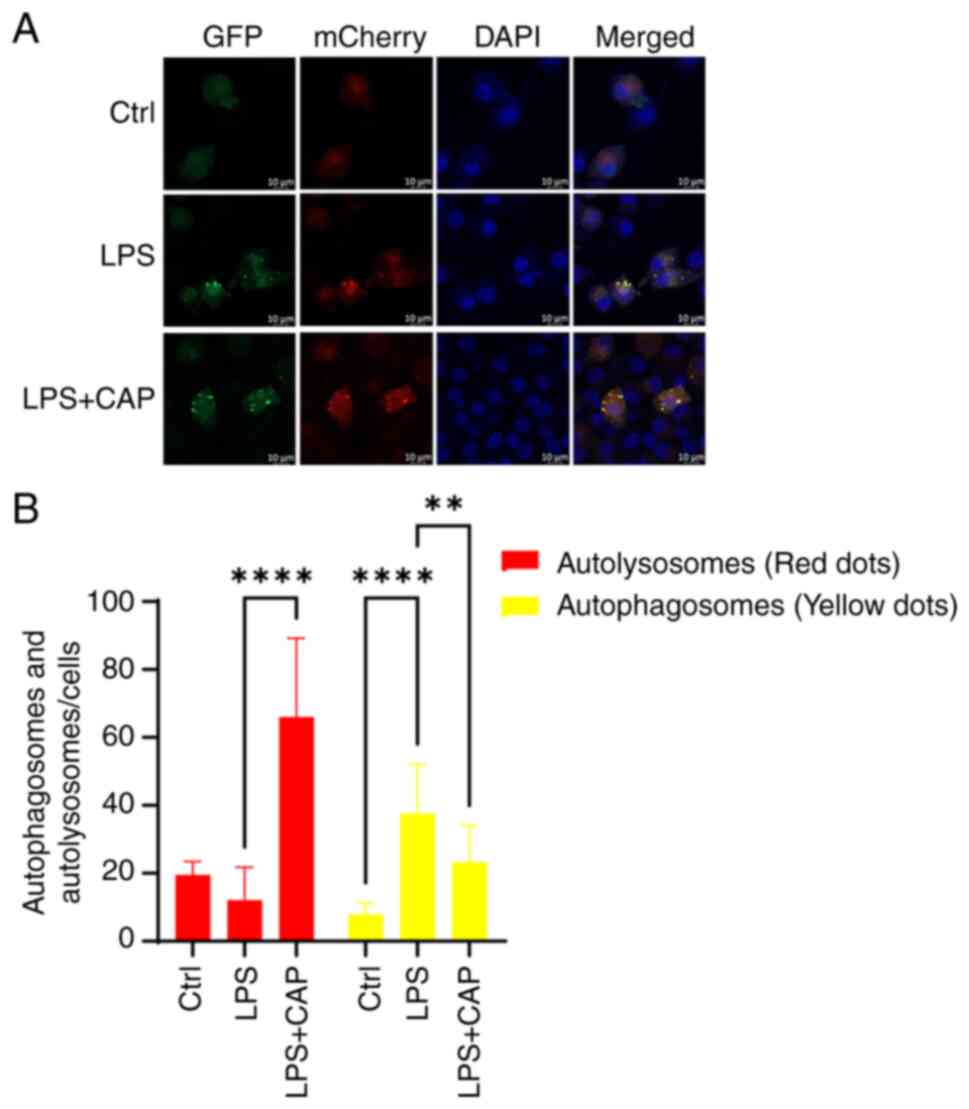
The observed increase in both p62 expression, an
autophagy substrate, and LC3-II induced by CLP indicates a blockage
in autophagic flux. Therefore, autophagic flux was next measured
using the LC3-GFP-mCherry fluorescent reporter method in
vitro. As shown in Fig. 6, the
relative quantities of yellow puncta were significantly increased
in the LPS group, indicating that autophagic flux was blocked;
however, in the LPS + capsaicin group, the red puncta were markedly
increased, suggesting that autophagic flux was activated. Taken
together, these findings suggested that capsaicin application may
promote mitophagy.
To provide further insights into the molecular
mechanisms through which capsaicin can promote mitophagy, the
expression levels of proteins associated with this process were
assessed. The results revealed that there were no significant
changes in the expression levels of PINK1 and Parkin (Fig. 7A-C), whereas BNIP3 and NIX
expression were significantly increased after CLP surgery compared
with that in the sham group (Fig. 7A,
D and E). Notably, capsaicin application further enhanced the
expression levels of BNIP3 and NIX induced by CLP. These findings
suggested that capsaicin acted through the BNIP3/NIX signaling
pathway to activate mitophagy in CLP-induced mice.
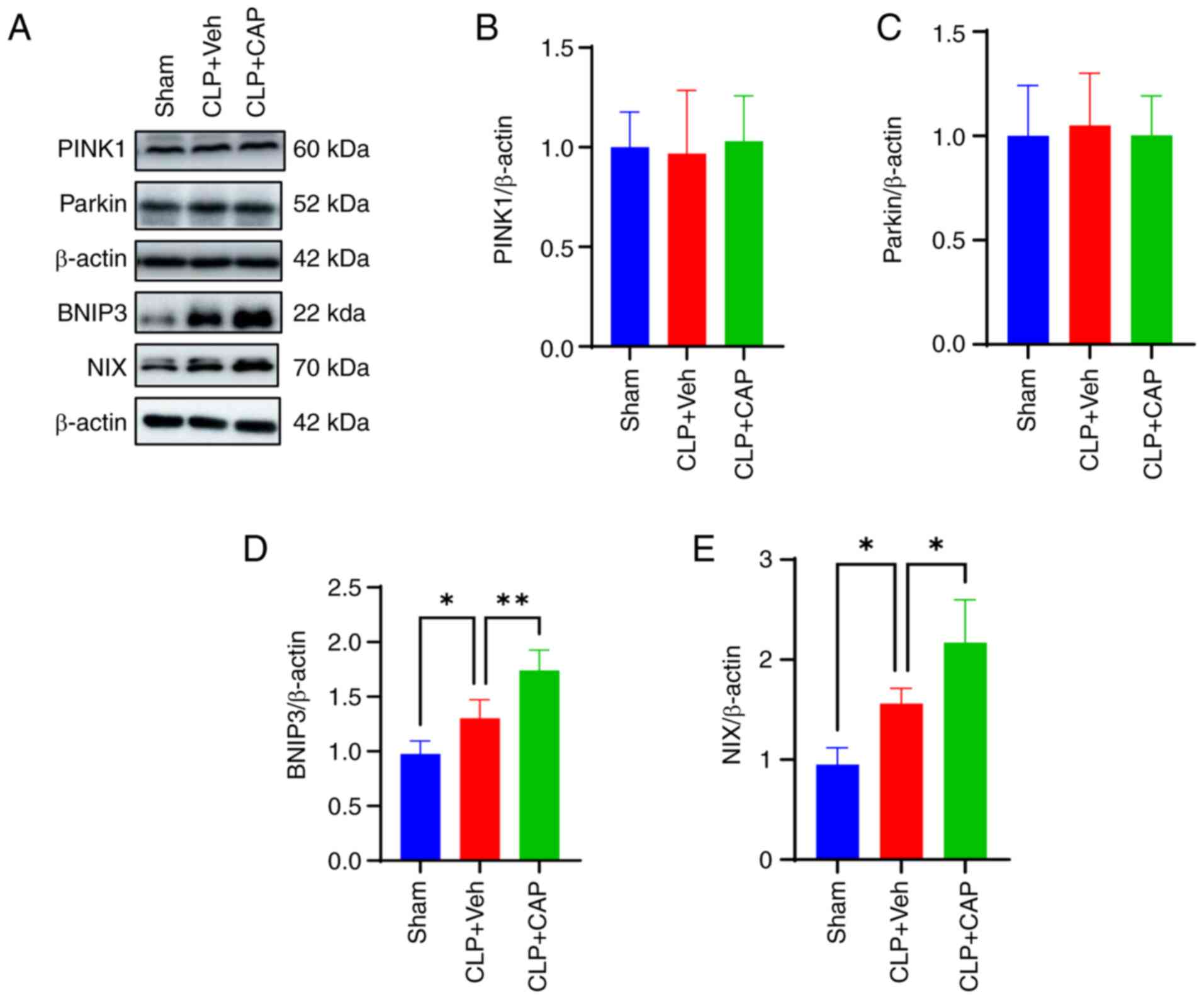 | Figure 7.Capsaicin promotes mitophagy via the
BNIP3/NIX pathway. (A) PINK1, Parkin, BNIP3 and NIX proteins were
detected by western blotting. Relative density of (B) PINK1, (C)
Parkin, (D) BNIP3 and (E) NIX expression; n=4 mice/group. (one-way
ANOVA with Bonferroni post hoc test; BNIP3: F(2,9)=22.79; P=0.0003; NIX:
F(2,9)=22; P=0.0003). Data are presented
as the mean ± standard deviation. *P<0.05 and **P<0.01.
BNIP3, Bcl-2-interacting protein 3; CAP, capsaicin; CLP, cecal
ligation and puncture; NIX, BNIP3-like; PINK1, PTEN-induced kinase
1; Veh, vehicle. |
Discussion
The present study investigated the role of capsaicin
in SAE. Initially, the expression levels of TRPV1 in mice following
CLP were shown to be significantly increased. Subsequently, s.c.
injection of capsaicin at an appropriate dose (1 mg/kg; 3 times),
revealed that a single injection was ineffective. This study
demonstrated that three doses, however, significantly improved
survival rate, alleviated cognitive dysfunction and promoted
hippocampal mitophagy, by increasing autophagic flux. In addition,
hippocampal microglia were observed to be activated in mice with
SAE (assumed when mice exhibited cognitive impairment in behavioral
tests), whereas capsaicin administration reduced microglial
activation, release of the proinflammatory cytokine TNF-α,
expression of the apoptosis-related protein cleaved caspase 3 and
brain tissue destruction, thereby exerting a neuroprotective effect
overall. Therefore, capsaicin may protect against SAE by regulating
BNIP3/NIX-mediated mitophagy. To the best of our knowledge, the
present study was the first to reveal the neuroprotective effects
of capsaicin against SAE.
The development of multiorgan dysfunction is the
predominant clinical event in sepsis, because of its association
with patient morbidity and mortality. Sepsis-induced brain
dysfunction is a prevalent condition that manifests in the early
stages (39). Sepsis has been
demonstrated to result in long-term cognitive or emotional
impairment, in addition to an elevated risk of mortality (9,40).
The CLP mouse model used in the present study has been shown to
mimic cognitive dysfunction in previous studies (16,41,42).
The presence of long-term cognitive impairment, induced by sepsis,
has been observed to be associated with neuroinflammation and
pathological tissue changes (43).
In the present study, neuroinflammation, apoptosis and brain
destruction in the hippocampal brain region were detected, which is
consistent with previous studies (44,45).
Previous studies have largely focused on the effects
of antagonists, agonists or TRPV1 gene manipulation in the context
of sepsis (46,47). However, to the best of our
knowledge, only a limited number of studies have examined TRPV1
expression in peripheral tissues or cells, and there is also a
paucity of data regarding the expression and function of TRPV1 in
the brain. In the present study, TRPV1 expression was shown to be
reduced in the hippocampal tissues of mice with sepsis. In previous
studies, capsaicin treatment was reported to confer a protective
effect against unfavorable outcomes associated with sepsis
(23,48). In the present study, capsaicin was
shown to markedly improve the survival rate and alleviate cognitive
deficits in mice with sepsis. A comparable protective impact of
capsaicin was previously documented in studies examining the
effects of LPS or CLP-induced multi-organ damage (26,27,49).
Therefore, the present findings provide support for the
consideration of capsaicin as a promising therapeutic agent for
SAE.
Mitochondrial dysfunction serves an important role
in SAE pathogenesis (50). A
previous study has shown that mitochondria are damaged in SAE, with
the release of large quantities of reactive oxygen species
(16). Mitophagy removes damaged
mitochondria and maintains mitochondrial mass. Dysfunctional
mitophagy has been proposed to lead to the accumulation of
pathogenic proteins, which in turn induce neurological diseases
(51). In the present study, the
expression levels of mitophagy-related proteins, including LC3 and
p62, were detected in mice following CLP. LC3 expression was
revealed to be upregulated by SAE induction and p62 expression was
also upregulated, suggesting that autophagy was disturbed. The
occurrence of mitophagy was supported by observations from TEM.
Under normal conditions, the autophagy receptor p62 is
ubiquitinated and subsequently degraded by lysosomes; however, when
autophagic flow is blocked, p62 levels increase (52). In the present study, the autophagic
flux was monitored using the LC3-GFP-mCherry fluorescent reporter
method, which found that the red dots (autolysosomes) were markedly
increased after capsaicin treatment, suggesting that capsaicin can
promote mitophagy in vivo. The present findings
substantiated the impact of capsaicin on mitophagy through in
vitro cell line investigations.
Mitophagy is mediated by the PINK1/Parkin signaling
pathway or mitophagic receptors (53). To date, the majority of previous
studies have centered on PINK1/Parkin-mediated mitophagy and its
role in the clearance of depolarized mitochondria (18,54).
The present findings indicated that the PINK1/Parkin pathway was
not implicated in the protective effects of capsaicin on SAE.
Instead, BNIP/NIX-mediated mitophagy more likely served the
important role in this process. At present, it remains unclear
whether capsaicin can regulate BNIP3/NIX through TRPV1 channels. In
addition, to the best of our knowledge, no studies have explored
the potential interaction between TRPV1 and BNIP3/NIX. Therefore,
it is necessary to further study the component immediately
downstream of capsaicin.
Notably, there are limitations in the present study
that must be acknowledged. The present study does not exclude the
notion that capsaicin can exert an ameliorative effect on SAE
through TRPV1 in a non-dependent manner. Therefore, TRPV1 knockout
mice should be used in the future. The present study also
exclusively utilized 8–10-week-old male mice, neglecting the
potential variations in sex and age. Previous studies have
demonstrated that male patients afflicted with sepsis exhibit an
elevated mortality rate, whereas individuals aged ≥65 years also
demonstrate a 13× increased likelihood of developing sepsis and
encountering a higher mortality rate (55,56).
Consequently, it is imperative that future studies take into
account the effects of both sex and age on the outcomes under
investigation. In addition, in the present study, the mechanism
through which capsaicin can promote the BNIP3/NIX pathway was not
explored. In the future, the interaction between TRPV1 and
BNIP3/NIX or the binding domains in which capsaicin acts with
BNIP3/NIX should be investigated.
In conclusion, the present study highlighted the
role of capsaicin in SAE. Capsaicin application could effectively
improve the survival rate of septic mice, improved cognitive
function, reduced neuroinflammation and apoptosis, and promoted
mitophagy. The mechanism of protection may involve
BNIP3-NIX-mediated mitophagy. Capsaicin may be as a novel treatment
strategy for SAE.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science, Technology &
Innovation Project of Xiongan New Area (grant no. 2023XAGG0070),
the National Natural Science Foundation of China (grant no.
82001138), the Guangdong Basic and Applied Basic Research
Foundation (grant nos. 2023A1515011649 and 2025A1515012639) and the
Sanming Project of Medicine in Shenzhen (grant no.
SZSM202211007).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YLi performed and analyzed the study. YLi and SZ
constructed figures. NL and SZ performed the experiments. HW
contributed to the establishment of the sepsis model. HW and SZ
performed and analyzed the western blotting and ELISA. JC and YJ
contributed to cell culture. SZ performed the immunofluorescence
staining. LX and HL analyzed the data. YLiu and SZ wrote the paper.
YLi, QX, YLu, BY and ZL designed the study. YLi and ZL supervised
the research. NL and SZ confirm the authenticity of all the raw
data. YLi, QX and ZL acquired the funding. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal study was reviewed and approved by the
Committee on the Animal Research Ethics of the China Technology
Industry Holdings (Shenzhen) Co., Ltd. (approval no.
202200105).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu D, Sheeja Prabhakaran H, Zhang YY, Luo
G, He W and Liou YC: Mitochondrial dysfunction in sepsis:
Mechanisms and therapeutic perspectives. Crit Care. 28:2922024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990–2017: Analysis for the Global Burden of Disease
Study. Lancet. 395:200–211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borges A and Bento L: Organ crosstalk and
dysfunction in sepsis. Ann Intensive Care. 14:1472024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slooter AJC, Otte WM, Devlin JW, Arora RC,
Bleck TP, Claassen J, Duprey MS, Ely EW, Kaplan PW, Latronico N, et
al: Updated nomenclature of delirium and acute encephalopathy:
Statement of ten Societies. Intensive Care Med. 46:1020–1022. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mazeraud A, Righy C, Bouchereau E,
Benghanem S, Bozza FA and Sharshar T: Septic-associated
encephalopathy: A comprehensive review. Neurotherapeutics.
17:392–403. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sonneville R, Benghanem S, Jeantin L, de
Montmollin E, Doman M, Gaudemer A, Thy M and Timsit JF: The
spectrum of Sepsis-associated encephalopathy: A clinical
perspective. Crit Care. 27:3862023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Widmann CN and Heneka MT: Long-term
cerebral consequences of sepsis. Lancet Neurol. 13:630–636. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lund-Sorensen H, Benros ME, Madsen T,
Sørensen HJ, Eaton WW, Postolache TT, Nordentoft M and Erlangsen A:
A nationwide cohort study of the association between
hospitalization with infection and risk of death by suicide. JAMA
Psychiatry. 73:912–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li D, Zhang X, Lu Y, Jing L, Hu H, Song Y,
Wu S and Zhu W: Post-sepsis psychiatric disorder: Pathophysiology,
prevention, and treatment. Neurol Sci. 45:3093–3105. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Bai L, Tang W, Yang W and Sun L:
Research progress in the pathogenesis of sepsis-associated
encephalopathy. Heliyon. 10:e334582024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Denver P and Cunningham C: Microglial
activation and neuroinflammation in acute and chronic cognitive
deficits in sepsis. Neuropharmacology. 267:1102852025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu YX, Yu Y, Liu JP, Liu WJ, Cao Y, Yan
RM and Yao YM: Neuroimmune regulation in Sepsis-associated
encephalopathy: The interaction between the brain and peripheral
immunity. Front Neurol. 13:8924802022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashrafi G and Schwarz TL: The pathways of
mitophagy for quality control and clearance of mitochondria. Cell
Death Differ. 20:31–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma K, Chen G, Li W, Kepp O, Zhu Y and Chen
Q: Mitophagy, mitochondrial homeostasis, and cell fate. Front Cell
Dev Biol. 8:4672020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Picca A, Faitg J, Auwerx J, Ferrucci L and
D'Amico D: Mitophagy in human health, ageing and disease. Nat
Metab. 5:2047–2061. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Yang H, Luo N, Fu Y, Qiu F, Pan Z,
Li X, Jian W, Yang X, Xue Q, et al: An Fgr kinase inhibitor
attenuates sepsis-associated encephalopathy by ameliorating
mitochondrial dysfunction, oxidative stress, and neuroinflammation
via the SIRT1/PGC-1α signaling pathway. J Transl Med. 21:4862023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan P, Wang X and Liu D: The potential
mechanism of mitochondrial dysfunction in septic cardiomyopathy. J
Int Med Res. 46:2157–2169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu CL, Yao RQ, Li LX, Li P, Xie J, Wang
JF and Deng XM: Mechanism of mitophagy and its role in sepsis
induced organ dysfunction: A review. Front Cell Dev Biol.
9:6648962021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maximiano TKE, Carneiro JA, Fattori V and
Verri WA: TRPV1: Receptor structure, activation, modulation and
role in neuro-immune interactions and pain. Cell Calcium.
119:1028702024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
In: TRP Ion Channel Function in Sensory
Transduction and Cellular Signaling Cascades. Liedtke WB and Heller
S: CRC Press/Taylor & Francis; Boca Raton, FL: 2007
|
|
21
|
Tyagi S, Shekhar N and Thakur AK:
Protective role of capsaicin in neurological disorders: An
overview. Neurochem Res. 47:1513–1531. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pasierski M and Szulczyk B: Beneficial
effects of capsaicin in disorders of the central nervous system.
Molecules. 27:24842022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghorbanpour A, Salari S,
Baluchnejadmojarad T and Roghani M: Capsaicin protects against
septic acute liver injury by attenuation of apoptosis and
mitochondrial dysfunction. Heliyon. 9:e142052023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han J, Wu J, Liu H, Huang Y, Ju W, Xing Y,
Zhang X and Yang J: Inhibition of pyroptosis and apoptosis by
capsaicin protects against LPS-induced acute kidney injury through
TRPV1/UCP2 axis in vitro. Open Life Sci. 18:202206472023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiao Y, Wang L, Hu T, Yin D, He H and He
M: Capsaicin protects cardiomyocytes against
lipopolysaccharide-induced damage via 14-3-3gamma-mediated
autophagy augmentation. Front Pharmacol. 12:6590152021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang R, Li Q, Wu P, Ren K, Li Y, Wang Y,
Zhu H and Lv C: Fe-capsaicin nanozymes attenuate Sepsis-induced
acute lung injury via NF-κB signaling. Int J Nanomedicine.
19:73–90. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Q, Luo P, Xia F, Tang H, Chen J,
Zhang J, Liu D, Zhu Y, Liu Y, Gu L, et al: Capsaicin ameliorates
inflammation in a TRPV1-independent mechanism by inhibiting
PKM2-LDHA-mediated Warburg effect in sepsis. Cell Chem Biol.
29:1248–1259.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan J, Liu H, Zhang H, Wang T, Zheng Q
and Li Z: Controlled activation of TRPV1 Channels on microglia to
boost their autophagy for clearance of Alpha-synuclein and enhance
therapy of Parkinson's disease. Adv Mater. 34:e21084352022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin Y, Huang T, Shen W, Pang Q, Xie Q,
Chen X and Tu F: TRPV1 suppressed NLRP3 through regulating
autophagy in microglia after Ischemia-reperfusion injury. J Mol
Neurosci. 72:792–801. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang C, Huang W, Lu J, Chen H and Yu Z:
TRPV1-mediated microglial autophagy attenuates Alzheimer's
Disease-associated pathology and cognitive decline. Front
Pharmacol. 12:7638662021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beider K, Rosenberg E, Dimenshtein-Voevoda
V, Sirovsky Y, Vladimirsky J, Magen H, Ostrovsky O, Shimoni A,
Bromberg Z, Weiss L, et al: Blocking of transient receptor
potential vanilloid 1 (TRPV1) promotes terminal mitophagy in
multiple myeloma, disturbing calcium homeostasis and targeting
ubiquitin pathway and bortezomib-induced unfolded protein response.
J Hematol Oncol. 13:1582020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shibata M, Kayama Y, Takizawa T, Ibata K,
Shimizu T, Yuzaki M, Suzuki N and Nakahara J: Resilience to
capsaicin-induced mitochondrial damage in trigeminal ganglion
neurons. Mol Pain. 16:17448069209608562020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Biol. 18:e30004102020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu J, Zhang M, Hao S, Jia M, Ji M, Qiu L,
Sun X, Yang J and Li K: Mitochondria-targeted peptide reverses
mitochondrial dysfunction and cognitive deficits in
Sepsis-Associated encephalopathy. Mol Neurobiol. 52:783–791. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kelliny S, Lin L, Deng I, Xiong J, Zhou F,
Al-Hawwas M, Bobrovskaya L and Zhou XF: A new approach to model
sporadic alzheimer's disease by intracerebroventricular
streptozotocin injection in APP/PS1 mice. Mol Neurobiol.
58:3692–3711. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawase M, Murakami K, Fujimura M,
Morita-Fujimura Y, Gasche Y, Kondo T, Scott RW and Chan PH:
Exacerbation of delayed cell injury after transient global ischemia
in mutant mice with CuZn superoxide dismutase deficiency. Stroke.
30:1962–1968. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gofton TE and Young GB: Sepsis-associated
encephalopathy. Nat Rev Neurol. 8:557–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sonneville R, de Montmollin E, Poujade J,
Garrouste-Orgeas M, Souweine B, Darmon M, Mariotte E, Argaud L,
Barbier F, Goldgran-Toledano D, et al: Potentially modifiable
factors contributing to sepsis-associated encephalopathy. Intensive
Care Med. 43:1075–1084. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xin Y, Tian M, Pei X, Deng S, Wang Y, Zhao
F, Behnisch T, Gao Y and Gong Y: Optimized mouse model of
Sepsis-associated encephalopathy: A rational standard based on
modified SHIRPA score and neurobehaviors in mice. CNS Neurosci
Ther. 31:e703652025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liao H, Li H, Bao H, Jiang L, Du J, Guo Y
and Si Y: Short chain fatty acids protect the cognitive function of
sepsis associated encephalopathy mice via GPR43. Front Neurol.
13:9094362022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sekino N, Selim M and Shehadah A:
Sepsis-associated brain injury: Underlying mechanisms and potential
therapeutic strategies for acute and long-term cognitive
impairments. J Neuroinflammation. 19:1012022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu XE, Liu L, Wang YC, Wang CT, Zheng Q,
Liu QX, Li ZF, Bai XJ and Liu XH: Caspase-1 inhibitor exerts
brain-protective effects against sepsis-associated encephalopathy
and cognitive impairments in a mouse model of sepsis. Brain Behav
Immun. 80:859–870. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tian M, Wang W, Wang K, Jin P, Lenahan C,
Wang Y, Tan J, Wen H, Deng S, Zhao F and Gong Y: Dexmedetomidine
alleviates cognitive impairment by reducing Blood-brain barrier
interruption and neuroinflammation via regulating Th1/Th2/Th17
polarization in an experimental sepsis model of mice. Int
Immunopharmacol. 101:1083322021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bodkin JV and Fernandes ES: TRPV1 and SP:
Key elements for sepsis outcome? Br J Pharmacol. 170:1279–1292.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bryant P, Shumate M, Yumet G, Lang CH,
Vary TC and Cooney RN: Capsaicin-sensitive nerves regulate the
metabolic response to abdominal sepsis. J Surg Res. 112:152–161.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Q, Liu J, Shen J, Ou J, Wong YK, Xie
L, Huang J, Zhang C, Fu C, Chen J, et al: Single-cell RNA
sequencing reveals the effects of capsaicin in the treatment of
sepsis-induced liver injury. MedComm (2020). 4:e3952023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen H, Li N, Zhan X, Zheng T, Huang X,
Chen Q, Song Z, Yang F, Nie H, Zhang Y, et al: Capsaicin protects
against lipopolysaccharide-induced acute lung injury through the
HMGB1/NF-κB and PI3K/AKT/mTOR pathways. J Inflamm Res.
14:5291–5304. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Haileselassie B, Joshi AU, Minhas PS,
Mukherjee R, Andreasson KI and Mochly-Rosen D: Mitochondrial
dysfunction mediated through dynamin-related protein 1 (Drp1)
propagates impairment in blood brain barrier in septic
encephalopathy. J Neuroinflammation. 17:362020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang L, Dai L and Li D: Mitophagy in
neurological disorders. J Neuroinflammation. 18:2972021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Klionsky DJ, Abdel-Aziz AK, Abdelfatah S,
Abdellatif M, Abdoli A, Abe S, Abeliovich H, Abildgaard MH,
Princely Abudu Y, Acevedo-Arozena A, et al: Guidelines for the use
and interpretation of assays for monitoring autophagy (4th
edition)1. Autophagy. 17:1–382. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang S, Long H, Hou L, Feng B, Ma Z, Wu Y,
Zeng Y, Cai J, Zhang DW and Zhao G: The mitophagy pathway and its
implications in human diseases. Signal Transduct Target Ther.
8:3042023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cui Y, Liu J, Song Y, Chen C, Shen Y and
Xie K: High Concentration hydrogen protects Sepsis-associated
encephalopathy by enhancing pink1/Parkin-Mediated mitophagy and
inhibiting cGAS-STING-IRF3 pathway. CNS Neurosci Ther.
31:e703052025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Min SY, Yong HJ and Kim D: Sex or gender
differences in treatment outcomes of sepsis and septic shock. Acute
Crit Care. 39:207–213. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nasa P, Juneja D and Singh O: Severe
sepsis and septic shock in the elderly: An overview. World J Crit
Care Med. 1:23–30. 2012. View Article : Google Scholar : PubMed/NCBI
|















![Effects of subcutaneous CAP
administration on mouse survival rate and cognitive function after
CLP surgery. (A) Western blot analysis and semi-quantitative
analysis of TRPV1 protein expression levels in hippocampal tissues
after CLP surgery (n=6 mice/group; unpaired Student's t-test;
t(10)=3.221;
P<0.01). (B) Survival rate of sham (n=10), CLP + Veh (n=15) and
CLP + 1 mg/kg CAP (n=15) mice were monitored for 7 days. Log-rank
Mantel-Cox test, P=0.035. (C) Recognition index of the novel
objective in the novel object recognition test [Sham (n=8), CLP
(n=6) and CLP + CAP (n=7) mice; one-way ANOVA with Bonferroni post
hoc test; F(2,18) =3.670; P=0.046]. (D) Time
spent in the target quadrant in the Morris water maze test phase
[Sham (n=8), CLP (n=10) and CLP + CAP (n=12) mice]. Data are
presented as the mean ± standard deviation. *P<0.05 and
**P<0.01 vs. sham. CAP, capsaicin; CLP, cecal ligation and
puncture; TRPV1, transient receptor potential vanilloid 1; Veh,
vehicle; CAP-1*1, capsaicin 1 mg/kg * 1 application.](/article_images/mmr/32/6/mmr-32-06-13686-g00.jpg)
![CAP-induced protection in mice with
sepsis. (A) Recognition index of the novel object in the novel
object recognition test [Sham (n=10), CLP (n=12) and CLP + CAP
(n=11) mice; n=10-12 mice/group; one-way ANOVA with Bonferroni post
hoc test; F(2,30)=12.45; P=0.0001]. (B) Escape
latency in the MWM during the training phase [Sham (n=10), CLP
(n=15) and CLP + CAP (n=14) mice]. (C) Time spent in the target
quadrant in the MWM during the test phase (one-way ANOVA with
Bonferroni post hoc test; F(2,35)=8.925; P=0.0007). (D)
Representative swimming traces in the MWM during the learning and
test phase. (E) Average swimming speed in the MWM during the test
phase. Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and ***P<0.001. CAP, capsaicin; CLP,
cecal ligation and puncture; MWM, Morris water maze; Veh, vehicle;
CAP-1*3, capsaicin 1 mg/kg * 3 application.](/article_images/mmr/32/6/mmr-32-06-13686-g01.jpg)