Introduction
Globally, colorectal cancer (CRC) is a leading
oncological burden, positioned as the third most frequently
diagnosed malignancy and the second principal contributor to
cancer-associated mortality (1).
The World Health Organization estimated that in 2022, there were
>1.9 million new cases of CRC and 904,000 CRC-associated deaths
worldwide, with China accounting for ~30% of these cases (2). Projections indicate that by 2040, the
number of new cases and deaths to will increase to 3.2 million and
1.6 million, respectively, posing a notable public health challenge
(3). Despite 60% of patients being
diagnosed with resectable CRC, ~50% of those who undergo curative
surgery alone and 20–25% of those who receive adjuvant chemotherapy
experience cancer relapse, metastatic disease and eventual death
(4). This underscores the
inadequacy of current treatment options for this fatal
malignancy.
Due to the toxicity concerns and high costs
associated with modern therapies, there is an increasing interest
in discovering potential natural products (5,6).
Curcumin, one of the primary curcuminoids found in the root of the
Curcuma longa (turmeric) plant, has been extensively studied
in the treatment of a wide range of diseases, including CRC
(7). A phase IIa open-label
randomized controlled trial showed that adjuvant treatment with
curcumin significantly increased the objective response rate (53.3%
vs. 11.1%, P=0.039) and prolonged median survival time (502 vs. 200
days, P=0.02) compared with single chemotherapy (8). Additionally, incorporating curcumin
into standard chemotherapeutic regimens for metastatic CRC can help
reduce side effects, overcome chemotherapy resistance and
ultimately enhance the quality of life for patients (9). Due to the potential therapeutic
benefits, several other clinical trials have already been
registered and conducted, assessing curcumin either in combination
with chemotherapy or as a single agent for the prevention of colon
cancer (10–12).
Several studies have investigated and revealed the
potential mechanisms of curcumin in treating cancer, such as
inducing apoptosis and senescence, suppressing migration and
invasion (13), blocking the cell
cycle (14), activating
ferroptosis (15), regulating the
gut microbiota, and exerting anti-inflammatory and antioxidant
effects (16). However, the
underlying mechanisms of curcumin against CRC are still not fully
elucidated.
Pyroptosis, a form of inflammatory programmed cell
death that differs from apoptosis, has emerged as a focal area of
research due to its implications in various diseases, including CRC
(17). This process is primarily
triggered by inflammatory stimuli that activate caspases,
particularly caspase-1 (18),
which induces the formation of perforation-active gasdermin
(GSDM)D. Following proteolytic activation, the N-terminal of GSDMD
oligomerizes into transmembrane pores on the plasma membrane,
mediating: i) Non-selective ion flux causing osmotic imbalance and
cytolysis, and ii) regulated release of canonical inflammatory
mediators (IL-1β and IL-18); these collectively execute the
terminal phase of pyroptotic cell death (19).
Investigative findings have revealed that malignant
cells demonstrate heightened pyroptotic susceptibility compared
with normal cells (20).
Pyroptosis has demonstrated notable antitumor potential in
controlling cancer progression, including in CRC (17). A previous study reported that
caspase-1 activation could mediate a ‘cold’ to ‘hot’ transformation
of the tumor microenvironment (TME) in CRC (21). By contrast, Fusobacterium
nucleatum could inhibit the caspase-3/GSDME pyroptosis-related
pathway induced by chemotherapy drugs, thereby mediating CRC cell
chemo-resistance (22).
Consequently, pyroptosis induction could serve as a dual-function
mechanism for both tumor suppression and therapeutic intervention
in CRC.
Given the increasing incidence of CRC, the
development of targeted therapeutic interventions is of paramount
importance. The diverse mechanisms of curcumin, particularly its
role in inducing caspase-1-mediated pyroptosis, warrant further
investigation as a potential therapeutic strategy for CRC. The
present study aimed to elucidate the mechanisms through which
curcumin modulates caspase-1 activity and its impact on pyroptosis
in CRC cells, thereby advancing the understanding of CRC and the
therapeutic potential of natural compounds.
Materials and methods
Materials
The human CRC cell lines HCT-116 and SW480 (cat.
nos. SCSP-5076 and SCSP-5033) were acquired from The Cell Bank of
Type Culture Collection of The Chinese Academy of Sciences. For
comparative analysis, the normal colon epithelial cell line FHC was
procured from the American Type Culture Collection (cat. no.
CRL-1831). Cell culture media components including RPMI 1640, DMEM
and fetal bovine serum (FBS) (cat. nos. 11875093, 12491015 and
A5670501) were commercially obtained from Thermo Fisher Scientific,
Inc. Apoptosis detection reagents (In Situ Cell Death
Detection Kit) were sourced from Roche Diagnostics (cat. no.
11684795910). The caspase-1 inhibitor VX-765 was supplied by
MedChemExpress (cat. no. HY-13205). Western blot analysis employed
specific antibodies targeting caspase-1/3 (both precursor and
cleaved forms), IL-1β, IL-18, GSDMD and nucleotide-binding
oligomerization domain (NOD)-like receptor protein (NLRP)3
inflammasome components (cat. nos. ab138483, ab184787, ab2302,
ab283818, ab207323, ab209845 and ab263899; Abcam; cat. no.
HY-P80622; MedChemExpress). All the materials were maintained under
manufacturer-specified storage conditions.
Cell culture and experimentation
SW480 and HCT116 cell lines were propagated in RPMI
1640 medium containing 10% FBS, whereas FHC cells were cultured in
DMEM with equivalent serum supplementation. All cell populations
were maintained under standard culture conditions (37°C, 5%
CO2). For experimental interventions, both SW480 and
HCT116 cells were stratified into four treatment cohorts: i)
Curcumin monotherapy (10 µM), ii) caspase-1 inhibitor VX-765 (10 µM
for SW480; 20 µM for HCT116) (23), iii) combination therapy with
curcumin (10 µM) and VX-765 (20 µM), and iv) untreated control
group. Pharmacological treatments were administered following
established dosing protocols for 48 h at 37°C with 5%
CO2.
Cell viability assay
Cell viability was quantitatively assessed using the
CCK-8 colorimetric assay (cat. no. CK04-20; Dojindo Laboratories,
Inc.) to evaluate curcumin-induced cytotoxicity. Specifically,
exponentially growing cells were plated in 96-well culture plates
at a standardized density of 5×103 cells/well. After 24
h adherence, cells were exposed to curcumin (cat. no. C1386;
Sigma-Aldrich; Merck KGaA) dissolved in DMSO, with concentrations
titrated from 0 to 200 µM. Following 48 h pharmacological exposure,
10 µl CCK-8 detection reagent was administered per well and
incubated for 2 h at 37°C, followed by optical density
quantification using a microplate reader (BioTek Synergy H1;
Agilent Technologies, Inc.) at 450 nm with a reference wavelength
of 650 nm. Experimental design incorporated triplicate technical
replicates across three independent biological experiments.
Cell migration and invasion
assays
Cell migration under curcumin treatment was assessed
via scratch assay. Cells (5×105/well) in 12-well plates
were cultured to 95% confluence in medium containing 10% FBS.
Uniform wounds were created using sterile pipette tips, washed with
serum-free medium, and then cultured in serum-free medium at 37°C.
Images were captured at 0 and 48 h using phase-contrast microscopy
to monitor closure (Leica DMi8; Leica Microsystems, Inc.). Wound
closure was quantified using Digimizer software v4.5.1 (MedCalc
Software Ltd.) with migration rate (%) calculated as: (Initial
width-final width)/initial width ×100. Triplicate experiments were
performed.
Invasion assays utilized 24-well Transwell plates
pre-coated with Matrigel at 37°C for 1 h (pore size, 8 µm; BD
Biosciences). A total of 1×105 cells in serum-deprived
medium were introduced into the upper chamber, with 500 µl medium
containing 10% FBS placed in the lower chamber. After 48 h
incubation at 37°C, invasive cells retained on the membrane were
fixed with 4% paraformaldehyde and stained with 0.5% (w/v) crystal
violet for 20 min, whereas non-invasive cells were removed with a
cotton swab. Three random microscopic fields per well were
quantified for statistical analysis.
Microscopy
Pyroptotic cellular dynamics were investigated using
24-well culture systems with an initial seeding density of
5×104 cells/well. Morphological evaluation was performed
through bright-field imaging (Olympus IX53; Olympus Corporation)
following experimental interventions.
Lactate dehydrogenase (LDH) release
assay
Pyroptotic cell death was quantitatively assessed
through LDH release analysis (cat. no. EL-H0866; Wuhan Elabscience
Biotechnology Co., Ltd.). Culture supernatants collected
post-treatment were centrifuged at 300 × g for 5 min at 4°C, and
the clarified supernatants processed for LDH quantification
according to the manufacturer protocols, with absorbance measured
at 490 nm using a microplate reader.
TUNEL staining
Apoptotic nuclei were detected via TUNEL assay
(In Situ Cell Death Detection Kit). Cells seeded at
2×104 cells/well on sterilized coverslips were fixed in
4% paraformaldehyde at 4°C for 30 min, grown on coverslips,
permeabilized with 0.1% Triton X-100/0.1% sodium citrate solution
at room temperature (RT) for 10 min and subsequently incubated with
TUNEL reaction mixture at 37°C for 1 h. Nuclear counterstaining was
achieved with DAPI (1 µg/ml; at RT for 5 min). Samples were mounted
with ProLong™ Gold Antifade Mountant (cat. no. P36930;
Thermo Fisher Scientific, Inc.) and imaged using a Zeiss Axio
Observer fluorescence microscope. Quantitative analysis was
performed on ≥3 random fields per sample (×200 magnification). All
procedures strictly followed manufacturer-specified protocols,
including negative controls processed without terminal
transferase.
Western blotting
Cellular proteins were extracted using RIPA buffer
(cat. no. 89900; Thermo Fisher Scientific, Inc.) and quantified via
the BCA assay. Equal aliquots (30 µg) were separated by SDS-PAGE on
12% gels and were transferred to PVDF membranes. After blocking
with 5% BSA at RT for 1 h (cat. no. E-IR-R107; Wuhan Elabscience
Biotechnology Co., Ltd.), the membranes were incubated with primary
antibodies (1:500 for caspase-1, cleaved-caspase-1 and
cleaved-caspase-3; 1:2,000 for caspase-3; 1:1,000 for IL-1β, IL-18,
GSDMD and NLRP3) at 4°C for 16 h, and then with HRP-conjugated
rabbit anti-rat IgG(H+L) secondary antibody (1:5,000; cat. no.
ab6734; Abcam) at RT for 2 h. β-actin (1:5,000; cat. no. ab20272;
Abcam) was used as the internal control. Protein bands were
visualized using ECL reagent (cat. no. E422-01; Vazyme Biotech Co.,
Ltd.) with chemiluminescent detection.
Immunofluorescence staining
HCT116 and SW480 cells (2×104 cells/well)
were seeded in 24-well plates containing round coverslips and fixed
in 4% paraformaldehyde at RT for 15 min. The cells were then
membrane-permeabilized by incubation in 0.1% Triton-X 100 (cat. no.
T9284; MilliporeSigma), followed by blocking with 5% BSA (cat. no.
E-IR-R107; Wuhan Elabscience Biotechnology Co., Ltd.) for 1 h at
RT. Cells were incubated with primary antibody (GSDMD: 1:500, cat.
no. HY-P85810; caspase-1: 1:500, cat. no. HY-P81232;
MedChemExpress) at 4°C for 16 h, and incubation with goat
anti-mouse IgG H&L Alexa Fluor 488 secondary antibody (cat. no.
ab150117; Abcam) at RT for 1 h. Fluorescence signals were captured
using confocal microscopy (Leica SP8; Leica Microsystems, Inc.)
with DAPI nuclear counterstaining for 24 h at RT for signal
normalization.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 9.4.1 software (Dotmatics). All experiments were repeated
three times. Quantitative data are presented as the median (IQR).
All continuous variables were formally tested for normality using
the Shapiro-Wilk test (α=0.05). Due to the small sample size (only
in vitro experiments were performed), non-parametric
analysis was conducted through Kruskal-Wallis with Dunn's post hoc
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
Determination of curcumin
concentration
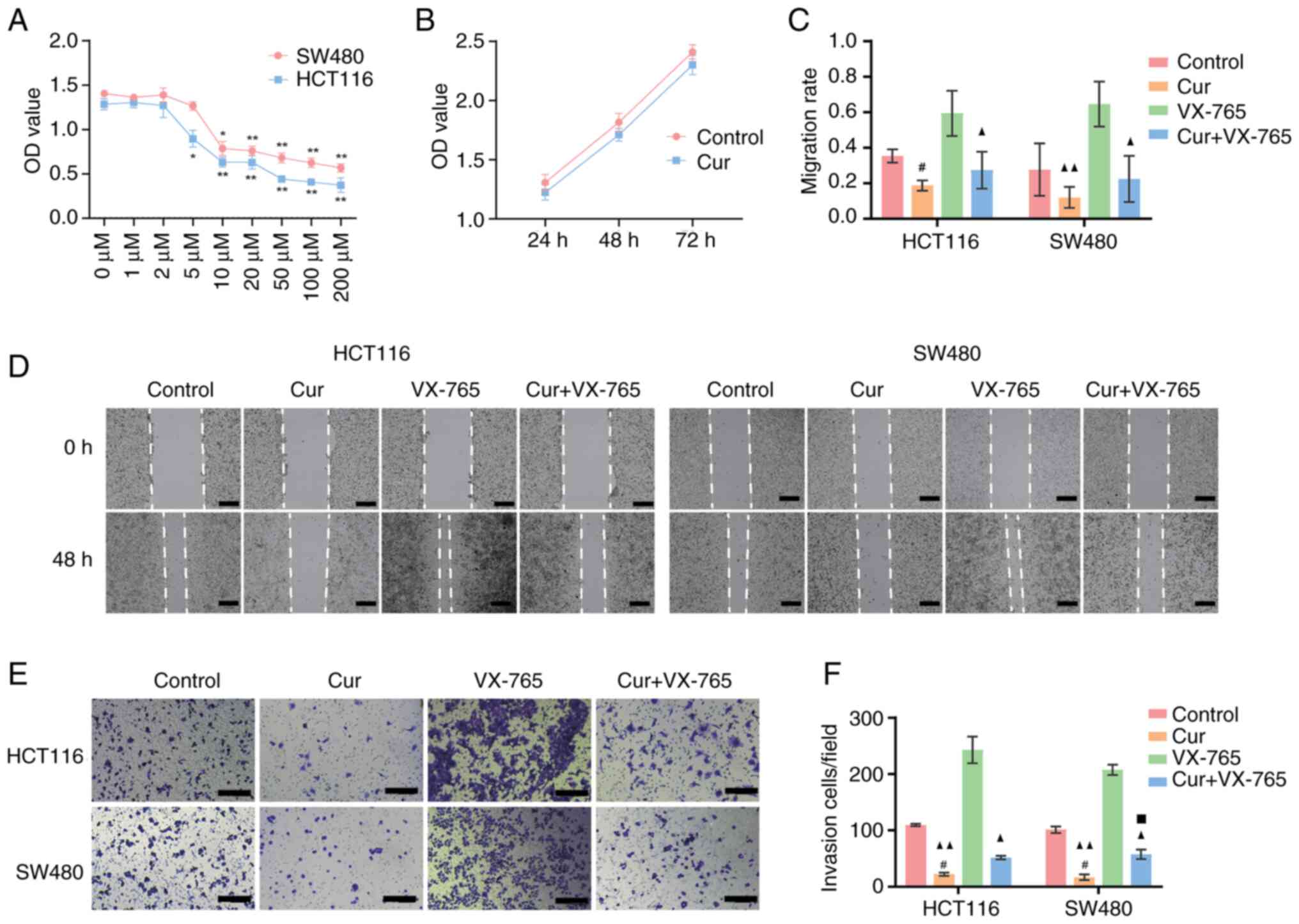
To determine the optimal dosage of curcumin, CRC
cell lines HCT-116 and SW480 were treated with curcumin at
concentrations ranging from 0 to 200 µM, and cell viability was
assessed using CCK-8 assays. As shown in Fig. 1A, curcumin reduced cell viability
in a dose-dependent manner. In response to 10 µM curcumin, both
cell lines exhibited statistically significant differences
(compared with 0 µM). Additionally, the cell viability assay
revealed no effect on FHC normal colon epithelial cells in response
to this concentration of curcumin for 72 h (Fig. 1B). Therefore, 10 µM curcumin was
selected for subsequent experiments.
Curcumin inhibits cell migration and
invasion
The migratory ability of CRC cell lines was assessed
using a wound healing assay. Cells were cultured in maintenance
medium for 48 h, and the scratch distance was measured for each
cell line. Curcumin treatment significantly reduced the extent of
wound closure and the migration rate (Fig. 1C and D). The effect of curcumin on
invasive capability was evaluated using a Matrigel-coated Transwell
system. As shown in Fig. 1E and F,
curcumin treatment significantly reduced the number of invasive
HCT-116 and SW480 cells compared with those in the control group.
These results demonstrated that curcumin may inhibit autonomous
migration and invasion in CRC cells.
Curcumin induces pyroptosis in CRC
cells
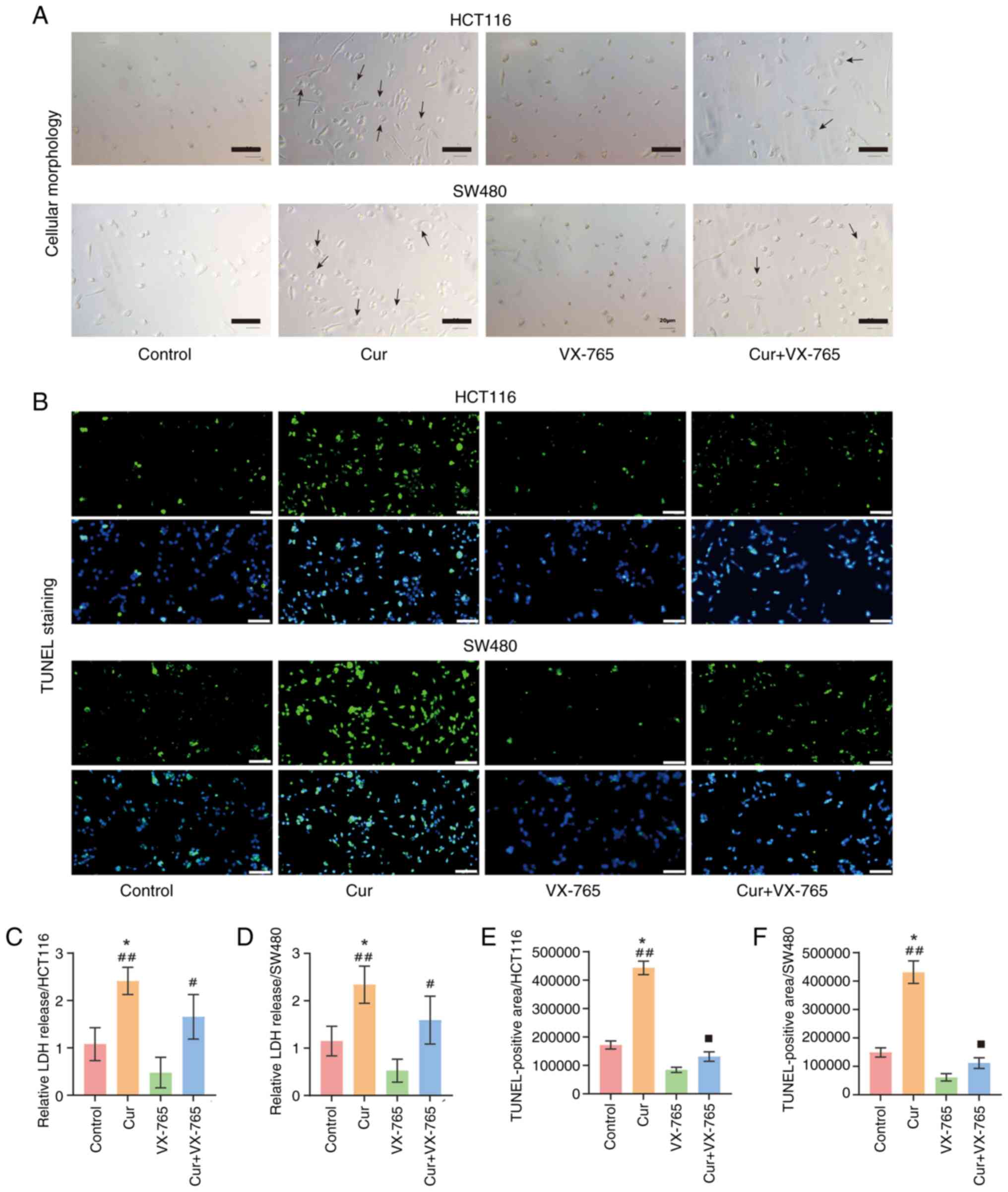
To assess cellular morphology, CRC cells were
examined under a microscope. Fig.
2A demonstrated that curcumin-treated cells manifested
characteristic pyroptotic morphology, including membrane swelling
and subsequent rupture with cytoplasmic content release. TUNEL
staining was then performed to assess cell death. The results
demonstrated that curcumin significantly increased cell death
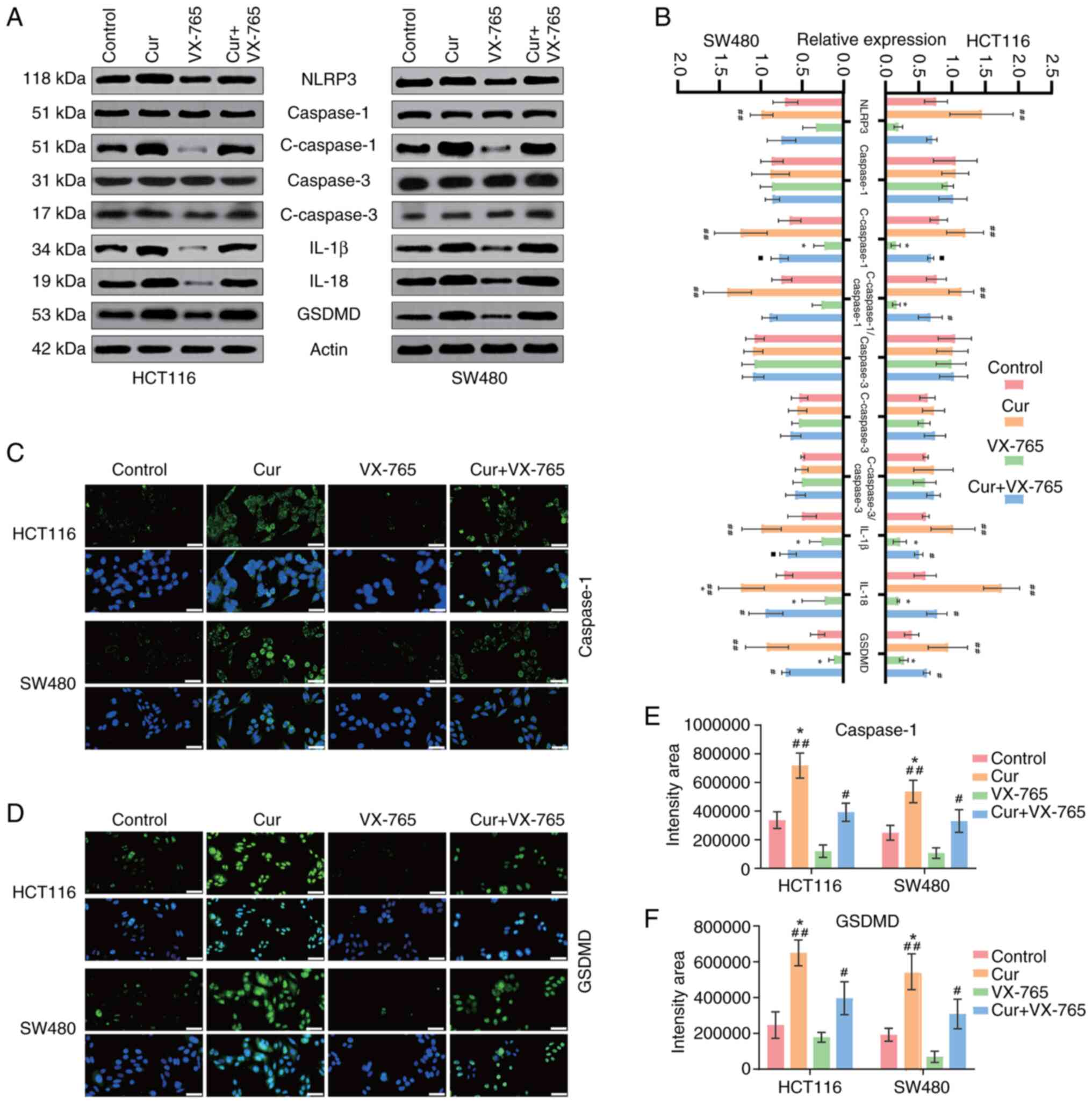
(Fig. 2B, E and F). Caspase-3, an
apoptosis-specific marker, was detected to distinguish between
pyroptosis and apoptosis. The results indicated that curcumin could
slightly increase cleaved-caspase-3 expression; however, this was
not significantly different (Fig. 3A
and B). Furthermore, cell membrane integrity is a key indicator
of pyroptosis. Therefore, LDH levels in the cell supernatant and
GSDMD expression were measured. The results indicated that
curcumin-treated cells exhibited elevated LDH levels (Fig. 2C and D) and increased GSDMD
expression (Fig. 3A, B and D).
Furthermore, the expression levels of pyroptosis-related factors,
including caspase-1, its upstream marker NLRP3, and the downstream
markers IL-18 and IL-1β, were analyzed (Fig. 3A-C and E). The results indicated
that curcumin significantly upregulated the expression of
pyroptosis-related proteins, suggesting that pyroptosis may
contribute to the anti-CRC effects of curcumin.
Curcumin induces pyroptosis by
activating the caspase-1 signal
To evaluate the role of the caspase-1 signaling
pathway in curcumin-induced pyroptosis, VX-765, a selective
caspase-1 inhibitor, was used as a negative control. VX-765
markedly attenuated abnormal cellular morphological changes and
cell death induced by curcumin (Fig.
2A and B). Additionally, the expression levels of caspase-1 and
the downstream factors IL-1β and IL-18 were significantly lower in
the curcumin + VX-765 group compared with those in the
curcumin-only group (Fig. 3A-C and
E). Meanwhile, reduced LDH levels and GSDMD expression
indicated that VX-765 attenuated curcumin-induced perforation
(Figs. 2C and D, and 3A, B, D and F).
Additionally, curcumin markedly reduced the
migratory capability of HCT116 and SW480 cells; however, the
inhibitory effect on migration was arrested when cells were
co-treated with curcumin and VX-765 (Fig. 1C and D). Similarly, the invasive
ability of CRC cells was significantly higher in the presence of
VX-765 compared with curcumin alone (Fig. 1E and F). These findings suggested
that curcumin inhibits CRC growth and metastasis by inducing
caspase-1-mediated pyroptosis.
Discussion
The present study demonstrated that curcumin may
exert antitumor effects on CRC cells, primarily through the
induction of caspase-1-mediated pyroptosis. The data revealed that
curcumin treatment not only suppressed tumor cell viability,
migration and invasion in a dose-dependent manner, but also
triggered pyroptosis, a lytic and immunogenic form of programmed
cell death distinct from apoptosis. This finding aligns with the
established literature indicating the ability of curcumin to engage
multiple cell death pathways in cancer, reinforcing its potential
as a therapeutic agent for CRC (24–26).
CRC remains a major global health burden and a
leading cause of cancer-related mortality. While notable advances
have been made in cancer immunotherapy, efforts continue to
identify strategies that elicit robust and sustained antitumor
immunity (27). Accumulating
evidence has indicated that specific regulated cell death
modalities, including pyroptosis, can function as potent
immunogenic switches. This occurs through the systemic release of
damage-associated molecular patterns and tumor-associated antigens,
which subsequently prime and activate antitumor immune responses
(28). Leveraging the inherent
immunogenicity of dying tumor cells thus represents a promising
avenue for enhancing immunotherapy efficacy. Pyroptosis,
classically recognized as a host defense mechanism against
pathogens, elicits a strong inflammatory response and activates
innate immunity (29). Critically,
pyroptosis-induced immunogenic cell death can transform
immunologically ‘cold’ tumors into ‘hot’ T cell-inflamed
microenvironments, establishing a dual-phase tumor suppressive
mechanism involving both primary tumor regression and disruption of
metastatic niches (30).
Consequently, strategies combining pyroptosis induction with
conventional anticancer therapies represent a viable treatment
approach (31).
The critical role of pyroptosis in CRC pathogenesis
and therapy is increasingly being recognized. Clinical studies have
reported dysregulation of inflammasome components, such as NLRP1,
NLRP3, NOD-like receptor family caspase recruitment
domain-containing protein (NLRC)4 and absent in melanoma 2 (AIM2),
in patients with CRC (32), with
several inflammasomes (NLRP1, NLRP6, AIM2, pyrin and NLRC4)
demonstrating anti-tumorigenic function (33). Pharmacological enhancement of NLRP3
activity, combined with chemotherapeutics such as 5-fluorouracil or
regorafenib, can effectively induce pyroptosis and suppress tumor
growth in preclinical CRC models (34). Inflammasome-regulated release of
IL-18 and IL-1β by immune cells within the TME holds therapeutic
potential by promoting inflammation and immune activation (31). For example, NLRP3-mediated
IL-18/IL-1β production enhances natural killer cell maturation and
cytotoxicity, thereby inhibiting CRC liver metastasis (35). Clinically, downregulated GSDMD
expression is associated with a poor prognosis in CRC (36), while cytoplasmic/nuclear GSDMB
expression may predict benefit from 5-fluorouracil-based
chemotherapy (37). Furthermore,
inducing pyroptosis via other mechanisms, such as
caspase-3-mediated GSDME cleavage during photodynamic therapy, can
sensitize microsatellite stable CRC cells to PD-1 blockade
(38). Targeted induction of
GSDMD-dependent pyroptosis has also shown efficacy in inhibiting
metastatic CRC (39).
The present mechanistic investigation revealed that
curcumin activates the NLRP3 inflammasome in CRC cells, leading to
the upregulation of key pyroptosis executioner proteins caspase-1
and GSDMD. This cascade consequently promotes the maturation and
release of the pro-inflammatory cytokines IL-18 and IL-1β.
Morphologically, curcumin-treated CRC cells exhibited
characteristic features of pyroptosis, including cellular swelling,
plasma membrane ballooning with protruding vesicles, and eventual
rupture leading to cytoplasmic content release. Crucially, the
specific caspase-1 inhibitor VX-765 significantly attenuated
curcumin-induced cell death and associated molecular changes,
strongly supporting the central role of caspase-1-mediated
pyroptosis in the anti-CRC effects of curcumin.
Several studies have explored the antitumor effects
of curcumin via pyroptosis. In non-small cell lung cancer cells,
curcumin inhibits ubiquitin ligase Smurf2 activity, promoting
NLRP3-dependent pyroptosis (40).
In mouse breast cancer 4T1 cells, curcumin induces Ca2+
overload, effectively triggering caspase-1/GSDMD-mediated
pyroptosis to suppress tumors (41). In U937 leukemia cells, curcumin
upregulates AIM2, IFI16 and NLRC4 inflammasomes, activating
caspase-1, cleaving GSDMD and inducing pyroptosis (42). However, evidence in CRC remains
limited. Prior research indicates that curcumin induces programmed
cell death and upregulates the NLRP3 inflammasome in CRC cells;
however, the underlying mechanism remains unresolved (26). The present findings established
curcumin as a potent inducer of caspase-1/GSDMD-dependent
pyroptosis in CRC cells, advancing this evidence base.
Notably, curcumin exhibits a cell type-dependent
duality in modulating pyroptosis, acting as both an inducer and
suppressor. In non-cancerous settings, such as models of
fenpropathrin-induced nephrotoxicity (39), aflatoxin B1-induced hepatotoxicity
(43) and intestinal inflammation
(44), curcumin protects tissues
by suppressing pyroptosis, often through downregulating NLRP3,
caspase-1, GSDMD and associated cytokines. Conversely, in various
cancer cell types, including leukemia (42), hepatocellular carcinoma (45), and as demonstrated in the present
study and in a previous study on CRC (26), curcumin consistently promotes
pyroptosis as a key antitumor mechanism. This pro-pyroptotic effect
in CRC extends beyond direct tumor cell killing; curcumin-induced
pyroptosis can also repolarize tumor-associated macrophages towards
the immunostimulatory M1 phenotype, counteracting TME
immunosuppression and enhancing overall antitumor efficacy
(46). This cell-type-specific
duality is not unique to pyroptosis, as similar contrasting effects
of curcumin have been observed in ferroptosis (47).
Despite these notable findings, several limitations
warrant attention in future studies. Firstly, the current
conclusions are based solely on in vitro cell line models.
Validation in in vivo animal models of CRC is essential to
confirm the antitumor efficacy of curcumin and its ability to
induce pyroptosis within the complex TME. Secondly, while
pharmacological inhibition with VX-765 strongly implicates
caspase-1, definitive genetic validation is needed. Employing
caspase-1 silencing (such as via small interfering RNA or short
hairpin RNA) or overexpression in CRC cells, coupled with studies
utilizing caspase-1-deficient transgenic animal models, would
provide more conclusive evidence for its indispensable role in
curcumin-induced pyroptosis. Additionally, alternative pathways
(such as non-canonical inflammasome activation or other
GSDM-dependent mechanisms) may concurrently mediate this process,
warranting systematic exploration in future studies.
In conclusion, the current study uncovered a novel
mechanism of curcumin in CRC treatment. By activating the caspase-1
pathway, curcumin may induce pyroptosis, and inhibit migration and
invasion in CRC cells, thereby exerting an anti-CRC effect.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant nos. 82004333 and 82205033), the Natural
Science Foundation of Zhejiang Province (grant no. LQ21H290002),
the State Administration of Traditional Chinese Medicine Science
and Technology Department-Zhejiang Provincial Administration of
Traditional Chinese Medicine Co-construction of Key Laboratory of
Research on Prevention and Treatment for Depression Syndrome (grant
no. GZY-ZJ-SY-2402), and the Zhejiang Traditional Chinese Medicine
Science and Technology Project (grant nos. 2022ZA044 and
2023ZR005).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JZ and LY contributed equally to this work and
shared the first authorship. JZ conceived the study, carried out
the investigation, administered the project, validated the results
and acquired funding. LY conceived and designed the study,
administered the project and wrote the main manuscript text. ZF and
JC carried out the investigation, and performed data collection and
statistical analysis. YW and HL conducted the formal analysis and
visualization using GraphPad Prism. SL conceived the study, and
contributed to writing, reviewing and editing the manuscript. BF
conceived and supervised the study, validated the results and
acquired funding. JZ and BF confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AIM2
|
absent in melanoma 2
|
|
CRC
|
colorectal cancer
|
|
FBS
|
fetal bovine serum
|
|
GSDM
|
gasdermin
|
|
LDH
|
lactate dehydrogenase
|
|
NOD
|
nucleotide-binding oligomerization
domain
|
|
NLRP
|
NOD-like receptor protein
|
|
NLRC
|
NOD-like receptor family caspase
recruitment domain-containing protein
|
|
TME
|
tumor microenvironment
|
References
|
1
|
Hossain MS, Karuniawati H, Jairoun AA,
Urbi Z, Ooi J, John A, Lim YC, Kibria KMK, Mohiuddin AKM, Ming LC,
et al: Colorectal cancer: A review of carcinogenesis, global
epidemiology, current challenges, risk factors, preventive and
treatment strategies. Cancers (Basel). 14:17322022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
3
|
Morgan E, Arnold M, Gini A, Lorenzoni V,
Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N and Bray F:
Global burden of colorectal cancer in 2020 and 2040: Incidence and
mortality estimates from GLOBOCAN. Gut. 72:338–344. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weng W and Goel A: Curcumin and colorectal
cancer: An update and current perspective on this natural medicine.
Semin Cancer Biol. 80:73–86. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y and Xie J: Targeting ferroptosis
regulators by natural products in colorectal cancer. Front
Pharmacol. 15:13747222024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su Z, Li Y, Zhou Z, Feng B, Chen H and
Zheng G: Herbal medicine for colorectal cancer treatment: Molecular
mechanisms and clinical applications. Cell Prolif. Jun 9–2025.(Epub
ahead of print). View Article : Google Scholar
|
|
7
|
Neira M, Mena C, Torres K and Simón L: The
potential benefits of curcumin-enriched diets for adults with
colorectal cancer: A systematic review. Antioxidants (Basel).
14:3882025. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Howells LM, Iwuji COO, Irving GRB, Barber
S, Walter H, Sidat Z, Griffin-Teall N, Singh R, Foreman N, Patel
SR, et al: Curcumin combined with FOLFOX chemotherapy is safe and
tolerable in patients with metastatic colorectal cancer in a
randomized phase IIa trial. J Nutr. 149:1133–1139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Layos L, Martínez-Balibrea E and Ruiz de
Porras V: Curcumin: A novel way to improve quality of life for
colorectal cancer patients? Int J Mol Sci. 23:140582022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Wu Y, Tian Y, Xu H, Lin ZX and
Xian YF: Chinese herbal medicine for the treatment of intestinal
cancer: Preclinical studies and potential clinical applications.
Mol Cancer. 23:2172024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gilad O, Rosner G, Ivancovsky-Wajcman D,
Zur R, Rosin-Arbesfeld R, Gluck N, Strul H, Lehavi D, Rolfe V and
Kariv R: Efficacy of wholistic turmeric supplement on adenomatous
polyps in patients with familial adenomatous polyposis-a
randomized, double-blinded, placebo-controlled study. Genes
(Basel). 13:21822022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Panahi Y, Saberi-Karimian M, Valizadeh O,
Behnam B, Saadat A, Jamialahmadi T, Majeed M and Sahebkar A:
Effects of curcuminoids on systemic inflammation and quality of
life in patients with colorectal cancer undergoing chemotherapy: A
randomized controlled trial. Adv Exp Med Biol. 1328:1–9. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu C, Rokavec M, Huang Z and Hermeking H:
Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress
colorectal cancer metastasis. Cell Death Differ. 30:1771–1785.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Han Y, Zhao NN and Zhao XF: Curcumin
exerts therapeutic effects on colorectal cancer by blocking the
cell cycle and regulating apoptosis. Asian J Surg. Aug
28–2024.(Epub ahead of print).
|
|
15
|
Miyazaki K, Xu C, Shimada M and Goel A:
Curcumin and andrographis exhibit anti-tumor effects in colorectal
cancer via activation of ferroptosis and dual suppression of
glutathione peroxidase-4 and ferroptosis suppressor protein-1.
Pharmaceuticals (Basel). 16:3832023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ojo OA, Adeyemo TR, Rotimi D, Batiha GE,
Mostafa-Hedeab G, Iyobhebhe ME, Elebiyo TC, Atunwa B, Ojo AB, Lima
CMG and Conte-Junior CA: Anticancer properties of curcumin against
colorectal cancer: A review. Front Oncol. 12:8816412022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Q, Xu Y, Tan X, Wu X, Li S, Yuan J,
Chen X, Huang Q, Fu K and Xiao S: The role and therapeutic
potential of pyroptosis in colorectal cancer: A review.
Biomolecules. 14:8742024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pang Q, Wang P, Pan Y, Dong X, Zhou T,
Song X and Zhang A: Irisin protects against vascular calcification
by activating autophagy and inhibiting NLRP3-mediated vascular
smooth muscle cell pyroptosis in chronic kidney disease. Cell Death
Dis. 13:2832022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Min R, Bai Y, Wang NR and Liu X:
Gasdermins in pyroptosis, inflammation, and cancer. Trends Mol Med.
Apr 29–2025.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng MZ, Yang BB, Zhan ZT, Lin SM, Fang
ZP, Gao Y and Zhou WJ: MACC1 and gasdermin-E (GSDME) regulate the
resistance of colorectal cancer cells to irinotecan. Biochem
Biophys Res Commun. 671:236–245. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Q, Peng B, Lin L, Chen J, Jiang Z,
Luo Y, Huang L, Li J, Peng Y, Wu J, et al: Chondroitin
sulfate-modified hydroxyapatite for caspase-1 activated induced
pyroptosis through Ca overload/ER stress/STING/IRF3 pathway in
colorectal cancer. Small. 20:e24032012024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang N, Zhang L, Leng XX, Xie YL, Kang ZR,
Zhao LC, Song LH, Zhou CB and Fang JY: Fusobacterium
nucleatum induces chemoresistance in colorectal cancer by
inhibiting pyroptosis via the Hippo pathway. Gut Microbes.
16:23337902024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Z, Ji L, Han M, Xie J, Zhong F, Zhang
X, Su Q, Yang Z, Liu Z, Gao H and Jiang G: Pyroptosis is involved
in the inhibitory effect of FL118 on growth and metastasis in
colorectal cancer. Life Sci. 257:1180652020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brockmueller A, Ruiz de Porras V and
Shakibaei M: Curcumin and its anti-colorectal cancer potential:
From mechanisms of action to autophagy. Phytother Res.
38:3525–3551. 2024. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou H, Zhuang Y, Liang Y, Chen H, Qiu W,
Xu H and Zhou H: Curcumin exerts anti-tumor activity in colorectal
cancer via gut microbiota-mediated CD8+ T Cell tumor
infiltration and ferroptosis. Food Funct. 16:3671–3693. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dal Z and Aru B: The role of curcumin on
apoptosis and NLRP3 inflammasome-dependent pyroptosis on colorectal
cancer in vitro. Turk J Med Sci. 53:883–893. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galluzzi L, Humeau J, Buqué A, Zitvogel L
and Kroemer G: Immunostimulation with chemotherapy in the era of
immune checkpoint inhibitors. Nat Rev Clin Oncol. 17:725–741. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Tang B, Luo J, Yang Y, Weng Q,
Fang S, Zhao Z, Tu J, Chen M and Ji J: Cuproptosis, ferroptosis and
PANoptosis in tumor immune microenvironment remodeling and
immunotherapy: Culprits or new hope. Mol Cancer. 23:2552024.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du T, Gao J, Li P, Wang Y, Qi Q, Liu X, Li
J, Wang C and Du L: Pyroptosis, metabolism, and tumor immune
microenvironment. Clin Transl Med. 11:e4922021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang M, Yu F, Zhang Y and Li P: Programmed
cell death in tumor immunity: Mechanistic insights and clinical
implications. Front Immunol. 14:13096352024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Q, Sun Y, Wang S and Xu J: New
prospects of cancer therapy based on pyroptosis and pyroptosis
inducers. Apoptosis. 29:66–85. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu R, Truax AD, Chen L, Hu P, Li Z, Chen
J, Song C, Chen L and Ting JP: Expression profile of innate immune
receptors, NLRs and AIM2, in human colorectal cancer: Correlation
with cancer stages and inflammasome components. Oncotarget.
6:33456–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma BR and Kanneganti TD: Inflammasome
signaling in colorectal cancer. Transl Res. 252:45–52. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guan X, Liu R, Wang B, Xiong R, Cui L,
Liao Y, Ruan Y, Fang L, Lu X, Yu X, et al: Inhibition of HDAC2
sensitises antitumour therapy by promoting NLRP3/GSDMD-mediated
pyroptosis in colorectal cancer. Clin Transl Med. 14:e16922024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Y, Hu H, Liu Z, Xu J, Gao Y, Zhan X,
Zhou S, Zhong W, Wu D, Wang P, et al: Macrophage STING signaling
promotes NK cell to suppress colorectal cancer liver metastasis via
4-1BBL/4-1BB co-stimulation. J Immunother Cancer. 11:e0064812023.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu LS, Liu Y, Wang XW, Xu B, Lin YL, Song
Y, Dong Y, Liu JL, Wang XJ, Liu S, et al: LPS enhances the
chemosensitivity of oxaliplatin in HT29 cells via GSDMD-mediated
pyroptosis. Cancer Manag Res. 12:10397–10409. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun L, Wang J, Li Y, Kang Y, Jiang Y,
Zhang J, Qian S and Xu F: Correlation of gasdermin B staining
patterns with prognosis, progression, and immune response in
colorectal cancer. BMC Cancer. 24:5672024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou Y, Zhang W, Wang B, Wang P, Li D, Cao
T, Zhang D, Han H, Bai M, Wang X, et al: Mitochondria-targeted
photodynamic therapy triggers GSDME-mediated pyroptosis and
sensitizes anti-PD-1 therapy in colorectal cancer. J Immunother
Cancer. 12:e0080542024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sala R, Rioja-Blanco E, Serna N,
Sánchez-García L, Álamo P, Alba-Castellón L, Casanova I,
López-Pousa A, Unzueta U, Céspedes MV, et al: GSDMD-dependent
pyroptotic induction by a multivalent CXCR4-targeted nanotoxin
blocks colorectal cancer metastases. Drug Deliv. 29:1384–1397.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xi Y, Zeng S, Tan X and Deng X: Curcumin
inhibits the activity of ubiquitin ligase Smurf2 to promote
NLRP3-dependent pyroptosis in non-small cell lung cancer cells. Int
J Oncol. 66:212025. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li L, Xing Z, Wang J, Guo Y, Wu X, Ma Y,
Xu Z, Kuang Y, Liao T and Li C: Hyaluronic acid-mediated targeted
nano-modulators for activation of pyroptosis for cancer therapy
through multichannel regulation of Ca2+ overload. Int J
Biol Macromol. 299:1401162025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou Y, Kong Y, Jiang M, Kuang L, Wan J,
Liu S, Zhang Q, Yu K, Li N, Le A and Zhang Z: Curcumin activates
NLRC4, AIM2, and IFI16 inflammasomes and induces pyroptosis by
up-regulated ISG3 transcript factor in acute myeloid leukemia cell
lines. Cancer Biol Ther. 23:328–335. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Wang Y, Yang Y, Zhao D, Liu R, Li
S and Zhang X: Proteomic analysis of ITPR2 as a new therapeutic
target for curcumin protection against AFB1-induced pyroptosis.
Ecotoxicol Environ Saf. 260:1150732023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pan H, Hu T, He Y, Zhong G, Wu S, Jiang X,
Rao G, You Y, Ruan Z, Tang Z and Hu L: Curcumin attenuates
aflatoxin B1-induced ileum injury in ducks by inhibiting NLRP3
inflammasome and regulating TLR4/NF-κB signaling pathway. Mycotoxin
Res. 40:255–268. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang WF, Gong YX, Li HF, Sun FL, Li WL,
Chen DQ, Xie DP, Ren CX, Guo XY, Wang ZY, et al: Curcumin activates
ROS signaling to promote pyroptosis in hepatocellular carcinoma
HepG2 cells. In Vivo. 35:249–257. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheng F, He L, Wang J, Lai L, Ma L, Qu K,
Yang Z, Wang X, Zhao R, Weng L and Wang L: Synergistic
immunotherapy with a calcium-based nanoinducer: Evoking pyroptosis
and remodeling tumor-associated macrophages for enhanced antitumor
immune response. Nanoscale. 16:18570–18583. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Foroutan Z, Butler AE, Zengin G and
Sahebkar A: Curcumin and ferroptosis: A promising target for
disease prevention and treatment. Cell Biochem Biophys. 82:343–349.
2024. View Article : Google Scholar : PubMed/NCBI
|