|
1
|
Salomão R, Ferreira BL, Salomão MC, Santos
SS, Azevedo LCP and Brunialti M: Sepsis: Evolving concepts and
challenges. Braz J Med Biol Res. 52:e85952019. View Article : Google Scholar
|
|
2
|
Deitch EA: Gut-origin sepsis: Evolution of
a concept. Surgeon. 10:350–356. 2012. View Article : Google Scholar
|
|
3
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990–2017: Analysis for the global burden of disease
study. Lancet. 395:200–211. 2020. View Article : Google Scholar
|
|
4
|
Clements TW, Tolonen M, Ball CG and
Kirkpatrick AW: Secondary peritonitis and intra-abdominal sepsis:
An increasingly global disease in search of better systemic
therapies. Scand J Surg. 110:139–149. 2021. View Article : Google Scholar
|
|
5
|
Rittirsch D, Flierl MA and Ward PA:
Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 8:776–787.
2008. View
Article : Google Scholar
|
|
6
|
Zarjou A and Agarwal A: Sepsis and acute
kidney injury. J Am Soc Nephrol. 22:999–1006. 2011. View Article : Google Scholar
|
|
7
|
Tadokoro T, Ikeda M, Ide T, Deguchi H,
Ikeda S, Okabe K, Ishikita A, Matsushima S, Koumura T, Yamada KI,
et al: Mitochondria-dependent ferroptosis plays a pivotal role in
doxorubicin cardiotoxicity. JCI Insight. 5:e1327472020. View Article : Google Scholar
|
|
8
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar
|
|
9
|
Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C,
Wu H, Deng W, Shen D and Tang Q: Ferritinophagy-mediated
ferroptosis is involved in sepsis-induced cardiac injury. Free
Radic Biol Med. 160:303–318. 2020. View Article : Google Scholar
|
|
10
|
Li J, Lu K, Sun F, Tan S, Zhang X, Sheng
W, Hao W, Liu M, Lv W and Han W: Panaxydol attenuates ferroptosis
against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1
pathway. J Transl Med. 19:962021. View Article : Google Scholar
|
|
11
|
Wang Z, Sun R, Wang G, Chen Z, Li Y, Zhao
Y, Liu D, Zhao H, Zhang F, Yao J and Tian X: SIRT3-mediated
deacetylation of PRDX3 alleviates mitochondrial oxidative damage
and apoptosis induced by intestinal ischemia/reperfusion injury.
Redox Biol. 28:1013432020. View Article : Google Scholar
|
|
12
|
Xu S, Liu Y, Yang S, Fei W, Qin J, Lu W
and Xu J: FXN targeting induces cell death in ovarian cancer
stem-like cells through PRDX3-Mediated oxidative stress. iScience.
27:1105062024. View Article : Google Scholar
|
|
13
|
Tang F, Fan K, Wang K and Bian C:
Atractylodin attenuates lipopolysaccharide-induced acute lung
injury by inhibiting NLRP3 inflammasome and TLR4 pathways. J
Pharmacol Sci. 136:203–211. 2018. View Article : Google Scholar
|
|
14
|
Koonrungsesomboon N, Na-Bangchang K and
Karbwang J: Therapeutic potential and pharmacological activities of
Atractylodes lancea (Thunb.) DC. Asian Pac J Trop Med.
7:421–428. 2014. View Article : Google Scholar
|
|
15
|
Yu C, Xiong Y, Chen D, Li Y, Xu B, Lin Y,
Tang Z, Jiang C and Wang L: Ameliorative effects of atractylodin on
intestinal inflammation and co-occurring dysmotility in both
constipation and diarrhea prominent rats. Korean J Physiol
Pharmacol. 21:1–9. 2017. View Article : Google Scholar
|
|
16
|
Xu L, Zhou Y, Xu J, Xu X, Lu G, Lv Q, Wei
L, Deng X, Shen X, Feng H and Wang J: Anti-inflammatory,
antioxidant and anti-virulence roles of atractylodin in attenuating
Listeria monocytogenes infection. Front Immunol. 13:9770512022.
View Article : Google Scholar
|
|
17
|
Lin YC, Yang CC, Lin CH, Hsia TC, Chao WC
and Lin CC: Atractylodin ameliorates ovalbumin-induced asthma in a
mouse model and exerts immunomodulatory effects on Th2 immunity and
dendritic cell function. Mol Med Rep. 22:4909–4918. 2020.
View Article : Google Scholar
|
|
18
|
Qu L, Lin X, Liu C, Ke C, Zhou Z, Xu K,
Cao G and Liu Y: Atractylodin attenuates dextran sulfate
sodium-induced colitis by alleviating gut microbiota dysbiosis and
inhibiting inflammatory response through the MAPK pathway. Front
Pharmacol. 12:6653762021. View Article : Google Scholar
|
|
19
|
Heo G, Kim Y, Kim EL, Park S, Rhee SH,
Jung JH and Im E: Atractylodin ameliorates colitis via PPARα
agonism. Int J Mol Sci. 24:8022023. View Article : Google Scholar
|
|
20
|
Vitzthum LK, Nalawade V, Riviere P, Marar
M, Furnish T, Lin LA, Thompson R and Murphy JD: Impacts of an
opioid safety initiative on US veterans undergoing cancer
treatment. J Natl Cancer Inst. 114:753–760. 2022. View Article : Google Scholar
|
|
21
|
Nullens S, Staessens M, Peleman C, Plaeke
P, Francque SM, Lammens C, Malhotra-Kumar S, De Man J and De Winter
BY: Su1190 effect of gastrointestinal barrier protection on
sepsis-induced changes of intestinal motility, inflammation and
colonic permeability. Gastroenterology. 150:S4912016. View Article : Google Scholar
|
|
22
|
Song GY, Kim SM, Back S, Yang SB and Yang
YM: Atractylodes lancea and its constituent, atractylodin,
ameliorates metabolic dysfunction-associated steatotic liver
disease via AMPK activation. Biomol Ther (Seoul). 32:778–792. 2024.
View Article : Google Scholar
|
|
23
|
Kuo WT, Odenwald MA, Turner JR and Zuo L:
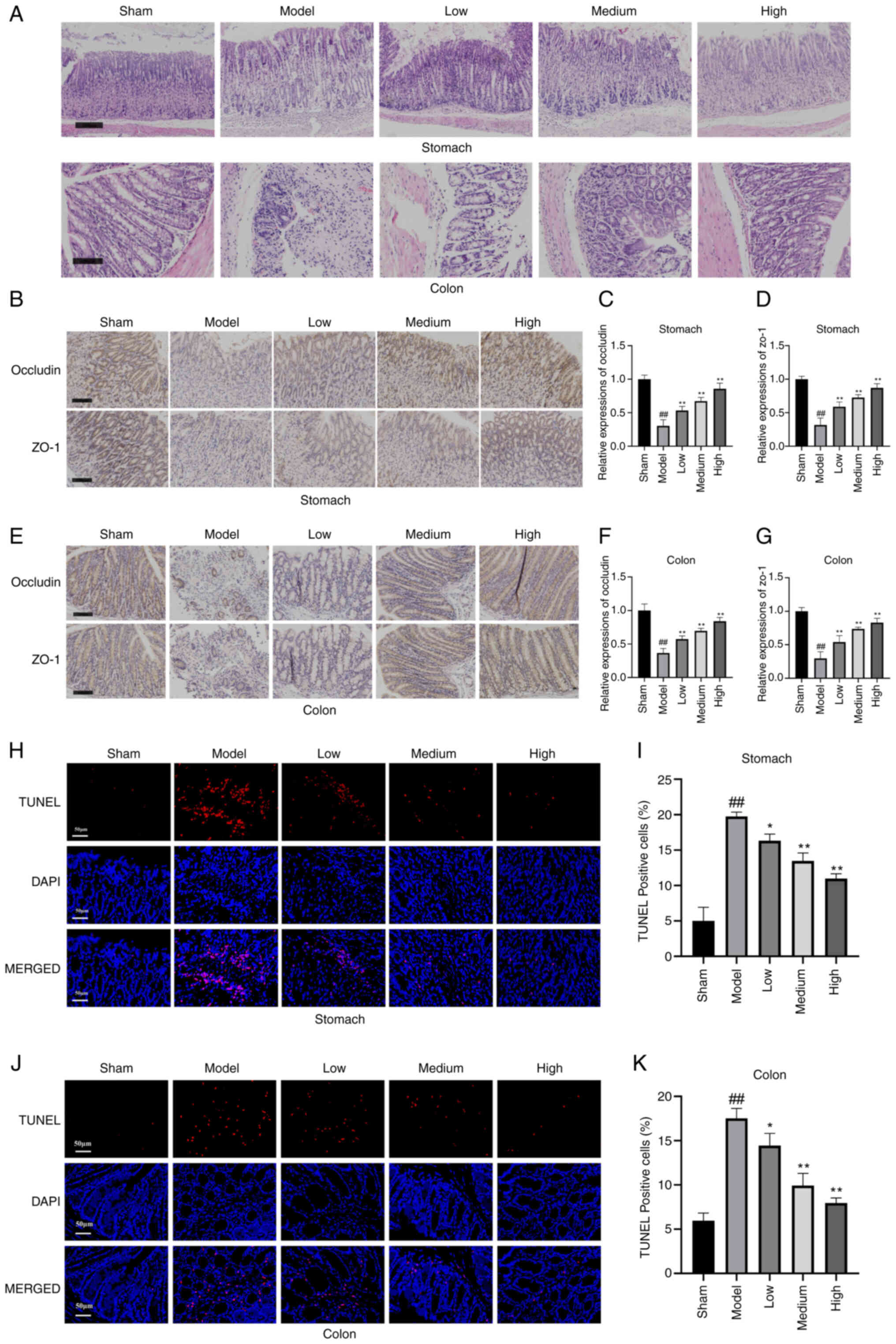
Tight junction proteins occludin and ZO-1 as regulators of
epithelial proliferation and survival. Ann N Y Acad Sci.
1514:21–33. 2022. View Article : Google Scholar
|
|
24
|
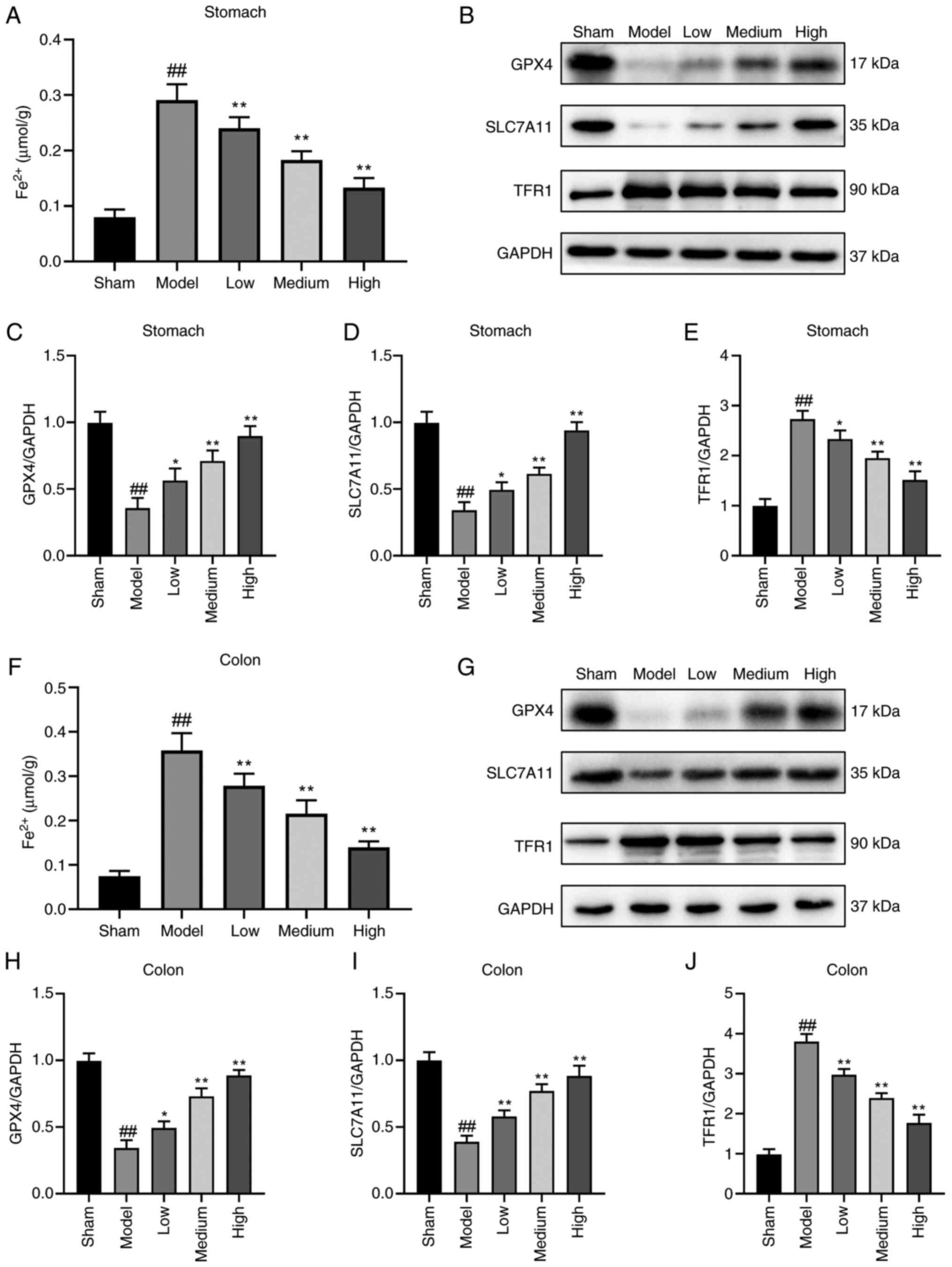
Li S and Huang Y: Ferroptosis: An
iron-dependent cell death form linking metabolism, diseases, immune
cell and targeted therapy. Clin Transl Oncol. 24:1–12. 2022.
View Article : Google Scholar
|
|
25
|
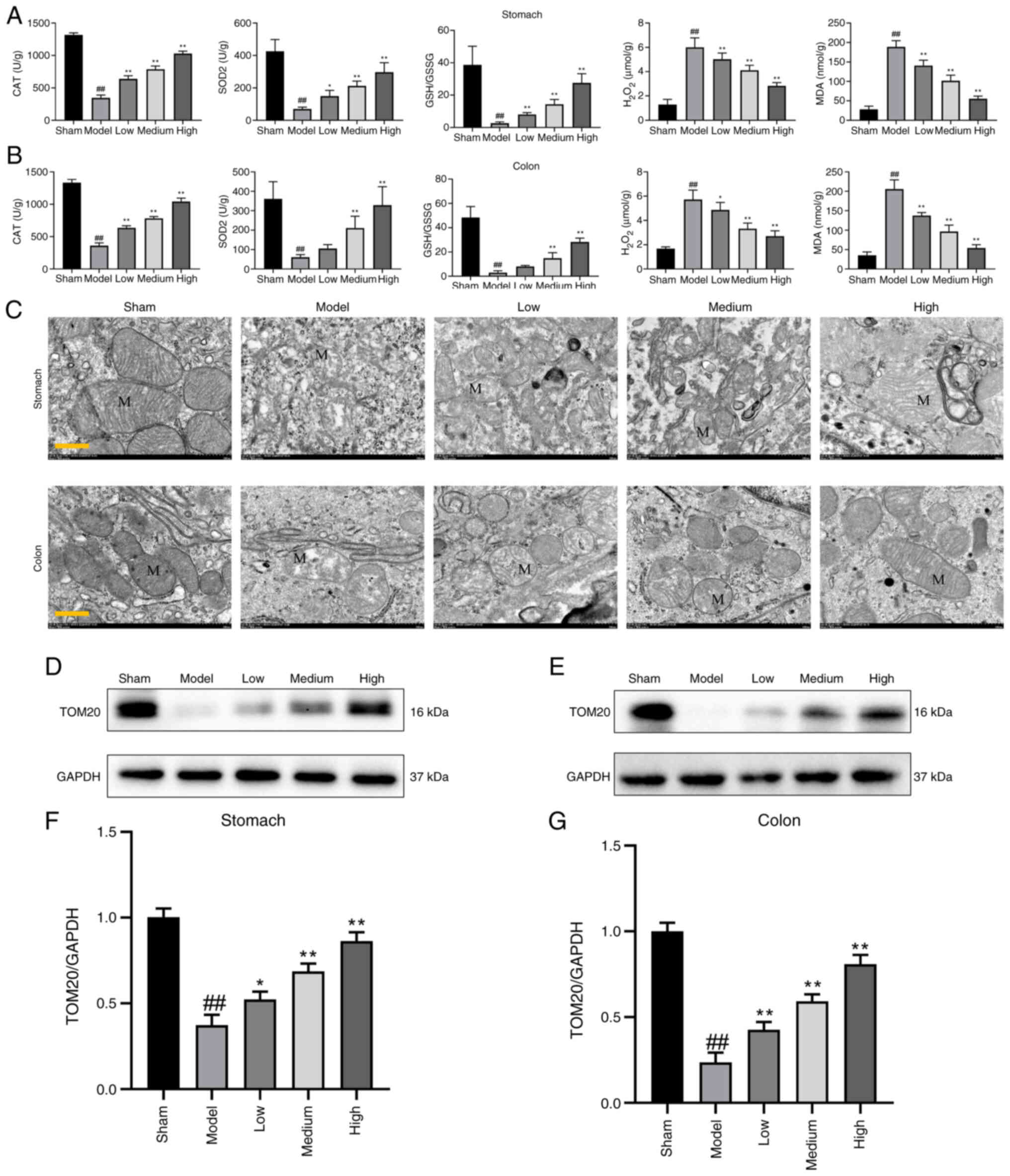
Wu H, Wang F, Ta N, Zhang T and Gao W: The
multifaceted regulation of mitochondria in ferroptosis. Life
(Basel). 11:2222021.
|
|
26
|
Gu J, Liu T, Guo R, Zhang L and Yang M:
The coupling mechanism of mammalian mitochondrial complex I. Nat
Struct Mol Biol. 29:172–182. 2022. View Article : Google Scholar
|
|
27
|
Ryu KW, Fung TS, Baker DC, Saoi M, Park J,
Febres-Aldana CA, Aly RG, Cui R, Sharma A, Fu Y, et al: Cellular
ATP demand creates metabolically distinct subpopulations of
mitochondria. Nature. 635:746–754. 2024. View Article : Google Scholar
|
|
28
|
Sazanov LA: A giant molecular proton pump:
structure and mechanism of respiratory complex I. Nat Rev Mol Cell
Biol. 16:375–388. 2015. View Article : Google Scholar
|
|
29
|
Lee HJ, Svahn E, Swanson JM, Lepp H, Voth
GA, Brzezinski P and Gennis RB: Intricate role of water in proton
transport through cytochrome c oxidase. J Am Chem Soc.
132:16225–16239. 2010. View Article : Google Scholar
|
|
30
|
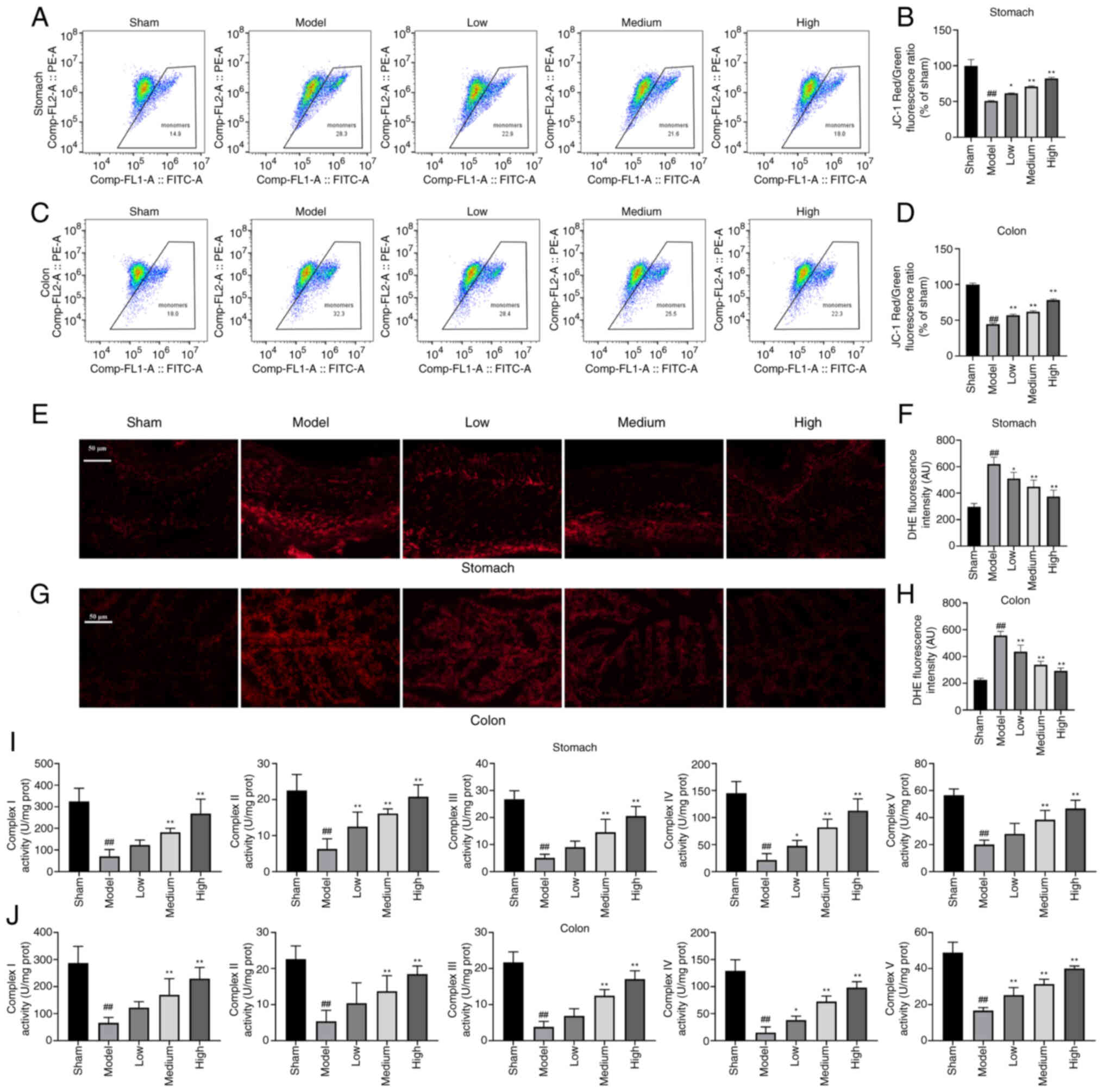
Chen Y, Guo X, Zeng Y, Mo X, Hong S, He H,
Li J, Fatima S and Liu Q: Oxidative stress induces mitochondrial
iron overload and ferroptotic cell death. Sci Rep. 13:155152023.
View Article : Google Scholar
|
|
31
|
Dixon SJ and Olzmann JA: The cell biology
of ferroptosis. Nat Rev Mol Cell Biol. 25:424–442. 2024. View Article : Google Scholar
|
|
32
|
Bryk R, Griffin P and Nathan C:
Peroxynitrite reductase activity of bacterial peroxiredoxins.
Nature. 407:211–215. 2000. View Article : Google Scholar
|
|
33
|
Kang SW, Chae HZ, Seo MS, Kim K, Baines IC
and Rhee SG: Mammalian peroxiredoxin isoforms can reduce hydrogen
peroxide generated in response to growth factors and tumor necrosis
factor-alpha. J Biol Chem. 273:6297–6302. 1998. View Article : Google Scholar
|
|
34
|
Cox AG, Winterbourn CC and Hampton MB:
Mitochondrial peroxiredoxin involvement in antioxidant defence and
redox signalling. Biochem J. 425:313–325. 2009. View Article : Google Scholar
|
|
35
|
Peskin AV, Low FM, Paton LN, Maghzal GJ,
Hampton MB and Winterbourn CC: The high reactivity of peroxiredoxin
2 with H(2)O(2) is not reflected in its reaction with other
oxidants and thiol reagents. J Biol Chem. 282:11885–11892. 2007.
View Article : Google Scholar
|
|
36
|
Nonn L, Berggren M and Powis G: Increased
expression of mitochondrial peroxiredoxin-3 (thioredoxin
peroxidase-2) protects cancer cells against hypoxia and
drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res.
1:682–689. 2003.
|
|
37
|
Lombard DB and Zwaans BM: SIRT3: As simple
as it seems? Gerontology. 60:56–64. 2014. View Article : Google Scholar
|
|
38
|
Shimazu T, Hirschey MD, Hua L,
Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt
FW, Denu JM, et al: SIRT3 deacetylates mitochondrial
3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body
production. Cell Metab. 12:654–661. 2010. View Article : Google Scholar
|
|
39
|
Hebert AS, Dittenhafer-Reed KE, Yu W,
Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ,
Higbee AJ, et al: Calorie restriction and SIRT3 trigger global
reprogramming of the mitochondrial protein acetylome. Mol Cell.
49:186–199. 2013. View Article : Google Scholar
|
|
40
|
Liang J, Zhou C, Zhang C, Liang S, Zhou Z,
Zhou Z, Wu C, Zhao H, Meng X, Zou F, et al: Nicotinamide
mononucleotide attenuates airway epithelial barrier dysfunction via
inhibiting SIRT3 SUMOylation in asthma. Int Immunopharmacol.
127:1113282024. View Article : Google Scholar
|
|
41
|
Wang T, Cao Y, Zheng Q, Tu J, Zhou W, He
J, Zhong J, Chen Y, Wang J, Cai R, et al: SENP1-Sirt3 signaling
controls mitochondrial protein acetylation and metabolism. Mol
Cell. 75:823–834.e5. 2019. View Article : Google Scholar
|
|
42
|
Wang X, Shen T, Lian J, Deng K, Qu C, Li
E, Li G, Ren Y, Wang Z, Jiang Z, et al: Resveratrol reduces
ROS-induced ferroptosis by activating SIRT3 and compensating the
GSH/GPX4 pathway. Mol Med. 29:1372023. View Article : Google Scholar
|
|
43
|
Na-Bangchang K, Kulma I, Plengsuriyakarn
T, Tharavanij T, Kotawng K, Chemung A, Muhamad N and Karbwang J:
Phase I clinical trial to evaluate the safety and pharmacokinetics
of capsule formulation of the standardized extract of
Atractylodes lancea. J Tradit Complement Med. 11:343–355.
2021. View Article : Google Scholar
|
|
44
|
Park SJ, Park J, Lee MJ, Seo JS, Ahn JY
and Cho JW: Time series analysis of delta neutrophil index as the
predictor of sepsis in patients with acute poisoning. Hum Exp
Toxicol. 39:86–94. 2020. View Article : Google Scholar
|
|
45
|
Geven C, Blet A, Kox M, Hartmann O,
Scigalla P, Zimmermann J, Marx G, Laterre PF, Mebazaa A and
Pickkers P: A double-blind, placebo-controlled, randomised,
multicentre, proof-of-concept and dose-finding phase II clinical
trial to investigate the safety, tolerability and efficacy of
adrecizumab in patients with septic shock and elevated
adrenomedullin concentration (AdrenOSS-2). BMJ Open. 9:e0244752019.
View Article : Google Scholar
|