Introduction
In recent years, the incidence of new global cancer
cases has been consistently increasing. As indicated in the World
Health Organization report, malignant neoplasms are among the
predominant causes of mortality worldwide, and it is estimated that
the global population of patients with cancer will reach 28.4
million by 2040 (1). The onset and
progression of malignant tumors are closely associated with the
tumor microenvironment, which comprises tumor cells, immune cells,
fibroblasts, the extracellular matrix and a variety of cytokines
that enhance the proliferation, migration and immune evasion
capabilities of tumor cells (2).
As research into the tumor microenvironment has increased, tumor
immunotherapy has emerged as a novel and promising therapeutic
modality for the management of cancer. This type of therapy
uniquely activates the immune microcirculation, thereby eliciting a
targeted immune response against tumor cells and facilitating their
destruction. Traditional Chinese Medicine (TCM) has been used in
the treatment of tumors, with an emphasis on individualized patient
care (3). Throughout the course of
tumor progression, TCM has demonstrated efficacy in bolstering
immunity, thereby restoring and maintaining normal immune
functions, preventing the invasion of exogenous pathogens and
mitigating the detrimental effects of tumor cells. These principles
are consistent with the modern medical concept of tumor
immunotherapy. Astragalus polysaccharide (APS) can modulate
various immune cells, including macrophages, dendritic cells (DCs),
myeloid-derived suppressor cells (MDSCs), T lymphocytes and natural
killer (NK) cells, thereby altering their functional activities and
enhancing immune responsiveness. Consequently, the tumor immune
microenvironment and the cytotoxic capability of immune cells
against tumor cells are improved (4). In the present study, research on the
antitumor immune properties of APS has been comprehensively
reviewed, and its regulatory effects on immune cells and the
underlying antitumor mechanisms are discussed, thereby providing a
theoretical foundation for the development of novel
Astragalus-based antitumor pharmacological agents.
Chemical structure of APS
Huangqi (Jianqi) is the dried root of the leguminous
plant Astragalus mongholicus or Astragalus
membranaceus (5). As a common
traditional Chinese herbal medicine, Astragalus has a history of
more than 2,000 years of use in China, is used extensively in
numerous countries and has been included in the pharmacopeias of
the United States, Japan, and South Korea (6). The constituents of Astragalus
encompass a variety of compounds such as APS, triterpenoid
saponins, alkaloids, flavonoids and different mineral elements
(7–10). APS, which serves as the principal
active constituent within Astragalus, is characterized by
its copious availability, affordability, and minimal toxic or
adverse effects (11). The
categorization of polysaccharides is performed in accordance with
the structural classification principles employed for proteins and
DNA. Investigating the constituent monosaccharides, relative
molecular mass and sequential arrangement of monosaccharide
residues is necessary to elucidate the structure of APS (12). APS is classified as an acidic
complex polysaccharide, predominantly comprising glucose (Glc),
galactose (Gal), arabinose (Ara), rhamnose (Rha) and mannose (Man).
Infrared spectroscopic analysis has revealed that APS possesses the
typical infrared absorption characteristics of sugar, along with
distinct signals that are attributed to the carboxyl group,
indicating the presence of glucuronic acid. The signals
corresponding to sugar rings indicate that APS constitutes a
pyranose ring structure with α and β configurations (13). Glucose is a monosaccharide based on
a glucopyranose structure, including α-D-Glu, β-D-Glu and α,
β-D-Glu, with β-D-Glu being the most extensively investigated
monosaccharide (14).
Given the presence of numerous branched chains and a
number of hydroxyl groups, APS exhibits a high degree of structural
diversity and complexity (Table
I). APS MAPS-5 is composed of an α-D-(1–4) Glc
backbone, where, on average, approximately two out of every 15
sugar residues at the C-6 position are substituted with terminal
glucose residues, with an approximate relative molecular weight of
1.32×104 (15).
Furthermore, three distinct polysaccharides, namely APS I, II and
III, have been isolated from the aqueous extract of
Astragalus roots sourced from Inner Mongolia. APS I is a
heteropolysaccharide composed of D-Glu, D-Gal, and L-Ara in a molar
ratio of 1.75:1.63:1, with an average relative molecular mass of
approximately 3.63×104. APS II and III are predominantly
composed of D-Glu, with average relative molecular weights of
~1.23×104 and 3.46×104, respectively. Their
structure primarily comprises α-(1→6)-D-Glc condensation linkages,
with a minor fraction of α-(1→6)-D-Glc condensation bonds (16). Through complete acid hydrolysis,
four types of APS have been differentiated, including two glucans
(AG-1 and AG-2) and two heteropolysaccharides (AH-1 and AH-2)
(17). AG-1 is a water-soluble
glucan, whereas AG-2 is water insoluble and is characterized as an
α-(1→4) glucan. AH-1 is an acidic, water-soluble polysaccharide;
after hydrolysis, AH-1 contains hexuronic acids (galacturonic and
glucuronic acids), glucose, rhamnose and arabinose, as identified
through paper chromatography, with a molar ratio of
1:0.04:0.02:0.01. AH-2 is also water soluble, and the presence of
glucose and arabinose can be confirmed via paper and gas
chromatography post-hydrolysis, with a molar ratio of 1:0.15
(17).
 | Table I.Chemical structure of APS. |
Table I.
Chemical structure of APS.
| First author,
year | Polysaccharide
name | Monosaccharide
composition | Relative molecular
mass | Quantity ratio of
matter | (Refs.) |
|---|
| Lin, 2009 | APS MAPS-5 | α-D-(1–4)
Glc |
1.32×104 | - | (15) |
| Fang, 1982 | APS I | D-Glu, D-Gal,
L-Ara |
3.63×104 | 1.75:1.63:1 | (16) |
| Fang, 1982 | APS II | D-Glu |
1.23×104 | - | (16) |
| Fang, 1982 | APS III | D-Glu |
3.46×104 | - | (16) |
| Huang, 1982 | AG-1 | Water-soluble
glucan | - | - | (17) |
| Huang, 1982 | AG-2 | α-(1→4) glucan | - | - | (17) |
| Huang, 1982 | AH-1 | Hexuronic acids
(Glc UA and Gal UA), | - |
1:0.04:0.02:0.01 | (17) |
|
|
| Glc, Rha and
Ara |
|
|
|
| Huang, 1982 | AH-2 | Glc and Ara | - | 1:0.15 | (17) |
| Tang, 2014 | APS-I | Man, Rha, Glc UA,
Gal UA, Glc, Gal |
1.06×104 |
29.12:1.89:4.00:1.35:1:81.97 | (18) |
| Tang, 2014 | APS-II | Man, Rha, Glc UA,
Gal UA, Glc, Gal, Xyl |
2.47×106 | 50.46:1.16:1:2.27:
2.66:15.72:7.86 | (18) |
| Cao, 2020 | APS | Rha, Gal UA, Glc,
Gal, Ara |
300-2×106 |
0.43:0.23:19.36:0.69:1 | (19) |
| Liu, 2020 | APS I | Gal, Glc | - | 1:49.76 | (20) |
| Liu, 2020 | APS II | Rha, Gal, Glc | - | 1:2.99:16.26 | (20) |
Tang et al (18) extracted and isolated
polysaccharides from Astragalus, and characterized their
physicochemical properties and structure. This previous study
revealed that the composition of monosaccharides in APS-I was
mannose (Man), rhamnose (Rha), Glc uronic acid (Glc UA), Gal UA,
Glc and Gal, with a substance amount ratio of
29.12:1.89:4.00:1.35:1:81.97, whereas the composition of
monosaccharides in APS-II was Man, Rha, Glc UA, Gal UA, Glc, Gal
and xylose, with a substance amount ratio of
50.46:1.16:1:2.27:2.66:15.72:7.86. The relative molecular mass of
APS-I was ~1.06×104, and the relative molecular mass of
APS-II was ~2.47×106. Cao et al (19) extracted from the experiment that
the total polysaccharide content of APS was 74.6%, the protein
content was 0.42%, and the relative molecular weight distribution
was 300–2×106. In addition, its monosaccharide
composition was Rha, Gal UA, Glc, Gal and Ara, with a substance
amount ratio of 0.43:0.23:19.36:0.69:1. Furthermore, a previous
study characterized the monosaccharide group of two polysaccharides
obtained from the isolation and purification of Astragalus
membranaceus from Mongolia using ion chromatography. The
results showed that APS I was composed of Gal and Glc, with a
substance amount ratio of 1:49.76, whereas APS II mainly consisted
of Rha, Gal and Glc, with a substance amount ratio of 1:2.99:16.26
(20). Although a number of
technical methods have been used to analyze APS, the specific
molecular structure of APS remains difficult to elucidate, which
results in certain obstacles in the study of the molecular
mechanism underlying APS bioactivity.
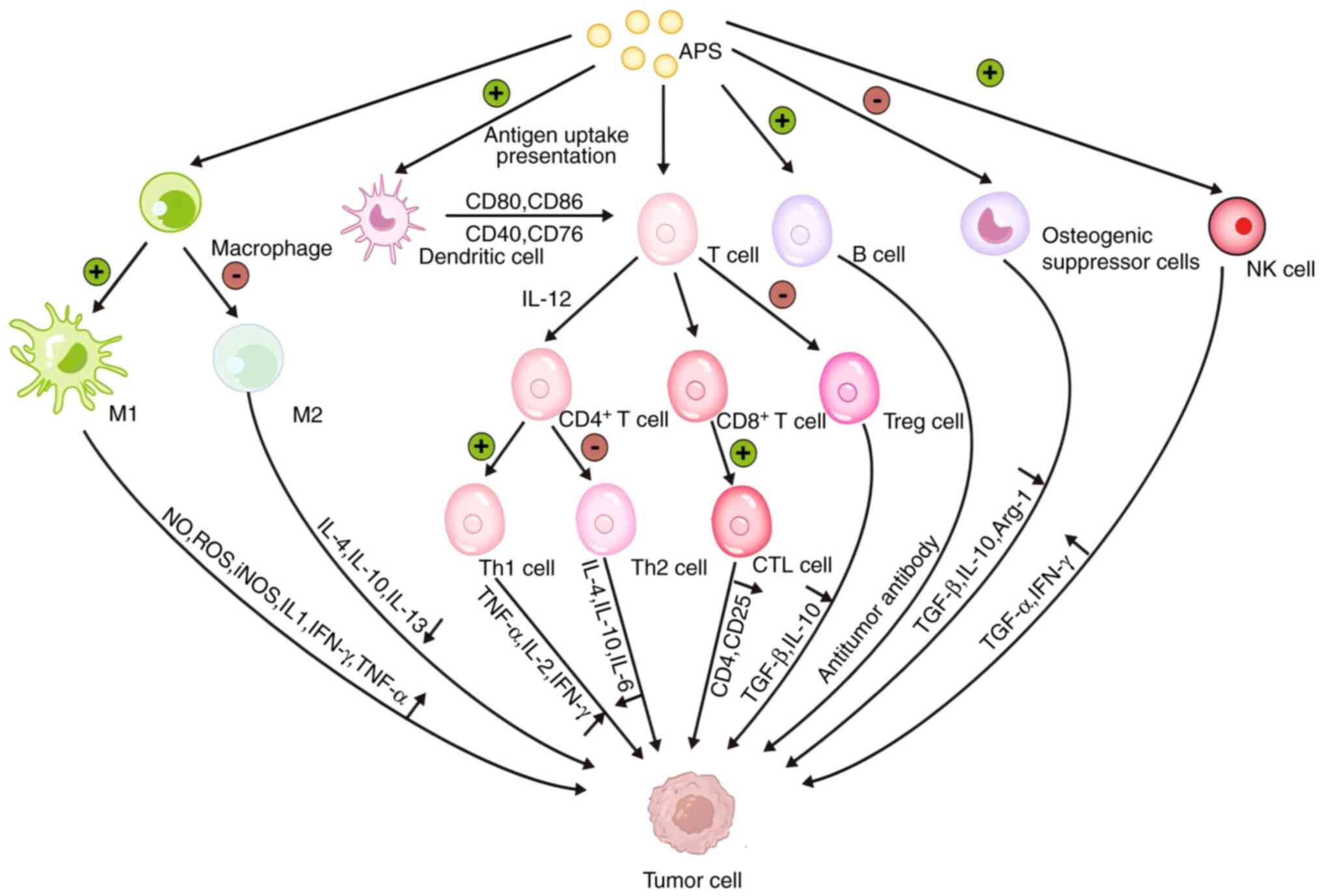
APS regulates tumor immune function
As academic research on tumor immunotherapy
continues, the successful development of immune checkpoint
inhibitors that target PD-1, CTLA-4 and PD-L1 has led to the
gradual emergence of immunotherapy as a potential method to treat
tumors (21). Body immunity is
divided into active and passive immunity. Tumor cells evade
elimination by the immune system, which not only promotes the state
of immunosuppression and the production of regulatory immune cells,
but also recruits a large number of protumor cells to establish a
tumor microenvironment (22). In
the tumor microenvironment, tumor cells inhibit the antigen
presentation function of DCs; promote the polarization of
tumor-associated macrophages from M1 type to M2 type; release the
immune-suppressive factors TGF-β, VEGF and IL-10; deliver the
abnormally expressed tumor-associated antigens to T cells; induce
T-cell apoptosis; and inhibit the function of cytotoxic T
lymphocytes, which together affect the immune system and result in
tumor immune escape and the acceleration of tumor progression
(23–26). In determining the mechanism
underlying tumor immune escape, current immunotherapeutic drugs,
including drugs that block negative regulatory signals,
immunostimulatory drugs, tumor vaccines and exogenous recombinant
cytokines, have been used (27),
among which immune checkpoint inhibitors, such as anti CTLA-4 and
anti-PD-1/PD-L1 antibodies, have shown good therapeutic effects in
clinical tumor therapy. A number of active ingredients of TCM have
shown effects on regulating immune cells and improving the tumor
microenvironment, indicating their therapeutic potential in
synergistic radiotherapy and chemotherapy against tumors (28).
APS, as a main active ingredient of
Astragalus, has important pharmacological effects, including
immune-regulatory and antitumor properties, which can regulate the
microenvironment of numerous solid tumors, improve the state of
immune suppression and inhibit tumor growth. APS inhibits the
M2-type polarization of tumor-associated macrophages, increases
cytotoxic T lymphocyte infiltration, and promotes the maturation of
DCs and the function of NK cells (29). In addition, APS not only reduces
the release of the immunosuppressive factors IL-10, TGF-β and VEGF
in the tumor microenvironment, but also increases the levels of the
immune activating factors TNF-α, IL-2 and IL-12 (30). In clinical trials for patients with
cancer, APS has been shown to relieve pain, nausea and vomiting
symptoms, enhance immune function, reduce the toxic side effects of
chemotherapeutic drugs and improve quality of life (31) (Table
II; Figs. 1 and 2).
 | Table II.Antitumor immune mechanism of
APS. |
Table II.
Antitumor immune mechanism of
APS.
| Pharmacological
action | Function | Intervention
model | (Refs.) |
|---|
| Regulation of
macrophage M1/M2 type polarization | Promoted the
production of cytokines, such as IL-6, TNF-α and inducible NO
synthase, through the Notch signaling pathway to activate M1
macrophages, while inhibiting macrophage M2-type polarization | Non-small cell lung
cancer; mouse macrophages | (36) |
|
| Influence cell-cell
interactions, specifically through modulation of macrophage M2
polarization and CD8+ | Macrophages | (37) |
|
| T-cell exhaustion,
thereby overcoming DDP resistance |
|
|
|
| Increased the
proportion of M1 macrophages in HCC tumor cell tissues and
decreased macrophage M2 type polarization | Macrophages in HCC
tumor cell tissues | (38) |
|
| Increased cellular
energy metabolism and enhanced phagocytosis of RAW 264.7 mouse
monocyte macrophages | RAW 264.7 monocyte
macrophages | (39,40) |
|
| Activated mouse
macrophages through TLR4-mediated signaling pathways, upregulated
the expression of p-p38, p-ERK1/2 and p-JNK, induced the
degradation of IκB-α and NF-κB translocation, and ultimately
produced TNF-α, IL-6 and nitric oxide | Macrophages | (41,42) |
|
| Enhanced the
proliferation and phagocytic function of mouse macrophages;
increased the content of IL-2, TNF-α and IFN-γ in the peripheral
blood of mice | Macrophages | (43) |
| Induces activation
of DCs | Induced the DC cell
surface molecule CD80 through the TLR2/4 receptor-mediated MyD88
signaling pathway, CD86 and IL-12 expression, increased T
lymphocyte activity and released more IL-2 | DCs | (49,50) |
|
| Activated DCs
through the TLR-mediated signaling pathway, increased the
expression of co-stimulatory molecules on the surface of DCs, and
enhanced the antigen-presenting ability of tumor cells, which led
to the rapid migration of DCs to lymph nodes, activated the initial
T lymphocytes in the lymph nodes and inhibited the growth of
secondary lymph node tumors | DCs | (51) |
|
| After APS
treatment, DCs promoted the proliferation of CD4+ and
CD8+ T cells in mice with breast cancer; DC-based
antitumor vaccine increased the expression of CD40, CD80 and CD86
molecules on the surface of DCs, and inhibited the proliferation
and metastasis of breast cancer tumor cells | DCs | (52) |
|
| APS activated BDCA1
and BDCA3 peripheral blood dendritic cells elicited proliferative
activation of autologous T cells | Monocyte-derived
DCs | (53) |
| Reduces the number
and activity of MDSCs | Suppress MDSC
generation and reduce the MDSC population in the spleens of
tumor-bearing mice. | MDSCs in the spleen
of tumor-bearing mice | (57) |
|
| Concurrently, APS
downregulates immunosuppressive cytokines (IL-10, TGF-β) while
upregulating pro-inflammatory cytokines (IFN-γ, TNF-α), thereby
enhancing overall immune function |
|
|
|
| Reduced the number
of MDSCs, decreased the immunosuppressive activity of MDSCs in mice
with melanoma, decreased the expression of Arg-1, IL-10 and TGF-β,
and enhanced the killing ability of CD8+ T cells on
tumor cells | MDSCs in mice with
melanoma | (58) |
|
| Reduced the level
of myeloid-derived suppressor cells in the peripheral circulation
of lung cancer, continuously improve imune function, and increas
teh overall clinical treatment efficacy | MDSCs | (59) |
|
| The protein and
gene expression of S1PR1, STAT3 and p-STAT3 in the S1PR1/STAT3
signaling pathway was inhibited by APS, which interfered with the
S1PR1/STAT3 signaling pathway and inhibited the accumulation of
MDSCs in the pre-metastatic niche | MDSCs | (61) |
| Reversal of Th1 to
Th2 conversion | Increased the
expression of Th1 cytokines IL-2 and IFN-γ, decreased the
expression of Th2 cytokines IL-4 and IL-10, regulated the ratio of
Th1/Th2 cells, reversed the conversion of Th1 to Th2 cells through
the TLR4/ MyD88/NF-κB signaling pathway | Th1 cells and Th2
cells in the spleen tissue of mice with lung cancer | (62) |
|
| Not only increased
the immune organ index in mice with lung cancer, but also increased
the ratio of CD4+/CD8+ cells in mouse serum
and significantly enhanced cellular immune activity |
CD4+/CD8+ T
cells | (63,64) |
|
| Protected the
immune organs and regulated the ratio of
CD4+/CD8+ T lymphocyte subpopulations |
CD4+/CD8+ T
lymphocyte subpopulations | (65) |
|
| Increases the
number of allogeneic T lymphocytes and the expression level of
IL-12 and IFN-γ. In addition, cytotoxic T lymphocytes activated by
sensitized DCs kill tumor cells | T lymphocyte | (66) |
| Suppression of Treg
cells | Reduced the number
of Treg cells, decreased the expression of | Treg cells | (69) |
|
| splenic TGF-β and
IL-10 in mice with malignant melanoma, |
|
|
|
| and inhibited tumor
cell metastasis of malignant melanoma in |
|
|
|
| mice Repaired
cytokine imbalance, inhibited forkhead box | Treg cells | (70) |
|
| protein P3
expression, and suppressed Treg cell activity and function |
|
|
|
| Inhibited the
expression of TGF-β by activating the TLR4-mediated signaling
pathway, which reduced the number of Treg cells | Treg cells | (71) |
|
| Reduced Treg
frequency, decreased anti-inflammatory cytokines including IL-10,
TGF-β, and VEGF-A, and incresaed the pro-inflammatory cytokine
IL-6 | Treg cells | (72) |
| Enhances NK cell
killing activity | Inhibited the
proliferation of leukemia cells by activating MHC class I
polypeptide-related sequence A molecules on the surface of leukemia
cells and enhanced the killing sensitivity of NK cells to HL-60
leukemia cells | NK cells | (78) |
|
| Protected immune
organs and stimulated NK cells to kill tumor cells; promoted the
proliferation of splenic lymphocytes, improved NK cell activity and
enhanced the immune response ability of rats with cancer | Lymphocytes and NK
cells | (79,80) |
| Inhibition of tumor
cell proliferation | Inhibited the
proliferation of ovarian cancer cells through the miR-27a/F-box and
WD-40 structural domain protein 7 signaling pathway | Ovarian cancer
cells | (81) |
|
| Inhibited the
proliferation and migration of NSCLC cells and its mechanism of
action was related to the regulation of the miR-195-5p signaling
pathway; improved the morphology of lung cancer cells by inhibiting
the lung cancer cell cycle | NSCLC cells | (83,84) |
|
| Inhibited
TGF-β1-induced EMT of A549/DDP cell xenografts of lung
adenocarcinoma, which was associated with the inhibition of PI3K/
Akt pathway protein activation | A549/DDP lung
adenocarcinoma cell graft tumor | (85) |
|
| APS had an
inhibitory effect on the cell proliferation of nasopharyngeal
carcinoma cell lines in a time-and dose-dependent manner; the
combination of APS and cisplatin inhibited the migration and
invasion of CNE-1 cells | CNE-1
nasopharyngeal carcinoma cells | (86) |
|
| Inhibited the
proliferation of SW620 human colorectal cancer cells, which were
subjected to cyclic inhibition, with a predominant G2/M
phase block | SW620 colorectal
cancer cells | (87) |
|
| Inhibited the
proliferation and invasion of PCa cells in a time-and
dose-dependence manner; inhibited tumorigenesis and lipid
metabolism by modulating the miR-138-5p/SIRT1/SREBP1 pathway in
PCa | PCa cells | (88) |
|
| Enhanced miR-133a
expression and inhibited the JNK signaling pathway in MG63
osteosarcoma cells, which suppressed proliferation, migration and
invasion, and induced apoptosis in MG63 cells | MG63 osteosarcoma
cells | (89) |
| Induces apoptosis
in tumor cells | Promotes the
anti-proliferative and apoptotic effects of cisplatin on
nasopharyngeal carcinoma cells, by regulating the expression levels
of Bax/Bcl-2, caspase-3, and caspase-9 | Nasopharyngeal
carcinoma cells | (94) |
|
| Decreased the
viability of GC cells and accelerated their apoptosis in a time-
and dose-dependent manner; MGC-803 cells treated with APS showed
typical apoptotic morphology and cell cycle arrest in S phase | GC cells | (95) |
|
| Promoted apoptosis
in NSCLC cell lines by inhibiting the expression of Notch1 and
Notch3, and upregulating tumor suppressors | NSCLC cells | (97–101) |
|
| Induced apoptosis
of tumor cells and prevented cell transformation from G1
phase to S phase of cell cycle | - | (102) |
|
| Decreased the
number of HepG2 liver cancer cells in S phase, and differentiated
the cells into G0/G1 and G2/M
phases, which blocked the cellular proliferation cycle and induceed
cancer cell apoptosis | HepG2 cells | (103,104) |
|
| Promoted the
apoptosis of HepG2 cells, increased the activity of caspase-3,
decreased the expression of ERK1/2, inhibited the ERK1/2 signaling
pathway and promoted the apoptosis of tumor cells | HepG2 cells | (105) |
|
| APS
dose-dependently promoted Dox-induced apoptosis and enhanced ER
stress; decreased O-GlcNAc transferase mRNA levels and protein
stability, and increased O-GlcNAcase expression; promoted
Dox-induced apoptosis in HCC cells by decreasing O-GlcNAcylation,
leading to increased ER stress and activation of apoptotic
pathways | HCC cells | (114) |
| Inhibits tumor cell
invasion and metastasis | Inhibited the
growth and metastasis of Lewis lung cancer in mice, improved the
function of the immune organs, and inhibited the expression of VEGF
and EGFR proteins in tumor tissues | Lewis lung
cancer | (116) |
|
| Downregulated NF-κB
transcriptional activity, and significantly inhibited the
proliferation and metastasis of A549 and NCI-H358 NSCLC cells | A549 and NCI-H358
NSCLC cells | (117,118) |
|
| Inhibited
angiogenesis of human colorectal cancer tumors and decreased VEGF
expression in tumor tissue | Colorectal cancer
tumor | (119) |
|
| Inhibited the
proliferation and invasion of HS-746T cells, suppress the
expression of miR-25 mRNA, and promote the expression of FBXW7
mRNA. It can also reverse the increase in MiR-25 and the
suppression of FBXW7 caused by transfection with miR-25 minic. APS
inhibits the proliferation and invasion of gastric cancer HS-746-T
cells through the miR-25/FBXW7 signaling pathway. | Gastric cancer
HS-746T cells | (120) |
|
| Promoted
inactivation of the JNK signaling pathway through the upregulation
of miR-133a, which inhibited the proliferation, metastasis and
invasion, and induced the apoptosis of MG63 osteosarcoma cells | MG63 osteosarcoma
cells | (89,121) |
|
| Inhibited the
Wnt/β-catenin signaling pathway and reduced breast cancer cell
proliferation and EMT-mediated migration and invasion | Breast cancer
cells | (122,123) |
|
| Moesin is the
target of miR-133a-3p, miR-133a-3p negatively regulates MSN; APS
attenuated PD-L1-mediated immunosuppression through the
miR-133a-3p/MSN pathway | HCC | (124) |
| Removal and
inhibition of free radicals | Increased
superoxide dismutase activity, reduced malondialdehyde content, and
scavenged reactive oxygen species and reactive nitrogen species
generated by intracellular oxidative stress, but also reduced free
radical generation | - | (127) |
| Reversal or escape
of MDR | Inhibited TGF-β1
overexpression-mediated EMT, and suppressed the proliferation of
cisplatin-resistant A549 cell xenograft tumors | A549-cell
transplanted tumors | (131) |
|
| Induced apoptosis
in GC cells and enhanced the pro-apoptotic effect of adriamycin on
GC cells; APS acted as a chemotherapeutic sensitizer to a certain
extent | GC cells | (95) |
|
| Induced apoptosis
in HL-60/A cells, activated the caspase cascade reaction,
significantly decreased the expression of MDR-associated protein
and increased the concentration of intracellular antitumor
drugs | HL-60/A cells | (132) |
|
| Improved the
sensitivity of tumor cells to chemotherapeutic drugs, such as
apatinib, escaped MDR and strengthened the antitumor effect | - | (133) |
|
| Increased the
expression levels of IL-6 and TNF-α, and decreased the expression
of IL-10, P-glycoprotein and MDR1. | H22 hepatocellular
carcinoma mice | (134) |
|
| APS enhanced the
antitumor effect of adriamycin and the sensitization effect of
chemotherapy drugs |
|
|
|
| Activated the JNK
signaling pathway and enhanced the sensitivity of SKOV3 cells to
cisplatin | SKOV3 cells | (135) |
|
| Promoted
gefitinib-resistant cell apoptosis and decreased cell proliferation
and migration; inhibited the PD-L1/SREBP-1/EMT signaling pathway,
increased E-cadherin expression, decreased N-cadherin and vimentin
protein expression, and reversed acquired resistance to gefitinib
in lung cancer cells | Lung cancer
cells | (136) |
Regulation of M1/M2 macrophage
polarization
Macrophages develop from bone marrow precursor cells
and serve an important role in the immune system. They are present
in almost all tissues of the body, and they directly phagocytose
and kill foreign pathogens. In addition, they can activate
lymphocytes or other immune cells in the body to indirectly respond
to pathogens, and protect tissues and organs from foreign pathogens
(32). Macrophages are classified
into M1 and M2 types. M1 macrophages, as classically activated
macrophages, produce nitric oxide (NO), reactive oxygen species
(ROS), inducible NO synthase and a large number of pro-inflammatory
cytokines, such as IL-1β, interferon (IFN)-γ and TNF-α, which
participate in the positive immune response. M1 macrophages also
have a role in phagocytosis and tumor cell killing (33).
By contrast, M2 macrophages, as alternatively
activated macrophages, produce a large number of anti-inflammatory
cytokines, such as IL-4, IL-10 and IL-13, which suppress the immune
response to a certain extent. Tumor cells selectively recruit bone
marrow-derived macrophages to differentiate into M2 macrophages,
that is, tumor-associated macrophages (34), which promote tumor cell
proliferation, neovascularization, invasion and metastasis
(35). Adjustment of M1/M2
macrophage polarization is key to improve the effectiveness of
antitumor therapy. In an experimental study in mice with non-small
cell lung cancer (NSCLC), APS modulates the Notch signaling
pathway, promotes the production of IL-6, TNF-α and iNOS cytokines,
activates M1 macrophages, inhibits macrophage M2-type polarization,
and enhances phagocytosis and tumor cell killing (36). Bioinformatics analysis indicates
that APS may remodel the tumor microenvironment (TME) and influence
cell-cell interactions, specifically through modulation of
macrophage M2 polarization and CD8+ T cell exhaustion, thereby
overcoming DDP resistance (37).
Li et al (38) reported
that APS can increase the proportion of M1 macrophages in
hepatocellular carcinoma cell-derived xenograft tumors, reduce
macrophage M2-type polarization and inhibit the proliferation of
hepatocellular carcinoma tumor cells. Furthermore, APS improves the
energy metabolism of mouse macrophages in vivo, and enhances
the phagocytic activity of RAW 264.7 monocyte macrophages and the
immune response in mice (39,40).
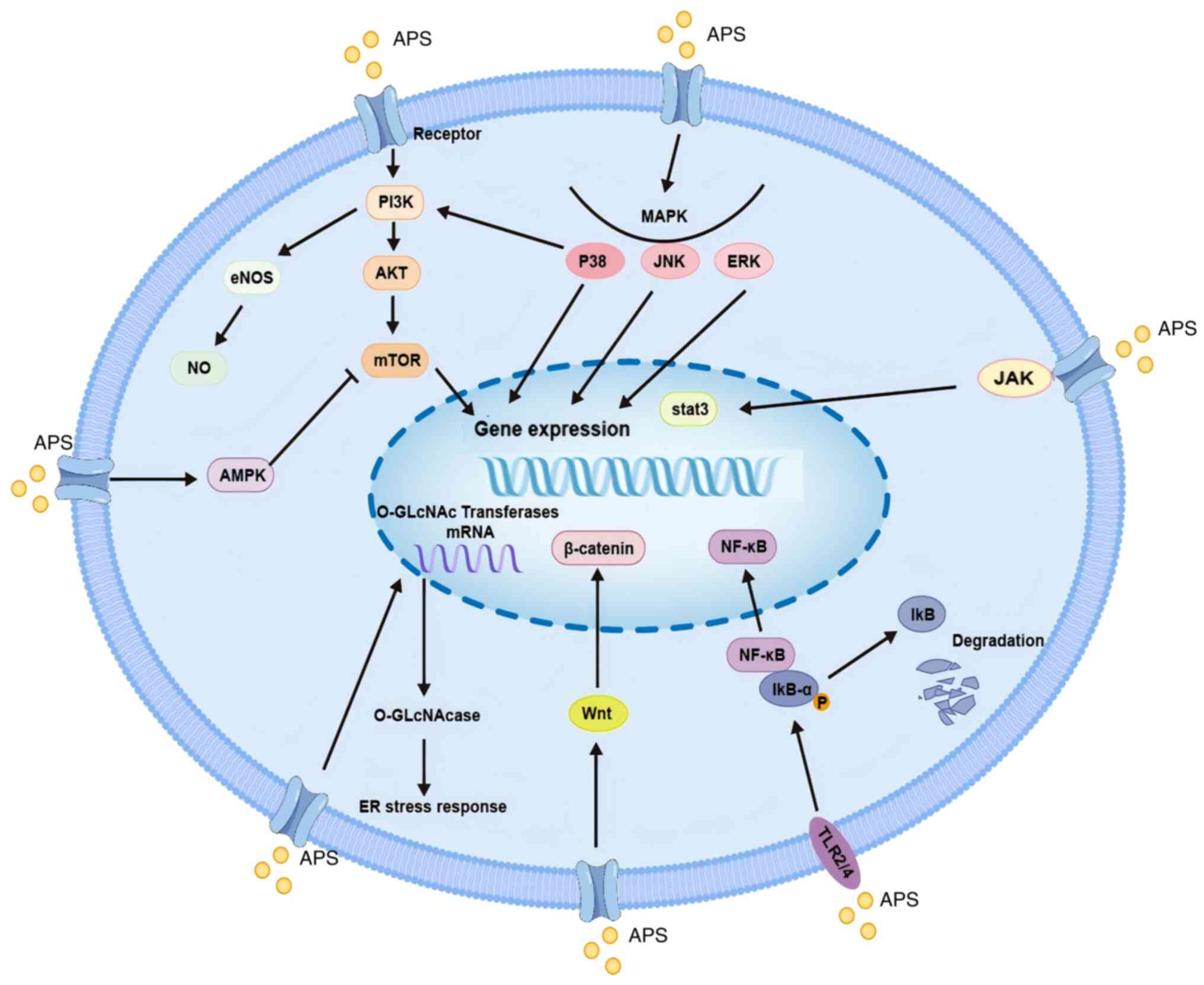
Mouse macrophages activated by APS are triggered through the
Toll-like receptor (TLR)4-mediated signaling pathway; this
activation event results in the upregulation of the levels of
phosphorylated (p)-p38, p-ERK1/2 and p-JNK. Concurrently, it
induces the degradation of IκB-α and the subsequent translocation
of NF-κB. Eventually, these molecular events culminate in the
production of TNF-α, IL-6 and NO, thereby exerting an inhibitory
effect on the proliferation of mouse tumor cells (41,42).
Li et al (43) demonstrated
that APS enhances the proliferation and phagocytic functions of
mouse macrophages, increases the peripheral blood levels of IL-2,
TNF-α and IFN-γ, and improves the ability of macrophages to
phagocytose tumor cells and inhibit the proliferation of tumor
cells. Therefore, APS may enhance macrophage phagocytosis and tumor
cell killing, and inhibit tumor cell proliferation and metastasis
by increasing M1 macrophage activity, regulating M1/M2-type
macrophage polarization and altering the levels of immune
cytokines.
Induced activation of DCs
DCs are derived from bone marrow CD34+
hematopoietic stem cells and are a specialized class of
antigen-presenting cells, which are divided into immature DCs
(imDCs), mature DCs (mDCs) and regulatory DCs, in accordance with
the different stages of cell differentiation (44). ImDCs capture tumor antigens and are
stimulated to differentiate into mature mDCs. The mDCs migrate to
draining lymph nodes via lymphatic vessels, where they present the
antigens to T cells. They secrete various co-stimulatory molecules
such as CD80, CD86, CD40 and CD70, which further activate effector
T cells, thereby inducing and maintaining anti-tumor immunity
(45,46). Enhancing the function of DCs and
promoting T-cell activation in patients with cancer are an
effective means of antitumor immunotherapy. APS promotes DC
maturation, enhances DC antigen-presenting ability, improves the
accuracy of tumor cell killing and inhibits tumor cell
proliferation (47). When DCs are
induced to mature further, the expression of the DC surface
co-radical molecules CD80 and CD86 are enhanced. DCs present
antigens to T lymphocytes, T lymphocytes increase IL-12 and IFN-γ
secretion, improve antitumor immunity and inhibit the proliferation
of tumor cells (48).
APS induces the DC cell surface molecule CD80, CD86
and IL-12 expression through the TLR2/4 receptor-mediated MyD88
signaling pathway, increases T lymphocyte activity, releases more
IL-2 and enhances antitumor ability (49,50).
APS nanoparticles have been shown to modulate the TLR-mediated
signaling pathway to activate DCs, to increase the expression
levels of co-stimulatory molecules on the surface of DCs and
enhance their tumor cell antigen-presenting ability. Subsequently,
DCs migrate rapidly to lymph nodes, activate the initial T
lymphocytes at lymph nodes and inhibit secondary lymph node tumor
growth (51). Furthermore, after
APS treatment, DCs have been shown to promote the proliferation of
CD4+ and CD8+ T cells in mice with breast
cancer, and a DC-based antitumor vaccine can increase the
expression levels of CD40, CD80 and CD86 molecules on the surface
of DCs, and inhibit the growth and metastasis of breast cancer
cells (52). APS induces
morphological changes, the expression of activation markers and the
upregulation of inflammatory cytokines in human blood
monocyte-derived DCs. In addition, APS promotes the activation of
blood DC antigen (BDCA)1 and BDCA3 peripheral blood dendritic cells
(pBDCs), which results in the proliferative activation of T cells
(53). Therefore, APS may induce
the activation of DCs, increased the expression levels of
co-stimulatory factors on the surface of DCs, and accelerate the
maturation of DCs and antigen presentation. Subsequently, this may
enhance the proliferation and activation of CD4+ and
CD8+ T cells, and thus the activation of immune function
under the stimulation of cytokines, thereby effectively inhibiting
the proliferation and metastasis of tumor cells.
Reduced activity of MDSCs
MDSCs are a group of heterogeneous cells derived
from the bone marrow; the number of MDSCs in the peripheral blood
and spleen is small, accounting for only 4% of cells (54). Under pathological conditions, these
immature myeloid-derived heterogeneous cells stop differentiating
and become immunosuppressive MDSCs (55,56).
MDSCs promote tumor cell survival, angiogenesis, and invasion and
metastasis to healthy tissues.
APS inhibit MDSC activity, promote MDSC
differentiation and reduce MDSC population. The underlying
mechanisms represent a current research hotspot in immunotherapy
(51). APS suppress MDSC
generation and reduce the MDSC population in the spleens of
tumor-bearing mice. Concurrently, APS downregulates
immunosuppressive cytokines (IL-10, TGF-β) while upregulating
pro-inflammatory cytokines (IFN-γ, TNF-α), thereby enhancing
overall immune function (57).
Ding et al (58) revealed
that APS exert a marked regulatory effect on gut microbiota. APS
effectively remodels the gut microbiota environment, and elevates
the levels of glutamate and creatine metabolites. Notably,
glutamate and creatine serve crucial roles in controlling tumor
growth. Simultaneously, APS contributes to the reduction in the
number of MDSCs. Furthermore, in mice with melanoma, APS has been
shown to curtail the immunosuppressive activity of MDSCs, as
manifested by decreased expression levels of Arg-1, IL-10 and
TGF-β; this series of changes bolsters the cytotoxic capacity of
CD8+ T cells against tumor cells. Consequently, this
previous study demonstrated that the tumor inhibition rate in mice
with melanoma was significantly increased, highlighting the
potential of APS in antitumor regulation. APS has been reported to
effectively reduce the level of myeloid-derived suppressor cells in
the peripheral circulation of lung cancer, continuously improve
immune function, and increase the overall clinical treatment
efficacy (59). As a common
malignant tumor, lung cancer has shown an increasing trend in
morbidity and mortality in recent years (60). Multi-targeted, low-toxicity Chinese
medicines intervene in the ‘pre-metastatic niche’, a potentially
effective means of preventing and treating tumor metastasis. APS
has been reported to inhibit the formation of the lung
pre-metastatic niche and suppress the recruitment of lung MDSCs.
Mechanistically, the expression of S1PR1, STAT3 and p-STAT3 in the
S1PR1/STAT3 signaling pathway have been shown to be inhibited by
APS, which interferes with the S1PR1/STAT3 signaling pathway and
inhibits the accumulation of MDSCs in the pre-metastatic niche to
achieve antitumor effects (61).
Therefore, APS promote the rapid differentiation of MDSCs, reduce
the number and activity of MDSCs, influence the release of immune
cytokines, enhance immune functions, and inhibit the proliferation
and metastasis of tumor cells.
Reversal of Th1 to Th2 conversion
T lymphocytes develop from bone marrow pluripotent
stem cells, differentiate and mature under the induction of thymic
hormone, and become immunologically active T cells, playing
antitumor specific immunity. T lymphocytes are classified into CD4+
T and CD8+ T cells in accordance with their surface molecular
types. CD4+ and CD8+ are the two functionally different
subpopulations of T lymphocytes. CD4+ T cells are also known as
helper T cells, which play a role in assisting the induction of
body immunity. Th cells are differentiated into functional
subpopulations such as Th1 cells, Th2 cells, and Th17 cells. The
main functional subpopulation of CD8+ T cells is cytotoxic T
lymphocytes. Th1 cells secrete IL-2 and TNF-α and stimulate the
development of cytotoxic T lymphocytes. TNF-α, which stimulates the
activation of macrophages and NK cells, also secretes IFN-γ to kill
tumor cells. Th2 cells secrete IL-4, IL-6, and IL-10, which assist
B lymphocytes to produce specific antibodies and participate in
humoral immunity. Under normal circumstances, the number of Th1 and
Th2 cells remains relatively balanced. However, in patients with
tumor, a transformation from Th1 cells to Th2 cells occurs, which
suppresses the body's cellular immunity. APS enhances the
expression of Th1 cytokines such as IL-2 and IFN-γ in the spleen
tissues of mice with lung cancer through the TLR4/MyD88/NF-κB
signaling pathway while reducing the expression of Th2 cytokines,
including IL-4 and IL-10. It also regulates the ratio of Th1/Th2
cells, reverses the transformation from Th1 to Th2, improves the
immunity of mice with lung cancer, and inhibits the growth of tumor
cells (62). APS not only improves
the immune organ index in mice with lung cancer, but also increases
the ratio of CD4+/CD8+ T cells in mouse serum and enhances cellular
immune activity (63,64). APS protects immune organs,
regulates the ratio of CD4+/CD8+ T lymphocyte subpopulations, and
inhibits the growth of mouse tumors in vivo; APS also
activates antitumor immune cells, promotes the tumor
microenvironment of anaerobic metabolism, and effectively induced
apoptosis in tumor cells (65).
APS increases the number of allogeneic T lymphocytes and the
expression level of IL-12 and IFN-γ. In addition, cytotoxic T
lymphocytes activated by sensitized DCs kill tumor cells (66). APS reverses Th1-to-Th2 conversion,
maintains Th1/Th2 cell balance, regulates CD4+/CD8+ T cell ratio
and T cell-related cytokine expression, enhances cellular immunity,
and inhibits tumor growth.
Suppression of regulatory T cells
(Tregs)
Tregs are a group of T lymphocytes that can regulate
the immune response and maintain immune tolerance. Tumor
immunotherapy reduces the number of Tregs, inhibits their cellular
activity, maintains immune function of the body and achieves the
killing effects against tumor cells. DCs are a key target for Tregs
to inhibit the tumor immune response (67), and Tregs interact with
tumor-associated CD11c+ DCs to reduce the expression of
DC co-stimulatory ligands and inhibit T-cell proliferation.
Conversely, the clearance of Tregs leads to the restoration of
CD11c+ DC immunogenicity, co-stimulatory ligand
expression and CD8+ T-cell activation, which in turn
inhibits tumor growth (68).
APS has been reported to reduce the number of Tregs,
decrease the expression levels of splenic TGF-β and IL-10, and
inhibit the proliferation and metastasis of malignant melanoma
tumor cells in mice (69). APS
also repairs cytokine imbalance, inhibits forkhead box protein P3
expression, and suppresses Treg activity and function (70). Through the CXCR4/CXCL12 signaling
pathway, APS has been shown to inhibit the recruitment of Tregs by
stromal cell-derived factor-1, and to attenuate the proliferation
and migration of Tregs. Furthermore, APS activates the
TLR4-mediated signaling pathway, thereby inhibiting the expression
of TGF-β, decreasing the number of Tregs and sustaining the killing
effect of immune cells on tumor cells (71). APS significantly enhanced the PBMC
proliferation, reduced Treg frequency, decreased anti-inflammatory
cytokines including IL-10, TGF-β, and VEGF-A, and increased the
pro-inflammatory cytokine IL-6 (72). Therefore, APS may inhibit Treg
activity by reducing the number of Tregs and their cytokine
secretion, thus restoring and maintaining the normal immune
function of T lymphocytes, achieving the killing effect of T cells
on tumor cells, and inhibiting tumor growth and metastasis.
Enhanced NK cell killing activity
NK cells are derived from bone marrow lymphoid stem
cells, which are important members of the lymphocyte family, and
they are not controlled by high-resolution antigen specificity. NK
cells can rapidly recognize and remove heterogeneous cells, and the
degree of attenuation in vivo is closely related to the
malignant progression of tumors (73). NK cells secrete cytokines, regulate
adaptive immunity and kill tumor cells, and they have killing and
inhibitory effects on hepatocellular carcinoma, NSCLC, breast
cancer and ovarian cancer cells (74–77).
APS has been reported to inhibit the proliferation of leukemia
cells by activating MHC class I polypeptide-related sequence A
molecules on the surface of leukemia cells, and enhances the
sensitivity of HL-60 cells to NK cell killing activity and inhibit
leukemia cell growth (78).
Low-molecular-weight APS can protect the immune organs of mice with
hepatocellular carcinoma and stimulate NK cells to kill tumor
cells. In addition, APS promotes the proliferation of splenic
lymphocytes, increases the activity of NK cells and enhances the
immune response of rats with cancer (79,80).
Antitumor effects of APS
Inhibition of tumor cell
proliferation
Tumor cell proliferation primarily aggravates cancer
progression, and pharmacological intervention in tumor cell
proliferation serves an important role in tumor therapy. F-box and
WD-40 structural domain protein 7 (FBXW7) is a classical tumor
suppressor, the translation of which is inhibited by microRNA
(miR)-27a in ovarian cancer cells. APS inhibits the proliferation
of ovarian cancer cells through the miR-27a/FBXW7 signaling
pathway, revealing the therapeutic potential of APS for ovarian
cancer (81). Lung cancer is the
second most common and the deadliest type of cancer worldwide.
Clinically, non-small cell lung cancer (NSCLC) is the most common
pathological type of lung cancer (82). APS can inhibit the proliferation
and migration of NSCLC cells, and its mechanism of action is
related to the regulation of the miR-195-5p signaling pathway; in
addition, APS inhibits the lung cancer cell cycle and improves the
morphology of lung cancer cells (83,84).
APS inhibit TGF-β1-induced epithelial-mesenchymal transition (EMT)
in A549/DDP cell xenografts of lung adenocarcinoma, which is
associated with inhibition of PI3K/Akt pathway protein activation
(85). In a study exploring the
synergistic inhibitory effect of APS and cisplatin on the growth of
human carcinoma nasopharyngeal cell (CNE), APS was shown to exert
an inhibitory effect on the proliferation of nasopharyngeal
carcinoma cells in a time- and dose-dependent manner, and the
combined application of APS and cisplatin could inhibit the
migration and invasion of CNE-1 cells, which was more effective
than the use of either drug alone with regard to the antitumor
effect of either drug (86). In
addition, the study demonstrated that APS at varying concentrations
inhibited the proliferation of human colorectal cancer SW620 cells,
inducing SW620 cell cycle arrest predominantly at the G2/M phase
(87). In addition, APS can
markedly inhibit the proliferation and invasion of prostate cancer
(PCa) cells in a time- and dose-dependent manner. Under APS
treatment, the cellular triacylglycerol and cholesterol levels of
patients were revealed to be markedly reduced, and APS can regulate
the miR-138-5p/SIRT1/SREBP1 pathway in PCa to inhibit lipid
metabolism and tumorigenesis (88). Furthermore, APS enhances the
expression of miR-133a in MG63 osteosarcoma cells, inhibits the JNK
signaling pathway, the proliferation, migration and invasion of
MG63 cells, and induces the apoptosis of MG63 cells (89).
Inducing tumor cell apoptosis
Apoptosis is a type of programmed cell death
regulated by various genes, which serves an important role in
tumorigenesis and development (90). The occurrence of malignant tumors
is due to the uncontrolled and excessive proliferation of tumor
cells. In recent years, promoting the apoptosis of tumor cells has
become a focus of research for the treatment of tumors. There are
two main apoptotic pathways: i) The extrinsic pathway, which is
mediated by the activation of caspases through the cell membrane
death receptor; and ii) the intrinsic pathway, which is mediated by
mitochondria in the cytoplasm that release apoptotic factors and
activate caspases. These activated caspases degrade the key
proteins in the cell, causing apoptotic cell death. The genes and
proteins that have been studied include caspase-3, caspase-8,
caspase-9, the Bcl-2 family and cyclooxygenase-2 (91). In the mitochondrion-mediated
intrinsic apoptotic pathway, proteins belonging to the Bcl-2 family
alter mitochondrial membrane permeability, regulate cytochrome
c release and modulate the apoptotic process (92). Bax and Bcl-2 belong to the Bcl-2
superfamily, which are key genes in the regulation of apoptosis,
and they are in a balanced state in normal organisms. Notably,
apoptosis can be induced by a decrease in the expression levels of
the apoptosis inhibitory protein Bcl-2, or an increase in the
expression levels of the pro-apoptotic protein Bax (93).
APS markedly promotes the anti-proliferative and
apoptotic effects of cisplatin on nasopharyngeal carcinoma cells,
by regulating the expression levels of Bax/Bcl-2, caspase-3, and
caspase-9 (94). APS also markedly
decreases gastric cancer (GC) cell viability, and accelerates GC
cell apoptosis in a time- and dose-dependent manner; these effects
are associated with an increase in p-AMPK. APS-treated MGC-803
cells have been shown to exhibit the typical morphological features
of apoptosis, alongside cell cycle arrest at the S phase (95). A novel cold-water-soluble
polysaccharide (APS4) was isolated from Astragalus
membranaceus, induce mitochondria-dependent apoptosis, which is
associated with intracellular ROS accumulation, mitochondrial
membrane potential imbalance, increased
pro-apoptotic/anti-apoptotic Bax/Bcl-2 ratio and cytochrome
c release (96).
The Notch signaling has an important role in the
transformation and proliferation of human malignant tumors, and it
is readily activated in human non-small cell lung carcinoma,
whereas the inhibitors of Notch signaling can attenuate cell
proliferation and induce apoptosis in lung cancer cell lines. APS
has been shown to inhibit the expression of Notch1 and Notch3 in
NSCLC cell lines to upregulate the pro-apoptotic Bax and caspase 8,
promoting apoptosis in NSCLC cell lines (97-101). APS may also inhibit tumor cells
of A549, MC38 and B16, induce apoptosis and prevent cell
transformation from the G1 phase to the S phase of the
cell cycle, and is associated with the expression of molecules
related to immunogenic cell death and the regulation of immune
cells (102). APS has also been
reported to decrease the number of HepG2 liver cancer cells in
S-phase, and cells were arrested at the G0-G1 and G2-M phases,
block the cellular proliferation cycle and induce apoptosis in
cancer cells (103,104). Wang (105) investigated the role of ERK1/2 in
the promotion of apoptosis in HepG2 cells by APS and found that
caspase-3 activity is increased, ERK1/2 expression is decreased and
APS inhibits the ERK1/2 signaling pathway to promote apoptosis in
tumor cells.
Protein O-GlcNAc modification is a unique
post-translational modification, and various nuclear and
cytoplasmic proteins can be modified by the O-GlcNAcylation
of free hydroxyl groups of selected serine and threonine residues
(106). The cell modification
cycle is mediated by O-GlcNAc transferase, which transfers
N-acetylglucosamine to the protein substrate, and the
O-GlcNAcase enzyme, which removes this modification from the
protein (107–109). O-GlcNAcylation affects a
wide range of protein functions, including transcription,
subcellular localization, protein-protein interactions and protein
stability (110). In numerous
types of cancer, O-GlcNAc modification is associated with
endoplasmic reticulum (ER) stress, and reduced
O-GlcNAcylation leads to the activation of the ER stress
response in a variety of cancer cells (111–113). APS promotes doxorubicin
(Dox)-induced apoptosis and ER stress response in a dose-dependent
manner. In addition, APS decreases O-GlcNAc transferase mRNA
levels and protein stability. and increases O-GlcNAcase
expression level. APS thus reduces O-GlcNAcylation and
promotes Dox-induced apoptosis in hepatocellular carcinoma cells,
leading to the exacerbation of ER stress and the activation of
apoptotic pathways (114).
Suppression of tumor cell invasion and
metastasis
Invasion and metastasis are crucial biological
characteristics of malignant tumors, as well as important factors
contributing to tumor recurrence following surgery. Tumor cells
disseminate to new organs and tissues through the blood and
lymphatic systems, subsequently proliferating. Notably, tumor
vasculature serves as a conduit for metastasizing tumor cells;
therefore, inhibiting tumor cell angiogenesis can impede the
progressive deterioration of tumors and aid in the treatment of
neoplastic diseases. Vascular endothelial growth factor (VEGF) is
widely recognized as a pivotal mediator of tumor angiogenesis
(115). APS effectively inhibits
tumor cell metastasis, with APS efficiently suppressing Lewis lung
cancer growth and metastasis in mice while enhancing immune organ
function. Moreover, it suppresses the protein expression levels of
VEGF and EGFR in tumor tissues, exhibiting a
concentration-dependent relationship (116). The NF-κB signaling pathway has a
critical role in lung cancer prevention and treatment; APS
significantly downregulates NF-κB transcriptional activity, thereby
inhibiting the proliferation and metastasis of A549 and NCI-H358
NSCLC cells (117,118). Furthermore, the angiogenesis of
human colorectal cancer tumors is markedly inhibited by APS, which
is closely associated with the reduction in VEGF expression within
tumor tissues (119). APS can
inhibit the proliferation and invasion of HS-746T cells, suppress
the expression of miR-25 mRNA, and promote the expression of FBXW7
mRNA. It can also reverse the increase in miR-25 and the
suppression of FBXW7 caused by transfection with miR-25 mimic. APS
can inhibit the proliferation and invasion of gastric cancer
HS-746T cells through the miR-25/FBXW7 signaling pathway (120). miR-133, which serves as a tumor
suppressor, inhibits proliferation, migration in tumor cells and
promotes their apoptosis. APS has been shown to upregulate the
expression levels of miR-133a, promote the inactivation of the JNK
signal transduction pathway, and inhibit the proliferation,
metastasis and invasion of MG63 osteosarcoma cells while inducing
apoptosis (89,121). EMT serves a crucial role in tumor
migration and invasion. APS can serve as a therapeutic agent for
breast cancer by inhibiting the Wnt/β-catenin signaling pathway to
reduce proliferation and the EMT-mediated migration and invasion of
breast cancer cells (122,123).
Moesin (MSN) serves as a potential biomarker associated with tumor
invasion and metastasis while maintaining PD-L1 stability. In
hepatocellular carcinoma, MSN is targeted by miR-133a-3p, which
negatively regulates its expression; notably, APS attenuates
PD-L1-mediated immunosuppression by modulating the miR-133a-3p/MSN
pathway (124).
Scavenging and inhibiting free
radicals
Under physiological conditions, free radicals
enhance immune function; by contrast, under pathological
conditions, free radicals cause damage to normal tissues and cells,
leading to diseases (125).
Scavenging free radicals is another important tool for tumor
prevention and treatment. The activities of free radical-scavenging
enzymes, such as catalase and superoxide dismutase (SOD), are
markedly decreased in patients with tumors, whereas the content of
lipid peroxides and lipid peroxidation products such as
malondialdehyde (MDA) are elevated (126). In addition, the anti-oxidative
stress function is decreased. APS have been reported to reduce free
radical production, increase SOD activity, decrease MDA content,
and scavenge ROS and reactive nitrogen species generated by
intracellular oxidative stress (127).
Reversal or escape of multidrug
resistance (MDR)
In patients with cancer who are receiving
chemotherapy, most treatment failures or deaths are attributed to
tumor MDR (128). Modern
pharmaceutical research focuses on drugs that can reverse or escape
MDR, and the key to reversing tumor drug resistance lies in
blocking the MDR pathway, reducing drug efflux and improving the
chemosensitivity of tumor cells (129). APS markedly increases tumor
response to chemotherapeutic drugs and reverses the MDR effect,
while reducing the toxicity response induced by chemotherapeutic
drugs (130). APS can also
inhibit TGF-β1 overexpression-mediated EMT, and inhibit the
proliferation of cisplatin-resistant A549 cell xenograft tumors
(131). In vitro
experiments confirmed that APS induces apoptosis in GC cells and
enhances the pro-apoptotic effect of Adriamycin on GC cells, and
that APS serves as a chemotherapeutic sensitizer to a certain
extent (95). In addition, in the
HL-60/A cell line with high drug resistance, APS induces apoptosis,
activates the caspase cascade reaction, decreases the expression
levels of MDR-associated protein and increases the concentration of
intracellular antitumor drugs (132). Furthermore, APS increases the
sensitivity of tumor cells to chemotherapeutic drugs, such as
apatinib, escapes MDR and strengthens the antitumor effect
(133). In the experimental study
using H22 hepatoma cell xenograft mouse models, the expression
levels of IL-6 and TNF-α were increased, whereas those of IL-10,
P-glycoprotein and MDR1 were decreased in the APS treatment group.
In addition, APS was shown to enhance the antitumor effect of
Adriamycin (134). APS can also
serve as a chemosensitive enhancer by activating the JNK signaling
pathway and enhancing the sensitivity of SKOV3 ovarian cancer cells
to cisplatin (135). Furthermore,
APS has been shown to enhance the sensitivity of HeLa cervical
cancer cells to cisplatin chemotherapy by upregulating the
autophagy-related protein beclin1, promoting the conversion of LC3
I to LC3 II, and downregulating the marker protein p62 to enhance
the autophagic activity of HeLa cells (133). APS promotes the apoptosis of
gefitinib-resistant cells, and reduces cell proliferation and
migration. Moreover, APS inhibits the PD-L1/SREBP-1/EMT signaling
pathway, increases the expression levels of E-cadherin, reduces the
expression levels of N-cadherin and vimentin, and reverses the
acquired resistance of lung cancer cells to gefitinib (136).
Summary and outlook
Astragalus membranaceus has a medicinal
history of >2,000 years in China. As an active ingredient with
the highest content in Astragalus membranaceus, APS has
shown great application potential in regulating the tumor
microenvironment and improving immunity. Numerous previous
experimental studies have confirmed that APS can regulate the
activation of immune cells and the release of cytokines in the
tumor microenvironment, as well as inhibit tumor immune escape. The
combination of APS and chemotherapeutic drugs may alleviate the
adverse reactions of patients after chemotherapy and improve their
quality of life, which provides great reference for the use of APS
as an adjuvant in clinical antitumor medication. APS also has
certain effects on the treatment of other diseases, such as
diabetes and inflammatory diseases (137). Therefore, the therapeutic use of
APS for the treatment of other diseases requires further
exploration.
The structure of APS is complex; thus, its
bioavailability can be further improved through chemical means,
such as structural modification, so that it can serve a better
targeted role in cancer. Although APS has been proven to have good
application potential in antitumor immunity, it still faces a
number of problems: i) The structure of APS is complex, and it
contains a large number of chemical components. Its extraction,
separation, purification and structural analysis are currently the
biggest challenges, which require further in-depth exploration. ii)
Tumor immune escape is a complex process in the tumor
microenvironment. Tumor cells can not only directly affect the
function of immune cells, but also indirectly inhibit immunity by
regulating immune checkpoints and cytokines. Although APS can
improve the function of immune cells in the tumor microenvironment,
the specific mechanism by which APS regulates immune cells remains
unclear. iii) At present, most of the studies on the antitumor
effect of APS immunotherapy are limited to cell experiments or
in vivo experiments using mice, and evidence from
clinical-related trials is lacking. Therefore, more abundant
clinical trials are needed for verification. In conclusion, the
research on the mechanism of action of APS in regulating immune
cells, improving the tumor immune microenvironment and resisting
tumors, as well as further research on the mechanism by which APS
serves a regulatory role in the immunotherapy of malignant tumors,
is of great importance to the research and development of clinical
antitumor drugs. This research may improve the treatment of
tumor-related diseases by combining traditional Chinese and Western
medicine.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82173996).
Availability of data and materials
Not applicable.
Authors' contributions
ZQ, ZW and YW conceived and designed the study. ZQ
wrote the manuscript. YW and ZW revised the manuscript. WC and XX
conducted literature organization and proofreading Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI
|
|
2
|
Liu Y, Zhao Y, Song H, Li Y, Liu Z, Ye Z,
Zhao J, Wu Y, Tang J and Yao M: Metabolic reprogramming in tumor
immune microenvironment: Impact on immune cell function and
therapeutic implications. Cancer Lett. 597:2170762024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie D, Wang N, Chen Z, Liu Z, Zhou Z, Zeng
J, Zhong Y and Wang Z: Medication Rules and Core Formula Analysis
of Famous National Traditional. Chinese Medicine Masters for Cancer
Treatment Based on Data Mining World Chin Med. 15:2726–2734.
2020.(In Chinese).
|
|
4
|
He Z, Liu X, Qin S, Yang Q, Na J, Xue Z
and Zhong L: Anticancer mechanism of Astragalus
polysaccharide and its application in cancer immunotherapy.
Pharmaceuticals (Basel). 17:6362024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
National Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China (Part 1).
Pharmaceutical Science and Technology Press. (Beijing, China).
3632020.(In Chinese).
|
|
6
|
Su HF, Shaker S, Kuang Y, Zhang M, Ye M
and Qiao X: Phytochemistry and cardiovascular protective effects of
Huang-Qi (Astragali Radix). Med Res Rev. 41:1999–2038. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tong X: The Pharmacology Study of Active
Constituents from Astragalus. Lishizhen Medicine and Materia
Medica Research. 22:1246–1249. 2011.(In Chinese).
|
|
8
|
Xiao M, Chen H, Shi Z and Rui W: Rapid and
reliable method for analysis of raw and honey-processed
Astragalus by UPLC/ESI-Q-TOF-MS using HSS T3 columns. Anal
Methods. 6:8045–8054. 2014. View Article : Google Scholar
|
|
9
|
Liao J, Huang J, Liu W, Liao S, Chen H and
Rui W: Effects of total flavonoids and astragalosides of
honey-processed Astragalus on NO secretion levels. J
Guangdong Pharm Univ. 33:162–166. 2017.(In Chinese).
|
|
10
|
Huang J, Liao J, Liu W, Liao S, Feng Y,
Chen H and Rui W: Establishment of determination method for three
main active ingredients of honey-processed Astragalus in rat urine
based on UPLC/Q-TOF-MS. Lishizhen Med Mater Med Res. 28:2564–2567.
2017.(In Chinese).
|
|
11
|
Zhong RZ, Yu M, Liu HW, Sun HX, Cao Y and
Zhou DW: Effects of dietary Astragalus polysaccharide and
Astragalus membranaceus root supplementation on growth
performance, rumen fermentation, immune responses, and antioxidant
status of lambs. Animal Feed Sci Technol. 174:60–67. 2012.
View Article : Google Scholar
|
|
12
|
Zeng P, Li J, Chen Y and Zhang L: The
structures and biological functions of polysaccharides from
traditional Chinese herbs. Prog Mol Biol Transl Sci. 163:423–444.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan X, Li K, Yang Y, Li H, Li X and Qin X:
Research Progress on structural characterization of astragalus
polysaccharides. J Shanxi Coll Tradit Chin Med. 23:260–267.
2022.(In Chinese).
|
|
14
|
Synytsya A and Novák M: Structural
diversity of fungal glucans. Carbohydr Polym. 92:792–809. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin MG, Yang YF and Xu HY: Chemical
structure of Astragalus polysaccharide MAPS-5 and its in
vitro lymphocyte proliferative activity. Chin Tradit Herbal Drugs.
40:1865–1868. 2009.(In Chinese).
|
|
16
|
Fang SD, Chen Y, Xu XY and Ye C: Studies
on the Active Principles of Astragalus Mongholicus Bunge I.
Isolation, Characterization and Biological Effect of its
Polysaccharaides. Chin J Org Chem. 2:26–31. 1982.(In Chinese).
|
|
17
|
Huang Q, Lu G, Li Y, Guo J and Wang R:
Studies on the polysaccharides of “Huang Qi” (Astragalus
Mongholicus Bunge.). Acta Pharm Sin. 17:200–206. 1982.(In
Chinese).
|
|
18
|
Tang Y, Zhang Y, Wang Y, Zhao H and Shen
Y: Isolation and Structural Feature Analysis of Astragalus
Polysaccharides. Lishizhen Med Mater Med Res. 25:1097–1100.
2014.(In Chinese).
|
|
19
|
Cao Y, Li K, Qin X, Jiao S, Du Y, Li S and
Li X: Quality evaluation of different areas of Astragali Radix
based on carbohydrate specific chromatograms and immune cell
activities. Acta Pharmaceutica Sinica. 54:1277–1287. 2019.(In
Chinese).
|
|
20
|
Liu W, Yu X, Xu J, et al: Isolation,
structural characterization and prebiotic activity of
polysaccharides from Astragalus membranaceus. Food Ferment
Ind. 46:50–56. 2020.(In Chinese).
|
|
21
|
Bergman PJ: Cancer immunotherapies. Vet
Clin North Am Small Anim Pract. 49:881–902. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rui R, Zhou L and He S: Cancer
immunotherapies: Advances and bottlenecks. Front Immunol.
14:12124762023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Helmy KY, Patel SA, Nahas GR and Rameshwar
P: Cancer immunotherapy: Accomplishments to date and future
promise. Ther Deliv. 4:1307–1320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ganesh K, Stadler ZK, Cercek A, Mendelsohn
RB, Shia J, Segal NH and Diaz LA Jr: Immunotherapy in colorectal
cancer: Rationale, challenges and potential. Nat Rev Gastroenterol
Hepatol. 16:361–375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J,
Li J, Li F and Tan HB: Immune cells within the tumor
microenvironment: Biological functions and roles in cancer
immunotherapy. Cancer Lett. 470:126–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katz JB, Muller AJ and Prendergast GC:
Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune
escape. Immunol Rev. 222:206–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Velcheti V and Schalper K: Basic overview
of current immunotherapy approaches in cancer. Am Soc Clin Oncol
Educ Book. 35:298–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Li M, Bi L, Hu X and Wang Y:
Traditional Chinese medicine in regulating tumor microenvironment.
Onco Targets Ther. 17:313–325. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong F, Chen T, Li X and Jia Y: The
current application and future prospects of Astragalus
polysaccharide combined with cancer immunotherapy: A review. Front
Pharmacol. 12:7376742021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou L, Liu Z, Wang Z, Yu S, Long T, Zhou
X and Bao Y: Astragalus polysaccharides exerts
immunomodulatory effects via TLR4-mediated MyD88-dependent
signaling pathway in vitro and in vivo. Sci Rep. 7:448222017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang WC, Kuo KT, Bamodu OA, Lin YK, Wang
CH, Lee KY, Wang LS, Yeh CT and Tsai JT: Astragalus
polysaccharide (PG2) ameliorates cancer symptom clusters, as well
as improves quality of life in patients with metastatic disease,
through modulation of the inflammatory cascade. Cancers (Basel).
11:10542019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bonnardel J and Guilliams M: Developmental
control of macrophage function. Curr Opin Immunol. 50:64–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li M, Yang Y, Xiong L, Jiang P, Wang J and
Li C: Metabolism, metabolites, and macrophages in cancer. J Hematol
Oncol. 16:802023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei W, Li ZP, Bian ZX and Han QB:
Astragalus polysaccharide RAP induces macrophage phenotype
polarization to M1 via the notch signaling pathway. Molecules.
24:20162019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen R, Li Y, Zuo L, Xiong H, Sun R, Song
X and Liu H: Astragalus polysaccharides inhibits tumor
proliferation and enhances cisplatin sensitivity in bladder cancer
by regulating the PI3K/AKT/FoxO1 axis. Int J Biological
Macromolecules. 311:1437392025. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li C, Pan XY, Ma M, Zhao J, Zhao F and Lv
YP: Astragalus polysacharin inhibits hepatocellular
carcinoma-like phenotypes in a murine HCC model through repression
of M2 polarization of tumour-associated macrophages. Pharm Biol.
59:1531–1537. 2021. View Article : Google Scholar
|
|
39
|
Li SY, Li K, Qin XM, Li XR and Du YG:
Study on active constituents of Astragalus polysaccharides
for injection to regulate metabolism of macrophages based on LC-MS
metabolomics. Chin Tradit Herb Drugs. 51:1575–1585. 2020.(In
Chinese).
|
|
40
|
Feng S, Ding H, Liu L, Peng C, Huang Y,
Zhong F, Li W, Meng T, Li J, Wang X, et al: Astragalus
polysaccharide enhances the immune function of RAW264.7 macrophages
via the NF-κB p65/MAPK signaling pathway. Exp Ther Med. 21:202021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shao BM, Xu W, Dai H, Tu P, Li Z and Gao
XM: A study on the immune receptors for polysaccharides from the
roots of Astragalus membranaceus, a Chinese medicinal herb.
Biochem Biophys Res Commun. 320:1103–1111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei W, Xiao HT, Bao WR, Ma DL, Leung CH,
Han XQ, Ko CH, Lau CB, Wong CK, Fung KP, et al: TLR-4 may mediate
signaling pathways of Astragalus polysaccharide RAP induced
cytokine expression of RAW264.7 cells. J Ethnopharmacol.
179:243–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li W, Hu X, Wang S, Jiao Z, Sun T, Liu T
and Song K: Characterization and anti-tumor bioactivity of
Astragalus polysaccharides by immunomodulation. Int J Biol
Macromol. 145:985–997. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin W, Liu T, Wang B and Bi H: The role of
ocular dendritic cells in uveitis. Immunol Lett. 209:4–10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Villar J and Segura E: Decoding the
heterogeneity of human dendritic cell subsets. Trends Immunol.
41:1062–1071. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chudnovskiy A, Pasqual G and Victora GD:
Studying interactions between Dendritic Cells and T cells in vivo.
Curr Opin Immunol. 58:24–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bamodu OA, Kuo KT, Wang CH, Huang WC, Wu
ATH, Tsai JT, Lee KY, Yeh CT and Wang LS: Astragalus
polysaccharides (PG2) enhances the M1 polarization of macrophages,
functional maturation of dendritic cells, and T cell-mediated
anticancer immune responses inpatients with lung cancer. Nutrients.
11:22642019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jing XN, Qiu B, Wang JF, Wu YG, Wu JB and
Chen DD: In vitro anti-tumor effect of human dendritic cells
vaccine induced by Astragalus polysacharin: An experimental
study. Zhongguo Zhong Xi Yi Jie He Za Zhi. 34:1103–1107. 2014.(In
Chinese). PubMed/NCBI
|
|
49
|
Gu Y, Pu X, Yin N and Wan C: The study on
mechanism of the anti-tumor effect of Fufang Huangqi Oral Solution
combined chemotherapy. J Tianjin Univ Tradit Chin Med. 41:85–89.
2022.(In Chinese).
|
|
50
|
Bian Y, Zhang J, Wang L, Wang X, Zhang K,
Wang X, Meng J and Li J: Progress of research on the effect of
Astragalus polysaccharide on the morphology and function of
dendritic cells. Prog Vet Med. 37:86–89. 2016.(In Chinese).
|
|
51
|
Pang G, Chen C, Liu Y, Jiang T, Yu H, Wu
Y, Wang Y, Wang FJ, Liu Z and Zhang LW: Bioactive polysaccharide
nanoparticles improve radiation-induced abscopal effect through
manipulation of dendritic cells. ACS Appl Mater Interfaces.
11:42661–42670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chang WT, Lai TH, Chyan YJ, Yin SY, Chen
YH, Wei WC and Yang NS: Specific medicinal plant polysaccharides
effectively enhance the potency of a DC-based vaccine against mouse
mammary tumor metastasis. PLoS One. 10:e01223742015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
An EK, Zhang W, Kwak M, Lee PC and Jin JO:
Polysaccharides from Astragalus membranaceus elicit T cell
immunity by activation of human peripheral blood dendritic cells.
Int J Biol Macromol. 223:370–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lasser SA, Ozbay Kurt FG, Arkhypov I,
Utikal J and Umansky V: Myeloid-derived suppressor cells in cancer
and cancer therapy. Nat Rev Clin Oncol. 21:147–164. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xia L, Zhao S, Wei Q and Xu X: Study on
changes of myeloid-derived suppressor cells in tumor tissues of
tumor-bearing mice and its significance. Curr Immunol. 34:232–236.
2014.(In Chinese).
|
|
56
|
Hao WB, Xiang FF, Liu QL, Yue HK and Kang
XD: Research adances of myeloid inhibitory cells in tumor
microenvironment. Immunol J. 33:729–732. 2017.(In Chinese).
|
|
57
|
Chai W, He X, Zhu J, Lv C, Zhao H, Lv A
and Yv C: Immunoregulatory effects of astragalus polysaccharides on
myeloid derived suppressor cells in B16-F10 tumor bearing mice.
Chin J Basic Med Tradit Chin Med. 18:63–65. 2012.(In Chinese).
|
|
58
|
Ding G, Gong Q, Ma J, Liu X, Wang Y and
Cheng X: Immunosuppressive activity is attenuated by
Astragalus polysaccharides through remodeling the gut
microenvironment in melanoma mice. Cancer Sci. 112:4050–4063. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mei Q, Zhang W, Ran R and Wang J: Effect
of astragalus polysaccharide on peripheral circulation MDSC in lung
cancer and its clinical effect. Modern Doctor. 55:10–13. 2017.(In
Chinese).
|
|
60
|
Li Y, Yan B and He S: Advances and
challenges in the treatment of lung cancer. Biomed Pharmacother.
169:1158912023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shen M, Wang YJ, Liu ZH, Chen YW, Liang
QK, Li Y and Ming HX: Inhibitory effect of Astragalus
polysaccharide on premetastatic niche of lung cancer through the
S1PR1-STAT3 signaling pathway. Evid Based Complement Alternat Med.
2023:40107972023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu Y, Yuan J, Guo M, Zhang Y, Lu J and
Cao W: Effect of astragalus polysaccharides based on
TLR4/MyD88/NF-κB signaling pathway on immune function of lung
cancer mice and its regulatory effect on Th1/ Th2. Chin J Immunol.
37:676–682. 2021.(In Chinese).
|
|
63
|
Li K, Li S, Wang D, Li X, Wu X, Liu X, Du
G, Li X, Qin X and Du Y: Extraction, characterization, antitumor
and immunological activities of hemicellulose polysaccharide from
Astragalus radix herb residue. Molecules. 24:36442019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou X, Liu Z, Long T, Zhou L and Bao Y:
Immunomodulatory effects of herbal formula of Astragalus
polysaccharide (APS) and polysaccharopeptide (PSP) in mice with
lung cancer. Int J Biol Macromol. 106:596–601. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu J, Dong XD, Jiao JS, Ji HY and Liu AJ:
Antitumor and immunoregulatory activities of a novel polysaccharide
from Astragalus membranaceus on S180 tumor-bearing mice. Int
J Biol Macromol. 189:930–938. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Deng M, Chen Z, Dou X, Shi Y and Wo X: The
Anti - leukemia Effect Mediated by DCs Derived from the Human Cord
Blood Monocyte Induced by the Astragalus Polysaccharides (APS) in
Vitro. Chin Arch Tradit Chin Med. 27:2360–2365. 2009.(In
Chinese).
|
|
67
|
Jang JE, Hajdu CH, Liot C, Miller G,
Dustin ML and Bar-Sagi D: Crosstalk between regulatory T cells and
tumor-associated dendritic cells negates anti-tumor immunity in
pancreatic cancer. Cell Rep. 20:558–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mentucci FM, Ferrara MG, Ercole A, Rumie
Vittar NB and Lamberti MJ: Interplay between cancer-associated
fibroblasts and dendritic cells: implications for tumor immunity.
Front Immunol. 16:15153902025. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sun S, He X, Chai W, Lv C, Wang P, Lv A
and Yu C: Effects of Astragalus Polysaccharide on Treg Cells
in Tumor-bearing Mice. Chin J Exp Tradit Med Formulae. 19:176–178.
2013.(In Chinese).
|
|
70
|
Li Q, Bao JM, Li XL, Zhang T and Shen XH:
Inhibiting effect of Astragalus polysaccharides on the
functions of CD4+CD25 highTreg cells in the tumor microenvironment
of human hepatocellular carcinoma. Chin Med J (Engl). 125:786–793.
2012.PubMed/NCBI
|
|
71
|
Du X, Chen X, Zhao B, Lv Y, Zhang H, Liu
H, Chen Z, Chen Y and Zeng X: Astragalus polysaccharides
enhance the humoral and cellular immune responses of hepatitis B
surface antigen vaccination through inhibiting the expression of
transforming growth factor β and the frequency of regulatory T
cells. FEMS Immunol Med Microbiol. 63:228–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shokati E, Safari E and Motalebnezhad M:
Astragalus Polysaccharide Mediates Immunomodulatory Effects on
Crosstalk between Human Peripheral Blood Mononuclear Cells and
Ovarian Cancer Cell Line. Iran J Allergy Asthma Immunol.
22:172–182. 2023.PubMed/NCBI
|
|
73
|
Guillerey C and Smyth MJ: NK cells and
cancer immunoediting. Curr Top Microbiol Immunol. 395:115–145.
2016.PubMed/NCBI
|
|
74
|
Zhou J, Peng H and Tian ZG: Research
progress on negative regulation of adaptive immune response by NK
cells. Chin J Immunol. 32:769–776. 2016.(In Chinese).
|
|
75
|
Wang W, Xi J, Zhao Y, He L, Zhou J, Fan Z,
Zhang B, Wang H, Zeng Q, et al: Specific cytotoxicity of natural
killer cells against hepatocellular carcinoma and potential
mechanism. Mil Med Sci. 41:775–783. 2017.(In Chinese).
|
|
76
|
Yang Y, Li H, Guo S, Li X, Yin J, Zhao P,
Gao L and Wu S: Killing effect of NK cells on non-small cell lung
cancer cells NCI-H292 and the effect on their β-catenin protein
expression. Shandong Med J. 57:33–35. 2017.(In Chinese).
|
|
77
|
Liu C, Zhang Y and Lu H: Efficient
amplification of NK cells and their anti-tumor cytotoxic activity
in vitro. Chin J Immunol. 33:1186–1190+1196. 2017.(In Chinese).
|
|
78
|
Zeng PY, Deng LL, Yue LL and Zhang L:
Effect of Astragalus Polysaccharide on Sensitivity of Leukemic Cell
Line HL- 60 to NK Cell Cytotoxicity and Its Mechanism. J Exp
Hematol. 20:880–883. 2012.(In Chinese).
|
|
79
|
Li H: Effects of Astragalus Polysaccharide
on Quality of Life and Cellular Immune Function in Elderly Patients
with Advanced Pancreatic Cancer. Chinese Journal of Traditional
Chinese Medicine and Pharmacy. 17:243–244. 2010.(In Chinese).
|
|
80
|
Li R, Chen WC, Wang WP, Tian WY and Zhang
XG: Extraction, characterization of Astragalus
polysaccharides and its immune modulating activities in rats with
gastric cancer. Carbohydr Polym. 78:738–742. 2009. View Article : Google Scholar
|
|
81
|
Guo Y, Zhang Z, Wang Z, Liu G, Liu Y and
Wang H: Astragalus polysaccharides inhibit ovarian cancer
cell growth via microRNA-27a/FBXW7 signaling pathway. Biosci Rep.
40:BSR201933962020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Miao D, Zhao J, Han Y, Zhou J, Li X, Zhang
T, Li W and Xia Y: Management of locally advanced non-small cell
lung cancer: State of the art and future directions. Cancer Commun
(Lond). 44:23–46. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tao X, Zhang X and Feng F:
Astragalus polysaccharide suppresses cell proliferation and
invasion by up-regulation of miR-195-5p in non-small cell lung
cancer. Biol Pharm Bull. 45:553–560. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang YM, Liu YQ, Liu D, Zhang L, Qin J,
Zhang Z, Su Y, Yan C, Luo YL, Li J, et al: The effects of
Astragalus polysaccharide on bone marrow-derived mesenchymal
stem cell proliferation and morphology induced by A549 lung cancer
cells. Med Sci Monit. 25:4110–4121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang Y, Wang CH, Yu D, Gao Y, Jing H,
Wang Y and Liu C: Effect of Astragalus Polysaccharide on EMT of
A549/DDP Lung Adenocarcinoma Xenograft: An Exploration Based on
PI3K/Akt Signaling Pathway. Chin J Exp Tradit Med Formulae.
27:51–58. 2021.(In Chinese).
|
|
86
|
Yang YL, Lin ZW, He PT, Nie H, Yao QY and
Zhang SY: Inhibitory effect of Astragalus polysaccharide
combined with cisplatin on cell cycle and migration of
nasopharyngeal carcinoma cell lines. Biol Pharm Bull. 44:926–931.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yan L, Hong T, Luo J, Cao X, Liu W, Gao Y,
Cui W and Wang F: Effect of Astragali Radix Polysaccharides on
Proliferation and Apoptosis of Human Colon Cancer Cell Line SW620.
Chin J Exp Tradit Med Formulae. 23:97–101. 2017.(In Chinese).
|
|
88
|
Guo S, Ma B, Jiang X, Li X and Jia Y:
Astragalus polysaccharides inhibits tumorigenesis and lipid
metabolism through miR-138-5p/SIRT1/SREBP1 pathway in prostate
cancer. Front Pharmacol. 11:5982020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chu Y, Fang Y, Chi J, Li J, Zhang D, Zou Y
and Wang Z: Astragalus polysaccharides decrease
proliferation, migration, and invasion but increase apoptosis of
human osteosarcoma cells by up-regulation of microRNA-133a. Braz J
Med Biol Res. 51:e76652018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mocan L, Matea C, Tabaran FA, Mosteanu O,
Pop T, Mocan T and Iancu C: Photothermal treatment of liver cancer
with albumin-conjugated gold nanoparticles initiates Golgi
Apparatus-ER dysfunction and caspase-3 apoptotic pathway activation
by selective targeting of Gp60 receptor. Int J Nanomedicine.
10:5435–5445. 2015.PubMed/NCBI
|
|
91
|
Moyer A, Tanaka K and Cheng EH: Apoptosis
in cancer biology and therapy. Annu Rev Pathol. 20:303–328. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhou Z, Meng M and Ni H: Chemosensitizing
effect of Astragalus polysaccharides on nasopharyngeal
carcinoma cells by inducing apoptosis and modulating expression of
Bax/Bcl-2 ratio and caspases. Med Sci Monit. 23:462–469. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Song J, Chen Y, He D, Tan W, Lv F, Liang
B, Xia T and Li J: Astragalus polysaccharide promotes
adriamycin-induced apoptosis in gastric cancer cells. Cancer Manag
Res. 12:2405–2414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yu J, Ji H, Dong X, Feng Y and Liu A:
Apoptosis of human gastric carcinoma MGC-803 cells induced by a
novel Astragalus membranaceus polysaccharide via intrinsic
mitochondrial pathways. Int J Biol Macromol. 126:811–819. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ohnuki H, Jiang K, Wang D, Salvucci O,
Kwak H, Sánchez-Martín D, Maric D and Tosato G: Tumor-infiltrating
myeloid cells activate Dll4/Notch/TGF-β signaling to drive
malignant progression. Cancer Res. 74:2038–2049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li C, Zhang S, Lu Y, Zhang Y, Wang E and
Cui Z: The roles of Notch3 on the cell proliferation and apoptosis
induced by CHIR99021 in NSCLC cell lines: A functional link between
Wnt and Notch signaling pathways. PLoS One. 8:e846592013.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Haruki N, Kawaguchi KS, Eichenberger S,
Massion PP, Olson S, Gonzalez A, Carbone DP and Dang TP:
Dominant-negative Notch3 receptor inhibits mitogen-activated
protein kinase pathway and the growth of human lung cancers. Cancer
Res. 65:3555–3561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Konishi J, Kawaguchi KS, Vo H, Haruki N,
Gonzalez A, Carbone DP and Dang TP: Gamma-secretase inhibitor
prevents Notch3 activation and reduces proliferation in human lung
cancers. Cancer Res. 67:8051–8057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang JX, Han YP, Bai C and Li Q: Notch1/3
and p53/p21 are a potential therapeutic target for APS-induced
apoptosis in non-small cell lung carcinoma cell lines. Int J Clin
Exp Med. 8:12539–12547. 2015.PubMed/NCBI
|
|
102
|
Sha X, Xu X, Liao S, Chen H and Rui W:
Evidence of immunogenic cancer cell death induced by
honey-processed Astragalus polysaccharides in vitro and in
vivo. Exp Cell Res. 410:1129482022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Feng T and Zhang BG: Inhibitory effect of
Astragalus polysaccharide on hepatocellular carcinoma HepG2
cells and its mechanism. Prac Geriatr. 6:486–488. 4952010.(In
Chinese).
|
|
104
|
Jiao J, Yu J, Ji H and Liu A: Synthesis of
macromolecular Astragalus polysaccharide-nano selenium
complex and the inhibitory effects on HepG2 cells. Int J Biol
Macromol. 211:481–489. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang HY: The Role of ERK1 /2 in Astragalus
Polysaccharide-induced Apoptosis in HepG2 Cell Line. Chin J Exp
Tradit Med Formulae. 7:235–238. 2012.(In Chinese).
|
|
106
|
Hart GW, Housley MP and Slawson C: Cycling
of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins.
Nature. 446:1017–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Dong DL and Hart GW: Purification and
characterization of an O-GlcNAc selective
N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J Biol
Chem. 269:19321–19330. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kreppel LK, Blomberg MA and Hart GW:
Dynamic glycosylation of nuclear and cytosolic proteins. Cloning
and characterization of a unique O-GlcNAc transferase with multiple
tetratricopeptide repeats. J Biol Chem. 272:9308–9315. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Haltiwanger RS, Holt GD and Hart GW:
Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins.
Identification of a uridine diphospho-N-acetylglucosamine: Peptide
beta-N-acetylglucosaminyltransferase. J Biol Chem. 265:2563–2568.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hart GW, Slawson C, Ramirez-Correa G and
Lagerlof O: Cross talk between O-GlcNAcylation and phosphorylation:
Roles in signaling, transcription, and chronic disease. Annu Rev
Biochem. 80:825–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lee SJ, Lee DE, Choi SY and Kwon OS:
OSMI-1 enhances TRAIL-induced apoptosis through ER stress and NF-κB
signaling in colon cancer cells. Int J Mol Sci. 22:110732021.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lee SJ and Kwon OS: O-GlcNAc transferase
inhibitor synergistically enhances doxorubicin-induced apoptosis in
HepG2 cells. Cancers (Basel). 12:31542020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ferrer CM, Lynch TP, Sodi VL, Falcone JN,
Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN and Reginato MJ:
O-GlcNAcylation regulates cancer metabolism and survival stress
signaling via regulation of the HIF-1 pathway. Mol Cell.
54:820–831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Li M, Duan F, Pan Z, Liu X, Lu W, Liang C,
Fang Z, Peng P and Jia D: Astragalus polysaccharide promotes
doxorubicin-induced apoptosis by reducing O-GlcNAcylation in
hepatocellular carcinoma. Cells. 12:8662023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kemse NG, Kale A and Joshi SR:
Supplementation of maternal omega-3 fatty acids to pregnancy
induced hypertension Wistar rats improves IL-10 and VEGF levels.
PLEFA. 43:276–279. 2015.
|
|
116
|
Zhao L, Zhong Y, Liang J, Gao H and Tang
N: Effect of Astragalus polysaccharide on the expression of
VEGF and EGFR in mice with lewis transplantable lung cancer. J Coll
Physicians Surg Pak. 29:392–394. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen W, Li Z, Bai L and Lin Y: NF-kappaB
in lung cancer, a carcinogenesis mediator and a prevention and
therapy target. Front Biosci (Landmark Ed). 16:1172–1185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wu CY, Ke Y, Zeng YF, Zhang YW and Yu HJ:
Anticancer activity of Astragalus polysaccharide in human
non-small cell lung cancer cells. Cancer Cell Int. 17:1152017.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Auyeung KK, Law PC and Ko JK: Novel
anti-angiogenic effects of formononetin in human colon cancer cells
and tumor xenograft. Oncol Rep. 28:2188–2194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li M, Zhao W, Geng L, Jiang J, Yang N, Gu
L, Wang L and Wang Y: Astragalus Polysaccharides Inhibiting Gastric
Cancer Cell Proliferation and Invasion via miR-25/FBXW7 Signaling
Pathway. New Chinese Medicine. 57:150–155. 2025.(In Chinese).
|
|
121
|
Ji F, Zhang H, Wang Y, Li M, Xu W, Kang Y,
Wang Z, Wang Z, Cheng P, Tong D, et al: MicroRNA-133a,
downregulated in osteosarcoma, suppresses proliferation and
promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone. 56:220–226.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Medici D, Shore EM, Lounev VY, Kaplan FS,
Kalluri R and Olsen BR: Conversion of vascular endothelial cells
into multipotent stem-like cells. Nat Med. 16:1400–1406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yang S, Sun S, Xu W, Yu B, Wang G and Wang
H: Astragalus polysaccharide inhibits breast cancer cell
migration and invasion by regulating epithelial-mesenchymal
transition via the Wnt/β-catenin signaling pathway. Mol Med Rep.
21:1819–1832. 2020.PubMed/NCBI
|
|
124
|
He L, Xu K, Niu L and Lin L:
Astragalus polysaccharide (APS) attenuated PD-L1-mediated
immunosuppression via the miR-133a-3p/MSN axis in HCC. Pharm Biol.
60:1710–1720. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Yang N, Jia X, Zhang Z, Sun E and Yan H:
Advance in studies on anti-cancer activity and mechanism of
flavonoids. China J Chin Mater Med. 40:373–381. 2015.(In
Chinese).
|
|
126
|
Glorieux C, Liu S, Trachootham D and Huang
P: Targeting ROS in cancer: Rationale and strategies. Nat Rev Drug
Discov. 23:583–606. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Pu X, Fan W, Yu S, Li Y, Ma X, Liu L, Ren
J and Zhang W: Polysaccharides from angelica and Astragalus
exert hepatoprotective effects against carbon-tetrachloride-induced
intoxication in mice. Can J Physiol Pharmacol. 93:39–43. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bukowski K, Kciuk M and Kontek R:
Mechanisms of multidrug resistance in cancer chemotherapy. IntJ Mol
Sci. 21:32332020. View Article : Google Scholar
|
|
129
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Cao Y, Ruan Y, Shen T, Huang X, Li M, Yu
W, Zhu Y, Man Y, Wang S and Li J: Astragalus polysaccharide
suppresses doxorubicin-induced cardiotoxicity by regulating the
PI3k/Akt and p38MAPK pathways. Oxid Med Cell Longev.
2014:6742192014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhang Y, Wang CH, Yu D, Gao Y, Jing H,
Tang Y and Liu C: Astragalus Polysaccharides Improve Cisplatin
Resistance by Inhibiting EMT of Lung Adenocarcinoma A549/DDP Cells
Transplanted into Nude Mice. Chin J Exp Tradit Med Formulae.
28:79–85. 2022.(In Chinese).
|
|
132
|
Peng H, Zhang X and Li F: Mechanisms of
Astragalus Polysaccharids Synergized with Doxorubicin against Drug
Resistance in HL-60/A Cells. J Exp Hemat. 25:743–748. 2017.(In
Chinese).
|
|
133
|
Zhai QL, Hu XD, Xiao J and Yu DQ:
Astragalus polysaccharide may increase sensitivity of
cervical cancer HeLa cells to cisplatin by regulating cell
autophagy. Zhongguo Zhong Yao Za Zhi. 43:805–812. 2018.(In
Chinese). PubMed/NCBI
|
|
134
|
Tian Q: The study on the adjunct
anticancer mechanism of astragalus polysaccharide in vivo and in
vitro. PhD Thesis. Central South University. 26–49. 2013.(In
Chinese).
|
|
135
|
Li C, Hong L, Liu C, Min J, Hu M and Guo
W: Astragalus polysaccharides increase the sensitivity of
SKOV3 cells to cisplatin. Arch Gynecol Obstet. 297:381–386. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wei J, Li Y, Xu B and Yu J:
Astragalus polysaccharides reverse gefitinib resistance by
inhibiting mesenchymal transformation in lung adenocarcinoma cells.
Am J Transl Res. 12:1640–1657. 2020.PubMed/NCBI
|
|
137
|
Zhang Y, Chen Z, Chen L, Dong Q, Yang DH,
Zhang Q, Zeng J, Wang Y, Liu X, Cui Y, et al: Astragali radix
(Huangqi): A time-honored nourishing herbal medicine. Chin Med.
19:1192024. View Article : Google Scholar : PubMed/NCBI
|