Recent research has indicated that Y-box binding
protein 1 (YBX1/YB-1) has multifaceted functions in regulating drug
resistance, PANoptosis, stress response, ferroptosis and cell
proliferation (1–5). YBX1 is identified as an oncogene
which is overexpressed and positively associated with poor outcomes
and survival in numerous cancers including liver, endometrial,
non-small-cell lung cancer (NSCLC), ovarian, breast, gastric
cancer, kidney renal papillary and clear cell renal cell carcinoma
(4–11). YBX1 performs the roles through the
regulation of nucleotide metabolism, pre-mRNA splicing, DNA repair,
stress granule formation, transcription and translation, and
sorting of microRNAs (miRNAs) into exosomes (12–14).
Previous studies have shown that YBX1 also plays a crucial role in
regulating RNA methylation modification which affects mRNA
stability and translation, including N6-methyladenine
(m6A) and 5-methylcytosine (m5C) RNA
modifications (5,15). The performance of these functions
depends on the structure and location of YBX1. The structural
features of YBX1 are crucial for its diverse functions, mediating
interactions with DNA, RNA and protein molecules (3,16).
SU056 has been demonstrated to specifically target
and inhibit the YBX1 protein, rendering it a valuable tool for
investigating YBX1 inhibition (17–20).
Multiple studies have demonstrated that SU056 effectively hinders
tumor progression in diverse types of cancer including pancreatic
cancer (18), triple-negative
breast cancer (17) and ovarian
cancer (19,20).
In the present review, the focus is mainly on the
function of YBX1 in RNA modification. The mechanisms by which YBX1
is involved in RNA modification, including m5C,
m6A and RNA editing are clarified, and YBX1 is proposed
as a potential therapeutic target. Furthermore, the roles of YBX1
in regulating anticancer drug resistance through RNA modification
are discussed, and its functions on tumor progression are
encompassed. This provides a research foundation for the
development of anticancer drugs targeting YBX1.
YBX1 was initially isolated using double-stranded
oligonucleotides in a phage λgt11 library screening as the
interacting molecule of the MHC class II gene Y-box element
(21). In 1992, the murine (m)
CCAAT-binding protein was identified and termed mYB-1 (22). The amino acid sequence of mYB-1 has
a 95% homology with human YB-1 (22). In addition, YBX1 cDNA was isolated
by screening the binding site through the enhancer oligonucleotide
of type 18 human papillomavirus in an expression library of HeLa
(23). Researchers found that YBX1
is a nuclear protein with a molecular weight of 42 kDa (23). YBX1 is expressed in most tissues,
including the liver, spleen, lung and heart (23). Shortly thereafter, it was shown
that YBX1 acted as a binding protein of human multidrug resistance
1 (MDR1) gene and regulated MDR1 gene expression in response to
adverse environments (24). In
humans, YBX1 genes are located on chromosome 1p34 as identified by
in situ fluorescence hybridization (25).
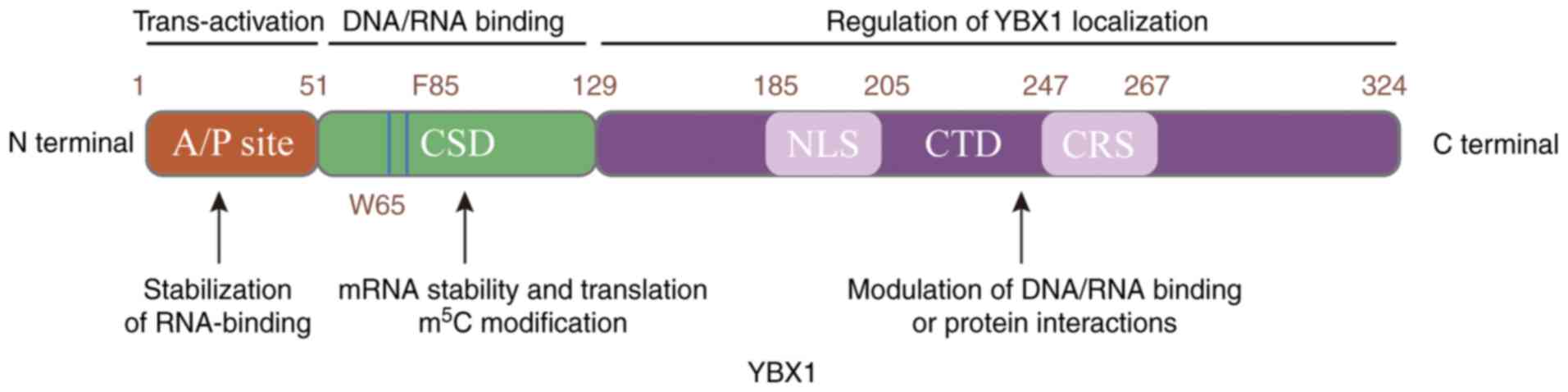
YBX1 belongs to the superfamily of the cold shock
proteins and is considered to be evolutionarily conserved (26). The structure of YBX1 consists of
three distinct domains, the alanine/proline-rich domain (A/P site)
in the N-terminal, the cold shock domain (CSD) in the central
region and the C-terminal domain (CTD; Fig. 1). The A/P site domain is involved
in protein-protein interactions and mediates the assembly of
ribonucleoprotein complexes. Additionally, the A/P site domain
functions as a regulatory element, modulating the stabilization of
RNA-binding proteins (27). The
highly conserved CSD facilitates both DNA and RNA binding, plays a
crucial role in mRNA translation, and regulates the adaptation of
bacteria to low temperatures (28). In previous studies, CSD was
identified to contribute to the identification of m5C
modification, enhancing mRNA stability and promoting translation
(29,30). The CTD contains two important
domains that mediate the localization of YBX1, the cytoplasmic
retention site (CRS) and the nuclear localization signals (NLS),
regulating the location of YBX1 between the cell nucleus and
cytoplasm in response to cellular stimuli. Furthermore, CTD has
been shown to mediate interactions with diverse protein partners
and DNA/RNA-binding proteins (27,28).
YBX1 is usually localized in the cell cytoplasm due to the stronger
influence of CRS compared with that of NLS. The various functions
of YBX1 stem from its diverse domains, making it a key
DNA/RNA-binding protein.
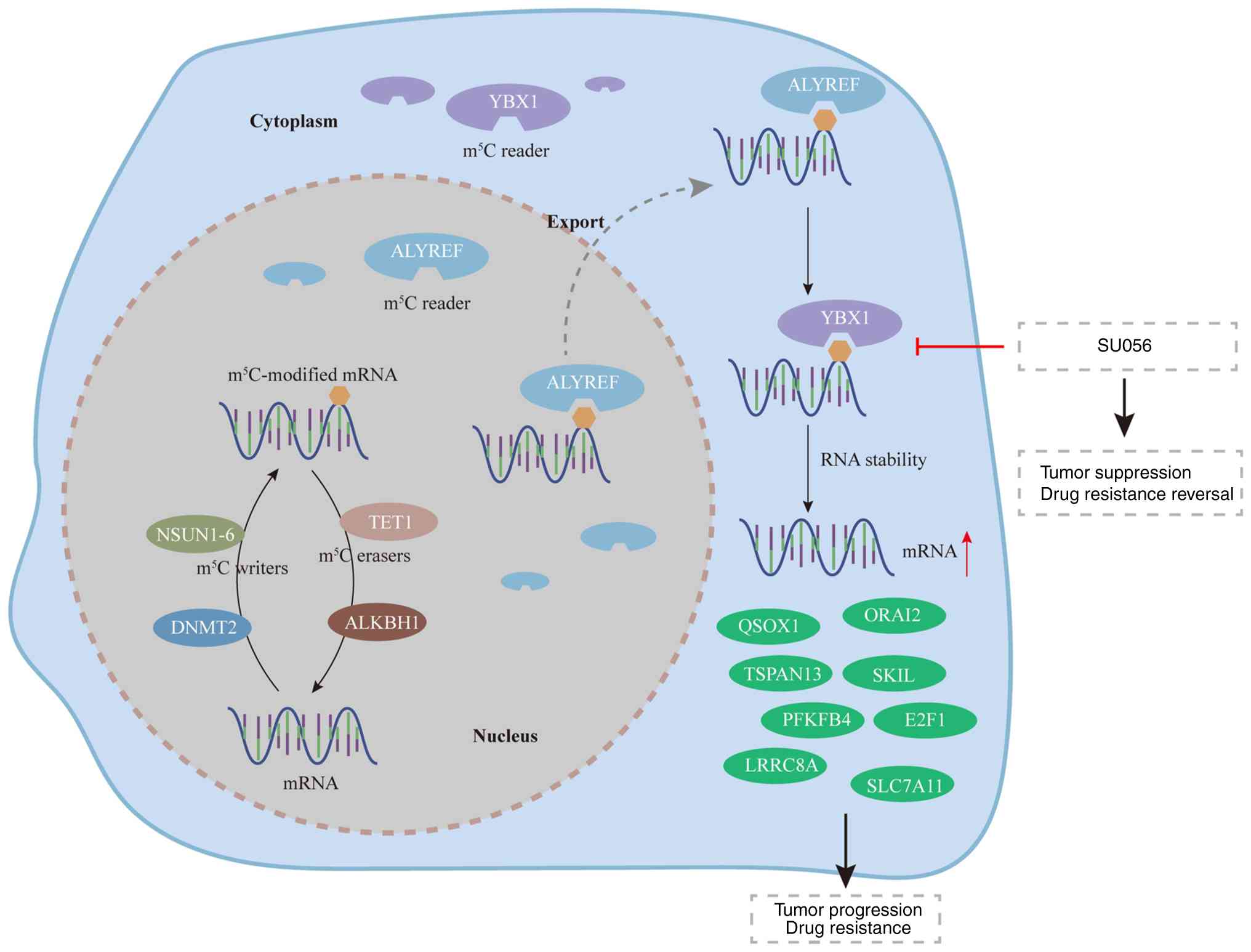
Research has revealed that the cytidine residues at
position five of RNA could be methylated by m5C
methyltransferases, such as the NOP2/Sun RNA methyltransferase
(NSUN) protein family (31).
During the m5C modification, numerous methyltransferases
and proteins are involved in this process, including m5C
writers, m5C erasers and m5C readers
(31,32). In humans, m5C writers
consist of the NSUN protein family 1–6 and DNA methyltransferase 2,
which catalyze cytosine-5 methylation (32). Among them, NSUN2 is considered the
most prominent m5C methyltransferase (32). The m5C erasers contain
the ten-eleven translocation (TET) protein family and AlkB homolog
1, histone H2A dioxygenase (32,33).
However, the functions of the m5C eraser and those of
additional eraser proteins require further investigation (33). The m5C readers include
YBX1, YTH N6-methyladenosine RNA binding protein F2 (YTHDF2) and
Aly/Ref export factor (ALYREF), and assume the recognition of the
m5C modification (34,35).
In addition, ALYREF is required for the nuclear export of
m5C-modified mRNA (34). Furthermore, YBX1 can identify
m5C-modified mRNA in the cytoplasm and maintain mRNA
stability (36,37). YTHDF2 can regulate the maturation
of m5C-modified ribosomal RNA (rRNA) (35).
Although YBX1 is mainly studied in cancers, it has
also been demonstrated to regulate bone formation and metabolic
diseases through m5C modification (52,53).
Li et al (52) demonstrated
that elevated YBX1 could facilitate osteogenesis and reduce bone
loss. YBX1 deletion could repress the morphology of CD31-high,
endomucin-high (CD31highEMCNhigh) endothelial
cells, leading to decreased bone mass in an
m5C-dependent manner. YBX1 could modulate the stability
of EMCN, CD31 and bone morphogenetic protein 4 (BMP4), and
influence the release of BMP4, thereby controlling bone formation
(52). Furthermore, YBX1 could
promote adipogenesis and autophagy in an m5C-dependent
manner, thus leading to obesity (45). In a previous study it was found
that YBX1 directly recognized m5C-modified Unc-51 like
autophagy activating kinase 1 (ULK1) mRNA and improved its
stability. In addition, YBX1 enhanced ULK1-mediated autophagy and
increased obesity formation (53).
These findings indicate that YBX1-mediated m5C
modification assumes a crucial function in various diseases.
YBX1 can serve as a transcription factor or
DNA-binding protein that regulates the expression of multiple genes
(75). Accumulating evidence has
revealed that YBX1 is a regulatory protein involved in the
m6A modification. In 2021, Feng et al (15) demonstrated the significance of YBX1
in myeloid leukemia cell survival via m6A modification
of BCL2 apoptosis regulator (BCL2). It was found that YBX1 was
notably upregulated, promoting proliferation in myeloid leukemia
cells. Mechanistically, YBX1 stabilized m6A-modified
BCL2 and MYC proto-oncogene, BHLH transcription factor by
cooperating with IGF2BP1 and IGF2BP3. Furthermore, the loss of YBX1
led to an accelerated decay of m6A-tagged BCL2 mRNA,
suggesting that YBX1 can regulate BCL2 expression in an
m6A-dependent manner, thus playing a critical role in
the survival of myeloid leukemia cells (15). Similarly, YBX1 is required for the
survival of leukemia stem cells (94). YBX1 was revealed to interact with
the IGF2BP protein to enhance the stability of
m6A-tagged tyrosine 3-monooxygenase/tryptophan
5-monooxygenase activation protein zeta (YWHAZ) mRNA. YBX1
deficiency downregulated the expression of YWHAZ by promoting YWHAZ
mRNA degradation in an m6A-dependent manner, thereby
maintaining leukemia cell survival (94). Additionally, YBX1 has been shown to
affect embryonic development (95,96)
and ischemic stroke (97) in an
m6A-dependent manner. YBX1 was found to influence the
gene expression of zygotic genome activation by
m6A-modified RNA, thereby regulating early embryonic
development. YBX1 fine-tuned polycomb repressive complex 2 activity
to regulate embryonic neural development (95) Peng et al (97) also observed that NSC-derived
exosomes loaded with YBX1 inhibited neuronal pyroptosis, thereby
mitigating the development of ischemic stroke. YBX1 interacted with
IGF2BP1 to heighten the stability of m6A-tagged G
protein-coupled receptor 30 (GPR30) mRNA, which increased the
expression of GPR30. GPR30 then facilitated the degradation of
NLRP3 by ubiquitination, alleviating the progression of ischemic
stroke. The inhibition of IGF2BP1 decreased YBX1 binding to GPR30,
which contributed to the progression of ischemic stroke (97). YBX1 is a regulatory protein
involved in the m6A modification of RNA. It stabilizes
the mRNA of target genes, thereby enhancing their expression.
Unlike the YBX1 regulation model in the m5C
modification, the IGF2BP protein is required for YBX1 to maintain
mRNA stability in the m6A modification. Furthermore,
YBX1 can function as an m6A reader, dependent on the
IGF2BP protein (98–100). The functions of YBX1 in
N1methyladenosine (m1A) and 7methylguanosine
(m7G) remain unknown. Therefore, further investigation
is needed to explore the contributions and potential mechanisms of
YBX1 in RNA methylation modifications.
Research has shown that YBX1 upregulation could
promote tumor development by regulating m5C and
m6A modifications. SU056 is a small molecule of
azopodophyllotoxin that inhibits YBX1, and helps reverse drug
resistance and inhibit tumor development (17–20).
YBX1 knockdown was shown to reduce gemcitabine resistance, and
SU056 in combination with gemcitabine overcame gemcitabine
resistance in pancreatic cancer (18). Additionally, SU056 was demonstrated
to inhibit triple-negative breast cancer growth in preclinical
models by targeting YB-1 to disrupt protein translation mechanisms
(17). SU056 also reduced the
progression of ovarian cancer while sensitizing to
paclitaxel-mediated cytotoxicity (20). Furthermore, platinum-induced cell
stress enhanced YBX1, which was expressed at high levels in
platinum-resistant ovarian cancer. YBX1 recognized and stabilized
CHD3 mRNA through m5C modification. By targeting YBX1,
SU056 reversed platinum resistance and enhanced tumor cell killing
(19). In addition, another YBX1
inhibitor, 2,4-dihydroxy-5-pyrimidinyl imidothiocarbomate, also
exerted similar antitumor effects, although its mechanism of action
remains unexplored (106). The
activities and efficacy profiles of other YBX1 inhibitors also
remain incompletely characterized.
Collectively, SU056 has been demonstrated to exert
antitumor effects and reverse drug resistance by targeting YBX1,
with its effectiveness dependent on the cellular expression of YBX1
(20). Additionally, YBX1 has been
shown to facilitate tumor progression and confer drug resistance
through its regulation of RNA m5C and m6A
modifications (5,107–109). Therefore, it is hypothesized that
the antitumor effect and reversal of drug resistance exerted by
SU056 may be attributed to its inhibition of RNA m5A and
m6A methylation.
YBX1, a DNA/RNA-binding protein, is implicated in
DNA repair, mRNA transcription, pre-mRNA splicing, mRNA stability
regulation, translation and exosome sorting (28,110), influencing the development of
multiple diseases. Notably, extensive evidence has shown that YBX1
plays multifunctional roles in cancer progression and drug
resistance (111,112). Furthermore, RNA methylation
modifications are dysregulated in cancers and serve as potential
targets of tumor therapy (31).
Although YBX1 is implicated in various cancer hallmarks, its
underlying mechanisms, particularly those related to RNA
methylation modifications during cancer development such as
m1A, m5C, m6A and m7G
require further investigation. Understanding the functional role of
YBX1 in RNA modifications could provide novel insights into the
regulation of gene expression, cellular homeostasis and molecular
pathogenesis. This knowledge may also lead to promising therapeutic
strategies that target RNA modifications for cancer treatment.
Given its critical roles in cancer pathogenesis and
treatment resistance, YBX1 has emerged as an attractive therapeutic
target for anticancer therapy. Several approaches have been
explored to modulate YBX1 activity, including the use of small
molecule inhibitors. The YBX1 inhibitor SU056 has shown promising
efficacy in inhibiting tumor growth, promoting cell apoptosis and
sensitizing cancer cells to chemotherapy. Despite the significance
of YBX1 in cancer, the clinical application of YBX1 inhibitors
remains limited. Further investigations should focus on the
regulatory networks between YBX1 and RNA modifications, which will
broaden the understanding of the role of YBX1 in cellular activity
and tumorigenesis.
In summary, YBX1 plays a pivotal role in regulating
RNA modifications specifically m6A and m5C
RNA modifications influencing a variety of cellular processes and
diseases (Table I). Understanding
the underlying mechanisms of YBX1 in RNA modification is crucial
for developing effective strategies to treat cancers. Targeting
YBX1 and RNA modifications may help overcome the challenges of drug
resistance in the clinic, and the YBX1 inhibitor SU056 exhibits
great potential as an antitumor agent.
Not applicable.
Funding: No funding was received.
Not applicable.
XX conceived and designed the review, as well as
drafted and provided overall supervision of the manuscript. GX
performed and contributed to specific sections of the review and
participated in manuscript revision. YX was involved in revising
the manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Lin C, Lin P, Yao H, Liu S, Lin X, He R,
Teng Z, Zuo X, Li Y, Ye J and Zhu G: Modulation of YBX1-mediated
PANoptosis inhibition by PPM1B and USP10 confers chemoresistance to
oxaliplatin in gastric cancer. Cancer Lett. 587:2167122024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao S, Xie C, Liu Y, Zhao Y, Li M, Gao H,
Xiao Y, Zou Y, Zheng Z, Gao Y, et al: Apurinic/apyrimidinic
endodeoxyribonuclease 1 (APE1) promotes stress granule formation
via YBX1 phosphorylation in ovarian cancer. Cell Mol Life Sci.
81:1132024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Fan JS, Li S, Yang Y, Sun P, Zhu
Q, Wang J, Jiang B, Yang D and Liu M: Structural basis of DNA
binding to human YB-1 cold shock domain regulated by
phosphorylation. Nucleic Acids Res. 48:9361–9371. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen SJ, Zhang J, Zhou T, Rao SS, Li Q,
Xiao LY, Wei ST and Zhang HF: Epigenetically upregulated NSUN2
confers ferroptosis resistance in endometrial cancer via m(5)C
modification of SLC7A11 mRNA. Redox Biol. 69:1029752024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Wei J, Feng L, Li O, Huang L, Zhou
S, Xu Y, An K, Zhang Y, Chen R, et al: Aberrant m5C
hypermethylation mediates intrinsic resistance to gefitinib through
NSUN2/YBX1/QSOX1 axis in EGFR-mutant non-small-cell lung cancer.
Mol Cancer. 22:812023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu J, Ji L, Liang Y, Zheng W, Song X,
Gorshkov K, Sun Q, Lin H, Zheng X, Chen J, et al: CircRNA-SORE
mediates sorafenib resistance in hepatocellular carcinoma by
stabilizing YBX1. Signal Transduct Target Ther. 5:2982020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Wei Q, Yang C, Zhao H, Xu J, Mobet
Y, Luo Q, Yang D, Zuo X, Chen N, et al: RNA m(5)C modification
upregulates E2F1 expression in a manner dependent on YBX1 phase
separation and promotes tumor progression in ovarian cancer. Exp
Mol Med. 56:600–615. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song S, He X, Wang J, Song H, Wang Y, Liu
Y, Zhou Z, Yu Z, Miao D and Xue Y: A novel long noncoding RNA,
TMEM92-AS1, promotes gastric cancer progression by binding to YBX1
to mediate CCL5. Mol Oncol. 15:1256–1273. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu T, Xiong M, Hong Q, Pan B, Xu M, Wang
Y, Sun Y, Sun H, Pan Y, Wang S and He B: Hsa_circ_0007990 promotes
breast cancer growth via inhibiting YBX1 protein degradation to
activate E2F1 transcription. Cell Death Dis. 15:1532024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Cao C, Zhou CL, Li X, Miao C,
Shen L, Singla RK and Lu X: Identification of a novel
5-methylcytosine-related signature for prognostic prediction of
kidney renal papillary cell carcinoma and a Putative target for
drug repurposing. Transl Oncol. 36:1017412023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Yin H, Chen Y, Pan C, Hang H, Lu
Y, Ma W, Li X, Gan W, Guo H and Li D: Low expression of PEBP1P2
promotes metastasis of clear cell renal cell carcinoma by
post-transcriptional regulation of PEBP1 and KLF13 mRNA. Exp
Hematol Oncol. 11:872022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gandhi M, Groß M, Holler JM, Coggins SA,
Patil N, Leupold JH, Munschauer M, Schenone M, Hartigan CR,
Allgayer H, et al: The lncRNA lincNMR regulates nucleotide
metabolism via a YBX1-RRM2 axis in cancer. Nat Commun. 11:32142020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lyabin DN, Eliseeva IA and Ovchinnikov LP:
YB-1 protein: Functions and regulation. Wiley Interdiscip Rev RNA.
5:95–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XM, Ma L and Schekman R: Selective
sorting of microRNAs into exosomes by phase-separated YBX1
condensates. Elife. 10:e719822021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng M, Xie X, Han G, Zhang T, Li Y, Li Y,
Yin R, Wang Q, Zhang T, Wang P, et al: YBX1 is required for
maintaining myeloid leukemia cell survival by regulating BCL2
stability in an m6A-dependent manner. Blood. 138:71–85. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Budkina K, El Hage K, Clément MJ,
Desforges B, Bouhss A, Joshi V, Maucuer A, Hamon L, Ovchinnikov LP,
Lyabin DN and Pastré D: YB-1 unwinds mRNA secondary structures in
vitro and negatively regulates stress granule assembly in HeLa
cells. Nucleic Acids Res. 49:10061–10081. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dheeraj A, Garcia Marques FJ, Tailor D,
Bermudez A, Resendez A, Pandrala M, Grau B, Kumar P, Haley CB,
Honkala A, et al: Inhibition of protein translational machinery in
triple-negative breast cancer as a promising therapeutic strategy.
Cell Rep Med. 5:1015522024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B, Xing F, Wang J, Wang X, Zhou C, Fan
G, Zhuo Q, Ji S, Yu X, Xu X, et al: YBX1 as a therapeutic target to
suppress the LRP1-β-catenin-RRM1 axis and overcome gemcitabine
resistance in pancreatic cancer. Cancer Lett. 602:2171972024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng H, Miao H, Zhang Y, Chen T, Yuan L,
Wan Y, Jiang Y, Zhang L and Cheng W: YBX1 promotes homologous
recombination and resistance to platinum-induced stress in ovarian
cancer by recognizing m5C modification. Cancer Lett.
597:2170642024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tailor D, Resendez A, Garcia-Marques FJ,
Pandrala M, Going CC, Bermudez A, Kumar V, Rafat M, Nambiar DK,
Honkala A, et al: Y box binding protein 1 inhibition as a targeted
therapy for ovarian cancer. Cell Chem Biol. 28:1206–1220.e6. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Didier DK, Schiffenbauer J, Woulfe SL,
Zacheis M and Schwartz BD: Characterization of the cDNA encoding a
protein binding to the major histocompatibility complex class II Y
box. Proc Natl Acad Sci USA. 85:7322–7326. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gai XX, Lipson KE and Prystowsky MB:
Unusual DNA binding characteristics of an in vitro translation
product of the CCAAT binding protein mYB-1. Nucleic Acids Res.
20:601–606. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spitkovsky DD, Royer-Pokora B, Delius H,
Kisseljov F, Jenkins NA, Gilbert DJ, Copeland NG and Royer HD:
Tissue restricted expression and chromosomal localization of the
YB-1 gene encoding a 42 kD nuclear CCAAT binding protein. Nucleic
Acids Res. 20:797–803. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asakuno K, Kohno K, Uchiumi T, Kubo T,
Sato S, Isono M and Kuwano M: Involvement of a DNA binding protein,
MDR-NF1/YB-1, in human MDR1 gene expression by actinomycin D.
Biochem Biophys Res Commun. 199:1428–1435. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Makino Y, Ohga T, Toh S, Koike K, Okumura
K, Wada M, Kuwano M and Kohno K: Structural and functional analysis
of the human Y-box binding protein (YB-1) gene promoter. Nucleic
Acids Res. 24:1873–1878. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuwano M, Shibata T, Watari K and Ono M:
Oncogenic Y-box binding protein-1 as an effective therapeutic
target in drug-resistant cancer. Cancer Sci. 110:1536–1543. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun X, Gao C, Xu X, Li M, Zhao X, Wang Y,
Wang Y, Zhang S, Yan Z, Liu X and Wu C: FBL promotes cancer cell
resistance to DNA damage and BRCA1 transcription via YBX1. EMBO
Rep. 24:e562302023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bates M, Boland A, McDermott N and
Marignol L: YB-1: The key to personalised prostate cancer
management? Cancer Lett. 490:66–75. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Li A, Sun BF, Yang Y, Han YN, Yuan
X, Chen RX, Wei WS, Liu Y, Gao CC, et al: 5-methylcytosine promotes
pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell
Biol. 21:978–990. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Wang L, Han X, Yang WL, Zhang M,
Ma HL, Sun BF, Li A, Xia J, Chen J, et al: RNA 5-methylcytosine
facilitates the maternal-to-Zygotic transition by preventing
maternal mRNA. Decay Mol Cell. 75:1188–1202.e11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barbieri I and Kouzarides T: Role of RNA
modifications in cancer. Nat Rev Cancer. 20:303–322. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nombela P, Miguel-López B and Blanco S:
The role of m(6)A, m(5)C and Ψ RNA modifications in cancer: Novel
therapeutic opportunities. Mol Cancer. 20:182021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo G, Pan K, Fang S, Ye L, Tong X, Wang
Z, Xue X and Zhang H: Advances in mRNA 5-methylcytosine
modifications: Detection, effectors, biological functions, and
clinical relevance. Mol Ther Nucleic Acids. 26:575–593. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang C, Hou X, Guan Q, Zhou H, Zhou L, Liu
L, Liu J, Li F, Li W and Liu H: RNA modification in cardiovascular
disease: Implications for therapeutic interventions. Signal
Transduct Target Ther. 8:4122023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dai X, Gonzalez G, Li L, Li J, You C, Miao
W, Hu J, Fu L, Zhao Y, Li R, et al: YTHDF2 Binds to
5-Methylcytosine in RNA and modulates the maturation of ribosomal
RNA. Anal Chem. 92:1346–1354. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang G, Liu L, Li J, Chen Y, Wang Y,
Zhang Y, Dong Z, Xue W, Sun R and Cui G: NSUN2 stimulates tumor
progression via enhancing TIAM2 mRNA stability in pancreatic
cancer. Cell Death Discov. 9:2192023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu W, Wan F, Xu W, Liu Z, Wang J, Zhang
H, Huang S and Ye D: Positive epigenetic regulation loop between AR
and NSUN2 promotes prostate cancer progression. Clin Transl Med.
12:e10282022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han X, Wang M, Zhao YL, Yang Y and Yang
YG: RNA methylations in human cancers. Semin Cancer Biol.
75:97–115. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cui L, Ma R, Cai J, Guo C, Chen Z, Yao L,
Wang Y, Fan R, Wang X and Shi Y: RNA modifications: Importance in
immune cell biology and related diseases. Signal Transduct Target
Ther. 7:3342022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang N, Tang H, Wang X, Wang W and Feng J:
Homocysteine upregulates interleukin-17A expression via
NSun2-mediated RNA methylation in T lymphocytes. Biochem Biophys
Res Commun. 493:94–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zou S, Huang Y, Yang Z, Zhang J, Meng M,
Zhang Y, Feng J, Sun R, Li W, Wang W, et al: NSUN2 promotes
colorectal cancer progression by enhancing SKIL mRNA stabilization.
Clin Transl Med. 14:e16212024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen B, Deng Y, Hong Y, Fan L, Zhai X, Hu
H, Yin S, Chen Q, Xie X, Ren X, et al: Metabolic recoding of
NSUN2-Mediated m(5)C modification promotes the progression of
colorectal cancer via the NSUN2/YBX1/m(5)C-ENO1 positive feedback
loop. Adv Sci (Weinh). 11:e23098402024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Y, Xue M, Deng X, Nguyen LXT, Ren L,
Han L, Li C, Xue J, Zhao Z, Li W, et al: TET2-mediated mRNA
demethylation regulates leukemia stem cell homing and self-renewal.
Cell Stem Cell. 30:1072–1090.e10. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng S, Hu C, Lin Q, Li T, Li G, Tian Q,
Zhang X, Huang T, Ye Y, He R, et al: Extracellular vesicle-packaged
PIAT from cancer-associated fibroblasts drives neural remodeling by
mediating m5C modification in pancreatic cancer mouse models. Sci
Transl Med. 16:eadi01782024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang L, Zhang J, Su Y, Maimaitiyiming Y,
Yang S, Shen Z, Lin S, Shen S, Zhan G, Wang F, et al: Distinct
roles of m(5)C RNA methyltransferase NSUN2 in major gynecologic
cancers. Front. Oncol. 12:7862662022.PubMed/NCBI
|
|
46
|
Chen Y, Jiang Z, Zhang C, Zhang L, Chen H,
Xiao N, Bai L, Liu H and Wan J: 5-Methylcytosine transferase NSUN2
drives NRF2-mediated ferroptosis resistance in non-small cell lung
cancer. J Biol Chem. 300:1067932024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao W, Chen L, Lin L, Yang M, Li T, Wei H,
Sha C, Xing J, Zhang M, Zhao S, et al: SIAH1 reverses
chemoresistance in epithelial ovarian cancer via ubiquitination of
YBX-1. Oncogenesis. 11:132022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu T, Zhang Q, Yu SK, Nie FQ, Zhang ML,
Wang Q and Lu KH: THOC3 interacts with YBX1 to promote lung
squamous cell carcinoma progression through PFKFB4 mRNA
modification. Cell Death Dis. 14:4752023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yin H, Huang Z, Niu S, Ming L, Jiang H, Gu
L, Huang W, Xie J, He Y and Zhang C: 5-Methylcytosine (m(5)C)
modification in peripheral blood immune cells is a novel
non-invasive biomarker for colorectal cancer diagnosis. Front
Immunol. 13:9679212022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu K, Xu P, Lv J, Ge H, Yan Z, Huang S,
Li B, Xu H, Yang L, Xu Z and Zhang D: Peritoneal high-fat
environment promotes peritoneal metastasis of gastric cancer cells
through activation of NSUN2-mediated ORAI2 m5C modification.
Oncogene. 42:1980–1993. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Y, Zuo X, Wei Q, Xu J, Liu X, Liu S,
Wang H, Luo Q, Wang Y, Yang Y, et al: Upregulation of LRRC8A by
m(5)C modification-mediated mRNA stability suppresses apoptosis and
facilitates tumorigenesis in cervical cancer. Int J Biol Sci.
19:691–704. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li YJ, Guo Q, Ye MS, Cai G, Xiao WF, Deng
S and Xiao Y: YBX1 promotes type H vessel-dependent bone formation
in an m5C-dependent manner. JCI Insight. 9:e1723452024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu R, Feng S, Li F, Shu G, Wang L, Gao P,
Zhu X, Zhu C, Wang S and Jiang Q: Transcriptional and
post-transcriptional control of autophagy and adipogenesis by YBX1.
Cell Death Dis. 14:292023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Boo SH and Kim YK: The emerging role of
RNA modifications in the regulation of mRNA stability. Exp Mol Med.
52:400–408. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Delaunay S and Frye M: RNA modifications
regulating cell fate in cancer. Nat Cell Biol. 21:552–559. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Y, Yang D, Liu T, Chen J, Yu J and Yi
P: N6-methyladenosine-mediated gene regulation and therapeutic
implications. Trends Mol Med. 29:454–467. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fazi F and Fatica A: Interplay between N
(6)-Methyladenosine (m(6)A) and non-coding RNAs in cell development
and cancer. Front Cell Dev Biol. 7:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Olazagoitia-Garmendia A, Rojas-Márquez H,
Sebastian-delaCruz M, Agirre-Lizaso A, Ochoa A, Mendoza-Gomez LM,
Perugorria MJ, Bujanda L, Madrigal AH, Santin I and
Castellanos-Rubio A: m(6) a methylated long noncoding RNA LOC339803
regulates intestinal inflammatory response. Adv Sci (Weinh).
11:e23079282024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang R, Xu X, Yang J, Chen W, Zhao J, Wang
M, Zhang Y, Yang Y, Huang W and Zhang H: BPDE exposure promotes
trophoblast cell pyroptosis and induces miscarriage by
up-regulating lnc-HZ14/ZBP1/NLRP3 axis. J Hazard Mater.
455:1315432023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Q, Wei T, Yan L, Zhu S, Jin W, Bai
Y, Zeng Y, Zhang X, Yin Z, Yang J, et al: Hypoxia-Responsive lncRNA
AC115619 encodes a micropeptide that suppresses m6A modifications
and hepatocellular carcinoma progression. Cancer Res. 83:2496–2512.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zheng H, Zhu M, Li W, Zhou Z and Wan X:
m(5) C and m(6) A modification of long noncoding NKILA accelerates
cholangiocarcinoma progression via the miR-582-3p-YAP1 axis. Liver
Int. 42:1144–1157. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yan J, Liu J, Huang Z, Huang W and Lv J:
FOXC2-AS1 stabilizes FOXC2 mRNA via association with NSUN2 in
gastric cancer cells. Hum. Cell. 34:1755–1764. 2021.
|
|
63
|
Li H, Lin R, Zhang Y, Zhu Y, Huang S, Lan
J, Lu N, Xie C, He S and Zhang W: N6-methyladenosine-modified
circPLPP4 sustains cisplatin resistance in ovarian cancer cells via
PIK3R1 upregulation. Mol Cancer. 23:52024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zeng K, Peng J, Xing Y, Zhang L, Zeng P,
Li W, Zhang W, Pan Z, Zhou C and Lin J: A positive feedback circuit
driven by m(6)A-modified circular RNA facilitates colorectal cancer
liver metastasis. Mol Cancer. 22:2022023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu S, Tang W, Liu L, Wei K, Tang Y, Ma J,
Li H and Ao Y: Obesity-induced downregulation of miR-192
exacerbates lipopolysaccharide-induced acute lung injury by
promoting macrophage activation. Cell Mol Biol Lett. 29:362024.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
van den Homberg DAL, van der Kwast R, Quax
PHA and Nossent AY: N-6-Methyladenosine in Vasoactive microRNAs
during Hypoxia; A novel role for METTL4. Int J Mol Sci.
23:10572022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou L, Jiang J, Huang Z, Jin P, Peng L,
Luo M, Zhang Z, Chen Y, Xie N, Gao W, et al: Hypoxia-induced lncRNA
STEAP3-AS1 activates Wnt/β-catenin signaling to promote colorectal
cancer progression by preventing m(6)A-mediated degradation of
STEAP3 mRNA. Mol Cancer. 21:1682022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Qi YN, Liu Z, Hong LL, Li P and Ling ZQ:
Methyltransferase-like proteins in cancer biology and potential
therapeutic targeting. J Hematol Oncol. 16:892023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wu Y, Li J, Li C, Lu S, Wei X, Li Y, Xia
W, Qian C, Wang Z, Liu M, et al: Fat mass and obesity-associated
factor (FTO)-mediated N6-methyladenosine regulates spermatogenesis
in an age-dependent manner. J Biol Chem. 299:1047832023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jin KX, Zuo R, Anastassiadis K, Klungland
A, Marr C and Filipczyk A: N6-methyladenosine (m(6)A) depletion
regulates pluripotency exit by activating signaling pathways in
embryonic stem cells. Proc Natl Acad Sci USA. 118:e21051921182021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dou X, Xiao Y, Shen C, Wang K, Wu T, Liu
C, Li Y, Yu X, Liu J, Dai Q, et al: RBFOX2 recognizes
N(6)-methyladenosine to suppress transcription and block myeloid
leukaemia differentiation. Nat Cell Biol. 25:1359–1368. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ma L, Zhou X, Yao S, Zhang X, Mao J, Vona
B, Fan L, Lou S, Li D, Wang L and Pan Y: METTL3-dependent m(6)A
modification of PSEN1 mRNA regulates craniofacial development
through the Wnt/β-catenin signaling pathway. Cell Death Dis.
15:2292024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li B, Xiong X, Xu J, Peng D, Nie G, Wen N,
Wang Y and Lu J: METTL3-mediated m(6)A modification of lncRNA
TSPAN12 promotes metastasis of hepatocellular carcinoma through
SENP1-depentent deSUMOylation of EIF3I. Oncogene. 43:1050–1062.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ou X, Tan Y, Xie J, Yuan J, Deng X, Shao
R, Song C, Cao X, Xie X, He R, et al: Methylation of GPRC5A
promotes liver metastasis and docetaxel resistance through
activating mTOR signaling pathway in triple negative breast cancer.
Drug Resist Updat. 73:1010632024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jiang T, Qi J, Xue Z, Liu B, Liu J, Hu Q,
Li Y, Ren J, Song H, Xu Y, et al: The m(6)A modification
mediated-lncRNA POU6F2-AS1 reprograms fatty acid metabolism and
facilitates the growth of colorectal cancer via upregulation of
FASN. Mol Cancer. 23:552024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang Y, Geng X, Li Q, Xu J, Tan Y, Xiao
M, Song J, Liu F, Fang C and Wang H: m6A modification in RNA:
biogenesis, functions and roles in gliomas. J Exp Clin Cancer Res.
39:1922020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
van Tran N, Ernst FGM, Hawley BR, Zorbas
C, Ulryck N, Hackert P, Bohnsack KE, Bohnsack MT, Jaffrey SR,
Graille M and Lafontaine DLJ: The human 18S rRNA m6A
methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids
Res. 47:7719–7733. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang L, Li Y, Zhou L, Zhou H, Ye L, Ou T,
Hong H, Zheng S, Zhou Z, Wu K, et al: The m6A Reader YTHDF2
promotes bladder cancer progression by suppressing RIG-I-Mediated
immune response. Cancer Res. 83:1834–1850. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ghazi T, Nagiah S and Chuturgoon AA:
Fusaric acid decreases p53 expression by altering promoter
methylation and m6A RNA methylation in human hepatocellular
carcinoma (HepG2) cells. Epigenetics. 16:79–91. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shulman Z and Stern-Ginossar N: The RNA
modification N6-methyladenosine as a novel regulator of the immune
system. Nat Immunol. 21:501–512. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cheng W, Li M, Zhang L, Zhou C, Yu S, Peng
X and Zhang W and Zhang W: New roles of N6-methyladenosine
methylation system regulating the occurrence of non-alcoholic fatty
liver disease with N6-methyladenosine-modified MYC. Front.
Pharmacol. 13:9731162022.PubMed/NCBI
|
|
82
|
Jin S, Li M, Chang H, Wang R, Zhang Z,
Zhang J, He Y and Ma H: The m6A demethylase ALKBH5 promotes tumor
progression by inhibiting RIG-I expression and interferon alpha
production through the IKKε/TBK1/IRF3 pathway in head and neck
squamous cell carcinoma. Mol Cancer. 21:972022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ye G, Li J, Yu W, Xie Z, Zheng G, Liu W,
Wang S, Cao Q, Lin J, Su Z, et al: ALKBH5 facilitates CYP1B1 mRNA
degradation via m6A demethylation to alleviate MSC senescence and
osteoarthritis progression. Exp Mol Med. 55:1743–1756. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fu Y and Zhuang X: m(6)A-binding YTHDF
proteins promote stress granule formation. Nat Chem Biol.
16:955–963. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Khan D, Ramachandiran I, Vasu K, China A,
Khan K, Cumbo F, Halawani D, Terenzi F, Zin I, Long B, et al:
Homozygous EPRS1 missense variant causing hypomyelinating
leukodystrophy-15 alters variant-distal mRNA m(6)A site
accessibility. Nat Commun. 15:42842024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sun R, Tian X, Li Y, Zhao Y, Wang Z, Hu Y,
Zhang L, Wang Y, Gao D, Zheng S and Yao J: The m6A reader
YTHDF3-mediated PRDX3 translation alleviates liver fibrosis. Redox
Biol. 54:1023782022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Alarcón CR, Goodarzi H, Lee H, Liu X,
Tavazoie S and Tavazoie SF: HNRNPA2B1 is a mediator of
m(6)A-dependent nuclear RNA processing events. Cell. 162:1299–1308.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jiang L, Lin W, Zhang C, Ash PEA, Verma M,
Kwan J, van Vliet E, Yang Z, Cruz AL, Boudeau S, et al: Interaction
of tau with HNRNPA2B1 and N(6)-methyladenosine RNA mediates the
progression of tauopathy. Mol Cell. 81:4209–4227.e12. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang L, Wen M and Cao X: Nuclear hnRNPA2B1
initiates and amplifies the innate immune response to DNA viruses.
Science. 365:eaav07582019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Miller C, Ealy A, Gregory A, Janarthanam
C, Albers W, Richardson G, Jin H, Zenitsky G, Anantharam V,
Kanthasamy A and Kanthasamy AG: Pathological α-synuclein
dysregulates epitranscriptomic writer METTL3 to drive
neuroinflammation in microglia. Cell Rep. 44:1156182025. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang T, Zhang SW, Zhang SY and Ma QQ:
m(6)Aexpress-Reader: Prediction of m(6)A regulated expression genes
by integrating m(6)A sites and reader binding information in
specific-context. Methods. 203:167–178. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Boriack-Sjodin PA, Ribich S and Copeland
RA: RNA-modifying proteins as anticancer drug targets. Nat Rev Drug
Discov. 17:435–453. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wei J, Harada BT, Lu D, Ma R, Gao B, Xu Y,
Montauti E, Mani N, Chaudhuri SM, Gregory S, et al: HRD1-mediated
METTL14 degradation regulates m(6)A mRNA modification to suppress
ER proteotoxic liver disease. Mol Cell. 81:5052–5065.e6. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chai J, Wang Q, Qiu Q, Han G, Chen Y, Li W
and Zhang H: YBX1 regulates the survival of chronic myeloid
leukemia stem cells by modulating m(6)A-mediated YWHAZ stability.
Cell Oncol (Dordr.). 46:451–464. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Evans MK, Matsui Y, Xu B, Willis C, Loome
J, Milburn L, Fan Y, Pagala V and Peng JC: Ybx1 fine-tunes PRC2
activities to control embryonic brain development. Nat Commun.
11:40602020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jiang WJ, Sun MH, Li XH, Lee SH, Heo G,
Zhou D and Cui XS: Y-box binding protein 1 influences zygotic
genome activation by regulating N6-methyladenosine in porcine
embryos. J Cell Physiol. 238:1592–1604. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Peng J, He J, Lin L, Li Y and Xia Y:
Neural stem cell extracellular vesicles carrying YBX1 inhibited
neuronal pyroptosis through increasing m6A-modified GPR30 stability
and expression in ischemic stroke. Transl Stroke Res. 16:262–279.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Huang H, Weng H, Sun W, Qin X, Shi H, Wu
H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al: Recognition of RNA
N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and
translation. Nat Cell Biol. 20:285–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ramesh-Kumar D and Guil S: The IGF2BP
family of RNA binding proteins links epitranscriptomics to cancer.
Semin Cancer Biol 86(Pt 3). 18–31. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ying Y, Wu Y, Zhang F, Tang Y, Yi J, Ma X,
Li J, Chen D, Wang X, Liu X, et al: Co-transcriptional
R-loops-mediated epigenetic regulation drives growth retardation
and docetaxel chemosensitivity enhancement in advanced prostate
cancer. Mol Cancer. 23:792024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li W, Li W, Leng Y, Xu H, Xia Z and Wang
Y: ALKBH5-Mediated M(6)A demethylation of G3BP1 attenuates
ferroptosis via cytoplasmic retention of YBX1/p53 in diabetic
myocardial ischemia-reperfusion injury. Adv Sci (Weinh).
12:e072542025. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang C, Shen S, Kang J, Sugai-Munson A,
Xiao X, Zhang Y, Zhu J, Liu Z, McKay TB, Akeju O, et al: METTL3 is
essential for exercise benefits in diabetic cardiomyopathy.
Circulation. 152:327–345. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xi Q, Yang G, He X, Zhuang H, Li L, Lin B,
Wang L, Wang X, Fang C, Chen Q, et al: M(6)A-mediated upregulation
of lncRNA TUG1 in liver cancer cells regulates the antitumor
response of CD8(+) T cells and phagocytosis of macrophages. Adv Sci
(Weinh). 11:e24006952024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yin H, Chen L, Piao S, Wang Y, Li Z, Lin
Y, Tang X, Zhang H, Zhang H and Wang X: M6A RNA
methylation-mediated RMRP stability renders proliferation and
progression of non-small cell lung cancer through regulating
TGFBR1/SMAD2/SMAD3 pathway. Cell Death Differ. 30:605–617. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang H, Han Y, Wu C, Wang S, Chen M, Xu
Q, Wei H, Zhou X and Wang G: m6A-modified LINC02418 induces
transcriptional and post-transcriptional modification of CTNNB1 via
interacting with YBX1 and IGF2BP1 in colorectal cancer. Cell Death
Discov. 11:1012025. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gunasekaran VP, Nishi K, Sivakumar D,
Sivaraman T and Mathan G: Identification of
2,4-dihydroxy-5-pyrimidinyl imidothiocarbomate as a novel inhibitor
to Y box binding protein-1 (YB-1) and its therapeutic actions
against breast cancer. Eur J Pharm Sci. 116:2–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
He M, Li T, Wang A, Liu Y, Wang X, Liu Z,
Xie J, Wang Y, Wang Y, Ren Z, et al: MARCH8/NSUN6/ROS-mediated DNA
damage positive feedback loop regulates cisplatin resistance in
osteosarcoma. Cell Death Differ. Jul 19–2025.(Epub ahead of print).
View Article : Google Scholar
|
|
108
|
Huang H, Fang L, Zhu C, Lv J, Xu P, Chen
Z, Zhang Z, Wang J, Wang W and Xu Z: YBX1 promotes 5-Fluorouracil
resistance in gastric cancer via m5C-dependent ATG9A mRNA
stabilization through autophagy. Oncogene. 44:2357–2371. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li D, Chu X, Liu W, Ma Y, Tian X and Yang
Y: The regulatory roles of RNA-binding proteins in the tumour
immune microenvironment of gastrointestinal malignancies. RNA Biol.
22:1–14. 2025. View Article : Google Scholar
|
|
110
|
Ma L, Singh J and Schekman R: Two
RNA-binding proteins mediate the sorting of miR223 from
mitochondria into exosomes. Elife. 12:e858782023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Dinh NTM, Nguyen TM, Park MK and Lee CH:
Y-Box binding protein 1: Unraveling the multifaceted role in cancer
development and therapeutic potential. Int J Mol Sci. 25:7172024.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Alkrekshi A, Wang W, Rana PS, Markovic V
and Sossey-Alaoui K: A comprehensive review of the functions of
YB-1 in cancer stemness, metastasis and drug resistance. Cell
Signal. 85:1100732021. View Article : Google Scholar : PubMed/NCBI
|