Introduction
Among solid tumors, colorectal cancer (CRC) ranks
among the most common causes of morbidity and mortality, and its
prevalence continues to rise (1).
The 5-year survival rate of patients with early-stage localized CRC
is >90%, whereas that of patients with metastatic CRC is only
14% (2). There is evidence to
suggest that CRC development is influenced by both intrinsic and
extrinsic factors. In particular, dysregulated gut microbiota
composition has been demonstrated to influence the immune
microenvironment and promote distant metastases, and gut microbiota
are increasingly recognized as pathogenic contributors to CRC
metastasis (3).
Patients with cancer often have a poor prognosis and
experience ongoing psychological distress following cancer
diagnosis, surgery, chemotherapy and other cancer-related
complications (4). Prolonged
stress can impair the immune system by changing the metabolic
characteristics and composition of the gut microbiota (5), and is a major risk factor that can
accelerate CRC development (6). In
addition, long-term stress in patients with cancer may reduce their
ability to respond to treatments that rely on antitumor immune
activity, particularly immunotherapies (7). Therefore, strategies to reduce the
effect of psychological stress and depression on treatment outcomes
are crucial.
The association between long-term stress and the
growth of tumors is becoming increasingly evident, particularly
through neuroimmune interactions (6). Aside from cancer, chronic stress has
been shown in numerous studies to stimulate the sympathetic nervous
system (8,9), leading to the release of
neurotransmitters or hormones that regulate the activity of
macrophages, dendritic cells (DCs) and cytotoxic T lymphocytes
(10). DCs are immune cells that
play a key role in influencing the tumor microenvironment (TME),
processing and transmitting antigens, and activating
tissue-specific T-cell responses (11). In mice, prolonged psychological
stress causes the significant dysregulation of T-cell functions,
including reduced cytokine production and impaired cytotoxicity
(12), ultimately contributing to
metastasis. Disturbances of the microbiota-gut-brain axis and gut
microbial balance have been implicated in both depression and CRC
pathogenesis. This axis has emerged as a novel target for
depression since it mediates communication between the brain and
intestinal flora (13).
Traditional Chinese medicine has been widely used to
treat CRC, where it may reduce treatment resistance and adverse
effects, promote cancer cell death and inhibit metastasis (14). Ginseng, which is widely used in
traditional Chinese medicine, contains a variety of active
components, including polyacetylenes, ginsenosides and
polysaccharides (15). Among
these, ginsenosides have been shown to strengthen resistance to
stress, boost immunity, and protect the nervous system, with
ginsenoside Rh1 (Rh1) having been shown to improve memory and
learning by promoting cell viability in the hippocampal region
(16). Rh1 is the main metabolite
of protopanaxatriol-type ginsenoside Rg1 (17). Rg1 is a natural steroidal saponin
extracted from Panax ginseng with potent neuroprotective,
anti-inflammatory and antidepressant effects (18,19).
Notably, Rh1 exhibits stronger effects than its precursor Rg1 in
the enhancement of memory and hippocampus excitability (20). These findings suggest that the
antidepressive ability of Rh1 is closely associated with the in
vivo metabolism of Rg1.
Rh1 has demonstrated anti-inflammatory and antitumor
properties, mediated by the inhibition of angiogenesis and
metastasis (15,21). In particular, Rh1 has been reported
to prevent the growth of colon and breast cancers (22,23).
However, few studies have investigated the mechanisms by which Rh1
impacts CRC via modulation of the gut microbiota or immune
responses, and the impact of Rh1 on CRC under chronic stress
conditions remains unclear. In the present study, a CRC mouse model
subjected to chronic restraint stress (CRS) was used to explore the
antidepressant effect of Rh1, and to evaluate the relationship
between Rh1 treatment and the microbial composition of the gut.
Furthermore, the study aimed to determine whether the underlying
mechanism involves the promotion of DC maturation, thereby
activating T cells and enhancing the antitumor immune response to
retard CRC progression.
Materials and methods
Cell culture
The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences supplied the CT26 murine colon
adenocarcinoma cell line (cat. no. SCSP-523). The cells were
cultivated in RPMI-1640 complete medium containing 10% fetal bovine
serum (both Jiangsu KeyGEN BioTECH Co., Ltd.), supplemented with 1%
penicillin/streptomycin at 37°C with 5% CO2.
Animal care
A total of 90 male SPF BALB/c mice (age, ~8 weeks;
weight, 20–22 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. The mice were kept in a climate-controlled
environment at a temperature of 22±2°C and a relative humidity of
50±10%, with a 12-h light/dark cycle and free access to food and
water. Humane endpoints were established, including >20% weight
loss, tumor volume >2,000 mm3, ulceration, necrosis
or infection on the surface of the tumor, abnormal posture or
dyspnea. However, no mice were humanely sacrificed or found dead
during the study. The maximum weight loss observed was 6.1%. At the
end of the experiment, all mice were anesthetized with an
intraperitoneal injection of 50 mg/kg pentobarbital sodium. Then,
300 µl blood was collected from the mice by retro-orbital puncture.
Afterwards, the mice were sacrificed by cervical dislocation, and
death was confirmed by the absence of breathing and heartbeat for
>5 min.
Experimental design and drug
administration protocols
The CRS model was used to induce depression-like
behaviors in mice (24). All mice
were randomly assigned to five groups on day 0, following 7 days of
adaptation (n=5/group): i) Control group (CRC); ii) model group
(CRC + CRS); iii) low dose Rh1 group (CRC + CRS + Rh1 10 mg/kg);
iv) high dose Rh1 group (CRC + CRS + Rh1 20 mg/kg); and v)
fluoxetine group (CRC + CRS + fluoxetine 10 mg/kg). The doses of
Rh1 (Shanghai Yuanye Biotechnology Co., Ltd.) were selected on the
basis of a preliminary experiment, in which it was found that 10
and 20 mg/kg Rh1 are able to inhibit both tumor growth and
depression. Fluoxetine (Sigma-Aldrich) was employed as a positive
antidepressant control. The 10 mg/kg dose of fluoxetine
hydrochloride powder was administered by gavage following dilution
with distilled water (25). CRS
was used to induce depression in the mice prior to tumor
implantation. The mice subjected to CRS endured physical
restriction for 8 h/day (from 9:00 to 17:00), starting 21 days
prior to the start of CT26 cell injection and continued for a total
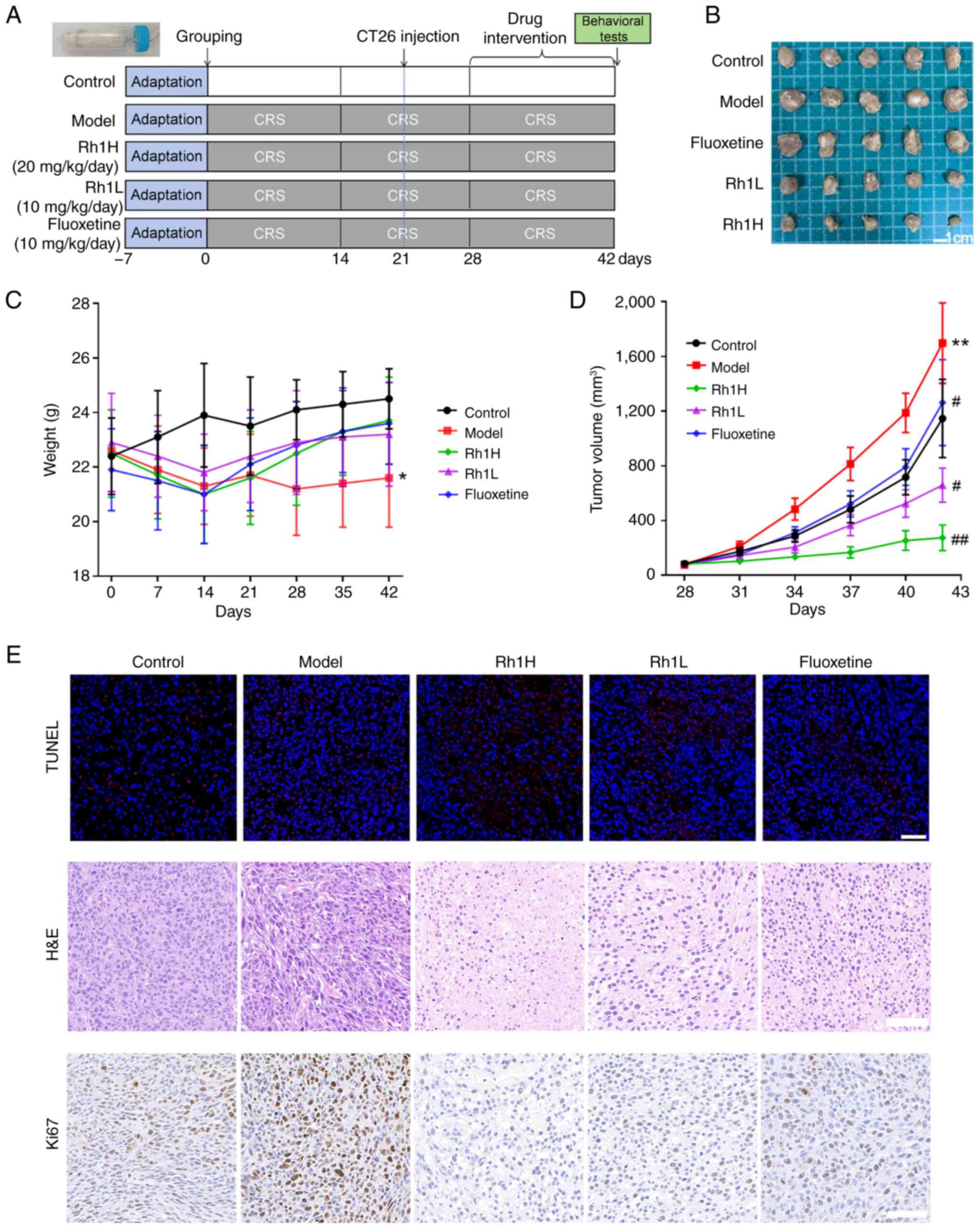
of 42 days (Fig. 1A).
CRS model establishment
All mice except those in the control group were put
in a 50-ml conical tube with adequate ventilation to enable easy
breathing for 8 h daily, continuously for 42 days in a row, thereby
establishing the CRS model. By contrast, the mice in the control
group were undisturbed in their cages, without food and water, over
the same time periods. After the CRS procedure, the animals were
returned to their home cages. At the end of the experiment, on day
42, behavioral assessments were performed, namely the open field
test (OFT), the sucrose preference test (SPT) and the elevated plus
maze test (EPMT).
CRC tumor model establishment
To establish the CRC model, 1×106 CT26
cells suspended in 0.1 ml PBS were subcutaneously injected into the
right flank of each mouse on day 21. The tumors reached ~100
mm3 in volume 7 days after CT26 cell inoculation. The
mice were then subjected to treatment with 10 or 20 mg/kg Rh1, or
10 mg/kg fluoxetine. Both Rh1 and fluoxetine were orally
administered for 2 weeks, from day 28 to 42. The control and model
groups both received the vehicle orally over the same time period.
A caliper was used to measure the volume of the tumor every 3 days,
and the tumor volume was calculated using the following formula:
V=(L × W2)/2, where V, L and W represent the volume,
length and width of the tumor, respectively. Photographs of the
tumors were captured after immersion in formalin.
OFT
The OFT was conducted as described previously
(26). Mice were placed in a
50×50×50-cm open-field arena with a black floor and blue Plexiglas
walls. Each mouse was allowed to spontaneously explore the arena
for 5 min. Following each trial, 75% ethanol was used to clean the
apparatus to remove odors. The following parameters were measured:
Total distance traveled, distance traveled in the central area and
dwelling time in the central area (27).
EPMT
The EPMT was performed as previously described, with
refinements (28). The plus-maze
apparatus consisted of four arms arranged in a cross, elevated 90
cm above the floor. Two of the arms were closed and two were open.
Mice were placed on the central platform facing an open arm and
allowed to explore the apparatus for 5 min. During this period, the
behavior of the mice was recorded, and the time spent and distance
traveled in the open arms was examined.
SPT
The SPT was conducted as previously described
(29). Mice were individually
housed in a cage containing two bottles of 1% sucrose solution for
24 h. This was followed by 24 h of food and water deprivation.
After adaption, the mice were given free access to two identical
bottles, one containing a 1% sucrose solution and the other
containing water. The volumes of water and sucrose solution
consumed were measured, and the percentage of sucrose solution to
total fluid consumed was calculated to indicate sucrose
preference.
Antibiotics (ABX) treatment
Pseudo germ-free BALB/c mice were established by the
administration of ABX solution to depleting the endogenous gut
microbiota and to investigate the causal role of gut microbiota.
The ABX solution contained neomycin (1 g/l), ampicillin (1 g/l),
metronidazole (1 g/l) and vancomycin (500 mg/l) in autoclaved water
(30). This combination was
selected for its broad-spectrum activity (covering Gram-positive
and -negative bacteria, and anaerobes) to ensure maximal depletion
of gut microbial communities. The ABX solution was orally
administered to the mice for consecutive 21 days starting on day
21, with replacement of the drinking water every 2 days. The ABX
treatment was designed to establish a causal link between gut
microbiota and the Rh1 treatment response and to explore whether
the effect of Rh1 was microbiota-dependent.
Assessments of inflammatory factors
and hormones
The blood collected by retro-orbital puncture was
centrifuged 3,500 × g for 12 min at 4°C to isolate the serum. In
addition, mouse brains were collected, homogenized in saline on
ice, and the homogenates were centrifuged 6,000 × g for 15 min at
4°C. The supernatant was carefully transferred to a sterile tube
and stored at −20° until required for analysis. Noradrenaline (NE),
adrenaline, cortisol (CORT), corticotropin-releasing hormone (CRH),
interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and C-X-C
motif chemokine ligand 1 (CXCL1) in the serum and
5-hydroxytryptamine (5-HT) in the brain were measured using the
following ELISA kits: NE (cat. no. E-EL-0047; Wuhan Elabscience
Biotechnology Co., Ltd.), adrenaline (cat. no. E-EL-0045; Wuhan
Elabscience Biotechnology Co., Ltd.), CORT (cat. no. AD3213Mo; Andy
Gene Biotechnology Co., Ltd.), CRH (cat. no. E-EL-M0351; Wuhan
Elabscience Biotechnology Co., Ltd.), IL-6 (cat. no. PI326;
Beyotime Institute of Biotechnology), TNF-α (cat. no. PT512;
Beyotime Institute of Biotechnology), CXCL1 (cat. no. PC173;
Beyotime Institute of Biotechnology) and 5-HT (cat. no. E-EL-0033;
Wuhan Elabscience Biotechnology Co., Ltd.).
Histopathological analysis and
immunohistochemical staining
Tumors were excised from the animals and immediately
fixed in 4% paraformaldehyde at room temperature for 48 h. For
hematoxylin and eosin (H&E) staining, deparaffinized and
rehydrated 4-µm-thick sections were stained with hematoxylin
solution at room temperature for 5 min. Sections were stained with
0.5% eosin Y solution at room temperature for 3 min. After
staining, sections were dehydrated, cleared in xylene, and mounted
with neutral balsam. Immunohistochemical staining was also
performed. The 4-µm-thick tumor sections were blocked with 3% BSA
(Sigma-Aldrich) for 30 min at 37°C, subjected to peroxidase
quenching with 3% hydrogen peroxide for 10 min at room temperature
and then incubated with an anti-Ki67 primary antibody (cat. no.
ab15580; Abcam, 1:500) overnight at 4°C. Subsequently, the sections
were incubated with HRP-labeled secondary antibody (cat. no.
ab6721; Abcam, 1:1,000 dilution) for 1 h at 37°C, and then
counterstained with hematoxylin (5 min at room temperature). Both
H&E and Ki67 staining were observed under a bright-field light
microscope (Olympus BX53).
TUNEL assays
Paraffin sections (3 µm thick) were prepared from
the tumors. An In Situ Cell Death Detection Kit (cat. no.
11684817910; Roche Diagnostics GmbH) was used to detect apoptotic
cells in the sections. A total of 50 µl TUNEL reaction mixture was
added at 37°C for 45 min. After incubation, sections were washed
with PBS. Nuclei were counterstained with DAPI (cat. no. ab104139;
Abcam) at a concentration of 2 µg/ml at room temperature for 8 min,
while red fluorescence indicated the apoptotic nuclei. Tissue
sections were visualized under a fluorescence microscope (Zeiss
Axio Imager Z2, Carl Zeiss AG). For quantification, four random
fields were chosen from each section and the average percentage of
positive cells in each field was calculated.
Immunofluorescence staining
3 µm-thick tumor sections were subjected to
deparaffinization and rehydration. Sections were immersed in 0.01 M
sodium citrate buffer, and heated in a microwave. Endogenous
peroxidase activity was quenched with 3% hydrogen peroxide for 10
min at room temperature. Sections were then covered with 5% goat
serum (cat. no. GB25004; Wuhan Servicebio Technology Co., Ltd.) to
block non-specific binding at room temperature for 30 min. For
CD11c/CD80 dual staining, tumor sections were first incubated with
anti-CD11c antibody (cat. no. ab254183; Abcam, 1:200) at 4°C
overnight, followed by anti-mouse IgG H&L (HRP) (cat. no.
GB23301; Wuhan Servicebio Technology Co., Ltd., 1:500 dilution) at
room temperature for 1 h. Cyanine 3 tyramide (cat. no. G1233;
Servicebio, 1:50 dilution) was then incubated at room temperature
for 10 min to amplify the HRP signals, producing red fluorescence.
Sections were subsequently incubated with FITC-conjugated anti-CD80
antibody (cat. no. ZB115640; Wuhan Servicebio Technology Co., Ltd.,
1:100 dilution) at room temperature for 1 h, which generated green
fluorescence. For CD8 staining, sections were incubated with
anti-CD8 antibody (cat. no. GB115692; Wuhan Servicebio Technology
Co., Ltd., 1:200 dilution) at 4°C overnight, followed by
FITC-conjugated IgG (H+L) secondary antibody (cat. no. GB22303;
Wuhan Servicebio Technology Co., Ltd., 1:500) at room temperature
for 1 h. After staining, all sections were counterstained with DAPI
(working concentration: 2 µg/ml, cat. no. ab104139; Abcam) at room
temperature for 8 min. The stained sections were observed using a
fluorescence microscope (Zeiss Axio Imager Z2, Carl Zeiss AG).
16S rRNA sequencing and data
analysis
Mice fecal samples were collected on day 42, at the
end of the experiment, and were snap frozen in liquid nitrogen and
stored at −80°C until use. Total DNA was extracted from the fecal
samples using the FastPure Feces DNA Isolation Kit (Shanghai Major
Yuhua Co., Ltd.) according to the manufacturer's instructions. DNA
concentration and purity were assessed using a NanoDrop2000
spectrophotometer (Themo Fisher Scientific, Inc.). The V3-V4
variable region of the 16S rRNA gene was amplified using PrimeSTAR
Max DNA Polymerase (cat. no. R045A; Takara Bio Inc., Shiga,
Japan),with the universal bacterial primer pair: Forward primer
(338F): 5′-ACTCCTACGGGAGGCAGCAG-3′; Reverse primer (806R):
5′-GGACTACHVGGGTWTCTAAT-3′. The thermocycling condition was set as
follows: Pre-denaturation: 98°C for 3 min; Denaturation: 98°C for
10 sec, 55°C for 30 sec; Extension: 72°C for 45 sec; Steps 2–4
repeated for 30 cycles; Final extension: 72°C for 5 min. Amplicon
visualization was verified by 2% agarose gel electrophoresis
stained with 0.5 µg/ml ethidium bromide (cat. no. E1510;
Sigma-Aldrich, St. Louis, MO, USA). Amplified products were
purified using the AMPure XP Beads (cat. no. A63881; Beckman
Coulter, Inc., Brea, CA, USA). Then, the Nextera XT DNA Library
Prep Kit (cat. no. FC-131-1096; Illumina, Inc., San Diego, CA, USA)
was used to construct sequencing libraries. Sequencing was then
performed using the MiSeq PE250 system (Illumina, Inc.) with 250 bp
paired-end (PE250) sequencing. The MiSeq Reagent Kit v3 (600
cycles, cat. no. MS-102-3003; Illumina, Inc.) was used. After
library quality control, qualified libraries were pooled and
diluted to a loading concentration of 6 pM. Raw sequencing reads
were quality-controlled using FastQC (Version 0.11.9; Babraham
Bioinformatics, Cambridge, UK). Paired-end reads were merged into
full-length sequences using FLASH (Version 1.2.11). Amplicon
sequence variations were generated using Deblur-denoised sequences
(Version 1.1.0; github.com/biocore/deblur. The Simpson (measuring
community evenness), abundance-based coverage estimator (ACE,
estimating species richness) and Shannon (combining richness and
evenness) indices (31–33) were determined to evaluate α
diversity of the data and principal coordinate analysis (PCoA) was
performed to evaluate the b diversity of the data. PCoA
visualization was performed using the q2-emperor plugin in QIIME 2
(qiime2.org).
Flow cytometry analysis
The spleen and tumor-draining lymph nodes were each
mechanically filtered using a 200-mesh screen. Red blood cells in
the resulting cell suspensions were lysed using ACK lysis buffer
(cat. no. C3702; Beyotime Institute of Biotechnology). Tumor
tissues were mechanically minced and digested in RPMI-1640 medium
containing DNase, collagenase IV and hyaluronidase (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C for 1 h. The tissues were filtered
through a sieve to prepare a single-cell suspension. Isolated cells
were plated and cultured for 4 h in a medium containing Cell
Activation Cocktail (cat. no. 423303; BioLegend, Inc.) to analyze
the release of cytokines by tumor-infiltrating T cells. Cells were
then aliquoted into tubes and surface stained in the dark for 30
min at 4°C with Fixable Viability Dye eFluor 780 (cat. no. 2633409;
eBioscience; Thermo Fisher Scientific, Inc.), anti-CD3-PE-cy7 (cat.
no. 552774; BD Biosciences), anti-CD4-APC (cat. no. 100412;
BioLegend, Inc.), anti-CD45-Percy-cy5 (cat. no. 103131; BioLegend,
Inc.) and anti-CD8-FITC (cat. no. 100706; BioLegend, Inc.). The
anti-CD45 antibody is used to gate the ‘leukocyte population’, and
the anti-CD3 antibody is used to identify T cells from the
CD45+ leukocyte population. For intracellular staining,
cells were fixed with fixation buffer (4% paraformaldehyde, cat.
no. 420801; BioLegend, Inc.) in the dark for 30 min at room
temperature and washed with permeabilization buffer (cat. no.
421002; BioLegend, Inc.) twice. Cells were incubated with
anti-granzyme B-BV421 (cat. no. 396413; BioLegend, Inc.) and
anti-IFN-γ-PE (cat. no. 505807; BioLegend, Inc.) in the dark for 30
min at 4°C.
After blocking with anti-mouse CD16/32 (Fc block,
cat. no. 553141, BD Pharmingen), MDSC was examined by staining with
Fixable Viability Dye eFluo 780 (eBioscience), anti-CD45-Percy-cy5
(cat. no. 103131; BioLegend, Inc.), anti-CD11b-FITC (cat. no.
101206; BioLegend, Inc.) and anti-Gr-1-APC (cat. no. 108411;
BioLegend, Inc.) in the dark for 30 min at 4°C. After blocking with
anti-mouse CD16/32, M-MDSCs was examined by staining with Fixable
Viability Dye eFluo 780 (eBioscience), anti-CD45-Alexa Fluor700
(cat. no. 103127; BioLegend, Inc.), anti-CD11b-FITC (cat. no.
101206; BioLegend, Inc.), anti-Ly-6C-PerCP cy5.5 (cat. no. 128011;
BioLegend, Inc.) and anti-Ly-6G-PE (cat. no. 127607; all BioLegend,
Inc.) in the dark for 30 min at 4°C. After blocking with anti-mouse
CD16/32, DC maturation was examined by staining with Fixable
Viability Dye eFluo 780 (eBioscience, USA), anti-CD45-Percy-cy5,
anti-CD11c-APC (cat. no. 117309; BioLegend, Inc.), and
anti-MHCII-FITC (cat. no. 107605; BioLegend, Inc.) antibodies, or
an isotype IgG control (cat. no. 400605; BioLegend, Inc.) in the
dark for 30 min at 4°C. Flow cytometry analysis was performed using
an Beckman CytoFLEX flow cytometer (Beckman Coulter Inc.). The flow
cytometry data were analyzed using FlowJo software V10.8.1 (BD
Biosciences).
Statistical analysis
Data are presented as the mean ± standard deviation
for normally distributed data. Non-normally distributed data are
presented as the median and 25th and 75th percentile). Statistical
analyses were performed using GraphPad Prism 9.0 software
(Dotmatics). When the data displayed a normal distribution and
homogeneous variance, one-way ANOVA was employed, followed by
Bonferroni post-hoc multiple comparisons tests. When the data
displayed a normal distribution and heterogeneous variance, Welch
ANOVA was used; no post-hoc test was performed as the results were
not statistically significant. When the data were non-normally
distributed, Kruskal-Wallis H test was applied, followed by
post-hoc pairwise comparisons using Bonferroni-corrected
Mann-Whitney U tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Rh1 inhibits tumor growth in mice with
CRC and CRS
The experimental plan is displayed in Fig. 1A. CRS was applied to induce
depression in all mice, with the exception of those in the control
group. A CT26 cell-bearing tumor model was established after 3
weeks of CRS. The mean body weight of the mice in the model group
at the end of the study was significantly less than that of mice in
the control group. Although mean body weight exhibited an ascending
tendency in the Rh1 and fluoxetine treatment groups compared with
that in the model group, the difference between these groups was
not found to be statistically significant (Fig. 1C). Tumor growth in the model group
was greater than that in the control group (Fig. 1B). By day 42, the tumor volume in
the stressed mice of the model group was significantly higher than
that in the non-stressed mice of the control group, but
significantly lower in the two Rh1 groups compared with the model
group (Fig. 1D).
H&E staining revealed that the tumor cells in
the model group were densely packed, exhibiting irregular
morphologies, nuclear dimorphism and mitotic figures. In the Rh1
and fluoxetine groups, features of cell necrosis and apoptosis were
observed. Necrosis was indicated by dispersed lower-density cells,
cytoplasmic leakage, cellular rupture and multiple vacuolar
structures, while apoptosis was characterized by nuclear
condensation, fragmentation and dissolution (Fig. 1E). In addition, the number of TUNEL
positive cells in the Rh1 groups was increased markedly compared
with that in the model group (Fig.
1E). Furthermore, Rh1 treatment clearly reduced Ki67 expression
in tumors to lower levels than those observed in the model group.
These findings indicate that Rh1 attenuates tumor development in
mice with CRC and CRS.
Rh1 ameliorates depressive-like behaviors and
enhances cognitive function in mice with CRC and CRS. The
therapeutic impact of low and high dosages of Rh1 was assessed
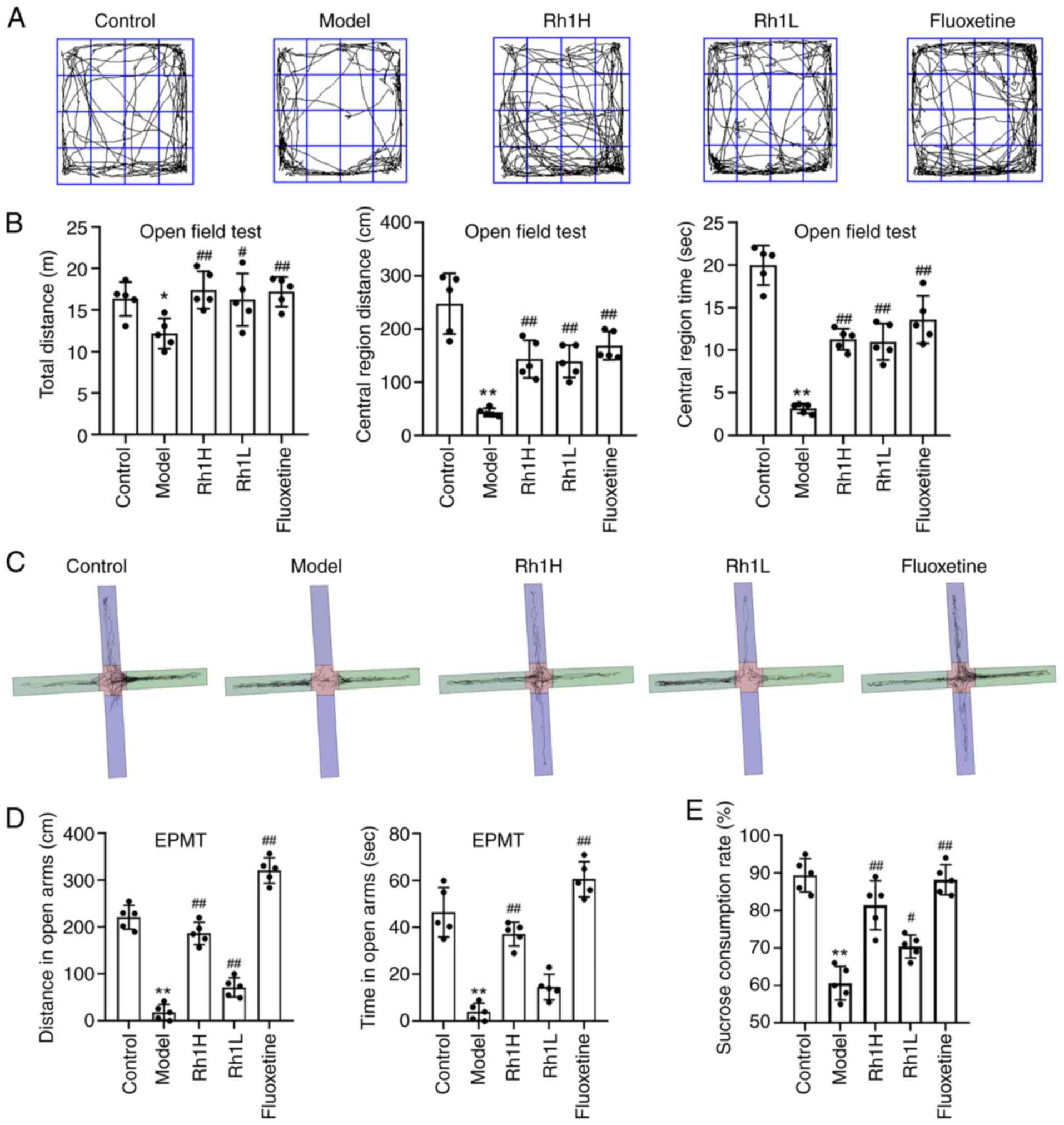
using the stress-associated cancer model mice (Fig. 2). Depression- and anxiety-like
behaviors were evaluated using the SPT, EPMT and OFT. In the OFT,
the depressed mice in the model group exhibited a reduced capacity
for spontaneous exploration and tended to remain in the corners and
edges of the field (Fig. 2A). The
total distance traveled, distance traveled in the central area and
dwelling time in the central area of the model mice were
significantly decreased compared with those of the control group
(Fig. 2B). However, these measures
were significantly increased in the mice treated with fluoxetine or
Rh1 compared with those in the model group.
The results of the EPMT assay revealed that the time
spent and distance traveled in the open arms by the model mice were
significantly lower compared with those of the control mice
(Fig. 2C and D). However,
following Rh1 or fluoxetine intervention, significant increases in
the time spent and distance traveled in the open arms were
observed.
The SPT is regarded as an index of anhedonia.
Sucrose preference decreased significantly in model group compared
with that in the control group. However, the Rh1H and fluoxetine
groups exhibited significantly increase sucrose preference rates
compared with that of the model group (Fig. 2E). These results indicate that Rh1
treatment attenuated the abnormal behaviors of CRS rats in the SPT,
EPMT and OFT, and suggest that both fluoxetine and Rh1 alleviate
depression-like behaviors in mice.
Rh1 regulates cytokine expression and hormone
levels in mice with CRC and CRS. Cytokines are essential
contributors to the regulation of inflammatory processes. ELISA
results revealed that the levels of TNF-α, IL-6 and CXCL1 were
significantly higher in CRS-exposed mice with CRC compared with
those in control mice with CRC, indicating that CRS increased the
inflammatory response in mice with CT26 tumors (Fig. 3A). The levels of TNF-α, CXCL1 and
IL-6 in the Rh1 and fluoxetine groups were considerably lower
compared with those in the model group. Reductions in the levels of
the monoamine neurotransmitters 5-HT and NE, and increases in CORT
levels are associated with depression (34). In particular, alterations in the
release or reuptake of 5-HT are associated with depressive
disorder. Mice in the CRS-stimulated CRC-bearing model group
exhibited significantly lower brain 5-HT and serum NE levels
compared with those in the control group, indicating that CRS
stimulation disrupts monoamine neurotransmitter homeostasis.
Treatment with Rh1 or fluoxetine resulted in significant increases
in these levels (Fig. 3).
Adrenaline is a key catecholamine hormone released during stress
and may influence cancer progression (35). The serum levels of adrenaline, CRH
and CORT in the model group were significantly higher compared with
those in the control group; however, treatment with a high dose of
Rh1 or fluoxetine resulted in a significant reduction in these
levels (Fig. 3B).
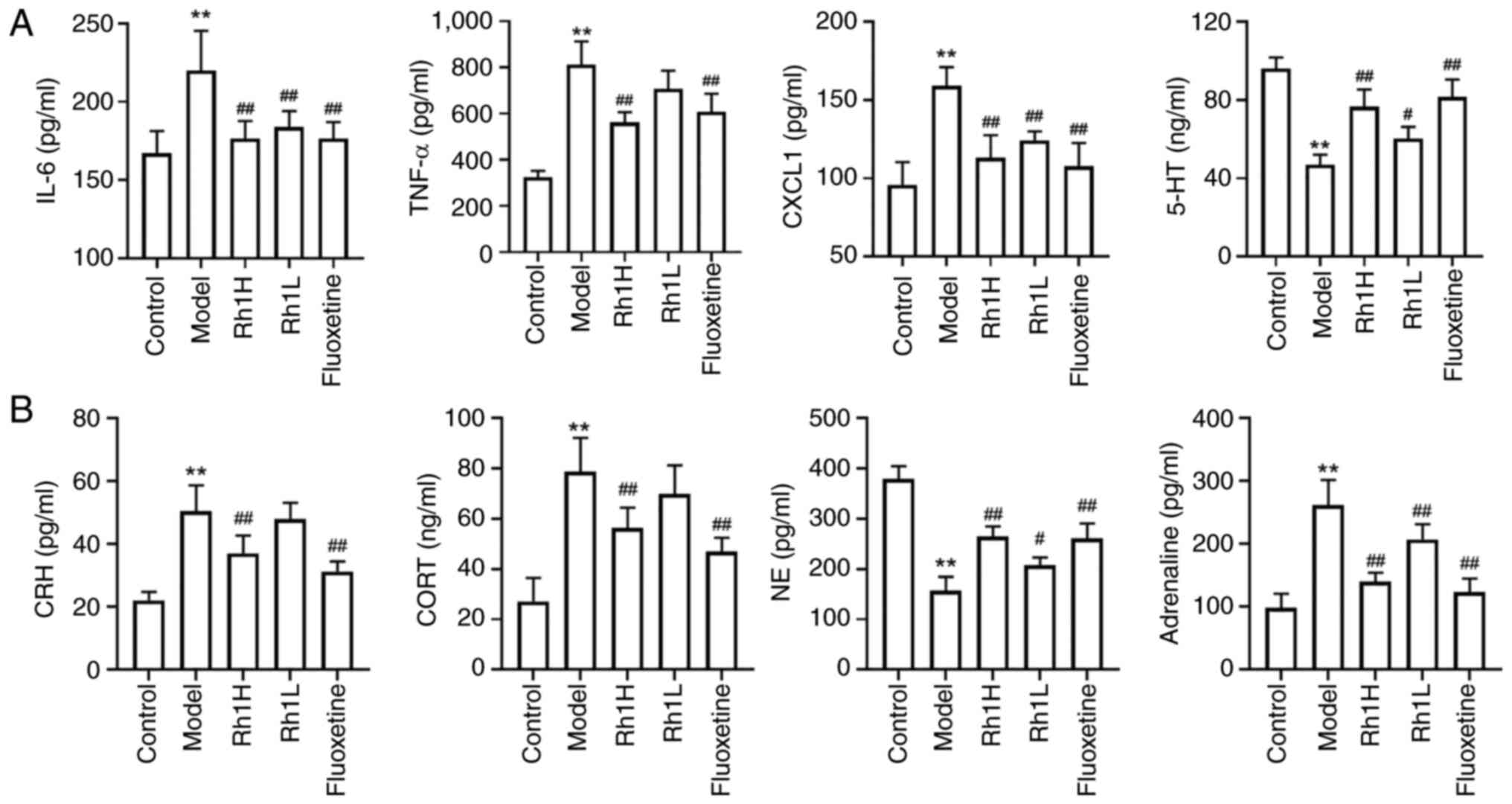 | Figure 3.Rh1 regulates the levels of hormones
and inflammatory factors in the brain and serum of mice bearing
colorectal tumors and subjected to chronic restraint stress. (A)
Brain levels of 5-HT and serum levels of TNF-α, IL-6 and CXCL1. (B)
Serum levels of adrenaline, NE, CRH and CORT. Results are presented
as the mean ± SD (n=5). **P<0.01 vs. the control group;
#P<0.05 and ##P<0.01 vs. the model
group. Rh1, ginsenoside Rh1; Rh1H, high dose Rh1; Rh1L, low dose
Rh1; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; CXCL1,
C-X-C motif chemokine ligand 1; 5-HT, 5-hydroxytryptamine; CRH,
corticotropin-releasing hormone; CORT, cortisol; NE,
noradrenaline. |
Rh1 rebalances gut dysbiosis in mice with CRC and
CRS. The results of α-diversity analyses indicated that the gut
microbial communities in all five groups were rich and diverse. In
the model group, the Simpson index was significantly higher and the
ACE and Shannon indices were significantly lower compared with
those in the control group. ACE indices in the Rh1 or fluoxetine
groups were not significantly different from that of the model
group. The Simpson indices of the Rh1 and fluoxetine groups were
significantly lower than that of the model group. The Shannon
indices of the Rh1 and fluoxetine groups were significantly higher
than the Shannon index of the model group (Fig. 4A). Furthermore, b diversity was
analyzed using PCoA based on various distance measures, including
Bray-Curtis distances. When plotted against the relative
contributions of each group to each principal component (PC), the
first two principal components, which together accounted for 39.80%
of the total microbial factor loadings (PC1, 22.76%; PC2, 17.04%),
revealed notable differences in the gut microbiota communities
among the groups. In the Rh1-treated groups, clear shifts in
bacterial composition towards that of the control group were
observed (Fig. 4B), indicating
partial restoration of the gut microbial community. Proteobacteria,
Firmicutes, Bacteriodota, Actinobacteriota and Desulfobacterota
accounted for the majority of the sequencing reads among the phyla
that were detected (Fig. 4C). When
compared with the microbial composition of the control group, the
model group exhibited a significantly higher abundance of
Firmicutes and Actinobacteriota, lower abundance of Bacteroidota
and Proteobacteria (Fig. 4D), and
a higher Firmicutes/Bacteroidota ratio (Fig. 4E). Exposure to a high dose of Rh1
significantly increased the abundance of Bacteroidota and reduced
the abundance of Firmicutes. Mice treated with a low dose of Rh1
also exhibited a significantly reduced abundance of Firmicutes.
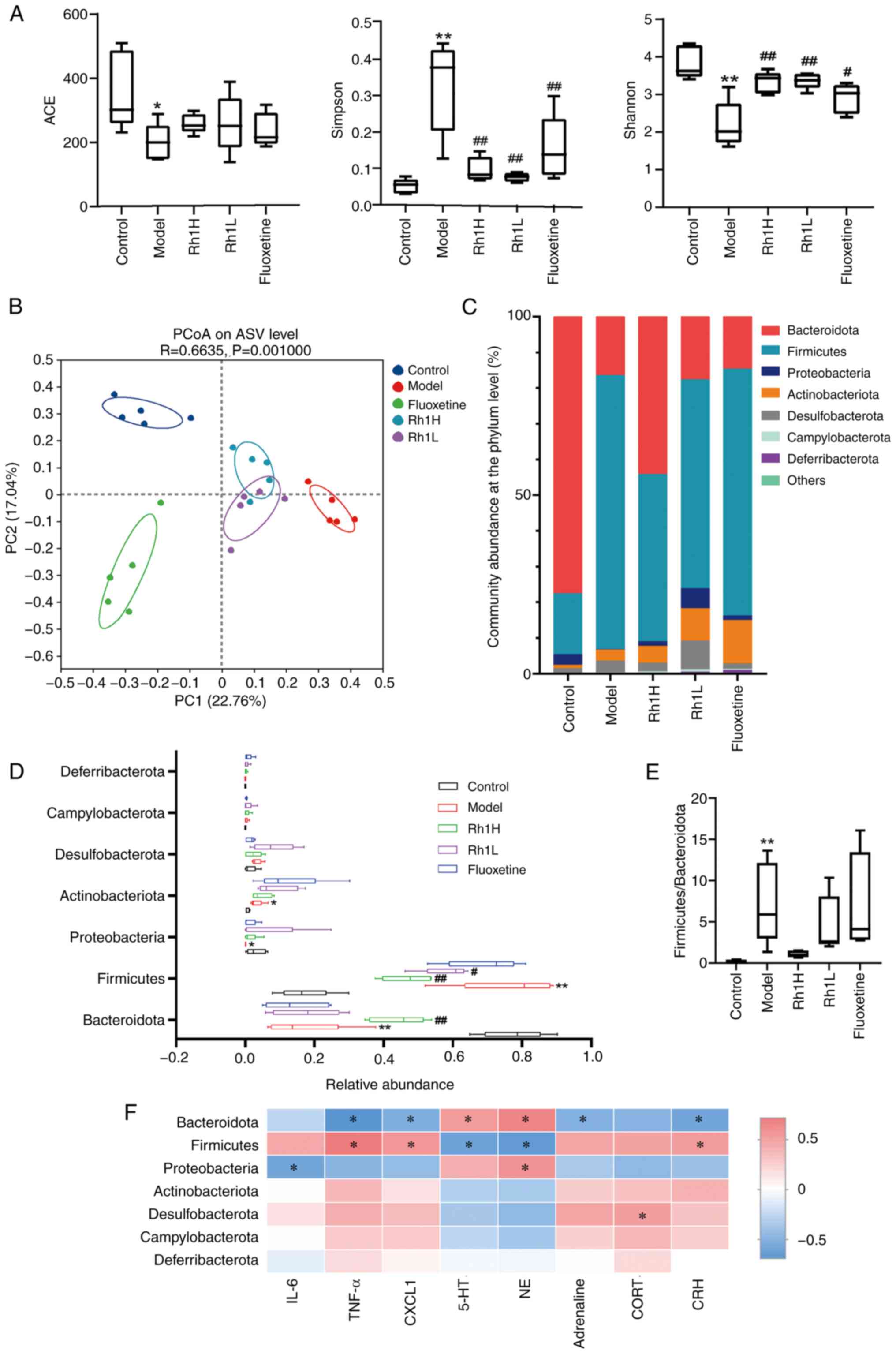 | Figure 4.Rh1 treatment regulates the gut
microbial balance in mice bearing colorectal tumors and subjected
to chronic restraint stress. (A) ACE, Simpson and Shannon indices
of α-diversity and (B) PCoA analysis of the β-diversity of the gut
microbiota. (C) Community distribution and (D) richness of the
intestinal flora at the phylum level. (E) Firmicutes/Bacteroidota
ratios. (F) Heat map showing the Spearman correlation coefficients
for hormone or inflammatory factors with phylum abundance. n=5).
*P<0.05 and **P<0.01 vs. the control group;
#P<0.05 and ##P<0.01 vs. the model
group. Rh1, ginsenoside Rh1; Rh1H, high dose Rh1; Rh1L, low dose
Rh1; ACE, abundance-based coverage estimator; PCoA, principal
coordinate analysis; PC, principal component; ASV, amplicon
sequence variant; IL-6, interleukin-6; TNF-α, tumor necrosis
factor-α; CXCL1, C-X-C motif chemokine ligand 1; 5-HT,
5-hydroxytryptamine; NE, noradrenaline; CORT, cortisol; CRH,
corticotropin-releasing hormone. |
A Spearman correlation heatmap revealed that
Bacteroidota abundance positively correlated with 5-HT and NE
(Fig. 4F), and negatively
correlated with TNF-α, CXCL1, adrenaline and CRH. It also revealed
that Firmicutes abundance negatively correlated with 5-HT and NE,
and positively correlated with TNF-α, CXCL1 and CRH. In addition,
the inflammatory factor IL-6 and Proteobacteria exhibited a
negative correlation, while the endocrine hormone CORT and
Desulfobacterota exhibited a positive correlation.
Gut microbiota mediates the antitumor
effect of Rh1 in mice with CRC and CRS
We hypothesized that the efficacy of Rh1 in treating
cancer comorbid with depression may be mediated by gut bacteria,
given the importance of intestinal flora in both carcinogenesis and
depression (36). ABX treatment
was administered to decrease the resident microbiota in mice
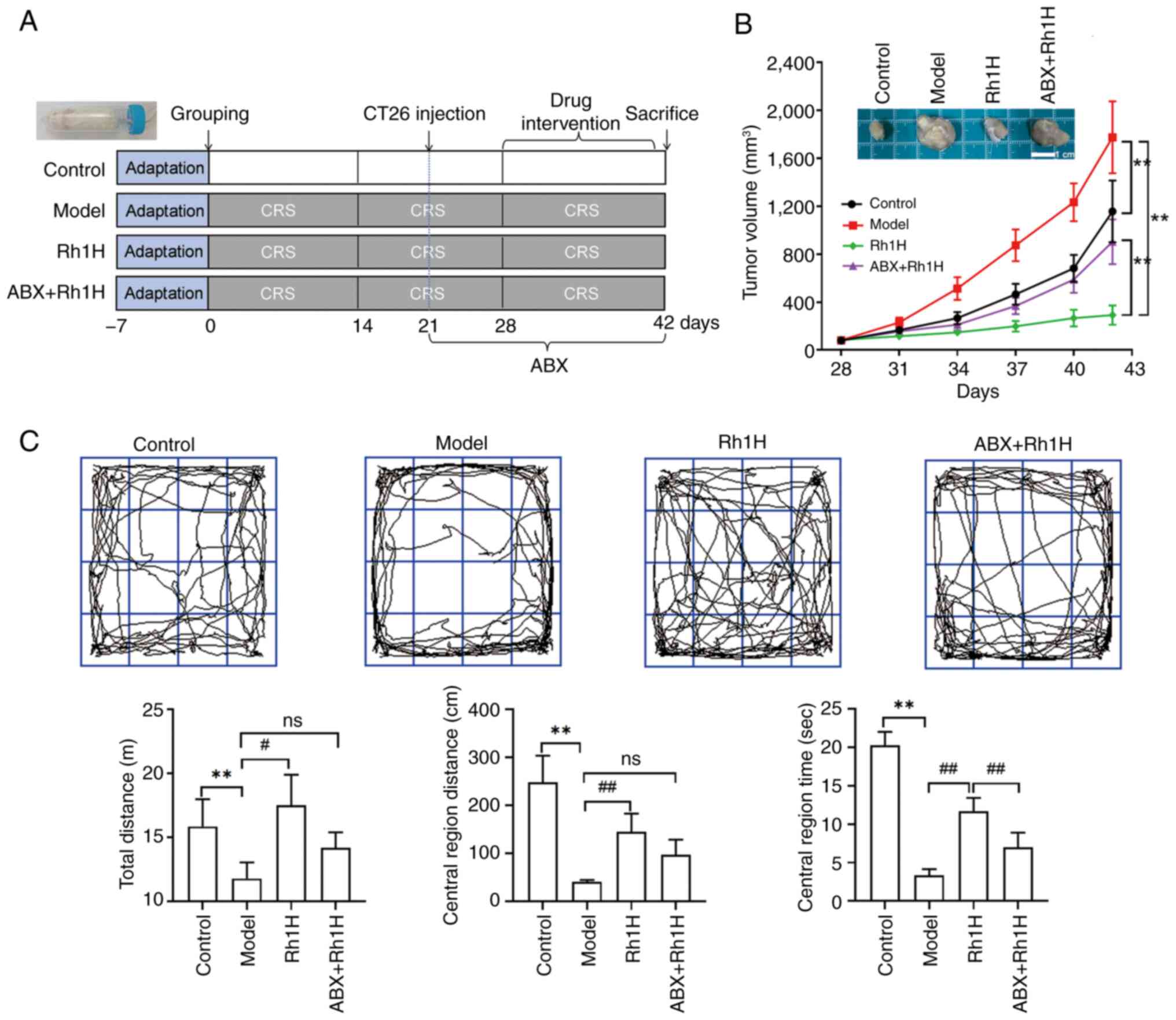
subjected to CRS during Rh1 intervention (Fig. 5A). The amelioration of
depression-like behaviors observed in the OFT for the high dose Rh1
group was attenuated when the mice also received ABX treatment
(Fig. 5C). No significant
differences were observed between the mice in the model group and
those treated with both ABX and high dose Rh1 with regard to the
total distance traveled and the distance traveled in the central
area. The time in the central region in ABX + Rh1H group were
considerably lower than that of Rh1H group. The antitumor effect of
Rh1 in mice with CRS and CRC may be weakened by the reduction of
gut flora, as suggested by the significantly larger mean tumor
volume in the mice treated with ABX and high dose Rh1 compared with
that in mice treated with high dose Rh1 alone (Fig. 5B).
Rh1 improves T-cell activation in mice
with CRC and CRS
The effect of Rh1 on immune cell infiltration and
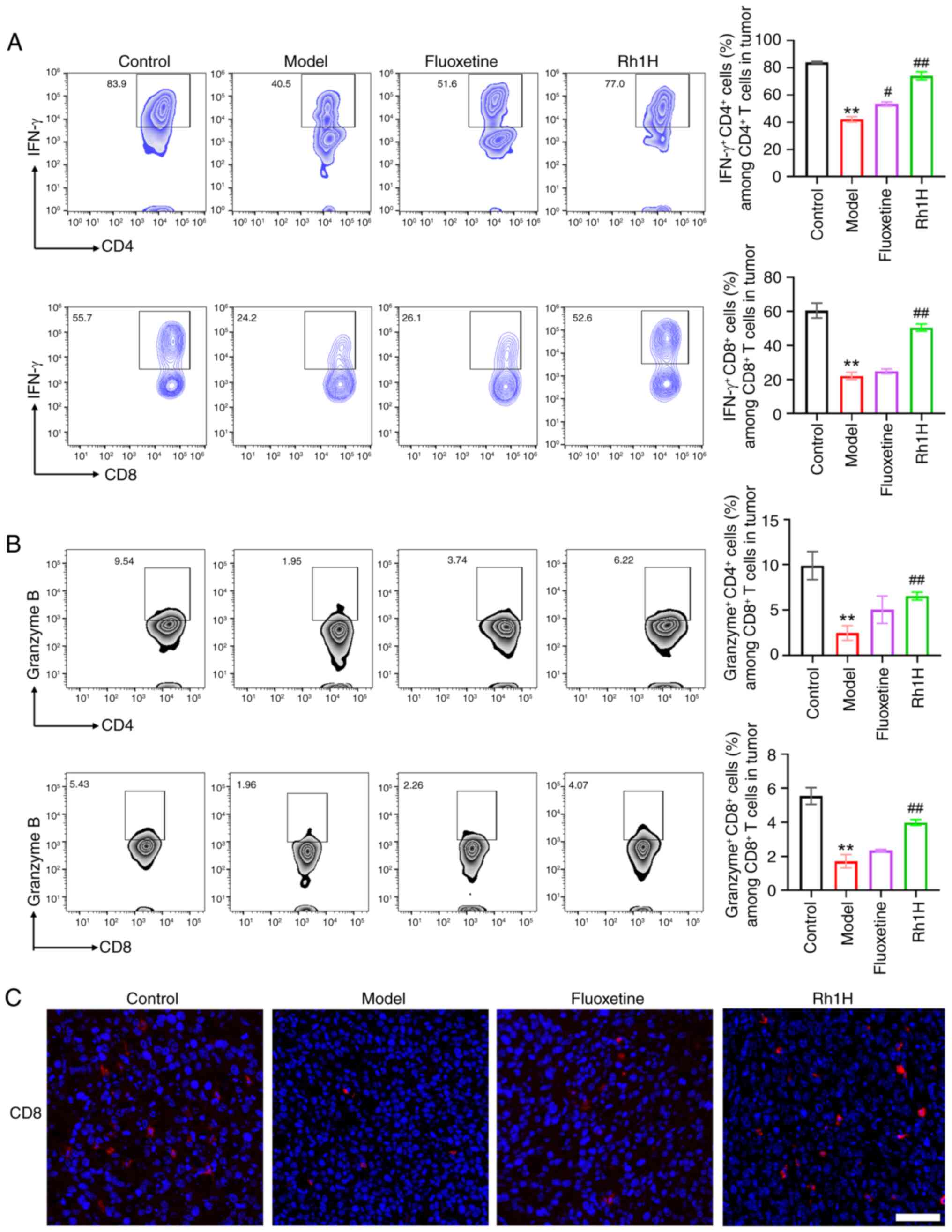
activation in mice with CRC and CRS was investigated (Fig. 6). Examination of CD8+T
and CD4+T cell subsets in the tumors revealed that mice
in the high dose Rh1 group had a significantly higher population of
effector T cells (IFN-γ+CD8+T cells and
IFN-γ+CD4+T cells) compared with that in the
model group (Fig. 6A). These data
indicate that increased activation of CD8+ and
CD4+ T cells increases IFN-γ production in tumor
tissues, which may serve as a key mediator of the tumor rejection
response. In addition, by significantly increasing the percentage
of granzyme B+ cells within the CD8+T and
CD4+T cell population, Rh1 may restore the functional
activity of tumor-infiltrating CD8+T and
CD4+T cells (Fig. 6B).
Furthermore, immunofluorescence staining indicated that
CD8-positive T cells exhibited weaker red fluorescence in the model
group compared with that in the control group, while CD8-positive T
cells were markedly increased in the high dose Rh1 group (Fig. 6C).
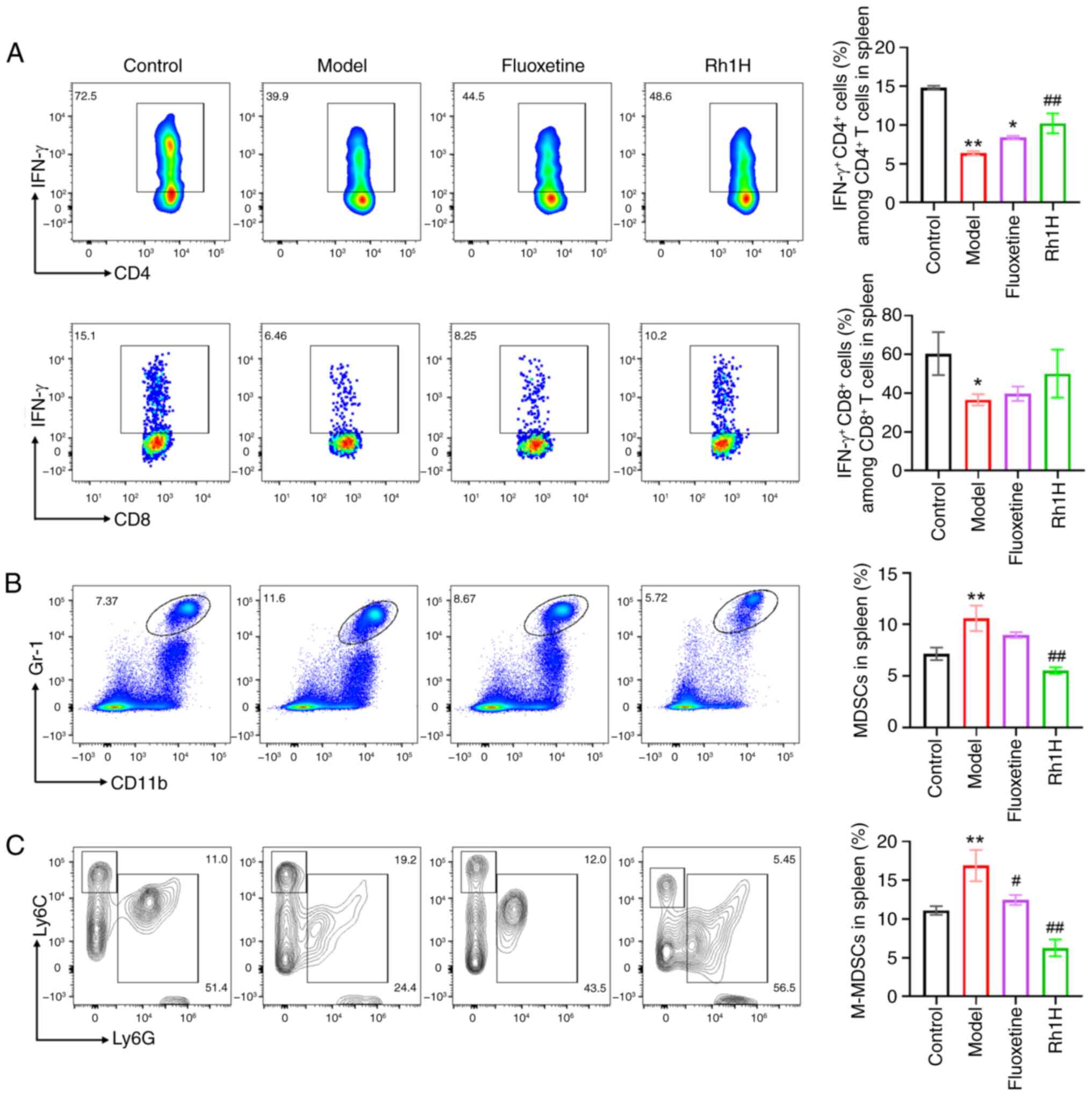
The spleens of mice treated with a high dose of Rh1
had a significantly higher frequency of
IFN-γ+CD4+T cells than that observed in the
model group (Fig. 7A). Treatment
with a high dose of Rh1 also raised the proportion of
CD8+T cells co-expressing IFN-γ; however, this increase
was not statistically significant. Myeloid-derived suppressor cells
(MDSCs) isolated from the spleen were also analyzed by flow
cytometry. The number of tumor-infiltrating
CD11b+Gr-1+ cells, considered to represent
MDSCs, was analyzed. As shown in Fig.
7B, Rh1 significantly decreased the proportion of total MDSCs
in the spleen compared with that in the model group (Fig. 7B). Additionally, the percentages of
different MDSC subtypes were evaluated, and the results revealed
that after treatment with a high dose of Rh1, the frequency of
monocytic MDSCs (M-MDSC,
CD11b+Ly6G−Ly6Chigh) cells was
significantly reduced (Fig. 7C).
These findings suggest that CD8+T, CD4+T and
MDSC cells may contribute to the antitumor response of Rh1, with
Rh1 reducing MDSC counts and promoting the recovery of T
cell-dependent antitumor responses.
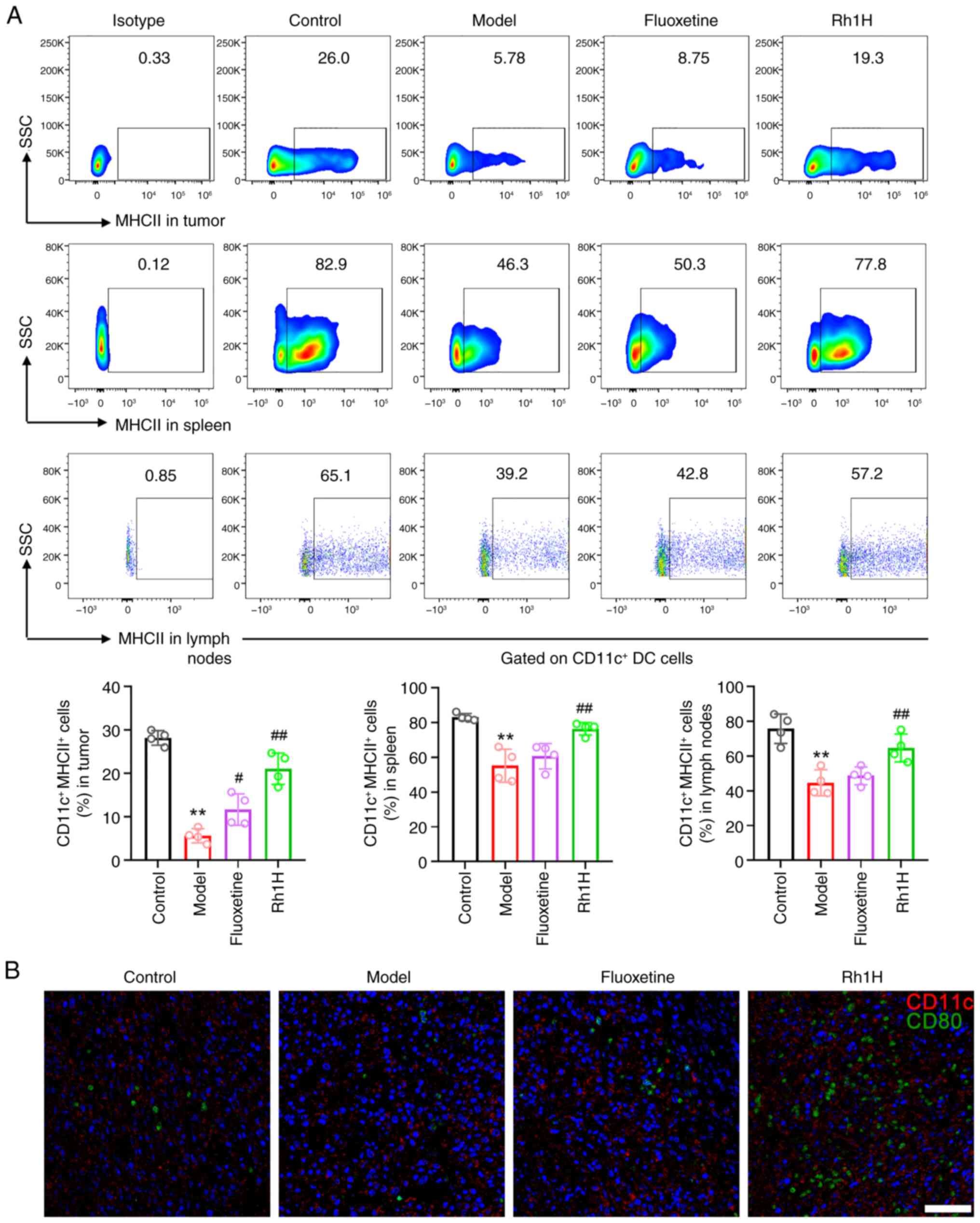
Rh1 promotes DC maturation in mice
with CRC and CRS
Naive or memory CD8+ and CD4+T
cells are exposed to antigens by mature DCs through MHCI and MHCII
molecules, respectively (37). To
verify whether DCs contributed to the immune response, the
maturation of DCs was detected in Rh1-treated CRC tissues,
tumor-draining lymph nodes and the spleen. Flow cytometric analysis
confirmed that the expression of MHCII, a DC maturation marker, was
significantly increased in the spleen, lymph node and tumor tissue
following Rh1 treatment compared with that in the model group
(Fig. 8A), suggesting the robust
accumulation and maturation of DCs in the TME following Rh1
treatment. In addition, immunofluorescence staining indicated that
CD80-positive DCs exhibited only weak green fluorescence in the
model group (Fig. 8B), but
markedly higher green fluorescence in the high dose Rh1 group.
These results demonstrate that Rh1 effectively stimulates DCs to
cross-present antigens, which in turn activate CD8+T
cells to enhance the immune response. In addition, Rh1 may have
markedly boosted the antitumor immune response by increasing the
capacity of activated T cells to eradicate tumor cells.
Discussion
Depression is a prevalent psychological disorder
that is a threat to mental well-being worldwide and has resulted in
a growing suicide rate during the 21st century (38). CRC is the second most common cause
of cancer-related deaths (39).
Depression and chronic psychological stress negatively impact the
quality of life of patients with CRC. In addition, an increased
risk of depression has been observed in patients with CRC following
prognosis (40). Therefore, the
effective management of comorbid depression has attracted
considerable attention.
The Rg1 metabolite Rh1 has demonstrated strong
inhibitory effects on the proliferation of lung and breast cancer
cells (41), and has been reported
to suppress the invasion of CRC and breast cancer cells (22). Research on Rh1 has focused on its
anticancer, anti-inflammatory and immune modulating properties
(11,42). However, its potential
antidepressant effects and the underlying mechanisms remain
unclear. Cognitive impairments and depression-like behaviors have
been noted in mice subjected to CRS, and ginsenoside Rb1 was shown
to prevent these depression-like behaviors (43). Similarly, Rg1 exhibits
antidepressant-like effects in rats subjected to CRS (44). Rb1 also significantly attenuated
azoxymethane/dextran sodium sulfate-induced colon carcinogenesis in
mice (45), while Rg1 demonstrated
antitumor activity in a CT26 CRC xenograft mouse model (46). However, these protective effects of
Rb1 or Rg1 were not evaluated in models combining depression with
cancer. Therefore, the present study investigated the anticancer
and antidepressant potential of Rh1 in CRS-exposed CRC-bearing
mice. Previous studies have shown that Rh1 (20 mg/kg) inhibits
tumor growth in mice bearing gastric cancer and CRC xenografts
(22,47). In addition, fluoxetine (10 mg/kg)
was used as a positive control in a previous study on a combined
CRS and CRC xenograft model (48).
Therefore, it was selected for use as a positive control in the
present study. Notably, Rh1 (20 mg/kg) significantly mitigated the
depression-like behaviors of mice subjected to CRS in the present
study. In addition, the findings of the study indicate that Rh1
prevents CRC tumor growth in mice subjected to CRS.
There is a significant association between
depression and the brain-gut axis. Therefore, the preservation and
restoration of the normal state of the intestinal flora is
considered beneficial for the prevention and treatment of mental
disorders (49). In the present
study, the results indicated that CRS is positively associated with
the progression of CRC, likely by affecting the balance of
intestinal flora. Consistent with previous reports, Firmicutes and
Bacteroidota were the most common phyla identified across all
groups (50). An increased
abundance of Firmicutes and a decreased proportion of Bacteroidota
were observed in the CRS-exposed xenografted mice in the present
study, which is consistent with previous observations (51). However, treatment with a high dose
of Rh1 attenuated these changes, lowering the abundance of
Firmicutes and increasing that of Bacteroidota compared with the
respective abundances in the CRS model mice.
Due to the complexity of depression, the
relationship between the Firmicutes/Bacteriodota ratio and
depression remains unclear. However, the oral administration of
probiotics or fecal microbiota transplantation (FMT) has been shown
to restore the microbial balance of the gut and alleviate dysbiosis
(52). For example, the
introduction of a probiotic mixture containing six
Bifidobacteria and Lactobacillus strains to patients
following CRC surgery lead to a significant reduction in the serum
levels of pro-inflammatory cytokines and improved the intestinal
microenvironment (53).
The findings of the present study suggest that gut
flora at least partially mediate the effects of Rh1 in mice with
CRS and CRC, supporting the notion that changes in the gut flora
may play a role in the pathophysiology of patients with CRC and
comorbid depression. This aligns with clinical evidence that
dysregulation of the gut microbiota contributes to CRC development,
metastasis and treatment resistance (54). In patients with CRC who have a
bacterial infection and require treatment with antibiotics,
probiotics supplementation or FMT from a healthy donor may
represent a supportive strategy (55). However, future research is
necessary to validate these findings and address current
limitations.
Immunological dysregulation contributes to the
development of depression and can hinder antidepressant treatment
outcomes. A study demonstrated that chronic stress increases the
proportions of tumor-associated macrophages and MDSCs in the TME,
while decreasing the activity of CD8+T cells. In
addition, the study revealed that splenic
CD11b+Gr-1intLy6Chi myeloid cells
induce tolerance in memory CD8+T cells (56). Notably, patients with major
depressive disorder exhibit altered numbers of monocytes,
CD4+ and CD8+ T cells in the peripheral
circulation compared with those in healthy individuals (57). The most potent antigen-presenting
cells are DCs, which are crucial components of the adaptive immune
response. During maturation, DCs increase the secretion of
inflammatory or anti-inflammatory cytokines and upregulate MHCII
expression, while mature DCs provide processed antigens to naïve T
cells in adjacent lymphatic organs (58). In the present study, a reduction in
the quantity of CD8+T and CD4+T cells
infiltrating the CRC tissue and producing IFN-γ and granzyme B was
observed, which was attenuated by Rh1 treatment. These findings
suggest that the functions of DC and T cells were suppressed in CRC
tumor-bearing mice subjected to CRS, and that such a repression was
mitigated by treatment with Rh1.
Depression and cancer progression have both been
associated with MDSC proliferation, which suppresses the immune
response (59). Consistent with
this, the CRS model mice in the present study displayed a
significantly elevated percentage of MDSCs than those in the
control group, whereas treatment with Rh1 reduced the frequency of
monocytic MDSCs.
Immune dysregulation and heightened inflammatory
responses are recognized as contributors to depression,
particularly in vulnerable individuals (60). Consistent with this,
anti-inflammatory treatments decrease depressive symptoms (61–63).
Elevated levels of the proinflammatory cytokines IL-6, IL-1β and
TNF-α have been reported in patients with depression (64); therefore, peripheral blood levels
of these cytokines have been proposed as indicators of depression
(65). In addition, chronically
stressed tumor-bearing animals also exhibit elevated levels of
TNF-α, IFN-γ and IL-6, which is consistent with the inflammatory
hypotheses of depression and may accelerate the development of
cancer (66). In the present
study, persistent CRS stress increased the release of IL-6, TNF-α
and CXCL1 into the serum of tumor-bearing mice, potentially
contributing to the progression of CRS. Notably, mice treated with
Rh1 had significantly reduced serum levels of these proinflammatory
cytokines compared with those in the model group, suggesting that
Rh1 exerts anti-inflammatory and antitumor effects under CRS
conditions.
The present study has several limitations. Firstly,
no electroencephalogram (EEG) activity data were collected to
detect if any changes in brain electrical activity associated with
depressive symptoms occurred. In future studies, active
collaboration with an institution equipped with EEG detection
facilities will be sought to investigate the
neuroelectrophysiological mechanisms of Rh1, with the aim of
obtaining more comprehensive evidence for its therapeutic effects.
Second, the 16S rRNA sequencing used to analyze gut microbiota has
a relatively low resolution and cannot distinguish closely related
species. The results revealed differences in the microbiota of
certain groups at the phylum level, as exemplified by the
abundances of Proteobacteria, Firmicutes, Bacteriodota and
Actinobacteriota. Some data obtained on bacterial genera displayed
non-normal distributions and heterogeneous variance. After using
appropriate statistical tests, namely Welch's ANOVA or
Kruskal-Wallis, non-significant differences in bacterial genera
were detected among groups for certain genera, including
Bifidobacterium, Romboutsia and Alistipes. In future
studies, the sample size will be expanded and metagenomic
sequencing performed to explore the effect of Rh1 on microorganisms
more deeply. Metagenomic sequencing can also be used to gain
whole-genome information and achieve species- or strain-level
resolution, with high sensitivity in the identification of
low-abundance species.
In conclusion, Rh1 shows promise as an adjunctive
therapy for CRC by reducing tumor growth, alleviating
depression-like behaviors, and restoring intestinal flora and
immunological homeostasis in CRC-xenografted mice under CRS. These
findings reveal a novel bioactivity of Rh1 derived from ginseng,
which has potential as a novel strategy to improve the outcomes of
patients with CRC and associated depressive symptoms. To obtain
direct evidence of its interaction with existing therapies, future
research should prioritize preclinical trials combining Rh1 with
chemotherapy, immunotherapy or targeted agents to evaluate their
potential effects on tumor growth and survival.
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Project of TCM Science and
Technology Development Plan of Jiangsu Province (grant nos.
ZD202415 and ZD202320), Nanjing Medical Institution Traditional
Chinese Medicine Preparation Research Project (grant no.
NJCC-ZJ-202426), Key Research Project of Jiangsu Provincial Health
Commission (grant no. K2023033), Jiangsu Clinical Innovation Center
of Digestive Cancer of Traditional Chinese Medicine (grant no.
2021.6), Jiangsu Provincial Association for Maternal and Child
Health Studies (grant no. JSFY202202), Traditional Chinese Medicine
Clinical Transformation of Public Service Platform in Jiangsu
Province (grant no. BM2023005), Postgraduate Research &
Practice Innovation Program of Jiangsu Province (grant no.
SJCX24_0881) and Post-subsidy Project for High-Quality Research
Achievements of Jiangsu Province Academy of Traditional Chinese
Medicine (grant nos. HBZ202501 and HBZ202405).
Availability of data and materials
The high-throughput 16S rRNA sequencing data
generated in the present study may be found in the NCBI Sequence
Read Archive under the accession number PRJNA1288220 and may be
accessed using the following URL: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1288220.
All other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
TD, SS and SL conceived and designed the
experiments. TD, LL and JS performed the experiments. TD, LL and JL
revised the manuscript. JL, TD, XT, XW and YL analyzed and
interpreted the data. LL wrote the manuscript. All authors read and
approved the final version of the manuscript. TD and SS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All experimental procedures were authorized by the
Institutional Animal Care and Use Committee of Jiangsu Province
Academy of Traditional Chinese Medicine (approval nos.
AEWC-20230902-327 and AEWC-20240301-391). Jiangsu Province Academy
of Traditional Chinese Medicine is an alternative name for Nanjing
Integrated Traditional Chinese and Western Medicine Hospital
Affiliated with Nanjing University of Chinese Medicine. All animal
experiments were performed in compliance with the National
Institutes of Health Guide for the Care and Use of Laboratory
animals (NIH Publication no. 8023, amended 1978) and described in
compliance with the ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salvucci M, Crawford N, Stott K, Bullman
S, Longley DB and Prehn JHM: Patients with mesenchymal tumours and
high Fusobacteriales prevalence have worse prognosis in colorectal
cancer (CRC). Gut. 71:1600–1612. 2022.PubMed/NCBI
|
|
2
|
Zhao N, Lai C, Wang Y, Dai S and Gu H:
Understanding the role of DNA methylation in colorectal cancer:
Mechanisms, detection, and clinical significance. Biochim Biophys
Acta Rev Cancer. 1879:1890962024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng Z, Hou X, Bian Z, Jia W and Zhao L:
Gut microbiota and colorectal cancer metastasis. Cancer Lett.
555:2160392023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J and Liao ZX: Research progress of
microrobots in tumor drug delivery. Food Med Homol. 1:94200252024.
View Article : Google Scholar
|
|
5
|
Ye L, Hou Y, Hu W, Wang H, Yang R, Zhang
Q, Feng Q, Zheng X, Yao G and Hao H: Repressed Blautia-acetate
immunological axis underlies breast cancer progression promoted by
chronic stress. Nat Commun. 14:61602023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang S, Li Y, Zhang Y and Wang Y: Impact
of chronic stress on intestinal mucosal immunity in colorectal
cancer progression. Cytokine Growth Factor Rev. 80:24–36. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otun S, Achilonu I and Odero-Marah V:
Unveiling the potential of Muscadine grape Skin extract as an
innovative therapeutic intervention in cancer treatment. J Funct
Foods. 116:1061462024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urakami H, Yoshikawa S, Nagao K, Miyake K,
Fujita Y, Komura A, Nakashima M, Umene R, Sano S, Hu Z, et al:
Stress-experienced monocytes/macrophages lose anti-inflammatory
function via β2-adrenergic receptor in skin allergic inflammation.
J Allergy Clin Immunol. 155:865–879. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Globig AM, Zhao S, Roginsky J, Maltez VI,
Guiza J, Avina-Ochoa N, Heeg M, Araujo Hoffmann F, Chaudhary O,
Wang J, et al: The β1-adrenergic receptor links sympathetic nerves
to T cell exhaustion. Nature. 622:383–392. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Yu F, Che A, Tan B, Huang C, Chen
Y, Liu X, Huang Q, Zhang W, Ma C, et al: Neuroendocrine regulation
of stress-induced T cell dysfunction during lung cancer
immunosurveillance via the kisspeptin/GPR54 signaling pathway. Adv
Sci (Weinh). 9:e21041322022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XH, Fu YL, Xu YN, Zhang PC, Zheng TX,
Ling CQ and Feng YL: Ginsenoside Rh1 regulates the immune
microenvironment of hepatocellular carcinoma via the glucocorticoid
receptor. J Integr Med. 22:709–718. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sommershof A, Scheuermann L, Koerner J and
Groettrup M: Chronic stress suppresses anti-tumor T(CD8+) responses
and tumor regression following cancer immunotherapy in a mouse
model of melanoma. Brain Behav Immun. 65:140–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou M, Fan Y, Xu L, Yu Z, Wang S, Xu H,
Zhang J, Zhang L, Liu W, Wu L, et al: Microbiome and tryptophan
metabolomics analysis in adolescent depression: Roles of the gut
microbiota in the regulation of tryptophan-derived
neurotransmitters and behaviors in human and mice. Microbiome.
11:1452023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou Y, Wang S, Zhang H, Gu Y, Chen H,
Huang Z, Yang F, Li W, Chen C, Men L, et al: The triangular
relationship between traditional Chinese medicines, intestinal
flora, and colorectal cancer. Med Res Rev. 44:539–567. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng W, Shen P, Yu C, Tang Y, Qian C,
Yang C, Gao M, Wu Y, Yu S, Tang W, et al: Ginsenoside Rh1, a novel
casein kinase II subunit alpha (CK2α) inhibitor, retards metastasis
via disrupting HHEX/CCL20 signaling cascade involved in tumor cell
extravasation across endothelial barrier. Pharmacol Res.
198:1069862023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou J, Xue J, Lee M, Yu J and Sung C:
Long-term administration of ginsenoside Rh1 enhances learning and
memory by promoting cell survival in the mouse hippocampus. Int J
Mol Med. 33:234–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Gao X, Yang C, Liang Z, Guan D,
Yuan T, Qi W, Zhao D, Li X, Dong H and Zhang H: Structural
characters and pharmacological activity of protopanaxadiol-type
saponins and protopanaxatriol-type saponins from ginseng. Adv
Pharmacol Pharm Sci. 2024:90967742024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Yang Y, Yang S, Ren S, Feng J, Liu
Y, Chen H and Chen N: Ginsenoside Rg1 ameliorates neuroinflammation
via suppression of connexin43 ubiquitination to attenuate
depression. Front Pharmacol. 12:7090192021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han D, Zhao Z, Mao T, Gao M, Yang X and
Gao Y: Ginsenoside Rg1: A neuroprotective natural dammarane-type
triterpenoid saponin with anti-depressive properties. CNS Neurosci
Ther. 30:e701502024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YZ, Chen J, Chu SF, Wang YS, Wang XY,
Chen NH and Zhang JT: Improvement of memory in mice and increase of
hippocampal excitability in rats by ginsenoside Rg1′s metabolites
ginsenoside Rh1 and protopanaxatriol. J Pharmacol Sci. 109:504–510.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nahar J, Boopathi V, Murugesan M, Rupa EJ,
Yang DC, Kang SC and Mathiyalagan R: Investigating the anticancer
activity of G-Rh1 using in silico and in vitro studies (A549 Lung
Cancer Cells). Molecules. 27:83112022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lyu X, Xu X, Song A, Guo J and Zhang Y and
Zhang Y: Ginsenoside Rh1 inhibits colorectal cancer cell migration
and invasion in vitro and tumor growth in vivo. Oncol Lett.
18:4160–4166. 2019.PubMed/NCBI
|
|
23
|
Jin Y, Huynh DTN and Heo KS: Ginsenoside
Rh1 inhibits tumor growth in MDA-MB-231 breast cancer cells via
mitochondrial ROS and ER stress-mediated signaling pathway. Arch
Pharm Res. 45:174–184. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Y, Hu Y, Yang Y, Yan R, Zheng L, Fu
X, Xiao C and You F: Tong-Xie-Yao-Fang promotes dendritic cells
maturation and retards tumor growth in colorectal cancer mice with
chronic restraint stress. J Ethnopharmacol. 319((Pt 1)):
1170692024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olescowicz G, Neis VB, Fraga DB, Rosa PB,
Azevedo DP, Melleu FF, Brocardo PS, Gil-Mohapel J and Rodrigues
ALS: Antidepressant and pro-neurogenic effects of agmatine in a
mouse model of stress induced by chronic exposure to
corticosterone. Prog Neuropsychopharmacol Biol Psychiatry.
81:395–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Liu L, Yang X, Gao H, Tang QK, Yin
LY, Yin XY, Hao JR, Geng DQ and Gao C: Ketamine improved
depressive-like behaviors via hippocampal glucocorticoid receptor
in chronic stress induced-susceptible mice. Behav Brain Res.
364:75–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu X, Shetty AK and Reddy DS: Long-term
changes in neuroimaging markers, cognitive function and psychiatric
symptoms in an experimental model of Gulf War Illness. Life Sci.
285:1199712021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Liu Y, Li R, Teng Y, Zhao S, Chen
J, Li C, Hu X and Sun L: Identification of critical signature in
post-traumatic stress disorder using bioinformatics analysis and in
vitro analyses. Brain Behav. 15:e702432025. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Zhang T, Yu L, Huang S, Yang Y, Yu
S, Li J, Cao Y, Wei Z, Li X, et al: Zhile capsule exerts
antidepressant-like effects through upregulation of the BDNF
signaling pathway and neuroprotection. Int J Mol Sci. 20:1952019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sui H, Zhang L, Gu K, Chai N, Ji Q, Zhou
L, Wang Y, Ren J, Yang L, Zhang B, et al: YYFZBJS ameliorates
colorectal cancer progression in Apc(Min/+) mice by remodeling gut
microbiota and inhibiting regulatory T-cell generation. Cell Commun
Signal. 18:1132020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simpson EH: Measurement of diversity.
Nature. 163:6881949. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chao A and Lee SM: Estimating the number
of classes via sample coverage. J Am Stat Assoc. 87:210–217. 1992.
View Article : Google Scholar
|
|
33
|
Shannon CE: Shannon CE. A mathematical
theory of communication. Bell System Technical Journal. 27:379–423.
1948. View Article : Google Scholar
|
|
34
|
Ren Q, He C, Sun Y, Gao X, Zhou Y, Qin T,
Zhang Z, Wang X, Wang J, Wei S and Wang F: Asiaticoside improves
depressive-like behavior in mice with chronic unpredictable mild
stress through modulation of the gut microbiota. Front Pharmacol.
15:14618732024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao L, Zhu X, Ni Y, You J and Li A:
Xiaoyaosan, a traditional Chinese medicine, inhibits the chronic
restraint stress-induced liver metastasis of colon cancer in vivo.
Pharm Biol. 58:1085–1091. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan X, Shi L, Zhu X, Zhao Y, Luo J, Li Q,
Xu Z and Zhao J: From microbial homeostasis to systemic
pathogenesis: A narrative review on gut flora's role in
neuropsychiatric, metabolic, and cancer disorders. J Inflamm Res.
18:8851–8873. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chu Y, Qian L, Ke Y, Feng X, Chen X, Liu
F, Yu L, Zhang L, Tao Y, Xu R, et al: Lymph node-targeted
neoantigen nanovaccines potentiate anti-tumor immune responses of
post-surgical melanoma. J Nanobiotechnology. 20:1902022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hao Y, Ge H, Sun M and Gao Y: Selecting an
appropriate animal model of depression. Int J Mol Sci. 20:48272019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding H, Lin J, Xu Z, Wang HHX, Huang L,
Huang J and Wong MCS: The association between organised colorectal
cancer screening strategies and reduction of its related mortality:
A systematic review and meta-analysis. BMC Cancer. 24:3652024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He L, Tian Y, Liu Q, Bao J and Ding RB:
Antidepressant sertraline synergistically enhances paclitaxel
efficacy by inducing autophagy in colorectal cancer cells.
Molecules. 29:37332024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huynh DTN, Jin Y, Myung CS and Heo KS:
Ginsenoside Rh1 induces MCF-7 cell apoptosis and autophagic cell
death through ROS-Mediated Akt signaling. Cancers (Basel).
13:18922021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mathiyalagan R, Wang C, Kim YJ,
Castro-Aceituno V, Ahn S, Subramaniyam S, Simu SY, Jiménez-Pérez
ZE, Yang DC and Jung SK: Preparation of polyethylene
glycol-ginsenoside Rh1 and Rh2 conjugates and their efficacy
against lung cancer and inflammation. Molecules. 24:43672019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo Y, Xie J, Zhang L, Yang L, Ma J, Bai
Y, Ma W, Wang L, Yu H, Zeng Y, et al: Ginsenoside Rb1 exerts
antidepressant-like effects via suppression inflammation and
activation of AKT pathway. Neurosci Lett. 744:1355612021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li J, Gao W, Zhao Z, Li Y, Yang L, Wei W,
Ren F, Li Y, Yu Y, Duan W, et al: Ginsenoside Rg1 reduced
microglial activation and mitochondrial dysfunction to alleviate
depression-like behaviour via the GAS5/EZH2/SOCS3/NRF2 axis. Mol
Neurobiol. 59:2855–2873. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang L, Zhang QQ, Xu YY, Zhang R, Zhao Q,
Zhang YQ, Huang XH, Jiang B and Ni M: Ginsenoside Rb1 Suppresses
AOM/DSS-induced colon carcinogenesis. Anticancer Agents Med Chem.
23:1067–1073. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu R, Zhang B, Zou S, Cui L, Lin L and Li
L: Ginsenoside Rg1 induces autophagy in colorectal cancer through
inhibition of the Akt/mTOR/p70S6K pathway. J Microbiol Biotechnol.
34:774–782. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang Z, Wu X, Shen J, Gudamu A, Ma Y,
Zhang Z and Hou M: Ginsenoside Rh1 regulates gastric cancer cell
biological behaviours and transplanted tumour growth in nude mice
via the TGF-β/Smad pathway. Clin Exp Pharmacol Physiol.
49:1270–1280. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Z, Shao S, Zhang Y, Jia R, Hu X, Liu
H, Sun M, Zhang B, Li Q and Wang Y: Xiaoyaosan slows cancer
progression and ameliorates gut dysbiosis in mice with chronic
restraint stress and colorectal cancer xenografts. Biomed
Pharmacother. 132:1109162020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gao Y: Inflammation and gut microbiota in
the alcoholic liver disease. Food Med Homol. 1:94200202024.
View Article : Google Scholar
|
|
50
|
Wang J, Fan L, Teng T, Wu H, Liu X, Yin B,
Li X, Jiang Y, Zhao J, Wu Q, et al: Adolescent male rats show
altered gut microbiota composition associated with depressive-like
behavior after chronic unpredictable mild stress: Differences from
adult rats. J Psychiatr Res. 173:183–191. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shao S, Jia R, Zhao L, Zhang Y, Guan Y,
Wen H, Liu J, Zhao Y, Feng Y, Zhang Z, et al: Xiao-Chai-Hu-Tang
ameliorates tumor growth in cancer comorbid depressive symptoms via
modulating gut microbiota-mediated TLR4/MyD88/NF-κB signaling
pathway. Phytomedicine. 88:1536062021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu JY, Liu MT, Tao T, Zhu X and Fei FQ:
The role of gut microbiota in tumorigenesis and treatment. Biomed
Pharmacother. 138:1114442021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zaharuddin L, Mokhtar NM, Muhammad Nawawi
KN and Raja Ali RA: A randomized double-blind placebo-controlled
trial of probiotics in post-surgical colorectal cancer. BMC
Gastroenterol. 19:1312019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Piccinno G, Thompson KN, Manghi P, Ghazi
AR, Thomas AM, Blanco-Míguez A, Asnicar F, Mladenovic K, Pinto F,
Armanini F, et al: Pooled analysis of 3,741 stool metagenomes from
18 cohorts for cross-stage and strain-level reproducible microbial
biomarkers of colorectal cancer. Nat Med. 31:2416–2429. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jamal R, Messaoudene M, de Figuieredo M
and Routy B: Future indications and clinical management for fecal
microbiota transplantation (FMT) in immuno-oncology. Semin Immunol.
67:1017542023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ugel S, Peranzoni E, Desantis G, Chioda M,
Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S and
Bronte V: Immune tolerance to tumor antigens occurs in a
specialized environment of the spleen. Cell Rep. 2:628–639. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Foley ÉM, Parkinson JT, Mitchell RE,
Turner L and Khandaker GM: Peripheral blood cellular
immunophenotype in depression: A systematic review and
meta-analysis. Mol Psychiatry. 28:1004–1019. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shi H, He J, Li X, Han J, Wu R, Wang D,
Yang F and Sun E: Isorhamnetin, the active constituent of a Chinese
herb Hippophae rhamnoides L, is a potent suppressor of
dendritic-cell maturation and trafficking. Int Immunopharmacol.
55:216–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lu YT, Li J, Qi X, Pei YX, Shi WG and Lin
HS: Effects of Shugan Jianpi Formula () on myeloid-derived
suppression cells-mediated depression breast cancer mice. Chin J
Integr Med. 23:453–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bai Y, Cai Y, Chang D, Li D, Huo X and Zhu
T: Immunotherapy for depression: Recent insights and future
targets. Pharmacol Ther. 257:1086242024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Du Y, Dou Y, Wang M, Wang Y, Yan Y, Fan H,
Fan N, Yang X and Ma X: Efficacy and acceptability of
anti-inflammatory agents in major depressive disorder: A systematic
review and meta-analysis. Front Psychiatry. 15:14075292024.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Baghdadi LR: Tocilizumab reduces
depression risk in rheumatoid arthritis patients: A systematic
review and meta-analysis. Psychol Res Behav Manag. 17:3419–3441.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Iyengar RL, Gandhi S, Aneja A, Thorpe K,
Razzouk L, Greenberg J, Mosovich S and Farkouh ME: NSAIDs are
associated with lower depression scores in patients with
osteoarthritis. Am J Med. 126:1017.e11–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Drago A, Crisafulli C, Calabrò M and
Serretti A: Enrichment pathway analysis. The inflammatory genetic
background in Bipolar Disorder. J Affect Disord. 179:88–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Argue BMR, Casten LG, McCool S, Alrfooh A,
Richards JG, Wemmie JA, Magnotta VA, Williams AJ, Michaelson J,
Fiedorowicz JG, et al: Immune dysregulation in bipolar disorder. J
Affect Disord. 374:587–597. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou
M, Li X, Li Y, Xiong W, Li G, et al: Chronic stress promotes cancer
development. Front Oncol. 10:14922020. View Article : Google Scholar : PubMed/NCBI
|